Abstract
Objective:
To evaluate the diagnostic value of diffusion weighted magnetic resonance imaging (DW-MRI) in assessment of metastases in axillary lymph nodes (ALNs) in a sample of Iranian women with breast cancer.
Methods:
A total of 50 axillary lymph nodes from 30 female patients with histologically verified breast cancer were assessed by 1.5 T MRI. DWI was implemented at b-values of 50, 400 and 800 s/mm2. Short axis diameter, presence of fatty hilum and apparent diffusion coefficient (ADC) values (min, max and mean) of metastatic and non-metastatic ALNs was compared. Cutoff ADC values to discriminate between benign and malignant axillary lymph nodes were analyzed with receiver coefficient characteristic (ROC) curves.
Result:
The final histopathological examination revealed 46% (n=23) metastatic and 54% (n=27) non-metastatic ALNs. There was no statistically significant difference in short axis diameter between the two groups (p = 0.537). However there was significantly correlation between loss of fatty hilum and presence of metastases (p < 0.001) and ADC values (0.255 ± 0.19×10-3 mm2/s vs 0.616 ±0.3×10-3 mm2/s (ADC min), 1.088 ± 0.22×10-3 mm2/s vs 1.497 ± 0.24×10-3 mm2/s (ADC max) and 0.824 ± 0.103 ×10-3 mm2/s vs 1.098 ± 0.23 ×10-3 mm2/s (ADC mean)) of metastatic ALNs were significantly lower than those of non-metastatic ALNs (p < 0.001). The optimal mean ADC cut-off value for differentiation between metastatic and non-metastatic ALNs was 0.904×10-3 mm2/s which had a higher specificity (88.9%) and accuracy (91.8%) as compared with ADC min and ADC max.
Conclusion:
DWI-MRI and ADC values are promising imaging methods which can assess metastatic ALNs in breast cancer with high sensitivity, specificity and accuracy.
Keywords: DWI-MRI, axillary lymph nodes, breast cancer, metastasis
Introduction
Evaluation of the axillary lymph nodes in patients with breast cancer has increased greatly in recent decades (Yamaguchi et al., 2015). Due to the presence of metastases in the axillary lymph nodes is the only important prediction of long-term survival of patients with early-stage breast cancer, so accurate prediction and early diagnosis of the metastatic status of ALNs is very important to create a plan of treatment and grading for patients with early stages of breast cancer (Chung et al., 2014; Faeghi et al., 2014). There are various methods for the detection and grading of axillary lymph node disease, but their preoperative diagnostic accuracy, sensitivity and specificity are low and unsatisfactory (Kamitani et al., 2013).
ALNs dissection and biopsy are invasive methods that have long and short-term complications on patients with breast cancer and also negatively affect the quality of her life (Harnan et al., 2011; Mullen et al., 2013; Kim et al., 2014; Zaiton et al., 2016). Ultrasonography and mammography are also operator dependent and have limited ability for detecting deep lymph nodes (Cools-Lartigue and Meterissian, 2012; Yamaguchi et al., 2015). Computed tomography, CT perfusion and PET associated with CT-scan, didn’t report more accurate diagnostic results about metastatic axillary lymph node, and due to ionization risks don’t use in longitudinal studies (Nasu et al., 2010; An et al., 2014). Nowadays, MRI as a noninvasive method used for preoperative grading of metastatic axillary lymph nodes which has high sensitivity and specificity (Kim et al., 2014). Among MRI techniques, the features of DCE have not proven reliable for differentiation of abnormal nodes because many normal nodes show kinetics features typical of breast malignancy such as: delayed washout (Mortellaro et al., 2009).
