Abstract
Coronary artery disease (CAD) is a leading cause of morbidity and mortality worldwide1,2. Although 58 genomic regions have been associated with CAD to date3–9, most of the heritability is unexplained9, indicating additional susceptibility loci await identification. An efficient discovery strategy may be larger-scale evaluation of promising associations suggested by genome-wide association studies (GWAS). Hence, we genotyped 56,309 participants using a targeted gene array derived from earlier GWAS results and meta-analysed results with 194,427 participants previously genotyped to give a total of 88,192 CAD cases and 162,544 controls. We identified 25 new SNP-CAD-associations (P < 5x10-8, in fixed effects meta-analysis) from 15 genomic regions, including SNPs in or near genes involved in cellular adhesion, leucocyte migration and atherosclerosis (PECAM1, rs1867624), coagulation and inflammation (PROCR, rs867186 [p.Ser219Gly]) and vascular smooth muscle cell differentiation (LMOD1, rs2820315). Correlation of these regions with cell type-specific gene expression and plasma protein levels shed light on potential novel disease mechanisms.
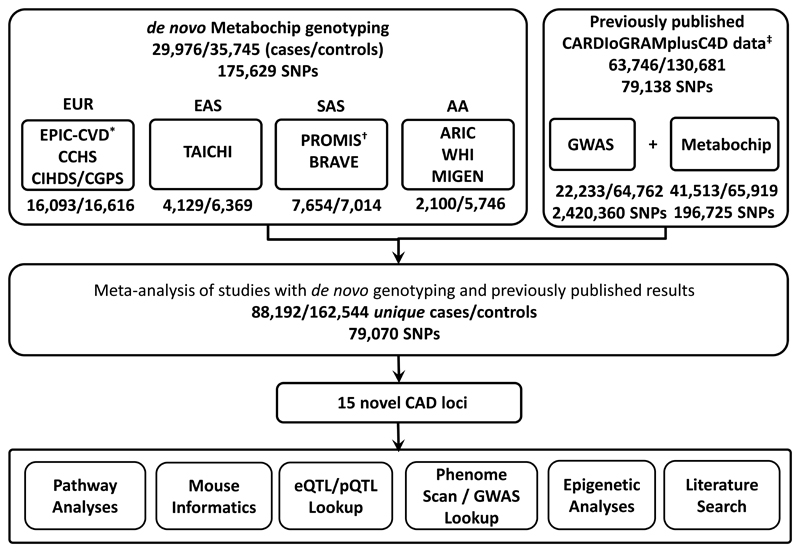
The CardioMetabochip is a genotyping array that contains 196,725 variants of confirmed or suspected relevance to cardiometabolic traits derived from earlier GWAS.10 A previous meta-analysis by the CARDIoGRAMplusC4D consortium of 79,138 SNPs common to the CardioMetabochip and GWAS arrays, identified 15 new loci associated with CAD3. Using the CardioMetabochip, we genotyped 56,309 additional samples of European (EUR; ~52%), South Asian (SAS; ~23%), East Asian (EAS; ~17%) and African American (AA; ~8%) ancestries (Supplementary Information; Supplementary Tables 1, 2, 3; Supplementary Fig. 1). The results from our association analyses of these additional samples were meta-analysed with those reported by CARDIoGRAMplusC4D at 79,070 SNPs in two fixed effects meta-analyses, one in EUR participants and a second across all four ancestries (Figure 1 and 2). (Over-lapping samples were removed prior to meta-analysis [Methods]). A genome-wide significance threshold (P≤5x10-8 in the fixed effects meta-analysis) was adopted to minimise false positive findings. However, even at this strict P-value threshold, there is still a small chance of a false-positive result. The EUR fixed effects meta-analysis identified 15 SNPs associated with CAD at genome-wide significance (P<5x10-8) from nine distinct genomic regions that are not established CAD-associated loci (Table 1; Supplementary Table 4; Supplementary Fig. 2). An additional six distinct novel CAD-associated regions were identified in the all ancestries fixed effects meta-analysis (Table 1; Figure 2; Supplementary Table 4). In total, 15 novel CAD-associated genomic regions (25 SNPs) were identified (Supplementary Fig. 3 and 4). The lead SNPs had at least nominal evidence of association (P<0.05) in either a fixed effects meta-analysis of the EUR studies with de novo genotyping, or in a fixed effects meta-analysis of all the studies with de novo genotyping (Supplementary Table 5, Supplementary Fig. 5). Within the CARDIoGRAMplusC4D results for these SNPs, there was no evidence of heterogeneity of effects (P≥0.10) and allele frequencies were consistent with our EUR studies (Supplementary Table 5). Tests for enrichment of CAD-associations within sets of genes11 and Ingenuity Pathway Analysis confirmed known CAD pathways (Supplementary Information; Supplementary Tables 6, 7, 8).
Figure 1.
Schematic of the study design. The sample-size information is provided as number of cases/number of controls. Note, samples with de novo genotyping that were also in the CARDIoGRAMplusC4D study were removed prior to meta-analysis.* 1,826 CAD cases and 449 controls from EPIC-CVD with de novo genotyping were also included in CARDIoGRAMplusC4D and were therefore excluded from the larger meta-analysis. The actual number of EUR individuals contributed to the meta-analysis of our studies with de novo genotyping and CARDIoGRAMplusC4D was 14,267 CAD cases and 16,167 controls.†3,704 CAD cases and 3,433 controls from PROMIS with de novo genotyping were also included in CARDIoGRAMplusC4D and were therefore excluded from the larger meta-analysis. The actual number of SAS samples contributed to the meta-analysis of our studies with de novo genotyping and CARDIoGRAMplusC4D was 3,950 CAD cases and 3,581 controls.
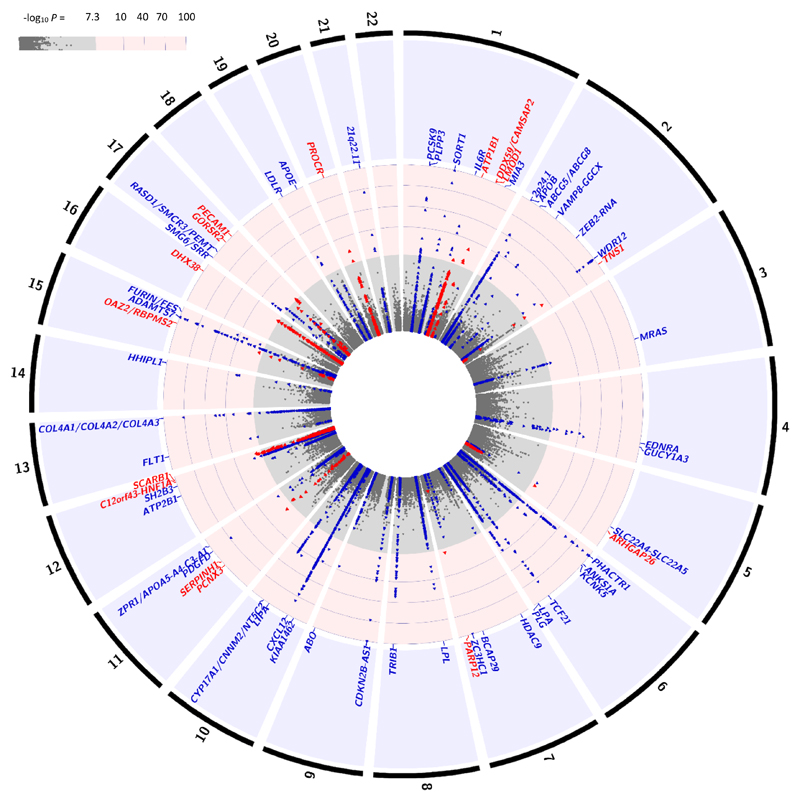
Figure 2.
