Abstract
Background
Neuropathic pain represents the major public health burden with a strong impact on quality life in multiple sclerosis patients. Although some advances have been obtained in the last years, the conventional therapies remain poorly effective. Thus, the discovery of innovative approaches to improve the outcomes for multiple sclerosis patients is a goal of primary importance. With this aim, we investigated the efficacy of the 4-(α−L-rhamnopyranosyloxy)benzyl isothiocyanate (moringin), purified from Moringa oleifera seeds and ready-to-use as topical treatment in experimental autoimmune encephalomyelitis, murine model of multiple sclerosis. Female C57BL/6 mice immunized with myelin oligodendrocyte glycoprotein (MOG35–55) were topically treated with 2% moringin cream twice daily from the onset of the symptoms until the sacrifice occurred about 21 days after experimental autoimmune encephalomyelitis induction.
Results
Our observations showed the efficacy of 2% moringin cream treatment in reducing clinical and histological disease score, as well as in alleviating neuropathic pain with consequent recovering of the hind limbs and response to mechanical stimuli. In particular, Western blot analysis and immunohistochemical evaluations revealed that 2% moringin cream was able to counteract the inflammatory cascade by reducing the production of pro-inflammatory cytokines (interleukin-17 and interferon-γ) and in parallel by increasing the expression of anti-inflammatory cytokine (interleukin-10). Interestingly, 2% moringin cream treatment was found to modulate the expression of voltage-gated ion channels (results focused on P2X7, Nav 1.7, Nav 1.8 KV4.2, and α2δ-1) as well as metabotropic glutamate receptors (mGluR5 and xCT) involved in neuropathic pain initiation and maintenance.
Conclusions
Finally, our evidences suggest 2% moringin cream as a new pharmacological trend in the management of multiple sclerosis-induced neuropathic pain.
Keywords: Neuropathic pain, moringa isothiocyanate, multiple sclerosis, neuroinflammation, voltage-gated ion channels
Introduction
Multiple sclerosis (MS) is a demyelinating autoimmune disease of the central nervous system (CNS) causing motor, sensory, and cognitive impairment in more than 2 million of young adults worldwide.1 Although MS etiology is not yet been entirely known, several lines of evidence demonstrate that its development is due to an aberrant autoimmune response against myelin sheath surrounding the neurons.2,3 This ultimately causes a wide range of clinical symptoms and neurological deficits observed in MS patients. Among these, chronic neuropathic pain is a common and disabling symptom with a prevalence of 50–85% and with a strong impact on patients’ quality of life.4,5 Neuropathic pain occurs usually as a result of demyelination, neuroinflammation, and axonal damage in the CNS. It is often accompanied by hypersensitivity to non-noxious (allodynia) and noxious (hyperalgesia) stimulation.6,7 To date, neuropathic pain represents the most serious and difficult type of pain to treat, reflecting both an inadequate understanding of the disease and an absence of selective and definitive cure. The conventional therapies including non-steroidal anti-inflammatory drugs, opioid analgesics, anti-epileptics, and anti-depression drugs8,9 are often times ineffective or limited due to undesirable side effects.10,11 Thus, innovative therapeutic strategies to relieve and counteract completely neuropathic pain need to be developed. To date, pharmacological studies are still looking at natural compounds as a source of powerful and effective therapeutic agents. In this context, the curative effects of some plants containing phenolic compounds, cannabinoids, alkaloids, and vanilloids have already been explored. The encouraging results obtained have led to the development of a topical formulation, like Qutenza (patches containing capsaicin), as new treatment for neuropathic pain.
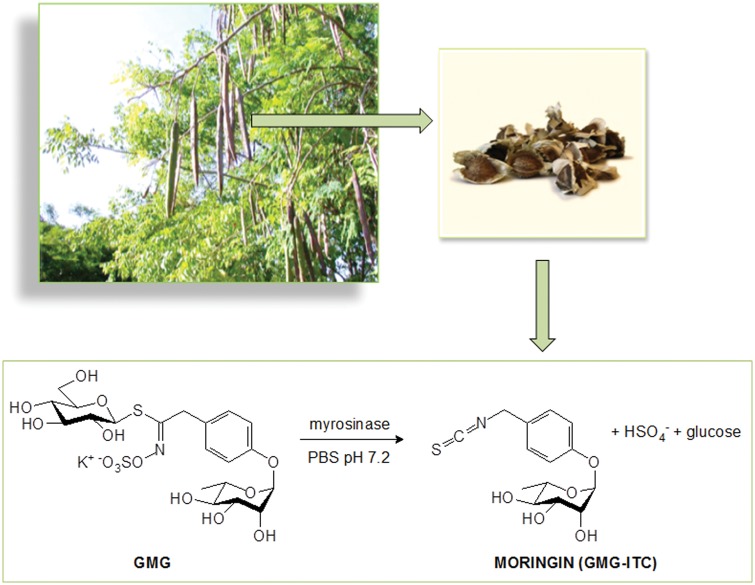
In the last decades, the isothiocyanate (ITC) compounds obtained from the enzymatic hydrolysis of the corresponding glucosinolates, found in Brassicaceae as well as in Moringaceae plants, have been demonstrated to exert therapeutic effects in the management of chronic pain due to several conditions.12,13 A recent study of 2017 showed the anti-nociceptive and anti-inflammatory effects of sulforaphane (SFN), a derivative of glucoraphanin present in cruciferous vegetables, in chronic constriction injury-induced neuropathic pain mice.14 In line of this, other studies have already confirmed the efficacy of SFN in alleviating the nitroglycerin-induced hyperalgesia in rats15 as well as mechanical allodynia and thermal hyperalgesia in spinal nerve transection-injured mice.12 In parallel with the well-recognized properties of SFN, the attention of researchers has focused in the last decades to other ITCs. Among these, the glycosylated compound 4-(α−L-rhamnopyranosyloxy)benzyl ITC (moringin) resulting from myrosinase hydrolysis of 4-(α−L-rhamnopyranosyloxy)benzyl glucosinolate (glucomoringin) has been recently characterized and proven as modulator of peripheral neuropathic pain in in vivo experimental models of diabetes and chemotherapy-induced neuropathy.16 Moringin is a solid, odorless, and stable compound that can be purified in gram amounts mainly from seeds of the most widely distributed Moringa oleifera Lam17,18 (Figure 1). Commonly known as drumstick tree, M. oleifera is a small-sized tree belonging to the Moringaceae family, widely cultivated in Asia, Africa, and other tropical parts of the world for its high nutritional and medicinal value.19 A large number of research studies involving M. oleifera published in recent years indicate that various preparations of M. oleifera possess a wide range of physiological and pharmacological activities, including antitumor, antibacterial and antifungal, antipyretic, antispasmodic, hypotensive, hypolipidemic, anti-oxidant, anti-inflammatory, and antinociceptive activities.18,20–22
Figure 1.
Moringin was produced by myrosinase-catalyzed hydrolysis of the glucosinolate glucomoringin (GMG). This glucosinolate was purified from Moringa oleifera (Moringaceae) seeds.
As like most ITCs, moringin is very poorly soluble in water and unstable in buffered solutions, due to the high electrophilic character of the N=C=S function. Therefore, alternative delivery routes are necessary to achieve successful therapeutic effects.
The present study was designed both to provide new evidences in the field of neuropathic pain and also to investigate the efficacy of moringin, directly purified and ready-to-use as topical treatment in experimental autoimmune encephalomyelitis (EAE), the murine model of MS. As already demonstrated,23 this new formulation can provide an easy and effective method of treatment without side effects. More in detail, here, we examined whether treatment with a topical 2% moringin cream, applied at the EAE symptomatic onset, could counteract neuropathic pain, promoting this treatment as a new trend in management of MS-related neuropathic pain.
Material and methods
Plant material
Moringin production and purification
Moringin was produced by myrosinase-catalyzed hydrolysis of the glucosinolate glucomoringin. This glucosinolate was purified at the Bologna laboratory (CREA-AA) from M. oleifera (Moringaceae) seeds in two sequential steps, isolation through anion exchange followed by gel filtration, as previously reported.24,25 Moringin was produced via myrosinase-catalyzed hydrolysis of glucomoringin in phosphate buffer (pH 6.5) at 37℃ and purified by reverse-phase chromatography as reported.24 The identity and purity of moringin was confirmed by nuclear magnetic resonance spectroscopy.25
Preparation of 2% moringin cream
In order to obtain a preparation of 2% of moringin cream, moringin has been solubilized in non-ionic oil in water (O/W) cream base canaletto, commonly used topical preparations. This contains aqua q.s. to 100%, cetearyl ethylhexanoate and isopropyl myristate 10%, cetearyl/stearyl alcohol 4%, decyl oleate 2%, glycerin 2%, cetearyl glucoside 1%, sodium cetearyl sulfate 0.5%, phenoxyethanol 0.5%; sodium benzoate 0.4%; xanthan gum 0.3%, potassium sorbate 0.3%, tocopheryl acetate 0.1%, lecithin 0.025%, tocopherol 0.006%, ascorbyl palmitate 0.004%, citric acid q.s. to pH required (6.0–6.3). The achieved cream has been subjected to microbiological investigations to verify any contamination by the most common pathogens. It was found negative for Candida albicans, Pseudomonas aeruginosa, Staphylococcus Aureus, and Escherichia coli.
Animals
Female C57BL/6 mice weighing 20–25 g were used for this experiment. Animals were acquired from Harlan Milan, Italy and housed in independently ventilated cages and maintained under 12 h light/dark cycles at 21 ± 1℃ and 50–55% humidity with food and water ad libitum.
