Summary
Background
Cross-resistance after first-line antiretroviral therapy (ART) failure is expected to impair activity of nucleoside reverse-transcriptase inhibitors (NRTIs) in second-line therapy for patients with HIV, but evidence for the effect of cross-resistance on virological outcomes is limited. We aimed to assess the association between the activity, predicted by resistance testing, of the NRTIs used in second-line therapy and treatment outcomes for patients infected with HIV.
Methods
We did an observational analysis of additional data from a published open-label, randomised trial of second-line ART (EARNEST) in sub-Saharan Africa. 1277 adults or adolescents infected with HIV in whom first-line ART had failed (assessed by WHO criteria with virological confirmation) were randomly assigned to a boosted protease inhibitor (standardised to ritonavir-boosted lopinavir) with two to three NRTIs (clinician-selected, without resistance testing); or with raltegravir; or alone as protease inhibitor monotherapy (discontinued after week 96). We tested genotypic resistance on stored baseline samples in patients in the protease inhibitor and NRTI group and calculated the predicted activity of prescribed second-line NRTIs. We measured viral load in stored samples for all patients obtained every 12–16 weeks. This trial is registered with Controlled-Trials.com (number ISRCTN 37737787) and ClinicalTrials.gov (number NCT00988039).
Findings
Baseline genotypes were available in 391 (92%) of 426 patients in the protease inhibitor and NRTI group. 176 (89%) of 198 patients prescribed a protease inhibitor with no predicted-active NRTIs had viral suppression (viral load <400 copies per mL) at week 144, compared with 312 (81%) of 383 patients in the protease inhibitor and raltegravir group at week 144 (p=0·02) and 233 (61%) of 280 patients in the protease inhibitor monotherapy group at week 96 (p<0·0001). Compared with results with no active NRTIs, 95 (85%) of 112 patients with one predicted-active NRTI had viral suppression (p=0·3) and 20 (77%) of 26 patients with two or three active NRTIs had viral suppression (p=0·08). Over all follow-up, greater predicted NRTI activity was associated with worse viral load suppression (global p=0·0004).
Interpretation
Genotypic resistance testing might not accurately predict NRTI activity in protease inhibitor-based second-line ART. Our results do not support the introduction of routine resistance testing in ART programmes in low-income settings for the purpose of selecting second-line NRTIs.
Funding
European and Developing Countries Clinical Trials Partnership, UK Medical Research Council, Institito de Salud Carlos III, Irish Aid, Swedish International Development Cooperation Agency, Instituto Superiore di Sanita, WHO, Merck.
Introduction
More than 17 million people receive antiretroviral therapy (ART) for HIV infection, mainly delivered with the WHO-recommended public health approach, characterised by use of standardised sequential regimens.1, 2 Standardised first-line and second-line regimens both include nucleoside reverse-transcriptase inhibitors (NRTIs), combined with a non-NRTI (NNRTI) in first-line and a boosted protease inhibitor in second-line therapy.3 Treatment guidelines for individualised therapy in high-income settings generally recommend that resistance testing be done at the time of first-line failure, because of the assumption that choosing more active NRTI drugs (those with less predicted cross-resistance) will probably optimise outcomes.4, 5, 6 However, in programmes that have adopted the public health approach, in which patients often have prolonged exposure to a failing first-line regimen (because diagnosis of failure is delayed by limited access to viral load monitoring) with extensive cross-resistance to second-line NRTIs (which is usually undetected, because resistance testing is not done routinely), treatment outcomes seem excellent.7, 8 This suggests that, in the setting of protease inhibitor-based second-line therapy, greater cross-resistance and lower predicted activity of NRTIs do not greatly affect outcomes. Such a scenario might reduce the potential benefit that could be gained from the introduction of resistance testing into the public health approach for second-line NRTI drug selection. We aimed to assess the association between the activity, predicted by resistance testing, of the NRTIs used in second-line therapy and treatment outcomes for patients with HIV.
Research in context.
Evidence before this study
We searched PubMed with no start date or language restrictions for articles published until Aug 1, 2016, with the terms “second-line therapy”, “protease inhibitors”, “resistance testing”, and the individual drug names, and we also reviewed relevant HIV conference abstracts. This search identified studies that examined the association between nucleoside reverse-transcriptase inhibitor (NRTI) resistance and virological outcomes for NRTI plus protease inhibitor second-line therapy after failure on a first-line non-NRTI (NNRTI)-containing regimen. We found two cohort studies done in sub-Saharan Africa (243 and 101 participants, follow-up 12 months) and one in Asia (105 participants, follow-up 12 months) and analyses of the NRTI plus protease inhibitor arm in two randomised controlled trials (254 participants, follow-up 48 weeks; 270 participants, follow-up 96 weeks). These studies either reported no association or an inverse association between predicted activity of prescribed NRTIs and viral load suppression.
Added value of this study
In the EARNEST trial, we prospectively followed up a large group of HIV-infected patients taking a protease inhibitor and NRTI second-line regimen and collected adherence data and relevant outcome data, including viral load, with greater frequency and with lower incidence of loss-to-follow-up than in preceding cohort studies. This trial was also larger (426 patients on protease inhibitor and NRTI) than the other two randomised controlled trials of second-line therapy and had longer follow-up (144 weeks). Our analysis shows that a regimen containing NRTIs with no or limited predicted activity achieved viral load suppression in a high proportion of patients, which was sustained throughout longer-term follow-up. The size and duration of our study also allows for a more robust model for analysing predictors of outcome than previous studies: we found a paradoxical inverse association between extent of baseline resistance and viral suppression (noted in several, but not all, previous analyses) but were also able to show more clearly that this inverse association persisted after adjustment for other relevant factors including baseline viral load and CD4 cell count and adherence during follow-up (adherence was proposed as the probable explanation for the inverse association by several previous studies). The main added value of this analysis is the availability of two comparison groups within the same trial, allowing us to show the contribution of the NRTIs to regimen activity. In the group with no predicted active NRTIs in the prescribed regimen, viral load suppression was at least as good as that of a regimen containing a predicted fully active drug from a new drug class (raltegravir) and clearly superior to that obtained with the protease inhibitor used alone. Thus the good outcomes obtained with a protease inhibitor and NRTI second-line regimen in programme settings are not solely attributable to the high potency of protease inhibitors (proposed as the explanation in some earlier studies) but also reflect a substantial additional contribution from NRTIs predicted to have limited or no activity as a result of cross-resistance.