Plot showing the association of ~79,000 variants with CAD (-log10P-value) in up to 88,192 cases and 162,544 controls from the all ancestry fixed effects meta-analysis. SNPs are ordered in physical position. No adjustments to P-values to account for multiple testing have been made. The outer track represents the chromosomal number. Blue dots represent known loci and red dots are the new loci identified in the current study. Each association peak is labeled with the name of the closest gene(s) to the sentinel SNP. GWAS significance (-log10(P) ~ 7.3).
Table 1. Newly identified CAD-associated genomic regions.
CAD-association results for the lead SNPs from the European and the all ancestry meta-analyses are reported. Note, SNP allele frequencies for each ancestry are provided in, Supplementary Table 5 and in Supplementary Fig. 3 for each of the studies with de novo genotyping.
| Closest gene(s) | Variant/alleles | Chr:Position (EA AF) | European | All Ancestries | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | [95% CI] | P | N | OR | [95%CI] | P | log10BF | N | |||
| ATP1B1 | rs1892094C>T | 1:169094459 (T 0.50) | 0.96 | [0.94-0.97] | 3.99x10-8 | 217,782 | 0.96 | [0.94-0.97] | 2.25x10-8 | 6.33 | 243,623 |
| DX59/CAMSAP2 | rs6700559C>T | 1:200646073 (T 0.47) | 0.96 | [0.94-0.97] | 2.50x10-8 | 221,073 | 0.96 | [0.95-0.97] | 1.13x10-8 | 6.68 | 246,913 |
| LMOD1 | rs2820315C>T | 1:201872264 (T 0.30) | 1.05 | [1.03-1.07] | 4.14x10-9 | 214,844 | 1.05 | [1.03-1.07] | 7.70x10-10 | 7.72 | 240,685 |
| TNS1a | rs2571445G>A | 2:218683154 (A 0.39) | 1.04 | [1.02-1.06] | 3.58x10-6 | 194,254 | 1.05 | [1.03-1.06] | 4.55x10-10 | 8.41 | 220,047 |
| ARHGAP26 | rs246600C>T | 5:142516897 (T 0.48) | 1.05 | [1.03-1.06] | 1.29x10-8 | 210,380 | 1.04 | [1.03-1.06] | 1.51x10-8 | 6.39 | 236,223 |
| PARP12 | rs10237377G>T | 7:139757136 (T 0.35) | 0.95 | [0.93-0.97] | 1.70x10-7 | 181,559 | 0.95 | [0.93-0.97] | 1.75x10-8 | 6.32 | 207,399 |
| PCNX3 | rs12801636G>A | 11:65391317 (A 0.23) | 0.95 | [0.93-0.97] | 1.00x10-7 | 211,152 | 0.95 | [0.94-0.97] | 9.71x10-9 | 6.64 | 236,985 |
| SERPINH1 | rs590121G>T | 11:75274150 (T 0.30) | 1.05 | [1.03-1.07] | 1.54x10-8 | 207,426 | 1.04 | [1.03-1.06] | 9.32x10-8 | 5.80 | 233,249 |
| C12orf43/HNF1A | rs2258287C>A | 12:121454313 (A 0.34) | 1.05 | [1.03-1.06] | 6.00x10-9 | 221,068 | 1.04 | [1.03-1.06] | 2.18x10-8 | 6.40 | 246,901 |
| SCARB1 | rs11057830G>A | 12:125307053 (A 0.16) | 1.07 | [1.05-1.10] | 5.65x10-9 | 177,550 | 1.06 | [1.04-1.09] | 1.34x10-8 | 6.49 | 203,394 |
| OAZ2, RBPMS2 | rs6494488A>G | 15:65024204 (G 0.18) | 0.95 | [0.93-0.97] | 1.43x10-6 | 205,410 | 0.95 | [0.93-0.97] | 2.09x10-8 | 6.41 | 228,578 |
| DHX38 | rs1050362C>A | 16:72130815 (A 0.38) | 1.04 | [1.03-1.06] | 2.32x10-7 | 216,025 | 1.04 | [1.03-1.06] | 3.52x10-8 | 6.16 | 241,858 |
| GOSR2 | rs17608766T>C | 17:45013271 (C 0.14) | 1.07 | [1.04-1.09] | 4.14x10-8 | 215,857 | 1.06 | [1.04-1.09] | 2.10x10-7 | 5.30 | 231,213 |
| PECAM1 | rs1867624T>C | 17:62387091 (C 0.39) | 0.96 | [0.94-0.97] | 1.14x10-7 | 220,831 | 0.96 | [0.95-0.97] | 3.98x10-8 | 6.03 | 246,674 |
| PROCRa | rs867186A>G | 20:33764554 (G 0.11) | 0.93 | [0.91-0.96] | 1.26x10-8 | 213,505 | 0.93 | [0.91-0.96] | 2.70x10-9 | 7.11 | 239,340 |
These are nonsynonymous SNPs.
EA, Effect allele. AF, Effect allele frequency in Europeans. N, Number of individuals in the analysis. Log10BF, log base 10 of the Bayes factor obtained from the MANTRA analyses (log10BF>6 is considered significant). There was no convincing evidence of heterogeneity at the new CAD-associated SNPs, Phet ≥ 0.01. P-value for heterogeneity across meta-analysed datasets are provided in Supplementary Table 4 and I2 statistics in Supplementary Fig. 3.
To prioritize candidate causal genes at the new loci, we defined regions encompassing the novel CAD-associated SNPs based on recombination rates (Supplementary Table 9) and cross referenced them with expression quantitative trait loci (eQTL) databases including GTEx12, MuTHER13 and STARNET14 (Methods). Twelve of the 15 novel CAD-associated SNPs were identified as potential eQTLs in at least one tissue (P<5x10-8; Table 2, Supplementary Table 10). Haploreg analysis15 (Methods) showed CAD-associated SNPs were enriched for H3K27ac enhancer marks (P < 5.1x10-4) in multiple heart related tissues (left ventricle, right atrium, aorta) in the EUR results and in one heart related tissue (right atrium) and liver in the all ancestry analyses (Supplementary Table 11). We next tested for protein quantitative trait loci (pQTL) in plasma on the aptamer-based Somalogic platform (Methods). Twenty-four proteins from the newly identified CAD regions were assayed and passed QC. Of our 15 novel CAD-associated SNPs, two associated with plasma protein abundance in trans: rs867186 (NP_006395.2:p.Ser219Gly), a missense variant in PROCR was a trans-pQTL for protein C (P=10-10, discussed below) and rs1050362 (NP_054722.2:p.Arg140=) a synonymous variant in DHX38 was a trans-pQTL for the apolipoprotein L1 (P=5.37x10-29; Methods) which is suggested to interact with HPR in the DHX38 region (string database).