Ethics statement
All animal care and use was accomplished according to the European Organization Guidelines for Animal Welfare. The study has been authorized by the Ministry of Health “General Direction of animal health and veterinary drug” (Authorization A79E4.8 – 05/08/2016- D.lgs 26/2014). The experiments were planned in such a way to minimize the total number of mice necessary for the study.
Induction of experimental autoimmune encephalomyelitis
Mice were anesthetized with a combination of tiletamine and xylazine (10 ml/kg, intraperitoneal (i.p.)). Afterwards, EAE was induced in mice using myelin oligodendrocyte glycoprotein (MOG)35–55 peptide (MEVGWYRSPFSRVVHLYRNGK; % peak area by HPLC ≥ 95, AnaSpec, EGT Corporate Headquarters, Fremont, CA, USA) as reported by Paschalidis et al.26 Briefly, mice were immunized subcutaneously with 300 µl/flank of the emulsion consisting of 300 µg of (MOG)35–55 in phosphate-buffered saline (PBS) mixed with an equal volume of complete Freund’s adjuvant containing 300 µg heat-killed Mycobacterium tubercolosis H37Ra (Difco Laboratories, Sparks, MD,USA). Immediately after (MOG)35–55 injection, mice received an intraperitoneal (i.p.) injection of 100 µl of Bordetella pertussis toxin (Sigma-Aldrich, Milan, Italy) (500 ng/100 µl, i.p.), repeated 48 h later. The disease follows a course of progressive degeneration, with visible signs of pathology consisting of flaccidity of the tail and loss of motion of the hind legs.
Experimental design
Mice were arbitrarily distributed into the following groups (N = 30 total animals): Naive group (N = 5): mice did not receive injection of (MOG)35–55 or other drugs, used only as control; EAE group (N = 15): mice subjected to EAE with no other treatment; EAE + 2% moringin cream group (N = 10): EAE mice were subjected to topical treatment of lower limbs with the 2% moringin cream 2 times daily. The treatment was started after the onset of disease signs and then protracted until the sacrifice.
Of note, before the beginning of topical treatment, all mice were exposed to shaving of both hind limbs in outer thigh (area of 1 cm2) to allow the complete absorption of cream. Indeed, at each treatment, cream was spread until totally absorbed, and to avoid that animal could eat or lick cream, all mice were placed in their cages only after full absorption.
Animals of the EAE vehicle cream (devoid of moringin) group were not provided here, because in our previous observations we did not found any effects due to vehicle cream alone. Likewise, naive + 2% moringin cream and naive + vehicle cream (devoid of moringin) were observed only to verify if any beneficial effect was ascribed to the method of administration of the cream, such as spreading, or whether treatment with 2% moringin cream or vehicle cream could cause some allergic reaction in mice either at cutaneous or at systemic level. For this reason and also to minimize the number of animals used for experiment, we have decided not to include these groups in our experimental design.
Finally, on 21st day after EAE induction, mice were euthanized with Tanax (5 ml/kg body weight; i.p.). Spinal cord tissues were collected and processed for subsequent evaluations. The entire in vivo experimental design was repeated twice and that all results achieved with different techniques have been calculated from three independent experiments.
Clinical disease score and body weight assessment
Approximately 14 days after EAE immunization, MS pathological symptoms such as hind limb paralysis, tail tonus reduction, and body weight loss. The severity of encephalitogenic responses was estimated using a standardized 0–6 scoring system27 as follows: 0 = no signs; 1 = partial flaccid tail; 2 = complete flaccid tail; 3 = hind limb hypotonia; 4 = partial hind limb paralysis; 5 = complete hind limb paralysis; and 6 = moribund or dead animal. Animals with a score ≥ 5 were sacrificed to avoid animal suffering. In addition, the following clinical disease parameters were evaluated: incidence, day of onset of clinical signs, peak disease score, cumulative disease score (CDS) and mortality (Table 1). The first assessment of clinical disease score as well as body weight was measured on the day of EAE induction (day zero), and all the successive measurements were recorded every 24 h until the sacrifice. The daily variation of the clinical score of the disease and body weight has been expressed in comparison with a day of EAE induction (day zero). The value day has been expressed as mean ± standard error of mean (SEM) of all animals for each experimental group.
Table 1.
Clinical parameters of EAE.
| Group | Incidence (%) | Day of disease onset (avg) ± SD | Peak disease (avg) ± SD | Cumulative disease score ± SD | Mortality |
|---|---|---|---|---|---|
| Naive | 0 | 0 | 0 | 0 | 0/5 |
| EAE | 100 | 12.3 ± 0.22 | 4.5 ± 0.52 | 4.0 ± 0.56 | 0/15 |
| EAE + 2% moringin cream | 100 | 13.1 ± 0.50 | 3.10 ± 0.82* | 2.21 ± 0.31* | 0/10 |
Incidence was calculated as a percent of mice that displayed any clinical signs of disease. Day of onset: the first day mice showed clinical signs. Peak of disease: the maximum value score observed between days 0 and 21. Cumulative disease score: the mean of the sum of daily score observed between days 0 and 21. One-way multiple comparisons with Tukey’s test was used to determine the statistical significance of differences. All data have been calculated from two experiments.
SD: standard deviation; EAE: experimental autoimmune encephalomyelitis.
p < 0.005 compared to EAE group.
Needle test
The test was designed to assess mice’s responsiveness to a mechanical stimulus according to Chaplan et al.28 with some modifications. It starts with the filament of 0.02 g, applying force to the left paw 3 times for a total period of 30 s (about 2 s to the stimulus) and to evaluate the response of the mouse after each application. The same treatment is repeated on the right paw. Response to two on three stimuli is regarded as a positive reaction. Specifically, a positive response is a paw withdrawal from the stimulus. The maximum score for both paws has a value of 6. The tests were performed every 24 h in 1 week from the first administration of 2% moringin cream. The values are expressed as mean ± SEM of each group.
Light microscopy
Spinal cord tissues (fixed in10% (w/v) PBS-buffered formaldehyde) were embedded in paraffin and sectioned into 7 µm thin slices. After processing into xylene deparaffinization and subsequent rehydration steps, sections were stained with eosin and hematoxylin (E/H). Sections were visualized under optical microscope (Leica microscope ICC50HD).
Immunohistochemistry
Parrafin-embedded tissue slices were deparaffinized with xylene, rehydrated with alcohol series, and incubated in 0.01 M citrate buffer (pH 6) for 4 min to retrieve antigen. Then, the slices were incubated with 0.3% (v/v) hydrogen peroxide in 60% (v/v) methanol for 30 min in order to quench endogenous peroxidase and blocked with normal goat serum in PBS (2% v/v) for 20 min. Afterwards, slices were incubated with selective primary antibodies for overnight at 4℃. The primary antibodies applied for immunohistochemical analysis are as follows: anti-myelin basic protein (MBP) antibody (1:250 in PBS v/v; Millipore, Vimodrone, Milan, Italy) and anti-interleukin (IL)-17 polyclonal antibody (1:100 in PBS v/v; Santa Cruz Biotechnology, Inc., Dallas, Texas, USA). Then, the slices were washed with PBS and incubated with avidin/biotin blocking reagent (DBA, Italy) to block endogenous avidin and biotin binding sites. Afterwards, slices were incubated with universal biotinylated secondary antibody followed by avidin-HRP-conjugated solution (Vectastain ABC kit, Vector Laboratories, Burlingame, California, USA) according to manufacturer’s instructions. Slices were then incubated with hydrogen peroxide/DAB kit 3,3′-diaminobenzidine-tetrahydrochloride-dihydrate (DAB) kit (Vector Laboratories, USA) according to manufacturer’s instructions. Counterstaining was performed with nuclear fast red (Vector Laboratories, Burlingame, California, USA) (pink background). To verify non-specific background immunostaining, slices were incubated with either primary or secondary antibody alone. No staining was noticed in these controls, proving that the immunoreactions were positive in all the experiments performed. Immunohistochemical pictures (N = 5 photos from each samples collected from all mice in each experimental group) were acquired using light microscopy (LEICA DM 2000 combined with LEICA ICC50 HD camera) and assessed by densitometric analysis by Leica Application Suite V4.2.0 software. Values shown are mean ± SEM expressed for all mice of each group.