Implications of all the available evidence
Despite extensive cross-resistance, NRTIs make a major contribution to viral load suppression in second-line therapy when combined with a protease inhibitor. Predictions based on genotypic resistance testing appear to overestimate the detrimental effect of resistance mutations on the activity of NRTIs and should be re-examined for use in the context of second-line switch to a protease inhibitor-based regimen and possibly for other specific regimen changes. Taken together, the data support WHO treatment guidelines that do not recommend resistance testing at switch to second-line therapy. Adherence seems to be an important determinant of outcomes in second-line therapy and NRTI selection might best be based on minimising toxicity and maximising tolerability, in view of the effect that these might have on adherence.
Methods
Study design and patients
We did an observational analysis within an open-label, randomised trial of second-line ART (EARNEST) in sub-Saharan Africa. The EARNEST trial,7 done from 2010–14 in 14 clinical sites in Uganda, Zimbabwe, Malawi, Kenya, and Zambia, enrolled adults and adolescents older than 12 years infected with HIV in whom first-line NNRTI-based regimens had failed (by WHO clinical, immunological, or virological criteria, all confirmed by viral load >400 copies per mL; and with no more than three missed ART doses reported in the previous 1 month). The EARNEST trial protocol (including viral load and genotyping sub-studies described here) was approved by ethics committees in participating countries and the UK. All patients or caregivers provided written informed consent.
Eligible patients were randomly assigned using computer-generated randomisation list with variable block size to receive a protease inhibitor (standardised to ritonavir-boosted lopinavir) together with two or three NRTIs (protease inhibitor and NRTI group), or with raltegravir (protease inhibitor and raltegravir group), or alone as monotherapy (following an initial induction with 12 weeks' raltegravir; protease inhibitor monotherapy group). In the protease inhibitor and NRTI group, NRTIs were selected by clinicians (without resistance testing) on the basis of NRTIs used first-line, side-effects, local standard-of-care, drug availability, and WHO guidelines (tenofovir and lamivudine or emtricitabine used if zidovudine or stavudine was used in first-line; zidovudine and lamivudine used if tenofovir was used in first-line).
Procedures
Patients were followed for 144 weeks with clinic visits every 1–2 months. Adherence was assessed by structured questions and intensive adherence counselling was given when lapses were identified. Treatment was monitored clinically and with CD4 cell counts every 12–16 weeks.
We measured viral load on stored samples in a central laboratory (JCRC, Kampala, Uganda) with the Abbott RealTime HIV-1 assay (Abbott Laboratories, Chicago, IL, USA) at weeks 4, 12, 24, 36, 48, 60, 80, 96, 110, 126 and 144. In the protease inhibitor monotherapy group, viral load was tested at all timepoints to week 48, and at week 96, after which the group was discontinued. Viral load results were reviewed by a data monitoring committee, but not returned to managing physicians.7
We genotyped reverse transcriptase on all available baseline samples in the protease inhibitor and NRTI group using a WHO-accredited in-house assay9 at JCRC. We used the Stanford algorithm (version 7) to predict drug susceptibility.
We calculated the number of predicted-active NRTIs in the prescribed second-line regimen by counting the NRTI drugs initially prescribed that had no more than low-level resistance (ie, not intermediate or high level resistance) predicted from baseline genotyping. We also calculated the genotypic susceptibility score10 for the prescribed second-line NRTIs by assigning a score of 0 (high-level resistance), 0·25 (intermediate resistance), 0·50 (low-level resistance), 0·75 (potential low-level resistance), and 1 (susceptible) to each prescribed NRTI.
Statistical analysis
Analyses were by intention-to-treat (ie, including patients regardless of subsequent treatment changes) and based on observed viral loads (ie, not imputing missing viral load data). We used generalised estimating equations (independent working correlation structure, binomial distribution, and robust variance) to test differences between treatment groups in the proportion of patients with virological suppression across the whole follow-up period. Risk differences were used to compare groups at the latest timepoint in the study (week 144; or week 96 for the protease inhibitor monotherapy group because this group was discontinued early). We used logistic multivariable regression with fractional polynomials and backwards elimination (exit p>0·05, stata command mfp) to identify independent predictors of a viral load of less than 400 copies per mL at week 144 in complete cases in the protease inhibitor and NRTI group. Variables included in the analysis were age (continuous), sex, viral subtype, proportion of visits missed or when non-adherence was self-reported (continuous), years on first-line ART (continuous), history of ever smoking, ever drinking alcohol, years of education, job status (employed, student, or unemployed), hours worked if employed (continuous), household income (<US$50, $50–200, or >$200 per month), availability of food for regular meals, clinical history (diabetes, tuberculosis, CNS disease, cardiovascular disease), and, at switch to second-line, viral load, CD4 cell count, haemoglobin, blood glucose, and estimated glomerular filtration rate (eGFR; all continuous). We tested the additional effect of individual mutations, pairs of mutations with more than 10% prevalence, and the presence of mutations in the well recognised thymidine analogue mutations type 1 (TAM1; ie, 41Leu, 210Trp, or 215Tyr) and type 2 (TAM2; ie, 67Asn, 70Arg, 215Phe, or 219Gln) pathways one at a time in the selected model. We also did two sensitivity analyses, first without backwards elimination including all factors with p<0·2 on univariable analysis (appendix p 6–7), and second with backwards elimination with exit p>0·2. We used Stata (version 13.1) for all analyses.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Between April 12, 2010, and April 29, 2011, 1277 patients were randomly assigned (426 to protease inhibitor and NRTI, 433 to protease inhibitor and raltegravir, 418 to protease inhibitor monotherapy). Baseline characteristics were similar between the groups (42% had viral load >100 000 copies per mL, 62% had CD4 cell count <100 cells per mL).7 At 144 weeks, 106 (8%) had died and 30 (2%) had withdrawn or were lost to follow-up. The protease inhibitor monotherapy group changed to combination therapy (mostly adding NRTIs) following review of the week 96 results by the data monitoring committee; two participants in the protease inhibitor and NRTI group switched for failure by week 144; no patients in the protease inhibitor and raltegravir group switched for failure. Viral load results were available from 12 327 (91%) of 13 481 scheduled follow-up timepoints until death, last follow-up, or lost to follow-up.
