Abstract
Background:
DNA damage accumulation has been linked to the cancer phenotype. The purpose of this study was to compare the levels of DNA base 8-hydroxy-2’-deoxyguanosine (8-OHdG) and C-reactive protein (CRP) inflammatory markers in healthy controls and pancreatic cancer patients from a hospital-based case-control study.
Materials and Methods:
Fifty-five pancreatic cancer patients and 55 healthy controls were enrolled from a pool of patients referred to the Endoscopic Ultrasound (EUS) center. Analysis of DNA content of peripheral blood cells was conducted for 8-OHdG with the 32P-postlabelling assay. Serum CRP levels were measured by high-sensitivity assays and demographic data for comparison were collected from individual medical records.
Results:
The group of cases showed significant increased median (IQR) 8-OHdG DNA adducts/106 nucleotides and CRP compared to the controls (208.8 (138.0-340.8) vs 121.8 (57.7-194.8) RAL value; P<0.001) and (3.5 (1.5-8.6) vs 0.5 (0.2-1.5) mg/L P<0.001). A number of conditional regression models confirmed associations of pancreatic cancer with oxidative DNA damage in peripheral leukocytes.
Conclusions:
Our findings suggest the importance of leukocyte 8-OHdG adducts as an indicator for systemic oxidative DNA damage in pancreatic cancer patients. In addition to increase in the CRP inflammatory marker, this supports the impact of inflammation in the occurrence of pancreatic cancer as well as inflammatory responses during cancer development.
Keywords: Pancreatic cancer, 8-hydroxy-2’-deoxyguanosine (8-OHdG), oxidative DNA damage
Introduction
Pancreatic ductal cell carcinoma is comparatively a rare cancer, however it has remained one of the most causative of cancer-related deaths in the world in spite of novel treatment improvement (Ahmadloo et al., 2010; Sepanlou et al., 2015). The risk factors from individual-interpersonal, environmental and health are attributed to the development of this cancer (Hadizadeh et al., 2014). With some consistent results diabetes is known as potential risk factor for pancreatic cancer and its mortality among individuals (Ahmadloo et al., 2010). Mutations of cationic trypsinogen gene (PRSS1) have been recognized in hereditary or idiopathic chronic pancreatitis patients (Ahmadloo et al., 2010; Gasiorowska et al.,, 2011). Likewise genes such as BRCA1 and BRCA2, TP53, ataxia-telangiectasia-mutated (ATM) and the mismatch repair (MMR) that carrying inherited mutations are associated with an increased risk of developing pancreatic cancer (Li et al., 2002; Petronzelli et al., 2000). However, Mutations related to antioxidant defense enzymes are accountable for cancer susceptibility (Mohamadkhani et al., 2015; Yu and Kim 2014).
Reactive oxygen species (ROS) that are made continuously in the courses of cells breathing are important for inducing endogenous DNA damage. An imbalance between the production of oxidants ROS in the course of mitochondrial ATP synthesis and the antioxidant defense results in the oxidative stress (Closa and Folch-Puy 2004; Gackowski et al., 2002). Oncogenes also increase ROS production and contribute to the DNA damage and malignant transformation (Li et al., 2002). Therefore, in dual roles, ROS induce carcinogenesis and cancer progression in intermediate levels, while too much release of ROS affectedly damages cancer cells. In human cancers, ROS productions are increased by macrophages and neutrophils. Therefore, ROS production is the etiological basis of aging, cancer and degenerative diseases that induce DNA damage and consequently malignant transformation (Closa and Folch-Puy, 2004). The mismatch repair system, mainly through DNA glycosylase (hOGG1), is the principal mechanism of protecting the reliability of human DNA particularly in the subject of 8-hydroxy-2’ -deoxyguanosine (8-OHdG) (Petronzelli et al., 2000). 8-OHdG is one of the main free radical-induced oxidative constructs that generate GC to TA transversion by mispairing with adenine in subsequent replications (Devanaboyina and Gupta 1996; Ock et al., 2012). It is one of the most widely used biomarkers of oxidative stress, especially because of its abundance in DNA and also because of its reliable detectability. The chromatographic system analysis of 8-OHdG, one of the main forms of free radical-induced oxidative lesions, has been widely used as a biomarker of oxidative stress and cancer risk and known to be effective for the detection and quantitative analysis of such oxidative lesions. This procedure specifically detects 8-OHdG, which is the only adduct retained in chromatograms developed in acidic medium (Balansky et al., 2014; Devanaboyina and Gupta 1996; Izzotti et al., 2001; Izzotti et al., 2007).
In general exposure to carcinogenic agents, like tobacco smoke, asbestos, polycyclic aromatic hydrocarbons, and heavy metals are responsible for 8-OHdG formation. Moreover, variety of p53 tumor suppressor mutations are related to the formation of 8-OHdG in DNA (von Brevern et al., 1996). Previous studies from cancers showed that the tumor tissues harbor higher levels of 8-OHdG in comparison to normal tissue, therefore it is a valuable mutagenesis biomarker. 8-OHdG is largely measured in leukocytic DNA, however, its urinary excretion is also pointing to oxidative stress in humans (Ock et al., 2012; Peluso et al., 1991; Peluso et al., 1990; Peluso et al., 2010; Vulimiri et al., 2000).
