Abstract
The internal face of the detrusor smooth muscle wall of the urinary bladder is covered by a mucosa, separating muscle from the hostile environment of urine. However, the mucosa is more than a very low permeability structure and offers a sensory function that monitors the extent of bladder filling and composition of the urine. The mucosa may be considered as a single functional structure and comprises a tight epithelial layer under which is a basement membrane and lamina propria. The latter region itself is a complex of afferent nerves, blood vessels, interstitial cells and in some species including human beings a muscularis mucosae. Stress on the bladder wall through physical or chemical stressors elicits release of chemicals, such as ATP, acetylcholine, prostaglandins and nitric oxide that modulate the activity of either afferent nerves or the muscular components of the bladder wall. The release and responses are graded so that the mucosa forms a dynamic sensory structure, and there is evidence that the gain of this system is increased in pathologies such as overactive bladder and bladder pain syndrome. This system therefore potentially provides a number of drug targets against these conditions, once a number of fundamental questions are answered. These include how is mediator release regulated; what are the intermediate roles of interstitial cells that surround afferent nerves and blood vessels; and what is the mode of communication between urothelium and muscle – by diffusion of mediators or by cell-to-cell communication?
The urinary bladder has two functions: to store urine, up to 500 ml in the normal adult, and to completely void its content when expeditious. Storage is associated with very little increase in intravesical pressure and low bladder wall tension, whilst voiding occurs with a sustained rise of pressure, sufficient to overcome out-flow resistance, due to contraction of detrusor smooth muscle. This two-state system is controlled by the central nervous system but modulated by interaction between different cell types in the layers of the bladder wall. In pathological conditions such as overactive bladder, this on-off process may be disrupted by uncontrolled activity that could elicit unpleasant sensations of urinary urgency or pain and also contractions that may be powerful enough to cause involuntary loss of urine. It is therefore important to understand how storage and voiding modalities of the bladder are controlled to provide therapies that minimize these pathologies.
Structure of the Bladder Wall
The smooth muscle (detrusor) of the bladder wall is protected by an external serosa and on the vesical face overlain by a mucosa that itself consists of a tight transitional epithelium (urothelium), basement membrane and sub-urothelium (fig. 1A). The urothelium itself is covered by a mucopolysaccharide glycocalyx that offers protection for the urothelium from the hostile medium of urine. The urothelium is made up of three layers: a basal cell layer attached to a basement membrane, an intermediate layer and a superficial or apical layer composed of large hexagonal cells known as the ‘umbrella cells’. An essential function of the urothelium is to offer an effective barrier between urine and underlying tissues, achieved by tight junctions between umbrella cells, severely limiting solute and water movement across the barrier [1,2]. Damage to the urothelium, evident on exposure to noxious agents or associated with pathologies such as spinal cord injury [3], is accompanied by irritative lower urinary tract symptoms. However, the urothelium has transport functions as demonstrated by the development of a finite membrane potential, solute and water movement and the presence of aquaporins, urea transporters, ion channels (e.g. ENaC) and mineralocorticoid receptors [4–6]. Moreover, the different composition of urine sampled from the bladder lumen and renal pelvis is consistent with post-renal urinary tract salt and water exchange [7].
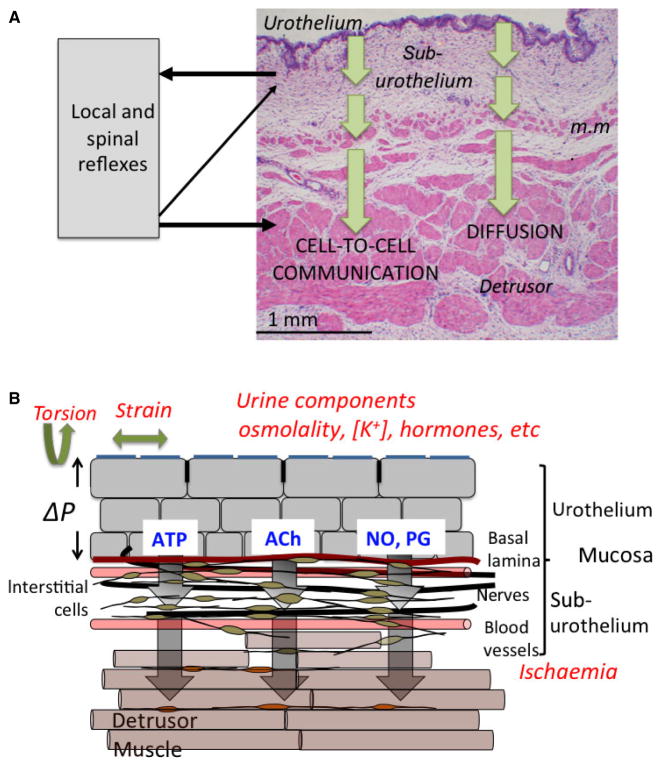
Fig. 1.
