Abstract
Background
Plant-based diets are recommended for coronary heart disease (CHD) prevention. However, not all plant foods are necessarily beneficial for health.
Objectives
To examine associations between plant-based diet indices and CHD incidence.
Methods
We included 73,710 women in Nurses’ Health Study (NHS) (1984–2012), 92,329 women in NHS2 (1991–2013), and 43,259 men in Health Professionals Follow-up Study (1986–2012), free of chronic diseases at baseline. We created an overall plant-based diet index (PDI) from repeated semi quantitative food-frequency questionnaire data, by assigning positive scores to plant foods and reverse scores to animal foods. We also created a healthful PDI (hPDI) where healthy plant foods (whole grains, fruits/vegetables, nuts/legumes, oils, tea/coffee) received positive scores, while less-healthy plant foods (juices/sweetened beverages, refined grains, potatoes/fries, sweets) and animal foods received reverse scores. To create an unhealthful PDI (uPDI), we gave positive scores to less-healthy plant foods and reverse scores to animal and healthy plant foods.
Results
Over 4,833,042 person-years of follow-up, we documented 8,631 incident CHD cases. In pooled multivariable analysis, higher adherence to PDI was independently inversely associated with CHD (HR comparing extreme deciles: 0.92, 95% CI: 0.83–1.01; p trend=0.003). This inverse association was stronger for hDPI (HR: 0.75, 95% CI: 0.68–0.83; p trend<0.001). Conversely, uPDI was positively associated with CHD (HR: 1.32, 95% CI: 1.20–1.46; p trend<0.001).
Conclusions
Higher intake of a plant-based diet index rich in healthier plant foods is associated with substantially lower CHD risk, while a plant-based diet index that emphasizes less-healthy plant foods is associated with higher CHD risk.
Keywords: Diet, nutrition, dietary pattern, coronary heart disease, prospective cohort study, epidemiology
Introduction
Plant-based diets have been associated with a lower risk of various diseases,(1–3) including coronary heart disease (CHD),(4–9) the leading global cause of death.(10) However, these studies suffer from key limitations. With the exception of a recent investigation,(3) prior studies(4–9) have defined plant-based diets as ‘vegetarian’ diets, which constitute a family of dietary patterns that exclude some or all animal foods. As recommendations based on incremental dietary changes are easier to adopt, it is important to understand how gradual reductions in animal food intake with concomitant increases in consumption of plant foods affect cardiovascular health. Additionally, in studies of vegetarian diets all plant foods are treated equally, even though certain plant foods, such as refined grains and sugar-sweetened beverages (SSBs) are associated with higher cardio-metabolic risk.(11–13)
To overcome these limitations, we have created three versions of plant-based diet indices using a graded approach – an overall plant-based diet index (PDI) which emphasizes consumption of all plant food while reducing animal food intake; a healthful plant-based diet index (hPDI) which emphasizes intake of healthy plant foods associated with improved health outcomes such as whole grains, fruits, and vegetables; and an unhealthful plant-based diet index (uPDI) which emphasizes consumption of less healthy plant foods known to be associated with a higher risk of several diseases.(14) In three US cohorts, we previously documented that the PDI was inversely associated with type 2 diabetes risk with a stronger inverse association for hPDI, and a positive association for uPDI(14). In the present study, we examined the associations of these plant-based diet indices with CHD incidence in more than 200,000 male and female health professionals in the US.
Methods
Study population
The Nurses’ Health Study (NHS) started in 1976 with 121,701 female registered nurses (aged 30–55 years), the NHS2 started in 1989 with 116,686 female registered nurses (aged 25–42 years), and the Health Professionals Follow-Up Study (HPFS) started in 1986 with 51,529 male health professionals (aged 40–75 years). Participants receive a follow-up questionnaire every two years on lifestyle, health behaviors, and medical history, with a response rate of ~90% at each cycle. Participants with CHD at baseline were excluded. Participants with cancer (except nonmelanoma skin cancer), stroke, and coronary artery surgery at baseline were also excluded, as diagnosis with these conditions can change diet. Lastly, individuals with implausible energy intake at baseline (<600 or >3500 kcal/day for women and <800 or >4200 kcal/day for men) were excluded. The final baseline sample included 73,710 women in NHS, 92,320 women in NHS2, and 43,259 men in HPFS (1984 for NHS, 1991 for NHS2, and 1986 for HPFS).
Study protocols for all cohorts were approved by the institutional review boards of Brigham and Women’s Hospital and the Harvard T.H. Chan School of Public Health; completion of the self-administered questionnaire was considered to imply informed consent.
Dietary assessment and the plant-based diet indices
Dietary data were collected using a semi-quantitative food frequency questionnaire every 2–4 years. Participants were asked how often, on average, they consumed a defined portion of ~130 food items over the previous year. There were 9 response categories ranging from “never or less than once/month” to “≥6 times/day”. The reliability and validity of the questionnaires have been described previously.(15–18)
Using this dietary data, we created three versions of a plant-based diet for each food frequency questionnaire cycle for each cohort: an overall plant-based diet index (PDI), a healthful plant-based diet index (hPDI), and an unhealthful plant-based diet index (uPDI).(14) We created 18 food groups based on nutrient and culinary similarities within the larger categories of healthy plant foods, less healthy plant foods, and animal foods (Table 1). Given that alcoholic beverages have different directions of association for various health outcomes, and margarine’s fatty acid composition has changed over time from high trans to high unsaturated fats, we did not include these foods in the indices, but adjusted for them in the analysis. Food groups were ranked into quintiles, and given positive or reverse scores. With positive scores, participants above the highest quintile of a food group received a score of 5, following on through to participants below the lowest quintile who received a score of 1. With reverse scores, this pattern of scoring was inversed. For creating PDI, plant food groups were given positive scores, while animal food groups were given reverse scores. For creating hPDI, positive scores were given to healthy plant food groups, and reverse scores to less healthy plant food groups and animal food groups. Finally, for uPDI, positive scores were given to less healthy plant food groups, and reverse scores to healthy plant food groups and animal food groups. The 18 food group scores were summed to obtain the indices. Higher intake of all indices reflected lower animal food intake (e.g. 5–6 vs. 3 servings/day comparing extreme PDI deciles).
Table 1.
