Abstract
Estrogens suppress feeding in part by enhancing the response to satiation signals. Glucagon-like peptide 1 (GLP-1) acts on receptor populations both peripherally and centrally to affect food intake. We hypothesized that modulation of the central GLP-1 system is one of the mechanisms underlying the effects of estrogens on feeding. We assessed the anorexic effect of 0, 1, and 10 μg doses of GLP-1 administered into the lateral ventricle of bilaterally ovariectomized (OVX) female rats on a cyclic regimen of either 2 μg β-estradiol-3-benzoate (EB) or oil vehicle 30 min prior to dark onset on the day following hormone treatment. Central GLP-1 treatment significantly suppressed food intake in EB-treated rats at both doses compared to vehicle, whereas only the 10 μg GLP-1 dose was effective in oil-treated rats. To follow up, we examined whether physiologic-dose cyclic estradiol treatment influences GLP-1-induced c-Fos in feeding-relevant brain areas of OVX females. GLP-1 significantly increased c-Fos expression in the area postrema (AP) and nucleus of the solitary tract (NTS), and the presence of estrogens may be required for this effect in the paraventricular nucleus of the hypothalamus (PVN). Together, these data suggest that modulation of the central GLP-1 system may be one of the mechanisms by which estrogens suppress food intake, and highlight the PVN as a region of interest for future investigation.
Keywords: GLP-1, estrogens, food intake, meal pattern, c-Fos
INTRODUCTION
Sex differences in food intake have been reported in the literature as far back as the early twentieth century (Wang, 1925). In intact cycling female rats, the peri-ovulatory rise in estradiol secretion is followed by a reduction in food intake that extends throughout the subsequent dark phase, when the rat is in estrus (Blaustein and Wade, 1976). Elimination of endogenous estrogens by ovariectomy results in an increase in food intake and body weight compared to intact rats (McElroy and Wade, 1987), whereas cyclic estradiol treatment after ovariectomy attenuates these increases (Asarian and Geary, 2002). Estradiol, a common estrogen produced by the ovaries, acts to selectively suppress meal size through interactions with factors that directly promote satiation during a meal (Eckel, 2004).
Research on interactions between estrogens and factors that influence meal size has been limited, but a few candidates have emerged. Of these interactions, the most is known about cholecystokinin (CCK) and 5-hydroxytryptamine (5-HT) (Asarian and Geary, 1999; Eckel et al., 2002; Eckel and Geary, 1999; Geary and Asarian, 1999; Rivera et al., 2012; Rivera and Eckel, 2005; Tecott et al., 1995). These findings suggest that modulation of central mechanisms controlling food intake may underlie estradiol-induced hypophagia in females. Here, we focused on the potential interaction between estrogens and glucagon-like peptide 1 (GLP-1), which acts both peripherally as a hormone and centrally as a neurotransmitter to affect food intake, body weight, and glucose homeostasis (Larsen and Holst, 2005).
Peripherally, GLP-1 is an incretin hormone secreted by L cells in the distal intestine, where it acts on nearby receptors to promote satiation and satiety, thus reducing food intake (Williams et al., 2009). Centrally, GLP-1 is produced by neurons in the caudal NTS, which project throughout the brain to several feeding-relevant regions and are activated by nutrient ingestion, gastric distension, CCK, and vagal afferent stimulation (Larsen et al., 1997; Rinaman, 2010). Pharmacologic studies support the role of central GLP-1 in the control of food intake. Stimulation of GLP-1 receptors (GLP-1R) in the brain suppresses food intake, whereas blockade of the receptor with GLP-1R antagonist Exendin-(9–39) (Ex9) or NTS GLP-1R mRNA knockdown increases food intake, revealing the endogenous action of central GLP-1 (Barrera et al., 2011; Turton et al., 1996). Given that GLP-1 in the brain is a central mediator of incoming satiation and satiety signals from nutrient presence in the gut, we asked whether estradiol might interact with the central GLP-1 system to influence feeding.
Recent findings suggest that estradiol may interact with the GLP-1 system to influence feeding. The satiating potency of endogenous peripheral GLP-1 was tested in OVX female rats maintained on a cyclic regimen of either estradiol or sesame oil, that had also undergone Roux-en-Y gastric bypass (RYGB) surgery or sham bypass surgery (Asarian et al., 2012). RYGB is a highly effective weight loss surgery, after which food intake is strongly suppressed. Clinical and basic research studies report that one of the consequences of RYGB is an increase in intestinal GLP-1 release (Morínigo et al., 2006; Pournaras et al., 2012). Current speculation in the field is that this elevation in GLP-1 is at least partially responsible for the RYGB-induced suppression of food intake. Interestingly, there was no effect of bypass surgery on GLP-1-mediated satiation, yet there was a significant effect of estradiol treatment. In both RYGB and sham bypass rats, the effect of peripheral GLP-1R blockade on Ensure intake in a 1-h feeding test was greater in estradiol-treated rats compared to oil-treated rats, suggesting an interaction between estradiol and the endogenous peripheral GLP-1 system in the control of food intake. In another recent study, Finan and colleagues found that in gonadally intact male and female mice, an estradiol/GLP-1 conjugate molecule was more effective at suppressing food intake and body weight than both GLP-1 in its native form and a dissociated conjugate mixture of estradiol and GLP-1 (2012). Furthermore, new evidence has emerged suggesting that estrogens may be involved in the effect of central GLP-1R activation on food-motivated behavior (Richard et al., 2016). However, given that these studies were not designed to control for estrous cycle stage when testing females, and utilized the GLP-1R agonist Ex4, rather than GLP-1 itself, the conclusions that can be drawn regarding an interaction between estrogens and GLP-1 are limited. Taken together, these studies suggest that estrogens may potentiate the feeding effects of GLP-1, and here we assess this hypothesis in a more physiologic context.
To evaluate the potential for the GLP-1 system to mediate EB-induced hypophagia, we examined the effect of physiologic-dose cyclic estradiol treatment on sensitivity to the anorexic effect of central GLP-1 in an OVX female rat model. Additionally, we assessed whether cyclic estradiol treatment modulates GLP-1-induced c-Fos in several feeding-relevant brain areas. Our data indicate that modulation of the central GLP-1 system may be one of the mechanisms underlying the effect of estrogens on food intake and highlight the paraventricular nucleus (PVN) as a potential contributor to this effect.
METHODS
Subjects
Naïve female Wistar rats weighing 175–200 g (Harlan, Indianapolis, IN) were single-housed in a temperature-controlled vivarium on a 12 h light: 12 h dark cycle in plastic chambers fitted with the Research Diets BioDaq continuous food intake monitoring system (Experiment 1) or standard plastic cages (Experiment 2). Rats had ad libitum access to distilled water and rat chow (Purina 5001) except where otherwise noted. Animals were handled daily and underwent habituation to experimental procedures prior to study onset. All experimental procedures were approved by the Florida State University Institutional Animal Care and Use Committee and conformed to the standard of the Guide for the Care and Use of Laboratory Animals (National Research Council, 2011).
Surgery
Under 2–4% isoflurane delivered at a rate of 1 L/min, rats underwent bilateral ovariectomy using an intra-abdominal approach, and were implanted with unilateral 26G guide cannulas (Plastics One, Roanoke, VA) targeting the lateral ventricle (LV). Coordinates for the LV were: 1.5 mm lateral to midline, 0.9 mm posterior to bregma, and 2.27 mm ventral to skull surface. Injectors extending 2.5 mm below the end of the cannula guide were used. Carprofen (10 mg/kg sc) (Butler Schein Animal Health Supply, Dublin, OH) was administered with the onset of surgery and for the first two days of post-operative recovery. Cannula placements were verified prior to experiment onset via successful induction of water intake (greater than 5 ml ingested during a 45 min test) induced by 40 μg Angiotensin II (Sigma-Aldrich, St. Louis, MO) administered intra-LV.
