Abstract
The formation of CoIIITMPyP(CN)2 at pH 7.4 has been shown to be completely cooperative (αH = 2) with an association constant of 2.1 (± 0.2) × 1011. The kinetics were investigated by stopped-flow spectrophotometry and revealed a complicated net reaction exhibiting 4 phases at pH 7.4 under conditions where cyanide was in excess. The data suggest molecular HCN (rather than CN−) to be the attacking nucleophile around neutrality. The two slower phases do not seem to be present when cyanide is not in excess and the other two phases have rates comparable to that observed for cobalamin, a known effective cyanide scavenger. Addition of bovine serum albumin (BSA) did not affect the cooperativity of cyanide binding to CoIIITMPyP, only lowered the equilibrium constant slightly to 1.2 (± 0.2) × 1011 and had an insignificant effect on the observed rate. A sub-lethal mouse model was used to assess the effectiveness of CoIIITMPyP as a potential cyanide antidote. The administration of CoIIITMPyP to sodium cyanide intoxicated mice resulted in the time required for the surviving mice to right themselves from a supine position being significantly decreased (9 ± 2 min.) compared to the controls (33 ± 2 min.). All observations were consistent with the demonstrated antidotal activity of CoIIITMPyP operating through a cyanide-binding (i.e. scavenging) mechanism.
Keywords: antidote, cobalamin, decorporation, scavenging, respiratory poison
Table of Contents graphic
INTRODUCTION
Cases of cyanide poisoning in which successful clinical intervention was possible have frequently involved very high doses of cyanogenic material (multiples of the LD50) being slowly absorbed and distributed systemically. The antidotal use of cyanide-scavenging agents is an effective part of the therapy in such cases.1-3 While only recently approved for use in the U.S., cobalamin (i.e. hydroxocobalamin, a vitamin B12 derivative) has been accepted to be a safe and effective cyanide antidote for some years in Europe, with the central cobalt(III) ion directly binding cyanide anion. However, cobalamin is a less than ideal cyanide antidote requiring intravenous administration in gram quantities.1 Its immediate biological precursor, cobinamide, presently under development, is 3 to 10 times more efficacious in vivo.4,5 Unfortunately, from the pharmaceutical perspective, both of these cyanide scavengers are complicated molecules, costly to produce, cobinamide significantly more so than cobalamin. The alternative sodium nitrite-thiosulfate combination therapy is more cost effective, but there are toxicity issues beginning to emerge in relation to this treatment. 6,7 It follows that there remains a need to find improved cyanide antidotes that can be produced at reasonable cost and, ideally, stored at ambient temperatures.
Both cobalamin and cobinamide contain cobalt(III) chelated within the same macrocyclic corrin-ring structure, but cobinamide lacks the 5,6-dimethylbenzimidazole ribonucleotide tail normally occupying the fifth ligand position in cobalamin. Clearly, it is advantageous that each cobinamide molecule has two axial coordination positions at the cobalt(III) ion available to bind two cyanide anions compared with only one by cobalamin. Based upon this observation, it is reasonable to assert that cobalt(III) complexes of other more easily synthesized macrocycles, like certain porphyrins and phthalocyanines, should exhibit cyanide-binding properties suitable for their application to antidotal cyanide scavenging. Consequently, the results of an earlier study 8 showing cobalt(III) porphyrins to be ineffective as cyanide antidotes when given to mice prophylactically are surprising.
Hypothesizing that there may be one or more pathways through which the putative antidote could become slowly deactivated in vivo, we have undertaken an investigation into the possible therapeutic use of a water-soluble cobalt(III)-containing porphyrin as cyanide-scavenging antidote when given after the toxin. Cobalt(III)meso-tetra(4-N-methylpyridinyl)porphine (CoIIITMPyP, Figure 2A, insert) can be synthesized in three steps from commercially available starting materials in reasonable yield. CoIIITMPyP is monomeric over a wide range of pH 9 and thus, has the required two axial ligand positions available to bind cyanide anions. In this paper, we show that the association and rate constants for cyanide binding by CoIIITMPyP are such that this macrocyclic compound should work as a cyanide scavenger and provide proof-of-concept data supporting this assertion in mice.
Figure 2. Titrations of CoIIITMPyP(OH)(H2O) with cyanide at pH 7.4 and 25°C.
Small aliquots of sodium cyanide solution in 5 mM sodium tetraborate buffer (pH 11) were titrated into a solution of CoIIITMPyP(OH)(H2O) (3.48 μM in 50 mM sodium phosphate buffer, 1 mM EDTA pH 7.4) using gas-tight syringes and a 1.00 cm pathlength septum-sealed cuvette at 25°C (see Experimental for further details). (A) Electronic absorption spectra of CoIIITMPyP(OH)(H-2O) species titrated with NaCN, 1.00 cm pathlength. Main panel: Initial CoIIITMPyP(OH)(H-2O) (dotted trace); subsequent absorbance changes observed during the addition of cyanide to concentrations of 5, 10, and 15 μM (solid traces); final spectrum of CoIIITMPyP(CN)2 (dashed trace) after addition of cyanide to 100 μM. Inset: Structure of CoIIITMPyP. (B) Titration of 3.48 μM CoIIITMPyP(OH)(H2O) with cyanide following the absorbance changes at 454 nm. The solid line represents a nonlinear least squares fit to the data using the Hill equation. (C) Scatchard plot (number of cyanide molecules bound per CoIIITMPyP(OH)(H2O) molecule (ν) versus ν/cyanide concentration) of the data from B.
EXPERIMENTAL METHODS
Reagents
All reagents were ACS grade, or better, used without further purification and, unless otherwise noted, purchased from Aldrich or Sigma. Argon gas (99.998%) was acquired from Matheson. Concentrations of bovine serum albumin (BSA) solutions were determined spectrophotometrically using the average extinction coefficient ε280 = 43 mM-1cm-1 calculated from several reported values 10 (10 mg/mL absorption = 6.57 cm-1, n = 14, σ = 0.22, s = 0.06, median = 6.6).