Examples of food items constituting the 18 food groups (from the 1984 NHS FFQ)
| PDI | hPDI | uPDI | ||
|---|---|---|---|---|
| Plant Food Groups | ||||
| Healthy | ||||
| Whole grains | Whole grain breakfast cereal, other cooked breakfast cereal, cooked oatmeal, dark bread, brown rice, other grains, bran, wheat germ, popcorn | Positive scores | Positive scores | Reverse scores |
| Fruits | Raisins or grapes, prunes, bananas, cantaloupe, watermelon, fresh apples or pears, oranges, grapefruit, strawberries, blueberries, peaches or apricots or plums | Positive scores | Positive scores | Reverse scores |
| Vegetables | Tomatoes, tomato juice, tomato sauce, broccoli, cabbage, cauliflower, Brussels sprouts, carrots, mixed vegetables, yellow or winter squash, eggplant or zucchini, yams or sweet potatoes, spinach cooked, spinach raw, kale or mustard or chard greens, iceberg or head lettuce, romaine or leaf lettuce, celery, mushrooms, beets, alfalfa sprouts, garlic, corn | Positive scores | Positive scores | Reverse scores |
| Nuts | Nuts, peanut butter | Positive scores | Positive scores | Reverse scores |
| Legumes | String beans, tofu or soybeans, beans or lentils, peas or lima beans | Positive scores | Positive scores | Reverse scores |
| Vegetable oils | Oil-based salad dressing, vegetable oil used for cooking | Positive scores | Positive scores | Reverse scores |
| Tea & Coffee | Tea, coffee, decaffeinated coffee | Positive scores | Positive scores | Reverse scores |
| Less healthy | ||||
| Fruit juices | Apple cider (non-alcoholic) or juice, orange juice, grapefruit juice, other fruit juice | Positive scores | Reverse scores | Positive scores |
| Refined grains | Refined grain breakfast cereal, white bread, English muffins or bagels or rolls, muffins or biscuits, white rice, pancakes or waffles, crackers, pasta | Positive scores | Reverse scores | Positive scores |
| Potatoes | French fries, baked or mashed potatoes, potato or corn chips | Positive scores | Reverse scores | Positive scores |
| Sugar sweetened beverages | Colas with caffeine & sugar, colas without caffeine but with sugar, other carbonated beverages with sugar, non-carbonated fruit drinks with sugar | Positive scores | Reverse scores | Positive scores |
| Sweets and Desserts | Chocolates, candy bars, candy without chocolate, cookies (home-baked & ready-made), brownies, doughnuts, cake (home-baked & ready-made), sweet roll (home-baked & ready-made), pie (home-baked & ready-made), jams or jellies or preserves or syrup or honey | Positive scores | Reverse scores | Positive scores |
| Animal Food Groups | ||||
| Animal fat | Butter added to food, butter or lard used for cooking | Reverse scores | Reverse scores | Reverse scores |
| Dairy | Skim low fat milk, whole milk, cream, sour cream, sherbet, ice cream, yogurt, cottage or ricotta cheese, cream cheese, other cheese | Reverse scores | Reverse scores | Reverse scores |
| Egg | Eggs | Reverse scores | Reverse scores | Reverse scores |
| Fish or Seafood | Canned tuna, dark meat fish, other fish, shrimp or lobster or scallops | Reverse scores | Reverse scores | Reverse scores |
| Meat | Chicken or turkey with skin, chicken or turkey without skin, bacon, hot dogs, processed meats, liver, hamburger, beef or pork or lamb mixed dish, beef or pork or lamb main dish | Reverse scores | Reverse scores | Reverse scores |
| Misc. animal-based foods | Pizza, chowder or cream soup, mayonnaise or other creamy salad dressing | Reverse scores | Reverse scores | Reverse scores |
Abbreviations: hPDI, Healthful Plant-based Diet Index; PDI, Overall Plant-based Diet Index; uPDI, Unhealthful Plant-based Diet Index
Outcome ascertainment
CHD was defined as non-fatal myocardial infarction and fatal CHD. Participants self-reporting newly diagnosed CHD on the biennial questionnaires were asked permission to access their medical records to confirm diagnosis, which was done through blinded review by study physicians. To confirm diagnosis of nonfatal MI, we used the World Health Organization criteria(19) of the presence of typical symptoms plus either elevated enzymes or diagnostic electrocardiographic findings. Cases that required hospital admission and were confirmed by interview or letter but for which medical records were unobtainable were included in the analysis as “probable”.
Reports from next of kin or postal authorities were used to identify deaths, in addition to searching the National Death Index. Classification of CHD as the cause of death was done by examining autopsy reports, hospital records, or death certificates, using International Classification of Diseases, ninth revision(20) codes 410–412. CHD deaths were considered confirmed if fatal CHD was established through medical records or autopsy reports, or if CHD was listed as the cause of death on the death certificate with prior medical record of CHD. If CHD was listed as the cause of death on the death certificate, but medical records were unavailable and no prior knowledge of CHD existed, the CHD death was included in the analysis as “probable”.
Assessment of covariates
We obtained updated information on participants’ smoking status, multivitamin use, CHD family history, and physical activity through self-report on the biennial questionnaires. Among women, information was assessed on menopausal status, post-menopausal hormone use, and oral contraceptive use (NHS2 only). Self-reported data on height were collected at baseline, with updated information on weight assessed every two years through the questionnaires. We also collected updated information on self-reported diagnosis of diseases such as hypertension, hypercholesterolemia, and diabetes, and on medication use.
Statistical analysis
We used Cox proportional-hazards regression to estimate hazard ratios and 95% confidence intervals evaluating, separately, the associations of deciles of each index with CHD. Person-time was calculated from questionnaire return date till CHD diagnosis, death, or end of follow-up (30th June 2012 in NHS, 30th June 2013 in NHS2, and 1st January 2012 in HPFS). We used age (in years) as the time scale, with stratification by calendar time (in 2-year intervals). We adjusted for time-varying covariates including smoking status, alcohol intake, physical activity, CHD family history, multivitamin use, aspirin use, energy intake, margarine intake, body mass index (BMI), postmenopausal status and hormone use (women), and oral contraceptive use (NHS2). We additionally adjusted for baseline self-reported hypertension, hypercholesterolemia, and diabetes.
Indices were cumulatively averaged over follow-up to better capture long-term diet; for instance, for the 2001–2003 risk set, plant-based diet index scores in 1991, 1995, and 1999 were averaged to predict CHD risk Because diagnosis of conditions such as type 2 diabetes, stroke, and cancer could change an individual’s diet and potentially be associated with the underlying risk of CHD, we stopped updating diet upon diagnosis of these conditions. Values of other covariates were updated every 2 years to account for changes over time. A continuous variable for each index was created by assigning the median value to each decile and conducting tests for linear trend. To examine potential deviation from linearity, we fit restricted cubic splines to the fully adjusted model with the indices entered as continuous variables. The proportional hazards assumption was tested by including interaction terms between the indices, and age and calendar year. We examined potential effect modification by gender, BMI, physical activity, family history of CHD, and smoking status. We also evaluated the independent associations of the three food categories which constituted the diet indices (healthy plant foods, less healthy plant foods, animal foods) with CHD risk by entering all three simultaneously into the model in place of the diet indices. We also created a healthy omnivorous diet, by assigning positive scores to healthy plant foods and healthy animal foods [dairy products (except ice cream), egg, fish], and reverse scores to less healthy plant foods and less healthy animal foods [animal fat, ice cream, meat, miscellaneous animal-based foods]. The analysis was carried out separately for each cohort, and combined using a fixed effects model; heterogeneity was examined using the Cochrane Q statistic(21) and the I2 statistic.(22) All analyses were performed using SAS software (version 9.2; SAS Institute Inc.), and statistical significance was set at a 2-tailed p value<0.05.
Results
At baseline, the indices ranged from a median of 42–44 in the lowest decile, to 66–68 in the highest decile (Online Table 1). Participants with higher scores on PDI and hPDI were older, more active, leaner, and less likely to smoke than participants with lower scores. Conversely, high consumers of uPDI were younger, less active, and more likely to smoke then low consumers. The proportion of participants with a history of diabetes decreased with increasing deciles of PDI and uPDI, but increased with higher hPDI intake. Animal food intake ranged from 5–6 servings/day in the highest decile to 3–4 servings/day in the lowest decile of the indices.
