Abstract
Background:
The Oxaliplatin plus 5-Fluorouracil /Leucovorin (FOLFOX4) regimen have been approved by Chinese Food and Drug Administration (CFDA), and covered by health insurance for patients with advanced hepatocellular carcinoma (HCC) in China. However, the efficacy of FOLFOX4 for HCC patients is still under debate. In this study, we aimed to establish a nomogram to identify HCC patients who might benefit from FOLFOX4 chemotherapy base on individual profile.
Methods:
A total of 184 patients from the EACH study who were treated with FOLFOX4 were included in this analysis. Backward Cox proportional hazards regression combined with clinical experience was used to select variables for construction of the nomogram. The nomogram performance was assessed in terms of discrimination and calibration. The results were validated using bootstrap resampling.
Results:
Six variables were included in the prognostic models based on their clinical relevance: age, maximum tumor diameter, lymph node status, aspartate aminotransferase (AST), total bilirubin (TBIL) and alpha-fetoprotein (AFP). The calibration curve showed that the predicted survival probabilities closely matched the actual observations. The C-index of the model was 0.75 (95% CI:0.71-0.80). This value was significantly superior to the one for the following staging systems: BCLC (0.67, P=0.004), CUPI (0.66, P<0.001), AJCC seventh edition (0.63, P=0.002), GRETCH (0.63, P<0.001).
Conclusions:
The proposed nomogram showed accurate prognostic prediction for 6-month overall survival of patients treated with FOLFOX4 and could be useful for clinicians counseling patients and making treatment decisions.
Keywords: FOLFOX regimen, hepatocellular carcinoma, nomogram, oxaliplatin, systemic chemotherapy
Introduction
Hepatocellular carcinoma (HCC) is the third leading cause of cancer-related death worldwide and represents a major health problem (Jemal et al., 2011). More than 700,000 new HCC cases are diagnosed worldwide each year and approximately 80% of these occur in Asia due to the high prevalence of hepatitis B and C viral infections (Jemal et al., 2011).
Most of HCC patients present with an advanced disease stage at diagnosis, and a large number of patients diagnosed with early-stage disease eventually experience recurrence (Ferlay et al., 2013). Sorafenib, a tyrosine kinase inhibitor targeting the vascular endothelial growth factor and RAS/RAF/MEK/ERK pathway, was the first systemic therapy and the sole molecular target agent to demonstrate a statistically significant improvement of overall survival (OS) in patients with advanced HCC (Llovet et al., 2008; Cheng et al., 2012; Hollebecque et al., 2015). However, the high cost of sorafenib have limited its widely application in developing courtries. Recently, the Oxaliplatin (OXA) plus 5-Fluorouracil (5-FU)/Leucovorin (LV) (FOLFOX4) compared with single-agent doxorubicin (Adriamycin) as palliative chemotherapy in advanced Hepatocellular carcinoma patients ineligible for curative resection or local treatment (EACH) study showed that the FOLFOX4 regimen was associated with a trend toward improved OS as compared with doxorubicin (6.4 months in the FOLFOX4 group vs. 4.9 months in the doxorubicin group, p=0.06) and may confer some benefit to Asian patients with advanced HCC (Qin et al., 2013). A significant benefit of this therapy in terms of OS was observed in the Chinese patients who accounted for 75% of the patients in the EACH study (Qin et al., 2014). The subgroup analysis showed that Chinese patients with advanced HCC treated with FOLFOX4 had a significantly longer median OS (5.7 vs. 4.3 months, p=0.03), progression free survival (2.4 months vs. 1.7 months, p=0.0002), RR (8.6% vs. 1.4%, p=0.006) and disease control rate (47.1% vs. 26.6%, p=0.0004) than those treated with doxorubicin (Qin et al., 2014). Based on this study, oxaliplatin has been approved by China Food and Drug Administration (CFDA), and covered by health insurance for patients with advanced HCC in China.
Despite this, the efficacy of FOLFOX4 for HCC patients is still under debate due to lack of sufficient evidence. Hence, an accurate prognostic system to predict the outcome of patients starting FOLFOX4 therapy is needed to help clinician make treatment decisions. Compared with conventional staging systems such as the Barcelona Clinic Liver Cancer (BCLC) and American Joint Committee on Cancer (AJCC) systems (Llovet et al., 1999; Vauthey et al., 2002), nomograms can provide individualized rather than group estimation for cancer prognosis. It is a graphic representation of complex models that generate the probability of a particular outcome (such as survival) based on the individual profile of each patient (Iasonos et al., 2008; Apolo et al., 2013; Halabi et al., 2013; Hyder et al., 2014). The aim of the retrospective analysis presented here was to develop a nomogram for estimation of individualized survival probabilities for advanced HCC patients receiving FOLFOX4 using the EACH study data. Such a model can serve as a useful clinical aid for counseling patients and optimizing therapeutic approaches.
