Abstract
How does visual attention affect spatial resolution? In texture-segmentation tasks, exogenous (involuntary) attention automatically increases resolution at the attended location, which improves performance where resolution is too low (at the periphery) but impairs performance where resolution is already too high (at central locations). Conversely, endogenous (voluntary) attention improves performance at all eccentricities, which suggests a more flexible mechanism. Here, using selective adaptation to spatial frequency, we investigated the mechanism by which endogenous attention benefits performance in resolution tasks. Participants detected a texture target that could appear at several eccentricities. Adapting to high or low spatial frequencies selectively affected performance in a manner consistent with changes in resolution. Moreover, adapting to high, but not low, frequencies mitigated the attentional benefit at central locations where resolution was too high; this shows that attention can improve performance by decreasing resolution. Altogether, our results indicate that endogenous attention benefits performance by modulating the contribution of high-frequency information in order to flexibly adjust spatial resolution according to task demands.
Keywords: attention, spatial resolution, texture segmentation, spatial frequency, adaptation
The visual system is limited by spatial resolution—the ability to discriminate two nearby points in space, which is highest at the fovea and declines with eccentricity. An attentional mechanism that increases resolution is beneficial in most situations (Anton-Erxleben & Carrasco, 2013; Carrasco & Yeshurun, 2009). However, heightened resolution can be detrimental when high-resolution information is sparse (e.g., navigating in fog) or when a global assessment of the scene is required (e.g., viewing Impressionist paintings). This is illustrated by texture-segmentation tasks, in which performance is constrained by spatial resolution (Gurnsey, Pearson, & Day, 1996; Kehrer, 1989; Morikawa, 2000; Talgar & Carrasco, 2002; Yeshurun & Carrasco, 1998, 2000). In these tasks, the aim is to detect a target (a patch of oriented lines) within a larger array of differently oriented lines (Fig. 1). The visual system’s resolution limits performance in an eccentricity-specific manner: Performance peaks where resolution is optimal (at the midperiphery) and falls off where resolution is either too low (at the periphery) or too high (at central locations) for the texture scale. This central performance drop (CPD) is ascribed to the mismatch between the texture scale and the spatial characteristics of second-order filters selective to texture-defined information (Kehrer, 1997; Kehrer & Meinecke, 2003; Yeshurun & Carrasco, 2000). The inverted-U shape of performance on these tasks makes them ideal for exploring how attention affects resolution (Carrasco, Loula, & Ho, 2006; Yeshurun & Carrasco, 1998, 2000; Yeshurun, Montagna, & Carrasco, 2008).
Fig. 1.
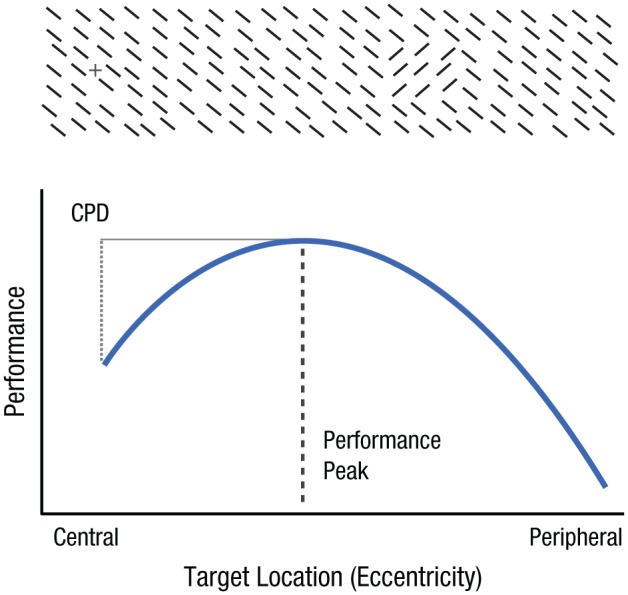
Typical performance on a texture-segmentation task. The task is to detect a target (a patch of oriented lines) within a larger array of differently oriented lines. Performance peaks at the target eccentricity where resolution matches the scale of the texture and drops where resolution is too low (at peripheral locations) or too high (at central locations). The decrease in performance at central locations is known as the central performance drop (CPD).
There is compelling evidence for resolution enhancement with both endogenous and exogenous attention at varying eccentricities in tasks that benefit from increased resolution (Anton-Erxleben & Carrasco, 2013), such as visual search (Carrasco & Yeshurun, 1998), acuity and hyperacuity (Carrasco, Williams, & Yeshurun, 2002; Golla, Ignashchenkova, Haarmeier, & Thier, 2004; Montagna, Pestilli, & Carrasco, 2009), crowding (Yeshurun & Rashal, 2010), and texture segmentation (Carrasco et al., 2006; Talgar & Carrasco, 2002; Yeshurun & Carrasco, 1998, 2000; Yeshurun et al., 2008). Furthermore, attention enhances perceived spatial frequency (SF; Abrams, Barbot, & Carrasco, 2010; Gobell & Carrasco, 2005) and size (Anton-Erxleben, Henrich, & Treue, 2007). Exogenous covert attention—involuntary attention to a location without moving one’s eyes—is an automatic, inflexible mechanism (Carrasco, 2011) that increases resolution at the attended area, even when it is detrimental to the task at hand. In texture-segmentation tasks, exogenous attention improves performance where resolution is too low for a given texture scale but hinders performance where resolution is already too high for that texture scale (i.e., where the CPD occurs; Carrasco et al., 2006; Talgar & Carrasco, 2002; Yeshurun & Carrasco, 1998, 2000). Conversely, endogenous covert attention—the voluntary orienting of attention without moving one’s eyes—improves performance at all eccentricities, regardless of whether the texture scale is too large or too small for the visual system’s resolution (Yeshurun et al., 2008). These differential effects indicate that endogenous attention is more flexible and may actually decrease resolution when such a decrease is beneficial for the task at hand. Here, we directly tested this hypothesis.
