Abstract
Background
Oxidized high-density lipoprotein (oxHDL) has reduced capacity for cholesterol efflux and some of other anti-atherogenic properties of HDL, but the role of oxHDL in the pathogenesis of cardiometabolic disease has not been fully demonstrated. This study investigated the association of oxHDL with plasma glucose (PG) and the other atherosclerotic risk variables in non-diabetic dyslipidemic subjects.
Methods
Conventional atherosclerotic markers and LDL particle size (LDL-PS), as determined by a gel electrophoresis, were measured in 155 non-diabetic subjects (mean age of 57 years) with dyslipidemia. Serum oxHDL levels were quantified using an antibody against oxidized human apoA-I in a sandwich ELISA format.
Results
Multiple regression analysis adjusted for possible confounders revealed that HDL-cholesterol was independently, significantly and positively correlated with LDL-PS and oxHDL. By multiple regression analysis, oxHDL was independently, significantly and positively correlated with fasting PG (β = 0.19, P = 0.01). Subjects in the highest PG tertile group had approximately 30% higher oxHDL levels than the lowest PG tertile group.
Conclusions
These results suggest that high PG levels may contribute to the HDL oxidation, irrespective of HDL-cholesterol levels, even in non-diabetic subjects with dyslipidemia, and that the measurement of oxHDL may be a useful marker of dysfunctional HDL.
Keywords: atherosclerosis, hyperlipidemia, oxidative stress, small dense LDL, LDL particle size
1. Introduction
Cardiovascular disease (CVD) is a major cause of moribidity and mortality, and the identification of patients at risk by biomarkers is critical in the clinical management of these patients [1]. High-density lipoprotein (HDL) particles play a critical role in the reverse cholesterol transport pathway, and also exert anti-oxidant, anti-inflammatory and anti-coagulant actions [2]. Because of its beneficial anti-atherogenic functions, HDL-cholesterol (HDL-C), which is a marker for HDL levels, is strongly inversely related to CVD risk [2]. Recently, it has been demonstrated that HDL can undergo a variety of modifications, including oxidation, making it dysfunctional and potentially even pro-atherogenic [2,3]. Oxidized HDL (oxHDL) has been detected in endothelial cells overlying atherosclerotic lesions [4], and unlike native HDL can be taken up by macrophage, thus possibly contributing to foam cell formation [5]. Unlike oxidized low-density lipoprotein (LDL) [6], however, there is much less known about the role of oxHDL in atherogenesis.
HDL contains a wide variety of lipids and proteins that can potentially be modified by oxidation [7]. Furthermore, HDL particles are considered to be the principal vehicle for circulating lipid hydroperoxides [8]. Apolipoprotein A-I (apoA-I), the major protein component of HDL, is known to be modified at several specific residues [9], particularly at methionine and tyrosine residues [10,11], leading to its loss of function in cholesterol efflux [3]. In fact, apoA-I is described to be more susceptible to oxidation than proteins on LDL [8,12–14]. Results from a clinical study of patients, who were assessed by coronary angiography, proposed that oxHDL, rather than oxLDL, may be more sensitively related to coronary disease (i.e., artery spasm) [15]. The pathophysiologic conditions, however, that lead to the oxidation of HDL are not well understood, but like LDL [16–19] glucose may modify and lead to the oxidation of HDL even in non-diabetic subjects.
We have recently developed an ELISA system for measuring oxHDL that utilizes an antibody against oxidized human apoA-I generated by the in vitro treatment with H2O2 [20]. This assay has been tested in several clinical settings, and increased oxHDL has been found in diabetic patients [20] and was reported to be predictive of CVD outcomes in chronic renal failure patients treated by hemodialysis [21,22]. The aim of the present study is to investigate the relationship between fasting plasma glucose (PG) and oxHDL, as well as other cardiovascular lipid/lipoprotein risk factors, in non-diabetic, dyslipidemic subjects to determine if the oxHDL assay can be potentially be applied in a more general population.