Materials and Methods
Patient Population
The EACH study was a randomized, international, multicenter, open-label phase III study (NCT00471965) enrolling patients with advanced HCC from mainland China, Taiwan, Korea, and Thailand. The inclusion and exclusion criteria and treatment were previously described by Qin et al (Qin et al., 2013). Briefly, 371 patients aged 18 to 75 years with histologically, cytologically, or clinically diagnosed unresectable HCC, ineligible for local invasive treatment, were enrolled and randomly assigned (1:1) to receive either FOLFOX4 (OXA 85 mg/m2 intravenously [IV] on day 1, LV 200 mg/m2 IV from hour 0 to 2 on days 1 and 2, and 5-FU 400 mg/m2 IV bolus at hour 2, then 600 mg/m2 over 22 hours on days 1 and 2, once every two weeks) or doxorubicin (50 mg/m2 IV, once every 3 weeks). Treatment was continued until disease progression, intolerable toxicity, or until the patient became eligible for surgical resection or withdrew consent, whichever occurred first. Once patients terminated the treatment phase, they were followed until death or study termination. Tumor evaluation, by CT and/or MRI scans and assessment of serum alpha-fetoprotein (AFP) levels, was performed at the screening visit, at randomization, every 6 weeks during the treatment phase and at each study visit during the follow-up. At each study visit blood samples were collected for hematology and biochemistry evaluations: hemoglobin, whole blood cell count, sodium, potassium, calcium, albumin, alkaline phosphatase (AKP), aspartate aminotransferase (AST), alanine aminotransferase (ALT), total bilirubin (TBIL), serum creatinine, glucose, creatinine clearance. For the analysis presented here only the baseline values were considered.
The objective of this retrospective analysis was to identify potential prognostic factors and construct an effective prognostic nomogram to predict the OS in advanced HCC patients treated with FOLFOX4. For reaching this aim, we analyzed the 184 patients allocated to the FOLFOX4 group.
Statistical Methods
(OS), the only endpoint used in the analysis, was calculated from the date when the patient was enrolled in the EACH study to either the date of death or the date of the last follow-up. If death was not confirmed, survival time was censored at the last time point in which the patient was known to have been alive or at the cut-off date, whichever came first. OS was assessed on all randomized patients, regardless of the number of treatment cycles received. All clinically relevant baseline variables from the study database were considered. Continuous predictors were transformed using restricted cubic splines with the aim to relax the linearity assumptions. A multivariate Cox proportional hazards regression model including the transformed continuous predictors as covariates was applied on the OS as dependent variable. A reduced model was constructed using a backward stepdown selection process, which the Akaike’s information criterion used as a stopping rule (Harrell et al., 1996). In this final model, coefficients of the predictors, hazard ratios (HRs) and their 95% confidence intervals (CIs) were estimated. The nomogram was based on this Cox model.
The performance of the nomogram was evaluated in two steps. First, the model’s discriminative ability was quantified by the Harrell Concordance index (C-index), which measures the capacity to discriminate patients with different outcomes. The higher the C-index, the more accurate the model is for a specific patient (Huitzil-Melendez et al., 2010). The C-indices of other staging systems were also calculated and compared with the new model according to Newson (Newson, 2010). To internally validate the predictive accuracy of the nomogram, 1000 bootstrap resamples were used to estimate the bias-corrected C-index and the extent of “over fitting” (Harrell et al., 1996). Patients were split into three subgroups (low, intermediate and high risk for predicted survival) based on the nomogram score which can be calculated by the model. To assess differences in survival of the subgroups, Kaplan-Meier curves were built.
Finally, we examined the nomogram calibration -i.e. the concordance between predicted and observed outcomes. This was performed by a visual inspection of a calibration plot comparing the predicted and actual survival probability, stratified by the nomogram score. Again, the bootstrapping correction was used for this activity (Steyerberg, 2009). Perfect calibration was considered to be achieved when the predicted probabilities were identical with the actual probabilities -i.e. the plots display a 45° line.
The descriptive statistics for demographic and baseline clinical characteristics as well as prior medications were computed. Numbers and percentages of patients were also presented per category of the American Joint Committee on Cancer Tumor, Node, Metastasis classification (AJCC TNM), BCLC, Chinese University Prognostic Index (CUPI), Group d’Etude de Traitement du Carcinoma Hepatocellullarire (GRETCH), Cancer of the Liver Italian Program (CLIP) staging systems.
Statistical analyses were performed using R, version 2.15.3 with software packages (http://www. r-project. org/), and Stata, version 10.0.
Ethical considerations
The EACH study was approved by Ethics Committees of the Bayi Hospital, Nanjing, China, in Dec 2006. Participants gave written informed consent and the confidentiality was ensured. In addition, the protocol was registered at ClinicalTrials.gov (Identifier NCT00471965).
Results
The demographic, baseline clinical characteristics and prior medications for the 184 patients allocated to the FOLFOX4 group are displayed in Table 1. Overall, the majority of patients were male (90.2%) and the median age was 50 years (range 18 to 73 years). 102 (55.4%) patients had cirrhosis and 163 (88.6%) of these were Child-Pugh A. Of all patients included in the analysis, 146 (79.3%) had more than one tumor nodule and the median of the longest diameter was 7.85 cm (range from 5 cm to 12 cm); 72.8% of the patients had tumors of 5 cm or larger. Forty-six (25%) patients had lymph node metastasis and 104 (56.5%) had extrahepatic metastases. Twelve patients (6.5%) had prior radiotherapy and 48 (26.1 %) had surgical resection. The median OS was 6.43 months, and the 6-month and 1-year survival rates were 52.8% and 19.9%, respectively.