Western blot analysis
Spinal cord tissues were homogenized using ice-cold lysis buffer with following ingredients: 10 mM Tris-HCl pH 7.4, 0.32 M Sucrose, 2 mM EDTA,1 mM EGTA, 50 mM NaF, 5 mM NaN3, 10 mM 2-mercaptoethanol, and protease inhibitor tablets (Roche Applied Science, Monza, Italy). Homogenates were clarified by centrifugation at 1000 g for 10 min at 4℃ and the resulting supernatant was served as cytoplasmic fraction. The pellets were further lysed using ice-cold extraction buffer consists of 10 mM Tris-HCl pH 7.4, 150 mM NaCl,1 mM EDTA, 1 mM EGTA, 1% Triton X-100, and protease inhibitors. Homogenates were clarified by centrifugation at 15,000 g for 30 min at 4℃. The resulting supernatant was served as nuclear fraction. Protein concentration was assayed using Bio-Rad Protein Assay (Bio-Rad, Segrate, Italy). Proteins were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis, followed by blotting with polyvinylidene fluoride membranes (Immobilon-P transfer membrane, Millipore, Vimodrone, Milan, Italy). Then, membranes were incubated in blocking solution (5% skimmed milk in 1X PBS) for 45 min at room temperature. Subsequently, membranes were incubated with selective primary antibodies overnight at 4℃. The primary antibodies used were: interferon (IFN)-γ (1:250; Santa Cruz Biotechnology, Inc., Dallas, Texas, USA), IL-10 (1:250; Santa Cruz Biotechnology, Inc., Dallas, Texas, USA), P2X7 (1:1000; Thermo Scientific, Waltham, MA USA), Nav 1.7 (1:1000 in PBS v/v; Abcam, Cambridge, UK), Nav 1.8 (1:1000 in PBS v/v; Abcam, Cambridge, UK); KV4.2 (1:1000; Santa Cruz Biotechnology, Inc., Dallas, Texas, USA), CACNA2D1 (1:1000; Aviva System Biology, San Diego, CA, USA), cystine/glutamic acid transporter (xCT) (1:500; Trans genic Inc., Chuo ku Kobe, Japan), mGluR5 (1:500; Santa Cruz Biotechnology, Inc., Dallas, Texas, USA) in 1 × PBS, 5% (w/v) non-fat dried milk, 0.1% Tween-20). Then, the membranes were incubated with HRP-conjugated goat anti-mouse IgG, goat anti-rabbit IgG, or anti-rat as secondary antibody (1:2000; Santa Cruz Biotechnology, Inc., Dallas, Texas, USA) for 1 h at room temperature. In order to assess equal loading of proteins, membranes were stripped and reprobed with HRP-conjugated glyceraldehyde 3-phosphate dehydrogenase antibody (1:1000; Cell Signaling Technology, Leiden, The Netherlands). Images of protein bands were visualized using an enhanced chemiluminescence system (Luminata Western HRP Substrates, Millipore, Vimodrone, Milan, Italy) and then acquired and quantified with ChemiDoc™ MP System (Bio-Rad, Segrate, Milan, Italy) and a computer program (ImageJ software), respectively. The figure showing Western blot analysis is representative of three separate experiments. In detail, a representative blot of samples obtained from two naive mice, three EAE mice, and three EAE + 2% moringin cream mice are shown and densitometry analysis of all animals is reported. Data are expressed as mean ± SEM.
Tumor necrosis factor-α assay
Enzyme-linked immunosorbent assay (ELISA) kit testing for (tumor necrosis factor) TNF-α (eBioscience ELISA Ready-SET-Go!, Waltham, MA, USA) was used to measure cytokine levels in spinal cord samples. The kits were used according to the manufacturer’s protocol. Experiment was repeated for 3 times.
Statistical evaluation
GraphPad Prism version 6.0 program (GraphPad Software, La Jolla, CA, USA) was used for statistical analysis of the data. The results were statistically analyzed using one-way analysis of variance and post hoc Bonferroni test for multiple comparisons. A p value less than or equal to 0.05 was considered significant. All values are expressed as mean ± SEM of N experiments.
Results
Moringin cream improves histopathology and clinical disease features
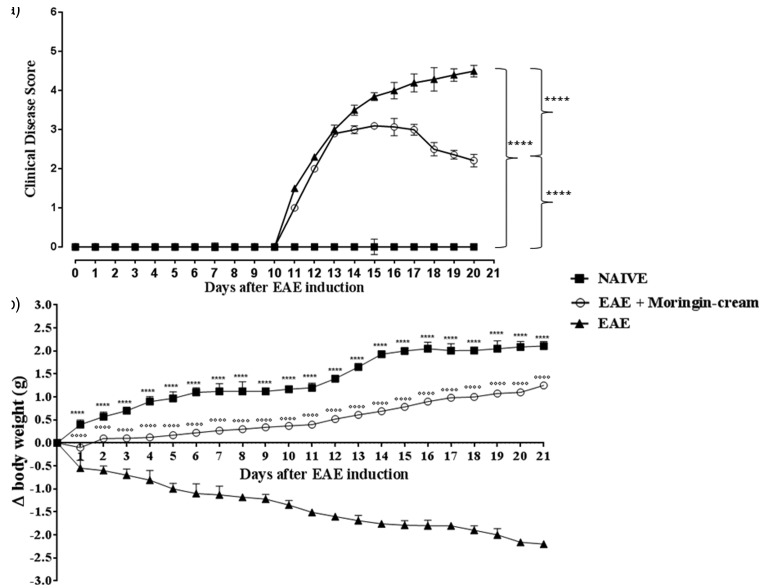
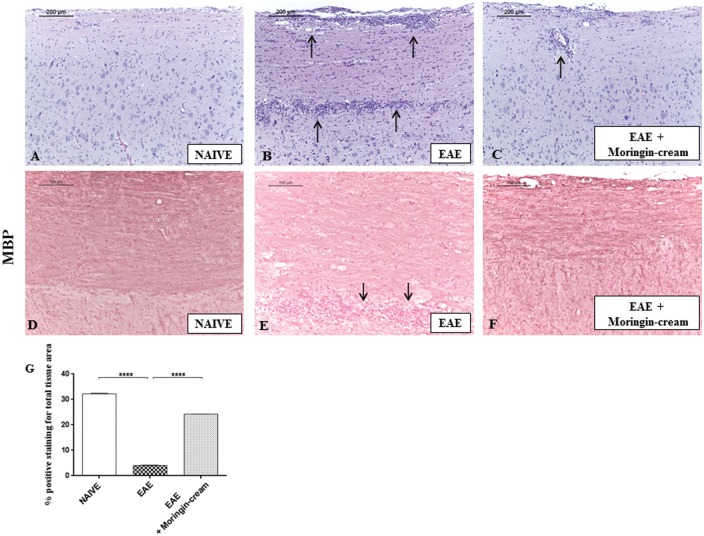
EAE is a well-recognized experimental model of MS that mimics similar clinical (i.e. paralysis and body weight loss) and neuropathological (i.e. demyelination, infiltration of inflammatory cells into the CNS) signs of human pathology. Clinical disease score assessment revealed the active encephalitogenic challenges occurred in EAE mice. We found that EAE induction caused severe phenotypic impairments. EAE-related clinical disease parameters such as severe problem in gait, righting reflex deficiency, and loss of tail and limb tonicity were observed in EAE mice. In specific, mice belonging to the EAE group exhibited a grading of disease with a mean clinical score of 4.0. On the contrary, EAE mice treated with 2% moringin cream showed significant improvement with a clinical score as mean of 2.21 (Figure 2(a)). In addition, compared to EAE group, 2% moringin cream-treated mice exhibited a significant reduction in disease incidence and average CDS. The lower CDS in EAE + 2% moringin cream group was also reflected as significant amelioration of disease severity (Table 1). Of note, mice subjected to topical treatment with 2% moringin cream showed an amelioration of clinical disease score that coincides with an increase in the body weight (+1.25 g). Moreover, significant body weight loss was observed in EAE mice (−2.20). As expected, mice belonging to naive group exhibited a regular increase in body weight (+2.10) as well as absence of motor anomalies (Figure 1(b)). Interestingly, EAE model allows evaluating the typical inflammatory frame occurring mainly in the spinal cord, which in turn resulted in chronic demyelination. E/H staining revealed no histological damage in naive mice (Figure 3(a)). Conversely, a marked infiltration of lymphocytes and polymorphonuclear cells in white matter of spinal cord samples from EAE (Figure 3(b)) was found. Surprisingly, treatment with 2% moringin cream led to a resolution of inflammatory cells infiltration, with some rare infiltrated cell (Figure 3(c), see arrow). In addition, in the EAE model, demyelination has occurred from myelin degradation, including the cleavage of MBP to smaller peptides for presentation to T cells, thus sensitizing them to myelin impairment.29 Therefore, 2% moringin cream was also evaluated for the protective action on myelin sheath integrity by looking to the expression of MBP, the main component of the myelin sheath. By immunohistochemical evaluations, we found a positive staining for MBP in naive mice (Figure 3(d)), whereas EAE mice showed negative staining with evident reduction in myelin and axonal structures in spinal cord sections (Figure 3(e), as shown by arrows). The positive staining for MBP in EAE + 2% moringin cream mice revealed a marked remyelination following topical treatment (Figure 3(f); see densitometric analysis in Figure 3(g)).
Figure 2.
(a) Clinical disease score. Naive vs. EAE ****p < 0.0001; EAE vs. EAE + moringin cream ****p < 0.0001; naive vs. EAE + moringin cream ****p < 0.0001. (b) Body weigh evaluations. Naive vs. EAE ****p < 0.0001; EAE vs. EAE + moringin cream °°°°p < 0.0001. The measure of clinical disease score and body weight variations are expressed as mean ± SEM of all measurements of each experimental group. Naive group (N = 5), EAE group (N = 15), and EAE + moringin cream mice (N = 10). Results were analyzed by one-way ANOVA followed by a Bonferroni test for multiple comparisons. EAE: experimental autoimmune encephalomyelitis.
Figure 3.
Eosin and hematoxylin (E/H) staining. Naive mice (a) did not show histological alterations in the spinal cord tissues, whereas EAE mice (b) displayed a wide area of infiltrating cells (see arrows). Topical treatment with 2% moringin cream led to a complete resolution of inflammatory cells infiltration (c). All images were acquired with 10× objective (size bar 200 µm). A positive staining for MBP was observed in naive (d), whereas a negative tissue localization was found in EAE mice (e) with evident reduction in myelin and axonal structures in spinal cord sections (as shown by arrows). A positive staining for MBP was noticed in EAE + moringin cream with a marked remyelination (f) Densitometric analysis for MBP (g). Naive vs. EAE ****p < 0.0001; EAE vs. EAE + moringin cream ****p < 0.0001. Immunohistochemical pictures (N = 5 photos from each samples collected from all mice in each experimental group) were acquired using light microscopy (LEICA DM 2000 combined with LEICA ICC50 HD camera) and assessed by densitometric analysis by Leica Application Suite V4.2.0 software. All images were acquired with 20× objective (size bar 100 µm).Values shown are mean ± SEM expressed of all mice for each group. All results were analyzed by one-way ANOVA followed by a Bonferroni test for multiple comparisons. EAE: experimental autoimmune encephalomyelitis.
