Abstract
BACKGROUND
A protein that is expressed on capillary endothelial cells, called GPIHBP1 (glycosylphosphatidylinositol-anchored high-density lipoprotein binding protein 1), binds lipoprotein lipase and shuttles it to its site of action in the capillary lumen. A deficiency in GPIHBP1 prevents lipoprotein lipase from reaching the capillary lumen. Patients with GPIHBP1 deficiency have low plasma levels of lipoprotein lipase, impaired intravascular hydrolysis of triglycerides, and severe hypertriglyceridemia (chylomicronemia). During the characterization of a monoclonal antibody–based immunoassay for GPIHBP1, we encountered two plasma samples (both from patients with chylomicronemia) that contained an interfering substance that made it impossible to measure GPIHBP1. That finding raised the possibility that those samples might contain GPIHBP1 autoantibodies.
METHODS
Using a combination of immunoassays, Western blot analyses, and immunocytochemical studies, we tested the two plasma samples (as well as samples from other patients with chylomicronemia) for the presence of GPIHBP1 autoantibodies. We also tested the ability of GPIHBP1 autoantibodies to block the binding of lipoprotein lipase to GPIHBP1.
RESULTS
We identified GPIHBP1 autoantibodies in six patients with chylomicronemia and found that these autoantibodies blocked the binding of lipoprotein lipase to GPIHBP1. As in patients with GPIHBP1 deficiency, those with GPIHBP1 autoantibodies had low plasma levels of lipoprotein lipase. Three of the six patients had systemic lupus erythematosus. One of these patients who had GPIHBP1 autoantibodies delivered a baby with plasma containing maternal GPIHBP1 autoantibodies; the infant had severe but transient chylomicronemia. Two of the patients with chylomicronemia and GPIHBP1 autoantibodies had a response to treatment with immunosuppressive agents.
CONCLUSIONS
In six patients with chylomicronemia, GPIHBP1 autoantibodies blocked the ability of GPIHBP1 to bind and transport lipoprotein lipase, thereby interfering with lipoprotein lipase–mediated processing of triglyceride-rich lipoproteins and causing severe hypertriglyceridemia.
A protein in the lymphocyte antigen 6 (Ly6) superfamily, called GPIHBP1 (glycosylphosphatidylinositol-anchored high-density lipoprotein binding protein 1), is expressed on the surface of capillary endothelial cells. GPIHBP1 binds lipoprotein lipase in the interstitial spaces (where the lipase is secreted by myocytes and adipocytes) and shuttles it to its site of action in the capillary lumen.1,2 In patients with GPIHBP1 deficiency, lipoprotein lipase is mislocalized in the interstitial spaces and never reaches the capillary lumen. The absence of intraluminal lipoprotein lipase prevents the lipolytic processing of triglyceride-rich lipoproteins and results in severe hypertriglyceridemia (chylomicronemia, defined as a triglyceride level of >1000 mg per deciliter [>11.3 mmol per liter]).1,2 Many GPIHBP1 missense mutations that cause chylomicronemia have been identified.3–8 All these mutations disrupt the folding of the Ly6 domain of GPIHBP1 (the domain that binds lipoprotein lipase with high affinity) and block the ability of GPIHBP1 to bind lipoprotein lipase and transport it to the capillary lumen.3–8 A signature of GPIHBP1 deficiency in humans is low levels of lipoprotein lipase in plasma obtained either before or after the intravenous administration of heparin (preheparin and postheparin, respectively), a finding that reflects a virtual absence of lipoprotein lipase inside capillaries.3,4,7,9
We recently used monoclonal antibodies against human GPIHBP1 to create an enzyme-linked immunosorbent assay (ELISA) that could detect GPIHBP1 in human plasma.10 We encountered two plasma samples, both obtained from patients with chylomicronemia, that contained an interfering substance that prevented the measurement of GPIHBP1 in those samples or even the detection of recombinant GPIHBP1 that had been spiked into those samples. We hypothesized that such interference on ELISA might be caused by GPIHBP1 autoantibodies. We further hypothesized that these autoantibodies would prevent the binding of lipoprotein lipase to GPIHBP1 (i.e., the GPIHBP1-autoantibody syndrome) and thereby cause chylomicronemia. In this study, we report the presence of specific, high-titer GPIHBP1 autoantibodies in six patients with chylomicronemia and show that these antibodies block the binding of lipoprotein lipase to GPIHBP1.
METHODS
STUDY PATIENTS
The initial study cohort, which was selected to assist in the development of the ELISA analysis for GPIHBP1, included 23 patients who were known to have mutations in GPIHBP1 or LPL (the gene encoding lipoprotein lipase), 8 patients who had hypertriglyceridemia without mutations in GPIHBP1 or LPL, and 9 controls. After the identification of 2 patients who had GPIHBP1 autoantibodies (1 with systemic lupus erythematosus [SLE] and both with hypertriglyceridemia) in this initial cohort of 40 patients, we then screened another 162 patients for the presence of such autoantibodies. This group included 40 patients with SLE from the rheumatology clinic at the University of California, Los Angeles (half of whom were receiving immunosuppressive therapy), and 122 patients from lipid clinics (all with hypertriglyceridemia of unknown cause).
The 202 plasma samples from study patients were either archived specimens that had been obtained under institutional approval or samples without identifiers that were sent from other investigators to the last author. Thus, the university ethics committee deemed that the use of these samples was exempt from approval by institutional review boards. The numbers that are used to identify the patients throughout this report are the sample numbers recorded for specimen identification by the laboratory of the last author.
ELISA ANALYSES
To measure GPIHBP1 levels in plasma samples obtained from the study patients, we used a monoclonal antibody–based sandwich ELISA that is similar to those used to measure lipoprotein lipase and apolipoprotein B levels.11,12 In these analyses, 96-well ELISA plates were coated with the GPIHBP1-specific monoclonal antibody RF4.10 After the addition of plasma samples or known amounts of recombinant human GPIHBP1, bound GPIHBP1 was detected with horseradish peroxidase (HRP)–labeled GPIHBP1-specific monoclonal antibody RE3.10
To detect GPIHBP1 autoantibodies in plasma, 96-well plates were coated with human GPIHBP1 or GPIHBP1 containing an amino-terminal urokinase-type plasminogen activator receptor tag.8 After blocking with bovine serum albumin, plasma samples were added. After the plates were washed, antibody binding to GPIHBP1 was detected with HRP-labeled goat antihuman IgG and IgM. We determined the amount of GPIHBP1 autoantibodies (in arbitrary units) by comparing ELISA signals with those in a parallel ELISA in which plates were coated with known amounts of human IgG. We also performed ELISA analyses to test for autoantibodies against three other Ly6 proteins (CD177, C4.4A, and CD59) that were available for testing in our laboratory.