Synthesis of 4,4’,4”,4”’-porphyrin-5,10,15,20-tetrayltetrakis(1-methylpyridinium) tetrakis(4-methylbenzenesulfonate) (H2TMPyP)
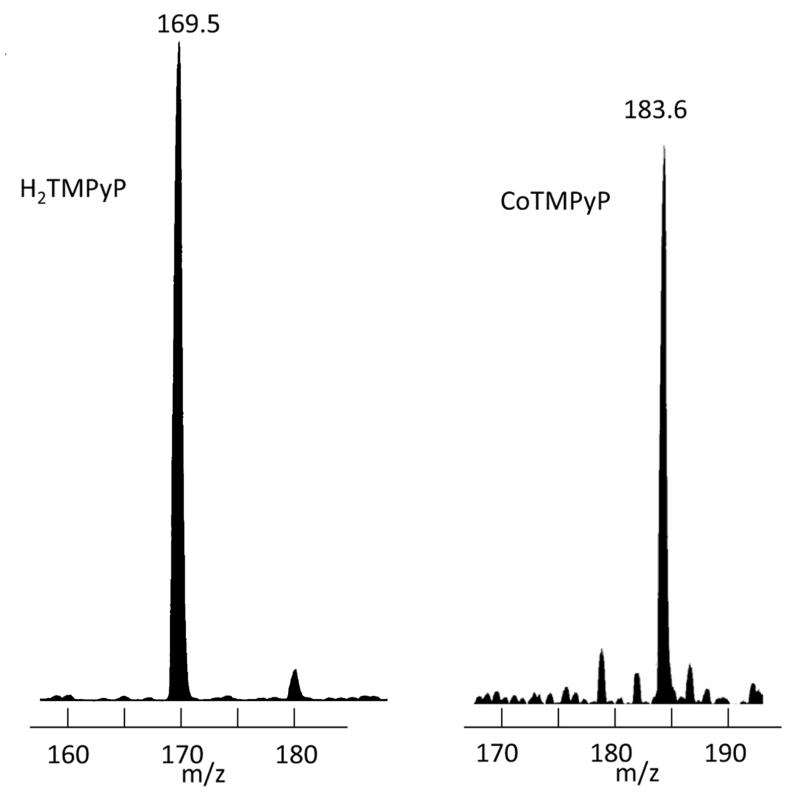
H2TMPyP was synthesized by the method of Hambright et al. 11. Electronic absorption spectrum in 1 M HCl: 446 nm, 592 nm, 643 nm (literature11); 445 nm, 591 nm, 642 nm (found). Elemental analysis (C72H66N8O12•5H2O): C, 59.50; H, 5.23; N, 7.71 (calculated); C, 59.85; H, 5.25; N, 8.00; (found). These preparations can contain variable amounts of water, Hambright et al. 11 reporting a single water of crystallization to be present. Consequently, we verified the presence of the target macrocyclic structure by electrospray mass spectrometry: 169.5 calculated for m/z = C44H38N8/4+; 169.5 found (Figure 1A, insert).
Figure 1. Electrospray ionization (ESI) mass spectra of H2TMPyP (A) and CoTMPyP (B).
Samples in 50:50 (v/v) acetonitrile:water containing 0.1% acetic acid. Sheath flow 5 μL/min, voltage differential 2.3-3.5 keV in the positive ion mode, source temperature 70 °C.
Synthesis of [[4,4’,4”,4”’-porphyrin-5,10,15,20-tetrayltetrakis(1-methyl-pyridiniumato)](2-)] cobalt(III) pentaiodide (“CoIII meso tetra(4-N-methylpyridinyl) porphine,” CoIIITMPyP)
Cobalt was inserted into the macrocycle using the method of Pasternack et al. 13. Briefly, CoCl2 was refluxed under argon with aqueous H2-TMPyP overnight. Upon cooling in air, the resultant CoIIITMPyP was precipitated with excess potassium iodide. The flat brown flakes of precipitated metalloporphyrin iodide were then dissolved in a 5% acetone aqueous solution and re-precipitated with excess potassium iodide. The final solid product was filtered from the mixture, washed with dilute aqueous potassium iodide, and dried in an oven at 100°C for 1 hour. Insertion of the metal ion was monitored by a shift in the Soret electronic absorption band from 421 nm (pH ~7) to 437 nm as the reaction proceeded. This was confirmed by the electrospray mass spectrum: 183.7 calculated for m/z = CoC44H36N8/4+; 183.6 found (Figure 1B). The synthesized complex has previously been determined to be diamagnetic13 and, therefore, to contain Co(III). As prepared, the product was confirmed to contain Co(III) by electron paramagnetic resonance (EPR). Initially, there were no EPR signals to be observed, but upon reduction of the complex with sodium ascorbate, Co(II) EPR signals became readily detectable (not shown). However, the mass spectral data indicates the presence of Co(II) rather than Co(III), so reduction must have occurred on the probe during introduction to the mass spectrometer. In all cases (animal, kinetic and titration procedures) concentrations of CoIIITMPyP solutions were determined using the reported extinction coefficient ε437 = 1.7 × 105 M-1cm-1 15. Comparing concentrations of solutions estimated by weighing with those determined spectrophotometrically suggested that the purity of CoIIITMPyP preparations was typically 85 – 90%; not surprising given that the product was obtained as a precipitate, different preparations exhibiting some variation in the amounts of residual water and potassium iodide.
Titrations
For determinations of cyanide binding equilibria all solutions were maintained in “capped” (septum sealed, with head volumes minimized) vessels and transfers made with gas-tight syringes. Small aliquots of relatively concentrated cyanide solutions (buffered with 50 mM sodium borate; pH 11) were titrated into larger volumes of relatively dilute solutions of CoIIITMPyP (buffered with 50 mM sodium phosphate and 1 mM EDTA; pH 7.4) to maintain neutrality at both 25°C and 37°C. As multiple fast and slow cyanide binding forms are feasible, solutions were allowed to equilibrate for ~10 minutes after the addition of cyanide prior to recording the resultant absorption changes, by which time constant readings were obtained. Binding constants were determined by the construction of Hill plots. The saturation of the CoIIITMPyP with cyanide was determined from the absorbance changes, where Y represents the proportion of sites occupied by cyanide (or fractional saturation) was plotted against the concentration of the free cyanide concentrations. Fits of the data yielded cooperativity values and binding constants for the association of cyanide to CoIIITMPyP.
Animals, Exposures and Righting Recovery Determinations
All animal procedures were approved by the University of Pittsburgh Institutional Animal Care and Use Committee (Protocol Number 1008725). Veterinary care was provided by the Division of Laboratory Animal Research of the University of Pittsburgh. Male Swiss Webster (CFW) mice weighing 35-45 g were purchased from Charles River Laboratories, Wilmington, MA. All animals were 16-20 weeks old and were housed four per cage. The mice were allowed access to food and water ad lib. and experiments commenced after the animals were allowed to adapt to their new environment for one week. All reagents were diluted in saline and administered through ~0.1 mL intraperitoneal (i.p.) injections. At the end of exposures and tests, mice were euthanized with 150 mg/kg (i.p.) sodium pentobarbital followed by cervical dislocation. Righting recovery times were assessed based on the method of Crankshaw et al. 7 with minor changes as described by Cambal et al. 6. Following injections, mice were placed in clear plastic tubes in a supine position. The time from the cyanide injection until the mouse flipped from a supine to a prone position in the plastic tube was taken as the endpoint. After righting, the tube was rolled to make sure the mouse could maintain the prone position, thereby avoiding false positive results.