DWI-MRI is a noninvasive technique that works on Brownian motion of water molecules. Apparent Diffusion Coefficient (ADC) value calculated by using two or more diffusion images which is depended on certain parameters such as size of cell, inside and outside cellular volume fraction and cell membrane permeability (Edelman et al., 2006). In many organs, increase of cellularity of malignant cells to benign causes reduces the amount of ADC. So metastatic ALNs have a limited diffusion (Fornasa et al., 2012). Diffusion-weighted MR imaging correlation with prognostic parameters is used for staging of breast cancer and monitoring of patient after therapy. In addition, DWI is used for the assessment of mediastina, Cervical, abdominal and pelvic lymph nodes (Razek et al., 2016). There are few studies on the role of this type of imaging in the evaluation of axillary lymph nodes. The aim of this study is to evaluate the diagnostic value of DWI in the assessment of metastatic axillary lymph node in a sample of Iranian women with breast cancer.
Materials and Methods
This study was prospectively performed from 20 October 2015 to 21 September 2016 at the MRI department of Bahman hospital, and carried out 30 female patients with histologically proven breast cancer.
The study was approved by the local ethical committee of the university, our patients were informed of the study aims and an informed written consent was obtained before being included.
The main cancer types consisted of 18 invasive ductal carcinoma, 12 invasive lobular carcinoma. None of these patients had received axillary clearance surgery, biopsy, chemotherapy or radiotherapy to axillary LNs before MRI examination. Patients’ age ranged between 39-78 years with mean age of 51.06 ± 9.61 and the affected side was left in 11 patients, right in 16 patients and bilateral affection in 3 cases.
After an MRI examination (2 weeks range from 2 to 15 days) all patients underwent axillary lymph node biopsy or surgical and received a definite pathological diagnosis.
MRI Examination
Breast and axillary MRI examination were performed using a 1.5-T system (Magneto Avanto Siemens, Erlangen Germany Trim [32×8]). All patients were scanned with dedicated breast coils (Breast Matrix A. trim coil) in the standard prone position. The MR protocol is as follows:
STIR-axial: TR: 8540 ms, TE: 59 ms, TI: 170 ms, slice thickness: 3.5 mm, flip angel: 142 d, matrix: 320×314, NEX: 1
T2W-TSE-axail-high resolution: TR: 3840 ms, TE: 81 ms, Flip angel: 180 d, slice thickness: 3.5 mm, Matrix: 448×448, NEX: 1
STIR-sagittal-high resolution: TR: 4500 ms, TE: 59 ms, TI: 170 ms, flip angel: 142 d, slice thickness: 3.5 mm, Matrix: 173×256, NEX:1
T1W-axial-non-fat sat: TR: 8.6 ms, TE: 4.7 ms, flip angel: 20 d, slice thickness: 1.3 mm, Matrix: 320×320, NEX:1
Dynamic MR Images were sequentially implemented before 0, 90, 180 and 270s after injection of 0.1–0.2 mmol/kg body weight of Gadopentatedimeglumine. T1W Axial fat sat (3D gradient echo) images were obtained with the following parameters: TR/TE: 4.4/1.2 ms, matrix: 384×276, slice thickness: 1.1 mm, NEX: 1
Diffusion Weighted Images was performed using single shot spin echo-echo planer image (SE EPI) sequence with parallel imaging (reduction factor = 2) and spectral, spatial fat suppression, in the axial plane on both breasts before contrast medium injection. Sensitizing diffusion gradients were done with b-values of 50, 400 & 800 s/mm2 with the following parameters: TR: 7700ms, TE: 89 ms, FOV: 380 mm, Flip angle: 90, NEX: 4, matrix: 192×192 pixels, Slice thickness: 5 mm. To reduce motion artifact, phase encoding direction was AP in the axial plane, Otherwise detection of lymph nodes due to artifact was difficult.
Image Analysis
All MR images were reviewed by one radiologist experience in the breast MRI interpretation. She was blinded to the pathology results of the ALNs.
We selected the ALNs with short axis larger than 5 mm. In our study, morphological MR image analysis also was performed on T2W image and dynamic 3D T1W image. Suspicious morphological criteria of metastatic ALNs included: short-axis diameter >10 mm, loss of fatty hilum, irregular margins or apparent speculation, rim or heterogeneous enhancement and diffusion restriction (Razek et al., 2016; Zaiton et al., 2016).