(A) Section of the sheep bladder wall. The section shows the urothelium, suburothelium and detrusor smooth muscle layers. The suburothelium is a complex structure of blood vessels, interstitial cells, afferent nerves and in this species a muscularis mucosae (m.m.). External physical and chemical agents can cause release of mediators (arrows) from the urothelium that could influence suburothelium structures to elicit nervous responses, changes to blood vessel tone and contractile responses of detrusor and possibly muscularis mucosae. Contractile responses could be mediated either by diffusion of mediators and/or by cell-to-cell communication. (B) A schematic drawing of the bladder wall, illustrating the cell types in different layers, as well as the stresses that may induce mediator release.
The sub-urothelium that separates the urothelium from the detrusor layer is composed of an extracellular matrix containing interstitial cells, fibroblasts, adipocytes, afferent and efferent nerve endings, blood vessels and, in some species including human beings, a more ill-defined muscular layer –the muscularis mucosae. The functional interaction of these different cells and how they communicate with the urothelium and detrusor layers is crucial to understand how this layer has essential roles to sense bladder filling as well as exert control over detrusor contractile activity.
The detrusor layer itself constitutes the mass of the bladder wall and consists of smooth muscle bundles separated by connective tissue and interstitial cells. Parasympathetic post-ganglionic nerves provide the excitatory input.
The Release of Mediators and Sensations Arising From the Bladder Wall
Physical or chemical stressors applied to the bladder itself, isolated sections of the bladder wall, strips of mucosa dissected free of detrusor or isolated urothelial cells evoke release of several small molecules including: ATP, acetylcholine (ACh), prostaglandins or nitric oxide [4,8–10] – fig. 1B. The fact that all these preparations release these compounds assumes that the source is the urothelium, although the contribution from other cells has not been systematically evaluated. Physical stressors include longitudinal strain or tension; the rate of change of these variables; trans-mural pressure changes; osmotic swelling; or shear stresses to cells; chemical or cellular stressors include extracellular acidosis [11], noxious compounds such as doxorubicin [12] and inflammatory conditions [13]. Primary sensory neurons also release several neuropeptides such as calcitonin gene-related peptide (CGRP) and substance P that may mediate local inflammatory responses [14]. However, this is beyond the scope of this article and will not be considered further.
The pathways for release and their signalling roles have been mostly investigated for ATP and ACh. Overall, their action will be largely autocrine or paracrine as extracellular ATPases (eNTPDases) and cholinesterases will limit their half-time. In principle, these mediators can either affect local afferent nerves to convey sensations of filling to the central nervous system, regulate local blood flow by affecting vessel resistance or modulate detrusor contractile function. Mucosa afferents express a number of receptors that include P2X and P2Y purinergic families; transient receptor potential channel TRP-V, TRP-M and TRP-A families; as well as pituitary adenylate cyclase type 1 activating polypeptide (PACAP)-selective receptors. P2X2/3 receptors are understood to mediate the excitatory effects of locally released ATP. P2X3 knockout mice showed a diminished micturition reflex whereby greater stretch of the bladder wall was required to elicit a given degree of afferent signalling. However, activity was not abolished [15] completely, which may suggest additional roles for CGRP, TRPV1 and PACAP receptors [15,16] although their functional ligands are yet to be fully elucidated. The life-time and extent of the effect for ATP released from the urothelium will be limited due to the presence of ectoATPases (E-NTPDase3) on the basal surfaces of urothelial cells [17]. This would be anticipated for a dynamic sensory modulator but also raises the question of the roles of ADP, AMP and adenosine in also modulating signalling responses.
