Abstract
The discordance between genome size and the complexity of eukaryotes can partly be attributed to differences in repeat density. The Muller F element (∼5.2 Mb) is the smallest chromosome in Drosophila melanogaster, but it is substantially larger (>18.7 Mb) in D. ananassae. To identify the major contributors to the expansion of the F element and to assess their impact, we improved the genome sequence and annotated the genes in a 1.4-Mb region of the D. ananassae F element, and a 1.7-Mb region from the D element for comparison. We find that transposons (particularly LTR and LINE retrotransposons) are major contributors to this expansion (78.6%), while Wolbachia sequences integrated into the D. ananassae genome are minor contributors (0.02%). Both D. melanogaster and D. ananassae F-element genes exhibit distinct characteristics compared to D-element genes (e.g., larger coding spans, larger introns, more coding exons, and lower codon bias), but these differences are exaggerated in D. ananassae. Compared to D. melanogaster, the codon bias observed in D. ananassae F-element genes can primarily be attributed to mutational biases instead of selection. The 5′ ends of F-element genes in both species are enriched in dimethylation of lysine 4 on histone 3 (H3K4me2), while the coding spans are enriched in H3K9me2. Despite differences in repeat density and gene characteristics, D. ananassae F-element genes show a similar range of expression levels compared to genes in euchromatic domains. This study improves our understanding of how transposons can affect genome size and how genes can function within highly repetitive domains.
Keywords: Drosophila, genome size, heterochromatin, retrotransposons, Wolbachia
An unusual feature of eukaryotic genomes is their large variations in genome size. The size of animal genomes can range from 0.03 pg in the rice root knot nematode (Meloidogyne graminicola), to 3.5 pg in human, and up to 133 pg in marbled lungfish (Protopterus aethiopicus) (Gregory et al. 2007; Gregory 2016), where 1 pg corresponds to ∼978 Mb of DNA (Dolezel et al. 2003). The sizes of eukaryotic genomes can vary substantially even among closely related species (Bosco et al. 2007; Fierst et al. 2015). Flow cytometry analyses show that the genome sizes of 67 species within Drosophilidae range from 0.14 pg in Drosophila mauritiana to 0.40 pg in Chymomyza amoena, while maintaining a comparable number of protein-coding genes (Gregory and Johnston 2008). At broader evolutionary timescales, this lack of correlation between genome size and the genetic complexity of an organism is known as the C-value paradox (reviewed in Patrushev and Minkevich 2008; Eddy 2012).
One of the potential explanations for the large variations in genome sizes among different strains of the same species and between closely related species is the amount of satellite DNA and transposable elements (Bosco et al. 2007; Craddock et al. 2016; reviewed in Kidwell 2002). A previous study of 26 Drosophila species has shown a strong positive correlation between genome size and transposon density (Sessegolo et al. 2016). Because active transposons can lead to genome instability and deleterious mutations, most transposons are silenced via epigenetic and post-transcriptional silencing mechanisms (reviewed in Slotkin and Martienssen 2007; Castañeda et al. 2011). These silencing mechanisms could allow transposons and other repetitive sequences to persist in the genome.
DNA packaged as heterochromatin generally has higher transposon density than regions that are packaged as euchromatin (Smith et al. 2007). The original dichotomy between heterochromatin and euchromatin is based on the cytological staining patterns in interphase nuclei (Heitz 1928), where regions that are densely stained throughout the cell cycle are classified as heterochromatin while regions that are lightly stained in the interphase nucleus are classified as euchromatin. In addition to exhibiting higher transposon density, heterochromatic regions are late replicating, have lower gene density and lower rates of recombination, and are enriched in histone modifications such as histone 3 lysine 9 di-/trimethylation (H3K9me2/3) and chromosomal proteins such as heterochromatin protein 1a (HP1a) (reviewed in Grewal and Elgin 2007). Results from recent high-throughput chromatin immunoprecipitation (ChIP) studies of the epigenomic landscapes of metazoan genomes suggest there are multiple subtypes of heterochromatin and euchromatin (Kharchenko et al. 2011; Riddle et al. 2011; Ho et al. 2014).
The Muller F element in D. melanogaster (also known as the “dot” or the fourth chromosome in that species) is unusual in that the chromosome as a whole exhibits properties of heterochromatin (e.g., late replication and low rates of recombination), but the distal portion of its long arm has a euchromatic gene density (reviewed in Riddle et al. 2009). The chromosome overall has an estimated size of 5.2 Mb (Locke and McDermid 1993). Its 79 genes, all located in the distal 1.3 Mb of the F element, exhibit a gene expression pattern that is similar to those of genes in euchromatin; ∼50% of the genes are active in the D. melanogaster S2 and BG3 cell lines across a similar range of expression levels (Riddle et al. 2009, 2012).
Similar to D. melanogaster, the F elements in other Drosophila species generally appear as a small “dot” chromosome (Schaeffer et al. 2008). Among the different Drosophila species, the D. ananassae genome is unusual; despite having only diverged from D. melanogaster ∼15 MYA (Obbard et al. 2012), the D. ananassae F element has undergone a substantial expansion compared to D. melanogaster. The estimated sizes of the D. ananassae and D. melanogaster genomes are similar [0.20 pg vs. 0.18 pg (Gregory and Johnston 2008)]. However, cytological studies have shown that the F element in D. ananassae is much larger than the small dot chromosome in D. melanogaster, appearing as a metacentric chromosome similar in size to the Muller A element in D. ananassae (Schaeffer et al. 2008). The D. ananassae F element appears to be uniformly heterochromatic in mitotic chromosome preparations (Hinton and Downs 1975), and appears as a large heterochromatic mass located near the chromocenter with a few distinct bands in polytene chromosome preparations (Kaufmann 1937). Based on the placement of the putative orthologs of D. melanogaster F-element genes in 16 scaffolds of the D. ananassae Comparative Analysis Freeze 1 (CAF1) assembly (Drosophila 12 Genomes Consortium 2007), Schaeffer and colleagues estimated that the D. ananassae F element is at least 17.8 Mb with a transposon density of 32.5% (Schaeffer et al. 2008).
A potential contributor to the expansion of the D. ananassae F element is horizontal gene transfer from the genome of the Wolbachia endosymbiont of D. ananassae (wAna) into the D. ananassae genome (reviewed in Dunning Hotopp 2011). Wolbachia is an intracellular bacterium that infects a large number of arthropods and nematodes (Dunning Hotopp et al. 2007; reviewed in Werren et al. 2008); a previous meta-analysis estimates that Wolbachia are found in ∼66% of arthropod species (Hilgenboecker et al. 2008). Wolbachia are transmitted vertically from infected females to progeny through the germline (Serbus and Sullivan 2007). To facilitate its spread, Wolbachia alters the reproductive system of the host to favor infected female hosts, resulting in sperm–egg cytoplasmic incompatibility, feminization of infected male hosts, and male killing (reviewed in Serbus et al. 2008). However, the interactions between Wolbachia and its hosts can also be mutualistic (Weeks et al. 2007; reviewed in Zug and Hammerstein 2015).
Previous studies have demonstrated that the Wolbachia genome is integrated into the genomes of the beetle Callosobruchus chinensis (Nikoh et al. 2008), the mosquito Aedes aegypti (Klasson et al. 2009a), and the filarial nematode Brugia malayi (Ioannidis et al. 2013). Cytological and bioinformatic analyses have indicated that the wAna genome is integrated into the genomes of some strains of D. ananassae (Klasson et al. 2014; Choi et al. 2015), including the Hawaiian strain used to construct the D. ananassae CAF1 assembly (Drosophila Species Stock Center stock number 14024-0371.13). Klasson and colleagues have previously estimated that the horizontal gene transfers and the subsequent duplications of wAna sequences account for ∼5 Mb (20%) of the D. ananassae F element (Klasson et al. 2014).
In this study, we performed manual sequence improvement and gene annotations of two putative D. ananassae F-element scaffolds. We also improved and annotated a portion of the D. ananassae Muller D element and used it as a reference euchromatic region in this comparative analysis. These resources have enabled us to conclude that the D. ananassae F element has a much higher transposon density (78.6%) than the previous estimate [32.5% (Schaeffer et al. 2008)]. We find that LTR and LINE retrotransposons are the major contributors to the expansion of the assembled portions of the D. ananassae F element, with the horizontal gene transfer of wAna playing only a minor role. We also examined the impact of this expansion on gene characteristics and on the epigenomic landscape of the D. ananassae F element in comparison to that of D. melanogaster. We find numerous similarities between the F elements in D. ananassae and D. melanogaster, but also detect differences in codon bias and in chromatin packaging of Polycomb-regulated genes.
Materials and Methods
General overview
The D. ananassae sequence improvement and gene annotations were organized using the framework provided by the Genomics Education Partnership (GEP) (Shaffer et al. 2010). Additional details on the sequence improvement and gene annotation protocols, and on the tools and tool parameters used in the bioinformatic analyses, are available in Supplemental Material, “Supplemental Methods” in File S7. The version information for the tools used in this study is available in Table S1 in File S7.
Sequence improvement
The sequence improvement protocol and the quality standards for the improved regions have been described previously (Slawson et al. 2006; Leung et al. 2010). The D. ananassae CAF1 assembly (Drosophila 12 Genomes Consortium 2007) was obtained from the AAA: 12 Drosophila Genomes Web site (http://eisenlab.org/AAA/index.html). The fosmid clones for the D. ananassae analysis regions improved_13010, improved_13034_2, and improved_13337 were obtained from the Drosophila Genomics Resource Center at Indiana University. These fosmid clones were used as templates for additional sequencing reactions and to produce the restriction digests for verifying the final assembly. Additional sequencing data for the improved_13034_1 region was produced by sequencing genomic PCR products. The D. ananassae genomic DNA (derived from the same strain used in the original sequencing project) was obtained from the Drosophila Species Stock Center at the University of California, San Diego (stock number 0000-1005.01).
The final assemblies for the improved_13010, improved_13034_2, and improved_13337 regions were confirmed by multiple restriction digests of the overlapping fosmids (Figure S2A in File S7). The improved_13034_1 assembly was confirmed by subreads produced by the Pacific Biosciences (PacBio) RS II sequencer (Figure S2B in File S7). In collaboration with the McDonnell Genome Institute, the Single Molecule, Real Time (SMRT) sequencing of the D. ananassae genome was performed using eight SMRT cells with the DNA Template Prep Kit 2.0 (3–10 kb), the DNA/Polymerase Binding Kit 2.0 (24 Rxn), the C2 chemistry, and a movie duration of 120 min.
Gene annotations
The gene annotation protocol has been described previously (Shaffer et al. 2010; Leung et al. 2015). For quality control purposes, each annotation project was completed by two or more GEP students working independently. Experienced students working under the supervision of GEP staff used Apollo (Lewis et al. 2002) to reconcile these gene annotations, and to create the gene models analyzed in this study. The annotations of the D. ananassae genes used the release 6.06 D. melanogaster gene annotations produced by FlyBase as a reference (Attrill et al. 2016). Release 1.04 of the D. ananassae gene predictions were obtained from FlyBase, and the UCSC liftOver utility was used to migrate these predictions to the improved D. ananassae assembly. Seven gene predictions (FBtr0115589, FBtr0115686, FBtr0128102, FBtr0128153, FBtr0381268, FBtr0384375, and FBtr0385219) could not be transferred to the improved assembly because of changes to the genomic sequences as a result of sequence improvement.
Repeat density analysis
Repeat Detector (Red) (Girgis 2015) and WindowMasker (Morgulis et al. 2006) were run against the improved D. ananassae assembly using default parameters. For the Tallymer analysis (Kurtz et al. 2008), the word sizes (k) of 17 and 19 were used to analyze the D. melanogaster and the D. ananassae assemblies, respectively (see “Supplemental Methods” in File S7 for the procedures used to select these word sizes). Simple and low complexity repeats were identified using tantan (Frith 2011) with the default masking rate (−r 0.005). Tandem repeats were identified using Tandem Repeats Finder (TRF) (Benson 1999) with the parameters: Match = 2, Mismatch = 7, Delta = 7, Match Probability = 80, Mismatch Probability = 10, Minscore = 50, and MaxPeriod = 2000.
Transposon remnants were identified using RepeatMasker (Smit et al. 2013) with the RepBase library [release 20150807 (Jurka et al. 2005)]. RepeatMasker was run on the D. melanogaster and D. ananassae assemblies using the WU BLAST search engine (-e wublast) at the most sensitive setting (-s) against Drosophila sequences in the RepBase library (-species drosophila), without masking low complexity and simple repeats (-nolow). Overlapping repeats were merged into a single interval if they belonged to the same class of transposon, and reclassified as the “overlapping” class if they belonged to difference classes of transposons.
Estimating the density of Wolbachia fragments
The assemblies for the Wolbachia endosymbionts of D. ananassae [wAna (Salzberg et al. 2005)], D. simulans strain Riverside [wRi (Klasson et al. 2009b)], and D. melanogaster [wMel (Wu et al. 2004)] were obtained from the NCBI BioProject database using the accession numbers PRJNA13365, PRJNA33273, and PRJNA272, respectively. Each Wolbachia assembly was compared against the D. melanogaster and the improved D. ananassae assemblies using RepeatMasker with the following parameters: -e wublast -s -nolow. The RepeatMasker results against the three Wolbachia assemblies were filtered using cutoff scores (255.6–263.4 for D. melanogaster and 250.7–256.0 for D. ananassae) to reduce spurious matches.
The Wolbachia protein sequences for wAna, wRi, and wMel were obtained from the NCBI Protein database using the BioProject accession numbers PRJNA13365, PRJNA33273, and PRJNA272, respectively. These protein sequences were compared against the D. melanogaster and D. ananassae assemblies using CENSOR (Kohany et al. 2006) with the WU BLAST (Gish 1996) tblastn module (-bprg tblastn) at the sensitive (-s) setting. The results were filtered by cutoff scores (112.0–116.2 for D. melanogaster and 94.8–106.2 for D. ananassae) to reduce the number of spurious matches (see “Supplemental Methods” in File S7 for the protocol used to determine the cutoff scores for the RepeatMasker and CENSOR searches).
Analysis of the wAna assembly
The wAna assembly was aligned against the wRi and wMel assemblies using LAST (Kiełbasa et al. 2011) with default parameters. These alignments were filtered and chained together using the UCSC Chain and Net alignment protocol (Kent et al. 2003). The regions of the wAna assembly that aligned to the improved D. ananassae assembly were extracted from the RepeatMasker results produced as part of the Estimating the density of Wolbachia fragments analysis. Regions within the wAna assembly that showed sequence similarity to Drosophila transposons in the RepBase library [release 20150807 (Jurka et al. 2005)] were identified using RepeatMasker (Smit et al. 2013) with the parameters: -s -e wublast -nolow -species drosophila.
Gene characteristics analysis
The isoform with the largest total coding exon size (i.e., the most comprehensive isoform) was used in the analysis of gene characteristics. Because the analysis focused only on the coding regions, the total intron size metric for D. ananassae and D. melanogaster only included the introns that were located between coding exons. Similarly, only the internal coding exons and the translated portions of the first and last coding exons were included in the analysis of coding exon sizes, even though the transcribed exons might be larger because of untranslated regions.
The statistical analyses of gene characteristics were performed using R (R Core Team 2015). Violin plots were created using a modified version of the vioplot function in the R package vioplot (Adler 2005). Modifications to the vioplot function were made to highlight the interquartile range (IQR) in each violin plot. For violin plots using the log10 scale, a pseudocount of 1 was added to all the values.
