Abstract
Background
The objective of this retrospective study was to determine whether lymph node metastasis has a prognostic impact on patients with stage IV breast cancer.
Patients and methods
Seven thousand three hundred and seventy-nine patients with de novo stage IV breast cancer diagnosed from 2004 to 2013 were identified. Kaplan-Meier estimate method was fitted to measure overall survival and breast cancer-specific survival (BCSS). Cox proportional hazard analysis was used to evaluate the association between N stage and BCSS after controlling variables such as other patient/tumor characteristics.
Results
The primary site of M1 tumors was mainly upper-outer quadrant and overlapping lesion of the breast. Patients with N1 disease had better overall survival and BCSS than did those without lymph node metastasis. The overall survival and BCSS of M1 patients with N3 disease were significantly lower than that of those with N0, N1 and N2 disease, whereas patients with N2 and N0/N1 involvement showed no significant difference with survival. Multivariate analysis showed that lymph node metastasis was an important prognostic factor for M1 patients (N1 versus N0, hazard ratio [HR] = 0.902, 95% confidence interval [CI]: 0.825–0.986, p = 0.023; N3 versus N0, HR = 1.161, 95% CI: 1.055–1.276, p = 0.002). For M1 patients, age, race, marital status, primary site, ER, PR and HER2 were the independent prognostic factors.
Conclusions
The cohort study provides an insight into de novo stage IV breast cancer with lymph node metastasis. Our results indicated that accurate lymph node evaluation for stage IV patients is still necessary to obtain important prognostic information.
Introduction
Breast cancer is the most common cancer in women worldwide, and 1.68 million cases are newly diagnosed annually [1]. Among them, about 6%–10% of women present with de novo stage IV breast cancer at diagnosis [2–4]. The median survivals of the disease with distant metastasis are ranging between 18 and 24 months [4–6]. Once metastatic disease is diagnosed, treatment is usually palliative with systemic therapy. This practice is based on prior studies, which have shown stage IV breast cancer to be an incurable disease [7–8].
The combination of primary tumor (T), regional lymph nodes (N) and metastases (M) is the cornerstone of the breast cancer staging system of the American Joint Committee on Cancer (AJCC). Based on the presence of distant metastasis, all patients are divided into two groups—staging M0 and M1 [9]. For M0 patients, lymph node metastasis is an important demarcation criterion, which is also regarded as one of the most important prognostic factors in clinical practice [9–12]. However, M1 patients are all categorized as stage IV regardless of any N status [9]. Up to date, the clinical value of N descriptors has been neglected in various cancers of M1 staging, including breast cancer. Recently, Dai et.al reported that lymph node involvement is an independent prognostic factor for M1 patients with lung cancer [13], whereas the clinical effect of lymph node status on patients with M1 breast cancer has not been studied extensively. Thus, the present study aims to identify whether accurate identification of lymph node status in M1 patients with breast cancer is of clinical value.
Materials and methods
Patients
The Surveillance Epidemiology and End Results (SEER) database (2004–2013) was used for the study. The National Cancer Institute’s SEER*Stat software (Version 8.2.0) was used to identify patients. All patients had a pathologically confirmed diagnosis of stage IV breast cancer according to the 6th and 7th edition of the AJCC criteria. Patients for whom breast cancer was not the first tumor were excluded. Demographics, including age, gender, race and marital status at diagnosis were retrieved. Tumor variables included location of the primary tumor, T staging, N staging, histological type, estrogen receptor (ER) status, progesterone receptor (PR) status and human epidermal growth factor receptor 2 (HER2) status. Data of HER2 status is available since 2010. Survival data were extracted at 1 mo intervals for a follow-up period between 1 mo and 120 mo.
