Abstract
A common complaint among pain patients is that they lose their appetite. These accounts are anecdotal, however, and the neural mechanism underlying pain-induced loss of appetite remains unknown. In this study, we documented the occurrence of appetite loss in patients under migraine attack and investigated the neuronal substrate of pain-induced anorexia in our animal model of intracranial pain. We found that loss of appetite during the migraine attack in humans coincided strongly with the onset and duration of the head pain in 32/39 cases, and that brief noxious stimulation of the dura in conscious rats produced a transient suppression of food intake. Mapping of neuronal activation in the rat showed that noxious dural stimulation induced a 3- to 4-fold increase in the number of Fos-positive neurons in medullary dorsal horn areas that process nociceptive signals (laminae I, V) and in parabrachial and hypothalamic neurons positioned to suppress feeding behavior. In the parabrachial area, activated neurons were localized in the superior-lateral subnucleus, and 40% of them expressed the mRNA encoding the anorectic neuropeptide cholecystokinin. In the hypothalamus, activated Fos-positive neurons were found in the dorsomedial area of the ventromedial nucleus, and 76% of them expressed the mRNA for cholecystokinin type-B receptor. Based on these findings, we suggest that at least one of several groups of hypothalamic neurons that normally inhibit appetite in response to metabolic cues is positioned to mediate the suppression of food intake by pain signals.
Anecdotal accounts by physicians and patients suggest that the experience of pain may be associated with decreased or loss of appetite. However, no systematic studies have been carried out to characterize the patient's state of appetite under different classes of pain (e.g., inflammatory or neuropathic, deep or superficial, acute, or chronic). Perhaps the best documented association between pain and loss of appetite comes from migraine patients (1–3). Considering that migraine attacks are also associated with nausea and vomiting (1–3), our first goal was to establish whether the loss of appetite is driven by pain per se or, alternatively, by nausea. We therefore asked a group of 39 patients to describe their state of appetite during migraine and characterize whether the occurrence and timing of appetite loss coincides with the head pain, nausea, or both. Thirty-two of the patients (82%) reported a loss of appetite during migraine, 29 of which described a partial or total suppression of food intake throughout the attack (in 5 cases, as long as 2–3 days). An overwhelming 94% of those experiencing appetite loss (30/32) reported that their desire to eat decreases in unison with the development of head pain, often well before they experience nausea, or even in the absence of any nausea. This strong association between head pain and loss of appetite prompted us to turn to our animal model of intracranial pain and examine whether noxious stimulation of the meninges would evoke neuronal activation in brainstem and diencephalic areas known to be involved in the regulation of feeding and appetite.
Noxious stimulation of the meninges in our rat model of intracranial pain was produced chemically and mechanically by exposing the dura to an “inflammatory soup” (IS) solution (4, 5) while repeatedly indenting the dura with a monofilament of measured force to mimic blood vessel pulsation. This paradigm was developed on the basis of our electrophysiological evidence that chemical stimulation of the dura activates and sensitizes meningeal-nociceptors in the trigeminal ganglion and nociceptive neurons in the medullary dorsal horn, and that mechanical dural stimulation further enhances the firing rate of these neurons (6, 7). The noxious nature of these dural stimuli was determined not only by their ability to activate and sensitize peripheral and central nociceptive neurons but also by their ability to induce pressor responses (i.e., acute increases in blood pressure) (8), because pressor responses are induced by visceral and cutaneous stimuli which damage tissue and cause pain (9–11).
The goal of this study was to identify an anatomical and biochemical connection between neurons that transmit nociceptive signals from the dura and neurons that inhibit appetite. Using Fos immunocytochemistry (ICC) combined with in situ hybridization, we report here that noxious stimulation of the rat dura selectively activates neurons involved in the transmission of nociceptive signals and the inhibition of appetite. Consistent with these findings, dural stimulation was found to induce an acute decrease in food intake.
Materials and Methods
Mapping of Neuronal Activation by Dural Stimulation.
To identify specific groups of neurons in brainstem and diencephalic areas activated by noxious stimulation of the meninges and their potential role in feeding behavior, we used Fos ICC in combination with in situ hybridization for the anorectic neuropeptide cholecystokinin (CCK) and its type B receptor (CCK-RB). All studies were approved by the Institutional Animal Care and Use Committees of Harvard Medical School.
Male Sprague–Dawley rats (280–350 g, Taconic Farms) were fitted with a cranial chamber and an i.v. catheter under deep chloral hydrate anesthesia (350 mg/kg i.p.). To fit the cranial chamber, a 3-mm-wide craniotomy was performed above the transverse sinus, and a plastic cylinder with removable cap was affixed to the bone around the opening in the skull (Fig. 1). For chronic i.v. cannulation, silastic tubing (Dow-Corning, no. T57152) was inserted into the femoral vein and attached to polyethylene tubing (Clay Adams) that continued under the skin and emerged at the nape of the neck (12). These procedures allowed direct access to the dura with minimal handling, using brief i.v. anesthesia on the day of the experiment (see below). After surgery, rats were housed individually in a temperature- (21–22°C) and noise-controlled environment under a 12-h light/dark cycle and given food and water ad libitum.
