Abstract
Background:
Salivary gland tumors are rare head and neck tumors with lymphoepithelial carcinoma (LEC) as a particularly infrequent variant. This study was an evaluation of the incidence of EBV infection in malignant salivary gland tumors with the emphasis on tumor type and geographical area.
Methods:
Five databases (PubMed, ScienceDirect, Scopus, Web of Science and Cochrane library) were searched for data on the prevalence of EBV in malignant salivary gland tumors. A random-effects meta-analysis was conducted with Comprehensive Meta-Analysis software version 2.0 (CMA 2.0) using the event rate (ER) for estimation of the incidence of EBV in the salivary gland tumor patients. Publication bias was lacking as assessed through funnel plot analysis with the Begg’s and Egger’s tests (P>0.05).
Results:
Out of 618 studies searched in databases, 19 reported the prevalence of EBV in malignant salivary gland tumors and were included in the present meta-analysis. The pooled ER of all studies was 44% [95%CI=21.5-69.2%] with extreme heterogeneity that for the studies in America was 44.2% [95%CI=4.1-93.6%], in Asia (249 patients) was 70% [95%CI= 33.4-91.6%] and in Europe was 11.8% [95%CI=7.4-85.5%] with extreme heterogeneity for three subgroups. The pooled ER for patients with undifferentiated carcinoma was 86.7% [95%CI=71.5-94.4%] compared with 6.6% [95%CI=2.5-16.5%] for other carcinomas.
Conclusions:
The incidence of EBV infection in malignant salivary gland tumors in Asia was greater than in Europe and America and the higher presence of EBV infection in LEC cases implies that EBV may be a major factor in its etiology or pathogenesis. Genetic, environmental and other geographic factors may also be involved.
Keywords: Salivary gland, malignant tumor, Epstein-Barr virus
Introduction
Benign and malignant salivary gland tumors belong to rare head and neck tumors, which most of them are benign and only 20% are malignant (To et al., 2012). The incidence of these tumors is more in men and the most common location of them is the parotid gland (Rezaei et al., 2016). Lymphoepithelial carcinoma (LEC) or the preferred term, lymphoepithelioma-like carcinoma is a rare malignancy (Terada, 2013; Schneider and Rizzardi; 2008). It occurs mainly in East Asia population and only rarely in western countries (Terada, 2013), accounting for less than 1% of all salivary gland tumors (Schneider and Rizzardi;2008). Morphological features are similar to undifferentiated nasopharyngeal carcinoma and most of the cases have been reported in South China and Eskimos (Iezzoni et al.,1995). Epithelial malignancies of the head and neck region such as undifferentiated nasopharyngeal carcinoma and LELC of the salivary gland have been linked to EBV infection (Iezzoni et al.,1995). Epstein-Barr virus (EBV) is detected by EBER in-situ hybridization (ISH) and by polymerase chain reaction (PCR) to detect latent membrane protein-1 (LMP-1) gene with formalin-fixed, paraffin-embedded tissues (Kuo and Tsang, 2001). The aim of this study was to assess the incidence of EBV infection in malignant salivary gland tumors with emphasis on tumor type and geographical area.
Materials and Methods
Search strategies and Study criteria
The studies were searched in five databases (PubMed, ScienceDirect, Scopus, Web of Science and Cochrane library) from 1980 to 2016 for publications with English abstract using the keywords Epstein Barr virus or Epstein-Barr virus or EBV and salivary gland and tumor or carcinoma.
Study selection
One author (M.S) searched the articles and then the second author (M.R) blinded to the first reviewer. If there was any disagreement between two reviewers, third reviewer (H.R.M) resolved the problem. All studies were searched for evaluation of the prevalence of EBV in salivary gland tumors. The inclusion criteria for the studies selected were as follows: I) studies reporting the prevalence of EBV based on ISH or PCR; II) studies including only malignant salivary gland; III) studies reporting only the prevalence of EBV in salivary gland; IV) only studies with English-language abstract could be included; The exclusion criteria: I) reporting both malignant and non-malignant salivary gland; II) reporting the prevalence of EBV in salivary gland and other oral areas; III) data from case reports, incomplete reports (not sufficient information), and letters were not eligible for this study.