The quantity of ATP released during imposition of stressors alters with the age and the pathology of the parent tissue, suggesting an underlying cause of pathological lower urinary tract sensations. Thus, ATP release is raised in bladder wall tissue from: old animals and human beings compared to younger counterparts [18,19], tissue biopsies of patients with overactive bladders [20] and cultured urothelial cells of patients with painful bladder syndrome/interstitial cystitis [21].
Urothelial cells also have the capacity to synthesize and exhibit stretch-activated release of acetylcholine (ACh) [8,22]. There is inconsistent evidence as to whether release is enhanced [23] or diminished [18] with age, but several other agents including the cytotoxic drug, doxorubicin and lipopolysaccharide reduced ACh release stimulated by cell stretch [23,24]. Comparison of ACh and ATP release reveals some interesting differences: stretch-activated release of ACh is much greater than ATP per unit mass of tissue; the magnitude of stresses required to release ACh is much smaller, as is the dynamic range of stresses that release ACh [25]. Moreover, the release of ATP is modulated by muscarinic receptor activation independently of physical stressors; muscarinic receptor agonists increase ATP release whilst antagonists, particularly to M2 but not M3 receptors, inhibit it. Thus, it has been suggested that ACh release is the first step in a sensory transducer system that itself regulates the further release of ATP with consequent downstream effects [25,26]. Two observations follow which question perceived wisdom about the use of antimuscarinic agents to manage overactive bladder (OAB) symptoms: firstly, their site of action may not solely be on detrusor M3 receptors at the efferent nerve/smooth muscle junction, as assumed, but also on the mucosa; secondly, drugs with a mixed M2/M3 profile may be more effective than selective M3 receptor antagonists. Certainly, antimuscarinic agents increase cystometric capacity in patients with OAB, which can be explained by their action on storage rather than solely on voiding mechanisms.
Stretch-induced prostaglandin (PGE2) release from the mucosa has also been measured and may exert direct effects on detrusor contractile function or, via an EP1 receptor, enhance local ATP release to increase afferent activation [27]. Moreover, a positive feedback process is suggested by the ability of ATP to augment PGE2 release [28]. Urothelial cells contain the enzymatic machinery to synthesize nitric oxide (NO) [29] and there is evidence that it suppresses afferent nerve activity [30]. Increase in NO production, as occurs, for example, in a cat model of bladder pain syndrome, is also associated with a loss of barrier function [31] that in turn will augment afferent activity by allowing noxious components of urine more direct access to suburothelial structures.
Pathways for Mediator Release
Significant effort has been expended to identify the cellular routes for mediator release and suggests the involvement of several pathways. ATP release has been identified via hemichannels of connexin or pannexin proteins, or even through vesicles [32,33]. However, these conclusions are generally based on inhibitors of hemichannel proteins or vesicular transport and there is debate about the specificity of these agents [34]. In addition, release is enhanced by an increase in intracellular [Ca2+] that may underlie the augmentation of release by TRPV1 channel activation and extracellular acidosis [11] and is attenuated by extracellular Ca2+ that is consistent with involvement of connexin hemichannels. However, the mode of action of P2Y receptor agonists that increase release of ATP, as well as of adenosine (A1) receptor agonists that reduce release, have not been clarified. Of interest is that ATP release is reduced from the tissue of patients who have received botulinum toxin type A (BnTx-A) injections to reduce overactive bladder symptoms [35]. Moreover, direct application of BnTx-A attenuates stress-dependent ATP release, and the binding targets for BnTx-A have been identified on urothelial cells [36]. This also raises the question whether BnTx-A as an agent to reduce OAB contractions, does so by reducing transmitter release from efferent nerves, as it has assumed to work, or by dampening the sensory responses to bladder filling, as suggested by these observations. Release of ACh is via different routes: it is unaffected by reduction in vesicular formation, blockade of hemichannels or botulinum toxin. The only effective modulator identified was an inhibitor of CFTR channels, which reduced release by about 50% [25].