Drugs and Injections
β-estradiol-3-benzoate (EB) (Sigma-Aldrich, St. Louis, MO) was dissolved in sesame oil (Sigma-Aldrich, St. Louis, MO). One week following surgery, rats were separated into weight-matched groups and placed on a cyclic regimen of either 2 μg β-estradiol-3-benzoate (EB) delivered in 0.1 ml sesame oil or oil vehicle alone. This dose of EB was chosen because it has been shown to produce plasma estradiol levels comparable to that which is achieved physiologically during proestrus in intact cycling rats (Geary and Asarian, 1999). Furthermore, this dose has been shown to induce a transient decrease in food intake, similar to the decrease observed on estrus in cycling rats (Asarian and Geary, 2002). Angiotensin II (Sigma-Aldrich, St. Louis, MO), and GLP-1 (American Peptide, California, USA) were each dissolved in sterile 0.9% saline. Intra-LV injections were administered using a 10-μl syringe (Hamilton, Reno, NV) connected to a 33-G injector extending 2.5 mm past the guide cannula (Plastics One, Roanoke, VA) via Tygon tubing (VWR, Radnor, PA). Injections were delivered at a rate of 1.0 μl/min to the LV, and injections were 2.0 μl in volume.
Experiment 1: Feeding effects of central GLP-1
To assess EB’s ability to enhance the anorexic effect of central GLP-1, we conducted feeding tests the day after rats received their weekly estradiol or oil injection (Figure 1A). This allowed us to examine EB’s effect on GLP-1 sensitivity at a time during which EB-induced suppression of food intake is expressed. The weekly EB treatment timing was selected to accommodate the scheduling requirements of the experimenters, and has been previously shown to be an effective regimen (Asarian and Geary, 1999; Geary et al., 1994; Geary and Asarian, 1999; Santollo and Eckel, 2008). From days 1–4, this regimen mimics the changes in plasma estradiol levels across the ovarian cycle in gonadally intact females (Asarian & Geary, 2002). That is, estradiol levels are low, then peak on the day of EB treatment, and rapidly fall the subsequent day, which models estrus (Becker et al., 2005).
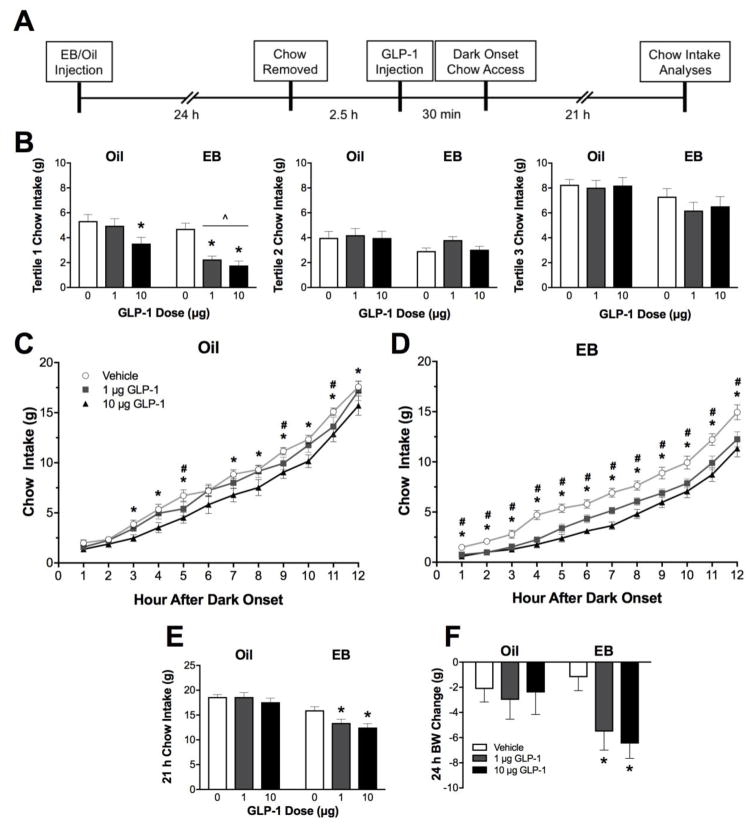
Figure 1. Food intake effects of central GLP-1.
A. Experimental timeline. B. Non-cumulative food intake. During the first tertile of the dark phase, EB-treated rats show greater suppression of chow intake relative to oil-treated rats following both 1 μg and 10 μg GLP-1 treatment. EB and GLP-1 treatments did not significantly influence chow intake during the second and third tertiles of the dark phase. C. In oil-treated rats, only the 10 μg dose of GLP-1 suppressed cumulative food intake throughout the 12 h dark phase. D. In EB-treated rats, both doses of GLP-1 significantly suppressed chow intake throughout the 12 h dark phase. E. 21 h chow intake is significantly suppressed by GLP-1 treatment in only EB-treated rats. F. Body weight change measured the day after vehicle or GLP-1 treatment. Data are means ± SEM. For C and D, #p<0.05 vehicle vs. 1 μg GLP-1 and *p<0.05 vehicle vs. 10 μg GLP-1 within respective hormone treatment groups. For B, E, and F, ^p<0.05 relative to respective oil-treated group GLP-1 condition and *p<0.05 relative to respective hormone group vehicle condition.
A within-subjects counterbalanced design was used to assess the feeding response to intra-LV GLP-1 in oil- and EB-treated rats (n = 10 oil & 12 EB). On test days, food was removed from the rats’ cages 3 h before dark onset. Thirty min before dark onset, rats received an intra-LV injection of 0, 1, or 10 μg of GLP-1 in 2 μl of saline. These doses are supra-threshold for an effect when administered into the LV of male rats, with the 10 μg significantly suppressing intake and the 1 μg dose being near the threshold for significant effect (Kinzig et al., 2002). Food was returned to the rats’ cages immediately before dark onset, and food intake was measured continuously by the BioDaq system for the next 21 h.
Analysis of meal pattern was performed using BioDaq Data Viewer Software (Research Diets, New Brunswick, NJ). A meal was defined as any feeding episode measuring at least 0.25 g separated from subsequent intake by at least 15 min (Eckel et al., 2000, 1998; Eckel and Geary, 1999). Rats ate very little chow during the light phase, therefore, we only analyzed dark phase meal patterns. From this meal pattern analysis, we obtained data on the following meal pattern variables: latency to begin the first meal, meal size, number of meals, meal duration, and inter-meal intervals (IMI). Latency to begin the first meal was defined as the number of min between the time when food access resumed and the start of the first meal, and inter-meal interval was defined as the time (sec) between the end of one meal and the onset of the subsequent meal. To examine intake over time, we analyzed hourly cumulative intake throughout the 12 h dark phase, as well as non-cumulative intake during each 4-h tertile of the dark phase.
Throughout the experiment, body weights and food intake were measured daily. Drug treatments were separated by seven days.
Experiment 2: GLP-1-induced c-Fos
OVX female rats (n = 4–5 per group) with cannulas targeting the LV were maintained on a cyclic regimen of either 2 μg EB or oil, administered subcutaneously in 0.1 ml volumes for 5–6 cycles. The day following the final EB or oil injection, access to food was removed mid-light phase and 2 h later rats received an intra-LV injection of 0, 1, or 10 μg of GLP-1 in 2 μl of saline. Ninety min post-injection, rats were deeply anesthetized (5% isoflurane in 1L oxygen/minute) and transcardially perfused with 10 mM PBS and 4% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA). Brains were removed, cryoprotected in 30% sucrose, and frozen in 2-methylbutane on dry ice.