To test the ability of GPIHBP1 autoantibodies to block the binding of lipoprotein lipase to GPIHBP1, 96-well plates that were coated with GPIHBP1 were incubated with dilutions of plasma samples overnight at 4°C. Plates were then incubated for 1 hour at 4°C with V5-tagged human lipoprotein lipase (200 ng per well) (see Methods Section S1 in the Supplementary Appendix, available with the full text of this article at NEJM.org). After washing, an HRP-V5 antibody was added to quantify the amount of bound lipoprotein lipase. In parallel, an HRP-labeled goat antihuman IgG was added to document autoantibody binding to GPIHBP1. The presence of GPIHBP1 on the plates was verified with the HRP-labeled monoclonal antibody 11A12. As a control, we tested the ability of the monoclonal antibody RE310 to block the binding of lipoprotein lipase to GPIHBP1. Details regarding the ELISA analyses are provided in Methods Sections S2 through S5 in the Supplementary Appendix.
WESTERN BLOT ASSAY OF LIPOPROTEIN LIPASE BINDING TO GPIHBP1
Cells expressing S-protein–tagged GPIHBP1 were incubated with V5-tagged human lipoprotein lipase. After washing and lysis, cell extracts were fractionated according to size by means of sodium dodecyl sulfate–polyacrylamide-gel electrophoresis (SDS-PAGE), and the amount of lipoprotein lipase that was bound to cells was determined on Western blot analysis with a V5 antibody.13 To determine whether GPIHBP1 autoantibodies blocked the binding of lipoprotein lipase to GPIHBP1, the same assay was performed, except that transfected cells were preincubated with plasma samples containing GPIHBP1 autoantibodies. Additional details about the Western blot assays are provided in Methods Section S6 in the Supplementary Appendix.
IMMUNOCYTOCHEMICAL ANALYSIS
To visualize binding of GPIHBP1 autoantibodies, Chinese hamster ovary (CHO) cells expressing S-protein–tagged GPIHBP1 were incubated for 2 hours at 4°C with human plasma samples. After washing, cells were incubated with Alexa Fluor 488–conjugated goat antihuman IgG and IgM for 1 hour at 4°C and then fixed with paraformaldehyde. GPIHBP1 expression was assessed with the use of monoclonal antibody RG310 or with an antibody against the S-protein tag.
To test the ability of the GPIHBP1 autoantibodies to block lipoprotein lipase binding, S-protein–tagged GPIHBP1-transfected cells were incubated for 1 hour at 4°C with human plasma samples. After washing, the cells were incubated for 1 hour at 4°C with V5-tagged human lipoprotein lipase. After washing and fixation with methanol, the cells were stained with an Alexa Fluor 488–conjugated goat antihuman IgG and IgM, a rabbit antibody against the S-protein tag followed by an Alexa Fluor 647–conjugated donkey antirabbit IgG, and an Alexa Fluor 555–conjugated mouse anti-V5 antibody. Experimental details are provided in Methods Sections S7 and S8 in the Supplementary Appendix.
RESULTS
ELISA INTERFERENCE AND GPIHBP1 AUTOANTIBODIES
On the sandwich ELISA, GPIHBP1 levels in control plasma samples ranged from 239 to 1110 pg per milliliter, values that were much higher than those in two patients with a homozygous GPIHBP1 C89X mutation (3 pg per milliliter in Patient 11 and 6 pg per milliliter in Patient 15) and in a patient with a homozygous GPIHBP1 deletion7 (36 pg per milliliter in Patient 3) (Table S1 in the Supplementary Appendix).
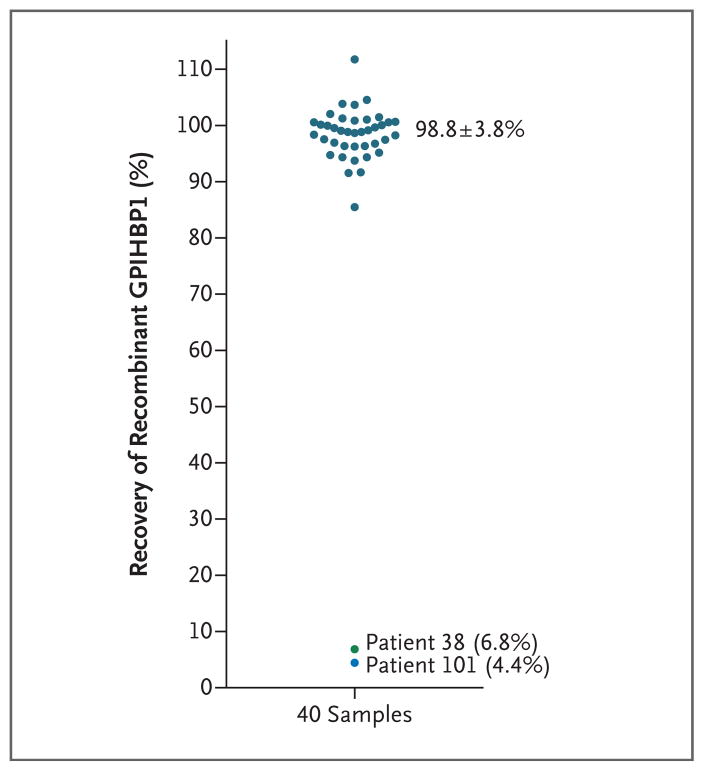
To validate the ELISA analysis, we spiked recombinant GPIHBP1 into 40 plasma samples. In 38 samples, the mean (±SD) recovery of spiked GPIHBP1 was 98.8±3.8%. However, in samples from two patients with chylomicronemia and low plasma GPIHBP1 levels (Patient 38 with 85 pg per milliliter and Patient 101 with 29 pg per milliliter), the recovery of spiked GPIHBP1 was extremely low (6.8% and 4.4%, respectively), which indicated assay interference (Fig. 1). Patient 38 was a 26-year-old man5 with severe hypertriglyceridemia (highest recorded triglyceride level, 5572 mg per deciliter [62.9 mmol per liter]) complicated by pancreatitis. Patient 101 was a 53-year-old Japanese woman who had a 25-year history of SLE and severe hypertriglyceridemia (triglyceride range, 500 to 5000 mg per deciliter [5.6 to 56.5 mmol per liter]) (Table S1 in the Supplementary Appendix). No LPL mutations were identified in either patient. Additional clinical information is provided in the Results section in the Supplementary Appendix.
Figure 1. GPIHBP1 Immunoassay Interference.
To validate the enzyme-linked immunosorbent assay (ELISA) analysis, recombinant GPIHBP1 was spiked into 40 plasma samples; the results of that analysis are shown here. (Details are provided in Methods Section S2 in the Supplementary Appendix.) Briefly, GPIHBP1 levels in 1:20 dilutions of plasma were measured before and after spiking of the sample with 62.5 pg of recombinant GPIHBP1. In 38 of 40 plasma samples, the mean (±SD) recovery of spiked recombinant GPIHBP1 was nearly complete (98.8±3.8%). In these samples, the mean GPIHBP1 values were 21.0±3.0 pg per milliliter before spiking with recombinant GPIHBP1 and 82.5±3.0 pg per milliliter after spiking. The recovery of recombinant GPIHBP1 in samples obtained from Patients 38 and 101 (two patients with chylomicronemia and low plasma GPIHBP1 levels) was very low (6.8% and 4.4%, respectively). The GPIHBP1 level in the plasma sample obtained from Patient 38 was 3.0 pg per milliliter in the 1:20 dilution and 4.5 pg per milliliter after the sample was spiked; in the sample obtained from Patient 101, the GPIHBP1 levels before and after spiking were 1.0 pg per milliliter and 3.0 pg per milliliter, respectively. Because of the low coefficient of variation in the ELISA analysis, a few of the samples had an apparent recovery rate that was slightly more than 100%.