Baseline genotypes were available for 391 (92%) of 426 participants in the protease inhibitor and NRTI group. 95% had at least one NRTI mutation (table 1). 230 patients (59%) had no predicted-active NRTIs in their prescribed second-line regimen: regimen included tenofovir plus lamivudine or emtricitabine in 176 (77%); patients had median five NRTI mutations; 75% with TAM1 and 69% with TAM2. 128 (33%) of 391 had one predicted-active NRTI prescribed: regimen included tenofovir plus lamivudine or emtricitabine in 113 (88%); median two mutations; 30% with TAM1 and 47% with TAM2. 33 (8%) of 391 had at least two predicted-active NRTIs prescribed: regimen included tenofovir plus lamivudine or emtricitabine in 26 (79%); median zero mutations.
Table 1.
Resistance at first-line failure and predicted activity of NRTIs prescribed in second-line protease inhibitor and NRTI regimen
| Total |
Number of predicted-active NRTIs in second-line regimen |
||||
|---|---|---|---|---|---|
| 0 | 1 | ≥2 | |||
| Baseline genotype results available | 391 | 230 | 128 | 33 | |
| GSS of prescribed NRTIs | 0·25 (0–1; 0–3) | 0 (0–0·25; 0–0·5) | 1 (0·5–1; 0·5–1·25) | 2 (2–2; 1–3) | |
| Country | |||||
| Malawi* | 37 (9%) | 17 (7%) | 8 (6%) | 12 (36%) | |
| Uganda | 253 (65%) | 154 (67%) | 87 (68%) | 12 (36%) | |
| Zimbabwe | 84 (21%) | 56 (24%) | 21 (16%) | 7 (21%) | |
| Kenya | 17 (4%) | 3 (1%) | 12 (9%) | 2 (6%) | |
| Age (years) | 37 (31–43) | 37 (31–43) | 37 (31–45) | 36 (24–41) | |
| Years on first line ART | 4·0 (2·8–5·4) | 4·1 (2·9–5·5) | 4·2 (2·7–5·3) | 2·8 (2·3–5·0) | |
| Viral load (copies per mL) | 68 359 (23 200–181 887) | 83 634 (34 800–222 514) | 47 733 (13 683–94 293) | 113 982 (10 683–325 122) | |
| >100 000 | 158 (40%) | 110 (48%) | 29 (23%) | 19 (58%) | |
| CD4 (cells per μL) | 69 (28–136) | 47 (21–98) | 116 (64–183) | 91 (50–177) | |
| ≤100 | 247 (63%) | 174 (76%) | 54 (42%) | 19 (58%) | |
| Number of NRTI mutations | 4 (2–5) | 5 (4–5) | 2 (2–3) | 0 (0–1) | |
| Any NRTI mutation | 370 (95%) | 230 (100%) | 128 (100%) | 12 (36%) | |
| Any TAM1 mutations | 211 (54%) | 173 (75%) | 38 (30%) | 0 | |
| Any TAM2 mutations | 220 (56%) | 159 (69%) | 60 (47%) | 1 (3%) | |
| Most common NRTI mutations | |||||
| Met184Val | 355 (91%) | 217 (94%) | 126 (98%) | 12 (36%) | |
| Met41Leu | 170 (43%) | 158 (69%) | 12 (9%) | 0 | |
| Thr215Tyr | 167 (43%) | 137 (60%) | 30 (23%) | 0 | |
| Asp67Asn | 150 (38%) | 126 (55%) | 24 (19%) | 0 | |
| Lys70Arg | 125 (32%) | 81 (35%) | 43 (34%) | 1 (3%) | |
| Leu210Trp | 118 (30%) | 110 (48%) | 8 (6%) | 0 | |
| Thr215Phe | 97 (25%) | 75 (33%) | 22 (17%) | 0 | |
| Lys219Gln | 66 (17%) | 42 (18%) | 24 (19%) | 0 | |
| Lys219Glu | 44 (11%) | 40 (17%) | 4 (2%) | 0 | |
| Lys65Arg | 29 (7%) | 25 (11%) | 4 (2%) | 0 | |
| Most common NRTI mutation pattern | |||||
| 41Leu, 184Val, 210Trp, 215Tyr | 40 (11%) | 40 (17%) | 0 | 0 | |
| 184Val | 38 (10%) | 1 | 27 | 10 | |
| 41Leu, 67Asn, 184Val, 210Trp, 215Tyr | 26 (7%) | 26 | 0 | 0 | |
| 67Asn, 70Arg, 184Val, 219Gln | 18 (5%) | 1 | 17 | 0 | |
| 184Val, 215Tyr | 15 (4%) | 0 | 15 | 0 | |
| First-line NRTIs used | |||||
| Zidovudine | 275 (70%) | 177 (80%) | 86 (67%) | 12 (36%) | |
| Tenofovir | 39 (10%) | 19 (8%) | 16 (13%) | 4 (12%) | |
| Initial second-line NRTIs prescribed | |||||
| Tenofovir, lamivudine/emtricitabine | 279 (71%) | 159 (69%) | 105 (82%) | 15 (45%) | |
| Tenofovir, zidovudine, lamivudine/emtricitabine* | 36 (9%) | 17 (7%) | 8 (6%) | 11 (33%) | |
| Abacavir, didanosine | 51 (13%) | 40 (17%) | 5 (4%) | 6 (18%) | |
| Abacavir, lamivudine/emtricitabine | 12 (3%) | 9 (4%) | 2 (2%) | 1 (3%) | |
| Zidovudine, lamivudine/emtricitabine | 10 (3%) | 2 (1%) | 8 (6%) | 0 | |
| Didanosine, lamivudine/emtricitabine | 2 (1%) | 2 (1%) | 0 | 0 | |
| Zidovudine, didanosine | 1 (<1%) | 1 (<1%) | 0 | 0 | |
Data are median (IQR) or median (IQR; range), and number of patients and % of those with baseline genotypes available in each group. Predicted-active NRTIs are prescribed NRTIs with no more than low-level resistance on baseline genotype. ART=antiretroviral therapy. NRTI=nucleoside reverse-transcriptase inhibitor. GSS=genotypic susceptibility score. TAM=thymidine analogue mutation.