Over 4,833,042 person-years of follow-up, 8,631 participants developed CHD (3,233 cases over 1,876,942 person-years in NHS; 667 cases over 1,999,945 person-years in NHS2; and 4731 cases over 956,155 person-years in HPFS). In the fully adjusted model, PDI was modestly inversely associated with CHD incidence (HR comparing extreme deciles: 0.92, 95% CI: 0.83–1.01; HR per 10-unit increase: 0.93, 95% CI: 0.90–0.97; p trend=0.003) (Table 2). When we analyzed hPDI (Table 3) and uPDI (Table 4) separately, we found a stronger inverse association between hPDI and CHD incidence (HR comparing extreme deciles: 0.75, 95% CI: 0.68–0.83; HR per 10-unit increase: 0.88, 95% CI: 0.85–0.91; p trend<0.001) and a positive association for uPDI (HR comparing extreme deciles: 1.32, 95% CI: 1.20–1.46; HR per 10-unit increase: 1.10, 95% CI: 1.06–1.14; p trend<0.001). The association of uPDI with CHD was non-linear (p for test of curvature=0.01; p for non-linear association<0.001) (Central Illustration panel A and Online Figure 1). We found no evidence of deviation from linearity for PDI and hPDI (p for test of curvature>0.20 for both; p for linearity=0.001 for PDI, and <0.001 for hPDI). Further adjustment for ethnicity, marital status, recent physical exam, diet beverage intake, and indicators of socioeconomic status did not appreciably alter the results [pooled HR for extreme deciles of (PDI, 0.93; 95% CI, 0.84–1.03; p trend=0.01) (hPDI, 0.76; 95% CI, 0.69–0.84; p trend<0.001) (uPDI, 1.30; 95% CI, 1.18–1.44; p trend<0.001)].
Table 2.
Hazard Ratios (95% CI) for Coronary Heart Disease according to deciles of the Overall Plant-based Diet Index
| Decile 1 | Decile 2 | Decile 3 | Decile 4 | Decile 5 | Decile 6 | Decile 7 | Decile 8 | Decile 9 | Decile 10 | HR (95% CI) per 10 units | P Trend* | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Nurses’ Health Study
| ||||||||||||
| Median | 45.3 | 48.7 | 50.7 | 52.2 | 53.7 | 55.0 | 56.5 | 58.0 | 60.0 | 63.5 | ||
| Cases/PY | 354/187576 | 345/182392 | 342/188258 | 295/175859 | 352/200856 | 272/183715 | 337/192344 | 298/184899 | 326/190404 | 312/190640 | ||
| Age-adjusted | 1.00 | 0.96 (0.83, 1.11) |
0.92 (0.80, 1.07) |
0.83 (0.71, 0.97) |
0.86 (0.75, 1.00) |
0.71 (0.61, 0.84) |
0.82 (0.70, 0.95) |
0.74 (0.64, 0.87) |
0.78 (0.67, 0.90) |
0.70 (0.60, 0.82) |
0.81 (0.76, 0.87) |
<0.001 |
| Multivariable adjusted | 1.00 | 1.02 (0.88, 1.18) |
1.04 (0.90, 1.21) |
0.97 (0.83, 1.13) |
1.01 (0.87, 1.18) |
0.88 (0.75, 1.03) |
0.97 (0.83, 1.13) |
0.92 (0.79, 1.09) |
0.95 (0.81, 1.12) |
0.87 (0.74, 1.03) |
0.92 (0.85, 0.98) |
0.04 |
|
Nurses’ Health Study 2 | ||||||||||||
| Median | 45.0 | 48.5 | 50.7 | 52.3 | 53.8 | 55.0 | 56.8 | 58.5 | 60.7 | 64.0 | ||
| Cases/PY | 91/195183 | 75/194826 | 75/204890 | 73/197879 | 63/187964 | 67/202075 | 56/215822 | 51/201307 | 60/200824 | 56/199175 | ||
| Age-adjusted | 1.00 | 0.84 (0.62, 1.15) |
0.77 (0.57, 1.05) |
0.78 (0.58, 1.07) |
0.67 (0.48, 0.92) |
0.69 (0.51, 0.95) |
0.54 (0.39, 0.76) |
0.51 (0.36, 0.71) |
0.59 (0.43, 0.82) |
0.54 (0.39, 0.75) |
0.69 (0.60, 0.79) |
<0.001 |
| Multivariable adjusted | 1.00 | 0.95 (0.70, 1.29) |
0.91 (0.67, 1.24) |
0.95 (0.69, 1.29) |
0.80 (0.58, 1.12) |
0.89 (0.64, 1.23) |
0.71 (0.50, 1.01) |
0.66 (0.46, 0.95) |
0.80 (0.56, 1.13) |
0.77 (0.54, 1.11) |
0.81 (0.70, 0.95) |
0.02 |
|
Health Professionals Follow-Up Study | ||||||||||||
| Median | 45.0 | 48.0 | 50.4 | 52.0 | 54.0 | 55.3 | 57.0 | 58.5 | 60.8 | 64.2 | ||
| Cases/PY | 492/86581 | 441/87892 | 409/88955 | 471/97460 | 434/86993 | 449/94437 | 369/80989 | 435/89147 | 397/92546 | 463/92145 | ||
| Age-adjusted | 1.00 | 0.90 (0.79, 1.02) |
0.80 0.70, 0.91) | 0.83 (0.73, 0.94) |
0.86 (0.75, 0.97) |
0.81 (0.71, 0.92) |
0.73 (0.64, 0.84) |
0.79 (0.69, 0.90) |
0.69 (0.60, 0.79) |
0.79 (0.70, 0.90) |
0.88 (0.84, 0.92) |
<0.001 |
| Multivariable adjusted | 1.00 | 0.97 (0.85, 1.10) |
0.88 (0.77, 1.01) |
0.91 (0.80, 1.04) |
0.98 (0.86, 1.12) |
0.92 (0.81, 1.05) |
0.85 (0.74, 0.98) |
0.91 (0.80, 1.04) |
0.82 (0.71, 0.94) |
0.95 (0.83, 1.09) |
0.96 (0.90, 1.01) |
0.10 |
|
Pooled results (fixed effects model) | ||||||||||||
| Age-adjusted | 1.00 | 0.92 (0.84, 1.01) |
0.85 (0.78, 0.94) |
0.83 (0.76, 0.91) |
0.85 (0.78, 0.93) |
0.77 (0.70, 0.84) |
0.76 (0.69, 0.84) |
0.75 (0.68, 0.82) |
0.71 (0.65, 0.78) |
0.75†‡ (0.68, 0.82) |
0.84†‖ (0.81, 0.87) |
<0.001†‖ |
| Multivariable adjusted | 1.00 | 0.99 (0.91, 1.09) |
0.96 (0.87, 1.05) |
0.94 (0.86, 1.04) |
0.99 (0.90, 1.08) |
0.91 (0.83, 1.00) |
0.90 (0.82, 0.99) |
0.90 (0.82, 0.99) |
0.87 (0.79, 0.96) |
0.92 (0.83, 1.01) |
0.93 (0.90, 0.97) |
0.003 |
Multivariable adjusted model: Adjusted for age (years), smoking status (never, past, current [1–14, 15–24, or ≥25 cigarettes/day]), physical activity (<3, 3–8.9, 9–17.9, 18–26.9, or ≥27 metabolic equivalent task hours/week), alcohol intake (0, 0.1–4.9, 5–9.9, 10–14.9, or ≥15 g/day), multivitamin use (yes/no), aspirin use (yes/no), family history of CHD (yes/no), margarine intake (quintiles), energy intake (quintiles), baseline hypertension, hypercholesterolemia, and diabetes (yes/no), and updated body mass index (<21, 21–22.9, 23–24.9, 25–26.9, 27–29.9, 30–32.9, 33–34.9, 35–39.9, or ≥40 kg/m2). Also adjusted for postmenopausal hormone use in NHS & NHS2 (premenopausal, postmenopausal current, past or never user), and for oral contraceptive use in NHS2 (never, past, or current user).
P value when we assigned the median value to each decile and entered this as a continuous variable in the model
P value for Q-statistic for heterogeneity <0.05, indicating statistically significant heterogeneity in HRs among the three studies
I2 statistic=60–69%;
I2 statistic=70–79%;
I2 statistic=80–89%
Table 3.