Data Extraction
The name of author, year of publication, country of region, number of patients, tumor type, method of viral detection and number of patients with EBV infection were the relevant data extracted from every study.
Statistical analysis
A random-effects meta-analysis was used by Comprehensive Meta-Analysis software version 2.0 (CMA 2.0). The event rate (ER) of the studies was calculated for estimation of the incidence of EBV in the salivary gland tumor patients. Heterogeneity between estimates was assessed by the Q and I2 statistic that for the Q statistic, heterogeneity was considered for P<0.1. Confidence interval (CI) was 95% and 2-sided p-value<0.05 was considered to be statistically significant in this meta-analysis study. The I2 statistic yields results ranging from zero to 100% (I2: 0 to 25%, no heterogeneity; I2: 25 to 50%, moderate heterogeneity; I2: 50 to75%, large heterogeneity; I2: 75 to 100%, extreme heterogeneity) (Egger et al.,1997). Also, the publication bias was assessed through funnel plot analysis with the Begg’s and Egger’s tests.
Results
Out of 618 studies searched in databases, 49 studies were evaluated for eligibility. Of 49 studies, three studies reported polymorphisms of EBV, eighteen were case report study, four reported benign tumors, one didn’t report the number of patients, two studies didn’t have sufficient information and two were the letter to editor study that excluded from the study (Figure 1). Therefore, 19 studies reported the prevalence of EBV in malignant salivary gland tumors and included in the meta-analysis study (Table 1).
Figure 1.
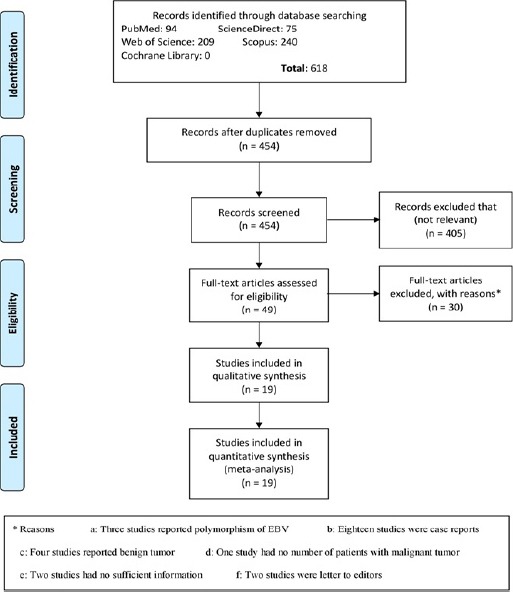
The Flow Chart of Study
Table 1.
The Characteristics of Studies in Meta- Analysis (n=19)
| Study (year) | Country | Number of patients (N) | Tumor type of salivary gland | Method of detection of EBV | Number of EBV+ patients (N) |
|---|---|---|---|---|---|
| Hamilton-dutoit (1991) | Denmark | 13 | Lymphoepithelial carcinomas | ISH | 11 |
| Lanier (1991) | Alaska | 6 | Lymphoepithelial carcinoma | ISH | 6 |
| Taira (1992) | Japan | 5 | Mucoepidermoid carcinomas | PCR | 3 |
| Gallo (1994) | Italy | 7 | Undifferentiated carcinomas | ISH | 3 |
| Leung (1995) | China | 10 | Lymphoepithelial carcinomas | ISH | 10 |
| Nagao (1996) | Japan | 5 | Undifferentiated carcinoma with lymphoid stroma | ISH | 5 |
| Tsai (1996) | Taiwan | 56 | Mucoepidermoid carcinomas: 14; Adenoid cystic carcinomas: 13; Malignant mixed tumors: 7; Adenocarcinomas: 4; Salivary duct carcinomas: 4; Acinic cell carcinomas: 2; Undifferentiated carcinomas without lymphoid stroma: 2; Lymphoepithelioma-like carcinomas: 7; Squamous cell carcinomas: 2; Small cell carcinoma: 1 | ISH | 7 |
| Wen (1997) | Japan | 80 | Undifferentiated carcinomas: 6; T-cell lymphomas: 3; B-cell lymphomas: 3; Mucoepidermoid carcinomas: 15; Adenoid cystic carcinomas: 20; Acinic cell carcinomas: 15; Carcinomas in pleomorphic adenoma: 8; Adenocarcinoma: 5; Squamous cell carcinomas: 3; Papilloadenocarcinomas: 2 | ISH | 9 |
| Lin (1997) | China | 18 | Lymphoepithelial carcinomas | PCR/ISH* | 18 |
| Wolvius (1997) | Netherlands | 18 | B-cell lymphoma | ISH | 0 |
