Abstract
Circadian clocks orchestrate daily activity patterns and free running periods of locomotor activity under constant conditions. While the first often depends on temperature, the latter is temperature-compensated over a physiologically relevant range. Here, we explored the locomotor activity of the temperate housefly Musca domestica. Under low temperatures, activity was centered round a major and broad afternoon peak, while high temperatures resulted in activity throughout the photophase with a mild midday depression, which was especially pronounced in males exposed to long photoperiods. While period (per) mRNA peaked earlier under low temperatures, no temperature-dependent splicing of the last per 3ʹ end intron was identified. The expression of timeless, vrille, and Par domain protein 1 was also influenced by temperature, each in a different manner. Our data indicated that comparable behavioral trends in daily activity distribution have evolved in Drosophila melanogaster and M. domestica, yet the behaviors of these two species are orchestrated by different molecular mechanisms.
Keywords: temperature compensation of circadian rhythms, locomotor activity, transcription, mRNA splicing, circadian clock genes
Circadian clocks are ubiquitous adaptations to periodic day/night alternations and orchestrate metabolic and behavioral activities of many organisms. For instance, Drosophila melanogaster displays a bimodal locomotor activity pattern with morning and evening peaks separated by a siesta under a constant temperature (Rosato and Kyriacou 2006) or afternoon activity bursts under natural environmental conditions (Vanin et al. 2012; Green et al. 2015). Under elevated temperatures, the morning peak is advanced and the evening peak is delayed. In contrast, the overall free running period under constant conditions is temperature-compensated over a physiologically relevant range. Circadian genes drive both of these activities.
Temperature strongly affects the weak splice site of the last period (per) gene intron (dmpi8) and locomotor activity pattern of D. melanogaster (Majercak et al. 1999; Collins et al. 2004; Majercak et al. 2004). However, a comparison of two tropical Drosophila species, D. yakuba and D. santomea, revealed that temperature has no effect on the splicing efficiency of the last per intron, nor on the daily activity of the flies (Low et al. 2008). Transgenic constructs specifically addressed the structure of this intron and established its splicing efficiency as an important molecular mechanism adjusting behavior to the temperature. The impact of temperature on the transcription of per and timeless (tim) was explored both in D. melanogaster exposed to a laboratory-controlled environment (Boothroyd et al. 2007) and more recently, under natural conditions (Montelli et al. 2015).
Notably, the locomotor activity of northern Drosophila species, including D. littoralis and D. montana, is tightly linked to weather, and their behavior becomes arrhythmic under constant dark conditions (Lankinen 1986; Kauranen et al. 2012; D. Dolezel and J.C. Hall, unpublished data). Given these interspecific differences within the Drosophila genus, we decided to explore the impact of temperature on the locomotor activity of the housefly, Musca domestica, a dipteran species using a characterized circadian toolkit (Codd et al. 2007), including rescue experiments of the D. melanogaster per0 mutant (Piccin et al. 2000) and numerous anatomical data (Pyza and Meinertzhagen 1997; Miskiewicz et al. 2008).
Materials and Methods
Fly maintenance
M. domestica larvae were raised on wheat bran (55 g), heat-inactivated yeast (3 g), milk (150 ml), and antimycotic nipagin (0.35 g; Sigma) until pupariation. After eclosion, adult flies were fed water, sugar, and dried milk. Fly stocks were maintained at 25° under either 12 hr light: 12 hr dark cycles (LD 12:12) or 16 hr light: 8 hr dark cycles (LD 16:8). In our study, we mainly used homozygous strains of white eye and apterous mutations (gift from Daniel Bopp, University of Zurich) (Hediger et al. 2001; Codd et al. 2007). For behavioral experiments, we also collected a wild-type strain in České Budějovice (Czech Republic), which was amplified for three to five generations, and used it for locomotor activity recordings. The species identity was verified by sequencing the intron of the per gene.
Locomotor activity
Male and female flies, 3–5 d old, were individually housed in glass test tubes (24 × 150 mm, PYREX; Sigma) with a sugar lump wrapped in a mesh bag on one side and water reservoir on the other side. The movements of the flies were automatically detected using an infrared photosensor (Large Activity Monitors, LAM; TriKinetics, Waltham, MA) and recorded at 5 min intervals. Monitors were placed under a controlled regime in MIR 153 or 253 incubators (SANYO/Panasonic) with a 15 W cool white lamp (ZS15; Top Light) generating light at ∼400–800 lux.
To determine the free running period, flies were entrained for 4 d at either LD 16:8, LD 12:12, or LD 8:16 (at 15, 25, or 35°) and released into a constant, dark (DD) environment of the same temperature for another 10 d. The activity data were evaluated using ActogramJ software (Schmid et al. 2011).
Daily patterns of locomotor activity were recorded at three different, constant temperatures (15, 25, or 35°) under either LD 16:8 or LD 12:12 regimes. Data were collected for at least 14 d, but the last 4 d served as a control of survivor and were omitted from the analysis. To compare daily activity distribution patterns, data were collected in 5 min bins, smoothed by factor 2 in Actogram J (Schmid et al. 2011), and an activity histogram was plotted as mean ± SEM in Graphpad Prism. To compare absolute activity levels under different conditions for each individual fly, the total average activity at 24 hr intervals was calculated from day 5 to 14 and presented as one point (Graphpad Prism).
Gene cloning
To collect samples for cDNA cloning, the flies were snap-frozen and their heads were either stored at −80°, or immediately used for total RNA isolation with the TRIzol-reagent (Ambion). Using the oligo (dT) primer (24mer) and Superscript III reverse transcriptase (Invitrogen), 5 μg of total RNA was reverse transcribed. Sequences for M. domestica circadian clock genes, casein kinase 2beta (Md_ck 2beta), clockwork orange (Md_cwo), and Par domain protein 1 (Md_Pdp1), were obtained using PCR with degenerate primers designed according to the conserved protein regions of insect (D. melanogaster, Apis melifera, Culex pipiens, Aedes aegypti, Danaus plexipus, Tribolium castaneum), amphibian (Xenopus laevis), and mammalian (Rattus norvegicus, Homo sapiens sapiens) clock gene orthologs. Short fragments (200–700 bp) were amplified, cloned, and sequenced. Gene sequences were further extended by primer walking 3ʹ - RACE (rapid amplification of 3ʹ ends) and 5ʹ - RACE (Ambion). The sequences of the Musca pigment dispersing factors (Md_pdf) (Matsushima et al. 2004), period (Piccin et al. 2000), vrille (Md_vri), Clock (Md_Clk), cycle (Md_cyc), and timeless (Md_tim) (Codd et al. 2007) have been previously described. The intron sequences close to the coding sequence (CDS) 3ʹ end of the cwo, ck 2beta, and pdf genes and close to the start codon of the Pdp 1epsilon gene were obtained from genomic DNA using specific primers. No intron sequences were identified in the double time gene. All primers used are listed in Supplemental Material, Table S1.
Phylogenetic analysis
To clarify which cryptochromes are encoded in the Musca genome, CRY proteins were aligned and trimmed using the MAFFT program (Geneious; Biomatters) and substitution models were tested in the ProTest 2.4 program (Abascal et al. 2005). Phylogenetic trees were constructed under the WAG and LG models (RaXML) and in the Fasttree algorithm (Geneious 8.1; Biomatters). Since all trees shared the same topology, we only presented phylogenetic analyses performed in RaXML 7.2.8 under the LG+Gamma model with 500 bootstrap replications.
As a reference, we used insects containing both mammalian- and Drosophila-type CRYs: Anopheles gambiae, D. plexippus (Yuan et al. 2007), Blattella germanica (Bazalova et al. 2016), Pogonus chalceus, insect with only mammalian CRYs: A. mellifera (Rubin et al. 2006) and Pyrrhocoris apterus (Bajgar et al. 2013b), and D. melanogaster representing insects with only the photosensitive CRY (Stanewsky et al. 1998).
Expression analysis
Three independent animal groups were reared and killed for each experiment. For each experiment, 5- to 10-d-old males and females were collected separately, and their heads were stored at −80° until needed for RNA extraction. Total RNA was isolated from 25 heads per time-point using the TRIzol-reagent (Ambion) according to the manufacturer’s instructions. Using oligo (dT) primers and Superscript III reverse transcriptase (Invitrogen), 1 μg of total RNA was reverse transcribed. To safeguard against the amplification of possible contaminant genomic DNA, the primers were designed either to anneal only to a template corresponding to the spliced transcript, or (in the case of dbt) the genomic DNA was removed from the sample using TURBO DNase (TURBO DNA-free Kit; Ambion), according to the manufacturer’s instructions, or (in the case of tim) the primers were designed to include a large (2.5 kb) intron in the genomic DNA template (Codd et al. 2007).