The concepts of anti-inflammatory therapies as a preventive approach for pancreatic cancer have been demonstrated that patients with acute or recurrent non-familial pancreatitis are at high risk of cancer (Baron and Sandler 2000; Mohamadkhani et al., 2015). This implies the significance of inflammation in accumulation of DNA damage and genetic defects (Farrow and Evers 2002; Mohamadkhani et al., 2015). Persistent infiltration of inflammatory cells in the context of chronic pancreatitis is related to the release of cytokines and free radicals and consequent acinar cell injury (Closa and Folch-Puy 2004; Lowenfels et al., 1993). C-reactive protein (CRP) is highly considered as prognostic indicators in physiological response to inflammation and acute-phase reactant. The significance of this protein as an indicator in advanced pancreatic cancer has been reported in several studies (Kishi et al., 2015; Mitsunaga et al., 2016).
Herein, we examined the association between the amounts of DNA base adduct 8-OHdG in peripheral leukocytes as a marker of oxidative stress and subsequent risk of pancreatic cancer. We also determined the amounts of CRP to study the link between inflammation and pancreatic cancer.
Materials and Methods
Study population and sample collection
A total of 55 pancreatic cancer cases and 55 controls from individuals referred to the Endoscopic Ultrasound (EUS) center of Shariati Hospital, which is a referral center for EUS in Tehran, Iran, were enrolled during January 2012 to March 2015. A structured, valid and reliable questionnaire was used for the study (Farrokhzad et al., 2014; Mohamadkhani and Poustchi 2015). Accordingly, cases were new incident cases with a histopathological diagnosis of adenocarcinoma when the tissue was obtained directly from the pancreas. Controls were those who had normal pancreas in their EUS exam and no history or current diagnosis of liver or renal failure or any cancer, there were no adherence to special diets and absence of pancreatic disease or any cancer one year after the initial visit. EUS was done by Pentax linear EUS device (EG 3830 UT) for all potential cases and controls. If any solid or cystic lesion was detected in the pancreas by EUS, Fine Needle Aspiration (FNA) biopsy was offered. A pathologist evaluated histopathology of all FNA biopsies and surgical tissues of the patients, when available. A written informed consent was obtained from each study participant. Blood samples were collected according to the standardized protocol for each participant and processed by centrifugation on the same day of collection, and divided into several aliquots to be stored in -80 centigrade. The protocol for this study was approved by the ethics committee of the Digestive Disease Research Institute of Tehran University of Medical Sciences in Shariati Hospital.
DNA extraction
Leukocyte DNA was extracted and purified using the Gentra Pure gene kit according to the manufacturer recommendation (Qiagen, Alameda, CA, USA). DNA concentration and purity were determined by Nano drop. DNA samples were subsequently stored at –80°C until laboratory analyses.
32P-DNA post-labeling assay
DNA (2 μg) was hydrolyzed by incubation with micrococcal nuclease (21.45 mU/μl) and spleen phosphodiesterase (6.0 mU/μl) (Sigma Aldrich, MO, and Worthington, New Jersey, USA) in 5.0 mM Na succinate, 2.5 mM calcium chloride, pH 6.0 at 37°C for 4.5 h (Munnia et al., 2007; Peluso et al., 2010). Digest DNA was then diluted with ultrapure water to 20 ng/µl. Diluted DNA digest was incubated with 10 μCi of carrier-free [γ-32P] ATP (3000 Ci/mM) (Amersham, UK) and 2 U of polynucleotide kinase T4 (10 U/μl) (Roche Diagnostics, Indianapolis, IN, USA) to generate 32P-labeled adducts in the reaction buffer (10 x) at room temperature for 45 min (Munnia et al., 2004; Peluso et al., 2010). 32P-labeled samples were treated with 1.2 U of nuclease P1 (1.9 U/μl) (Sigma Aldrich, MO, USA) in 62.5 mM sodium acetate, pH 5.0, and 0.27 mM ZnCl2 at 37°C for 60 min (final volume 10 μl) (Izzotti et al., 2001; Munnia et al., 2007). 32P-labeled samples were applied on polyethyleneimine cellulose thin-layer chromatography plates (Macherey-Nagel, Postfach, Germany) for chromatographic analysis of 8-OHdG, which is the only adduct retained in chromatograms developed in acidic medium (Balansky et al., 2014; Devanaboyina and Gupta 1996; Izzotti et al., 2001; Izzotti et al., 2007). Specifically, an aliquot (2.5 µl) of the labeled solution was applied to the origin of a chromatogram and developed overnight onto a 3 cm-long Whatman 1 paper wick with 1.5 M formic acid for the first dimensional chromatography. For the second dimensional chromatography, chromatographic plates were developed at the right angle to the previous development with 0.6 M ammonium formate, pH 6.0.