The Kruskal–Wallis rank sum test (KW test) (Kruskal and Wallis 1952) (the kruskal.test function in R) was used to determine if the differences in gene characteristics among the four analysis regions are statistically significant [type I error (α) = 0.05]. Following the rejection of the null hypothesis by the KW test, post hoc Dunn tests (DT) (Dunn 1964) were performed using the dunn.test function in the R package dunn.test (Dinno 2016) to identify the pairs of analysis regions that show significantly different distributions. The Holm–Bonferroni method (method=“holm”) was used by the DT to control the family-wise error rate (Holm 1979). Rejection of the null hypothesis using the Holm–Bonferroni method depends on both the adjusted p-values (adjp) and the order of the unadjusted p-values.
Codon bias analysis
The codon bias analysis protocol has previously been described (Leung et al. 2015). The effective number of codons (Nc) and the codon adaptation index (CAI) for each gene were determined by the chips and cai programs in the EMBOSS package (Rice et al. 2000), respectively (see “Supplemental Methods” in File S7 for the procedure used to construct the reference gene set for the CAI analysis). The violin plots and the KW tests were performed using the procedure described in the Gene characteristics analysis section. The codon frequency and the codon GC content for the genes in the D. melanogaster and D. ananassae analysis regions were determined by the cusp program in the EMBOSS package.
Locally estimated scatterplot smoothing (LOESS) (Cleveland and Devlin 1988) was applied to each Nc vs. CAI scatterplot to delineate the major trends. The span parameter for the LOESS regression model was determined by generalized cross-validation using the loess.as function in the R package fANCOVA with the parameters: degree = 1, criterion=“gcv,” family=“symmetric.” The scatterplot and the LOESS curve were created using the scatter.smooth function in R.
Melting temperature metagene profile
The protocol for creating the melting temperature (Tm) metagene profile has previously been described (Leung et al. 2015). The Tm in the D. melanogaster and the D. ananassae analysis regions were determined using the dan program in the EMBOSS package with a 9-bp sliding window (-windowsize = 9), a step size of one (-shiftincrement = 1), and the following parameters: -dnaconc = 50 -saltconc = 50 -mintemp = 55. The Tm for the coding span was standardized to 3 kb. The metagene consisted of the standardized 3-kb coding span and the 2-kb region upstream and downstream of the coding span. The median Tm at each position of the metagene was calculated, and a cubic smoothing spline was fitted to the median Tm profile using the smooth.spline function in R with ordinary cross-validation (cv = TRUE).
Chromatin immunoprecipitation sequencing analysis
Detailed descriptions of the chromatin immunoprecipitation sequencing analysis (ChIP-Seq) read mapping and peak calling protocols are available in “Supplemental Methods” in File S7. The D. ananassae ChIP-Seq data were produced using samples from the third instar larval stage of development. The chromatin isolation and immunoprecipitation protocols have previously been described (Riddle et al. 2012). The antibodies (Abcam ab1220 for H3K9me2, Millipore 07-030 for H3K4me2, and Abcam ab6002 for H3K27me3) have previously been validated by the modENCODE project (Egelhofer et al. 2011). Eight samples (two biological replicates of the ChIP for the three histone modifications and input DNA) were sequenced by the Genome Technology Access Center at Washington University in St. Louis using a single lane on the Illumina HiSeq 2000 sequencer, producing paired-end reads with read lengths of 101 bp. The ChIP-Seq reads were mapped against the improved D. ananassae assembly using BWA-MEM (Li 2013) with default parameters.
The third instar larvae ChIP-Seq data for the Oregon-R strain of D. melanogaster were produced by the modENCODE project (Landt et al. 2012; Ho et al. 2014). These ChIP-Seq data and input DNA controls were obtained from the European Nucleotide Archive (ENA) using the accession numbers SRP023384 (H3K9me2), SRP023385 (H3K4me2), and SRP028497 (H3K27me3). The ChIP-Seq samples were sequenced using the Illumina Genome Analyzer, producing unpaired reads with a read length of 50 bp. The ChIP-Seq reads were mapped against the D. melanogaster assembly using BWA-backtrack (Li and Durbin 2009) with default parameters.
Regions enriched in these three histone modifications were identified using MACS2 (Zhang et al. 2008). The log-likelihood-enrichment ratios (LLR) of the ChIP samples compared to DNA input controls were calculated by the bdgcmp subprogram in MACS2 using the following parameters: -m logLR -p 0.00001. The LLR metagene profiles were created using the technique described in the Melting temperature metagene profile section.
RNA-Seq expression analysis
In addition to the D. ananassae adult males and adult females RNA-Seq samples [SRP006203 (Graveley et al. 2011)] used in gene annotations, RNA-Seq data from other developmental stages and tissues were obtained from the ENA. The 76-bp, paired-end RNA-Seq data produced using the Illumina HiSeq 2500 sequencer for D. ananassae virgin female carcass, male carcass, female ovaries, and male testes were obtained from the ENA using the accession number SRP058321 (Rogers et al. 2014). The 75-bp, paired-end RNA-Seq data produced by the Illumina Genome Analyzer IIx sequencer from Wolbachia-cured D. ananassae embryos were obtained from the ENA using the accession number SRP007906 (Kumar et al. 2012).
The RNA-Seq reads from these seven samples were mapped against the improved D. ananassae assembly using two rounds of HISAT2 (Kim et al. 2015). The RNA-Seq read counts for each gene were determined by htseq-count (Anders et al. 2015) using default parameters. The D. ananassae gene predictions with no mapped reads in any of the seven RNA-Seq samples were omitted from the analysis. The regularized log (rlog) transformed expression level of each D. ananassae gene was calculated by the rlogTransformation function in DESeq2, where the experimental design information was used in the calculation of the gene-wise dispersion estimates (blind = FALSE). The distributions of rlog values for all D. ananassae genes, genes on the F element, and genes at the base of the D element were analyzed using violin plots and KW tests with the protocols described in the Gene characteristics analysis section.
Data availability
The D. ananassae ChIP-Seq data are available at the NCBI BioProject database with the accession number PRJNA369805. The D. ananassae PacBio whole-genome sequencing data are available at the NCBI BioProject database with the accession number PRJNA381646. The improved D. ananassae sequences and gene annotations, and the results of the bioinformatic analyses for D. ananassae and D. melanogaster, are available through the GEP UCSC Genome Browser Mirror (http://gander.wustl.edu).
Results
Sequence improvement of the D. ananassae F-element assembly
The D. ananassae CAF1 assembly was constructed by the Drosophila 12 Genomes Consortium through the reconciliation of the whole-genome shotgun (WGS) assemblies from multiple assemblers (Drosophila 12 Genomes Consortium 2007; Zimin et al. 2008). Earlier studies have shown that the F element has a higher repeat density compared to the other autosomes (reviewed in Riddle et al. 2009), which would likely result in a higher frequency of misassemblies (Salzberg and Yorke 2005). In this study, we performed manual sequence improvement of three regions from the D. ananassae F element (improved_13010, improved_13034_1, and improved_13034_2) and a euchromatic reference region near the base of the D element (improved_13337). These regions are labeled as “D. ana: F (improved)” and “D. ana: D (improved)” in the subsequent analysis, respectively (see Table S2 in File S7 for the coordinates of the improved regions and the corresponding comparison regions in D. melanogaster).
These sequence improvement efforts produce a 1.4-Mb, high-quality region for the D. ananassae F element (Table 1A) and a 1.7-Mb, high-quality region for the D element (Table 1B). The manual sequence improvement closed 35 gaps in the CAF1 assembly, adding a total of 26,266 bases. Net alignments between the improved regions and the corresponding regions in the CAF1 assembly show a total of 249 differences, with a total size difference of 79,735 bases. The large size differences could primarily be attributed to collapsed repeats in the CAF1 assembly. Some of the highly repetitive regions within the improved regions remained unresolved (6% in the F element and 2% in the D element). See the distributions of the problem regions in Figure S1 in File S7.
Table 1. Sequence improvement statistics for the D. ananassae F- and D-element regions studied.
| Region | Total (bp) | Gap (bp) | Unresolved (bp) | Low Quality (bp) |
|---|---|---|---|---|
| A | ||||
| improved_13010 | 597,760 | 495 | 7048 | 16 |
| improved_13034_1 | 490,783 | 50 | 70,695 | 944 |
| improved_13034_2 | 395,316 | 25 | 7204 | 392 |
| Total | 1,483,859 | 570 | 84,947 | 1352 |
| B | ||||
| improved_13337 | 1,708,805 | 0 | 30,512 | 709 |
Unresolved regions generally correspond to areas with large tandem or inverted repeats. Regions with Phred scores <30 (i.e., estimated error rate >1/1000 bases) are classified as low quality. Most low quality regions either overlap with unresolved regions or are located adjacent to gaps. (A) One region from the F-element scaffold improved_13010 and two regions from the F-element scaffold improved_13034 were improved, which results in a total of 1.4 Mb of high quality bases. (B) The region near the base of the D element (scaffold improved_13337) was improved, which results in 1.7 Mb high quality bases.
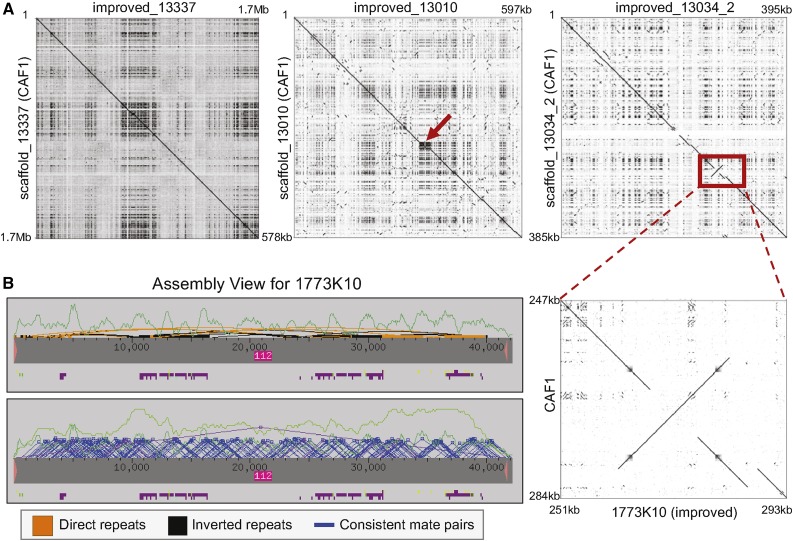
Dot plot alignments show that the improved scaffolds and the corresponding scaffolds in the CAF1 assembly are generally consistent with each other (Figure 1A, left and center). However, there is a major misassembly (inversion) in the second improved region within the scaffold improved_13034 (red box in Figure 1A right). This region is covered by the fosmid 1773K10 and manual sequence improvement revealed that the misassembled region contains a tandem repeat that is located adjacent to an inverted repeat (Figure 1B). The final assembly for the fosmid 1773K10 is supported by consistent forward-reverse mate pairs (Figure 1B, bottom) and the EcoRI and HindIII restriction digests (Figure S2A in File S7).
Figure 1.
Results of the manual sequence improvement of the D. ananassae D and F elements. (A) Dot plot comparisons of the scaffolds in the original CAF1 assembly (y-axis) vs. the scaffolds in the improved assembly (x-axis). Dots within each dot plot denote regions of similarity between the CAF1 assembly and the improved assembly. The diagonal lines in the dot plots for the D-element scaffold improved_13337 (left) and the F-element scaffold improved_13010 (middle) show that the overall CAF1 assemblies for these regions are consistent with the corresponding assemblies following manual sequence improvement. However, the high density of dots in the middle of the dot plot for improved_13010 corresponds to a collapsed repeat within the CAF1 assembly (red ←). Manual sequence improvement also identified a major misassembly in the second improved region (improved_13034_2) within the F-element scaffold_13034 (right), where part of the scaffold was inverted compared to the final assembly (red box). The misassembled region is part of the fosmid 1773K10 (bottom inset). (B) The Consed Assembly View for the improved fosmid project 1773K10 shows that the misassembled region contains multiple tandem and inverted repeats. (Top) The gray bar within the Assembly View corresponds to the improved fosmid assembly, and the pink Δ’s denote the ends of the fosmid. The purple and green boxes underneath the gray box correspond to tags (e.g., repeats and comments), and the dark green line corresponds to the read depth. The orange and black boxes above the gray bar correspond to tandem and inverted repeats, respectively. These orange and black boxes indicate that the improved assembly contains multiple tandem and inverted repeats that are located adjacent to each other. (Bottom) The improved assembly for this fosmid was supported by consistent forward-reverse mate pairs (blue Δ’s) and by multiple restriction digests (Figure S2A in File S7).
As part of the sequence improvement standards used in this study, the fosmid projects in the improved_13337, improved_13010, and improved_13034_2 regions are all supported by at least two restriction digests. The projects in the improved_13034_1 region are supported by long overlapping subreads produced by the PacBio sequencer (Figure S2B in File S7). In conjunction with the consistent forward-reverse mate pairs, these resources provide external confirmation of the final assembly and provide more accurate gap size estimates.
In addition to improving the assemblies, we also created a set of manual gene annotations for these improved regions, using the gene models in D. melanogaster as a reference. Collectively, we annotated 13 genes on the improved D. ananassae F-element regions and 125 genes at the base of the D element (see File S1 for the list of noncanonical features and differences in gene or isoform structures compared to the D. melanogaster orthologs).
Because of the low levels of sequence similarity between the untranslated regions of D. melanogaster genes and the D. ananassae assembly, we only annotated the coding regions of the D. ananassae genes and focused our analyses on the properties of the coding span (i.e., the region from start codon to stop codon, including introns). To avoid counting the same genomic region multiple times due to alternative splicing, we also restricted our analyses to the most comprehensive isoform for each gene (i.e., the isoform with the largest total coding exon size).
Identifying D. ananassae F element scaffolds
Earlier work has demonstrated that ∼95% of the D. melanogaster genes remain on the same Muller element across 12 Drosophila species that diverged >40 MYA (Bhutkar et al. 2008). To increase the statistical power of the comparative analysis with D. melanogaster, we identified additional D. ananassae F-element scaffolds based on sequence similarity to protein-coding genes on the D. melanogaster F element.
This analysis identified 64 complete D. ananassae F-element genes on 21 scaffolds [labeled “D. ana: F (all)” in the following analysis], with a total size of 18.7 Mb (see “Supplemental Methods” in File S7 and File S2 for details). These D. ananassae gene annotations are available through the GEP UCSC Genome Browser Mirror at http://gander.wustl.edu/ [D. ananassae (GEP/DanaImproved) assembly]. Using these resources and the orthologous regions from D. melanogaster [labeled “D. mel: F” and “D. mel: D (base)” in the following analysis, respectively], we performed a comparative analysis to examine the factors that contribute to the expansion of the D. ananassae F element and the impact of this expansion on gene characteristics.