Instrumentation
Shimadzu UV-1650PC and UV-2501PC spectrophotometers were employed for the measurement of electronic absorption spectra and photometric titratons. Mass spectral analysis was performed by direct infusion into a triple-quadrupole ESI mass spectrometer (Quattro II, Micromass UK Ltd., Manchester, England). Stopped-flow kinetics were measured with an Applied Photophysics stopped-flow/laser-flash spectrometer (LKS.60-SX.18MV-R system) and the resultant data was fit with the PC Pro-K software (!SX.18MV) provided by the manufacturer. All reactions (cyanide or azide) were run under pseudo first order conditions and the temperature regulated by a thermostat-controlled reaction chamber. The individual rates reported are the means of at least 3 runs. The average deviation of these runs was observed to be less than 5%. Rate constants were obtained from linear fits to the observed rates versus the ligand concentrations: total cyanide (CN− + HCN) or sodium azide.
Data Analysis
Titration data and animal experiments were analyzed using KaleidaGraph™ software. A p-value of < 0.05 was considered statistically significant. Where appropriate, unless specified to the contrary, data are presented as values ± standard error.
RESULTS
Binding of cyanide to CoIIITMPyP at physiological pH
In aqueous media at pH 7.4, CoIIITMPyP axially binds two water molecules one of which is deprotonated to form CoIIITMPyP(OH)(H2O) with a pKa1 of 6.013. The second deprotonation occurs at a much higher pH (pKa2= 10.0) and, therefore, at the physiological pH of 7.4, the aquo/hydroxo form of CoIIITMPyP predominates (95%). Previous work has also established that one molecule of CoIIITMPyP usually binds two axial ligands13. The formation of the bis(cyano) adduct occurs in a stepwise fashion, characterized by the following equations:
| (1) |
| (2) |
Hambright and Langley14 report an equilibrium constant for the second process (Kf2, monocyano to dicyano) to be about 5.6 (± 0.3) × 107. The equilibrium process in this and many other Co(III) macrocycles is thought to be completely cooperative, that is the second cyanide binds as soon as the first is attached to the cobalt atom. In order to obtain the overall equilibrium constant for the binding of two cyanides at physiological pH, we carried out a titration of CoIIITMPyP(OH)(H2O) with cyanide in 50 mM phosphate buffer, 1 mM EDTA, pH 7.4, using a spectrophotometric method. CoIIITMPyP(OH)(H2O) in a sealed cuvette with little to no headspace was monitored spectrophotometically as known amounts of cyanide (kept in borate buffer to prevent HCN loss) were added using a gas tight syringe and a time interval of 10 minutes was allowed between additions for the solution to equilibrate before recording spectra (see Methods). The electronic absorption spectra obtained during the titration of CoIIITMPyP(OH)(H2O) with cyanide at 25°C display well-maintained isosbestic points (Figure 2A) indicating the presence of only two species: the aquo/hydroxo and the dicyano species. As we wish to report a physiologically relevant reaction, an effective formation constant (K’β) can be defined as K’β = [CoIIITMPyP(CN)2] / ([CoIIITMPyP(H2O)2][HCN]2) at pH 7.4 where the hydrogen ion concentration is ignored. A further complication arises from the fact that cyanide has a pKa of 9.2 at 25°C, so that 98% of the cyanide free in solution is protonated, but the form bound to CoIIITMPyP is the anion. From the spectra (at 454 nm) the fraction of the CoIIITMPyP(CN)2 per total porphyrin (fractional saturation, Y) was determined, and thus the free cyanide concentration (protonated and unprotonated) could be calculated. In Figure 2B the free cyanide is plotted versus the fractional saturation (Y) and the data was fit using a nonlinear regression and the Hill equation:
Using αH = 2 gave the best fits of the data (αH = 1 is shown for comparison) confirming the complete cooperativity of the binding and K’β was found to be 2.1(±0.2) × 1011. A Scatchard plot (Figure 2C) of the data also shows the cooperative nature of the interaction by the convex-curve shape obtained. If independent, non-interacting sites of cyanide binding existed, the Scatchard plot obtained would be linear. Thus the data in Figure 2 confirms the all or none reaction of CoIIITMPyP(H2O)(OH) with cyanide to form the dicyano complex as has been noted for many other water soluble porphyrin and corrin systems. The same calculations were applied to the data obtained at 37°C resulting in a K’β37°C of 2.4(±0.2) × 1011.
Kinetics of cyanide binding to CoIIITMPyP
The substantial magnitude of the equilibrium binding constant of CoIIITMPyP to cyanide hints at its potential as a cyanide-chelating antidote. However, to be efficacious, it is also necessary that the reaction of CoIIITMPyP with cyanide is reasonably fast. In order to verify the suitably fast binding of cyanide to CoIIITMPyP(OH)(H2O), we conducted stopped-flow spectrophotometric experiments under pseudo-first order cyanide conditions (at least a 20-fold excess of total cyanide, CN− + HCN), monitoring the appearance of the CoIIITMPyP(CN)2 Soret maximum (454 nm) at two temperatures. At pH 7.4 and 25°C, the resulting data clearly did not represent a single process and was best fit by at least three exponentials (Figure 3A). It was apparent from the sum of the amplitudes of these three phases that the overall process was incomplete and, consequently, there must be at least one additional slower phase. The additional phase was inconveniently slow for measurement in the stopped-flow spectrometer, so these measurements were performed independently in a conventional spectrophotometer. At both 25°C and 37°C four rate constants were obtained that all showed a linear dependence on cyanide concentration with zero or near-zero intercepts (Figures 3B, 3C, 3D and 3E). As expected all four rate constants increased upon raising the temperature from 25°C to 37°C (Table I). However, the amplitude of the absorbance changes was increased for k1 and decreased for k2, while k3 and k4 remained roughly constant (Table I).
Figure 3. Kinetics of the reaction of cyanide with CoIIITMPyP(OH)(H2O) under pseudo first order conditions.