Detection and quantification of 8-OHdG and total nucleotides was obtained by storage phosphor imaging technique employing intensifying screens from Molecular Dynamics (Sunnyvale, California, USA). The screens were scanned using a Typhoon 9210 (Amersham, Buckinghamshire, United Kingdom). Software used to process the data was ImageQuant (Molecular Dynamics) (Peluso et al., 2005; Peluso et al., 2010). After background subtraction, the levels of 8-OHdG were expressed such as relative adduct labelling (RAL) = screen pixel in 8-OHdG spot / screen pixel in total normal nucleotides (Izzotti et al., 2001). To calculate the levels of total normal nucleotides, aliquots of hydrolysed DNA were appropriately diluted and reacted in the mixtures used for adduct labelling (Peluso et al., 1997; Peluso et al., 1996; Peluso et al., 1998). The obtained total nucleotides were separated on Merck plates using 280 mM (NH4)2SO4, 50 mM NaH2PO4 (Munnia et al., 2007). The values measured for the 8-OHdG spot were corrected across experiments based on the recovery of the internal standard, e.g. 8-OHdG (Sigma Aldrich, MO, USA). Higher specificity of the 32P-labeling technique is obtained using appropriated reference standards. The levels of 8-OHdG DNA adducts/106 nucleotides are expressed as Relative Adduct Labeling (RAL) values (Izzotti et al., 2001; Peluso et al., 1991; Peluso et al., 1990; Phillips and Castegnaro 1999).
Clinical and biochemical assessment
All participants were evaluated at baseline with a questionnaire describing their medical history, and smoking status. Height and weight were measured, and Body Mass Index (BMI) was calculated. Quantitative determination of CRP in serum was accomplished in Hitachi 747–200 analyzer using the latex particle-enhanced immunoturbidimetric assay (ITA).
Statistical analysis
Data of continuous variables were presented as mean and standard deviation (SD) or median and inter quartile range (IQR), as appropriate. Categorical variables were presented as frequency and percentage. Baseline characteristics between two groups were compared using independent t-test or Mann-Whitney U test for continues variables and chi square test for categorical variables. Normality assumption was assessed using the Shapiro-Wick test. The determinant of 8-OHdG was calculated by simple linear regression with log-transform on 8-OHdG. Since no variables had a p-value less than 0.1 upon 8-OHdG in univariate analysis, we didn’t complete any multivariate linear regression analysis. The relationship of 8-OHdG with pancreatic cancer was evaluated by logistic regression that was considered in various forms. All models were adjusted for age, gender, BMI, smoking, diabetes and CRP. Spearman’s rank correlation was obtained to assess the relation between 8-OHdG and CRP. Statistical analysis was performed using the Stata varsion12 (Stata Statistical Software: Release 12. College Station, TX: StataCorp LP). P values of less than 0.05 were considered statistically significant.
Results
General information of study subjects
Table 1 presented the characteristics of age, gender, BMI, diabetes history, smoking status (never used, former and current user) and the median (IQR) of 8-OHdG DNA adducts/106 nucleotides and CRP concentration for cases and controls, overall and according to selected individual characteristics. There was no significant difference in the means of age, diabetes incidence, BMI and smoking status between cases and control. However the CRP level was significantly different between groups with higher expression in pancreatic cancer patients (3.5 (1.5-8.6) vs 0.5 (0.2-1.5) mg/L P<0.001) (Figure 1). Variables 8-OHdG and CRP revealed strength and direct association in Spearman’s rank correlation analysis [0.22 (P-value=0.021)].
Table 1.
Baseline Characteristics
| Pancreatic cancer | Control group | P-value | |
|---|---|---|---|
| N=55 | N=55 | ||
| Age (y), Mean±SD | 65.2±11.1 | 65.5±12.1 | 0.666 |
| Gender, N (%) | 0.036 | ||
| Male | 34 (61.8) | 23 (41.8) | |
| Female | 21 (38.2) | 32 (58.2) | |
| BMI, Mean±SD | 22.8±4.8 | 24.4±5.0 | 0.08 |
| Smoking, N (%) | 0.482 | ||
| Never used | 10 (18.2) | 14 (25.5) | |
| Former | 30 (54.5) | 24 (43.6) | |
| Current user | 15 (27.3) | 17 (30.9) | |
| Diabetes, N (%) | 0.087 | ||
| No | 36 (65.5) | 44 (80.0) | |
| Yes | 19 (34.5) | 11 (20.0) | |
| CRP (mg/L), Median (IQR) | 3.5 (1.5-8.6) | 0.5 (0.2-1.5) | <0.001 |
| 8-OHdG DNA adducts/106 nucleotides (RAL values), Median(IQR) | 208.8 (138.0-340.8) | 121.8 (57.7-194.8) | <0.001 |
Figure 1.
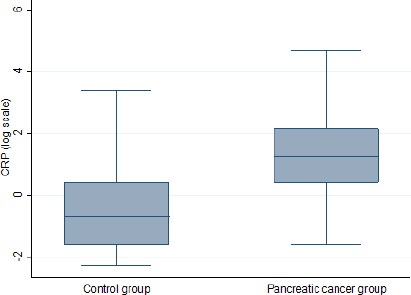
Distribution of CRP Inflammation Marker (Log Scale) between Pancreatic Cancer Patients Cases and Controls
The determinant values of 8-OHdG DNA base
The median (IQR) of 8-OHdG was 208.8 (138.0-340.8) in cases compare to 121.8 (57.7-194.8) in controls (P<0.001). Figure 2 represents the distribution plot of 8-OHdG within the two groups. The determinants of log-transform of 8-OHdG with 95% confidence interval (CI) from pancreatic cancer and corresponding control subjects are displayed in Table 2. Pancreatic cancer patients had the significant coefficient on 8-OHdG (0.74 (0.41, 1.07); P<0.001). However, no correlation was found between 8-OHdG and possible determinants, such as, age, smoking status, occupation, gender, BMI, diabetes and CRP.
