Abstract
Background
There is strong scientific evidence that human papillomavirus (HPV) vaccines, which protect against two oncogenic HPV types (16 and 18), can prevent cervical, vaginal, and vulvar cancers in women. In addition, recent research has established that the HPV vaccine can prevent anal cancer and has implied that it may also prevent oropharyngeal cancers.
Methods
A 2009 web-based survey of 1500 physicians from four specialties (pediatricians, family practitioners, internists, and obstetrician-gynecologists) explored knowledge about which female cancers the HPV vaccine was effective in preventing. Physician characteristics associated with the belief that the HPV vaccine prevents cervical, vaginal, vulvar, anal, and other cancers were examined using logistic regression models.
Results
Nearly all respondents (97.8%) identified cervical cancer as being prevented by the HPV vaccine; however, lower awareness that the vaccine prevents vaginal (23.8%), vulvar (27.8%), and anal cancer (28.4%) was found. Physician specialty was the most significant covariate identified, with obstetrician-gynecologists being more likely than other physicians to report that the HPV vaccine protected against vaginal (p < 0.001), vulvar (p < 0.001), and anal (p < 0.001) cancers.
Conclusions
Physicians may benefit from educational efforts clarifying which noncervical cancers can be prevented by the HPV vaccine. Education is needed across all medical specialties, but it is particularly important for pediatricians and family practitioners, the physicians most likely to administer the HPV vaccine to young adolescents.
Introduction
The relationship between human papillomavirus (HPV) 16/18 and cervical cancer has been known for several decades, with HPV infection considered necessary but not sufficient to cause cervical cancer.1 Less well known is the fact that these two oncogenic HPV types also are responsible for a significant portion of vulvar, vaginal, anal, and oropharyngeal cancers.2 Further, the HPV vaccines, which have proven > 98% efficacious in preventing high-grade cervical precancer (cervical intraepithelial neoplasia grade 2/3 [CIN2/3]) associated with the HPV types specified, have also demonstrated success in preventing certain other anogenital precancers, such as high-grade vulvar and vaginal lesions (VIN2/3 and VaIN2/3)3 and high-grade anal precursor lesions (AIN2/3).4–6 Given the long time from HPV infection to development of cancer, these precancers were considered acceptable end points in vaccine efficacy trials for the Food and Drug Administration (FDA) indications and eventual Advisory Committee on Immunization Practices (ACIP) recommendation. Although vaccine protection against HPV-associated oropharyngeal cancers has not been established, given the high prevalence of HPV16 in oropharyngeal cancers, there may well be some protection afforded by the HPV vaccine.7
Since 2006, the federal ACIP recommends HPV vaccination of females routinely for the prevention of cervical, vaginal, and vulvar cancer.8,9 In December 2010, the FDA also approved HPV vaccine use for the prevention of anal cancer, although this indication had not yet been approved at the time of the survey described here.4 Most recently, in October 2011, the ACIP recommended routine vaccination of 11–12 year old males for prevention of anal cancer.22 No studies, however, thus far have examined the full spectrum of HPV associated cancers that physicians think can be prevented by the HPV vaccine. This survey of 1500 physicians in pediatrics (PED), family medicine/general practice (FM/GP), internal medicine (IM), and obstetrics-gynecology (OB-GYN) is the first study we are aware of to assess physician knowledge of HPV vaccine utility in preventing noncervical cancers among women. This analysis was undertaken to help inform development of Inside Knowledge: Get the Facts About Gynecologic Cancer, a new public awareness campaign established by the Centers for Disease Control and Prevention (CDC) in collaboration with the Department of Health and Human Services’ Office on Women’s Health. This information may be instrumental in guiding CDC’s and others’ educational efforts aimed at improving physician knowledge of the HPV vaccine and communication of its benefits to parents and recipients of the HPV vaccine, thereby improving coverage of HPV vaccination.
Materials and Methods
Physicians from four specialties, PED, FM/GP, IM, and OB-GYN, were asked in the DocStyles 2009 survey which cancers they believed the HPV vaccine protected against. DocStyles is an annual web-based survey of U.S. physicians and allied health professionals that investigates attitudes and clinical practices related to a variety of health issues. To ensure that adequate representation of targeted provider specialties was obtained, the 2009 DocStyles survey used quota sampling, a form of nonprobability sampling in which the proportions of selected participant characteristics within the sample are deliberately set.10–12 The DocStyles survey has been used to explore a variety of topics, including chronic fatigue syndrome13 and immunodeficiency diseases.14 CDC licensed the results of the 2009 DocStyles survey from Porter Novelli (Washington, D.C.). As personal identifiers were not included in the data provided to CDC, analyses of 2009 DocStyles results were exempted from institutional review board approval.