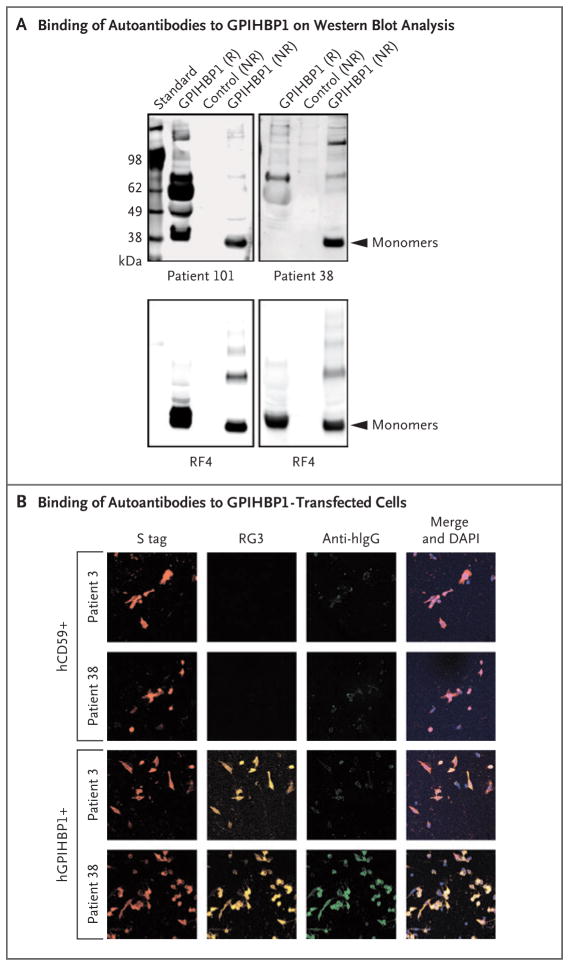
We suspected that the ELISA interference in samples obtained from Patients 38 and 101 was caused by GPIHBP1 autoantibodies. Indeed, GPIHBP1 autoantibodies were detected on Western blot analysis (Fig. 2A). ELISA analyses showed that the autoantibodies were IgG and not IgM (Fig. S1 in the Supplementary Appendix). The autoantibodies in the sample obtained from Patient 38 had avid binding to GPIHBP1-transfected CHO pgsA-745 cells but not to nontransfected cells nor to cells transfected with CD59 (another Ly6 protein); a control plasma sample (control sample 3) did not bind to GPIHBP1-transfected cells (Fig. 2B).
Figure 2. GPIHBP1 Autoantibodies in Plasma Samples Obtained from Patients 38 and 101.
Panel A shows the results of Western blot analysis of GPIHBP1 autoantibodies in plasma samples obtained from Patients 38 and 101. Proteins in the medium of GPIHBP1-transfected drosophila S2 cells were fractionated according to size by means of sodium dodecyl sulfate–polyacrylamide-gel electrophoresis (SDS-PAGE) under reducing (R) and nonreducing (NR) conditions. (Details are provided in Methods Section S9 in the Supplementary Appendix.) GPIHBP1 contains a tightly folded cysteine-rich domain (Ly6 domain) that is essential for the binding of lipoprotein lipase. The hypothesis was that some of the autoantibodies might bind to the Ly6 domain and that disrupting the disulfide bonds with reducing reagents might disrupt the epitope of some of the autoantibodies. The autoantibodies in plasma obtained from Patient 101 (containing 20 arbitrary units [AU] of GPIHBP1 autoantibodies per milliliter) bound to both reduced and nonreduced GPIHBP1; additional nonspecific binding was seen under reducing conditions. The autoantibodies in plasma obtained from Patient 38 (containing 20 AU of GPIHBP1 autoantibodies per milliliter) bound avidly only to nonreduced GPIHBP1. In the lower row, the panels show the same blots incubated with the GPIHBP1-specific monoclonal antibody RF4 (4 μg per milliliter) (see Methods Section S10 in the Supplementary Appendix). The monoclonal antibody RF4 binds to the acidic domain of GPIHBP1 and therefore binds both reduced and nonreduced human GPIHBP1; it also binds to GPIHBP1 dimers and multimers; the latter forms are artifacts of insect-cell overexpression and are due to inappropriate intermolecular disulfide bonds. The control culture medium was obtained from S2 cells that do not express human GPIHBP1. Panel B shows the results of an immunocytochemical experiment documenting that GPIHBP1 autoantibodies in plasma obtained from Patient 38 (1:20 dilution; green) bound to cells that had been transfected with an S-protein–tagged version of human GPIHBP1 (hGPIHBP1) but did not bind to nontransfected cells or to cells that were transfected with S-protein–tagged human CD59 (hCD59) (see Methods Section S7 in the Supplementary Appendix). CD59-transfected and GPIHBP1-transfected cells were detected with an antibody against the S-protein tag (red); GPIHBP1-expressing cells were also detected with a GPIHBP1-specific monoclonal antibody RG3 (orange). DNA was stained with 4,6-diamidino-2-phenylindole (DAPI, blue) to reveal all cells (both transfected and nontransfected) on the coverslip. The immunoglobulins in plasma obtained from Patient 3 (who was homozygous for a GPIHBP1 deletion) did not bind to GPIHBP1-transfected cells.
ADDITIONAL PLASMA SAMPLES WITH GPIHBP1 AUTOANTIBODIES
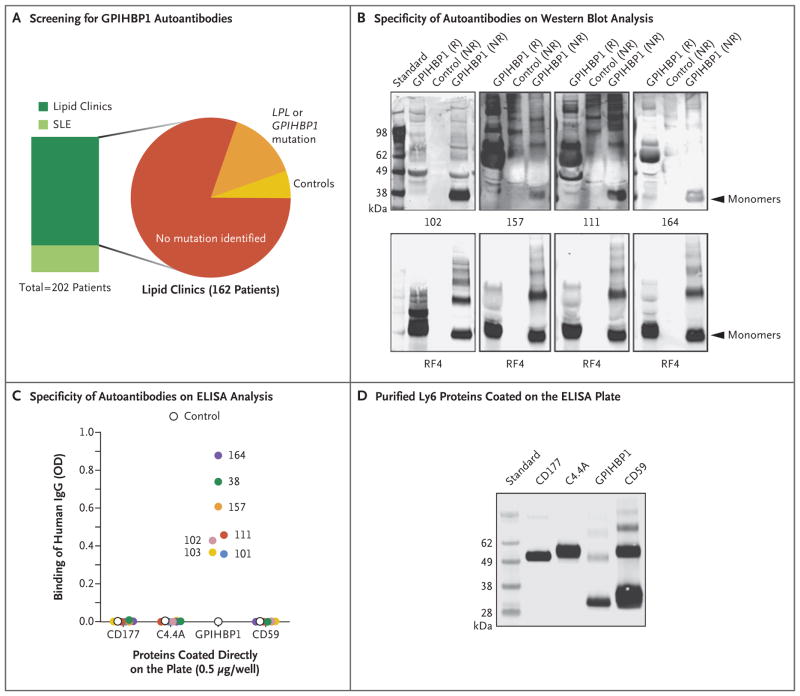
Using ELISA analysis, we screened 130 samples obtained from patients with unexplained hypertriglyceridemia (several of whom had SLE or Sjögren’s syndrome) for GPIHBP1 autoantibodies (Fig. 3A). We also screened plasma samples obtained from 40 patients with SLE, half of whom were receiving immunosuppressive therapy. None of the 40 patients with SLE had either severe hypertriglyceridemia or GPIHBP1 autoantibodies. Of the 130 patients with unexplained hypertriglyceridemia, 124 were negative for GPIHBP1 autoantibodies and 6 were positive (above-mentioned Patients 38 and 101, along with Patients 102, 111, 157, and 164).