An NRTI regimen of three NRTIs (tenofovir, zidovudine, and lamivudine) was initially prescribed in Malawi, but this was reduced to two NRTIs (usually tenofovir and lamivudine) after a median of 45 weeks from randomisation when national guidelines changed.
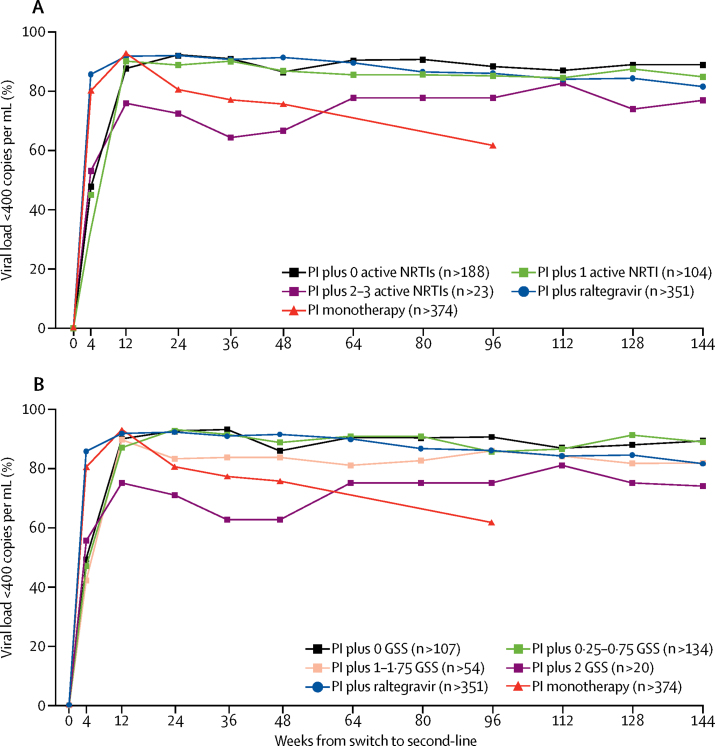
At week 144, 176 (89%) of 198 prescribed a second-line protease inhibitor and NRTI regimen containing no predicted-active NRTIs had viral suppression (viral load <400 copies per mL) compared with 312 (81%) of 383 in the protease inhibitor and raltegravir group (risk difference 7·4%; 95% CI 1·6 to 13·3; p=0·02). At week 96, 181 (88%) of 205 prescribed a regimen with no predicted-active NRTIs had viral suppression compared with 233 (61%) of 380 in the protease inhibitor monotherapy group (27·0%; 20·4 to 33·6; p<0·0001; figure). At week 144, 95 (85%) of 112 prescribed a regimen with one predicted-active NRTI had viral load suppression compared with 176 (89%) of 198 prescribed a regimen with no predicted-active NRTIs (−4·1%; −12·0 to 3·9; p=0·30). Furthermore, 20 (77%) of 26 with two to three predicted-active NRTIs had viral suppression (−12·0%; −28·7 to 4·8%; p=0·08 compared with no predicted-active NRTIs). Across the whole follow-up period, greater predicted activity of prescribed NRTIs was associated with worse viral load suppression (global generalised estimating equation p=0·0004; figure). A similar pattern of responses for all study weeks was seen with predicted activity of prescribed NRTIs calculated by genotypic susceptibility score (p=0·003; figure), and in analyses including only those failing on tenofovir first-line (p<0·0001), with viral load thresholds of less than 50 copies per mL (same rank, but difference attenuated, p=0·4; appendix p 3), or less than 1000 copies per mL (p=0·003; appendix p 4), and excluding patients in Malawi taking three NRTIs (p=0·03; appendix p 5).
Figure.
Viral load suppression by second-line regimen
Suppression defined as <400 copies per mL. Predicted active NRTIs are prescribed NRTIs with no more than low-level resistance on baseline genotype (A) By number of predicted-active NRTIs; global p<0·0001; within PI and NRTI group, global p<0·0004. (B) By genotypic susceptibility score; global p<0·0001; within PI and NRTI group, global p=0·003. GSS=genotypic susceptibility score. NRTI=nucleoside reverse-transcriptase inhibitor. n=minimum number of viral load values available at any follow-up timepoint in each group. PI=protease inhibitor.
In the protease inhibitor and NRTI group, increased viral load or reduced CD4 cell count at baseline, being unemployed or a student, working more hours if employed, and reporting being non-adherent at more visits were all independently associated with reduced probability of a viral load of less than 400 copies per mL at week 144 (table 2). Adjusting for these factors, increased genotypic susceptibility score remained associated with reduced probability of a viral load of less than 400 copies per mL (p=0·001); the effect remained (p=0·004) after restricting analysis to those with a genotypic susceptibility score of less than 2 (appendix p 8). Adjusting for these factors and genotypic susceptibility score, there was no evidence of an association between any individual mutation, TAM1, or TAM2 and a viral load of less than 400 copies per mL (p>0·05, data not shown). Results were very similar including all factors with p<0·2 on univariable analysis without backwards elimination, and using backward elimination with exit p>0·2 (appendix p 8).
Table 2.
