Abstract
Background:
In some jurisdictions, routine reporting of the estimated glomerular filtration rate (eGFR) has led to an increase in nephrology referrals and wait times.
Objective:
We describe the use of the Kidney Failure Risk Equation (KFRE) as part of a triage process for new nephrology referrals for patients with chronic kidney disease stages 3 to 5 in a Canadian province.
Design:
A quasi-experimental study design was used.
Setting:
This study took place in Manitoba, Canada.
Measurements:
Demographics, laboratory values, referral numbers, and wait times were compared between periods.
Methods:
In 2012, we adopted a risk-based cutoff of 3% over 5 years using the KFRE as a threshold for triage of new referrals. Referrals who did not meet other prespecified criteria (such as pregnancy, suspected glomerulonephritis, etc) and had a kidney failure risk of <3% over 5 years were returned to primary care with recommendations based on diabetes and hypertension guidelines. The average wait time and number of consults seen between the pretriage (January 1, 2011, to December 31, 2011) and posttriage period (January 1, 2013, to December 31, 2013) were compared using a general linear model.
Results:
In the pretriage period, the median number of referrals was 68/month (range: 44-76); this increased to 94/month (range: 61-147) in the posttriage period. In the posttriage period, 35% of referrals were booked as urgent, 31% as nonurgent, and 34% of referrals were not booked. The median wait times improved from 230 days (range: 126-355) in the pretriage period to 58 days (range: 48-69) in the posttriage period.
Limitations:
We do not have long-term follow-up on patients triaged as low risk. Our study may not be applicable to nephrology teams operating under capacity without wait lists. We did not collect detailed information on all referrals in the pretriage period, so any differences in our pretriage and posttriage patient groups may be unaccounted for.
Conclusions:
Our risk-based triage scheme is an effective health policy tool that led to improved wait times and access to care for patients at highest risk of progression to kidney failure.
Keywords: referral, risk, triage, wait time
Abrégé
Contexte:
Dans certaines régions administratives, la déclaration systématique des valeurs de débit de filtration glomérulaire estimé (DFGe) a conduit à une augmentation des recommandations de patients pour un suivi en néphrologie et, par conséquent, du temps d’attente pour obtenir une consultation.
Objectif de l’étude:
Dans cette étude, nous décrivons l’utilisation de l’équation de risque pour l’insuffisance rénale terminale (KFRE) dans le cadre du processus de triage des nouvelles recommandations pour un suivi en néphrologie de patients atteints d’insuffisance rénale chronique de stade 3 à stade 5 dans une province canadienne.
Type d’étude:
On a utilisé un modèle quasi expérimental pour cette étude.
Cadre de l’étude:
Cette étude a eu lieu dans la province du Manitoba au Canada.
Mesures:
Nous avons comparé les données démographiques et les valeurs de laboratoire des patients, de même que le nombre de nouvelles recommandations de patients en néphrologie et le temps d’attente pour obtenir une consultation entre les périodes choisies.
Méthodologie:
En 2012, à l’aide de la KFRE, nous avons établi un risque de défaillance rénale de 3 % sur 5 ans comme valeur seuil pour le triage des nouvelles recommandations de patients pour un suivi en néphrologie. Les patients redirigés qui ne respectaient pas les autres critères de triage spécifiés au préalable, notamment la grossesse ou une glomérulonéphrite soupçonnée, de même que ceux qui présentaient un risque de défaillance rénale inférieur à 3 % sur 5 ans ont été retournés vers les soins primaires avec une recommandation fondée sur les directives du diabète et de l’hypertension. Le délai d’attente moyen et le nombre de consultations observés entre la période prétriage (du 1er janvier 2011 au 31 décembre 2011) et la période suivant le triage (du 1er janvier 2013 au 31 décembre 2013) ont été comparés à l’aide d’un modèle linéaire général.
Résultats:
Au cours de la période de prétriage, le nombre médian de recommandations en néphrologie a été de 68 par mois (intervalle : 44 à 76 par mois). Ce nombre est passé à 94 par mois (intervalle : 61 à 147 par mois) dans la période suivant le triage. Au cours de cette période, 35 % des recommandations étaient identifiées comme étant urgentes, 31 % comme étant non urgents et 34 % ne portaient aucune mention. Le temps d’attente médian s’est amélioré, passant de 230 jours (intervalle : 126 à 355 jours) en période de prétriage à 58 jours (intervalle : 48 à 69 jours) dans la période post-triage.