The Mucosa and Contractile Functions of the Bladder
Contractile function in the bladder exists in two modalities: phasic contractions initiated by transmitters released from parasympathetic fibres that evoke large contractions to void urine and spontaneous contractions that are not primarily initiated by motor nerves. The origin and function of the latter remain unclear, but they have several properties that distinguish them from nerve-mediated contractions and imply they have a physiological and pathological role:
they are unaffected by neurotoxins, but are Ca2+ sensitive;
they are greatly augmented by the mucosa overlaying the detrusor;
they can manifest as micromotions – localized, non-propagating contractions on the bladder wall – that are mirrored as small intravesical pressure fluctuations;
they are enhanced in pathologies that manifest as overactive bladders.
Their normal function may be to maintain a significant tone in the bladder wall during filling to ensure it maintains a roughly spherical shape but not enough to reduce the natural compliance of the bladder in this phase. Several, not mutually exclusive, theories have been proposed that might also contribute to the large spontaneous contractions associated with a subtype of OAB called detrusor overactivity:
a myogenic theory, due to intrinsic spontaneous activity of detrusor myocytes
a neurogenic hypothesis whereby spontaneous nervous activity initiated in the central or peripheral nervous system drives contractions
spontaneous release of neurotransmitters
a urotheliogenic theory whereby the mucosa drives spontaneous detrusor contractions
the mucosa itself has significant, independent contractile function.
Of these, the urotheliogenic theory and an independently contractile mucosa are the most consistent with experimental evidence, although a neurogenic origin is likely in a subset of patients. However, the questions arise about the nature of the interaction between mucosa and detrusor, as well as how the mucosa itself generates significant contractile activity.
The Contractile Properties of the Mucosa
The mucosa, in most species, may be readily separated from the detrusor layer by blunt dissection and in vitro generates spontaneous contractions, as well as tonic responses to electrical field stimulation and cholinergic agonists [37–39]. Several origins, not mutually exclusive, have been proposed including interstitial cells with a contractile phenotype (myofibroblasts) and pericytes around blood vessels or the muscularis mucosae. It is evident that the pharmacological profile of mucosa spontaneous contractions is different from that of the detrusor layer, for example capsaicin augments detrusor activity whilst suppressing mucosal activity [38]. This would argue against the possibility that in dissecting the preparations there is residual contamination of detrusor smooth muscle. This phenomenon is of significance as the mucosa thickens in several conditions associated with overactive bladder [40] and this activity may be especially significant in these pathologies. There is also evidence that such contractile activity may be influenced by mucosal ATP release. Under resting conditions, mucosal ATP release is cyclical with a periodicity of about 10 min. and this is reflected in a similar periodicity of the integral of spontaneous contractility but with a delay of a few minutes [38]. It might be suggested that ATP release form urothelium diffuses within the mucosa to modulate contractility activity. It does not identify the cellular targets except that they probably have a receptor phenotype to ATP or its metabolites. The contractile behaviour of the mucosal layer under various pathological conditions has not yet been investigated; however, there is a change in the characteristics of spontaneous contractions of this layer with ageing [41].
Functional Interactions Between the Mucosa, Detrusor and Associated Vasculature
There is also convincing evidence of mucosa–detrusor interaction in generating spontaneous activity – the urotheliogenic theory. The most straightforward observation is that an in vitro bladder wall preparation of detrusor and attached mucosa generates substantial spontaneous contractions and these are dramatically reduced when the mucosa is removed [42,43]. This is complicated by the fact that an intact mucosa overlaying detrusor muscle also exerts a tonic negative inotropic effect [44]. This complex interaction can be by diffusion of mediators between the two layers or from a cellular interaction. The observation that simply placing a mucosa layer over previously denuded detrusor restores some contractile activity supports a role for a diffusive interaction. However, if this was the sole mode of interaction, it would be expected that the pharmacological profile of spontaneous contractions would be solely determined by the phenotype of detrusor and this is not the case. Apart from the opposite actions of capsaicin on mucosa and detrusor activity (above), the same is true of P2Y receptor agonists such as ADP, UDP and UDP. These agonists generally suppress or are at least neutral on detrusor function but they increase mucosa activity [38]. Moreover, they greatly enhance spontaneous contractions of bladder wall preparations when mucosa and detrusor are attached [45]. Optical imaging experiments that map intracellular [Ca2+] and membrane potential propagated waves across the bladder wall reveal not only that an intact mucosa required for such activity but it is augmented by the above P2Y agonists. Moreover, these experiments also show that such propagated activity is initiated in the suburothelium of the mucosa and actually propagates to the detrusor – again augmented by P2Y agonists [45]. These mapping experiments also suggest that local diffusion of agents is insufficient alone to explain mucosa–detrusor interaction as the propagation velocity of such waves is too rapid and moreover too extensive over the bladder wall and suggests cellular interaction is also likely.
