Abstract
Background:
Despite intense interest in molecular characterization and searches for novel therapeutic targets, the glioblastoma remains a formidable clinical challenge. Among many contributors to gliomagenesis, chemokines have drawn special attention due to their involvement in a plethora of biological processes and pathological conditions. In the present study we aimed to elucidate any pro-gliomagenic chemokine axis and probable roles in development of glioblastoma multiforme (GBM).
Method:
An array of 84 chemokines, chemokine receptors and related genes were studied by real time PCR with comparison between low grade astrocytoma (diffuse astrocytoma – grade II) and high grade astrocytoma (glioblastoma multiforme – grade IV). Gene ontology analysis and database mining were performed to funnel down the important axis in GBM followed by validation at the protein level by immunohistochemistry on tissue microarrays.
Results:
Gene expression and gene ontology analysis identified CXCL8 as an important chemokine which was more frequently up-regulated in GBM as compared to diffuse astrocytoma. Further we demonstrated localization of CXCL8 and its receptors in glioblastoma possibly affecting autocrine and paracrine signalling that promotes tumor cell proliferation and neovascularisation with vascular mimicry.
Conclusion:
From these results CXCL8 appears to be an important gliomagenic chemokine which may be involved in GBM growth by promoting tumor cell proliferation and neovascularization via vascular mimicry. Further in vitro and in vivo investigations are required to explore its potential candidature in anti-GBM therapy.
Keywords: Chemokines, glioblastoma, CXCL8, CXCR1, CXCR2
Introduction
Glioblastoma multiforme (GBM) is the most aggressive and fatal form of brain tumor with median survival ranging between 12-15 months (Nakada et al., 2011; Brigliadori et al., 2016). Due to its highly invasive nature and limited success with conventional treatment, intense research is focussed on exploring new molecular markers that may help in developing novel therapeutic targets. The pathological hall mark of glioblastoma is its heterogeneneity and vascular endothelial proliferation which helps it to invade and infiltrate the brain (Hambardzumyan et al., 2015). Several genetic alterations have been associated with these tumors including cell-cycle and tumor suppressor gene regulated (p53), growth factor receptor mediated (EGFR or PDGFR) or other signalling pathways like PTEN (The Cancer Genome Atlas (TCGA) Research Network. 2008; Ohgaki. 2005; Lo. 2010; Cenciarelli et al., 2016).
Chemokines are a family of small chemo attractant cytokines acting via G-protein coupled receptors (GPCR) classically known for lymphocyte trafficking and recruitment at the site of inflammation (Kufareva. 2016, Domanska et al., 2011; Cartier et al.,2005; Mukaida et al., 2014). Within the tumor microenvironment, inflammatory cells may exert both tumor suppressing and tumor promoting effects. Chemokine expression is not limited to lymphocytes but tumors cells also express these molecules. Thus chemokines act as key mediators of the crosstalk between inflammatory and tumor cells that dictate pro or anti-tumor response (Tlsty et al.,2006; Crusz et al., 2015). Pro tumorigenic activity of chemokines has been reported in many cancers. They either directly influence the tumor growth by activating pathways related to cell survival and cell proliferation or indirectly by promoting angiogenesis (Cartier et al., 2005; Mukaida et al., 2014). In past, many isolated chemokine axis have been explored and their involvement in GBM progression has been studied (Hattermann et al., 2014; Sharma et al., 2016; Domanska et al., 2011)
In present study, a compendium of chemokines and their receptors in low grade diffuse astrocytoma and glioblastoma multifome (GBM) was comparatively studied to identify differential expression pattern between these variants of astrocytoma. Statistically significantly up-regulated genes in GBM were further analysed and classified by gene ontology analysis and database mining. Key genes thus identified were validated by high-throughput tissue microarray based immunohistochemistry.
Materials and Methods
Tissue Samples
Patient sample collection was done after taking approval from the institutional ethical committee of Safdarjung Hospital. Ten postsurgical tissue samples of GBM, ten postsurgical tissue samples of diffuse astrocytoma and two non-neoplastic post-mortem brain tissues were included in this study. Samples were stabilized in RNA later (LifeTechnologies, Ambion Cat. No. AM7020) and stored at -70°C for further processing. A portion of each tissue sample was fixed in 10% buffered formalin which was later processed and embedded in paraffin. Formalin fixed paraffin embedded (FFPE) sections were evaluated independently by two pathologists and a consensus in the diagnosis as well as its WHO grade was achieved. For Tissue microarray (TMA), 160 archival FFPE tissues were taken to construct TMA block.
