Abstract
The human immune system is under constant challenge from many viruses, some of which the body is successfully able to clear. Other viruses have evolved to escape the host immune responses and thus persist, leading to the development of chronic diseases. Dendritic cells are professional antigen-presenting cells that play a major role in both innate and adaptive immunity against different pathogens. This review focuses on the interaction of different chronic viruses with dendritic cells and the viruses’ ability to exploit this critical cell type to their advantage so as to establish persistence within the host.
Keywords: dendritic cells, human chronic viral infections, HIV-1, HTLV-1, HBV/HCV, HSV, HCMV
INTRODUCTION
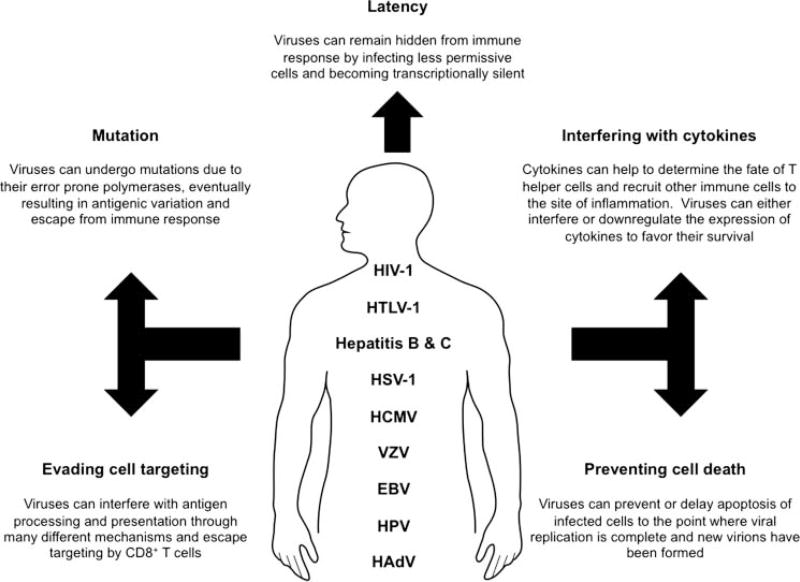
Viruses have been one of the major causes in tremendous loss of human lives and, thus, being an important matter of health and economic concern. It is for these reasons that viruses have garnered such considerable attention over the years and much research has focused at understanding the mechanisms of how viruses interact with the host. Humans have evolved a complex and diverse armory to defend against viruses in the form of innate and adaptive immunity. Our defense immunity is able to successfully protect us from a plethora of viruses. However, in this constant struggle of power between viruses and the host, there are some viruses that the body is not able to fully clear that persist and gradually develop into chronic infections. With chronic viral infections rising globally, persistent viruses have attracted much attention and most of our current understanding about them has emerged in the past decade. Some of the major human chronic viruses (Fig. 1) include human immunodeficiency virus 1 (HIV-1), human T-cell leukemia virus type 1 (HTLV-1), hepatitis viruses B and C (HBV and HCV), herpes simplex virus (HSV), human cytomegalovirus (HCMV), varicella-zoster virus (VZV), Epstein-Barr virus (EBV), papillomavirus (HPV), and adenovirus (HAdV). These chronic viruses can escape and subvert the immune response through a wide variety of unique mechanisms (Table 1). Following primary infection, most of these chronic viruses progress into a state of latency with minimal viral gene expression, allowing them to evade immune surveillance. Such individuals remain asymptomatic during the course of primary infection. Following conditions of stress, other physiological triggers, or even secondary pathogen infection, these viruses can come out of latency and progress to cause disease symptoms. Although viruses can differ in their tropism and interact with many different cell types, one major cell type that all viruses must initially challenge is immune cells that are involved in surveillance, recognition of foreign threats, and antigen processing and presentation to other key immune cell types. No wonder that chronic viruses have very smartly evolved to use this important cell type to their advantage.
FIGURE 1.
Human chronic viruses: The major mechanisms by which different human chronic viruses evade the host immune response and persist. EBV = Epstein-Barr virus; HAdV = human adenovirus; HCMV = human cytomegalovirus; HIV-1 = human immunodeficiency virus; HPV = human papillomavirus; HSV = herpes simplex virus; HTLV = human T-cell leukemia virus type 1; VZV = varicella-zoster virus.
TABLE I.
Human Chronic Viruses: Different Human Chronic Viruses with Their Virological and Clinical Features
| Feature | HIV-1 | HTLV-1 | HBV | HCV | HSV-1 | HCMV | VZV | EBV | HPV | HAdV |
|---|---|---|---|---|---|---|---|---|---|---|
| Family | Retroviridae | Retroviridae | Hepadnaviridae | Flaviviridae | Alpha-herpesviridae | Beta-herpesviridae | Alpha-herpesviridae | Gamma-herpesviridae | Papillomaviridae | Adenoviridae |
| Entry factor | CD4, CCR5, CXCR4, DC-SIGN | DC-SIGN, GLUT-1, NRP-1 | Unknown | CD81, caludin 1, occludin | DC-SIGN, PIR-α | DC-SIGN | Unknown | Unknown | Unknown | CAR, Lactoferrin, DC-SIGN |
| Transmission | IV drug use, blood transfusions, perinatal transfer, and sexual contact | IV drug use, blood transfusions, perinatal transfer, and sexual contact | IV drug use, blood transfusions, perinatal transfer, and sexual contact | IV drug use, blood transfusions, perinatal transfer, and sexual contact | IV drug use, blood transfusions, perinatal transfer, and sexual contact | IV drug use, blood transfusions, perinatal transfer, and sexual contact | Direct contact, airborne | Close contact with saliva of infected person | Sexual contact | Direct contact, fecal-oral route |
| Disease | AIDS | ATL and HAM/TSP | Hepatitis B | Hepatitis C | Herpes | Complications associated with immunocompromised patients | Chicken pox (in children) Shingles (in adults) | Burkitt’s lymphoma and SLE | Cervical cancer and genital warts | Upper respiratory infections |
| Vaccine | None | None | HBsAg based | None | None | None | None | None | Yes, to some HPV types | None |
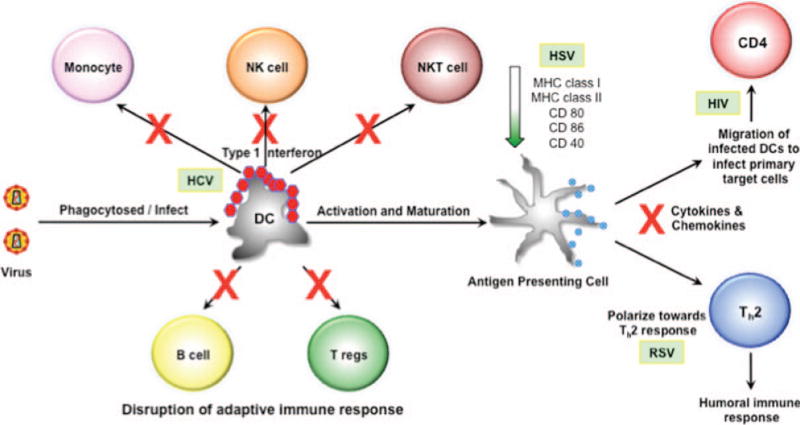
The human body is under constant threat from pathogens. Usually, the body is able to cope with the pathogenic challenge and overcome the threat. Certain pathogens, particularly viruses, have a long history of causing great harm to human populations, and the human body has evolved to develop an armory against these viral challenges. Foremost among the cells involved in the body’s first line of defense are the dendritic cells (DCs). The DCs are armed with a number of different pattern recognition receptors that can identify unique pathogenic molecular signatures called pathogen-associated molecular patterns. The major types of pattern recognition receptors include members of the Toll-like receptor (TLR) family as well as the cytoplasmic sensors retinoid acid–inducible gene-like receptors and nucleotide oligomerization domain-like receptors. These various receptors are capable of identifying unique components of the virion, such as the high repetition of capsomers and peplomers on virion surfaces as well as the viral nucleic acid and its various replication intermediates. The triggering of these receptors leads to activation of the innate immune response through the production of type 1 interferons (IFNs) as well as other cytokines and chemokines. While the cytokines help to determine the fate of T helper cells, the chemokines are crucial for the recruitment of effector cells in addition to aiding DCs to migrate to the sites of T-cell activation, where they can present processed antigenic peptides in the context of major histocompatibility complex (MHC) class I to the naive T cells. DCs also undergo activation and maturation through the increased upregulation of surface molecules, such as CD80, CD86, and MHC class II. These surface markers aid in the activation of naive T cells and thereby play an important role in the generation of the adaptive immune response. Thus, DCs are well placed to mount a successful immunologic response against viral challenge through their ability to bridge both innate and adaptive immune response (Fig. 2).
FIGURE 2.
Viral-mediated disruption of the immune response through dendritic cells (DCs). Once the virus has been taken in by DCs, it can use different mechanisms to overcome the host immune response. Hepatitis C virus (HCV) can blunt the production of the type 1 interferons (IFNs) and, thus, disrupt the activation of other cells of the innate immune response (monocytes, natural killer [NK] cells, and natural killer T [NKT] cells). Herpes simplex virus (HSV) can downregulate the expression of major histocompatibility complex (MHC) class I and II as well as costimulatory molecules required for the activation of T cells. Respiratory syncytia virus (RSV) can skew the production of cytokines to favor a T helper 2 (Th2) response rather than a T helper 1 response, which is needed for clearing viral infected cells. Other viruses can either reduce or block the production of different cytokines and chemokines. Human immunodeficiency virus 1 (HIV-1) can use the DCs themselves to facilitate infection of the CD4+ T cells.
In the human peripheral blood, depending upon the expression of CD11c, two subsets of DCs are recognized [1]. The CD11c+ myeloid DCs (mDCs) express myeloid markers, such as CD13, CD33 and CD11b, while the CD11c− lymphoid DCs do not express these markers but instead express CD123. The CD11c− lymphoid DCs exhibit a plasmacytoid morphology and, hence, are called plasmacytoid DCs (pDCs). The mDCs have been found in three different compartments—peripheral tissue DCs, secondary lymphoid organ-resident DCs, and circulating blood mDCs. Within the skin, three subsets of DCs have been identified and include the Langerhan cells (LCs) within the epidermis while the CD1a+ DCs and CD14+ DCs are present within the dermis [2]. The LCs express the C-type lectins, such as Langerin and dendritic cell immunoreceptor (DCIR) as well as TLR1, TLR2, TLR3, TLR6 and TLR10 [3, 4]. On the other hand, the dermal CD14+ DCs express a wide variety of lectins, such as dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin (DC-SIGN), CD205, CLEC-6, Dectin 1, and DCIR as well as TLR2, TLR4, TLR5, TLR6, TLR8 and TLR10 [3, 5]. However, the LCs produce only very few cytokines including interleukin (IL)-15 while the CD14+ DCs can produce IL-1β, IL-6, IL-8, IL-10, IL-12, granulocyte macrophage colony stimulating factor (GM-CSF), monocyte chemoattractant protein (MCP), and transforming growth factor (TGF-β) [6]. As a result of the different cytokine profile, both these subsets yield different immunologic end results. The LCs are highly efficient in their ability to prime naive CD8+ T cells and thereby induce potent cytotoxic T lymphocyte (CTL) response [6]. In contrast, the CD14+ DCs induce naive CD4+ T cells to differentiate into effector cells that share properties with the T-follicular helper cells (Tfh), a CD4+ T cell subset specialized in B cell assistance. The CD14+ DCs prime naive CD4+ T cells in order to assist naive B cells to produce largea mounts of IgM and switch isotypes towards IgG and IgA [6]. In human blood, the CD1c+ (blood dendritic cell antigen BDCA-1+) DCs form a major fraction of the Lin-1− HLA-DR+ CD11c+ mDCs [7].
The pDCs are categorized as Lin-1−CD11c−HLA-DR+ and express high levels of CD123 (IL-3Rα chain), BDCA-2, and immunoglobulin-like transcripts (ILT)-7 [8, 9]. The pDCs predominantly express very high levels of TLR7 and TLR9, thereby allowing them to act as viral sensors and produce large amounts of type 1 IFNs in response to viral threat [10]. In addition, the pDCs can also produce proinflammatory cytokines like TNF-α and IL-6 in response to virus stimulation as well as chemokines, such as C-X-C motif chemokine 10 (CXCL10) and C-C motif chemokine ligands 4 and 5 (CCL4 and CCL5) [11–13]. Upon exposure to viruses, the pDCs are able to elicit memory responses through the activation, proliferation, and differentiation of antigen-specific memory B and T lymphocytes into plasma cells and CTLs, respectively [14–16].There are two subsets of human pDCs that can be differentiated on the basis of expression of CD2 into CD2high and CD2low [17]. Both subsets can secrete IFN-α and express the cytotoxic molecules granzyme B and TNF-α related apoptosis inducing ligand (TRAIL). The CD2high pDCs are more efficient than the CD2low in their ability to induce allogeneic T cell proliferation. The two subsets are associated with distinct transcription profiles, differential secretion of IL-12p40, and the differential expression of the costimulatory molecule CD80.
Although numerous studies have highlighted, the importance of DCs in the generation of immune response and transmission of various viruses, the exact mechanism still remains to be elucidated. Most of our current understanding is from work done in vitro; however, when such results are compared to in vivo situations, the comparison is stark and in most cases contrary. We explore a few common discrepancies in the field of HIV-DC as well as HCV-DC research.
HUMAN IMMUNODEFICIENCY VIRUS 1 AND DENDRITIC CELLS
The human immunodeficiency virus was discovered in the early 1980s and became associated with acquired immunodeficiency syndrome (AIDS). The virus now has a worldwide distribution of almost 40 million infected individuals. HIV is transmitted mostly sexually but can also be transmitted either parenterally or prenatally. HIV is classified under the genus Lentivirus of the family Retroviridae. Initially, acute infection with HIV-1 causes flulike symptoms, but the chronic viral disease develops over many years. The virus employs multiple strategies for persistence, including stable latent proviral integration, very high replication capacity, and the ability to undergo rapid mutation. These are some of the key features that enable HIV-1 to evade the body’s immune response and eventually establish persistent infection within the host.