Hazard Ratios (95% CI) for Coronary Heart Disease according to deciles of the Healthful Plant-based Diet Index
| Decile 1 | Decile 2 | Decile 3 | Decile 4 | Decile 5 | Decile 6 | Decile 7 | Decile 8 | Decile 9 | Decile 10 | HR (95% CI) per 10 units | P trend* | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Nurses’ Health Study
| ||||||||||||
| Median | 44.3 | 48.0 | 50.5 | 52.4 | 54.0 | 55.8 | 57.5 | 59.3 | 61.7 | 65.5 | ||
| Cases/PY | 359/18835 2 | 323/186140 | 327/190716 | 327/187664 | 313/183877 | 322/191819 | 306/188145 | 330/187373 | 322/184367 | 304/188490 | ||
| Age-adjusted | 1.00 | 0.85 (0.73, 0.99) |
0.80 (0.69, 0.93) |
0.78 (0.67, 0.91) |
0.74 (0.64, 0.86) |
0.71 (0.61, 0.83) |
0.66 (0.57, 0.77) |
0.70 (0.60, 0.81) |
0.66 (0.57, 0.77) |
0.57 (0.49, 0.67) |
0.80 (0.75, 0.84) |
<0.001 |
| Multivariable adjusted | 1.00 | 0.91 (0.78, 1.06) |
0.90 (0.77, 1.05) |
0.88 (0.75, 1.02) |
0.87 (0.74, 1.01) |
0.83 (0.71, 0.97) |
0.76 (0.65, 0.90) |
0.83 (0.71, 0.98) |
0.78 (0.67, 0.92) |
0.68 (0.57, 0.80) |
0.86 (0.81, 0.91) |
<0.001 |
|
Nurses’ Health Study 2 | ||||||||||||
| Median | 44.0 | 48.0 | 50.5 | 52.3 | 54.0 | 55.8 | 57.3 | 59.2 | 61.6 | 65.6 | ||
| Cases/PY | 79/203121 | 72/192054 | 78/220042 | 61/187944 | 76/207405 | 70/201138 | 60/196640 | 65/199695 | 62/192381 | 44/199524 | ||
| Age-adjusted | 1.00 | 0.90 (0.66, 1.24) |
0.87 (0.64, 1.19) |
0.74 (0.53, 1.03) |
0.84 (0.61, 1.15) |
0.76 (0.55, 1.05) |
0.67 (0.47, 0.93) |
0.67 (0.48, 0.93) |
0.65 (0.47, 0.91) |
0.42 (0.29, 0.61) |
0.72 (0.64, 0.81) |
<0.001 |
| Multivariable adjusted | 1.00 | 0.98 (0.71, 1.35) |
0.97 (0.71, 1.34) |
0.85 (0.60, 1.19) |
0.98 (0.71, 1.35) |
0.87 (0.62, 1.21) |
0.78 (0.55, 1.11) |
0.80 (0.57, 1.13) |
0.77 (0.54, 1.09) |
0.53 (0.36, 0.79) |
0.79 (0.69, 0.90) |
0.001 |
|
Health Professionals Follow-Up Study | ||||||||||||
| Median | 43.0 | 47.2 | 50.0 | 52.0 | 53.8 | 55.5 | 57.2 | 59.2 | 62.0 | 66.0 | ||
| Cases/PY | 413/88274 | 452/89330 | 404/92920 | 486/93019 | 425/88417 | 448/89543 | 425/89922 | 431/85604 | 424/91479 | 452/88635 | ||
| Age-adjusted | 1.00 | 0.96 (0.84, 1.10) |
0.84 (0.73, 0.96) |
0.95 (0.83, 1.08) |
0.84 (0.74, 0.97) |
0.86 (0.75, 0.98) |
0.79 (0.69, 0.90) |
0.82 (0.72, 0.94) |
0.74 (0.65, 0.85) |
0.77 (0.67, 0.88) |
0.88 (0.84, 0.92) |
<0.001 |
| Multivariable adjusted | 1.00 | 0.99 (0.87, 1.14) |
0.87 (0.76, 1.00) |
0.99 (0.86, 1.13) |
0.89 (0.78, 1.03) |
0.91 (0.79, 1.04) |
0.84 (0.73, 0.97) |
0.89 (0.77, 1.02) |
0.80 (0.70, 0.93) |
0.84 (0.73, 0.97) |
0.90 (0.86, 0.95) |
<0.001 |
|
Pooled results (fixed effects model) | ||||||||||||
| Age-adjusted | 1.00 | 0.90 (0.82, 0.99) |
0.82 (0.74, 0.90) |
0.86 (0.78, 0.94) |
0.79 (0.72, 0.87) |
0.78 (0.71, 0.85) |
0.72 (0.66, 0.79) |
0.75 (0.68, 0.82) |
0.70 (0.63, 0.76) |
0.66†‖ (0.60, 0.73) |
0.84†‖ (0.81, 0.86) |
<0.001†‖ |
| Multivariable adjusted | 1.00 | 0.95 (0.86, 1.04) |
0.88 (0.80, 0.97) |
0.92 (0.84, 1.01) |
0.88 (0.80, 0.97) |
0.86 (0.78, 0.95) |
0.80 (0.73, 0.88) |
0.85 (0.77, 0.94) |
0.79 (0.71, 0.87) |
0.75 (0.68, 0.83) |
0.88 (0.85, 0.91) |
<0.001 |
Multivariable adjusted model: Adjusted for age (years), smoking status (never, past, current [1–14, 15–24, or ≥25 cigarettes/day]), physical activity (<3, 3–8.9, 9–17.9, 18–26.9, or ≥27 metabolic equivalent task hours/week), alcohol intake (0, 0.1–4.9, 5–9.9, 10–14.9, or ≥15 g/day), multivitamin use (yes/no), aspirin use (yes/no), family history of CHD (yes/no), margarine intake (quintiles), energy intake (quintiles), baseline hypertension, hypercholesterolemia, and diabetes (yes/no), and updated body mass index (<21, 21–22.9, 23–24.9, 25–26.9, 27–29.9, 30–32.9, 33–34.9, 35–39.9, or ≥40 kg/m2). Also adjusted for postmenopausal hormone use in NHS & NHS2 (premenopausal, postmenopausal current, past or never user), and for oral contraceptive use in NHS2 (never, past, or current user).
P value when we assigned the median value to each decile and entered this as a continuous variable in the model
P value for Q-statistic for heterogeneity <0.05, indicating statistically significant heterogeneity in HRs among the three studies
I2 statistic=60–69%;
I2 statistic=70–79%;
I2 statistic=80–89%
Table 4.