RNA extraction and RT2 ProfilerTM PCR array and RT-PCR
Total RNA was isolated from tissue samples stabilized in RNA later using Qiagen RNeasy Mini Kit as per manufacturer’s guidelines. For RT2 ProfilerTM PCR array 0.5ug RNA was converted to cDNA using RT2 First Strand Kit (Qiagen) as per manufacturer’s guidelines. cDNA was then used in RT2 ProfilerTM PCR array Human Chemokines and Receptors (cat. No. PAHS-022Z). The array comprised of 84 target genes, 5 houskeeping genes, 3 Positive PCR controls and 3 Reverse Transcriptase Controls. Array was setup as per manufacturer’s guidelines on StepOne Plus RealTime PCR (Applied Biosystems). Results were analysed using Qiagen’s online webportal –Data Analysis Center.
Tissue Microarray and Immunohistochemistry and Immunofluorescence
Tissue microarray was constructed and standard immunohistochemical technique was followed as described previously (Sharma et al., 2016). Antibodies against CXCL8, CXCR2 were purchased from Biorbyt (UK), CXCR1 from Boster Bio (USA), GFAP (DAKO, Denmark), EnVision polymer detection system (Dako, Denmark), Anti-Rabbit / PE (Abcam, UK), Anti-Mouse/FITC(Dako, Denmark) and fluoroshield with DAPI from Sigma Alderich, USA. IDH1 R132H (clone H09; Dianova, Hamburg, Germany)
Statistical analysis
Statistical analysis was done using SPSS v17.0, Chicago, IL, USA. P values were calculated using Student’s t-test with two tailed distribution and value less than 0.05 was considered statistically significant. Bar graphs and line graphs were made using Microsoft Excel 2010.
Results
Differential gene expression profile of chemokines and their receptors in GBM and Diffuse astrocytoma
RT2 Profiler TM PCR array based gene expression study was done in 10 GBM and 10 Diffuse Astrocytoma cases, with 2 normal post mortem brain tissues. Age range of GBM patients was 25-65 years which included 8/10 (80%) males and 2/10 (20%) females. Age range of Diffuse astrocytoma patients was 26-71 years which comprised of 6/10 (60%) males and 4/10 (40%) females. Gene expression study revealed differential expression pattern of chemokines, chemokine receptors and related genes between GBM and diffuse astrocytoma. Figure.1a and b) summarizes the chemokines and their receptor genes which were up regulated and down-regulated in GBM and diffuse astrocytoma respectively.
Figure 1.
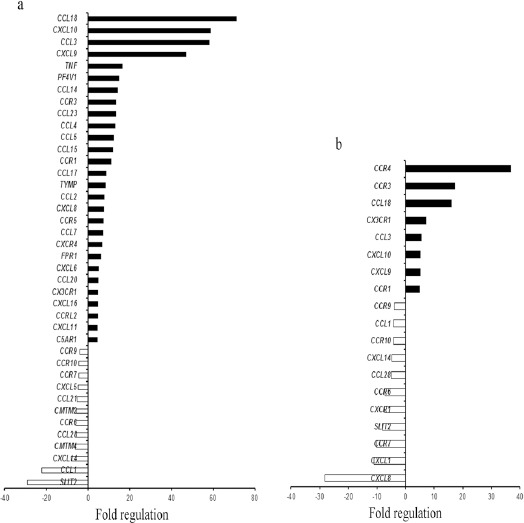
Chemokines and Their Receptors Gene Expression Profile in GBM and Diffuse Astrocytoma. Bar graph showing >4 fold gene regulation of chemokine and their receptors in a) GBM and b) Diffuse astrocytoma. Black bar indicate up-regulated genes and white bar indicates down-regulated genes.
Overall, a dramatic increase in expression of chemokines and chemokine receptor genes was observed in GBM when compared to diffuse astrocytoma. 28 genes were upregulated in GBM as compared to diffuse astrocytoma with 8 upregulated genes. Among these upregulated genes 7 were common in both GBM and diffuse astrocytoma. 12 genes exhibited negative regulation in GBM and 10 in diffuse astrocytoma while 8 genes were found to be common in both. Only one gene (CXCL8) displayed upregulation in GBM while downregulation in diffuse astrocytoma (Figure 2a). This gene expression pattern indicate an enhanced chemokine signalling in GBM in comparison to diffuse astrocytoma.