Multivariable model for viral load <400 copies per mL at week 144 in protease inhibitor and nucleoside reverse-transcriptase inhibitor group
| Unadjusted odds ratio (95% CI) | p value | Adjusted odds ratio (95% CI) | p value | |
|---|---|---|---|---|
| Genomic susceptibility score of second-line regimen (per 0·5 higher) | 0·78 (0·61–0·99) | 0·04 | 0·61 (0·46–0·81) | 0·001 |
| Proportion of non-adherent visits (per 10% higher)* | 0·66 (0·55–0·79) | <0·0001 | 0·61 (0·49–0·77) | <0·0001 |
| Unemployed or student vs employed | 0·39 (0·21–0·72) | 0·003 | 0·22 (0·07–0·63) | 0·005 |
| Hours worked by employed and students (per 10 h higher) | 1·03 (0·92–1·17) | 0·6 | 0·83 (0·70–0·99) | 0·04 |
| Baseline viral load per doubling | 0·82 (0·70–0·95) | 0·01 | 0·80 (0·67–0·97) | 0·02 |
| Baseline CD4 cell count per doubling | 1·24 (1·02–1·50) | 0·03 | 1·33 (1·04–1·71) | 0·02 |
n=317, excluding those with missing week 144 viral load, baseline genotype or baseline employment status. Baseline refers to switch to second-line therapy (enrolment into the trial). Univariable analyses are in the appendix. Adjusted odds ratio adjusted for the factors given in the table. Factors not selected (p>0·05): sex, age, viral subtype, years on first-line antiretroviral treatment, diabetes, family history of cardiovascular disease, previous CNS disease, previous tuberculosis, smoking, alcohol consumption, household income, food availability, years of education, estimated glomerular filtration rate, haemoglobin, glucose, presence of individual mutations in the baseline genotype (where >10% prevalence), presence of combinations of 2 mutations in the baseline genotype (where >10% prevalence).
Scheduled visit that was either missed or where the participant self-reported missing pills since the last visit.
Discussion
We found that NRTIs predicted by resistance testing to contribute limited or no antiviral activity to a protease inhibitor-containing second-line regimen still had substantial virological effect, at least as good as that obtained from a fully active drug from a new class (raltegravir). This suggests that in the context of a switch from a first-line NNRTI-based to a second-line protease-inhibitor-based regimen, predictions based on genotypic resistance testing might overestimate the detrimental effect of cross-resistance on the activity of NRTIs. Prediction of clinical outcomes from genotypic resistance tests is challenging,10, 11 and algorithms might be particularly poor at predicting the combined effects of NRTI mutations that could sometimes be antagonistic.
In addition to our finding of substantial efficacy of NRTIs that had limited or no predicted activity, the paradoxical association we found between greater predicted NRTI activity and worse viral load suppression also contrasts with standard assumptions underlying resistance. Our findings are consistent with other second-line studies that have also found an absent or paradoxical association between NRTI resistance and outcomes,12, 13, 14, 15, 16, 17 but several features of our study (especially the prospectively collected adherence data and availability of two comparison groups) allow for more robust interpretation. The inferior viral suppression observed in those individuals with two or more fully active NRTIs in the prescribed regimen is probably explained by treatment adherence: this very small group of patients had no resistance mutations detected at baseline and probably comprised those who concealed complete non-adherence at study entry and continued to have poor adherence during second-line. The high viral load suppression in those taking NRTIs with limited or no predicted activity is probably not explained by residual confounding by adherence, elevated viral load, or other baseline factors associated with resistance because the effects persisted after adjustment. Furthermore, the randomised comparison of the entire protease inhibitor and NRTI group versus the protease inhibitor monotherapy group (ie, with all confounding removed) showed viral load suppression was increased in the protease inhibitor and NRTI group overall, which can only be because of the presence of ongoing NRTI activity.7 Our interpretation is further supported by a randomised comparison of protease inhibitor monotherapy versus protease inhibitor plus lamivudine as maintenance in second-line therapy that showed clear additional contribution of lamivudine despite almost universal lamivudine resistance.18
The observed NRTI effect might, in part, arise from resistance mutations such as Met184Val and Lys65Arg affecting viral replicative capacity, with effects increasing with more mutations.19, 20, 21 The effect of resistance mutations on viral replication fidelity might also protect the protease inhibitor by limiting the development of new mutations to escape drug pressure, thus increasing regimen durability.22, 23 Pharmacokinetic considerations might enhance these benefits; NRTIs, such as tenofovir and lamivudine, have long intracellular half-lives that might help to maintain viral suppression when protease inhibitor levels are low (with late or occasional missed doses) and this effect might be independent of predicted activity. This potential effect could explain why we observed that the NRTIs were capable of matching the suppression obtained with raltegravir (a drug with a relatively short intracellular half-life) in combination with a protease inhibitor.
The strengths of our study include large numbers of patients, long follow-up, high retention, regular viral load testing at predetermined timepoints, centralised viral load and resistance testing, and the availability of two relevant treatment comparison groups (especially the protease inhibitor monotherapy group) that enable the contribution of NRTIs to the regimen to be delineated. Although patients were participating in a clinical trial, the trial eligibility criteria were kept broad and we followed the widely used approach of clinical and CD4 cell count monitoring (targeted viral load testing was done to confirm treatment failure before changing therapy, but there was no real-time viral load testing during the trial). The results are therefore probably generalisable to patients failing first-line therapy in programmes that follow the public health approach to ART delivery.
