Abstract
Background:
Catechol-O-methyltransferase (COMT) is an important estrogen-metabolizing enzyme. Numerous case-control studies have evaluated the role COMT Val 158Met (rs4680;472G->A) polymorphism in the risk of breast cancer and provided inconclusive results, hence present meta-analysis was designed to get a more reliable assessment in Asian population.
Methods:
A total of 26 articles were identified through a search of four electronic databases-PubMed, Google Scholar, Science Direct and Springer link, up to March, 2016. Pooled odds ratios (ORs) with 95% con¬fidence intervals (CIs) were used as association measure to find out relationship between COMT Val158Metpolymorphism and the risk of breast cancer. We also assessed between study heterogeneity and publication bias. All statistical analyses were done by Open Meta-Analyst.
Results:
Twenty six case-control studies involving 5,971 breast cancer patients and 7,253 controls were included in the present meta-analysis. The results showed that the COMT Val158Met polymorphism was significantly associated with breast cancer risk except heterozygote model(allele contrast odds ratio (ORAvsG)= 1.13, 95%CI=1.02-1.24,p=0.01; heterozygote/co-dominant ORGAvsGG= 1.03, 95%CI=0.96-1.11,p=0.34; homozygote ORAAvsGG= 1.38, 95%CI= 1.08-1.76,p=0.009; dominant model ORAA+GAvsGG= 1.08, 95%CI=1.01-1.16,p=0.02; and recessive model ORAAvsGA+GG= 1.35, 95%CI=1.07-1.71,p=0.01). In addition, we also performed subgroup analysis based on source of controls and menopausal state of patients.
Conclusions:
In conclusion, the COMT Val158Met polymorphism was related to increased breast cancer susceptibility in the Asian population.
Keywords: Catechol-O-methyltransferase, COMT, Val158Met, 472G->A, breast cancer-Asian population
Introduction
Breast cancer (BC)is the leading cause of cancer death among females (Jemal et al., 2011; Guo et al., 2012) and its development is a multifactorial complex process influenced by multiple genetic variants and environmental factors (Nathanson et al.,2001; Guo et al., 2012). Estrogen hormones affect the cell growth and proliferation during breast carcinogenesis and metabolized by several enzyme including COMT, which metabolized itinto biologically non-hazardous methoxyestrogens(Onay et al., 2008). COMT enzyme are found in two isoforms in the cells: a cytoplasmic smaller protein (S-COMT; 221 aa) and a membrane-bound longer protein (MB-COMT 271 aa) (Tenhunen et al. 1994).
COMT gene is present at chromosome 22q11.1 and a single base pair G-->A substitution at position 472(G472A/Val158Met)in exon 4, results in substitution of valine by methionine in COMT enzyme (Lotta et al., 1995; Lachman et al., 1996). The two alleles are referred to as Val(G) and Met(A). Val allele encodes the thermostable high activity COMT enzyme and Met allele encodes the thermolabile low activity COMT enzyme (Spielman and Weinshilboum, 1981; Lotta et al., 1995; Nobile et al., 2010). Both the alleles are co-dominant, i.e. heterozygous individuals (Val/Met) have an intermediate level of COMT activity (Lotta et al., 1995). The frequency of the mutant Met allele vary greatly among the populations studied, frequency of Met allele is reported as 0.56 in American (Vandenbergh et al., 1997), 0.5 in European (Kunugi et al., 1997), and 0.27 in Asian (Chen et al., 1997)populations. COMT gene Val158Met is a clinically functional polymorphism, and reported as risk factor for several disorders/diseases-schizophrenia (Kayahan et al., 2013), attention-deficit hyperactivity disorder (Retz et al., 2008), autism (Gadow et al., 2009), drug abuse (Vinkers et al., 2013), posttraumatic stress disorder (Valente et al., 2011), and cancer (Omrani et al., 2009) etc.
COMT enzyme metabolized estrogen and its carcinogenic derivatives, hence study of COMT gene polymorphisms as risk for cancer is of particular interest. In the past years, several case-control studies have been investigated the association between COMT Val158Met polymorphisms and breast cancer susceptibility (Kocabas et al.,2002; Wen et al., 2005;Chang et al.,2006; Wang et al., 2010; Naushad et al., 2011;Lajin et al.,2013). However, individual study limitations contributed to divergent conclusions among them. Aim of the present meta-analysis was to find out the relationship between COMT Val158Met polymorphism and breast cancer risk in Asian population.
Materials and Methods
Data Sources, Search Strategy, and Selection Criteria
The articles for the present meta-analysis were retrieved by searching the PubMed (http://www.ncbi.nlm.nih.gov/pubmed), Google Scholar (http://scholar.google.com), Science Direct (http://www.sciencedirect.com), and Springer Link (http://link.springer.com)databases up to March, 2016, using the keywords “breast cancer”, “Val158Met”, “Catechol-O-methyltransferase” and “COMT”.
Article selection for the present meta-analysis used the following inclusion criteria:(i) study should be case-control; (ii) sufficient genotype/allele data to calculate the odds ratios (ORs) with 95% confidence intervals should be reported (CIs). Exclusion criteria were the following: (i) only cases were analyzed; (ii) editorial, review articles etc.;(iii)not sufficient data/information to calculate odds ratio with 95%CI were reported; (v) other cancer type were investigated in the study and (vi) non-Asian breast cancer cases were investigated.
Data Extraction and Quality Assessment
Data extraction and quality assessment were performed by two investigators (PK and UY). From each included relevant article, the following data were extracted: the family name of first author, the publication year, journal name, country name, the study design i.e. source of controls, the sample size, and the genotype distribution for the participants. Method of Guo et al., (2012) was adopted for study quality assessment. The quality scores ranged from 0 to 10 and studies with score <5 was defined as low quality, and studies with score ≥7 was defined as high quality.