In total, 3 μl of 100× times (M. domestica) or 3 μl of 50× times (D. melanogaster) diluted complementary DNA (cDNA) was used for the 12 μl PCR reaction (either in IQ SYBRGreen Supermix from Bio-Rad; or with 2×TP SYBR Master Mix from Top-Bio; primers 0.4 μM each). Amplifications were carried out on the C1000 Thermal Cycler (Bio-Rad) in 96-well micro plates (Hard-Shell; Bio-Rad) according to the following protocol: initial denaturation (94°, 2 min) followed by 35 cycles of denaturation (94°, 10 sec), annealing primers (60°, 20 sec), and elongation (72°, 20 sec). Product size was always confirmed by the melting analysis. Each cDNA sample was amplified in triplicates for each primer combination, where rp49 served as a normalization reference. A list of the specifications of the primers used is given in Table S1. Data were analyzed and quantified using CFX Manager Software. Relative values were standardized to rp49 and normalized to the sample with the highest expression (Kobelkova et al. 2010). Values represent the mean of three independent biological replicates ± SD.
Promoter comparison
Promoters of D. melanogaster and M. domestica were explored manually to identify conserved cis-regulatory motifs that were subsequently highlighted as sequence annotations in Geneious 7.1 (Biomatters). Corresponding figures were manually redrawn in Adobe Illustrator CS5. The expected frequencies of particular cis-motifs were compared to observed frequencies in a Microsoft Excel 2010 spreadsheet, obtained values were color-coded (Conditional formatting in Excel), and the figure was finalized in Adobe Illustrator.
Data availability
Drosophila strain is available on request from our laboratory. Musca strain was obtained from D. Bopp (Hediger et al. 2001). Sequence data of D. melanogaster and M. domestica circadian genes can be found on FlyBase (http://flybase.org/) and VectorBase (https://www.vectorbase.org/) databases, respectively (see Table S2 for accession numbers).
Results
Temperature compensation of the free running period
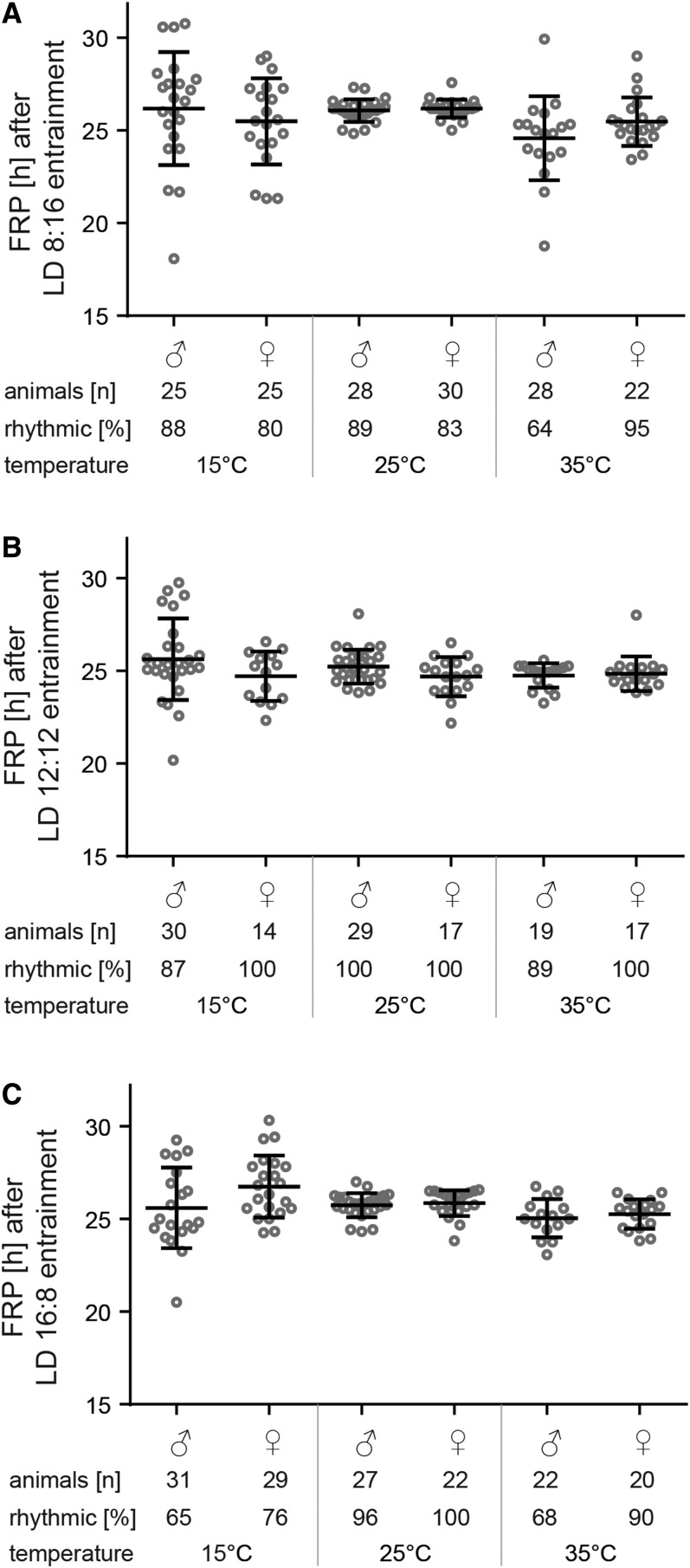
Our first goal was to verify if the circadian clock of M. domestica was functional at all temperatures. Flies were entrained to three different photoperiods, at three different temperatures (nine different combinations), and afterward released to DD. In general, behavioral rhythmicity is well temperature-compensated and free running period stayed constant at all three different temperatures (Figure 1). While the free running period was observed in nearly all flies exposed to 25°, both lower (15°) and higher (35°) temperatures resulted in a lower percentage of rhythmic individuals, particularly males, and a higher variability in the free running period of rhythmic individuals (Figure 1). Moreover, a short photoperiod entrainment (LD 8:16) slightly reduced the percentage of rhythmic individuals even at 25° (Figure 1A). Examples of double-plotted actograms are shown in Figure S1.
Figure 1.
Free running period of males and females (±SD) at 15, 25, and 35° after entrainment at three different photoperiods: (A) short day LD 8:16, (B) equinox LD 12:12, and (C) long day LD 16:8. Each dot corresponds to individual flies. Number of all animals measured and percent of rhythmic individuals is shown under each chart.
Locomotor activity distribution in LD
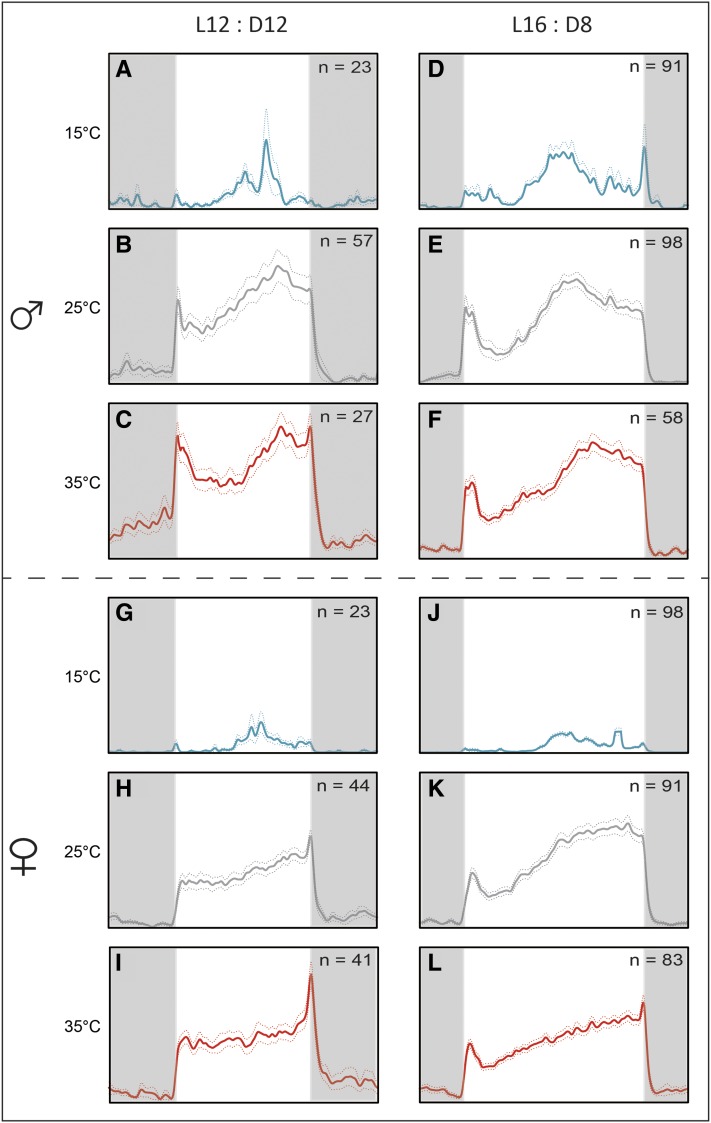
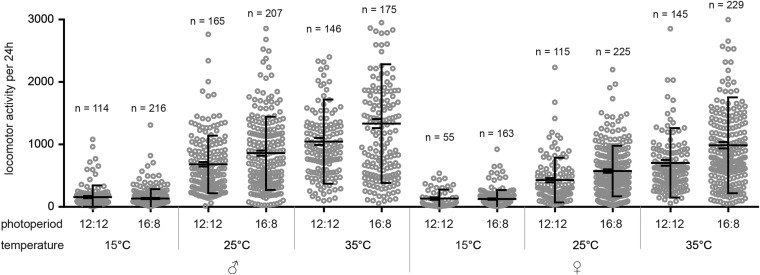
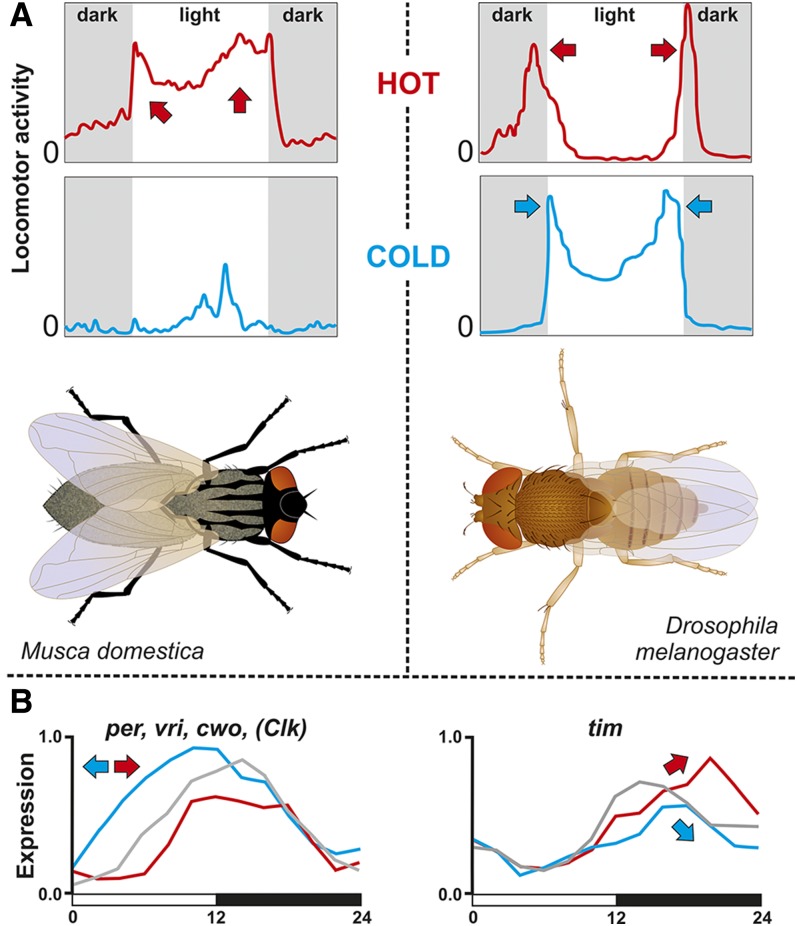