| Ioachim (1998) | USA | 6 | B-cell lymphoma | ISH | 3 |
| Atula (1998) | Finland | 19 | Acinic cell carcinomas: 4; Lymphomas: 4; Squamous cell carcinomas: 3; Mucoepidermoid carcinomas: 3; Adenocarcinomas: 2; Adenoid cystic carcinoma:1; Epithelial-myoepithelial carcinoma:1; Myoepithelial carcinoma: 1 | PCR/ISH* | 1/0 |
| Kim (1999) | Korea | 28 | Mucoepidermoid carcinoma: 13; Adenoid cystic carcinoma: 8; Malignant mixed tumor: 2; Acinic cell carcinoma: 2; Cystadenocarcinoma: 1; Poorly differentiated carcinoma: 1; Lymphoepithelial carcinoma: 1 | ISH | 1 |
| Pollock (1999) | Ireland | 13 | Mucoepidermoid carcinoma: 2; Salivary duct carcinoma: 2; Non-Hodgkin’s lymphoma: 2; Acinic cell carcinoma: 2; Adenocarcinoma arising in PSA: 2; Polymorphous low grade adenocarcinoma: 1; Undifferentiated carcinoma: 1; Epithelial-myoepithelial carcinoma: 1 | ISH | 0 |
| Venkateswaran (2000) | USA | 13 | Mucoepidermoid carcinoma: 7; Rhabdomyosarcoma: 3; Acinic cell carcinoma: 2; Malignant fibrous histiocytoma: 1 | ISH | 0 |
| Wu (2004a) | China | 14 | Lymphoepithelial carcinomas | PCR/ISH* | 12/14 |
| Wu (2004b) | China | 16 | lymphoepithelial carcinomas | ISH | 16 |
| Zhao (2014) | China | 21 | Primary lymphoepithelioma-like carcinomas | ISH | 21 |
| Hühns (2015) | Germany | 93 | Mucoepidermoid carcinoma: 17; Adenoid cystic carcinoma: 16; Adenocarcinoma NOS: 10; Salivary duct carcinoma: 9; Acinus cell carcinoma: 7; Adenoid basal-cell carcinoma: 5; Squamous cell carcinoma: 5; Nonkeratinized squamous cell carcinoma: 4; Keratinized squamous cell carcinoma: 4; Oncocytic carcinoma: 2; Lymphoepithelial carcinoma: 2; Micropapillary carcinoma: 2; Myoepithelial carcinoma: 5; Pseudo sarcomatoid carcinoma: 1; Polymorphic low-grade carcinoma: 1; Undifferentiated carcinoma: 1; Cystadenocarcinoma: 1; Malignant melanoma: 1 | ISH | 1 |
ISH, in situ hybridization; NOS, not otherwise specified; PCR, polymerase chain reaction.
in two methods, we used the number of the most detection of EBV in meta-analysis.
Study characteristics
The characteristics of 19 studies included in meta-analysis have been shown in Table 1. All studies were published from 1991 to 2015. One study was done in Denmark (Hamilton-Dutoit et al.,1991), one study in Alaska (Lanier et al.,1991), three studies in Japan (Taira et al.,1992; Nagao et al.,1996; Wen et al.,1997), one study in Italy (Gallo et al.,1994), five studies in China (Leung et al.,1995; Lin et al.,1997; Wu et al.,2004a; Wu et al.,2004b; Zhao et al.,2014), one study in Taiwan (Tsai et al.,1996), one study in Netherlands (Wolvius et al., 1997), two studies in the USA (Ioachim et al., 1998; Venkateswaran et al.,2000), one study in Finland (Atula et al., 1998), one study in Korea (Kim et al., 1999), one study in Ireland (Pollock et al.,1999) and one study in Germany (Hühns et al., 2015). Four-hundred and forty-one patients were included in the meta-analysis that 127 (28.8%) patients had EBV positivity. The tumor type and method of detection of EBV have been shown in Table 1. One study (Wen et al.,1997) was short communication that had sufficient information and therefore we selected it for meta-analysis study.