Hazard Ratios (95% CI) for Coronary Heart Disease according to deciles of the Unhealthful Plant-based Diet Index
| Decile 1 | Decile 2 | Decile 3 | Decile 4 | Decile 5 | Decile 6 | Decile 7 | Decile 8 | Decile 9 | Decile 10 | HR (95% CI) per 10 units | P trend* | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Nurses’ Health Study
| ||||||||||||
| Median | 43.5 | 47.6 | 50.0 | 52.0 | 53.7 | 55.5 | 57.3 | 59.3 | 62.0 | 66.0 | ||
| Cases/PY | 274/187546 | 311/195345 | 359/177785 | 267/183572 | 343/191298 | 334/191430 | 341/191659 | 322/187773 | 325/186951 | 357/183583 | ||
| Age-adjusted | 1.00 | 1.12 (0.95, 1.32) |
1.41 (1.20, 1.65) |
1.03 (0.87, 1.22) |
1.28 (1.10, 1.51) |
1.26 (1.07, 1.47) |
1.32 (1.13, 1.55) |
1.26 (1.08, 1.49) |
1.30 (1.11, 1.53) |
1.49 (1.27, 1.74) |
1.14 (1.08, 1.20) |
<0.001 |
| Multivariable adjusted | 1.00 | 1.20 (1.02, 1.41) |
1.52 (1.30, 1.78) |
1.13 (0.96, 1.34) |
1.41 (1.20, 1.66) |
1.36 (1.16, 1.61) |
1.43 (1.21, 1.68) |
1.34 (1.13, 1.58) |
1.34 (1.13, 1.58) |
1.49 (1.26, 1.76) |
1.13 (1.06, 1.19) |
<0.001 |
|
Nurses’ Health Study 2 | ||||||||||||
| Median | 43.5 | 47.5 | 50.0 | 52.0 | 54.0 | 56.0 | 58.0 | 60.0 | 62.5 | 66.5 | ||
| Cases/PY | 52/205047 | 77/197734 | 65/198432 | 71/214560 | 58/196690 | 71/205961 | 61/192014 | 80/194172 | 60/204436 | 72/190899 | ||
| Age-adjusted | 1.00 | 1.57 (1.10, 2.23) |
1.35 (0.93, 1.94) |
1.49 (1.04, 2.13) |
1.26 (0.87, 1.83) |
1.52 (1.06, 2.17) |
1.40 (0.97, 2.03) |
1.85 (1.30, 2.62) |
1.40 (0.96, 2.03) |
1.81 (1.26, 2.58) |
1.19 (1.06, 1.32) |
0.01 |
| Multivariable adjusted | 1.00 | 1.67 (1.17, 2.38) |
1.45 (1.01, 2.10) |
1.56 (1.09, 2.25) |
1.29 (0.88, 1.89) |
1.59 (1.10, 2.30) |
1.46 (0.99, 2.14) |
1.91 (1.32, 2.75) |
1.37 (0.93, 2.03) |
1.77 (1.21, 2.59) |
1.16 (1.03, 1.31) |
0.04 |
|
Health Professionals Follow-Up Study | ||||||||||||
| Median | 44.0 | 48.0 | 50.0 | 52.0 | 54.0 | 55.6 | 57.3 | 59.0 | 61.5 | 65.2 | ||
| Cases/PY | 456/90508 | 454/90758 | 415/86415 | 409/89136 | 461/92660 | 449/89599 | 447/94149 | 416/87472 | 410/87604 | 443/88847 | ||
| Age-adjusted | 1.00 | 1.01 (0.89, 1.15) |
1.02 (0.89, 1.16) |
0.98 (0.86, 1.12) |
1.10 (0.97, 1.26) |
1.11 (0.97, 1.26) |
1.07 (0.94, 1.22) |
1.10 (0.96, 1.25) |
1.11 (0.97, 1.27) |
1.22 (1.07, 1.40) |
1.09 (1.04, 1.14) |
<0.001 |
| Multivariable adjusted | 1.00 | 1.04 (0.92, 1.19) |
1.07 (0.94, 1.22) |
1.05 (0.91, 1.20) |
1.18 (1.04, 1.35) |
1.15 (1.01, 1.32) |
1.10 (0.96, 1.25) |
1.14 (0.99, 1.31) |
1.14 (0.99, 1.31) |
1.21 (1.05, 1.39) |
1.08 (1.03, 1.14) |
0.003 |
|
Pooled results (fixed effects model) | ||||||||||||
| Age-adjusted | 1.00 | 1.09 (0.99, 1.20) |
1.17†‖ (1.07, 1.29) |
1.05 (0.95, 1.15) |
1.17 (1.07, 1.29) |
1.18 (1.08, 1.30) |
1.20 (1.10, 1.32) |
1.19†§ (1.08, 1.31) |
1.19 (1.08, 1.31) |
1.35†‡ (1.22, 1.48) |
1.11 (1.08, 1.15) |
<0.001 |
| Multivariable adjusted | 1.00 | 1.14†‡ (1.04, 1.25) |
1.24†‖ (1.13, 1.37) |
1.12 (1.01, 1.23) |
1.26 (1.14, 1.39) |
1.25 (1.13, 1.37) |
1.25 (1.13, 1.38) |
1.23†§ (1.11, 1.36) |
1.21 (1.09, 1.34) |
1.32†§ (1.20, 1.46) |
1.10 (1.06, 1.14) |
<0.001 |
Multivariable adjusted model: Adjusted for age (years), smoking status (never, past, current [1–14, 15–24, or ≥25 cigarettes/day]), physical activity (<3, 3–8.9, 9–17.9, 18–26.9, or ≥27 metabolic equivalent task hours/week), alcohol intake (0, 0.1–4.9, 5–9.9, 10–14.9, or ≥15 g/day), multivitamin use (yes/no), aspirin use (yes/no), family history of CHD (yes/no), margarine intake (quintiles), energy intake (quintiles), baseline hypertension, hypercholesterolemia, and diabetes (yes/no), and updated body mass index (<21, 21–22.9, 23–24.9, 25–26.9, 27–29.9, 30–32.9, 33–34.9, 35–39.9, or ≥40 kg/m2).
Also adjusted for postmenopausal hormone use in NHS & NHS2 (premenopausal, postmenopausal current, past or never user), and for oral contraceptive use in NHS2 (never, past, or current user).
P value when we assigned the median value to each decile and entered this as a continuous variable in the model
P value for Q-statistic for heterogeneity <0.05, indicating statistically significant heterogeneity in HRs among the three studies
I2 statistic=60–69%;
I2 statistic=70–79%;
I2 statistic=80–89%
Central Illustration. Dose-response relationship of (A) the Plant-based Diet Indices and (B) animal, healthy plant, and less healthy plant foods with CHD incidence.
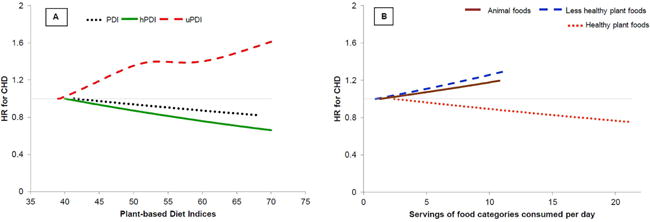
Analysis carried out after combining all three cohorts Adjusted for age, smoking status, physical activity, alcohol intake, multivitamin use, aspirin use, family history of CHD, margarine intake, baseline hypertension, hypercholesterolemia, and diabetes, and updated body mass index. Also adjusted for postmenopausal hormone use in NHS & NHS2, and for oral contraceptive use in NHS2. Energy intake was additionally adjusted when analyzing the plant-based diet indices. The three plant-based diet indices were examined in separate models. The three food categories (healthy and less healthy plant foods, and animal foods) were simultaneously included in the same model.
For uPDI, p for test of curvature=0.01 and p for non-linear association<0.001. P for test of curvature for PDI=0.25, for hPDI=0.82, for animal foods=0.58, for healthy plant foods=0.99, and for less healthy plant foods=0.74; P for linearity=0.004 for animal foods, 0.001 for PDI, and <0.001 for hPDI, less healthy plant foods, and healthy plant foods.
Abbreviations: CHD, Coronary Heart Disease; hPDI, Healthful Plant-based Diet Index; PDI, Overall Plant-based Diet Index; uPDI, Unhealthful Plant-based Diet Index
The associations of hPDI and uPDI with risk of CHD were consistently observed across strata defined by age, BMI, family history of CHD, and sex (Figure 1). Associations of both indices were significantly stronger among more active relative to less active participants (p interaction=0.002 for both); the association of uPDI with CHD was slightly stronger among ever smokers compared with never smokers (p interaction=0.04). There was no evidence of significant effect modification by calendar year in any of the cohorts for hPDI or uPDI (all p values for interaction>0.20).
Figure 1. Pooled HRs (95% CI) for CHD comparing extreme deciles of the plant-based diet indices, stratified by selected characteristics.