One potential cellular mediator of mucosa–detrusor interaction is the dense network of interstitial cells in the suburothelium – a network substantially increased in pathologies associated with enhanced spontaneous activity such as spinal cord injury [39]. These cells tend to have their cell bodies in the suburothelium nearest to the urothelium, but projections run towards the detrusor layer where much of the immunore-activity to the gap junction protein connexin 43 is found. These cells also have the attributes of forming an electrical functional syncytium: they are connected by connexin 43 gap junctions and also generate spontaneous depolarizations due to activation of a large density Ca2+-activated Cl− current, ICl,Ca [45]. Moreover, ICl,Ca is enhanced by interventions that accelerate Ca2+ wave propagation both across the bladder wall and between mucosa and detrusor, namely P2Y agonists and local reduction in pH. It may be proposed therefore that a function of suburothelial interstitial cells is to provide a cellular communication between the mucosa and detrusor that will augment contractile activity of the latter. The cells are ideally located below the urothelium to respond to mediators released from this layer, as well as their metabolites and their excitable nature means they can effectively propagate responses.
Moreover, interstitial cells might be involved in the local control of bladder tissue perfusion as a subpopulation of these cells is associated with the microvessels in the LP [46]. It is postulated that adjacent perivascular interstitial cells have a role in generating spontaneous vasoconstrictions of venules, which might be beneficial in maintaining blood flow during the filling phase of the micturition cycle [47]. Inadequate perfusion of the bladder and the resultant ischaemia can readily affect the urothelium and suburothelial cells, leading to altered urothelial signalling/barrier function and detrusor smooth muscle overactivity [48]. The relationship between suburothelial microvessels, interstitial cells and the urothelium needs to be further studied.
Conclusions
The mucosa lining the inner surface of the detrusor smooth muscle layer of the bladder has crucial roles other than providing an essential barrier function to protect detrusor from the unphysiological environment of urine. The urothelium acts as a sensor to bladder filling, although it has to be determined what is the actual physical stressor: wall stress, transmural pressure, acidosis from ischaemia, etc. The urothelium responds by releasing chemical mediators that eventually activate afferent nerves and/or locally influence muscle function. The role of intermediate cells, such as interstitial cells, remains to be determined. However, their electrically excitable nature gives them the capacity to modulate the function of nerves, detrusor muscle and even local blood vessels. Overall, the mucosa offers a dynamic sensory structure that allows the bladder to respond directly to the volume and composition of urine and thus optimize bladder contractile function. A major unanswered question is whether pathological changes to bladder function, such as overactive bladder and bladder pain syndrome, are determined by alterations to mucosa behaviour.