The ultimate number of axillary lymph nodes that selected in the analysis was 50 lymph nodes. For the assessment of ADC value a round or elliptical Region of Interest (ROI) was drawn manually with greatest diameter to covering nearly the inner margin of LN on the central slice of each lymph node on ADC map. The zone of the ROI in the selected LN ranged 31.02 ± 19.48 mm2, according to their different size. Trying to avoid addition of the margins at ROI; T2W-FSE images were selected as an anatomical reference, and Dynamic T1W-FS was used to detect any misidentification of blood vessels as lymph nodes and lastly at DWI. All the ADC values were averaged from three-time measurement and expressed as the mean ± standard deviation. Three ADC values (min ADC, max ADC, mean ADC) were calculated for all selected ALNs. All patients with detected axillary LNs by MRI examination were subjected to biopsy or surgical dissection.
Surgical Analysis
SLNB were implemented for all doubtful ALNs and in patients with metastatic ALNs on SLNB, ALN dissection was subsequently carried out. All pathological samples were interpreted by one expert pathologist in breast pathology, who was blinded to the MRI results. Correlation between the diffusion MR finding and pathological reports for these selected lymph nodes was performed.
Statistical analysis
The analysis of data was performed to assess statistical significant variances.
The loss of fatty hila, mean short axis and the ADC values (min, max and mean) were compared among benign and metastatic axillary lymph nodes by Chi-square and independent t-test.
ROC (Receiver operating characteristic) curves were plotted to find the optimal cutoff point of ADC values (min, max and mean) which are used to discriminate benign from metastatic axillary lymph nodes with calculation of accuracy, specificity, sensitivity, and area under the curve (AUC).
Accuracy, specificity, sensitivity, of data achieved by ADC-DWI examination in discriminating axillary LNs were evaluated using the histopathological results as the gold standard. Statistical analyses were performed by using SPSS, Version19.0.0.
Results
In this study, we examined the lymph nodes on node by node basis. Among the 50 surgically excised LNs, the final histopathological examination was 46% (n=23) metastatic and 54% (n=27) non-metastatic lymph nodes. High signal intensity at DWI images was significantly found in the metastatic nodes than in the non-metastatic nodes (p-value < 0.001). 26 ALNs (20 with and 6 without metastasis) were shown with high signal intensity on DWI image, while 24 ALNs (3 with and 21 without metastasis) was shown with low signal intensity on DWI images (Figure 1, 2 and 3).
Figure 1.
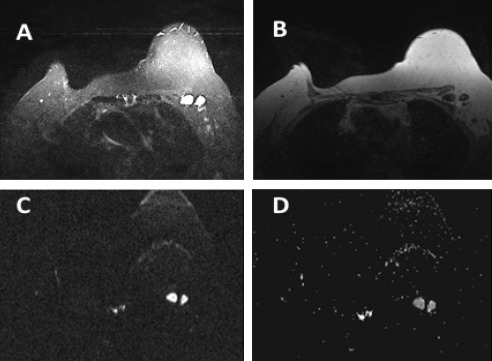
A 62 Year-Old Woman with Breast Cancer. A, STIR shows two enlarged left axillary LNs displaying round shape and attenuated fatty hila; B, T1W non-Fat Sat; C, DWI shows high signal intensity; D, ADC displays metastatic LNs with restricted diffusion and ADC value = 0.872 ×10-3 mm2/s
Figure 2.