Statistical analysis
The data were presented as median (range) and percent values. Overall survival and breast cancer-specific survival (BCSS) were evaluated using the Kaplan-Meier method and compared using the log-rank test for all M1 patients according to N staging. Overall survival was determined from the SEER record of survival time (total number of months) and vital status. Breast cancer–specific survival (BCSS) was defined as the interval from diagnosis of M1 disease until death due to breast cancer. In addition, multivariate the Cox proportional-hazard regression model was applied to adjust for potential confounders in the survival analysis for all M1 patients, with p-values < 0.05 considered statistically significant. All analyses were conducted using SPSS 19.0 software (IBM, Inc., Armonk, NY).
Results
A total of 7379 patients of M1 breast cancer were selected from the SEER database. The age of the patients ranged from 19 to 99 years, with a median age of 59 years. There were 117 male and 7262 female patients. Among them, the majority (approximately 95%) were ductal and lobular neoplasms. The primary site of M1 tumors was mainly upper-outer quadrant and overlapping lesion of the breast (26.3% and 21.8%, respectively). Table 1 showed the baseline characteristics of patients.
Table 1. Baseline characteristics of patients with breast cancer with M1 disease.
| Characteristic | M1 patients(N = 7379) |
|---|---|
| Median age (range), yrs | 59 (19–99) |
| Age | |
| ≤65 yrs | 5050 (68.4%) |
| >65 yrs | 2329 (31.6%) |
| Sex | |
| Male | 117 (1.6%) |
| Female | 7262 (98.4%) |
| Marriage | |
| Yes | 3558 (48.2%) |
| No | 3821 (51.8%) |
| Race | |
| White | 5643 (76.5%) |
| Black | 1162 (15.7%) |
| Other | 574 (7.8%) |