References
- 1.Acharya P, Beckel J, Ruiz WG, Wang E, Rojas R, Birder L, et al. Distribution of the tight junction proteins ZO-1, occludin, and claudin-4, -8, and -12 in bladder epithelium. Am J Physiol Renal Physiol. 2004;287:F305–18. doi: 10.1152/ajprenal.00341.2003. [DOI] [PubMed] [Google Scholar]
- 2.Lewis SA. Everything you wanted to know about the bladder epithelium but were afraid to ask. Am J Physiol Renal Physiol. 2000;278:F867–74. doi: 10.1152/ajprenal.2000.278.6.F867. [DOI] [PubMed] [Google Scholar]
- 3.Birder LA. Role of the urothelium in urinary bladder dysfunction following spinal cord injury. Prog Brain Res. 2006;152:135–46. doi: 10.1016/S0079-6123(05)52009-0. [DOI] [PubMed] [Google Scholar]
- 4.Ferguson DR, Kennedy I, Burton TJ. ATP is released from rabbit urinary bladder epithelial cells by hydrostatic pressure changes - a possible sensory mechanism? J Physiol. 1997;505:503–11. doi: 10.1111/j.1469-7793.1997.503bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ma HP, Eaton DC. Acute regulation of epithelial sodium channel by anionic phospholipids. J Am Soc Nephrol. 2005;16:3182–7. doi: 10.1681/ASN.2005040434. [DOI] [PubMed] [Google Scholar]
- 6.Rubenwolf PC, Georgopoulos NT, Kirkwood LA, Baker SC, Southgate J. Aquaporin expression contributes to human transurothelial permeability in vitro and is modulated by NaCl. PLoS ONE. 2012;7:e45339. doi: 10.1371/journal.pone.0045339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cahill DJ, Fry CH, Foxall PJ. Variation in urine composition in the human urinary tract: evidence of urothelial function in situ? J Urol. 2003;169:871–4. doi: 10.1097/01.ju.0000052404.42651.55. [DOI] [PubMed] [Google Scholar]
- 8.Hanna-Mitchell AT, Beckel JM, Barbadora S, Kanai AJ, de Groat WC, Birder LA. Non-neuronal acetylcholine and urinary bladder urothelium. Life Sci. 2007;80:2298–302. doi: 10.1016/j.lfs.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Downie JW, Karmazyn M. Mechanical trauma to bladder epithelium liberates prostanoids which modulate neurotransmission in rabbit detrusor muscle. J Pharmacol Exp Ther. 1984;230:445–9. [PubMed] [Google Scholar]
- 10.Munoz A, Gangitano DA, Smith CP, Boone TB, Somogyi GT. Removal of urothelium affects bladder contractility and release of ATP but not release of NO in rat urinary bladder. BMC Urol. 2010;10:10. doi: 10.1186/1471-2490-10-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sadananda P, Shang F, Liu L, Mansfield KJ, Burcher E. Release of ATP from rat urinary bladder mucosa: role of acid, vanilloids and stretch. Br J Pharmacol. 2009;158:1655–62. doi: 10.1111/j.1476-5381.2009.00431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kang SH, McDermott C, Farr S, Chess-Williams R. Enhanced urothelial ATP release and contraction following intravesical treatment with the cytotoxic drug, doxorubicin Naunyn Schmiedebergs. Arch Pharmacol. 2015;388:773–80. doi: 10.1007/s00210-015-1097-2. [DOI] [PubMed] [Google Scholar]
- 13.Smith CP, Vemulakonda VM, Kiss S, Boone TB, Somogyi GT. Enhanced ATP release from rat bladder urothelium during chronic bladder inflammation: effect of botulinum toxin A. Neurochem Int. 2005;47:291–7. doi: 10.1016/j.neuint.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 14.Gonzalez EJ, Merrill L, Vizzard MA. Bladder sensory physiology: neuroactive compounds and receptors, sensory transducers, and target-derived growth factors as targets to improve function. Am J Physiol Regul Integr Comp Physiol. 2014;306:R869–78. doi: 10.1152/ajpregu.00030.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vlaskovska M, Kasakov L, Rong W, Bodin P, Bardini M, Cockayne DA, et al. P2X3 knock-out mice reveal a major sensory role for urothelially released ATP. J Neurosci. 2001;21:5670–7. doi: 10.1523/JNEUROSCI.21-15-05670.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoshiyama M, de Groat WC. The role of vasoactive intestinal polypeptide and pituitary adenylate cyclase-activating polypeptide in the neural pathways controlling the lower urinary tract. J Mol Neurosci. 