Spatial resolution is directly related to receptive-field size and SF tuning, that is, the visual system’s sensitivity to different spatial scales. The ability of smaller receptive fields to resolve finer details is correlated with a reduction of the area over which receptive fields integrate information. Fine-scale analysis is mediated by the smaller-receptive-field, high-SF neurons: The smaller the neuron’s receptive field, the higher its preferred SF (DeValois & DeValois, 1988; Jones & Palmer, 1987). Modulating the contribution of either high SFs or low SFs affects texture-segmentation performance in a manner consistent with resolution changes: Selectively removing high SFs from the stimulus display eliminates the CPD (Morikawa, 2000). Likewise, adapting to high SFs reduces the CPD and shifts the performance peak toward central locations (Carrasco et al., 2006). Moreover, high-SF adaptation eliminates the central attentional impairment observed with exogenous attention, which indicates that exogenous attention automatically enhances resolution by increasing the sensitivity of high-SF filters (Carrasco et al., 2006). Computational models assuming lower preferred SF and larger receptive-field size with eccentricity can account for the CPD magnitude and the performance-peak eccentricity observed for different texture scales (Kehrer, 1997; Kehrer & Meinecke, 2003), which supports a direct link between the visual system’s preferred SF and resolution constraints at a given eccentricity.
To investigate whether and how endogenous attention affects resolution at central locations where resolution is too high (i.e., where the CPD occurs) and where exogenous and endogenous attention have differential effects, we combined SF adaptation with a texture-segmentation task while manipulating endogenous attention. SF adaptation allowed us to manipulate the visual system’s effective resolution by selectively reducing the contribution of low-SF or high-SF filters. Assessing whether and how SF adaptation modulates the effects of endogenous attention enabled us to pinpoint the underlying mechanism by which endogenous attention improves performance at the CPD, where heightened resolution is detrimental for the task at hand.
Method
Observers
Twelve observers (6 females, 6 males; age range = 20–32 years) with normal or corrected-to-normal vision participated in this study. Six were experienced psychophysical observers, and all (except one of the authors) were naive to the purposes of the study. A sample size of 12 is common for studies of visual attention and texture segmentation (e.g., Carrasco et al., 2006; Talgar & Carrasco, 2002; Yeshurun & Carrasco, 1998, 2000; Yeshurun et al., 2008). The New York University Institutional Review Board approved the study.
Apparatus
Stimuli were generated using MATLAB (The MathWorks, Natick, MA) and displayed on a 22-in. CRT monitor (1,600 × 1,200 resolution; 60 Hz) situated 114 cm from the observer. The displays were calibrated using a Photo Research (Syracuse, NY) PR-650 SpectraColorimeter to produce linearized look-up tables. Eye position was monitored at 1000 Hz with an EyeLink 1000 eye tracker (SR Research, Ottawa, Ontario, Canada).
Stimuli
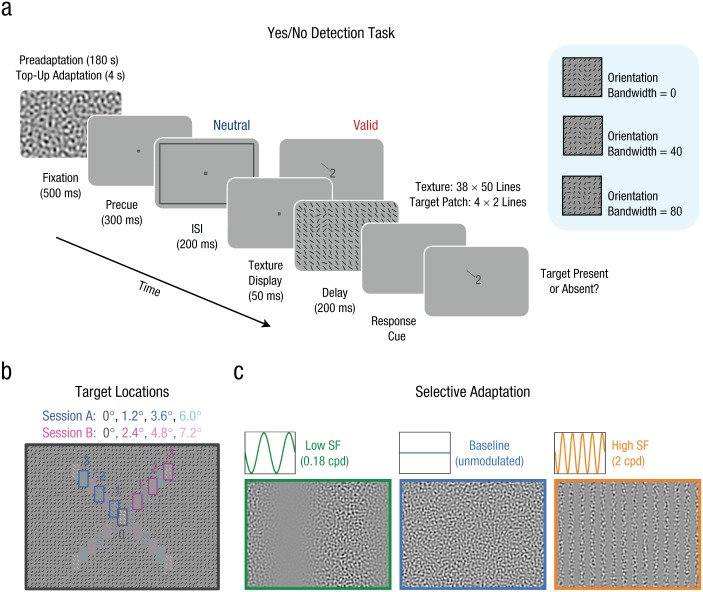
Each texture display consisted of 1,900 black lines (length: 0.24°) on a gray background arranged on a grid in 38 rows by 50 columns. Each line subtended 0.4° × 0.4°, and its position was jittered (±0–0.1°). For each trial, the average display orientation was ±45° from vertical, but each line’s orientation was randomly chosen from a uniform orientation distribution within a limited range. This range—the orientation bandwidth—was adjusted between experimental sessions to maintain an overall performance level of approximately 75% correct (mean orientation bandwidth: 89°, range: 60–120°). In half of the trials, the texture display contained a target patch comprising 2 columns × 4 rows of lines (subtending 0.8° × 1.6°); the average orientation of the lines in the target patch (±45°) was orthogonal to the average orientation of the lines in the background texture (Fig. 2a). The target patch could appear at any of seven possible eccentricities (0.0°, 1.2°, 2.4°, 3.6°, 4.8°, 6.0°, 7.2°) along the diagonal meridians (25 possible locations; Fig. 2b). Limiting performance by manipulating the variability in the orientation of the texture lines (i.e., orientation range of the lines around ±45° from vertical), rather than by using a backward mask, ensures that performance changes are constrained by spatial factors (Morikawa, 2000; Potechin & Gurnsey, 2003; Yeshurun et al., 2008). The diagonals prevented heterogeneities observed along the vertical and horizontal meridians in texture-segmentation tasks (Talgar & Carrasco, 2002).
Fig. 2.
Experimental procedure and design. An example trial sequence from the yes/no detection task is presented in (a). Each block began with a 180-s adaptation period, and each trial started with a 4-s top-up. This was followed by a fixation interval, after which a precue appeared. The precue could be either neutral (no information given regarding the target location) or valid (a diagonal line and a number from 0–3 indicating, respectively, the diagonal meridian and eccentricity of the target location). The precue was followed by an interstimulus interval (ISI) and then a texture display, in which a target patch could appear at the cued location. Regardless of whether a trial was neutral or valid, a response cue appeared after the texture display to indicate the relevant location where the target patch would have been, if present. The mean orientation of the target patch (±45º) was always orthogonal to the mean orientation of the background lines (±45°). Task difficulty was controlled by varying the orientation bandwidth of the texture (i.e., the orientation range of the uniform distribution from which the orientation of each line was randomly selected). The target patch, which appeared on 50% of both neutral and valid trials, could appear at any of 25 possible locations (b), at seven possible eccentricities along the diagonal meridians. The seven eccentricities were presented in two sessions, with only 0° eccentricity overlapping between sessions. Texture-defined second-order adaptors (c) consisted of a carrier noise (6 cycles/deg, or cpd) that was either unmodulated (baseline) or modulated in contrast by a vertical grating of low or high spatial frequency (SF). The images in this figure were altered to improve visibility.