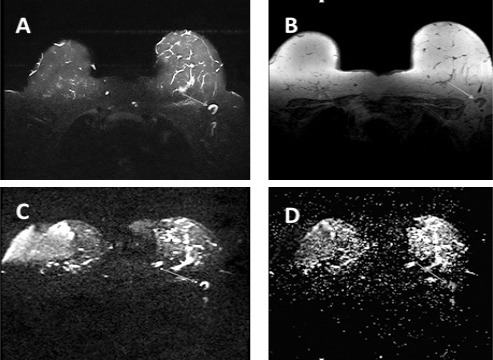
A 48 Year-Old Woman with IDC and ALN metastasis. A, STIR shows a hyper signal left ALN. B, T1W non-fat sat displays same ALN hypo signal (arrow); C, DWI shows the lymph node as an area of high signal intensity; D, Axial ADC shows restricted diffusion with low ADC value (0.653 ×10-3 mm2/s) which indicated a metastatic LN with a 0.904 ×10-3 mm2/s ADC cut-off
Figure 3.
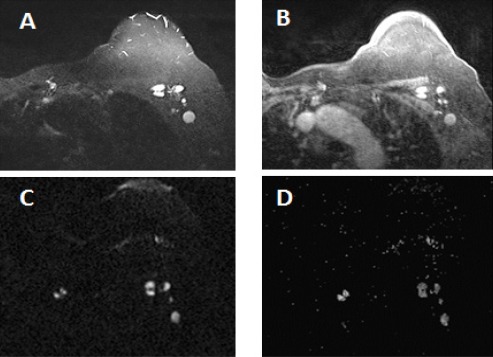
A 58 Year Old Woman with IDC and Multiple Left ALNs. A, STIR show high signal intensity of ALNs with variable shape, size and attenuated fatty hila; B, Dynamic breast MRI shows enhanced LNs; C, DWI shows high signal intensity; D, ADC displays restricted diffusion of LNs on ADC (0. 814 ×10-3 mm2/s).
The mean short-axis diameter of the lymph nodes with metastasis was 10.47 ± 2.91 mm and non-metastasis was 10.01 ± 2.35mm. This difference was not statistically significant with p-value = 0.537.
The loss of fatty hilum in non-metastatic ALNs was found statistically significant (p-value < 0.001). Fatty hilum existed in 24 (88.8%) non-metastatic LNs and 4 (17.39%) metastatic LNs and lost in 19 (82.60%0) metastatic and 3 (11.1%) non-metastatic LNs.
The mean ADC of metastatic ALNs was 0.824 ± 0.103 ×10-3 mm2/s and of non-metastatic ALNs was 1.098 ± 0.23 ×10-3 mm2/s. There was statistically difference in mean ADC values between metastatic and non-metastatic ALNs (p-value <0.001) (Figure 5).
Figure 4.
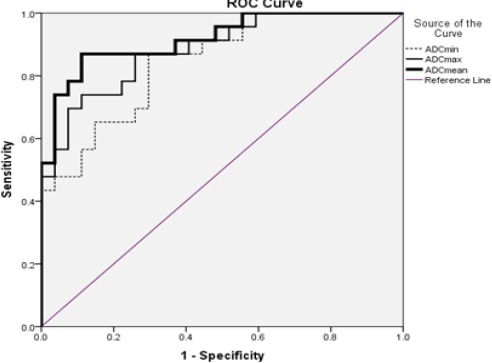
Receiver Operating Characteristic Curves for ADC Values (Min, Max and Mean) for Differentiation between Metastatic and Non-Metastatic ALNs
Figure 5.
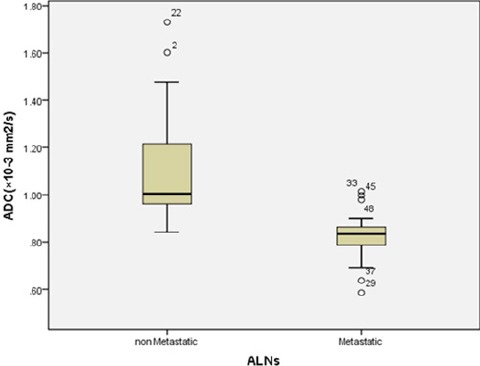
Box Plot of Mean ADC Value Demonstrate a Significant Difference (p-value < 0.001) between Metastatic and Non-Metastatic ALNs
In this study, statistical analysis of our results, based on ROC curve analysis, showed that optimal mean ADC cut off value for differentiation between malignant and benign lymph nodes was 0.904×10-3 mm2/s with accuracy 91.8%, sensitivity 87% and specificity 88.9%.