We explored the impacts of temperature and photoperiod on daily activity patterns. In general, a low temperature of 15° resulted in locomotor activity being centered around the middle of the photophase (Figure 2, A, D, G, and J) in both photoperiods, whereas at 25 and 35°, flies were active during the entire photophase (Figure 2, B, C, E, F, H, I, K, and L). There was also a mild difference between male and female activity at 25 and 35°. Males showed a slightly bimodal pattern with the light on peak followed by a mild trough in the first half of the photophase, then a gradual activity increase during the afternoon reaching a maximum ∼10 hr after the light on signal (Figure 2, B, C, E, and F). The afternoon activity peak was delayed with the temperature increase (compare Figure 2, A–C for the short day and Figure 2, D–F for the long day). The activity gradually decreased during the second half of the photophase under the long photoperiod of LD 16:8 (Figure 2, E and F). In contrast, the activity of females increased progressively during the photophase at 25 and 35° peaking either after the light off signal (Figure 2, H, I, and L) or just at the end of the photophase (Figure 2K). The overall activity at 15° was markedly lower than the activity detected at 25 or 35° in both sexes (Figure 3 and Table S3). At 25 and 35°, males were slightly more active than females (Figure 3 and Table S3).
Figure 2.
Locomotor activity pattern of M. domestica kept in LD 12:12 (left column) and LD 16:8 (right column). Blue, gray, and red lines represent 15° (low, panels A, D, G, J), 25° (normal, panels B, E, H, K), and 35° (high, panels C, F, I, L) temperatures, respectively. Flies were measured individually. The average ± SD is shown as dashed colored lines; n, numbers of animals used in the experiment. Vertical dashed gray line corresponds to ZT 12 under the LD 16:8 regime.
Figure 3.
Locomotor activity levels of M. domestica plotted as the number of laser beam crosses per 24 hr. The left side depicts the mean ± SEM; the right side depicts the average ± SD. Each circle corresponds to individual flies, and number of analyzed days is shown above each category. See Table S3 for statistics.
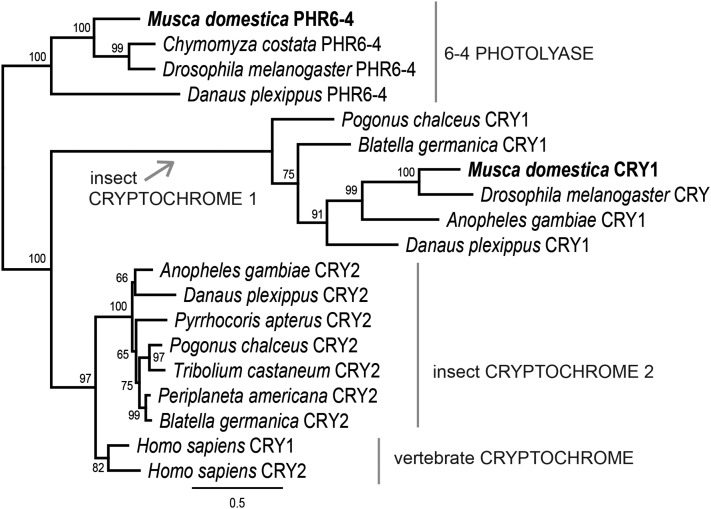
Cryptochrome gene in Musca: phylogenetic analysis
The initial search identified two cry-like genes in the M. domestica genome. Phylogenetic analysis revealed three clearly distinguished clusters: (i) 6–4 photolyase, (ii) insect cryptochrome 1 (Drosophila-type CRYs), and (iii) a group containing both human CRYs and insects CRY2 (Figure 4). This analysis, unambiguously supported by bootstrap values, completely agreed with the previously published phylogeny of CRYs (Yuan et al. 2007). Thus, M. domestica contained one CRY protein of Drosophila-type (Md_CRY1) and the second gene clearly belonged to 6–4 photolyases (Figure 4).
Figure 4.
Phylogeny of M. domestica CRY in context of insect CRYs. The tree was obtained from the RAxML 7.2.8 analysis of protein sequence alignment under the LG+Gamma substitutional model. Bootstrap support from 500 replicates is shown in percent under each node. 6–4 photolyases served as an outgroup.
Our phylogenetic analysis revealed an additional, interesting feature. In an attempt to add CRY proteins from relevant holometabolan insects, we sought transcriptomes of beetles (Coleoptera). Whereas in T. castaneum only one gene belonging to the CRY2 group was identified, in another beetle, P. chalceus, both the CRY1 (Drosophila-type) and CRY2 -coding genes were found (Figure 4). This finding supports the remarkable diversity of the circadian clock design, particularly the role of various CRY combinations reported in holometabola (Yuan et al. 2007) and recently in hemimetabola (Bazalova et al. 2016).
Given the noncyclical expression of Md_cry1 mRNA (Codd et al. 2007), which is in contrast with the expression of Drosophila cry (Dm_cry) (Emery et al. 1998), and the possible role of Dm_cry in temperature-dependent rhythmicity (Dolezelova et al. 2007), we sought to analyze the expression profile of both Md_cry1 and Md_phr6-4.
mRNA expression at different temperatures: M. domestica
qRT PCR was performed on samples isolated from male M. domestica heads collected every 2 hr. First, we explored expression of circadian clock genes under the LD and DD conditions at 25° (Figure S2). Cyclically expressed genes were further analyzed at three different temperatures.