Figure 2.
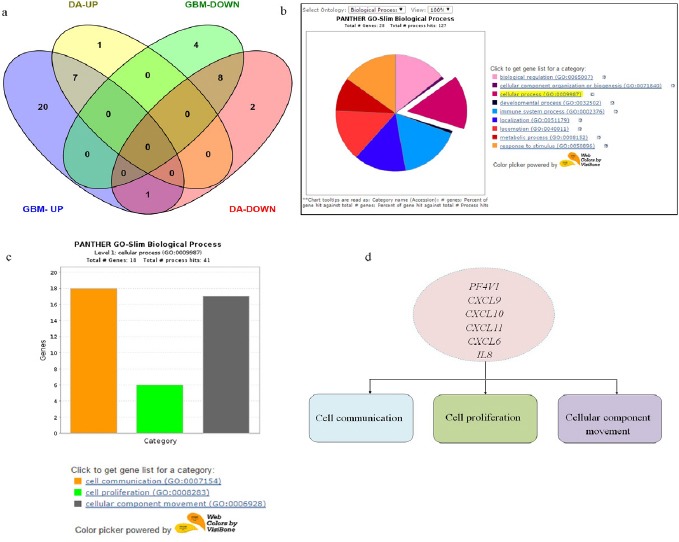
Gene Expression Summary and Gene Ontology Analysis. a) Venn diagram showing differentially expressed up regulated and down regulated genes between GBM and Diffuse astrocytoma. DA-UP (up regulated genes in Diffused Astrocytoma), GBM-UP (up regulated genes in GBM), DA-DOWN (down regulated genes in Diffused astrocytoma), GBM-DOWN (down regulated genes in GBM). b) Gene Ontology analysis of chemokines and their receptor genes upregulated in GBM-Pie chart of Biological processes associated with 28 chemokines and receptor genes upregulated in GBM. c) Bar graph showing 18 genes involved in three cellular processes-cell communication, cell proliferation and cellular component movement. d) Common genes involved in three cellular processes.
Functional classification of upregulated genes in GBM and comparison with other databases puts spotlight on CXCL8.
To funnel down important genes for further investigation we first classified upregulated genes in GBM based on gene ontology using PANTHER (Figure 2b) (Mi et al., 2013). Since chemokines are invloved in plethora of biological processes, genes involved in cellular processes such as cell communication, cell proliferation and cell movement were selected (Figure 2c). Further we queried the gene list in each of the processes. Supplementary 1, lists out the genes involved in cell proliferation, cell communication and cellular component movement. From these gene lists we next sorted genes which were common in all the three processes. These were PF4V1, CXCL6, CXCL9, CXCL10, CXCL11 and CXCL8 (IL8) (Figure 2d). Among these common genes, those which were up-regulated only in GBM and not in diffuse astrocytoma were further filtered out. These were PF4V1, CXCL11, CXCL6 and IL8.
Comparison with others datasets using ONCOMINE
Further we compared the expression pattern in other publically available databases. For this we used Oncomine database(https://www.oncomine.org/resource/main.html). For analysis we selected differential analysis type for Cancer Vs Cancer (to compare between different grades of astrocytoma). Further we selected cancer type i.e. Brain and CNS cancer. Under this we selected glioblastoma which displayed 27 datasets. Since we are looking for gene expression data in clinical samples, databases for cell lines or DNA copy number were excluded. After this filtration, only 8 datasets qualified for comparison. Based on number of datasets showing overexpression of genes in question and median gene rank, CXCL8 topped the list (Table 1).
Table 1.
Gene Ranking Based on Expression Level in Different Datasets Using ONCOMINE. Color Code Corresponds to Gene Rank (Ranging from Top 1% To Top 25%) in Each Dataset.