Figure 2.
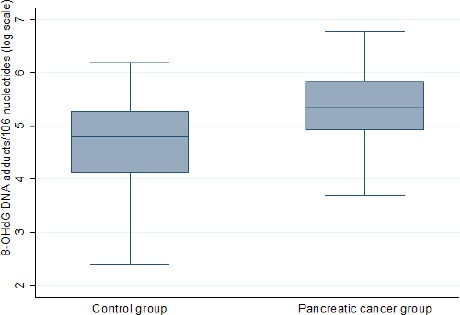
Scattering of RAL Values of 8-Ohdg DNA Adducts/106 Nucleotides in the Groups of Pancreatic Cancer and Corresponding Control Subjects
Table 2.
Determinants of 8-OHdG*
| Coefficient (95% CI) | Standardized coefficient | P-value | |
|---|---|---|---|
| Pancreatic cancer | |||
| Yes/No | 0.72 (0.39,1.07) | 0.379 | <0.001 |
| Age (year) | -0.002 (-0.02,0.01) | -0.018 | 0.848 |
| Gender | |||
| Male/Female | -0.05 (-0.41,0.32) | -0.024 | 0.803 |
| BMI | 0.01 (-0.03,0.05) | 0.057 | 0.556 |
| Smoking | |||
| Former/Never used | 0.22 (-0.24,0.69) | 0.117 | 0.347 |
| Current user/Never used | 0.02 (-0.50,0.53) | 0.008 | 0.948 |
| Diabetes, N (%) | |||
| Yes/No | 0.15 (-0.26,0.56) | 0.07 | 0.468 |
| CRP (mg/L) | 0.01 (-0.01,0.02) | 0.101 | 0.294 |
Log-transform of 8-OHdG DNA adducts/106 nucleotides was analyzed
8-OHdG and pancreatic cancer risk
There was a trend of higher median of 8-OHdG in conditional regression models in the group of pancreatic cancer patients. Table 3 displays the adjusted ORs for pancreatic cancer risk and 95% CIs in relation to the levels of 8-OHdG by the use of several conditional regression models and reference categories. The adjusted ORs (and 95% CIs) for pancreatic cancer in relation to 8-OHdG levels in continues scale by meant of 100 unit increase were statistically significant [1.78 (1.25,2.52) (P=0.001)]. When 8-OHdG was categorized in Quartile scale, the trend was also significant [1.50 (1.09,2.06) (P=0.011)]. Finally, significant association relationships were detected in the Dichotomous scale of 8-OHdG with pancreatic cancer [2.67 (1.09,6.58) (P=0.032)].
Table 3.
Adjusted ORs for Pancreatic Cancer and 95% CI in Relation to RAL Values of 8-OHdG DNA Adducts/106 Nucleotides
| Model* | 8-OHdG | Adjusted OR (95% CI) | P-value |
|---|---|---|---|
| 1 | Continues scale (for 100 unit increase) | 1.78 (1.25,2.52) | 0.001 |
| 2 | Quartile scale | ||
| 1 | 1 | ||
| 2 | 2.25 (0.63,8.10) | 0.214 | |
| 3 | 2.67 (0.74,9.64) | 0.134 | |
| 4 | 5.70 (1.57,20.73) | 0.008 | |
| 3 | Linear trend of quartiles | 1.50 (1.09,2.06) | 0.011 |
| 4 | Dichotomous scale | ||
| Below the median | 1 | ||
| Above the median | 2.67 (1.09,6.58) | 0.032 |
Discussion
The relationship of pancreatic cancer development with oxidative stress and inflammation has been proposed in previous studies. 8-OHdG is one of the most common oxidative stress-generated pre-mutagenic lesions in the genome. In this study, we showed the levels of peripheral leukocytes 8-OHdG as exclusive features of pancreatic cancer patients. As the main results of this study, pancreatic cancer cases showed a trend for a higher median of 8-OHdG as well as a significant trend when the level of 8-OHdG was categorized in Quartile scale [1.50 (1.09, 2.06) (P=0.01)]. Oxidative stress-generated damage to DNA may be due to a declining activity of the antioxidant defense and repair systems or increased level of pro-oxidant factors, or both (Jacob et al., 2013). Compared with other bases, guanine most readily undergoes an oxidative attack in the presence of ROS to form 8-OHdG. If these oxidative lesions are not repaired, particularly during DNA replication, they can become mutagenic (Liu et al., 2006).