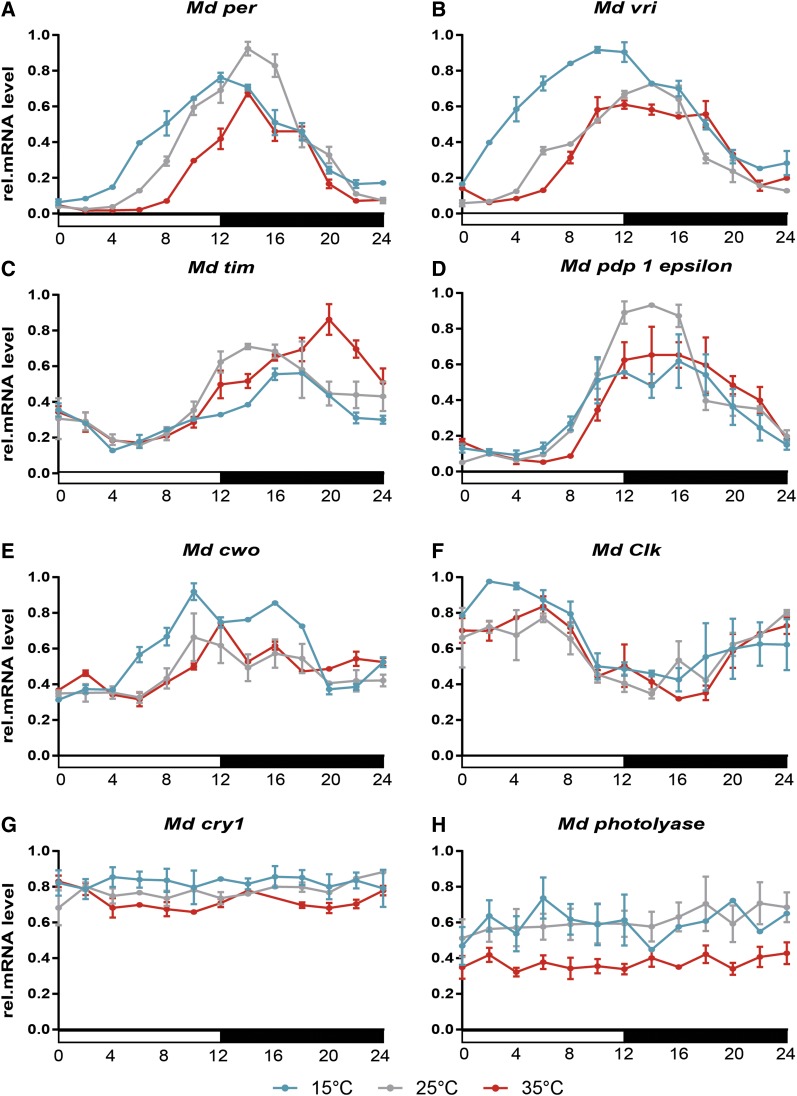
A previous study identified the peak of per abundance at Zeitgeber time (ZT) 16 in houseflies kept at 25° (Codd et al. 2007). Our data, relying on more detailed 2 hr resolutions, indicated the mRNA maximum at ZT 14 (Figure 5A). The expression rise and its peak were advanced by ∼2 hr at 15°. Consistently, Md_per accumulation started with ∼2 hr delay at 35°; however, the peak and descending arm was identical for both 35 and 25° (Figure 5A).
Figure 5.
Relative mRNA level of clock gene expression in M. domestica heads at three different constant temperatures (15° – blue line, 25° – gray line, and 35° – red line) under the short photoperiod (LD 12:12). The average of three independent biological replicates is shown for each temperature ±SD. (A) period, (B) vrille, (C) timeless, (D) Par domain protein 1 epsilon, (E) clockwork orange, (F) Clock, (G) cryptochrome1, and (H) photolyase. See Results section and Table S3 (A–H) for statistical analysis.
A similar trend was observed in Md_vri, where exposure to 15° resulted in 5–6 hr advancements in the mRNA expression profile (Figure 5B). In contrast to Md_per, no difference was observed in Md_vri expression at 25° Compared to 35° (Figure 5B gray vs. magenta). A comparable trend to Md_vri expression, a phase advancement at 15°, and no difference between expressions at 25 and 35° were found in Md_cwo (Figure 5E) and Md_Clk (Figure 5F). The main difference was in the phase of expression: Md_Clk started to accumulate during the scotophase and peaked early in the photophase, particularly at 15° (Figure 5F), whereas the Md_cwo peak lies at the end of the photophase (Figure 5E) similar to Md_per, Md_vri, and Md_Pdp1epsilon (Figure 5, A, B, and D).
The expressions of Md_tim were influenced differently with changing temperatures. Similar to the Md_per maximum, at 25°, mRNA peaked at ZT 14 (Figure 5C). A low temperature of 15° resulted in a phase delay (peak at ZT 16–18) and a slightly lower expression level. Moreover, a high temperature of 35° delayed the phase of the Md_tim maximum even more (peak at ZT 20) and the expression level was higher than that observed at 15 or 25° (Figure 5C).
Temperature did not affect the phase of Md_Pdp1epsilon, which peaked during the early photophase at all three temperatures (Figure 5D). The expression level of Md_cry was temperature-independent and noncyclical (Figure 5G), confirming results published previously (Codd et al. 2007). Similarly, the expression of Md_photolyase was noncyclical (Figure 5H).
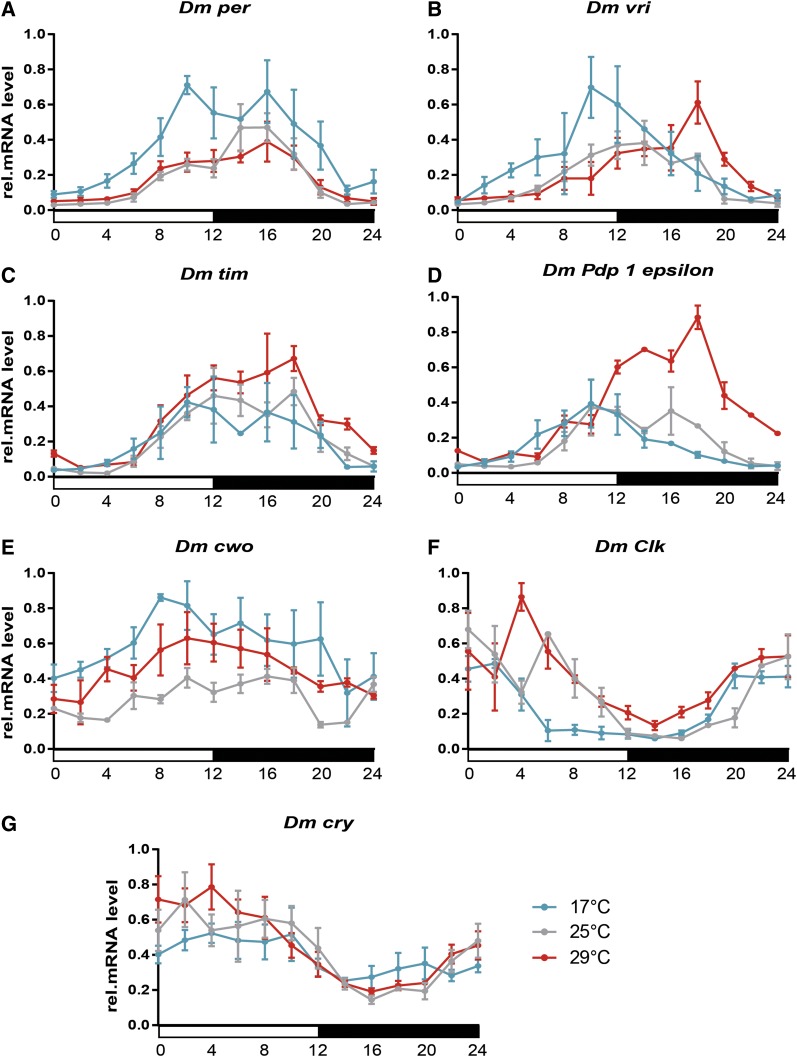
mRNA expression at different temperatures: D. melanogaster
To compare if similar trends were conserved in D. melanogaster, expression profiles at low (18°), ambient (25°), and high (29°) temperatures were defined with a 2 hr resolution. The Dm_per peak was phase advanced to ZT 10 at low temperatures (Figure 6A), whereas mRNA reached its maximum at ZT 14–16 at both ambient and high temperatures. Similarly, the Dm_vri peak was advanced and delayed at 18 and 29°, respectively (Figure 6B). Similar to Musca, the accumulation of Dm_tim started identically at all three temperatures (Figure 6C). The only differences, bordering on a statistically significant value (see Table S5), were observed later in the middle of the scotophase, where higher temperatures were associated with the higher Dm_tim levels in contrast to the low temperature samples (Figure 6C). The Dm_Pdp1epsilon was influenced by temperature in a different manner from that of Md_Pdp1epsilon (Figure 5D). The high temperature resulted in higher Dm_Pdp1epsilon levels that peaked at ZT 18, ∼8 hr later than the low temperature maximum (Figure 6D).
Figure 6.
Relative mRNA levels of clock gene expression in D. melanogaster heads at three different constant temperatures (17° – blue line, 25° – gray line, and 29° – red line) under the short photoperiod (LD 12:12). The average of three independent biological replicates is shown for each temperature ±SD (A) period, (B) vrille, (C) timeless, (D) Par domain protein 1 epsilon, (E) clockwork orange, (F) Clock, and (G) cryptochrome1. See Results section and Table S4 (A–G) for statistical analysis.