Table 1.
Baseline Characteristics and Staging Information of 184 Patients with Advanced HCC
| Characteristic | Parameter |
|---|---|
| Age (years) | 50 (42-58) |
| Sex | |
| Male | 166 (90.2%) |
| Female | 18 (9.8%) |
| Tumor number | 3 (1-11) |
| Maximum tumor diameter (cm) | 7.85 (4.75-11.7) |
| Extrahepatic metastases | 104 (56.5%) |
| Location | |
| Left | 23 (12.5%) |
| Right | 108 (58.7%) |
| Both | 50 (27.2%) |
| Unkown | 3 (1.6%) |
| Portal vein thrombosis | 112 (60.9%) |
| Cirrhosis | 102 (55.4%) |
| Ascites | 6 (3.3%) |
| Total bilirubin (µmol/L) | 15.49 (11.9-19.2) |
| ALT (U/L) | 38 (27.25-64.5) |
| AST (U/L) | 60.85 (40.4-88.5) |
| ALK (U/L) | 133.5 (94.0-201.0) |
| Platelet (/L) | 165 (122-229) |
| International normalized ratio | 1.09 (1.0-1.2) |
| Serum creatinine (µmol/L) | 62.2 (1-74) |
| Prothrombin time (s) | 12.9 (12.0-14.1) |
| AFP (ng/ml) | 1312 (98.2-14470) |
| History of surgery | 48 (26. 1%) |
| History of radiotherapy | 12 (6.5%) |
| History of chemotherapy | 38 (20.7%) |
| History of TACE | 65 (35.3%) |
| BCLC system | |
| B | 40 (21.7%) |
| C | 144 (78.3%) |
| CUPI system | |
| L | 97 (52.7%) |
| M | 85 (46.2%) |
| H | 2 (1.1%) |
| TNM system | |
| I | 8 (4.3%) |
| II | 7 (3.8%) |
| III | 65 (35.3%) |
| IV | 104 (56.5%) |
| GRETCH system | |
| A | 15 (8.2%) |
| B | 154 (83.7%) |
| C | 15 (8.2%) |
Median (IQR) and number (%) are displayed for quantitative and qualitative characteristics, respectively; BCLC, Barcelona Clinic Liver Cancer; CUPI, Chinese University Prognostic Index; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALK, alkaline phosphatase; AFP, alpha-fetoprotein; TACE, transarterial chemoembolization
Prognostic factors
Initially, 20 clinically relevant candidate variables were selected from the database: age, sex, ascites, lymph node status, number of nodules, maximum tumor diameter, extrahepatic metastases, portal vein thrombosis, AKP, AST, TBIL, cirrhosis, ALT, platelet numbers, international normalized ratio (INR), AFP, prior radiotherapy, prior surgical resection, prior chemotherapy and serum creatinine. AFP was log-transformed due to its markedly skewed distribution.
Univariate analysis showed that ascites, nodal status, maximum tumor diameter, alkaline phosphatase, AST, TBIL, INR and log10 AFP were significant baseline predictors of survival in patients with advanced HCC (Table 2). Furthermore, we also chose age, extrahepatic metastases, portal vein thrombosis and cirrhosis from the candidate variables, based on clinical experience. Among these candidates, certain continuous variables (AKP, AST, maximum tumor diameter, TBIL, INR, log10 AFP, age) were explored by restricted cubic splines to relax the linearity assumption. Only maximum tumor diameter and age had non-linear effects on the HR of mortality (Figures 1a and 1b). We did not divide continuous variables into groups, so as to maximize the exploitation of original data. Furthermore, we observed the non-linear effect of age and maximum tumor diameter on survival (Figures 1a and 1b). Considering the non-linear relationship, restricted cubic splines that can represent a wide range of curve shapes were used to avoid a misinterpretation of the influence of a predictor and an inaccurate prediction. The Wald test showed no linear relationship between the HR of mortality and the maximum tumor diameter (=11.93; P=0.007) or age (=13.06; P=0.004), when 5 knots were used. Subsequently, a backward stepdown selection using the Akaike’s information criterion as stopping rule was performed to construct the final model. Six variables were entered into the reduced model: age, lymph node status, maximum tumor diameter, AST, TBIL, and log10 AFP. HRs and P values of these variables are shown in Table 3. No significant interactions were noted among these variables.
Table 2.
Univariate Analysis of Baseline Predictors of Survival in 184 Patients With HCC
| Variable | P value |
|---|---|
| Age | 0.617 |
| Sex | 0.502 |
| Ascites | 0.003 |
| Lymph node status | <0.001 |
| Number of nodules | 0.368 |
| Maximum tumor diameter | 0.004 |
| Extrahepatic metastases | 0.47 |
| Portal vein thrombosis | 0.182 |
| Alkaline phosphatase | 0.004 |
| AST | <0.001 |
| Total bilirubin | 0.003 |
| Cirrhosis | 0.67 |
| ALT | 0.663 |
| Platelet | 0.665 |
| International normalized ratio | <0.001 |
| Serum creatinine | 0.686 |
| Log AFP | 0.001 |
| Prior radiotherapy | 0.236 |
| Prior surgical resection | 0.157 |
| Prior chemotherapy | 0.856 |
ALT, albumin, alanine aminotransferase; AST, aspartate aminotransferase; AFP, alpha-fetoprotein
Figure 1.