Previous studies have examined the amounts of oxidative DNA damage in various cancers and have found higher levels of 8-OHdG in the cases in comparison to the controls. Although substantial evidence have been describing the 8-OHdG as biomarker of various cancers and degenerative diseases, however 8-OHdG is rather more elucidates the importance of association of pancreatic cancer with oxidative DNA damage (Peluso et al., 2000; Saieva et al., 2011; Vulimiri et al., 2000). Serum CA 19-9 is clinically valuable biomarker that generally used in diagnosing pancreatic cancer (Hadizadeh et al., 2014).
Elevated levels of 8-OHdG have been reported from cancer patients compared with healthy subjects in lung cancer (Vulimiri et al., 2000), bladder cancer (Kaczmarek et al., 2005), colon-rectal cancer (Oliva et al., 1997), renal cell carcinoma (Okamoto et al., 1994), prostate cancer (Miyake et al., 2004) and gastric adenocarcinoma (Lee et al., 1998). Evidence from these studies suggests that elevated 8-OHdG levels would be a sign of increased oxidative stress, impaired antioxidant defense and/or inadequate repair of oxidative DNA damage. Furthermore, genetic variants within the genes of DNA repair system and p53, with description of ’guardian of the genome’, are predisposing factors of checkpoints ineffectiveness and subsequent increased formation of 8-OHdG (Li et al., 2002; Petronzelli et al., 2000; von Brevern et al., 1996). Nevertheless, there are also few reports that have observed no difference in the levels of 8-OHdG of cancer patients with healthy subjects (Gackowski et al., 2002; Jacob et al., 2013). With some controversial results, the effect of variable occupational and environmental exposures as well as a variety of confounding factors such as age, gender, smoking, diabetes and alcohol consumption on the level of this adduct have been reported (Peluso et al., 1998). Human bio monitoring studies showed that smoking and occupational exposure such as adjacent workplaces near the coke are confounding factor for higher levels of 8-OHdG (Liu et al., 2006), though in our recent study we revealed that cigarette smoking was not related to increased risk of pancreatic cancer (Shakeri et al., 2016). Moreover, in present study, diabetes and potential factors, such as, age, sex, smoking, BMI and CRP inflammation marker had no correlation with 8-OHdG.
The link between inflammation and pancreatic cancer has been proposed by epidemiological and experimental studies (Zahir et al., 2013). Inflammation is well-known reason in genomic damage and the development of solid tumor malignancies (Farrow and Evers 2002; Yu and Kim 2014). Both hereditary and sporadic forms of chronic pancreatitis have been shown to increase the risk of developing pancreatic cancer. Patients with chronic pancreatitis are 17 times more likely to develop pancreatic cancer compared to matched controls (Lowenfels et al., 1993). Chronic inflammation is stimulated by biochemical and physical factors and is in turn connected with an increased risk of several human cancers. Likewise persistent inflammation develops the malignant transformation of pancreatic ductal (Farrow and Evers 2002; Pinho et al., 2014). DNA damage induces systemic stress and triggers innate immune responses that promote inflammation (Yu and Kim 2014) and higher ROS production that in turn induce 8-OHdG formation (Ock et al., 2012). It has been identified that ROS production of neutrophils and macrophages is individual approach to kill tumor cells through (Segal and Shatwell 1997). CRP is one of the mediators of inflammation that is significantly increased in pancreatic cancer patients. It is also clinically appropriate index for systemic inflammation and oxidative stress (Kishi et al., 2015). A high level of serum CRP is associated with poor outcomes in various malignancies (Mitsunaga et al., 2016). Accordingly, patients with increased levels of CRP had higher distant metastases that point to the crucial treatment failure (Kishi et al., 2015). Mitsunaga (2016) represented CRP as an indicator in severs pancreatic cancer and showed that patients with higher CRP level had the larger burden of tumor (Mitsunaga et al., 2016). The result of the present study demonstrated the significant correlation of CRP and 8-OHdG in pancreatic cancer that is compatible with association between oxidative DNA damage and inflammation.
Along these lines, increased content of 8-OHdG in serum and isolated DNA from lymphocytes were recognized in colon carcinoma patients (Gackowski et al., 2002) This adduct gives valuable information regarding to the mutagenic effect of exposure to oxidative stress and persistent chronic inflammation (Peluso et al., 2000; Vulimiri et al., 2000). Therefore the results of this study are more consistent with higher exposure to ROS production in pancreatic cancer. On the other hand, the association between leukocytes DNA adducts and pancreatic cancer probably reveals lack of ability of patients in the DNA repair system as well as inefficient diet with anti-oxidative mediators. Prior studies established increased amounts of oxidative DNA damage in pancreatic tumors compared to benign pancreatic tissue (Dhillon et al., 2014; Li et al., 2002), despite the lack of the relationship between 8-OHdG and clinicopathological parameters or survival (Isohookana et al., 2015).
Therefore quantification of 8-OHdG in the appropriate pancreatic cancer tissues can give a more accurate representation of exposure to different carcinogenic compounds. To our knowledge, there are no similar data in the literature with which to compare this finding and further work is required to understand whether the 8-OHdG biomarker is obviously complicated with the occurrence of this cancer. However additional studies with longitudinal follow-up of subjects with higher levels of 8-OHdG in peripheral leukocytes are required to determine its precise impact on pancreatic cancer development. Our analyses have some limitations that need to be considered with larger sample size. Female subjects more agreed to participate as controls, nonetheless, the factor of gender had no significant effects on the levels of 8-OHdG in peripheral leukocytes as well as increasing risk of pancreatic cancer.