The first DCs to have been reported to be susceptible to HIV-1 infection were the LCs, the antigen presenting cells (APCs) of the epidermis [18]. Although the virus has a preference for infecting CD4+ T cells, it can nevertheless directly infect DCs (cis infection) but with a lower efficiency, and for this reason a very small percentage of DCs are positive for the virus in HIV-1-infected individuals [18]. DCs do express the primary HIV-1 receptor, CD4, as well as the two main coreceptors CXCR4 and CCR5, and it has been demonstrated in vitro that they can be infected with both the X4 and R5 strains of HIV-1 [19]. Compared with mature DCs, immature DCs have been shown to be more susceptible to HIV-1 infection due to their ability to pick up viral antigens [20]. Interestingly, pDCs infected with HIV-1 in vitro can transmit the virus to CD4+ T cells (trans infection) [21], thereby suggesting a plausible mechanism by which infected migrating DCs can transmit the virus to CD4+ T cells at sites of activation. However, pDCs are less efficient than mDCs in their ability to transmit the virus [21]. Conversely, the LCs seem to prevent HIV-1 transmission by degrading the captured virus particles [22]. But in another study, it was shown that in vitro activated CD34-derived LCs were found to mediate the trans infection of HIV [23].The cis infection of DCs is functionally different from the DC-mediated HIV-1 trans infection of CD4+ T cells, as the latter involves the trafficking of whole virus particles from DCs to T cells via a virologic synapse and is mediated by viral receptors, coreceptors, and DC-SIGN, or other C-type lectins [24–26]. This sequential endocytosis and exocytosis of whole HIV-1 particles without viral replication is known as the “Trojan horse”model. Using monocyte-derived DCs (MDDCs) it was shown that the productive replication of HIV-1 is dependent on fusion-mediated viral entry and the mature HIV-1 particles were localized to a specialized tetraspanin-enriched subcompartment within the DC cytoplasm [27, 28]. Another study demonstrated that the viral envelope protein gp120 can inhibit the activation of T cells [29].
Due to the unique ability of DCs to transfer the virus to CD4+ T cells, DCs may serve as reservoirs of the virus and continuously supply the T cells with virus over time. Depending on the DC subtype, DCs can serve in varying capacities as reservoirs. Follicular DCs from HIV-1-infected individuals have been shown to carry genetically diverse viral strains not found elsewhere in the body [30], suggesting that these cells may serve as a melting pot for the emergence of new mutations in infected individuals. Moreover, peripheral blood mDCs are not found to have the virus during antiretroviral therapy [31], suggesting that DCs in the lymph nodes serve as the long-term reservoir. A study demonstrating the presence of HIV-1 in mDCs isolated from lymph node biopsies of infected individuals on antiretroviral therapy [32] supports this notion. In addition, pDCs have been ruled out as serving as reservoirs due to their ability to produce IFN-α and an unidentified small molecule [21, 33].
Viral proteins can also play a major role in countering the antiviral effects of DCs in many ways. The HIV-1 protein, Vpr, is known to decrease the expression of CD40, CD80, CD83, and CD86 on MDDCs [34]. Immature MDDCs that were treated with HIV-1 Vpr were incapable of attaining high levels of costimulatory molecules and, thus, failed to mature [34]. Nef, a vital factor needed for viral replication and pathogenesis, has been verified to influence DC phenotype, morphology, and function, thus facilitating the spread of HIV [35].
One of the early studies with peripheral blood mononuclear cells (PBMCs) from HIV-1-infected individuals demonstrated their reduced ability to produce type 1 IFN (IFN-α) in response to HSV-infected fibroblasts or cell-free HSV virions even when corrected for the white blood count [36, 37]. In the absence of distinct phenotypic description of DCs at that time, the study showed that not only do HIV-1-infected individuals have fewer IFN-producing cells but also each cell makes less IFN-α on a per-cell basis in response to HSV-1 compared with controls [38]. Investigators later demonstrated that both the mDCS and pDCs, the key type 1 IFN-producing subset of DCs, were reduced in frequency and functional capacity in the peripheral blood of HIV-1 patients [39–42]. The same studies showed that the pDC numbers correlated with the CD4 numbers or were inversely correlated with the viral load. One of the ways by which the numbers of pDCs are reduced in such infected individuals is through the direct killing of these cells by HIV-1 [33]. Moreover, the virus can utilize the pDCs to block CD4+ T-cell proliferation or induce the differentiation of naive CD4+ T cells into T-regulatory cells. This can be achieved in pDCs through the viral-induced expression of indoleamine 2,3-deoxygenase, which is a CD4+ T-cell suppressor and regulatory T-cell activator [43, 44]. Because the pDCs are the primary cells equipped with major viral nucleic acid–sensing TLRs, HIV-1 can suppress the activation of TLR 7 and 8 as well as block the production of IFN-α [45, 46]. Moreover, the pDCs from HIV-infected individuals demonstrate defective stimulation in an allogeneic mixed lymphocyte reaction and a reduced activation phenotype [47, 48]. However, the role of IFN-α in HIV-1 infection is complex and numerous studies suggest it can play both protective and pathogenic roles. Both in vitro and in vivo data using severe combined immunodeficient human mouse models have demonstrated the protective role of IFN-α [49–54]. In addition, the negative correlation between the pDCs and the viral load confirms this notion [42, 47].However, during chronic infection, IFN-α has been found to contribute to TRAIL-induced and Fas/FasL-induced apoptosis of infected as well as uninfected CD4+ T cells, thereby suggesting the role of IFN-α in leading to the exacerbation of immunopathogenesis and disease progression [55–58]. Although the exact mechanism by which IFN-α leads to immunopathogenesis is still not clear but current studies suggest that during initial acute HIV infection, IFN-α may play a plausible protective role. However, as the infection progresses it may then lead to a state of chronic immune activation, thereby becoming detrimental to the immune system during chronic infection.
There has been considerable debate regarding the importance of DC-SIGN in facilitating virus entry, binding, and transmission. Early studies highlighted the crucial role that DC-SIGN played in the trans-infection of HIV, but subsequent studies have shown the trans-infection to occur by DC-SIGN independent mechanism as well [59–66]. Moreover, the macrophages express relatively more DC-SIGN than the DCs in the lymphoid tissues and blood, suggesting that the macrophages may in turn be responsible for the HIV trans-infection in vivo [61, 67, 68]. But this has not yet been demonstrated. In addition, it has been observed that the monocyte-derived macrophages, like the MDDCs, can also transmit the virus in a DC-SIGN independent manner [69, 70]. One of the biggest discrepancies has been the difference in expression of DC-SIGN observed on DCs isolated from tissues and the DC subsets observed in vivo [60, 61, 71–74]. These discrepancies possibly suggest that the role of DC-SIGN may be limited in DC-mediated transmission in vivo (Table II).
TABLE II.
Conflicting Issues Surrounding HIV-DCs and HCV-DCs
| HIV and DCs | Issues | References |
|---|---|---|
| Role of DC-SIGN | Studies suggesting that DC-SIGN is important to facilitate the trans-infection of HIV | [59, 60, 62] |
| Studies demonstrating trans-infection to occur in a DC-SIGN independent manner | [61, 63–66] | |
| Dendritic cells versus macrophages | Macrophages express more DC-SIGN than DCs in the lymphoid tissues and blood | [61, 67, 68] |
| Monocyte-derived macrophages can transmit virus in DC-SIGN independent manner like the monocyte-derived dendritic cells | [69, 70] | |
| Expression levels of DC-SIGN | The levels of DC-SIGN expressed by DCs differ between those isolated from tissues and those obtained in vivo | [60, 61, 71–74] |
| HCV and DCs | Issues | References |
| Quality of conventional DCs | Studies demonstrating the functional impairment of conventional DCs | [147, 148] |
| Studies demonstrating the presence of functional conventional dendritic cells during chronic HCV infection | [149–152] |
HUMAN T-CELL LEUKEMIA VIRUS TYPE 1 AND DENDRITIC CELLS
Human T-cell leukemia virus type 1 is classified in the genus Deltaretrovirus of the sub-family Orthoretrovirinae and was the first identified human retrovirus from a patient with cutaneous T-cell cancer almost three decades ago [75–78]. Approximately 10 to 20 million people are infected with HTLV-1 worldwide, mostly along the tropical belt. The infection is endemic in Japan, the Caribbean, parts of South America, and Central Africa [79]. Although a majority of HTLV-1-infected individuals remain asymptomatic carriers and only a very few (<5%) eventually develop the disease [80, 81], HTLV-1 thus represents a classic example of a persistent retroviral infection. However, why certain infected individuals develop the disease while others remain healthy is still not known. Etiologic studies have shown HTLV-1 to be associated with many different clinical syndromes, such as uveitis, alveolitis, dermatitis, thyroiditis, Sjögren’s syndrome, polymyositis, and arthritis [82] as well as two predominant immunologically distinct diseases, oncogenic adult T-cell leukemia (ATL) [83] and neuroinflammatory HTLV-1-associated myelopathy or tropical spastic paraparesis (HAM/TSP) [84].
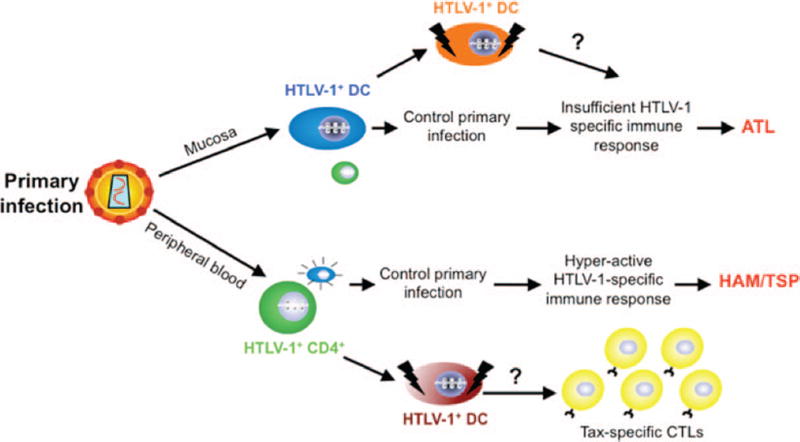
The role of DCs in HTLV-1-associated diseases has not been clearly elucidated, but studies suggest that DCs are of particular significance because the development of HAM/TSP is associated with rapid maturation of DCs whereas ATL involves a maturation defect in this critical cell population (Fig. 3) [85–88]. DCs obtained from patients with HAM/TSP were found to be infected with HTLV-1 and can also be infected in vitro [85, 89]. Autologous infected DCs, as well as those pulsed with inactivated HTLV-1 virions, have also been shown to lead to a strong proliferative response of both CD4+ and CD8+ T cells [90]. Moreover, cell-free HTLV-1 can infect freshly isolated mDCs and pDCs [91]. Such infected DCs can then rapidly transfer the virus to autologous primary CD4+ T cells, resulting in chronic infection as well as leading to their interleukin-2 (IL-2)-independent transformed state [91, 92]. These results suggest that DCs are susceptible to HTLV-1 infection and that their cognate interaction with T cells may contribute to the development of ATL and HAM/TSP. Using a murine DC line (JAWS II) and in vitro differentiated human MDDCs, it was shown that DCs once exposed to Tax undergo activation and maturation, exhibiting alterations in surface phenotype, secreting cytokines/chemokines, and leading to an allogenic and antigen (Tax)-specific immune response [93–96]. The genesis, priming, and maintenance of the Tax-specific CTL response are not completely characterized and a recent study suggested that the HTLV-1-infected CD4+CD25+ T cells stimulate and expand the Tax-specific CD8+ T cells in HAM/TSP patients [97]. However, this study did not demonstrate that infected CD4+ T cells can alone prime naive CD8s from normal donors, and it also did not consider the involvement of DCs or the costimulation pathway in the process. In vitro and in vivo priming assays demonstrated that compared with the CD4+CD25+ T cells, DCs were far more efficient in generating and maintaining a Tax-specific CTL response in peripheral blood lymphocytes from naive normal donors as well as in HHD II mice [98]. In addition, we showed that DCs do play a role in determining the degree of infection (proviral load, Tax mRNA expression, and cellular immune response) in cell-free versus cell-associated HTLV-1 infections using an in vivo transgenic murine model system that allowed for the selective transient depletion of DCs [99]. To further understand the intricate relationship between the DCs and cell-free HTLV-1, we studied the kinetics of interaction in isolation using murine bone marrow-derived dendritic cells and observed selective downregulation of many ISGs, chemokines, cytokines, and MHC class I molecules [100]. Our results demonstrate a clear viral-mediated strategy in targeted downregulation of key signaling molecules as well as those required for antigen presentation to the CTLs. Investigators have also recently demonstrated that free HTLV-1 induces TLR7-dependent innate immune response through pDCs by the production of IFN-α and by upregulating expression of TRAIL [101]. Moreover, such HTLV-1-stimulated pDCs were transformed into IFN-producing killer pDCs [101].
FIGURE 3.
Hypothesized disease outcome following entry of human T-cell leukemia virus type 1 (HTLV-1).The virus can enter the body through either the mucosa or peripheral blood. Following mucosal exposure, local resident dendritic cells (DCs) would be expected to pick up the cells and initially control the infection. However, some infected individuals develop compromised DC functions, leading to insufficient immune response and eventually to adult T-cell leukemia (ATL). In the case of peripheral blood exposure, blood DCs would be able to control the initial threat. However, as chronic infection develops, the DCs are in a state of constant stimulation leading to a hyperactive immune response and eventually to HTLV-1-associated myelopathy or tropical spastic paraparesis (HAM/TSP). CTL = cytotoxic T lymphocyte.
With respect to ATL, DCs are of particular significance as the development of ATL involves a maturation defect in this critical cell population [86–88]. Monocyte-derived immature DCs from patients with ATL were found to have reduced ability to take up antigen and to express surface antigens CD1a and CD86 [86]. In addition, mature DCs differentiated from such cells poorly stimulated the autologous proliferation of CD4+ and CD8+ T cells [86]. Another study showed that patients with ATL have reduced numbers of both mDCs and pDCs compared with controls [87]. Moreover, pDCs from such HTLV-1-infected individuals were found to have impaired IFN-α capacity [87]. The activation and maturation of DCs require the interaction of its CD40 receptor with the cognate CD40L (CD154) expressed on activated T cells [102]. However, CD40L expression was found to be blocked in T cells from patients with ATL but not from either patients with HAM/TSP or normal controls [103]. It was also shown that although the HTLV-1 transcriptional transactivating protein Tax upregulated CD40L gene expression, CD40L expression was absent in HTLV-1 Tax-expressing transformed cell lines through an epigenetic methylation mechanism [103].Moreover, cell-free HTLV-1 can infect freshly isolated mDCs and pDCs [91]. Such infected DCs can then rapidly transfer the virus to autologous primary CD4+ T cells, resulting in chronic infection as well as leading to their IL-2-independent transformed state [91, 92]. These results suggest that DCs are susceptible to HTLV-1 infection and are functionally impaired in cases of ATL, as well as being able to transfer the virus to CD4+ T cells leading to their eventual transformed state.
HEPATITIS VIRUSES B AND C AND DENDRITIC CELLS
Hepatitis viruses B and C are hepatotropic and primarily infect hepatocytes. Both these viruses can cause chronic liver disease and progressive liver injury that may eventually lead to liver cirrhosis, liver failure, and liver cancer [104, 105]. More than 500 million people worldwide are chronically infected with HBV or HCV [106]. HBV belongs to the Hepadnaviridae family whereas HCV is the only known member of the Hepacivirus genus in the family Flaviviridae. The lack of adequate immune response against these viruses is believed to explain their persistence and, thus, current research has been focused on understanding the immune escape strategies of these viruses.