Table 2. Summary of functional data implicating candidate causal genes in newly identified CAD regions.
Genes in region, provides genes in the LD block containing the CAD-associated SNP. Phenotype in murine model, lists the phenotype as provided in the mouse genome informatics database, genes are listed if the phenotype affects the cardiovascular system, inflammation or liver function. eQTLs are listed where the SNP or a proxy with r2>0.9 are an eQTL for the listed gene in one of the following refs: 12, 13, 26, 43, 44, 45, 46,38,47,48,14,49 (refer to Supplementary Table 10 for an extended listing where r2>0.8 between the CAD-associated SNP and the lead eQTL). Candidate genes are based on the most likely given the information ascertained on murine phenotype, eQTL, protein expression and any literature information described in the main text. Loci are further discussed in the Supplementary Information.
| SNP | Genes in region | Phenotype in murine model | Cis-eQTLs with SNP (or proxy r2>0.9) | Proteins expressed in SMC, heart, liver, blood+ | Candidate causal gene(s) |
|---|---|---|---|---|---|
| rs1892094C>T | ATP1B1, BLZF1, CCDC181, F5, NME7, SELP, SLC19A2 | ATP1B1 (cardiovascular, homeostasis, mortality/aging, muscle) F5 (blood coagulation) SELP (cardiovascular, coagulation, inflammatory response) | NME7*, ATP1B1* | ATP1B1, NME7, SELP | ATP1B1, NME7 |
| rs6700559C>T | CAMSAP2, DDX59, KIF14 | CAMSAP2*, DDX59* | CAMSAP2, DDX59, KIF14 | CAMSAP2, DDX59 | |
| rs2820315C>T | IPO9, LMOD1, NAV1, SHISA4, TIMM17A | LMOD1, IPO9* | LMOD1 | LMOD1 | |
| rs2571445G>A | CXCR2, RUFY4, TNS1 | CXCR2 (increased IL6, abnormal interleukin level) | TNS1* | TNS1, RUFY4 | TNS1 |
| rs246600C>T | ARHGAP26, FGF1 | None | |||
| rs10237377G>T | PARP12, TBXAS1 | TBXAS1 (increased bleeding, decreased platelet aggregation) | TBXAS1* | TBXAS1 | |
| rs12801636G>A | PCNX3, POLA2, RELA, RNASEH2C, SAC3D1, SCYL1, SIPA1, SLC22A20, SLC25A45, SNX15, SNX32, SPDYC, SSSCA1, SYVN1, TIGD3, TM7SF2, TMEM262, VPS51, ZFPL1, ZNHIT2 | CAPN1 (cardiovascular system), CDCA5 (decreased mean corpuscular volume), CFL1 (cardiovascular system), EFEMP2 (cardiovascular), MUS81 (cardiovascular system), RELA (CVD others), SCYL1 (small myocardial fiber), | SIPA1* | SIPA1 | |
| rs590121G>T | GDPD5, KLHL35, SERPINH1 | SERPINH1 (hemorrhage) | SERPINH1* | SERPINH1 | SERPINH1 |
| rs2258287C>A | SPPL3, HNF1A-AS1, HNF1A, C12orf43, OASL, P2RX7, P2RX4 | HNF1A (increased cholesterol, decreased liver function) P2RX4 (abnormal vascular endothelial cell physiology, abnormal vasodilation, abnormal common carotid artery morphology) | C12orf43, SPPL3, P2RX7, P2RX4 | ||
| rs11057830G>A | SCARB1, UBC | SCARB1 (increased susceptibility to atherosclerosis, reduced heart rate, abnormal lipoprotein metabolism abnormal vascular wound healing) | None | UBC | SCARB1 |
| rs6494488A>G | ANKDD1A, CSNK1G1, DAPK2, FAM96A, KIAA0101, OAZ2, PIF1, PLEKHO2, PPIB, RBPMS2, SNX1, SNX22, TRIP4, ZNF609 | PIF1 (abnormal telomere length) | ANKDD1A*, RBPMS2*, TRIP4* | TRIP4 | TRIP4 |
| rs1050362C>A | AP1G1, ATXN1L, CALB2, CHST4, DHODH, DHX38, HP, HPR | HP (renal, development of atherosclerosis25) | DHODH*, HP*, DHX38* | HP, DHX38, DHODH | HP |
| rs17608766T>C | ARL17A, CDC27, GOSR2, MYL4, WNT9B, WNT3 | GOSR2* | GOSR2 | ||
| rs1867624T>C | DDX5, MILR1, PECAM1, POLG2, TEX2 | DDX5 (abnormal vascular development), PECAM1 (cardiovascular system, liver inflammation) | PECAM1* | PECAM1, TEX2 | PECAM1 |
| rs867186A>G | RALY, EIF2S2, ASIP, AHCY, ITCH, DYNLRB1, MAP1LC3A,PIGU, HMGB3P1, GGT7, ACSS2, NCOA6, GSS, MYH7B, TRPC4AP, EDEM2, PROCR, MMP24, EIF6 | ASIP (cardiovascular system), NCOA6 (cardiovascular system), PROCR (abnormal circulatiung C-reactive protein and fibrinogen levels; thrombosis/blood coagulation), | PROCR*, EIF6*, ITGB4BP* | EIF6, ITGB4BP | PROCR |
| rs6088590 C>T | PROCR*, GGT7*, MAP1LC3A*, ACSS2*, TRPC4AP* | GGT7 |
indicates that the eQTL is identified in one of blood (including peripheral blood mononuclear cells) heart, aorta/coronary artery or live. Note the PCNX3 region also encompasses AP5B1, ARL2, CAPN1, CDC42EP2, CDCA5, CFL1, CTSW, DPF2, EFEMP2, EHBP1L1, FAM89B, FAU, FRMD8, KAT5, KCNK7, LTBP3, MAP3K11, MRPL49, MUS81, NAALADL1, OVOL1. The DHX38 region also encompasses, IST1, MARVELD3, PHLPP2, PKD1L3, PMFBP1, TAT, TXNL4B, ZFHX3, ZNF19, ZNF23, ZNF821. The PROCR region also includes: FAM83C, UQCC1, GDF5, SPAG4, CEP250, C20orf173, ERGIC3, FER1L4, CPNE1, RBM12, NFS1, ROMO1, RBM39, SCAND1, CNBD2, EPB41L1, LINC00657, AAR2, DLGAP4
To further help prioritize candidate genes, we also queried the mouse genome informatics database to discover phenotypes resulting from mutations in the orthologous genes for all genes in our 15 CAD-associated regions (Table 2). To understand the pathways by which our novel loci might be related to CAD risk, we examined the associations of the 15 novel CAD regions with a wide range of risk factors, molecular traits, and clinical disorders, using PhenoScanner16 (which encompasses the NHGRI-EBI GWAS catalogue and other genotype-phenotype databases).