Performance in texture-segmentation tasks has been shown to depend on second-order spatial filters that are selective to texture information (Gurnsey et al., 1996; Kehrer & Meinecke, 2003; Yeshurun & Carrasco, 2000). Moreover, attention affects texture segmentation at the second-order stage of visual processing (Yeshurun & Carrasco, 2000). Accordingly, we used second-order adaptors of different SFs to affect spatial filters selective to texture-defined information. Adaptors (Fig. 2c) were filtered noise stimuli (6 cycles/deg ±1 octave) subtending the entire screen, either not modulated (baseline) or modulated in contrast by a vertical grating of relatively high SF (2 cycles/deg) or relatively low SF (0.18 cycles/deg). Given that second-order adaptation is orientation specific (e.g., Hallum, Landy, & Heeger, 2011), the orientations of the adaptor stimuli and of the texture target patch were both vertical.
Procedure
We used a yes/no detection task and manipulated endogenous attention by precuing the potential target location with a central symbolic cue (Fig. 2a). Each block began with a 180-s adaptation period. To avoid low-level adaptation effects, we set the carrier noise to counter-flicker in phase at 4 Hz, while the vertical modulators with random phase flickered at 2 Hz. Each trial began with a 4-s top-up adaptation period. A 300-ms precue was presented, and following a 200-ms interstimulus interval, a texture appeared for 50 ms. After a 200-ms delay, a response cue at fixation indicated the location where the target could have been presented; this cue was intended to eliminate location uncertainty. The target patch was presented on 50% of the trials. Participants were instructed to maintain fixation during the entire trial sequence and to report, as accurately as possible, whether the texture display contained a target at the location indicated by the response cue. Auditory feedback indicated whether the response was correct or incorrect at the end of each trial.
On half of the trials (valid trials), the central cue consisted of a digit and a small line at fixation indicating with 100% validity the location where the texture target, if present, would be presented. The digit indicated the target eccentricity: 0 indicated the central location, and 1 through 3 progressively more eccentric locations. The line indicated the quadrant where the target, if present, would be displayed. Observers were told that the attentional cue was 100% valid. On the other half of the trials (neutral trials), a black contour framing the entire texture display indicated that the target, if present, was equally likely to appear at any of the 25 possible locations; this encouraged observers to distribute their attention across all possible locations. To allow observers to readily associate each symbolic cue with a specific target location, we placed the target center at one of these eccentricities: 0.0°, 1.2°, 3.6°, or 6.0° (Session A) and 0.0°, 2.4°, 4.8°, or 7.2° (Session B; Fig. 2b). Sessions A and B occurred with equal frequency and were interleaved. A schematic of the tested locations and their corresponding symbolic cues was presented at the beginning of each block. Performance at each eccentricity was averaged across the four quadrants. To equate the number of trials at all eccentricities, we tested the three eccentric locations twice more than the central location (0°) within a session.
During practice (one to two sessions), participants were familiarized with the task without adaptation and trained to associate the symbolic cues to each designated location. Each participant completed 16 blocks (42 trials/block) for each of the three adaptation conditions, for a total of 2,016 trials, which were completed in four experimental sessions. To avoid carryover effects of adaptation, we tested only one adaptation condition within each experimental session on separate days, and the order was counterbalanced across observers. There were 48 trials for each of the 42 conditions of attention (valid or neutral), adaptation (baseline, low SF, or high SF), and eccentricity (seven possibilities) conditions.
Analysis
For each observer, performance, d′ = z(hit rate) minus z(false alarm rate), was assessed across experimental sessions for each eccentricity, attention, and adaptation condition, and within-subjects analyses of variance (ANOVAs) were used to assess their effects on sensitivity (d′). In all cases in which Mauchly’s test of sphericity indicated a violation of the sphericity assumption, Greenhouse-Geisser-corrected values were used. We fitted the performance pattern using second-order polynomials and estimated the eccentricity at which performance peaked as well as the magnitude of the CPD from the peak eccentricity to the central location (0°). Supplementary analyses were also conducted on mean reaction time (a secondary measure) and response criterion (see the Supplemental Material available online). For the eye-position analysis, raw data were converted to eye position in degrees of visual angle. Eye-position samples around the time of blinks (100 ms preceding and following a blink) were excluded from further analysis. The mean eye position during the fixation interval at the beginning of each trial served as a baseline and was subtracted from the mean eye position in each following interval to compensate for any slow drift in the measurements during each block. Saccades were detected using the standard EyeLink algorithm (combined velocity 30°/s and acceleration criterion 8,000°/s2). For each trial, we computed the mean eye position and gaze-to-fixation distance during stimulus presentation (except for 1.4% of the eye-position data, which were missing because of technical problems).
Results
First, we describe the expected effects of SF adaptation on texture-segmentation performance and how SF adaptation could interact with the effects of endogenous attention, depending on the underlying hypothesized mechanism. Then, we report how adapting to high SFs and low SFs affected performance in our study relative to the baseline (control) condition. Finally, we examine whether and how SF adaptation modulated the effects of endogenous attention.
Predicted effects of SF adaptation on texture segmentation and attention
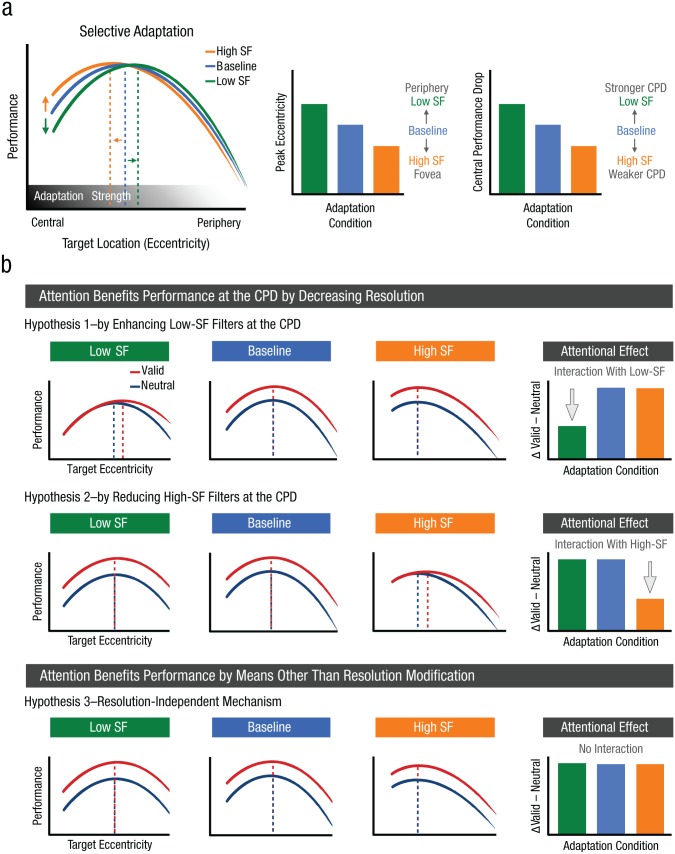
We predicted that when attention is distributed, adapting to high SFs should reduce sensitivity to high SFs and shift sensitivity toward lower frequencies, which should reduce the CPD and displace the performance peak closer to the fovea, consistent with decreased resolution of the visual system (Fig. 3a). Conversely, adapting to low SFs should reduce sensitivity to low SFs and shift sensitivity toward higher frequencies, which should exacerbate the CPD and displace the performance peak toward the periphery, consistent with increased resolution of the visual system. Adaptation effects should be informative at central locations, where the CPD and the differential effects of exogenous and endogenous attention occur. Given that texture-contrast sensitivity decreases with eccentricity because of the pronounced drop in sensitivity to the carrier noise (Hess, Baker, May, & Wang, 2008; Summers, Baker, & Meese, 2015), adaptation should become less effective at peripheral locations. Nonetheless, by altering the sensitivity to different SFs at central locations via adaptation, we were able to test whether and how endogenous attention benefits performance where resolution is too high (i.e., at the CPD).