Figure 3. Identification of Four Additional Plasma Samples with GPIHBP1 Autoantibodies.
Panel A summarizes the plasma samples screened for GPIHBP1 autoantibodies in 202 deidentified archived plasma samples from lipid clinics or a rheumatology clinic in which patients with systemic lupus erythematosus (SLE) were being treated. Forty patients with SLE, half of whom were receiving immunosuppressive therapy, were included; 10 of those patients had plasma triglyceride levels ranging from 350 to 750 mg per deciliter (4.0 to 8.5 mmol per liter). The 162 patients from various lipid clinics included 130 patients who had hypertriglyceridemia with no known genetic cause, 23 patients who had hypertriglyceridemia and a mutation in GPIHBP1 or LPL, and 9 controls. (Details are provided in Table S1 in the Supplementary Appendix.) Panel B shows Western blot analysis demonstrating that the immunoglobulins in plasma samples obtained from Patients 102, 157, 111, and 164 (containing 20 AU of GPIHBP1 autoantibody per milliliter) bind preferentially to nonreduced human GPIHBP1. The lower panels show the same blots incubated with the human GPIHBP1–specific monoclonal antibody RF4 (4 μg per milliliter). The monoclonal antibody RF4, which binds to reduced and nonreduced forms of GPIHBP1 and to GPIHBP1 multimers, was used as a loading control. The control culture medium was obtained from S2 cells that do not express human GPIHBP1. Panel C shows the results from ELISA analyses revealing that the immunoglobulin (IgG) in plasma samples obtained from Patients 38, 101, 102, 103, 111, 157, and 164 bind to wells of an ELISA plate coated with purified human GPIHBP1 but not to wells coated with other human Ly6 proteins (CD177, C4.4A, and CD59) (see Methods Section S4 in the Supplementary Appendix). The dilution was 1:12,500 for the sample obtained from Patient 102 and 1:500 for the other samples. A control plasma sample did not bind to any of the Ly6 proteins. The optical density (OD) was measured at a wavelength of 450 nm. Panel D shows Western blot analysis (performed with the use of monoclonal antibody R2416) of purified Ly6 proteins (CD177, C4.4A, GPIHBP1, and CD59) that had been fractionated according to size by means of SDS-PAGE. All four proteins had an amino-terminal urokinase-type plasminogen activator receptor (uPAR) tag that could be detected with monoclonal antibody R24. This Western blot analysis documents that similar amounts of all four Ly6 proteins were used to coat 96-well plates for the ELISA analysis shown in Panel C.
All the patients who were positive for GPIHBP1 antibodies had severe hypertriglyceridemia complicated by pancreatitis. (Clinical and laboratory details are provided in the Results section and Tables S1 and S2 in the Supplementary Appendix.) The hyperlipidemia in Patient 157, a 26-year-old woman with SLE, resolved with immunosuppressive drugs.14 When Patient 164, a 9-year-old girl with Sjögren’s syndrome,15 was treated with immunosuppressive drugs, the plasma triglyceride levels normalized and GPIHBP1 autoantibodies were no longer detectable in the plasma.15 Patient 102, a 38-year-old woman with SLE, delivered a baby girl; testing of blood samples from the baby (Patient 103) on the first day of life showed maternal GPIHBP1 autoantibodies and triglyceride levels of 9090 mg per deciliter (102.6 mmol per liter). By the time the child was 1 year of age, the plasma triglyceride levels had normalized to 72 mg per deciliter (0.8 mmol per liter).
Western blot analysis revealed GPIHBP1 autoantibodies in plasma samples obtained from Patients 102, 111, 157, and 164 (Fig. 3B). In these patients, the autoantibodies bound poorly to GPIHBP1 treated with reducing agents, which suggested that the epitopes for the autoantibodies required the proper conformation of the cysteine-rich Ly6 domain (the domain that mediates binding of lipoprotein lipase) in GPIHBP1. The plasma samples with GPIHBP1 autoantibodies that were obtained from Patients 38, 101, 102 (and her infant, Patient 103), 111, 157, and 164 were negative for autoantibodies against three other Ly6 proteins (CD177, C4.4A, and CD59) (Fig. 3C and 3D).16
GPIHBP1 AUTOANTIBODIES AND BINDING OF LIPOPROTEIN LIPASE
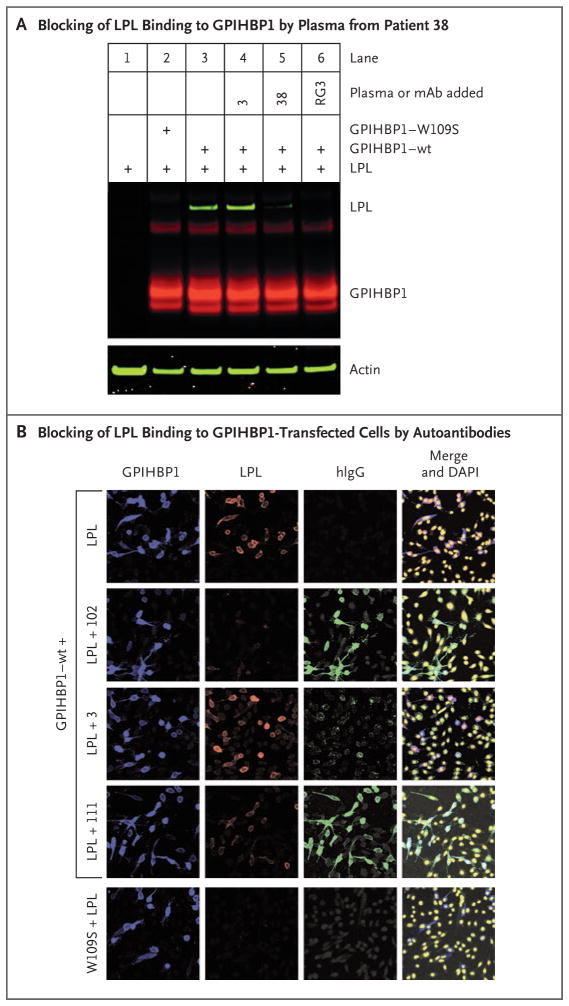
For GPIHBP1 autoantibodies to cause hypertriglyceridemia, they would need to block the binding of lipoprotein lipase to GPIHBP1. To explore that possibility, we tested the ability of one plasma sample containing GPIHBP1 autoantibody (from Patient 38) and a plasma sample without GPIHBP1 autoantibody (from Patient 3) as a control to block the binding of V5-tagged human lipoprotein lipase to GPIHBP1-expressing CHO cells. Only plasma from Patient 38 blocked binding of lipoprotein lipase, as judged by Western blot analyses of cell extracts (Fig. 4A). We also used immunocytochemical studies to test the ability of the GPIHBP1 autoantibodies to block the binding of lipoprotein lipase to the surface of GPIHBP1-transfected cells. Autoantibodies in plasma samples from Patients 102 and 111 blocked the binding of lipoprotein lipase to GPIHBP1 on the cell surface (Fig. 4B). The immunoglobulins in control plasma from Patient 3 had weak and nonspecific binding to cells and did not block lipoprotein lipase binding (Fig. 4B).