Limites de l’étude:
Nous n’avons aucun suivi à long terme pour les patients classés à faible risque de défaillance rénale sur 5 ans. De plus, il est possible que notre étude ne puisse s’appliquer aux équipes de néphrologues qui opèrent en dessous de leur capacité et donc, sans liste d’attente. Enfin, nous ne pouvons tenir compte des différences susceptibles d’être observées entre les groupes de patients vus en prétriage et en post-triage puisque nous n’avons pas recueilli de renseignements détaillés sur tous les patients recommandés.
Conclusions:
Notre système de triage axé sur les risques de défaillance rénale sur 5 ans est un outil efficace d’élaboration de politiques en santé. Ce système conduit à l’amélioration du temps d’attente et à un accès plus facile aux soins pour les patients à haut risque de voir leur état progresser vers l’insuffisance rénale.
What was known before
In 2011, the Kidney Failure Risk Equation (KFRE) was developed and has subsequently been shown to be highly accurate in predicting the progression of chronic kidney disease (CKD) to kidney failure.
What this adds
A threshold risk of 3% over 5 years for kidney failure, as determined by the KFRE, can be integrated into a triage process, and reduce wait times for nephrology care.
Background
Chronic kidney disease (CKD) is a major public health problem that is associated with increased morbidity and mortality.1,2 CKD is often a “silent” disease, whereby disease-related symptoms typically present in later stages.2,3 Identifying patients with CKD early in their disease trajectory and implementing specific treatments may prevent adverse complications such as progression to end-stage kidney disease (ESKD), acute kidney injury, and cardiovascular disease.2,4 As such, most international clinical practice guidelines recommend using estimated glomerular filtration rate (eGFR) and albuminuria for the identification, monitoring, and classification of CKD.5,6
Primary care providers usually test for kidney disease by estimating GFR using serum creatinine and by testing for protein in the urine. Automated eGFR reporting in the general population has led to a substantial increase in the recognition of CKD (eGFR <60 mL/min/1.73 m2) in older populations, generating an increase in referrals to specialized nephrology care teams.7 A substantial proportion of this newly recognized CKD population is considered at low risk of progression to kidney failure (reference companion paper). In many of these patients, nephrology referral may not necessarily lead to improvement of long-term outcomes.8,9 Concurrently, the increase in referrals in resource-limited settings has led to significant wait times, higher costs, and potentially less access to care for patients at higher risk of kidney failure.8,10-12 Our own experience with automatic eGFR reporting suggested a large increase in low-risk referrals who did not meet prespecified criteria for nephrology care.13
In 2011, the Kidney Failure Risk Equation (KFRE) was developed and has subsequently been shown to be highly accurate in predicting the progression of CKD to kidney failure.14 Since that time, the KFRE has been extensively validated in multiple diverse CKD populations worldwide.15-19 In 2012, as response to increased nephrology wait times, we arrived at 3% risk threshold over 5 years as a criterion for nephrology referrals through physician consensus. This threshold represents the risk for a 70-year-old male with an eGFR of 45 mL/min/1.73 m2 and 15 mg/mmol of albuminuria.
Subsequently, we validated the KFRE in our province and demonstrated that this risk threshold of 3% over 5 years is 97% sensitive and 62% specific for predicting progression to kidney failure, with a negative predictive value of 99% (reference companion paper). Here, we describe the results from our quality improvement initiative, using the KFRE and these thresholds as part of a triage tool for referrals, in an effort to improve timely access to specialist care through improved wait times.
Methods
Study Population
Manitoba (population 1.27 million) is a province situated in central Canada. It has the second highest incidence and prevalence of ESKD in Canada.20 Winnipeg, the provincial capital city, is where more than half of the provincial population is concentrated. The remainder of the population is dispersed over a large area of 649 950 km2.21 Manitoba is culturally diverse with almost 10% of the population being a member of a visible minority, and 14% of Aboriginal origin.22,23
Our initiative was exempt from institutional review board approval as it was deemed a quality improvement project, with less than minimal risk to patients and with all patients de-identified.
Manitoba Renal Program
The Manitoba Renal Program (MRP) is responsible for providing all CKD care for patients referred to nephrology, as well as the ESKD services in the province. In Winnipeg, there are 3 renal centers, which together care for and assess the majority of new nephrology referrals (CKD and dialysis) within the MRP. One other center in Brandon, Manitoba, a city of 46 000 people,24 sees a minority of total provincial referrals and primarily sees referrals from its catchment area only. Those consultations were not included in this study. The MRP provides referral pathways and standard referral forms for clinicians, which can be found at www.kidneyhealth.ca.
eGFR reporting in Manitoba began in October of 2010, and KFRE triage was started in January 1, 2012. We collected referral counts, wait times, and demographic data on all Winnipeg-directed referrals sent to 2 of the 3 renal centers in the MRP between January 1, 2011, and December 31, 2011, in the pretriage period, and January 1, 2013, and December 31, 2013, in the posttriage period. Wait times for each patient were defined as the time between referral and actual visit to a nephrologist, measured in days. A 12-month period immediately following implementation of triage was observed as a transition period to the new triage system and was excluded from the final analysis.