Fig. 3.
Schematic predictions of spatial-frequency (SF) adaptation effects on (a) texture segmentation and (b) how SF adaptation interacts with attention. Relative to a baseline condition, high-SF adaptation should reduce the central performance drop (CPD) and shift peak performance closer to the fovea (a). Conversely, low-SF adaptation should accentuate the CPD and shift peak performance toward the periphery. Adaptation should be stronger at central locations and fade with eccentricity because of reduced sensitivity to the adaptors at peripheral locations. The bar graphs illustrate predicted changes in peak eccentricity and CPD in each adaptation condition. The graphs in (b) illustrate the expected effects of low-SF, baseline, and high-SF adaptation on attention according to each of three hypothesized attention mechanisms. Endogenous attention could decrease resolution to benefit performance at the CPD via two mechanisms: by enhancing the sensitivity of low-SF filters (Hypothesis 1) or by reducing the sensitivity of high-SF filters (Hypothesis 2). Alternatively, attention could benefit performance without affecting resolution (e.g., by improving the signal-to-noise ratio at all eccentricities) and would not interact with adaptation (Hypothesis 3). The bar graphs (right-most column) show the predicted pattern of attentional benefits for the three adaptation conditions, which would differ for the three hypotheses. In both panels, the dashed vertical lines highlight the eccentricity at which peak performance would be expected.
Whether and how SF adaptation interacts with attention should reveal the mechanism by which endogenous attention benefits performance at the CPD (Fig. 3b). Enhanced spatial resolution is the only explanation that can account for the performance impairment caused by exogenous attention at the CPD (Carrasco et al., 2006; Carrasco & Yeshurun, 2009; Talgar & Carrasco, 2002; Yeshurun & Carrasco, 1998, 2000; Yeshurun et al., 2008). Here, we hypothesized two possible attentional mechanisms that can benefit performance at central locations by decreasing spatial resolution in different ways, as well as a third resolution-independent mechanism:
Hypothesis 1: Endogenous attention can decrease resolution at the CPD by enhancing the sensitivity of low-SF filters (Fig. 3b, top row). Adaptation to low SFs would reduce the availability of low-SF filters and reduce the attentional benefit at central locations. However, high-SF adaptation would not modulate the effect of attention on resolution. Thus, adapting to low SFs, but not to high SFs, will interact with attention and reduce the central attentional benefit.
Hypothesis 2: Endogenous attention can decrease resolution at the CPD by reducing the sensitivity of high-SF filters (Fig. 3b, middle row). Adaptation to high SFs would reduce the availability of high-SF filters and preclude the effect of attention at central locations. However, low-SF adaptation would not modulate the effect of attention on resolution. Hence, adapting to high SFs, but not to low SFs, will interact with attention and reduce the central attentional benefit.
Hypothesis 3: Endogenous attention affects performance by means other than resolution modulation (Fig. 3b, bottom row). This hypothesis posits that attention benefits performance via a resolution-independent mechanism (e.g., by increasing the signal-to-noise ratio) that would benefit performance regardless of the resolution constraints and of the SF-adaptation condition. Therefore, adaptation and attention would not interact, and attention would benefit performance similarly across adaptation conditions.
SF adaptation affects performance in a manner consistent with changes in resolution
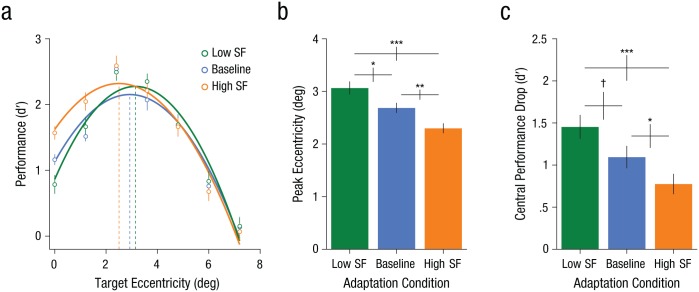
To assess the effects of adaptation, we examined how it affected performance for the distributed-attention condition (neutral trials only) in the three different adaptation conditions (Fig. 4a). In the baseline condition, we found the expected performance pattern: Performance peaked at midperiphery and diminished at both central and peripheral locations (Carrasco et al., 2006; Kehrer, 1989; Morikawa, 2000; Talgar & Carrasco, 2002; Yeshurun & Carrasco, 1998, 2000; Yeshurun et al., 2008). A two-way repeated measures ANOVA (3 adaptation conditions × 7 eccentricities) on neutral d′ revealed main effects of adaptation, F(1.31, 14.37) = 4.26, p = .049, ηp2 = .28, and eccentricity, F(6, 66) = 40.73, p < .001, ηp2 =.79, as well as a significant Adaptation × Eccentricity interaction, F(12, 132) = 2.85, p = .002, ηp2 = .21. The performance pattern across eccentricities was well described by second-order polynomials in all three adaptation conditions—baseline (neutral trials: R2 = .91, valid trials: R2 = .94), low SF (neutral trials: R2 = .94, valid trials: R2 = .97), and high SF (neutral trials: R2 = .96, valid trials: R2 = .95).
Fig. 4.