Coronal sections (20 μm) through the caudal brainstem and hypothalamus were slide-mounted and stored at −80° C. Anatomically matched sections from each rat that included the AP and caudal NTS in the brainstem, and the PVN in the forebrain were selected for c-Fos staining. For each subject, the AP was represented by ~9 sections approximately 13.7 to 14.3 mm posterior to bregma, and the NTS and PVN were represented by ~15 sections approximately 13.2 to 14.4 mm and 1.1 to 2.0 mm posterior to bregma, respectively. Slide-mounted sections were then washed with 10 mM phosphate buffered saline (PBS) at room temperature, and incubated with a blocking buffer of 5% normal donkey serum in 1% bovine serum albumin (BSA) in 10 mM PBS (1% BSA-PBS) for 1 h. After additional PBS washes, the slides incubated overnight at 4°C with rabbit anti-c-Fos primary antibody (Millipore, Temecula, CA; catalog # ABE457) at 1:3,000 in 1% BSA-PBS. Slides were then washed in 10 mM PBS, followed by a 1-h incubation at room temperature with donkey anti-rabbit IgG-Cy3 antibody (Jackson ImmunoResearch, West Grove, PA; catalog # 711-165-152) used at a 1:1,000 dilution in 1% BSA-PBS. Slides were washed in 10 mM PBS, then coverslipped using Aqua Polymount (Polysciences, Inc., Warrington, PA) mounting media.
Slides were examined using an Olympus BX41 epifluorescence microscope, and digital images of each area were captured with a Retiga EXI Aqua camera and Q-Capture software (Hunt Optics, Pittsburgh, PA). Adobe Photoshop CS4 (Adobe, San Jose, CA) was used to invert the images and adjust contrast/brightness where necessary. ImageJ (NIH, Bethesda, MD) was used to quantify c-Fos-like immunoreactivity bilaterally in the brain regions described above. Threshold criteria based on optical density, object shape, and object size were used to identify c-Fos-positive cells within the boundaries of each region as defined by Paxinos and Watson’s rat brain atlas (2006). We then calculated the average number of c-Fos-positive cell nuclei per section across all sections taken from each brain region.
Statistical Analysis
Data are reported as mean ± SEM. Three-way mixed-design ANOVA was used to assess effects of EB treatment group, GLP-1 dose, and time (hour) on non-cumulative food intake during the 12-h dark cycle after intra-LV injections. Two-way mixed-design ANOVA was used to analyze the effects of hormone treatment (EB) and drug treatment (GLP-1) on food intake at single timepoints, meal patterns, and body weight. For assessment of food intake and meal pattern variables, we split the 12-h dark phase into tertiles, based on previous data showing that the effects of EB on feeding are most robust early in the dark phase (Eckel et al., 2000). This was also supported by our analysis of cumulative food intake (see below). One-way repeated measures ANOVA were conducted as planned comparisons to assess our hypothesis that the effect of GLP-1 treatment is enhanced in EB-treated rats. Two-way ANOVA was used to analyze the effects of EB and GLP-1 on c-Fos induction. Holm-Bonferroni tests were conducted as pairwise planned comparisons to investigate differences between two conditions following significant (p < 0.05) main or interactive ANOVA effects, within- or between-subjects as appropriate. P-values of < 0.05 were considered significant. Cohen’s d and Eta squared (η2) effect size estimates were calculated for pairwise comparisons and ANOVA, respectively. The convention for classifying effect sizes based on Cohen’s d is: small ≥ 0.20, medium ≥ 0.50, and large ≥ 0.80, and effect sizes based on η2 are classified as: small ≥ 0.01, medium ≥ 0.06, and large ≥ 0.14 (Cohen, 1988).
For experiment 1, analysis of average meal size and duration across the first 4 h of the dark phase excluded subjects that failed to consume a meal during this period in any condition (1 rat excluded from EB group). Analysis of IMI across the first 4 h of the dark phase only included subjects that consumed 2 or more meals during this time, as that is the minimum meal requirement to obtain an IMI (1 rat excluded from oil group, 5 rats excluded from EB group).
RESULTS
Experiment 1: Feeding effects of central GLP-1
Non-cumulative Food Intake
Three-way ANOVA yielded significant main effects of EB [F(1,20) = 17.47, p < 0.001, η2 = 0.47], GLP-1 [F(2,40) = 11.35, p < 0.001, η2 = 0.01], and time [F(11,220) = 20.11, p < 0.001, η2 = 0.24]. There was also a significant GLP-1 x time interaction [F(22,440) = 1.71, p < 0.05, η2 = 0.04]. Because it is already known that EB affects feeding primarily in the early hours of the dark phase (Eckel et al., 2000), we used two-way ANOVA to examine hormone and drug effects in detail at individual time points, splitting the dark phase into thirds.
Chow intake during the first tertile of the dark phase (0 – 4 h) was influenced by both EB [F(1,20) = 13.27, p < 0.01, η2 = 0.40] and GLP-1 treatments [F(2,40) = 21.54, p < 0.001, η2 = 0.09]. In addition, there was a significant interaction between EB and GLP-1 to suppress chow intake [F(2,40) = 4.08, p < 0.05, η2 = 0.09]. Planned comparisons showed that there was no significant difference in intakes between oil- and EB-treated rats following vehicle treatment. However, GLP-1 treatment induced greater suppression of food intake during the first tertile of the dark phase in EB- versus oil-treated rats following both the 1 μg and 10 μg dose of GLP-1 [p’s < 0.01, d’s = −1.94 (1 μg) and −1.29 (10 μg)] (Figure 1B). Planned pairwise comparisons showed that in the oil-treated rats, 10 μg GLP-1 treatment significantly reduced intake relative to vehicle [p < 0.01, d = 1.12], whereas both 1 μg and 10 μg GLP-1 significantly reduced chow intake in the EB-treated rats during this time [p < 0.001, d’s = −1.87 (1 μg) and −2.1 (10 μg)] (Figure 1B).
Chow intake in the second tertile of the dark phase (4 – 8 h) was significantly influenced by EB treatment [F(2,22) = 4.397, p < 0.05, η2 = 0.18]. Pairwise planned comparisons between oil- and EB-treated rats after intra-LV vehicle and 10 μg GLP-1 revealed a trend towards decreased chow intake in EB-treated rats during this time, however, these differences failed to reach statistical significance (Figure 1B). There were no main effects of or interactions between EB or GLP-1 treatment on chow intake during the third tertile of the dark phase (8 – 12 h; Figure 1B) or the subsequent light phase (12 – 21 h).
Cumulative Food Intake
On test days, two-way mixed-design ANOVA revealed a main effect of EB treatment to suppress chow intake at each hourly timepoint throughout the 12 h dark phase [12 h: F(1,20) = 17.49, p < 0.01, η2 = 0.47); statistics for hourly time points 1 to 11 were similar], and this effect was still present at 21 h post-dark onset [F(1,20) = 31.11, p < 0.001, η2 = 0.61]. Intra-LV injection of GLP-1 also suppressed cumulative chow intake at each hourly timepoint throughout the 12 h dark phase [12 h: F(2,40) = 11.35, p < 0.01, η2 = 0.34; statistics for hourly time points 1 to 11 were similar], and this effect was also present at 21 h post-dark onset [F(2,40) = 6.51, p < 0.01, η2 = 0.22]. As noted above, two-factor mixed-design ANOVA also revealed a significant interaction between EB and GLP-1 to suppress chow intake at 4 h (the first tertile of the dark phase) [F(2,40) = 4.08, p < 0.05, η2 = 0.09], and a trend towards an interaction at 21 h [F(2,40) = 2.62, p = 0.085, η2 = 0.09].
