Abstract
Shammah is a preparation of smokeless tobacco (ST) that is frequently used in the Arabian Peninsula, especially in Saudi Arabia. A mixture of powdered tobacco, lime, ash, black pepper, oils and flavorings, shammah in is placed in the buccal cavity or lower labial vestibule of the mouth. The user (or dipper) spits out insoluble debris. ST is linked to a number of harmful effects such as dental disease, oral cancer, oesophagus cancer, and pancreas cancer. It also causes adverse reproductive effects including stillbirth, premature birth and low birth weight. The importation of ST products is prohibited in Saudi Arabia. Legislative action to combat the use of ST (moist snuff and chewing tobacco) in Saudi Arabia appeared in 1990. The actual percentage use may be higher than reported since shammah is illegal in Saudi Arabia and there may be some unwillingness to admit to its use. Data on ST use in the Arabian Peninsula are sparse. Most studies conducted there focused on the prevalence of shammah use among adolescents rather than among adults. This review paper aimed to understand the pattern of use of shammah and its adverse health effects. It also aimed to provide suitable epidemiological data for public health policy makers.
Keywords: Shammah, smokeless tobacco, health effects, cancer, Arabian peninsula
Introduction
The tobacco plant is thought to have originated somewhere between North and South America dating back to 1400–1000 BCE (Goodman, 2005). The American Indians were likely the first people to smoke, chew and snuff tobacco as early as the 1400s (Christen et al., 1982). The American Indians made use of a Y-shaped tube which they called tobago or tobaca to inhale the tobacco powder. At the time, Columbus discovered the Americas and the plant was introduced to Europe and used by the Portuguese and Spanish who changed the word Tabacum to tobacco (Christen et al., 1982). In the mid-16th century, Jean Nicot, the French Ambassador to Portugal, introduced tobacco and tobacco seeds to France, hence the name “Nicotiana” (Stewart, 1967). Later on, the tobacco plant became common throughout the Americas, and many civilizations discovered the effects of a self-administered dose of tobacco independently of each other. The recent history of tobacco began with the design of the first machine to manufacture cigars in the mid-19th century by James Bonsack. Table 1 provides the history of early tobacco cultivation and use.
Table 1.
The History of Early Tobacco Cultivation and Use*
| Date | Event |
|---|---|
| 1492 | Columbus sighted the home of the Arawaks and was offered dried tobacco leaves. |
| 1499 | Amerigo Vespucci recorded the use of chewing tobacco on an island off Venezuela. |
| 1545 | Iroquois Indians near Montreal, Canada, were found to have smoking habits. |
| 1556 | Tobacco was first grown or became known in France. |
| 1558 | Tobacco was used in Brazil and Portugal. |
| 1559 | Tobacco was used in Spain. |
| 1560 | Nicotiana rustica was used in Central Africa. |
| 1565 | Tobacco was used in England. |
| 1600 | Tobacco was introduced to Italy, Germany, Norway, Sweden, Russia, Persia, India, Indochina, Japan, China and the west coast of Africa. |
| 1612 | John Rolfe, at Jamestown, Virginia, was the first man known to grow tobacco commercially for export. |
| 1631 | Tobacco production extended to Maryland and then gradually to other areas. |
| 1650s | Portuguese took tobacco to South Africa and other countries. Spaniards distributed tobacco to the Philippines, Guatemala and other Central and South American countries and to the West Indies. |
| Tobacco cultivation was begun in Indonesia. | |
| Tobacco cultivation was extended in Europe. |
The Prevalence of ST Use
Tobacco use is familiar throughout the global due to its widespread marketing, addiction potential of nicotine, absence or weakness of warnings and legislation concerning tobacco use and low price (WHO, 2005).
More than 300 million adults in 70 countries across all WHO regions use ST. The largest share, 89%, are in South-East Asia. More than 250 million adult ST users are in low-and middle-income countries (WHO, 2011; CDCP, 2012). Figure 1 shows a large disparity in the prevalence of ST use globally. A prevalence of ST use of 10% or more is reported in Myanmar (29.6%), Bangladesh (27.2%), India (25.9%), Nepal (18.6%), Sweden (17%), Sri Lanka (15.8%), Micronesia (11.4%), Uzbekistan (11.3%), Yemen (10.7%) and Norway (10%). Countries with a prevalence of use ranging from 5-10% include Algeria, Mauritania and Tunisia (WHO, 2011).
Figure 1.