2008;36:227–40. doi: 10.1007/s12031-008-9090-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu W. Polarized ATP distribution in urothelial mucosal and serosal space is differentially regulated by stretch and ectonucleotidases. Am J Physiol Renal Physiol. 2015;309:F864–72. doi: 10.1152/ajprenal.00175.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daly DM, Nocchi L, Liaskos M, McKay NG, Chapple C, Grundy D. Age-related changes in afferent pathways and urothelial function in the male mouse bladder. J Physiol. 2014;592:537–49. doi: 10.1113/jphysiol.2013.262634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoshida M, Miyamae K, Iwashita H, Otani M, Inadome A. Management of detrusor dysfunction in the elderly: changes in acetylcholine and adenosine triphosphate release during aging. Urology. 2004;63:17–23. doi: 10.1016/j.urology.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 20.Munoz A, Smith CP, Boone TB, Somogyi GT. Overactive and underactive bladder dysfunction is reflected by alterations in urothelial ATP and NO release. Neurochem Int. 2011;58:295–300. doi: 10.1016/j.neuint.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun Y, Chai TC. Augmented extracellular ATP signaling in bladder urothelial cells from patients with interstitial cystitis. Am J Physiol Cell Physiol. 2006;290:C27–34. doi: 10.1152/ajpcell.00552.2004. [DOI] [PubMed] [Google Scholar]
- 22.Yoshida M, Masunaga K, Satoji Y, Maeda Y, Nagata T, Inadome A. Basic and clinical aspects of non-neuronal acetylcholine: expression of non-neuronal acetylcholine in urothelium and its clinical significance. J Pharmacol Sci. 2008;106:193–8. doi: 10.1254/jphs.fm0070115. [DOI] [PubMed] [Google Scholar]
- 23.McDermott C, Chess-Williams R, Mills KA, Kang SH, Farr SE, Grant GD, et al. Alterations in acetylcholine, PGE2 and IL6 release from urothelial cells following treatment with pyocyanin and lipopolysaccharide. Toxicol In Vitro. 2013;27:1693–8. doi: 10.1016/j.tiv.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 24.Kang SH, Chess-Williams R, Anoopkumar-Dukie S, McDermott C. Induction of inflammatory cytokines and alteration of urothelial ATP, acetylcholine and prostaglandin E2 release by doxorubicin. Eur J Pharmacol. 2013;700:102–9. doi: 10.1016/j.ejphar.2012.11.053. [DOI] [PubMed] [Google Scholar]
- 25.McLatchie LM, Young JS, Fry CH. Regulation of ACh release from guinea pig bladder urothelial cells: potential role in bladder filling sensations. Br J Pharmacol. 2014;171:3394–403. doi: 10.1111/bph.12682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kullmann FA, Artim DE, Birder LA, de Groat WC. Activation of muscarinic receptors in rat bladder sensory pathways alters reflex bladder activity. J Neurosci. 2008;28:1977–87. doi: 10.1523/JNEUROSCI.4694-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang X, Momota Y, Yanase H, Narumiya S, Maruyama T, Kawatani M. Urothelium EP1 receptor facilitates the micturition reflex in mice. Biomed Res. 2008;29:105–11. doi: 10.2220/biomedres.29.105. [DOI] [PubMed] [Google Scholar]
- 28.Nile CJ, de Vente J, Gillespie JI. Stretch independent regulation of prostaglandin E2 production within the isolated guinea-pig lamina propria. BJU Int. 2010;105:540–8. doi: 10.1111/j.1464-410X.2009.08705.x. [DOI] [PubMed] [Google Scholar]
- 29.Birder LA, Nealen ML, Kiss S, de Groat WC, Caterina MJ, Wang E, et al. Beta-adrenoceptor agonists stimulate endothelial nitric oxide synthase in rat urinary bladder urothelial cells. J Neurosci. 2002;22:8063–70. doi: 10.1523/JNEUROSCI.22-18-08063.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aizawa N, Igawa Y, Nishizawa O, Wyndaele JJ. Effects of nitric oxide on the primary bladder afferent activities of the rat with and without intravesical acrolein treatment. Eur Urol. 2011;59:264–71. doi: 10.1016/j.eururo.2010.10.035. [DOI] [PubMed] [Google Scholar]