HEPATITIS B VIRUS
Previous studies have demonstrated decreased frequency of circulating blood DC numbers in patients with chronic HBV infection compared with healthy individuals [107–113]. Inmost studies, increased serum alanine aminotransferase levels, an indicator of liver inflammation, were found to correlate reciprocally with the number of circulating mDCs [111, 114] and pDCs [115]. These studies correlate with earlier findings demonstrating increased frequencies of intrahepatic DCs in the portal areas of the liver in patients with chronic HBV infection [112, 114].
A major finding in patients with chronic HBV infection, in addition to reduced frequency of circulating DCs, is the functional impairment of DCs, which may contribute to the insufficient immune response against HBV [111]. Ex vivo patient studies on mDCs demonstrate no or very little reduced expression of the costimulatory molecules; however, expression levels of the inhibitory molecule B7-H1 were found to be highly upregulated [109, 111, 114, 116]. Unlike minor changes in phenotypic expression, DCs from such infected individuals were found to be severely compromised in terms of functional capacity. When the mDCs were stimulated with double-stranded RNA, the cells produced reduced amounts of IFN-β and TNF-α but no significant change in the levels of IL-6, IL-1β, and IL-12 [111, 117]. Such functional modulation of DCs may affect their ability to prime and elicit a T-cell response. mDCs from HBV-infected individuals are less efficient in inducing T-cell proliferation in vitro than those from healthy individuals [111, 116, 118]. Type 1 IFN production following viral challenge is the key feature of pDCs. In contrast to healthy individuals, pDCs from HBV-infected individuals are impaired in their ability to produce IFN-α when stimulated with Staphylococcus aureus Cowan [111], HSV [108], or DNA containing CpG motifs [112, 115, 119]. Not only was the production of IFN-α impaired in such individuals, but the type 1 IFN signaling pathway itself was found to be compromised, which would aid in the eventual replication and survival of the virus [120, 121].
The uptake or binding of HBV and hepatitis B surface antigen (HBsAg) by DCs has been shown but the receptor remains unknown [122–124]. When peripheral blood mDCs were mixed with physiologically relevant levels of either HBV or HBsAg, DCs were found to be reduced in their capacity to stimulate T cells. Moreover, only HBV and not HBsAg was found to significantly reduce the ability of these cells to produce IL-12 [123]. In line with the effect of HBV on blood mDCs, MDDCs were found to be similarly impaired in their ability to stimulate allogeneic T cells and produce IL-12 [122]. Overall, these data demonstrate that HBV or its viral protein is partially involved in disrupting DC function as seen in patients with chronic HBV infection. This is supported by the fact that when the viral load in such individuals is reduced by the administration of nucleoside analogues, mDCs then have increased IL-12 production and are also able to activate T cells ex vivo [110].
HEPATITIS C VIRUS
Previous studies have demonstrated reduced frequencies of both peripheral mDCs and pDCs in chronically infected HCV individuals [125, 126]. Strikingly, the numbers of such cells were found to be increased in the liver of HCV patients [127, 128]. However, it is difficult to ascertain whether the observed increase of DCs in the liver results from the decrease of these cells from the periphery. pDCs derived from HCV-infected subjects were observed to be deficient in IFN-α production upon stimulation by CpG [129]. Furthermore, mDCs from these patients showed a lessened capability to induce an allogeneic mixed lymphocyte reaction (AMLR), which is supported by the low levels of HLA-DR and CD86 expression as well as the high levels of IL-10 production in response to poly-IC stimulation [129]. Conversely, pDCs from HCV-infected subjects were characterized by an increased ability to stimulate an AMLR [129]. The reduced number of DCs is probably due to their induced apoptosis by the HCV core NS3 and NS5 proteins in vitro [130]. Although lower frequencies of mDCs and pDCs are usually detected in patients infected with chronic HCV, there appear to be no phenotypic or functional defects when compared with healthy subjects [131].The reduction in pDCs induced a lower production capacity of IFN-α [131]. Experimental data reveal that DC subsets are all functional in patients with chronic HCV infection, a finding that could be used for DC-based HCV immunotherapy trials, contrary to previous studies [131].
In a comparison of liver mDCs isolated from both noninfected inflamed liver and HCV-infected liver, HCV-infected livers were observed to have a higher expression of MHC class II, CD86, and CD123 [132]. Moreover, mDCs obtained from HCV-infected livers were better stimulators of allogeneic T cells and secreted significantly less IL-10 [132]. The reduction in IL-10 secretion is believed to contribute to the enhanced functional properties of mDCs from HCV-infected livers [132]. Additionally, depletion of IL-10 heightened the ability of mDCs from a noninfected liver to stimulate T cells [132]. In contrast, pDCs were present at much lower frequencies in a HCV-infected liver as well as expressing higher levels of regulatory receptor BDCA2 [132]. However, another study reported that HCV-infected patients had significantly lower numbers of circulating BDCA1+ and BDCA2+ DCs when compared with healthy donors [128]. Moreover, chronic HCV infection was shown to correlate with intrahepatic DC proliferation [128]. These characteristics of mDCs and pDCs are believed to contribute to the viral persistence of HCV [132].
When ex vivo mDCs from individuals with chronic HCV infection were analyzed for expression of surface costimulatory and MHC molecules, little difference was found from healthy individuals [126]. Moreover, mDCs from patients with chronic infection were reduced in their ability to produce IL-12 and TNF-α when stimulated with TLR agonists but the level of IL-6 remained the same [117, 126, 133]. Interestingly, levels of the regulatory cytokine IL-10 were increased compared with controls [129, 134]. As a result of this altered cytokine profile, the mDCs were dampened in their ability to induce proliferation of allogeneic T cells [129, 135, 136]. Similar studies with peripheral pDCs from patients with chronic HCV infection demonstrated their reduced ability to produce IFN-α upon stimulation with TLR agonists [125, 126], as well as their ability to activate T cells [136, 137]. There are many mechanisms by which HCV can disrupt the type 1 IFN signaling pathway [138–140]. Moreover, many HCV proteins can interfere with various molecules of the signaling pathway, such as TRIF (TIR-domain-containing adapter-inducing interferon-β) and Cardif, that are produced following recognition of viral RNA and thereby allow the virus to blunt the type 1 IFN response [140]. DCs can utilize either of their two C-type lectin receptors, DC-SIGN and L-SIGN, to bind to the HCV envelope glycoproteins E1 and E2 [141].
The HCV envelope glycoprotein E2 binding to DC was shown to lead to a shift from immature to mature phenotype through increased expression of cell surface molecules, such as CD83, CD80, CD11c, and MHC class II [142]. DCs that were E2-matured displayed a higher capacity to stimulate T cell proliferation and IFN-γ and IL-12 production in comparison to immature DCs. The promotion of DC E2-maturation was shown to be both time-dependent as well as dose-dependent and was capable of being inhibited by anti-E2 antibodies. Moreover, E2-matured DCs showed a declined activity in endocytosis and phagocytosis upon maturation. Thus, the results indicate that E2 proteins not only induce DC maturation, but may also play a role in immune regulation during HCV infection [142]. Furthermore, DC migration appears to be significantly affected by interactions of HCV E2 with CD81 [128].
DC specific C type lectins DC-SIGN and L-SIGN have been shown to be vital receptors for HCV envelope glycoproteins E1 and E2 [143, 144]. Both HCV E1 and E2 bind to the same binding site on DC-SIGN that HIV-1 binds to. A recent study has shown that C type lectins L-SIGN, expressed mostly on endothelial cells in liver sinusoids, and DC-SIGN, expressed on DCs, are not only able to capture HCV, but do so via specific binding to envelope glycoprotein E2 [145]. C-type lectins L-SIGN and DC-SIGN bind to the HCV E2 glycoprotein with high affinity and growing evidence indicates that liver sinusoidal endothelial cell-expressed L-SIGN may act as a HCV capture receptor and transmitter of infectious virions to neighboring hepatocytes [144, 146]. The HCV envelope glycoprotein E2 binds DC-SIGN and L-SIGN via high-mannose N-glycans [146]. HCV particle capture by SIGN+ cells may not only promote viral infection of hepatocytes, but may also be vital for persistence of infection [145].
However, there are few conflicting studies in the field of HCV-DC research (Table II). Early studies demonstrated the maturation and functional impairment of conventional DCs (cDCs) inHCV infection with decreased IL-12 and increased IL-10 production in vitro [147, 148]. Interestingly, the impaired allostimulatory potential of cDCs has been reported with mixed results [149–152]. This is particularly intriguing as clinical studies clearly demonstrate absence of any impairment of the cDCs in HCV infected individuals.
HERPES SIMPLEX VIRUS AND DENDRITIC CELLS
The term herpes is derived from the Greek word herpin, meaning creeping, and is used to explain the characteristic creeping nature of the eruptions caused by the virus. HSV belongs to the α-herpesviridae family. HSV affects more than one-third of the human population and is responsible for eczema herpeticatum in patients with atopic eczema or other severe life-threatening forms that can occur in immunosuppressed individuals. It enters the body through skin lesions and primarily infects epithelial cells. The most striking feature of HSV is its capability to establish latency after primary infection and, thus, persist in the host and then later reactivate throughout the lifetime of the individual. It was recently demonstrated that in case of Kaposi’s sarcoma-associated herpesvirus (KSHV/HHV8), a member of the γ-herpesviridae, secondary pathogen infection can lead to the reactivation of the latent virus [153].
The in vitro infection of immature DCs by HSV-1 has been shown to result in morphologic changes and in downregulated expression of various costimulatory molecules, such as CD80, CD86, and CD40; the DCmarker CD1a; adhesion molecule CD54 (ICAM1); and MHC I [154]. The selective targeting of MHC class I molecule by HSV-1 allows it to blunt the presentation of viral peptides by infected DCs to naive CD8+ T cells. The formation of the HSV gene product infected cell protein (ICP) 47 with TAP (transporter associated with antigen processing) to ICP47-TAP complex allows it to block the translocation of MHC class I peptide complex to the cell surface and thereby inhibits the MHC class I pathway [155–157]. In addition, HSV-infected DCs have reduced secretion of IL-12, a key cytokine produced by mature DCs, and of the maturation marker CD83, suggesting the ability of HSV-1 to interfere with the maturation process [158]. The HSV-dependent loss of CD83 surface expression has been observed to be due to the degradation of CD83 in the lysosomal compartment as well as by the proteosome and not due to reduced mRNA expression [159, 160]. Among all the viral-mediated strategies discussed regarding the persistence of HSV, one of the most striking was the selective downregulation in surface expression of CD40 and CD54 along with the reduced secretion of IL-12 by the HSV infected DCs [154]. Secretion of IL-12 is highly dependent on CD40-CD40L interaction and, therefore, the reduced secretion of IL-12 by infected DCs can hamper the ability of DCs to generate a successful Th1-mediated cellular immune response. The surface marker CD54 (ICAM1) is required for providing costimulation to the CD8+ T cells independently of CD80 and CD86. In addition, it also required the cells to extravasate across the transendothelial membrane as well as in inhibiting the secretion of IL-4 so as to favor a Th1 mediated immune response.
One of the major primary responses generated by the host against HSV involves DC-mediated T helper cell responses and antibody production. HSV can interfere with the MHC class II antigen presentation of the CD4+ T cells by reducing the expression of the invariant chain and through the interaction of viral envelope glycoprotein B with HLA-DR and HLA-DM polypeptides [161]. In addition, the presence of virus-derived antigen presenting DCs is critical to the generation of a successful CD8+ T-cell response to cutaneous HSV-1 infection [162].
In another approach to deter the host immune response, HSV has been shown to induce apoptosis in DCs in a two-step manner consisting of an early antiapoptotic and a late proapoptotic stage. In the first phase, the HSV glycoprotein D ensures the survival of the HSV-infected cell, through the induction of nuclear factor-κB and thereby protects against Fas-induced apoptosis through the reduction of caspase 8 activities as well as upregulation of apoptotic molecules [163]. During the second phase after sufficient viral replication has taken place, HSV induces apoptosis of immature DCs through the caspase 8 pathway by upregulating TNF-α, TRAIL, and p53 and down-regulating cellular FLICE-inhibitory protein (c-FLIP) [164]. Moreover, HSV has been demonstrated to downregulate the chemokine receptors CCR7 and CXCR4 on DCs, as well as the prostaglandin E(2) receptors EP2 and EP4, and thereby interfere with their migration [165, 166]. Recently it was demonstrated that pDCs found at sites of genital lesions were resistant to HSV infection in vitro and were able to proliferate autologous virus-specific T cells [167]. However, the blood-resident pDCs have been shown to secrete large amounts of type I IFN in response to the HSV antigen [168, 169]. The recognition of HSV by the pDCS was shown to be TLR9 dependent [170–172]. In addition, HSV can enter DCs through either DC-SIGN or the inhibitory receptor paired immunoglobulin-like type 2 receptor (PIR) α [173, 174].
Since HSV infection is restricted to the epidermal layers, studies have shown that the surface resident LCs can also be infected by HSV and that they may be compromised in their function to successfully elicit a fully functional anti-HSV CTL response [175]. However, the apoptosis of such compromised LCs and other infected cells may serve as a source for other resident bystander immature LCs or dermal DCs that can then phagocytose the apoptotic bodies without undergoing maturation. The phagocytosed particles can then be loaded onto MHC class I molecules to be cross-presented to the CD8+ T cells [176].
HUMAN CYTOMEGALOVIRUS AND DENDRITIC CELLS
Human cytomegalovirus is a member of the β-herpesviridae family with a large double-stranded DNA genome. Depending on the geography, the seroprevalence of HCMV can be 40% to 90% of the population. Primary HCMV infection occurs early in life through either saliva or breast milk, leading to latency, lifelong persistence and accompanied by periodic bouts of reactivation that are usually asymptomatic. However, under conditions of systemic immunosuppression, such as in AIDS patients and transplant recipients, HCMV infection can manifest with other complications or even lead to morbidity and mortality. It is, therefore, one of the most common types of opportunistic infections in such immunocompromised individuals.
