Abstract
Objectives:
To determine associations, if any, of bacterial vaginosis with cervical pre-neoplastic lesions and evaluate any effects of sub-categorization of smears with bacterial vaginosis.
Methods:
All cervico-vaginal smears reported as positive for bacterial vaginosis over a five-year period were reviewed and sub-categorized into ’type I (dysbacteriosis)’ and ’type II (pure Gardenerella infection)’ smears by two cytopathologists (PS, SG). The proportion of smears with healthy flora and pre-neoplastic lesions was compared with those having bacterial vaginosis in conjunction with such changes. In addition, a comparison was also attempted between the frequencies of pre-neoplastic lesions with the two categories of bacterial vaginosis smears.
Results:
Bacterial vaginosis was diagnosed in 28.6% (7017 of the 24,565) of the 24,565 smears received in the Institute during the study period. Of these 7,017 smears with bacterial vaginosis, 53% (3717) were categorized as type I and 42.7% (3000) as type II by both cytopathologists. Pre-neoplastic lesions were detected in 10.2% of smears with bacterial vaginosis compared to 5.7% of those with healthy flora (P<0.0001). Of the sub-categories of bacterial vaginosis, the risk of detecting precancerous lesion was higher for type II smears (P<0.001).
Conclusion:
Sub-categorization of bacterial vaginosis, as performed in the Dutch coding system, may be worthwhile due to the strikingly different risk of associated preneoplasia.
Keywords: Bacterial vaginosis, cervical intraepithelial neoplasia, association, dysbacteriosis
Introduction
Bacterial vaginosis (BV), one of the commonest vaginal disorders in females of reproductive age, is associated with a number of obstetric and gynaecologic complications (Morris et al., 2001). The prevalence of BV has varied in studies from 10 to 64% (Sodhani et al., 2005), depending on the method of detection used. Though the gold standard of diagnosis of BV is Nugent’s scoring of gram-stained smears, other criteria are being widely used because of the large number of asymptomatic women with BV infection (Schnadig et al., 1989; Amsel et al., 1983). Our initial study had demonstrated good sensitivity, specificity and positive predictive value for Pap smear in diagnosis of BV (Sodhani et al., 2005).
Though the Bethesda system for reporting of cervico-vaginal smears recognizes only one category of “shift in vaginal flora, suggestive of bacterial vaginosis”, studies have shown a distinction between “dysbacteriosis” and “pure Gardenerella infection” (Meisels and Morin, 2007; Bulk et al., 2004). Cervical cytology has been shown to be an effective tool for differentiation of these two entities (Klomp et al., 2009). These two sub-categories of bacterial vaginosis have been shown to exert differing impact on the risk of detection of pre-cancerous lesion in the same smear (Klomp et al., 2008; Roeters et al., 2010).
Cervical cancer and pre-cancerous lesion have been causatively linked to persistent infection by human papillomavirus (Huh, 2009). However, numerous factors, including other sexually transmitted diseases, have been postulated to influence the progression from infection to high grade lesion (Hawes and Kiviat, 2002). In this context, BV has also been implicated to play a role in cervical carcinogenesis (Platz-Christensen et al., 1989). Many authors have supported this association of BV with cervical pre-cancerous lesions (Watts et al., 2005; Verbruggen et al., 2006; Gillet et al., 2011). This possible association has been explained on the basis of higher vaginal pH in BV infection predisposing to persistence of HPV, production of carcinogenic nitrosamines and altered inflammatory cytokine profile (Pavić, 1984). However, other researchers have refuted this association of BV with cervical neoplasia (Peters et al., 1995; Jahic et al., 2013).
The present study was aimed at evaluating the association, if any, of bacterial vaginosis in cervical smear and detection of pre-neoplastic lesion in the same smear. Also, an attempt has been made to sub-categorize the smears with bacterial vaginosis into dysbacteriosis and Gardenerella infection with intent to study the difference in their respective association with pre-neoplastic lesion detection.
Materials and Methods
The study included all cervico-vaginal smears reported in the division over a five year period (2011-15). Smears reported as shift in vaginal flora suggestive of bacterial vaginosis were reviewed in a blinded fashion by two cytopathologists (PS, SG) and categorized into type I smear pattern (smears with shortage of lactobacilli and abundance of coccobacilli in background and surface of squamous cells, i.e. clue cells); and type II smears (complete lack of lactobacilli and all coccobacilli gathered on surface of squamous cells covering them completely, i.e. blue mountain cells in Dutch coding system) (Bulk et al., 2004). The sub-categorization performed by the two investigators was compared as concordant or discordant.