The max ADC value of metastatic ALNs (1.088 ± 0.22×10-3 mm2/s) lower than that of non-metastatic ALNs (1.497 ± 0.24×10-3mm2/s) (p-value < 0.001). The optimal maximum ADC cut-off value was 1.25×10-3mm2/s for differentiation between metastatic and non-metastatic LNs with sensitivity 78.35%, specificity 77.8% and accuracy 88.4%.
The min ADC of metastatic ALNs (0.255 ± 0.19×10-3 mm2/s) significantly lower than that of non-metastatic ALNs (0.616 ±0.3×10-3 mm2/s) (p-value <0.001). The optimal min ADC cut-off value was 0.444×10-3 mm2/s for differentiation between metastatic and non-metastatic LNs with sensitivity 87%, specificity 70.4% and accuracy 84.4% (Table 1 and 2).
Table 1.
Comparison of Sensitivity, Specificity and Accuracy of ADC Values
| Sensitivity | Specificity | Accuracy | Cut-off point a | |
|---|---|---|---|---|
| mean ADC value | 87.0% | 88.9% | 91.8% | 0.904 |
| min ADC value | 87.0% | 70.4% | 84.4% | 0.444 |
| Max ADC value | 78.3% | 77.8% | 88.4% | 1.258 |
a = ×10-3 mm2/s
Table 2.
Average ADC Values of Metastatic and Non-Metastatic ALNs
| min ADC a | max ADC a | mean ADC a | |
|---|---|---|---|
| Metastatic LNs | 0.255 ± 0.19 | 1.088 ± 0.22 | 0.824 ± 0.10 |
| Non-metastatic LNs | 0.616 ± 0.3 | 1.497 ± 0.24 | 1.098 ± 0.23 |
a = ×10-3 mm2/s
Discussion
Prediction the status of axillary lymph nodes is an important factor in the diagnosis and treatment planning in patients with breast cancer. Invasive methods such as; biopsy and dissection, and their side effects, also due to unsatisfactory sensitivity, specificity and accuracy of other imaging modalities of ALNs, Led the researchers to make more effort to solve these problems, aiming to decrease the cost, time and complicated risks of diagnosis (Chung et al., 2014; Zhou et al., 2015; Zaiton et al., 2016).
Axillary lymph nodes with short axis more than 10 mm are as a positive traditional indicator for malignancy. In studies of Razek (2016) and Chung (2014) they reported, metastatic ALNs are larger than non-metastatic types. While many other authors such as Zaiton (2016) and Azeem Ismail (2014) stated, there is no significant relationship between the size of the lymph nodes and the presence of metastasis. Also we found, the difference in the short axis diameter between metastatic (10.47 ± 2.91 mm) and benign nodes (10.01 ±2.35mm) is not statistically meaningful and there is an overlap in the diameter of the short axis between both groups (p-value = 0.537).
There are studies that have reported, the loss of fatty hilum as a predictor for metastatic ALNs (Mortellaro et al., 2009; Kaur et al., 2013; Zaiton et al., 2016). We also found similar results, therefor there was significantly correlation between loss of fatty hilum of ALNs and presence of metastatic nodes (p-value<0.001).
Differentiating between malignant and benign ALNs only on morphological criteria is constantly challenging (Chung et al., 2014; Razek et al., 2016). Diffusion Weighted Imaging is an unenhanced, simple, fast and non-invasive functional MRI sequence that has shown promising results in LN mapping in recent years (Azeem Ismail et al., 2014; Chung et al., 2014).