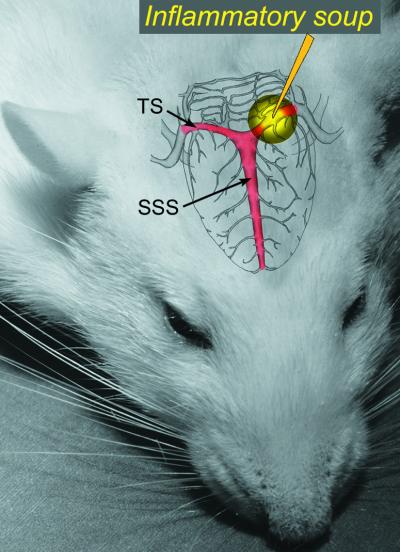
Figure 1.
Schematic illustration of dural stimulation applied to an area overlaying the left transverse sinus (TS) through a plastic chamber affixed to the skull 4–7 days earlier. SSS, superior sagittal sinus.
Six days later, rats received a bolus of propofol (10 mg/kg) infused via the femoral vein cannula to produce anesthesia for a defined interval of 15 min, during which the cranial chamber was opened and the exposed area of the dura was stimulated chemically with 20 μl of IS and mechanically by repeated indentations at 1 Hz frequency, using a von Frey monofilament under a stereomicroscope. Control rats received sham stimulation with 20 μl of synthetic interstitial fluid (SIF). The IS consisted of 1 mM histamine, serotonin, bradykinin, and 0.1 mM prostaglandin E2 in 10 mM Hepes buffer, pH 5.0 (4, 5). The von Frey monofilament was calibrated to 2.35 g (0.25-mm tip diameter, Stoelting). The SIF consisted of 10 mM Hepes, 5 mM KCl, 1 mM MgCl2, 5 mM CaCl2, and 135 mM NaCl, pH 7.3. The brief propofol anesthesia was necessary to allow delivery of the repeated mechanical stimulation of the dura. We found that, propofol anesthesia limited to 15 min, produced minimal alteration in baseline neuronal Fos labeling in limbic brain areas as compared to other anesthetics we tested (12).
At the end of propofol anesthesia, rats woke up and were allowed to move freely in their cages for 2 h before they were killed under deep pentobarbital anesthesia (100 mg/kg, i.p.) with perfusion of RNase-free fixative solution (10% formalin). The brain and upper cervical spinal segments were cut into 40-μm coronal sections and processed for either Fos ICC or Fos ICC combined with in situ hybridization.
Fos ICC was performed by using a rabbit primary antibody (Ab-5, Oncogene Science) specific to the Fos N-terminal domain. Free-floating brain sections were rinsed with 0.1 M PBS and incubated in 0.3% hydrogen peroxide for 30 min at room temperature. To block nonspecific binding, sections were rinsed in PBS and preincubated for 2 h in PBS containing 3% normal goat serum and 0.25% Triton X-100. Sections were then incubated for 48 h at 4°C with rabbit primary Fos antibody diluted 1:150,000 in PBS. After washing in PBS, sections were incubated for 2 h at room temperature with biotinylated goat anti-rabbit IgG (Vector Laboratories) diluted 1:600 in PBS. After additional washing in PBS, sections were reacted with avidin-biotin complex (Vector Elite kit; diluted 1:200 in PBS) for 1 h at room temperature. After rinsing, sections were incubated in 0.1 M PBS containing 0.04% 3,3′-diaminobenzidine tetrahydrochloride (Sigma), 0.02% nickel sulfate, 0.02% cobalt chloride (Fisher Scientific), and 0.01% hydrogen peroxide. The reaction was terminated after 6 min with two successive rinses in PBS. Sections were mounted onto gelatin-coated slides, dehydrated through ascending concentrations of ethanol, cleared in xylene, and coverslipped with Permount (Fisher Scientific).
For ICC combined with in situ hybridization, free-floating sections were first processed and stained for Fos as described above with three modifications: (i) pretreatment with hydrogen peroxide was excluded; (ii) 2% BSA and 5 mg/ml heparin were used in place of goat or donkey serum as a blocking agent, and (iii) the 3,3′-diaminobenzidine tetrahydrochloride reaction product was not enhanced with nickel. The sections were then mounted on glass slides and stored at −20°C until processed for in situ hybridization.