In summary, this study suggests that the quantitation of 8-OHdG in leukocytes DNA from peripheral blood can reflect the effect of oxidative stress on systemic DNA damage and could be present as a potential characteristic of pancreatic cancer patients. Moreover, increased level of CRP inflammatory marker, supposed the impact of inflammation in the occurrence of pancreatic cancer or inflammatory response, all through, cancer development. However, further studies are also expected to identify the effect of possible low level of anti-oxidants in various phases of this cancer as well as an inadequate function of DNA repair system in pancreatic cancer incidence.
Acknowledgments
Authors of this article take this chance to appreciate patients which participated in this research program.
Finding
This work was supported partially by the “Associazione Italiana per la Ricerca sul Cancro”, Milan, Italy, the Tuscany Region, Italy, and Digestive Diseases Research Institute, Tehran University of Medical Sciences, Tehran, Iran.
Authors and Contributions
All authors participated in the conception of the work, experimental and clinical analysis, and interpretation of data and writing of this paper, have seen and approved the final manuscript and come across the criteria for authorship.
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- Ahmadloo N, Bidouei F, Omidvari S, et al. Pancreatic cancer in southern Iran. Iran Red Crescent Med J. 2010;12:624–30. [Google Scholar]
- Balansky R, Izzotti A, D’Agostini F, et al. Assay of lapatinib in murine models of cigarette smoke carcinogenesis. Carcinogenesis. 2014;35:2300–7. doi: 10.1093/carcin/bgu154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron JA, Sandler RS. Nonsteroidal anti-inflammatory drugs and cancer prevention. Annu Rev Med. 2000;51:511–23. doi: 10.1146/annurev.med.51.1.511. [DOI] [PubMed] [Google Scholar]
- Closa D, Folch-Puy E. Oxygen free radicals and the systemic inflammatory response. IUBMB Life. 2004;56:185–91. doi: 10.1080/15216540410001701642. [DOI] [PubMed] [Google Scholar]
- Devanaboyina U, Gupta RC. Sensitive detection of 8-hydroxy-2’deoxyguanosine in DNA by 32P-postlabeling assay and the basal levels in rat tissues. Carcinogenesis. 1996;17:917–24. doi: 10.1093/carcin/17.5.917. [DOI] [PubMed] [Google Scholar]
- Dhillon H, Chikara S, Reindl KM. Piperlongumine induces pancreatic cancer cell death by enhancing reactive oxygen species and DNA damage. Toxicol Reports. 2014;1:309–18. doi: 10.1016/j.toxrep.2014.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrokhzad S, Nedjat S, Kamangar F, et al. Validity and reliability of a questionnaire designed to assess risk factors of pancreatic cancer in Iran. Arch Iran Med. 2014;17:102–5. [PubMed] [Google Scholar]
- Farrow B, Evers BM. Inflammation and the development of pancreatic cancer. Int J Surg Oncol. 2002;10:153–69. doi: 10.1016/s0960-7404(02)00015-4. [DOI] [PubMed] [Google Scholar]
- Gackowski D, Banaszkiewicz Z, Rozalski R, Jawien A, Olinski R. Persistent oxidative stress in colorectal carcinoma patients. Int J Cancer. 2002;101:395–7. doi: 10.1002/ijc.10610. [DOI] [PubMed] [Google Scholar]
- Gasiorowska A, Talar-Wojnarowska R, Czupryniak L, et al. The prevalence of cationic trypsinogen (PRSS1) and serine protease inhibitor, Kazal type 1 (SPINK1) gene mutations in Polish patients with alcoholic and idiopathic chronic pancreatitis. Dig Dis Sci. 2011;56:894–901. doi: 10.1007/s10620-010-1349-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadizadeh M, Padashi M, Mohammad Alizadeh AH, Zali MR. Clinical, laboratory biomarkers and imaging findings of pancreatic adenocarcinoma in Iran. Asian Pac J Cancer Prev. 2014;5:4349–52. doi: 10.7314/apjcp.2014.15.10.4349. [DOI] [PubMed] [Google Scholar]
- Isohookana J, Haapasaari KM, Soini Y, Karihtala P. Keap1 expression has independent prognostic value in pancreatic adenocarcinomas. Diagn Pathol. 2015;10:28. doi: 10.1186/s13000-015-0258-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izzotti A, Balansky RM, Dagostini F, et al. Modulation of biomarkers by chemopreventive agents in smoke-exposed rats. Cancer Res. 2001;61:2472–9. [PubMed] [Google Scholar]
- Izzotti A, De Flora S, Cartiglia C, et al. Interplay between Helicobacter pylori and host gene polymorphisms in inducing oxidative DNA damage in the gastric mucosa. Carcinogenesis. 2007;28:892–8. doi: 10.1093/carcin/bgl208. [DOI] [PubMed] [Google Scholar]