The 2009 DocStyles survey contained 133 questions, some limited to specific specialties, such as those reported here that were answered only by physicians specializing in PED, FM/GP, IM, and OB-GYN. The sample was drawn from Epocrates Honors Panel®, which included 156,000 physicians in 36 specialties and 425,000 allied healthcare professionals. At the time of panel registration, the identities of physicians were verified by comparing first name, last name, date of birth, medical school, and graduation date against the American Medical Association’s (AMA) Physician Masterfile®, an inventory of licensed U.S. physicians that includes both AMA members and nonmembers. Physicians were eligible to take part in the DocStyles 2009 survey if they practiced in the United States, treated at least 10 patients a week, worked in an individual, group, or hospital practice, and had practiced medicine for at least 3 years.
Sampling quotas for the 2009 DocStyles survey were set to reach 250 PEDs, 1000 primary care physicians (including FM/GPs and IMs), and 250 OB-GYNs. In July 2009, email invitations to take part in the survey were sent to 500 PEDs, 2325 primary care physicians, and 500 OB-GYNs who were randomly selected from the Epocrates Honors Panel to match the proportions for age, gender, and region of the AMA Physician Masterfile. Per the sampling quotas, completed responses were obtained from 250 PEDs, 1000 primary care physicians (609 FM/GPs and 391 IMs), and 250 OB-GYNs. Respondents who were excluded because they did not meet the screening criteria included 7 PEDs, 52 primary care physicians, and 5 OB-GYNs; 9 PEDs, 43 primary care physicians, and 11 OB-GYNs were eligible but did not complete the survey (incomplete surveys were discarded). Once a sampling quota was filled, the survey website locked out additional respondents in that specialty. As a result, response rates were capped around 50% for each specialty (response rates: PEDs 51%, primary care physicians 46%, OB-GYNs 51%).
Our outcome of interest was measured by the following item: “The HPV vaccine is effective in preventing which of the following cancers? Select all that apply.” Nine responses were listed: three correct responses (cervical cancer, vulvar cancer, and vaginal cancer); two responses associated with HPV for which vaccine effectiveness had not yet been determined at the time of the study (anal cancer and oropharyngeal cancer); two gynecologic cancers not associated wih HPV (ovarian cancer and uterine cancer); one nongynecologic cancer not associated with HPV (skin cancer), and none of these. Percentages of physicians in each specialty selecting the specified cancer responses and combinations of responses were calculated and compared using chi-square tests. Although evidence for anal cancer protection was established in December 2010, we reported correct responses as cervical, vulvar, and vaginal cancers, based on the evidence established at the time of the survey in 2009. Next, physician specialty, physician gender, years in practice, teaching hospital privileges, and hours per week spent treating patients in an office setting were entered into separate unadjusted logistic regression models with each cancer response of interest (vulvar, vaginal, anal, and oropharyngeal) as a binary dependent variable (selected vs. not selected). The cervical cancer response was not modeled because of the lack of variance. Finally, the covariates found to be significant in the unadjusted models were included in an adjusted, multivariate model for each cancer response. All analyses were done using IBM SPSS Statistics 19.0 software.
Results
The majority of participants were male (69.1%) and Caucasian (74.1%) (Table 1). The median age of participants was 44. Roughly a third of participants had practiced medicine for < 10 years (32.7%), 10–19 years (37.4%), and ≥ 20 years (29.9%). Just over half of participants had privileges at a teaching hospital (53.9%), and most worked in group practices (63.9%). More than 40% spent 40 or more hours per week treating patients in an office setting (43.5%). The vast majority (98.9%) reported that they treated patients who were eligible for HPV vaccine (females between the ages of 9 and 26 years).
Table 1.