Retrotransposons are the major contributors to the expansion of the D. ananassae F element
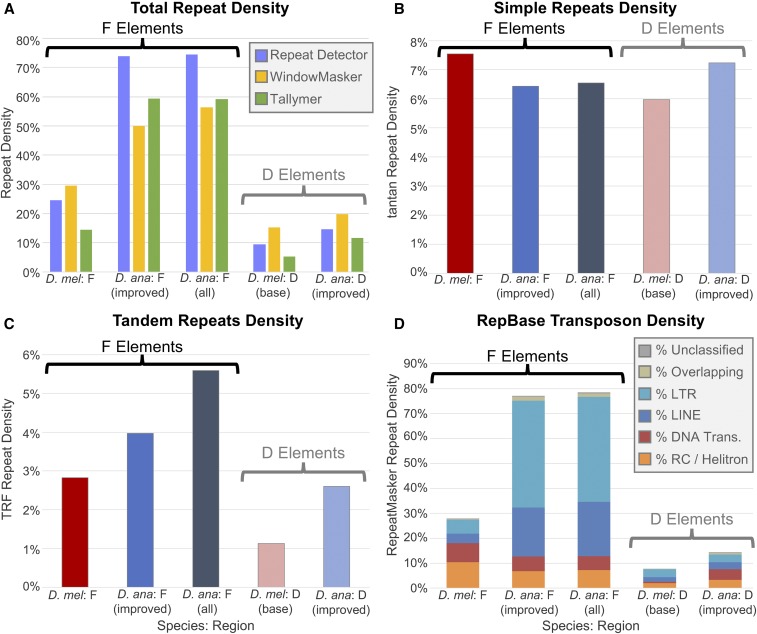
To ascertain the extent to which repetitive elements contribute to the expansion of the D. ananassae F element, we examined the analysis regions using three de novo repeat finders [Red (Girgis 2015), WindowMasker (Morgulis et al. 2006), and Tallymer (Kurtz et al. 2008)] that can identify novel repeat sequences. This analysis shows that repetitive sequences are major contributors to the expansion of the D. ananassae F element; the D. ananassae F element [D. ana: F (all)] has a repeat density of 56.4–74.5% compared to 14.4–29.5% for the D. melanogaster F element (Figure 2A). Within each species, the F element shows higher repeat density than the euchromatic region at the base of the D element.
Figure 2.
Expansion of the D. ananassae F element can primarily be attributed to the high density of LTR and LINE retrotransposons. (A) Total repeat density estimates from de novo repeat finders (Red, WindowMasker, and Tallymer) show that the D. ananassae F element has higher repeat density than the D. melanogaster F element (56.4–74.5% vs. 14.4–29.5%). Both F elements show higher repeat density than the euchromatic reference regions from the base of the D elements in D. ananassae (11.6–19.8%) and in D. melanogaster (5.4–15.2%). The repeat densities of the improved D. ananassae F-element scaffolds [D. ana: F (improved)] are similar to the repeat densities for all D. ananassae F-element scaffolds [D. ana: F (all)]. (B) Results from the tantan analysis show that the five analysis regions from D. melanogaster and D. ananassae have similar simple repeat density (6.0–7.5%). (C) TRF analysis shows that D. ananassae has higher tandem repeats density than D. melanogaster on both the F (5.6 vs. 2.8%) and the D elements (2.6 vs. 1.1%). (D) RepeatMasker analysis using the Drosophila RepBase library shows that the F element has higher transposon density than the D element both in D. melanogaster (28.0 vs. 7.7%) and in D. ananassae (78.6 vs. 14.4%). There is a substantial increase in the density of LTR and LINE retrotransposons on the D. ananassae F element compared to the D. melanogaster F element (42.1 vs. 5.5% and 21.8 vs. 3.8%, respectively). The D. ananassae euchromatic reference region also shows higher transposon density than D. melanogaster, but most of the difference can be attributed to the density of DNA transposons (4.3 vs. 0.6%). The region of overlap between two repeat fragments is classified as “Overlapping” if the two repeats belong to different repeat classes.
Subsequent analyses using tantan (Frith 2011), TRF (Benson 1999), and RepeatMasker with Drosophila transposons in the RepBase library (Jurka et al. 2005; Smit et al. 2013) show that transposons are major contributors to the expansion of the D. ananassae F element. The tantan analysis results show that the D. melanogaster and D. ananassae analysis regions have a similar density of simple repeats (6.0–7.5%; Figure 2B). However, the TRF analysis shows that the D. ananassae F element has a higher density of tandem repeats than the D. melanogaster F element (5.6 vs. 2.8%; Figure 2C). RepeatMasker analysis using the Drosophila RepBase library shows that the D. ananassae F element has a transposon density of 78.6%, compared to 28.0% on the D. melanogaster F element (Figure 2D). Most of the increase in transposon density can be attributed to LTR (42.1 vs. 5.5%) and LINE retrotransposons (21.8 vs. 3.8%). By contrast, while the total sizes of the Helitron and DNA transposon remnants on the D. ananassae F element are greater than those on the D. melanogaster F element, the Helitron and DNA transposon density are lower on the D. ananassae F element than the D. melanogaster F element (7.3 vs. 10.4% and 5.6 vs. 7.6%, respectively).
To mitigate the impact of potential biases in the RepBase repeat library on the results of the transposon density estimates, we performed an additional repeat analysis using a more comprehensive repeat library. This centroid repeat library is comprised of the Drosophila RepBase library; the consensus sequences for Drosophila helentrons and Helentron-associated INterspersed Elements (HINE) (Thomas et al. 2014); and consensus sequences derived from structural, assembly, alignment, and k-mer based de novo repeat finders (see “Supplemental Methods” in File S7 for details). RepeatMasker analysis of the D. ananassae F element using this centroid repeat library results in a transposon density estimate of 90.0%, an increase of 11.4% compared to the Drosophila RepBase library. However, the distributions of the different transposon classes are similar to the results obtained using the Drosophila RepBase library (Figure S3 in File S7) (RepeatMasker results for the centroid repeat library and for each de novo repeat library are available on the GEP UCSC Genome Browser).
Consistent with the results of the repeat density analysis, eight of the 10 most common transposons on the D. melanogaster F element are classified as rolling circle (RC) Helitrons or DNA transposons (Table 2). The 589 copies of DNAREP1 (DINE-1) RC/Helitron (from D. simulans, D. melanogaster, and D. yakuba) account for 32.1% of the transposon remnants on the D. melanogaster F element that were identified using RepeatMasker. The 84 copies of the 1360 DNA transposon (PROTOP) and its derivatives (i.e., PROTOP_A and PROTOP_B) account for 12.0% of all the transposon remnants on the D. melanogaster F element. The 1360 sequence has previously been demonstrated to be a target for heterochromatin formation and associated silencing (Sun et al. 2004; Haynes et al. 2006).
Table 2. The 10 most common transposons (by the cumulative size of the transposon fragments) on the D. melanogaster F element [i.e., the region between the most proximal (JYalpha) and the most distal (Cadps) genes].
| Repeat | Total Size (bp) | Repeat Count | Repeat Class | Total Region (%) | Total Repeat (%) |
|---|---|---|---|---|---|
| DNAREP1_DSim | 49,801 | 258 | RC/Helitron | 4.0 | 14.2 |
| DNAREP1_DM | 46,551 | 241 | RC/Helitron | 3.7 | 13.3 |
| PROTOP_A | 21,635 | 33 | DNA transposon | 1.7 | 6.2 |
| DNAREP1_DYak | 16,266 | 90 | RC/Helitron | 1.3 | 4.6 |
| DMCR1A | 12,993 | 40 | LINE | 1.0 | 3.7 |
| PROTOP | 11,769 | 18 | DNA transposon | 0.9 | 3.4 |
| FB4_DM | 10,290 | 36 | DNA transposon | 0.8 | 2.9 |
| TC1_DM | 8942 | 26 | DNA transposon | 0.7 | 2.6 |
| PROTOP_B | 8678 | 33 | DNA transposon | 0.7 | 2.5 |
| Gypsy-1_DSim-I | 8207 | 28 | LTR | 0.7 | 2.3 |
Eight of the 10 most common transposons on the D. melanogaster F element are RC/Helitrons and DNA transposons. The repeat count for each transposon corresponds to the total number of transposon fragments reported by the RepeatMasker annotation file.
By contrast, nine of the 10 most common transposons on the D. ananassae F element are LINE and LTR retrotransposons (Table 3). The CR1-2_DAn LINE retrotransposon is the most prominent type of transposon, with 2292 copies accounting for 9.6% of all transposon remnants on the D. ananassae F element. The second most common transposon is the Helitron-N1_DAn, with 2927 copies accounting for 6.4% of all transposon remnants and 68.9% of all RC/Helitrons identified on the D. ananassae F element. Seven of the most common transposons are LTR retrotransposons in the BEL and Gypsy families, accounting for 16.6% of the transposon remnants on the D. ananassae F element. Collectively, the 10 most common transposons account for 55.7% of all transposon remnants on the D. melanogaster F element and 35.6% of all transposon remnants on the D. ananassae F element.
Table 3. The 10 most common transposons (by the cumulative size of the transposon fragments) on all D. ananassae F-element scaffolds.
| Repeat | Total Size (bp) | Repeat Count | Repeat Class | Total Region (%) | Total Repeat (%) |
|---|---|---|---|---|---|
| CR1-2_DAn | 1,326,170 | 2292 | LINE | 7.5 | 9.6 |
| Helitron-N1_DAn | 882,145 | 2927 | RC/Helitron | 5.0 | 6.4 |
| BEL-14_DAn-I | 599,914 | 754 | LTR | 3.4 | 4.3 |
| Loa-1_DAn | 424,975 | 546 | LINE | 2.4 | 3.1 |
| BEL-1_DBp-I | 396,161 | 868 | LTR | 2.2 | 2.9 |
| BEL-10_DAn-I | 291,535 | 573 | LTR | 1.7 | 2.1 |
| BEL-18_DAn-LTR | 264,131 | 551 | LTR | 1.5 | 1.9 |
| Gypsy-30_DAn-I | 256,665 | 277 | LTR | 1.5 | 1.9 |
| Gypsy-23_DAn-I | 243,223 | 362 | LTR | 1.4 | 1.8 |
| Gypsy-23_DAn-LTR | 240,787 | 443 | LTR | 1.4 | 1.7 |
Nine of the 10 most common transposons on the D. ananassae F element are LINE and LTR retrotransposons. The repeat count for each transposon corresponds to the total number of transposon fragments reported by the RepeatMasker annotation file.
In addition to analyzing the overall transposon distributions, we also compared the transposon distributions within the introns of coding spans and within the intergenic regions (Table S3 in File S7). The intronic regions of D. melanogaster F-element genes have a higher total transposon density than the intergenic regions (38.9 vs. 31.7%). The increase in transposon density within the intronic regions of D. melanogaster F-element genes can primarily be attributed to RC/Helitrons (+6.3%). By contrast, the intronic and intergenic regions of D. ananassae F-element genes have similar transposon density (78.1 vs. 80.0%). The intronic regions of D. ananassae F-element genes show lower density of LTR retrotransposons (−6.4%) compared to the intergenic region but a higher density of RC/Helitrons (+2.3%) and DNA transposons (+1.6%).
In both D. ananassae and D. melanogaster, the intronic regions of D-element genes show lower transposon density than the intergenic regions (6.9 vs. 20.4% and 5.3 vs. 9.9%, respectively). For D. melanogaster, most of the differences can be attributed to the lower density of LTR retrotransposons (−4.7%) within the intronic regions of D-element genes. However, the density of LINE retrotransposons is greater within the intronic regions (+2.1%) of D. melanogaster D-element genes than within the intergenic regions. For D. ananassae, all the major classes of transposons show lower density within the intronic regions than the intergenic regions, particularly for DNA transposons (−5.2%), LTR retrotransposons (−3.3%), and RC/Helitrons (−2.6%).
Integration of Wolbachia sequences into the D. ananassae genome is a minor contributor to the expansion of the F element
Previous studies have shown that genomic sequences from wAna are integrated into the D. ananassae genome (Klasson et al. 2014; Choi et al. 2015). To ascertain the extent to which wAna integration has contributed to the expansion of the D. ananassae F element, we compared the D. ananassae improved assembly against the published assembly for wAna. Because the wAna assembly was based on Sanger sequencing reads recovered from the WGS sequencing of D. ananassae (Salzberg et al. 2005), the wAna assembly (GenBank assembly accession: GCA_000167475.1) is incomplete; it consists of 464 contigs with an N50 of 6730 bp (i.e., contigs this size and larger account for half of the total length of the assembly). Hence, we also compared the D. ananassae genome against two additional Wolbachia species that have been manually improved to a single high-quality scaffold: wRi (Klasson et al. 2009b) and wMel (Wu et al. 2004).
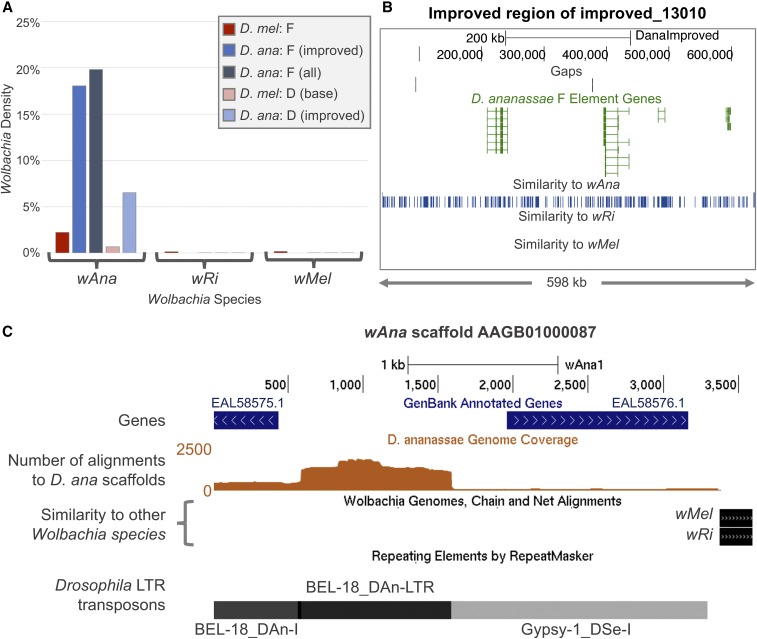
RepeatMasker comparison of the wAna genome assembly against the D. ananassae analysis regions suggest that Wolbachia sequences account for 19.79% of the D. ananassae F element and 6.51% of the base of the D. ananassae D element (Figure 3A). However, while earlier works have demonstrated that wRi is closely related to wAna (Klasson et al. 2009b; Choi and Aquadro 2014), RepeatMasker detected substantially fewer matches to wRi than to wAna on the D. ananassae F element (0.02%) and on the D element (0.05%). Similarly, we find substantially more matches to wAna than wMel on the D. melanogaster F and D elements (2.23 vs. 0.18% and 0.68 vs. 0.04%, respectively). Some of the matches to wRi and wMel can be attributed to Wolbachia genes that contain the same conserved domains (e.g., NADH dehydrogenase I, D subunit) as Drosophila genes (e.g., ND-49; Figure S4 in File S7).
Figure 3.
The high density of “Wolbachia” sequences in the D. ananassae F element can be attributed to Drosophila transposons in the wAna assembly. (A) RepeatMasker analysis shows that 19.8% of the D. ananassae F element matches the genome assembly for wAna. By contrast, 0.02% of the D. ananassae F element matches the genome assemblies for wRi and wMel. Similarly, the D. ananassae D element and the D. melanogaster F and D elements show a substantially higher density of regions that exhibit sequence similarity to the wAna assembly (0.68–6.51%) than the wRi and wMel assemblies (0.00–0.18%). (B) Distribution of regions with matches to the wAna, wRi, and wMel assemblies in the manually improved region of the D. ananassae F-element scaffold improved_13010. The matches to the wAna assembly are distributed throughout the improved scaffold (blue boxes) but there are no matches to either the wRi or the wMel assemblies. (C) The portions of the 3.6-kb wAna scaffold AAGB01000087 (x-axis) with large numbers of alignments to D. ananassae scaffolds show similarity to Drosophila transposons. The portions of the wAna scaffolds that show similarity to D. ananassae scaffolds were extracted from the RepeatMasker output and collated into an alignment coverage track relative to the wAna assembly (brown graph). Whole-genome Chain and Net alignments show that only the last 216 bp of this wAna scaffold has sequence similarity to the wRi and wMel assemblies. RepeatMasker analysis using the Drosophila RepBase library shows that the first 3.4 kb of this wAna scaffold has sequence similarity to the internal and long terminal repeat portions of the BEL-18 LTR retrotransposon (BEL-18_DAn-I and BEL-18_DAn-LTR), as well the internal portion of the Gypsy-1 LTR retrotransposon from D. sechellia (Gypsy-1_DSe-I). Most of the alignments can be attributed to BEL-18_DAn-LTR, with a maximum of 1835 alignments between the wAna scaffold AAGB01000087 and the D. ananassae scaffolds.