- Jacob KD, Noren Hooten N, Trzeciak AR, Evans MK. Markers of oxidant stress that are clinically relevant in aging and age-related disease. Mech Ageing Dev. 2013;134:139–57. doi: 10.1016/j.mad.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczmarek P, Blaszczyk J, Fijalkowski P, et al. Assessment of 8-hydroxy-2’-deoxyguanosine concentrations in bladder cancer patients treated with intravesical BCG instillation. Pol Merkur Lekarski. 2005;19:526–8. [PubMed] [Google Scholar]
- Kishi T, Nakamura A, Itasaka S, et al. Pretreatment C-reactive protein level predicts outcome and patterns of failure after chemoradiotherapy for locally advanced pancreatic cancer. Pancreatolog. 2015;15:694–700. doi: 10.1016/j.pan.2015.09.016. [DOI] [PubMed] [Google Scholar]
- Lee BM, Jang JJ, Kim HS. Benzo[a]pyrene diol-epoxide-I-DNA and oxidative DNA adducts associated with gastric adenocarcinoma. Cancer lett. 1998;125:61–8. doi: 10.1016/s0304-3835(97)00520-x. [DOI] [PubMed] [Google Scholar]
- Li D, Firozi PF, Zhang W, et al. DNA adducts, genetic polymorphisms, and K-ras mutation in human pancreatic cancer. Mutat Res. 2002;513:37–48. doi: 10.1016/s1383-5718(01)00291-1. [DOI] [PubMed] [Google Scholar]
- Liu AL, Lu WQ, Wang ZZ, et al. Elevated levels of urinary 8-hydroxy-2 -deoxyguanosine, lymphocytic micronuclei, and serum glutathione S-transferase in workers exposed to coke oven emissions. Environ Health Perspect. 2006;114:673–7. doi: 10.1289/ehp.8562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenfels AB, Maisonneuve P, Cavallini G, et al. Pancreatitis and the risk of pancreatic cancer. International pancreatitis study group. N Engl J Med. 1993;328:1433–7. doi: 10.1056/NEJM199305203282001. [DOI] [PubMed] [Google Scholar]
- Mitsunaga S, Ikeda M, Shimizu S, et al. C-Reactive protein level is an indicator of the aggressiveness of advanced pancreatic cancer. Pancreas. 2016;45:110–6. doi: 10.1097/MPA.0000000000000465. [DOI] [PubMed] [Google Scholar]
- Miyake H, Hara I, Kamidono S, Eto H. Oxidative DNA damage in patients with prostate cancer and its response to treatment. J Urol. 2004;171:1533–6. doi: 10.1097/01.ju.0000116617.32728.ca. [DOI] [PubMed] [Google Scholar]
- Mohamadkhani A, Akbari MR, Ghanbari R, et al. Direct sequencing of cyclooxygenase-2 (COX-2) revealed an intronic variant rs201231411 in Iranian patients with pancreatic cancer. Middle East J Dig Dis. 2015;7:14–8. [PMC free article] [PubMed] [Google Scholar]
- Mohamadkhani A, Poustchi H. Repository of human blood derivative biospecimens in biobank:Technical implications. Middle East J Dig Dis. 2015;7:61–8. [PMC free article] [PubMed] [Google Scholar]
- Munnia A, Amasio ME, Peluso M. Exocyclic malondialdehyde and aromatic DNA adducts in larynx tissues. Free Radic Biol Med. 2004;37:850–8. doi: 10.1016/j.freeradbiomed.2004.05.024. [DOI] [PubMed] [Google Scholar]
- Munnia A, Saletta F, Allione A, et al. 32P-Post-labelling method improvements for aromatic compound-related molecular epidemiology studies. Mutagenesis. 2007;22:381–5. doi: 10.1093/mutage/gem030. [DOI] [PubMed] [Google Scholar]
- Ock CY, Kim EH, Choi DJ, et al. 8-Hydroxydeoxyguanosine:not mere biomarker for oxidative stress, but remedy for oxidative stress-implicated gastrointestinal diseases. World J Gastroenterol. 2012;18:302–8. doi: 10.3748/wjg.v18.i4.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto K, Toyokuni S, Uchida K, et al. Formation of 8-hydroxy-2’-deoxyguanosine and 4-hydroxy-2-nonenal-modified proteins in human renal-cell carcinoma. Int J Cancer. 1994;58:825–9. doi: 10.1002/ijc.2910580613. [DOI] [PubMed] [Google Scholar]
- Oliva MR, Ripoll F, Muniz P, et al. Genetic alterations and oxidative metabolism in sporadic colorectal tumors from a Spanish community. Mol Carcinog. 1997;18:232–43. [PubMed] [Google Scholar]
- Peluso M, Airoldi L, Magagnotti C, et al. White blood cell DNA adducts and fruit and vegetable consumption in bladder cancer. Carcinogenesis. 2000;21:183–7. doi: 10.1093/carcin/21.2.183. [DOI] [PubMed] [Google Scholar]
- Peluso M, Amasio E, Bonassi S, et al. Detection of DNA adducts in human nasal mucosa tissue by 32P-postlabeling analysis. Carcinogenesis. 1997;18:339–44. doi: 10.1093/carcin/18.2.339. [DOI] [PubMed] [Google Scholar]