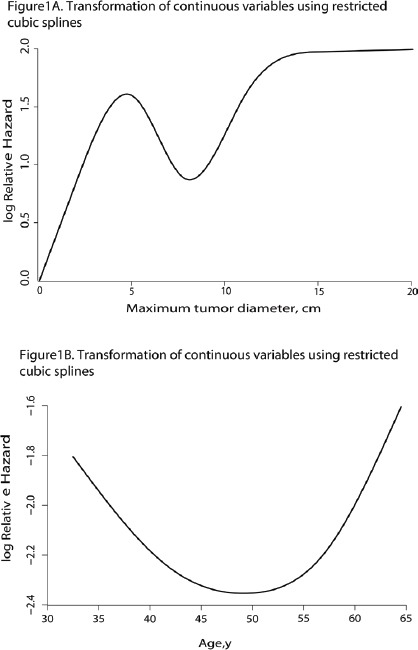
Transformation of Continuous Variables (a: maximum tumor diameter, b: age) using restricted cubic splines
Table 3.
Multivariate Cox Proportional Hazards Regression Model for Prediction of Survival
| Variable | Hazard Ratio (95% CI) | P value |
|---|---|---|
| Age | 1.014 (1.004, 1.024) | 0.183 |
| Lymph node status | ||
| N0 | 1.0 [Reference] | |
| N1 | 2.787 (2.232, 3.473) | <0.001 |
| NX | 1.539 (1.030, 2.314) | |
| TBIL | 1.036 (1.021, 1.049) | 0.022 |
| AST | 1.007 (1.005, 1.009) | 0.002 |
| Maximum tumor diameter | 1.045 (1.026, 1.063) | 0.009 |
| Log AFP | 1.173 (1.101, 1.246) | 0.007 |
AST, aspartate aminotransferase; AFP, alpha-fetoprotein; TBIL, total bilirubin
Prognostic Nomogram
A nomogram (Figure 2) was developed to predict the survival of advanced HCC patients with unresectable HCC treated with FOLFOX4 using the 6 independent prognostic predictors identified above. A prognostic score was assigned to each predictor. For example, the presence of N1 was associated with 25 points, whereas an AST of 200 U/L was associated with 40 points. A total score was calculated by summing all the scores corresponding to each independent predictor, and it can be used to estimate the probability of 6-month survival on the survival scales. A higher score implies a poorer survival outcome.
Figure 2.
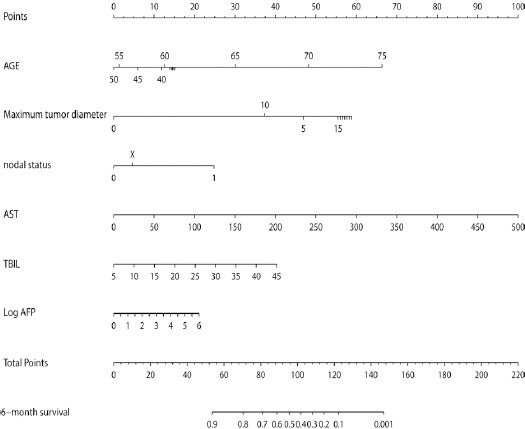
A nomogram to Predict 6-Month Survival of Patients with Advanced HCC.
To use the nomogram, each variable is located on the row and a straight line is drawn to correspond to the top line labeled “point”; after each point is obtained, a total score is calculated by summing the scores of each variable in the nomogram, located on the row labeled “total point”, which corresponds to the row labeled “6-month survival”.
Model performance
The C-index of the model for predicting the 6-month overall survival was 0.75 (0.71-0.80), which was significantly superior to the C-index for the following staging systems: BCLC (0.67, P=0.004), CUPI (0.66, P<0.001), AJCC seventh edition (0.63, P=0.002), and GRETCH (0.63, P<0.001). On the basis of the nomogram, patients were split into three groups according to their 6-month survival probability predicted by the model: low risk (survival rate >0.7), intermediate risk (0.3-0.7), high risk (survival rate <0.3). The Kaplan-Meier curve also showed a good discriminative ability of the nomogram (Figure 3). Figure 4 presents the 45-sample bootstrapped calibration curve of the nomogram for predicting the 6-month overall survival. The nomogram-predicted probabilities closely matched the actual probabilities, which suggests a good model calibration.
Figure 3.
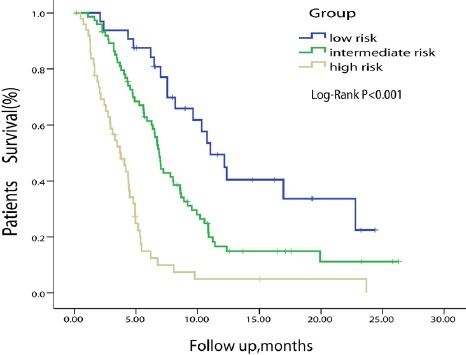
Kaplan-Meier Curve Split by Predicted Survival According to the Nomogram Score.