Physician Characteristics by Specialty—DocStyles Survey, 2009
| Pediatrics (n = 250) |
Family/general practice (n = 609) |
Internal medicine (n = 391) |
Obstetrics-gynecology (n = 250) |
Total (n = 1500) |
|
|---|---|---|---|---|---|
| Gender (%) | |||||
| Male | 64.0 | 73.9 | 68.5 | 63.6 | 69.1 |
| Female | 36.0 | 26.1 | 31.5 | 36.4 | 30.9 |
| Race (%) | |||||
| Caucasian | 73.2 | 80.5 | 59.3 | 82.8 | 74.1 |
| Asian | 16.8 | 11.5 | 27.9 | 6.4 | 15.8 |
| Black or African American | 4.0 | 4.9 | 4.1 | 5.2 | 4.6 |
| Other | 6.0 | 3.1 | 8.7 | 5.6 | 5.5 |
| Ethnicity (%) | |||||
| Hispanic or Latino | 8.4 | 4.6 | 4.3 | 5.2 | 5.3 |
| Median age (years) | 44.0 | 45.0 | 41.0 | 46.0 | 44.0 |
| Years in practice (%) | |||||
| < 10 | 31.6 | 31.7 | 39.1 | 26.0 | 32.7 |
| 10–19 | 34.8 | 37.1 | 37.9 | 40.0 | 37.4 |
| ≥ 20 | 33.6 | 31.2 | 23.0 | 34.0 | 29.9 |
| Teaching hospital privileges (%) | 72.8 | 41.9 | 55.5 | 62.0 | 53.9 |
| Practice type (%) | |||||
| Individual | 11.2 | 20.5 | 17.6 | 16.0 | 17.5 |
| Group | 66.4 | 67.8 | 51.9 | 70.4 | 63.9 |
| Hospital or clinic | 22.4 | 11.7 | 30.4 | 13.6 | 18.7 |
| Hours per week spent treating patients in office setting (%) | |||||
| < 20 | 16.8 | 6.4 | 22.3 | 10.0 | 12.9 |
| 20–39 | 43.6 | 45.5 | 37.6 | 48.8 | 43.7 |
| ≥ 40 | 39.6 | 48.1 | 40.2 | 41.2 | 43.5 |
| Treat patients eligible for HPV vaccine, females between 9 and 26 years | 99.2 | 99.2 | 97.7 | 99.6 | 98.9 |
| Percentage of female patients between 9 and 17 years old | |||||
| 0 | 0.8 | 4.6 | 62.4 | 6.0 | 19.3 |
| 1–24 | 28.8 | 81.1 | 33.2 | 88.4 | 61.1 |
| ≥ 25–49 | 70.4 | 14.3 | 4.3 | 5.6 | 19.6 |
| Percentage of female patients between 18 and 26 years old (%) | |||||
| 0 | 20.0 | 0.8 | 2.3 | 0.4 | 4.3 |
| 1–24 | 73.2 | 70.6 | 78.5 | 38.0 | 67.7 |
| ≥ 25 | 6.8 | 28.6 | 19.2 | 61.6 | 28.0 |
Across all specialties, 97.8% of physicians said that the HPV vaccine is effective in preventing cervical cancer (Table 2 and Fig. 1). Recognition that the HPV vaccine can prevent vulvar and vaginal cancer was lower among all specialties but highest among OB-GYNs (52.4%, p < 0.001 and 46.4%, p < 0.001, respectively) and lowest among IMs (18.2%, p < 0.001 and 15.6%, p < 0.001, respectively). The percentage of physicians indicating that the HPV vaccine can prevent anal cancer ranged from 52.4% of OB-GYNs to 19.2% of internists, and the percentage who reported the vaccine can prevent oropharyngeal cancer ranged from 27.6% of OB-GYNs to 9.2% of internists. Finally, < 5% of physicians in any specialty selected the answer choices uterine, ovarian, and skin cancers, which have no demonstrated association with HPV infection, or the none of these response.
Table 2.