- Peluso M, Castegnaro M, Malaveille C, et al. 32P Postlabelling analysis of urinary mutagens from smokers of black tobacco implicates 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) as a major DNA-damaging agent. Carcinogenesis. 1991;12:713–7. doi: 10.1093/carcin/12.4.713. [DOI] [PubMed] [Google Scholar]
- Peluso M, Castegnaro M, Malaveille C, et al. 32P-postlabelling analysis of DNA adducted with urinary mutagens from smokers of black tobacco. Carcinogenesis. 1990;11:1307–11. doi: 10.1093/carcin/11.8.1307. [DOI] [PubMed] [Google Scholar]
- Peluso M, Hainaut P, Airoldi L, et al. Methodology of laboratory measurements in prospective studies on gene-environment interactions:the experience of GenAir. Mutat Res. 2005;574:92–104. doi: 10.1016/j.mrfmmm.2005.01.025. [DOI] [PubMed] [Google Scholar]
- Peluso M, Merlo F, Munnia A, et al. (32)P-postlabeling detection of DNA adducts in peripheral white blood cells of greenhouse floriculturists from western Liguria, Italy. Cancer epidemiology, biomarkers and prevention :a publication of the American association for cancer research. Am J Clin Oncol. 1996;5:361–9. [PubMed] [Google Scholar]
- Peluso M, Merlo F, Munnia A, et al. 32P-postlabeling detection of aromatic adducts in the white blood cell DNA of nonsmoking police officers. Cancer epidemiology, biomarkers and prevention :a publication of the American Association for cancer research. Am Society Prev Oncol. 1998;7:3–11. [PubMed] [Google Scholar]
- Peluso M, Srivatanakul P, Munnia A, et al. Malondialdehyde-deoxyguanosine adducts among workers of a Thai industrial estate and nearby residents. Environ Health Perspect. 2010;118:55–9. doi: 10.1289/ehp.0900907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petronzelli F, Riccio A, Markham GD, et al. Investigation of the substrate spectrum of the human mismatch-specific DNA N-glycosylase MED1 (MBD4):fundamental role of the catalytic domain. J Cell Physiol. 2000;185:473–80. doi: 10.1002/1097-4652(200012)185:3<473::AID-JCP19>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Phillips DH, Castegnaro M. Standardization and validation of DNA adduct postlabelling methods:report of interlaboratory trials and production of recommended protocols. Mutagenesis. 1999;14:301–15. doi: 10.1093/mutage/14.3.301. [DOI] [PubMed] [Google Scholar]
- Pinho AV, Chantrill L, Rooman I. Chronic pancreatitis:a path to pancreatic cancer. Cancer lett. 2014;345:203–9. doi: 10.1016/j.canlet.2013.08.015. [DOI] [PubMed] [Google Scholar]
- Saieva C, Peluso M, Masala G, et al. Bulky DNA adducts and breast cancer risk in the prospective EPIC-Italy study. Breast Cancer Res Treat. 2011;129:477–84. doi: 10.1007/s10549-011-1472-8. [DOI] [PubMed] [Google Scholar]
- Segal AW, Shatwell KP. The NADPH oxidase of phagocytic leukocytes. Ann N Y Acad Sci. 1997;832:215–22. doi: 10.1111/j.1749-6632.1997.tb46249.x. [DOI] [PubMed] [Google Scholar]
- Sepanlou SG, Malekzadeh F, Naghavi M, et al. Trend of gastrointestinal and liver diseases in Iran:results of the global burden of disease study 2010. Middle East J Dig Dis. 2015;7:121–37. [PMC free article] [PubMed] [Google Scholar]
- Shakeri R, Kamangar F, Mohamadnejad M, et al. Opium use, cigarette smoking, and alcohol consumption in relation to pancreatic cancer. Medicine. 2016;95:e3922. doi: 10.1097/MD.0000000000003922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Brevern MC, Hollstein MC, Cawley HM, et al. Circulating anti-p53 antibodies in esophageal cancer patients are found predominantly in individuals with p53 core domain mutations in their tumors. Cancer Res. 1996;56:4917–21. [PubMed] [Google Scholar]
- Vulimiri SV, Wu X, Baer-Dubowska W, et al. Analysis of aromatic DNA adducts and 7, 8-dihydro-8-oxo-2’-deoxyguanosine in lymphocyte DNA from a case-control study of lung cancer involving minority populations. Mol Carcinog. 2000;27:34–46. doi: 10.1002/(sici)1098-2744(200001)27:1<34::aid-mc6>3.3.co;2-7. [DOI] [PubMed] [Google Scholar]
- Yu JH, Kim H. Oxidative stress and cytokines in the pathogenesis of pancreatic cancer. Int Cancer Prev. 2014;19:97–102. doi: 10.15430/JCP.2014.19.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahir ST, Arjmand A, Kargar S, Neishaboury M. Incidence and trends of malignant and benign pancreatic lesions in Yazd, Iran between 2001 and 2011. Asian Pac J Cancer Preve. 2013;14:2631–5. doi: 10.7314/apjcp.2013.14.4.2631. [DOI] [PubMed] [Google Scholar]