2. Subjects and methods
2.1. Studied subjects
The present cross-sectional hospital-based study included 155 dyslipidemic subjects (21 men and 134 women) between 30 to 80 years of age. Dyslipidemia was diagnosed, according to the guidelines of the Japan Atherosclerosis Society (circulating concentrations of LDL cholesterol [LDL-C] ≥ 3.64 mmol/l, triglycerides [TG] ≥ 1.69 mmol/l, HDL-C < 1.04 mmol/l) [23]. Inclusion criteria required all subjects to be non-medicated, non-smokers, abstainers from alcohol, and all subjects had a normal fasting PG levels (< 7.0 mmol/l [24,25]). Subjects were excluded from the study if they were pregnant, had an acute infections, such as the common cold, or if they had a positive history of diabetes, cardio/cerebrovascular, thyroid, collagen, severe kidney or liver diseases. The study was approved by the institutional ethics committees of Kyoto Medical Center and Jichi Medical University, and all subjects gave their informed consent.
2.2. Atherosclerotic risk variables
All analysis was done on serum after an overnight fast. Serum LDL-C, HDL-C, TG and PG concentrations were measured by enzymatic methods (Sekisui Co. Ltd., Tokyo, Japan). The mean LDL particle size (LDL-PS) was simultaneously measured with a high-resolution, nongradient polyacrylamide gel electrophoresis system (the Lipoprint system; Quantimetrix, Redondo Beach, CA, USA) [26], which was validated for lipoprotein particle size by NMR.
Body mass index (BMI) was calculated based on height and weight measured, while subjects wore light clothing without shoes. Blood pressure (BP) was measured in the subject’s right arm with a mercury sphygmomanometer, while the subject was in a seated position, and the mean BP (MBP) was calculated using the equation: Diastolic BP plus (systolic BP minus diastolic BP)/3.
2.3. OxHDL assay
Serum oxHDL levels were measured by ELISA, as described previously [20]. An anti-human oxidized apoA-I monoclonal antibody (clone No.7D3) was obtained by immunization with H2O2-oxidized apoA-I in mice [20]. The antibody was shown to react with oxHDL, but not native HDL. Serum samples were diluted in Tris-buffered saline (TBS: 100 mmol/l Tris-HCl pH 8.0, 150 mmol/l NaCl), and the monoclonal antibody against oxidized apoA-I was dissolved in 50 mmol/l Tris-HCl buffer (pH 8.4) to a final protein concentration of 10 µg/ml. A 100 µl aliquot of the monoclonal antibody solution was placed in each well of a 96-well microplate (Corning Inc., NY, USA) and passively immobilized after an overnight incubation at 4°C. The microplates were rinsed three times with distilled water and were then blocked with 1% casein in TBS at 100 µl/well. After the addition of samples (50 µl/well), microplates were incubated 1 hour at room temperature and rinsed three times with PBS containing 0.01% Tween 20. A biotinylated anti-human apoA-I monoclonal antibody was used as a secondary antibody and was diluted to 1 µg/ml in 1% casein in TBS and placed in the microplates at 100 µl/well. The mixture in the microplates was allowed to react for 1 hour at room temperature, and the microplates were then rinsed three times with PBS, containing 0.01% Tween 20. A peroxidase-labeled avidin conjugate (Vector Laboratories Inc., CA, USA) was diluted 20000-fold in 1% casein in TBS and added to the microplates at 100 µl/well, and the mixture was allowed to react for 30 minutes at room temperature. The microplates were rinsed three times with PBS, containing 0.01% Tween 20. Signal was measured in terms of the difference in optical absorbance between 450 and 620 nm after adding into each well 100 µl of substrate solution, containing 200 mg/l of tetramethylbenzidine, 0.1 mol/l Tris-HCl buffer, 35 mmol/l citrate and 5.5 mmol/l H2O2. The reaction was terminated by adding 1 mol/l phosphoric acid. The reference curves were constructed by plotting the known concentration of oxidized apoA-I standards versus absorbance. The intra- and inter-assay coefficient variations were 8.2 and 10%, respectively.