Six of our loci have previously been associated with known CAD risk factors, such as major lipids (PCNX3,17 C12orf43/HNF1A, SCARB1, DHX38)18 and blood pressure (GOSR2,19 PROCR20). The sentinel variants for the CAD and risk factor associations at PCNX3, GOSR2 and PROCR were the same, implicating them in known biological pathways. Two correlated SNPs (r2=0.93, D’=1.0 in 1000 genomes) rs11057830 and rs11057841 tag the CAD-association in the SCARB1 region (Table 1; Supplementary Table 4), a region reported previously to be associated with HDL (rs838876, β=-0.049, P=7.33x10-33)18. A rare nonsynonymous variant rs74830677 (NP_005496.4:p.Pro376Leu) in SCARB1 also associated with high levels of high-density lipoprotein cholesterol (HDL-C)21. Conditional analyses showed that the CAD-association was independent of the common variant HDL association (Supplementary Information, Supplementary Fig. 6). We found the CAD SNPs and the common HDL-C SNP, rs838880 overlap enhancers active in primary liver tissue (Supplementary Fig. 7). SCARB1 is highly expressed in liver and adrenal gland tissues (GTEx; Supplementary Fig. 7)12. These findings suggest that the discovered genetic variants most likely play a role in regulation of liver-restricted expression of SCARB1.
The DHX38 region has previously been associated with increased total and LDL cholesterol18. Both CAD-associated SNPs in DHX38, rs1050362 (NP_054722.2:p.Arg140=) and rs2072142 (synonymous and intronic respectively; Table 1, Supplementary Table 4) are in LD but not strongly correlated with the previously reported cholesterol increasing SNP, intronic in HPR, rs2000999, (r2=0.41, D’=1 in 1000 Genomes EUR). Deletions in the HP gene have recently been shown to drive the reported cholesterol association in this region22. The CAD SNPs are in strong LD with SNPs that increase haptoglobin levels23 (rs6499560, P=2.92x10-13, r2=0.97), and haptoglobin has been reported to be associated with increased CAD risk24. HP encodes an alpha-2-glycoprotein which is synthesised in the liver. It binds free haemoglobin and protects tissues from oxidative damage. Mouse models indicate the role of Hp with development of atherosclerosis25, where the underlying mechanism is disruption of the protective nature of the Hp protein against hemoglobin-induced injury of atherosclerotic plaque. While the CAD-associated SNPs are eQTLs (or in LD with eQTLs) for multiple genes in the region e.g. DHODH in aorta artery12 (rs1050362 A allele, β=0.41, P=1.4x10-9), DHX38 in peripheral blood26, atherosclerotic aortic root14 (P<8x10-26; Table 2, Supplementary Table 10), the A allele at rs1050362 is also associated with increased expression of HP in left ventricle heart (β=0.535, P=8.71x10-10)12 and decreased expression of HP in whole blood (β=-0.27, P=1.22x10-10)12. While there could be multiple causal genes in the region, together these findings suggest HP is a promising candidate gene.
PROCR encodes the endothelial protein C receptor (EPCR). We found the G allele at rs867186 (which codes for the glycine residue at p.Ser219Gly) in PROCR confers protection from CAD (OR[95%CI]=0.93[0.91-0.96]; Table 1, Supplementary Fig. 8). The same variant is also associated with increased circulating levels of soluble EPCR (which does not enhance protein C activation)27, increased levels of protein C28, increased factor VII levels29, and increased risk of venous thrombosis27. Consistent with these associations, the variant has also been demonstrated to render the EPCR more susceptible to proteolytic cleavage, resulting in increased shedding of membrane-bound EPCR from the endothelial surface30 causing elevated protein C levels in the circulation31. We found evidence of a second, independent CAD-association at rs6088590 (r2=0, D’=0.01 with rs867186 in 1000G EUR samples; Supplementary Fig. 8), an intronic SNP in NCOA6 with the T allele conferring increased risk of CAD (conditional on rs867186, conditional P=1.14x10-5, OR[95% CI]=0.97[0.95-0.98]). No additional SNPs were associated with CAD after conditioning on rs867186 and rs6088590 (P>0.01).
Five of the novel CAD regions identified in the current analysis include genes that encode proteins expressed in smooth muscle cells (LMOD1, SERPINH1, DDX59/CAMSAP2, TNS1, PECAM1)32,33. The CAD risk allele (T) of rs2820315, which is intronic in LMOD1, is associated with increased expression of LMOD1 in omental and subcutaneous adipose tissues13,34 (MuTHER, β=0.11, P=1.43x10-11). The protein is found in smooth muscle cells (SMC)32,33. In vitro and transgenic mouse studies demonstrate an essential requirement for CArG elements in the expression of LMOD1 through both serum response factor (SRF) and myocardin (MYOCD)35. Myocardin has emerged as an important molecular switch for the programs of SMC and cardiac myocyte differentiation36,37. The CAD-associated SNP (or tag) is an eQTL for IPO9 in peripheral blood mononuclear cells38, however, given the prior biological evidence LMOD1 would make the most plausible candidate gene.
rs1867624 is upstream of PECAM1, which encodes platelet/endothelial cell adhesion molecule 1, a protein found on platelet, monocyte and neutrophil surfaces. The C-allele is associated with reduced CAD risk (Table 1), increased expression of PECAM1 in peripheral blood mononuclear cells38 (β=0.1199, P=1.38x10-107) and is in LD with rs2070784 and rs6504218 (D’=1.0, r2>0.8 in 1000G EUR samples), which are eQTL for PECAM1 in aortic endothelial cells (P=4.35x10-13) and stimulated CD14+ monocytes39 respectively (P<1.7x10-24; Supplementary Table 10)39. PECAM-1 has been implicated in the maintenance of vascular barrier integrity, breach of which is a sign of inflammatory response. Failure to restore barrier function contributes to the development of chronic inflammatory diseases such as atherosclerosis. PECAM-1 expressing endothelial cell monolayers have been shown to exhibit increased steady-state barrier function, as well as more rapid restoration of barrier integrity following thrombin-induced perturbation compared to PECAM-1 deficient cells40. Expression of PECAM-1 has been shown to be correlated with increased plaque burden in athero-susceptible regions of the aorta in mice41 and also with decreased atherosclerotic area in the aorta overall42. Together, these findings prioritise PECAM1 as a candidate causal gene for this CAD-associated region in humans.
Of the 58 previously established CAD loci3–9, 47 were included on the CardioMetabochip. Forty-five regions were directionally concordant with the previous reports (two were neutral) and thirty-four of these 45 (42 SNPs) had at least nominal evidence of association in a fixed effects meta-analysis (P<0.05) in either our EUR or all ancestry studies with de novo genotyping (Supplementary Table 12). Twenty-three of these formally replicated at a Bonferroni significance level P=0.05/47=0.001). PHACTR1, CXCL12 and COL4A1-COL4A2 had more statistical support of association (smaller P-values despite fewer samples) in SAS compared with the other ancestries. The PHACTR1 SNP, rs9349379, is ancestrally informative, as the A allele frequency ranges between 0.29 in the Taiwanese and 0.91 in African Americans (Supplementary Table 12). In contrast, the COL4A1-COL4A2 SNP, rs4773144, had similar allele frequencies across ancestries (EAF=0.56-0.62). The stronger effect size in SAS (OR[95%CI]=0.91[0.86-0.95] versus 0.98[0.95-1.02] in EUR, heterogeneity P=0.0042) could suggest gene-environment or gene-gene interactions at this locus.