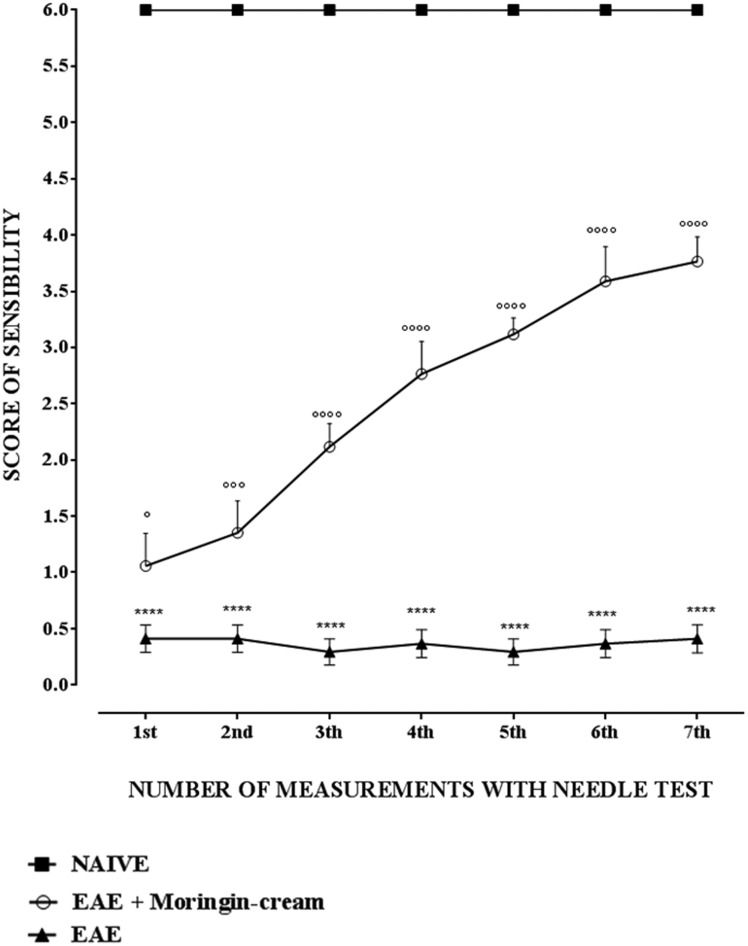
Moringin cream reduces mechanical allodynia in EAE mice
EAE model is widely used to investigate the mechanisms underlying to the development and maintenance of MS-associated central neuropathic pain. Interestingly, mice subjected to EAE exhibit pro-nociceptive behaviors mainly in the lower extremities, tail and hind limbs, as observed in MS patients suffering from neuropathic pain.30 Thus, by using EAE mice it may be conceivable to identify new potential targets for discovery of novel analgesics for improved relief of this chronic pain condition. Here, to assess whether topical treatment with 2% moringin cream can recover limbs sensitivity to mechanical stimuli, we evaluated by needle test mechanical allodynia that may result following nerve or tissue damage. To this objective, a stimulus was applied on the paw plantar surface, estimating the paw retraction response threshold. EAE mice topically administered with 2% moringin cream showed a significantly improved response to mechanical stimulus. In addition, EAE mice exhibited no response to mechanical stimulus applied to the paw plantar. As expected, naive mice responded constantly by withdrawing the paw (Figure 4). It is important to emphasize that the beneficial effects of this topical treatment are not due to the method of application (shaving and/or spreading of the cream). Mice subjected to topical treatment with cream and without pharmacological ingredient did not exhibit any improvement both in recovering paralysis of hind limbs and in relieving neuropathic pain.
Figure 4.
Needle test to evaluate mechanical allodynia. The measure of sensibility score is expressed as mean ± SEM of all measurements of each experimental group. Naive group (N = 5), EAE group (N = 15), EAE + moringin cream mice (N = 10). Naive vs. EAE ****p < 0.0001; EAE vs. EAE + moringin cream °°°°p < 0.0001. Results were analyzed by one-way ANOVA followed by a Bonferroni test for multiple comparisons. EAE: experimental autoimmune encephalomyelitis.
Effects of moringin cream on inflammatory pathway
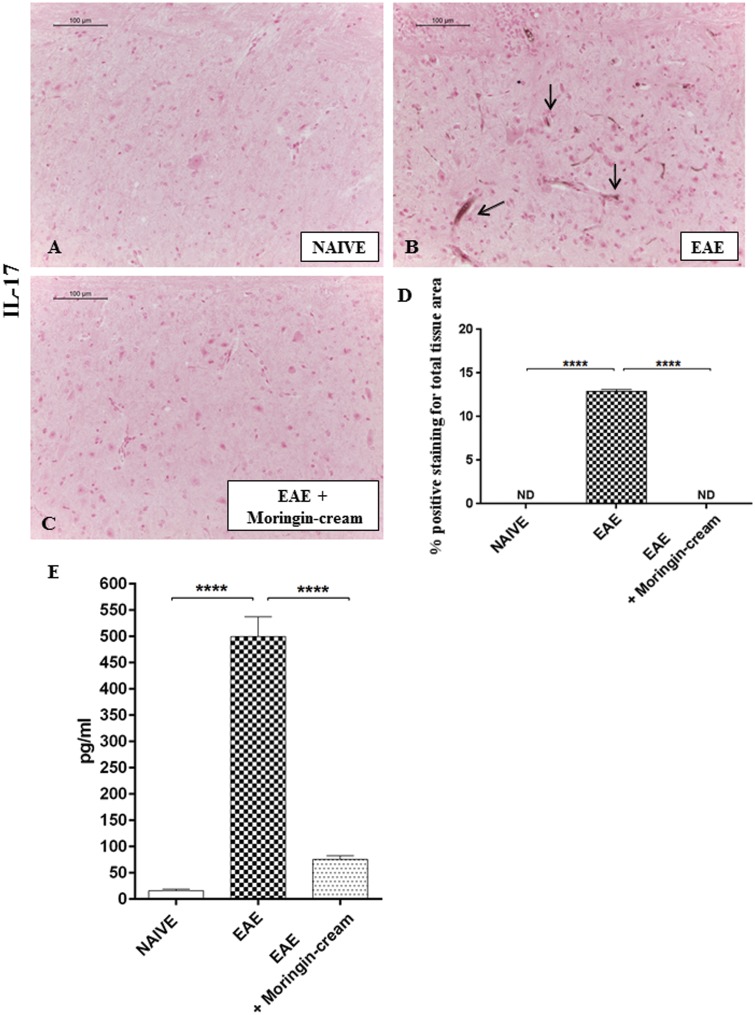
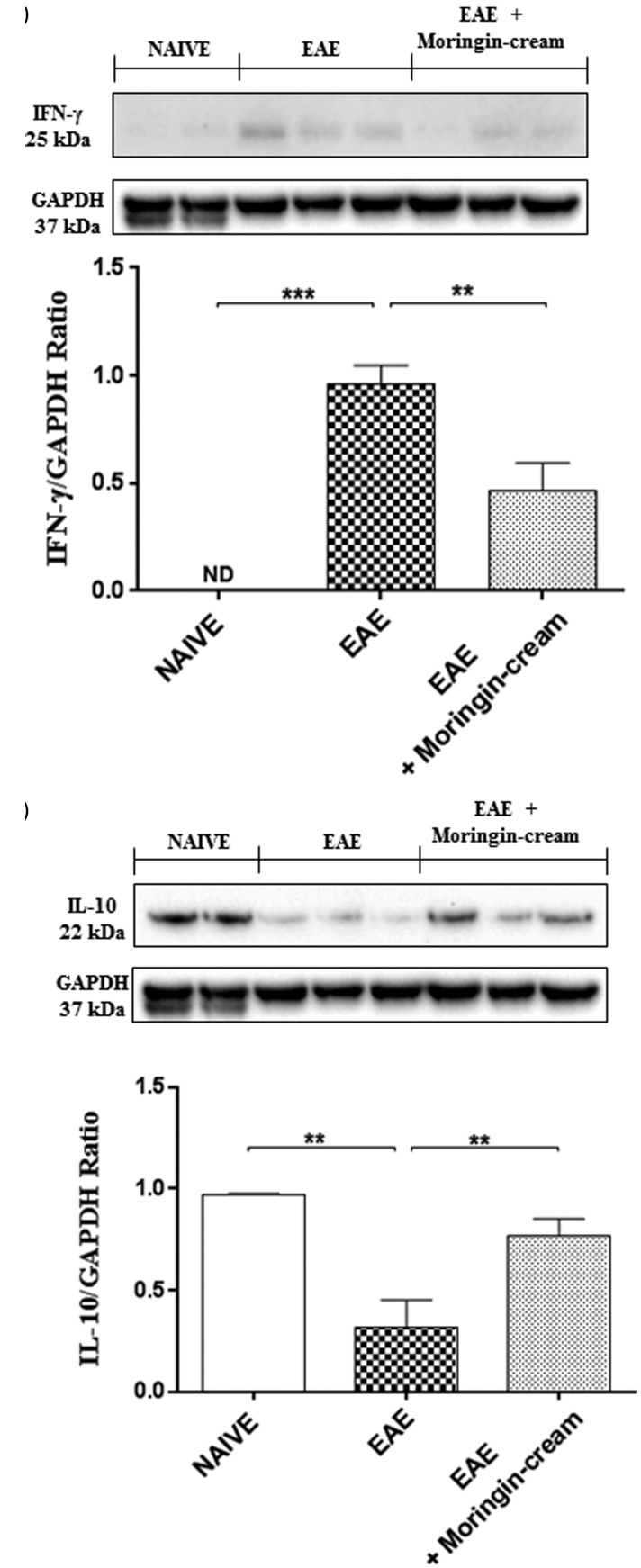
Numerous knowledge suggest that T cells specific for self-antigens mediate progression of this EAE disease. More in detail, two diverse subsets of autoreactive T cells have been principally implicated in the pathogenesis of both EAE and MS: the IFN-γ-producing CD4+ T helper (Th) 1 and IL-17-producing Th17 cells.31,32 Hence, we first evaluated whether these cytokines are modulated after topical treatment with 2% moringin cream in EAE mice. Immunohistochemical evaluations displayed a negative staining for IL-17 in spinal cord sections of naive mice (Figure 5(a)). Conversely, a positive staining for this pro-inflammatory mediator was observed in EAE group (Figure 5(b), arrows indicated positive inflammatory cells in vascular endothelium). No positive staining for IL-17 was found in EAE mice treated with 2% moringin cream (Figure 5(c), see densitometric analysis 5 D). ELISA results showed upregulation of pro-inflammatory cytokines TNF-α in EAE mice compared to naive ones. On the contrary, treatment with 2% moringin cream significantly reduced the activation of TNF-α in EAE mice (Figure 5(e)). In addition, Western blot analysis showed that IFN-γ expression was significantly increased in the spinal cord tissues from EAE mice, compared to naive group. On the contrary, IFN-γ expression was reduced following topical treatment with 2% moringin cream (Figure 6(a)). Besides, by Western blot analysis, we looked at the role of IL-10 as anti-inflammatory cytokine. As expected, naive mice did not show IL-10 expression, while a basal level expression of IL-10 was found in spinal cord samples obtained from EAE group. Topical treatment of mice with 2% moringin cream significantly enhanced its expression (Figure 6(b)).