The reaction was followed at 454 nm in 50 mM potassium phosphate buffer, pH 7.4, 1 mM EDTA, at 25°C and 37°C under conditions of excess cyanide. Cyanide solutions in the drive syringe were in 5 mM sodium tetraborate buffer (pH 11); cyanide concentrations after mixing were 20 to 2500 μM; upon completion of reactions, discharged solutions were verified to be pH < 7.45. (A) Solid circles indicate the observed data over a 1000 s time frame. The data could not be fit with a single exponential (dotted trace) and the best fit was obtained using 3 exponentials (solid trace). The insert shows the kinetic data associated with the two fast rate constants (k1 and k2, solid circles) and a two exponential fit (solid line) compared with a single exponential fit (dotted trace). Each section of the kinetic trace was fit to the total cyanide concentration and the resulting observed rates were calculated at 25°C and 37°C. All three were found to be linear in cyanide concentration. (B) k1; (C) k2; (D) k3; (E) k4 (determined subsequently by conventional time-resolved spectrophotometry). Rate constants (see Table I) were then obtained from the slopes of the plots.
Table 1.
Second order rate constants and absorbance amplitudes for the formation of CoIIITMPyP(CN)2 at 25-37°C, pH 7.4-8.4 in 0.1 M sodium phosphate buffers. The observed kinetics could be resolved into 4 exponential phases. Rate constants are calculated from linear fits of observed rates to the cyanide concentration as shown in Figure 3 (cyanide was in at least 20-fold excess over metalloporphyrin). The percent absorbance change (amplitude) for each phase is also given. Uncertainties shown in parentheses are standard deviations.
| Phase 1 | Phase 2 | Phase 3 | Phase 4 | |||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Conditions | k1 (M-1s-1) | ΔA% | k2 (M-1s-1) | ΔA% | k3 (M-1s-1) | ΔA% | k4 (M-1s-1) | ΔA% |
| pH 7.4, 25 °C | 111 (±7) | 6 | 29 (±1) | 40 | 9 (±1) | 8 | 0.35 (±.05) | 46 |
| pH 7.4, 37 °C | 270 (±24) | 14 | 93 (±9) | 25 | 20 (±3) | 7 | 0.7 (±.05) | 49 |
| pH 8.4, 25 °C | ----- | 0 | 30 (±5) | 39 | 5 (±1) | 18 | 0.4 (±.05) | 43 |
The reaction of sodium azide with CoIIITMPyP(OH)(H2O) was also examined (pH 7.4, 25°C) and only one exponential was observed (Figure 4, main panel) apparently in keeping with the results of an earlier study involving thiocyanate anion and this same metalloporphyrin 13. From the slope of the linear fit of the observed rates with the azide concentrations (Figure 4, insert) a rate constant of 16.2 (±0.8) M-1s-1 was calculated. This is less than the previously reported 15 value of ~102 M-1s-1 for the rate constant for the reaction of thiocyanate with CoIIITMPyP(OH)(H2O) at pH 8 (presumably at room temperature) and intermediate between the k2 (29 M-1 s-1) and k3 (9 M-1 s-1) found here for the reaction of cyanide with CoIIITMPyP(OH)(H2O) at pH 7.4 and 25°C (Table I). The observed monophasic azide-binding (Figure 4) and similar thiocyanate-binding 15 kinetics strongly suggest that the multiphasic nature of the cyanide-binding reaction (Figure 3A) is specific to the HCN/CN− system rather than the metalloporphyrin. The earlier observation of multiphasic kinetic behavior for the analogous cobinamide reaction by Baldwin et al. 16 support this assertion. It has previously been noted by Pezeshk et al. 17,18 that the available data regarding anion binding to metalloporphyrins suggests a dissociative mechanism; specifically in the present context, it is the dissociation of water molecules that is expected to be rate limiting and the identity of the incoming nucleophile should be relatively unimportant in determining the substitution rate. Therefore, the similarity of the rate constants we find for the reaction of CoIIITMPyP with N3−, cyanide, and that previously reported for SCN− 13 is (i) unremarkable and (ii) does not provide any unambiguous clues as to whether HCN or CN− might be the attacking species in any of the phases documented in Table I.
Figure 4. Kinetics of the reaction of azide with CoIIITMPyP(OH)(H2O) under pseudo first order conditions.

The reaction was followed at 454 nm in 50 mM potassium phosphate buffer, pH 7.4, 1 mM EDTA, 25°C under conditions of excess sodium azide (500 to 5000 μM). Main panel: Representative stopped flow kinetic transient for the reaction of azide with CoIIITMPyP(OH)(H2O) under pseudo first order conditions (solid circle) with a single exponential fit (solid line). Inset: Linear fit of the observed rates with increasing azide concentration, slope = 16.2(±0.8) M-1s-1.
Seeking clarification regarding the possible involvement of HCN and/or CN−, we examined the kinetics of cyanide binding at pH 8.4, where the porphyrin is still expected to be predominately (> 95%) in the aquo/hydroxo form (pKas = 6.0 and 10.015). At pH 8.4, in 50 mM phosphate buffer, only two exponential phases were revealed in the stopped-flow data (not shown) plus the slowest phase observed by conventional spectrophotometry remained. The fastest rate, k1, could no longer be found and the slower rate constants were essentially unchanged (Table I). As the fast rate, corresponding to k1 (Figure 2B) may have increased and been lost in the dead-time, we carefully examined the kinetics at lower cyanide concentrations, on a millisecond time scale, and even lowered the temperature (to 10°C) in an attempt to observe the “missing” phase. We found that the total absorbance changes were very similar to those observed at pH 7.4 (except slower-corresponding to the second rate constant) and thus our conclusion is that the “fast” rate, with rate constant k1, is not present at pH 8.4.
Cyanide binding to CoIIITMPyP in the presence of bovine serum albumin (BSA)
In vivo CoIIITMPyP will encounter many biomolecules potentially able to interfere with its cyanide-scavenging capability. Serum albumin is present at relatively high concentration in the bloodstream and thus a likely participant in such interference. Therefore, examination of the reaction of CoIIITMPyP with cyanide in the presence of a physiologically relevant amount of bovine serum albumin (10 μM BSA) should be instructive. In the presence of BSA the electronic absorption spectra of both CoIIITMPyP(OH)(H2O) and CoIIITMPyP(CN)2 (Figures 5A and B) exhibited very minor differences (red shifts and decreases in intensity) compared with the spectra of the same species in BSA-free buffer. BSA is a cysteine-rich protein and it is reasonable to expect more dramatic spectral changes than those evident (Figures 5A and B) if substantial displacement of the water-derived axial ligands in CoIIITMPyP(OH)(H2O) by cysteine thiols occurred. The small shifts and intensity differences observed most probably indicate an interaction between BSA and the metalloporphyrin primarily involving the macrocyclic moiety rather than the cobalt(III) ion. We then conducted a titration experiment to assess any effect of the presence of BSA on cooperativity and binding equilibrium in the reaction of cyanide with CoIIITMPyP. The titration curve (fractional saturation versus free cyanide, Figure 5C) indicated that the cooperativity of the cyanide binding was essentially maintained and the equilibrium was only slightly lowered; K’βBSA = 1.2 (±0.2) × 1011 at 25°C. Additionally, when we followed the rate of cyanide binding in the presence and absence of BSA, it was clear that the initial rates were virtually identical (Figure 5D). So, contrary to expectation, these observations collectively suggest that the presence of proteinaceous species (such as amines or thiols) as potential axial ligands does not, in fact, interfere significantly with the cyanide-scavenging ability of CoIIITMPyP.