To ensure that the discrepancies in the Wolbachia density estimates cannot be explained by misassemblies in the D. ananassae assembly, we examined the distribution of the regions that showed sequence similarity to the wAna, wRi, and wMel assemblies on the manually improved region of the D. ananassae F-element scaffold improved_13034. We find that regions that match the wAna assembly are distributed throughout the improved scaffold, but there are no matches to either the wRi or the wMel assemblies (Figure 3B). Collectively, these results indicate that the wAna assembly contains sequences that are classified as being part of the Wolbachia genome that have no sequence similarity to either wRi or wMel.
Comparison of the wAna matches with the Drosophila transposon matches identified using RepeatMasker shows that most of the wAna matches overlap with Drosophila transposons in the RepBase library. For D. ananassae, 85.0% of the wAna matches on the F element and 83.1% of the wAna matches on the D element overlap with Drosophila transposon remnants identified using RepeatMasker. The RC/Helitron Helitron-N1_DAn is the most prominent class of transposon that overlaps with the wAna matches, accounting for 25.0% of all wAna matches on the D. ananassae F element and 32.1% of all wAna matches on the D element. Similarly, 92.3% of the wAna matches on the D. melanogaster F element and 93.6% of the wAna matches on the D element overlap with Drosophila transposons remnants identified using RepeatMasker. The DNAREP1_DSim RC/Helitron most frequently overlaps with the wAna matches on the D. melanogaster F element (32.2%), while the BEL_I-int LTR retrotransposon most frequently overlaps with the wAna matches on the D element (19.9%).
Because the wAna assembly is constructed from D. ananassae WGS sequencing reads and the wAna genome is integrated into the D. ananassae genome (Klasson et al. 2014; Choi et al. 2015), Drosophila transposons could have been incorporated into the wAna assembly. Matches to these Drosophila transposons in the wAna assembly would inflate the apparent wAna density on the D. ananassae F and D elements compared to the densities of wRi and wMel. To test this hypothesis, we determined the portions of the wAna assembly that aligned to the D. ananassae assembly from the RepeatMasker output and then calculated the D. ananassae scaffold alignment coverage relative to the wAna assembly. We also searched the wAna assembly against the Drosophila RepBase library to identify Drosophila transposons within the wAna assembly.
The scaffold AAGB01000209 in the wAna assembly shows the highest D. ananassae alignment coverage among all the wAna scaffolds (maximum coverage = 4813). RepeatMasker analysis of this scaffold with the Drosophila RepBase library shows that 56% (818/1460 bp) of this scaffold exhibits sequence similarity to the RC/Helitron Helitron-N1_DAn. By contrast, this scaffold did not show any sequence similarity to either wRi or wMel. The presence of the Helitron-N1_DAn within scaffold AAGB01000209 would explain the large number of overlapping matches between the wAna scaffolds and Helitron-N1_DAn on the D. ananassae F and D elements.
Another wAna scaffold that shows high D. ananassae alignment coverage (max coverage = 1835) is AAGB01000087 (Figure 3C). RepeatMasker analysis using the Drosophila RepBase library shows that 91.5% (3292/3598 bp) of this scaffold has sequence similarity to the BEL and Gypsy LTR retrotransposons in Drosophila. Only the last 216 bp of this scaffold show sequence similarity to wRi and wMel; there is no D. ananassae alignment coverage in this region.
Collectively, our analyses suggest that the high density of wAna matches on the D. ananassae F and D elements can likely be attributed to Drosophila transposons in the wAna assembly. Using the manually improved wRi assembly as a reference, only 0.02% (3567 bp) of the D. ananassae F element and 0.05% (817 bp) of the base of the D element show similarity to wRi. Hence our results suggest that the integration of Wolbachia into the D. ananassae genome is not a major contributor to the expansion of the assembled portions of the D. ananassae F element.
D. ananassae F element genes show distinct characteristics
To examine the impact of the expansion of the F element on gene characteristics, we used violin plots (Hintze and Nelson 1998) and the KW test (Kruskal and Wallis 1952) to compare the characteristics of the coding regions of F- and D-element genes in D. ananassae and D. melanogaster. If the KW test rejects the null hypothesis that the gene characteristics in the four analysis regions are derived from the same distribution, then post hoc DT (Dunn 1964) are used to identify the pairs of analysis regions that show significant differences [type I error (α) = 0.05]. The Holm–Bonferroni method is used to correct for multiple testing in the DT (Holm 1979) (see File S3 for the p-values of all the KW tests and the adjP for the DT).
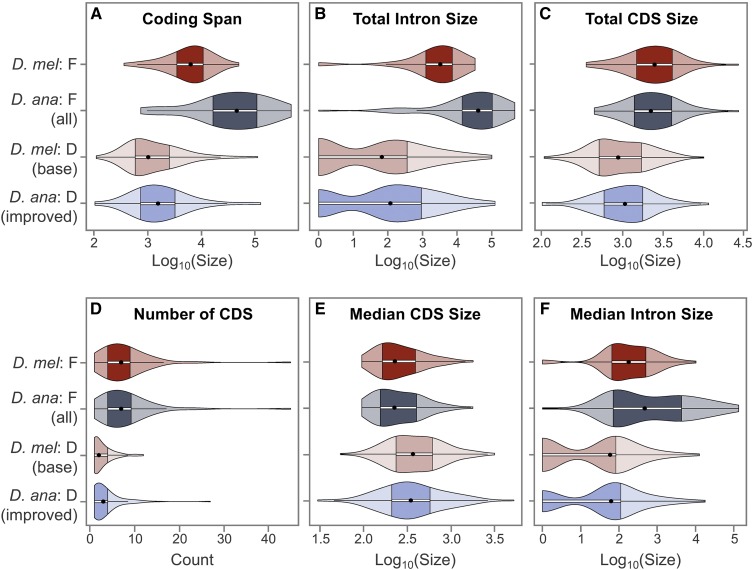
The violin plot and KW test show that the coding span sizes (i.e., distance from start codon to stop codon, including introns) of the genes in the four analysis regions have significantly different distributions (Figure 4A; KW test p-value = 1.40E−35). The DT show that the F-element genes in D. ananassae have significantly larger coding span sizes than in D. melanogaster (DT adjP = 6.82E−05). F-element genes in both D. melanogaster and D. ananassae have larger coding spans than D-element genes. Although the identity of the genes found at the base of the D. ananassae D element differs from those found at the base of the D. melanogaster D element (due to inversions and other rearrangements that have occurred within the element), DT shows that the difference in the distribution of coding span sizes is not statistically significant.
Figure 4.
F-element genes show distinct gene characteristics compared to D-element genes. Each violin plot is comprised of a box plot and a kernel density plot. The ● in each violin plot denotes the median and the darker region demarcates the IQR, which spans from the first (Q1) to the third (Q3) quartiles. The whiskers extending from the darker region spans from Q1 = −1.5 × IQR to Q3 = +1.5 × IQR; data points beyond the whiskers are classified as outliers. (A) D. ananassae F-element genes have larger coding spans (start codon to stop codon, including introns) than D. melanogaster F-element genes. (B) The D. ananassae F-element genes have larger coding spans because they have larger total intron sizes than D. melanogaster F-element genes. (C) F-element genes have larger total coding exon (CDS) sizes than D-element genes in both D. ananassae and D. melanogaster. (D) F-element genes have more CDS than D-element genes. (E) F-element genes have smaller median CDS size than D-element genes. (F) The median intron size for D. ananassae and D. melanogaster F-element genes shows a bimodal distribution; this distribution pattern indicates that the expansion of the coding spans of D. ananassae F-element genes compared to D. melanogaster F-element genes can be attributed to the substantial expansion of a subset of introns.
The difference in the distribution of coding span sizes can primarily be attributed to the differences in total intron sizes within the coding span (Figure 4B; p-value = 3.52E−37) and total coding exon (CDS) sizes (Figure 4C; p-value = 5.34E−17). DT shows that the coding spans of F-element genes in both D. melanogaster and D. ananassae are larger than D-element genes primarily because they have significantly larger total intron sizes (adjP range from 5.74E−30 to 2.88E−10) and total CDS sizes (adjP range from 3.69E−12 to 1.51E−07). Genes on the D. ananassae F element have significantly larger total intron sizes than D. melanogaster F element genes (adjP = 6.72E−05), while the differences in total CDS sizes are not statistically significant (adjP = 3.84E−01).
The larger total CDS sizes of F-element genes compared to D-element genes can be attributed to the greater number of CDS (Figure 4D; p-value = 5.08E−26; adjP range from 2.94E−17 to 1.26E−11). However, the median CDS sizes of F-element genes are significantly smaller than D-element genes (Figure 4E; p-value = 5.07E−06; adjP range from 1.07E−04 to 3.69E−03). In accordance with the larger total intron sizes in F-element genes, F-element genes have larger median intron sizes than D-element genes (Figure 4F; p-value = 5.68E−19; adjP range from 2.19E−11 to 1.39E−09). Although the violin plot shows that D. ananassae F-element genes have a larger IQR (first to third quartiles; IQR = 69–4286 bp) compared to D. melanogaster F-element genes (IQR = 64–499 bp), DT shows that this difference is not statistically significant (adjP = 4.11E−02). The kernel density component of the violin plots shows that the median intron sizes follow a bimodal distribution. Because the first quartile of the median intron sizes of D. ananassae F-element genes are similar to D. melanogaster F-element genes (69 vs. 64 bp) but the third quartile is 8.6 times larger (4286 vs. 499 bp), the expansion of the coding spans of D. ananassae F-element genes compared to D. melanogaster can primarily be attributed to the expansion of a subset of the introns.
In addition to comparing gene characteristics across the four analysis regions, we also compared the characteristics of D. ananassae F-element genes (minuend) with their orthologs in D. melanogaster (subtrahend) (D-element genes were omitted from this analysis because different sets of genes are found near the base of the D. ananassae and D. melanogaster D elements). These difference violin plots show that D. ananassae F-element genes generally have larger coding spans (median difference = +32,500 bp) than their D. melanogaster orthologs (Figure S5A in File S7) because they have larger total intron sizes (median difference = +32,570 bp; Figure S5B in File S7). By contrast, the total coding exon size is slightly smaller in D. ananassae F-element genes compared to their D. melanogaster orthologs (median difference = −15 bp; Figure S5C in File S7). In most cases, D. ananassae and D. melanogaster F-element genes have the same number of CDS (Q1 = median = Q3 = 0; Figure S5D in File S7). D. ananassae genes have similar median CDS sizes (median difference = 0 bp; Figure S5E in File S7) and larger median intron sizes (median difference = +213 bp; Figure S5F in File S7) compared to their D. melanogaster orthologs. These results indicate that the expansion of D. ananassae F-element genes compared to their D. melanogaster orthologs can be attributed to the increase in intron size.
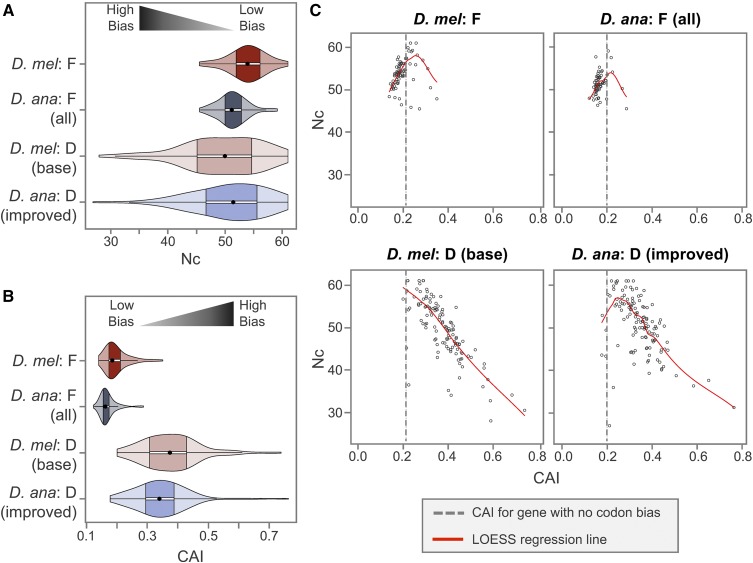
D. ananassae F-element genes show less optimal codon usage
As previously demonstrated, F-element genes in different Drosophila species show lower codon-usage bias than genes in other autosomes (Vicario et al. 2007; Leung et al. 2015). To assess how the expansion of the D. ananassae F element affects the usage of synonymous codons, we analyzed the distributions of codon usage bias for F- and D-element genes using two metrics: the Nc (Wright 1990) and the CAI (Sharp and Li 1987). Nc measures the deviations from the uniform usage of synonymous codons, and these deviations can be attributed to either mutational biases or selection. Nc values range from 20 to 61, where greater Nc values correspond to lower codon bias because it indicates that more synonymous codons were used. By contrast, CAI measures deviations from optimal codon usage, which reflects selection. CAI values range from 0.0 to 1.0, where greater values correspond to more optimal codon usage.
Violin plots of Nc show that D. ananassae F-element genes exhibit significantly more deviations from the uniform usage of synonymous codons (i.e., a lower Nc) than D. melanogaster F-element genes (Figure 5A; KW test p-value = 2.59E−06; DT adjP = 4.65E−04). The difference violin plot for Nc shows that most D. ananassae F-element genes have a lower Nc than their corresponding D. melanogaster orthologs (Figure S5G in File S7; median = −3.012). While the IQR for the Nc distribution of D. ananassae F-element genes (49.99–52.85) is smaller than the IQR for the D. melanogaster and the D. ananassae D-element genes (45.11–54.66 and 46.67–55.58, respectively), this difference is not statistically significant (adjP = 3.37E−01 and 3.83E−01, respectively).
Figure 5.
Codon bias in D. ananassae F-element genes can primarily be attributed to mutational biases instead of selection. (A) Violin plots of the Nc show that D. ananassae F-element genes exhibit stronger deviations from equal usage of synonymous codons (lower Nc) than D. melanogaster F-element genes. (B) Violin plots of the CAI show that D. ananassae F-element genes exhibit less optimal codon usage (lower CAI) than D. melanogaster F-element genes. F-element genes in both D. ananassae and D. melanogaster show less optimal codon usage (lower CAI) than D-element genes. (C) Scatterplot of Nc vs. CAI suggests that the codon bias in most D. ananassae and D. melanogaster F-element genes can be attributed to mutational bias instead of selection, as indicated by the placement of most of the genes in the portion of the LOESS regression line (red line) with a positive slope. By contrast, the codon bias in most D. ananassae and D. melanogaster D-element genes can be attributed to selection, as denoted by the LOESS regression line with a negative slope. The dotted line in each Nc vs. CAI scatterplot corresponds to the CAI value for a gene with equal codon usage relative to the species-specific reference gene sets constructed by the program scnRCA (0.200 for D. ananassae and 0.213 for D. melanogaster). Hence this species-specific threshold corresponds to the CAI value when the strengths of mutational bias and selection on codon bias are the same. A smaller percentage of F-element genes in D. ananassae (6/64; 9.4%) have CAI values above this species-specific CAI threshold compared to D. melanogaster (18/79; 22.8%).