Statistical Analysis
Odds ratios (ORs) with corresponding 95% confidence intervals (CIs) were used as the measure of association between the COMT Val158Met polymorphism and breast cancer susceptibility. Data were pooled using the fixed effect (Mantel and Haenszel,1959) and random effect (DerSimonian and Laird, 1987) methods. p<0.05 was considered as statistically significant. Heterogeneity between conducted by X2-based Q-test and quantified by I2 (Cochran,1954; Higgins and Thompson, 2002; Whitehead, 2002). For this polymorphism all five genetic models, the additive model (Met vs. Val; A v.s G), homozygote model (Met/Met vs.Val/Val; AA vs. GG), heterozygote model (Val/Met vs Val/Val; GA vs GG), dominant model (Met/Met+Val/Met vs Val/Val; AA+GA vs GG) and recessive model (Met/Met vs Val/Met + Val/Val; AAvs GA+GG) were chosen to calculate the pooled ORs. x2 test was done to evaluate Hardy-Weinberg Equilibrium (HWE) for control subjects in each study. Publication bias was assessed by Begg’s funnel plot and Egger’s linear regression test (Egger et al., 1997). All statistical analyses were performed by Open Meta-Analyst (Wallace et al., 2013).
Results
Literature Search
Initial search of four databases, 203 articles were retrieved, but 141 articles did not meet the inclusion criteria after reviewing abstract. The excluded articles include results of drug treatments of breast cancer, book chapter, comments, editorials, reviews, meta-analysis and articles investigated other genes. Out of remaining sixty two articles, we also excluded thirty nine articles, in which investigated subjects were not from Asian population. After applying inclusion/exclusion criteria, total 23articles (Figure 1) were suitable for the present meta-analysis (Huang et al., 1999; Hamajima et al., 2001; Yim et al., 2001; Kocabas et al., 2002; Tan et al., 2003; Wu et al., 2003; Sazci et al., 2004; Cheng et al., 2005; Lin SC et al., 2005; Lin WY et al., 2005; Wen et al., 2005; Chang et al., 2006; Akisik and Dalay, 2007; Fan et al., 2007; Hu et al., 2007; Sangrajrang et al., 2009; Yadav et al., 2009; Syamala et al., 2010; Xu et al., 2010; Naushad et al., 2011; Wang et al., 2011; Lajin et al., 2013; Li et al., 2013). These studies were published between 1999 to 2013. One author (Syamala et al.,2010) studied sporadic and familial cases both and reported separately in their article, so we also included both groups of data as separate studies. Wu et al., 2003 reported three individual populations (Chinese, Japanese and Filipino) so we included them as three separate studies. Hence total twenty six studies were included in the present meta-analysis. Characteristics of all the included studies were given in Table 1. These studies were carried out in different countries-China (Huang et al., 1999; Tan et al.,2003; Wu et al., 2003; Cheng et al., 2005; Lin WY et al.,2005; Wen et al., 2005; Chang et al., 2006; Fan et al., 2007; Hu et al., 2007; Xu et al., 2010; Wang et al., 2011; Li et al., 2013), India (Yadav et al., 2009; Syamala et al., 2010; Naushad et al., 2011), Japan (Hamajima et al., 2001; Yim et al., 2001; Wu et al., 2003), Philippines (Wu et al., 2003), Syria (Lajin et al., 2013), Taiwan (Lin SC et al., 2005), Thailand (Sangrajrang et al., 2009), Turkey (Kocabas et al., 2002; Sazci et al., 2004; Akisik and Dalay, 2007).
Figure 1.

Flow Diagram of Study Search and Selection Process
Table 1.
Characteristics of Twenty Four Studies Included in the Present Meta-Analysis
| Study | Country | Source of Control | Menopausal Status | Case/Control | Case | Genotypes | Control | Genotypes | HWE | Quality Score | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Val/Val (GG) | Val/Met (AG) | Met/Met (AA) | Val/Val (GG) | Val/Met (AG) | Met/Met (AA) | |||||||
| Huang et al., 1999 | China | HB | Pre-,Post- | 113/124 | 66 | 35 | 12 | 65 | 55 | 4 | 0.06 | 8.5 |
| Hamajima et al., 2001 | Japan | HB | Pre-,Post- | 150/165 | 60 | 72 | 18 | 79 | 63 | 23 | 0.08 | 8.5 |
| Yim et al., 2001 | Japan | HB | Pre-,Post- | 163/163 | 81 | 79 | 3 | 101 | 46 | 16 | 0.004* | 5.5 |
| Kocabas et al., 2002 | Turkey | HB | Pre-,Post- | 84/103 | 28 | 42 | 14 | 35 | 55 | 13 | 0.23 | 5.5 |
| Tan et al., 2003 | China | HB | Pre-,Post- | 250/250 | 121 | 103 | 26 | 132 | 105 | 13 | 0.17 | |
| Wu et al., 2003 | China | PB | Mixed | 178/199 | 97 | 67 | 14 | 106 | 78 | 15 | 0.9 | 8.0 |
| Japan | PB | Mixed | 193/197 | 88 | 89 | 16 | 86 | 87 | 24 | 0.78 | ||
| Philippines | PB | Mixed | 218/166 | 143 | 57 | 18 | 90 | 64 | 12 | 0.89 | ||
| Sazci et al., 2004 | Turkey | PB | Pre- | 130/224 | 33 | 69 | 28 | 62 | 146 | 16 | 0.00* | 6.0 |