Figure 5. Titrations and kinetics of CoIIITMPyP(OH)(H2O) with cyanide in the presence of BSA at pH 7.4 and 25°C.
(A) Electronic absorption spectra from 400 nm to 500 nm of 3.48 μM CoIIITMPyP(OH)(H2O) (solid trace), CoIIITMPyP(OH)(H2O) + 10 μM BSA (dotted trace). (B) Electronic absorption spectra from 500 nm to 700 nm of 3.48 μM CoIIITMPyP(CN)2 (solid trace) and CoIIITMPyP(CN)2 + 10 μM BSA in the presence of excess cyanide (dash-dot trace). (C) Small aliquots of sodium cyanide solution in 5 mM sodium tetraborate buffer (pH 11) were titrated into a solution of CoIIITMPyP(OH)(H2O) (3.48 μM in 50 mM sodium phosphate buffer, pH 7.4, 1 mM in EDTA) in the presence of 10 μM BSA using gas-tight syringes and a 1.00 cm pathlength septum-sealed cuvette at 25°C (see Experimental for further details). The solid line represents a nonlinear least squares fit to the data using the Hill equation. (D) The rate of cyanide binding in the presence (solid dots) and absence (solid trace) of BSA. Conditions: 3.48 μM CoIIITMPyP(OH)(H2O) in 50 mM sodium phosphate buffer, pH 7.4, 1 mM in EDTA and 0.1 mM in NaCN.
Animal Experiments
A previous study with mice in which CoIIITMPyP was given as a prophylactic, 15 and 60 minutes prior to the administration of lethal doses of cyanide, found no beneficial antidotal effect8. To the contrary, in the present work with Swiss Webster mice (males, 16-20 weeks of age) we have been able to demonstrate the effectiveness of CoIIITMPyP as a cyanide antidote when administered after the toxin. It is becoming increasingly difficult to obtain IACUC approval for protocols where death is the endpoint. To combat this problem, Crankshaw et al. 7 developed a procedure that we have slightly modified Cambal et al. 6 in which amelioration of cyanide toxicity may be assessed sub-lethally in mice. Intraperitoneal (i.p.) injection of mice with 0.1 mmol/kg (5 mg/kg) NaCN in saline results in loss of consciousness, with clear indications of the onset of narcosis (animals stagger, or are motionless) beginning at around 1 minute following administration of the toxin. Shortly thereafter, the animals may be placed on their backs and observed until they regain consciousness – at which time they turn themselves to an upright (prone) position. The “righting-recovery time” is the duration of this process measured from injection of the toxin to the animal returning itself to a prone position. Mice given only NaCN i.p. (controls) that survived the dose displayed righting recovery at 33 (± 2) min (n = 6). Surviving mice given 0.1 mmol/kg NaCN followed by 0.05 mmol/kg (68 mg/kg) CoIIITMPyP i.p. 1 min later exhibited righting recovery at 8 (± 2) min (n = 4) – clearly indicating amelioration of sub-lethal cyanide toxicity by CoIIITMPyP. For comparison, righting-recovery times were also measured for mice given the known antidote 6 sodium nitrite 1 min and 2 min following the NaCN dose. All of these data are summarized graphically (Figure 6).
Figure 6. The antidotal effect of CoIIITMPyP on cyanide-intoxicated mice.

Swiss-Webster mice (males, 16-20 weeks of age) were given NaCN (5.0 mg/kg i.p.). The administration of toxin (time = 0 min, n=6, designated control) was followed by the administration of antidote at the following times and doses: 68 mg/kg CoIIITMPyP (at t = 1 min, n = 4); 10 mg/kg NaNO2 (at t = 1 min, n = 5); or 4-16 mg/kg NaNO2 (at t = 2 min, n > 80 6); all i.p. The surviving mice were then placed on their backs and the times at which the animals righted were recorded (see Methods). The righting-recovery time of the surviving cyanide-intoxicated mice was decreased in those administered CoIIITMPyP, or sodium nitrite.
The method we have employed is primarily designed to provide data regarding sub-lethal intoxication, but we do experience collateral deaths and, therefore, some survival information is also obtained. A total of 18 control animals were used, divided into 3 groups of 6 mice each; the experiments with one group being performed at the same time as the CoIIITMPyP antidotal procedures, the other two being employed at the same time as the sodium nitrite antidotal procedures. At the experimental dose of 0.1 mmol/kg (5 mg/kg) NaCN i.p., with no antidote being administered, only 6 of 18 mice survived, whereas in the case of animals also given 0.05 mmol/kg (68 mg/kg) CoIIITMPyP ip 1 min after the toxin, 4 of 6 survived. The LD50 for NaCN given i.p. to the same kind of mice as employed in this work has been reported to be 5.7 mg/kg by Baskin et al. 19. Clearly, since 12 out of 18 control mice died, the 5.0 mg/kg NaCN used in the current experiments was greater than the LD50. We do not need to speculate here on the reasons for this discrepancy, since the “true” value for the LD50 is irrelevant to our findings. Suffice to say that the relevant controls were interspersed with the antidotal test measurements using the same NaCN solution.