Figure 5.
Immunohistochemical localization for IL-17. A negative staining for IL-17 was noticed in naive mice (a). An intense positive staining in the vascular endothelium of EAE mice was observed (b; see arrows), compared to EAE mice topically treated with 2% moringin cream (c) Densitometric analysis for IL-17 (d) Naive vs. EAE ****p < 0.0001; EAE vs. EAE + moringin cream ****p < 0.0001. Immunohistochemical pictures (N = 5 photos from each samples collected from all mice in each experimental group) were acquired using light microscopy (LEICA DM 2000 combined with LEICA ICC50 HD camera) and assessed by densitometric analysis by Leica Application Suite V4.2.0 software. All images were acquired with 20× objective (size bar 100 µm). Values shown are mean ± SEM expressed of all mice for each group. ELISA assay for TNF-α (E). Naive vs. EAE ****p < 0.0001; EAE vs. EAE + moringin cream ****p < 0.0001. The data are representative of three independent experiments. All results were analyzed by one-way ANOVA followed by a Bonferroni test for multiple comparisons. EAE: experimental autoimmune encephalomyelitis.
Figure 6.
Western blot analysis for IFN-γ (a). Naive vs. EAE ***p = 0.0004; EAE vs. EAE + moringin cream **p = 0.0054. Western blot analysis for IL-10 (b). Naive vs. EAE **p = 0.0024; EAE vs. EAE + moringin cream **p = 0.0080. GAPDH was used as the internal control. The figure is representative of three separate experiments. In detail, a representative blot of samples obtained from two naive, two EAE, and three EAE + moringin cream mice is shown and densitometry analysis of all animals is reported. Data are expressed as mean ± SEM. All results were analyzed by one-way ANOVA followed by a Bonferroni test for multiple comparisons. EAE: experimental autoimmune encephalomyelitis.
Moringin cream modulates voltage-gated ion channels involved in neuropathic pain
Generally, neuropathic pain is initiated by opening of sensory voltage-dependent ion channels within nociceptive terminals in response to damaging stimuli of sufficient strength, like inflammation. Voltage-gated ion channels involved in the perception of pain are placed on neurons critical for the transmission and modulation of noxious or potentially tissue damaging stimuli.33
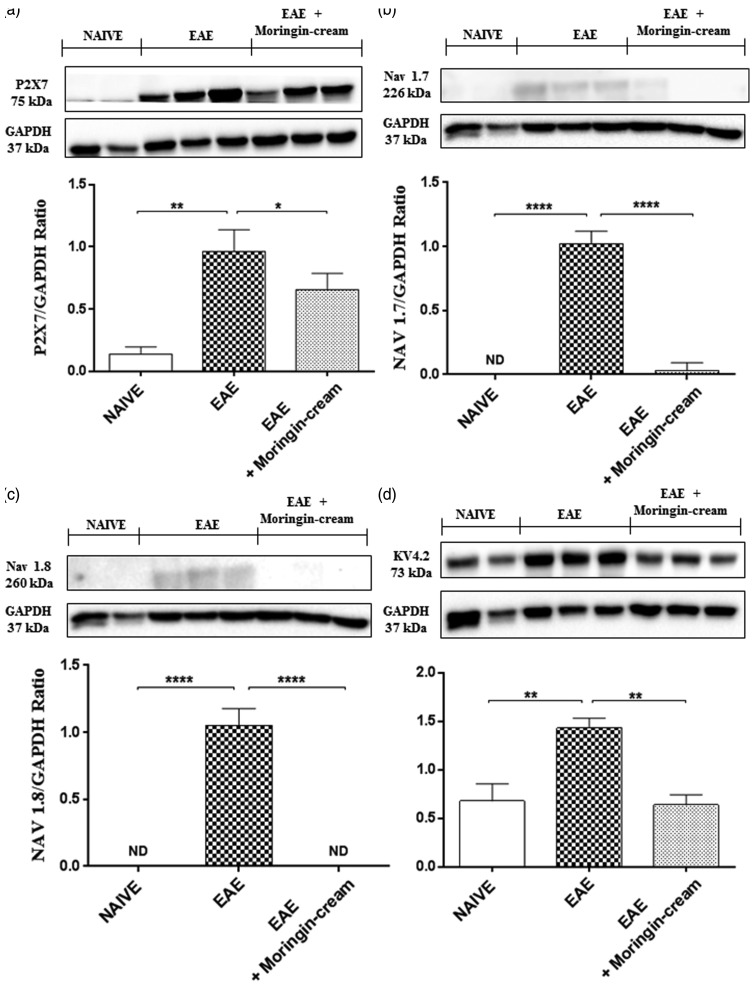
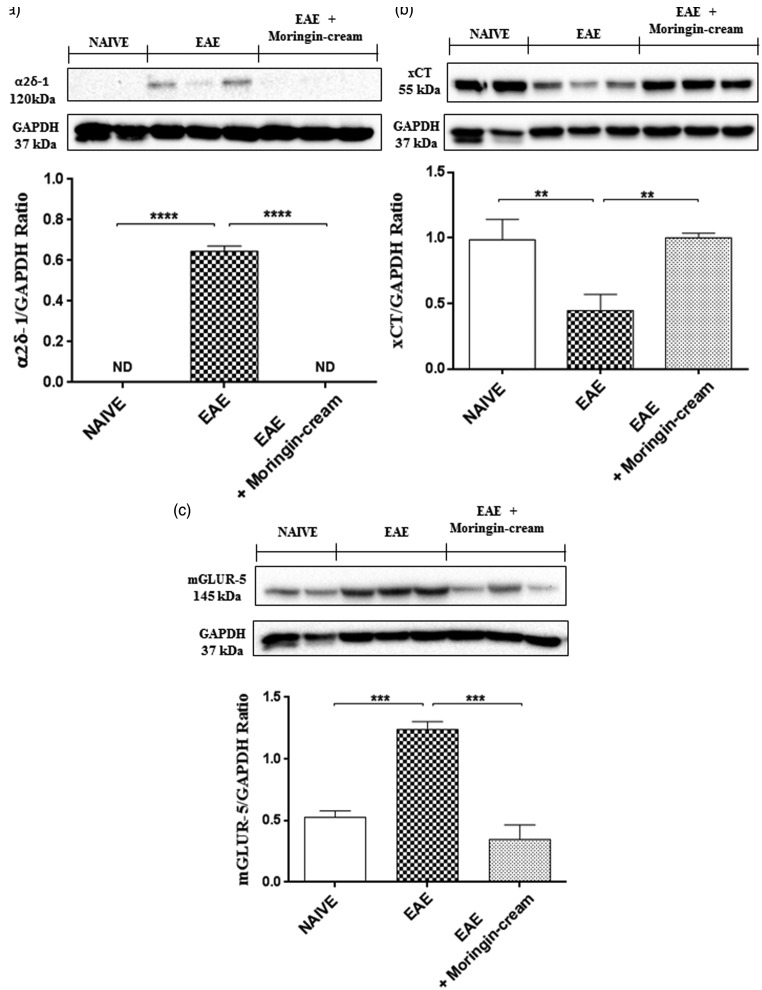
Firstly, we evaluated the expression of purinergic P2X7 receptor, a ligand-gated cation channel that opens in response to ATP binding and leads to cell depolarization. This receptor is involved both in inflammation and in neuropathic pain. By Western blot analysis, we found an increased expression in spinal cord samples from untreated EAE mice, while topically administered moringin reduced its expression (Figure 7(a)). Afterwards, we examined the expression of the main voltage-gated sodium (Na+), potassium (K+), and calcium channels (Ca2+), found to have a role in neuropathic pain. Our results achieved by Western blot evaluations, showed an upregulation of Nav 1.7 (Figure 7(b)) and Nav 1.8 (Figure 7(c)) voltage-gated Na+ channels in untreated EAE mice compared to naive ones. Conversely, topical treatment with 2% moringin cream completely abolished their expression levels. Moreover, by looking at voltage-gated K+ channels, we chose to observe the expression of Kv4.2 potassium channel subunit spinal cord following EAE induction. Our results showed a basal expression of Kv4.2increased in naive mice instead in EAE mice. On the contrary, the administration of topical treatment allows a reduction in its expression (Figure 7(d)). Likewise, we evaluated the involvement of the calcium voltage-gated channel auxiliary subunit α2δ-1, which represents an essential component of the voltage-gated calcium channel complex. Western blot analysis showed a significant expression of α2δ-1in spinal cord samples collected from EAE-mice, whereas topical treatment with 2% moringin cream inhibited its expression as observed in naive mice (Figure 8(a)).