Referrals in the pretriage period included data on patient name, provider name, and date of referral and appointment. In contrast, all referrals made in the posttriage period had data collected for demographics, comorbidities, serum biochemistry markers (GFR, creatinine, hemoglobin, calcium, phosphate, and albumin), and urine albumin:creatinine ratio (ACR).
Kidney Failure Risk Equation
The 4-variable KFRE was calculated for each referral using the variables age, sex, eGFR, and quantified urinary proteinuria (ACR; or protein:creatinine or 24-hour urine protein normalized to ACR). These values were entered by our physician assistant into the online calculator at uniform resource locator http://www.qxmd.com/calculate-online/nephrology/kidney-failure-risk-equation. The generated score as a 5-year risk of kidney failure was recorded on the initial referral for review by the nephrologist. A patient with a score ≥3% was labeled as high risk for progression to kidney failure, and a score <3% was classified as low risk.
If all values were not included in the original referral, a search was done on our provincial database (eCHART) and a request was made to the referring physician for more complete information. No consults were triaged unless all 4 variables were available, or another indication for nephrology referral was provided on the referral letter/form.
Triage Systems
Referrals in the pretriage period were sent to a specific site for either a specific physician or into a general pool to distribute among physicians at the site. Once referrals were received, they were triaged as urgent, nonurgent, or “do not book” by an individual rotating nephrologist. There were no set criteria or risk-based calculations applied in this period, and acuity was solely at the discretion of the nephrologist. We define referrals are consultations that are booked for a visit with a nephrologist.
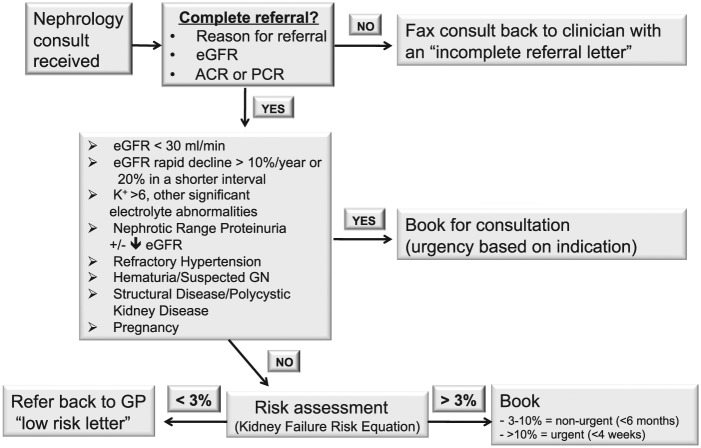
In the posttriage period, each referral was considered in the context of the triage scheme based on the KFRE, or if another indication was listed for specialist consultation (Figure 1). Referrals were deemed as appropriate if any of the significant criteria for referral were met, or if the 5-year risk score was greater than 3% for ESKD. If none of these criterions were present, patients were noted to have a low predicted risk and a letter was sent back to the referring provider explaining the relatively low risk for progression to kidney failure, and a brief outline of further management suggestions for the patient. In addition, referring clinicians were invited to contact the nephrologist for any questions, concerns, or if the clinician felt a consultation with a nephrologist was still warranted. These patients would then be booked without further discussion. Referrals were seen urgently if the patient had an eGFR of less than 15, whereas other significant indications were triaged primarily dependent on indication and clinical context.
Figure 1.
Process for triage of referrals.
Note. eGFR = estimated glomerular filtration rate; ACR = albumin:creatinine ratio; PCR = protein:Creatinine Ratio; GP = general Practitioner; GN = glomerulonephritis.
Data Analysis
The median wait time and number of consults were compared between the pretriage and posttriage periods using a general linear model. Each model contained a variable for reporting month, an indicator variable representing the posttriage period, and an interaction term between the reporting month and posttriage indicator. The change in slope following triage was represented by the coefficient of the interaction term between reporting month and the triage indicator variable. Demographic comparisons were made between the low- and high-risk groups in the posttriage period. Continuous variables are expressed as median and interquartile range (IQR) and compared between both groups using the Mann-Whitney U test. Categorical variables are expressed as frequencies and percentages and compared between both groups using a chi-square test. All statistical analysis was performed using SAS version 9.2 (Cary, North Carolina) for Microsoft Windows. Visual representations of these models were developed using Microsoft Excel 2010 (Seattle, Washington).