Percentage by Specialty Reporting the Human Papillomavirus Vaccine Is Effective in Preventing Specified Cancers—DocStyles Survey, 2009
| Accuracy | Response | Pediatrics (n = 250) |
Family/general practice (n = 609) |
Internal medicine (n = 391) |
Obstetrics-gynecology (n = 250) |
Total (n = 1500) |
|---|---|---|---|---|---|---|
| Correcta | Cervical cancer | 98.8 | 97.7 | 97.2 | 98.0 | 97.8 |
| Vulvar cancer* | 24.0 | 25.5 | 18.2 | 52.4 | 27.8 | |
| Vaginal cancer* | 20.8 | 21.0 | 15.6 | 46.4 | 23.8 | |
| Plausibleb | Anal cancer* | 26.8 | 25.1 | 19.2 | 52.4 | 28.4 |
| Oropharyngeal cancer* | 12.0 | 10.3 | 9.2 | 27.6 | 13.2 | |
| Incorrectc | Uterine cancer* | 4.4 | 2.3 | 3.6 | 0.0 | 2.6 |
| Skin cancer | 0.8 | 2.0 | 1.5 | 2.4 | 1.7 | |
| Ovarian cancer | 0.4 | 1.6 | 2.0 | 0.0 | 1.3 | |
| None of these | 0.0 | 1.3 | 0.8 | 1.2 | 0.9 |
Cancers that the human papillomavirus (HPV) vaccine had been found to be protective against at the time of the study.
Cancers known to be associated with HPV infection, but the protective benefit of the HPV vaccine had not been determined at the time of the study.
Cancers with no known association with HPV infection at the time of the study and none of these responses.
Significant differences (p < 0.01) by specialty; percents compared using Pearson chi-square asymptotic two-sided tests.
FIG. 1.
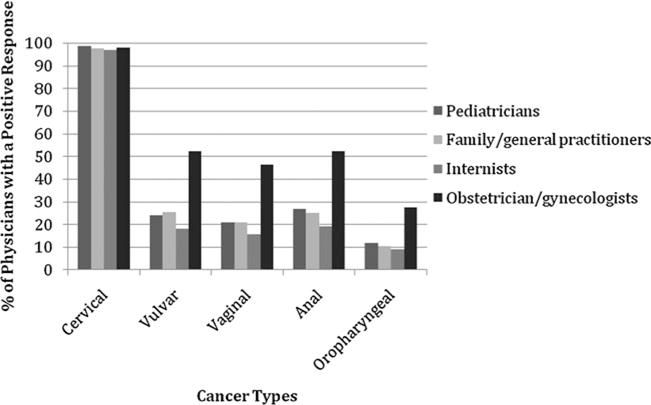
Cancers that U.S. physicians believe are prevented by the human papillomavirus (HPV) vaccine. Four other responses were offered: ovarian cancer, skin cancer, uterine cancer, and none of these. However, these responses were rarely selected, with < 5% of physicians in any specialty indicating that the HPV vaccine could prevent any of them.
When various mutually exclusive combinations of responses were analyzed, only 3.9% (FM/GPs) to 5.2% (PEDs and OB-GYNs) selected all the correct responses (cervical, vulvar, and vaginal cancers) with no other responses selected (Table 3). OB-GYNs were more likely to select the correct responses and anal cancer (18.4%, p < 0.001) as well as the correct responses and anal and oropharyngeal cancer (16.8%, p < 0.001). Most commonly across all specialties, however, was the response that the HPV vaccine is effective in preventing only cervical cancer, with the majority of PEDs (60.4%), FM/GPs (62.4%), and IMs (68.0%) and a third of OB-GYNs (33.6%) selecting only the cervical cancer response.
Table 3.
Percentage by Specialty Reporting Human Papillomavirus Vaccine Is Effective in Preventing Various Combinations of Cancers—DocStyles, 2009
| Response combination | Response selecteda | Pediatrics (n = 250) |
Family/general practice (n = 609) |
Internal medicine (n = 391) |
Obstetrics-gynecology (n = 250) |
Total (n = 1500) |
|---|---|---|---|---|---|---|
| All correct responsesb | Cervical, vulvar, and vaginal cancers | 5.2 | 3.9 | 4.1 | 5.2 | 4.4 |
| Incomplete combinations of correct responses | Cervical cancer* | 60.4 | 62.4 | 68.0 | 33.6 | 58.7 |
| Cervical and vulvar cancers | 1.2 | 2.6 | 1.0 | 2.4 | 1.9 | |
| Cervical and vaginal cancers | 1.2 | 0.5 | 0.8 | 2.0 | 0.9 | |
| All correct responses and plausible responsesc | Cervical, vulvar, vaginal, and anal cancers* | 7.2 | 6.6 | 4.3 | 18.4 | 8.1 |
| Cervical, vulvar, vaginal, and oropharyngeal cancers | 0.4 | 0.5 | 0.0 | 0.8 | 0.4 | |
| Cervical, vulvar, vaginal, and anal, and oropharyngeal cancers* | 3.2 | 4.1 | 2.8 | 16.8 | 5.7 | |
| Other | All other combinations of responsesd | 21.2 | 19.4 | 18.9 | 20.8 | 19.8 |
The “Responses selected” column lists all responses given; thus, the various combinations reported are mutually exclusive and sum to 100% for each specialty.