| Primary location | |
| Nipple | 51 (0.7%) |
| Central portion | 518 (7.0%) |
| Upper-outer quadrant | 1938 (26.3%) |
| Upper-inner quadrant | 455 (6.2%) |
| Lower-inner quadrant | 309 (4.2%) |
| Lower-outer quadrant | 439 (5.9%) |
| Overlapping lesion | 1605 (21.8%) |
| Other | 2064 (27.9%) |
| Histological type | |
| Ductal and lobular neoplasms | 6977 (94.6%) |
| Other | 402 (5.4%) |
| T stage | |
| T0 | 14 (0.2%) |
| T1 | 998 (13.5%) |
| T2 | 2649 (35.9%) |
| T3 | 1282 (17.4%) |
| T4 | 2198 (29.8%) |
| Tx | 238 (3.2%) |
| N stage | |
| N0 | 1550 (21.0%) |
| N1 | 2682 (36.3%) |
| N2 | 1485 (20.1%) |
| N3 | 1662 (22.5%) |
| ER status | |
| Negative | 2047 (27.7%) |
| Positive | 5008 (67.9%) |
| Other | 324 (4.4%) |
| PR status | |
| Negative | 3066 (41.6%) |
| Positive | 3934 (53.3%) |
| Other | 379 (5.1%) |
| HER2 status | |
| Negative | 1962 (26.6%) |
| Positive | 725 (9.8%) |
| Other | 4692 (63.6%) |
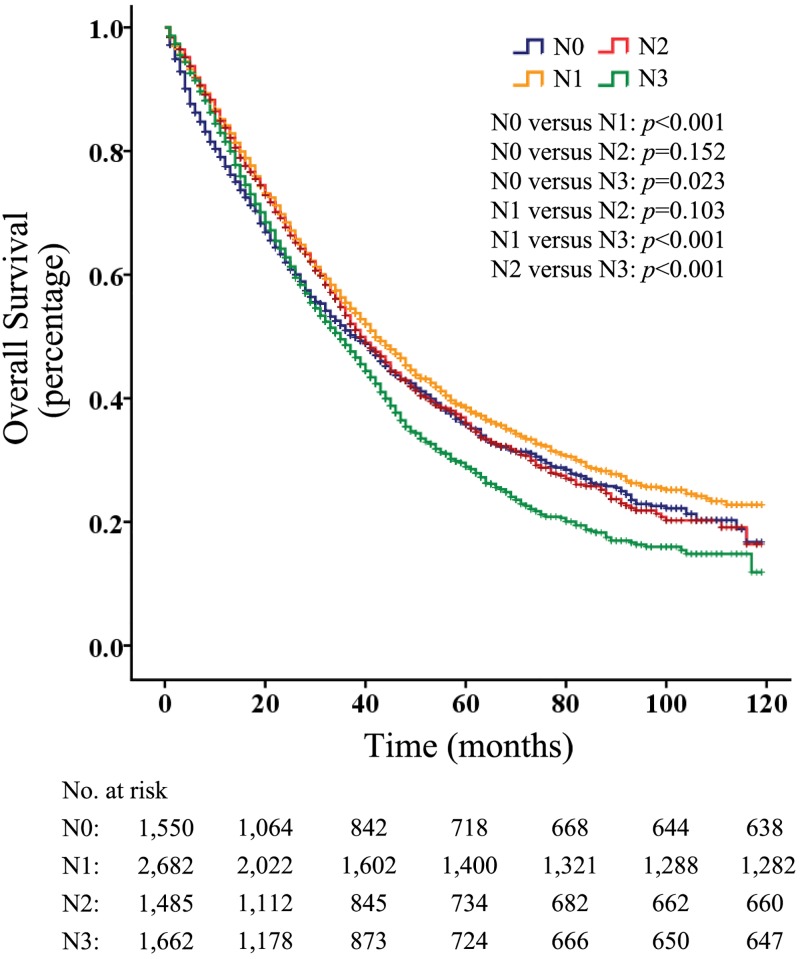
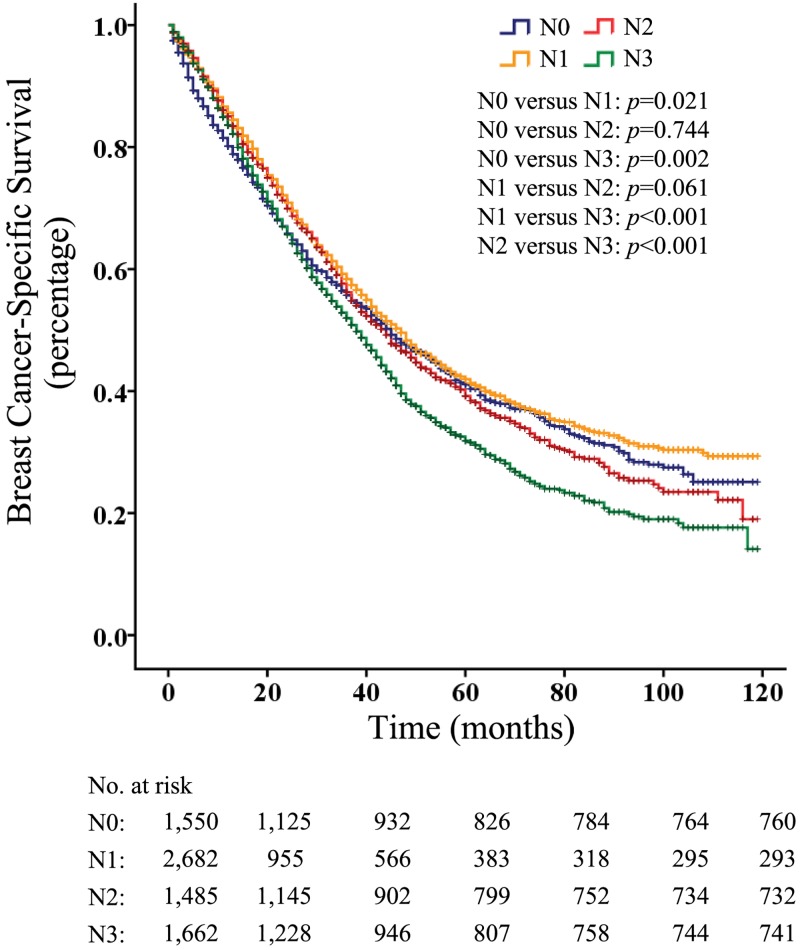
Of all the M1 patients, 1550 patients had their disease diagnosed as N0, 2682 as N1, 1485 as N2, and 1662 as N3. The survival analysis evaluating the entire cohort showed that patients with N1 disease had a better overall survival (p < 0.001) than did those without lymph node metastasis (Fig 1). In addition, the overall survival of M1 patients with N3 disease were significantly lower than that of those with N0, N1 and N2 disease (p < 0.05, p < 0.001 and p < 0.001, respectively) (Fig 1), whereas similar overall survival rates were observed between patients with N2 involvement and those with N0 or N1 involvement (p = 0.152 and p = 0.103, respectively) (Fig 1). Further, the BCSS rates of M1 patients were similar to the overall survival (Fig 2).