Axillary lymph nodes were shown higher signal intensity than muscle and surrounding normal vessels on DWI; so identification of nodes on DWI is easier than that on T2WI (Vandecaveye et al., 2009; Zaiton et al., 2016). Similar to Perrone (2011), Zaiton (2016) and Azeem Ismail (2014) we also found, there is a statistically meaningful relationship between high signal intensity on DWI and metastatic ALNs (p-value < 0.001). However, some other studies reported, may both inflammatory and malignant LNs have high signal intensity on DWI that make it difficult to identify (Wang et al., 2012; Vandecaveye et al., 2009).
ADC is a quantitative parameter that acquired by DWI images and can exclude the T2 shine-effect, also provide quantitative assessment of water diffusivity in the target tissues which makes it easier to differentiate between lesions (Azeem Ismail et al., 2014; Zaiton et al., 2016).
Similar to studies of Wang (2012) and Zaiton (2016) We also used high b-value of 800 s/mm2 to evaluate water diffusion more accurately and reduce the effects of capillary perfusion.
In our study, obtained mean ADC values were significantly lower for metastatic ALNs than non-metastatic type (0.824 ± 0.103 ×10-3 mm2/s vs 1.098 ± 0.23 ×10-3 mm2/s) that many other prior studies had similar results (Abdel Razek et al., 2006; Perrone et al., 2011; Wang et al., 2012; Azeem Ismail et al., 2014) (Figure 5). The cutoff value of mean ADC for discriminating benign from malignant was 0.904×10-3 mm2/s with a sensitivity of 87%, specificity of 88.9% and accuracy of 91.8%, which is virtually similar to the results of Yamaguchi (2015), Kim (2014) and Razek (2016). Min and max ADC values of metastatic ALNs (0.255± 0.19 ×10-3 mm2/s and 1.544 ± 0.27 ×10-3 mm2/s respectively) also were lower than those of non-metastatic ALNs (0.616 ±0.3 ×10-3 mm2/s and 1.214 ±0.21×10-3 mm2/s respectively).
In this study, we compared the diagnostic value of ADC min, max and mean. Based on ROC analysis, the mean and min ADC values showed same sensitivity (87%), but the mean ADC had higher accuracy (91.8%) and specificity (88.9%) compared with min and max ADC (Figure 4) and (Table 1).
There was a wide difference in the mean and cutoff value of ADC between the different studies. Some studies have demonstrated, this difference to that ADC value which had been affected by many factors, such as the magnetic field, MRI acquisition parameters, location, size and area of ROI, patient age as well as the body temperature (Zhang et al., 2013; Zaiton et al., 2016).
Few studies, such as Kamitani (2013) and Schipper (2015) reported opposite results; in their studies, the ADC value of metastatic ALNs was higher than that of non-metastatic one. These results may be explained by the difference in the histological types, the variation within lymph nodes at the cellular level and also heterogeneous ROI. In the necrotic tissue cell density is low, so the ADC value becomes higher. Also ADC value may increase in lymph nodes with inflammation due to edema if there is no inflammatory cell infiltration, reactive hyperplasia and fibrous connective tissue proliferation (Wang et al., 2012; Yamaguchi et al., 2015; Zaiton et al., 2016).
In this study we faced several limitations. First: sensitivity of MRI in detecting lymph nodes less than 5 mm is low due to the limited resolution of DWI-MR Imaging, so axillary lymph nodes smaller than 5mm were not evaluated. SLNB is currently the most accurate method to exclude small micro metastatic axillary lymph nodes in breast cancer (Schipper et al., 2015). Further improvement, to get higher spatial resolution DWI images and more anatomical details, 3.0T MRI is recommended to allow the study of smaller nodules. Second: unlike the breasts, axillary Lymph nodes were not located in the center of the field of the coil. Therefore, use of coils for axillary lymph nodes are recommended for better evaluation. Third: DW Images are very sensitive to artifacts such as motion, susceptibility, or chemical shift which makes it difficult to discover of lesions on DWI. The fourth limitation was the low number of patients. Additional studies with large number of subjects will increase the accuracy of the results of DWI-MRI and guide physicians involved in the diagnosis, treatment and follow up of breast cancer patients.