Figure 7.
Western blot analysis for P2X7 (a). Naive vs. EAE **p = 0.0039; EAE vs. EAE + moringin cream *p = 0.0293. Western blot analysis for Nav 1.7 (b). Naive vs. EAE ****p < 0.0001; EAE vs. EAE + moringin cream ****p < 0.0001. Western blot analysis for Nav 1.8 (c). Naive vs. EAE ****p < 0.0001; EAE vs. EAE + moringin cream ****p < 0.0001. Western blot analysis for KV4.2 (d). Naive vs. EAE **p = 0.0032; EAE vs. EAE + moringin cream **p = 0.0015. GAPDH was used as the internal control. The figure is representative of three separate experiments. In detail, a representative blot of samples obtained from two naive, two EAE, and three EAE + moringin cream mice is shown and densitometry analysis of all animals is reported. Data are expressed as mean ± SEM. All results were analyzed by one-way ANOVA followed by a Bonferroni test for multiple comparisons. EAE: experimental autoimmune encephalomyelitis; ND: not detectable.
Figure 8.
Western blot analysis for α2δ-1 (a). Naive vs. EAE **p = 0.0029. Western blot analysis for xCT (b). Naive vs. EAE ****p < 0.0001; EAE vs. EAE + moringin cream ****p < 0.0001. Western blot analysis for mGluR5 (c). Naive vs. EAE ***p = 0.0001; EAE vs. EAE + moringin cream ***p = 0.0001. GAPDH was used as the internal control. The figure is representative of three separate experiments. In detail, a representative blot of samples obtained from two naive, two EAE, and three EAE + moringin cream mice is shown and densitometry analysis of all animals is reported. Data are expressed as mean ± SEM. All results were analyzed by one-way ANOVA followed by a Bonferroni test for multiple comparisons. EAE: experimental autoimmune encephalomyelitis.
Moringin cream modulates metabotropic glutamate receptors
It is well recognized that dysregulated glutamate homeostasis, involving downregulation of glutamate transporters and upregulation of metabotropic glutamate receptors (mGluRs) is involved in neuropathic pain in both MS patients and in EAE mice. Here, we looked at the expression of cysteine–glutamate antiporter (xCT), which is involved in the exchange of extracellular l-cystine (l-Cys2) and intracellular l-glutamate (l-Glu) across the cellular plasma membrane. Western blot analysis showed a significant decrease in xCT expression in spinal cord tissues from untreated EAE mice compared to naive ones. Conversely, topical treatment with 2% moringin cream enhanced its levels (Figure 8(b)). In parallel, we observed a considerable increase in mGluR5 expression in EAE animals, compared to naive ones. mGluR5 expression was instead restored following topical application of 2% moringin cream (Figure 8(c)).
Discussion
Neuropathic pain is a severe and debilitating symptom, affecting the majority of MS patients with a strong impact on their quality of life.34,35 Although the mechanisms of neuropathic pain are still not fully understood, a growing number of studies showed the pivotal role of neuroinflammation in its generation and maintenance.36,37 Thus, targeting neuroinflammation could represent a new potential approach for treating this condition. Over the last years, moringin was at the center of an intense research activity for the management of neurodegenerative disorders, and MS in particular.17,38 Although, currently, there are no data regarding the efficacy of moringin in alleviating MS-associated neuropathic pain, its ability to counteract inflammation was widely demonstrated.17,38
The present study was aimed to provide a new application of topical 2% moringin cream, as a promising option in relieving MS-related neuropathic pain.
Our findings demonstrated that topical treatment with 2% moringin cream, given at the symptomatic onset of EAE, is able to relieve neuropathic pain by counteracting inflammatory pathway. Additionally, these data are consistent with a recent study where synthetic SFN, another widely examined ITC present in cruciferous vegetables, was proved to exert anti-nociceptive and anti-inflammatory effects in sciatic nerve chronic constriction injury mouse model.14
Overall, our results proved that moringin topically applied on the hind limbs of EAE mice was effective in alleviating mechanical allodynia, suggesting that this treatment attenuated also behavioral hypersensitivity in mice. Indeed, EAE + 2% moringin cream applied in EAE mice significantly increased response to mechanical stimulus, recovering the responsiveness of the hind limbs. The beneficial effects of topical moringin are reflected also in the significant improvement of disability score and reduction in body weight loss that are strictly associated with EAE severity. As EAE is a demyelinating disorder, we looked at MBP expression, considered an essential protein of myelinated axons.39 Our evidences showed that untreated EAE mice displayed reduced myelin along different white matter tracts of spinal cord. On the contrary, a marked remyelination was found following topical administration of 2% moringin cream cream. MBP degradation is an important step in both in MS and EAE pathology40 as causes impairment in blood–brain barrier permeability with consequent infiltration of inflammatory cells and exacerbation of the inflammatory cascade.40 Overall, our histological evaluations performed on spinal cord sections revealed that treatment with 2% moringin cream is able to reduce lymphocyte cells infiltration and axonal loss in EAE mice, thus improving neuroinflammation status. Accumulating evidences showed that following EAE induction, peripheral immune T cells (T helper (Th)),41 and glial cells are activated to produce a variety of pro-inflammatory cytokines, that, in turn, by activating nociceptive neurons contribute to neuropathic pain initiation.42–44 As expected, our observations indicated a considerable increase in IFN-γ and IL-17 expression in spinal cord from EAE mice, reduced following the topical application of 2% moringin cream. These observations were further corroborated by the reduction in pro-inflammatory cytokine TNF-α found in spinal cord from EAE mice following topical treatment. Thus, our results demonstrated that the beneficial effects of 2% moringin cream are due not only to its property to suppress inflammatory mediator production over the course of treatment but also to the increase in anti-inflammatory cytokines, like IL-10. Indeed, increased expression of IL-10 was observed in EAE mice after seven days of topical treatment. In addition, the enhanced secretion of pro-inflammatory mediators in damaged tissues seems to affect expression and activity of receptors and voltage-dependent ion channels located on axonal membrane, leading to alteration of the electrical properties of sensory neurons. Among receptors, we investigated the expression of the purinergic P2X7 receptor, a non-selective cation channel permeable to Ca2+, K+, and Na+ activated by high concentrations of ATP or by the release of pro-inflammatory mediators.45,46 Activation of P2X7 receptor in oligodendrocytes also renders these cells more vulnerable to ATP excitotoxicity exacerbating symptoms and progression of EAE.47 In particular, the role of P2X7 receptors in neuropathic pain was established on the basis of diminished pain sensitivity in P2X7 receptor-deficient mice and after its pharmacological block.48,49 In line with this, our observations showed an increase in P2X7 expression in EAE mice, reduced instead by topical application of 2% moringin cream. The transmission of pain signals from peripheral nociceptive afferents to neurons of the spinothalamic tract is critically dependent on the activity of several ion channels including voltage-gated sodium (Nav) channels as well as voltage-gated calcium (Cav) channels.50–52 These channels are involved in the regulation of cellular excitability of the sensory neurons or in the release of pro-nociceptive neurotransmitter by generating and conducting action potentials.53 Indeed, upon activation, individual ion channels transition from a closed to an open conformation to allow ions to pass down their electrochemical gradients, until the channels closes again. The current literature suggests that neuropathic pain is due to a dysregulation of these channels that could alter their specificity and make them more vulnerable to pharmacological modulation.54 Therefore, we investigated whether 2% moringin cream by inhibiting the expression of these channels may prove to be a potent analgesic against neuropathic pain. In particular, we evaluated the expression of two Na channel α-subunits, Nav 1.7 and Nav 1.8, predominantly expressed at high levels in nociceptor terminals and implicated in the development of neuropathic pain.55 More in detail, in experimental model of nerve sectioning or chronic constriction-induced neuropathic pain, it was demonstrated that nerve or tissues injuries increase expression of Nav channels in dorsal root ganglion and around the terminal injury site of peripheral axons allowing the generation and propagation of spikes that are finally warned in the CNS as pain.51 In addition, it has been proven that in animals knockout of Nav 1. 7 and Nav 1.8 or blockage of these channels led to a significant alleviation of neuropathic pain and also to a significant reduction in inflammation.56 Consistently with this, we found that the increased expression of Nav 1.7 and Nav 1.8 in spinal cord samples from EAE mice was surprisingly inhibited by topical administration of 2% moringin cream. Likewise, numerous studies reported that pharmacological inhibition of potassium (K+) channels at demyelinating axons could represent an interesting strategy to improve action potential generation in demyelinating disorders.57 Therefore, we looked at the expression of Kv4.2 potassium channel subunit, the activation of which was found to increase neuronal excitability and nociceptive behaviors in the superficial spinal cord dorsal horn.58 Our data showed increased expression of Kv4.2 following EAE induction, whereas topical treatment with 2% moringin cream diminished its levels. Therefore, by targeting K+ channels, control of the action potential frequency and threshold, action potential configuration, neurotransmitter release, and postsynaptic excitability underlying the development of the neuropathic pain could be possible.