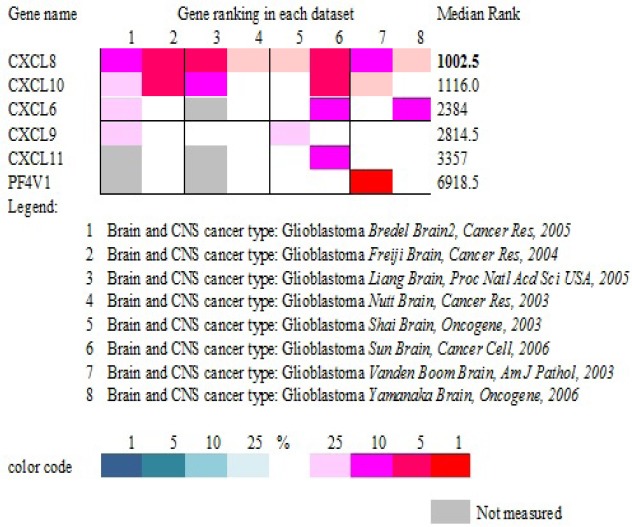
CXCL8 appeared to be an important gene requiring further investigation. This gene was significantly up-regulated in GBM while down regulated in diffuse astrocytoma. Furthermore, this gene is associated with cell proliferation, cell communication, cellular component movement and a known potent angiogenic factor which makes it even more interesting target to investigate its independent role in disease progression. This observation based on mRNA expression was further validated at protein level by tissue microarray (TMA) based immunohistochemical analysis.
Protein localization reveals CXCL8 and CXCR2 expression in tumor astrocytes while CXCR1 expression in tumor micro vessels.
A TMA block consisting of 160 tumor cores that included 5 non-neoplastic brain tissues, 60 diffuse astrocytoma and 95 primary GBM cores was constructed and stained with Anti-Human CXCL8. Mean age of GBM patients was 47.2 years where 72/95 were males and 23/95 were females. Mean age of diffuse astrocytoma patients was 39.2 years among which 39 were males and 21 were females. After processing only 55 cores of diffuse astrocytoma and 91 cores of GBM were suitable for immnunohistochemical evaluation. We also classified these samples based on their IDH mutation status. IDH-mutant and IDH-wildtype was assessed by immunohistochemical expression using monoclonal antibody IDH1 (R132). This has been shown to have high concordance with molecular testing (Pyo et al.2016). In GBM, 89.04% cases were IDH-wild type, while 10.96% exhibited IDH mutation. In diffuse astrocytoma, 58.7% cases were IDH-wild type while 41.30 % showed IDH mutation.
Further CXCL8-CXCR1/2 immunopositivity was assessed. In diffuse astrocytoma only 30.90% (17/55) cases stained positively for CXCL8 while 67.03% (61/91) positivity was observed in GBM. Difference in immunopositivity between GBM and diffuse astrocytoma for CXCL8 was found to be significant with p< 0.001 (Figure 4). However between IDH-wildtype and IDH1-mutated groups in both GBM and diffuse astrocytoma no significant difference was observed (data not shown). Expression was primarily cytoplasmic. (Figure 3)To understand the cell type expressing this chemokine, a double immunofluorescence was performed using antibodies against CXCL8 (red) and GFAP (Glial fibrillary acidic protein) (green) (Figure 5). Expression of CXCL8 was seen in cytoplasm of GFAP positive astrocytes.
Figure 3.
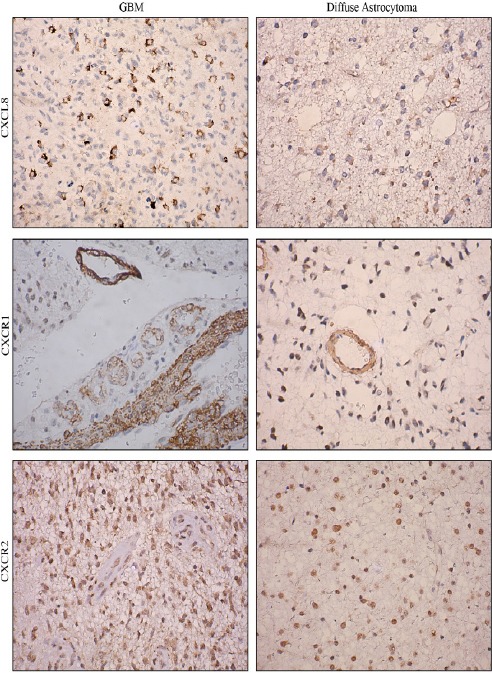
Immunohistochemical Expression of CXCL8, CXCR1 and CXCR2 in GBM and Diffuse Astrocytoma. Image Resolution Is X400.
Figure 4.