Fig 1. Overall survival according to N categories in patients with M1 breast cancer.
Fig 2. Breast cancer–specific survival according to N categories in patients with M1 Disease.
In the multivariate analysis, the results indicated that lymph node metastasis was an important prognostic factor for M1 patients (N1 versus N0, hazard ratio [HR] = 0.902, 95% confidence interval [CI]: 0.825–0.986, p = 0.023; N3 versus N0, HR = 1.161, 95% CI: 1.055–1.276, p = 0.002) (Table 2). In addition, the risk of death was lower for M1 patients aged ≤ 65 years than those aged > 65 years (HR = 1.417, 95% CI: 1.325–1.516, p < 0.001) (Table 2). For M1 patients, race, marital status, primary site, ER, PR and HER2 (negative vs. positive) were the independent prognostic factors (Table 2).
Table 2. Cox proportional hazards regression model for breast cancer–specific survival in patients with M1 disease.
| Characteristic | Hazard Ratios (95% CI) | p value |
|---|---|---|
| Age | ||
| ≤65 yrs | 1.00 (reference) | <0.001 |
| >65 yrs | 1.417 (1.325–1.516) | |
| Sex | ||
| Male | 1.00 (reference) | 0.08 |
| Female | 0.806 (0.633–1.026) | |
| Marriage | ||
| Yes | 1.00 (reference) | <0.001 |
| No | 1.315 (1.233–1.403) | |
| Race | ||
| White | 1.00 (reference) | |
| Black | 1.445 (1.328–1.572) | <0.001 |
| Other | 0.823 (0.722–0.938) | 0.004 |
| Primary location | ||
| Nipple | 1.00 (reference) | |
| Central portion | 0.691 (0.473–1.009) | 0.055 |
| Upper-outer quadrant | 0.770 (0.535–1.107) | 0.159 |
| Upper-inner quadrant | 0.671 (0.458–0.982) | 0.04 |
| Lower-inner quadrant | 0.635 (0.428–0.942) | 0.024 |
| Lower-outer quadrant | 0.665 (0.454–0.976) | 0.037 |
| Overlapping lesion | 0.740 (0.514–1.065) | 0.105 |
| Other | 0.821 (0.571–1.180) | 0.286 |
| Histological type | ||
| Ductal and lobular neoplasms | 1.00 (reference) | 0.02 |
| Other | 1.178 (1.026–1.351) | |
| T stage | ||
| T0 | 1.00 (reference) | |
| T1 | 1.827 (0.587–5.685) | 0.298 |
| T2 | 1.954 (0.629–6.066) | 0.246 |
| T3 | 2.484 (0.799–7.719) | 0.116 |
| T4 | 3.239 (1.043–10.053) | 0.042 |
| Tx | 2.163 (0.688–6.799) | 0.186 |
| N stage | ||
| N0 | 1.00 (reference) | |
| N1 | 0.902 (0.825–0.986) | 0.023 |
| N2 | 0.983 (0.890–1.087) | 0.74 |
| N3 | 1.161 (1.055–1.276) | 0.002 |
| ER status | ||
| Negative | 1.00 (reference) | |
| Positive | 1.571 (1.434–1.721) | <0.001 |
| Other | 1.224 (0.903–1.658) | 0.193 |
| PR status | ||
| Negative | 1.00 (reference) | |
| Positive | 1.574 (1.442–1.718) | <0.001 |
| Other | 1.435 (1.080–1.907) | 0.013 |
| HER2 status | ||
| Negative | 1.00 (reference) | |
| Positive | 1.915 (1.595–2.298) | <0.001 |
| Other | 1.797 (1.518–2.127) | <0.001 |
Discussion
The TNM classification system attempts to account for most basic parameters of cancer, and it has utility for determining the extent of disease, providing guidance for treatment planning and predicting the outcome. As the single most important prognostic factor in breast cancer [14–15], the nodal status in M0 patients has attracted much more attention than that in M1 patients. Remarkably, the results from the current study showed that lymph node metastasis was an important prognostic factor for patients with M1 breast cancer.