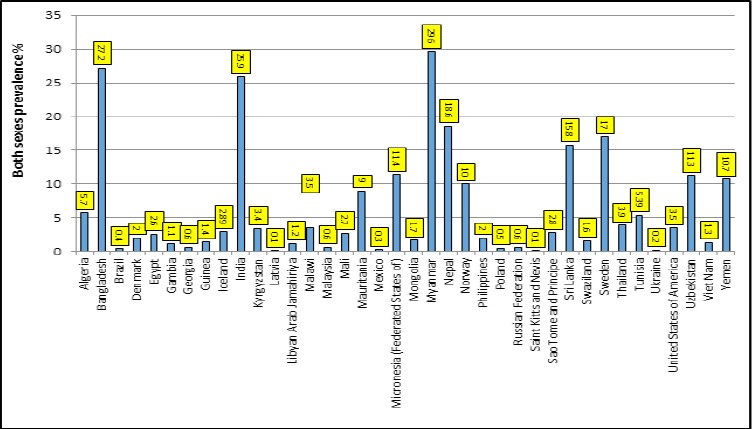
Prevalence of Smokeless Tobacco in Some Countries (6)
Oral tobacco, or moist snuff, is the only ST form that is used significantly in Europe, mainly in Sweden, Norway and Finland. Other ST products from India, Pakistan and Bangladesh are available on the UK market and are largely consumed by immigrants from those countries, which represent 4.5% of the UK population.
In a few countries of the Eastern Mediterranean Region, such as Sudan, Yemen, and Pakistan, locally made or produced ST products are widely consumed. In other countries such as Egypt, the most populous Arab country, ST use has markedly increased among adults, according to the Global Adult Tobacco Survey (GATS) (WHO, 2010 and CDCP, 2012). Data on ST use in the Arabian Peninsula are sparse (Agili and Park, 2012). Most studies conducted there focused on the prevalence of shammah use among adolescents rather than among adults (Moradi et al, 2015). The available national data show that the prevalence of smoking among people over 15 years of age was 12.2 %, and males were more likely to smoke than females (21.5 % vs. 1.1 %). Mean age of smoking initiation was 19.1 years (±6.5 years) with 8.9 % of smokers starting before the age of 15. Daily shisha smoking was reported by 4.3 % of the population (7.3 % of men and 1.3 % of women). Around 1.4 % of the population (2.6 % of men and 0.1 % of women) were daily smokers of cigarettes/cigars and shisha (Moradi et al, 2015). In one regional study, the prevalence of tobacco use among middle school students in Jeddah was 9.72% (12.4% in boys and 6.7% in girls), and only 2.0% of male students reported the use of local ST, shammah (Agili and Park, 2012). In Yemen, a cross-sectional study was carried out on adult outpatients aged 18 years and older. These outpatients attended dental clinics at the Al-Thawra Modern General Hospital in Sana. The study outcomes showed that 4.4 % of the subjects (23 patients out of 520 patients) were shammah users (Hajeb, 2015).
Adverse Health Effects of ST Use
ST use poses numeral health risks (Raute et al., 2011). The 2015 WHO report on the global tobacco epidemic stated that tobacco-related diseases caused more than 6 million deaths per year worldwide (WHO, 2015). This number is greater than the number of deaths caused by diseases such as AIDS, tuberculosis and malaria combined. By 2030, the death toll will exceed eight million a year. Unless urgent action is taken, tobacco could kill one billion people during this century (WHO, 2015).
Tobacco-related deaths and disease are attributed to the toxic and carcinogenic components present in tobacco products (WHO, 2015). ST use is linked to the development of cardiovascular disease, hypertension, and stroke (Piano et al., 2010). Furthermore, in addition to its systemic damage, ST use causes local damage depending on the method of consumption. ST is associated with the development of localized oral lesions that may develop into oral cancer. In addition, the swallowed ST juice may induce cancers of the esophagus, larynx, stomach, pancreas and prostate, according to various studies carried out globally (CDCP, 2004; Bhawna, 2013; Mishra, 2013 and Mishra, 2013). Other notable health disorders linked to tobacco consumption include erectile dysfunction (Wang, 2013) and problems in pregnancy, including stillbirth and low birth weight of a liveborn infant (England, 2010).
Cardiovascular Disease
A number of studies which compared cigarette smoking with ST revealed qualitatively similar effects on the sympathetic nervous system from nicotine (Savitz, 2006). Nicotine increases heart rate, which raises blood pressure. The nicotine in ST is absorbed into the bloodstream from the oral cavity or through the gastrointestinal tract when tobacco juice is swallowed. The rise in nicotine blood concentration levels is gradual and persists for a longer interval in case of snuff use compared to the intense rise and short interval of nicotine levels during smoking (Benowitz, 2009). The absorption of a single dose of chewing or snuffing tobacco were 4.5 and 3.6 mg, respectively (Benowitz, 2009). The use of oral and nasal ST for 30 minutes leads to a continuous increase in blood nicotine concentration levels that is maintained for up to 2 hours. Generally, and during most of the day, circadian heart rates are higher by approximately 10% among tobacco users than those who do not use any tobacco.
Smokeless tobacco consumption and the development of adverse cardiovascular events like myocardial infarction, stroke, and ischemic heart disease has been described in detail in western populations (Alsanosy, 2013). Observations from these studies paint a mixed picture with some showing increased incidence and others showing no such relation. Likewise, contradictory results have been noted in studies evaluating increased risk factors for cerebrovascular death (CVD) in ST consuming populations.
Several studies have examined ST use and the risk of ischemic stroke. The Cancer Prevention Studies found significant associations between current ST use and risk of CVD (Lyon, 2014). This risk varied according to ST subtype. Other studies, however, namely the population-based study from the Swedish survey of living conditions study of men aged 16–74, found that stroke incidence was not significantly elevated in daily ST users, nor was there was a risk of stroke death in this population (Duffy et al., 2007).