In situ hybridization for specific mRNAs was adapted from previous studies (13–15). In brief, single-stranded RNA probes labeled with 35S-[α]UTP were transcribed in vitro from linearized Bluescript plasmids containing the template cDNA clones, using T7 or SP6 polymerase according to the orientation of the insert. The rat cDNA clones of CCK (16), CCK-RB (17), and the long-form of leptin receptor (18) were generously provided by A. Blomqvist, S. Wank, and C. Bjorbaek, respectively. Sections were pretreated with 0.001% proteinase K (Roche Molecular Biochemicals) for 30 min, followed by 0.025% acetic anhydride for 10 min. Each slide then was incubated for 12–16 h at 56°C with 120 μl of hybridization solution containing 50% formamide, 10 mM Tris⋅HCl (pH 8), 5 mg/ml tRNA, 10 mM DTT, 10% dextran sulfate, 0.3 M NaCl, 1 mM EDTA (pH 8), 1× Denhardt's solution, and riboprobe (1,000 cpm/μl). Slides were washed four times in 4× SSC and treated for 30 min at 37°C with 0.002% RNase A (Roche Molecular Biochemicals), 0.5 M NaCl, 10 mM Tris⋅HCl (pH 8), and 1 mM EDTA. Next, slides were rinsed through 2×, 1×, 0.5×, and 0.1× SSC and incubated for 30 min at 60°C in 0.1× SSC and 0.25% DTT. Dehydrated sections were dipped in Kodak NTB2 photographic emulsion, dried, and stored with desiccant at 4°C in complete darkness for 2–4 wk. The emulsion-coated slides were processed with Kodak D-19 developer, dehydrated in graded ethanols, cleared in xylene, and coverslipped with Permount.
Cell nuclei showing brown 3,3′-diaminobenzidine tetrahydrochloride staining that were surrounded (occasionally also covered) by clusters of black silver grains at a density greater than five times the background level were scored as neurons positive for both Fos and the specific mRNA. Double-labeled neurons were counted “blindly” by using image analysis software (image-pro plus 4.0, Media Cybermetics, Silver Spring, MD). Mean numbers of Fos-positive neurons/section were calculated from the cell counts of six sections from the spinal trigeminal nucleus and cervical spinal cord (corresponding to Vc, C1/Vc, and C2 in Fig. 2a); one section from the superior lateral parabrachial nucleus (corresponding to Fig. 3b); and one section from the ventromedial hypothalamus (corresponding to Fig. 4b); one representative section from other areas as listed in Results. Comparisons between groups were performed by using the Mann–Whitney rank-sum test. Least squares regression analyses were carried out to test for correlation between the numbers of Fos-positive cell nuclei in the different brain areas.
Figure 2.
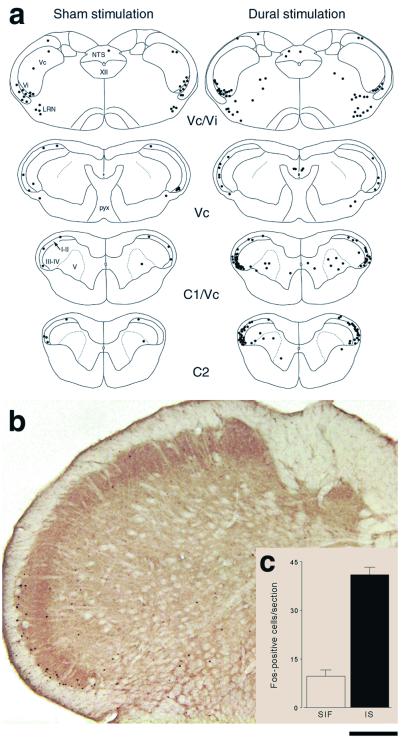
Noxious stimulation of the dura activates Fos expression in the spV. (a) Schematic representations of Fos-positive neurons in nucleus caudalis (Vc) and upper cervical spinal cord segments (C1 and C2), illustrating that dural stimulation increases neuronal activation along the transition zone between Vc and C1 and in C2. Note that the stimulated rat and the sham-stimulated one showed increased baseline Fos expression in the transition zone between subnuclei caudalis and interpolaris (Vc/Vi), indicating nonspecific activation. (b) Photomicrograph showing selective activation of laminae I/II and V neurons in the ventrolateral region of the medullary dorsal horn after dural stimulation. (c) Quantitative analysis showing a 4-fold increase in spV Fos expression in rats receiving dural stimulation (n = 10) compared to sham stimulation (n = 6). LRN, lateral reticular nucleus; NTS, nucleus of the solitary tract; pyx, pyramidal decussation; XII, hypoglossal nucleus; I–V, laminae of dorsal horn. (Bar = 2,000 μm in a and 630 μm in b.)
Figure 3.