DISCUSSION
Antidotal effectiveness of CoIIITMPyP
Equilibrium constants for the binding of cyanide to various cobaltic macrocycles have been reported, but the numerical values depend on assumptions made about the binding model – in particular, (i) whether [CN−] or [HCN] is written on the left hand side of equations 1 & 2 and (ii) should the hydrogen-ion concentration be included8,20-24. For example, the formation constant for the (mono)cyano adduct of the FDA approved cyanide-scavenging agent cobalamin (or vitamin B12) was originally (1960) evaluated in terms of a model assuming CN− to be the incoming nucleophile24:
| (3) |
However, in a seminal paper almost 20 years later (1979) Reenstra and Jencks21 showed that HCN is the relevant nucleophile around neutrality:
| (4) |
Here, in the case of CoIIITMPyP, we agree that two cyanide molecules displace the water-derived axial ligands from the aquo/hydroxo form of CoIIITMPyP, but make no other assertion as to the species of cyanide (HCN or CN−) involved, or whether a proton is consumed/released. That is, to facilitate comparison with ligand-binding constants as normally reported for metalloproteins, we adopt an essentially biochemical convention and present the result as K’β with a value of 2.1(±0.2) × 1011, where the equilibrium constant has been evaluated in terms of total cyanide (= [HCN + CN−] ≈ [HCN] at pH 7.4) and the product [H3O+] has simply been left out. Consequently, to meaningfully compare the present result with the earlier formation constant for cyanocobalamin of George et al. 24, we should multiply the earlier result by [CN−]/[HCN] at neutrality: 108 × (~10-2) ≈ 106. Therefore, inspection of the relevant equilibrium constants suggests that CoIIITMPyP could be a significantly better cyanide scavenger than cobalamin.
Hambright et al. have reported14 a formation constant of 5.6 × 107 for the binding of a second cyanide anion to the monocyanoCoIIITMPyP complex. Re-calculating this result to reflect total cyanide rather than the anion concentration (as per the earlier authors) gives K’f2 = 9.0 × 105, again a significantly smaller numerical value, confirming that some care needs to be exercised in comparing results from different studies. It follows that Kf1 = K’β – K’f2 = 2.1 × 1011 – 9.0 × 105 ≈ 105. These estimates for the two formation constants (i.e. Kf1 and K’f2 ≈ 105 and 106 respectively) are in keeping with the binding of cyanide to CoIIITMPyP being cooperative and, indeed, a Hill constant of 2.0 fits the equilibrium data much better than a constant of 1.0 (Figure 2B) or any intermediate number. Titrations performed in the presence of one equivalent BSA (Figure 5C), a possible source of interference in serum, show no alteration in the cooperativity of cyanide binding to CoIIITMPyP and only modest changes in the equilibrium constant (1.2 × 1011 in the presence of BSA vs. 2.1 × 1011 without). Moreover, a kinetic comparison (Figure 5D) shows that there is virtually no difference in the rate of cyanide binding to CoIIITMPyP in the presence and absence of BSA. These findings appear to confirm the promising candidacy of CoIIITMPyP as a potentially useful cyanide antidote.
The second order rate constant for the binding of cyanide to cobalamin at 25°C and approximately neutral pH has been reported to be 80 M-1s-1 21. This is comparable to the faster phases of the reaction we observe for cyanide with CoIIITMPyP (Table I). Therefore, there does not seem to be any overriding kinetic reason why CoIIITMPyP should not, by virtue of its cyanide-scavenging ability, have reasonable antidotal activity toward cyanide intoxication. Consequently, the reported 8 lack of any such antidotal activity in mice is troublesome, as based upon these findings the accepted cyanide-scavenging mechanism by which cobalamin surely works could logically be questioned.
In the previous study8 the CoIIITMPyP (and other metalloporphyrins) were given prophylactically, 15 and 60 min. before the (lethal) cyanide dose, with no detectable beneficial effect. In the present proof-of-concept study we gave the CoIIITMPyP soon (1 min.) after the cyanide and found readily measurable protection in terms of quicker recovery of survivors (Figure 6) for mice given the antidote compared to controls. The failure of CoIIITMPyP as a prophylactic cyanide antidote must be due to its inactivation within 1-15 min. in vivo. The possible mechanism(s) responsible for this inactivation are currently under investigation and will be presented in due course. We attempted a few experiments in which the CoIIITMPyP was given 2 min following the cyanide, obtaining righting recovery times that were comparable to controls, but less reproducible (not shown) and these were not continued to statistical significance. In contrast, in a previous study6 we showed that sodium nitrite was markedly antidotal if given 2 min after the cyanide (reproduced as the last column in Figure 6). Collateral deaths in these experiments occurred between 1.5 and 3 min and behavioral indications of toxicity were already apparent 1 min after injection of the cyanide, suggesting the toxic dose to have become systemically distributed by 1 min onwards. We interpret the more rapid action of sodium nitrite to be a consequence of its ability to directly protect cytochrome c oxidase from inhibition within the mitochondrion.6 Scavengers like CoIIITMPyP must work more passively by binding available cyanide in the circulating bloodstream, or as may have been the case to some extent in the present experiments, any remaining within the intraperitoneal cavity.
Complexity of the reaction between CoIIITMPyP and cyanide
The kinetics and mechanisms of the substitution reactions of various anionic ligands with water-soluble porphyrins have been studied by several groups, but in particular, Pasternack & Cobbs 15 found that the addition of thiocyanate (SCN−) and a solvent proton to CoIIITMPyP(OH)(H2O) resulted in an intermediate, CoIIITMPyP(H2O)(SCN), which then quite rapidly aquired a second thiocyanate ion to form CoIIITMPyP(SCN)2. The presence of the thiocyanate group was found to exert a trans influence in the CoIIITMPyP(H2O)(SCN) intermediate resulting in the fast addition of the second thiocyanate, so that the addition of the first thiocyanate is the rate-determining step. The limited data set that we obtained for the reaction of CoIIITMPyP with azide (N3−) (Figure 4) appears to be in keeping with an analogous mechanism. Based upon such observations, it is reasonable to expect that cyanide should behave similarly and, indeed, in the case of another water-soluble metalloporphyrin, cobalt(III)-tetrakis(4-sulfonatophenyl)porphyrin (CoIIITSPP), a two-step reaction in which the first step is rate limiting was found14. In their report primarily on the CoIIITSPP-cyanide reaction, Hambright and Langley14 briefly noted that the first cyanide addition to CoIIITMPyP(OH)(H2O) had a rate constant of 1.1 × 103 M-1s-1 at pH 7.4 and 25°C – but then made no further mention of CoIIITMPyP, did not show any of the relevant data and their opinion regarding the attacking species, cyanide anion or HCN, was unclear.