The strongest risks of CVD were found in the US studies, whereas the risk for heart disease and stroke were substantially weaker when results were limited to Swedish studies. If ST use were to increase blood pressure or cholesterol levels, there may be higher risk of circulatory disease death with ST use. However, evidence of a causal association between ST use and circulatory disease is not well supported, as no dose–response has been found between ST use and CVD (Colilla, 2010).
Cancer
ST has 28 known carcinogens, and tobacco-specific N-nitrosamines (TSNA) are among the main carcinogens in ST. The IARC noted a strong probability between the geographical distribution of ST-related diseases and the concentration of carcinogenic compounds in ST products used in these areas (Lyon, 2014). The concentration of TSNA and their probability of increasing the risk of oral cancer depends on many factors, including the plant type, its nicotine content, nitrate and nitrite content, procedures in ST production (curing, fermentation, ageing), storage and PH. These factors together strongly influence the concentration of unprotonated nicotine (Smoke, 2004). The use of ST tobacco causes metabolic activation of TSNA to form covalent bonds with DNA leading to the formation of DNA adducts. This results in accumulation of permanent somatic mutations in critical genes, which is the major established pathway of cancer development.
The metabolic activation of NNN and NNK involves α and methyl hydroxylation, respectively, to form pyridyloxo¬butyl (POB), active intermediate electrophiles agents, which react with DNA nucleophilic sites to form (POB)-DNA (Colilla, 2010). These DNA adducts have miscoding properties and can interfere with the normal processes of replication. The presence of DNA adducts in proto-oncogenes can lead to their activation and the presence of DNA adducts in tumor suppressor genes can lead to their inactivation. Both processes are involved in the transformation of normal cells to neoplastic cells and the eventual development of tumors.
Oral Precancerous Lesions
Nicotine in ST products is absorbed into the blood through the buccal mucosa in the oral cavity. It then reaches the brain where it induces most of its behavioral, psychological, and toxic effects. The ability of nicotine to be absorbed readily into the bloodstream is largely dependent on the PH of the product. Adding an alkaline substance like lime increases the PH and subsequently the level of unprotonated nicotine which has the ability to diffuse through the cell lipoprotein. The type of alkaline substances that are added to ST and the duration of use are the main factors in the development of oral mucosal lesions. The severity of these lesions is strongly related to the frequency and duration of ST use.
Oral lesions are usually found at the site where ST is frequently placed (Axell, 1976). Axéll et al. classified the clinical oral lesions (leukoplakia) associated with ST use into four types (Axell, 1996):
Degree 1: A superficial lesion with a color similar to the surrounding mucosa and with slight wrinkling. No obvious mucosal thickening.
Degree 2: A superficial, whitish or yellowish lesion with wrinkling. No obvious mucosal thickening.
Degree 3: A whitish-yellowish to brown, wrinkling lesion with intervening furrows of normal mucosal color. Obvious mucosal thickening is present.
Degree 4: A marked yellowish to brown and heavy wrinkled lesion with intervening deep reddened furrows and/or heavy thickening. These lesions are generally reversible on quitting ST. A study of 302 ST users from a US army camp reported that 39.4% of these users had oral leukoplakia lesions that were detected 2-6 days after last use. Upon reexamination 6 weeks after last use, 97% of these lesions completely resolved (Hebert, 1999). The danger, however, lies in the possibility of evolution of leukoplakia lesions into abnormal epithelial cell growth (dysplasia). There is evidence that up to 5% of these lesions may develop into cancer (Hebert, 1999).
The prevalence of oral leukoplakia among 661 shammah users in Jazan, South of Saudi Arabia, was 86% (Salem, 1992). The prevalence of leukoplakia and preleukoplakia in Jazan was studied in a population of 16,400 people. The prevalence of oral leukoplakia and preleukoplakia was 11.4% and 4.3%, respectively. The study found a strong association between preleukoplakia and leukoplakia with shammah use, where 99% of affected subjects were shammah users and the oral lesions were localized at the site where shammah was habitually held (Salem, 1984).
Oral Cancer
Oral cancer is the eleventh most common cancer in the world with an estimated 267,000 cases and 128,000 deaths in 2000, two-thirds of which occurred in developing countries (Khan, 2012 and Honarmand, 2013). This percentage rises up to 50% in countries such as India and Southeast Asian countries where about 90% of oral cancer is attributable to tobacco use, alcohol use, and Human Papilloma Virus (HPV) infection. Worldwide, the estimated 5-year prevalence of oral and lip cancer cases in both sexes was 48% in Asia, 24% in Europe, 14% in North America, and the lowest prevalence (2%) was reported in Oceania (Figure 2).
Figure 2.