In addition, patients without lymph node metastasis had worse overall survival and BCSS than did those with N1 disease, which was confusing indeed. For one hand, T stage has not been taken into consideration. For example, patients with T1N1 should have better survival than those with T3N0. For another, the invasion of tumor cell into lymph nodes can activate an antitumor immune response, which may benefit patients with lymph node metastasis[16].
Patients with N2 and N0/N1 involvement showed no significant difference with survival (P > 0.05). We speculate that the abnormality may be related to the site of metastasis, such as visceral metastases, bone metastases and brain metastases. Several studies have reported a range of prognostic factors for women with metastatic breast cancer including factors such as age at diagnosis, ER, PR, HER2 and site of metastases [5,17]. The current study showed that for M1 patients, age, race, marital status and primary site were the independent prognostic factors. Thus, further studies or more clinical data are required to evaluate the impact of the site of metastasis on survival in M1 patients with different N stages. Additionally, several studies have reported improvement in survival of women with metastatic breast cancer, such as partial or total mastectomy [18–19]. Hence, different clinical treatments may have influence on survival in M1 patients.
Prognostic factors combining clinical and laboratory variables with physician’s estimates have been developed in recent years [20]. However, in this study, we just selected patients from the SEER database to analyze the prognostic factors. It is necessary for us to include more detailed information using our own patient database to verify the results.
In conclusion, accurate lymph node staging is utilized mainly to estimate prognosis, and it also contributes to determining treatment strategies. Our results supported that the prognostic value of lymph node staging extends even to M1 patients and indicated that accurate lymph node evaluation for M1 patients is still necessary to obtain important prognostic information.
Supporting information
(XLSX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E86. doi: 10.1002/ijc.29210 [DOI] [PubMed] [Google Scholar]
- 2.Donegan WL, Spratt JS. Cancer of the breast. 5th ed Philadelphia: Saunders; 2002. xvi, 1050 p. p. [Google Scholar]
- 3.Ries LAG MD, Krapcho M, Mariotto A, Miller BA, Feuer EJ, Clegg L, et al. (eds). SEER Cancer Statistics Review, 1975–2004, National Cancer Institute; Bethesda, MD, http://seercancergov/csr/1975_2004/, based on November 2006 SEER data submission, posted to the SEER web site. 2007. [Google Scholar]
- 4.Dawood S, Broglio K, Ensor J, Hortobagyi GN, Giordano SH. Survival differences among women with de novo stage IV and relapsed breast cancer. Annals Of Oncology. 2010;21(11):2169–74. doi: 10.1093/annonc/mdq220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cardoso F, Di Leo A, Lohrisch C, Bernard C, Ferreira F, Piccart MJ. Second and subsequent lines of chemotherapy for metastatic breast cancer: what did we learn in the last two decades? Annals Of Oncology. 2002;13(2):197–207. doi: 10.1093/annonc/mdf101 [DOI] [PubMed] [Google Scholar]
- 6.Norton L. Metastatic breast cancer. Length and quality of life. The New England journal of medicine. 1991;325(19):1370–1. doi: 10.1056/NEJM199111073251909 . [DOI] [PubMed] [Google Scholar]