Effects of spatial-frequency (SF) adaptation in the neutral attention condition. Mean performance (a) is shown at each of the seven target eccentricities as a function of adaptation condition. For each condition, the curves correspond to the best-fitting second-order polynomials (all R2s > .9), and the dashed vertical lines highlight the eccentricity at which the peak performance occurred. Estimates for mean peak eccentricity (b) and mean central performance drop (c) are shown as a function of adaptation condition. Symbols indicate significant (*p < .05, **p < .01, ***p < .001) and marginal (†p < .10) differences between conditions. Error bars for means represent ±1 SEM within subjects (Morey, 2008), and error bars for the differences between condition means represent ±1 SEM.
We estimated the eccentricity of the performance peak and the magnitude of the CPD for each participant using second-order polynomial fits. Adaptation significantly affected the peak eccentricity, F(2, 22) = 19.71, p < .001, ηp2 = .64 (Fig. 4b). Relative to the baseline condition (2.69°), adapting to high SFs shifted the peak closer to the fovea (2.30°), t(11) = 4.10, p = .0018, Cohen’s d = 1.26, whereas adapting to low SFs shifted the peak toward the periphery (3.07°), t(11) = 2.81, p = .017, Cohen’s d = 1.07. The effects of low- and high-SF adaptation differed significantly from each other, t(11) = 5.78, p < .001, Cohen’s d = 1.83. Moreover, adaptation significantly affected the magnitude of the CPD, F(2, 22) = 9.93, p = .001, ηp2 = .47 (Fig. 4c). The CPD was estimated from the fits by taking the difference in performance (d′) between the peak eccentricity (maximum d′) and 0° eccentricity. Adapting to high SFs and low SFs had a differential effect on the magnitude of the CPD, t(11) = 4.47, p < .001, Cohen’s d = 1.35. Relative to the baseline condition (∆d′ = −1.10), the CPD was reduced with high-SF adaptation (∆d′ = −0.78), t(11) = 2.28, p = .0438, Cohen’s d = 0.67, and was more pronounced with low-SF adaptation (∆d′ = −1.46), t(11) = 2.18, p = .052, Cohen’s d = 0.63.
To summarize, when attention was distributed across the visual field, selective SF adaptation affected performance in accordance with changes in the visual system’s resolution, shifting the performance peak and modulating the magnitude of the CPD. These findings are consistent with the predicted effects of adaptation on texture segmentation at central locations where resolution is too high (Fig. 3a).
Selective adaptation to high SFs interacts with attention
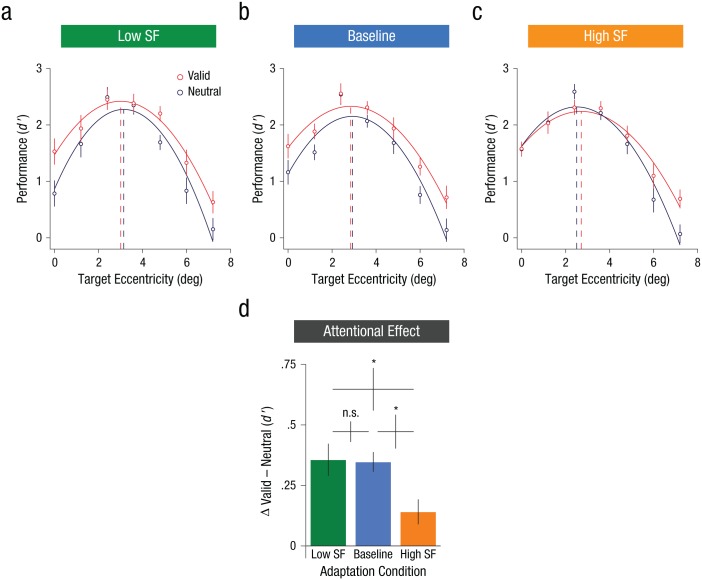
A within-subjects three-way ANOVA (3 adaptation conditions × 2 attention conditions × 7 eccentricities) on d′ indicated that all the two-way interactions were significant: Adaptation had a stronger effect at central than peripheral locations, F(12, 132) = 2.14, p = .018, ηp2 = .16; attention varied across locations, F(6, 66) = 2.84, p = .016, ηp2 = .21; and more important, attention and adaptation interacted, F(1.33, 14.6) = 6.16, p = .019, ηp2 = .36), with attention resulting in stronger improvements for both the baseline and the low-SF adaptation conditions than for the high-SF adaptation condition.
To explore how adaptation modulated the effects of attention, we compared performance in the valid and neutral conditions, separately for the three adaptation conditions (Fig. 5). In the baseline condition (Fig. 5b), performance varied as a function of eccentricity, F(3.05, 33.6) = 34.22, p < .001, ηp2 = .76, and attention significantly benefited performance across eccentricities, shifting the performance function upward, F(1, 11) = 72.03, p < .001, ηp2 = .87, without interacting with eccentricity, F(6, 66) = 1.08, p = .38, ηp2 = .09, which confirms previous findings (Yeshurun et al., 2008). Similarly, in the low-SF adaptation condition (Fig. 5a), there were significant main effects of eccentricity, F(3.52, 38.75) = 28.79, p < .001, ηp2 = .72, and attention, F(1, 11) = 28.76, p < .001, ηp2 = .72, with no interaction between them, F(3.42, 37.67) = 1.91, p = .137, ηp2 = .15. However, in the high-SF adaptation condition (Fig. 5c), there were significant effects of eccentricity, F(6, 66) = 40.38, p < .001, ηp2 = .79, and attention, F(1, 11) = 7.40, p = .02, ηp2 = .40, as well as a significant interaction between them, F(6, 66) = 2.98, p = .012, ηp2 = .21. Specifically, after observers adapted to high SFs, there was no effect of attention at central or parafoveal locations, only at the most peripheral locations, where adaptation was less effective. Overall, the attentional benefit across eccentricities was significantly reduced after observers adapted to high SFs relative to both the baseline condition, t(11) = 2.95, p = .013, Cohen’s d = 0.86, and the low-SF condition, t(11) = 2.45, p = .032, Cohen’s d = 0.71, which did not differ from each other, t(11) = 0.21, p = .84 (Fig. 5d). Supplementary analyses indicated that eye movements, reaction times, and response criterion were not responsible for these findings (see the Supplemental Material).
Fig. 5.