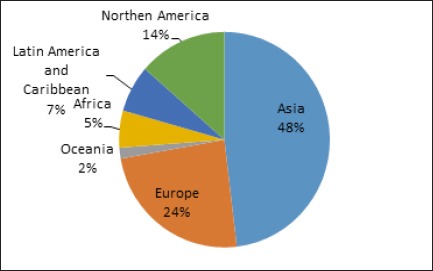
Estimated 5-Year Prevalence of Lip and Mouth Cancer Cases in Both Sexes
In 2008, there were more than 260,000 newly diagnosed lip and oral cavity cancer cases worldwide in both sexes. Numerous studies conducted in different parts of the world concluded that ST use causes oral cancer. Some studies reported that oral cancer lesions occur where the ST product was placed in the mouth during use, supporting a link between ST use and oral cancer. Other studies found a significant positive association between the prevalence of ST use and the rate of oral cancer. Estimated deaths from oral cancer in 2004 total 4,830 men and 2,400 women.
In Saudi Arabia, the statistical report of the Saudi Arabia Cancer Registry showed that cancer of the lip, mouth and tongue in Jazan (south of Saudi Arabia) were ranked first in males (13%) and second after breast cancer in females (21%) among all cancer cases in the region.
Allard et al. reported a relationship between ST product (shamma), frequency of oral cancer, and Jizan province: oral cancer appears to be more common in this province where shamma is also common (Allard, 1999). Esam et al. examined Yemeni patients treated for oral and pharyngeal cancers in Saudi Arabia and found that the relative frequencies of oral and pharyngeal cancers in Yemen are quite high (Halboub, 2011). Their prevalence was largely attributed to the excessive use of Shammah by its residents. Figure 3 shows the prevalence of oral cancer in Saudi Arabia.
Figure 3.
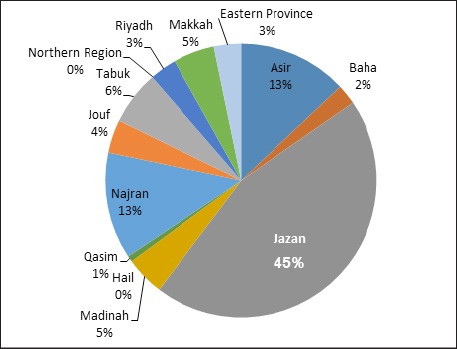
The Prevalence of Oral Cancer in Saudi Arabia
Pancreatic Cancer
A potential association between increased risk of pancreatic cancer and ST use has been reported in several studies. The risk of pancreatic cancer increased by about twofold in ST users compared to non-ST users (Hung, 2005).
Lung Cancer
The risk of lung cancer is low in ST users compared to non-users as expected considering its route of administration. However, some studies reported significant associations between lung cancer and ST use (Acortt et al. 2002; Ezzati et al, 2005). Other study reports have gone even further and assumed that the TSNAs (i.e., NNAL) that exist in ST products are lung cancer-specific carcinogens (Keshet et al., 2006).
Cancer at Other Sites
Several published studies supported a causal role of ST use in the etiology of esophageal cancer despite controlling for tobacco smoking and alcohol use (Anand et al, 2008 and Vioque et al., 2008). Furthermore, in one study from Sweden among users of moist snuff, an increased overall risk of head and neck cancer was not detected. However, an increased risk was observed among a small subgroup of never-smokers (Gartner et al, 2007).
Different Types of ST
ST includes a large variety of commercially or non-commercially available products and mixtures that contain tobacco as the principle constituents and is used either orally or nasally without combustions. ST mixtures differ according to their geographic location of use and the nature of chemicals added to them (Samman et al., 1998). The two main forms of ST are snuff, or dipping tobacco, and chewing tobacco. Snuff consists of dry or moist ground cured tobacco leaves which are placed in the mouth (dipped or sucked) or nasal passages (inhaled). Chewing tobacco is made up of cured tobacco leaves in the form of a plug, twist or loose leaves and is chewed in the mouth.
ST is used in Africa, North America, South-East Asia, Europe and the Middle East. A ’chaw’ is a portion of tobacco the size of a golf ball that is generally chewed whereas a ’quid’ is much smaller in size and is generally sucked, not chewed. Dry snuff and liquid snuff are used nasally in many regions of the world. Betel quid with tobacco, naswar, shammah, and toombak are among the common varieties of oral ST used in the Eastern Mediterranean Region (EMR) of the world. Dry snuff is another variety that is used both orally and nasally in the EMR. Table 2 shows the different varieties of ST products used worldwide and their method of use.
Table 2.