There are many levels at which HCMV can usurp the activity of DCs in their ability to launch a strong innate and adaptive immune response against the virus and its proteins. HCMV can interfere with the antigen processing and presenting capacity of DCs through the action of various viral proteins. The tegument protein pp65 prevents processing of the viral intermediate early antigen-1 for presentation [177]. The US2 and US11 genes downregulate the surface expression of MHC class I by translocating them to the cytosol for proteosomal degradation [178, 179]. US2 can also target the MHC class II and nonclassic MHC class I molecule HFE [180, 181]. Immunoevasive genes, such as US3, can retain MHC class I and interfere with MHC class II while US6 can block TAP functions, thereby blunting the ability of viral peptides to be loaded within MHC class I molecules [182–184]. US10 has been demonstrated to be involved in delaying intracellular MHC class I trafficking [185].The viral protein pp71 encoded by UL82 can inhibit the transport of MHC class I molecules between the endoplasmic reticulum and cis-Golgi [186]. Other strategies employed by HCMV involve the use of viral homologues for IL-10, chemokine receptors, and products involved in regulating cell-cycle progression or inhibiting apoptosis [187–192].
Recent studies have utilized MDDCs as a primary cell surrogate to understand the interaction between HCMV and DCs. Studies have shown that MDDCs can be infected with fresh isolates of HCMV [193, 194]. The virus utilizes the viral glycoprotein B to bind to DC-SIGN on the MDDCs [195]. It then utilizes the UL128-UL131A genes to facilitate entry [196]. When the expression of various surface markers was analyzed following infection with HCMV, the immature MDDCs matured rapidly and upregulated the costimulatory molecules CD40, CD80, and CD86 but downregulated both MHC class I and II molecules [197]. Such mature MDDCs were also able to upregulate expression of CD95L (FasL) and TRAIL, allowing them to target both PBMCs and T-cell lines for lysis [197]. When different viral strains were used, however, expression of costimulatory molecules was downregulated, DC maturation was inhibited, IL-12 and TNF-α production in response to lipopolysaccharide or CD40L were reduced, but earlier findings about inefficient induction of T-cell proliferation by infected DCs were supported [198]. In addition, MDDCs generated from PBMCs of immunocompetent patients with symptomatic HCMV disease were found to be impaired in their ability to proliferate T cells [199]. The HCMV protein UL18 when bound to immature MDDCs results in the expression of CD83 but no other maturation marker; increased IL-10 and IL-6 secretion; and reduced migration in response to RANTES (regulated upon activation, normal T-cell expressed, and secreted) [200]. HCMV has also been shown to induce the expression of MIP-1α (macrophage inflammatory protein, CCL3), MIP-1β (CCL4), and RANTES (CCL5) to allow the downregulation of CCR1 and CCR5 receptors on immature DCs, thereby interfering with the migratory ability of MDDCs [201].
With regard to the pDCs, HCMV can also lead to the production of type 1 IFNs from these cells but is unable to productively infect the pDCs [202, 203]. Very little viral gene expression was observed in such infected pDCs. However, the pDCs underwent dramatic changes in phenotype and function following infection through the robust production of IFN-α, TNF-α, and IL-6 [203]. Although the soluble factors released from such infected pDCs was unable to proliferate T cells in AMLR, the soluble signals were, nevertheless, able to cause the activation and proliferation of B cells [203]. In addition, in the presence of CD4+ assistance, the soluble factors are able to mediate antibody production in B cell receptor stimulated B cells [203]. In another separate study utilizing pDCs derived from umbical cord blood, no type I IFN production was observed from such pDCs following infection with HCMV [204].
OTHER CHRONIC VIRUSES AND DENDRITIC CELLS
Varicella zoster virus (VZV) is the etiologic agent of varicella (chickenpox) and infects up to 90% of the human population. After the primary infection has been cleared, the virus establishes a lifelong latent infection in the dorsal root and cranial nerve ganglia, the reactivation of which causes herpes zoster (shingles). The cellular immune response is critical for controlling the primary infection as well as preventing the reactivation. DCs are important for controlling and mounting cellular immune response against VZV [205]. Both immature and mature DCs can be infected by VZV [206, 207]. VZV-infected immature DCs do not undergo apoptosis and can also transfer virus to fibroblasts or autologous T cells [206]. VZV infection using mature DCs resulted in the downregulated expression of key surface markers as well as reduced capacity to stimulate the proliferation of allogeneic T cells, suggesting the ability of VZV to alter DC functions [207].
Epstein-Barr virus (EBV) is a γ-herpes virus that infects 95% of adults in the United States and is responsible for mononucleosis, Burkitt’s lymphoma, nasopharyngeal carcinoma, Sézary syndrome, and systemic lupus erythematosus [208, 209]. Following initial infection of mature B cells, EBV undergoes a latent chronic phase with periodic reactivation and production of new virus and large quantities of EBV-encoded RNAs (EBER) [210]. Using immature DCs, researchers demonstrated that EBER could be recognized by TLR3 [211]. Moreover, such activation of TLR3 can cause the DCs to undergo maturation through the upregulation of CD86 and CD83, produce type I IFNs and proinflammatory cytokines, and stimulate allogeneic T cells in a mixed lymphocyte reaction [211]. Recently, human pDCs have been shown to produce IFN-α in response to TLR9 and TLR7 triggering by EBV nucleic acid and EBER, respectively [212].
Human papillomaviruses (HPVs) are a family of about 120 double-stranded DNA viruses that have been linked with a wide variety of epithelial cancers in humans. HPV has been found to be associated with more than 90% of uterine cervical cancers. Of the large number of asymptomatic women with clinically detectable infection, less than 1% develop the chronic malignancy [213]. The viral oncoprotein E7 can block the DC-produced IFN-α-mediated antiviral effects by inhibiting the induction of IFN-α-inducible genes as well as by inhibiting the activation of the IFN-β promoter [214, 215]. In addition, the expression of E7 can downregulate the activation of both E7- and non-E7-specific CD8+ T cells by manipulating the immunogenic property of DCs into tolerogenic property [216]. Similarly, HPV E6 can downregulate the expression of IL-18, which is required for the generation of the CD8+ T-cell response [217].The other viral protein (E5) mediated acidification of endosomes affects antigen processing and presentation in APCs [218]. Both LCs and DCs have been shown to recognize and target tumor cells expressing HPV E6 and E7 for lysis [219].
Human adenoviruses (HAdVs) are nonenveloped viruses with a 30- to 40-kb linear double-stranded DNA genome and belong to the family Adenoviridae. These viruses lead to upper respiratory infections and are a significant cause of morbidity in immunocompetent individuals and mortality in those who are immunocompromised. In addition, HAdVs can also lead to various ocular adenoviral infections [220, 221]. Both mDCs and pDCs can be infected with HAdV through either the coxsackievirus and adenovirus receptor (CAR) or independently of CAR by using lactoferrin and DC-SIGN [222, 223]. In addition, DCs upon infection with HAdV produce early and late viral antigens aswell as altered expression of surfacemarkers but can still strongly stimulate CD8+ T cells [224]. However, infection of monocytes with HAdV prevents their differentiation into DCs [224].
CONCLUSIONS AND FUTURE DIRECTIONS
The burgeoning effect of chronic viral infections has made us delve deeper into the intricate host–pathogen relationship, particularly between viruses and DCs. As the professional antigen presenting cells that serve at the crucial junction between innate and adaptive immunity, the role of dendritic cells is very critical. From serving on the first line of defense to determining the fate of the adaptive immune response, dendritic cells serve as the conductor of the immune system. Hence, their role in the generation and maintenance of a successful immune response is pivotal.
Viruses have identified the important link that DCs play and have evolved mechanisms to evade and exploit the defense tactics of DCs. The growing number of chronic viral infections worldwide is a serious health crisis. Most of the chronic human viruses, such as HIV-1, HTLV-1, HBV and HCV, HSV-1, HCMV, VZV, EBV, HPV, and HAdV, have developed unique strategies to subvert and hijack the functions of DCs to their advantage to allow them to persist in the host. It is, therefore, imperative that future studies should address and explain the mechanistic approach of viral mediated infection strategy of DCs as well as identify the key viral and host proteins/receptors that are involved in subverting the functions of these cells. To successfully fight chronic viruses, future vaccines must depend on restoring the functional capacity of DCs to elicit a successful innate and adaptive immune response. Therefore, a full understanding of the different tactics utilized by viruses to overcome the host immune response and in particular the dendritic cells is crucial.
GLOSSARY OF TERMS
- AMLR
allogeneic mixed lymphocyte reaction
- APC
antigen presenting cell
- ATL
adult T cell leukemia
- BDCA
blood dendritic cell antigen
- CAR
coxsackievirus and adenovirus receptor
- c-FLIP
cellular FLICE-inhibitory protein
- cDCs
conventional dendritic cells
- CTL
cytotoxic T lymphocyte
- CCL
C-C motif chemokine ligand
- CXCL
C-X-C motif chemokine ligand
- DC
dendritic cells
- DCIR
dendritic cell immunoreceptor
- DC-SIGN
dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin
- EBER
EBV-encoded RNAs
- EBV
Epstein-Barr virus
- GM-CSF
granulocyte macrophage colony stimulating factor
- HAM/TSP
HTLV-1 associated myelopathy/tropical spastic paraparesis
- HAdV
human adenovirus
- HBV
human hepatitis B virus
- HCV
human hepatitis C virus
- HCMV
human cytomegalovirus
- HIV
human immunodeficiency virus
- HPV
human papillomavirus
- HSV
herpes simplex virus
- HTLV
human T cell leukemia virus
- ICAM
intercellular adhesion molecule
- ICP
infected cell protein
- IL
interleukin
- ILT
immunoglobulin like transcripts
- LCs
Langerhan cells
- mDCs
myeloid DCs
- MCP
monocyte chemoattractant protein
- MDDC
monocyte derived dendritic cells
- MIP
macrophage inflammatory protein
- pDCs
plasmacytoid dendritic cells
- PBMC
peripheral blood mononuclear cells
- PIR
paired immunoglobulinlike type 2 receptor
- TAP
transporter associated with antigen processing
- Tax
HTLV-1 transcription transactivating protein
- TLR
Toll like receptor
- TGF-β
transforming growth factor
- TRAIL
TNF-α related apoptosis inducing ligand
- VZV
varicella-zoster virus
Footnotes
Declaration of Interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.O’Doherty U, Peng M, Gezelter S, et al. Human blood contains two subsets of dendritic cells, one immunologically mature and the other immature. Immunology. 1994;82:487–493. [PMC free article] [PubMed] [Google Scholar]
- 2.Valladeau J, Saeland S. Cutaneous dendritic cells. Semin Immunol. 2005;17:273–283. doi: 10.1016/j.smim.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 3.van Der Aar AM, Sylva-Steenland RM, Bos JD, et al. Loss of TLR2, TLR4, and TLR5 on Langerhans cells abolishes bacterial recognition. J Immunol. 2007;178:1986–1990. doi: 10.4049/jimmunol.178.4.1986. [DOI] [PubMed] [Google Scholar]
- 4.Flacher V, Bouschbacher M, Verronese E, et al. Human Langerhans cells express a specific TLR profile and differentially respond to viruses and Gram-positive bacteria. J Immunol. 2006;177:7959–7967. doi: 10.4049/jimmunol.177.11.7959. [DOI] [PubMed] [Google Scholar]
- 5.Klechevsky E, Liu M, Morita R, et al. Understanding human myeloid dendritic cell subsets for the rational design of novel vaccines. Hum Immunol. 2009;70:281–288. doi: 10.1016/j.humimm.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klechevsky E, Morita R, Liu M, et al. Functional specializations of human epidermal Langerhans cells and CD14 dermal dendritic cells. Immunity. 2008;29:497–510. doi: 10.1016/j.immuni.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dzionek A, Fuchs A, Schmidt P, et al. BDCA-2, BDCA-3, and BDCA-4: Three markers for distinct subsets of dendritic cells in human peripheral blood. J Immunol. 2000;165:6037–6046. doi: 10.4049/jimmunol.165.11.6037. [DOI] [PubMed] [Google Scholar]
- 8.Dzionek A, Sohma Y, Nagafune J, et al. BDCA-2, a novel plasmacytoid dendritic cell-specific type II C-type lectin, mediates antigencaptureandis a potent inhibitor of interferon alpha/beta induction. J Exp Med. 2001;194:1823–1834. doi: 10.1084/jem.194.12.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cao W, Rosen DB, Ito T, et al. Plasmacytoid dendritic cell-specific receptor ILT7-Fc epsilonRI gamma inhibits Toll-like receptor-induced interferon production. J Exp Med. 2006;203:1399–1405. doi: 10.1084/jem.20052454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siegal FP, Kadowaki N, Shodell M, et al. The nature of the principal type 1 interferon-producing cells in human blood. Science. 1999;284:1835–1837. doi: 10.1126/science.284.5421.1835. [DOI] [PubMed] [Google Scholar]
- 11.Megjugorac NJ, Young HA, Amrute SB, et al. Virally stimulated plasmacytoid dendritic cells produce chemokines and induce migration of T and NK cells. J Leukoc Biol. 2004;75:504–514. doi: 10.1189/jlb.0603291. [DOI] [PubMed] [Google Scholar]