The proportion of cervical smears with normal flora and pre-neoplastic lesion as well as that of smears with bacterial vaginosis and pre-neoplastic lesion were calculated and compared. Also, the frequency of association of cervical intraepithelial neoplasia with the two types of smears with bacterial vaginosis was also compared.
Results
Over the study period, a total of 24,565 women were screened for cervical cancer and precancerous lesions at our Institute. Of these, 7017 (28.6%) smears showed features of bacterial vaginosis (BV) on Pap smear examination. The results of sub-categorization of the smears with BV are tabulated in Table 1. As seen in the table, concordance of sub-categorization of BV smears was obtained in 95.7% (6717/7017) of smears. A mild predominance of type I smear (type I: type II 1.2:1) was noted.
Table 1.
Result of Concordance of Sub-Categorization of BV Smears
| Smear Pattern | Concordant diagnosis | Discordant diagnosis |
|---|---|---|
| Type I | 3,717 | 300 |
| Type II | 3,000 | 300 |
| 6,717 |
Among the smears with BV, 719 of 7017 (10.2%) smears had pre-neoplastic lesions compared to 1000 of 17,548 (5.7%) of the smears with normal flora, as depicted in Table 2. The frequency of detecting cervical intraepithelial neoplasia in smears with BV was significantly higher (OR 1.88, 95% CI 1.71-2.08) with P<0.0001.
Table 2.
Frequency of Pre-Neoplastic Lesion in Smears with BV vs Healthy Flora
| Type of bacterial flora on smear | No. of smears | No. of pre-neoplastic lesion (%) |
|---|---|---|
| Bacterial vaginosis | 7,017 | 719 (10.2) |
| Healthy flora | 17,548 | 1,000 (5.7) |
Figure 1.
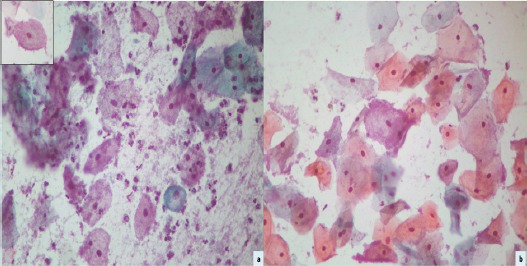
Photomicrograph of Cervical Smear Depicting ’Clue Cells’ (Inset) in a Background of Coccobacilli (Papanicolaou’s Stain x400). Another smear displaying ’blue mountain cell’ covered by coccobacilli (Papanicolaou’s stain x400).
Of the smears (719) with co-existing BV and pre-neoplastic lesion, 39.7% (286) were sub-categorized as type I and 61.3% (433) as type II smears (Table 3). The risk of detecting pre-neoplastic lesion was higher for type II smears (2.79, 95% CI 2.47-3.14) compared to type I smears (1.38, 95% CI 1.20-1.58) with P<0.001.
Table 3.
Comparison of Frequency of Pre-Neoplastic Lesion in Type of BV Smear
| Pre-neoplastic lesion | Type I smear (n=3,717) | Type II smear (n=3,000) |
|---|---|---|
| Present | 286 (7.7%) | 433 (14.4%) |
| Absent | 3,431 (92.3%) | 2,567 (85.6%) |
Discussion
Bacterial vaginosis (BV) is a syndrome of replacement of lactobacilli-dominated flora by a complete mix of strict and facultative anaerobic bacteria. The altered flora in BV is constituted mainly by gardenerella as also micrococci, streptococci and staphylococci (Frega et al., 1997). This is known to be associated with obstetric and gynaecologic complications such as preterm labour, chorioamnionitis, pelvic inflammatory disease, post-operative cuff infections and post-operative endometritis (Morris et al., 2001; Peipert et al., 1997). The prevalence of BV in different clinical settings has been reported to variably range from 10% to 64% (Kenyon et al., 2013). An earlier study by the authors reported the prevalence of BV in urban slum of Delhi as 41.5% (Sodhani et al., 2005). In the present report, BV was detected in 28.5% of smears from women attending gynaecology outpatient department. The gold standard for diagnosis of BV is considered to be Nugent’s scoring of a gram-stained smear (Nugent et al., 1991). However, since about 50% of patients with BV infection are asymptomatic, Nugent’s scoring may not be performed on all women. Other criteria like Schnadig et al (20% or more clue cells in Pap smear) and modified Amsel’s criteria have been used in various studies for diagnosis of BV (Schnadig et al., 1989; Amsel et al., 1983). An earlier study from our Institute had demonstrated the sensitivity of Pap smear as 78% and specificity and positive predictive value to be 86.9% for diagnosis of BV (Sodhani et al., 2005). Hence, we have adopted Pap smear as our standard test for diagnosis of BV
The Bethesda system for reporting of cervico-vaginal smears identifies one category of “shift in flora suggestive of bacterial vaginosis” (Nayar and Wilbur, 2015). However, as per Meisels and Morin, dysbacteriosis is a cytological subdivision of this category and represents microscopic diagnosis of shift in vaginal flora (Naylor, 2007). Hence, the Dutch coding system (KOPAC) introduced in 1988 recognized two categories of “dysbacteriosis O3” and “Gardenerella vaginalis O5”, the former showing clue cells along with coccobacilli in background while the latter displays blue mountain cells which are completely covered by a mountain of blue staining round bacteria (Bulk et al., 2004). Earlier studies have demonstrated that cervical cytology can well be exploited to differentiate between these two categories, the significance being the clinical importance of pure Gardenerella infection (Klomp et al., 2009). Our results in the present study confirm the utility of Pap smear in distinction of dysbacteriosis from pure Gardenerella infection.