ST Products Used Worldwide and Their Method of Use*
| Mode of use | Tobacco product | WHO Region | ||||||
|---|---|---|---|---|---|---|---|---|
| AFRO | AMRO | EMRO | EURO | SEARO | WPRO | |||
| Oral use | Sucking | Chimo | X | |||||
| Dry snuff | X | X | X | |||||
| Gutka | X | |||||||
| Khaini | X | |||||||
| Looose-leaf | X | X | ||||||
| Maras | X | |||||||
| Mishri | X | |||||||
| Moist snuff | X | X | ||||||
| Naswar | X | X | X | |||||
| Shammah | X | X | ||||||
| Snus | ||||||||
| Tobacco tablet | X | |||||||
| Toombak | X | |||||||
| Chewing | Betel quid | X | ||||||
| Gutka | X | |||||||
| Iq`mik | X | |||||||
| Khaini | X | |||||||
| Khiwam | X | |||||||
| Loose-leaf | X | X | ||||||
| Mawa | X | |||||||
| Plug chewing tobacco | X | |||||||
| Tobacco chewing gum | X | |||||||
| Twist or roll | X | |||||||
| Zarda | X | X | ||||||
| Other oral use | Creamy stuff | X | ||||||
| Gudhaku | X | |||||||
| Gul | X | |||||||
| Mishri | X | |||||||
| Red tooth powder | X | |||||||
| Tuibur | X | |||||||
| Nasal use | Dry snuff | X | X | X | X | |||
| Liquid snuff | X | |||||||
This table is adapted from IARC MONOGRAPHS (IARC, 2008)
More recently, new spitless forms of oral ST products are available which eliminate the need to spit, such as lozenges or small pouches. Mint or fruit flavors have been added to them to make them more attractive and to improve taste.
Shammah
Shammah is a snuff-dipping form of ST prepared by mixing powdered tobacco, calcium oxide, ash, black pepper, oils, and flavors (Scheifele et al., 2007). This form of ST is obtainable in various varieties and is grouped according to color and composition. Some forms of shammah are used as white, black, and gray powder (Agili and Park, 2012). The gray powder is locally known as toombak and is the most frequently used form in South Yemen. Toombak is composed of powdered tobacco leaf and ash.
Shammah Use and Oral Health
A case report study found that shammah use was linked to a leukoplakia-like lesion in a 73-year-old male patient from Algeria. The lesion was located in the anterior mandibular vestibule and lower lip (Bethke and Reichart, 2004). Another case report study described a shammah-induced oral leukoplakia-like lesion in a 44-year-old patient who used shammah for 33 years. Follow-up of the patient for 2 years showed that whenever the patient changed the location of application, the white lesion regressed or disappeared within 4-6 weeks. The study authors indicated that the mucosal burn was due to the composition of shammah (Zhang et al., 2004).
A survey of oral mucosal lesions in various locations in the Jazan region showed that mucosal lesions compatible with oral leukoplakia were found in 68% of 661 users of shammah. The lesions were almost always found at the sites where shammah was held in the mouth. The results of the study pointed to a possible causal relationship between the use of shammah and the development of oral premalignant and malignant lesions among shammah users in Jazan (Scheifele et al., 2007). Data from a study of an in vitro test of genotoxicity of shammah indicated that a direct-acting mutagen was present in a chloroform extract of shammah (Hannan et al., 1986).
A review of the Tumor Registry data from King Faisal Hospital and Research Center in Riyadh over a 20-year period revealed that 35.4% of oral cancer cases were referred from Jazan. The authors suggested a relationship between shammah use, frequency of oral cancer, and the Jazan province (Halboub et al., 2011). Without any doubt, the ability of ST to cause head, neck, and throat cancers is due to the carcinogens it contains (Hatsukami and Severson, 1999).
The literature review reveals that the quality and findings of available studies investigating links between the risk of oral cancer and the use of ST are variable. However, published results do support the need for well-controlled studies which include clearly defined measures to diagnose oral cancer patients and to categorize them as users of ST, and which include a dependable way to obtain information from subjects and an understandable method to match cases and controls or the use of statistical methods to regulate some important variables like age, cigarette smoking, and alcohol consumption.
The sites in which shammah is placed inside the oral cavity have been reported as being the buccal cavity, the vestibule of the mouth, the gingivobuccal sulcus, and the lower labial or buccal vestibules. The use of ST in KSA is most frequently observed in Jazan, although it is not restricted to this province. Shammah is also used in Yemen, which borders Jazan. The oral use of ST in Jazan has been reported to begin as young as 10 to 13 years of age. It is also reported to be used with infants to reduce pain caused by erupting teeth. The real percentage may be higher and there may be some unwillingness to disclose information about its use (Halboub et al., 2011).
Periodontal disease has emerged as a major public health problem and is among the most prevalent chronic diseases leading to tooth loss (Petersen and Ogawa, 2000). Data reporting the risk factors linked with periodontal disease in Yemen show a high occurrence of the disease among users of shammah. The occurrence of periodontitis Saudi Arabia is comparable with a study conducted in China (25.9 %) (Zhang et al., 2014). However, a higher prevalence of periodontitis (44.69 %) has been recorded in Libya (Peeran et al., 2013), but a lower prevalence (15.8 %) was reported among adults in India (Kumar et al., 2009). Multiple logistic regression analysis revealed that shammah use is a factor that effects the development of periodontal pockets. Current shammah users are at more risk of developing periodontal pockets than former and non-shammah users. The onset of periodontal pockets varies considerably between current and former shammah users. Thus, we conclude that the previous and current use of shammah plays an analogous role in the disappearance of periodontal disease once the shammah use is stopped. Shammah users report a significant association between periodontal disease and tobacco use (James et al., 1999 and Mohammed et al., 2013). Kumar et al., (2008) examined 513 Indian dentate adult males to determine the effect of tobacco use on the severity of periodontal disease and showed that ST users are more likely to develop periodontal disease than smokers. By contrast, a study carried out in Saudi Arabia revealed that shammah is not a significant risk indicator in the development of periodontal disease. Such opposite observations may be ascribed to several factors such as differences in the trends of oral ST practices and in the type of ST products used by the respective populations.