Mdpi11 and Mdpi12 splicing
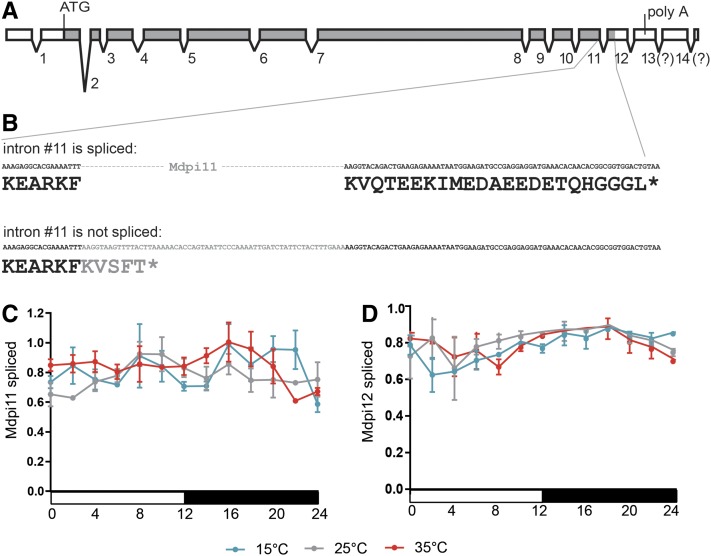
Two PER-immunoreactive bands were identified in the head extracts, whereas only a single band had previously been recognized in the thorax (figure 4 in Codd et al. 2007). We failed to identify any introns in the 3ʹ UTR of the Musca per gene corresponding to dmpi8 (Majercak et al. 1999) either by the in silico search in the published Musca genome (Scott et al. 2014), or using a PCR-based approach. Nevertheless, the alternative retention of mdpi11, the last intron within the coding sequence, would result in the PER protein being shorter by 18 amino acids (Figure 7, A and B). Therefore, we measured the expression of both transcript variants using a splice isoform-specific primer combination. Clearly, the expression of mdpi11 was not affected by temperature (Figure 7C). Similarly, temperature had no influence on splicing of the last intron, mdpi12, which is localized in 3ʹ UTR (Figure 7D).
Figure 7.
(A) Structure of M. domestica per gene with exons (rectangles) and introns (lines). Gray fill corresponds to coding sequence; position of the start codon highlighted (ATG) and stop codon (*… stop codon) are shown. Although the genome annotation predicts 14 introns within the per gene, our 3ʹ RACE identified the poly A tail (poly A) in mRNAs corresponding to the 13th exon. (B) Details of intron 11 (in gray color) and surrounding exons with corresponding protein sequence shown below DNA sequence. Both intron Mdpi11 (C) and Mdpi12 (D) are spliced effectively at all three tested temperatures.
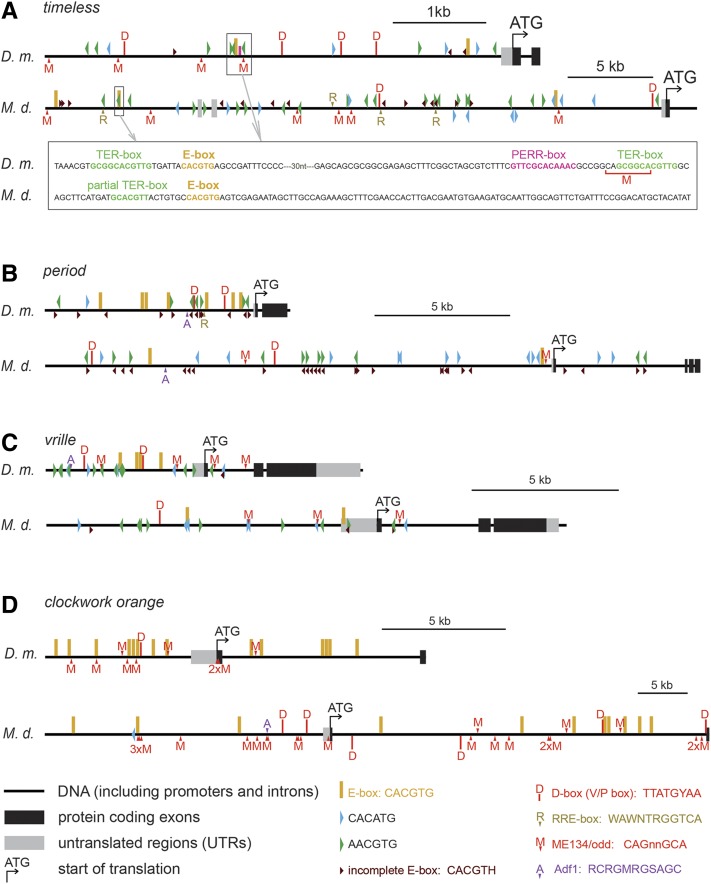
timeless splicing in Musca
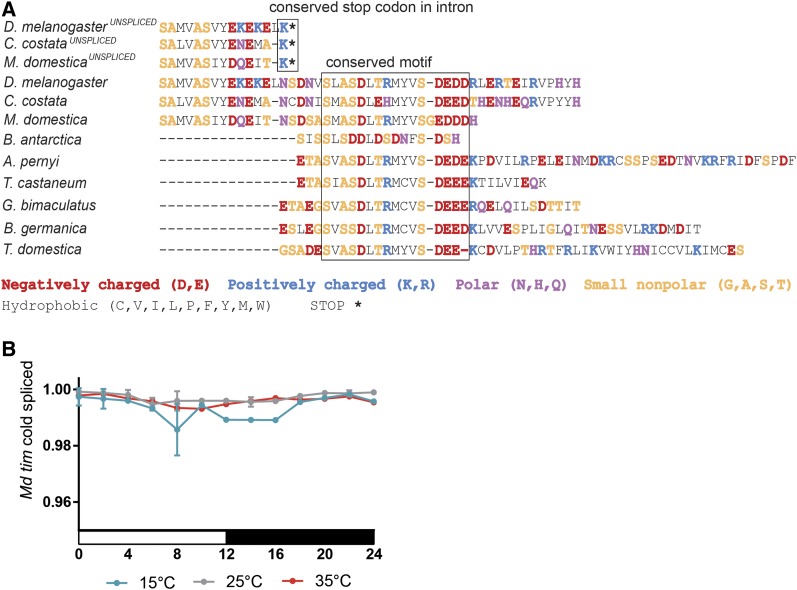
Interspecific comparisons of TIM proteins across all insect species identified 14–15 conserved amino acid motifs containing 3–4 acidic residues near the C terminus (Figure 8A), whereas its preceding sequences were highly variable between various species. Low temperatures result in the unspliced last intron of D. melanogaster tim containing an earlier stop codon completely removing this conserved TIM motif (Boothroyd et al. 2007; Montelli et al. 2015). A comparison of the genomic sequence identified the existence of identical intronic premature stop codons in C. costata and M. domestica (Figure 8A). Therefore, we explored the splicing frequency of the last intron by measuring the levels of both isoforms: the spliced transcript, resulting in a full protein with a conserved motif, and the nonspliced isoform, resulting in a shorter protein, called TIMUNSPLICED. Low temperatures resulted in a ∼1% reduction in the splicing efficiency of Md_tim (Figure 8A). Although this difference is statistically significant, low temperatures have a minimal effect when compared to the ∼10-fold splicing difference in the homologous tim intron of D. melanogaster (Montelli et al. 2015).
Figure 8.
(A) Details of C terminal region alignment of TIM proteins with a highlighted conserved motif in all insect species and conserved stop codon resulting from putative unspliced transcripts in D. melanogaster, C. costata, and M. domestica. (B) This last tim intron is spliced effectively in M. domestica at all three tested temperatures.
Promoter comparison
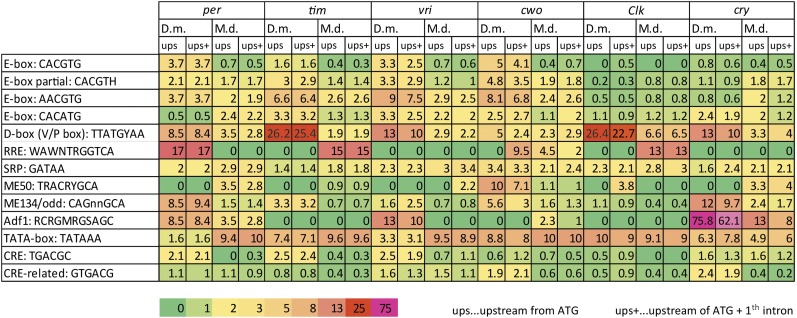
Having the M. domestica genome in hand (Scott et al. 2014), we explored the promoter regions for the presence of putative cis-regulatory sequences and compared them with promoters in D. melanogaster. No experimental data defined the length of the promoter regions in M. domestica, and only some D. melanogaster circadian genes were functionally explored using systematic promoter truncation experiments. Therefore, we analyzed the DNA region upstream of the start codon (“ups” in Figure 9) and also separately analyzed regions including the first intron after the start codon (“ups+” in Figure 9).