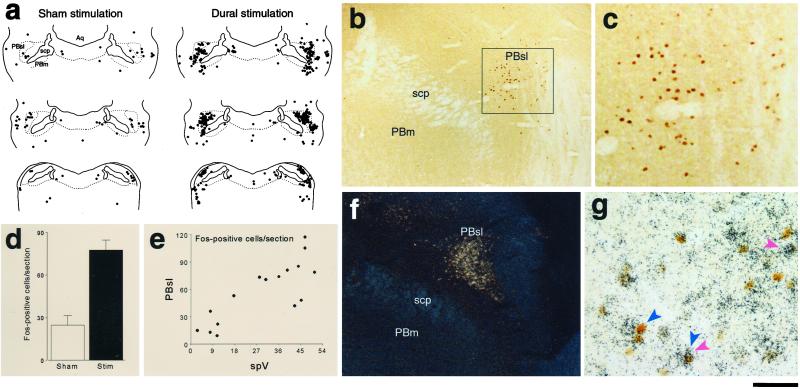
Noxious stimulation of the dura activates Fos expression in CCK-positive neurons of the PBsl nucleus. (a) Schematic representation of Fos-positive neurons in the parabrachial complex illustrating that dural stimulation increases neuronal activation within the PBsl nucleus. (b) Photomicrograph showing selective activation of PBsl neurons after dural stimulation. (c) Magnification of the Inset in b. (d) Quantitative analysis showing a 3-fold increase in PBsl Fos expression in rats receiving dural stimulation (n = 10) compared to sham stimulation (n = 6). (e) Scatter plots illustrating correlation between the number of Fos-positive neurons in spV and PBsl. (f) Darkfield photomicrograph of CCK mRNA in situ hybridization autoradiography defining the anatomical borders of the PBsl nucleus. (g) Brightfield photomicrograph of Fos immunoreactivity (blue arrows) combined with CCK mRNA in situ hybridization autoradiographic grains (red arrows) showing single- and double-labeled neurons. Aq, cerebral aqueduct; PBm, medial parabrachial complex; scp, superior cerebellar peduncle. (Bar = 2,100 μm in a, 330 μm in b, 100 μm in c, 420 μm in f, and 50 μm in g.)
Figure 4.
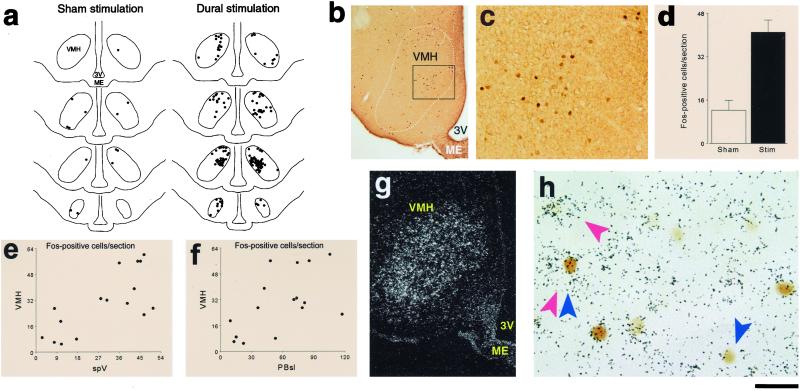
Noxious stimulation of the dura activates Fos expression in ventromedial hypothalamic neurons expressing CCK-RB. (a) Schematic representation of Fos-positive neurons in the VMH, illustrating that dural stimulation increases neuronal activation through the entire length of the nucleus. (b) Photomicrograph showing selective activation of dorsomedial VMH neurons after dural stimulation. (c) Magnification of the Inset in b. (d) Quantitative analysis showing a 3-fold increase in VMH Fos expression in rats receiving dural stimulation (n = 10) compared to sham stimulation (n = 6). (e and f) Scatter plots illustrating correlation between the number of Fos-positive neurons in spV and VMH (e) and between the number of Fos-positive neurons in PBsl and VMH (F). (g) Darkfield photomicrograph of CCK receptor type B mRNA in situ hybridization autoradiography delineating the borders of the ventromedial hypothalamic nucleus. (h) Brightfield photomicrograph of Fos immunoreactivity (blue arrows) combined with CCK-RB mRNA in situ hybridization autoradiographic grains (red arrows) showing single- and double-labeled neurons. ME, median eminence; 3V, third ventricle. (Bar = 1,500 μm in a, 485 μm in b, 120 μm in c, 375 μm in g, and 20 μm in h.)
Food Intake After Dural Stimulation.
In this study, we examined whether noxious stimulation of the dura can acutely suppress food intake in awake, freely moving rats. To allow for meaningful measurement of uninterrupted food intake, the paradigm of dural stimulation was modified as follows: (i) stimulation was delivered without the propofol infusion described above, thus eliminating potential anesthesia-induced drop in food consumption; (ii) in the absence of anesthesia, the 15-min mechanical stimulation of the dura was not feasible and therefore excluded.
In preparation for noxious stimulation of the dura, male Sprague–Dawley rats were surgically fitted with a plastic chamber affixed to the skull above the transverse sinus as described above. Rats were individually housed in cages designed for measuring food intake (Harvard Apparatus, model 52–6756). Pulverized standard rat chow (Purina Mills, no. 50110) and water were provided ad libitum, and food intake was measured by weighing the food dish. Daily food intake was measured from day 4 to 7 after surgery to confirm that it had returned to normal, as compared to nonoperated controls. Rats were handled daily for removal of the chamber cap to minimize potential stress effects on food intake at the time of dural stimulation.
On the day of dural stimulation, the chamber was opened and 20 μl of IS (noxious stimulation) or SIF (sham stimulation) was applied onto the exposed area of the dura. The chamber was then closed, the rats returned to their cages, and food intake was measured 4, 12, and 24 h later. To maximize the probability of detecting an acute drop in food intake, noxious or sham stimulation was applied at the onset of the dark cycle—the time when rats normally wake up and begin eating. Comparisons between groups on the day of dural stimulation (hours 0–4 and 4–12) were performed by using Mann–Whitney rank-sum test. Total daily (24 h) food intake on the day before, day of, and day after stimulation was analyzed by using two-factor ANOVA with repeated measurements.