This review paper is an initial step to understand the pattern of use of shammah and its harmful effects on health. It provides adequate epidemiological data of value to health authorities.
References
- Accortt NA, Waterbor JW, Beall C, Howard G. Chronic disease mortality in a cohort of smokeless tobacco users. Am J Epidemiol. 2002;156:730–7. doi: 10.1093/aje/kwf106. [DOI] [PubMed] [Google Scholar]
- Al Agili D, Park H. Oral health status of male adolescent smokeless tobacco users in Saudi Arabia. East Mediterr Health J. 2013;19:711, 9. [PubMed] [Google Scholar]
- Al Agili DE, Park HK. The prevalence and determinants of tobacco use among adolescents in Saudi Arabia. J Sch Health. 2012;82:131–8. doi: 10.1111/j.1746-1561.2011.00676.x. [DOI] [PubMed] [Google Scholar]
- Allard WF, DeVol EB, Te OB. Smokeless tobacco (shamma) and oral cancer in Saudi Arabia. Community Dent Oral Epidemiol. 1999;27:398–405. doi: 10.1111/j.1600-0528.1999.tb02038.x. [DOI] [PubMed] [Google Scholar]
- Alsanosy RM. Smokeless tobacco (shammah) in Saudi Arabia:a review of its pattern of use, prevalence, and potential role in oral cancer. Asian Pac J Cancer Prev. 2013;15:6477–83. doi: 10.7314/apjcp.2014.15.16.6477. [DOI] [PubMed] [Google Scholar]
- Anand P, Kunnumakara AB, Sundaram C, et al. Cancer is a preventable disease that requires major lifestyle changes. Pharm Res. 2008;25:2097–116. doi: 10.1007/s11095-008-9661-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axéll T, Mörnstad H, Sundström B. The relation of the clinical picture to the histopathology of snuff dipper’s lesions in a Swedish population. J Oral Pathol Med. 1976;5:229–36. [PubMed] [Google Scholar]
- Axéll T, Pindborg JJ, Smith CJ, et al. Oral white lesions with special reference to precancerous and tobacco-related lesions:conclusions of an international symposium held in Uppsala, Sweden, May 18-21 1994. International collaborative group on oral white lesions. J Oral Pathol Med. 1996;25:49–54. doi: 10.1111/j.1600-0714.1996.tb00191.x. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Hukkanen J, Jacob P., III Nicotine chemistry, metabolism, kinetics and biomarkers. InNicotine psychopharmacology. 2009;10:29–60. doi: 10.1007/978-3-540-69248-5_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethke G, Reichart PA. Shammah-associated oral leukoplakia-like lesions. Mund Kiefer Gesichtschir. 2004;8:261–3. doi: 10.1007/s10006-004-0553-9. [DOI] [PubMed] [Google Scholar]
- Bhawna G. Burden of smoked and smokeless tobacco consumption in India-results from the global adult tobacco survey India (GATS-India)-2009-2010. Asian Pac J Cancer Prev. 2013;14:3323–9. doi: 10.7314/apjcp.2013.14.5.3323. [DOI] [PubMed] [Google Scholar]
- Centers for disease control and prevention. Percentage of adults who currently use smokeless tobacco. Global Tobacco Surveillance System data [Internet database]. Atlanta:U.S. Department of Health and Human Services, Centers for Disease Control and Prevention. Available from:Centers for disease control and prevention. Global adult tobacco survey 2008–2010. Percentage of adults who currently use smokeless tobacco. Global Tobacco Surveillance System data [Internet database] Atlanta: Centers for Disease Control and Prevention; [[no date] [cited 2012 Jan 25], [no date] [cited 2012 Jan 25]]. Global Adult Tobacco Survey 2008–2010. Available from: http://apps.nccd.cdc.gov/GTSSData/default/IndicatorResults.aspx? [Google Scholar]
- Christen AG, Swanson BZ, Glover ED, Henderson AH. Smokeless tobacco:The folklore and social history of snuffing, sneezing, dipping, and chewing. J Am dent Assoc. 1982;105:821, 9. doi: 10.14219/jada.archive.1982.0453. [DOI] [PubMed] [Google Scholar]
- Colilla SA. An epidemiologic review of smokeless tobacco health effects and harm reduction potential. Regul Toxicol Pharmacol. 2010;56:197–211. doi: 10.1016/j.yrtph.2009.09.017. [DOI] [PubMed] [Google Scholar]
- Duffy MJ, Van Dalen A, Haglund C, et al. Tumour markers in colorectal cancer:European group on tumour markers (EGTM) guidelines for clinical use. Eur J Cancer. 2007;43:1348–60. doi: 10.1016/j.ejca.2007.03.021. [DOI] [PubMed] [Google Scholar]
- England LJ, Kim SY, Tomar SL, et al. Non-cigarette tobacco uses among women and adverse pregnancy outcomes. Acta Obstet Gynecol Scand. 2010;89:454–64. doi: 10.3109/00016341003605719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezzati M, Henley SJ, Thun MJ, Lopez AD. Role of smoking in global and regional cardiovascular mortality. Circulation. 2005;112:489–97. doi: 10.1161/CIRCULATIONAHA.104.521708. [DOI] [PubMed] [Google Scholar]
- Gartner CE, Hall WD, Vos T, et al. Assessment of Swedish snus for tobacco harm reduction:an epidemiological modelling study. Lancet. 2007;369:2010, 4. doi: 10.1016/S0140-6736(07)60677-1. [DOI] [PubMed] [Google Scholar]
- Goodman Jordan. Tobacco in history and culture:An Encyclopedia (Detroit:Thomson Gale) Granite Hill Publishers. 2005 [Google Scholar]
- Hajeb R. Prevalence of oral mucosal lesions and related risk habits in outpatient dental clinics in Malaysia and Yemen 2010. 2015. [Accessed 22 Feb 2015]. http://studentsrepo.um.edu.my/3670/2/SECOND_PART._DR._RAJI_MANSOOR._DISSERTATION.pdf .