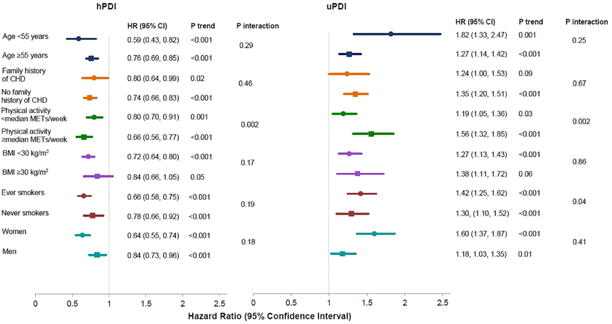
The HRs and P values for men and women were obtained after combining all three cohorts. All other HRs and P values were obtained by pooling estimates from the three cohorts using a fixed-effects model.
Adjusted for age, smoking status, physical activity, alcohol intake, multivitamin use, aspirin use, family history of CHD, margarine intake, energy intake, baseline hypertension, hypercholesterolemia, and diabetes, and updated body mass index. Also adjusted for postmenopausal hormone use in NHS & NHS2, and for oral contraceptive use in NHS2. Abbreviations: BMI, Body Mass Index; CHD, Coronary Heart Disease; hPDI, Healthful Plant-based Diet Index; MET, Metabolic Equivalent Task; uPDI, Unhealthful Plant-based Diet Index
When, in place of the indices, we entered variables for the three food categories together into the fully adjusted model, we found an inverse association for healthy plant foods, and positive associations for animal foods and less healthy plant foods (Central Illustration panel B and Online Table 2). To quantify the benefit of hPDI that was due to lower intake of red meat or SSBs, we individually adjusted for these foods in the final model. The results were largely unchanged upon red meat adjustment: [pooled HR for extreme deciles of (PDI, 0.93; 95% CI, 0.84–1.03; p trend=0.01) (hPDI, 0.76; 95% CI, 0.68, 0.84; p trend<0.001) (uPDI, 1.32; 95% CI, 1.19, 1.46; p trend<0.001)], and changed in expected directions with SSB adjustment [pooled HR for extreme deciles of (PDI, 0.90; 95% CI, 0.81–0.99; p trend=0.001) (hPDI, 0.79; 95% CI, 0.71, 0.88; p trend<0.001) (uPDI, 1.22; 95% CI, 1.10, 1.36; p trend=0.005)]. Given the previously observed inverse association between fish intake and CHD,(23) we modified hPDI to score fish intake positively, and found similar results (pooled HR for extreme deciles, 0.74; 95% CI, 0.67–0.81; p trend<0.001). The results were slightly attenuated when we modified hPDI to score healthy animal foods positively (dairy except ice cream, egg, and fish) (HR comparing extreme deciles: 0.78, 95% CI: 0.71–0.86; HR per 10-unit increase: 0.91, 95% CI: 0.89–0.94; p trend<0.001).
Sensitivity analyses
The associations of PDI, hPDI and uPDI with risk of CHD did not vary based on how we modeled diet. For example, we found similar results when we continuously updated the indices throughout follow-up, used baseline values of the indices, used the most recent index scores before CHD diagnosis, and stopped updating the indices once intermediate conditions such as hypertension and hypercholesterolemia developed (Online Table 3). When we created the plant-based diet indices with quintiles of energy-adjusted food groups (instead of with quintiles of unadjusted food groups as we had originally done), the association of PDI with CHD became slightly stronger, but that of uPDI with CHD was slightly attenuated (Online Table 4). Removing potential intermediates (BMI and aspirin use) from the model strengthened the association of PDI with CHD [pooled HR for extreme deciles of (PDI, 0.86; 95% CI, 0.78–0.95; p trend<0.001) (hPDI, 0.73; 95% CI, 0.66–0.81; p trend<0.001) (uPDI, 1.27; 95% CI, 1.15, 1.40; p trend<0.001)]. Adjustment for additional potential intermediates in the causal pathway, (updated history of hypertension, hypercholesterolemia, and diabetes instead of baseline history) slightly attenuated associations of hPDI and uPDI with CHD [pooled HR for extreme deciles of (PDI, 0.92; 95% CI, 0.83–1.02; p trend=0.003) (hPDI, 0.80; 95% CI, 0.73–0.89; p trend<0.001) (uPDI, 1.24; 95% CI, 1.12, 1.37; p trend=0.001); proportion of the association with hPDI explained by these intermediates ranged from 9.5% in NHS to 4.9% in HPFS, with all p values<0.01]. Finally, the results did not change when we excluded participants who had diabetes at baseline [pooled HR for extreme deciles of (PDI, 0.93; 95% CI, 0.84–1.03; p trend=0.002) (hPDI, 0.74; 95% CI, 0.66–0.82; p trend<0.001) (uPDI, 1.35; 95% CI, 1.21, 1.50; p trend<0.001)], or when we pooled results across the cohorts using a random-effects model [pooled HR for extreme deciles of (PDI, 0.92; 95% CI, 0.83–1.01; p trend=0.01) (hPDI, 0.71; 95% CI, 0.57–0.88; p trend<0.001) (uPDI, 1.40; 95% CI, 1.13, 1.73; p trend<0.001)].
Discussion
In three ongoing prospective cohort studies, higher adherence to an overall plant-based diet index (PDI) was modestly associated with lower CHD incidence [HR comparing extreme deciles: 0.92 (0.83–1.01)]. This inverse association was considerably stronger for adherence to a healthier version (hPDI) [0.75 (0.68–0.83)], but positive for adherence to a less healthy version (uPDI) [1.32 (1.20–1.46)] of a plant-based diet index. These associations remained robust to adjustment for multiple confounders and were consistently observed in various subgroups.
In a previous analysis,(14) we found similar associations of these three indices with type 2 diabetes. Our current analysis extends the potentially protective association with hPDI to CHD. The mechanisms through which hPDI could reduce CHD risk are likely shared with the mechanisms for type 2 diabetes risk reduction.(2, 24–32) Specifically, greater adherence to hPDI would lead to diets high in dietary fiber, antioxidants, unsaturated fat, and micronutrient content, and low in saturated fat and heme iron content (Online Table 1), all of which could aid in weight loss/maintenance, enhance glycemic control and insulin regulation, improve lipid profile, reduce blood pressure, improve vascular health, decrease inflammation, and foster more favorable diet- gut microbiome interactions (e.g. through lowered levels of trimethylamine N-oxide), thereby lowering CHD risk. Greater adherence to uPDI, on the other hand, leads to diets with higher glycemic load and index, and added sugar, and lower levels of dietary fiber, unsaturated fats, micronutrients, and antioxidants, which could result in higher CHD risk through the above-mentioned pathways. This is also illustrated in the fact that the associations of hPDI and uPDI with CHD incidence were slightly attenuated upon adjustment for some of these pathways, specifically hypercholesterolemia, hypertension, and diabetes.
Prospective cohort studies examining the association of plant-based diets with CHD have focused on CHD mortality. Most of these studies have been carried out in Europe, with only three studies in the US (Adventist Health Studies(7)). A pooled analysis of five of the above cohorts found a 24% lower risk of CHD mortality (95% CI: 6%–38%) comparing vegetarians with non-vegetarians.(5) A recent meta-analysis found similar results with vegetarians experiencing a 29% lower risk of CHD mortality (95% CI: 13%–43%) relative to non-vegetarians.(6) The EPIC-Oxford study, one of the few studies to examine the association of a vegetarian diet with CHD incidence in addition to mortality, found a 32% lower 11-year CHD incidence (95% CI: 19%–42%) among vegetarians relative to non-vegetarians.(8)
The above studies have defined plant-based diets dichotomously as being vegetarian or not. Our study adds to the evidence base by examining the association of gradations of adherence to an overall plant-based diet index with CHD incidence. For instance, those in the lowest decile of PDI consumed 5–6 servings of animal foods per day, while those in the highest decile consumed 3 servings of animal foods per day. This approach has the advantage of being easily translatable, as we found that even a slightly lower intake of animal foods combined with higher intake of healthy plant foods is associated with lower CHD risk. One other studies adopted this approach with respect to cardiovascular disease mortality and found similar results.(3) However, these studies have examined plant-based diets at a single time point, making it difficult to fully capture the association of a time-varying exposure such as diet on the development of CHD which has a long etiologic period. Our study adds to the existing literature by demonstrating the associations of long-term cumulative intake of a plant-based diet index with more than 20-year CHD incidence.