- 12.Penna G, Vulcano M, Roncari A, et al. Cutting edge: Differential chemokine production by myeloid and plasmacytoid dendritic cells. J Immunol. 2002;169:6673–6676. doi: 10.4049/jimmunol.169.12.6673. [DOI] [PubMed] [Google Scholar]
- 13.Penna G, Vulcano M, Sozzani S, Adorini L. Differential migration behavior and chemokine production by myeloid and plasmacytoid dendritic cells. Hum Immunol. 2002;63:1164–1171. doi: 10.1016/s0198-8859(02)00755-3. [DOI] [PubMed] [Google Scholar]
- 14.Jego G, Palucka AK, Blanck JP, et al. Plasmacytoid dendritic cells induce plasma cell differentiation through type I interferon and interleukin 6. Immunity. 2003;19:225–234. doi: 10.1016/s1074-7613(03)00208-5. [DOI] [PubMed] [Google Scholar]
- 15.Fonteneau JF, Gilliet M, Larsson M, et al. Activation of influenza virus-specific CD4 and CD8 T cells: A new role for plasmacytoid dendritic cells in adaptive immunity. Blood. 2003;101:3520–3526. doi: 10.1182/blood-2002-10-3063. [DOI] [PubMed] [Google Scholar]
- 16.Di Pucchio T, Chatterjee B, Smed-Sorensen A, et al. Direct proteasome-independent cross-presentation of viral antigen by plasmacytoid dendritic cells on major histocompatibility complex class I. Nat Immunol. 2008;9:551–557. [Google Scholar]
- 17.Matsui T, Connolly JE, Michnevitz M, et al. CD2 distinguishes two subsets of human plasmacytoid dendritic cells with distinct phenotype and functions. J Immunol. 2009;182:6815–6823. doi: 10.4049/jimmunol.0802008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu L, KewalRamani VN. Dendritic-cell interactions with HIV: Infection and viral dissemination. Nat Rev Immunol. 2006;6:859–868. doi: 10.1038/nri1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patterson S, Rae A, Hockey N, et al. Plasmacytoid dendritic cells are highly susceptible to human immunodeficiency virus type 1 infection and release infectious virus. J Virology. 2001;75:6710–6713. doi: 10.1128/JVI.75.14.6710-6713.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Canque B, Bakri Y, Camus S, et al. The susceptibility to X4 and R5 human immunodeficiency virus-1 strains of dendritic cells derived in vitro from CD34( ) hematopoietic progenitor cells is primarily determined by their maturation stage. Blood. 1999;93:3866–3875. [PubMed] [Google Scholar]
- 21.Groot F, van Capel TM, Kapsenberg ML, et al. Opposing roles of blood myeloid and plasmacytoid dendritic cells in HIV-1 infection of T cells: Transmission facilitation versus replication inhibition. Blood. 2006;108:1957–1964. doi: 10.1182/blood-2006-03-010918. [DOI] [PubMed] [Google Scholar]
- 22.de Witte L, Nabatov A, Pion M, et al. Langerin is a natural barrier to HIV-1 transmission by Langerhans cells. Nat Med. 2007;13:367–371. doi: 10.1038/nm1541. [DOI] [PubMed] [Google Scholar]
- 23.Fahrbach KM, Barry SM, Ayehunie S, et al. Activated CD34-derived Langerhans cells mediate transinfection with human immunodeficiency virus. J Virology. 2007;81:6858–6868. doi: 10.1128/JVI.02472-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dong C, Janas AM, Wang JH, et al. Characterization of human immunodeficiency virus type 1 replication in immature and mature dendritic cells reveals dissociable cis- and trans-infection. J Virology. 2007;81:11352–11362. doi: 10.1128/JVI.01081-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Donald D, Wu L, Bohks SM, et al. Recruitment of HIV and its receptors to dendritic cell-T cell junctions. Science. 2003;300:1295–1297. doi: 10.1126/science.1084238. [DOI] [PubMed] [Google Scholar]
- 26.Wang JH, Janas AM, Olson WJ, Wu L. Functionally distinct transmission of human immunodeficiency virus type 1 mediated by immature and mature dendritic cells. J Virology. 2007;81:8933–8943. doi: 10.1128/JVI.00878-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garcia E, Nikolic DS, Piguet V. HIV-1 replication in dendritic cells occurs through a tetraspanin-containing compartment enriched in AP-3. Traffic. 2008;9:200–214. doi: 10.1111/j.1600-0854.2007.00678.x. [DOI] [PubMed] [Google Scholar]
- 28.Janas AM, Dong C, Wang JH, Wu L. Productive infection of human immunodeficiency virus type 1 in dendritic cells requires fusion-mediated viral entry. Virology. 2008;375:442–451. doi: 10.1016/j.virol.2008.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chougnet C, Gessani S. Role of gp120 in dendritic cell dysfunction in HIV infection. J Leukoc Biol. 2006;80:994–1000. doi: 10.1189/jlb.0306135. [DOI] [PubMed] [Google Scholar]
- 30.Keele BF, Tazi L, Gartner S, et al. Characterization of the follicular dendritic cell reservoir of human immunodeficiency virus type 1. J Virology. 2008;82:5548–5561. doi: 10.1128/JVI.00124-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Otero M, Nunnari G, Leto D, et al. Peripheral blood dendritic cells are not a major reservoir for HIV type 1 in infected individuals on virally suppressive HAART. AIDS Res Hum Retrovir. 2003;19:1097–1103. doi: 10.1089/088922203771881194. [DOI] [PubMed] [Google Scholar]
- 32.Haase AT, Henry K, Zupancic M, et al. Quantitative image analysis of HIV-1 infection in lymphoid tissue. Science. 1996;274:985–989. doi: 10.1126/science.274.5289.985. [DOI] [PubMed] [Google Scholar]
- 33.Meyers JH, Justement JS, Hallahan CW, et al. Impact of HIV on cell survival and antiviral activity of plasmacytoid dendritic cells. PLoS One. 2007;2:e458. doi: 10.1371/journal.pone.0000458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muthumani K, Hwang DS, Choo AY, et al. HIV-1 Vpr inhibits the maturation and activation of macrophages and dendritic cells in vitro. Int Immunol. 2005;17:103–116. doi: 10.1093/intimm/dxh190. [DOI] [PubMed] [Google Scholar]
- 35.Quaranta MG, Mattioli B, Giordani L, Viora M. The immunoregulatory effects of HIV-1 Nef on dendritic cells and the pathogenesis of AIDS. Faseb J. 2006;20:2198–2208. doi: 10.1096/fj.06-6260rev. [DOI] [PubMed] [Google Scholar]
- 36.Lopez C, Fitzgerald PA, Siegal FP. Severe acquired immune deficiency syndrome in male homosexuals: Diminished capacity to make interferon-alpha in vitro associated with severe opportunistic infections. J Infect Dis. 1983;148:962–966. doi: 10.1093/infdis/148.6.962. [DOI] [PubMed] [Google Scholar]
- 37.Siegal FP, Lopez C, Fitzgerald PA, et al. Opportunistic infections in acquired immune deficiency syndrome result from synergistic defects of both the natural and adaptive components of cellular immunity. J Clin Invest. 1986;78:115–123. doi: 10.1172/JCI112539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Howell DM, Feldman SB, Kloser P, Fitzgerald-Bocarsly P. Decreasedfrequencyoffunctionalnatural interferon-producing cells in peripheral blood of patients with the acquired immune deficiency syndrome. Clin Immunol Immunopathol. 1994;71:223–230. doi: 10.1006/clin.1994.1076. [DOI] [PubMed] [Google Scholar]
- 39.Donaghy H, Pozniak A, Gazzard B, et al. Loss of blood CD11c( ) myeloid and CD11c( ) plasmacytoid dendritic cells in patients with HIV-1 infection correlates with HIV-1 RNA virus load. Blood. 2001;98:2574–2576. doi: 10.1182/blood.v98.8.2574. [DOI] [PubMed] [Google Scholar]
- 40.Feldman S, Stein D, Amrute S, et al. Decreasedinterferon-alpha productionin HIV-infectedpatients correlates with numerical and functional deficiencies in circulating type 2 dendritic cell precursors. Clin Immunol. 2001;101:201–210. doi: 10.1006/clim.2001.5111. [DOI] [PubMed] [Google Scholar]
- 41.Pacanowski J, Kahi S, Baillet M, et al. Reduced blood CD123 (lymphoid) and CD11c (myeloid) dendritic cell numbers in primary HIV-1 infection. Blood. 2001;98:3016–3021. doi: 10.1182/blood.v98.10.3016. [DOI] [PubMed] [Google Scholar]
- 42.Soumelis V, Scott I, Gheyas F, et al. Depletion of circulating natural type 1 interferon-producing cells in HIV-infected AIDS patients. Blood. 2001;98:906–912. doi: 10.1182/blood.v98.4.906. [DOI] [PubMed] [Google Scholar]
- 43.Boasso A, Herbeuval JP, Hardy AW, et al. HIV inhibits CD4 T-cell proliferation by inducing indoleamine 2,3-dioxygenase in plasmacytoid dendritic cells. Blood. 2007;109:3351–3359. doi: 10.1182/blood-2006-07-034785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Manches O, Munn D, Fallahi A, et al. HIV-activated human plasmacytoid DCsinduce Tregs through an indoleamine 2,3-dioxygenase-dependent mechanism. J Clin Invest. 2008;118:3431–3439. doi: 10.1172/JCI34823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Martinson JA, Roman-Gonzalez A, Tenorio AR, et al. Dendritic cells from HIV-1 infected individuals are less responsive to toll-like receptor (TLR) ligands. Cell Immunol. 2007;250:75–84. doi: 10.1016/j.cellimm.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tilton JC, Manion MM, Luskin MR, et al. Human immunodeficiency virus viremia induces plasmacytoid dendritic cell activation in vivo and diminished alpha interferon production in vitro. J Virology. 2008;82:3997–4006. doi: 10.1128/JVI.01545-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Donaghy H, Gazzard B, Gotch F, Patterson S. Dysfunction and infection of freshly isolated blood myeloid and plasmacytoid dendritic cells in patients infected with HIV-1. Blood. 2003;101:4505–4511. doi: 10.1182/blood-2002-10-3189. [DOI] [PubMed] [Google Scholar]
- 48.Dillon SM, Robertson KB, Pan SC, et al. Plasmacytoid and myeloid dendritic cells with a partial activation phenotypeaccumulate inlymphoidtissue during asymptomatic chronic HIV-1 infection. J Acquir Immune Defic Syndr. 2008;48:1–12. doi: 10.1097/QAI.0b013e3181664b60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bednarik DP, Mosca JD, Raj NB, Pitha PM. Inhibition of human immunodeficiency virus (HIV) replication by HIV-trans-activated alpha 2-interferon. Proc Natl Acad Sci U S A. 1989;86:4958–4962. doi: 10.1073/pnas.86.13.4958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gendelman HE, Baca LM, Turpin J, et al. Regulation of HIV replication in infected monocytes by IFN-alpha. Mechanisms for viral restriction. J Immunol. 1990;145:2669–2676. [PubMed] [Google Scholar]
- 51.Shirazi Y, Pitha PM. Alpha interferon inhibits early stages of the human immunodeficiency virus type 1 replication cycle. J Virology. 1992;66:1321–1328. doi: 10.1128/jvi.66.3.1321-1328.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Poli G, Orenstein JM, Kinter A, et al. Interferon-alpha but not AZT suppresses HIV expression in chronically infected cell lines. Science. 1989;244:575–577. doi: 10.1126/science.2470148. [DOI] [PubMed] [Google Scholar]
- 53.Vieillard V, Jouveshomme S, Leflour N, et al. Transfer of human CD4( ) T lymphocytes producing beta interferon in Hu-PBL-SCIDmice controls human immunodeficiency virus infection. J Virology. 1999;73:10281–10288. doi: 10.1128/jvi.73.12.10281-10288.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lapenta C, Santini SM, Proietti E, et al. Type I interferon is a powerful inhibitor of invivo HIV-1 infection and preserves human CD4( ) T cells from virus-induced depletion in SCID mice transplanted with human cells. Virology. 1999;263:78–88. doi: 10.1006/viro.1999.9869. [DOI] [PubMed] [Google Scholar]
- 55.Lum JJ, Pilon AA, Sanchez-Dardon J, et al. Induction of cell death in human immunodeficiency virus-infected macrophages and resting memory CD4 T cells by TRAIL/Apo2l. J Virology. 2001;75:11128–11136. doi: 10.1128/JVI.75.22.11128-11136.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Herbeuval JP, Boasso A, Grivel JC, et al. TNF-related apoptosis-inducing ligand (TRAIL) in HIV-1-infected patients and its in vitro production by antigen-presenting cells. Blood. 2005;105:2458–2464. doi: 10.1182/blood-2004-08-3058. [DOI] [PubMed] [Google Scholar]
- 57.Miura Y, Misawa N, Maeda N, et al. Critical contribution of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) to apoptosis of human CD4 T cells in HIV-1-infected hu-PBL-NOD-SCID mice. J Exp Med. 2001;193:651–660. doi: 10.1084/jem.193.5.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lichtner M, Maranon C, Vidalain PO, et al. HIV type 1-infected dendritic cells induce apoptotic death in infected and uninfected primary CD4 T lymphocytes. AIDS Res Hum Retrovir. 2004;20:175–182. doi: 10.1089/088922204773004897. [DOI] [PubMed] [Google Scholar]
- 59.Geijtenbeek TB, Kwon DS, Torensma R, et al. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell. 2000;100:587–597. doi: 10.1016/s0092-8674(00)80694-7. [DOI] [PubMed] [Google Scholar]
- 60.Geijtenbeek TB, Torensma R, van Vliet SJ, et al. Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell. 2000;100:575–585. doi: 10.1016/s0092-8674(00)80693-5. [DOI] [PubMed] [Google Scholar]
- 61.Granelli-Piperno A, Pritsker A, Pack M, et al. Dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin/CD209 is abundant on macrophages in the normal human lymph node and is not required for dendritic cell stimulation of the mixed leukocyte reaction. J Immunol. 2005;175:4265–4273. doi: 10.4049/jimmunol.175.7.4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Trumpfheller C, Park CG, Finke J, et al. Cell type-dependent retention and transmission of HIV-1 by DC-SIGN. Int Immunol. 2003;15:289–298. doi: 10.1093/intimm/dxg030. [DOI] [PubMed] [Google Scholar]