EBV infection and all malignant tumors
The incidence of EBV in malignant tumors of salivary gland has been reported in Figure 1 by the ER. Pooled ER of the studies (441 patients) was 44% [95%CI=21.5-69.2%] with extreme heterogeneity [I2=85.53%; P<0.001].
EBV infection and geographical area
The incidence of EBV in malignant tumors of salivary gland based on geographical areas has been shown in Figure 2. Three studies were in America (Lanier et al., 1991; Ioachim et al., 1998; Venkateswaran et al., 2000), ten studies in Asia (Taira et al.,1992; Nagao et al.,1996; Wen et al.,1997; Leung et al., 1995; Lin et al., 1997; Wu et al., 2004a; Wu et al., 2004b; Zhao et al., 2014; Tsai et al., 1996; Kim et al., 1999) and six studies in Europe (Hamilton-Dutoit et al., 1991; Gallo et al.,1994; Wolvius et al., 1997; Atula et al., 1998; Pollock et al., 1999; Hühns et al., 2015). Pooled ER of the articles for the incidence of EBV in malignant tumors of the salivary gland in America (25 patients) was 44.2% [95%CI=4.1-93.6%] with extreme heterogeneity [I2=75.69%; P=0.016], in Asia (249 patients) was 70% [95%CI= 33.4-91.6%] with extreme heterogeneity [I2=88.70%; P<0.001] and in Europe (167 patients) was 11.8% [95%CI=7.4-85.5%] with extreme heterogeneity [I2=85.39%; P<0.001].
Figure 2.

Forest Plot of the Incidence of EBV in Malignant Tumors of Salivary Gland
EBV infection and tumor type
Figure 4 shows the incidence of EBV in malignant tumors of salivary gland based on tumor type (LEC/LELC or undifferentiated carcinoma versus other carcinomas). Pooled ER for the patients with undifferentiated carcinoma (128 patients) was 86.7% [95%CI=71.5-94.4%] with moderate heterogeneity [I2=43.40%; P=0.054] and for other carcinomas (313 patients) was 6.6% [95%CI=2.5-16.5%] with large heterogeneity [I2=68.33%; P=0.001].
Figure 3.

Forest Plot of the Incidence of EBV in Malignant Tumors of Salivary Gland Based on Geographical Areas
Figure 4.
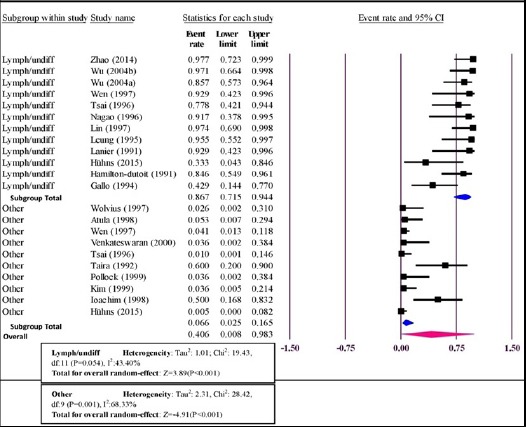
Forest Plot of the Incidence of EBV in Malignant Tumors of Salivary Gland Based on Tumor Type (Lymphoepithelial (-like) or Undifferentiated Carcinomas Versus other Carcinomas (Lymph/undiff versus other))
Publication bias
The funnel plot analysis of random effect of the studies in meta-analysis has been shown in Figure 5. The Begg’s and Egger’s tests didn’t show publication bias (P=0.327 and P=0.068, respectively).
Figure 5.