Results
Identification of Neurons Activated by Dural Stimulation.
We identified two brainstem structures and one hypothalamic nucleus in which dural stimulation (chemical plus mechanical) consistently induced a 3- to 4-fold increase in the number of Fos-positive neurons compared to sham stimulation. Brainstem neuronal activation was found in the caudal part of the spinal trigeminal nucleus (spV) and the lateral parabrachial complex (PB), specifically its superior lateral subnucleus (PBsl). Within the spV (Fig. 2), laminae I/II and V of nucleus caudalis and C1–2 exhibited 41.0 ± 2.3 (mean ± SE) Fos-positive neurons per section in rats receiving dural stimulation vs. 9.6 ± 2.0 in controls (P < 0.005). In the PBsl (Fig. 3 a–d), the number of Fos-positive neurons was significantly higher in rats receiving dural stimulation compared to controls (77.6 ± 7.2 vs. 24.7 ± 6.8 cells/section; P < 0.005). Hypothalamic neuronal activation was found in the ventromedial nucleus (VMH), specifically its dorsomedial subdivision (Fig. 4 a–d), which contained 41.1 ± 4.5 Fos-positive neurons/section after dural stimulation compared to 12.3 ± 3.6 in controls (P < 0.005).
Inconsistent neuronal activation was found in two hypothalamic areas, the paraventricular (PVH) and dorsomedial (DMH) nuclei, where the number of Fos-labeled neurons/section was elevated in 50% of the rats that received dural stimulation (PVH, 70.4 ± 23.2; DMH, 39.0 ± 9.5) and unchanged in the remaining rats (PVH, 3.6 ± 0.9; DMH, 13.2 ± 3.5) as compared to sham-stimulated controls (PVH, 2.0 ± 1.0; DMH, 14.0 ± 4.6). Examination of other brainstem and hypothalamic areas that are commonly associated with feeding (19, 20) revealed no significant group differences (P > 0.05) in the number of Fos-labeled neurons: area postrema (1.7 ± 0.6 vs. 0.7 ± 0.5); medial PB area (1.1 ± 0.4 vs. 0.3 ± 0.2); arcuate nucleus (1.3 ± 1.2 vs. 0); supraoptic nucleus (7.8 ± 6.4 vs. 0); and lateral hypothalamic area (15.8 ± 11.8 vs. 12.5 ± 12.4). Both groups exhibited no Fos-positive neurons in the hypoglossal nucleus.
Correlation analyses indicated that the numbers of Fos-positive neurons were significantly correlated (P < 0.001) between the PBsl and spV (Fig. 3e), VMH and spV (Fig. 4e), and VMH and PBsl (Fig. 4f), suggesting that the activation of VMH neurons by dural stimulation may be mediated by nociceptive signals relayed via the PBsl and spV. Fos labeling in the PVH and DMH was not correlated with Fos labeling in any of the areas above.
Histochemical Identity of Neurons Activated by Dural Stimulation.
To examine whether VMH and PBsl neurons activated by dural stimulation are functionally equipped to suppress food intake, we determined the chemical identity of the activated neurons by using Fos ICC combined with in situ hybridization for the mRNAs encoding the anorectic neuropeptide CCK (Fig. 3 f and g) or its type B receptor (Fig. 4 g and h). In the PBsl, the number of neurons double-labeled for Fos antigen and CCK mRNA was 3-fold higher in rats receiving dural stimulation (n = 16) compared to controls (n = 7) (58.4 ± 7.0 vs. 16.7 ± 4.6 cells/section; P < 0.001). The percentage of Fos-positive neurons that expressed CCK mRNA remained similar between rats receiving dural stimulation vs. controls (41.1 ± 8.0% vs. 46.1 ± 3.4%). In the VMH, the number and percentage of Fos-positive neurons that expressed CCK-RB mRNA were both significantly higher (P < 0.02) in rats receiving dural stimulation (n = 8) compared to controls (n = 3): 55.8 ± 6.4 vs. 22.3 ± 5.2 cells/section and 60.5 ± 5.0% vs. 35.3 ± 5.5%, respectively. Based on these numbers, we estimate that 37% of the PBsl neurons and 76% of the VMH neurons that were recruited by the dural stimulation expressed CCK and CCK-RB mRNA, respectively.
We also used Fos ICC combined with in situ hybridization for the receptor of leptin, a hormone that suppresses food intake (21, 22) and activates VMH neurons expressing leptin receptors and PBsl neurons containing CCK (23, 24). Leptin receptor expression could be detected in Fos-positive VMH neurons but the cellular mRNA level was too low to permit rigorous quantitative analysis.
Dural Stimulation Induces Acute Suppression of Food Intake.
Based on the activation of PBsl and VMH neurons that express the anorectic neuropeptide CCK and its receptor, respectively, we tested whether noxious dural stimulation would also produce a measurable suppression of food intake. We found that a single application of IS to the dura (without any mechanical stimulation) produced a transient decrease in food intake between 0 and 4 h.