We have now shown that the kinetics of cyanide binding to CoIIITMPyP are remarkably complicated with 3 to 4 rate constants (depending on pH, see Table I) all of which depend on the cyanide concentration (Figure 3). It is highly unlikely that unidentified impurities in the CoIIITMPyP preparations are responsible for any of these phases as the otherwise analogous azide reaction exhibits only a single phase (Figure 4). Similarly, Baldwin et al. 16 observed three exponential phases in their study of the rate of reaction of cyanide with cobinamide, a CoIII-containing corrin, where the fastest phase was lost on raising the pH from 7 to 8. Therefore, the observed kinetics of the association reaction between cyanide and CoIIITMPyP (Figure 2, Table I) has more features in common with the analogous cobinamide reaction 16 than with the CoIIITSPP reaction 14. The pKa of HCN is 9.24 at 25°C25 and it follows that in the kinetic experiments at pH 8.4 the concentration of cyanide anion was an order of magnitude greater than at pH 7.4. Therefore, if CN− were the incoming nucleophile in any of the kinetic phases, there should be a significant increase in the observed rate for that phase at pH 8.4 compared to pH 7.4. No such effect was observed and, consequently, the data indicate molecular HCN to be the more important attacking nucleophile. This is in keeping with the previously reported findings for cobalamin where HCN was also shown to be the attacking nucleophilic species around neutral pH rather than the anion21. To the contrary, the reaction of cyanide with CoIIITSPP does appear to involve both HCN and CN− under neutral conditions, as the observed reaction rate increases by 30% between pH ~7 and ~814. At pH 7.4, a small portion of CoIIITMPyP (~4%, pKa = 6) exists as the diaquo complex (CoIIITMPyP(OH2)2) whereas at pH 8.4, a small portion (~ 3%, pKa = 10) is present as the dihydroxo complex (CoIIITMPyP(OH)2). In the case of both cobalt(III) corrins 16 and porphyrins14, displacement of the axial aquo ligands in the bis(aquo) complexes by cyanide is very slow and, consequently, the presence of the rapid phase 1 at pH 7.4 (and its absence at pH 8.4) (Table I) cannot be explained on the basis of this phase involving reaction of the bis(aquo) CoIIITMPyP.
It is apparent in the kinetic traces (e.g. Figure 3A) that the reaction was still continuing more than 15 min. after it was initiated. This was not the case in the titration experiments where absorbance changes had ceased within 10 min. of cyanide additions being made and suggests that at least the phase associated with k4 (and perhaps also that associated with k3) is(are) only present when cyanide is in large (> 20-fold) excess over CoIIITMPyP. There are plausible explanations for such behavior. For example, cyanide may self-associate to form complex species (such as NC—H---NCH, [NC—H---NC]−, etc.) that could form metastable complexes with CoIIITMPyP, inhibiting its final conversion to CoIIITMPyP(CN)2. Supporting this argument, the amplitude of phase 4 was observed to diminish at high ionic strength (0.3 M KCl, not shown). However, since the focus of the present study was the potential use of CoIIITMPyP as an antidotal cyanide scavenger, we viewed the details of reactions in which cyanide is in large excess as being of marginal interest and did not investigate the slow phases further.
We are confronted with two quicker phases that must be considered in relation to cyanide scavenging in vivo, where the cyanide cannot be in large excess over the antidote if the intervention is to be successful. The kinetics of the reaction between pyridine and CoIIITMPyP have previously been shown to be biphasic at pH 8 and explained on the basis of rapid substitution by the first pyridine followed by slower substitution of the second to yield the bis(pyridyl) product12. The cyanide results, monitored at the absorption maximum for CoIIITMPyP(CN)2 (454 nm), are inconsistent with a similar biphasic mechanism at pH 8.4. If phase 2 were to represent formation of the mono(cyano) adduct followed by phase 3 leading to the final bis(cyano) product, then the amplitude of phase 3 would have to be the same or greater than the phase 2 amplitude, whereas the reverse situation was actually observed (Table I). The amplitudes of phase 2 and phase 4 (Table I) are consistent with a two-step mechanism similar to the pyridine reaction, but if this were the case, phase 4 would have to dominate the later stages of any titration procedures rather than be absent as already stated. We conclude that the presence of more than one independent phase in the cyanide-reaction kinetics necessarily means there are multiple mechanistic pathways to product formation and suggest the following scheme to account for the two faster processes (phases 1 and 2):
Scheme 1.

A plausible scheme for the two fastest phases of the reaction between CoIIITMPyP and HCN at pH 7.4-8.4.
The key features of this scheme are (i) interconversion of liganded HCN and CN− in the intermediate, with an associated pKa somewhat less than 7.4, accounts for the absence of phase 1 under mildly alkaline conditions (Table I) and (ii) the differing trans effects of HCN and CN− account for the two distinct rate constants (k1 and k2). Of course, our assertion that HCN is the most important attacking nucleophile only applies to the experimentally detected rate-determining processes. For reasons discussed above, we have not been able to reconcile any of our findings with substitution of the first cyanide being rate limiting in the reaction with CoIIITMPyP. Consequently, in the initial fast step leading to formation of the mono(cyano) intermediate, the nucleophile could be CN− rather than HCN (as drawn) or a combination of both.
Concluding Remarks
However perplexing the mechanistic details may be, the rate of the cyanide reaction with CoIIITMPyP and the magnitude of the association constant are large enough to render this metalloporphyrin an effective antidote to cyanide intoxication in experimental animals (Figure 6). The multiple positive charges on the molecule (Figure 2A, inset) lead us to suspect that it may partition into mitochondria and have an undesirable toxicity not readily apparent in the present proof-of-concept study. Nevertheless, the results are encouraging in so far as they suggest that many cobaltic macrocycles should be antidotal to cyanide intoxication, broadening the range of potential candidate structures to include simpler, less expensive molecules than the corrinoids currently either available, or under development.
Acknowledgments
Supported by the CounterACT Program, National Institutes of Health Office of the Director (NIH OD), and the National Institute of Neurological Disorders and Stroke (NINDS), Grant Number NS063732 to J.P. and L.L.P. and Bruce R. Pitt.