The main limitation of our study is that most patients were taking zidovudine-based first-line regimens; whereas, contemporary WHO guidelines recommend tenofovir-based first-line ART. The similar results seen in the analysis of the subgroup failing on first-line tenofovir, however, support the generalisability of our findings. The protease inhibitor was standardised in this trial to ritonavir-boosted lopinavir, which is recommended as a preferred protease inhibitor in WHO guidelines3 and remains widely used in treatment programmes following the WHO public health approach. Whether NRTIs with limited or no predicted activity would contribute in a similar way to a regimen with atazanavir (WHO preferred protease inhibitor) or darunavir (alternative protease inhibitor) is unclear, although this seems probable at least for darunavir on the basis of its activity with NRTI resistance in other second-line studies.18, 24 We analysed our data with the Stanford algorithm, the most widely used. Although alternative algorithms might give slightly different weight to individual resistance mutations, they are based on the same mutations and would probably provide similar outcome predictions.10 Patients had non-subtype-B virus, whereas the reference algorithms in the Stanford database are for subtype-B virus. Mutations conferring NRTI drug resistance differ little between subtypes, however, and do not seem to affect outcomes.25, 26 A cohort study17 in a subtype-B population drew similar conclusions with respect to predicted NRTI activity and outcomes. For our binary classification of NRTI drugs as active or inactive we used a cutoff (NRTI considered active if no more than low-level resistance) that corresponds to the way many clinicians interpret resistance tests. Although some researchers might prefer alternative cutoffs, we found the same results with an analysis based on genotypic susceptibility score that gives more discrimination at intermediate values of resistance, and on a linear regression analysis of the genotypic susceptibility score that dispensed entirely with cutoffs. Furthermore, arguments on cutoffs are irrelevant for the group in which there was high-level resistance to both NRTI drugs selected (in which genotypic susceptibility score=0). In this situation, most would expect little benefit from the prescribed drugs and yet there was clear evidence of a major contribution to regimen activity.
Our findings are relevant to discussions about the merit of introducing routine resistance testing into the public health approach to ART for patients switching to the standard protease inhibitor-based second-line regimen. Although genotypic resistance testing is recommended in treatment guidelines for individualised therapy and widely used by clinicians in high-income countries for ART drug selection at treatment failure,4, 5, 6 the randomised controlled trials on which this practice is based generally showed modest short-term benefit and, of particular relevance to the present discussion, enrolled mainly patients who had failed on protease inhibitor-based regimens.27 None of these trials specifically examined the benefit of genotypic resistance testing in patients not previously treated with a protease inhibitor switching from a failing NNRTI-based first-line regimen to a protease inhibitor-based second-line regimen. The lack of association between predicted NRTI activity and outcomes shown in our study and other observational studies of protease inhibitor-based second-line therapy supports WHO treatment guidelines that do not recommend resistance testing at switch to second-line ART in the public health approach.3 The findings also suggest that NRTI selection might best be based on minimising toxicity and maximising tolerability in view of the effect of these on adherence. Introducing resistance testing in programme settings could theoretically provide additional benefit by identifying patients without resistance mutations who might be managed with more adherence counselling rather than switching to second-line. Our data suggest the effect of this would also be limited, however, because such patients were uncommon in our population (less than 2% lacked either an NRTI or NNRTI mutation).22 The proportion without resistance mutations detected at failure might be higher in programmes that succeed in implementing the WHO recommendation of annual viral load testing, but in some of these cases the resistance mutations might be present but archived (ie, would re-emerge upon resumption of regular therapy). Furthermore, such patients might not be responsive to additional adherence counselling: in our trial we provided universal adherence counselling at first-line failure and during second-line, but such patients still had inferior outcomes on second-line therapy. The NRTI drug class will probably remain in widespread use for millions of people in treatment programmes that use the public health approach (and elsewhere). Our results should stimulate further work to validate resistance-testing algorithms against NRTI clinical response in second-line protease inhibitor-based therapy and in other drug regimens. More generally, our findings emphasise the need for critical thinking around the benefits to be gained, if any, before new elements of care are introduced into the public health approach, even if they are considered as standard practice in high-income settings.
Acknowledgments
Acknowledgments
This trial was presented in part at the Conference on Retroviruses and Opportunistic Infections (Seattle, WA, Feb 23–26, 2015). We thank all the patients and staff from all the centres participating in the EARNEST trial. We thank Keith Fairbrother and David Dolling from the UK Drug Resistance Database for processing the genotyping data. The EDCTP (grant IP.2007.33011.003) funded the main trial with contributions from Medical Research Council (MRC), Institito de Salud Carlos III (grant A107/90015), Irish Aid, Swedish International Development Cooperation Agency (SIDA), Instituto Superiore di Sanita (ISS), WHO, and Merck. Substantive in-kind contributions were made by MRC Clinical Trials Unit, CINECA, Janssen Diagnostics, GlaxoSmithKline and ViiV Healthcare Ltd, and Abbott Laboratories. Trial medication was donated by AbbVie, Merck, Pfizer, GlaxoSmithKline, and Gilead. The Malawi-Liverpool-Wellcome Trust Clinical Research Programme, University of Malawi College of Medicine receives core funding from the Wellcome Trust. Additional funding for resistance testing and viral load testing for this sub-study was provided by WHO and GlaxoSmithKline.
Contributors
NIP, CK, AH, and ASW designed the study. CK, JH, AK, JJvO, MK, PJE, and PM enrolled patients into the study. IN and LB did the laboratory analyses with advice from SB. JT and ASW did the statistical analyses. All authors interpreted data. NIP, JT, and ASW drafted the report. All authors provided input into the report and approved the final version of the report.
Declaration of interests
NIP, CK, JT, IN, LB, AH, JH, AK, JJvO, MK, PJE, PM, and ASW report grants from European and Developing Countries Clinical Trials Partnership (EDCTP), non-financial support from AbbVie, non-financial support from Janssen laboratories, grants and non-financial support from GlaxoSmithKline and ViiV Healthcare, grants and non-financial support from Merck, non-financial support from Abbott Laboratories, grants from WHO, and non-financial support from Gilead during the conduct of the study. NIP reports personal fees from AbbVie, Janssen, and Roche outside the submitted work. JH reports personal fees from Gilead Scientific, Johnson and Johnson, and Mylan Pharmaceuticals outside the submitted work; ASW reports money paid to institution from Tibotec and Gilead Sciences outside the submitted work. SB declares no competing interests.
Contributor Information
Nicholas I Paton, Email: nick_paton@nuhs.edu.sg.