| Cheng et al., 2005 | China | HB | Mixed | 469/740 | 237 | 197 | 35 | 420 | 262 | 58 | 0.06 | 7.5 |
| Lin WY et al., 2005 | China | PB | Mixed | 87/341 | 51 | 31 | 5 | 190 | 133 | 18 | 0.39 | 7.5 |
| Lin SC et al., 2005 | Taiwan | PB | Mixed | 99/366 | 58 | 35 | 6 | 205 | 138 | 23 | 0.97 | 6.5 |
| Wen et al., 2005 | China | PB | Pre-,Post- | 1120/1191 | 612 | 425 | 83 | 628 | 470 | 93 | 0.69 | 9.5 |
| Chang et al., 2006 | China | HB | Mixed | 189/320 | 103 | 77 | 9 | 131 | 159 | 30 | 0.06 | 6.5 |
| Akisik et al., 2007 | Turkey | NR | Mixed | 114/108 | 29 | 59 | 26 | 34 | 53 | 21 | 0.96 | 3.0 |
| Fan et al., 2007 | China | NR | Mixed | 200/100 | 96 | 75 | 29 | 51 | 44 | 5 | 0.24 | 7.5 |
| Hu et al., 2007 | China | HB | Pre-,Post- | 112/110 | 65 | 36 | 11 | 66 | 41 | 3 | 0.25 | 6.5 |
| Sangrajrang et al., 2009 | Thailand | HB | Mixed | 565/486 | 290 | 233 | 42 | 266 | 190 | 30 | 0.6 | 9.0 |
| Yadav et al., 2009 | India | HB | Pre-,Post- | 59/99 | 23 | 30 | 6 | 32 | 53 | 14 | 0.28 | 7.0 |
| Syamala et al., 2010a | India | PB | Mixed | 140 /367 | 48 | 64 | 28 | 138 | 164 | 65 | 0.18 | 7.0 |
| Syamala et al., 2010b | India | PB | Mixed | 219 / 367 | 74 | 104 | 41 | 138 | 164 | 65 | 0.18 | 7.0 |
| Xu et al., 2010 | China | NR | Mixed | 140/122 | 60 | 42 | 38 | 68 | 44 | 10 | 0.45 | 6.5 |
| Naushad et al., 2011 | India | HB | Mixed | 212/233 | 71 | 94 | 47 | 115 | 103 | 15 | 0.2 | 6.0 |
| Wang et al., 2011 | China | PB | Pre-,Post- | 400/400 | 187 | 145 | 68 | 208 | 156 | 36 | 0.39 | 7.0 |
| Lajin et al., 2013 | Syria | PB | Pre-,Post- | 135/107 | 34 | 70 | 31 | 23 | 54 | 30 | 0.88 | 6.5 |
| Li et al., 2013 | China | HB | Mixed | 120/120 | 58 | 45 | 17 | 73 | 42 | 5 | 0.73 | 7.0 |
HB, hospital-based; PB, population-based; NR, not reported; Pre, premenopausal; Post, postmenopausal
Study Characteristics
All twenty six studies were published between 1999 (Huang et al., 1999) to 2013 (Li et al., 2013). Smallest sample size of cases studied was 59 (Yadav et al., 2009) and largest sample size was 1,120 (Wen et al., 2005). In twelve studies, age and sex matched controls are selected from hospital and in eleven studies controls were selected from population. In three studies source of controls were not given. Control population of two studies (Yim et al., 2001; Sazciet al., 2004) were not in HWE. In eleven studies, selected patients were of premenopausal state and in ten studies cases were at postmenopausal state. In remaining studies menopausal status of patients was not given. Total cases were 5,971 with GG (2,844), GA (2,432) and AA (695) genotypes and controls were 7,253 with GG (3,584), GA (2,998) and AA (671) genotypes. In total cases, genotypes percentage of GG, GA and AA were 47.63%, 40.73% and 11.64% respectively. In controls, genotypes percentage of GG, GA and AA were 49.41, 41.34% and 9.25%respectively. Out of twenty six studies, six studies did not report any association between COMT Val158Met and breast cancer (Lin SC et al., 2005; Lin WY et al., 2005;Wen et al., 2005; Chang et al., 2006; Yadav et al., 2009; Lajin et al., 2013).
Meta-analysis
Meta-analysis with allele contrast (A vs. G) showed significant association with both fixed effect (ORAvsG= 1.10; 95%CI= 1.05-1.17; p= <0.001) and random effect model (ORAvsG= 1.13; 95% CI= 1.02-1.24; p= 0.01) (Table 2, Figure 2). There was observed an increased risk of breast cancer using homozygote model (AA vs GG; homozygote model), with both fixed (ORAAvsGG= 1.32; 95%CI= 1.17-1.49; p= <0.001) and random (ORAAvsGG= 1.38; 95%CI= 1.08-1.76; p=0.009) effect models with high statistical heterogeneity between studies (Table 2, Figure 3). Association of mutant heterozygous genotype (GAvs.GG; co-dominant model) was not observed significant with both fixed (ORGAvsGG= 1.03; 95%CI= 0.96-1.11; p= 0.34) and random (ORGAvsGG= 1.03; 95%CI= 0.93-1.14; p= 0.48) effect models. Combined mutant genotypes (AA+GA vs GG; dominant model) showed positive association with breast cancer using fixed (ORAA+GAvsGG= 1.08; 95%CI= 1.01-1.16; p= 0.02)effect model (Table 2). Similarly the recessive genotypes model (AA vs. GA+GG) also showed significant strong association with breast cancerusing both fixed (ORAAvsGA+GG= 1.29; 95%CI= 1.15-1.45; p= <0.001) and random (ORAAvsGA+GG= 1.35; 95%CI= 1.07-1.71; p= 0.01) effect models (Table 2, Figure 4).
Table 2.
Summary Estimates for the Odds Ratio (OR) in Various Allele/Genotype Contrasts, the Significance Level (P Value) of Heterogeneity Test (Q Test), and the I2 Metric: Overall Analysis, and Subgroup Analyses.