Results
Patient Characteristics
Baseline characteristics of the posttriage referral groups are shown in Table 1. The median age of the patients was 68, with an equal number of males and females. The median eGFR at referral was 39 mL/min/1.73 m2 (IQR: 27.4-55.2), and the median urine ACR was 9.2 mg/mmol (IQR: 1.6-65.5). A low eGFR was the primary reason for referral by the referring clinician for 66% of the patients, followed by proteinuria in 20.4% of referrals. Other indications including suspected glomerulonephritis and electrolyte disorders made up less than 15% of all referrals.
Table 1.
Posttriage Period Patient Demographics.
| Total | Low-risk strata not booked | Low-risk strata booked | High-risk strata | P value | |
|---|---|---|---|---|---|
| Age | 67.7 (56.3-77.9) | 67.7 (56.3-74.6) | 59.1 (43.8-68.7) | 72.2 (61.8-80.8) | <.0001 |
| Gender | .4395 | ||||
| Male | 50.3% | 46.8% | 52.4% | 51.2% | |
| Female | 49.7% | 53.0% | 47.6% | 48.8% | |
| Indication | <.0001 | ||||
| 0 (not indicated) | 2.0% | 4.0% | 0.7% | 1.3% | |
| 1 (decreased eGFR/increased creatinine) | 65.8% | 62.4% | 31.4% | 79.5% | |
| 2 (hematuria with eGFR <60 mL/min/1.73 m2) | 0.7% | 0.8% | 0.7% | 0.7% | |
| 3 (hematuria with eGFR >60 mL/min/1.73 m2) | 1.3% | 2.8% | 2.6% | 0.0% | |
| 4 (proteinuria with eGFR <60 mL/min/1.73 m2) | 10.8% | 4.0% | 4.6% | 16.6% | |
| 5 (proteinuria with eGFR >60 mL/min/1.73 m2) | 9.6% | 14.8% | 27.5% | 0.2% | |
| 6 (suspected glomerulonephritis) | 2.9% | 0.0% | 13.7% | 0.9% | |
| 7 (others) | 7.0% | 11.2% | 19.0% | 0.9% | |
| Triage | <.0001 | ||||
| Urgent | 35.2% | 0.0% | 34.7% | 55.0% | |
| Nonurgent | 31.0% | 0.0% | 65.3% | 37.8% | |
| Not booked | 33.8% | 100.0% | 0.0% | 7.3% | |
| Urinalysis available | 63.4% | 65.8% | 72.9% | 59.6% | |
| SCr, µmol/L | 135.0 (98.0-182.0) | 108.0 (84.0-124.0) | 92.0 (68.0-123.0) | 176.0 (149.6-224.0) | <.0001 |
| Calcium, mmol/L | 2.33 (2.27-2.42) | 2.33 (2.29-2.44) | 2.32 (2.22-2.42) | 2.32 (2.26-2.40) | .3611 |
| Phosphate, mmol/L | 1.17 (1.06-1.32) | 1.10 (0.99-1.24) | 1.14 (1.05-1.29) | 1.26 (1.14-1.46) | <.0001 |
| Albumin, g/L | 38.0 (35.0-42.0) | 40 (37.0-44.0) | 38.0 (33.0-42.0) | 36.0 (33.0-40.0) | <.0001 |
| HCO3 (TCO2), mmol/L | 26.0 (23.0-28.0) | 28.0 (26.0-30.0) | 26.0 (24.0-28.0) | 24.0 (21.0-27.0) | <.0001 |
| Urine ACR, mg/mmol | 9.2 (1.6-65.5) | 1.5 (0.4-12.8) | 17.6 (1.0-89.4) | 22.6 (5.8-165.1) | <.0001 |
| eGFR, mL/min/1.73 m2 | 38.6 (27.4-55.2) | 52.4 (43.7-60.0) | 60.0 (47.3-60.0) | 28.8 (21.8-36.4) | <.0001 |
Note. eGFR = estimated glomerular filtration rate; SCr = serum creatinine; ACR = albumin:creatinine ratio.
Effect of Triage on Referrals and Wait Times
In the posttriage period, 35% of referrals were booked as urgent, 31% as nonurgent, and 34% of referrals were not booked (Table 1). Low-risk patients were younger, had a higher eGFR (57 vs 29 mL/min/1.73 m2, P < .001), and lower urinary albumin excretion (3.4 vs 22.6 mg/mmol, P < .01) compared with the high-risk patients.