Figure 4. Blocking of Binding of Lipoprotein Lipase to GPIHBP1 by Autoantibodies.
Panel A shows a Western blot analysis of cell extracts from Chinese hamster ovary (CHO) cells transfected with S-protein–tagged wild-type (wt) GPIHBP1 or a mutant GPIHBP1 (W109S), which lacks the ability to bind lipoprotein lipase (LPL) (see Methods Section S6 in the Supplementary Appendix). The lipoprotein lipase that was used in these experiments was V5-tagged. Western blot analyses were performed with a goat antibody against the S-protein tag (Abcam, 5 μg per milliliter), followed by infrared dye (IRDye) 680–donkey antigoat IgG at 1:2000 dilution (red) and an IRDye 800-V5 antibody at 1:500 dilution (green). Actin was used as a loading control and was detected with a rabbit antibody against actin (Abcam, 5 μg per milliliter) followed by an IRDye 800–donkey antirabbit IgG at 1:2000 dilution (green). In lane 3, binding of lipoprotein lipase to wild-type GPIHBP1 is shown. In lane 4, a control plasma sample from Patient 3 did not block binding of lipoprotein lipase to wild-type GPIHBP1. In lane 5, preincubation of cells with plasma obtained from Patient 38 (1:20 dilution) blocked lipoprotein lipase binding. In lane 6, the GPIHBP1-specific monoclonal antibody (mAb) RG3 (20 μg per milliliter) also blocked the binding of lipoprotein lipase to wild-type GPIHBP1. In lane 2, mutant W109S was included as a control. Panel B shows an immunocytochemical study indicating that the binding of immunoglobulins (green) in plasma samples obtained from Patients 102 and 111 blocked the binding of lipoprotein lipase (red) to GPIHBP1-expressing cells (blue). Under the same conditions, the immunoglobulins in a control plasma sample from Patient 3 did not block binding of lipoprotein lipase to GPIHBP1-transfected cells. Cells expressing the W109S mutant did not bind lipoprotein lipase. DNA was stained with DAPI (yellow).
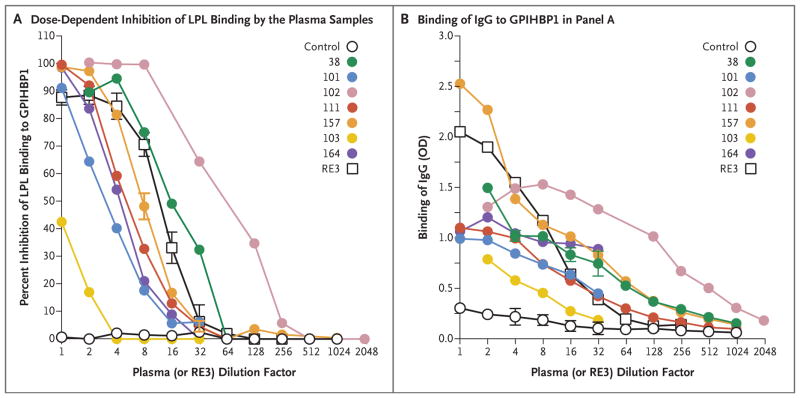
We performed ELISA analyses to assess the ability of GPIHBP1 autoantibodies to block the binding of lipoprotein lipase to GPIHBP1 and simultaneously determined autoantibody titers. Plasma samples obtained from Patients 38, 101, 102, 111, 157, and 164 blocked the binding of lipoprotein lipase in a dose-dependent fashion (Fig. 5A). Plasma from Patient 102 had the highest titers of autoantibodies against GPIHBP1 (Fig. 5B) and was most potent in blocking lipoprotein lipase binding. The sample from the infant of Patient 102 (Patient 103) also blocked the binding of lipoprotein lipase to GPIHBP1 (Fig. 5A), even though the titer of GPIHBP1 autoantibodies was less than 5% of that in plasma obtained from the infant’s mother (Fig. 5B, and Table S1 in the Supplementary Appendix).
Figure 5. Blocking of Binding of Lipoprotein Lipase to GPIHBP1 by Autoantibodies in a Dose-Dependent Manner.
Panel A shows the results of a solid-phase assay of binding of lipoprotein lipase to GPIHBP1 (see Methods Section S5 in the Supplementary Appendix). Briefly, ELISA plates were coated with the monoclonal antibody R24 and incubated with uPAR-tagged human GPIHBP1, followed by an overnight incubation with serial dilutions of human plasma samples or the GPIHBP1-specific monoclonal antibody RE3. The next day, after incubating the plates with V5-tagged human lipoprotein lipase, the amount of GPIHBP1-bound lipoprotein lipase was detected with a horseradish peroxidase (HRP)–labeled V5 antibody and compared with the amount of bound lipoprotein lipase in the absence of human plasma or monoclonal antibody RE3 (set at 100% binding). These ELISA studies showed that GPIHBP1 autoantibodies in the plasma of six adult patients with chylomicronemia (Patients 38, 101, 102, 111, 157, and 164) and the plasma of an infant of Patient 102 (Patient 103) blocked binding of lipoprotein lipase to GPIHBP1, as did the monoclonal antibody RE3. (The 1:1 dilution for monoclonal antibody RE3 corresponds to 20 μg per milliliter.) A control plasma sample did not block binding of lipoprotein lipase to GPIHBP1. Panel B shows the amount of IgG binding to GPIHBP1 for each of the dilutions tested in Panel A. Plasma samples obtained from Patients 38, 102, and 157 had the highest titers of GPIHBP1 autoantibody.