The high, intermediate and low risk groups were split by the 6-month survival rates predicted by the nomogram (high risk: <0.3, intermediate risk: 0.3-0.7, low risk: >0.7)
Figure 4.
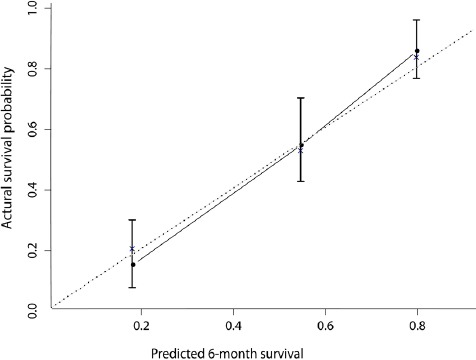
Calibration Plot for Predicting Patient Survival at 6 Months.
The dotted line represents the perfect predicting line, which means that predicted probabilities are identical with the actual probabilities.
The model was internally validated by using bootstrap method with 1,000 iterations. The bias-corrected C-index was 0.73, and the extend of “over-optimism” was small (2.6%), indicating that this nomogram will also show a good performance for future patients.
Discussion
This analysis, based on a sample size of patients with advanced HCC, aimed to establish a nomogram that is able to predict survival probabilities following FOLFOX4 treatment using objective parameters that are usually evaluated before chemotherapy initiation. Based on the multivariate analysis, a total of six pre-chemotherapy factors related to tumor extent (maximum tumor diameter, lymph node status and AFP), liver function (AST and TBIL) and patient age were identified as having a strong effect on the survival outcome and were selected in the final nomogram model. These predictors can be clearly defined, are reproducible and need less subjective interpretation, which makes the established nomogram relatively easy to use and enhanced the generalizability of the model to clinical practice. Using objective predictors, the nomogram performed well in terms of calibration and discrimination (C-index = 0.75) and it showed good predictive accuracy on bootstrap validation with a C statistic of 0.73.
Although the efficacy of FOLFOX4 regimen for HCC patients is still under debate, this regimen has been not only approved for advanced HCC from CFDA, but also covered by insurance in China. For patients who cannot afford or tolerate sorafenib, FOLFOX4 regimen might be an alternative (Lee, 2008; Je et al., 2009; Yau et al., 2009; Chen et al., 2010; Cheng et al., 2011; Cheng et al., 2012; Zhang et al., 2014b). Hence, it is important to create a prognostic model for HCC patients treated by FOLFOX4 and to help clinicians and patients in evaluating the odds of survival for a period of time given specific characteristics.
Nomograms, as weighted statistical models, can predict an accurate survival outcome for individual patients by evaluating multiple relevant variables simultaneously and the impact of each of those on the probability of survival (Iasonos et al., 2008; Apolo et al., 2013; Halabi et al., 2013; Hyder et al., 2014). In contrast to the widely used conventional staging system that assigns prognosis based on risk groups, nomograms take into account variation within each prognostic group, such as patient characteristics and treatment regimens (Iasonos et al., 2008; Apolo et al., 2013; Halabi et al., 2013; Hyder et al., 2014). This allows for a more individualized prediction of survival outcomes (Iasonos et al., 2008). Multiple studies have shown the superiority of prognostic nomograms in providing improved predictive accuracy compared with traditional staging systems (Kattan, 2003; Bochner et al., 2006; Wang et al., 2013; Hyder et al., 2014).
The predictor variables that we identified – age, TBIL, AFP, tumor diameter, and lymph nodes involvement -were identified as significant prognostic predictors in previous studies too (Stuart et al., 1996; Chen et al., 2006; Jun et al., 2013; Zhang et al., 2014b). Using these predictors, the nomogram developed here to tailor the assessment for a specific patient in regards to the FOLFOX4 therapy recommendation identifies three prognostic categories: a so called low risk population with a 6-months survival probability of >70% following FOLFOX4 and in which this therapy can provide the highest benefits in terms of survival; an intermediate group (6-months survival probability 30–70%) in which further refinement of prognostic on clinical judgment is needed; and a high risk population with a poor prognosis (survival rate at 6 months <30%) and for which FOLFOX4 chemotherapy may add little benefits in terms of OS.