Violin plots of CAI show that D. ananassae F-element genes exhibit significantly less optimal codon usage (i.e., lower CAI) than D. melanogaster F-element genes (Figure 5B; p-value = 7.32E−55; adjP = 1.24E−02). The difference violin plot for CAI shows that this difference can be seen on a gene-by-gene basis; most D. ananassae F-element genes have a lower CAI than their corresponding D. melanogaster orthologs (Figure S5H in File S7; median = −0.025). Genes on the F element show significantly lower CAI compared to D-element genes in both D. melanogaster and D. ananassae (adjP range from 8.41E−38 to 1.96E−20).
To further investigate the factors that contribute to the differences in codon bias between D. ananassae and D. melanogaster F-element genes, we constructed Nc vs. CAI scatterplots for the four analysis regions (Vicario et al. 2007) and then applied LOESS (Cleveland and Devlin 1988) to capture the trends in each scatterplot (Figure 5C). Deviations from the uniform usage of synonymous codons (i.e., lower Nc) can be attributed either to selection or mutational biases. By contrast, optimal codon usage (i.e., higher CAI) can primarily be attributed to selection. Hence, the codon bias for genes that are placed in the part of the LOESS regression line with a positive slope can primarily be attributed to mutational biases, while the codon bias for genes that are placed in the part of the LOESS regression line with a negative slope can be attributed to selection (see Vicario et al. 2007 for details on this analysis technique).
The LOESS regression lines for the Nc vs. CAI scatterplots for the D. melanogaster and D. ananassae F elements both show an inverted-V pattern (Figure 5C, top). Most of the F-element genes are placed in the part of the LOESS regression line with a positive slope, which suggests that the codon bias in these genes can primarily be attributed to mutational biases. By contrast, most of the genes on the D. ananassae and D. melanogaster D elements are placed in the part of the LOESS regression line with a negative slope, which suggests that most of the codon bias for these genes can be attributed to selection (Figure 5C, bottom). Consistent with the results of the violin plot, D. ananassae F-element genes (Figure 5C, top right) show lower Nc values than D. melanogaster F-element genes (Figure 5C, top left). This pattern suggests that the additional codon bias in D. ananassae F-element genes can primarily be attributed to mutational biases instead of selection.
Earlier works have shown that mutational bias favors A/T while selection favors G/C at synonymous sites in Drosophila (Moriyama and Powell 1997; Vicario et al. 2007). Examination of the GC content of codons shows that F-element genes have a lower GC content than D-element genes (38.9–41.9% vs. 54.7–55.3%, Table 4). The most prominent difference in GC content is at the third position of the codon, where the GC content is 30.1% lower in D. melanogaster F-element genes and 34.4% lower in D. ananassae F-element genes, compared to the D-element genes in each species. The codons for D. ananassae F-element genes have the lowest GC content among all the analysis regions. In particular, the GC content of the third position of the codon is 5.8% lower in D. ananassae F-element genes compared to D. melanogaster F-element genes. By contrast, the GC content of the third codon position is only 1.5% lower in D. ananassae D-element genes compared to D. melanogaster D-element genes. Similarly, the GC content of the fourfold degenerate sites is 5.0% lower in D. ananassae F-element genes compared to D. melanogaster F-element genes, but only 2.0% lower in D-element genes. This pattern of lower G/C content of the wobble base in D. ananassae F-element genes compared to D. melanogaster F-element genes is seen in all amino acids that are encoded by two or more codons. The biggest difference in codon usage between D. ananassae and D. melanogaster F-element genes is histidine; with an 8.6% increase in the usage of CAT (69.6 vs. 61.0%) instead of CAC (30.4 vs. 39.0%) in D. ananassae (see “Supplemental Methods” in File S7 and File S4 for the comparisons of the codon frequencies for all codons).
Table 4. Codon GC content for D. melanogaster and D. ananassae F- and D-element genes.
| Metric | D. mel: F (%) | D. ana: F (All) (%) | D. mel: D (%) | D. ana: D (Improved) (%) |
|---|---|---|---|---|
| Coding GC | 41.9 | 38.9 | 55.3 | 54.7 |
| First letter GC | 48.8 | 46.9 | 56.8 | 55.9 |
| Second letter GC | 40.2 | 39.0 | 42.7 | 42.9 |
| Third letter GC | 36.5 | 30.7 | 66.6 | 65.1 |
| 4D sites GCa | 33.2 | 28.2 | 62.5 | 60.5 |
F-element genes show lower GC content than D-element genes in both D. melanogaster and D. ananassae. D. ananassae F-element genes show the lowest overall GC content among the genes in the four analysis regions, particularly at the third position of the codon.
The “4D sites GC” corresponds to the GC content at fourfold degenerate sites (i.e., the GC content at the third position of the codons that code for alanine, glycine, proline, threonine, and valine).
To further explore the relationships between CAI and other gene characteristics, we analyzed the gene characteristics of the subset of F-element genes that show high CAI values. Among the 64 D. ananassae F-element genes analyzed in this study, six genes (Arf102F, ATPsynbeta, CG31997, Crk, ND-49, and RpS3A) have higher CAI values than that of a gene with equal codon usage (i.e., CAI > 0.200). Only five D. ananassae F-element genes (Arf102F, ATPsynbeta, Crk, onecut, and RpS3A) exhibit smaller total intron size compared to their D. melanogaster orthologs. Four of these genes (Arf102F, ATPsynbeta, Crk, and RpS3A) are present in both groups (Figure S6 in File S7, top right). Hence, the subset of D. ananassae F-element genes with a smaller total intron size compared to their D. melanogaster orthologs is significantly enriched in genes with higher CAI values (hypergeometric distribution p-value = 1.15E−04).
By contrast, of the 18 D. melanogaster F-element genes with CAI values greater than the CAI for a gene with equal codon usage (CAI > 0.213), only three genes (ATPsynbeta, onecut, and RpS3A) show a smaller total intron size in D. ananassae than in D. melanogaster (Figure S6 in File S7, top left; hypergeometric distribution p-value = 7.49E−02). The 16 D. ananassae F-element genes that show the largest increase in total intron size (i.e., genes in the fourth quartile; size difference ≥ +97,610 bp) all have lower CAI values than a gene with uniform codon usage in D. melanogaster (Figure S6 in File S7, bottom left) and in D. ananassae (Figure S6 in File S7, bottom right). These results suggest that there is a small group of D. ananassae F-element genes that are under selection for more optimal codon usage and smaller coding span size.
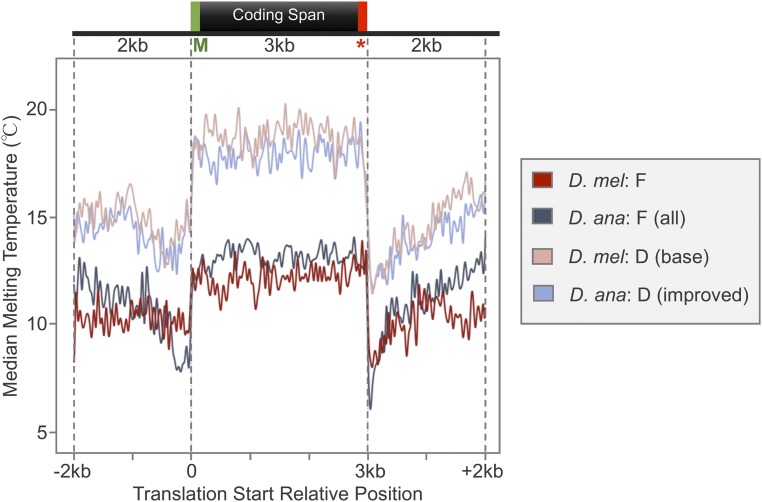
F element genes have lower Tm than D-element genes
Given the prior observations of low Tm across the coding spans of F-element genes (Leung et al. 2015), we constructed median Tm metagene profiles for the coding spans of the genes in all four analysis regions (Figure 6). The metagene profiles show that F-element genes have lower Tm than D-element genes in D. ananassae (median = 13.15 vs. 18.00°) and in D. melanogaster (median = 12.27 vs. 18.75°). Despite the lower codon GC content and the higher transposon density within the introns of D. ananassae F-element genes (both of which have been inferred to lower Tm), the coding spans of D. ananassae F-element genes show slightly higher median Tm than the median Tm of D. melanogaster F-element genes (median = 13.15 vs. 12.27°; IQR = 12.54–13.74° vs. 11.50–13.00°). Hence these two characteristics are not the primary determinant of the Tm of the coding span. The metagene profiles also show that the 2-kb regions upstream and downstream of the coding spans have lower Tm than the coding span in all four analysis regions.
Figure 6.
Metagene analysis shows that the coding spans of F-element genes have lower median 9-bp Tm than the genes at the base of the D element. The Tm profiles were determined using a 9-bp sliding window with a step size of 1 bp. The metagene consists of the 2-kb region upstream and downstream of the coding span, with the length of the coding spans normalized to 3 kb. While the codons in D. ananassae F-element genes have lower GC content, D. ananassae F-element genes exhibit a Tm profile that is similar to the D. melanogaster F-element genes. The green “M” below the coding span denotes the Methionine at the translation start site, and the red star denotes the stop codon.
F-element genes maintain distinct histone modification profiles
To explore the epigenomic landscape of the D. ananassae F element, we generated ChIP-Seq data sets for three histone modifications using third instar larvae: dimethylation of lysine 4 on histone 3 (H3K4me2), associated with transcription start sites during gene activation; dimethylation of lysine 9 on histone 3 (H3K9me2), associated with heterochromatin formation and gene silencing; and trimethylation of lysine 27 on histone 3 (H3K27me3), associated with gene silencing through the Polycomb system. The modENCODE project has previously produced ChIP-Seq data for these histone modifications in D. melanogaster (Ho et al. 2014), thereby enabling us to compare the epigenomic landscape of the D. ananassae and D. melanogaster F elements.
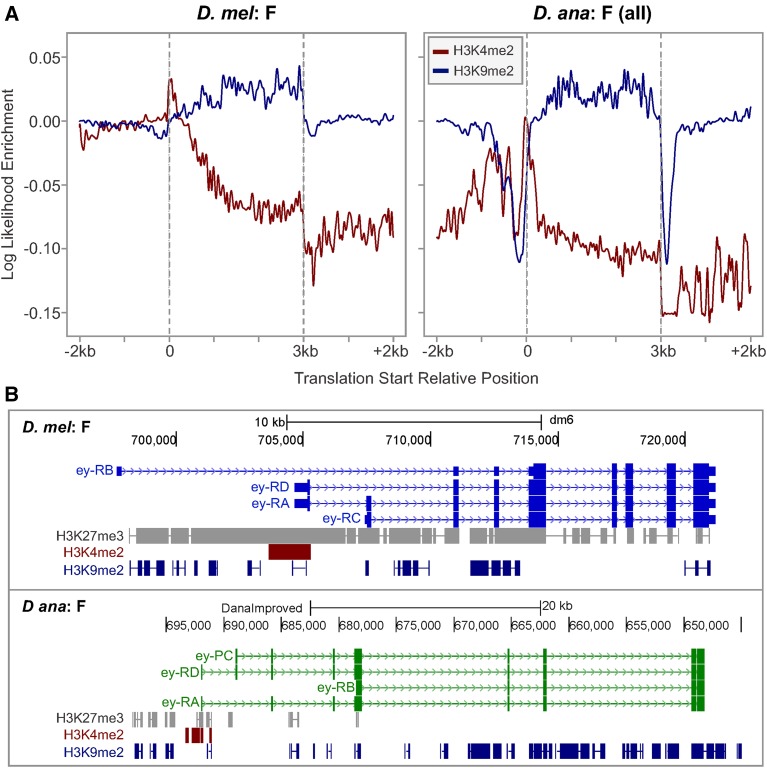
Because the gene annotations for D. ananassae only include the coding regions, the analysis of histone-modification enrichment patterns are relative to the translated regions instead of the transcribed regions, even though the H3K4me2 modification typically shows enrichment near the transcription start sites of active genes (reviewed in Boros 2012). Metagene analysis of the H3K4me2 and H3K9me2 log-enrichment profiles produced by MACS2 (Zhang et al. 2008) show that D. ananassae and D. melanogaster F-element genes exhibit similar H3K4me2 and H3K9me2 enrichment profiles (Figure 7A). The 5′ end of F-element genes are enriched in H3K4me2 while the coding span is enriched in H3K9me2. By contrast, consistent with a previous report on the epigenomic landscape of euchromatic genes in D. melanogaster (Riddle et al. 2011), D-element genes in both species show H3K4me2 enrichment near the 5′ end of the gene but the coding span generally does not show H3K9me2 enrichment.
Figure 7.
Histone modification profiles for D. ananassae and D. melanogaster F-element genes at the third instar larval stage of development. (A) Metagene analysis shows that the region surrounding the 5′ end of F-element genes is enriched in H3K4me2 while the body of the coding span is enriched in H3K9me2. The values in the y-axis within each metagene plot correspond to the log-likelihood ratio between each ChIP sample and input control (assuming a dynamic Poisson model) as determined by MACS2. (B) Differences in the H3K27me3 enrichment patterns for the D. melanogaster ey gene and its ortholog in D. ananassae. The entire coding span of the ey gene is enriched in H3K27me3 in D. melanogaster (top) (for the D. melanogaster gene models, the thick boxes denote the coding exons and the thin boxes denote the untranslated regions). By contrast, only the region surrounding the 5′ end of the ey ortholog in D. ananassae shows H3K27me3 enrichment. The 5′ ends of the A and D isoforms of ey shows enrichment of H3K4me2 and H3K27me3 in both D. melanogaster and D. ananassae. These bivalent domains suggest that these two isoforms of ey are poised for activation at the third instar larval stage of development in both species.
A subset of Drosophila genes show enrichment of H3K27me3; the expression of these genes is typically regulated by Polycomb group (PcG) proteins (reviewed in Lanzuolo and Orlando 2012). Eight of the D. melanogaster F-element genes (dati, ey, fd102C, fuss, Sox102F, sv, toy, and zfh2) show H3K27me3 enrichment at the third instar larval stage, with this histone mark being broadly distributed throughout the coding span of each gene (Figure S7 in File S7, left). Four of these genes (dati, fuss, sv, and zfh2) show stronger H3K27me3 enrichment near the 5′ end of the gene. The corresponding orthologs of these eight genes on the D. ananassae F element also show H3K27me3 enrichment. However, in all cases, the H3K27me3 enrichment tends to be restricted to the region near the 5′ end of the gene while the body of the coding span is enriched in H3K9me2 (Figure S7 in File S7, right).
Six of the eight F-element genes (ey, fd102C, Sox102F, sv, toy, and zfh2) that show H3K27me3 enrichment also show H3K4me2 enrichment in the region surrounding the 5′ end of the gene in both D. melanogaster and D. ananassae (Figure S7 in File S7). These “bivalent promoters” (i.e., promoters with both active and repressive histone modifications) are often found in developmentally regulated genes that are poised to be activated upon differentiation (reviewed in Voigt et al. 2013). For the D. melanogaster gene ey and its D. ananassae ortholog, H3K4me2 is only enriched in the region surrounding the 5′ ends of the A and D isoforms (Figure 7B), which suggests that these two isoforms of ey are poised to be activated at the third instar larval stage of development in both species.
D. ananassae F-element and D-element genes exhibit similar expression profiles
To assess whether the unusual genomic landscape of the D. ananassae F element would affect the levels of gene expression, we analyzed the RNA-Seq data from adult females and adult males (Chen et al. 2014), female carcass, male carcass, female ovaries, male testes (Rogers et al. 2014), and embryos (Kumar et al. 2012). This expression analysis is based on release 1.04 of the D. ananassae Gnomon gene predictions from FlyBase (Attrill et al. 2016), which include predictions of untranslated regions and alternative isoforms.