LIPOPROTEIN LIPASE IN PLASMA SAMPLES WITH GPIHBP1 AUTOANTIBODIES
The levels of lipoprotein lipase in control preheparin plasma samples range from 50 to 77 ng per milliliter.11 We measured preheparin plasma levels of lipoprotein lipase in patients who were homozygous for missense or nonsense mutations in GPIHBP1 and found them to be low in most cases (range, 7.3 to 70.6 ng per milliliter) (Table S1 in the Supplementary Appendix). These findings are similar to those in earlier studies3,4,7,9 and are consistent with impaired transport of lipoprotein lipase to the capillary lumen. One would predict that plasma levels of lipoprotein lipase in patients with GPIHBP1 autoantibodies would also be low if the GPIHBP1 autoantibodies truly caused the chylomicronemia (i.e., if the autoantibodies blocked lipoprotein lipase binding and transport). Indeed, the lipoprotein lipase levels in plasma samples containing the autoantibodies were low (range, 4.6 to 32.1 ng per milliliter) (Table S1 in the Supplementary Appendix), a finding that is consistent with impaired delivery of lipoprotein lipase to the capillary lumen.
DISCUSSION
We describe the discovery of autoantibodies against GPIHBP1, the protein that shuttles lipoprotein lipase to the capillary lumen, in the plasma of six patients with severe hypertriglyceridemia. Our data provide strong support for the conclusion that GPIHBP1 autoantibodies were the cause of the observed hypertriglyceridemia. First, the autoantibodies blocked the binding of lipoprotein lipase to GPIHBP1. Second, the plasma levels of lipoprotein lipase were very low in the patients with GPIHBP1 autoantibodies, which is consistent with impaired transport of lipoprotein lipase to the capillary lumen. Third, immunosuppressive drug therapy normalized the triglyceride levels in two of the six patients, Patients 157 and 164.14,15 Fourth, Patient 102 delivered a baby (Patient 103) who was born with GPIHBP1 autoantibodies. The infant had a plasma triglyceride level of 9090 mg per deciliter on the first day of life, but the hyperlipidemia steadily waned, which is consistent with a gradual disappearance of maternal IgGs.
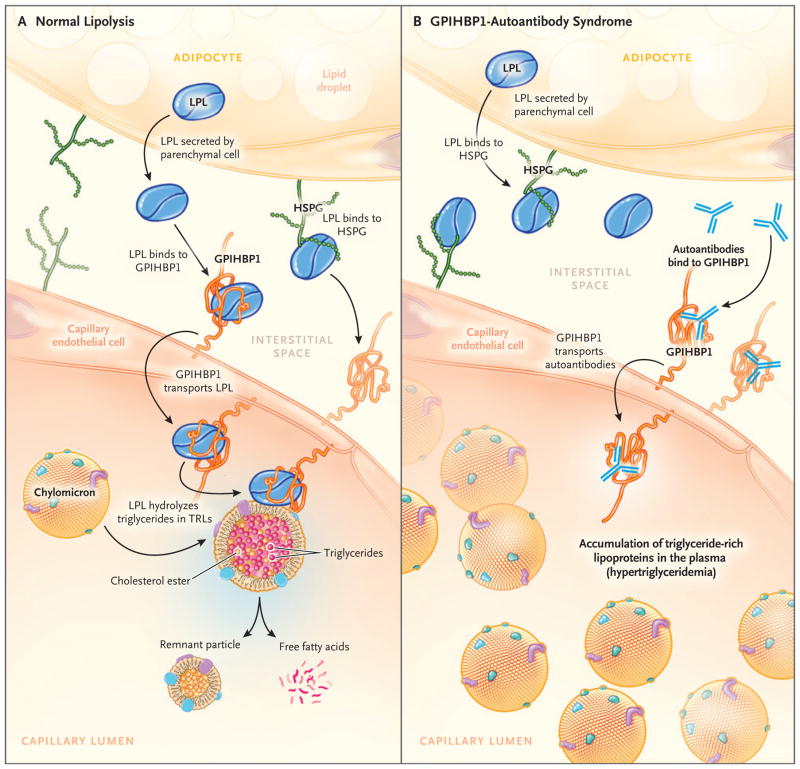
GPIHBP1, which binds lipoprotein lipase in the interstitial spaces and transports it to the capillary lumen,1,2 is a long-lived protein that moves bidirectionally across capillary endothelial cells.17 In murine models, GPIHBP1 has been shown to transport a GPIHBP1-specific monoclonal antibody from the capillary lumen to the basolateral surface of endothelial cells2; GPIHBP1 transported the same monoclonal antibody from the interstitial spaces to the capillary lumen18 (Fig. 6A). In our study, among the patients with GPIHBP1 autoantibodies, we found that the autoantibodies were able to bind to GPIHBP1 in capillaries, and it is likely (given the antibody-transport studies in mice) that the GPIHBP1–autoantibody complexes move back and forth across endothelial cells. Because GPIHBP1 autoantibodies block binding of lipoprotein lipase, we further assume that the transport of lipoprotein lipase to the capillary lumen in patients with autoantibodies is negligible and that the lipoprotein lipase is mislocalized in the interstitial spaces, as it is in Gpihbp1-deficient mice (Fig. 6B).2
Figure 6. Normal Lipolysis and Defective Triglyceride Processing in the GPIHBP1-Autoantibody Syndrome.
Panel A shows normal intravascular processing of triglycerides in a healthy person, and Panel B shows defective triglyceride processing in a patient with the GPIHBP1-autoantibody syndrome. Normally, the lipoprotein lipase that is secreted by parenchymal cells (e.g., adipocytes and myocytes) is captured by GPIHBP1 on the basolateral surface of endothelial cells. GPIHBP1 then transports lipoprotein lipase across endothelial cells to the capillary lumen, where the lipoprotein lipase hydrolyzes triglycerides in triglyceride-rich lipoproteins (e.g., very-low-density lipoproteins and chylomicrons). GPIHBP1 autoantibodies block the binding of lipoprotein lipase to GPIHBP1 and therefore block the transport of lipoprotein lipase to the capillary lumen, resulting in an accumulation of triglyceride-rich lipoproteins in the plasma (hypertriglyceridemia). HSPG denotes heparan sulfate proteoglycan, and TRL triglyceride-rich lipoprotein.
Previous studies have shown that chylomicronemia can be caused by autoantibodies against lipoprotein lipase.14,15,19–22 In an earlier study,14 investigators suspected that Patient 157 would also have autoantibodies against lipoprotein lipase,14 but we found none. The initial suspicion that lipoprotein lipase autoantibodies would be present was based on spiking the patient’s plasma into a postheparin control plasma sample and finding reduced amounts of triglyceride hydrolysis. That type of assay is problematic because of the inherent instability of lipoprotein lipase and because of competition from plasma lipoproteins. Also, Patient 164 was reported to have lipoprotein lipase autoantibodies on Western blot analysis,15 but we found none, and in hindsight the initial Western blot analysis was not definitive.
Four of the six patients with GPIHBP1 autoantibodies who had chylomicronemia had received a diagnosis of an autoimmune disease (SLE or Sjögren’s syndrome). In such patients, autoantibodies against many proteins can develop, and our data indicate that GPIHBP1 is one of those proteins. Because of the transfer of maternal autoantibodies, some infants who are born to mothers with SLE are found to have neonatal lupus (characterized by cutaneous lesions and cardiac conduction abnormalities).23 On the basis of our data, chylomicronemia is a potential finding in infants born to mothers with SLE. Of the six patients with GPIHBP1 autoantibodies, two had no evidence of rheumatologic disease. Thus, we believe that testing for GPIHBP1 autoantibodies should be considered in patients with acquired chylomicronemia regardless of whether autoimmune disease is diagnosed.