Cancers that the HPV vaccine had been found to be protective against at the time of the study.
Cancers known to be associated with HPV for which vaccine effectiveness had not yet been determined at the time of the study.
Nine possible responses were offered: anal cancer, cervical cancer, oropharyngeal cancer, ovarian cancer, skin cancer, uterine cancer, vaginal cancer, vulvar cancer, and none of these.
Significant differences (p < 0.001) by specialty; percents compared using Pearson chi-square asymptotic two-sided tests.
Physician specialty, physician gender, years in practice, teaching hospital privileges, and hours per week spent treating patients in an office setting were entered into separate unadjusted logistic regression models, with each cancer known to be associated with the HPV infection at the time of the study (vulvar, vaginal, anal, and oropharyngeal) as a binary dependent variable (selected vs. not selected). The cervical cancer response was not modeled because of the lack of variance (97.8% of physician participants across all specialties said that the HPV vaccine is effective in preventing cervical cancer). In the unadjusted models, OB-GYNs were significantly more likely to select the response for vulvar cancer (p < 0.001), vaginal cancer (p < 0.001), anal cancer (p < 0.001), and oropharyngeal cancer (p < 0.001) compared with physicians in all other specialties (results not shown). Female physicians were significantly more likely to select the responses of anal cancer (p = 0.007) and oropharyngeal cancer (p = 0.022) compared with male physicians. Physicians in practice for at least 20 years were significantly more likely to select oropharyngeal cancer (p = 0.001) compared with physicians practicing < 20 years. Having privileges at a teaching hospital and hours per week spent treating patients in an office setting were not significant covariates for any of the responses modeled.
Next, adjusted logistic regression models were run for the vulvar, vaginal, anal, and oropharyngeal responses. These models included the covariates found to be statistically significant in the unadjusted models: physician specialty, physician gender, and years in practice. In the adjusted models for the vulvar and vaginal cancer responses, physician specialty remained a significant covariate, and no other significant covariates emerged; OB-GYNs were more likely to report that the HPV vaccine prevented vulvar (p < 0.001) and vaginal cancers (p < 0.001) (Table 4). For the anal cancer response, physician specialty and gender remained statistically significant covariates in the adjusted model; OB-GYNs (p < 0.001) and female physicians (p = 0.04) were more likely to report that the HPV vaccine effectively prevents anal cancer. Likewise, the significant covariates in the unadjusted models of the oropharyngeal cancer response (physician specialty, physician gender, and years in practice) remained significant in the adjusted model; OB-GYNs (p < 0.001), female physicians (p = 0.03), and physicians in practice > 20 years (p = 0.008) were more likely to report that the HPV vaccine effectively prevents oropharyngeal cancer.
Table 4.