In the low-risk group, 64% of the low-risk patients were not booked. The remaining 36% met other indications for nephrology consultation, with 13% booked as urgent and 23% as nonurgent. Of high-risk referrals, 55% were booked as urgent. In all, 7% of the high-risk consultations were not booked as they were related to community-acquired acute kidney injury that had already resolved by the time of referral.
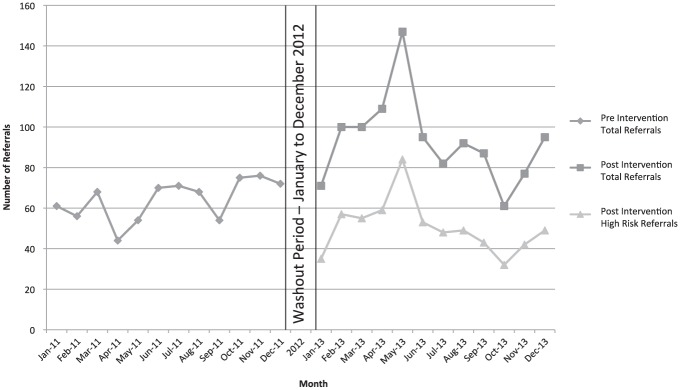
In 2011, the monthly number of referrals ranged from 44 to 76, with a median number of monthly referrals being 68/month. In the posttriage period, the monthly referrals ranged from 61 to 147, with a median number of monthly referrals of 94/month. This was a monthly referral increase of 45%. The median number of high-risk referrals in the posttriage period was 49/month (Figure 2).
Figure 2.
Number of referrals in pretriage and posttriage periods.
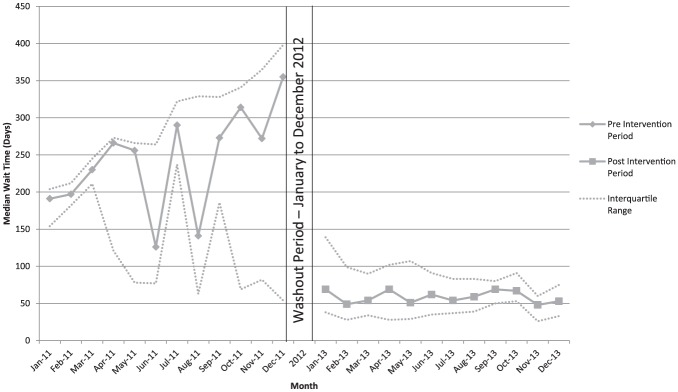
The median wait times for nephrology care in the pretriage period ranged from 126 to 355 days, with a median wait time of 230 days for the entire year. In the posttriage period, the monthly median wait time range decreased to 48 to 69 days, with a median wait time of 58 days (Figure 3).
Figure 3.
Median wait time in pretriage and posttriage periods.
Note. Intervention resulted in statistically significant change in wait time (P < .001) and change in wait time trend (slope) post intervention (P = .029).
Discussion
Our risk-based triage scheme led to an overall improvement in wait times. In turn, this expedited access to care for the patients at highest risk of progression to ESKD. Reduced consult volumes allow interprofessional teams and nephrologist time and resources to be concentrated on patients at highest risk of progression to ESKD. Interventions to slow the progression of CKD are demonstrated as more cost-effective in this cohort of patients.25,26 Our proposed triage scheme, if implemented in other jurisdictions, could provide better value for money at a system-wide level.
Many areas within the health care system have implemented triage systems to access services with limited resources (eg, specialized operative skills), higher cost (eg, certain medications for orphan diseases) or variable, and unpredictable demand (eg, emergency departments). In many nephrology practices, triage schemes have typically relied on ad hoc criteria and are rarely evaluated in a prospective manner.27,28 Our triage system acknowledges that not every patient with CKD is at high risk of progression to ESKD, and may not require specialist- or interdisciplinary team–based care as a result. It also provides a standardized, validated metric on which to focus limited health care resources providing greater value for money. Other medical subspecialties have carried similar principles of risk-based triage to ensure high-risk patients are seen in a timely manner.6,17,29,30
Our novel approach provides a standardized pathway to triaging low-risk patients and objectively allows higher risk referrals to be identified. When referrals are not felt to be indicated, we provide individualized correspondences back to referring providers on suggested management and continued surveillance of patients, as well as when a referral to nephrology should be reinitiated. It is important to note that the KFRE is only a part of our triage process, and other indications and the broader clinical context are equally weighted when triaging referrals. As such, our approach has a built-in safety check to ensure that patients who have other indications to see a nephrologist despite a low 5-year risk of progression are still seen. For instance, a 30-year-old patient with hematuria and suspected glomerulonephritis has a low 5-year risk using the KFRE. However, this patient would be seen by our program on the account of their high lifetime risk, as would a low-risk patient with a significant metabolic or electrolyte abnormality.