We have reported 15 novel CAD-associations, which, together with previous efforts, brings the total number of CAD-associated regions to 73. In addition to implicating atherosclerosis and traditional risk factors as mechanisms in the pathobiology of CAD, our discoveries highlight the potential importance of biological processes active in the arterial wall involving endothelial, smooth muscle and white blood cells and promote coronary atherogenesis.
Online Methods
Study participants
A full description of the component studies with de novo genotyping is given in the Supplementary Information and Supplementary Table 1. In brief, the European (EUR) studies comprised 16,093 CAD cases and 16,616 controls from EPIC-CVD (a case-cohort study embedded in the pan-European EPIC prospective study), the Copenhagen City Heart Study (CCHS), the Copenhagen Ischemic Heart Disease Study (CIHDS) and the Copenhagen General Population Study (CGPS) all recruited within Copenhagen, Denmark. The South Asian (SAS) studies comprised up to 7,654 CAD cases and 7,014 controls from the Pakistan Risk of Myocardial Infarction Study (PROMIS) a case-control study that recruited samples from 9 sites in Pakistan, and the Bangladesh Risk of Acute Vascular Events (BRAVE) study based in Dhaka, Bangladesh. The East Asian (EA) studies comprised 4,129 CAD cases and 6,369 controls recruited from 7 studies across Taiwan that collectively comprise the TAIwan metaboCHIp (TAICHI) Consortium. The African American (AA) studies comprised 2,100 CAD cases and 5,746 controls from the Atherosclerosis Risk in Communities Study (ARIC), Women’s Health Initiative (WHI) and six studies from the Myocardial Infarction Genetics Consortium (MIGen).
Ethical approval was obtained from the appropriate ethics committees and informed consent was obtained from all participants.
Genotyping and quality control in studies with de novo genotyping
Samples from EPIC-CVD, CCHS, CIHDS, CGPS, BRAVE and PROMIS were genotyped on a customised version of the Illumina CardioMetabochip (referred to as the “Metabochip+”, Illumina, San Diego, USA), in two Illumina-certified laboratories located in Cambridge, UK, and Copenhagen, Denmark, by technicians masked to the phenotypic status of samples. The remaining studies were genotyped using the standard CardioMetabochip10 in Hudson-Alpha and Cedars Sinai (TAICHI50, WHI, ARIC51) and the Broad Institute (MIGen).
Each collection was genotyped and underwent QC separately (Supplementary Tables 1 and 2). In brief, studies genotyped on the Metabochip+ had genotypes assigned using the Illumina GenCall software in Genome Studio. Samples were removed if they had a call rate < 0.97, average heterozygosity >±3 standard deviations away from the overall mean heterozygosity or their genotypic sex did not match their reported sex. One of each pair of duplicate samples and first degree relatives (assessed with a kinship co-efficient > 0.2) were removed.
Across all studies, SNP exclusions were based on minor allele frequency (MAF) < 0.01, P < 1x10-6 for Hardy Weinberg Equilibrium or call rate (CR) less than 0.97 (full details are given in Supplementary Table 2). These exclusions were also applied centrally to studies genotyped on the CardioMetabochip, namely the ARIC, WHI, MIGen and TAICHI studies. Principal component analysis (PCA) was applied to identify and remove ancestral outliers. More stringent thresholds were adopted for SNPs used in the PCA for TAICHI and those studies genotyped on the Metabochip+, namely, CR < 0.99, PHWE < 1x10-4 and MAF < 0.05. In addition, one of each pair of SNPs in LD (r2> 0.2) was removed, as were variants in regions known to be associated with CAD.
SNP association analyses and meta-analyses
Statistical analyses were performed in R or PLINK 52 unless otherwise stated.
We collected sufficient samples, to ensure the study was well powered to detect effect sizes in the range of OR=1.05-1.10 which have typically been reported for CAD. With 88,000 cases the study would have 88% power to detect an OR=1.05 for a SNP with MAF=0.2 at α=5x10-8, assuming a multiplicative model on the OR scale. For a lower MAF of 0.1 the study would have 0.93 power to detect OR=1.07 at α=5x10-8, assuming a multiplicative model. Power calculations were performed using Quanto.
Association with CAD was assessed in studies with de novo genotyping from EUR, SAS, and EA, using the Genome-wide Efficient mixed model analysis (GEMMA) approach53. This model includes both fixed effects and random effects of genetic inheritance. CAD (coded 0/1) was the outcome variable, up to five principal components and the test SNP, coded additively, were included as fixed effects. P-values from the score test are reported. The AA studies were analysed using a logistic model in PLINK, with CAD as the outcome variable and SNP coded additively as predictor. The covariates used by each study, including the number of principal components are reported in the Supplementary Information. Genomic inflation was at most 5% for any given study (Supplementary Table 3, Supplementary Fig. 1). A subset of the PROMIS study and EPIC-CVD consortium were contributed to the CARDIoGRAMplusC4D 2013 report. To avoid any overlap of individuals in our studies with those in CARDioGRAMplusC4D, two analyses of these two studies were performed. One analysis included all the samples. A second analysis of the PROMIS and EPIC-CVD studies was performed after excluding all samples that had been contributed to the CARDIoGRAMplusC4D study and before meta-analyzing our results with the results from CARDIoGRAMplusC4D consortium. The CARDIoGRAMplusC4D SNP association results were converted onto the plus strand of GRh37, checked for heterogeneity and checked to ensure allele frequencies were consistent with EUR populations.
Fixed effects inverse variance weighted meta-analysis was used to combine results across studies in METAL54. Heterogeneity P-values and I2 values were calculated and any SNP with P < 0.0001 for heterogeneity was removed. We performed two meta-analyses, the first involved just the European studies with de novo genotyping and the CARDIoGRAMplusC4D results to minimize ancestral diversity. The second involved all studies with de novo genotyping and the CARDIoGRAMplusC4D results to maximize sample size and statistical power. Given the ancestral diversity of the component studies with de novo genotyping, we also implemented meta-analyses with MANTRA55, a meta-analysis approach designed to handle trans-ethnic study designs. However, for our studies the data were broadly consistent with the results from METAL (Table 1, Supplementary Table 4) and we therefore primarily report the fixed effect meta-analysis.
Conditional association analyses
Analyses to test for secondary association signals across seven regions with potential for independent signals were performed using GCTA56. GCTA implements a method for conducting conditional analyses using summary-level statistics (effect size, standard error, P-value, effective sample size) and LD information (r2) between SNPs estimated from a reference panel56. Conditional analyses were performed in CARDIoGRAMplusC4D, EUR, SAS, and EAS respectively and the results were combined using an inverse-variance-weighted fixed effects meta-analysis approach. The conditional analyses were not performed in AA, because the SNP-level case-control counts were not made available for ARIC, MIGen, and WHI. 1000Genome Phase3 v5 ethnic-specific reference panel was used to provide LD information (r2) for the conditioned SNPs and other SNPs in the test regions for each of the 3 ancestries considered in the analyses. As approximately 9% of CARDIoGRAMplusC4D samples were SAS and the remainder EUR, in order to calculate LD for this dataset, we sampled with replacement the genotypes of 50 individuals from the 1000Genome SAS reference panel and combined them with the genotypes of the 503 EUR individuals available in 1000 Genomes. To identify SNPs that are associated with CAD independently of the lead SNP in the test region, the association of each SNP in the region was tested conditioning on the most significant SNP in the overall meta-analysis of EUR, SAS, EAS and CARIoGRAMplusC4D. The SNPs were identified as independent signals for a specific region, if the conditional P≤1x10-4. In each region, we performed several rounds of conditional analyses until the conditional P-values >1x10-4 for all SNPs in the region.
eQTL and epigenetic analyses
The MuTHER dataset contains gene expression data from 850 UK twins for 23,596 probes and 2,029,988 (HapMap 2 imputed) SNPs. All cis–associated SNPs with FDR<1%, within each of the 14 newly identified CAD regions (IMPUTE info score >0.8) were extracted from the MuTHER project dataset for each of the tissues, LCL (n=777), adipose (n=776) and skin (n=667).