Europe Africa Research Network for Evaluation of Second-line Therapy (EARNEST) Trial Team:
E Agweng, P Awio, G Bakeinyaga, C Isabirye, U Kabuga, S Kasuswa, M Katuramu, C Kityo, F Kiweewa, H Kyomugisha, E Lutalo, P Mugyenyi, D Mulima, H Musana, G Musitwa, V Musiime, M Ndigendawan, H Namata, J Nkalubo, P Ocitti Labejja, P Okello, P Olal, G Pimundu, P Segonga, F Ssali, Z Tamale, D Tumukunde, W Namala, R Byaruhanga, J Kayiwa, J Tukamushaba, S Abunyang, D Eram, O Denis, R Lwalanda, L Mugarura, J Namusanje, I Nankya, E Ndashimye, E Nabulime, D Mulima, O Senfuma, G Bihabwa, E Buluma, P Easterbrook, A Elbireer, A Kambugu, D Kamya, M Katwere, R Kiggundu, C Komujuni, E Laker, E Lubwama, I Mambule, J Matovu, A Nakajubi, J Nakku, R Nalumenya, L Namuyimbwa, F Semitala, B Wandera, J Wanyama, H Mugerwa, A Lugemwa, E Ninsiima, T Ssenkindu, S Mwebe, L Atwine, H William, C Katemba, S Abunyang, M Acaku, P Ssebutinde, H Kitizo, J Kukundakwe, M Naluguza, K Ssegawa, Namayanja, F Nsibuka, P Tuhirirwe, M Fortunate, J Acen, J Achidri, A Amone, M Chamai, J Ditai, M Kemigisa, M Kiconco, C Matama, D Mbanza, F Nambaziira, M Owor Odoi, A Rweyora, G Tumwebaze, H Kalanzi, J Katabaazi, A Kiyingi, M Mbidde, M Mugenyi, R Mwebaze, P Okong, I Senoga, M Abwola, D Baliruno, J Bwomezi, A Kasede, M Mudoola, R Namisi, F Ssennono, S Tuhirwe, G Abongomera, G Amone, J Abach, I Aciro, B Arach, P Kidega, J Omongin, E Ocung, W Odong, A Philliam, H Alima, B Ahimbisibwe, E Atuhaire, F Atukunda, G Bekusike, A Bulegyeya, D Kahatano, S Kamukama, J Kyoshabire, A Nassali, A Mbonye, T M Naturinda, Ndukukire, A Nshabohurira, H Ntawiha, A Rogers, M Tibyasa, S Kiirya, D Atwongyeire, A Nankya, C Draleku, D Nakiboneka, D Odoch, L Lakidi, R Ruganda, R Abiriga, M Mulindwa, F Balmoi, S Kafuma, E Moriku, J Hakim, A Reid, E Chidziva, G Musoro, C Warambwa, G Tinago, S Mutsai, M Phiri, S Mudzingwa, T Bafana, V Masore, C Moyo, R Nhema, S Chitongo, Robert Heyderman, Lucky Kabanga, Symon Kaunda, Aubrey Kudzala, Linly Lifa, Jane Mallewa, Mike Moore, Chrissie Mtali, George Musowa, Grace Mwimaniwa, Rosemary Sikwese, Joep van Oosterhout, Milton Ziwoya, H Chimbaka, B Chitete, S Kamanga, T Kayinga E Makwakwa, R Mbiya, M Mlenga, T Mphande, C Mtika, G Mushani, O Ndhlovu, M Ngonga, I Nkhana, R Nyirenda, P Cheruiyot, C Kwobah, W Lokitala Ekiru, M Mokaya, A Mudogo, A Nzioka, A Siika, M Tanui, S Wachira, K Wools-Kaloustian, P Alipalli, E Chikatula, J Kipaila, I Kunda, S Lakhi, J Malama, W Mufwambi, L Mulenga, P Mwaba, E Mwamba, A Mweemba, M Namfukwe, E Kerukadho, B Ngwatu, J Birungi, N Paton, J Boles, A Burke, L Castle, S Ghuman, L Kendall, A Hoppe, S Tebbs, M Thomason, J Thompson, S Walker, J Whittle, H Wilkes, N Young, M Spyer, C Kapuya, F Kyomuhendo, D Kyakundi, N Mkandawire, S Mulambo, S Senyonjo, B Angus, A Arenas-Pinto, A Palfreeman, F Post, D Ishola, J Arribas, R Colebunders, M Floridia, M Giuliano, P Mallon, P Walsh, M De Rosa, E Rinaldi, I Weller, C Gilks, J Hakim, A Kangewende, S Lakhi, E Luyirika, F Miiro, P Mwamba, P Mugyenyi, S Ojoo, N Paton, S Phiri, J van Oosterhout, A Siika, S Walker, A Wapakabulo, T Peto, N French, J Matenga, G Cloherty, J van Wyk, M Norton, S Lehrman, P Lamba, K Malik, J Rooney, W Snowden, and J Villacian
Supplementary Material
References
- 1.Gilks CF, Crowley S, Ekpini R. The WHO public-health approach to antiretroviral treatment against HIV in resource-limited settings. Lancet. 2006;368:505–510. doi: 10.1016/S0140-6736(06)69158-7. [DOI] [PubMed] [Google Scholar]
- 2.Joint United Nations Programme on HIV/AIDS Global AIDS Update. 2016. http://www.unaids.org/en/resources/documents/2016/Global-AIDS-update-2016 (accessed Nov 26, 2016). [PubMed]
- 3.WHO . Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. World Health Organization; Geneva: 2016. [PubMed] [Google Scholar]
- 4.Panel on Antiretroviral Guidelines for Adults and Adolescents United States Department of Health and Human Services. http://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf (accessed Feb 21, 2017).
- 5.European AIDS Clinical Society Guidelines 8.0. 2015. http://www.eacsociety.org/files/2015_eacsguidelines_8_0-english_rev-20160124.pdf (accessed Feb 21, 2017).