Adjusted Logistic Regression Results: Characteristics of Physicians Who Reported the Human Papillomavirus Vaccine Is Effective in Preventing Specified Cancers, DocStyles Survey, 2009
| n | Odds ratio (95% confidence interval)
|
||||
|---|---|---|---|---|---|
| Vulvar cancer | Vaginal cancer | Anal cancer | Oropharyngeal cancer | ||
| Specialty | |||||
| Pediatrics | 250 | 0.29 (0.20–0.42) | 0.30 (0.20–0.45) | 0.33 (0.23–0.48) | 0.36 (0.22–0.57) |
| Family/general medicine | 609 | 0.31 (0.23–0.43) | 0.30 (0.22–0.42) | 0.31 (0.23–0.42) | 0.32 (0.22–0.46) |
| Internal medicine | 391 | 0.20 (0.14–0.29) | 0.21 (0.15–0.31) | 0.21 (0.15–0.31) | 0.29 (0.18–0.44) |
| Obstetrics-gynecology | 250 | Reference | Reference | Reference | Reference |
| Gender | |||||
| Female | 463 | 1.13 (0.88–1.46) | 0.94 (0.72–1.24) | 1.30 (1.01–1.67) | 1.44 (1.04–2.00) |
| Male | 1,037 | Reference | Reference | Reference | Reference |
| Years in practice | |||||
| < 10 | 490 | 0.95 (0.70–1.28) | 0.98 (0.72–1.34) | 1.09 (0.81–1.46) | 0.58 (0.39–0.86) |
| 10–19 | 561 | 0.90 (0.68–1.21) | 0.84 (0.62–1.13) | 0.89 (0.67–1.19) | 0.62 (0.43–0.89) |
| ≥ 20 | 449 | Reference | Reference | Reference | Reference |
Discussion
Recognition that the HPV vaccine can prevent cervical cancer was nearly universally understood across all special- ties of physicians included in our study. However, significant gaps in knowledge remain regarding HPV protection against noncervical cancers. We found that the majority of physicians in all specialties, except OB-GYNs, reported that the HPV vaccine was effective in preventing cervical cancer alone (60%–68%). Despite increasing evidence demonstrating that the HPV vaccine confers protection against vaginal, vulvar, and anal cancers,3–6 most physicians in this survey were unaware of this additional protection. OB-GYNs were the best informed, with identification of noncervical cancer protection at least twice as often as other specialties, which is consistent with prior research.15 Even among OB-GYNs, however, only about half were aware of the added protection of the vaccine against noncervical cancers.
Pediatricians are the physicians most likely to see patients targeted for vaccination, and they play a role in educating parents about the protective role of the HPV vaccine. Still, pediatricians recognized vaccine protection against these anogenital cancers only about a quarter of the time. It is understandable that OB-GYNs are the best informed because they see the results of chronic HPV infection. However, because HPV vaccination is most effective before onset of sexual activity, pediatricians need to be better informed. In the United States, 2010 coverage among 13-year-old girls was only 48.7% for the first dose and 32% for all three doses.16 Improving pediatricians’ or other physicians’ knowledge about the HPV vaccine is desirable to communicate comprehensive information, but it is unclear if physician-targeted educational efforts will translate into higher coverage.
In 2007, the annual numbers of vulvar, vaginal, anal cancers in the United States were 4159, 1149, and 4317 cases per year, respectively, with approximately half of vulvar and vaginal cases and 90% of anal cases being HPV16/18-associated.17,18 With the advent of the Pap test, the incidence of cervical cancer has been declining. However, there remains no widespread screening tool for the other anogenital cancers, and, in fact, the incidence of anal cancer and vulvar cancer has been increasing over the past few decades.19,20 Although the combination of noncervical cancers comprises a substantial portion of the HPV-associated cancer burden and although protection against anal cancer is a key reason for vaccination of boys, only a small minority of physicians surveyed had knowledge of the range of protection offered by the HPV vaccine. Increasing knowledge among physicians about HPV vaccine protection against these cancers is an important component of patient education, with the potential outcome of increasing vaccine uptake among girls and boys and, ultimately, decreasing the burden of disease. Additional research is needed to examine the relationship between physician knowledge of the protective benefits of the HPV vaccine and their vaccine recommendations.
Oropharyngeal cancer presents a more complicated case. There have been no studies demonstrating HPV vaccine efficacy against oropharyngeal cancer, and the vaccine is not indicated for protection against this type of cancer. Oropharyngeal cancer has long been associated with smoking and alcohol use, but more recently, HPV infection has also been implicated in a certain subset of these cancers. There is considerable debate over what percentage of these cancers is attributable to HPV infection, with estimates being as high as 63% in the United States for recent years.18,21 The HPV vaccine has the potential to prevent a substantial number of oropharyngeal cancers, but the evidence will only be borne out in trends and genotyping results of future diagnoses. It is particularly interesting that OB-GYNs, who are the best informed about anogenital cancers prevented by the HPV vaccine, are also the group with the highest positive response rate for oropharyngeal cancer, even though scientific evidence about the efficacy of the HPV vaccine in preventing these cancers is lacking.