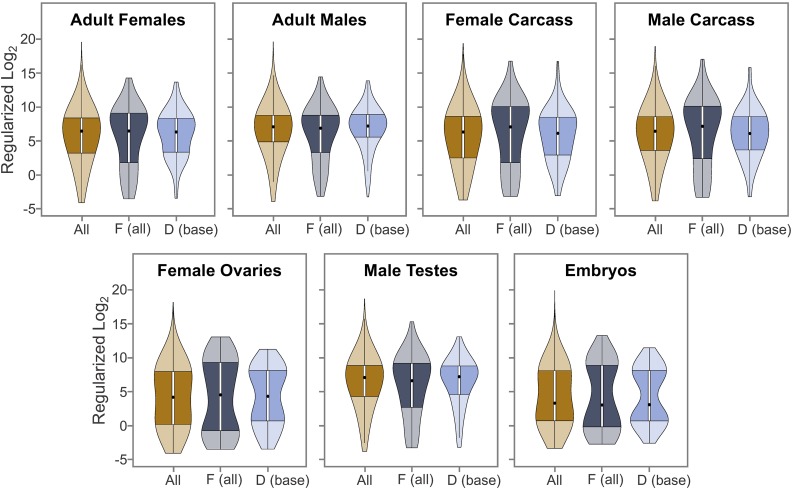
Violin plots of the rlog-transformed expression values show that the Gnomon-predicted genes on the D. ananassae F element exhibit similar expression patterns compared to genes on all scaffolds and genes at the base of the D element (Figure 8). The KW test shows that the differences in expression patterns among these three regions are not statistically significant in each of the seven developmental stages and tissues (p-values range from 1.30E−01 in female carcass to 9.50E−01 in embryos). Thus, despite the unusual genomic and epigenomic landscape, D. ananassae F-element genes show a range of expression patterns that is similar to those of genes in euchromatic regions.
Figure 8.
D. ananassae F-element genes show similar expression patterns compared to genes on other Muller elements. RNA-Seq reads from seven samples (adult females, adult males, female carcass, male carcass, female ovaries, male testes, and embryos) were mapped against the improved D. ananassae genome assembly and the read counts for the Gnomon gene predictions were tabulated by htseq-count. The read counts for the seven samples were normalized by library size and then transformed using Tikhonov/ridge regularization in the DESeq2 package to stabilize the variances among the samples. The violin plots compare the distributions of the regularized log2 expression values for the D. ananassae Gnomon gene predictions on all scaffolds (All), on the F-element scaffolds [F (all)], and on the base of the D element [D (base)] for these different developmental stages and tissues.
However, preliminary analyses of the seven RNA-Seq samples indicate a negative Spearman’s rank correlation coefficient between CAI and rlog expression values for D. ananassae F-element genes, but a positive correlation for all D. ananassae genes (“Supplemental Results,” Figure SM1, and Table SM1 in File S7). These results are consistent with the hypothesis that mutational biases (rather than selection) are the primary contributors to the codon biases observed in D. ananassae F-element genes. However, the results are considered preliminary because of the small number of replicates available for each RNA-Seq sample, and potential issues with the Gnomon gene predictions (e.g., GF22695; Figure SM2 in File S7).
Our preliminary results also suggest that D. ananassae and D. melanogaster F-element genes exhibit similar ranges of expression values despite the substantial expansion of most D. ananassae F-element genes compared to their D. melanogaster orthologs (“Supplemental Results,” Figure SM3, and Table SM2 in File S7). Collectively, these preliminary results suggest that D. ananassae F-element genes have adapted to their local environment in some way to maintain their expression in a heterochromatic domain with high repeat density. See “Supplemental Results” and “Supplemental Methods” in File S7 for additional details on these analyses.
Discussion
This study describes an initial survey of the characteristics of the D. ananassae F element through a comparative analysis with the D. melanogaster F element and the base of the D. ananassae and D. melanogaster D elements. In concordance with the results of previous comparative analysis of D. virilis (Leung et al. 2010) and of D. erecta, D. mojavensis, and D. grimshawi (Leung et al. 2015), we find that the D. ananassae F element generally exhibits distinct characteristics compared to the base of the D element. However, the contrasts between the characteristics of the D. ananassae F and D elements tend to be larger than the contrasts seen in the other Drosophila species, presumably because of the higher density of repetitious elements in the D. ananassae F element.
Given the propensity for repetitive sequences to be misassembled in whole-genome assemblies (Salzberg and Yorke 2005; Phillippy et al. 2008), we first assessed the veracity of the F-element scaffolds in the D. ananassae CAF1 assembly prior to the analysis of the repeat and gene characteristics. Manual sequence improvement of 1.4 Mb of the D. ananassae F element and 1.7 Mb of the base of the D element resolved 35 gaps in the CAF1 assembly (Table 1) and resolved a major misassembly within the D. ananassae F-element scaffold improved_13034 (Figure 1). In conjunction with the manually curated gene models, we have generated data that provide an important metric for assessing the quality of the F-element scaffolds in the D. ananassae CAF1 assembly and provide more accurate measures of repeat and gene characteristics. Because the comparisons of the improved regions and the corresponding regions in the CAF1 assembly show that they are generally in congruence with each other (Figure 1A), where possible we expanded the general analysis to include all putative F-element scaffolds in the D. ananassae CAF1 assembly to produce a more comprehensive overview of the properties of the D. ananassae F element.
Repeat analysis of the F element and the base of the D element from D. ananassae and D. melanogaster show that the expansion of the D. ananassae F element can primarily be attributed to repetitive sequences (Figure 2A). The D. ananassae F element shows a similar density of simple repeats compared to the other analysis regions (Figure 2B), and a higher density of tandem repeats (Figure 2C). However, most of the increase in total repeat density can be attributed to transposons, particularly LTR and LINE retrotransposons (Figure 2D).
The transposon density estimate for the D. ananassae F element (78.6%) is substantially higher than the previous estimate of 32.5% (Schaeffer et al. 2008). However, older versions of the Drosophila RepBase library has been shown to have a strong bias toward transposons in D. melanogaster, which would result in an underestimate of the transposon density in the other Drosophila species (Drosophila 12 Genomes Consortium 2007; Leung et al. 2010). Hence, the difference in the transposon-density estimates can be attributed to the use of a newer version of the Drosophila RepBase library (release 20150807), which includes transposons from other Drosophila species, including D. ananassae. Repeat analysis of the D. ananassae F element using the centroid repeat library (comprised of Drosophila repeats in the RepBase library, helentrons and HINE sequences, and repeats from de novo repeat finders) results in a repeat density estimate of 90.0% (Figure S3 in File S7), which suggests that there might be additional D. ananassae transposons that are not part of the RepBase library.
The repeat analysis also shows substantial differences in the repeat composition of the D. melanogaster and D. ananassae F elements. In contrast to the D. melanogaster F element where eight out of 10 of the most common transposons are RC/Helitrons and DNA transposons (Table 2), nine out of 10 of the most common transposons on the D. ananassae F element are LTR and LINE retrotransposons (Table 3). The two most prominent transposons on the D. ananassae F element are the LINE element CR1-2_DAn and the RC/Helitron Helitron-N1_DAn, which account for 7.5 and 5.0% of the D. ananassae F-element scaffolds, respectively.
An unusual aspect of the expansion of the D. ananassae F element is that the expansion appears to be mostly limited to a single Muller element, as D. melanogaster and D. ananassae have similar total genome sizes (Gregory and Johnston 2008). Previous analyses have shown that the accumulation of retrotransposons can result in a substantial increase in genome size (Kidwell 2002), particularly in plant genomes (Piegu et al. 2006; reviewed in Lee and Kim 2014). However, a recent invasion of retrotransposons that results in the expansion of the D. ananassae F element should also result in a concomitant increase in the size of the euchromatic portion of the genome. While the base of the D. ananassae D element shows a 6.7% increase in transposon density compared to the D. melanogaster D element (+6.7%; 14.4 vs. 7.7%), this increase in transposon density is much smaller than the increase of 50.6% seen on the F element (78.6 vs. 28.0%). Because the amplifications of transposons could impact the fitness of the organism (reviewed in Pritham 2009), one possible explanation for this dichotomy is that the F element already has the necessary mechanisms in place for silencing transposons and for allowing gene function in a region with a high density of transposons (Sun et al. 2004; Riddle et al. 2012). These mechanisms would mitigate the impact of a local expansion of transposons on the fitness of D. ananassae and enable the F element to accumulate a higher density of transposons than the euchromatic regions.
Two earlier studies have shown that the wAna genome is integrated into the genome of multiple strains of D. ananassae via horizontal gene transfer (Klasson et al. 2014; Choi et al. 2015). However, our analyses indicate that the integration of wAna into the D. ananassae genome is unlikely to be a major contributor to the expansion of the assembled portions of the D. ananassae F element (Figure 3). RepeatMasker analysis shows that the D. ananassae F element has a high density of regions that show sequence similarity to the wAna assembly (Figure 3A). However, while previous studies have shown that wAna is closely related to wRi (Klasson et al. 2009b; Choi and Aquadro 2014), we find that most of the regions within the D. ananassae F element that show strong sequence similarity to the published wAna assembly do not show sequence similarity to either the wRi or the wMel assemblies (Figure 3B).
The large disparity in the density of matches to the wAna assembly compared to the wRi and wMel assemblies on the D. ananassae F element could be attributed to the different strategies used to construct these Wolbachia assemblies. The subset of genomic reads from the WGS sequencing of D. ananassae that shows sequence similarity to wMel and their corresponding mate-pair reads was used to construct the wAna assembly (Salzberg et al. 2005). By contrast, the wRi (Klasson et al. 2009b) and the wMel (Wu et al. 2004) assemblies have been manually improved to high quality. A previous study has identified Drosophila retrotransposons within wAna fragments that are integrated into the D. ananassae genome (Choi et al. 2015). Hence, constructing the wAna assembly based on the D. ananassae WGS reads would likely result in the incorporation of Drosophila transposons into the wAna assembly. Consistent with this hypothesis, we find that most of the matches between the wAna assembly and the D. ananassae assembly show sequence similarity to transposons in the Drosophila RepBase library (Figure 3C). However, given the potential for Wolbachia to act as a vector for the horizontal transfer of transposable elements (Dupeyron et al. 2014), it is still possible that these Drosophila transposons are actually part of the wAna genome.
Using sequence similarity to wRi and wMel as the metric, we found only a low density of Wolbachia sequences within the 1.4-Mb manually improved region of the D. ananassae F element and the collection of all 18.7-Mb F-element scaffolds from the D. ananassae assembly (Figure 3A). However, because of gaps and misassemblies in the improved assembly, the possibility remains that wAna is integrated into the unassembled portions of the D. ananassae F element. In contrast to wAna, both wRi and wMel appear to infect but are not integrated into the D. simulans and D. melanogaster genomes. Hence the wAna genome might contain additional sequences that facilitate horizontal gene transfer that are not in either wRi or wMel. However, an improved wAna assembly would be required to identify these novel sequences. Based on the data currently available, we conclude that the expansion of the assembled portions of the D. ananassae F element can be explained by the increase in the density of Drosophila transposons, and that the integration of wAna is only a minor contributor to the expansion of the D. ananassae F element.
Previous work has demonstrated that F-element genes exhibit distinct characteristics compared to genes in the euchromatic reference regions in D. melanogaster, D. erecta, D. virilis, D. mojavensis, and D. grimshawi (Leung et al. 2010, 2015). Part of the difference in gene characteristics can be attributed to the low rates of recombination on the F element, which reduces the effectiveness of selection (Hill and Robertson 1966; Betancourt et al. 2009; Arguello et al. 2010). To assess the impact of the expansion of the D. ananassae F element on gene characteristics, we compared the characteristics of the genes on the D. melanogaster and D. ananassae F elements and on a euchromatic reference region at the base of the D elements. The base of the D element is used as a comparison region to mitigate the confounding effects caused by the proximity to the centromere, where there is a lower rate of homologous recombination and most of the homologous recombination can be attributed to gene conversions instead of crossing over (Shi et al. 2010; reviewed in Talbert and Henikoff 2010).
Our analyses show that D. ananassae F-element genes generally maintain the same contrast to D-element genes as seen in D. melanogaster (Figure 4), but for some features the differences are more pronounced. F-element genes have larger coding spans, primarily because they have larger total intron size, and this is particularly true for the D. ananassae F-element genes. This result is consistent with previous analysis that shows large introns tend to appear in regions with low rates of recombination (Carvalho and Clark 1999). The bimodal median intron size distribution suggests that the larger coding spans of D. ananassae F-element genes can be attributed to the expansion of a subset of introns. Most of the increase in total intron size for D. ananassae F-element genes compared to D. melanogaster F-element genes can be attributed to the higher density of transposable elements within introns.
Previous analysis has shown that D. melanogaster genes located in regions that exhibit lower rates of recombination have longer coding regions and more coding exons (Comeron and Kreitman 2000). Hence, our results are consistent with the hypothesis that, similar to the D. melanogaster F element, the D. ananassae F element has a low rate of recombination. We also find that, despite having smaller median CDS sizes, genes on both the D. melanogaster and D. ananassae F element have larger total CDS size because they have more coding exons than D element genes.
Low rates of recombination have also been associated with more uniform usage of synonymous codons (i.e., lower codon bias) in D. melanogaster (Kliman and Hey 1993). Codon bias can primarily be attributed to mutational bias and selection (reviewed in Hershberg and Petrov 2008; Plotkin and Kudla 2011). Earlier studies have suggested that highly expressed genes tend to show the strongest codon bias because selection tends to favor codons that pair with the most abundant tRNAs (Moriyama and Powell 1997; reviewed in Angov 2011). More recent studies suggest that, in addition to affecting gene expression, codon bias could also affect secondary structures in mRNA, the efficacy of protein translation, and protein folding (reviewed in Novoa and Ribas de Pouplana 2012).
Using the analysis technique developed by Vicario et al. (2007), we compared the codon usage patterns of F- and D-element genes in D. melanogaster and D. ananassae using two metrics: the Nc and the CAI. Our results show that F-element genes exhibit more uniform usage of synonymous codons (higher Nc) (Figure 5A) and less optimal codon usage (lower CAI) than D-element genes (Figure 5B). The codon bias for most F-element genes can primarily be attributed to mutational bias instead of selection (Figure 5C). D. ananassae F-element genes show stronger deviations from uniform usage of synonymous codons than D. melanogaster F-element genes, but this bias can primarily be attributed to mutational biases instead of selection.
Previous studies have demonstrated that mutation in Drosophila has a bias toward A/T, whereas selection tends to favor G/C at synonymous sites (Powell and Moriyama 1997; Vicario et al. 2007). Consistent with these observations, we find that the F-element genes have a lower GC codon content than D-element genes, particularly at the wobble base (Table 4). In concordance with the results of the Nc and CAI analysis, we find that codons in D. ananassae F-element genes have a lower GC content than D. melanogaster F-element genes. The lower GC content supports the hypothesis that the lower Nc exhibited by D. ananassae F-element genes can primarily be attributed to mutational bias.
Analysis of the five genes (Arf102F, ATPsynbeta, Crk, onecut, and RpS3A) on the D. ananassae F element that have smaller total intron sizes than their D. melanogaster orthologs shows that they tend to have a higher CAI than the CAI for a gene with uniform usage of synonymous codons (Figure S6 in File S7). Hence while most of the D. ananassae F-element genes are under weak selective pressure, a small subset of F-element genes is under stronger selective pressure to maintain their coding span size and to maintain a more optimal pattern of codon usage. These results are consistent with previous analysis in D. melanogaster, which shows a negative correlation between gene length and codon bias (Moriyama and Powell 1998).