- Halboub ES, Al-Anazi YM, Al-Mohaya MA. Characterization of Yemeni patients treated for oral and pharyngeal cancers in Saudi Arabia. Saudi Med J. 2011;32:1177–82. [PubMed] [Google Scholar]
- Hannan MA, El-Yazigi A, Paul M, Gibson DP, Phillips RL. Genotoxicity of ’shamma’, a chewing material suspected of causing oral cancer in Saudi Arabia. Mutat Res Genet Toxicol Environ Mutagen. 1986;169:41–6. doi: 10.1016/0165-1218(86)90016-9. [DOI] [PubMed] [Google Scholar]
- Hatsukami DK, Severson HH. Oral spit tobacco:addiction, prevention and treatment. Nicotine Tob Res. 1999;1:21–44. doi: 10.1080/14622299050011131. [DOI] [PubMed] [Google Scholar]
- Hébert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. N Engl J Med. 1999;340:409–17. doi: 10.1056/NEJM199902113400601. [DOI] [PubMed] [Google Scholar]
- Honarmand M, Farhadmollashahi L, Bekyghasemi M. Use of smokeless tobacco among male students of Zahedan universities in Iran:a cross sectional study. Asian Pac J Cancer Prev. 2013;14:6385–8. doi: 10.7314/apjcp.2013.14.11.6385. [DOI] [PubMed] [Google Scholar]
- http://apps.nccd.cdc.gov/GTSSData/default/IndicatorResults.aspx?
- Hung RJ, Hall J, Brennan P, Boffetta P. Genetic polymorphisms in the base excision repair pathway and cancer risk:A HuGE review. Am J Epidemiol. 2005;162:925–42. doi: 10.1093/aje/kwi318. [DOI] [PubMed] [Google Scholar]
- Smokeless Tobacco and Some Tobacco-specific N-Nitrosamines. 2008:89. [PMC free article] [PubMed] [Google Scholar]
- James JA, Sayers NM, Drucker DB, Hull PS. Effects of tobacco products on the attachment and growth of periodontal ligament fibroblasts. J Periodontol. 1999;70:518, 25. doi: 10.1902/jop.1999.70.5.518. [DOI] [PubMed] [Google Scholar]
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- Keshet I, Schlesinger Y, Farkash S, et al. Evidence for an instructive mechanism of de novo methylation in cancer cells. Nature Genet. 2006;38:49–53. doi: 10.1038/ng1719. [DOI] [PubMed] [Google Scholar]
- Khan ZU. An overview of oral cancer in Indian subcontinent and recommendations to decrease its Incidence. Webmed Centarl Cancer. 2012;3:WMC003626. [Google Scholar]
- Kumar S, Dagli RJ, Chandrakant D, Prabu D, Suhas K. Periodontal status of green marble mine labourers in Kesariyaji, Rajasthan. India Oral Health Prev Dent. 2008;6:217, 21. [PubMed] [Google Scholar]
- Kumar S, Dagli RJ, Dhanni C, Duraiswamy P. Relationship of body mass index with periodontal health status of green marble mine laborers in Kesariyaji. India Braz Oral Res. 2009;23:365, 9. doi: 10.1590/s1806-83242009000400003. [DOI] [PubMed] [Google Scholar]
- Mishra A, Meherotra R. Head and neck cancer:global burden and regional trends in India. Asian Pac J Cancer Prev. 2013;15:537–50. doi: 10.7314/apjcp.2014.15.2.537. [DOI] [PubMed] [Google Scholar]
- Mohamed S, Janakiram C. Periodontal status among tobacco users in Karnataka. India Indian J Public Health. 2013;57:105–8. doi: 10.4103/0019-557X.115006. [DOI] [PubMed] [Google Scholar]
- Moradi-Lakeh M, El Bcheraoui C, Tuffaha M, et al. Tobacco consumption in the Kingdom of Saudi Arabia 2013:findings from a national survey. BMC Public Health. 2015;15:1. doi: 10.1186/s12889-015-1902-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeran SW, Singh AR, Alagamuthu G, Kumar PN. Periodontal status and its risk factors among young adults of the Sebha city (Libya) Dent Res J (Isfahan) 2013;10:533, 8. [PMC free article] [PubMed] [Google Scholar]
- Petersen PE, Ogawa H. The global burden of periodontal disease:towards integration with chronic disease prevention and control. Periodontol. 2000;60:15–39. doi: 10.1111/j.1600-0757.2011.00425.x. [DOI] [PubMed] [Google Scholar]
- Piano MR, Benowitz NL, FitzGerald GA, et al. American heart association council on cardiovascular nursing. Impact of smokeless tobacco products on cardiovascular disease:implications for policy, prevention, and treatment a policy statement from the American Heart Association. Circulation. 2010;122:1520–44. doi: 10.1161/CIR.0b013e3181f432c3. [DOI] [PubMed] [Google Scholar]