We also found that a healthier version of a plant-based diet index, which emphasizes plant foods known to be associated with improved health outcomes, is associated with substantially lower CHD risk. Contrarily, when intake of less healthy plant foods is emphasized, the opposite association was observed. When we examined associations of the three food categories with CHD risk, less healthy plant foods and animal foods were both associated with increased risk, with a potentially stronger association for less healthy plant foods. This highlights the wide variation in nutritional quality of plant foods, making it crucial to consider the quality of plant foods consumed in plant-rich diets.
When we examined a diet which emphasized both healthy plant and healthy animal foods, the association with CHD was only slightly attenuated relative to that with hPDI. Thus, the moderate reductions in animal foods suggested here can be largely achieved by lowering intake of less healthy animal foods such as red and processed meats. The results of this study are in line with the recently released 2015 Dietary Guidelines for Americans,(33) which recommends higher consumption of high quality plant foods. Dietary recommendations based on the hPDI would also be environmentally sustainable, as plant-based food systems use fewer resources than food systems that are heavily reliant on animal foods.(34)
Potential study limitations
This is one of the largest prospective investigations of plant-based diet indices and incident CHD in the world, with periodic data on diet, lifestyle, and medical history collected over more than two decades. However, measurement error in diet assessment is likely, although evaluating cumulatively averaged intake reduces random errors(17) while allowing for the examination of long-term dietary intake. Given the observational nature of the study, residual and unmeasured confounding are possible; thus, we should interpret modest effect sizes such as those we observed for PDI with caution. However, the results were largely unchanged when we adjusted for additional covariates, including markers of socio-economic status. Additionally, randomized controlled trial evidence showing the protective effect of plant-based diets on intermediate outcomes, including weight change, lipid profile, glycemic control, and blood pressure lends further support to our findings.(35–38)
Conclusions
We found a modest inverse association of higher adherence to an overall plant-based diet index with CHD incidence in three prospective cohort studies in the US. While this inverse association was stronger for a plant-based diet index that emphasized healthy plant foods, CHD risk was significantly elevated for a plant-based diet index that emphasized less healthy plant foods.
Dietary guidelines and lifestyle interventions could recommend increasing intake of healthy plant foods, while reducing intake of less healthy plant foods and certain animal foods for improved cardio-metabolic health.
Supplementary Material
Condensed abstract.
Plant-based diets are recommended for coronary heart disease (CHD) prevention, but not all plant foods have health benefits. We examined prospective associations of graded plant-based diet indices [overall (PDI), healthful (hPDI), and unhealthful (uPDI)] with CHD in 209,298 participants. Higher adherence to PDI was inversely associated with CHD [HR comparing extreme deciles: 0.92 (0.83–1.01)], with a stronger inverse association for hDPI [0.75 (0.68–0.83)], but a positive association for uPDI [1.32 (1.20–1.46)]. Patients should be encouraged to increase healthy plant food intake (e.g. whole grains, fruits, vegetables), while reducing intake of animal foods and less-healthy plant foods (e.g. refined grains, sweets).
PERSPECTIVES.
Competency in patient care
Medical and health professionals should guide patients to increase intake of healthy plant foods such as whole grains, fruits, vegetables, and nuts, and reduce intake of animal foods and less healthy plant foods such as sugar-sweetened beverages for CHD prevention.
Translational outlook
Future research should replicate these findings in other racial/ethnic, occupational, and socio-economic groups, and explore biological mechanisms involved in the potentially cardio-protective effects of healthful plant-based diet indices to identify personalized clinical interventions and therapies for CHD prevention.
Acknowledgments
Financial support: This work was supported by research grants UM1 CA186107, UM1 CA176726, UM1 CA167552, HL034594, HL60712, and HL35464 from the National Institutes of Health
Relationships with Industry: Dr. Rimm received a research grant from the USDA/Blueberry Highbush Council. Dr. Hu received research support from the California Walnut Commission. All other authors have reported that they have no relevant relationships with industry to disclose.
ABBREVIATIONS & ACRONYMS
- BMI
Body Mass Index
- CHD
Coronary Heart Disease
- CI
Confidence Interval
- hPDI
Healthful Plant-based Diet Index
- HPFS
Health Professionals Follow-up Study
- HR
Hazard Ratio
- NHS
Nurses’ Health Study
- PDI
Overall Plant-based Diet Index
- SSB
Sugar-Sweetened Beverages
- uPDI
Unhealthful Plant-based Diet Index
References
- 1.Fraser GE. Vegetarian diets: what do we know of their effects on common chronic diseases? Am J Clin Nutr [Research Support, NIH, Extramural Review] 2009 May;89(5):1607S–12S. doi: 10.3945/ajcn.2009.26736K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McEvoy CT, Temple N, Woodside JV. Vegetarian diets, low-meat diets and health: A review. Public Health Nutr [Review] 2012 Dec;15(12):2287–94. doi: 10.1017/S1368980012000936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martinez-Gonzalez MA, Sanchez-Tainta A, Corella D, Salas-Salvado J, Ros E, Aros F, et al. A provegetarian food pattern and reduction in total mortality in the Prevencion con Dieta Mediterranea (PREDIMED) study. Am J Clin Nutr. 2014 Jul;100(Suppl 1):320S–8S. doi: 10.3945/ajcn.113.071431. [DOI] [PubMed] [Google Scholar]
- 4.Huang T, Yang B, Zheng J, Li G, Wahlqvist ML, Li D. Cardiovascular Disease Mortality and Cancer Incidence in Vegetarians: A Meta-Analysis and Systematic Review. Ann Nutr Metab. 2012;60(4):233–40. doi: 10.1159/000337301. [DOI] [PubMed] [Google Scholar]
- 5.Key TJ, Fraser GE, Thorogood M, Appleby PN, Beral V, Reeves G, et al. Mortality in vegetarians and nonvegetarians: detailed findings from a collaborative analysis of 5 prospective studies. Am J Clin Nutr. 1999 Sep;70(3 Suppl):516S–24S. doi: 10.1093/ajcn/70.3.516s. [DOI] [PubMed] [Google Scholar]
- 6.Kwok CS, Umar S, Myint PK, Mamas MA, Loke YK. Vegetarian diet, Seventh Day Adventists and risk of cardiovascular mortality: a systematic review and meta-analysis. Int J Cardiol. 2014 Oct 20;176(3):680–6. doi: 10.1016/j.ijcard.2014.07.080. [DOI] [PubMed] [Google Scholar]
- 7.Orlich MJ, Singh P, Sabaté J, et al. Vegetarian dietary patterns and mortality in adventist health study 2. JAMA Internal Medicine. 2013;173(13):1230–8. doi: 10.1001/jamainternmed.2013.6473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crowe FL, Appleby PN, Travis RC, Key TJ. Risk of hospitalization or death from ischemic heart disease among British vegetarians and nonvegetarians: results from the EPIC-Oxford cohort study. Am J Clin Nutr. 2013;97(3):597–603. doi: 10.3945/ajcn.112.044073. [DOI] [PubMed] [Google Scholar]