- 6.Churchill D, Waters L, Ahmed N. British HIV Association guidelines for the treatment of HIV-1-positive adults with antiretroviral therapy 2015. HIV Med. 2016;17(suppl 4):s2–s104. doi: 10.1111/hiv.12426. [DOI] [PubMed] [Google Scholar]
- 7.Paton NI, Kityo C, Hoppe A. Assessment of second-line antiretroviral regimens for HIV therapy in Africa. N Engl J Med. 2014;371:234–247. doi: 10.1056/NEJMoa1311274. [DOI] [PubMed] [Google Scholar]
- 8.Manasa J, Lessells RJ, Skingsley A. High-levels of acquired drug resistance in adult patients failing first-line antiretroviral therapy in a rural HIV treatment programme in KwaZulu-Natal, South Africa. PLoS One. 2013;8:e72152. doi: 10.1371/journal.pone.0072152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kyeyune F, Nankya I, Metha S. Treatment failure and drug resistance is more frequent in HIV-1 subtype D versus subtype A-infected Ugandans over a 10-year study period. AIDS. 2013;27:1899–1909. doi: 10.1097/QAD.0b013e3283610ec7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frentz D, Boucher CA, Assel M. Comparison of HIV-1 genotypic resistance test interpretation systems in predicting virological outcomes over time. PLoS One. 2010;5:e11505. doi: 10.1371/journal.pone.0011505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prosperi MC, De Luca A. Computational models for prediction of response to antiretroviral therapies. AIDS Rev. 2012;14:145–153. [PubMed] [Google Scholar]
- 12.Boyd MA, Moore CL, Molina JM. Baseline HIV-1 resistance, virological outcomes, and emergent resistance in the SECOND-LINE trial: an exploratory analysis. Lancet HIV. 2015;2:e42–e51. doi: 10.1016/S2352-3018(14)00061-7. [DOI] [PubMed] [Google Scholar]
- 13.Hosseinipour MC, Kumwenda JJ, Weigel R. Second-line treatment in the Malawi antiretroviral programme: high early mortality, but good outcomes in survivors, despite extensive drug resistance at baseline. HIV Med. 2010;11:510–518. doi: 10.1111/j.1468-1293.2010.00825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiamsakul A, Sungkanuparph S, Law M. HIV multi-drug resistance at first-line antiretroviral failure and subsequent virological response in Asia. J Int AIDS Soc. 2014;17:19053. doi: 10.7448/IAS.17.1.19053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.La Rosa AM, Harrison LJ, Taiwo B. Raltegravir in second-line antiretroviral therapy in resource-limited settings (SELECT): a randomised, phase 3, non-inferiority study. Lancet HIV. 2016;3:e247–e258. doi: 10.1016/S2352-3018(16)30011-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sigaloff KC, Hamers RL, Wallis CL. Second-line antiretroviral treatment successfully resuppresses drug-resistant HIV-1 after first-line failure: prospective cohort in Sub-Saharan Africa. J Infect Dis. 2012;205:1739–1744. doi: 10.1093/infdis/jis261. [DOI] [PubMed] [Google Scholar]
- 17.Waters L, Bansi L, Asboe D. Second-line protease inhibitor-based antiretroviral therapy after non-nucleoside reverse transcriptase inhibitor failure: the effect of a nucleoside backbone. Antiviral Ther. 2013;18:213–219. doi: 10.3851/imp2329. [DOI] [PubMed] [Google Scholar]
- 18.Ciaffi L, Koulla-Shiro S, Sawadogo A, et al. Dual therapy with a boosted protease inhibitor plus lamivudine is an effective maintenance strategy in patients on second-line antiretroviral therapy in Africa: the ANRS 12286/MOBIDIP trial. HIV Drug Therapy Conference; Glasgow; Oct 23–26, 2016. Abstract 0122.
- 19.Ross L, Parkin N, Lanier R. Short communication: the number of HIV major NRTI mutations correlates directly with other antiretroviral-associated mutations and indirectly with replicative capacity and reduced drug susceptibility. AIDS Res Hum Retroviruses. 2008;24:617–620. doi: 10.1089/aid.2007.0188. [DOI] [PubMed] [Google Scholar]
- 20.Deeks SG, Martin JN. Reassessing the goal of antiretroviral therapy in the heavily pre-treated HIV-infected patient. AIDS. 2001;15:117–119. doi: 10.1097/00002030-200101050-00017. [DOI] [PubMed] [Google Scholar]
- 21.Deeks SG, Hoh R, Neilands TB. Interruption of treatment with individual therapeutic drug classes in adults with multidrug-resistant HIV-1 infection. J Infect Dis. 2005;192:1537–1544. doi: 10.1086/496892. [DOI] [PubMed] [Google Scholar]
- 22.Lloyd SB, Kent SJ, Winnall WR. The high cost of fidelity. AIDS Res Hum Retroviruses. 2014;30:8–16. doi: 10.1089/aid.2013.0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beerenwinkel N, Montazeri H, Schuhmacher H. The individualized genetic barrier predicts treatment response in a large cohort of HIV-1 infected patients. PLoS Comput Biol. 2013;9:e1003203. doi: 10.1371/journal.pcbi.1003203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ciaffi L, Koulla-Shiro S, Sawadogo A. Efficacy and safety of three second-line antiretroviral regimens in HIV-infected patients in Africa. AIDS. 2015;29:1473–1481. doi: 10.1097/QAD.0000000000000709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kityo C, Walker S, Nankya I, et al. Differences in resistance mutations in non-B subtypes at first-line failure in Africa. Conference on Retroviruses and Opportunistic Infections; Seattle; Feb 23–26, 2015. Abstract 595.
- 26.White E, Smit E, Churchill D. No evidence that HIV-1 subtype C infection compromises the efficacy of tenofovir-containing regimens: cohort study in the United Kingdom. J Infect Dis. 2016;214:1302–1308. doi: 10.1093/infdis/jiw213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Panidou ET, Trikalinos TA, Ioannidis JP. Limited benefit of antiretroviral resistance testing in treatment-experienced patients: a meta-analysis. AIDS. 2004;18:2153–2161. doi: 10.1097/00002030-200411050-00007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.