In addition, it was demonstrated that impairment in Nav as well as in K channels due to demyelination, not only causes loss of capacity to maintain physiological ion gradients but also an increase in the calcium influx in neurons or axons that contributes to axonal/neural degeneration.54 Indeed, the CaV channels have a role in neuropathic pain development by reducing the threshold of activation and controlling the bursting activity of nociceptors.59 More in detail, these channels are involved in electrical activity, by mediating calcium-dependent enzyme activation, gene expression, and release of neurotransmitter. Moreover, Cav channels are largely expressed in the spinal cord, where primary afferent nociceptors form synapses with intrinsic dorsal horn neurons.60 Due to both localization and physiological role, these channels were proposed as potential targets in the management of neuropathic pain.59 In this context, we examined the expression of the calcium voltage-gated channel auxiliary subunit α2δ-1, an essential component of the voltage-gated calcium channel complex.61 α2δ-1 also represents the substrate for the anticonvulsant gabapentin and pregabalin, commonly used for neuropathic pain management.62 Overexpression of α2δ-1 subunit was found in active EAE lesions where affecting presynaptic function contribute to axonal degeneration and neuropathic pain development.63 In agreement with this, we found that mice subjected to EAE displayed a significant augmentation in α2δ-1 expression level, abolished by treatment with 2% moringin cream.
According to current knowledge, in addition to the blockers of the voltage-gated ion channel, the blockers of glutamate receptors (mGluRs) could offer another exciting opportunity for the clinical management of neuropathic pain. Indeed, glutamate is well recognized to play a fundamental role in the initiation and maintenance of neuropathic pain.64,65 In particular, mGluR5 involved in glutamate-induced plasticity of pain-related processes, like nociceptive hypersensitivity after tissue damage was found to be over-expressed in experimental models of spinal nerve injury.66 Moreover, upregulation of mGluRs is involved in neuropathic pain in both MS patients67,68 and EAE mice.69 Here, we firstly looked at the expression of cysteine–glutamate antiporter (xCT) that mediates the exchange of extracellular l-cystine (l-Cys2) and intracellular l-glutamate (l-Glu) across the cellular plasma membrane.70 By Western blot analysis, we observed that the increased expression of mGluR5 found EAE mice may be linked to the compromised function of xCT, as proven by its decrease in EAE samples. Two percent moringin cream treatment instead was found to restore xCT expression with consequent decrease in mGluR5.
In the light of the described findings, we can assess that the effectiveness of 2% moringin cream treatment in relieving neuropathic pain is due to the inhibition of inflammatory pathway as well as to blockade of voltage-gated ion channels. Therefore, the moringa ITC as topical cream could be an innovative, safe, and non-invasive pharmacological trend for many MS patients in which neuropathic pain is resistant to conventional therapies.
Author contributions
SG wrote the manuscript, performed in vivo experiments, molecular biology analysis, and the statistical analysis; RI performed isolation of moringin and supervised manuscript; PB conceived and designed the experiments and was involved in revising the manuscript; and EM performed in vivo experiments, immunohistochemical evaluations, and revised the manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by current research funds 2016 of IRCCS “Centro Neurolesi Bonino-Pulejo”, Messina, Italy.
References
- 1.Compston A, Coles A. Multiple sclerosis. Lancet 2008; 372: 1502–1517. [DOI] [PubMed] [Google Scholar]
- 2.Siffrin V, Brandt AU, Herz J, et al. New insights into adaptive immunity in chronic neuroinflammation. Adv Immunol 2007; 96: 1–40. [DOI] [PubMed] [Google Scholar]
- 3.Goldenberg MM. Multiple sclerosis review. Pharm Therapeut 2012; 37: 175–184. [PMC free article] [PubMed] [Google Scholar]
- 4.Archibald CJ, McGrath PJ, Ritvo PG, et al. Pain prevalence, severity and impact in a clinic sample of multiple sclerosis patients. Pain 1994; 58: 89–93. [DOI] [PubMed] [Google Scholar]
- 5.Osterberg A, Boivie J, Thuomas KA. Central pain in multiple sclerosis—Prevalence and clinical characteristics. Eur J Pain 2005; 9: 531–542. [DOI] [PubMed] [Google Scholar]
- 6.Brola W, Mitosek-Szewczyk K, Opara J. Symptomatology and pathogenesis of different types of pain in multiple sclerosis. Neurol Neurochir Pol 2014; 48: 272–279. [DOI] [PubMed] [Google Scholar]
- 7.Siddall PJ, Cousins MJ. Neurobiology of pain. Int Anesthesiol Clin 1997; 35: 1–26. [DOI] [PubMed] [Google Scholar]
- 8.Truini A, Galeotti F, Cruccu G. Treating pain in multiple sclerosis. Expert Opin Pharmacother 2011; 12: 2355–2368. [DOI] [PubMed] [Google Scholar]
- 9.Dworkin RH, O’Conno AB, Backonja M, et al. Pharmacologic management of neuropathic pain: evidence-based recommendations. Pain 2007; 132: 237–251. [DOI] [PubMed] [Google Scholar]
- 10.O’Connor AB, Schwid SR, Herrmann DN, et al. Pain associated with multiple sclerosis: systematic review and proposed classification. Pain 2008; 137: 96–111. [DOI] [PubMed] [Google Scholar]
- 11.Finnerup NB, Otto M, McQuay HJ, et al. Algorithm for neuropathic pain treatment: an evidence based proposal. Pain 2005; 118: 289–305. [DOI] [PubMed] [Google Scholar]
- 12.Kim D, You B, Jo EK, et al. NADPH oxidase 2-derived reactive oxygen species in spinal cord microglia contribute to peripheral nerve injury-induced neuropathic pain. Proc Natl Acad Sci U S A 2010; 107: 14851–14856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Negi G, Kumar A, Sharma SS. Nrf2 and NF-kappaB modulation by sulforaphane counteracts multiple manifestations of diabetic neuropathy in rats and high glucose-induced changes. Curr Neurovasc Res 2011; 8: 294–304. [DOI] [PubMed] [Google Scholar]
- 14.Wang C, Wang C. Anti-nociceptive and anti-inflammatory actions of sulforaphane in chronic constriction injury-induced neuropathic pain mice. Inflammopharmacology 2017; 25: 99–106. [DOI] [PubMed] [Google Scholar]
- 15.Di W, Shi X, Lv H, et al. Activation of the nuclear factor E2-related factor 2/anitioxidant response element alleviates the nitroglycerin-induced hyperalgesia in rats. J Headache Pain 2016; 17: 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Use of isothiocyanate derivatives as modulators of peripheral and neuropathic pain EP 3078371 A1, https://www.google.com/patents/EP3078371A1?cl=en (accessed 26 July 2017).