Figure 9.
Relative abundance of cis-regulatory motifs in promoters of D. melanogaster and M. domestica. The values indicate the actual occurrence of a particular cis-motif presented as a fold change when 1 is the expected frequency of a particular motif in a random DNA sequence. Heat map was used for easier orientation in the table. Two regions of the genes were analyzed: the region upstream of ATG (ups) and the upstream region with the first intron (ups+). The actual position and distribution of selected cis-motifs in DNA is shown in Figure 10 and Figure 11 and full maps are presented for cwo genes in Figure S3.
In general, M. domestica genes were longer than D. melanogaster homologs, often with larger intronic sequences. The frequency of E-box-related motifs (CACGTG, CACGTH, AACGTG) was lower in M. domestica than in D. melanogaster for the per, tim, vrille, and cwo promoters (Figure 9). The frequency of the canonical E-box (CACGTG) was even lower than expected for random occurrences of this motif in Md_per, Md_tim, Md_vri, and Md_cwo.
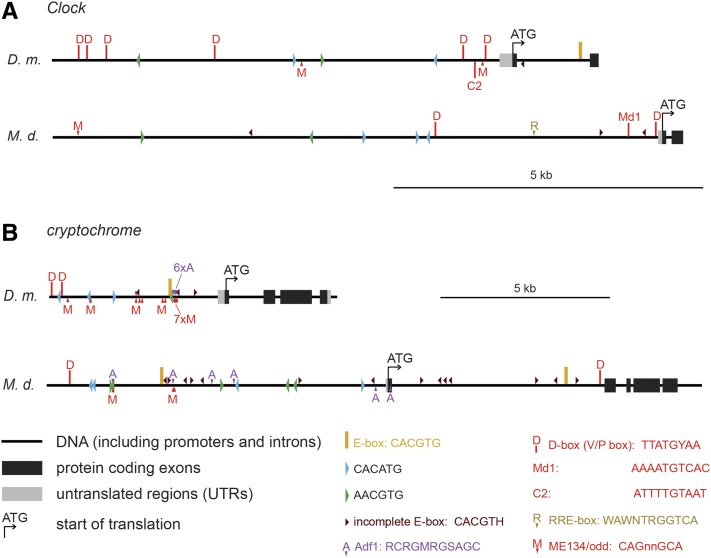
Remarkably, frequent occurrences of the D-box (known also as the V/P box) were found in Dm_tim (26-fold), Dm_Clk (26 fold), Dm_cry (13-fold), and Dm_vri (13-fold). In M. domestica, the D-box motif was less abundant (i.e., 6.6-fold in Md_Clk, 3.3-fold in Md_cry). An RRE-element (Harding and Lazar 1993) was found in Dm_per (17-fold), Md_tim (15-fold), and Md_Clk (13-fold). The Adf1 motif (Meireles-Filho et al. 2014) was strikingly abundant in Dm_cry (76-fold) and also frequent in Dm_per (8.5-fold), Dm_vri (13-fold), and Md_cry (13-fold) (see Figure 9 for full comparison of cis-motif frequency, Figure 10 for position of motifs in tim, per, vri and cwo promoters, and Figure 11 for Clk and cry promoters).
Figure 10.
Schematic depictions of D. melanogaster (D.m.) and M. domestica (M.d.) promoters with highlighted positions of putative cis-regulatory motifs in (A) timeless, (B) period, (C) vrille, and (D) clockwork orange. In the case of cwo (D), E-box and related cis-regulatory motifs were omitted for picture clarity. For the complete map see Figure S3. Note different scales (shown on the right for each gene).
Figure 11.
Schematic depictions of D. melanogaster (D.m.) and M. domestica (M.d.) promoters with highlighted positions of putative cis-regulatory motifs in (A) Clock, and (B) cryptochrome. C2 motif (Cyran et al. 2003) and Md1 motif are similar to D-box. Note different scales (shown on the right for each gene).
Discussion
The expression of circadian genes was explored in many insect species. In general, expression patterns often differ among tissues (Beaver et al. 2003; Iwai et al. 2006; Tomioka et al. 2012; Damulewicz et al. 2015), is affected by the reproductive/diapause status of the animal (Dolezel et al. 2008; Kostal et al. 2008; Bajgar et al. 2013a,b), and depends on the animals’ age and access to nutrition (Dolezel et al. 2007; Rakshit et al. 2012).
The temporal expression of circadian genes also remarkably differs between species. The strong cyclical expression characteristic of several circadian genes in Drosophila is not the rule for all insect species. For instance, the expression of circadian clock gene homologs per and Clk show no cyclical changes either in heads of the linden bug, P. apterus (Syrova et al. 2003), or even in dissected brains (J. Kotwica-Rolinska and D. Dolezel, unpublished data). More importantly, expression changes differ between different brain regions of D. melanogaster, including the prominent amplitude of many transcripts in PDF cells (Kula-Eversole et al. 2010). Notably, the expression in compound eyes contributes remarkably to whole head mRNA levels of several circadian genes. Indeed, the identification of the cryptochromeb mutation in a luciferase-reporter screen (Stanewsky et al. 1998) was made possible by this strong expression in the eye. Therefore, correlating expression patterns in the whole head with the locomotor activity profile is only an approximation and overlooks cell-specific expressions. However, the lack of suitable antibodies recognizing Musca circadian genes prevents rigorous analysis with anatomical resolution. At the same time, expression profiles on entire D. melanogaster heads were used extensively; thus, interspecific comparisons with solid data are feasible.
In this study, we addressed the impact of temperature on the expression of circadian genes in Musca compared with that of D. melanogaster homologs. Despite certain differences between these two species, a few general patterns were observed (Figure 12). Low temperatures resulted in the phase advancement of period, vrille, and cwo in both species (Figure 5 and Figure 6). However, the phase of Pdp1epsilon expression was not affected in Musca, whereas the expression of Dm_Pdp1epsilon was regulated similarly to Dm_tim (see below). Furthermore, the expression of Md_tim was clearly delayed by both high and low temperatures in M. domestica and temperature affected also the amplitude of Md_tim. However, Dm_tim differed only in the amplitude with no phase shift. Our Dm_tim expression is in agreement with results observed previously (Kidd et al. 2015), where tim mRNA and TIM protein expression served as a case support for a so-called “pathway” model of temperature compensation (Kidd et al. 2015). This model expects that “the concentration of the protein and mRNA components of the clock should scale in a simple fashion with temperature. In particular, the overall amplitude or average value of an oscillation in any given component can change with temperature, but the shape of the oscillation and the phase relationships between different oscillating components should remain approximately the same at any temperature…” (Kidd et al. 2015). Although our experiment did not address temperature compensation directly and data were obtained under an LD regime only, clear intraspecific difference in tim expression suggests further validation of the proposed pathway model. This caution is further strengthened by the more complex impact of temperature on expression of additional circadian clock genes, where phase was also affected.
Figure 12.
Summary of response to temperature in (A) M. domestica (left) and D. melanogaster (right). Although the locomotor activity pattern is different, both species display bimodal activity patterns at hot temperatures. Under cold temperatures, M. domestica reduces its activity to a single peak in the center of the photophase, whereas the activity of D. melanogaster is still bimodal; however, the position of both peaks moves toward the photophase. (B) Low temperatures result in the phase advancement of per, vri, cwo, and Clk expression in both species (left panel). In Musca, both hot and cold temperatures result in the amplitude change of tim expression and also in phase shift (right panel).
The transcription of per, tim, Pdp1epsilon, vri, and cwo requires the CLK-CYC heterodimer (Cyran et al. 2003); therefore, it was surprising to see different expression patterns in these genes. One possible explanation is the different temperature-dependent transcription. Indeed, CLK and CYC tissue-specific expression of Drosophila is enhanced by the transcription factors ODD PAIRED and SERPENT (Meireles-Filho et al. 2014). Similar alterations and redirections might contribute to the temperature-adjustment of circadian gene expression. Alternatively, posttranscriptional regulation might contribute to altered mRNA stability and, therefore, affect its accumulation. Indeed, three circadian genes, vri, cwo, and Clk, are regulated by microRNAs in D. melanogaster (Kadener et al. 2009). Most likely, the combination of both phenomena contributes to the temperature compensation mechanism.
The most striking difference was found in cry expression, which is flat in Md_cry (Codd et al. 2007, and Figure 5), whereas Dm_cry expression is cyclical with a peak during the photophase (Emery et al. 1998, and Figure 6). Although some weak phenotypes are found at nonambient temperatures for cry-null mutants (Dolezelova et al. 2007), cry is not a key component of the circadian clock under constant dark conditions. Instead, the CRY protein serves as a sensitive light receptor for the entrainment of the clock (Emery et al. 1998, 2000).