Interventions in conjunction with the KFRE could aid further in CKD identification and management. In low-risk patients, multidisciplinary nephrology care is unlikely to change treatment or prognosis, as this population generally has a low prevalence of CKD-related complications.31 There is limited evidence linking patients at low risk of progression managed by nephrology teams to improved health outcomes.32 A previous study in England evaluated patients with CKD in their national primary care registry, and used National Institute for Health and Clinical Excellence (NICE) guidelines to prompt treatment for all patients and nephrology referral for only high-risk patients. They found that the majority of patients with CKD usually are being treated for 1 modifiable CKD risk factor and that most interventions could be delivered efficiently in a primary care setting. Only 6% of higher risk or more complex patients required nephrology intervention.33 Educational programs on the importance of risk-based management, targeted at primary care physicians, can help these physicians better recognize and manage CKD.34 Risk prediction for cardiovascular disease is already well integrated in primary care. Similarly, effective knowledge translation strategies can help to integrate risk-based management of CKD for most patients. The KFRE with its existing point-of-care smartphones and web platforms is effectively positioned for this purpose. Further integration of this tool into electronic medical records or automated lab reporting could further enhance efficient clinical decision making for appropriate nephrology referrals.
Using the KFRE as a triage tool has several clinical implications. First, where nephrology resources are constrained, we have demonstrated that risk-based triage is an effective health policy tool to improve wait times and access to care for the patients at highest risk of progression to end stage. Our previous study reported a median wait time of 150 days for urgent referrals in June 2011, which is almost 3 times the median wait time in the posttriage period. Through estimating ESKD risk, the allocation of resources can be focused on higher risk patients. Our example for referral triage only shows 1 potential use of the KFRE. Other thresholds at different stages of CKD could inform different decisions. For example, vascular access planning at 20% per year and multidisciplinary clinic enrollment at 5% per year could be potential cutoff points. While these are currently in clinical use, their utilization effecting patient outcomes remains to be studied. In settings without significant referral wait times, the KFRE could still be used to determine priority of referral, and intensity of follow-up.
Our study has several strengths, including using a validated risk prediction model that has been validated in multiple populations.15-19 The KFRE uses common, widely available laboratory and demographic variables rendering this a low-cost implementation.14,19 In addition, as our triage approach combined information on risk (KFRE) with additional “red flags” based on clinical judgment, it was able to capture those with other important indications for nephrology referral despite a low 5-year risk. Our study also has limitations. We do not have long-term follow-up on patients triaged as low-risk in this era. However, we have previously confirmed, in an earlier cohort of patients in Manitoba (reference companion manuscript), that patients identified as low risk according to the KFRE do indeed have very low observed rates of kidney failure over 5 years (<1%). Our study may not be applicable to all nephrology teams, especially those operating under capacity without wait lists. We did not collect detailed information on all referrals in the pretriage period, so any differences in our pretriage and posttriage patient groups may be unaccounted for. However, our previous manuscript on wait times following eGFR implementation in 201113 used this same cohort and reported similar group characteristics. Also, we were not able to account for a potential Hawthorne effect on wait times, and other additional changes may have led to our improved wait times. This included additional capacity to see new referrals by 1 additional nephrologist being hired in September 2013, and a physician assistant–led clinic being started in September 2012. The physician assistant saw the same number of patients in both periods, and with the addition of a nephrologist lowered the average number of new referrals per nephrologist from 8.2 to 6.9 (16% decrease). Given that wait times decreased by 75%, we believe that the increased clinician capacity and a potential Hawthorne effect cannot fully explain the improved wait times. These certainly had an impact on our wait times; the risk-based triage process eliminated the need for one-third of all referrals to be seen.
Conclusions
In conclusion, a threshold risk of 3% over 5 years for kidney failure, as determined by the KFRE, can be integrated into a triage process, and reduce wait times for nephrology care and help direct resources to those patients at highest risk of progression to ESKD. Studies examining the effect of risk-based care on other important clinical decisions in CKD are needed.