Effects of attention on performance as a function of spatial-frequency (SF) adaptation. Mean performance at each of the seven target eccentricities is shown as a function of trial type, separately for (a) the low-SF adaptation condition, (b) the baseline adaptation condition, and (c) the high-SF adaptation condition. For each condition, the curves correspond to the best-fitting second-order polynomials (all R2s > .9), and the dashed vertical lines highlight the eccentricity at which the peak performance occurred. Change in performance between valid and neutral trials (d) is shown as a function of adaptation condition. The asterisks indicate significant differences between conditions (p < .05). Error bars for means represent ±1 SEM within subjects (Morey, 2008), and error bars for the differences between condition means represent ±1 SEM.
These results show that adapting to high SFs but not to low SFs modulated the effects of endogenous attention, which supports the idea that high-SF filters mediate the effects of attention on resolution. We tested whether a simpler model that allows only upward and downward shifts of the neutral performance pattern—without any change in the shape of the second-order polynomial function—could account for the attentional effects as well as a full model could. Consistent with a benefit at all eccentricities, nested F tests showed that the effects of attention in both the baseline and low-SF adaptation conditions could be explained by a simple upward shift of the neutral function with attention (baseline: p = .363; low SF: p = .145). However, this restricted model was ruled out in the high-SF condition (p = .039), which indicates that adapting to high SFs disrupted the effects of endogenous attention unevenly across eccentricities.
To summarize, our results support Hypothesis 2: Endogenous attention benefited performance at central locations, where resolution was too high, by reducing the sensitivity of high-SF filters and thus decreasing the visual system’s resolution (Fig. 3b).
Discussion
This study advances the field’s understanding of the mechanisms by which attention modulates perception. Using selective SF adaptation, we provided new evidence that texture-segmentation performance depends on resolution constraints at varying eccentricities (i.e., the mismatch between the scale of the texture and the preferred SF of the filters mediating texture analysis at a given eccentricity) and that endogenous attention affects resolution by selectively modulating high-SF filters. Consistent with Yeshurun et al.’s (2008) study, our results showed that endogenous attention improved performance across eccentricities in the baseline condition. This attentional benefit was mitigated at central and parafoveal locations after observers adapted to high SFs, but not after they adapted to low SFs. This finding reveals that endogenous attention benefits performance at the CPD, where resolution is too high, by decreasing resolution and does so by modulating high-SF filters (Fig. 3b, Hypothesis 2). An attentional benefit mediated by means other than resolution changes cannot explain this result, which rules out Hypothesis 3 (Fig. 3b). These findings can qualitatively account for a flexible attentional mechanism that benefits performance by increasing or decreasing the visual system’s resolution depending on task demands.
Texture-segmentation tasks that probe specific resolution constraints at varying eccentricities enable a critical test of the hypothesis that endogenous attention can adjust resolution to benefit performance. Exogenous attention automatically enhances resolution, improving performance at central locations where the resolution is too low (small texture scales) but impairing performance at the exact same eccentricity for larger texture scales for which enhancing resolution is detrimental (Carrasco et al., 2006; Talgar & Carrasco 2002; Yeshurun & Carrasco, 1998, 2000). Endogenous attention, however, improves performance regardless of whether increased or decreased resolution is beneficial. Our findings support an attentional-resolution mechanism that not only can increase resolution but also can decrease resolution when beneficial for the task at hand. This finding reveals that endogenous attention operates via a flexible mechanism that adjusts its operation to different resolution constraints, increasing or decreasing resolution depending on task demands. Specifically, high-SF filters mediate the effects of endogenous attention on resolution: Weakening high-SF filters via adaptation affected performance similarly to a decrease in resolution and concurrently mitigated the attention benefits at central locations. These results suggest that when high-SF filters are available, endogenous attention can increase or decrease resolution by, respectively, enhancing or suppressing their contribution. This finding provides the first behavioral evidence that attention can benefit performance by decreasing the visual system’s resolution.
The adaptation protocol we used effectively manipulated SF availability at central locations, allowing us to pinpoint the underlying mechanism by which endogenous attention benefits performance in resolution tasks, even when resolution is too high for the task at hand. As second-order sensitivity strongly depends on the sensitivity to the luminance-defined carrier (Hess et al., 2008; Summers et al., 2015), adaptation effects declined with eccentricity. The use of a high-SF (6 cycles/deg) luminance-defined carrier, for which sensitivity is reduced at peripheral locations, could explain the effectiveness drop of the second-order adaptors at eccentric locations. Future investigations could attempt to equate second-order adaptation effects across eccentricities by scaling both first- and second-order signals with eccentricity (Vakrou, Whitaker, & McGraw, 2007). This inherent difficulty to equate second-order signals across eccentricity limited our ability to assess the mechanisms of endogenous attention in the periphery using adaptation. Nonetheless, endogenous attention seems to operate at peripheral locations by increasing the sensitivity of high-SF filters. There is compelling evidence that both exogenous and endogenous attention increase resolution at peripheral locations in tasks that benefit from heightened resolution, such as visual search (Carrasco & Yeshurun, 1998), Landolt acuity (Carrasco et al., 2002; Montagna et al., 2009), and texture segmentation (Carrasco et al., 2006; Talgar & Carrasco, 2002; Yeshurun & Carrasco, 1998, 2000; Yeshurun et al., 2008). Given that poor resolution with eccentricity limits performance in most visual tasks, it would be parsimonious for higher-SF filters to be primarily responsible for the attentional-resolution enhancement in the periphery, which reduces differences in perceptual representations across the visual field.
Our findings uncover important differences between exogenous and endogenous attention, which are mediated by partially distinct neural substrates (Corbetta, Patel, & Shulman, 2008). Both types of attention affect resolution by modulating high-SF filters, but the difference lies in the flexibility of their mechanisms. Exogenous attention automatically increases resolution by enhancing high SFs at the attended area (Carrasco et al., 2006; Megna, Rocchi, & Baldassi, 2012; Yeshurun & Levy, 2003; Yeshurun & Sabo, 2012). Like exogenous attention, endogenous attention affects resolution by modulating high-SF filters. However, unlike exogenous attention, endogenous attention can actually reduce their contribution to decrease resolution when beneficial. By preferentially adjusting the activity of the high-SF subpopulation at a given eccentricity, endogenous attention can optimize resolution according to the resolution constraints. This result provides further evidence for a division of labor between a fast and automatic exogenous component and a slower but more flexible endogenous mechanism that adjusts its operation to stimulus constraints and task demands (Barbot, Landy, & Carrasco, 2012; Giordano, McElree, & Carrasco, 2009; Hein, Rolke, & Ulrich, 2006; Yeshurun et al., 2008).