| Genetic Contrast | Fixed effect OR (95% CI), p | Random effect OR (95% CI), p | Heterogeneity p-value (Q test) | I2 (%) | Publication Bias (p of Egger’s test) | |
|---|---|---|---|---|---|---|
| All | Allele Contrast (A vs. G) | 1.10 (1.05-1.17), <0.001 | 1.13 (1.02-1.24), 0.01 | <0.001 | 63.71 | 0.36 |
| Dominant (AA+AG vs. GG) | 1.08 (1.01-1.16), 0.02 | 1.09 (0.99-1.21), 0.07 | 0.009 | 43.9 | 0.61 | |
| Homozygote (AA vs. GG) | 1.32 (1.17-1.49), <0.001 | 1.38 (1.08-1.76), 0.009 | <0.001 | 68.73 | 0.33 | |
| Co-dominant (GA vs. GG) | 1.03 (0.96-1.11), 0.34 | 1.03 (0.93-1.14), 0.48 | 0.04 | 35.21 | 0.87 | |
| Recessive (GG+GA vs. AA) | 1.29 (1.15-1.45), <0.001 | 1.35 (1.07-1.71), 0.01 | <0.001 | 70.64 | 0.27 | |
| Pre | Allele Contrast (A vs. G) | 1.11 (1.00-1.23), 0.03 | 1.17 (1.01-1.36), 0.03 | 0.09 | 38.27 | 0.05 |
| Dominant (AA+AG vs. GG) | 1.06 (0.93-1.22), 0.36 | 1.06 (0.92-1.22), 0.36 | 0.45 | 0.00 | 0.02 | |
| Homozygote (AA vs. GG) | 1.38 (1.09-1.75), 0.006 | 1.53 (0.98-2.40), 0.05 | 0.004 | 61.58 | 0.29 | |
| Co-dominant (GA vs. GG) | 1.00 (0.86-1.15), 0.96 | 1.02 (0.86-1.21), 0.77 | 0.34 | 10.84 | 0.13 | |
| Recessive (GG+GA vs. AA) | 1.38 (1.11-1.71), 0.003 | 1.48 (0.95-2.29), 0.07 | <0.001 | 66.84 | 0.45 | |
| Post | Allele Contrast (A vs. G) | 1.04 (0.92-1.18), 0.46 | 1.04 (0.90-1.21), 0.53 | 0.25 | 20.18 | 0.58 |
| Dominant (AA+AG vs. GG) | 1.04 (0.88-1.22), 0.62 | 1.04 (0.86-1.25), 0.65 | 0.32 | 13.04 | 0.61 | |
| Homozygote (AA vs. GG) | 1.11 (0.83-1.47), 0.47 | 1.05 (0.65-1.70), 0.81 | 0.03 | 49.89 | 0.53 | |
| Co-dominant (GA vs. GG) | 1.01 (0.85-1.21), 0.83 | 1.01 (0.81-1.26), 0.88 | 0.2 | 26.09 | 0.77 | |
| Recessive (GG+GA vs. AA) | 1.11 (0.85-1.44), 0.43 | 1.07 (0.68-1.67), 0.76 | 0.02 | 52.54 | 0.59 | |
| Mixed | Allele Contrast (A vs. G) | 1.13 (1.05-1.21), <0.001 | 1.14 (0.98-1.32), 0.08 | <0.001 | 73.06 | 0.58 |
| Dominant (AA+AG vs. GG) | 1.11 (1.01-1.22), 0.02 | 1.10 (0.95-1.29), 0.19 | 0.004 | 56.41 | 0.61 | |
| Homozygote (AA vs. GG) | 1.37 (1.16-1.62), <0.001 | 1.40 (1.01-1.95), 0.04 | <0.001 | 69.87 | 0.53 | |
| Co-dominant (GA vs. GG) | 1.06 (0.96-1.17), 0.23 | 1.04 (0.91-1.18), 0.52 | 0.1 | 32.85 | 0.77 | |
| Recessive (GG+GA vs. AA) | 1.31 (1.12-1.54), <0.001 | 1.35 (1.00-1.83), 0.04 | <0.001 | 67.98 | 0.59 | |
| Hospital based | Allele Contrast (A vs. G) | 1.17 (1.07-1.27), <0.001 | 1.18 (1.01-1.36), 0.02 | <0.001 | 65.22 | 0.77 |
| Dominant (AA+AG vs. GG) | 1.19 (1.07-1.32), 0.001 | 1.18 (1.00-1.40), 0.04 | 0.01 | 52.18 | 0.86 | |
| Homozygote (AA vs. GG) | 1.38 (1.14-1.68), 0.001 | 1.44 (0.93-2.24), 0.09 | <0.001 | 75.07 | 0.68 | |
| Co-dominant (GA vs. GG) | 1.15 (1.03-1.29), 0.01 | 1.13 (0.95-1.35), 0.15 | 0.01 | 52.21 | 0.58 | |
| Recessive (GG+GA vs. AA) | 1.30 (1.08-1.57), 0.005 | 1.37 (0.89-2.11), 0.14 | <0.001 | 75.94 | 0.59 | |
| Population based | Allele Contrast (A vs. G) | 1.02 (0.94-1.10), 0.59 | 1.02 (0.91-1.14), 0.71 | 0.03 | 48.22 | 0.87 |
| Dominant (AA+AG vs. GG) | 0.97 (0.88-1.07), 0.60 | 0.97 (0.87-1.08), 0.64 | 0.39 | 5.11 | 0.85 | |
| Homozygote (AA vs. GG) | 1.15 (0.97-1.36), 0.09 | 1.15 (0.87-1.51), 0.30 | 0.01 | 53.52 | 0.99 | |
| Co-dominant (GA vs. GG) | 0.94 (0.84-1.04), 0.25 | 0.94 (0.84-1.04), 0.25 | 0.62 | 0.00 | 0.71 | |
| Recessive (GG+GA vs. AA) | 1.17 (0.99-1.37), 0.05 | 1.18 (0.89-1.55), 0.23 | 0.005 | 60.11 | 0.87 | |
| Not reported | Allele Contrast (A vs. G) | 1.49 (1.20-1.85), <0.001 | 1.49 (1.08-2.05), 0.13 | 0.11 | 53.64 | NA |
| Dominant (AA+AG vs. GG) | 1.36 (1.01-1.83), 0.03 | 1.36 (1.01-1.83), 0.03 | 0.52 | 0.00 | NA | |
| Homozygote (AA vs. GG) | 2.66 (1.66-4.25), <0.001 | 2.63 (1.32-5.22), 0.006 | 0.13 | 49.87 | NA | |
| Co-dominant (GA vs. GG) | 1.05 (0.77-1.45), 0.72 | 1.05 (0.77-1.45), 0.72 | 0.66 | 0.00 | NA | |
| Recessive (GG+GA vs. AA) | 2.36 (1.54-3.61), <0.001 | 2.45 (1.08-5.56), 0.03 | 0.03 | 69.44 | NA |
Figure 2.