Pairwise planned comparisons revealed that in the oil-treated rats, the 10 μg dose of GLP-1 significantly reduced chow intake relative to saline vehicle at 3, 4, 5, 7, 8, 9, 10, 11, and 12 h post-dark onset, while intake after the lower dose, 1 μg GLP-1, differed from saline at 5, 9, and 11 h post-dark onset [p’s < 0.05; d’s = −0.65 to −0.75 (1 μg) and −0.75 to −1.32 (10 μg)] (Figure 1C). In contrast, both doses of GLP-1 significantly suppressed chow intake relative to saline vehicle at every hour of the 12 h dark phase in the EB-treated rats [p’s < 0.05; d’s = −1.00 to −1.88 (1 μg) and −1.27 to −2.33 (10 μg)] (Figure 1D). The effects observed for 4 h cumulative intake (the first tertile) were detailed above. At 21 h post-dark onset, treatment with both 1 μg and 10 μg GLP-1 significantly reduced chow intake in the EB-treated rats relative to vehicle [p’s<0.01, d’s = −1.02 (1 μg) and −1.38 (10 μg)], but there was no effect of GLP-1 treatment in the oil-treated rats at this timepoint (Figure 1E).
Meal Size
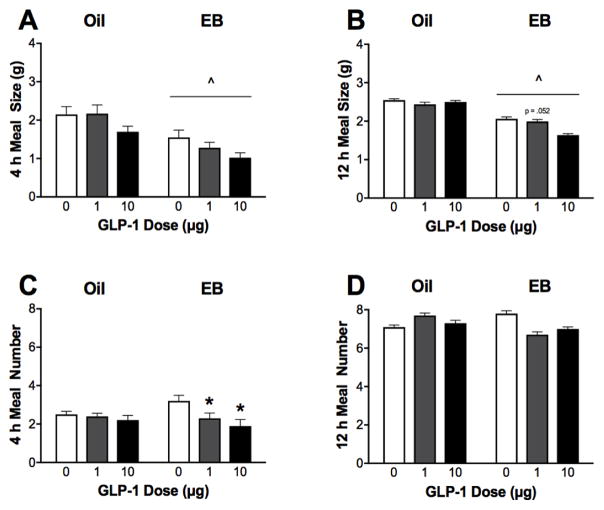
Average meal size during the first 4 h of the dark phase was influenced by both EB [F(1,19) = 22.18, p < 0.001, η2 = 0.54] and GLP-1 [F(2,38) = 4.55, p < 0.05, η2 = 0.19]. Planned comparisons revealed that for all GLP-1 conditions, 4 h average meal size was significantly smaller in EB-treated rats than oil-treated rats [p’s<0.05; d’s = −0.94 (vehicle), −1.48 (1 μg), and −1.52 (10 μg)] (Figure 2A). Repeated-measures ANOVA failed to reveal any significant effects of GLP-1 to suppress meal size in either the oil-treated or EB-treated groups assessed separately. As noted above in the Methods, this 4 h analysis excluded 1 EB rat that did not take a meal during this period.
Figure 2. Effect of central GLP-1 on meal patterns.
A, B. EB treatment alone suppressed average meal size during the first 4 h post-dark onset1 and persists throughout the 12 h dark phase. C. Central injection of GLP-1 reduced average meal number during the first 4 h post-dark onset in EB-treated rats. D. Neither EB nor GLP-1 treatment affect total meal number throughout the 12 h dark phase. Data are means ± SEM. ^p<0.05 relative to respective oil-treated group GLP-1 condition and *p<0.05 relative to respective hormone group vehicle condition. 1Analysis only includes rats that consumed ≥ 1 meal during the first 4 h of the dark phase (n = 10 oil- and 11 EB-treated rats).
Average meal size across the 12 h dark phase was also influenced by a significant main effect of EB [F(1,20) = 13.792, p < 0.001, η2 = 0.41]. Planned comparisons revealed that EB-treated rats had significantly smaller meal sizes than oil-treated rats following both vehicle and 10 μg GLP-1 [p’s<0.05; d’s = −0.94 (vehicle), and −2.08 (10 μg)], and a trend towards smaller meal size in the EB-treated rats following 1 μg GLP-1 [p = 0.052; d = −0.73] (Figure 2B). There was no main effect of GLP-1 treatment on average meal size for the entire 12 h dark phase.
Meal Number
The number of meals taken during the first 4 h of the dark phase was influenced by a significant main effect of GLP-1 [F(2,40) = 5.45, p < 0.01, η2 = 0.19] (Figure 2C). Follow-up repeated measures ANOVA showed that EB-treated rats took significantly fewer meals during the first 4 h of the dark phase following GLP-1 treatment [F(2,22) = 5.20, p < 0.05, η2 = 0.32], but there was no effect of GLP-1 treatment in the oil-treated rats. Both 1 μg and 10 μg doses of GLP-1 significantly reduced 4 h meal number in the EB-treated rats [p’s < 0.05, d’s = −0.91 (1 μg) and −1.19 (10 μg)]. There was no main effect of EB or GLP-1 treatment or interactions on average 12 h dark phase meal number evaluated by two-factor mixed ANOVA (Figure 2D).
Latency to First Meal
The latency to begin the first meal was influenced by a significant main effect of EB treatment [F(1,20) = 5.64, p < 0.05, η2 = 0.22] (Table 1). There is a tendency for increased latency to first meal following GLP-1 treatments in the EB-treated rats, but these trends did not reach statistical significance for any pairwise oil vs. EB comparisons due to the considerable variability in the EB-treated rats, particularly after GLP-1 treatment.
Table 1. Meal pattern variables measured in Experiment 1.
Data are means ± SEM. In Experiment 1, dark phase meal patterns were analyzed in oil- and EB-treated OVX female rats treated with intra-LV vehicle, 1 μg GLP-1, and 10 μg GLP-1.
| Meal Pattern Variable | Group | GLP-1 Dose | ||
|---|---|---|---|---|
| Vehicle | 1 μg | 10 μg | ||
| Latency to first meal, min | ||||
| Oil | 2.7 ± 1.27 | 0.2 ± 0.13 | 3.1 ± 2.07 | |
| EB | 8.9 ± 4.78 | 62.8 ± 26.80 | 58.5 ± 27.64 | |
| 4 h average meal duration1, sec | ||||
| Oil | 1273.1 ± 162.8 | 1111.8 ± 216.6 | 1404.0 ± 285.4 | |
| EB | 1960.4 ± 561.4 | 1760.6 ± 647.2 | 1403.1 ± 456.7 | |
| 12 h average meal duration, sec | ||||
| Oil | 1657.4 ± 234.3 | 1711.3 ± 453.5 | 1498.5 ± 228.5 | |
| EB | 2341.7 ± 513.8 | 2158.3 ± 496.9 | 1861.0 ± 439.6 | |
| 4 h average IMI2, sec | ||||
| Oil | 6054.1 ± 713.3 | 6435.9 ± 604.0 | 6997.3 ± 712.8 | |
| EB | 4292.1 ± 603.6 | 4857.3 ± 1158.7 | 4754.2 ± 884.8 | |
| 12 h average IMI, sec | ||||
| Oil | 5508.3 ± 313.6 | 5543.8 ± 631.0 | 7275.9 ± 575.7 * | |
| EB | 3864.3 ± 307.4 | 4880.4 ± 452.9 | 4821.2 ± 500.5 | |
IMI = inter-meal interval; sec = seconds. Bolded group identifiers indicate significant main effect of hormone treatment.
p<0.05 relative to respective hormone group vehicle condition.