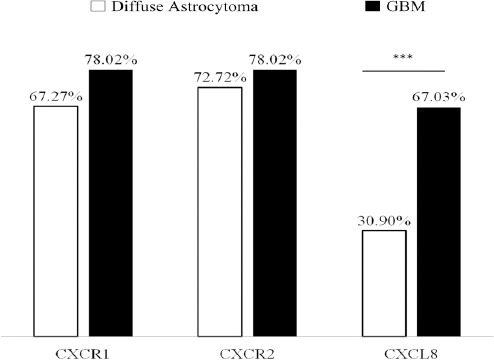
Bar Graph Representing Percentage Immuno Positivity for CXCL8, CXCR1 and CXCR2 in GBM and Diffuse Astrocytoma. *** Indicates P<0.001.
Figure 5.
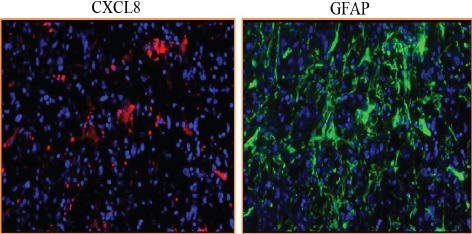
Double Immunofluorescence Staining of CXCL8 and GFAP in GBM (X200)
Since CXCL8 exerts its effect via receptors CXCR1 and CXCR2, their expression was also evaluated. CXCR1 expression was seen primarily in tumor associated vasculature with 33.3% cases showing expression in tumor cells as well (Figure 3). Both diffuse astrocytoma and GBM showed positivity for CXCR1 in tumor micro vessels, with higher CXCR1+ micro vessel density in GBM. However there was no significant difference in overall percentage positivity between diffuse astrocytoma and GBM (Figure 4).
CXCR2 was expressed by the tumor cells in both diffuse astrocytoma and GBM while no expression was seen in tumor micro vessels (Figure 3). There was no significant difference in its expression between diffuse astrocytoma and GBM (Figure 4).
Similar to CXCL8, no significant differences was observed between IDH1-wildtype and IDH1-mutated GBM and diffuse astrocytoma for CXCR1and CXCR2 expression.
A comparison of CXCR1 and CD34 immunostaining revealed CXCR1+/CD34 – vessels indicating VM (vascular mimicry) structures.
Extensive neovascularization is hallmark of GBM. In tumors, among various modes of neo vascularization, vascular mimicry is a mechanism where tumor cells acquire endothelial like phenotype and form tumor cell-lined channels for fluid transport. This obliges us to speculate that all CXCR1+ micro vessels which were observed in clinical tissue samples may not be of endothelial origin alone. To reconfirm the origin of CXCR1 positive vessels, we compared CXCR1 and CD34 staining in 10 GBM cases. Figure 6 shows existence of CXCR1+/CD34-vessels in addition to CXCR1+/CD34+ vessels. This suggests that CXCL8-CXCR1 axis promotes GBM growth by promoting angiogenesis by classical mode as well as by mode of vascular mimicry.
Figure 6.
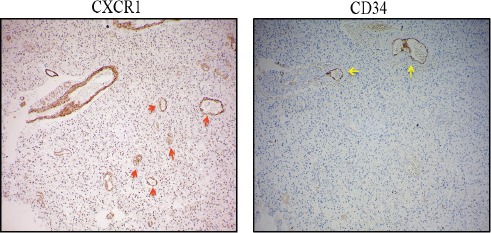
Anti-CXCR1 Staining in GBM, Red Arrow Indicates CXCR1+ / CD34-Micro Vessel and Yellow Arrow Indicates CXCR1+/CD34+ Vessel. Image Resolution Is100x.
Discussion
Current investigation in GBM is focussed on chemokines present in the tumor microenvironment and their ability to promote tumor cell proliferation and angiogenesis through the chemokine mediated signalling. We compared the gene expression of all chemokines and their cognate receptors between, GBM and diffuse astrocytoma taking normal brain tissue as reference. Differential gene expression pattern of chemokines and their receptors revealed PF4V1, CXCL11, and CXCL8 as important genes overexpressed more frequently in GBM than diffuse astrocytoma which are involved in biological processes such as cell communication, cell proliferation and cellular component movement. A comparison with other gene expression datasets suggested overexpression of CXCL8 is reported in most databases in GBM. Its role has been elucidated in other cancers (Vandercappellen et al. 2008), however in GBM there are limited reports.