Numerous staging systems and treatment guidelines are used to support therapeutic decision in patients with HCC. Compared with other staging systems (BCLC, CUPI, AJCC seventh edition and GRETCH), our nomogram showed higher values of the C-index of the model for predicting the 6-month OS thus having the ability to provide a more accurate prediction in terms of patient survival. This is important because our nomogram was specifically developed for evaluating the survival of HCC patients under FOLFOX4 therapy and a higher accuracy as compared to other staging systems was aimed. Most of the staging systems were developed for determining the prognosis without taking into account treatment and thus their prognostic accuracy for survival under specific treatment conditions is limited. Additionally it has been shown that the prognostic performances of the staging systems may vary between geographic regions and it has been postulated that differences in etiology, and variations in treatment approach may explain these discrepancies (Chan et al., 2014). Therefore, if a staging system was developed for a western population it may have lower accuracy in the East Asian patients, where the main etiological factor is HBV infection (Chan et al., 2014). The CUPI staging system, although initially developed and subsequently validated in the Chinese population (Leung et al., 2002), scored 3rd in terms of accuracy in predicting the survival in patients receiving FOLFOX4. Previously, CUPI was shown to be superior to BCLC in predicting survival in Chinese patients with either unresectable HCC or with HBV infection as the predominant etiology of HCC (Leung et al., 2002). However the usage of ALK as a marker of hepatic function in CUPI, although it has been shown to have low sensitivity (Leung et al., 2002), may explain the higher performance of our nomogram (which includes AST and not ALK). The BCLC system, which includes as predictors measures of liver function, tumor staging and performance status (Llovet et al., 1999) is considered the most comprehensive system available at this moment (Zhang et al., 2014a) and has the ability to provide treatment options based on different stages of the disease (Llovet et al., 2003). The potential reason for the advantage of our nomogram over the BCLB is that the former one was developed and further validated in western populations; additionally it has been shown not to be widely used for the therapy selection in Asian countries (Llovet et al., 1999; Liu et al., 2014).
Our study is not devoid of limitations. First, the data were derived from an international clinical trial. Despite the high quality of data, it is unclear whether this prognostic model is applicable to patients with different characteristics and backgrounds, because of the strict eligibility criteria used for the trial. Likewise, we have considered Karnofsky Performance Status (KPS) as one of the candidate variables in the planning stage of this study, however, one of the eligibility criteria is that KPS should be ≥70. Thus, all included patients had similar KPS that limited the distinguishing ability of KPS for survival in our study. Secondly, although a rigorous validation was performed using the bootstrap method, future work is still needed to validate this model, both externally and in a prospective manner. Thirdly, in spite of having achieved an accuracy superior to other conventional staging systems, our nomogram still might make a 27% incorrect prediction, leaving ample room for improvement in predictive ability. Indeed, this flaw can also be seen in virtually all predictive models, for which 100% correct predictions are virtually impossible to achieve (Kattan et al., 2002; Cindolo et al., 2005; Sorbellini et al., 2005; Chun et al., 2006; Steuber et al., 2006; Karakiewicz et al., 2007; Yau et al., 2009; Hyder et al., 2014). It is worth noting that we did not include these conventional disease staging systems in the nomogram because these staging systems are comprehensive in nature which are calculated by using single factors such as nodal status, maximum tumor diameter, thrombosis and so on. Our nomogram is also constructed based on multiple independent predictors. So it is inappropriate to include disease staging systems in the nomogram because they are not independent predictors and may cause some problems such as multicollinearity. Finally, until now, FOLFOX4 regimen is not regarded as a standard treatment for advanced HCC in any other country than China, which may limit the application of this nomogram. However, China has the most HCC patients and a useful predictive tool may helpful for chinese clinicians to get rid of the dilemma of whether to use the therapy still under debate.
In conclusions, the nomogram constructed in this study can provide a more accurate prediction of survival for HCC patients treated with FOLFOX 4 systemic chemotherapy. It can serve as a useful clinical aid for counseling patients and for planning an individualized treatment for the patient. Future studies are required to externally validate this model and to determine its applicability in other groups of patients.
Statement conflict of Interest
Lichuang Men is employed by Sanofi. The other authors have nothing to declare.
Abbreviations
AFP, alpha-fetoprotein
ALK, Alkaline phosphatase
ALT, Alanine aminotransferase
AJCC, the American Joint Committee on Cancer
AST, Aspartate aminotransferase
BCLC, Barcelona Clinic Liver Cancer system
CFDA, China Food and Drug Administration
CI, confidence interval
CLIP, Cancer of the Liver Italian Program
CUPI, Chinese University Prognostic Index
FOLFOX4, Oxaliplatin (OXA) plus 5-Fluorouracil (5-FU)/Leucovorin (LV)
HBV, Hepatitis B virus
HCC, Hepatocellular carcinoma
HCV, Hepatitis C virus
HR, Hazard Ratio
GETCH, Groupe d’Etude et de Traitement du Carcinome Hepatocellulaire
OS, Overall Survival
TACE, Transarterial chemoembolization
TBIL, Total bilirubin
Author contributions
Shukui Qin: participated in the design and coordination of the study, reviewed the manuscript, and contributed to the interpretation of the data. Xinji Zhang, Wei Guo and Jian Feng: participated in the design and coordination of the study, drafted the manuscript, performed the statistical analysis, and contributed to the interpretation of the data. Tianyi Zhang, Lichuang Men: collected and/or assembled of data, helped to draft the manuscript, and contributed to the interpretation of the results. Jia He: participated in the design of the study, reviewed the manuscript, and contributed to the interpretation of the data. All authors read and approved the final manuscript.
Acknowledgements
This study was sponsored by Sanofi. The statistical methods, data analysis and interpretation, as well as manuscript development were supported by the National Nature Science Foundation of China (NO. 81502895, 81373105), a grant from the key discipline for construction of evidence-based public health in Shanghai (NO. 12GWZX0602) and the Fourth Round of Three-year Action Plan on Public Health Discipline and Talent Program: Evidence-based Public Health and Health Economics (No. 15GWZK0901).