- 7.Hortobagyi GN. Drug therapy—Treatment of breast cancer. New Engl J Med. 1998;339(14):974–84. [DOI] [PubMed] [Google Scholar]
- 8.Bernard-Marty C, Cardoso F, Piccart MJ. Facts and controversies in systemic treatment of metastatic breast cancer. Oncologist. 2004;9(6):617–32. doi: 10.1634/theoncologist.9-6-617 [DOI] [PubMed] [Google Scholar]
- 9.Amin MB, Edge SB, Greene FL, Byrd DR, Brookland RK, Washington MK, Gershenwald JE, et al. AJCC Cancer Staging Manual. 8th ed New York:Springer; 2016. [Google Scholar]
- 10.Cianfrocca M, Goldstein LJ. Prognostic and predictive factors in early-stage breast cancer. Oncologist. 2004;9(6):606–16. doi: 10.1634/theoncologist.9-6-606 [DOI] [PubMed] [Google Scholar]
- 11.Soerjomataram I, Louwman MWJ, Ribot JG, Roukema JA, Coebergh JWW. An overview of prognostic factors for long-term survivors of breast cancer. Breast Cancer Research And Treatment. 2008;107(3):309–30. doi: 10.1007/s10549-007-9556-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salama JK, Heimann R, Lin F, Mehta N, Chmura SJ, Singh R, et al. Does the number of lymph nodes examined in patients with lymph node-negative breast carcinoma have prognostic significance? Cancer. 2005;103(4):664–71. doi: 10.1002/cncr.20830 [DOI] [PubMed] [Google Scholar]
- 13.Dai C, Ren Y, Xie D, Zheng H, She Y, Fei K, et al. Does Lymph Node Metastasis Have a Negative Prognostic Impact in Patients with NSCLC and M1a Disease? Journal Of Thoracic Oncology. 2016;11(10):1745–54. doi: 10.1016/j.jtho.2016.06.030 [DOI] [PubMed] [Google Scholar]
- 14.Fisher ER, Land SR, Fisher B, Mamounas E, Gilarski L, Wolmark N. Pathologic findings from the national surgical adjuvant breast and bowel project—Twelve-year observations concerning lobular carcinoma in situ. Cancer. 2004;100(2):238–44. doi: 10.1002/cncr.11883 [DOI] [PubMed] [Google Scholar]
- 15.Dent DM. Axillary lymphadenectomy for breast cancer—Paradigm shifts and pragmatic surgeons. Arch Surg-Chicago. 1996;131(11):1125–7. [DOI] [PubMed] [Google Scholar]
- 16.da Cunha A, Michelin MA, Candid Murta EF. Phenotypic profile of dendritic and T cells in the lymph node of Balb/C mice with breast cancer submitted to dendritic cells immunotherapy. Immunology Letters. 2016;177:25–37. doi: 10.1016/j.imlet.2016.07.009 [DOI] [PubMed] [Google Scholar]
- 17.Largillier R, Ferrero JM, Doyen J, Barriere J, Namer M, Mari V, et al. Prognostic factors in 1038 women with metastatic breast cancer. Annals Of Oncology. 2008;19(12):2012–9. doi: 10.1093/annonc/mdn424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dawood S, Broglio K, Gonzalez-Angulo AM, Buzdar AU, Hortobagyi GN, Giordano SH. Trends in Survival Over the Past Two Decades Among White and Black Patients With Newly Diagnosed Stage IV Breast Cancer. Journal Of Clinical Oncology. 2008;26(30):4891–8. doi: 10.1200/JCO.2007.14.1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andre F, Slimane K, Bachelot T, Dunant A, Namer M, Barrelier A, et al. Breast cancer with synchronous metastases: Trends in survival during a 14-year period. Journal Of Clinical Oncology. 2004;22(16):3302–8. doi: 10.1200/JCO.2004.08.095 [DOI] [PubMed] [Google Scholar]
- 20.Hauser CA, Stockler MR, Tattersall MHN. Prognostic factors in patients with recently diagnosed incurable cancer: a systematic review. Supportive Care in Cancer. 2006;14(10):999–1011. doi: 10.1007/s00520-006-0079-9 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.