In the present investigation, we observed significant upsurge of this chemokine in GBM while its receptor expression (both mRNA and protein) remained more or less same in low grade astrocytoma (diffuse astrocytoma) and high grade astrocytoma (GBM) signifying association of CXCL8 with tumor aggressiveness and its possible involvement in cell proliferation and cell survival pathways. Localization of its receptor CXCR1 on tumor micro vessels suggests its active role in promoting angiogenesis via paracrine mechanism since its ligand CXCL8 is secreted by tumor astrocyte, demonstrated in double immunofluorescence staining in present work. Although in fraction of cases (33.3%) expression of CXCR1 was also seen in tumor cells which leads us to speculate possibility of autocrine mode of action as well. Contrariwise CXCR2, second cognate receptor of CXCL8 is expressed by tumor cells and not tumor associated micro vessels in GBM, suggestive of autocrine activity via CXCL8-CXCR2 axis possibly promoting tumor cell proliferation. Gene expression of other CXCR2 ligands-CXCL2, CXCL3 and CXCL5 remained unaltered in GBM in comparison to normal brain tissue.
Despite its known angiogenic potency most of the angiogenesis research in GBM is focused on VEGF. The mechanism of angiogenesis is complex interplay of several factors, though VEGF has been considered as pivotal and has garnered maximum attention. The addition of bevacizumab (avastin) to upfront treatment with radiation and temozolomide in GBM patients conferred no benefit in terms of overall survival while progression free survival was improved by few months (Weathers et al. 2015). This limited success suggests existence of compensatory factors taking over role of VEGF in GBM angiogenesis. CXCL8 is an important pro-angiogenic factor known to be present in tumor microenvironment promoting endothelial cell survival and proliferation (Vandercappellen et al. 2008; Brat et al.2005). Of the two receptors of VEGF-A, VEGFR2 has been shown to be trans-activated by CXCL8 leading to vascular permeability (Dwyer et al.2012), thus indicating that CXCL8 might act in an additive or alternative manner to VEGF in GBM angiogenesis.
Other than this neovascularization in tumors is achieved via several modes. An alternative and unexpected mode is formation of tumor cell-lined channels capable of fluid transport independent of endothelial cells, known as vascular mimicry. Vascular mimicry (VM) aids molecular and phenotypic reprograming of tumor cells into endothelial-like cells in GBM and other hyper-vascular tumors (Arbab et al. 2015). Anti-angiogenic therapies such as avastin have failed to curb VM and could possibly be the reason for its limited success. In the present study we observed CXCR1 expression in tumor associated vessels while CXCL8 was limited to tumor astrocytes. This localization pattern gave us clue of its possible involvement in angiogenesis. A comparison of CD34 and CXCR1 staining exhibited presence of CD34-/CXCR1+ tumor associated microvessels which could well be speculated as VM structures and expression of CXCR1 on these highlight the involvement of CXCL8-CXCR1 in development and maintenance of these very structures. Formation of these structures has been positively correlated with poor prognosis in GBM (Mao et al. 2015; Wang et al. 2013). Therefore, targets such as CXCL8-CXCR1 axis may be explored for counteracting neovascularization in GBM tumors in addition to anti-VEGF therapy.
Ahn et al., (2016) demonstrated role of CXCL8 in migration and invasion of glioblastoma via NF-κB/AP-1-regulation. CXCL8 dependent synergistic interaction between endothelial cells and cancer stem cells in GBM has been shown to promote pro-tumor effects (Infanger et al., 2013). Zhou et al. 2014 also demonstrated both autocrine and paracrine signalling of CXCL8 in GBM stem cells which were critical for the maintenance of stem-like traits in GSCs.
Presence of other cognate receptor CXCR2 of CXCL8 in tumor cells indicates autocrine activity which might be driving tumor cell proliferation. Mitogenic effect of CXCL8 has been shown in melanoma, ovarian cancer, colon cancer prostate and lung cancer (Xie, 2001). Detailed underlying mechanism driving cell proliferation has not yet been deciphered. Although several signal pathways has been implicated to be involved in CXCL8 signal transduction. There are three primary downstream signalling cascades which are activated on stimulation of CXCR1/2 by CXCL8: phosphatidyl-inositol 3’ kinase/Akt (PI3K/Akt), phospholipase C/protein kinase C (PLC/PKC), and Ras/Raf/extracellular signal-regulated protein kinases 1 and 2 (Erk1/2). In addition to these, focal adhesion kinase, Rho, Rac, and Janus kinase/signal transducers and activators of transcription (JAK/STAT) pathways have also been shown to get activated by CXCL8 binding to its receptors (Xie, 2001).