- 31.Birder L, Andersson KE. Urothelial signaling. Physiol Rev. 2013;93:653–80. doi: 10.1152/physrev.00030.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beckel JM, Daugherty SL, Tyagi P, Wolf-Johnston AS, Birder LA, Mitchell CH, et al. Pannexin 1 channels mediate the release of ATP into the lumen of the rat urinary bladder. J Physiol. 2015;593:1857–71. doi: 10.1113/jphysiol.2014.283119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sui G, Fry CH, Montgomery B, Roberts M, Wu R, Wu C. Purinergic and muscarinic modulation of ATP release from the urothelium and its paracrine actions. Am J Physiol Renal Physiol. 2014;306:F286–98. doi: 10.1152/ajprenal.00291.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Verselis VK, Srinivas M. Connexin channel modulators and their mechanisms of action. Neuropharmacology. 2013;75:517–24. doi: 10.1016/j.neuropharm.2013.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith CP, Gangitano DA, Munoz A, Salas NA, Boone TB, Aoki KR, et al. Botulinum toxin type A normalizes alterations in urothelial ATP and NO release induced by chronic spinal cord injury. Neurochem Int. 2008;52:1068–75. doi: 10.1016/j.neuint.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hanna-Mitchell AT, Wolf-Johnston AS, Barrick SR, Kanai AJ, Chancellor MB, de Groat WC, et al. Effect of botulinum toxin A on urothelial-release of ATP and expression of SNARE targets within the urothelium. Neurourol Urodyn. 2015;34:79–84. doi: 10.1002/nau.22508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moro C, Leeds C, Chess-Williams R. Contractile activity of the bladder urothelium/lamina propria and its regulation by nitric oxide. Eur J Pharmacol. 2012;674:445–9. doi: 10.1016/j.ejphar.2011.11.020. [DOI] [PubMed] [Google Scholar]
- 38.Kushida N, Fry CH. On the origin of spontaneous activity in the bladder. BJU Int. 2015;117:982–92. doi: 10.1111/bju.13240. [DOI] [PubMed] [Google Scholar]
- 39.Moro C, Chess-Williams R. Non-adrenergic, non-cholinergic, non-purinergic contractions of the urothelium/lamina propria of the pig bladder. Auto Autocoid Pharmacol. 2012;32:53–9. doi: 10.1111/aap.12000. [DOI] [PubMed] [Google Scholar]
- 40.Ikeda Y, Fry C, Hayashi F, Stolz D, Griffiths D, Kanai A. Role of gap junctions in spontaneous activity of the rat bladder. Am J Physiol Renal Physiol. 2007;293:F1018–25. doi: 10.1152/ajprenal.00183.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vahabi B, Sellers D, Bijos D, Drake MJ. Phasic Contractions in Urinary Bladder from Juvenile versus Adult Pigs. PLoS ONE. 2013;8:e58611. doi: 10.1371/journal.pone.0058611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sui GP, Wu C, Roosen A, Ikeda Y, Kanai AJ, Fry CH. Modulation of bladder myofibroblast activity: implications for bladder function. Am J Physiol Renal Physiol. 2008;295:F688–97. doi: 10.1152/ajprenal.00133.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kanai A, Roppolo J, Ikeda Y, Zabbarova I, Tai C, Birder L, et al. Origin of spontaneous activity in neonatal and adult rat bladders and its enhancement by stretch and muscarinic agonists. Am J Physiol Renal Physiol. 2007;292:F1065–72. doi: 10.1152/ajprenal.00229.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hawthorn MH, Chapple CR, Cock M, Chess-Williams R. Urothelium-derived inhibitory factor(s) influences on detrusor muscle contractility in vitro. Br J Pharmacol. 2000;129:416–9. doi: 10.1038/sj.bjp.0703068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fry CH, Young JS, Jabr RI, McCarthy C, Ikeda Y, Kanai AJ. Modulation of spontaneous activity in the overactive bladder: the role of P2Y agonists. Am J Physiol Renal Physiol. 2012;302:F1447–54. doi: 10.1152/ajprenal.00436.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Johnston L, Woolsey S, Cunningham RM, O’Kane H, Duggan B, Keane P, et al. Morphological expression of KIT positive interstitial cells of Cajal in human bladder. J Urol. 2010;184:370–7. doi: 10.1016/j.juro.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hashitani H, Takano H, Fujita K, Mitsui K, Suzuki H. Functional Properties of Suburothelial microvessels in the rat bladder. J Urol. 2011;185:2382–91. doi: 10.1016/j.juro.2011.02.046. [DOI] [PubMed] [Google Scholar]
- 48.Azadzoi KM, Heim VN, Tarcan T, Siroky MB. Alteration of urothelial-mediated tone in the ischemic bladder: role of eicosanoids. Neurourol Urodyn. 2004;23:258–64. doi: 10.1002/nau.20029. [DOI] [PubMed] [Google Scholar]