Analysis only includes rats that consumed ≥ 1 meal during the first 4 h of the dark phase (n = 10 oil- and 11 EB-treated rats).
Analysis only includes rats that initiated ≥ 2 meals during the first 4 h of the dark phase (n = 9 oil- and 7 EB-treated rats).
Inter-meal Interval
There was a significant main effect of EB treatment to decrease average inter-meal interval (IMI) during the first 4 h of the dark phase [F(1,14) = 6.30, p < 0.05, η2 = 0.31], but pairwise oil vs. EB comparisons failed to reach statistical significance due to sizable variability (Table 1). As noted above, this 4 h analysis excluded rats that did not take at least 2 meals during this period, to yield an IMI within this time. The number of rats excluded for only taking 1 meal during this period are as follows: n = 1 oil-treated rats (n = 1 after 10 μg GLP-1) and n = 5 EB-rats (n = 2 after 10 μg GLP-1, and n = 3 after both GLP-1 conditions).
Average IMI across the 12-h dark phase was influenced by both EB [F(1,20) = 9.16, p < 0.01, η2 = 0.31] and GLP-1 [F(2,40) = 7.21, p < 0.01, η2 = 0.24], with a trend toward an interaction [F(2,40) = 3.07, p = 0.057, η2 = 0.10] (Table 1). Within the oil-treated group, GLP-1 treatment increased 12 h IMI [F(2,18) = 9.60, p < 0.001, η2 = 0.91], and this was due to a significant increase at the 10 μg dose of GLP-1 compared to vehicle [p < 0.001, d = 1.21]. There was no significant main effect of GLP-1 on 12 h IMI in the EB-treated group.
Meal Duration
There was no effect of EB or GLP-1 treatment or interactions on 4 h or 12 h average meal duration (Table 1).
Body Weight
Body weight was significantly reduced by GLP-1 treatment when measured the day following treatment [F(2,40) = 4.01, p < 0.05, η2 = 0.15], driven by an effect of GLP-1 to reduce body weight in only the EB-treated group [F(2,22) = 6.50, p < 0.01, η2 = 0.37] (Figure 1F). Both the 1 μg and 10 μg doses of GLP-1 significantly reduced body weight in the EB-treated group [p’s < 0.05; d’s = −1.07 (1 μg) and −1.49 (10 μg)]. There was no effect of GLP-1 treatment on body weight in the oil-treated group. Over the course of our feeding experiment, body weights diverged such that at the end of the experiment the mean weight of the oil-treated group was 353.73 ± 8.62 g, whereas the mean weight of the EB-treated group was 285.13 ± 5.33 g [F(1,20)=37.27, p < 0.0001, η2 = 0.65].
Experiment 2: GLP-1-induced c-Fos
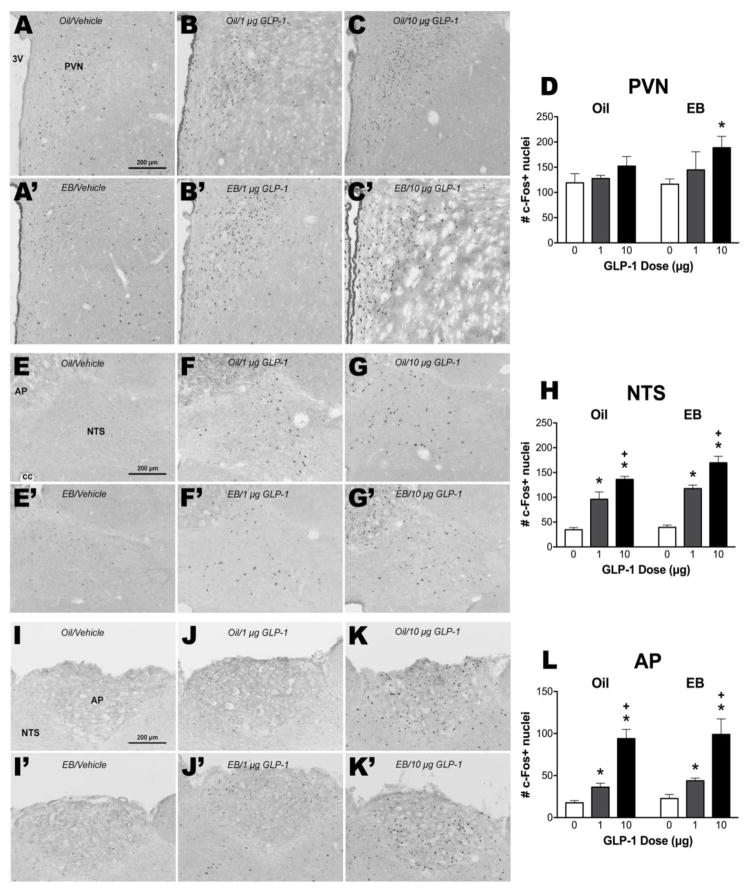
There was a significant main effect of GLP-1 treatment on number of c-Fos-positive cells in the AP [F(2,21) = 56.35, p < 0.001, η2 = 0.84], NTS [F(2,21) = 94.81, p < 0.001, η2 = 0.86] and PVN [F(2,20) = 4.55, p < 0.05, η2 = 0.29], but no main effects of EB or interactions (Figure 3). Planned comparisons revealed that in the PVN, a significant effect of the 10 μg dose of GLP-1 was observed only in the EB group [p < 0.05, d = 2.40] (Figure 3D). In the NTS and AP, pairwise comparisons revealed significant differences in the number of c-Fos-positive cells following central treatment with vehicle, 1 μg GLP-1 and 10 μg GLP-1 in both oil- and EB-treated rats [NTS: p’s < 0.001, d‘s = Oil: 2.93 (1 μg), 9.99 (10 μg), and 4.01 (1 vs 10 μg), EB: 6.80 (1 μg), 7.63 (10 μg), and 2.41 (1 vs 10 μg); AP: p’s < 0.001, d’s = Oil: 2.33 (1 μg), 6.05 (10 μg), and 1.70 (1 vs 10 μg), EB: 3.03 (1 μg), 3.27 (10 μg), 2.76 (1 vs 10 μg)] (Figure 3H and 3L, respectively).
Figure 3. Effect of GLP-1 on c-Fos induction in oil- or EB-treated female rats.
Representative images of c-Fos induction responses to intra-LV injection of vehicle, 1 μg GLP-1, or 10 μg GLP-1 in the PVN (A–C, A′–C′), the NTS (E–G, E′–G′), and the AP (I–K, I′–K′) from oil-treated and EB-treated rats. Panels D, H, and L show mean ± SEM c-Fos-positive cell nuclei per section through each brain region. *p<0.05 relative to respective hormone group vehicle condition, +p<0.05 relative to respective hormone group 1 μg GLP-1 condition.
DISCUSSION
Here, we demonstrated that estradiol enhances responsiveness to central GLP-1. In EB-treated OVX rats, pharmacologic activation of GLP-1R in the brain significantly suppressed cumulative chow intake throughout the dark phase at the 1 μg dose, which had limited effects on feeding in the oil-treated group (Figure 1). The suppressive effect of higher-dose GLP-1 was present in both hormone groups, showing that lack of ovarian hormones does not render rats non-responsive to GLP-1. Based on these data, we suggest that EB shifts sensitivity to GLP-1. These findings support the hypothesis that EB-induced suppression of food intake involves potentiation of the anorexic response to GLP-1.