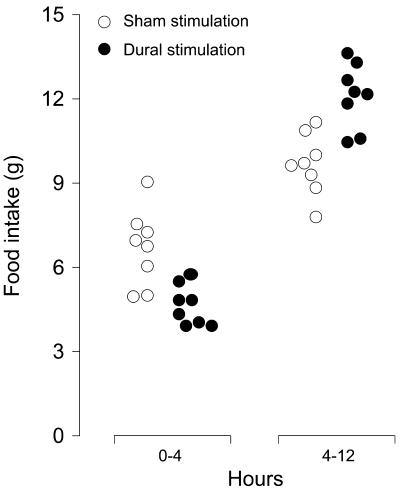
Food intake in the first 4 h after dural stimulation with IS was significantly lower compared to sham-stimulation with SIF (4.6 ± 0.2 vs. 6.7 ± 0.5 g; P < 0.004; Fig. 5). During hours 4–12, however, food intake was significantly higher in rats that received IS compared to SIF (12.1 ± 0.4 vs. 9.7 ± 0.4 g, P < 0.004; Fig. 5), suggesting that the stimulated rats were compensating for the initial inhibition of feeding. The amount of food consumed by sham-stimulated rats was similar to that consumed by another group of three control rats that were fitted with cranial chambers but received no handling or stimulation (7.1 ± 0.4 g during 0–4 h and 11.0 ± 0.8 g during 4–12 h), confirming that the manipulation involved with the sham stimulation per se had no significant stressful effect on food intake.
Figure 5.
Transient suppression of food intake over the first 4 h after noxious chemical stimulation of the rat dura.
Total daily (24 h) food intake remained constant between the IS and SIF groups (P > 0.1) and also from day to day (P > 0.07): 20.7 ± 0.9 vs. 18.3 ± 0.9 g on the day before stimulation; 20.5 ± 0.6 vs. 19.7 ± 0.6 g on the day of stimulation; and 21.6 ± 0.7 vs. 20.2 ± 0.9 g on the day after stimulation. These values were very similar (P > 0.3) to the normal daily food intake measured repeatedly over three consecutive days in a group of eight intact untreated rats (20.8 ± 0.9, 19.4 ± 1.2, and 21.1 ± 0.5 g, respectively).
Discussion
Using our animal model of migraine pain, we have demonstrated that food intake can be suppressed acutely by noxious stimulation of the dura. Implicit in the neural mechanism underlying this behavior was the correlated activation of (1) nociceptive trigeminovascular spV neurons (2), PBsl neurons that express the anorectic neuropeptide CCK, and (3) a specific group of VMH neurons that express the type B receptor for CCK. Based on these findings, it is tempting to hypothesize that loss of appetite and suppression of food intake occur during migraine attacks when intracranial pain activates nociceptive trigeminovascular neurons in spV, which then trigger the activation of dorsomedial VMH neurons via CCK fibers of PBsl origin (25, 26): an hypothesis that may potentially be tested by using CCK-BR knockout mice.
We have previously shown that our chemical stimulation of the rat dura is a nociceptive stimulus as it activates and sensitizes meningeal C and Aδ nociceptors (6) and nociceptive trigeminovascular neurons in the ventrolateral areas of laminae I and V of spV (7). It also produces acute increases in mean arterial pressure, or pressor responses (8), which, in humans, are usually associated with pain perception. Finally, chemical stimulation renders the dura hypersensitive to mild mechanical stimuli because it potentiates large neuronal and pressor responses to otherwise subthreshold mechanical stimuli (7, 8). It was therefore necessary in the present study to verify that dural stimulation with chemical and mechanical stimuli indeed produced robust Fos expression in the same ventrolateral areas of spV laminae I and V. In the discussion below, we shall consider the possibility of our nociceptive stimuli being stressful as well.
We interpret the acute decrease in food intake between 0 and 4 h after noxious stimulation of the dura in the rat as a response to intracranial pain and the potential stress associated with it. This acute decrease in food intake is in line with the duration of sensitization of second-order meningeal-sensitive neurons induced by a similar paradigm (7) and with the accounts of our migraine patients associating their loss of appetite with their head pain. The subsequent increase in food intake between 4 and 12 h after dural stimulation indicates that the initial suppression, however brief, was sufficient to release a compensatory mechanism in feeding behavior. Because the amounts of food eaten by sham-stimulated rats during hours 0–4 and 4–12 were equivalent to those eaten by control rats with cranial chambers that received no treatment, we conclude that our measurements were free of side effects of potential pain/stress related to surgery, or cranial chamber attachment, or the manipulation involved in the sham stimulation. Because total daily food intake in the days before and after surgery remained unchanged in all rats, we conclude that the suppressing effects of dural stimulation on feeding dissipated completely by the end of the night.