Examining combinations of responses, it becomes evident that most physicians who correctly selected cervical, vulvar, and vaginal cancers also selected anal cancer or selected both anal and oropharyngeal cancers. Although the evidence for anal cancer protection was not formally established at the time of this study and there are no studies to date demonstrating protection against oropharyngeal cancer, physicians appear to be lumping these cancers with the vulvar and vaginal cancers, for which there has long been strong evidence of protection. It is difficult to determine whether these physicians believe erroneously that the HPV vaccine has been shown to protect against oropharyngeal cancer or are making a logical speculation based on evidence of HPV pathogenesis in oropharyngeal cancer. Although the HPV vaccine has the potential to prevent certain oropharyngeal cancers, it is important to accurately convey to physicians and other vaccine providers that (1) this potential benefit of the vaccine has not yet been proven, (2) HPV is associated with only a subset of oropharyngeal cancer cases, and 3) vaccination will not preclude the importance of reducing other important risk factors, such as tobacco and alcohol use.
The representativeness of our sample is an issue of concern, given the use of quota sampling; however, Porter Novelli found that 2009 DocStyles participants were demographically comparable with physicians in the AMA Masterfile on gender, average age, and average years in practice (unpublished data, Porter Novelli, 258 DocStyles 2009 Methods, Washington DC, 2009). Also, the manner in which quotas were filled introduces bias, as participants were recruited through email on a first-come, first-served basis, with the survey website locking out potential respondents once the quota for their specialty was filled. Thus, participants may be more technologically connected than the aggregate U.S. physician population, as they were not only email users but most likely email users who check their messages frequently.
This survey offers information for directing future educational efforts and initiatives to improve physician knowledge about the HPV vaccine. In particular, this survey informed the development of the Inside Knowledge: Get the Facts About Gynecologic Campaign, created by the CDC, in collaboration with the U.S. Department of Health and Human Services’ Office on Women’s Health. The campaign is designed to raise awareness among women and healthcare providers about the signs, symptoms, risk factors, and prevention strategies associated with the five main types of gynecologic cancers: cervical, ovarian, uterine, vaginal, and vulvar cancers. However, given the new ACIP recommendation for routine male vaccination22, future surveys should explore the knowledge of the vaccine to prevent other HPV-associated cancers (such as penile cancers) or other types of cancers that may be linked to HPV but require stronger evidence (oral cavity, laryngeal cancers).2 Future studies should also survey physicians and other providers that are more likely to see males and vaccinate males. Clearly, an emphasis needs to be placed on increasing physician awareness about the protection provided by the HPV vaccine against vulvar, vaginal, and anal cancers. Efforts should also be made to provide clear, accurate information about the potential role of the HPV vaccine in preventing certain oropharyngeal cancers.
Acknowledgments
This project has been funded in part with federal funds from the Centers for Disease Control and Prevention. The findings and conclusions in this report are those of the authors and do not necessarily reflect the official position of the Department of Health and Human Services or the Centers for Disease Control and Prevention, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Footnotes
Disclosure Statement
The authors have no financial relationships relevant to this article or conflicts of interest to disclose.
References
- 1.Munoz N, Bosch FX, de Sanjose S, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348:518–527. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- 2.Cogliano V. International Agency for Research on Cancer. Monographs on the evaluation of carcinogenic risks to humans: Human papillomavirus. Lyon: IARC; 2007. [PMC free article] [PubMed] [Google Scholar]
- 3.Kjaer SK, Sigurdsson K, Iversen O, et al. A pooled analysis of continued prophylactic efficacy of quadrivalent human papillomavirus (types 6/11/16/18) vaccine against high-grade cervical and external genital lesions. Cancer Prev Res (Phila) 2009;2:868–878. doi: 10.1158/1940-6207.CAPR-09-0031. [DOI] [PubMed] [Google Scholar]