In conclusion, our results indicated that DWI-MRI and ADC values are promising imaging methods which can assess metastatic ALNs in breast cancer with higher sensitivity, specificity and accuracy.
References
- Abdel Razek AA, Soliman S, Elkhamary, et al. Role of diffusion-weighted MR imaging in cervical lymphadenopathy. Eur Radiol. 2006;16:1468–77. doi: 10.1007/s00330-005-0133-x. [DOI] [PubMed] [Google Scholar]
- An YS, Lee JK, Yoon SJ, et al. Diagnostic performance of 18F-FDG PET/CT, ultrasonography and MRI. Detection of axillary lymph node metastasis in breast cancer patients. Nuklearmedizin. 2014;53:89–94. doi: 10.3413/Nukmed-0605-13-06. [DOI] [PubMed] [Google Scholar]
- Azeem Ismail AA, Hasan DI, Abd-Alshakor H. Diagnostic accuracy of apparent diffusion coefficient value in differentiating metastatic form benign axillary lymph nodes in cancer breast. Egypt J Radiol Nucl Med. 2014;45:1011–16. [Google Scholar]
- Chung JJ, Youk JA, Kim HM, et al. Role of diffusion-weighted MRI:predicting axillary lymph node metastases in breast cancer. Acta Radiol. 2014;55:909–16. doi: 10.1177/0284185113509094. [DOI] [PubMed] [Google Scholar]
- Cools-Lartigue J, Meterissian S. Accuracy of axillary ultrasound in the diagnosis of nodal metastasis in invasive breast cancer:a review. World J Surg. 2012;36:46–54. doi: 10.1007/s00268-011-1319-9. [DOI] [PubMed] [Google Scholar]
- Edelman RR, Hesselink JR, Zlatkin MB, et al. Clinical magnetic resonance imaging, Diffusion weighted imaging. Philadelphia, PA, US: Saunders Elsevier; 2006. p. 342. [Google Scholar]
- Faeghi F, Baniasadipour B, Jalalshokouhi J. Comparative investigation of single voxel magnetic resonance spectroscopy and dynamic contrast enhancement MR imaging in differentiation of benign and malignant breast lesions in a sample of Iranian women. Asian Pac J cancer Prev. 2014;16:8335–8. doi: 10.7314/apjcp.2015.16.18.8335. [DOI] [PubMed] [Google Scholar]
- Fornasa F, Nesoti MV, Bovo C, et al. Diffusion-weighted magnetic resonance imaging in the characterization of axillary lymph nodes in patients with breast cancer. J Magn Reson Imaging. 2012;36:858–64. doi: 10.1002/jmri.23706. [DOI] [PubMed] [Google Scholar]
- Harnan SE, Cooper KL, Meng Y, et al. Magnetic resonance for assessment of axillary lymph node status in early breast cancer:a systematic review and meta-analysis. Eur J Surg Oncol. 2011;37:928–36. doi: 10.1016/j.ejso.2011.07.007. [DOI] [PubMed] [Google Scholar]
- Kamitani T, Hatakenaka M, Yabuuchi H, et al. Detection of axillary node metastasis using diffusion-weighted MRI in breast cancer. Clin Imaging. 2013;37:56–61. doi: 10.1016/j.clinimag.2012.02.014. [DOI] [PubMed] [Google Scholar]
- Kaur N, Sharma P, Garg A, et al. Accuracy of individual descriptors and grading of nodal involvement by axillary ultrasound in patients of breast cancer. Int J Breast Cancer. 2013;2013:1–6. doi: 10.1155/2013/930596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EJ, Kim SH, Kang BJ, et al. Diagnostic value of breast MRI for predicting metastatic axillary lymph nodes in breast cancer patients:diffusion-weighted MRI and conventional MRI. Magn Reson Imaging. 2014;32:1230–6. doi: 10.1016/j.mri.2014.07.001. [DOI] [PubMed] [Google Scholar]