Cervical cancer and precancer has been causatively linked to infections by oncogenic types of human papillomavirus (HPV) (Huh, 2009). However, numerous risk factors like age at first intercourse, multiplicity of sexual partners, cigarette smoking, high parity and low socioeconomic status have been found to contribute to development of cervical cancer (Juneja et al., 2003). Other sexually transmitted infections such as Chlamydia, herpes simplex virus and human immunodeficiency virus (HIV) have been proposed to influence progression from HPV infection to high grade lesion (Hawes and Kiviat, 2002). Similarly, BV has been suggested to play a role in cervical carcinogenesis. Platz-Christensen et al reported a significantly increased risk of CIN if BV was present (Platz-Christensen et al., 1989). This finding has been supported by other authors as well (Watts et al., 2005; Verbruggen et al., 2006). A study from Netherlands reported that women with dysbacteriosis had a higher risk of development of pre-neoplastic lesion (low-grade or high-grade squamous intraepithelial lesion) in follow-up smear while those with Candida infection had no increased risk of squamous intraepithelial lesion (Engberts et al., 2007). A meta-analysis by Gillet et al (2011) reported a positive association between BV and cervical HPV infection (OR 1.43, 95% CI 1.11-1.84) (Gillet et al., 2011). Several hypotheses have been proposed to explain the role of BV in HPV infection and cervical carcinogenesis. It has been suggested that the higher vaginal pH in BV may lead to arrest of squamous metaplasia and prolong the period of vulnerability of the transformation zone to oncogenic agents like HPV (Hudson et al., 1997). In addition, the volatile amines released by BV-associated anaerobes can form nitrosamines, which are carcinogenic capable of forming DNA adducts (Pavić, 1984). An alternative hypothesis proposed is the alteration in inflammatory cytokine profile that promotes development of cervical precancerous lesions (Cauci, 2004). A co-factor is the lack of hydrogen-peroxide (H2O2)-producing lactobacilli. H2O2-hypochlorous acid generation is a natural antitumor immune mechanism of vaginal environment. Absence of this mechanism may lead to persistence of transformed cells with subsequent progression (Bauer, 2001). More recent studies, including a meta-analysis by Gillet et al., (2012) have also reported a positive association between BV and presence of CIN (Nam et al., 2009; Roeters et al., 2010; Gillet et al., 2012). Our results are in consonance with these published studies.
However, few authors have refuted this association of BV with cervical neoplasia. Peters et al (1995) did not find any association between BV and dyskaryosis or severity of CIN (Peters et al., 1995). Study by Jahic et al also showed no significant difference in the frequency of BV in LSIL and normal cervical smear (Jahic et al., 2013). Hence, this question of any plausible association between BV and cervical precancer and cancer is still unanswered with conflicting results.
Though the Bethesda system does not differentiate between dysbacteriosis and pure Gardenerella infection, the Dutch coding system recognizes this distinction. An earlier study using the Dutch coding showed a higher risk of high-grade lesion in smears with dysbacteriosis compared to those with healthy flora (OR 2.0, 95% CI 1.8-2.3). On the other hand, the odds ratio for preneoplasia with pure Gardenerella infection was higher (OR 10.3, 95% CI 6.6-16.1) (Klomp et al., 2008). Similar results have been reported by Roeters et al who have found higher ORs for Gardenerella vaginalis (11.8, 95% CI 7.4-19.0) compared to dysbacteriosis (1.8, 95% CI 1.5-2.2) in screening smears (Roeters et al., 2010). In the present study, we attempted to classify our smears with B.V in a similar mechanism as Type I (representing dysbacteriosis) and type II (pure Gardenerella infection). Similar to previous studies, we also found a higher OR for detection of pre-neoplastic lesion in type II smears (2.79, 95% CI 2.47-3.14) compared to type I smears (1.38, 95% CI 1.20-1.58). The basis of this difference has not been outlined as yet. We hypothesize that the difference in the bacterial load between dysbacteriosis and Gardenerella infection with consequent variation in the level of carcinogenic substance production might underlie the observed disparity in the association of the two types of smears with cervical preneoplasia. A limitation of the present study was the inability to perform high-risk HPV testing in our cases due to financial constraints.