To further shed light on a possible mechanistic explanation of transcriptional regulation, extensive comparison of cis-regulatory motifs was performed. We were hoping to recognize some clear pattern that would define further functional tests. A few findings are worthy of attention: It is tempting to speculate that different expression of tim between these two fly species results from remarkably different frequency of D-box motif in tim promoters (4× D-box/5 kb in D. melanogaster vs. 2× D-box/30 kb in M. domestica). PDP1epsilon and VRI, two basic Leucine Zipper Domain (bZIP) circadian proteins, are known to bind to D-box motif in D. melanogaster (Cyran et al. 2003).
Another gene, with remarkably different expression between compared species was cry, which was cycling in D. melanogaster, but flat in M. domestica. While the frequency of E-box motifs was comparable in cry promoters of both species, D. melanogaster cry promoter was enriched for Adf1 motif and for D-box. Although this analysis was purely computational and hence identified only putative binding motifs, rigorous functional tests are easy in D. melanogaster and now even possible in Musca thanks to gene editing technology (Sharma et al. 2017).
Our locomotor activity experiments identified the clear impact of temperature on the activity distribution pattern in M. domestica. While at low temperatures houseflies showed a single activity peak located at the middle of the photophase, their activity was distributed throughout the entire photophase under ambient and high temperatures. Although one could expect that houseflies exposed to high temperatures will try to avoid dry midday and afternoon, minimal differences were observed between ambient and high temperatures. Notably, houseflies successfully colonized remarkably different environments including dry and humid regions. Since each experimental tube contained a water reservoir, we expect that the humidity was relatively high. It would be interesting to explore behavior under more natural conditions (see also text below).
The activity profile of males at ambient and high temperatures contained mild troughs between the light on and off peaks, exhibiting similarities with morning and evening activity peaks of D. melanogaster. It is believed that lower midday activity, the “siesta,” is an adaptation protecting D. melanogaster from dry environments experienced at noon. However, this behavior was observed under constant temperatures in laboratory experiments. Locomotor activities obtained from D. melanogaster recorded under natural conditions, with temperature cycles during the day (together with the light intensity), revealed more complex patterns with an additional noon peak (Vanin et al. 2012; Green et al. 2015). Despite these discrepancies, the daily activity pattern is clearly affected by either constant or cyclical temperature profiles in D. melanogaster.
Temperature-dependent splicing of the last per intron (Dmpi8) is established as the key factor influencing the timing of morning and evening activity peaks (Majercak et al. 1999, 2004; Cao and Edery 2017). Therefore, we searched for a homolog of this 3ʹ UTR intron in Musca. However, the expression analysis of both introns localized at the 3ʹ did not reveal any temperature-influenced splicing, suggesting a different molecular mechanism may be involved in M. domestica. This mechanism might include the splicing of introns localized in the open reading frame. Alternatively, the expression profile of additional circadian genes might be important. The latter explanation is supported by conserved temperature-dependent expression trends between D. melanogaster and M. domestica.
In D. melanogaster, low temperatures result in the timUNSPLICED isoform removing the last 34 amino acid of the TIM protein including the conserved 15 amino acid motif. The importance of this motif is suggested by its presence in Thermobia domestica, an insect species that shared a common ancestor with Drosophila >400 MYA (Misof et al. 2014). Surprisingly, the motif is highly modified in Belgica antarctica, a dipteran insect living in extreme and generally cold environments (Kelley et al. 2014). Nevertheless, stop codon positions are conserved in tim introns of D. melanogaster, Chymomyza costata, and M. domestica. However, only a few percent reductions in splicing were found at 15° in M. domestica. Perhaps even lower temperatures are required for this temperate fly to change its splicing pattern dramatically.
Functional experiments addressing the role of particular genes in Musca are needed to fully define underlying biological mechanisms. Alternatively, further comparative studies might point to conserved features across taxa. Interestingly, bimodal activity was found even in distantly related insects, for example in Lepidoptera where the flight activity of Ephestia kuehniella is restricted to early and late night peaks (Zavodska et al. 2012; Kobelkova et al. 2015). It would be interesting to see how this nocturnal insect changes its activity pattern at different temperatures, if the expression of circadian genes is affected by the temperature, and how. Similar comparative experiments might identify general patterns, which need to be verified functionally. These reverse genetics experiments are technically demanding in nonmodel insects, yet current gene editing tools are already reaching chronobiology (Markert et al. 2016; Sharma et al. 2017).
Supplementary Material
Supplemental material is available online at www.g3journal.org/lookup/suppl/doi:10.1534/g3.117.042374/-/DC1.
Acknowledgments
We thank Ing. Roman Neuzil for his excellent house fly husbandry,Daniel Bopp (University of Zurich) for M. domestica strains, and Martin Pivarci for the phylogenetic analyses. We thank Joanna Kotwica-Rolinska and Milena Damulewicz for the critical reading of the manuscript and Martina Hajduskova (www.biographix.cz) for Figure 12. This work was supported by the National Science Foundation (grant no. 17-01003S to D.D.) and locomotor activity monitors were purchased from the European Union program FP7/2007–2013 (grant no. 316304).
Footnotes
Communicating editor: B. H. Reed
Literature Cited
- Abascal F., Zardoya R., Posada D., 2005. ProtTest: selection of best-fit models of protein evolution. Bioinformatics 21: 2104–2105. [DOI] [PubMed] [Google Scholar]
- Bajgar A., Dolezel D., Hodkova M., 2013a Endocrine regulation of non-circadian behavior of circadian genes in insect gut. J. Insect Physiol. 59: 881–886. [DOI] [PubMed] [Google Scholar]
- Bajgar A., Jindra M., Dolezel D., 2013b Autonomous regulation of the insect gut by circadian genes acting downstream of juvenile hormone signaling. Proc. Natl. Acad. Sci. USA 110: 4416–4421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazalova O., Kvicalova M., Valkova T., Slaby P., Bartos P., et al. , 2016. Cryptochrome 2 mediates directional magnetoreception in cockroaches. Proc. Natl. Acad. Sci. USA. 113: 1660–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaver L. M., Rush B. L., Gvakharia B. O., Giebultowicz J. M., 2003. Noncircadian regulation and function of clock genes period and timeless in oogenesis of Drosophila melanogaster. J. Biol. Rhythms 18: 463–472. [DOI] [PubMed] [Google Scholar]
- Boothroyd C. E., Wijnen H., Naef F., Saez L., Young M. W., 2007. Integration of light and temperature in the regulation of circadian gene expression in Drosophila. PLoS Genet. 3: e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao W., Edery I., 2017. Mid-day siesta in natural populations of D. melanogaster from Africa exhibits an altitudinal cline and is regulated by splicing of a thermosensitive intron in the period clock gene. BMC Evol. Biol. 17: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codd V., Dolezel D., Stehlik J., Piccin A., Garner K. J., et al. , 2007. Circadian rhythm gene regulation in the housefly Musca domestica. Genetics 177: 1539–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins B. H., Rosato E., Kyriacou C. P., 2004. Seasonal behavior in Drosophila melanogaster requires the photoreceptors, the circadian clock, and phospholipase C. Proc. Natl. Acad. Sci. USA 101: 1945–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyran S. A., Buchsbaum A. M., Reddy K. L., Lin M. C., Glossop N. R. J., et al. , 2003. vrille, Pdp1, and dClock form a second feedback loop in the Drosophila circadian clock. Cell 112: 329–341. [DOI] [PubMed] [Google Scholar]
- Damulewicz M., Loboda A., Bukowska-Strakova K., Jozkowicz A., Dulak J., et al. , 2015. Clock and clock-controlled genes are differently expressed in the retina, lamina and in selected cells of the visual system of Drosophila melanogaster. Front. Cell. Neurosci. 9: 353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolezel D., Sauman I., Kost’al V., Hodkova M., 2007. Photoperiodic and food signals control expression pattern of the clock gene, period, in the linden bug, Pyrrhocoris apterus. J. Biol. Rhythms 22: 335–342. [DOI] [PubMed] [Google Scholar]