- Mortellaro VE, Marshall J, Singer L, et al. Magnetic resonance imaging for axillary staging in patients with breast cancer. J Magn Reson Imaging. 2009;30:309–12. doi: 10.1002/jmri.21802. [DOI] [PubMed] [Google Scholar]
- Mullen R, Purdie CA, Jordan LB, et al. Can additional histopathological examination of ultrasound-guided axillary lymph node core biopsies improve preoperative diagnosis of primary breast cancer nodal metastasis? Clin Radiol. 2013;68:704–7. doi: 10.1016/j.crad.2013.01.020. [DOI] [PubMed] [Google Scholar]
- Nasu Y, Shikishima H, Miyasaka Y, et al. A study of the assessment of axillary lymph nodes before surgery for breast cancer using multidetector-row computed tomography. Surg Today. 2010;40:1023–6. doi: 10.1007/s00595-009-4185-4. [DOI] [PubMed] [Google Scholar]
- Perrone A, Guerrisi P, Izzo L, et al. Diffusion-weighted MRI in cervical lymph nodes:differentiation between benign and malignant lesions. Eur J Radiol. 2011;77:281–6. doi: 10.1016/j.ejrad.2009.07.039. [DOI] [PubMed] [Google Scholar]
- Razek AA, Lattif MA, Denewer A, et al. Assessment of axillary lymph nodes in patients with breast cancer with diffusion-weighted MR imaging in combination with routine and dynamic contrast MR imaging. Breast Cancer. 2016;23:525–32. doi: 10.1007/s12282-015-0598-7. [DOI] [PubMed] [Google Scholar]
- Schipper RJ, Paiman ML, Beets-Tan RG, et al. Diagnostic performance of dedicated axillary T2-and diffusion-weighted MR Imaging for Nodal Staging in Breast Cancer. Radiol. 2015;275:345–55. doi: 10.1148/radiol.14141167. [DOI] [PubMed] [Google Scholar]
- Vandecaveye V, De Keyzer F, Vander Poorten V, et al. Head and neck squamous cell carcinoma:value of diffusion-weighted MR imaging for nodal staging. Radiol. 2009;251:134–46. doi: 10.1148/radiol.2511080128. [DOI] [PubMed] [Google Scholar]
- Wang J, Liao Q, Zhang Y, et al. Differential diagnosis of axillary inflammatory and metastatic lymph nodes in rabbit models by using diffusion-weighted imaging:compared with conventional magnetic resonance imaging. Korean J Radiol. 2012;13:458–66. doi: 10.3348/kjr.2012.13.4.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi K, Schacht D, Nakazono T, et al. Diffusion weighted images of metastatic as compared with nonmetastatic axillary lymph nodes in patients with newly diagnosed breast cancer. J Magn Reson Imaging. 2015;42:771–8. doi: 10.1002/jmri.24829. [DOI] [PubMed] [Google Scholar]
- Zaiton F, Shehata SM, Abo Warda MH, et al. Diagnostic value of MRI for predicting axillary lymph nodes metastasis in newly diagnosed breast cancer patients:Diffusion-weighted MRI. Egypt J Radiol Nucl Med. 2016;47:659–67. [Google Scholar]
- Zhang F, Zhu L, Huang X, et al. Differentiation of reactive and tumor metastatic lymph nodes with diffusion-weighted and SPIO-enhanced MRI. Mol Imaging Biol. 2013;15:40–7. doi: 10.1007/s11307-012-0562-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M, Lu B, Lv G, et al. Differential diagnosis between metastatic and non-metastatic lymph nodes using DW-MRI:a meta-analysis of diagnostic accuracy studies. J Cancer Res Clin Oncol. 2015;141:1119–30. doi: 10.1007/s00432-014-1895-9. [DOI] [PubMed] [Google Scholar]