Consistent with its short half-life, central GLP-1 treatment typically induces a brief anorexic effect in male rats where an early suppression in food intake is compensated for later in the dark phase, resulting in no 24 h feeding effects (Donahey et al., 1998). However, we observed significant GLP-1-induced intake suppression in female rats throughout the 21-h post-treatment period. This long-lasting suppression results from the early (first tertile of dark phase) reduction in feeding after GLP-1 relative to vehicle, along with a failure to compensate by consuming more chow later in the 21-h measurement period. A previous LV GLP-1 dose response study performed in our laboratory in male rats found smaller effects at 2 h into the dark phase, and no effect on overnight intake (Dossat et al., 2011). Although we did not perform a direct male-female comparison in the current study, these and other data (Richard et al., 2016) suggest that sizable sex differences in GLP-1 responsiveness exists. This effect may require the presence of ovarian hormones at the time of treatment, because OVX oil-treated female rats in the present study did not show the long-lasting intake suppression after GLP-1 (Figure 1E).
Meal pattern analyses provide insight into the behavioral mechanisms of the cumulative food intake effects of EB and GLP-1. EB is known to suppress food intake by enhancing responsiveness to satiation signals, which manifests as a selective reduction in meal size (Blaustein and Wade, 1977). We replicated this well-established effect here, where LV vehicle-injected OVX rats receiving EB treatment had significantly smaller average meal sizes than the oil-treated group after LV vehicle, both during the first 4 h of the dark phase, a time during which EB’s feeding effects have previously been shown to be most robust, and persisting throughout the 12 h dark phase (Eckel, Houpt, and Geary, 2000) (Figures 2A–B). Within the brain, several GLP-1R populations have been shown to influence meal patterns in male rats. For example, stimulation of GLP-1Rs in the ventral tegmental area with Ex4 decreases high-fat diet (HFD) intake primarily through a reduction in meal size, and lateral septum Ex4 treatment reduces both meal size and meal number in HFD-fed rats (Mietlicki-Baase et al., 2013; Terrill et al., 2016). Additionally, hindbrain GLP-1R stimulation decreases food intake through reduction in meal number, with no effect on meal size (Hayes et al., 2011). Given that EB treatment and GLP-1R activation have been independently shown to influence meal size, we predicted that central GLP-1 treatment in EB-treated females might affect within-meal processes by reducing meal size. While GLP-1 treatment did influence average meal size during the first 4 h of the dark phase, our findings suggest that this effect may not be potentiated by EB treatment.
Interestingly, meal number during the first 4 h of the dark phase was influenced by GLP-1 and EB treatments (Figure 2C). This early reduction in meal number in EB-treated rats following GLP-1 treatment is also complemented by an increased latency to begin the first meal (Table 1). However, meal number across the 12 h dark phase remained unaffected by both EB and GLP-1, indicating that rats later compensated for the early reduction in meal number (Figure 2D). These data suggest that EB and GLP-1 may interact to reduce likelihood to take a meal in the early dark phase.
The current data support our hypothesis that EB enhances the anorexic response to central GLP-1, but one potential confounding factor here is the significant body weight difference between the EB and oil-treated groups. Previous studies have demonstrated that high fat diet-induced obesity impairs response to peripheral GLP-1, raising the possibility that increased body mass and adiposity could do the same for central GLP-1 (Mul et al., 2013; Williams et al., 2011). This was assessed in an experiment testing responsiveness to third ventricular infusion of Ex4 in animals fed a low fat chow diet and obese animals maintained on a high fat diet (Mul et al., 2013). The effects of central Ex4 were equivalent across groups, indicating that responsiveness to central GLP-1R stimulation is not altered by increased body weight and adiposity, making it unlikely that these factors are responsible for the behavioral difference in our study of EB- and oil-treated OVX rats. However, it is important to note that the described study only tested one dose of Ex4, and it is possible that there is a change in sensitivity at lower doses.
To begin to investigate the neural mechanisms by which EB enhances the feeding effects of central GLP-1, we asked whether EB treatment would increase GLP-1-induced neuronal activation, as measured by c-Fos expression, in feeding-relevant brain areas. We focused on the NTS and PVN, brain areas that have been implicated in the control of food intake by GLP-1 (Hayes et al., 2009; Katsurada et al., 2014). We also assessed c-Fos induction in the AP, an area that expresses c-Fos following central GLP-1 treatment in male rats (Rowland et al., 1997). Consistent with the reported effects in males, GLP-1 dose-dependently induced c-Fos expression in the AP and NTS of female subjects. Our data are the first demonstration of this effect in female subjects. Contrary to our hypothesis, there was no difference in the degree of c-Fos induction between oil- and EB-treated OVX rats in either of those regions. Changes in c-Fos expression are taken to reflect changes in neuronal activity; thus, these data suggest that the differential feeding effects we observed were not mediated by EB effects on GLP-1-induced neuronal activation in the AP and NTS. In the PVN, however, GLP-1 treatment significantly increased c-Fos expression only in the EB-treated group, with no effect of GLP-1 treatment in the oil-treated group. The relatively modest effect of GLP-1 on c-Fos induction in the PVN of female rats is surprising, given that in males, central GLP-1 treatment has been shown to potently induce c-Fos expression in this brain area (Rowland et al., 1997). However, c-Fos expression in our LV vehicle-injected female rats was elevated in the PVN relative to typical baseline c-Fos observed in this nucleus, and it is possible that this masked any potentially greater effect of GLP-1. Despite this, our findings suggest that neuronal activity within the PVN may be involved in an interaction between EB and GLP-1.
There are a number of potential underlying molecular mechanisms for the effects observed here. EB may act within GLP-1R-bearing cells to modulate GLP-1R expression or second messenger signaling. It is also possible that the mechanism involves interactions among multiple different cells. This would not be unprecedented, as EB interacts with orexigenic peptide melanin-concentrating hormone (MCH) in an apparently indirect manner (Muschamp and Hull, 2007; Santollo and Eckel, 2013). Perhaps estrogen-sensitive cells synapse onto GLP-1R-expressing cells, or vice versa, or the two cell types converge onto a third postsynaptic cell. Future studies will address the neurobiological mechanisms of this interaction between EB and GLP-1.
Peripheral administration of GLP-1 or degradation-resistant GLP-1R agonists such as liraglutide and Ex4 reduce blood glucose and food intake in humans, with chronic use resulting in weight loss (Poon et al., 2005). Such drugs are currently FDA-approved treatments for type 2 diabetes and obesity. The effectiveness of these treatments is in part due to their action on central GLP-1R, as they readily cross the blood-brain barrier. Blockade of central GLP-1R with Ex9 attenuates the suppressive effect of peripheral liraglutide on food intake (Kanoski et al., 2011). Undoubtedly, women make up a large portion of the individuals taking these treatments, but there has been no systematic investigation on whether estrogen status in females alters the effectiveness of such treatments. Previous findings demonstrate that a novel estradiol/GLP-1 conjugate molecule was more effective at suppressing food intake in rodents than both GLP-1 in its native form and a dissociated conjugate mixture of estradiol and GLP-1, an effect hypothesized to be due to estrogens guiding GLP-1 to certain cell populations (Finan et al., 2012; Vogel et al., 2016). A recent examination of sex differences in the effect of GLP-1R activation on food reward-motivated behavior suggest an interaction with estrogens (Richard et al., 2016). Our data indicate that estrogens, specifically EB, enhance sensitivity to the anorexic effects of central GLP-1R activation. Future studies assessing the extent to which estrogens may interact with GLP-1 or related GLP-1R agonists to affect glucose metabolism and food intake across varying estrogen conditions, such as pre- and post-menopause, are warranted.