In conclusion, the association of bacterial vaginosis and cervical preneoplasia is still an unsettled issue. Nevertheless, careful screening is warranted in cases of such inflammatory events, since these are influenced by the same factors that are postulated to play significant role in cervical carcinogenesis. In addition, it seems worthwhile to classify the smears as dysbacteriosis and pure Gardenerella infection, since the risk of co-existing pre-neoplastic lesion differs between the two types of smears.
References
- Amsel R, Totten PA, Spiegel CA, et al. Nonspecific vaginitis. Diagnostic criteria and microbial and epidemiologic associations. Am J Med. 1983;74:14–22. doi: 10.1016/0002-9343(83)91112-9. [DOI] [PubMed] [Google Scholar]
- Bauer G. Lactobacilli-mediated control of vaginal cancer through specific reactive oxygen species interaction. Med Hypotheses. 2001;57:252–7. doi: 10.1054/mehy.2000.1285. [DOI] [PubMed] [Google Scholar]
- Bulk S, Van Kemenade FJ, Rozendaal L, Meijer CJ. The Dutch CISOE-A framework for cytology reporting increases efficacy of screening upon standardisation since 1996. J Clin Pathol. 2004;57:388–93. doi: 10.1136/jcp.2003.011841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauci S. Vaginal immunity in bacterial vaginosis. Curr Infect Dis Rep. 2004;6:450–6. doi: 10.1007/s11908-004-0064-8. [DOI] [PubMed] [Google Scholar]
- Engberts MK, Verbruggen BS, Boon ME, van Haaften M, Heintz AP. Candida and dysbacteriosis:a cytologic, population-based study of 100,605 asymptomatic women concerning cervical carcinogenesis. Cancer. 2007;111:269–74. doi: 10.1002/cncr.22947. [DOI] [PubMed] [Google Scholar]
- Frega A, Stentella P, Spera G. Cervical intraepithelial neoplasia and bacterial vaginosis:correlation or risk factor? Eur J Gynaecol Oncol. 1997;18:76–7. [PubMed] [Google Scholar]
- Gillet E, Meys JF, Verstraelen H, et al. Bacterial vaginosis is associated with uterine cervical human papillomavirus infection:a meta-analysis. BMC Infect Dis. 2011;11:10. doi: 10.1186/1471-2334-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillet E, Meys JF, Verstraelen H, et al. Association between bacterial vaginosis and cervical intraepithelial neoplasia:systematic review and meta-analysis. PLoS One. 2012;7:e45201. doi: 10.1371/journal.pone.0045201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawes SE, Kiviat NB. Are genital infections and inflammation cofactors in the pathogenesis of invasive cervical cancer? J Natl Cancer Inst. 2002;94:1592–3. doi: 10.1093/jnci/94.21.1592. [DOI] [PubMed] [Google Scholar]
- Hudson MM, Tidy JA, McCulloch TA, Rogstad KE. When is bacterial vaginosis not bacterial vaginosis?-a case of cervical carcinoma presenting as recurrent vaginal anaerobic infection. Genitourin Med. 1997;73:306–7. doi: 10.1136/sti.73.4.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh WK. Human papillomavirus infection:a concise review of natural history. Obstet Gynecol. 2009;114:139–43. doi: 10.1097/AOG.0b013e3181ab6878. [DOI] [PubMed] [Google Scholar]
- Jahic M, Mulavdic M, Hadzimehmedovic A, Jahic E. Association between aerobic vaginitis, bacterial vaginosis and squamous intraepithelial lesion of low grade. Med Arch. 2013;67:94–6. doi: 10.5455/medarh.2013.67.94-96. [DOI] [PubMed] [Google Scholar]
- Juneja A, Sehgal A, Mitra AB, Pandey A. A survey on risk factors associated with cervical cancer. Indian J Cancer. 2003;40:15–22. [PubMed] [Google Scholar]
- Kenyon C, Colebunders R, Crucitti T. The global epidemiology of bacterial vaginosis:a systematic review. Am J Obs Gynecol. 2013;209:505–23. doi: 10.1016/j.ajog.2013.05.006. [DOI] [PubMed] [Google Scholar]