The present study lends psychological reality to the neurophysiological evidence that voluntary attention affects resolution (Anton-Erxleben & Carrasco, 2013). Attention modulates neuronal responses and alters receptive fields’ position by shifting their centers toward the attended location. Attention also modulates receptive field’s effective size, with smaller receptive fields when attention is directed inside the receptive field than when attention is directed outside the receptive field (Anton-Erxleben, Stephan, & Treue, 2009; Womelsdorf, Anton-Erxleben, Pieper, & Treue, 2006). Receptive-field shift and shrinkage lead to more and smaller receptive fields at the attended location than at unattended locations, which could enhance resolution (Anton-Erxleben & Carrasco, 2013). Conversely, when attention is directed next to a receptive field, the receptive field expands (Anton-Erxleben et al., 2009), which potentially decreases resolution. Furthermore, attention inside the receptive field modulates length tuning in an eccentricity-dependent manner in V1, which decreases the preferred length of parafoveal receptive fields and increases the preferred length of peripheral receptive fields (Roberts, Delicato, Herrero, Gieselmann, & Thiele, 2007).
In human functional MRI studies, attention decreases the spatial overlap in blood-oxygen-level-dependent responses of adjacent locations, narrowing the population’s integration area and heightening resolution at the attended location (Fischer & Whitney, 2009). Conversely, withdrawing attention from the periphery results in larger peripheral population receptive fields, which could result in blurrier perceptual representations (de Haas, Schwarzkopf, Anderson, & Rees, 2014). Moreover, attention attracts population receptive fields toward the attentional focus across the visual field and throughout cortical areas (Klein, Harvey, & Dumoulin, 2014). Our study is also relevant for several computational models in which ways that attention can affect resolution have been implemented on the basis of neurophysiological and psychophysical findings (e.g., Miconi & VanRullen, 2016; Womelsdorf, Anton-Erxleben, & Treue, 2008).
Interestingly, a preferential gain enhancement of high-SF V1 neurons was observed in mice during locomotion (Mineault, Tring, Trachtenberg, & Ringach, 2016) and linked to heightened resolution brought about by attention in humans (Barbot, 2016; Carrasco & Yeshurun, 2009). V1 neurons tuned to different SFs had different operating points, which resulted in larger gain modulations for high-SF than low-SF neurons and in enhanced resolution during locomotion. This finding may account for the fact that both exogenous and endogenous attention operate on resolution by preferentially modulating high-SF neurons (Barbot, 2016).
To conclude, the present study illustrates how psychophysics methods, such as selective adaptation, are capable of revealing specific underlying perceptual mechanisms as well as how cognitive processes, such as visual attention, modulate them. Consistent with the finding that humans can selectively attend to the most informative neuronal population (Verghese, Kim, & Wade, 2012), our results portray endogenous attention as an adaptive mechanism that can swiftly modify resolution according to task demands. Whether resolution becomes finer or coarser with attention depends on the resolution constraints—that is, the relation between the scale of the relevant information and the visual system’s resolution at that location. When beneficial, endogenous attention can decrease resolution by reducing the contribution of high-SF filters, which supports the existence of an attentional mechanism that optimizes the visual system’s resolution and benefits performance according to task demands.
Supplementary Material
Acknowledgments
We thank B. Montagna and J. Gill for preliminary work, and N. Graham, M. Landy, S. Offen, J. Winawer, Y. Yeshurun, and members of the Carrasco lab for comments.
Footnotes
Action Editor: Edward S. Awh served as action editor for this article.
Declaration of Conflicting Interests: The authors declared that they had no conflicts of interest with respect to their authorship or the publication of this article.
Funding: M. Carrasco was supported by Grant No. NIH-R01-EY016200 from the National Institutes of Health.
Supplemental Material: Additional supporting information can be found at http://journals.sagepub.com/doi/suppl/10.1177/0956797616679634
References
- Abrams J., Barbot A., Carrasco M. (2010). Voluntary attention increases perceived spatial frequency. Attention, Perception, & Psychophysics, 72, 1510–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton-Erxleben K., Carrasco M. (2013). Attentional enhancement of spatial resolution: Linking behavioural and neurophysiological evidence. Nature Reviews Neuroscience, 14, 188–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton-Erxleben K., Henrich C., Treue S. (2007). Attention changes perceived size of moving visual patterns. Journal of Vision, 7(11), Article 5. doi: 10.1167/7.11.5 [DOI] [PubMed] [Google Scholar]
- Anton-Erxleben K., Stephan V. M., Treue S. (2009). Attention reshapes center-surround receptive field structure in macaque cortical area MT. Cerebral Cortex, 19, 2466–2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbot A. (2016). How attention enhances spatial resolution: Preferential gain enhancement of high spatial frequency neurons. Journal of Neuroscience, 36, 12080–12082. doi: 10.1523/JNEUROSCI.2691-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbot A., Landy M. S., Carrasco M. (2012). Differential effects of endogenous and exogenous attention on second-order texture contrast sensitivity. Journal of Vision, 12(8), Article 6. doi: 10.1167/12/8/6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco M. (2011). Visual attention: The past 25 years. Vision Research, 51, 1484–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco M., Loula F., Ho Y. X. (2006). How attention enhances spatial resolution: Evidence from selective adaptation to spatial frequency. Perception & Psychophysics, 68, 1004–1012. [DOI] [PubMed] [Google Scholar]
- Carrasco M., Williams P. E., Yeshurun Y. (2002). Covert attention increases spatial resolution with or without masks: Support for signal enhancement. Journal of Vision, 2(6), Article 4. doi: 10.1167/2.6.4 [DOI] [PubMed] [Google Scholar]
- Carrasco M., Yeshurun Y. (1998). The contribution of covert attention to the set-size and eccentricity effects in visual search. Journal of Experimental Psychology: Human Perception and Performance, 24, 673–692. [DOI] [PubMed] [Google Scholar]
- Carrasco M., Yeshurun Y. (2009). Covert attention effects on spatial resolution. Progress in Brain Research, 176, 65–86. [DOI] [PubMed] [Google Scholar]