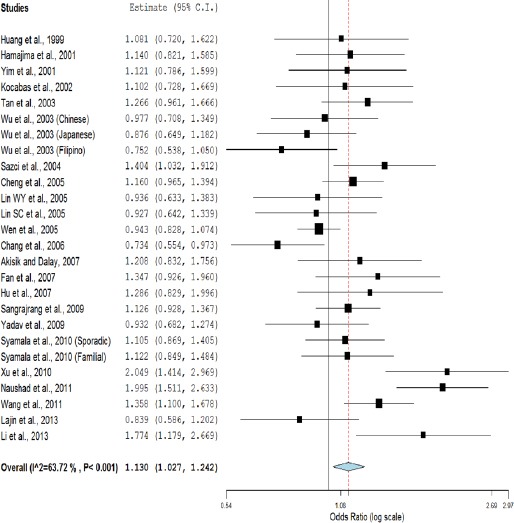
Random Effect Forest Plot of Allele Contrast Model (A vs. G) of COMT G472A Polymorphism
Figure 3.
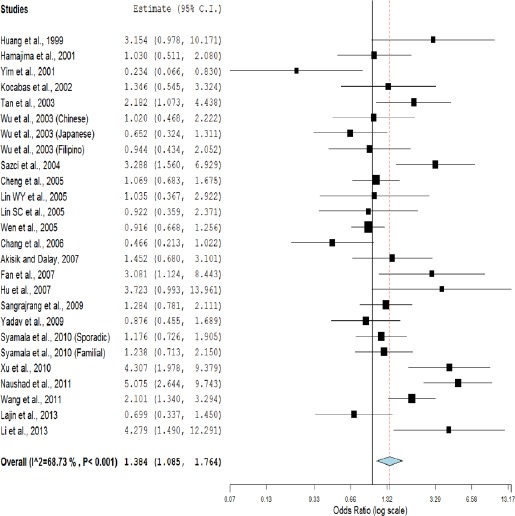
Random Effect Forest Plot of Homozygote Model (AA vs. GG) of COMT G472A Polymorphism
Figure 5.

Funnel Plots a-f, a. Precision by log odds ratio for additive model; b, standard error by log odds ratio for additive model; c, precision by log odds ratio for homozygote model; d, standard error by log odds ratio for homozygote model; e, precision by log odds ratio for recessive model; f, standard error by log odds ratio for recessive model.
Figure 4.

Random Effect Forest Plot of Recessive Model (GG+GA vs. AA) of COMT G472A Polymorphism
Subgroup analysis
Subgroup analysis were done on the basis of source of control (i.e. hospital based or population based) and status of menopause (i.e. premenopause and postmenopause). In total 26 studies, in 12 studies controls were selected from hospital and in remaining studies control samples were selected from population. In allele contrast meta-analysis with twelve studied of hospital based, showed significant association between COMT Val158Met polymorphism and breast cancer (ORAvsG = 1.18; 95%CI= 1.01-1.36; p= 0.02; I2=65.2%) and meta-analysis of eleven population based studies did not show any association(ORAvsG = 1.02; 95%CI= 0.94-1.10; p= 0.59; I2=48.22%). In three studies details of control samples were not given.
In eleven studies, subject were of premenopausal state, allele contrast meta-analysis showed meager association between COMT Val158Met polymorphism and breast cancer (ORAvsG= 1.11; 95%CI= 1.00-1.23; p= 0.03; I2= 38.27%), but meta-analysis of ten studies analysed postmenopausal subject did not show any association (ORAvsG= 1.04; 95%CI= 0.92-1.18; p= 0.46; I2= 20.18%). In four studies menopausal state of subjects were not mentioned.
Sensitivity analysis
In allele contrast meta-analysis, sensitivity analysis performed by exclusion of studies in which control population was not in HWE. Exclusion of two studies not in HWE (Yim et al., 2001;Sazciet al., 2004) did not affect heterogeneity but increased odds ratio (OR= 1.12; 95%CI= 1.01-1.23; p= 0.02).
Publication bias
Publication bias was absent in all five genetic models and P value of Egger’s test was greater than 0.05 (A vs G, p= 0.36; AG vs GG, p= 0.87; AA vs GG, p= 0.33; AA+AG vs GG, p= 0.61; AA vs AG+GG, p= 0.27). Funnel plots using standard error and precision were also symmetrical (Figure 5).
Discussion
Present meta-analysis of the association of the COMT Val158Met polymorphism with BC investigated 5,971 BC patients and 7,253 controls from 26 Asian case–control studies. The overall meta-analysis detected significant genetic association between the COMT Val158Met polymorphism and BC in Asian population. Five meta-analysis studies have been published so far on COMT Val 158Met polymorphism and breast cancer risk (Ding et al.,2010; He et al., 2012; Qin et al., 2012; Li et al., 2014; Wan et al., 2014) and reported no significant association. In all these five meta-analyses, information of Asian population is incomplete, hence present meta-analysis was conducted on case-control reports on Asian population and results suggested that the COMT Val158Met polymorphism is a risk factor for breast cancer development in the Asian population.
The COMT enzyme catalyzes the transfer of a methyl group from the S-adenosyl-L-methionine to the m-hydroxy group of catechol compounds, rendering the catechol estrogens more water soluble and enhancing excretion from the body (Service,1998; Ahsan et al., 2004). Several studies suggested protective role to COMT higher activity isoform, which protect reactive oxygen induced DNA damage, that are produced by estrogen oxidation(Onay et al., 2008). Prolonged exposure to estrogen is a risk factor for breast carcinoma (Hoffman et al., 1979; Amin et al., 1983; Lajin et al., 2013). Catechol estrogen metabolitesare genotoxic andcapable of initiating mammary tumors through their reactive metabolites by formation of depurinatingDNA adducts which are capable of creating de novo oncogenic mutations (Jan et al., 1998; Ahsan et al., 2004; Lajin et al., 2013).
Meta-analysis is a powerful statistical tool for analyzing cumulative data of case-control studies wherein the individual sample sizes are small and potentially investigates a large number of individuals and can estimate the effect of a genetic factor on the risk of the disease (Liwei et al., 2009; Li et al., 2013; Rai et al., 2014; Kumar et al., 2015). Several meta-analyses investigating the association of COMT Val158Met polymorphism with various disease/disorders have been published, like-attention-deficit/hyperactivity disorder (Sun et al., 2014), schizophrenia (Munafo et al., 2005), prostate cancer (Xiao et al., 2013; Zou et al., 2013) etc.