Previous studies have shown that the stability of the 9-bp RNA–DNA hybrid within the elongation complex affects the efficacy of transcript elongation (Palangat and Landick 2001). Our previous analysis of the Tm metagene profiles in D. melanogaster, D. erecta, D. mojavensis, and D. grimshawi has shown that F-element genes exhibit lower Tm than D-element genes (Leung et al. 2015). Given that GC content is correlated with Tm (Frank-Kamenetskii 1971), and that D. ananassae F-element genes exhibit low codon GC content, we compared the Tm metagene profiles of F- and D-element genes in D. ananassae with the corresponding Tm metagene profiles from D. melanogaster. The analysis shows that D. ananassae and D. melanogaster F-element genes have similar Tm metagene profiles, such that F-element genes have similarly lower Tm compared to D-element genes in both species (Figure 6). These results indicate that neither the shift in codon GC content nor the increased transposon density within and around the F-element genes in D. ananassae are sufficient to shift the Tm metagene profiles, which suggests that the Tm must reflect other features of sequence organization. The lower Tm could facilitate the transcription of F-element genes and is consistently observed in the five Drosophila species studied to date.
Another unusual characteristic of the D. melanogaster F element is its distinct epigenomic landscape compared to the other autosomes (Kharchenko et al. 2011; Riddle et al. 2012). ChIP-Seq analysis of H3K4me2 and H3K9me2 from third instar larvae shows that most D. ananassae F-element genes exhibit histone-modification enrichment patterns similar to those seen in D. melanogaster F-element genes, where the region surrounding the 5′ end of the gene is enriched in H3K4me2 while the body of the coding span is enriched in H3K9me2 (Figure 7A). This histone modification pattern is consistent with the pattern seen in D. melanogaster heterochromatic genes (Yasuhara and Wakimoto 2008), and could enable the transcription machinery to access the core promoters of D. ananassae F-element genes while maintaining silencing of the transposons that reside within introns.
Many key genes involved in Drosophila development are activated by the Trithorax group of proteins and silenced by PcG proteins (reviewed in Grimaud et al. 2006; Schuettengruber et al. 2007). Regions enriched in H3K27me3 are recognized by the chromodomain of Polycomb (Min et al. 2003), resulting in gene silencing (reviewed in Blackledge et al. 2015). ChIP-Seq analyses of H3K27me3 show that the eight D. melanogaster F-element genes that exhibit H3K27me3 enrichment at the third instar larval stage also show H3K27me3 enrichment in the D. ananassae orthologs (Figure S7 in File S7). The H3K27me3 enrichment tends to be limited to the region surrounding the 5′ end of D. ananassae F-element genes, while the body of the coding span are enriched in H3K9me2 (Figure 7B).
Of the eight F-element genes that show H3K27me3 enrichment, six genes (ey, fd102C, Sox102F, sv, toy, and zfh2) also show H3K4me2 enrichment near the 5′ ends of the genes in both D. melanogaster and D. ananassae. Past studies of mammalian embryonic stem (ES) cells have identified promoters that show enrichments of both H3K27me3 and H3K4me3 (i.e., bivalent domains). These genes are poised for activation upon the differentiation of the ES cells (Young et al. 2011; Nair et al. 2012; reviewed in Voigt et al. 2013). Hence, the histone-modification enrichment patterns for these six F-element genes suggest that they are poised for activation in both D. melanogaster and D. ananassae at the third instar larval stage of development.
Despite residing in a domain with high repeat density, D. melanogaster F-element genes show a similar fraction of active genes in S2 cells (∼50%) compared to genes in euchromatin (Riddle et al. 2012). Analysis of D. ananassae gene expression levels using RNA-Seq data from seven developmental stages and tissues shows that D. ananassae F-element genes exhibit a similar range of expression levels compared to all D. ananassae genes and compared to genes at the base of the D element (Figure 8). Thus, these genes are able to function while in the midst of a heterochromatic domain with high repeat density.
Past studies have shown that heterochromatic genes in D. melanogaster, such as lt (Wakimoto and Hearn 1990) and rl (Eberl et al. 1993), depend on the properties of the heterochromatic environment for proper expression. Inversely, euchromatic genes that are relocated to a heterochromatic region exhibit partial gene silencing (i.e., position effect variegation; reviewed in Elgin and Reuter 2013). Comparative analysis of genes (e.g., lt and Yeti) that have moved between heterochromatic and euchromatic domains in different Drosophila species show that the structure of the core promoter for these genes is typically conserved (Yasuhara et al. 2005; Moschetti et al. 2014). These observations suggest that the expression of heterochromatic genes might depend on specialized enhancers within heterochromatic domains (reviewed in Yasuhara and Wakimoto 2006; Corradini et al. 2007).
This study demonstrates that transposons can be an important contributor to the expansion of a chromosome, and provides insights into the impact on the resident genes. Our study shows that D. ananassae F-element genes can continue to be expressed at similar levels despite the large increase in repetitive content. Understanding the mechanisms that enable D. ananassae F-element genes to be expressed within a highly repetitive heterochromatic domain could provide insights into the factors that regulate gene expression in other eukaryotic genomes with high repeat density, such as in plant and mammalian genomes.
Supplementary Material
Supplemental material is available online at www.g3journal.org/lookup/suppl/doi:10.1534/g3.117.040907/-/DC1.
Acknowledgments
The authors would like to thank the students who worked together as a class to produce high-quality sequences and gene annotations for a given D. ananassae project. We also thank the additional students who contributed data analysis to this project, but for various reasons declined to participate in manuscript critique and revision. These students were enrolled in one of the courses listed in File S5, which includes all courses that contributed sequence improvement and annotation data to the D. ananassae project. We thank the McDonnell Genome Institute at Washington University for generating the raw sequences reported here, and for providing training and support to many of the coauthors. We thank the Genome Technology Access Center at Washington University in St. Louis for generating the D. ananassae ChIP-Seq data. This work was supported by grant #52007051 from the Howard Hughes Medical Institute (HHMI) Precollege and Undergraduate Science Education Professors Program to Washington University (to S.C.R.E.), the National Science Foundation (NSF) IUSE program under grant no. 1431407 (to S.C.R.E.), and the National Science Foundation grant MCB-1517266 (to S.C.R.E.). The support provided by the McDonnell Genome Institute was funded by grant 2U54 HG00307910 from the National Human Genome Research Institute (Richard K. Wilson, principal investigator). The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of HHMI, the NSF, the National Human Genome Research Institute, or the National Institute of General Medical Sciences.
Footnotes
Communicating editor: B. Oliver
Literature Cited
- Adler, D., 2005 vioplot: Violin plot. Available at: https://CRAN.R-project.org/package=vioplot.
- Anders S., Pyl P. T., Huber W., 2015. HTSeq — a python framework to work with high-throughput sequencing data. Bioinformatics 31: 166–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angov E., 2011. Codon usage: nature’s roadmap to expression and folding of proteins. Biotechnol. J. 6: 650–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arguello J. R., Zhang Y., Kado T., Fan C., Zhao R., et al. , 2010. Recombination yet inefficient selection along the Drosophila melanogaster subgroup’s fourth chromosome. Mol. Biol. Evol. 27: 848–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attrill H., Falls K., Goodman J. L., Millburn G. H., Antonazzo G., et al. , 2016. FlyBase: establishing a gene group resource for Drosophila melanogaster. Nucleic Acids Res. 44: D786–D792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson G., 1999. Tandem repeats finder: a program to analyze DNA sequences. Nucleic Acids Res. 27: 573–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betancourt A. J., Welch J. J., Charlesworth B., 2009. Reduced effectiveness of selection caused by a lack of recombination. Curr. Biol. 19: 655–660. [DOI] [PubMed] [Google Scholar]
- Bhutkar A., Schaeffer S. W., Russo S. M., Xu M., Smith T. F., et al. , 2008. Chromosomal rearrangement inferred from comparisons of 12 Drosophila genomes. Genetics 179: 1657–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackledge N. P., Rose N. R., Klose R. J., 2015. Targeting Polycomb systems to regulate gene expression: modifications to a complex story. Nat. Rev. Mol. Cell Biol. 16: 643–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boros I. M., 2012. Histone modification in Drosophila. Brief. Funct. Genomics 11: 319–331. [DOI] [PubMed] [Google Scholar]
- Bosco G., Campbell P., Leiva-Neto J. T., Markow T. A., 2007. Analysis of Drosophila species genome size and satellite DNA content reveals significant differences among strains as well as between species. Genetics 177: 1277–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho A. B., Clark A. G., 1999. Intron size and natural selection. Nature 401: 344. [DOI] [PubMed] [Google Scholar]
- Castañeda J., Genzor P., Bortvin A., 2011. piRNAs, transposon silencing, and germline genome integrity. Mutat. Res. 714: 95–104. [DOI] [PubMed] [Google Scholar]
- Chen Z.-X., Sturgill D., Qu J., Jiang H., Park S., et al. , 2014. Comparative validation of the D. melanogaster modENCODE transcriptome annotation. Genome Res. 24: 1209–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J. Y., Aquadro C. F., 2014. The coevolutionary period of Wolbachia pipientis infecting Drosophila ananassae and its impact on the evolution of the host germline stem cell regulating genes. Mol. Biol. Evol. 31: 2457–2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J. Y., Bubnell J. E., Aquadro C. F., 2015. Population genomics of infectious and integrated Wolbachia pipientis genomes in Drosophila ananassae. Genome Biol. Evol. 7: 2362–2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland W. S., Devlin S. J., 1988. Locally weighted regression: an approach to regression analysis by local fitting. J. Am. Stat. Assoc. 83: 596. [Google Scholar]
- Comeron J. M., Kreitman M., 2000. The correlation between intron length and recombination in Drosophila. Dynamic equilibrium between mutational and selective forces. Genetics 156: 1175–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corradini N., Rossi F., Giordano E., Caizzi R., Verní F., et al. , 2007. Drosophila melanogaster as a model for studying protein-encoding genes that are resident in constitutive heterochromatin. Heredity 98: 3–12. [DOI] [PubMed] [Google Scholar]
- Craddock E. M., Gall J. G., Jonas M., 2016. Hawaiian Drosophila genomes: size variation and evolutionary expansions. Genetica 144: 107–124. [DOI] [PubMed] [Google Scholar]
- Dinno, A., 2016 dunn.test: Dunn’s Test of Multiple Comparisons Using Rank Sums. Available at: https://CRAN.R-project.org/package=dunn.test.
- Dolezel J., Bartos J., Voglmayr H., Greilhuber J., 2003. Nuclear DNA content and genome size of trout and human. Cytometry A 51: 127–128; author reply 129. [DOI] [PubMed] [Google Scholar]
- Drosophila 12 Genomes Consortium , 2007. Evolution of genes and genomes on the Drosophila phylogeny. Nature 450: 203–218. [DOI] [PubMed] [Google Scholar]
- Dunn O. J., 1964. Multiple comparisons using rank sums. Technometrics 6: 241–252. [Google Scholar]
- Dunning Hotopp J. C., 2011. Horizontal gene transfer between bacteria and animals. Trends Genet. 27: 157–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunning Hotopp J. C., Clark M. E., Oliveira D. C. S. G., Foster J. M., Fischer P., et al. , 2007. Widespread lateral gene transfer from intracellular bacteria to multicellular eukaryotes. Science 317: 1753–1756. [DOI] [PubMed] [Google Scholar]
- Dupeyron M., Leclercq S., Cerveau N., Bouchon D., Gilbert C., 2014. Horizontal transfer of transposons between and within crustaceans and insects. Mob. DNA 5: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberl D. F., Duyf B. J., Hilliker A. J., 1993. The role of heterochromatin in the expression of a heterochromatic gene, the rolled locus of Drosophila melanogaster. Genetics 134: 277–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddy S. R., 2012. The C-value paradox, junk DNA and ENCODE. Curr. Biol. 22: R898–R899. [DOI] [PubMed] [Google Scholar]
- Egelhofer T. A., Minoda A., Klugman S., Lee K., Kolasinska-Zwierz P., et al. , 2011. An assessment of histone-modification antibody quality. Nat. Struct. Mol. Biol. 18: 91–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgin S. C. R., Reuter G., 2013. Position-effect variegation, heterochromatin formation, and gene silencing in Drosophila. Cold Spring Harb. Perspect. Biol. 5: a017780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fierst J. L., Willis J. H., Thomas C. G., Wang W., Reynolds R. M., et al. , 2015. Reproductive mode and the evolution of genome size and structure in Caenorhabditis Nematodes. PLoS Genet. 11: e1005323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank-Kamenetskii F., 1971. Simplification of the empirical relationship between melting temperature of DNA, its GC content and concentration of sodium ions in solution. Biopolymers 10: 2623–2624. [DOI] [PubMed] [Google Scholar]
- Frith M. C., 2011. A new repeat-masking method enables specific detection of homologous sequences. Nucleic Acids Res. 39: e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girgis H. Z., 2015. Red: an intelligent, rapid, accurate tool for detecting repeats de-novo on the genomic scale. BMC Bioinformatics 16: 227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gish, W., 1996 WU BLAST. Available at: https://blast.advbiocomp.com/licensing/.
- Graveley B. R., Brooks A. N., Carlson J. W., Duff M. O., Landolin J. M., et al. , 2011. The developmental transcriptome of Drosophila melanogaster. Nature 471: 473–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory, T. R., 2016 Animal Genome Size Database. Available at: http://www.genomesize.com/.