- Raute LJ, Sansone G, Pednekar MS, et al. Knowledge of health effects and intentions to quit among smokeless tobacco users in India:Findings from the international tobacco control policy evaluation (ITC) India pilot survey. Asian Pac J Cancer Prev. 2011;12:1233–8. [PubMed] [Google Scholar]
- Salem G. Leukoplakia and tobacco habits in Jazan, Saudi Arabia. Saudi Dent J. 1992;4:50–4. [Google Scholar]
- Salem G, Juhl R, Schiodt T. Oral malignant and premalignant changes in ‘Shammah’-users from the Jazan region, Saudi Arabia. Acta Odontologica. 1984;42:41–5. doi: 10.3109/00016358409041130. [DOI] [PubMed] [Google Scholar]
- Samman MA, Bowen ID, Taiba K, Antonius J, Hannan MA. Mint prevents shamma induced carcinogenesis in hamster cheek pouch. Carcinogenesis. 1998;19:1795, 801. doi: 10.1093/carcin/19.10.1795. [DOI] [PubMed] [Google Scholar]
- Savitz DA, Meyer RE, Tanzer JM, Mirvish SS, Lewin F. Public health implications of smokeless tobacco use as a harm reduction strategy. Am J Public Health. 2006;96:1934–9. doi: 10.2105/AJPH.2005.075499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheifele C, Nassar A, Reichart P. Prevalence of oral cancer and potentially malignant lesions among shammah users in Yemen. Oral Oncol. 2007;43:42, 50. doi: 10.1016/j.oraloncology.2005.12.028. [DOI] [PubMed] [Google Scholar]
- Smoke T, Smoking I. IARC monographs on the evaluation of carcinogenic risks to humans. Lyon: IARC; 2004. pp. 1–452. [Google Scholar]
- Stewart GG. A history of the medicinal use of tobacco 1492-1860. Medical History. 1967;11:228. doi: 10.1017/s0025727300012333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US department of health and human services. The health consequences of smoking:a report of the Surgeon General. Atlanta, GA: US department of health and human services, Centers for disease control and prevention, National center for chronic disease prevention and health promotion, Office on smoking and health; 2004. p. 62. [Google Scholar]
- Vioque J, Barber X, Bolumar F, et al. Esophageal cancer risk by type of alcohol drinking and smoking:a case-control study in Spain. BMC Cancer. 2008;8:1. doi: 10.1186/1471-2407-8-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Yang X, Liang J, et al. Cigarette smoking has a positive and independent effect on testosterone levels. Hormones. 2013;12:567–77. doi: 10.14310/horm.2002.1445. [DOI] [PubMed] [Google Scholar]
- World Health Organization. WHO report on the global tobacco epidemic 2015:the MPOWER package. 2005 [Google Scholar]
- World Health Organization. Global adult tobacco survey (GATS):Egypt country report. Cairo: World Health Organization, Regional Office of the Eastern Mediterranean 2010; 2009. Available from: http://www.who.int/tobacco/surveillance/gats_rep_egypt.pdf . [Google Scholar]
- World Health Organization. WHO report on the global tobacco epidemic, 2011. Appendix VIII—Table 8.2:Crude smokeless tobacco prevalence in WHO member states. Geneva:World Health Organization. 2011. Available from: http://www.who.int/tobacco/global_report/2011/en_tfi_global_report_2011_appendix_VIII_table_2.pdf .
- World Health Organization. WHO report on the global tobacco epidemic, 2011. Appendix VIII—Table 8.2:crude smokeless tobacco prevalence in WHO member states. Geneva: World Health Organization; 2011. Available from: http://www.who.int/tobacco/global_report/2011/en_tfi_global_report_2011_appendix_VIII_table_2.pdf . [Google Scholar]
- Zhang Q, Li Z, Wang C, et al. Prevalence and predictors for periodontitis among adults in China 2010. Glob Health Action. 2014;7:24503. doi: 10.3402/gha.v7.24503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Schmitz W, Gelderblom HR, Reichart PA. Shammah-induced oral leukoplakia-like lesions. Oral Oncol. 2001;37:609–12. doi: 10.1016/s1368-8375(00)00123-8. [DOI] [PubMed] [Google Scholar]