ABBREVIATIONS
- H2-TMPyP
4,4’,4”,4”’-porphyrin-5,10,20-tetrayltetrakis (1-methylpyridinium) tetrakis (4-methylbenzenesulfonate)
- CoTMPyP
[[4,4’,4”,4”’-porphyrin-5,10,20-tetrayltetrakis (1-methylpyridiniumato] (2-)] -cobalt(III) pentaiodide
- EDTA
ethylenediaminetetraacetate
- BSA
bovine serum albumin
References
- 1.Borron SW, Stonerook M, Reid F. Efficacy of hydroxocobalamin for the treatment of acute cyanide poisoning in adult beagle dogs. Clin Toxicol (Phila) 2006;44(Suppl 1):5–15. doi: 10.1080/15563650600811672. [DOI] [PubMed] [Google Scholar]
- 2.Corral Torres E, Suarez Bustamante R, Gomez Granizo E, Casado Florez MI, Gimenez Mediavilla JJ, de Elas Hernandez R. Hydroxocobalamin and lactate concentration in patients suspected of having cyanuric acid poisoning related to smoke inhalation syndrome. Emergencias. 2010;22:9–14. [Google Scholar]
- 3.Fortin JL, Waroux S, Giocanti JP, Capellier G, Ruttimann M, Kowalski JJ. Hydroxocobalamin for poisoning caused by ingestion of potassium cyanide: a case study. J Emerg Med. 2010;39:320–4. doi: 10.1016/j.jemermed.2008.04.040. [DOI] [PubMed] [Google Scholar]
- 4.Brenner M, Mahon SB, Lee J, Kim J, Mukai D, Goodman S, Kreuter KA, Ahdout R, Mohammad O, Sharma VS, Blackledge W, Boss GR. Comparison of cobinamide to hydroxocobalamin in reversing cyanide physiologic effects in rabbits using diffuse optical spectroscopy monitoring. J Biomed Opt. 2010;15:017001. doi: 10.1117/1.3290816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan A, Balasubramanian M, Blackledge W, Mohammad OM, Alvarez L, Boss GR, Bigby TD. Cobinamide is superior to other treatments in a mouse model of cyanide poisoning. Clin Toxicol (Phila) 2010;48:709–17. doi: 10.3109/15563650.2010.505197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cambal LK, Swanson MR, Yuan Q, Weitz AC, Li H-H, Pitt BR, Pearce LL, Peterson J. Acute, sublethal cyanide poisoning in mice is ameliorated by nitrite alone: complications arising from concomitant administration of nitrite and thiosulfate as an antidotal combination. Chem Res Toxicol. 2011;24:1104–12. doi: 10.1021/tx2001042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crankshaw DL, Goon DJ, Briggs JE, Delong D, Kuskowski M, Patterson SE, Nagasawa HT. A novel paradigm for assessing efficacies of potential antidotes against neurotoxins in mice. Toxicol Lett. 2007;175:111–7. doi: 10.1016/j.toxlet.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hambright P. Anti-cyanide drugs. Annual Report (91-06852): U S Army Medical Research and Development Command 1991 [Google Scholar]
- 9.Pasternack RF, Frances CL, Raff D, Spiro E. Aggregation of Nickel(II), Copper(II), and Zinc(II) Derivatives of Water-Soluble Porphyrins. Inorg Chem. 1973;12:2606–2611. [Google Scholar]
- 10.Fasman GD, editor. Practical Handbook of Biochemistry and Molecular Biology. CRC Press, Inc.; Boca Raton: 1989. [Google Scholar]
- 11.Hambright P, Adeymo A, Shamim A, Lemelle S, Lavallee DK, Miller D, White A. 4,4’,4”,4”’-Porphryin-5,10,15,20-tetratltetrakis(1-methylpyridiniumato](2-)]-indium(III) In: Kirshner S, editor. Inorganic Syntheses. Vol. 23. John Wiley & Sons, Inc.; Hoboken, NJ USA: 2007. [Google Scholar]
- 12.Pasternack RF, Cobb MA, Sutin N. Substitution and Oxidation-Reduction Reactions of a Water-Soluble Porphyrin: (Tetrakis( 4-N-methyl pyridyl) porphinecobalt(III)-Pyridine System. Inorg Chem. 1975;14:866–873. [Google Scholar]
- 13.Pasternack RF, Cobb MA. Substitution-Reactions of a Water-Soluble Cobalt(III) Porphyrin with Thiocyanate as a Function of pH. J Inorg Nucl Chem. 1975;35:4327–4339. [Google Scholar]
- 14.Hambright P, Langley R. Cyanide scavengers: kinetics of the reactions of cyanide with a water soluble cobalt(III) porphyrin. J Inorg Biochem. 1988;32:197–205. doi: 10.1016/0162-0134(88)80027-8. [DOI] [PubMed] [Google Scholar]
- 15.Pasternack RF, Cobb MA. Reactions of a Water-Soluble Cobalt Porphyrin with Thiocyanate. Biochem Biophys Res Comm. 1973;51:507–511. doi: 10.1016/0006-291x(73)91343-0. [DOI] [PubMed] [Google Scholar]
- 16.Baldwin DA, Betterton EA, Pratt JM. The chemistry of vitamin BI2. Part 20.1 Diaquocobinamide : pK values and evidence for conformational isomers. J Chem Soc Dal Trans. 1983:217–223. [Google Scholar]
- 17.Morris DE, Basolo F. Kinetics and Mechanism of Substitution Reactions of Dinitrosyldicarbonyliron(O) J Am Chem Soc. 1968;90:2531–&. [Google Scholar]
- 18.Fleischer EB, Jacobs S, Mestichelli L. The Kinetics of the Reaction of cobalt (III) and iron (III) hematoporphyrin with cyanide and thiocyanate. Evidence for a dissociative mechanism. J Amer Chem Soc. 1968;90:2527–2531. [Google Scholar]
- 19.Baskin SI, Nealley EW, Lempka JC. Cyanide toxicity in mice pretreated with diethylamine nitric oxide complex. Hum Exp Toxicol. 1996;15:13–18. doi: 10.1177/096032719601500103. [DOI] [PubMed] [Google Scholar]
- 20.Broderick KE, Potluri P, Zhuang S, Scheffler IE, Sharma VS, Pilz RB, Boss GR. Cyanide detoxification by the cobalamin precursor cobinamide. Exp Biol Med (Maywood) 2006;231:641–9. doi: 10.1177/153537020623100519. [DOI] [PubMed] [Google Scholar]
- 21.Reenstra WW, Jencks WP. Reactions of cyanide with cobalamins. J of the Am Chem Soc. 1979;101:5780–5791. [Google Scholar]
- 22.Marques HM, Baldwin DA, Pratt JM. Hemes and hemoproteins. 3. The reaction of microperoxidase-8 with cyanide: comparison with aquocobalamin and hemoproteins. J Inorg Biochem. 1987;29:677–91. doi: 10.1016/0162-0134(87)80014-4. [DOI] [PubMed] [Google Scholar]
- 23.Hayward GC, Hill HA, Pratt JM, Vanston NJ, Williams RJ. The chemistry of vitamin B 12. IV. The thermodynamic trans-effect. J Chem Soc Perkin. 1965;1:6485–93. [PubMed] [Google Scholar]
- 24.George P, Irvine DH, Glauser SC. The influence of chelation in determining the reactivity of the iron in hemoproteins. and the cobalt in vitamin B12 derivatives. Ann N Y Acad Sci. 1960;88:393–415. doi: 10.1111/j.1749-6632.1960.tb20038.x. [DOI] [PubMed] [Google Scholar]