GPIHBP1 is one of approximately 25 Ly6 proteins, each of which is characterized by one or more three-fingered Ly6 domains containing 8 or 10 cysteines that are all disulfide-bonded. The autoantibodies in our patients bound to properly folded GPIHBP1 monomers with intact disulfide bonds, and the autoantibodies did not cross-react with other Ly6 proteins, including CD59. It is intriguing that other autoantibodies associated with human disease bind to cysteine-rich domains in proteins. For example, autoantibodies against the cysteine-rich region of ADAMTS13 cause thrombotic thrombocytopenic purpura.24 In addition, autoantibodies against a cysteine-rich domain of the thyrotropin receptor have been identified in Graves’ disease,25–27 and autoantibodies against a cysteine-rich domain of the phospholipase A2 receptor (PLA2R) have been identified in membranous nephropathy.28
In our studies, immunoassay interference was the clue that led to our identification of GPIHBP1 autoantibodies. Immunoassay interference from autoantibodies is well known to clinical chemists. For example, autoantibodies against troponin can interfere with troponin assays used to diagnose myocardial infarction,29 and autoantibodies against thyrotropin, thyroxine, and triiodothyronine can interfere with immunoassays used to diagnose thyroid diseases.30
Our study does not allow us to estimate the incidence with which GPIHBP1 antibodies occur or lead to the clinical syndrome of chylomicronemia. In addition, our study provides limited information regarding effective forms of therapy for the syndrome. Further research will be necessary to address these important issues.
In conclusion, we identified autoantibodies against GPIHBP1 that block the ability of this protein to bind to lipoprotein lipase and transport it from the interstitial space to the capillary lumen. These autoantibodies, which were identified in six patients, interfered with lipoprotein lipase–mediated processing of triglyceride-rich lipoproteins and caused severe hypertriglyceridemia.
Supplementary Material
Acknowledgments
Funded by the National Heart, Lung, and Blood Institute and the Leducq Foundation.
Supported by grants (HL090553, HL087228, and HL125335) from the National Heart, Lung, and Blood Institute and a Transatlantic Network Grant (12CVD04) from the Leducq Foundation.
We thank Dr. Helen Hobbs for providing several plasma samples that were used in the study.
APPENDIX
The authors’ full names and academic degrees are as follows: Anne P. Beigneux, Ph.D., Kazuya Miyashita, B.Sc., Michael Ploug, Ph.D., Dirk J. Blom, M.D., Ph.D., Masumi Ai, M.D., Ph.D., MacRae F. Linton, M.D., Weerapan Khovidhunkit, M.D., Ph.D., Robert Dufour, M.D., Abhimanyu Garg, M.D., Maureen A. McMahon, M.D., Clive R. Pullinger, Ph.D., Norma P. Sandoval, B.Sc., Xuchen Hu, B.A., Christopher M. Allan, Ph.D., Mikael Larsson, Ph.D., Tetsuo Machida, M.T., Masami Murakami, M.D., Ph.D., Karen Reue, Ph.D., Peter Tontonoz, M.D., Ph.D., Ira J. Goldberg, M.D., Philippe Moulin, M.D., Ph.D., Sybil Charrière, M.D., Ph.D., Loren G. Fong, Ph.D., Katsuyuki Nakajima, Ph.D., and Stephen G. Young, M.D.
The authors’ affiliations are as follows: the Departments of Medicine (A.P.B., M.A.M., N.P.S., X.H., C.M.A., M.L., L.G.F., S.G.Y.), Rheumatology (M.A.M.), Human Genetics (K.R., S.G.Y.), and Pathology and Laboratory Medicine (P.T.), David Geffen School of Medicine, University of California, Los Angeles, Los Angeles, and the Cardiovascular Research Institute and Department of Physiological Nursing, University of California, San Francisco, San Francisco (C.R.P.); the Department of Clinical Laboratory Medicine, Gunma University Graduate School of Medicine, Maebashi (K.M., T.M., M.M., K.N.), and the Department of Insured Medical Care Management, Graduate School of Medical and Dental Sciences, Tokyo Medical and Dental University, Tokyo (M.A.) — both in Japan; the Finsen Laboratory, Rigshospitalet, Copenhagen (M.P.); the Department of Medicine, University of Cape Town, Cape Town, South Africa (D.J.B.); the Departments of Medicine and Pharmacology, Vanderbilt University Medical Center, Nashville (M.F.L.); the Department of Medicine, Faculty of Medicine, Chulalongkorn University and Thai Red Cross Society, Bangkok, Thailand (W.K.); Clinique de Prévention Cardiovasculaire, Institut de Recherches Cliniques de Montréal, University of Montreal, Montreal (R.D.); the Department of Medicine, University of Texas Southwestern Medical Center, Dallas (A.G.); the Department of Medicine, Division of Endocrinology, Diabetes, and Metabolism, New York University School of Medicine, New York (I.J.G.); and Fédération d’Endocrinologie, Groupement Hospitalier Est, Hospices Civils de Lyon, INSERM UMR-1060 Carmen, Université de Lyon, Lyon, France (P.M., S.C.).