Footnotes
List of Abbreviations: eGFR, estimated glomerular filtration rate; KFRE, Kidney Failure Risk Equation; CKD, chronic kidney disease; ESKD, end-stage kidney disease; MRP, Manitoba Renal Program; ACR, albumin:creatinine ratio.
Ethics Approval and Consent to Participate: Not applicable.
Consent for Publication: Not applicable.
Availability of Data and Materials: Not applicable.
Author Contributions: JH participated in the conception and design, provided intellectual content, and wrote the manuscript. PW and CR provided intellectual content and helped draft the manuscript. BH is a statistician who provided intellectual content and helped analyze the data. JB participated in the conception and design, interpretation of the data, and in drafting the manuscript. PK helped to conceive and design the study, provided intellectual content of critical importance, and helped to draft the manuscript. NT provided guidance in the study design, interpretation of the data, drafting the manuscript, and with intellectual content with his expertise with the Kidney Failure Risk Equation.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Connolly JO, Woolfson RG. A critique of clinical guidelines for detection of individuals with chronic kidney disease. Nephron Clin Pract. 2009;111:c69-c73. [DOI] [PubMed] [Google Scholar]
- 2. Vassalotti JA, Stevens LA, Levey AS. Testing for chronic kidney disease: a position statement from the National Kidney Foundation. Am J Kidney Dis. 2007;50:169-180. [DOI] [PubMed] [Google Scholar]
- 3. Coresh J, Byrd-Holt D, Astor BC, et al. Chronic kidney disease awareness, prevalence, and trends among U.S. adults, 1999 to 2000. J Am Soc Nephrol. 2005;16:180-188. [DOI] [PubMed] [Google Scholar]
- 4. Black C, Sharma P, Scotland G, et al. Early referral strategies for management of people with markers of renal disease: a systematic review of the evidence of clinical effectiveness, cost-effectiveness and economic analysis. Health Technol Assess. 2010;14:1-184. [DOI] [PubMed] [Google Scholar]
- 5. Levey AS, Atkins R, Coresh J, et al. Chronic kidney disease as a global public health problem: approaches and initiatives—a position statement from Kidney Disease Improving Global Outcomes. Kidney Int. 2007;72:247-259. [DOI] [PubMed] [Google Scholar]
- 6. Magin P, Lasserson D, Parsons M, et al. Referral and triage of patients with transient ischemic attacks to an acute access clinic: risk stratification in an Australian setting. Int J Stroke. 2013;8(Suppl A100):81-89. [DOI] [PubMed] [Google Scholar]
- 7. Akbari A, Grimshaw J, Stacey D, et al. Change in appropriate referrals to nephrologists after the introduction of automatic reporting of the estimated glomerular filtration rate. CMAJ. 2012;184:E269-E276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Naimark DM, Harel Z, Moineddin R, Bergman A. The impact of estimated glomerular filtration rate reporting on nephrology referral pattern, patient characteristics and outcome. Nephron Clin Pract. 2012;121:c10-c15. [DOI] [PubMed] [Google Scholar]
- 9. Hemmelgarn BR, Manns BJ, Lloyd A, et al. Relation between kidney function, proteinuria, and adverse outcomes. JAMA. 2010;303:423-429. [DOI] [PubMed] [Google Scholar]
- 10. Noble E, Johnson DW, Gray N, et al. The impact of automated eGFR reporting and education on nephrology service referrals. Nephrol Dial Transplant. 2008;23:3845-3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hemmelgarn BR, Zhang J, Manns BJ, et al. Nephrology visits and health care resource use before and after reporting estimated glomerular filtration rate. JAMA. 2010;303:1151-1158. [DOI] [PubMed] [Google Scholar]
- 12. Jain A, Hemmelgarn BR. Impact of estimated glomerular filtration rate reporting on nephrology referrals: a review of the literature. Curr Opin Nephrol Hypertens. 2011;20:218-223. [DOI] [PubMed] [Google Scholar]
- 13. Hingwala J, Bhangoo S, Hiebert B, et al. Evaluating the implementation strategy for estimated glomerular filtration rate reporting in Manitoba: the effect on referral numbers, wait times, and appropriateness of consults. Can J Kidney Health Dis. 2014;1:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tangri N, Stevens LA, Griffith J, et al. A predictive model for progression of chronic kidney disease to kidney failure. JAMA. 2011;305:1553-1559. [DOI] [PubMed] [Google Scholar]