The GTEx Project provides expression data from up to 449 individuals for 52,576 genes annotated in Gencode v12 (including pseudo genes) and 6,820,472 genotyped SNPs (using the Human Omni5-Quad array).
From each resource, we report eQTL signals, which reach the resource-specific thresholds for significance described above, for SNPs that are in LD (r2>0.8) with our sentinel SNP.
In addition to the publicly available MuTHER and GTeX databases imputed to HapMap and 1000Genomes, respectively, we used a curated database of over 100 distinct eQTL datasets to determine whether our lead CAD-associated SNPs or SNPs in high LD with them (r2 > 0.8 in Europeans from HapMap or 1000G) were associated with the expression of one or more nearby genes in cis57. Our collated eQTL datasets meet criteria for statistical thresholds for SNP-gene transcript associations as described in the original studies. 57 In total, more than 30 different cells/tissues were queried including, circulating white blood cells of various types, liver, adipose, skin, brain, breast, heart and lung tissues. Complete details of the datasets and tissues queried in the current work can be found in the Supplement Information and Supplementary Table 10, and a general overview of a subset of over 50 eQTL studies has been published57. We first identified all sets of eQTLs in perfect LD (r2=1 among Europeans in HapMap or 1000G) with each other for each unique combination of study, tissue, and transcript. We then determined whether any of these sets of eQTL were either in perfect (r2 = 1) or high LD (1>r2> 0.8) with our lead CAD SNP (Supplementary Table 10).
We required that any eQTL had P<5x10-8 for association with expression levels to be included in the eQTL tables.
We examined chromatin state maps of 23 relevant primary cell types and tissues. Chromatin states are defined as spatially coherent and biologically meaningful combinations of specific chromatin marks. These are computed by exploiting the correlation of such marks, including DNA methylation, chromatin accessibility, and several histone modifications58,59.
pQTL analyses
We conducted plasma protein assays in 3,301 healthy blood donors from the INTERVAL study60 who had all been genotyped on the Affymetrix Axiom UK Biobank genotyping array and imputed to a combined 1000Genomes + UK10K haplotype reference panel61. Proteins were assayed using the SomaLogic SomaScan platform, which uses high-specificity aptamer-binding to provide relative protein abundances. Proteins passing stringent QC (e.g. coefficient of variation<20%) were log transformed and age, sex, duration between venepuncture and sample processing and the first 3 principal components of genetic ancestry were regressed out. Residuals were then rank-inverse normalized before genomewide association testing using an additive model accounting for imputation uncertainty.
Enrichment analyses
Ingenuity pathway analyses
We used the Core Analysis' function in the Ingenuity Pathway Analysis (IPA) software (Ingenuity Systems, Redwood City) to identify canonical pathways enriched with one or more SNPs with a low P-value in the all ancestry meta-analysis.
Modified MAGENTA
Given the Metabochip comprises a select set of SNPs and lacks complete genomic coverage10, MAGENTA, which assumes random sampling of variants from across the genome, could not be directly implemented. Therefore a modified version of MAGENTA involving a hypergeometric test to account for the chip design was used to test for pathways that were enriched with CAD-associated variants11. This approach requires defining two sets of variants; a null set of variants that are not associated with CAD and a set that are associated with CAD, referred to as the “associated set”. Multiple variants can map to the same gene and still be included in the test. SNPs in LD were pruned out of the association results such that r2 < 0.2 for all pairs of SNPs (based on 1,000 Genomes Project data62; Supplementary Table 6) prior to implementation of the modified MAGENTA. The null set was defined as the 1,000 remaining QT interval SNPs with the largest P-values (least evidence) for association with CAD. The associated set was defined as variants (after LD pruning) that showed evidence of association P < 1x10-6. This approach was adopted to select the null and associated sets so as to limit the number of variants included in the hypergeometric cumulative mass function, as a large number of variants results in an intractable calculation for the binomial coefficients. The observed P-value from the hypergeometric test is compared to the P-values obtained from 10,000 random sets to compute an empirical enrichment P-value.
Haploreg: H3K27ac-based tissue enrichment analysis
The associated set as defined for MAGENTA was used for Haploreg analyses and compared to a background set of 12,000 SNPs previously associated with any trait at P<1x10-5 (taken from sources such as NHGRI-EBI GWAS catalogue). Using data from HaploReg15 we counted the number of SNPs with an H3K27ac annotation, or in high LD (r2 > 0.8 from the SNiPA63 EUR 1000 Genomes maps) with a SNP with an H3K27ac annotation. The significance of the enrichment in H3K27ac marks from a particular tissue was determined by comparing the fraction of associated SNPs with that mark, to the fraction of background SNPs with that same mark. A hypergeometric test was used to assign a P-value to the enrichment.
Data availability
The full set of results data from the trans-ancestry meta-analysis and the EUR meta-analysis from this report is available through www.phenoscanner.medschl.cam.ac.uk upon publication.
Supplementary Material
Acknowledgements
J Danesh is a British Heart Foundation Professor, European Research Council Senior Investigator, and NIHR Senior Investigator. J.D. Eicher and A.D. Johnson were supported by NHLBI Intramural Research Program funds. N Franceschini is supported by R21HL123677-01 and R56 DK104806-01A1. N Samani is supported by the British Heart Foundation and is a NIHR Senior Investigator. T.L. Assimes is supported by an NIH career development award K23DK088942. This work was funded by the UK Medical Research Council (G0800270), British Heart Foundation (SP/09/002), UK National Institute for Health Research Cambridge Biomedical Research Centre, European Research Council (268834), European Commission Framework Programme 7 (HEALTH-F2-2012-279233) and Pfizer. The eQTL database construction was supported by NHLBI intramural funds. The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the U.S. Department of Health and Human Services.
A full list of acknowledgements for the studies contributing to this work are provided in the Supplementary Information.