- 17.Galuppo M, Giacoppo S, De Nicola GR, et al. Antiinflammatory activity of glucomoringin isothiocyanate in a mouse model of experimental autoimmune encephalomyelitis. Fitoterapia 2014; 95: 160–174. [DOI] [PubMed] [Google Scholar]
- 18.Biswas SK, Chowdhury A, Das J, et al. Pharmacological potentials of Moringa oleifera Lam.: a review. Int J Pharm Sci Res 2012; 47: 305–310. [Google Scholar]
- 19.Anwar F, Latif S, Ashraf M, et al. Moringa oleifera: a food plant with multiple medicinal uses. Phytother Res 2007; 21: 17–25. [DOI] [PubMed] [Google Scholar]
- 20.Stohs SJ, Hartman MJ. Review of the Safety and Efficacy of Moringa oleifera. Phytother Res 2015; 29: 796–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saini RK, Sivanesan I, Keum YS. Phytochemicals of Moringa oleifera: a review of their nutritional, therapeutic and industrial significance. 3 Biotech 2016; 6: 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abdull Razis AF, Ibrahim MD, Kntayya SB. Health benefits of Moringa oleifera. Asian Pac J Cancer Prev 2014; 15: 8571–8576. [DOI] [PubMed] [Google Scholar]
- 23.Giacoppo S, Galuppo M, Pollastro F, et al. A new formulation of cannabidiol in cream shows therapeutic effects in a mouse model of experimental autoimmune encephalomyelitis. Daru 2015; 23: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brunelli D, Tavecchio M, Falcioni C, et al. The isothiocyanate produced from glucomoringin inhibits NF-kB and reduces myeloma growth in nude mice in vivo. Biochem Pharmacol 2010; 79: 1141–1148. [DOI] [PubMed] [Google Scholar]
- 25.Muller C, van Loon J, Ruschioni S, et al. Taste detection of the non-volatile isothiocyanate moringin results in deterrence to glucosinolate-adapted insect larvae. Phytochemistry 2015; 118: 139–148. [DOI] [PubMed] [Google Scholar]
- 26.Paschalidis N, Iqbal AJ, Maione F, et al. Modulation of experimental autoimmune encephalomyelitis by endogenous annexin A1. J Neuroinflammation 2009; 6: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rodrigues DH, Vilela MC, Barcelos L.dS, et al. Absence of PI3Kgamma leads to increased leukocyte apoptosis and diminished severity of experimental autoimmune encephalomyelitis. J Neuroimmunol 2010; 222: 90–94. [DOI] [PubMed] [Google Scholar]
- 28.Chaplan SR, Bach FW, Pogrel JW, et al. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 1994; 53: 55–63. [DOI] [PubMed] [Google Scholar]
- 29.Cross AH, Dolich S, Raine CS. Antigen processing of myelin basic protein is required prior to recognition by T cells inducing EAE. Cell Immunol 1990; 129: 22–31. [DOI] [PubMed] [Google Scholar]
- 30.Svendsen KB, Jensen TS, Hansen HJ, et al. Sensory function and quality of life in patients with multiple sclerosis and pain. Pain 2005; 114: 473–481. [DOI] [PubMed] [Google Scholar]
- 31.Komiyama Y, Nakae S, Matsuki T, et al. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J Immunol 2006; 177: 566–573. [DOI] [PubMed] [Google Scholar]
- 32.Fletcher JM, Lalor SJ, Sweeney CM, et al. T cells in multiple sclerosis and experimental autoimmune encephalomyelitis. Clin Exp Immunol 2010; 162: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li CY, Zhang XL, Matthews EA, et al. Calcium channel α2δ1 subunit mediates spinal hyperexcitability in pain modulation. Pain 2006; 125: 20–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moulin DE. Pain in central and peripheral demyelinating disorders. Neurol Clin 1998; 16: 889–898. [DOI] [PubMed] [Google Scholar]
- 35.Jensen TS, Madsen CS, Finnerup NB. Pharmacology and treatment of neuropathic pains. Curr Opin Neurol 2009; 22: 467–474. [DOI] [PubMed] [Google Scholar]
- 36.Watkins LR, Maier SF. Beyond neurons: evidence that immune and glial cells contribute to pathological pain states. Physiol Rev 2002; 82: 981–1011. [DOI] [PubMed] [Google Scholar]
- 37.Jha MK, Jeon S, Suk K. Glia as a link between neuroinflammation and neuropathic pain. Immune Netw 2012; 12: 41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Giacoppo S, Soundara Rajan T, De Nicola GR, et al. Moringin activates Wnt canonical pathway by inhibiting GSK3beta in a mouse model of experimental autoimmune encephalomyelitis. Drug Des Devel Ther 2016; 10: 3291–3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baron W, Hoekstra D. On the biogenesis of myelin membranes: sorting, trafficking and cell polarity. FEBS letters 2010; 584: 1760–1770. [DOI] [PubMed] [Google Scholar]
- 40.D’Aversa TG, Eugenin EA, Lopez L, et al. Myelin basic protein induces inflammatory mediators from primary human endothelial cells and blood-brain barrier disruption: implications for the pathogenesis of multiple sclerosis. Neuropathol Appl Neurobiol 2013; 39: 270–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lovett-Racke AE, Yang Y, Racke MK. Th1 versus Th17: are T cell cytokines relevant in multiple sclerosis? Biochim Biophys Acta 2011; 1812: 246–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tracey DJ, Walker JS. Pain due to nerve damage: are inflammatory mediators involved? Inflamm Res 1995; 44: 407–411. [DOI] [PubMed] [Google Scholar]
- 43.Zhang JM, An J. Cytokines, inflammation, and pain. Int Anesthesiol Clin 2007; 45: 27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moalem G, Xu K, Yu L. T lymphocytes play a role in neuropathic pain following peripheral nerve injury in rats. Neuroscience 2004; 129: 767–777. [DOI] [PubMed] [Google Scholar]
- 45.North RA. Molecular physiology of P2X receptors. Physiol Rev 2002; 82: 1013–1067. [DOI] [PubMed] [Google Scholar]
- 46.Inoue K, Tsuda M. [The role of microglia and ATP receptors in a mechanism of neuropathic pain]. Nihon Yakurigaku Zasshi 2006; 127: 14–17. [DOI] [PubMed] [Google Scholar]
- 47.Matute C. P2X7 receptors in oligodendrocytes: a novel target for neuroprotection. Mol Neurobiol 2008; 38: 123–128. [DOI] [PubMed] [Google Scholar]
- 48.Chessell IP, Hatcher JP, Bountra C, et al. Disruption of the P2X7 purinoceptor gene abolishes chronic inflammatory and neuropathic pain. Pain 2005; 114: 386–396. [DOI] [PubMed] [Google Scholar]
- 49.Dell’Antonio G, Quattrini A, Cin ED, et al. Relief of inflammatory pain in rats by local use of the selective P2X7 ATP receptor inhibitor, oxidized ATP. Arthritis Rheum 2002; 46: 3378–3385. [DOI] [PubMed] [Google Scholar]
- 50.Hargus NJ, Patel MK. Voltage-gated Na+ channels in neuropathic pain. Expert Opin Investig Drugs 2007; 16: 635–646. [DOI] [PubMed] [Google Scholar]
- 51.Moldovan M, Alvarez S, Romer Rosberg M, et al. Axonal voltage-gated ion channels as pharmacological targets for pain. Eur J Pharmacol 2013; 708: 105–112. [DOI] [PubMed] [Google Scholar]
- 52.Perret D, Luo ZD. Targeting voltage-gated calcium channels for neuropathic pain management. Neurotherapeutics 2009; 6: 679–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Watanabe K, Larsson K, Rydevik B, et al. Increase of sodium channels (nav 1.8 and nav 1.9) in rat dorsal root ganglion neurons exposed to autologous nucleus pulposus. Open Orthop J 2014; 8: 69–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bittner S, Meuth SG. Targeting ion channels for the treatment of autoimmune neuroinflammation. Ther Adv Neurol Disord 2013; 6: 322–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang W, Gu J, Li YQ, et al. Are voltage-gated sodium channels on the dorsal root ganglion involved in the development of neuropathic pain? Mol Pain 2011; 7: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gold MS, Weinreich D, Kim CS, et al. Redistribution of Na(V)1.8 in uninjured axons enables neuropathic pain. J Neurosci 2003; 23: 158–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Beeton C, Wulff H, Barbaria J, Clot-Faybesse O, et al. Selective blockade of T lymphocyte K(+) channels ameliorates experimental autoimmune encephalomyelitis, a model for multiple sclerosis. Proc Natl Acad Sci U S A 2001; 98: 13942–13947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hu HJ, Carrasquillo Y, Karim F, et al. The kv4.2 potassium channel subunit is required for pain plasticity. Neuron 2006; 50: 89–100. [DOI] [PubMed] [Google Scholar]
- 59.Todorovic SM, Jevtovic-Todorovic V. T-type voltage-gated calcium channels as targets for the development of novel pain therapies. Br J Pharmacol 2011; 163: 484–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bourinet E, Francois A, Laffray S. T-type calcium channels in neuropathic pain. Pain 2016; 157: S15–S22. [DOI] [PubMed] [Google Scholar]
- 61.Dolphin AC. Voltage-gated calcium channels and their auxiliary subunits: physiology and pathophysiology and pharmacology. J Physiol 2016; 594: 5369–5390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hendrich J, Van Minh AT, Heblich F, et al. Pharmacological disruption of calcium channel trafficking by the alpha2delta ligand gabapentin. Proc Natl Acad Sci U S A 2008; 105: 3628–3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kornek B, Storch MK, Bauer J, et al. Distribution of a calcium channel subunit in dystrophic axons in multiple sclerosis and experimental autoimmune encephalomyelitis. Brain 2001; 124: 1114–1124. [DOI] [PubMed] [Google Scholar]
- 64.Dray A. Neuropathic pain: emerging treatments. Br J Anaesth 2008; 101: 48–58. [DOI] [PubMed] [Google Scholar]
- 65.Chiechio S, Nicoletti F. Metabotropic glutamate receptors and the control of chronic pain. Curr Opin Pharmacol 2012; 12: 28–34. [DOI] [PubMed] [Google Scholar]
- 66.Hudson LJ, Bevan S, McNair K, et al. Metabotropic glutamate receptor 5 upregulation in A-fibers after spinal nerve injury: 2-methyl-6-(phenylethynyl)-pyridine (MPEP) reverses the induced thermal hyperalgesia. J Neurosci 2002; 22: 2660–2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Werner P, Pitt D, Raine CS. Multiple sclerosis: altered glutamate homeostasis in lesions correlates with oligodendrocyte and axonal damage. Ann Neurol 2001; 50: 169–180. [DOI] [PubMed] [Google Scholar]
- 68.Newcombe J, Uddin A, Dove R, Patel B, Turski L, Nishizawa Y, Smith T. Glutamate receptor expression in multiple sclerosis lesions. Brain Pathol 2008; 18: 52–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Olechowski CJ, Parmar A, Miller B, et al. A diminished response to formalin stimulation reveals a role for the glutamate transporters in the altered pain sensitivity of mice with experimental autoimmune encephalomyelitis (EAE). Pain 2010; 149: 565–572. [DOI] [PubMed] [Google Scholar]
- 70.Bridges RJ, Natale NR, Patel SA. System xc cystine/glutamate antiporter: an update on molecular pharmacology and roles within the CNS. Br J Pharmacol 2012; 165: 20–34. [DOI] [PMC free article] [PubMed] [Google Scholar]