In conclusion, our behavioral data suggest a potential role for the GLP-1 system in mediating estrogen-induced suppression of food intake. Moreover, these data provide the first demonstration of central GLP-1, itself, as opposed to other GLP-1R agonists, influencing food intake in female subjects. EB treatment potentiates the anorexic response to central GLP-1, and it will now be important to further investigate the mechanisms underlying this effect.
Highlights.
Estradiol-treated OVX female rats show enhanced sensitivity to central GLP-1.
GLP-1 dose-dependently induces c-Fos expression in the AP and NTS in female rats.
Estrogens may be required for GLP-1-induced c-Fos in the PVN in female rats.
Acknowledgments
We thank Nicole Lilly, Lauren Philmus, and Dr. Gregory Loney for technical assistance during portions of the behavioral experiments. This work was funded by the NIH grant R01DK095757 to D.L.W.
Footnotes
Conflict of Interest The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Asarian L, Abegg K, Geary N, Schiesser M, Lutz TA, Bueter M. Estradiol increases body weight loss and gut-peptide satiation after Roux-en-Y gastric bypass in ovariectomized rats. Gastroenterology. 2012;143:325–7. e2. doi: 10.1053/j.gastro.2012.05.008. [DOI] [PubMed] [Google Scholar]
- Asarian L, Geary N. Cyclic estradiol treatment normalizes body weight and restores physiological patterns of spontaneous feeding and sexual receptivity in ovariectomized rats. Horm Behav. 2002;42:461–471. doi: 10.1006/hbeh.2002.1835. [DOI] [PubMed] [Google Scholar]
- Asarian L, Geary N. Cyclic estradiol treatment phasically potentiates endogenous cholecystokinin’s satiating action in ovariectomized rats. Peptides. 1999;20:445–450. doi: 10.1016/S0196-9781(99)00024-8. [DOI] [PubMed] [Google Scholar]
- Barrera JG, Jones KR, Herman JP, D’Alessio DA, Woods SC, Seeley RJ. Hyperphagia and increased fat accumulation in two models of chronic CNS glucagon-like peptide-1 loss of function. J Neurosci. 2011;31:3904–3913. doi: 10.1523/JNEUROSCI.2212-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Arnold AP, Berkley KJ, Blaustein JD, Eckel LA, Hampson E, Herman JP, Marts S, Sadee W, Steiner M, Taylor J, Young E. Strategies and methods for research on sex differences in brain and behavior. Endocrinology. 2005;146:1650–1673. doi: 10.1210/en.2004-1142. [DOI] [PubMed] [Google Scholar]
- Blaustein J, Wade G. Ovarian influences on the meal patterns of female rats. Physiol Behav. 1976;17:201–208. doi: 10.1016/0031-9384(76)90064-0. [DOI] [PubMed] [Google Scholar]
- Blaustein JD, Wade GN. Ovarian hormones and meal patterns in rats: effects of progesterone and role of gastrointestinal transit. Physiol Behav. 1977;19:23–27. doi: 10.1016/0031-9384(77)90153-6. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. Stat Power Anal Behav Sci. 1988 doi: 10.1234/12345678. [DOI] [Google Scholar]
- Donahey JC, van Dijk G, Woods SC, Seeley RJ. Intraventricular GLP-1 reduces short- but not long-term food intake or body weight in lean and obese rats. Brain Res. 1998;779:75–83. doi: 10.1016/S0006-8993(97)01057-3. [DOI] [PubMed] [Google Scholar]
- Dossat AM, Lilly N, Kay K, Williams DL. Glucagon-like peptide 1 receptors in nucleus accumbens affect food intake. J Neurosci. 2011;31:14453–7. doi: 10.1523/jneurosci.3262-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckel LA. Estradiol: a rhythmic, inhibitory, indirect control of meal size. Physiol Behav. 2004;82:35–41. doi: 10.1016/j.physbeh.2004.04.023. [DOI] [PubMed] [Google Scholar]
- Eckel LA, Geary N. Endogenous cholecystokinin’s satiating action increases during estrus in female rats. Peptides. 1999;20:451–456. doi: 10.1016/S0196-9781(99)00025-X. [DOI] [PubMed] [Google Scholar]
- Eckel LA, Houpt TA, Geary N. Estradiol treatment increases CCK-induced c-Fos expression in the brains of ovariectomized rats. Am J Physiol Regul Integr Comp Physiol. 2002;283:R1378–R1385. doi: 10.1152/ajpregu.00300.2002. [DOI] [PubMed] [Google Scholar]
- Eckel LA, Houpt TA, Geary N. Spontaneous meal patterns in female rats with and without access to running wheels. Physiol Behav. 2000;70:397–405. doi: 10.1016/s0031-9384(00)00278-x. [DOI] [PubMed] [Google Scholar]
- Eckel LA, Langhans W, Kahler A, Campfield LA, Smith FJ, Geary N. Chronic administration of OB protein decreases food intake by selectively reducing meal size in female rats. Am J Physiol. 1998;275:R186–R193. doi: 10.1152/ajpregu.1998.275.1.R186. [DOI] [PubMed] [Google Scholar]
- Finan B, Yang B, Ottaway N, Stemmer K, Müller TD, Yi C-X, Habegger K, Schriever SC, García-Cáceres C, Kabra DG, Hembree J, Holland J, Raver C, Seeley RJ, Hans W, Irmler M, Beckers J, de Angelis MH, Tiano JP, Mauvais-Jarvis F, Perez-Tilve D, Pfluger P, Zhang L, Gelfanov V, DiMarchi RD, Tschöp MH. Targeted estrogen delivery reverses the metabolic syndrome. Nat Med. 2012;18:1847–56. doi: 10.1038/nm.3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geary N, Asarian L. Cyclic estradiol treatment normalizes body weight and test meal size in ovariectomized rats. Physiol Behav. 1999;67:141–147. doi: 10.1016/S0031-9384(99)00060-8. [DOI] [PubMed] [Google Scholar]
- Geary N, Trace D, McEwen B, Smith GP. Cyclic estradiol replacement increases the satiety effect of CCK-8 in ovariectomized rats. Physiol Behav. 1994;56:281–289. doi: 10.1016/0031-9384(94)90196-1. [DOI] [PubMed] [Google Scholar]
- Hayes MR, Leichner TM, Zhao S, Lee GS, Chowansky A, Zimmer D, De Jonghe BC, Kanoski SE, Grill HJ, Bence KK. Intracellular signals mediating the food intake-suppressive effects of hindbrain glucagon-like peptide-1 receptor activation. Cell Metab. 2011;13:320–330. doi: 10.1016/j.cmet.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes MR, Bradley L, Grill HJ. Endogenous hindbrain glucagon-like peptide-1 receptor activation contributes to the control of food intake by mediating gastric satiation signaling. Endocrinology. 2009;150:2654–2659. doi: 10.1210/en.2008-1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanoski SE, Fortin SM, Arnold M, Grill HJ, Hayes MR. Peripheral and central GLP-1 receptor populations mediate the anorectic effects of peripherally administered GLP-1 receptor agonists, liraglutide and exendin-4. Endocrinology. 2011;152:3103–3112. doi: 10.1210/en.2011-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsurada K, Maejima Y, Nakata M, Kodaira M, Suyama S, Iwasaki Y, Kario K, Yada T. Endogenous GLP-1 acts on paraventricular nucleus to suppress feeding: projection from nucleus tractus solitarius and activation of corticotropin-releasing hormone, nesfatin-1 and oxytocin neurons. Biochem Biophys Res Commun. 2014;451:276–81. doi: 10.1016/j.bbrc.2014.07.116. [DOI] [PubMed] [Google Scholar]