The present meta-analysis has few limitations like-(i) meta-analysis based on unadjusted data, (ii)there is marked heterogeneity among studies, and (iii)owing to the lack of information, gene-gene interactions were not done.
In conclusion, the results of present meta-analysis support significant association between the COMT Val158Met polymorphism and breast cancer risk in Asian population. The results should be interpreted cautiously due to presence of high heterogeneity. In future, case control studies from different ethnic populations with larger sample sizes should be carried out to confirm the association between COMT Val158Met polymorphism and breast cancer. Further, gene-gene and gene-environmental interactions should also be investigated.
Conflict of interest
None.
Acknowledgements
Authors are highly grateful to Leon Bax (Chief Scientific Officer at BiostatXL, UMC Utrecht) for his valuable suggestions which help us in statistical analysis. We also thank all authors of the included studies for their cooperation.
References
- Ahsan H, Chen Y, Whittemore AS, et al. A family-based genetic association study of variants in estrogen-metabolism genes COMT and CYP1B1 and breast cancer risk. Breast Cancer Res Treat. 2004;85:121, 31. doi: 10.1023/B:BREA.0000025401.60794.68. [DOI] [PubMed] [Google Scholar]
- Akisik E, Dalay N. Functional polymorphism of thymidylate synthase, but not of the COMT and IL-1B genes, is associated with breast cancer. J Clin Lab Anal. 2007;21:97–102. doi: 10.1002/jcla.20139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin A, Creveling C, Lowe M. Immunohistochemical localization of catechol methyltransferase in normal and cancerous breast tissues of mice and rats. J Natl Cancer Inst. 1983;70:337–42. [PubMed] [Google Scholar]
- Chang TW, Wang SM, Guo YL, et al. Glutathione S-transferase polymorphisms associated with risk of breast cancer in southern Taiwan. Breast J. 2006;15:754–61. doi: 10.1016/j.breast.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Chen CH, Lee YR, Wei FC, et al. Association study of NlaIII and MspI genetic polymorphismsof catechol-O-methyltransferase gene and susceptibilityto schizophrenia. Biol Psychiatry. 1997;41:985, 7. doi: 10.1016/S0006-3223(97)00045-0. [DOI] [PubMed] [Google Scholar]
- Cheng TC, Chen ST, Huang CS, et al. Breast cancer risk associated with genotype polymorphism of the catechol estrogen-metabolizing genes:a multigenic study on cancer susceptibility. Int J Cancer. 2005;113:345, 53. doi: 10.1002/ijc.20630. [DOI] [PubMed] [Google Scholar]
- Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10:101–29. [Google Scholar]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177, 88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- Ding HX, Fu YY, Chen WX, Wang ZW. COMT Val158Met polymorphism and breast cancer risk:evidence from. 26 case–control studies. Breast Cancer Res Treat. 2010;123:265–70. doi: 10.1007/s10549-010-0759-5. [DOI] [PubMed] [Google Scholar]
- Egger M, Dave Smith G, Schneider M, Minde C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Feng Y, Wang L, Wang Y, Fu L. The relationship between catechol-O-methyltransferase (COMT) polymorphisms and the development of breast cancer. Chin J Clin Oncol. 2007;34:430–3. [Google Scholar]
- Gadow KD, Roohi J, DeVincent CJ, Kirsch S, Hatchwell E. Association of COMT (Val158Met) and BDNF (Val66Met) gene polymorphisms with anxiety, ADHD and Tics in children with Autism spectrum disorder. J Autism Dev Disord. 2009;39:1542, 51. doi: 10.1007/s10803-009-0794-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Ming J, Liu C, et al. A common polymorphism near the ESR1 Gene is associated with risk of breast cancer:Evidence from a case-control study and a meta-analysis. Plos One. 2012;7:e524455. doi: 10.1371/journal.pone.0052445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamajima N, Matsuo K, Tajima K, Mizutani M, Iwata H, Iwase T, et al. Limited association between a catechol-O-methyltransferase (COMT) polymorphism and breast cancer risk in Japan. Int J Clin Oncol. 2001;6:13, 8. doi: 10.1007/pl00012073. [DOI] [PubMed] [Google Scholar]
- He XF, Wei W, Li SX, et al. Association between the COMT Val158Met polymorphism and breast cancer risk:a meta-analysis of 30,199 cases and 38,922 controls. Mol Biol Rep. 2012;39:6811, 23. doi: 10.1007/s11033-012-1506-2. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Thompson SE. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539, 58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- Hoffman A, Paul S, Axelrod J. Catechol estrogen synthesis and metabolism by human breast tumors in vitro. Cancer Res. 1979;39:4584, 7. [PubMed] [Google Scholar]
- Hu Z, Song CG, Lu JS, et al. A multigenic study on breast cancer risk associated with genetic polymorphisms of ER Alpha, COMT and CYP19 gene in BRCA1/BRCA2 negative Shanghai women with early onset breast cancer or affected relatives. J Cancer Res Clin Oncol. 2007;133:969, 78. doi: 10.1007/s00432-007-0244-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CS, Chern HD, Chang KJ, et al. Breast cancer risk associated with genotype polymorphism of the estrogen-metabolizing genes CYP17, CYP1A1, and COMT:a multigenic study on cancer susceptibility. Cancer Res. 1999;59:4870, 75. [PubMed] [Google Scholar]