The interrelationship between the activation of nociceptive trigeminal neurons and the acute decrease in food intake may be considered in terms of inhibition of hypothalamic “phagic neurons” and/or activation of hypothalamic “satiety neurons.” Because Fos ICC is unsuitable for detecting neuronal inhibition, suppression of phagic neurons as a mechanism for decreased feeding after dural stimulation remains to be explored by using more suitable techniques (e.g., single-unit recording). On the other hand, we found that noxious dural stimulation consistently coactivated neurons in ventrolateral spV, PBsl, and dorsomedial VMH, and that the activated neurons expressed the anorectic neuropeptide CCK in the PBsl and the CCK-RB in the VMH. Considering the anatomical evidence that CCK afferents to the VMH originate exclusively from the PBsl (24, 25) and behavioral evidence that administration of exogenous CCK into the VMH suppresses food intake (27, 28), we suggest that the suppression of feeding by intracranial pain is mediated at least by one group of satiety neurons located in the dorsomedial subdivision of the VMH. Although the role of the VMH in feeding has been controversial, recent anatomical and functional evidence has clearly implicated the dorsomedial VMH in feeding regulation (20).
Another potential target for neuronal activation by dural stimulation was found in the PVH and DMH, two hypothalamic nuclei known to be involved in feeding (20) and stress (29, 30). However, the effect of dural stimulation on activation of PVH and DMH neurons was unpredictable: In one-half of the rats, it induced robust Fos expression, whereas the other one-half showed little or no Fos labeling, as did sham controls. As this split pattern was not correlated with the uniform coactivation of neurons in the spV or PBsl, we cannot assess the significance of these findings in the context of pain-induced suppression of food intake. Although the data clearly indicate that neither surgery nor animal handling produced any neuronal activation in the PVH or DMH, the possibility remains that the induction of Fos in half of the animals that received dural stimulation was a reflection of a stress response to painful dural stimuli.
Understanding the differences and similarities between our findings in the rat and the human experience of migraine can allow us to identify brain areas that may be involved in the pathophysiology of loss of appetite induced by head pain. The suppression of food intake in the rat after a brief stimulation of a limited area of dura was only short-lived compared to the long-lasting loss of appetite associated with prolonged migraine pain in humans. By prolonging and intensifying noxious stimulation of the rat dura, we might be able to produce a more profound suppression of food intake that mimics more closely migraine-induced loss of appetite in humans. The potential involvement of CCK in suppression of food intake in our rat model of intracranial pain calls attention to human studies that have demonstrated a significant drop in appetite and food consumption after systemic administration of CCK analogs to healthy human subjects (31–33). The CCK-induced loss of appetite in humans was reported to be independent of accompanying nausea (31), a finding remarkably consistent with the accounts of the migraine patients in the present study.
Acknowledgments
We thank C. Lee and M. Mousavi for assistance with in situ hybridization and Dr. B. Ransil for help with statistical analysis. This study was supported by National Institutes of Health Grants DE-13347, DE-10904 (National Institutes of Dental and Craniofacial Research), and NS-35611-01 (National Institutes of Neurological Disorder and Stroke) to R.B., by the Education Fund of the Department of Anesthesia and Critical Care at Beth Israel Deaconess Medical Center, the Boston Foundation, the Goldfarb family, and the Fink family.
Abbreviations
- CCK
cholecystokinin
- CCK-RB
CCK-type-B receptor
- ICC
immunocytochemistry
- IS
inflammatory soup
- PBsl
superior lateral parabrachial nucleus
- spV
spinal trigeminal nucleus
- VMH
ventromedial hypothalamic nucleus
- PVH
paraventricular hypothalamic nucleus
- DMH
dorsomedial hypothalamic nucleus
- SIF
synthetic interstitial fluid
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Olesen J. Headache. 1978;18:268–271. doi: 10.1111/j.1526-4610.1978.hed1805268.x. [DOI] [PubMed] [Google Scholar]
- 2.Blau J N. Cephalalgia. 1993;13:293–295. doi: 10.1046/j.1468-2982.1993.1304293.x. [DOI] [PubMed] [Google Scholar]