- Kinzig KP, Alessio DAD, Seeley RJ. The diverse roles of specific GLP-1 receptors in the control of food intake and the response to visceral illness. J Neurosci. 2002;22:10470–10476. doi: 10.1523/JNEUROSCI.22-23-10470.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen PJ, Holst JJ. Glucagon-related peptide 1 (GLP-1): Hormone and neurotransmitter. Regul Pept. 2005;128:97–107. doi: 10.1016/j.regpep.2004.08.026. [DOI] [PubMed] [Google Scholar]
- Larsen PJ, Tang-Christensen M, Holst JJ, Ørskov C. Distribution of glucagon-like peptide-1 and other preproglucagon-derived peptides in the rat hypothalamus and brainstem. Neuroscience. 1997;77:257–270. doi: 10.1016/S0306-4522(96)00434-4. [DOI] [PubMed] [Google Scholar]
- McElroy JF, Wade GN. Short- and long-term effects of ovariectomy on food intake, body weight, carcass composition, and brown adipose tissue in rats. Physiol Behav. 1987;39:361–365. doi: 10.1016/0031-9384(87)90235-6. [DOI] [PubMed] [Google Scholar]
- Mietlicki-Baase EG, Ortinski PI, Rupprecht LE, Olivos DR, Alhadeff AL, Pierce RC, Hayes MR. The food intake-suppressive effects of glucagon-like peptide-1 receptor signaling in the ventral tegmental area are mediated by AMPA/kainate receptors. Am J Physiol - Endocrinol Metab. 2013;305:E1367–E1374. doi: 10.1152/ajpendo.00413.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morínigo R, Moizé V, Musri M, Lacy AM, Navarro S, Marín JL, Delgado S, Casamitjana R, Vidal J. Glucagon-like peptide-1, peptide YY, hunger, and satiety after gastric bypass surgery in morbidly obese subjects. J Clin Endocrinol Metab. 2006;91:1735–1740. doi: 10.1210/jc.2005-0904. [DOI] [PubMed] [Google Scholar]
- Mul JD, Begg DP, Barrera JG, Li B, Matter EK, D’Alessio DA, Woods SC, Seeley RJ, Sandoval DA. High-fat diet changes the temporal profile of GLP-1 receptor-mediated hypophagia in rats. Am J Physiol Regul Integr Comp Physiol. 2013;305:R68-77. doi: 10.1152/ajpregu.00588.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muschamp J, Hull E. Melanin concentrating hormone and estrogen receptor-alpha are coextensive but not coexpressed in cells of male rat hypothalamus. Neurosci Lett. 2007;427:123–126. doi: 10.1016/j.neulet.2007.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council. Guide for the Care and Use of Laboratory Animals. 8. 2011. Guide for the Care and Use of Laboratory Animals. [DOI] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 6. Vol. 170. Acad Press; 2006. p. 547612. [DOI] [Google Scholar]
- Poon T, Nelson P, Shen L, Mihm M, Taylor K, Fineman M, Kim D. Exenatide improves glycemic control and reduces body weight in subjects with type 2 diabetes: a dose-ranging study. Diabetes Technol Ther. 2005;7:467–477. doi: 10.1089/dia.2005.7.467. [DOI] [PubMed] [Google Scholar]
- Pournaras DJ, Aasheim ET, Bueter M, Ahmed AR, Welbourn R, Olbers T, Le Roux CW. Effect of bypassing the proximal gut on gut hormones involved with glycemic control and weight loss. Surg Obes Relat Dis. 2012;8:371–374. doi: 10.1016/j.soard.2012.01.021. [DOI] [PubMed] [Google Scholar]
- Richard JE, Anderberg RH, López-Ferreras L, Olandersson K, Skibicka KP. Sex and estrogens alter the action of glucagon-like peptide-1 on reward. Biol Sex Differ. 2016;7:6. doi: 10.1186/s13293-016-0059-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaman L. Ascending projections from the caudal visceral nucleus of the solitary tract to brain regions involved in food intake and energy expenditure. Brain Res. 2010 doi: 10.1016/j.brainres.2010.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera HM, Eckel LA. The anorectic effect of fenfluramine is increased by estradiol treatment in ovariectomized rats. Physiol Behav. 2005;86:331–7. doi: 10.1016/j.physbeh.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Rivera HM, Santollo J, Nikonova L, Eckel LA. Estradiol increases the anorexia associated with increased 5-HT2c receptor activation in ovariectomized rats. Physiol Behav. 2012;105:188–194. doi: 10.1016/j.physbeh.2011.08.018.Estradiol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland NE, Crews EC, Gentry RM. Comparison of Fos induced in rat brain by GLP-1 and amylin. Regul Pept. 1997;71:171–174. doi: 10.1016/S0167-0115(97)01034-3. [DOI] [PubMed] [Google Scholar]
- Santollo J, Eckel LA. Oestradiol Decreases Melanin-Concentrating Hormone (MCH) and MCH Receptor Expression in the Hypothalamus of Female Rats. J Neuroendocrinol. 2013;25:570–579. doi: 10.1111/jne.12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santollo J, Eckel LA. Estradiol decreases the orexigenic effect of neuropeptide Y, but not agouti-related protein, in ovariectomized rats. Behav Brain Res. 2008;191:173–177. doi: 10.1016/j.bbr.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tecott LH, Sun LM, Akana SF, Strack AM, Lowenstein DH, Dallman MF, Julius D. Eating disorder and epilepsy in mice lacking 5-HT2c serotonin receptors. Nature. 1995;374:542–546. doi: 10.1038/374542a0. [DOI] [PubMed] [Google Scholar]
- Terrill S, Jackson C, Greene H, Lilly N, Maske C, Vallejo S, Williams D. Role of lateral septum glucagon-like peptide 1 receptors in food intake. Am J Phyiol Regul Integr Comp Physiol. 2016;311:R124–32. doi: 10.1152/ajpregu.00460.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turton MD, O’Shea D, Gunn I, Beak SA, Edwards CMB, Meeran K, Choi SJ, Taylor GM, Heath MM, Lambert PD, Wilding JPH, Smith DM, Ghatei MA, Herbert J, Bloom SR. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature. 1996;379:69–72. doi: 10.1038/379069a0. [DOI] [PubMed] [Google Scholar]
- Vogel H, Wolf S, Rabasa C, Rodriguez-Pacheco F, Babaei CS, Stöber F, Goldschmidt J, DiMarchi RD, Finan B, Tschöp MH, Dickson SL, Schürmann A, Skibicka KP. GLP-1 and estrogen conjugate acts in the supramammillary nucleus to reduce food-reward and body weight. Neuropharmacology. 2016;110:396–406. doi: 10.1016/j.neuropharm.2016.07.039. [DOI] [PubMed] [Google Scholar]
- Wang GH. Age and sex differences in the daily food-intake of the albino rat. Am J Physiol. 1925;71:729–735. [Google Scholar]
- Williams DL, Baskin DG, Schwartz MW. Evidence that intestinal glucagon-like peptide-1 plays a physiological role in satiety. Endocrinology. 2009;150:1680–1687. doi: 10.1210/en.2008-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DL, Hyvarinen N, Lilly N, Kay K, Dossat A, Parise E, Torregrossa AM. Maintenance on a high-fat diet impairs the anorexic response to glucagon-like-peptide-1 receptor activation. Physiol Behav. 2011;103:557–564. doi: 10.1016/j.physbeh.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]