- Jan ST, Devanesan PD, Stack DE, et al. Metabolic activation and formation of DNA adducts of hexestrol, a synthetic nonsteroidal carcinogenic estrogen. Chem Res Toxicol. 1998;11:412, 9. doi: 10.1021/tx970141n. [DOI] [PubMed] [Google Scholar]
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69, 90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- Kayahan B, Kaymaz BT, Altıntoprak AE, et al. The lack of association between catechol-O-methyltransferase (COMT) Val108/158Met and brain-derived neurotrophic factor (BDNF) Val66Met polymorphisms and schizophrenia in a group of Turkish population. Neurol Psychiatry Brain Res. 2013;19:102, 8. [Google Scholar]
- Kocabas NA, Sardas S, Cholerton S, Daly AK, Karakaya AE. Cytochrome P450 CYP1B1 and catechol-O-methyltransferase (COMT) genetic polymorphisms and breast cancer susceptibility in a Turkish population. Arch Toxicol. 2002;76:643–9. doi: 10.1007/s00204-002-0387-x. [DOI] [PubMed] [Google Scholar]
- Kumar P, Yadav U, Rai V. Methylenetetrahydrofolate reductase gene C677T polymorphism and breast cancer risk:Evidence for genetic susceptibility. Meta Gene. 2015;6:72–84. doi: 10.1016/j.mgene.2015.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunugi H, Vallada HP, Hoda F, et al. No evidence for an association ofaffective disorders with high-or low-activity allele ofcatechol-O-methyltransferase gene. Biol Psychiatry. 1997;42:282, 5. doi: 10.1016/S0006-3223(96)00366-6. [DOI] [PubMed] [Google Scholar]
- Lachman HM, Papolos DF, Saito T, et al. Human catechol-O-methyltransferase pharmacogenetics:description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics. 1996;6:243, 50. doi: 10.1097/00008571-199606000-00007. [DOI] [PubMed] [Google Scholar]
- Lajin B, Hamzeh AR, Ghabreau L, et al. Catechol-O-methyltransferase Val 108/158 Met polymorphism andbreast cancer risk:a case control study in Syria. Breast Cancer. 2013;20:62–6. doi: 10.1007/s12282-011-0309-y. [DOI] [PubMed] [Google Scholar]
- Li J, Liu K, Jiang SF, et al. A case–control study of the relationship among environmental factors, COMT genetic polymorphisms and breast cancer. Hu Bei Wen Li Xue Yuan Xue Bao. 2013;34:82, 8. [Google Scholar]
- Li K, Li W, Zou H. Catechol-O-methyltransferase Val158Met polymorphism and breast cancer risk in Asian population. Tumour Biol. 2014;35:2343–50. doi: 10.1007/s13277-013-1310-1. [DOI] [PubMed] [Google Scholar]
- Lin SC, Chou YC, Wu MH, et al. Genetic variants of myeloperoxidase and catechol-O-methyltransferase and breast cancer risk. Eur J Cancer Prev. 2005;14:257, 61. doi: 10.1097/00008469-200506000-00010. [DOI] [PubMed] [Google Scholar]
- Lin WY, Chou YC, Wu MH, et al. Polymorphic catechol-O-methyltransferase gene, duration of estrogen exposure, and breast cancer risk:a nested case–control study in Taiwan. Cancer Detect Prev. 2005;29:427, 32. doi: 10.1016/j.cdp.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Liwei L, Chunyu L, Ruifa H. Association between manganese superoxide dismutase gene polymorphism and risk of prostate cancer:a meta-analysis. Urology. 2009;74:884, 8. doi: 10.1016/j.urology.2008.11.016. [DOI] [PubMed] [Google Scholar]
- Lotta T, Vidgren J, Tilgmann C, et al. Kinetics of human soluble and membrane bound catechol O-methyltransferase:a revised mechanism and description of the thermolabile variant of the enzyme. Biochemistry. 1995;34:4202, 10. doi: 10.1021/bi00013a008. [DOI] [PubMed] [Google Scholar]
- Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–48. [PubMed] [Google Scholar]
- Munafò MR, Bowes L, Clark TG, Flint J. Lack of association of the COMT (Val158/108 Met) gene and schizophrenia:a meta-analysis of case–control studies. Mol Psychiatry. 2005;10:765, 70. doi: 10.1038/sj.mp.4001664. [DOI] [PubMed] [Google Scholar]
- Nathanson KL, Wooster R, Weber BL. Breast cancer genetics:what we know and what we need. Nat Med. 2001;7:552, 6. doi: 10.1038/87876. [DOI] [PubMed] [Google Scholar]
- Naushad SM, Pavani A, Rupasree Y, et al. Modulatory effect of plasma folate and polymorphisms in one-carbon metabolism on catecholamine methyltransferase (COMT) H108L associated oxidative DNA damage and breast cancer risk. Indian J Biochem Biophysics. 2011;48:283, 9. [PubMed] [Google Scholar]
- Nobile M, Rusconi M, Bellina M, et al. COMT Val158Met polymorphism and socioeconomic status interact to predict attention deficit/hyperactivity problems in children aged 10–14. Eur Child Adolesc Psychiatry. 2010;19:549, 57. doi: 10.1007/s00787-009-0080-1. [DOI] [PubMed] [Google Scholar]
- Omrani MD, Bazargani S, Bagheri M, Yazdan-Nejad H. Association of catechol-o-methyl transferase gene polymorphism with prostate cancer and benign prostatic hyperplasia. J Res Med Sci. 2009;14:217, 22. [PMC free article] [PubMed] [Google Scholar]
- Onay UV, Aaltonen K, Briollais L, et al. Combined effect of CCND1 andCOMT polymorphisms and increased breast cancer risk. BMC Cancer. 2008;8:6. doi: 10.1186/1471-2407-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin X, Peng Q, Qin A, et al. Association of COMT Val158Met polymorphism and breast cancer risk:an updated meta-analysis. Diag Pathol. 2012;7:136. doi: 10.1186/1746-1596-7-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai V, Yadav U, Kumar P, Yadav SK, Mishra OP. Maternal methylenetetrahydrofolate reductase C677T polymorphism and down syndrome risk:a meta-analysis from 34 studies. PLoS One. 2014;9:e108552. doi: 10.1371/journal.pone.0108552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retz W, Reosler M, Kissling C, et al. Norepinephrine transporter and catecholamine-O-methyltransferase gene variants and attention-deficit/hyperactivity disorder symptoms in adults. J Neural Transm. 2008;115:323, 9. doi: 10.1007/s00702-007-0822-5. [DOI] [PubMed] [Google Scholar]