- 63.Wu L, Bashirova AA, Martin TD, et al. Rhesus macaque dendritic cells efficiently transmit primate lentiviruses independently of DC-SIGN. Proc Natl Acad Sci U S A. 2002;99:1568–1573. doi: 10.1073/pnas.032654399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu L, Martin TD, Vazeux R, et al. Functional evaluation of DC-SIGN monoclonal antibodies reveals DC-SIGN interactions with ICAM-3 do not promote human immunodeficiency virus type 1 transmission. J Virology. 2002;76:5905–5914. doi: 10.1128/JVI.76.12.5905-5914.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Baribaud F, Pohlmann S, Leslie G, et al. Quantitative expression and virus transmission analysis of DC-SIGN on monocyte-derived dendritic cells. J Virology. 2002;76:9135–9142. doi: 10.1128/JVI.76.18.9135-9142.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gummuluru S, Rogel M, Stamatatos L, Emerman M. Binding of human immunodeficiency virus type 1 to immature dendritic cells can occur independently of DC-SIGN and mannose binding C-type lectin receptors via a cholesterol-dependent pathway. J Virology. 2003;77:12865–12874. doi: 10.1128/JVI.77.23.12865-12874.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Soilleux EJ, Morris LS, Leslie G, et al. Constitutive and induced expression of DC-SIGN on dendritic cell and macrophage subpopulations in situ and in vitro. J Leukoc Biol. 2002;71:445–457. [PubMed] [Google Scholar]
- 68.Krutzik SR, Tan B, Li H, et al. TLR activation triggers the rapid differentiation of monocytes into macrophages and dendritic cells. Nat Med. 2005;11:653–660. doi: 10.1038/nm1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nguyen DG, Hildreth JE. Involvement of macrophage mannose receptor in the binding and transmission of HIV by macrophages. Eur J Immunol. 2003;33:483–493. doi: 10.1002/immu.200310024. [DOI] [PubMed] [Google Scholar]
- 70.Chehimi J, Luo Q, Azzoni L, et al. HIV-1 transmission and cytokine-induced expression of DC-SIGN in human monocyte-derived macrophages. J Leukoc Biol. 2003;74:757–763. doi: 10.1189/jlb.0503231. [DOI] [PubMed] [Google Scholar]
- 71.Soilleux EJ, Morris LS, Lee B, et al. Placental expression of DC-SIGN may mediate intrauterine vertical transmission of HIV. J Pathol. 2001;195:586–592. doi: 10.1002/path.1026. [DOI] [PubMed] [Google Scholar]
- 72.Jameson B, Baribaud F, Pohlmann S, et al. Expression of DC-SIGN by dendritic cells of intestinal and genital mucosae in humans and rhesus macaques. J Virology. 2002;76:1866–1875. doi: 10.1128/JVI.76.4.1866-1875.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Engering A, van Vliet SJ, Hebeda K, et al. Dynamic populations of dendritic cell-specific ICAM-3 grabbing nonintegrin-positive immature dendritic cells and liver/lymph node-specific ICAM-3 grabbing nonintegrin-positive endothelial cells in the outer zones of the paracortex of human lymph nodes. Am J Pathol. 2004;164:1587–1595. doi: 10.1016/S0002-9440(10)63717-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Soilleux EJ, Coleman N. Langerhans cells and the cells of Langerhans cell histiocytosis do not express DC-SIGN. Blood. 2001;98:1987–1988. doi: 10.1182/blood.v98.6.1987. [DOI] [PubMed] [Google Scholar]
- 75.Poiesz BJ, Ruscetti FW, Gazdar AF, et al. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci U S A. 1980;77:7415–7419. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Poiesz BJ, Ruscetti FW, Reitz MW, et al. Isolation of a new type C retrovirus (HTLV) in primary uncultured cells of a patient with Sezary T-cell leukaemia. Nature. 1981;294:268–271. doi: 10.1038/294268a0. [DOI] [PubMed] [Google Scholar]
- 77.Gallo RC. The discovery of the first human retrovirus: HTLV-1 and HTLV-2. Retrovirology. 2005;2:17. doi: 10.1186/1742-4690-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gallo RC. History of the discoveries of the first human retroviruses: HTLV-1 and HTLV-2. Oncogene. 2005;24:5926–5930. doi: 10.1038/sj.onc.1208980. [DOI] [PubMed] [Google Scholar]
- 79.Proietti FA, Carneiro-Proietti AB, Catalan-Soares BC, Murphy EL. Global epidemiology of HTLV-I infection and associated diseases. Oncogene. 2005;24:6058–6068. doi: 10.1038/sj.onc.1208968. [DOI] [PubMed] [Google Scholar]
- 80.Kaplan JE, Osame M, Kubota H, et al. The risk of development of HTLV-I-associated myelopathy/tropical spastic paraparesis among persons infected with HTLV-I. J Acquir Immune Defic Syndr. 1990;3:1096–1101. [PubMed] [Google Scholar]
- 81.Murphy EL, Hanchard B, Figueroa JP, et al. Modelling the risk of adult T-cell leukemia/lymphoma in persons infected with human T-lymphotropic virus type I. Intl J Cancer. 1989;43:250–253. doi: 10.1002/ijc.2910430214. [DOI] [PubMed] [Google Scholar]
- 82.Ohshima K. Pathological features of diseases associated with human T-cell leukemia virus type I. Cancer Sci. 2007;98:772–778. doi: 10.1111/j.1349-7006.2007.00456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Uchiyama T, Yodoi J, Sagawa K, et al. Adult T-cell leukemia: Clinical and hematologic features of 16 cases. Blood. 1977;50:481–492. [PubMed] [Google Scholar]
- 84.Osame M, Usuku K, Izumo S, et al. HTLV-I associated myelopathy, a new clinical entity. Lancet. 1986;1:1031–1032. doi: 10.1016/s0140-6736(86)91298-5. [DOI] [PubMed] [Google Scholar]
- 85.Ali A, Patterson S, Cruickshank K, et al. Dendritic cells infected in vitro with human T cell leukaemia/lymphoma virus type-1 (HTLV-1); enhanced lymphocytic proliferation and tropical spastic paraparesis. Clin Exp Immunol. 1993;94:32–37. doi: 10.1111/j.1365-2249.1993.tb05973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Makino M, Wakamatsu S, Shimokubo S, et al. Production of functionally deficient dendritic cells from HTLV-I-infected monocytes: Implications for the dendritic cell defect in adult T cell leukemia. Virology. 2000;274:140–148. doi: 10.1006/viro.2000.0445. [DOI] [PubMed] [Google Scholar]
- 87.Hishizawa M, Imada K, Kitawaki T, et al. Depletion and impaired interferon-alpha-producing capacity of blood plasmacytoid dendritic cells in human T-cell leukaemia virus type I-infected individuals. Br J Haematol. 2004;125:568–575. doi: 10.1111/j.1365-2141.2004.04956.x. [DOI] [PubMed] [Google Scholar]
- 88.Makino M, Utsunomiya A, Maeda Y, et al. Association of CD40 ligand expression on HTLV-I-infected T cells and maturation of dendritic cells. Scand J Immunol. 2001;54:574–581. doi: 10.1046/j.1365-3083.2001.01003.x. [DOI] [PubMed] [Google Scholar]
- 89.Macatonia SE, Cruickshank JK, Rudge P, Knight SC. Dendritic cells from patients with tropical spastic paraparesis are infected with HTLV-1 and stimulate autologous lymphocyte proliferation. AIDS Res Hum Retrovir. 1992;8:1699–1706. doi: 10.1089/aid.1992.8.1699. [DOI] [PubMed] [Google Scholar]
- 90.Makino M, Shimokubo S, Wakamatsu SI, et al. The role of human T-lymphotropic virus type 1 (HTLV-1)-infected dendritic cells in the development of HTLV-1-associated myelopathy/tropical spastic paraparesis. J Virology. 1999;73:4575–4581. doi: 10.1128/jvi.73.6.4575-4581.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jones KS, Petrow-Sadowski C, Huang YK, et al. Cell-free HTLV-1 infects dendritic cells leading to transmission and transformation of CD4( ) T cells. Nat Med. 2008;14:429–436. [Google Scholar]
- 92.Jain P, Manuel SL, Khan ZK, et al. DC-SIGN mediates cell-free infection and transmission of human T-cell lymphotropic virus type 1 by dendritic cells. J Virology. 2009;83:10908–10921. doi: 10.1128/JVI.01054-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mostoller K, Norbury CC, Jain P, Wigdahl B. Human T-cell leukemia virus type I Tax induces the expression of dendritic cell markers associated with maturation and activation. J Neurovirol. 2004;10:358–371. doi: 10.1080/13550280490521104. [DOI] [PubMed] [Google Scholar]
- 94.Jain P, Ahuja J, Khan ZK, et al. Modulation of dendritic cell maturation and function by the Tax protein of human T cell leukemia virus type 1. J Leukoc Biol. 2007;82:44–56. doi: 10.1189/jlb.1006641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ahuja J, Kampani K, Datta S, et al. Use of human antigen presenting cell gene array profiling to examine the effect of human T-cell leukemia virus type 1 Tax on primary human dendritic cells. J Neurovirol. 2006;12:47–59. doi: 10.1080/13550280600614981. [DOI] [PubMed] [Google Scholar]
- 96.Ahuja J, Lepoutre V, Wigdahl B, et al. Induction of pro-inflammatory cytokines by human T-cell leukemia virus type-1 Tax protein as determined by multiplexed cytokine protein array analyses of human dendritic cells. Biomed Pharmacother. 2007;61:1–8. doi: 10.1016/j.biopha.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yamano Y, Cohen CJ, Takenouchi N, et al. Increased expression of human T lymphocyte virus type I (HTLV-I) Tax11-19 peptide-human histocompatibility leukocyte antigen A*201 complexes on CD4 CD25 T Cells detected by peptide-specific, major histocompatibility complex-restricted antibodies in patients with HTLV-I-associated neurologic disease. J Exp Med. 2004;199:1367–1377. doi: 10.1084/jem.20032042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Manuel SL, Schell TD, Acheampong E, et al. Presentation of human T cell leukemia virus type 1 (HTLV-1) Tax protein by dendritic cells: The underlying mechanism of HTLV-1-associated neuroinflammatory disease. J Leukoc Biol. 2009;86:1205–1216. doi: 10.1189/jlb.0309172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rahman S, Manuel SL, Khan ZK, et al. Depletion of dendritic cells enhances susceptibility to cell-free infection of human T cell leukemia virus type 1 in CD11c-diphtheria toxin receptor transgenic mice. J Immunol. 2010;184:5553–5561. doi: 10.4049/jimmunol.0903226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rahman S, Khan ZK, Wigdahl B, et al. Murine FLT3 ligand-derived dendritic cell-mediated early immune responses are critical to controlling cell-free human T cell leukemia virus type 1 infection. J Immunol. 2011;186:390–402. doi: 10.4049/jimmunol.1002570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Colisson R, Barblu L, Gras C, et al. Free HTLV-1 induces TLR7-dependent innate immune response and TRAIL relocalization in killer plasmacytoid dendritic cells. Blood. 2010;115:2177–2185. doi: 10.1182/blood-2009-06-224741. [DOI] [PubMed] [Google Scholar]
- 102.Clarke SR. The critical role of CD40/CD40L in the CD4-dependent generation of CD8 T cell immunity. J Leukoc Biol. 2000;67:607–614. doi: 10.1002/jlb.67.5.607. [DOI] [PubMed] [Google Scholar]
- 103.Harhaj NS, Janic B, Ramos JC, et al. Deregulated expression of CD40 ligand in HTLV-I infection: Distinct mechanisms of downregulation in HTLV-I-transformed cell lines and ATL patients. Virology. 2007;362:99–108. doi: 10.1016/j.virol.2006.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ganem D, Prince AM. Hepatitis B virus infection—Natural history and clinical consequences. N Engl J Med. 2004;350:1118–1129. doi: 10.1056/NEJMra031087. [DOI] [PubMed] [Google Scholar]
- 105.Lauer GM, Walker BD. Hepatitis C virus infection. N Engl J Med. 2001;345:41–52. doi: 10.1056/NEJM200107053450107. [DOI] [PubMed] [Google Scholar]
- 106.Lavanchy D. Chronic viral hepatitis as a public health issue in the world. Best Pract Res Clin Gastroenterol. 2008;22:991–1008. doi: 10.1016/j.bpg.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 107.Beckebaum S, Cicinnati VR, Dworacki G, et al. Reduction in the circulating pDC1/pDC2 ratio and impaired function of ex vivo-generated DC1 in chronic hepatitis B infection. Clin Immunol. 2002;104:138–150. doi: 10.1006/clim.2002.5245. [DOI] [PubMed] [Google Scholar]
- 108.Duan XZ, Wang M, Li HW, et al. Decreased frequency and function of circulating plasmocytoid dendritic cells (pDC) in hepatitis B virus infected humans. J Clin Immunol. 2004;24:637–646. doi: 10.1007/s10875-004-6249-y. [DOI] [PubMed] [Google Scholar]
- 109.Tavakoli S, Mederacke I, Herzog-Hauff S, et al. Peripheral blood dendritic cells are phenotypically and functionally intact in chronic hepatitis B virus (HBV) infection. Clin Exp Immunol. 2008;151:61–70. doi: 10.1111/j.1365-2249.2007.03547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Van Der Molen RG, Sprengers D, Biesta PJ, et al. Favorable effect of adefovir on the number and functionality of myeloid dendritic cells of patients with chronic HBV. Hepatology. 2006;44:907–914. doi: 10.1002/hep.21340. [DOI] [PubMed] [Google Scholar]
- 111.Van Der Molen RG, Sprengers D, Binda RS, et al. Functional impairment of myeloid and plasmacytoid dendritic cells of patients with chronic hepatitis B. Hepatology. 2004;40:738–746. doi: 10.1002/hep.20366. [DOI] [PubMed] [Google Scholar]
- 112.Zhang Z, Chen D, Yao J, et al. Increased infiltration of intrahepatic DC subsets closely correlate with viral control and liver injury in immune active pediatric patients with chronic hepatitis B. Clin Immunol. 2007;122:173–180. doi: 10.1016/j.clim.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 113.Zhang Z, Zhang H, Chen D, et al. Response to interferon-alpha treatment correlates with recovery of blood plasmacytoid dendritic cells in children with chronic hepatitis B. J Hepatology. 2007;47:751–759. doi: 10.1016/j.jhep.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 114.Kunitani H, Shimizu Y, Murata H, et al. Phenotypic analysis of circulating and intrahepatic dendritic cell subsets in patients with chronic liver diseases. J Hepatology. 2002;36:734–741. doi: 10.1016/s0168-8278(02)00062-4. [DOI] [PubMed] [Google Scholar]
- 115.Wang K, Fan X, Fan Y, et al. Study on the function of circulating plasmacytoid dendritic cells in the immunoactive phase of patients with chronic genotype B and C HBV infection. J Viral Hepat. 2007;14:276–282. doi: 10.1111/j.1365-2893.2006.00819.x. [DOI] [PubMed] [Google Scholar]
- 116.Chen L, Zhang Z, Chen W, et al. B7-H1 up-regulation on myeloid dendritic cells significantly suppresses T cell immune function in patients with chronic hepatitis B. J Immunol. 2007;178:6634–6641. doi: 10.4049/jimmunol.178.10.6634. [DOI] [PubMed] [Google Scholar]
- 117.Miyazaki M, Kanto T, Inoue M, et al. Impaired cytokine response in myeloid dendritic cells in chronic hepatitis C virus infection regardless of enhanced expression of Toll-like receptors and retinoic acid inducible gene-I. J Med Virol. 2008;80:980–988. doi: 10.1002/jmv.21174. [DOI] [PubMed] [Google Scholar]