References
- Apolo AB, Ostrovnaya I, Halabi S, et al. Prognostic model for predicting survival of patients with metastatic urothelial cancer treated with cisplatin-based chemotherapy. J Natl Cancer Inst. 2013;105:499–503. doi: 10.1093/jnci/djt015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochner BH, Kattan MW, Vora KC. Postoperative nomogram predicting risk of recurrence after radical cystectomy for bladder cancer. J Clin Oncol. 2006;24:3967–72. doi: 10.1200/JCO.2005.05.3884. [DOI] [PubMed] [Google Scholar]
- Chan SL, Johnson PJ, Mo F, et al. International validation of the Chinese university prognostic index for staging of hepatocellular carcinoma:a joint United Kingdom and Hong Kong study. Chin J Cancer. 2014;33:481–91. doi: 10.5732/cjc.014.10133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CH, Chang TT, Cheng KS, et al. Do young hepatocellular carcinoma patients have worse prognosis? The paradox of age as a prognostic factor in the survival of hepatocellular carcinoma patients. Liver Int. 2006;26:766–73. doi: 10.1111/j.1478-3231.2006.01309.x. [DOI] [PubMed] [Google Scholar]
- Chen PJ, Furuse J, Han KH, et al. Issues and controversies of hepatocellular carcinoma-targeted therapy clinical trials in Asia:experts’opinion. Liver Int. 2010;30:1427–38. doi: 10.1111/j.1478-3231.2010.02292.x. [DOI] [PubMed] [Google Scholar]
- Cheng A, Kang Y, Lin D, et al. Phase III trial of sunitinib (Su) versus sorafenib (So) in advanced hepatocellular carcinoma (HCC) ASCO Annual Meeting Proceedings. 2011;2011(suppl) Abstr 4000. [Google Scholar]
- Cheng AL, Guan Z, Chen Z, et al. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma according to baseline status:subset analyses of the phase III Sorafenib Asia-Pacific trial. Eur J Cancer. 2012;48:1452–65. doi: 10.1016/j.ejca.2011.12.006. [DOI] [PubMed] [Google Scholar]
- Chun FK, Steuber T, Erbersdobler A, et al. Development and internal validation of a nomogram predicting the probability of prostate cancer Gleason sum upgrading between biopsy and radical prostatectomy pathology. Eur Urol. 2006;49:820–6. doi: 10.1016/j.eururo.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Cindolo L, Patard JJ, Chiodini P, et al. Comparison of predictive accuracy of four prognostic models for nonmetastatic renal cell carcinoma after nephrectomy:a multicenter European study. Cancer. 2005;104:1362–71. doi: 10.1002/cncr.21331. [DOI] [PubMed] [Google Scholar]
- Ferlay J, Soerjomataram I, Ervik M, et al. Cancer incidence and mortality worldwide:IARC cancer base No.11 [Internet] Lyon, France: International agency for research on cancer; 2013. [accessed on 06/08/2014]. Available from: http://globocan.iarc.fr . [Google Scholar]
- Halabi S, Lin CY, Small EJ, et al. Prognostic model predicting metastatic castration-resistant prostate cancer survival in men treated with second-line chemotherapy. J Natl Cancer Inst. 2013;105:1729–37. doi: 10.1093/jnci/djt280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrell FE, Jr, Lee KL, Mark DB. Multivariable prognostic models:issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–87. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Hollebecque A, Malka D, Ferte C, Ducreux M, Boige V. Systemic treatment of advanced hepatocellular carcinoma:from disillusions to new horizons. Eur J Cancer. 2015;51:327–39. doi: 10.1016/j.ejca.2014.12.005. [DOI] [PubMed] [Google Scholar]
- Huitzil-Melendez FD, Capanu M, O’Reilly EM, et al. Advanced hepatocellular carcinoma:which staging systems best predict prognosis? J Clin Oncol. 2010;28:2889–95. doi: 10.1200/JCO.2009.25.9895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyder O, Marques H, Pulitano C, et al. A nomogram to predict long-term survival after resection for intrahepatic cholangiocarcinoma:an Eastern and Western experience. JAMA Surg. 2014;149:432–8. doi: 10.1001/jamasurg.2013.5168. [DOI] [PubMed] [Google Scholar]
- Iasonos A, Schrag D, Raj GV, Panageas KS. How to build and interpret a nomogram for cancer prognosis. J Clin Oncol. 2008;26:1364–70. doi: 10.1200/JCO.2007.12.9791. [DOI] [PubMed] [Google Scholar]
- Je Y, Schutz FA, Choueiri TK. Risk of bleeding with vascular endothelial growth factor receptor tyrosine-kinase inhibitors sunitinib and sorafenib:a systematic review and meta-analysis of clinical trials. Lancet Oncol. 2009;10:967–74. doi: 10.1016/S1470-2045(09)70222-0. [DOI] [PubMed] [Google Scholar]
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- Jun CH, Sim DW, Kim SH, et al. Predictive factors for recurrence and survival in hepatocellular carcinoma in South Korea. Anticancer Res. 2013;33:4129–34. [PubMed] [Google Scholar]