- Sangrajrang S, Sato Y, Sakamoto H, et al. Genetic polymorphisms of estrogen metabolizing enzyme and breast cancer risk in Thai women. Int J Cancer. 2009;125:837–43. doi: 10.1002/ijc.24434. [DOI] [PubMed] [Google Scholar]
- Sazci A, Ergul E, Utkan NZ, Canturk NZ, Kaya G. Catechol-O-methyltransferase Val 108/158 Met polymorphism in premenopausal breast cancer patients. Toxicology. 2004;204:197–202. doi: 10.1016/j.tox.2004.06.026. [DOI] [PubMed] [Google Scholar]
- Service RF. New role for estrogen in cancer? Science. 1998;279:1631, 3. doi: 10.1126/science.279.5357.1631. [DOI] [PubMed] [Google Scholar]
- Spielman RS, Weinshilboum RM. Genetics of red cell COMT activity:analysis of thermal stability and family data. Am J Med Genet. 1981;10:279–90. doi: 10.1002/ajmg.1320100311. [DOI] [PubMed] [Google Scholar]
- Sun Yuan F, Shen X, Xiong G, Wu J. Role of COMT in ADHD:a systematic meta-analysis. Mol Neurobiol. 2014;49:251, 61. doi: 10.1007/s12035-013-8516-5. [DOI] [PubMed] [Google Scholar]
- Syamala VS, Syamala V, Sheeja VR, et al. Possible risk modification by polymorphisms of estrogen metabolizinggenes in familial breast cancer susceptibility in an Indian population. Cancer Invest. 2010;28:304–11. doi: 10.3109/07357900902744494. [DOI] [PubMed] [Google Scholar]
- Tan W, Qi J, Xing DY, et al. Relation between single nucleotide polymorphism in estrogen-metabolizing genes COMT, CYP17 and breast cancer risk among Chinese women. Zhonghua Zhong Liu Za Zhi. 2003;25:453, 6. [PubMed] [Google Scholar]
- Tenhunen J, Salminen M, Lundström K, et al. Genomic organization of the human catechol O-methyltransferase gene and its expression from two distinct promoters. Eur J Biochem. 1994;223:1049, 59. doi: 10.1111/j.1432-1033.1994.tb19083.x. [DOI] [PubMed] [Google Scholar]
- Valente NLM, Vallada H, Cordeiro Q, et al. Catechol-O-methyltransferase (COMT) val158met Polymorphism as a Risk Factor for PTSD After Urban Violence. J Mol Neurosci. 2011;43:516, 23. doi: 10.1007/s12031-010-9474-2. [DOI] [PubMed] [Google Scholar]
- Vandenbergh DJ, Rodriguez LA, Miller IT, Uhl GR, Lachman HM. High-activity catechol-O-methyltransferase alleleis more prevalent in polysubstance abusers. Am J Med Genet. 1997;74:439, 42. [PubMed] [Google Scholar]
- Vinkers CH, Van Gastel WA, Schubart CD, et al. The effect of childhood maltreatment and cannabis use on adultpsychotic symptoms is modified by the COMT Val158Met polymorphism. Schizophr Res. 2013;150:303–11. doi: 10.1016/j.schres.2013.07.020. [DOI] [PubMed] [Google Scholar]
- Wallace BC, Dahabreh IJ, Trikalinos TA, et al. Closing the Gap Between Methodologists and End-Users:R as a Computational Back-end. J Stat Software. 2013;49:1–15. [Google Scholar]
- Wan GX, Cao YW, Li WQ, Li YC, Li F. The Catechol-O-Methyltransferase Val158Met Polymorphism Contributes to the Risk of Breast Cancer in the Chinese Population:An Updated Meta-Analysis. J Breast Cancer. 2014;17:149–56. doi: 10.4048/jbc.2014.17.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Li H, Tao P, et al. Soy isoflavones, CYP1A1, CYP1B1, and COMT polymorphisms, and breast cancer:a case-control study in southwestern China. DNA Cell Biol. 2011;30:585–95. doi: 10.1089/dna.2010.1195. [DOI] [PubMed] [Google Scholar]
- Wen W, Cai Q, Shu XO, et al. Cytochrome P450 1B1 and catechol-O-methyltransferase genetic polymorphisms and breast cancer risk in Chinese women:results from the shanghai breast cancer study and a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2005;14:329, 35. doi: 10.1158/1055-9965.EPI-04-0392. [DOI] [PubMed] [Google Scholar]
- Whitehead A. Meta-Analysis of Controlled Clinical Trials. West Sussex, England: John Wiley and Sons Ltd:Chichester; 2002. [Google Scholar]
- Wu AH, Tseng CC, Van Den Berg D, Yu MC. Tea intake, COMT genotype, and breast cancer in Asian–American women. Cancer Res. 2003;63:7526–9. [PubMed] [Google Scholar]
- Xiao L, Tong M, Jin Y, Huang W, Li Z. The l58Val/Met polymorphism of catechol-O-methyl transferase gene and prostate cancer risk:a meta-analysis. Mol Biol Rep. 2013;40:1835, 41. doi: 10.1007/s11033-012-2238-z. [DOI] [PubMed] [Google Scholar]
- Xu YJ, Ge YL, Zhang JY, Li FN, Zheng Z. Polymorphism of COMT, p21 and NuMA in sporadic breast cancer. Chin J Clin. 2010;4:591, 6. [Google Scholar]
- Yadav S, Singhal NK, Singh V, et al. Association of single nucleotide polymorphisms in CYP1B1 and COMT genes with breast cancer susceptibility in Indian women. Dis Markers. 2009;27:203, 10. doi: 10.3233/DMA-2009-0663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yim DS, Parkb SK, Yoo KY, et al. Relationship between the Val108/158Met polymorphism of catechol-O-methyl transferase and breast cancer. Pharmacogenetics. 2001;11:279, 86. doi: 10.1097/00008571-200106000-00001. [DOI] [PubMed] [Google Scholar]
- Zou LW, Xu XJ, Liu T, et al. No association between COMT Val158Met polymorphism and prostate cancer risk:A meta-analysis. Genet Test Mol Biomarkers. 2013;17:78–84. doi: 10.1089/gtmb.2012.0216. [DOI] [PubMed] [Google Scholar]