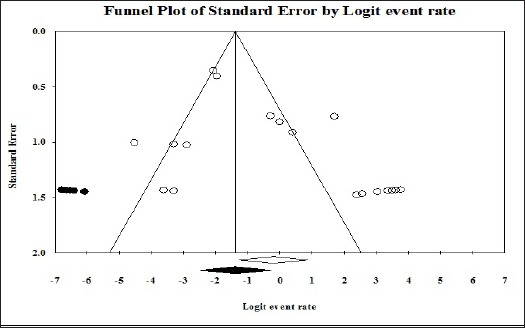
Funnel Plot of Random Effect of the Studies for the Incidence of EBV in Malignant Tumors of the Salivary Gland
Discussion
This study reported that the incidence of EBV infection in malignant tumors of salivary gland was 45.1% that this incidence in American patients was 44.2%, Asian patients 70% and European patients 11.8%. Also, this incidence in the patients with undifferentiated carcinomas was 86.7% compared with 6.6% for other carcinomas. Because nasopharyngeal lymphoepithelioma has been demonstrated to be strongly associated with EBV infection, the role of EBV in LELC of salivary glands has also been investigated (Zhao et al., 2014). EBV has been studied as an etiologic agent in adult salivary gland tumors (Venkateswaran et al., 2000). The oncogenic role of EBV is elicited by its products that among these, LMP-1 has a driving role (Lu et al., 2006). The expression of this protein was observed only in few EBV-linked salivary LELC from Asian patients in some studies. We know that different strains of EBV could be involved in the pathogenesis of salivary LELC in different geographic areas (Jen et al., 2003). Ampinder et al., (1994) indicated that EBV does not have a role in most of the tumor types, but the PCR method showed that EBV is strongly associated with lymphomas. Also, Hühns et al., (2015) indicated that infection with EBV does not play a major role in salivary gland neoplasm. One study in China (Wu et al., 2004a), showed that EBV plays an important role in the pathogenesis of LEC of salivary glands in Sichuan Chinese. The EBV has been studied as an etiologic agent in adult salivary gland tumors and was demonstrated that EBV infection in LELC of the salivary gland in certain ethnic groups with a striking racial and geographic predilection affecting the Greenland Eskimos, Alaskan natives, and Asian patients (Hamilton-Dutoit et al., 1991; Nagao et al., 1996; Sheen et al., 1997). The EBV infection does not appear to play a major role in the pathogenesis of pediatric salivary gland tumors (Venkateswaran et al., 2000). The results of two studies reported that that EBV infection could have some relationship with the genesis of malignant lymphoepithelial lesions (Lanier et al., 1991; Lin et al., 1997). Two studies (Ioachim et al., 1998; Nadal et al., 1994) reported that non-Hodgkin lymphoma and precursor lymphoproliferative lesions of the salivary glands in immunocompromised individuals also have a strong association with EBV infection. Therefore, the racial/genetic, environmental or geographic factors may impact on the incidence of EBV in malignant salivary tumors, especially undifferentiated carcinomas.
Limitations
1) The number of patients in most studies was low. 2) The method of detection of EBV was different in the studies. 3) The tumor type and number of patients for each tumor type were different. 4) The heterogeneity between the studies was high.
In conclusions, the incidence of EBV infection in Asian patients with malignant salivary gland tumors was more than European and American patients and the higher presence of EBV infection in LEC cases implies that EBV may be a major factor in the etiology or pathogenesis of LEC and also genetic, environmental or geographic factors may be involved in the etiology or pathogenesis.