These results underscore important role of CXCL8 in glioblastoma. It has the ability to influence tumor growth directly via autocrine stimulation of CXCL8-CXCR1/2 axis and indirectly via paracrine signalling through CXCL8-CXCR1 axis possibly influencing neovascularization by promoting VM structure formation. Effective inhibition of CXCL8-CXCR1/2 axis may prove to be a useful strategy in curbing GBM growth. However further in vitro and in vivo investigations will give more useful insights into its potential as novel chemotherapeutic target in treatment of GBM.
Conflict of interest
None.
Acknowledgements
Authors would like to acknowledge India Council of Medical Research (ICMR), New Delhi, India for research grant for this study.
References
- Ahn SH, Park H, Ahn YH, et al. Necrotic cells influence migration and invasion of glioblastoma via NF-κB/AP-1-mediated IL-8 regulation. Sci Rep. 2016;6:24552. doi: 10.1038/srep24552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbab AS, Jain M, Achyut B. Vascular mimicry:The next big glioblastoma target. Physiol Biochem. 2015;4:e410. doi: 10.4172/2168-9652.1000e140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brat DJ, Bellail AC, Van Meir EG. The role of interleukin-8 and its receptors in gliomagenesis and tumoral angiogenesis. Neuro Oncol. 2005;7:122–33. doi: 10.1215/S1152851704001061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigliadori G, Foca F, Dall’Agata M, et al. Defining the cutoff value of MGMT gene promoter methylation and its predictive capacity in glioblastoma. J Neurooncol. 2016;128:333–9. doi: 10.1007/s11060-016-2116-y. [DOI] [PubMed] [Google Scholar]
- Cancer genome atlas research network. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455:1061–8. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartier L, Hartley O, Dubois-Diffuse astrocytomauphin M, et al. Chemokine receptors in the central nervous system:role in brain inflammation and neurodegenerative diseases. Brain Res Brain Res Rev. 2005;48:16–42. doi: 10.1016/j.brainresrev.2004.07.021. [DOI] [PubMed] [Google Scholar]
- Cenciarelli C, Marei HE, Felsani A, et al. PDGFRαdepletion attenuates glioblastoma stem cells features by modulation of STAT3, RB1 and multiple oncogenic signals. Oncotarget. 2016;7:53047–63. doi: 10.18632/oncotarget.10132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi K-C, Auersperg N, Leung PC. Mitogen-activated protein kinases in normal and (pre)neoplastic ovarian surface epithelium. Reprod Biol Endocrinol. 2003;1:71. doi: 10.1186/1477-7827-1-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crusz SM, Balkwill FR. Inflammation and cancer:advances and new agents. Nat Rev Clin Oncol. 2015;12:584–96. doi: 10.1038/nrclinonc.2015.105. [DOI] [PubMed] [Google Scholar]
- Domanska UM, Kruizinga RC, den Dunnen WF, et al. The chemokine network, a newly discovered target in high grade gliomas. Crit Rev Oncol Hematol. 2011;79:154–63. doi: 10.1016/j.critrevonc.2010.07.006. [DOI] [PubMed] [Google Scholar]
- Dwyer J, Hebda JK, Le Guelte A, et al. Glioblastoma cell-secreted interleukin-8 induces brain endothelial cell permeability via CXCR2. PLoS One. 2012;7:e45562. doi: 10.1371/journal.pone.0045562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hambardzumyan D, Bergers G. Glioblastoma:Defining tumor niches. Trends Cancer. 2015;1:252–65. doi: 10.1016/j.trecan.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattermann K, Sebens S, Helm O, et al. Chemokine expression profile of freshly isolated human glioblastoma-associated macrophages/microglia. Oncol Rep. 2014;32:270–6. doi: 10.3892/or.2014.3214. [DOI] [PubMed] [Google Scholar]
- Huang M, Ke Y, Sun X, et al. Mammalian target of rapamycin signaling is involved in the vasculogenic mimicry of glioma via hypoxia-inducible factor-1α. Oncol Rep. 2014;32:1973–80. doi: 10.3892/or.2014.3454. [DOI] [PubMed] [Google Scholar]