- 15. Peeters MJ, van Zuilen AD, van den Brand JA, et al. ; for MASTERPLAN Study Group. Validation of the kidney failure risk equation in European CKD patients. Nephrol Dial Transplant. 2013;28:1773-1779. [DOI] [PubMed] [Google Scholar]
- 16. Acedillo RR, Tangri N, Garg AX. The kidney failure risk equation: on the road to being clinically useful? Nephrol Dial Transplant. 2013;28:1623-1624. [DOI] [PubMed] [Google Scholar]
- 17. Grams ME, Li L, Greene TH, et al. Estimating time to ESRD using kidney failure risk equations: results from the African American Study of Kidney Disease and Hypertension (AASK). Am J Kidney Dis. 2015;65:394-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kaushal A, Naimark D, Tangri N. Use of the kidney failure risk equation to reduce uncertainty in predicting time to ESRD. Am J Kidney Dis. 2015;65:369-371. [DOI] [PubMed] [Google Scholar]
- 19. Tangri N, Levey AS, Grams M, et al. ; for CKD Prognosis Consortium. Validation of the kidney failure risk equation in an international consortium. J Am Soc Nephrol. 2013;24:164-174. [Google Scholar]
- 20. Registry COR. Treatment of End-Stage Organ Failure in Canada,2000 to 2009. Ottawa, Ontario: Canadian Institute for Health Information; 2011. [Google Scholar]
- 21. Authority Winnipeg Regional Health Authority. Community Health Assessment 2009/2010. Winnipeg, Manitoba: WRHA; 2010. [Google Scholar]
- 22. Aboriginal peoples in Canada: First Nations people. Government of Canada; 2011. http://www12.statcan.gc.ca/nhs-enm/2011/as-sa/99-011-x/99-011-x2011001-eng.cfm. Accessed March 1, 2014.
- 23. Visible minority population, by province and territory (2006 census). Government of Canada; 2009. http://www.statcan.gc.ca/tables-tableaux/sum-som/l01/cst01/demo52b-eng.htm. Accessed March 1, 2014.
- 24. Statistics Canada. Population and dwelling counts, for Canada, provinces and territories, and census subdivisions (municipalities), 2011 and 2006 censuses. Manitoba, Canada; 2012. http://www12.statcan.gc.ca/census-recensement/2011/dp-pd/hlt-fst/pd-pl/Table-Tableau.cfm?LANG=Eng&T=302&PR=46&S=51&O=A&RPP=25 [Google Scholar]
- 25. Curtis BM, Barrett BJ, Djurdjev O, Singer J, Levin A. Evaluation and treatment of CKD patients before and at their first nephrologist encounter in Canada. Am J Kidney Dis. 2007;50:733-742. [DOI] [PubMed] [Google Scholar]
- 26. Lee B, Turley M, Meng D, et al. Effects of proactive population-based nephrologist oversight on progression of chronic kidney disease: a retrospective control analysis. BMC Health Serv Res. 2012;12:252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chronic Kidney Disease (CKD) Clinical Pathway. 2014. http://www.ckdpathway.ca. Accessed February 2, 2014.
- 28. Chronic kidney disease—identification, evaluation and management of patients. 2014. http://www2.gov.bc.ca/gov/content/health/practitioner-professional-resources/bc-guidelines/chronic-kidney-disease
- 29. Horsfall L, Macdonald G, Scott I, et al. Use of standardised assessment forms in referrals to hepatology outpatient services: implications for accurate triaging of patients with chronic hepatitis C. Aust Health Rev. 2013;37:218-222. [DOI] [PubMed] [Google Scholar]
- 30. Petkar S, Bell W, Rice N, et al. Initial experience with a rapid access blackouts triage clinic. Clin Med. 2011;11:11-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Clase CM, Kiberd BA, Garg AX. Relationship between glomerular filtration rate and the prevalence of metabolic abnormalities: results from the Third National Health and Nutrition Examination Survey (NHANES III). Nephron Clin Pract. 2007;105:c178-c184. [DOI] [PubMed] [Google Scholar]
- 32. Blantz RC. Handing out grades for care in chronic kidney disease: nephrologists versus non-nephrologists. Clin J Am Soc Nephrol. 2007;2:193-195. [DOI] [PubMed] [Google Scholar]
- 33. McIntyre NJ, Fluck R, McIntyre C, Taal M. Treatment needs and diagnosis awareness in primary care patients with chronic kidney disease. Br J Gen Pract. 2012;62:e227-e232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Akbari A, Swedko PJ, Clark HD, et al. Detection of chronic kidney disease with laboratory reporting of estimated glomerular filtration rate and an educational program. Arch Intern Med. 2004;164:1788-1792. [DOI] [PubMed] [Google Scholar]