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Beigneux AP, Davies BS, Gin P, et al. Glycosylphosphatidylinositol-anchored high-density lipoprotein-binding protein 1 plays a critical role in the lipolytic processing of chylomicrons. Cell Metab. 2007;5:279–91. doi: 10.1016/j.cmet.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davies BS, Beigneux AP, Barnes RH, II, et al. GPIHBP1 is responsible for the entry of lipoprotein lipase into capillaries. Cell Metab. 2010;12:42–52. doi: 10.1016/j.cmet.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Franssen R, Young SG, Peelman F, et al. Chylomicronemia with low postheparin lipoprotein lipase levels in the setting of GPIHBP1 defects. Circ Cardiovasc Genet. 2010;3:169–78. doi: 10.1161/CIRCGENETICS.109.908905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Olivecrona G, Ehrenborg E, Semb H, et al. Mutation of conserved cysteines in the Ly6 domain of GPIHBP1 in familial chylomicronemia. J Lipid Res. 2010;51:1535–45. doi: 10.1194/jlr.M002717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Charrière S, Peretti N, Bernard S, et al. GPIHBP1 C89F neomutation and hydrophobic C-terminal domain G175R mutation in two pedigrees with severe hyperchylomicronemia. J Clin Endocrinol Metab. 2011;96:E1675–E1679. doi: 10.1210/jc.2011-1444. [DOI] [PubMed] [Google Scholar]
- 6.Coca-Prieto I, Kroupa O, Gonzalez-Santos P, et al. Childhood-onset chylomicronaemia with reduced plasma lipoprotein lipase activity and mass: identification of a novel GPIHBP1 mutation. J Intern Med. 2011;270:224–8. doi: 10.1111/j.1365-2796.2011.02361.x. [DOI] [PubMed] [Google Scholar]
- 7.Rios JJ, Shastry S, Jasso J, et al. Deletion of GPIHBP1 causing severe chylomicronemia. J Inherit Metab Dis. 2012;35:531–40. doi: 10.1007/s10545-011-9406-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beigneux AP, Fong LG, Bensadoun A, et al. GPIHBP1 missense mutations often cause multimerization of GPIHBP1 and thereby prevent lipoprotein lipase binding. Circ Res. 2015;116:624–32. doi: 10.1161/CIRCRESAHA.116.305085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Plengpanich W, Young SG, Khovidhunkit W, et al. Multimerization of glycosylphosphatidylinositol-anchored high density lipoprotein-binding protein 1 (GPIHBP1) and familial chylomicronemia from a serine-to-cysteine substitution in GPIHBP1 Ly6 domain. J Biol Chem. 2014;289:19491–9. doi: 10.1074/jbc.M114.558528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu X, Sleeman MW, Miyashita K, et al. Monoclonal antibodies that bind to the Ly6 domain of GPIHBP1 abolish the binding of LPL. J Lipid Res. 2017;58:208–15. doi: 10.1194/jlr.M072462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Machida T, Miyashita K, Sone T, et al. Determination of serum lipoprotein lipase using a latex particle-enhanced turbidimetric immunoassay with an automated analyzer. Clin Chim Acta. 2015;442:130–5. doi: 10.1016/j.cca.2015.01.016. [DOI] [PubMed] [Google Scholar]
- 12.Young SG, Smith RS, Hogle DM, Curtiss LK, Witztum JL. Two new monoclonal antibody-based enzyme-linked assays of apolipoprotein B. Clin Chem. 1986;32:1484–90. [PubMed] [Google Scholar]
- 13.Beigneux AP, Davies BSJ, Tat S, et al. Assessing the role of the glycosylphosphatidylinositol-anchored high density lipoprotein-binding protein 1 (GPIHBP1) three-finger domain in binding lipoprotein lipase. J Biol Chem. 2011;286:19735–43. doi: 10.1074/jbc.M111.242024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blom DJ, Marais AD. Severe hypertriglyceridemia in a patient with lupus. Am J Med. 2005;118:443–4. doi: 10.1016/j.amjmed.2004.10.023. [DOI] [PubMed] [Google Scholar]
- 15.Ashraf AP, Beukelman T, Pruneta-Deloche V, Kelly DR, Garg A. Type 1 hyperlipoproteinemia and recurrent acute pancreatitis due to lipoprotein lipase antibody in a young girl with Sjogren’s syndrome. J Clin Endocrinol Metab. 2011;96:3302–7. doi: 10.1210/jc.2011-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gårdsvoll H, Hansen LV, Jørgensen TJ, Ploug M. A new tagging system for production of recombinant proteins in Drosophila S2 cells using the third domain of the urokinase receptor. Protein Expr Purif. 2007;52:384–94. doi: 10.1016/j.pep.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 17.Olafsen T, Young SG, Davies BSJ, et al. Unexpected expression pattern for glycosylphosphatidylinositol-anchored HDL-binding protein 1 (GPIHBP1) in mouse tissues revealed by positron emission tomography scanning. J Biol Chem. 2010;285:39239–48. doi: 10.1074/jbc.M110.171041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davies BS, Goulbourne CN, Barnes RH, II, et al. Assessing mechanisms of GPIHBP1 and lipoprotein lipase movement across endothelial cells. J Lipid Res. 2012;53:2690–7. doi: 10.1194/jlr.M031559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pruneta V, Moulin P, Labrousse F, Bondon PJ, Ponsin G, Berthezene F. Characterization of a new case of autoimmune type I hyperlipidemia: long-term remission under immunosuppressive therapy. J Clin Endocrinol Metab. 1997;82:791–6. doi: 10.1210/jcem.82.3.3835. [DOI] [PubMed] [Google Scholar]
- 20.Yoshimura T, Ito M, Sakoda Y, Kobori S, Okamura H. Rare case of autoimmune hyperchylomicronemia during pregnancy. Eur J Obstet Gynecol Reprod Biol. 1998;76:49–51. doi: 10.1016/s0301-2115(97)00154-1. [DOI] [PubMed] [Google Scholar]
- 21.Kihara S, Matsuzawa Y, Kubo M, et al. Autoimmune hyperchylomicronemia. N Engl J Med. 1989;320:1255–9. doi: 10.1056/NEJM198905113201906. [DOI] [PubMed] [Google Scholar]
- 22.Pruneta-Deloche V, Marçais C, Perrot L, et al. Combination of circulating antilipoprotein lipase (Anti-LPL) antibody and heterozygous S172 fsX179 mutation of LPL gene leading to chronic hyperchylomicronemia. J Clin Endocrinol Metab. 2005;90:3995–8. doi: 10.1210/jc.2005-0205. [DOI] [PubMed] [Google Scholar]
- 23.Izmirly PM, Llanos C, Lee LA, Askanase A, Kim MY, Buyon JP. Cutaneous manifestations of neonatal lupus and risk of subsequent congenital heart block. Arthritis Rheum. 2010;62:1153–7. doi: 10.1002/art.27333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ostertag EM, Kacir S, Thiboutot M, et al. ADAMTS13 autoantibodies cloned from patients with acquired thrombotic thrombocytopenic purpura: 1. Structural and functional characterization in vitro. Transfusion. 2016;56:1763–74. doi: 10.1111/trf.13584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen CR, Tanaka K, Chazenbalk McLachlan SM, Rapoport B. A full biological response to autoantibodies in Graves’ disease requires a disulfide-bonded loop in the thyrotropin receptor N terminus homologous to a laminin epidermal growth factor-like domain. J Biol Chem. 2001;276:14767–72. doi: 10.1074/jbc.M008001200. [DOI] [PubMed] [Google Scholar]
- 26.Schwarz-Lauer L, Pichurin PN, Chen CR, et al. The cysteine-rich amino terminus of the thyrotropin receptor is the immunodominant linear antibody epitope in mice immunized using naked deoxyribonucleic acid or adenovirus vectors. Endocrinology. 2003;144:1718–25. doi: 10.1210/en.2002-0069. [DOI] [PubMed] [Google Scholar]
- 27.Hamidi S, Chen CR, Murali, McLachlan SM, Rapoport B. Probing structural variability at the N terminus of the TSH receptor with a murine monoclonal antibody that distinguishes between two receptor conformational forms. Endocrinology. 2013;154:562–71. doi: 10.1210/en.2012-1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fresquet M, Jowitt TA, Gummadova J, et al. Identification of a major epitope recognized by PLA2R autoantibodies in primary membranous nephropathy. J Am Soc Nephrol. 2015;26:302–13. doi: 10.1681/ASN.2014050502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eriksson S, Hellman J, Pettersson K. Autoantibodies against cardiac troponins. N Engl J Med. 2005;352:98–100. doi: 10.1056/NEJM200501063520123. [DOI] [PubMed] [Google Scholar]
- 30.Després N, Grant AM. Antibody interference in thyroid assays: a potential for clinical misinformation. Clin Chem. 1998;44:440–54. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.