- Corbetta M., Patel G., Shulman G. L. (2008). The reorienting system of the human brain: From environment to theory of mind. Neuron, 58, 306–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Haas B., Schwarzkopf D. S., Anderson E. J., Rees G. (2014). Perceptual load affects spatial tuning of neuronal populations in human early visual cortex. Current Biology, 24(2), R66–R67. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- DeValois R. L., DeValois K. K. (1988). Spatial vision. New York, NY: Oxford University Press. [Google Scholar]
- Fischer J., Whitney D. (2009). Attention narrows position tuning of population responses in V1. Current Biology, 19, 1356–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano A. M., McElree B., Carrasco M. (2009). On the automaticity and flexibility of covert attention: A speed-accuracy trade-off analysis. Journal of Vision, 9(3), Article 30. doi: 10.1167/9.3.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobell J., Carrasco M. (2005). Attention alters the appearance of spatial frequency and gap size. Psychological Science, 16, 644–651. [DOI] [PubMed] [Google Scholar]
- Golla H., Ignashchenkova A., Haarmeier T., Thier P. (2004). Improvement of visual acuity by spatial cueing: A comparative study in human and non-human primates. Vision Research, 44, 1589–1600. [DOI] [PubMed] [Google Scholar]
- Gurnsey R., Pearson P., Day D. (1996). Texture segmentation along the horizontal meridian: Nonmonotonic changes in performance with eccentricity. Journal of Experimental Psychology: Human Perception and Performance, 22, 738–757. [DOI] [PubMed] [Google Scholar]
- Hallum L. E., Landy M. S., Heeger D. J. (2011). Human primary visual cortex (V1) is selective for second-order spatial frequency. Journal of Neurophysiology, 105, 2121–2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein E., Rolke B., Ulrich R. (2006). Visual attention and temporal discrimination: Differential effects of automatic and voluntary cueing. Visual Cognition, 13, 29–50. [Google Scholar]
- Hess R. F., Baker D. H., May K. A., Wang J. (2008). On the decline of 1st and 2nd order sensitivity with eccentricity. Journal of Vision, 8(1), Article 19. doi: 10.1167/8.1.19 [DOI] [PubMed] [Google Scholar]
- Jones J. P., Palmer L. A. (1987). An evaluation of the two-dimensional Gabor filter model of simple receptive fields in cat striate cortex. Journal of Neurophysiology, 58, 1233–1258. [DOI] [PubMed] [Google Scholar]
- Kehrer L. (1989). Central performance drop on perceptual segregation tasks. Spatial Vision, 4, 45–62. [DOI] [PubMed] [Google Scholar]
- Kehrer L. (1997). The central performance drop in texture segmentation: A simulation based on a spatial filter model. Biological Cybernetics, 77, 297–305. [Google Scholar]
- Kehrer L., Meinecke C. (2003). A space-variant filter model of texture segregation: Parameter adjustment guided by psychophysical data. Biological Cybernetics, 88, 183–200. [DOI] [PubMed] [Google Scholar]
- Klein B. P., Harvey B. M., Dumoulin S. O. (2014). Attraction of position preference by spatial attention throughout human visual cortex. Neuron, 84, 227–237. [DOI] [PubMed] [Google Scholar]
- Megna N., Rocchi F., Baldassi S. (2012). Spatio-temporal templates of transient attention revealed by classification images. Vision Research, 54, 39–48. [DOI] [PubMed] [Google Scholar]
- Miconi T., VanRullen R. (2016). A feedback model of attention explains the diverse effects of attention on neural firing rates and receptive field structure. PLoS Computational Biology, 12(2), Article e1004770. doi: 10.1371/journal.pcbi.1004770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineault P. J., Tring E., Trachtenberg J. T., Ringach D. L. (2016). Enhanced spatial resolution during locomotion and heightened attention in mouse primary visual cortex. Journal of Neuroscience, 36, 6382–6392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagna B., Pestilli F., Carrasco M. (2009). Attention trades off spatial acuity. Vision Research, 49, 735–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morey R. D. (2008). Confidence intervals from normalized data: A correction to Cousineau (2005). Tutorial in Quantitative Methods for Psychology, 4(2), 61–64. [Google Scholar]
- Morikawa K. (2000). Central performance drop in texture segmentation: The role of spatial and temporal factors. Vision Research, 40, 3517–3526. [DOI] [PubMed] [Google Scholar]
- Potechin C., Gurnsey R. (2003). Backward masking is not required to elicit the central performance drop. Spatial Vision, 16, 393–406. [DOI] [PubMed] [Google Scholar]
- Roberts M., Delicato L. S., Herrero J., Gieselmann M. A., Thiele A. (2007). Attention alters spatial integration in macaque V1 in an eccentricity-dependent manner. Nature Neuroscience, 10, 1483–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers R. J., Baker D. H., Meese T. S. (2015). Area summation of first- and second-order modulations of luminance. Journal of Vision, 15(1), Article 12. doi: 10.1167/15.1.12 [DOI] [PubMed] [Google Scholar]
- Talgar C. P., Carrasco M. (2002). Vertical meridian asymmetry in spatial resolution: Visual and attentional factors. Psychonomic Bulletin & Review, 9, 714–722. [DOI] [PubMed] [Google Scholar]
- Vakrou C., Whitaker D., McGraw P. V. (2007). Extrafoveal viewing reveals the nature of second-order human vision. Journal of Vision, 7(14), Article 13. doi: 10.1167/7.14.13 [DOI] [PubMed] [Google Scholar]
- Verghese P., Kim Y. J., Wade A. R. (2012). Attention selects informative neural populations in human V1. Journal of Neuroscience, 32, 16379–16390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womelsdorf T., Anton-Erxleben K., Pieper F., Treue S. (2006). Dynamic shifts of visual receptive fields in cortical area MT by spatial attention. Nature Neuroscience, 9, 1156–1160. [DOI] [PubMed] [Google Scholar]
- Womelsdorf T., Anton-Erxleben K., Treue S. (2008). Receptive field shift and shrinkage in macaque middle temporal area through attentional gain modulation. Journal of Neuroscience, 28, 8934–8944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeshurun Y., Carrasco M. (1998). Attention improves or impairs visual performance by enhancing spatial resolution. Nature, 396, 72–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeshurun Y., Carrasco M. (2000). The locus of attentional effects in texture segmentation. Nature Neuroscience, 3, 622–627. [DOI] [PubMed] [Google Scholar]
- Yeshurun Y., Levy L. (2003). Transient spatial attention degrades temporal resolution. Psychological Science, 14, 225–231. [DOI] [PubMed] [Google Scholar]
- Yeshurun Y., Montagna B., Carrasco M. (2008). On the flexibility of sustained attention and its effects on a texture segmentation task. Vision Research, 48, 80–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeshurun Y., Rashal E. (2010). Precueing attention to the target location diminishes crowding and reduces the critical distance. Journal of Vision, 10(10), Article 16. doi: 10.1167/10.10.16 [DOI] [PubMed] [Google Scholar]
- Yeshurun Y., Sabo G. (2012). Differential effects of transient attention on inferred parvocellular and magnocellular processing. Vision Research, 74, 21–29. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.