Footnotes
Author Contributions
Central analysis group: JMMH, WZ, DRB, TLA, ASB, DS. Writing group: JMMH, WZ, DRB, DSP, TLA, ASB, JD. Study analysts: JMMH, W-KH, RY, LLW, ELS, SFN, W-YL, RD, NF, AJ, APR, CLC, KY, MG, DA, CAH, Y-HC, XG, TLA. Study PIs and co-PIs: WH-HS; PD, JE, SK, NJS, HS, HJW, DJR, JJ, SH, AQ, JS, CJP, KEN, CK, UP, CAH, W-JL, I-TL, R-HC, Y-JH, JIR, J-MJJ, TQ, T-DW, DSA, AalSM, EDA, RC, Y-DIC, BGN, TLA, JD, ASB, DS, AR, PF. Bioinformatics, eQTL, pQTL and pathway analyses: DSP, WZ, DRB, DFF, TLA, EBF, AM, JBW, ELS, BBS, ASB, JDE, ADJ, PS, TLA, JMMH. Genotyping: SB, LAH, CK, EB, UP, DA, KDT, TQ, TLA. Phenotyping: WH-HS, AT-H, KLR, PRK, KEN, CK, CAH, W-JL, I-TL, R-HC, Y-JH, J-MJJ, TQ, Y-DIC
Competing Financial Interests
AM, EBF and JBW are full time employees of Pfizer. DFF is now a full time employee of Bayer AG, Germany. JD reports personal fees and non-financial support from Merck Sharp & Dohme UK Atherosclerosis, Novartis Cardiovascular & Metabolic Advisory Board, Pfizer Population Research Advisory Panel, Sanofi Advisory Board.
URLs
Data on coronary artery disease / myocardial infarction have been contributed by CARDIoGRAMplusC4D investigators and have been downloaded from www.cardiogramplusc4d.org; String database: http://string-db.org; GTEx expression data were obtained from: www.gtexportal.org; the mouse genome informatics database: http://www.informatics.jax.org; protein atlas: http://www.proteinatlas.org/; phenoscanner: www.phenoscanner.medschl.cam.ac.uk; R: www.R-project.org; linkage disequilibrium information: www.1000genomes.org, http://snipa.helmholtz-muenchen.de/; Gene information: http://www.ncbi.nlm.nih.gov/gene/5175
References
- 1.Roth GA, et al. Demographic and epidemiologic drivers of global cardiovascular mortality. N Engl J Med. 2015;372:1333–41. doi: 10.1056/NEJMoa1406656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.G. B. D. Mortality & Causes of Death Collaborators Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385:117–71. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.CARDIoGRAMplusC4D Consortium et al. Large-scale association analysis identifies new risk loci for coronary artery disease. Nat Genet. 2013;45:25–33. doi: 10.1038/ng.2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Myocardial Infarction Genetics Consortium et al. Genome-wide association of early-onset myocardial infarction with single nucleotide polymorphisms and copy number variants. Nat Genet. 2009;41:334–41. doi: 10.1038/ng.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.IBC 50K CAD Consortium Large-scale gene-centric analysis identifies novel variants for coronary artery disease. PLoS Genet. 2011;7:e1002260. doi: 10.1371/journal.pgen.1002260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Samani NJ, et al. Genomewide association analysis of coronary artery disease. N Engl J Med. 2007;357:443–53. doi: 10.1056/NEJMoa072366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schunkert H, et al. Large-scale association analysis identifies 13 new susceptibility loci for coronary artery disease. Nat Genet. 2011;43:333–8. doi: 10.1038/ng.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Erdmann J, et al. New susceptibility locus for coronary artery disease on chromosome 3q22.3. Nat Genet. 2009;41:280–2. doi: 10.1038/ng.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.CARDIoGRAMplusC4D Consortium. A comprehensive 1000 Genomes-based genome-wide association meta-analysis of coronary artery disease. Nat Genet. 2015;47:1121–30. doi: 10.1038/ng.3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Voight BF, et al. The metabochip, a custom genotyping array for genetic studies of metabolic, cardiovascular, and anthropometric traits. PLoS Genet. 2012;8:e1002793. doi: 10.1371/journal.pgen.1002793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Segre AV, et al. Pathways targeted by antidiabetes drugs are enriched for multiple genes associated with type 2 diabetes risk. Diabetes. 2015;64:1470–83. doi: 10.2337/db14-0703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.GTEx Consortium Human genomics. The Genotype-Tissue Expression (GTEx) pilot analysis: multitissue gene regulation in humans. Science. 2015;348:648–60. doi: 10.1126/science.1262110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grundberg E, et al. Mapping cis- and trans-regulatory effects across multiple tissues in twins. Nat Genet. 2012;44:1084–9. doi: 10.1038/ng.2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franzen O, et al. Cardiometabolic risk loci share downstream cis- and trans-gene regulation across tissues and diseases. Science. 2016;353:827–30. doi: 10.1126/science.aad6970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ward LD, Kellis M. HaploReg: a resource for exploring chromatin states, conservation, and regulatory motif alterations within sets of genetically linked variants. Nucleic Acids Res. 2012;40:D930–4. doi: 10.1093/nar/gkr917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Staley JR, et al. PhenoScanner: a database of human genotype-phenotype associations. Bioinformatics. 2016;32:3207–3209. doi: 10.1093/bioinformatics/btw373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Global Lipids Genetics Consortium et al. Discovery and refinement of loci associated with lipid levels. Nat Genet. 2013;45:1274–83. doi: 10.1038/ng.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teslovich TM, et al. Biological, clinical and population relevance of 95 loci for blood lipids. Nature. 2010;466:707–13. doi: 10.1038/nature09270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.International Consortium for Blood Pressure Genome-Wide Association Studies et al. Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature. 2011;478:103–9. doi: 10.1038/nature10405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Surendran P, et al. Trans-ancestry meta-analyses identify rare and common variants associated with blood pressure and hypertension. Nat Genet. 2016 doi: 10.1038/ng.3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zanoni P, et al. Rare variant in scavenger receptor BI raises HDL cholesterol and increases risk of coronary heart disease. Science. 2016;351:1166–71. doi: 10.1126/science.aad3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boettger LM, et al. Recurring exon deletions in the HP (haptoglobin) gene contribute to lower blood cholesterol levels. Nat Genet. 2016;48:359–66. doi: 10.1038/ng.3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johansson A, et al. Identification of genetic variants influencing the human plasma proteome. Proc Natl Acad Sci U S A. 2013;110:4673–8. doi: 10.1073/pnas.1217238110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holme I, Aastveit AH, Hammar N, Jungner I, Walldius G. Haptoglobin and risk of myocardial infarction, stroke, and congestive heart failure in 342,125 men and women in the Apolipoprotein MOrtality RISk study (AMORIS) Ann Med. 2009;41:522–32. doi: 10.1080/07853890903089453. [DOI] [PubMed] [Google Scholar]
- 25.Levy AP, et al. Haptoglobin genotype is a determinant of iron, lipid peroxidation, and macrophage accumulation in the atherosclerotic plaque. Arterioscler Thromb Vasc Biol. 2007;27:134–40. doi: 10.1161/01.ATV.0000251020.24399.a2. [DOI] [PubMed] [Google Scholar]
- 26.Westra HJ, et al. Systematic identification of trans eQTLs as putative drivers of known disease associations. Nat Genet. 2013;45:1238–43. doi: 10.1038/ng.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dennis J, et al. The endothelial protein C receptor (PROCR) Ser219Gly variant and risk of common thrombotic disorders: a HuGE review and meta-analysis of evidence from observational studies. Blood. 2012;119:2392–400. doi: 10.1182/blood-2011-10-383448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang W, et al. Genome-wide association study identifies novel loci for plasma levels of protein C: the ARIC study. Blood. 2010;116:5032–6. doi: 10.1182/blood-2010-05-283739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith NL, et al. Novel associations of multiple genetic loci with plasma levels of factor VII, factor VIII, and von Willebrand factor: The CHARGE (Cohorts for Heart and Aging Research in Genome Epidemiology) Consortium. Circulation. 2010;121:1382–92. doi: 10.1161/CIRCULATIONAHA.109.869156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qu D, Wang Y, Song Y, Esmon NL, Esmon CT. The Ser219-->Gly dimorphism of the endothelial protein C receptor contributes to the higher soluble protein levels observed in individuals with the A3 haplotype. J Thromb Haemost. 2006;4:229–35. doi: 10.1111/j.1538-7836.2005.01676.x. [DOI] [PubMed] [Google Scholar]