- 118.Zheng BJ, Zhou J, Qu D, et al. Selective functional deficit in dendritic cell—T cell interaction is a crucial mechanism in chronic hepatitis B virus infection. J Viral Hepat. 2004;11:217–224. doi: 10.1111/j.1365-2893.2004.00497.x. [DOI] [PubMed] [Google Scholar]
- 119.Xie Q, Shen HC, Jia NN, et al. Patients with chronic hepatitis B infection display deficiency of plasmacytoid dendritic cells with reduced expression of TLR9. Microbes Infect. 2009;11:515–523. doi: 10.1016/j.micinf.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 120.Christen V, Duong F, Bernsmeier C, et al. Inhibition of alpha interferon signaling by hepatitis B virus. J Virology. 2007;81:159–165. doi: 10.1128/JVI.01292-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wu M, Xu Y, Lin S, et al. Hepatitis B virus polymerase inhibits the interferon-inducible MyD88 promoter by blocking nuclear translocation of Stat1. J Gen Virol. 2007;88:3260–3269. doi: 10.1099/vir.0.82959-0. [DOI] [PubMed] [Google Scholar]
- 122.Beckebaum S, Cicinnati VR, Zhang X, et al. Hepatitis B virus-induced defect of monocyte-derived dendritic cells leads to impaired T helper type 1 response in vitro: Mechanisms for viral immune escape. Immunology. 2003;109:487–495. doi: 10.1046/j.1365-2567.2003.01699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Op den Brouw ML, Binda RS, van Roosmalenv MH, et al. Hepatitis B virus surface antigen impairs myeloid dendritic cell function: A possible immune escape mechanism of hepatitis B virus. Immunology. 2009;126:280–289. doi: 10.1111/j.1365-2567.2008.02896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Untergasser A, Zedler U, Langenkamp A, et al. Dendritic cells take up viral antigens but do not support the early steps of hepatitis B virus infection. Hepatology. 2006;43:539–547. doi: 10.1002/hep.21048. [DOI] [PubMed] [Google Scholar]
- 125.Albert ML, Decalf J, Pol S. Plasmacytoid dendritic cells move down on the list of suspects: in search of the immune pathogenesis of chronic hepatitis C. J Hepatology. 2008;49:1069–1078. doi: 10.1016/j.jhep.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 126.Liu B, Woltman AM, Janssen HL, Boonstra A. Modulation of dendritic cell function by persistent viruses. J Leukoc Biol. 2009;85:205–214. doi: 10.1189/jlb.0408241. [DOI] [PubMed] [Google Scholar]
- 127.Lau DT, Fish PM, Sinha M, et al. Interferon regulatory factor-3 activation, hepatic interferon-stimulated gene expression, and immune cell infiltration in hepatitis C virus patients. Hepatology. 2008;47:799–809. doi: 10.1002/hep.22076. [DOI] [PubMed] [Google Scholar]
- 128.Nattermann J, Zimmermann H, Iwan A, et al. Hepatitis Cvirus E2 and CD81 interaction may be associated with altered trafficking of dendritic cells in chronic hepatitis C. Hepatology. 2006;44:945–954. doi: 10.1002/hep.21350. [DOI] [PubMed] [Google Scholar]
- 129.Averill L, Lee WM, Karandikar NJ. Differential dysfunction in dendritic cell subsets during chronic HCV infection. Clin Immunol. 2007;123:40–49. doi: 10.1016/j.clim.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Siavoshian S, Abraham JD, Thumann C, et al. Hepatitis C virus core, NS3, NS5A, NS5B proteins induce apoptosis in mature dendritic cells. J Med Virol. 2005;75:402–411. doi: 10.1002/jmv.20283. [DOI] [PubMed] [Google Scholar]
- 131.Longman RS, Talal AH, Jacobson IM, et al. Normal functional capacity in circulating myeloid and plasmacytoid dendritic cells in patients with chronic hepatitis C. J Infect Dis. 2005;192:497–503. doi: 10.1086/431523. [DOI] [PubMed] [Google Scholar]
- 132.Lai WK, Curbishley SM, Goddard S, Alabraba E, Shaw J, Youster J, McKeating J, Adams DH. Hepatitis C is associated with perturbation of intrahepatic myeloid and plasmacytoid dendritic cell function. Journal of Hepatology. 2007;47:338–347. doi: 10.1016/j.jhep.2007.03.024. [DOI] [PubMed] [Google Scholar]
- 133.Rodrigue-Gervais IG, Jouan L, Beaule G, et al. Poly(I:C) and lipopolysaccharide innate sensing functions of circulating human myeloid dendritic cells are affected in vivo in hepatitis C virus-infected patients. J Virology. 2007;81:5537–5546. doi: 10.1128/JVI.01741-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Della Bella S, Crosignani A, Riva A, et al. Decrease and dysfunction of dendritic cells correlate with impaired hepatitis C virus-specific CD4 T-cell proliferation in patients with hepatitis C virus infection. Immunology. 2007;121:283–292. doi: 10.1111/j.1365-2567.2007.02577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kanto T, Hayashi N. Immunopathogenesis of hepatitis C virus infection: Multifaceted strategies subverting innate and adaptive immunity. Intern Med. 2006;45:183–191. doi: 10.2169/internalmedicine.45.1530. [DOI] [PubMed] [Google Scholar]
- 136.Kanto T, Inoue M, Miyatake H, et al. Reduced numbers and impaired ability of myeloid and plasmacytoid dendritic cells to polarize T helper cells in chronic hepatitis C virus infection. J Infect Dis. 2004;190:1919–1926. doi: 10.1086/425425. [DOI] [PubMed] [Google Scholar]
- 137.Yonkers NL, Rodriguez B, Milkovich KA, et al. TLR ligand-dependent activation of naive CD4 T cells by plasmacytoid dendritic cells is impaired in hepatitis C virus infection. J Immunol. 2007;178:4436–4444. doi: 10.4049/jimmunol.178.7.4436. [DOI] [PubMed] [Google Scholar]
- 138.Cheng G, Zhong J, Chisari FV. Inhibition of dsRNA-induced signaling in hepatitis C virus-infected cells by NS3 protease-dependent and -independent mechanisms. Proc Natl Acad Sci U S A. 2006;103:8499–8504. doi: 10.1073/pnas.0602957103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.de Lucas S, Bartolome J, Carreno V. Hepatitis C virus core protein down-regulates transcription of interferon-induced antiviral genes. J Infect Dis. 2005;191:93–99. doi: 10.1086/426509. [DOI] [PubMed] [Google Scholar]
- 140.Gale M, Jr, Foy EM. Evasion of intracellular host defence by hepatitis C virus. Nature. 2005;436:939–945. doi: 10.1038/nature04078. [DOI] [PubMed] [Google Scholar]
- 141.Cocquerel L, Voisset C, Dubuisson J. Hepatitis C virus entry: Potential receptors and their biological functions. J Gen Virol. 2006;87:1075–1084. doi: 10.1099/vir.0.81646-0. [DOI] [PubMed] [Google Scholar]
- 142.Zhou Y, Lukes Y, Andersonv J, et al. Hepatitis C virus E2 envelope protein induces dendritic cell maturation. J Viral Hepat. 2007;14:849–858. doi: 10.1111/j.1365-2893.2007.00879.x. [DOI] [PubMed] [Google Scholar]
- 143.Ludwig IS, Lekkerkerker AN, Depla E, et al. Hepatitis C virus targets DC-SIGN and L-SIGN to escape lysosomal degradation. J Virology. 2004;78:8322–8332. doi: 10.1128/JVI.78.15.8322-8332.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Gardner JP, Durso RJ, Arrigale RR, et al. L-SIGN (CD 209L) is a liver-specific capture receptor for hepatitis C virus. Proc Natl Acad Sci U S A. 2003;100:4498–4503. doi: 10.1073/pnas.0831128100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Cormier EG, Durso RJ, Tsamis F, et al. L-SIGN (CD209L) and DC-SIGN (CD209) mediate transinfection of liver cells by hepatitis C virus. Proc Natl Acad Sci U S A. 2004;101:14067–14072. doi: 10.1073/pnas.0405695101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lozach PY, Amara A, Bartosch B, et al. C-type lectins L-SIGN and DC-SIGN capture and transmit infectious hepatitis C virus pseudotype particles. J Biol Chem. 2004;279:32035–32045. doi: 10.1074/jbc.M402296200. [DOI] [PubMed] [Google Scholar]
- 147.Auffermann-Gretzinger S, Keeffe EB, Levy S. Impaired dendritic cell maturation in patients with chronic, but not resolved, hepatitis C virus infection. Blood. 2001;97:3171–3176. doi: 10.1182/blood.v97.10.3171. [DOI] [PubMed] [Google Scholar]
- 148.Dolganiuc A, Kodys K, Kopasz A, et al. Hepatitis C virus core and nonstructural protein 3 proteins induce pro- and anti-inflammatory cytokines and inhibit dendritic cell differentiation. J Immunol. 2003;170:5615–5624. doi: 10.4049/jimmunol.170.11.5615. [DOI] [PubMed] [Google Scholar]
- 149.Kanto T, Hayashi N, Takehara T, et al. Impaired allostimulatory capacity of peripheral blood dendritic cells recovered from hepatitis C virus-infected individuals. J Immunol. 1999;162:5584–5591. [PubMed] [Google Scholar]
- 150.Bain C, Fatmi A, Zoulim F, et al. Impaired allostimulatory function of dendritic cells in chronic hepatitis C infection. Gastroenterology. 2001;120:512–524. doi: 10.1053/gast.2001.21212. [DOI] [PubMed] [Google Scholar]
- 151.Longman RS, Talal AH, Jacobson IM, et al. Presence of functional dendritic cells in patients chronically infected with hepatitis C virus. Blood. 2004;103:1026–1029. doi: 10.1182/blood-2003-04-1339. [DOI] [PubMed] [Google Scholar]
- 152.Rollier C, Drexhage JA, Verstrepen BE, et al. Chronic hepatitis C virus infection established and maintained in chimpanzees independent of dendritic cell impairment. Hepatology. 2003;38:851–858. doi: 10.1053/jhep.2003.50426. [DOI] [PubMed] [Google Scholar]
- 153.Gregory SM, West JA, Dillon PJ, et al. Toll-like receptor signaling controls reactivation of KSHV from latency. Proc Natl Acad Sci U S A. 2009;106:11725–11730. doi: 10.1073/pnas.0905316106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Mikloska Z, Bosnjak L, Cunningham AL. Immature monocyte-derived dendritic cells are productively infected with herpes simplex virus type 1. J Virology. 2001;75:5958–5964. doi: 10.1128/JVI.75.13.5958-5964.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Jugovic P, Hill AM, Tomazin R, et al. Inhibition of major histocompatibility complex class I antigen presentation in pig and primate cells by herpes simplex virus type 1 and 2 ICP47. J Virology. 1998;72:5076–5084. doi: 10.1128/jvi.72.6.5076-5084.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Tomazin R, Hill AB, Jugovic P, et al. Stable binding of the herpes simplex virus ICP47 protein to the peptide binding site of TAP. EMBO J. 1996;15:3256–3266. [PMC free article] [PubMed] [Google Scholar]
- 157.York IA, Roop C, Andrews DW, et al. A cytosolic herpes simplex virus protein inhibits antigen presentation to CD8 T lymphocytes. Cell. 1994;77:525–535. doi: 10.1016/0092-8674(94)90215-1. [DOI] [PubMed] [Google Scholar]
- 158.Kruse M, Rosorius O, Kratzer F, et al. Mature dendritic cells infected with herpes simplex virus type 1 exhibit inhibited T-cell stimulatory capacity. J Virology. 2000;74:7127–7136. doi: 10.1128/jvi.74.15.7127-7136.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hock BD, Kato M, McKenzie JL, Hart DN. Asoluble form of CD83 is released from activated dendritic cells and B lymphocytes, and is detectable in normal human sera. Int Immunol. 2001;13:959–967. doi: 10.1093/intimm/13.7.959. [DOI] [PubMed] [Google Scholar]
- 160.Kummer M, Turza NM, Muhl-Zurbes P, et al. Herpes simplex virus type 1 induces CD83 degradation in mature dendritic cells with immediate-early kinetics via the cellular proteasome. J Virology. 2007;81:6326–6338. doi: 10.1128/JVI.02327-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Neumann J, Eis-Hubinger AM, Koch N. Herpes simplex virus type 1 targets the MHC class II processing pathway for immune evasion. J Immunol. 2003;171:3075–3083. doi: 10.4049/jimmunol.171.6.3075. [DOI] [PubMed] [Google Scholar]
- 162.Mueller SN, Jones CM, Smith CM, et al. Rapid cytotoxic T lymphocyte activation occurs in the draining lymph nodes after cutaneous herpes simplex virus infection as a result of early antigen presentation and not the presence of virus. J Exp Med. 2002;195:651–656. doi: 10.1084/jem.20012023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Medici MA, Sciortino MT, Perri D, et al. Protection by herpes simplex virus glycoprotein D against Fas-mediated apoptosis: Role of nuclear factor kappaB. J Biol Chem. 2003;278:36059–36067. doi: 10.1074/jbc.M306198200. [DOI] [PubMed] [Google Scholar]
- 164.Jones CA, Fernandez M, Herc K, et al. Herpes simplex virus type 2 induces rapid cell death and functional impairment of murine dendritic cells in vitro. J Virology. 2003;77:11139–11149. doi: 10.1128/JVI.77.20.11139-11149.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Prechtel AT, Turza NM, Kobelt DJ, et al. Infection of mature dendritic cells with herpes simplex virus type 1 dramatically reduces lymphoid chemokine-mediated migration. J Gen Virol. 2005;86:1645–1657. doi: 10.1099/vir.0.80852-0. [DOI] [PubMed] [Google Scholar]
- 166.Theodoridis AA, Prechtel AT, Turza NM, et al. Infectionofhumandendriticcellswithherpessimplex virus type 1 dramatically diminishes the mRNA levels of the prostaglandin E(2) receptors EP2 and EP4. Immunobiology. 2007;212:827–838. doi: 10.1016/j.imbio.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 167.Donaghy H, Bosnjak L, Harman AN, et al. Role for plasmacytoid dendritic cells in the immune control of recurrent human herpes simplex virus infection. J Virology. 2009;83:1952–1961. doi: 10.1128/JVI.01578-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Sandberg K, Matsson P, Alm GV. A distinct population of nonphagocytic and low level CD4 null lymphocytes produce IFN-alpha after stimulation by herpes simplex virus-infected cells. J Immunol. 1990;145:1015–1020. [PubMed] [Google Scholar]
- 169.Fitzgerald-Bocarsly P, Feldman M, Mendelsohnv M, et al. Human mononuclear cells which produce interferon-alpha during NK(HSV-FS) assays are HLA-DR positive cells distinct from cytolytic natural killer effectors. J Leukoc Biol. 1988;43:323–334. doi: 10.1002/jlb.43.4.323. [DOI] [PubMed] [Google Scholar]
- 170.Hochrein H, Schlatter B, O’Keeffe M, et al. Herpes simplex virus type-1 induces IFN-alpha production via Toll-like receptor 9-dependent and -independent pathways. Proc Natl Acad Sci U S A. 2004;101:11416–11421. doi: 10.1073/pnas.0403555101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Krug A, Luker GD, Barchet W, et al. Herpes simplex virus type 1 activates murine natural interferon-producing cells through toll-like receptor 9. Blood. 2004;103:1433–1437. doi: 10.1182/blood-2003-08-2674. [DOI] [PubMed] [Google Scholar]