- 3.Rasmussen B K, Jensen R, Olesen J. Cephalalgia. 1991;11:129–134. doi: 10.1046/j.1468-2982.1991.1103129.x. [DOI] [PubMed] [Google Scholar]
- 4.Steen K H, Reeh P W, Anton F, Handwerker H O. J Neurosci. 1992;12:86–95. doi: 10.1523/JNEUROSCI.12-01-00086.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steen K H, Steen A E, Reeh P W. J Neurosci. 1995;15:3982–3989. doi: 10.1523/JNEUROSCI.15-05-03982.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strassman A M, Raymond S A, Burstein R. Nature (London) 1996;384:560–564. doi: 10.1038/384560a0. [DOI] [PubMed] [Google Scholar]
- 7.Burstein R, Yamamura H, Malick A, Strassman A M. J Neurophysiol. 1998;79:964–982. doi: 10.1152/jn.1998.79.2.964. [DOI] [PubMed] [Google Scholar]
- 8.Yamamura H, Malick A, Chamberlin N L, Burstein R. J Neurophysiol. 1999;81:479–493. doi: 10.1152/jn.1999.81.2.479. [DOI] [PubMed] [Google Scholar]
- 9.Woodworth R S, Sherrington C S. J Physiol (London) 1904;31:234–243. doi: 10.1113/jphysiol.1904.sp001034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cervero F. Pain. 1982;13:137–151. doi: 10.1016/0304-3959(82)90024-0. [DOI] [PubMed] [Google Scholar]
- 11.Ness T J, Gebhart G F. Brain Res. 1988;450:153–169. doi: 10.1016/0006-8993(88)91555-7. [DOI] [PubMed] [Google Scholar]
- 12.Elmquist J K, Saper C B. J Comp Neurol. 1996;374:315–331. doi: 10.1002/(SICI)1096-9861(19961021)374:3<315::AID-CNE1>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 13.Elias C F, Lee C, Kelly J, Aschkenasi C, Ahima R S, Couceyro P R, Kuhar M J, Saper C B, Elmquist J K. Neuron. 1998;21:1375–1385. doi: 10.1016/s0896-6273(00)80656-x. [DOI] [PubMed] [Google Scholar]
- 14.Chan R K, Sawchenko P E. Neuroscience. 1995;66:377–390. doi: 10.1016/0306-4522(94)00600-a. [DOI] [PubMed] [Google Scholar]
- 15.Kelly A B, Watts A G. J Comp Neurol. 1996;370:231–246. doi: 10.1002/(SICI)1096-9861(19960624)370:2<231::AID-CNE7>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 16.Deschenes R J, Lorenz L J, Haun R S, Roos B A, Collier K J, Dixon J E. Proc Natl Acad Sci USA. 1984;81:726–730. doi: 10.1073/pnas.81.3.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wank S A, Pisegna J R, de Weerth A. Proc Natl Acad Sci USA. 1992;89:8691–8695. doi: 10.1073/pnas.89.18.8691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elmquist J K, Bjorbaek C, Ahima R S, Flier J S, Saper C B. J Comp Neurol. 1998;395:535–547. [PubMed] [Google Scholar]
- 19.Hoebel B G. Appetite. 1997;29:119–133. doi: 10.1006/appe.1997.0126. [DOI] [PubMed] [Google Scholar]
- 20.Elmquist J K, Elias C F, Saper C B. Neuron. 1999;22:221–232. doi: 10.1016/s0896-6273(00)81084-3. [DOI] [PubMed] [Google Scholar]
- 21.Halaas J L, Gajiwala K S, Maffei M, Cohen S L, Chait B T, Rabinowitz D, Lallone R L, Burley S K, Friedman J M. Science. 1995;269:543–546. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- 22.Schwartz M W, Seeley R J, Campfield L A, Burn P, Baskin D G. J Clin Invest. 1996;98:1101–1106. doi: 10.1172/JCI118891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elmquist J K, Ahima R S, Maratos-Flier E, Flier J S, Saper C B. Endocrinology. 1997;138:839–842. doi: 10.1210/endo.138.2.5033. [DOI] [PubMed] [Google Scholar]
- 24.Elias C F, Kelly J F, Lee C E, Ahima R S, Drucker D J, Saper C B, Elmquist J K. J Comp Neurol. 2000;423:261–281. [PubMed] [Google Scholar]
- 25.Zaborszky L, Beinfeld M C, Palkovits M, Heimer L. Brain Res. 1984;303:225–231. doi: 10.1016/0006-8993(84)91208-3. [DOI] [PubMed] [Google Scholar]
- 26.Fulwiler C E, Saper C B. Neurosci Lett. 1985;53:289–296. doi: 10.1016/0304-3940(85)90553-1. [DOI] [PubMed] [Google Scholar]
- 27.Schick R R, Harty G J, Yaksh T L, Go V L. Neuropharmacology. 1990;29:109–18. doi: 10.1016/0028-3908(90)90050-2. [DOI] [PubMed] [Google Scholar]
- 28.Blevins J E, Stanley B G, Reidelberger R D. Brain Res. 2000;860:1–10. doi: 10.1016/s0006-8993(99)02477-4. [DOI] [PubMed] [Google Scholar]
- 29.Sawchenko P E, Li H Y, Ericsson A. Prog Brain Res. 2000;122:61–78. doi: 10.1016/s0079-6123(08)62131-7. [DOI] [PubMed] [Google Scholar]
- 30.Cullinan W E, Herman J P, Battaglia D F, Akil H, Watson S J. Neuroscience. 1995;64:477–505. doi: 10.1016/0306-4522(94)00355-9. [DOI] [PubMed] [Google Scholar]
- 31.Greenough A, Cole G, Lewis J, Lockton A, Blundell J. Physiol Behav. 1998;65:303–310. doi: 10.1016/s0031-9384(98)00169-3. [DOI] [PubMed] [Google Scholar]
- 32.Rayner C K, Park H S, Doran S M, Chapman I M, Horowitz M. Am J Physiol. 2000;278:G98–G104. doi: 10.1152/ajpgi.2000.278.1.G98. [DOI] [PubMed] [Google Scholar]
- 33.Lam W F, Gielkens H A, de Boer S Y, Lamers C B, Masclee A A. Physiol Behav. 1998;65:505–511. doi: 10.1016/s0031-9384(98)00189-9. [DOI] [PubMed] [Google Scholar]