- Dolezel D., Zdechovanova L., Sauman I., Hodkova M., 2008. Endocrine-dependent expression of circadian clock genes in insects. Cell. Mol. Life Sci. 65: 964–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolezelova E., Dolezel D., Hall J. C., 2007. Rhythm defects caused by newly engineered null mutations in Drosophila’s cryptochrome gene. Genetics 177: 329–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery P., So W. V., Kaneko M., Hall J. C., Rosbash M., 1998. CRY, a Drosophila clock and light-regulated cryptochrome, is a major contributor to circadian rhythm resetting and photosensitivity. Cell 95: 669–679. [DOI] [PubMed] [Google Scholar]
- Emery P., Stanewsky R., Helfrich-Forster C., Emery-Le M., Hall J. C., et al. , 2000. Drosophila CRY is a deep brain circadian photoreceptor. Neuron 26: 493–504. [DOI] [PubMed] [Google Scholar]
- Green E. W., O’Callaghan E. K., Hansen C. N., Bastianello S., Bhutani S., et al. , 2015. Drosophila circadian rhythms in seminatural environments: summer afternoon component is not an artifact and requires TrpA1 channels. Proc. Natl. Acad. Sci. USA 112: 8702–8707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding H. P., Lazar M. A., 1993. The orphan receptor Rev-ErbA alpha activates transcription via a novel response element. Mol. Cell. Biol. 13: 3113–3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hediger M., Niessen M., Wimmer E. A., Dubendorfer A., Bopp D., 2001. Genetic transformation of the housefly Musca domestica with the lepidopteran derived transposon piggyBac. Insect Mol. Biol. 10: 113–119. [DOI] [PubMed] [Google Scholar]
- Iwai S., Fukui Y., Fujiwara Y., Takeda M., 2006. Structure and expressions of two circadian clock genes, period and timeless in the commercial silkmoth, Bombyx mori. J. Insect Physiol. 52: 625–637. [DOI] [PubMed] [Google Scholar]
- Kadener S., Menet J. S., Sugino K., Horwich M. D., Weissbein U., et al. , 2009. A role for microRNAs in the Drosophila circadian clock. Genes Dev. 23: 2179–2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauranen H., Menegazzi P., Costa R., Helfrich-Forster C., Kankainen A., et al. , 2012. Flies in the north: locomotor behavior and clock neuron organization of Drosophila montana. J. Biol. Rhythms 27: 377–387. [DOI] [PubMed] [Google Scholar]
- Kelley J. L., Peyton J. T., Fiston-Lavier A. S., Teets N. M., Yee M. C., et al. , 2014. Compact genome of the Antarctic midge is likely an adaptation to an extreme environment. Nat. Commun. 5: 4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd P. B., Young M. W., Siggia E. D., 2015. Temperature compensation and temperature sensation in the circadian clock. Proc. Natl. Acad. Sci. USA 112: E6284–E6292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobelkova A., Bajgar A., Dolezel D., 2010. Functional molecular analysis of a circadian clock gene timeless promoter from the Drosophilid fly Chymomyza costata. J. Biol. Rhythms 25: 399–409. [DOI] [PubMed] [Google Scholar]
- Kobelkova A., Zavodska R., Sauman I., Bazalova O., Dolezel D., 2015. Expression of clock genes period and timeless in the central nervous system of the Mediterranean flour moth, Ephestia kuehniella. J. Biol. Rhythms. 30: 104–116. [DOI] [PubMed] [Google Scholar]
- Kostal V., Tollarova M., Dolezel D., 2008. Dynamism in physiology and gene transcription during reproductive diapause in a heteropteran bug, Pyrrhocoris apterus. J. Insect Physiol. 54: 77–88. [DOI] [PubMed] [Google Scholar]
- Kula-Eversole E., Nagoshi E., Shang Y. H., Rodriguez J., Allada R., et al. , 2010. Surprising gene expression patterns within and between PDF-containing circadian neurons in Drosophila. Proc. Natl. Acad. Sci. USA 107: 13497–13502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lankinen P., 1986. Geographical variation in circadian eclosion rhythm and photoperiodic adult diapause in Drosophila-littoralis. J. Comp. Physiol. A 159: 123–142. [DOI] [PubMed] [Google Scholar]
- Low K. H., Lim C., Ko H. W., Ederyl I., 2008. Natural variation in the splice site strength of a clock gene and species-specific thermal adaptation. Neuron 60: 1054–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majercak J., Sidote D., Hardin P. E., Edery I., 1999. How a circadian clock adapts to seasonal decreases in temperature and day length. Neuron 24: 219–230. [DOI] [PubMed] [Google Scholar]
- Majercak J., Chen W. F., Edery I., 2004. Splicing of the period gene 3’-terminal intron is regulated by light, circadian clock factors, and phospholipase C. Mol. Cell. Biol. 24: 3359–3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markert M. J., Zhang Y., Enuameh M. S., Reppert S. M., Wolfe S. A., et al. , 2016. Genomic access to monarch migration using TALEN and CRISPR/Cas9-mediated targeted mutagenesis. G3 6: 905–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushima A., Sato S., Chuman Y., Takeda Y., Yokotani S., et al. , 2004. cDNA cloning of the housefly pigment-dispersing factor (PDF) precursor protein and its peptide comparison among the insect circadian neuropeptides. J. Pept. Sci. 10: 82–91. [DOI] [PubMed] [Google Scholar]
- Meireles-Filho A. C., Bardet A. F., Yanez-Cuna J. O., Stampfel G., Stark A., 2014. cis-regulatory requirements for tissue-specific programs of the circadian clock. Curr. Biol. 24: 1–10. [DOI] [PubMed] [Google Scholar]
- Miskiewicz K., Schurmann F. W., Pyza E., 2008. Circadian release of pigment-dispersing factor in the visual system of the housefly, Musca domestica. J. Comp. Neurol. 509: 422–435. [DOI] [PubMed] [Google Scholar]
- Misof B., Liu S., Meusemann K., Peters R. S., Donath A., et al. , 2014. Phylogenomics resolves the timing and pattern of insect evolution. Science 346: 763–767. [DOI] [PubMed] [Google Scholar]
- Montelli S., Mazzotta G., Vanin S., Caccin L., Corra S., et al. , 2015. period and timeless mRNA splicing profiles under natural conditions in Drosophila melanogaster. J. Biol. Rhythms 30: 217–227. [DOI] [PubMed] [Google Scholar]
- Piccin A., Couchman M., Clayton J. D., Chalmers D., Costa R., et al. , 2000. The clock gene period of the housefly, Musca domestica, rescues behavioral rhythmicity in Drosophila melanogaster. Evidence for intermolecular coevolution? Genetics 154: 747–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyza E., Meinertzhagen I. A., 1997. Neurites of period-expressing PDH cells in the fly’s optic lobe exhibit circadian oscillations in morphology. Eur. J. Neurosci. 9: 1784–1788. [DOI] [PubMed] [Google Scholar]
- Rakshit K., Krishnan N., Guzik E. M., Pyza E., Giebultowicz J. M., 2012. Effects of aging on the molecular circadian oscillations in Drosophila. Chronobiol. Int. 29: 5–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosato E., Kyriacou C. P., 2006. Analysis of locomotor activity rhythms in Drosophila. Nat. Protoc. 1: 559–568. [DOI] [PubMed] [Google Scholar]
- Rubin E. B., Shemesh Y., Cohen M., Elgavish S., Robertson H. M., et al. , 2006. Molecular and phylogenetic analyses reveal mammalian-like clockwork in the honey bee (Apis mellifera) and shed new light on the molecular evolution of the circadian clock. Genome Res. 16: 1352–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. G., Warren W. C., Beukeboom L. W., Bopp D., Clark A. G., et al. , 2014. Genome of the house fly, Musca domestica L., a global vector of diseases with adaptations to a septic environment. Genome Biol. 15: 466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid B., Helfrich-Forster C., Yoshii T., 2011. A new ImageJ plug-in “ActogramJ” for chronobiological analyses. J. Biol. Rhythms 26: 464–467. [DOI] [PubMed] [Google Scholar]
- Sharma A., Heinze S. D., Wu Y., Kohlbrenner T., Morilla I., et al. , 2017. Male sex in houseflies is determined by Mdmd, a paralog of the generic splice factor gene CWC22. Science 356: 642–645. [DOI] [PubMed] [Google Scholar]
- Stanewsky R., Kaneko M., Emery P., Beretta B., Wager-Smith K., et al. , 1998. The cry(b) mutation identifies cryptochrome as a circadian photoreceptor in Drosophila. Cell 95: 681–692. [DOI] [PubMed] [Google Scholar]
- Syrova Z., Dolezel D., Saumann I., Hodkova M., 2003. Photoperiodic regulation of diapause in linden bugs: are period and clock genes involved? Cell. Mol. Life Sci. 60: 2510–2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomioka K., Uryu O., Kamae Y., Umezaki Y., Yoshii T., 2012. Peripheral circadian rhythms and their regulatory mechanism in insects and some other arthropods: a review. J. Comp. Physiol. B 182: 729–740. [DOI] [PubMed] [Google Scholar]
- Vanin S., Bhutani S., Montelli S., Menegazzi P., Green E. W., et al. , 2012. Unexpected features of Drosophila circadian behavioural rhythms under natural conditions. Nature 484: 371–375. [DOI] [PubMed] [Google Scholar]
- Yuan Q., Metterville D., Briscoe A. D., Reppert S. M., 2007. Insect cryptochromes: gene duplication and loss define diverse ways to construct insect circadian clocks. Mol. Biol. Evol. 24: 948–955. [DOI] [PubMed] [Google Scholar]
- Zavodska R., Fexova S., von Wowern G., Han G. B., Dolezel D., et al. , 2012. Is the sex communication of two pyralid moths, Plodia interpunctella and Ephestia kuehniella, under circadian clock regulation? J. Biol. Rhythms 27: 206–216. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Drosophila strain is available on request from our laboratory. Musca strain was obtained from D. Bopp (Hediger et al. 2001). Sequence data of D. melanogaster and M. domestica circadian genes can be found on FlyBase (http://flybase.org/) and VectorBase (https://www.vectorbase.org/) databases, respectively (see Table S2 for accession numbers).