- 172.Lund J, Sato A, Akira S, et al. Toll-like receptor 9-mediated recognition of herpes simplex virus-2 by plasmacytoid dendritic cells. J Exp Med. 2003;198:513–520. doi: 10.1084/jem.20030162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.de Jong MA, de Witte L, Bolmstedt A, et al. Dendritic cells mediate herpes simplex virus infection and transmission through the C-type lectin DC-SIGN. J Gen Virol. 2008;89:2398–2409. doi: 10.1099/vir.0.2008/003129-0. [DOI] [PubMed] [Google Scholar]
- 174.Satoh T, Arase H. HSV-1 infection through inhibitory receptor, PILRalpha. Uirusu. 2008;58:27–36. doi: 10.2222/jsv.58.27. [DOI] [PubMed] [Google Scholar]
- 175.Puttur FK, Fernandez MA, White R, et al. Herpes simplex virus infects skin gamma delta T cells before Langerhans cells and impedes migration of infected Langerhans cells by inducing apoptosis and blocking E-cadherin downregulation. J Immunol. 2010;185:477–487. doi: 10.4049/jimmunol.0904106. [DOI] [PubMed] [Google Scholar]
- 176.Bosnjak L, Miranda-Saksena M, Koelle DM, et al. Herpes simplex virus infection of human dendritic cells induces apoptosis and allows cross-presentation via uninfected dendritic cells. J Immunol. 2005;174:2220–2227. doi: 10.4049/jimmunol.174.4.2220. [DOI] [PubMed] [Google Scholar]
- 177.Gilbert MJ, Riddell SR, Plachter B, Greenberg PD. Cytomegalovirus selectively blocks antigen processing and presentation of its immediate-early gene product. Nature. 1996;383:720–722. doi: 10.1038/383720a0. [DOI] [PubMed] [Google Scholar]
- 178.Wiertz EJ, Jones TR, Sun L, et al. The human cytomegalovirus US11 gene product dislocates MHC class I heavy chains from the endoplasmic reticulum to the cytosol. Cell. 1996;84:769–779. doi: 10.1016/s0092-8674(00)81054-5. [DOI] [PubMed] [Google Scholar]
- 179.Wiertz EJ, Tortorella D, Bogyo M, et al. Sec61-mediated transfer of a membrane protein from the endoplasmic reticulum to the proteasome for destruction. Nature. 1996;384:432–438. doi: 10.1038/384432a0. [DOI] [PubMed] [Google Scholar]
- 180.Ben-Arieh SV, Zimerman B, Smorodinsky NI, et al. Human cytomegalovirus protein US2 interferes with the expression of human HFE, a nonclassical class I major histocompatibility complex molecule that regulates iron homeostasis. J Virology. 2001;75:10557–10562. doi: 10.1128/JVI.75.21.10557-10562.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Tomazin R, Boname J, Hegde NR, et al. Cytomegalovirus US2 destroys two components of the MHC class II pathway, preventing recognition by CD4 T cells. Nat Med. 1999;5:1039–1043. doi: 10.1038/12478. [DOI] [PubMed] [Google Scholar]
- 182.Ahn K, Gruhler A, Galocha B, et al. The ER-luminal domain of the HCMV glycoprotein US6 inhibits peptide translocation by TAP. Immunity. 1997;6:613–621. doi: 10.1016/s1074-7613(00)80349-0. [DOI] [PubMed] [Google Scholar]
- 183.Hegde NR, Tomazin RA, Wisner TW, et al. Inhibition of HLA-DR assembly, transport, and loading by human cytomegalovirus glycoprotein US3: A novel mechanism for evading major histocompatibility complex class II antigen presentation. J Virology. 2002;76:10929–10941. doi: 10.1128/JVI.76.21.10929-10941.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Jones TR, Wiertz EJ, Sun L, et al. Human cytomegalovirus US3 impairs transport and maturation of major histocompatibility complex class I heavy chains. Proc Natl Acad Sci U S A. 1996;93:11327–11333. doi: 10.1073/pnas.93.21.11327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Furman MH, Dey N, Tortorella D, Ploeghv HL. The human cytomegalovirus US10 gene product delays trafficking of major histocompatibility complex class I molecules. J Virology. 2002;76:11753–11756. doi: 10.1128/JVI.76.22.11753-11756.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Trgovcich J, Cebulla C, Zimmerman P, Sedmak DD. Human cytomegalovirus protein pp71 disrupts major histocompatibility complex class I cell surface expression. J Virology. 2006;80:951–963. doi: 10.1128/JVI.80.2.951-963.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Goldmacher VS, Bartle LM, Skaletskaya A, et al. A cytomegalovirus-encoded mitochondria-localized inhibitor of apoptosis structurally unrelated to Bcl-2. Proc Natl Acad Sci U S A. 1999;96:12536–12541. doi: 10.1073/pnas.96.22.12536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Kledal TN, Rosenkilde MM, Schwartz TW. Selective recognition of the membrane-bound CX3C chemokine, fractalkine, by the human cytomegalovirus-encoded broad-spectrum receptor US28. FEBS Lett. 1998;441:209–214. doi: 10.1016/s0014-5793(98)01551-8. [DOI] [PubMed] [Google Scholar]
- 189.Kotenko SV, Saccani S, Izotova LS, et al. Human cytomegalovirus harbors its own unique IL-10 homolog (cmvIL-10) Proc Natl Acad Sci U S A. 2000;97:1695–1700. doi: 10.1073/pnas.97.4.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Mocarski ES., Jr Immunomodulation by cytomegaloviruses: Manipulative strategies beyond evasion. Trends Microbiol. 2002;10:332–339. doi: 10.1016/s0966-842x(02)02393-4. [DOI] [PubMed] [Google Scholar]
- 191.Penfold ME, Dairaghi DJ, Duke GM, et al. Cytomegalovirus encodes a potent alpha chemokine. Proc Natl Acad Sci U S A. 1999;96:9839–9844. doi: 10.1073/pnas.96.17.9839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Skaletskaya A, Bartle LM, Chittenden T, et al. A cytomegalovirus-encoded inhibitor of apoptosis that suppresses caspase-8 activation. Proc Nat Acad Sci U S A. 2001;98:7829–7834. doi: 10.1073/pnas.141108798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Hahn G, Jores R, Mocarski ES. Cytomegalovirus remains latent in a common precursor of dendritic and myeloid cells. Proc Natl Acad Sci U S A. 1998;95:3937–3942. doi: 10.1073/pnas.95.7.3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Beck K, Meyer-Konig U, Weidmann M, et al. Human cytomegalovirus impairs dendritic cell function: A novel mechanism of human cytomegalovirus immune escape. Eur J Immunol. 2003;33:1528–1538. doi: 10.1002/eji.200323612. [DOI] [PubMed] [Google Scholar]
- 195.Halary F, Amara A, Lortat-Jacob H, et al. Human cytomegalovirus binding to DC-SIGN is required for dendritic cell infection and target cell trans-infection. Immunity. 2002;17:653–664. doi: 10.1016/s1074-7613(02)00447-8. [DOI] [PubMed] [Google Scholar]
- 196.Gerna G, Percivalle E, Lilleri D, et al. Dendritic-cellinfectionbyhumancytomegalovirusisrestricted to strains carrying functional UL131-128 genes and mediates efficient viral antigen presentation to CD8 T cells. J Gen Virol. 2005;86:275–284. doi: 10.1099/vir.0.80474-0. [DOI] [PubMed] [Google Scholar]
- 197.Raftery MJ, Schwab M, Eibert SM, et al. Targeting the function of mature dendritic cells by human cytomegalovirus: a multilayered viral defense strategy. Immunity. 2001;15:997–1009. doi: 10.1016/s1074-7613(01)00239-4. [DOI] [PubMed] [Google Scholar]
- 198.Moutaftsi M, Mehl AM, Borysiewicz LK, Tabi Z. Human cytomegalovirus inhibits maturation and impairs function of monocyte-derived dendritic cells. Blood. 2002;99:2913–2921. doi: 10.1182/blood.v99.8.2913. [DOI] [PubMed] [Google Scholar]
- 199.Frascaroli G, Varani S, Mastroianni A, et al. Dendritic cell function in cytomegalovirus-infected patients with mononucleosis. J Leukoc Biol. 2006;79:932–940. doi: 10.1189/jlb.0905499. [DOI] [PubMed] [Google Scholar]
- 200.Wagner CS, Walther-Jallow L, Buentke E, et al. Human cytomegalovirus-derived protein UL18 alters the phenotype and function of monocyte-derived dendritic cells. J Leukoc Biol. 2008;83:56–63. doi: 10.1189/jlb.0307181. [DOI] [PubMed] [Google Scholar]
- 201.Varani S, Frascaroli G, Homman-Loudiyi M, et al. Human cytomegalovirus inhibits the migration of immature dendritic cells by down-regulating cell-surface CCR1 and CCR5. J Leukoc Biol. 2005;77:219–228. doi: 10.1189/jlb.0504301. [DOI] [PubMed] [Google Scholar]
- 202.Kvale EO, Dalgaard J, Lund-Johansen F, et al. CD11c dendritic cells and plasmacytoid DCs are activated by human cytomegalovirus and retain efficient T cell-stimulatory capability upon infection. Blood. 2006;107:2022–2029. doi: 10.1182/blood-2005-05-2016. [DOI] [PubMed] [Google Scholar]
- 203.Varani S, Cederarv M, Feld S, et al. Human cytomegalovirus differentially controls B cell and T cell responses through effects on plasmacytoid dendritic cells. J Immunol. 2007;179:7767–7776. doi: 10.4049/jimmunol.179.11.7767. [DOI] [PubMed] [Google Scholar]
- 204.Danis B, George TC, Goriely S, et al. Interferon regulatory factor 7-mediated responses are defective in cord blood plasmacytoid dendritic cells. Eur J Immunol. 2008;38:507–517. doi: 10.1002/eji.200737760. [DOI] [PubMed] [Google Scholar]
- 205.Jenkins DE, Yasukawa LL, Bergen R, et al. Comparison of primary sensitization of naive human T cells to varicella-zoster virus peptides by dendritic cells in vitro with responses elicited in vivo by varicella vaccination. J Immunol. 1999;162:560–567. [PubMed] [Google Scholar]
- 206.Abendroth A, Morrow G, Cunningham AL, Slobedman B. Varicella-zoster virus infection of human dendritic cells and transmission to T cells: Implications for virus dissemination in the host. J Virology. 2001;75:6183–6192. doi: 10.1128/JVI.75.13.6183-6192.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Morrow G, Slobedman B, Cunningham AL, Abendroth A. Varicella-zoster virus productively infects mature dendritic cells and alters their immune function. J Virology. 2003;77:4950–4959. doi: 10.1128/JVI.77.8.4950-4959.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Tsokos GC, Magrath IT, Balow JE. Epstein-Barr virus induces normal B cell responses but defective suppressor T cell responses in patients with systemic lupus erythematosus. J Immunol. 1983;131:1797–1801. [PubMed] [Google Scholar]
- 209.Young LS, Rickinson AB. Epstein-Barr virus: 40 years on. Nat Rev Cancer. 2004;4:757–768. doi: 10.1038/nrc1452. [DOI] [PubMed] [Google Scholar]
- 210.Lerner MR, Andrews NC, Miller G, Steitz JA. Two small RNAs encoded by Epstein-Barr virus and complexed with protein are precipitated by antibodies from patients with systemic lupus erythematosus. Proc Natl Acad Sci U S A. 1981;78:805–809. doi: 10.1073/pnas.78.2.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Iwakiri D, Zhou L, Samanta M, et al. Epstein-Barr virus (EBV)-encoded small RNA is released from EBV-infected cells and activates signaling from Toll-like receptor 3. J Exp Med. 2009;206:2091–2099. doi: 10.1084/jem.20081761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Quan TE, Roman RM, Rudenga BJ, et al. Epstein-Barr virus promotes interferon-alpha production by plasmacytoid dendritic cells. Arthritis Rheum. 2010;62:1693–1701. doi: 10.1002/art.27408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Tindle RW. Immune evasion in human papillomavirus-associated cervical cancer. Nat Rev Cancer. 2002;2:59–65. doi: 10.1038/nrc700. [DOI] [PubMed] [Google Scholar]
- 214.Barnard P, Payne E, McMillan NA. The human papillomavirus E7 protein is able to inhibit the antiviral and anti-growth functions of interferon-alpha. Virology. 2000;277:411–419. doi: 10.1006/viro.2000.0584. [DOI] [PubMed] [Google Scholar]
- 215.Park JS, Kim EJ, Kwon HJ, et al. Inactivation of interferon regulatory factor-1 tumor suppressor protein by HPV E7 oncoprotein. Implication for the E7-mediated immune evasion mechanism in cervical carcinogenesis. J Biol Chem. 2000;275:6764–6769. doi: 10.1074/jbc.275.10.6764. [DOI] [PubMed] [Google Scholar]
- 216.Tindle RW, Herd K, Doan T, et al. Nonspecific down-regulation of CD8 T-cell responses in mice expressing human papillomavirus type 16 E7 oncoprotein from the keratin-14 promoter. J Virology. 2001;75:5985–5997. doi: 10.1128/JVI.75.13.5985-5997.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Cho YS, Kang JW, Cho M, et al. Down modulation of IL-18 expression by human papillomavirus type 16 E6 oncogene via binding to IL-18. FEBS Lett. 2001;501:139–145. doi: 10.1016/s0014-5793(01)02652-7. [DOI] [PubMed] [Google Scholar]
- 218.Straight SW, Herman B, McCance DJ. The E5 oncoprotein of human papillomavirus type 16 inhibits the acidification of endosomes in human keratinocytes. J Virology. 1995;69:3185–3192. doi: 10.1128/jvi.69.5.3185-3192.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Le Poole IC, ElMasri WM, Denman CJ, et al. Langerhans cells and dendritic cells are cytotoxic towards HPV16 E6 and E7 expressing target cells. Cancer Immunol Immunother. 2008;57:789–797. doi: 10.1007/s00262-007-0415-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Ford E, Nelson KE, Warren D. Epidemiology of epidemic keratoconjunctivitis. Epidemiol Rev. 1987;9:244–261. doi: 10.1093/oxfordjournals.epirev.a036304. [DOI] [PubMed] [Google Scholar]
- 221.Kaufman HE. Treatment of viral diseases of the cornea and external eye. Prog Retin Eye Res. 2000;19:69–85. doi: 10.1016/s1350-9462(99)00004-x. [DOI] [PubMed] [Google Scholar]
- 222.Adams WC, Bond E, Havenga MJ, et al. Adenovirus serotype 5 infects human dendritic cells via a coxsackievirus-adenovirus receptor-independent receptor pathway mediated by lactoferrin and DC-SIGN. J Gen Virology. 2009;90:1600–1610. doi: 10.1099/vir.0.008342-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Lore K, Adams WC, Havenga MJ, et al. Myeloid and plasmacytoid dendritic cells are susceptible to recombinant adenovirus vectors and stimulate polyfunctional memory T cell responses. J Immunol. 2007;179:1721–1729. doi: 10.4049/jimmunol.179.3.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Kessler T, Hamprecht K, Feuchtinger T, Jahn G. Dendritic cells are susceptible to infection with wild-type adenovirus, inducing a differentiation arrest in precursor cells and inducing a strong T-cell stimulation. J Gen Virol. 2010;91:1150–1154. doi: 10.1099/vir.0.013920-0. [DOI] [PMC free article] [PubMed] [Google Scholar]