References
- Ampinder RF, Mann RB. Detection and characterization of Epstein-Barr virus in clinical specimens. Am J Pathol. 1994;145:239–52. [PMC free article] [PubMed] [Google Scholar]
- Atula T, Grénman R, Klemi P, Syrjänen S. Human papillomavirus, Epstein-Barr virus, human herpesvirus 8 and human cytomegalovirus involvement in salivary gland tumours. Oral Oncol. 1998;34:391–5. doi: 10.1016/s1368-8375(98)00023-2. [DOI] [PubMed] [Google Scholar]
- Egger M, Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo O, Santucci M, Calzolari A, Storchi OF. Epstein-Barr virus (EBV) infection and undifferentiated carcinoma of the parotid gland in Caucasian patients. Acta Otolaryngol. 1994;114:572–5. doi: 10.3109/00016489409126107. [DOI] [PubMed] [Google Scholar]
- Hamilton-Dutoit SJ, Therkildsen MH, Neilsen NH, et al. Undifferentiated carcinoma of the salivary gland in Greenlandic Eskimos:demonstration of Epstein-Barr virus DNA by in situ nucleic acid hybridization. Hum Pathol. 1991;22:811–5. doi: 10.1016/0046-8177(91)90210-g. [DOI] [PubMed] [Google Scholar]
- Hühns M, Simm G, Erbersdobler A, Zimpfer A. HPV infection, but not EBV or HHV-8 infection, is associated with salivary gland tumours. Biomed Res Int. 2015;2015:829349. doi: 10.1155/2015/829349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iezzoni JC, Gaffey MJ, Weiss LM. The role of Epstein-Barr virus in lymphoepithelioma-like carcinomas. Am J Clin Pathol. 1995;103:308–15. doi: 10.1093/ajcp/103.3.308. [DOI] [PubMed] [Google Scholar]
- Ioachim HL, Antonescu C, Giancotti F, Dorsett B. EBV-associated primary lymphomas in salivary glands of HIV-infected patients. Pathol Res Pract. 1998;194:87–95. doi: 10.1016/S0344-0338(98)80075-7. [DOI] [PubMed] [Google Scholar]
- Jen KY, Cheng J, Li J, et al. Mutational events in LMP1 gene of Epstein-Barr virus in salivary gland lymphoepithelial carcinomas. Int J Cancer. 2003;105:654–60. doi: 10.1002/ijc.11100. [DOI] [PubMed] [Google Scholar]
- Kim KI, Kim YS, Kim HK, et al. The detection of Epstein-Barr virus in the lesions of salivary glands. Pathol Res Pract. 1999;195:407–12. doi: 10.1016/S0344-0338(99)80014-4. [DOI] [PubMed] [Google Scholar]
- Kuo T, Tsang NM. Salivary gland type nasopharyngeal carcinoma:a histologic, immunohistochemical, and Epstein-Barr virus study of 15 cases including a psammomatous mucoepidermoid carcinoma. Am J Surg Pathol. 2001;25:80–6. doi: 10.1097/00000478-200101000-00009. [DOI] [PubMed] [Google Scholar]
- Lanier AP, Clift SR, Bornkamm G, et al. Epstein-Barr virus and malignant lymphoepithelial lesions of the salivary gland. Arctic Med Res. 1991;50:55–61. [PubMed] [Google Scholar]
- Leung SY, Chung LP, Yuen ST, et al. Lymphoepithelial carcinoma of the salivary gland:in situ detection of Epstein-Barr virus. J Clin Pathol. 1995;48:1022–7. doi: 10.1136/jcp.48.11.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H, Rao H, Saku The significance of detecting EB virus and its products in benign and malignant lymphoepithelial lesions of the salivary glands. Zhonghua Bing Li Xue Za Zhi. 1997;26:225–8. [PubMed] [Google Scholar]
- Lu SY, Huang CC, Hsiung CY, Eng HL, Huang HY. Primary lymphoepithelioma-like carcinoma of minor salivary gland:a case report with immunohistochemical and in situ hybridization studies. Head Neck. 2006;28:182–6. doi: 10.1002/hed.20312. [DOI] [PubMed] [Google Scholar]