- Karakiewicz PI, Briganti A, Chun FK, et al. Multi-institutional validation of a new renal cancer-specific survival nomogram. J Clin Oncol. 2007;25:1316–22. doi: 10.1200/JCO.2006.06.1218. [DOI] [PubMed] [Google Scholar]
- Kattan MW. Nomograms are superior to staging and risk grouping systems for identifying high-risk patients:preoperative application in prostate cancer. Curr Opin Urol. 2003;13:111–6. doi: 10.1097/00042307-200303000-00005. [DOI] [PubMed] [Google Scholar]
- Kattan MW, Leung DH, Brennan MF. Postoperative nomogram for 12-year sarcoma-specific death. J Clin Oncol. 2002;20:791–6. doi: 10.1200/JCO.2002.20.3.791. [DOI] [PubMed] [Google Scholar]
- Lee HC. Systemic chemotherapy of hepatocellular carcinoma–Korean experience. Oncology. 2008;75:114–8. doi: 10.1159/000173432. [DOI] [PubMed] [Google Scholar]
- Leung TW, Tang AM, Zee B, et al. Construction of the Chinese university prognostic index for hepatocellular carcinoma and comparison with the TNM staging system, the Okuda staging system, and the cancer of the liver Italian program staging system:a study based on 926 patients. Cancer. 2002;94:1760–9. doi: 10.1002/cncr.10384. [DOI] [PubMed] [Google Scholar]
- Liu C, Duan LG, Lu WS, et al. Prognosis evaluation in patients with hepatocellular carcinoma after hepatectomy:comparison of BCLC, TNM and Hangzhou criteria staging systems. PLoS One. 2014;9:e103228. doi: 10.1371/journal.pone.0103228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma:the BCLC staging classification. Semin Liver Dis. 1999;19:329–38. doi: 10.1055/s-2007-1007122. [DOI] [PubMed] [Google Scholar]
- Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–17. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–90. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- Newson RB. Comparing the predictive powers of survival models using Harrell’s C or Somers’D. Stata J. 2010;10:339–58. [Google Scholar]
- Qin S, Bai Y, Lim HY, et al. Randomized, multicenter, open-label study of oxaliplatin plus fluorouracil/leucovorin versus doxorubicin as palliative chemotherapy in patients with advanced hepatocellular carcinoma from Asia. J Clin Oncol. 2013;31:3501–8. doi: 10.1200/JCO.2012.44.5643. [DOI] [PubMed] [Google Scholar]
- Qin S, Cheng Y, Liang J, et al. Efficacy and safety of the FOLFOX4 regimen versus doxorubicin in Chinese patients with advanced hepatocellular carcinoma:a subgroup analysis of the EACH study. Oncologist. 2014;19:1169–78. doi: 10.1634/theoncologist.2014-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorbellini M, Kattan MW, Snyder ME, et al. A postoperative prognostic nomogram predicting recurrence for patients with conventional clear cell renal cell carcinoma. J Urol. 2005;173:48–51. doi: 10.1097/01.ju.0000148261.19532.2c. [DOI] [PubMed] [Google Scholar]
- Steuber T, Graefen M, Haese A, et al. Validation of a nomogram for prediction of side specific extracapsular extension at radical prostatectomy. J Urol. 2006;175:939–44. doi: 10.1016/S0022-5347(05)00342-3. [DOI] [PubMed] [Google Scholar]
- Steyerberg E. In ’clinical prediction models’. Springer; 2009. Case study on survival analysis:prediction of secondary cardiovascular events; pp. 427–46. [Google Scholar]
- Stuart KE, Anand AJ, Jenkins RL. Hepatocellular carcinoma in the United States. Prognostic features, treatment outcome, and survival. Cancer. 1996;77:2217–22. doi: 10.1002/(SICI)1097-0142(19960601)77:11<2217::AID-CNCR6>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Vauthey JN, Lauwers GY, Esnaola NF, et al. Simplified staging for hepatocellular carcinoma. J Clin Oncol. 2002;20:1527–36. doi: 10.1200/JCO.2002.20.6.1527. [DOI] [PubMed] [Google Scholar]
- Wang Y, Li J, Xia Y, et al. Prognostic nomogram for intrahepatic cholangiocarcinoma after partial hepatectomy. J Clin Oncol. 2013;31:1188–95. doi: 10.1200/JCO.2012.41.5984. [DOI] [PubMed] [Google Scholar]
- Yau T, Chan P, Cheung F, et al. 47LBA Phase II trial of sorafenib with capecitabine and oxaliplatin (SECOX) in patients with locally advanced or metastatic hepatocellular carcinoma. Eur J Cancer Care. 2009;7:20–1. [Google Scholar]
- Zhang JF, Shu ZJ, Xie CY, et al. Prognosis of unresectable hepatocellular carcinoma:comparison of seven staging systems (TNM, Okuda, BCLC, CLIP, CUPI, JIS, CIS) in a Chinese cohort. PLoS One. 2014a;9:e88182. doi: 10.1371/journal.pone.0088182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XJ, Zhang TY, Yu FF, et al. Risk of treatment-related mortality with sorafenib in patients with cancer. Asian Pac J Cancer Prev. 2014b;14:6681–6. doi: 10.7314/apjcp.2013.14.11.6681. [DOI] [PubMed] [Google Scholar]