- 31.Reiner AP, et al. PROC, PROCR and PROS1 polymorphisms, plasma anticoagulant phenotypes, and risk of cardiovascular disease and mortality in older adults: the Cardiovascular Health Study. J Thromb Haemost. 2008;6:1625–32. doi: 10.1111/j.1538-7836.2008.03118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uhlen M, et al. Towards a knowledge-based Human Protein Atlas. Nat Biotechnol. 2010;28:1248–50. doi: 10.1038/nbt1210-1248. [DOI] [PubMed] [Google Scholar]
- 33.Uhlen M, et al. Proteomics. Tissue-based map of the human proteome. Science. 2015;347:1260419. doi: 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- 34.Greenawalt DM, et al. A survey of the genetics of stomach, liver, and adipose gene expression from a morbidly obese cohort. Genome Res. 2011;21:1008–16. doi: 10.1101/gr.112821.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nanda V, Miano JM. Leiomodin 1, a new serum response factor-dependent target gene expressed preferentially in differentiated smooth muscle cells. J Biol Chem. 2012;287:2459–67. doi: 10.1074/jbc.M111.302224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen J, Kitchen CM, Streb JW, Miano JM. Myocardin: a component of a molecular switch for smooth muscle differentiation. J Mol Cell Cardiol. 2002;34:1345–56. doi: 10.1006/jmcc.2002.2086. [DOI] [PubMed] [Google Scholar]
- 37.Wang Z, Wang DZ, Pipes GC, Olson EN. Myocardin is a master regulator of smooth muscle gene expression. Proc Natl Acad Sci U S A. 2003;100:7129–34. doi: 10.1073/pnas.1232341100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kirsten H, et al. Dissecting the genetics of the human transcriptome identifies novel trait-related trans-eQTLs and corroborates the regulatory relevance of non-protein coding locidagger. Hum Mol Genet. 2015;24:4746–63. doi: 10.1093/hmg/ddv194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fairfax BP, et al. Innate immune activity conditions the effect of regulatory variants upon monocyte gene expression. Science. 2014;343:1246949. doi: 10.1126/science.1246949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Privratsky JR, et al. Relative contribution of PECAM-1 adhesion and signaling to the maintenance of vascular integrity. J Cell Sci. 2011;124:1477–85. doi: 10.1242/jcs.082271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harry BL, et al. Endothelial cell PECAM-1 promotes atherosclerotic lesions in areas of disturbed flow in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 2008;28:2003–8. doi: 10.1161/ATVBAHA.108.164707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goel R, et al. Site-specific effects of PECAM-1 on atherosclerosis in LDL receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2008;28:1996–2002. doi: 10.1161/ATVBAHA.108.172270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lappalainen T, et al. Transcriptome and genome sequencing uncovers functional variation in humans. Nature. 2013;501:506–11. doi: 10.1038/nature12531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zeller T, et al. Genetics and beyond--the transcriptome of human monocytes and disease susceptibility. PLoS One. 2010;5:e10693. doi: 10.1371/journal.pone.0010693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schroder A, et al. Genomics of ADME gene expression: mapping expression quantitative trait loci relevant for absorption, distribution, metabolism and excretion of drugs in human liver. Pharmacogenomics J. 2013;13:12–20. doi: 10.1038/tpj.2011.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schadt EE, et al. Mapping the genetic architecture of gene expression in human liver. PLoS Biol. 2008;6:e107. doi: 10.1371/journal.pbio.0060107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lin H, et al. Gene expression and genetic variation in human atria. Heart Rhythm. 2014;11:266–71. doi: 10.1016/j.hrthm.2013.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Narahara M, et al. Large-scale East-Asian eQTL mapping reveals novel candidate genes for LD mapping and the genomic landscape of transcriptional effects of sequence variants. PLoS One. 2014;9:e100924. doi: 10.1371/journal.pone.0100924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Innocenti F, et al. Identification, replication, and functional fine-mapping of expression quantitative trait loci in primary human liver tissue. PLoS Genet. 2011;7:e1002078. doi: 10.1371/journal.pgen.1002078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Assimes TL, et al. Genetics of Coronary Artery Disease in Taiwan: A Cardiometabochip Study by the Taichi Consortium. PLoS One. 2016;11:e0138014. doi: 10.1371/journal.pone.0138014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Franceschini N, et al. Prospective associations of coronary heart disease loci in African Americans using the MetaboChip: the PAGE study. PLoS One. 2014;9:e113203. doi: 10.1371/journal.pone.0113203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Purcell S, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–75. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou X, Stephens M. Genome-wide efficient mixed-model analysis for association studies. Nat Genet. 2012;44:821–4. doi: 10.1038/ng.2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Willer CJ, Li Y, Abecasis GR. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics. 2010;26:2190–1. doi: 10.1093/bioinformatics/btq340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morris AP. Transethnic meta-analysis of genomewide association studies. Genet Epidemiol. 2011;35:809–22. doi: 10.1002/gepi.20630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang J, et al. Conditional and joint multiple-SNP analysis of GWAS summary statistics identifies additional variants influencing complex traits. Nat Genet. 2012;44:369–75. doi: 10.1038/ng.2213. S1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang X, et al. Synthesis of 53 tissue and cell line expression QTL datasets reveals master eQTLs. BMC Genomics. 2014;15:532. doi: 10.1186/1471-2164-15-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ernst J, Kellis M. Discovery and characterization of chromatin states for systematic annotation of the human genome. Nat Biotechnol. 2010;28:817–25. doi: 10.1038/nbt.1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ernst J, Kellis M. ChromHMM: automating chromatin-state discovery and characterization. Nat Methods. 2012;9:215–6. doi: 10.1038/nmeth.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moore C, et al. The INTERVAL trial to determine whether intervals between blood donations can be safely and acceptably decreased to optimise blood supply: study protocol for a randomised controlled trial. Trials. 2014;15:363. doi: 10.1186/1745-6215-15-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Astle WJ, et al. The Allelic Landscape of Human Blood Cell Trait Variation and Links to Common Complex Disease. Cell. 2016;167:1415–1429 e19. doi: 10.1016/j.cell.2016.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Genomes Project C et al. A map of human genome variation from population-scale sequencing. Nature. 2010;467:1061–73. doi: 10.1038/nature09534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Arnold M, Raffler J, Pfeufer A, Suhre K, Kastenmuller G. SNiPA: an interactive, genetic variant-centered annotation browser. Bioinformatics. 2015;31:1334–6. doi: 10.1093/bioinformatics/btu779. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The full set of results data from the trans-ancestry meta-analysis and the EUR meta-analysis from this report is available through www.phenoscanner.medschl.cam.ac.uk upon publication.