- Nadal D, Caduff R, Frey E, et al. Non-Hodgkin’s lymphoma in four children infected with the human immunodeficiency virus:association with Epstein-Barr virus and treatment. Cancer. 1994;73:224, 30. doi: 10.1002/1097-0142(19940101)73:1<224::aid-cncr2820730138>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Nagao T, Ishida Y, Sugano I, et al. Epstein-Barr virus-associated undifferentiated carcinoma with lymphoid stroma of the salivary gland in Japanese patients. Comparison with benign lymphoepithelial lesion. Cancer. 1996;78:695–703. doi: 10.1002/(SICI)1097-0142(19960815)78:4<695::AID-CNCR1>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Pollock AM, Toner M, McMenamin M, Walker J, Timon CI. Absence of Epstein-Barr virus encoded RNA and latent membrane protein (LMP1) in salivary gland neoplasms. J Laryngol Otol. 1999;113:906–8. doi: 10.1017/s0022215100145542. [DOI] [PubMed] [Google Scholar]
- Rezaei F, Tavakoli P, Mozaffari HR, Azari M. Prevalence of salivary gland tumors in patients referred-pathology departments of Kermanshah hospitals, Iran 2007-2012. Res J Med Sci. 2016;10:487–91. [Google Scholar]
- Schneider M, Rizzardi C. Lymphoepithelial carcinoma of the parotid glands and its relationship with benign lymphoepithelial lesions. Arch Pathol Lab Med. 2008;132:278–82. doi: 10.5858/2008-132-278-LCOTPG. [DOI] [PubMed] [Google Scholar]
- Sheen TS, Tsai CC, Ko JY, Chang YL, Hsu MM. Undifferentiated carcinoma of the major salivary glands. Cancer. 1997;80:357–63. [PubMed] [Google Scholar]
- Taira S, Okuda M, Osato T, Mizuno F. Detection of Epstein-Barr virus DNA in salivary gland tumors. Nihon Jibiinkoka Gakkai Kaiho. 1992;95:860–8. doi: 10.3950/jibiinkoka.95.860. [DOI] [PubMed] [Google Scholar]
- Terada T. Epstein-Barr virus associated lymphoepithelial carcinoma of the esophagus. Int J Clin Exp Med. 2013;6:219–26. [PMC free article] [PubMed] [Google Scholar]
- To VSH, Chan JYW, Tsang RKY, Wei WI. Review of salivary gland neoplasms. ISRN Otolaryngol. 2012;2012:1–6. doi: 10.5402/2012/872982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai CC, Chen CL, Hsu HC. Expression of Epstein-Barr virus in carcinomas of major salivary glands:a strong association with lymphoepithelioma-like carcinoma. Hum Pathol. 1996;27:258–62. doi: 10.1016/s0046-8177(96)90066-0. [DOI] [PubMed] [Google Scholar]
- Venkateswaran L, Gan YJ, Sixbey JW, Santana VM. Epstein-Barr virus infection in salivary gland tumors in children and young adults. Cancer. 2000;89:463–6. doi: 10.1002/1097-0142(20000715)89:2<463::aid-cncr35>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Wen S, Mizugaki Y, Shinozaki F, Takada K. Epstein-Barr virus (EBV) infection in salivary gland tumors:lytic EBV infection in nonmalignant epithelial cells surrounded by EBV-positive T-lymphoma cells. J Virol. 1997;227:484–7. doi: 10.1006/viro.1996.8352. [DOI] [PubMed] [Google Scholar]
- Wolvius EB, Jiwa NM, van der Valk P, Horstman A, van der Waal I. Adenolymphoma and non-Hodgkin’s lymphoma of the salivary glands and oral cavity in immunocompetent patients are not associated with latent Epstein-Barr virus. Oral Oncol. 1997;33:119–23. doi: 10.1016/s0964-1955(96)00046-2. [DOI] [PubMed] [Google Scholar]
- Wu LY, Cheng J, Lu Y, Zhou ZY, Saku T. Epstein-Barr virus infection in benign lymphoepithelial lesions with malignant transformation of salivary glands. Zhonghua Kou Qiang Yi Xue Za Zhi. 2004a;39:291–3. [PubMed] [Google Scholar]
- Wu LY, Cheng J, Lu Y, Zhou ZY, Saku T. Epstein-Barr virus infection in lymphoepithelial carcinoma of salivary glands in Sichuan Chinese. Sichuan Da Xue Xue Bao Yi Xue Ban. 2004b;35:506–7. [PubMed] [Google Scholar]
- Zhao W, Deng N, Gao X, et al. Primary lymphoepithelioma-like carcinoma of salivary glands:a clinicopathological study of 21 cases. Int J Clin Exp Pathol. 2014;7:7951–6. [PMC free article] [PubMed] [Google Scholar]