- 9.Fraser GE, Lindsted KD, Beeson WL. Effect of risk factor values on lifetime risk of and age at first coronary event. The Adventist Health Study. Am J Epidemiol. 1995 Oct 1;142(7):746–58. doi: 10.1093/oxfordjournals.aje.a117706. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization. The global burden of disease 2004 update. Geneva: World Health Organization; 2008. Available from: http://www.who.int/healthinfo/global_burden_disease/2004_report_update/en/ [Google Scholar]
- 11.Huang C, Huang J, Tian Y, Yang X, Gu D. Sugar sweetened beverages consumption and risk of coronary heart disease: a meta-analysis of prospective studies. Atherosclerosis. 2014 May;234(1):11–6. doi: 10.1016/j.atherosclerosis.2014.01.037. [DOI] [PubMed] [Google Scholar]
- 12.Yang Q, Zhang Z, Gregg EW, Flanders WD, Merritt R, Hu FB. Added sugar intake and cardiovascular diseases mortality among US adults. JAMA Intern Med. 2014 Apr;174(4):516–24. doi: 10.1001/jamainternmed.2013.13563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu EA, Pan A, Malik V, Sun Q. White rice consumption and risk of type 2 diabetes: meta-analysis and systematic review. BMJ. 2012 Mar 15;344:e1454. doi: 10.1136/bmj.e1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Satija A, Bhupathiraju SN, Rimm EB, Spiegelman D, Chiuve SE, Borgi L, et al. Plant-Based Dietary Patterns and Incidence of Type 2 Diabetes in US Men and Women: Results from Three Prospective Cohort Studies. PLoS Med. 2016 Jun;13(6):e1002039. doi: 10.1371/journal.pmed.1002039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992 May 15;135(10):1114–26. doi: 10.1093/oxfordjournals.aje.a116211. 1992. [DOI] [PubMed] [Google Scholar]
- 16.Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985 Jul;122(1):51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 17.Willett W. Nutritional epidemiology. 3rd. USA: Oxford University Press; 2013. [Google Scholar]
- 18.Yuan C, Spiegelman D, Rimm EB, Rosner BA, Stampfer MJ, Barnett JB, et al. Validity of a Dietary Questionnaire Assessed by Comparison With Multiple Weighed Dietary Records or 24-Hour Recalls. Am J Epidemiol. 2016 doi: 10.1093/aje/kww104. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nomenclature and criteria for diagnosis of ischemic heart disease. Report of the Joint International Society and Federation of Cardiology/World Health Organization task force on standardization of clinical nomenclature. Circulation. 1979 Mar;59(3):607–9. doi: 10.1161/01.cir.59.3.607. [DOI] [PubMed] [Google Scholar]
- 20.Halton TL, Willett WC, Liu S, Manson JE, Albert CM, Rexrode K, et al. Low-Carbohydrate-Diet Score and the Risk of Coronary Heart Disease in Women. N Engl J Med. 2006;355(19):1991–2002. doi: 10.1056/NEJMoa055317. [DOI] [PubMed] [Google Scholar]
- 21.Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10(1):101–29. [Google Scholar]
- 22.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zheng J, Huang T, Yu Y, Hu X, Yang B, Li D. Fish consumption and CHD mortality: An updated meta-analysis of seventeen cohort studies. Public Health Nutr. 2012 Apr;15(4):725–37. doi: 10.1017/S1368980011002254. [DOI] [PubMed] [Google Scholar]
- 24.Jenkins DJ, Kendall CW, Marchie A, Jenkins AL, Augustin LS, Ludwig DS, et al. Type 2 diabetes and the vegetarian diet. Am J Clin Nutr [Research Support, Non-US Gov’t Review] 2003 Sep;78(3 Suppl):610S–6S. doi: 10.1093/ajcn/78.3.610S. [DOI] [PubMed] [Google Scholar]
- 25.Hu FB. Plant-based foods and prevention of cardiovascular disease: an overview. Am J Clin Nutr. 2003 Sep 1;78(3):544S–51S. doi: 10.1093/ajcn/78.3.544S. 2003. [DOI] [PubMed] [Google Scholar]
- 26.Jenkins DA, Kendall CC, Marchie A, et al. Effects of a dietary portfolio of cholesterol-lowering foods vs lovastatin on serum lipids and c-reactive protein. JAMA. 2003;290(4):502–10. doi: 10.1001/jama.290.4.502. [DOI] [PubMed] [Google Scholar]
- 27.Levine Morgan E, Suarez Jorge A, Brandhorst S, Balasubramanian P, Cheng C-W, Madia F, et al. Low Protein Intake Is Associated with a Major Reduction in IGF-1, Cancer, and Overall Mortality in the 65 and Younger but Not Older Population. Cell Metabolism. 2014;19(3):407–17. doi: 10.1016/j.cmet.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salonen JT, Nyyssönen K, Korpela H, Tuomilehto J, Seppänen R, Salonen R. High stored iron levels are associated with excess risk of myocardial infarction in eastern Finnish men. Circulation. 1992;86(3):803–11. doi: 10.1161/01.cir.86.3.803. [DOI] [PubMed] [Google Scholar]
- 29.Hunnicutt J, He K, Xun P. Dietary Iron Intake and Body Iron Stores Are Associated with Risk of Coronary Heart Disease in a Meta-Analysis of Prospective Cohort Studies. The Journal of Nutrition. 2014 Mar 1;144(3):359–66. doi: 10.3945/jn.113.185124. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang WHW, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, et al. Intestinal Microbial Metabolism of Phosphatidylcholine and Cardiovascular Risk. N Engl J Med. 2013;368(17):1575–84. doi: 10.1056/NEJMoa1109400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baer HJ, Glynn RJ, Hu FB, Hankinson SE, Willett WC, Colditz GA, et al. Risk Factors for Mortality in the Nurses’ Health Study: A Competing Risks Analysis. Am J Epidemiol. 2011;173(3):319–29. doi: 10.1093/aje/kwq368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Song M, Fung TT, Hu FB, et al. Association of animal and plant protein intake with all-cause and cause-specific mortality. JAMA Internal Medicine. 2016;176(10):1453–63. doi: 10.1001/jamainternmed.2016.4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.U.S. Department of Health and Human Services and U.S. Department of Agriculture. 2015 – 2020 Dietary Guidelines for Americans. 2015 Available from: http://health.gov/dietaryguidelines/2015/guidelines/
- 34.Pimentel D, Pimentel M. Sustainability of meat-based and plant-based diets and the environment. Am J Clin Nutr. 2003 Sep 1;78(3):660S–3S. doi: 10.1093/ajcn/78.3.660S. 2003. [DOI] [PubMed] [Google Scholar]
- 35.Yokoyama Y, Nishimura K, Barnard ND, Takegami M, Watanabe M, Sekikawa A, et al. Vegetarian diets and blood pressure: A meta-analysis. JAMA Intern Med. 2014 Apr;174(4):577–87. doi: 10.1001/jamainternmed.2013.14547. [DOI] [PubMed] [Google Scholar]
- 36.Ferdowsian HR, Barnard ND. Effects of plant-based diets on plasma lipids. Am J Cardiol. 2009;104(7):947–56. doi: 10.1016/j.amjcard.2009.05.032. [DOI] [PubMed] [Google Scholar]
- 37.Barnard ND, Levin SM, Yokoyama Y. A systematic review and meta-analysis of changes in body weight in clinical trials of vegetarian diets. J Acad Nutr Diet. 2015 Jun;115(6):954–69. doi: 10.1016/j.jand.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 38.Yokoyama Y, Barnard ND, Levin SM, Watanabe M. Vegetarian diets and glycemic control in diabetes: a systematic review and meta-analysis. Cardiovascular Diagnosis and Therapy. 2014;4(5):373–82. doi: 10.3978/j.issn.2223-3652.2014.10.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