- Infanger DW, Cho Y, Lopez BS, et al. Glioblastoma stem cells are regulated by interleukin-8 signaling in a tumoral perivascular niche. Cancer Res. 2013;73:7079–89. doi: 10.1158/0008-5472.CAN-13-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kufareva I. Chemokines and their receptors:insights from molecular modeling and crystallography. Curr Opin Pharmacol. 2016;30:27–37. doi: 10.1016/j.coph.2016.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo H-W. EGFR-targeted therapy in malignant glioma:Novel aspects and mechanisms of drug resistance. Curr Mol Pharmacol. 2010;3:37–52. doi: 10.2174/1874467211003010037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao JM, Liu J, Guo G, et al. Glioblastoma vasculogenic mimicry:signaling pathways progression and potential anti-angiogenesis targets. Biomark Res. 2015;3:8. doi: 10.1186/s40364-015-0034-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi H, Muruganujan A, Casagrande JT, et al. Large-scale gene function analysis with the PANTHER classification system. Nat Protoc. 2013;8:1551–66. doi: 10.1038/nprot.2013.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukaida N, Sasaki S, Baba T. Chemokines in cancer development and progression and their potential as targeting molecules for cancer treatment. Mediators Inflamm. 2014;2014:170381. doi: 10.1155/2014/170381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakada M, Kita D, Watanabe T, et al. Aberrant signaling pathways in glioma. Cancers. 2011;3:3242–78. doi: 10.3390/cancers3033242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohgaki H. Genetic pathways to glioblastomas. Neuropathology. 2005;25:1–7. doi: 10.1111/j.1440-1789.2004.00600.x. [DOI] [PubMed] [Google Scholar]
- Pyo JS, Kim NY, Kim RH, Kang G. Concordance analysis and diagnostic test accuracy review of IDH1 immunohistochemistry in glioblastoma. Brain Tumor Pathol. 2016;33:248–54. doi: 10.1007/s10014-016-0272-6. [DOI] [PubMed] [Google Scholar]
- Sharma I, Siraj F, Sharma KC, et al. Immunohistochemical expression of chemokine receptor CXCR3 and its ligand CXCL10 in low-grade astrocytomas and glioblastoma multiforme:A tissue microarray-based comparison. J Cancer Res Ther. 2016;12:793–7. doi: 10.4103/0973-1482.153657. [DOI] [PubMed] [Google Scholar]
- The cancer genome atlas (TCGA) research network. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455:1061–8. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tlsty TD, Coussens LM. Tumor stroma and regulation of cancer development. Annu Rev Pathol. 2006;1:119–50. doi: 10.1146/annurev.pathol.1.110304.100224. [DOI] [PubMed] [Google Scholar]
- Vandercappellen J, Van Damme J, Struyf S. The role of CXC chemokines and their receptors in cancer. Cancer Lett. 2008;267:226–44. doi: 10.1016/j.canlet.2008.04.050. [DOI] [PubMed] [Google Scholar]
- Wang S, Ke Y, Lu G, et al. Vasculogenic mimicry is a prognostic factor for postoperative survival in patients with glioblastoma. J Neurooncol. 2013;112:339–45. doi: 10.1007/s11060-013-1077-7. [DOI] [PubMed] [Google Scholar]
- Waugh DJ, Wilson C. The interleukin-8 pathway in cancer. Clin Cancer Res. 2008;14:6735–41. doi: 10.1158/1078-0432.CCR-07-4843. [DOI] [PubMed] [Google Scholar]
- Weathers SP, de Groot J. VEGF manipulation in glioblastoma. Oncology (Williston Park) 2015;29:720–7. [PubMed] [Google Scholar]
- Xie K. Interleukin-8 and human cancer biology. Cytokine Growth Factor Rev. 2001;12:375–91. doi: 10.1016/s1359-6101(01)00016-8. [DOI] [PubMed] [Google Scholar]
- Zhou J, Yi L, Ouyang Q, et al. Neurotensin signaling regulates stem-like traits of glioblastoma stem cells through activation of IL-8/CXCR1/STAT3 pathway. Cell Signal. 2014;26:2896–902. doi: 10.1016/j.cellsig.2014.08.027. [DOI] [PubMed] [Google Scholar]
- Zhu YM, Woll PJ. Mitogenic effects of interleukin-8/CXCL8 on cancer cells. Future Oncol. 2005;1:699–704. doi: 10.2217/14796694.1.5.699. [DOI] [PubMed] [Google Scholar]