- Gregory T. R., Johnston J. S., 2008. Genome size diversity in the family Drosophilidae. Heredity 101: 228–238. [DOI] [PubMed] [Google Scholar]
- Gregory T. R., Nicol J. A., Tamm H., Kullman B., Kullman K., et al. , 2007. Eukaryotic genome size databases. Nucleic Acids Res. 35: D332–D338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grewal S. I. S., Elgin S. C. R., 2007. Transcription and RNA interference in the formation of heterochromatin. Nature 447: 399–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimaud C., Nègre N., Cavalli G., 2006. From genetics to epigenetics: the tale of Polycomb group and trithorax group genes. Chromosome Res. 14: 363–375. [DOI] [PubMed] [Google Scholar]
- Haynes K. A., Caudy A. A., Collins L., Elgin S. C. R., 2006. Element 1360 and RNAi components contribute to HP1-dependent silencing of a pericentric reporter. Curr. Biol. CB 16: 2222–2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitz E., 1928. Das heterochromatin der moose. Jahrb Wiss Bot. 69: 762–818. [Google Scholar]
- Hershberg R., Petrov D. A., 2008. Selection on codon bias. Annu. Rev. Genet. 42: 287–299. [DOI] [PubMed] [Google Scholar]
- Hilgenboecker K., Hammerstein P., Schlattmann P., Telschow A., Werren J. H., 2008. How many species are infected with Wolbachia? — a statistical analysis of current data. FEMS Microbiol. Lett. 281: 215–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill W. G., Robertson A., 1966. The effect of linkage on limits to artificial selection. Genet. Res. 8: 269–294. [PubMed] [Google Scholar]
- Hinton C. W., Downs J. E., 1975. The mitotic, polytene, and meiotic chromosomes of Drosophila ananassae. J. Hered. 66: 353–361. [DOI] [PubMed] [Google Scholar]
- Hintze J. L., Nelson R. D., 1998. Violin plots: a box plot-density trace synergism. Am. Stat. 52: 181–184. [Google Scholar]
- Ho J. W. K., Jung Y. L., Liu T., Alver B. H., Lee S., et al. , 2014. Comparative analysis of metazoan chromatin organization. Nature 512: 449–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm S., 1979. A simple sequentially rejective multiple test procedure. Scand. J. Stat. 6: 65–70. [Google Scholar]
- Ioannidis P., Johnston K. L., Riley D. R., Kumar N., White J. R., et al. , 2013. Extensively duplicated and transcriptionally active recent lateral gene transfer from a bacterial Wolbachia endosymbiont to its host filarial nematode Brugia malayi. BMC Genomics 14: 639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurka J., Kapitonov V. V., Pavlicek A., Klonowski P., Kohany O., et al. , 2005. Repbase update, a database of eukaryotic repetitive elements. Cytogenet. Genome Res. 110: 462–467. [DOI] [PubMed] [Google Scholar]
- Kaufmann, B. P., 1937 Morphology of the Chromosomes of Drosophila ananassae, pp. 1043–1055 in Cytologia. FujiiJubilaei, Tokyo, Japan. [Google Scholar]
- Kent W. J., Baertsch R., Hinrichs A., Miller W., Haussler D., 2003. Evolution’s cauldron: duplication, deletion, and rearrangement in the mouse and human genomes. Proc. Natl. Acad. Sci. USA 100: 11484–11489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharchenko P. V., Alekseyenko A. A., Schwartz Y. B., Minoda A., Riddle N. C., et al. , 2011. Comprehensive analysis of the chromatin landscape in Drosophila melanogaster. Nature 471: 480–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidwell M. G., 2002. Transposable elements and the evolution of genome size in eukaryotes. Genetica 115: 49–63. [DOI] [PubMed] [Google Scholar]
- Kiełbasa S. M., Wan R., Sato K., Horton P., Frith M. C., 2011. Adaptive seeds tame genomic sequence comparison. Genome Res. 21: 487–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D., Langmead B., Salzberg S. L., 2015. HISAT: a fast spliced aligner with low memory requirements. Nat. Methods 12: 357–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klasson L., Kambris Z., Cook P. E., Walker T., Sinkins S. P., 2009a Horizontal gene transfer between Wolbachia and the mosquito Aedes aegypti. BMC Genomics 10: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klasson L., Westberg J., Sapountzis P., Näslund K., Lutnaes Y., et al. , 2009b The mosaic genome structure of the Wolbachia wRi strain infecting Drosophila simulans. Proc. Natl. Acad. Sci. USA 106: 5725–5730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klasson L., Kumar N., Bromley R., Sieber K., Flowers M., et al. , 2014. Extensive duplication of the Wolbachia DNA in chromosome four of Drosophila ananassae. BMC Genomics 15: 1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliman R. M., Hey J., 1993. Reduced natural selection associated with low recombination in Drosophila melanogaster. Mol. Biol. Evol. 10: 1239–1258. [DOI] [PubMed] [Google Scholar]
- Kohany O., Gentles A. J., Hankus L., Jurka J., 2006. Annotation, submission and screening of repetitive elements in Repbase: RepbaseSubmitter and Censor. BMC Bioinformatics 7: 474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruskal W. H., Wallis W. A., 1952. Use of ranks in one-criterion variance analysis. J. Am. Stat. Assoc. 47: 583–621. [Google Scholar]
- Kumar N., Creasy T., Sun Y., Flowers M., Tallon L. J., et al. , 2012. Efficient subtraction of insect rRNA prior to transcriptome analysis of Wolbachia-Drosophila lateral gene transfer. BMC Res. Notes 5: 230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz S., Narechania A., Stein J. C., Ware D., 2008. A new method to compute K-mer frequencies and its application to annotate large repetitive plant genomes. BMC Genomics 9: 517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landt S. G., Marinov G. K., Kundaje A., Kheradpour P., Pauli F., et al. , 2012. ChIP-seq guidelines and practices of the ENCODE and modENCODE consortia. Genome Res. 22: 1813–1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzuolo C., Orlando V., 2012. Memories from the polycomb group proteins. Annu. Rev. Genet. 46: 561–589. [DOI] [PubMed] [Google Scholar]
- Lee S.-I., Kim N.-S., 2014. Transposable elements and genome size variations in plants. Genomics Inform. 12: 87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung W., Shaffer C. D., Cordonnier T., Wong J., Itano M. S., et al. , 2010. Evolution of a distinct genomic domain in Drosophila: comparative analysis of the dot chromosome in Drosophila melanogaster and Drosophila virilis. Genetics 185: 1519–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung W., Shaffer C. D., Reed L. K., Smith S. T., Barshop W., et al. , 2015. Drosophila Muller F elements maintain a distinct set of genomic properties over 40 million years of evolution. G3 5: 719–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis S. E., Searle S. M. J., Harris N., Gibson M., Lyer V., et al. , 2002. Apollo: a sequence annotation editor. Genome Biol. 3: research0082.1–research0082.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, H., 2013 Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. arXiv arXiv:1303.3997.
- Li H., Durbin R., 2009. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25: 1754–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locke J., McDermid H. E., 1993. Analysis of Drosophila chromosome 4 using pulsed field gel electrophoresis. Chromosoma 102: 718–723. [DOI] [PubMed] [Google Scholar]
- Min J., Zhang Y., Xu R.-M., 2003. Structural basis for specific binding of Polycomb chromodomain to histone H3 methylated at Lys 27. Genes Dev. 17: 1823–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgulis A., Gertz E. M., Schäffer A. A., Agarwala R., 2006. WindowMasker: window-based masker for sequenced genomes. Bioinformatics 22: 134–141. [DOI] [PubMed] [Google Scholar]
- Moriyama E. N., Powell J. R., 1997. Codon usage bias and tRNA abundance in Drosophila. J. Mol. Evol. 45: 514–523. [DOI] [PubMed] [Google Scholar]
- Moriyama E. N., Powell J. R., 1998. Gene length and codon usage bias in Drosophila melanogaster, Saccharomyces cerevisiae and Escherichia coli. Nucleic Acids Res. 26: 3188–3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moschetti R., Celauro E., Cruciani F., Caizzi R., Dimitri P., 2014. On the evolution of Yeti, a Drosophila melanogaster heterochromatin gene. PLoS One 9: e113010.25405891 [Google Scholar]
- Nair N. U., Sahu A. D., Bucher P., Moret B. M. E., 2012. ChIPnorm: a statistical method for normalizing and identifying differential regions in histone modification ChIP-seq libraries. PLoS One 7: e39573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikoh N., Tanaka K., Shibata F., Kondo N., Hizume M., et al. , 2008. Wolbachia genome integrated in an insect chromosome: evolution and fate of laterally transferred endosymbiont genes. Genome Res. 18: 272–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novoa E. M., Ribas de Pouplana L., 2012. Speeding with control: codon usage, tRNAs, and ribosomes. Trends Genet. 28: 574–581. [DOI] [PubMed] [Google Scholar]
- Obbard D. J., Maclennan J., Kim K.-W., Rambaut A., O’Grady P. M., et al. , 2012. Estimating divergence dates and substitution rates in the Drosophila phylogeny. Mol. Biol. Evol. 29: 3459–3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palangat M., Landick R., 2001. Roles of RNA:DNA hybrid stability, RNA structure, and active site conformation in pausing by human RNA polymerase II. J. Mol. Biol. 311: 265–282. [DOI] [PubMed] [Google Scholar]
- Patrushev L. I., Minkevich I. G., 2008. The problem of the eukaryotic genome size. Biochemistry (Mosc). 73: 1519–1552. [DOI] [PubMed] [Google Scholar]
- Phillippy A. M., Schatz M. C., Pop M., 2008. Genome assembly forensics: finding the elusive mis-assembly. Genome Biol. 9: R55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piegu B., Guyot R., Picault N., Roulin A., Sanyal A., et al. , 2006. Doubling genome size without polyploidization: dynamics of retrotransposition-driven genomic expansions in Oryza australiensis, a wild relative of rice. Genome Res. 16: 1262–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotkin J. B., Kudla G., 2011. Synonymous but not the same: the causes and consequences of codon bias. Nat. Rev. Genet. 12: 32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell J. R., Moriyama E. N., 1997. Evolution of codon usage bias in Drosophila. Proc. Natl. Acad. Sci. USA 94: 7784–7790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritham E. J., 2009. Transposable elements and factors influencing their success in eukaryotes. J. Hered. 100: 648–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team , 2015. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- Rice P., Longden I., Bleasby A., 2000. EMBOSS: the European molecular biology open software suite. Trends Genet. 16: 276–277. [DOI] [PubMed] [Google Scholar]
- Riddle N. C., Shaffer C. D., Elgin S. C. R., 2009. A lot about a little dot — lessons learned from Drosophila melanogaster chromosome 4. Biochem. Cell Biol. 87: 229–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle N. C., Minoda A., Kharchenko P. V., Alekseyenko A. A., Schwartz Y. B., et al. , 2011. Plasticity in patterns of histone modifications and chromosomal proteins in Drosophila heterochromatin. Genome Res. 21: 147–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle N. C., Jung Y. L., Gu T., Alekseyenko A. A., Asker D., et al. , 2012. Enrichment of HP1a on Drosophila chromosome 4 genes creates an alternate chromatin structure critical for regulation in this heterochromatic domain. PLoS Genet. 8: e1002954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers R. L., Shao L., Sanjak J. S., Andolfatto P., Thornton K. R., 2014. Revised annotations, sex-biased expression, and lineage-specific genes in the Drosophila melanogaster group. G3 4: 2345–2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzberg S. L., Yorke J. A., 2005. Beware of mis-assembled genomes. Bioinformatics 21: 4320–4321. [DOI] [PubMed] [Google Scholar]
- Salzberg S. L., Dunning Hotopp J. C., Delcher A. L., Pop M., Smith D. R., et al. , 2005. Serendipitous discovery of Wolbachia genomes in multiple Drosophila species. Genome Biol. 6: R23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer S. W., Bhutkar A., McAllister B. F., Matsuda M., Matzkin L. M., et al. , 2008. Polytene chromosomal maps of 11 Drosophila species: the order of genomic scaffolds inferred from genetic and physical maps. Genetics 179: 1601–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuettengruber B., Chourrout D., Vervoort M., Leblanc B., Cavalli G., 2007. Genome regulation by polycomb and trithorax proteins. Cell 128: 735–745. [DOI] [PubMed] [Google Scholar]
- Serbus L. R., Sullivan W., 2007. A cellular basis for Wolbachia recruitment to the host germline. PLoS Pathog. 3: e190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serbus L. R., Casper-Lindley C., Landmann F., Sullivan W., 2008. The genetics and cell biology of Wolbachia-host interactions. Annu. Rev. Genet. 42: 683–707. [DOI] [PubMed] [Google Scholar]
- Sessegolo C., Burlet N., Haudry A., 2016. Strong phylogenetic inertia on genome size and transposable element content among 26 species of flies. Biol. Lett. 12: 20160407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer C. D., Alvarez C., Bailey C., Barnard D., Bhalla S., et al. , 2010. The genomics education partnership: successful integration of research into laboratory classes at a diverse group of undergraduate institutions. CBE Life Sci. Educ. 9: 55–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. M., Li W. H., 1987. The codon Adaptation Index — a measure of directional synonymous codon usage bias, and its potential applications. Nucleic Acids Res. 15: 1281–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J., Wolf S. E., Burke J. M., Presting G. G., Ross-Ibarra J., et al. , 2010. Widespread gene conversion in centromere cores. PLoS Biol. 8: e1000327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slawson E. E., Shaffer C. D., Malone C. D., Leung W., Kellmann E., et al. , 2006. Comparison of dot chromosome sequences from D. melanogaster and D. virilis reveals an enrichment of DNA transposon sequences in heterochromatic domains. Genome Biol. 7: R15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin R. K., Martienssen R., 2007. Transposable elements and the epigenetic regulation of the genome. Nat. Rev. Genet. 8: 272–285. [DOI] [PubMed] [Google Scholar]
- Smit, A. F. A., R. Hubley, and P. Green, 2013 RepeatMasker Open-4.0. Available at: http://www.repeatmasker.org/.
- Smith C. D., Shu S., Mungall C. J., Karpen G. H., 2007. The Release 5.1 annotation of Drosophila melanogaster heterochromatin. Science 316: 1586–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun F.-L., Haynes K., Simpson C. L., Lee S. D., Collins L., et al. , 2004. cis-Acting determinants of heterochromatin formation on Drosophila melanogaster chromosome four. Mol. Cell. Biol. 24: 8210–8220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbert P. B., Henikoff S., 2010. Centromeres convert but don’t cross. PLoS Biol. 8: e1000326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J., Vadnagara K., Pritham E. J., 2014. DINE-1, the highest copy number repeats in Drosophila melanogaster are non-autonomous endonuclease-encoding rolling-circle transposable elements (Helentrons). Mob. DNA 5: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicario S., Moriyama E. N., Powell J. R., 2007. Codon usage in twelve species of Drosophila. BMC Evol. Biol. 7: 226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigt P., Tee W.-W., Reinberg D., 2013. A double take on bivalent promoters. Genes Dev. 27: 1318–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakimoto B. T., Hearn M. G., 1990. The effects of chromosome rearrangements on the expression of heterochromatic genes in chromosome 2L of Drosophila melanogaster. Genetics 125: 141–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeks A. R., Turelli M., Harcombe W. R., Reynolds K. T., Hoffmann A. A., 2007. From parasite to mutualist: rapid evolution of Wolbachia in natural populations of Drosophila. PLoS Biol. 5: e114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werren J. H., Baldo L., Clark M. E., 2008. Wolbachia: master manipulators of invertebrate biology. Nat. Rev. Microbiol. 6: 741–751. [DOI] [PubMed] [Google Scholar]
- Wright F., 1990. The “effective number of codons” used in a gene. Gene 87: 23–29. [DOI] [PubMed] [Google Scholar]
- Wu M., Sun L. V., Vamathevan J., Riegler M., Deboy R., et al. , 2004. Phylogenomics of the reproductive parasite Wolbachia pipientis wMel: a streamlined genome overrun by mobile genetic elements. PLoS Biol. 2: E69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuhara J. C., Wakimoto B. T., 2006. Oxymoron no more: the expanding world of heterochromatic genes. Trends Genet. 22: 330–338. [DOI] [PubMed] [Google Scholar]
- Yasuhara J. C., Wakimoto B. T., 2008. Molecular landscape of modified histones in Drosophila heterochromatic genes and euchromatin-heterochromatin transition zones. PLoS Genet. 4: e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuhara J. C., DeCrease C. H., Wakimoto B. T., 2005. Evolution of heterochromatic genes of Drosophila. Proc. Natl. Acad. Sci. USA 102: 10958–10963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young M. D., Willson T. A., Wakefield M. J., Trounson E., Hilton D. J., et al. , 2011. ChIP-seq analysis reveals distinct H3K27me3 profiles that correlate with transcriptional activity. Nucleic Acids Res. 39: 7415–7427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Liu T., Meyer C. A., Eeckhoute J., Johnson D. S., et al. , 2008. Model-based analysis of ChIP-Seq (MACS). Genome Biol. 9: R137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimin A. V., Smith D. R., Sutton G., Yorke J. A., 2008. Assembly reconciliation. Bioinformatics 24: 42–45. [DOI] [PubMed] [Google Scholar]
- Zug R., Hammerstein P., 2015. Bad guys turned nice? A critical assessment of Wolbachia mutualisms in arthropod hosts. Biol. Rev. Camb. Philos. Soc. 90: 89–111. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The D. ananassae ChIP-Seq data are available at the NCBI BioProject database with the accession number PRJNA369805. The D. ananassae PacBio whole-genome sequencing data are available at the NCBI BioProject database with the accession number PRJNA381646. The improved D. ananassae sequences and gene annotations, and the results of the bioinformatic analyses for D. ananassae and D. melanogaster, are available through the GEP UCSC Genome Browser Mirror (http://gander.wustl.edu).