- 4.U.S. Food and Drug Administration. Gardasil. Rockville, MD: U.S. Department of Health & Human Services; updated August 5, 2011. Available at www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm094042.htm Accessed 2011. [Google Scholar]
- 5.Kreimer AR, Gonzalez P, Katki HA, et al. Efficacy of a bivalent HPV 16/18 vaccine against anal HPV 16/18 infection among young women: A nested analysis within the Costa Rica Vaccine Trial. Lancet Oncol. 2011;12:862–870. doi: 10.1016/S1470-2045(11)70213-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palefsky JM, Giuliano AR, Goldstone S, Moreira ED, Jr, Aranda C, Jessen H, Hillman R, Ferris D, Coutlee F, Stoler MH, Marshall JB, Radley D, Vuocolo S, Haupt RM, Guris D, Garner EI. HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. N Engl J Med. 2011 Oct 27;365:1576–1585. doi: 10.1056/NEJMoa1010971. [DOI] [PubMed] [Google Scholar]
- 7.Ryerson AB, Peters ES, Coughlin SS, et al. Burden of potentially human papillomavirus-associated cancers of the oropharynx and oral cavity in the US, 1998–2003. Cancer. 2008;113(Suppl 10):2901–2909. doi: 10.1002/cncr.23745. [DOI] [PubMed] [Google Scholar]
- 8.FDA licensure of quadrivalent human papillomavirus vaccine (HPV4, Gardasil) for use in males and guidance from the Advisory Committee on Immunization Practices (ACIP) MMWR. 2010;59:630–632. [PubMed] [Google Scholar]
- 9.FDA licensure of bivalent human papillomavirus vaccine (HPV2, Cervarix) for use in females and updated HPV vaccination recommendations from the Advisory Committee on Immunization Practices (ACIP) MMWR. 2010;59:626–629. [PubMed] [Google Scholar]
- 10.Morrow KM, Vargas S, Rosen RK, et al. The utility of non-proportional quota sampling for recruiting at-risk women for microbicide research. AIDS Behav. 2007;11:586–595. doi: 10.1007/s10461-007-9213-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cumming RG. Is probability sampling always better? A comparison of results from a quota and a probability sample survey. Community Health Stud. 1990;14:132–137. doi: 10.1111/j.1753-6405.1990.tb00033.x. [DOI] [PubMed] [Google Scholar]
- 12.Im EO, Chee W. Quota sampling in internet research: Practical issues. Comput Inform Nurs. 2011;29:381–385. doi: 10.1097/NCN.0b013e3181f9dc45. [DOI] [PubMed] [Google Scholar]
- 13.Brimmer DJ, Fridinger F, Lin JM, Reeves WC. U.S. health-care providers’ knowledge, attitudes, beliefs, and perceptions concerning chronic fatigue syndrome. BMC Fam Pract. 2010;11:28. doi: 10.1186/1471-2296-11-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waltenburg R, Kobrynski L, Reyes M, Bowen S, Khoury MJ. Primary immunodeficiency diseases: Practice among primary care providers and awareness among the general public, United States, 2008. Genet Med. 2010;12:792–800. doi: 10.1097/GIM.0b013e3181f3e2c9. [DOI] [PubMed] [Google Scholar]
- 15.Irwin K, Montano D, Kasprzyk D, et al. Cervical cancer screening, abnormal cytology management, and counseling practices in the United States. Obstet Gynecol. 2006;108:397–409. doi: 10.1097/01.AOG.0000230258.07737.fa. [DOI] [PubMed] [Google Scholar]
- 16.National and state vaccination coverage among adolescents aged 13 through 17 years—United States, 2010. MMWR. 2011;60:1117–1123. [PubMed] [Google Scholar]
- 17.United States Cancer Statistics: 1999–2007. Incidence and mortality web-based report, 2010. Accessed February 27, 2011. [Google Scholar]
- 18.Saraiya M. Burden of HPV-associated cancers in the United states. Presented at Advisory Committee on Immunization Practices. 2011 Feb 24; [Google Scholar]
- 19.Joseph DA, Miller JW, Wu X, et al. Understanding the burden of human papillomavirus-associated anal cancers in the US. Cancer. 2008;113(Suppl 10):2892–2900. doi: 10.1002/cncr.23744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Watson M, Saraiya M, Wu X. Update of HPV-associated female genital cancers in the United States, 1999–2004. J Womens Health. 2009;18:1731–1738. doi: 10.1089/jwh.2009.1570. [DOI] [PubMed] [Google Scholar]
- 21.Gillison ML, Chaturvedi AK, Lowy DR. HPV prophylactic vaccines and the potential prevention of noncervical cancers in both men and women. Cancer. 2008;113(Suppl 10):3036–3046. doi: 10.1002/cncr.23764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dunne E, Markowitz L, Chesson H, Curtis R, Saraiya M, Unger E. Recommendations on the Use of Quadrivalent Human Papillomavirus Vaccine in Males—Advisory Committee on Immunization Practices (ACIP), 2011. 2011 Dec 23;60(50):1705–1708. [PubMed] [Google Scholar]