- Klomp JM, Boon ME, Van Haaften M, Heintz AP. Cytologically diagnosed Gardnerella vaginalis infection and cervical (pre)neoplasia as established in population-based cervical screening. Am J Obstet Gynecol. 2008;199:480–5. doi: 10.1016/j.ajog.2008.04.036. [DOI] [PubMed] [Google Scholar]
- Klomp JM, Ouwerkerk-Noordam E, Boon ME, van Haaften M, Heintz AP. Gardnerella infection as distinguished from cervical dysbacteriosis:the advantageous spin-off of cervical screening. Acta Cytol. 2009;53:389–95. doi: 10.1159/000325338. [DOI] [PubMed] [Google Scholar]
- Morris M, Nicoll A, Simms I, Wilson J, Catchpole M. Bacterial vaginosis:a public health review. BJOG. 2001;108:439–50. doi: 10.1111/j.1471-0528.2001.00124.x. [DOI] [PubMed] [Google Scholar]
- Nam KH, Kim YT, Kim SR, et al. Association between bacterial vaginosis and cervical intraepithelial neoplasia. J Gynecol Oncol. 2009;20:39–43. doi: 10.3802/jgo.2009.20.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayar R, Wilbur DC. The bethesda system for reporting cervical cytology. Definitions, criteria and explanatory notes. Switzerland: Springer international publishing; 2015. pp. 79–82. [Google Scholar]
- Naylor B. Cytopathology of the uterus:Historical presepctives. In: Meisels A, Morin C, editors. Modern uterine cytopathology. Moving to the molecular smear. Vol. 2007. Chicago IL: ASCP Press; 2007. p. 18. [Google Scholar]
- Nugent RP, Krohn MA, Hillier S. Reliability of diagnosing BV is improved by a standardized method of gram stain interpretation. J Clin Microbiol. 1991;29:297, 301. doi: 10.1128/jcm.29.2.297-301.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavić N. Is there a local production of nitrosamines by the vaginal microflora in anaerobic vaginosis/trichomoniasis? Med Hypotheses. 1984;15:433–6. doi: 10.1016/0306-9877(84)90159-2. [DOI] [PubMed] [Google Scholar]
- Peipert JF, Montagno AB, Cooper AS, Sung CJ. Bacterial vaginosis as a risk factor for upper genital tract infection. Am J Obstet Gynecol. 1997;177:1184–7. doi: 10.1016/s0002-9378(97)70038-3. [DOI] [PubMed] [Google Scholar]
- Peters N, Van Leeuwen AM, Pieters WJ. Bacterial vaginosis is not important in the etiology of cervical neoplasia:a survey on women with dyskaryotic smears. Sex Transm Dis. 1995;22:296–302. doi: 10.1097/00007435-199509000-00005. [DOI] [PubMed] [Google Scholar]
- Platz-Christensen JJ, Larsson PG, Sundström E, Bondeson L. Detection of bacterial vaginosis in Papanicolaou smears. Am J Obstet Gynecol. 1989;160:132–3. doi: 10.1016/0002-9378(89)90104-x. [DOI] [PubMed] [Google Scholar]
- Roeters AM, Boon ME, van Haaften M, et al. Inflammatory events as detected in cervical smears and squamous intraepithelial lesions. Diagn Cytopathol. 2010;38:85–93. doi: 10.1002/dc.21169. [DOI] [PubMed] [Google Scholar]
- Schnadig VJ, Davie KD, Shafer SK, et al. The cytologist and bacterioses of the vaginal-ectocervical area Clues, commas and confusion. Acta Cytol. 1989;33:287–97. [PubMed] [Google Scholar]
- Sodhani P, Garg S, Bhalla P, et al. Prevalence of bacterial vaginosis in a community setting and role of the pap smear in its detection. Acta Cytol. 2005;49:634–8. doi: 10.1159/000326251. [DOI] [PubMed] [Google Scholar]
- Verbruggen BS, Boon ME, Boon LM. Dysbacteriosis and squamous (pre)neoplasia of immigrants and Dutch women as established in population-based cervical screening. Diagn Cytopathol. 2006;34:377–81. doi: 10.1002/dc.20374. [DOI] [PubMed] [Google Scholar]
- Watts DH, Fazzari M, Minkoff H, et al. Effects of bacterial vaginosis and other genital infections on the natural history of human papillomavirus infection in HIV-1-infected and high-risk HIV-1-uninfected women. J Infect Dis. 2005;191:1129–39. doi: 10.1086/427777. [DOI] [PubMed] [Google Scholar]