2.4. Proportion of oxidation of HDL particles
An immunoprecipitation method was used for determining the percentage of oxidized HDL particles, as previously described [27]. In brief, HDL fraction was isolated from 3 non-diabetic subjects (one male and two females) by ultracentrifugation [28]. 0.1 ml of isolated HDL (1.5 mg protein/ml) and 0.3 ml of anti-oxidized apoA-I monoclonal antibody (clone No.7D3) with immunomagnetic beads (JSR Co. Ltd., Tokyo, Japan) were incubated for 1 hour at room temperature in TBS (100 mmol/l Tris-HCl pH 8.0, 150 mmol/l NaCl) with 1% casein. Then, after collecting a pellet by a magnetic separator for 3 minutes, the pellet containing the immunoprecipitate was washed on the magnetic separator with PBS (0.01% EDTA-2K, 2 ml), followed by additional 3 washes. The glycine buffer (0.2 ml pH 2.5) was then added to the pellet and reacted for 10 minutes. Particles with immunomagnetic beads were collected by the magnetic separator for 3 minutes. The supernatants were collected, and Tris solution (1 mol/l, 0.01 ml) was added to the supernatants. An ELISA for apoA-I (Dako, Glostrup, Denmark) was then applied to the supernatants in order to determine the percent of oxHDL. The same ELISA was also applied to the original HDL fraction isolated in the first step in order to determine the total level of apoA-I on HDL. Finally, the percentage of the oxHDL-containing apoA-I was calculated by dividing by the total apoA-I level and multiplying by 100.
2.5. Statistical analysis
Unless otherwise indicated, all data are expressed as the means ± standard deviations or the medians plus interquartile ranges. A Pearson’s correlation test was used to observe a simple relationship of HDL-C, oxHDL or oxHDL/HDL-C levels with all of the other atherosclerotic risk variables, including the LDL-PS. Subsequently, a multiple linear regression analysis for HDL-C, oxHDL or oxHDL/HDL-C levels (as outcome variables), adjusted for the other atherosclerotic risk variables (as predictive variables), was utilized to observe their independent relationships. The oxHDL levels among three groups according to the tertiles of PG levels were compared, using one-way ANOVA with multiple comparison tests. The values of TG and oxHDL were log-transformed for all the analyses because of their skewed distributions. A P-value of < 0.05 was considered to be statistically significant.
3. Results
Clinical features and laboratory parameters of the study subjects are listed in Table 1. The subjects had a mean age of 57 years and all had relatively low BMI levels and were non-hypertensive. They also had a normal fasting PG level but were dyslipidemic [23] (elevated LDL-C [n = 110, 71%], decreased HDL-C [n = 4, 3%] and elevated TG [n = 57, 37%], respectively). The median level of serum oxHDL was 136 U/ml, which is not significantly lower than that previously observed in chronic renal failure patients [21,22].
Table 1.
Subject characteristics
| Variable | Mean/median levels |
|---|---|
| Age, years | 57 ± 11 |
| Gender, male/female | 21/134 |
| Body mass index, kg/m2 | 23.9 ± 3.8 |
| Mean blood pressure, mmHg | 96 ± 12 |
| Plasma glucose, mmol/l | 5.48 ± 0.65 |
| LDL-cholesterol, mmol/l | 4.04 ± 0.90 |
| Triglyceride, mmol/l | 1.39 (0.96–1.85) |
| LDL particle size, nm | 26.5 ± 0.6 |
| HDL-cholesterol, mmol/l | 1.78 ± 0.45 |
| Oxidized HDL, U/ml | 136 (107–167) |
LDL: low-density lipoprotein, HDL: high-density lipoprotein.
The data are presented as the means ± standard deviation, medians (interquartile range) or the number (%).
A simple and multiple linear regression analyses of the main outcome variables are listed in Table 2. As expected, an inverse relationship was observed between the HDL-C level and male gander, BMI, MBP or TG level, whereas HDL-C was positively correlated with LDL-PS and oxHDL. By multiple linear regression analysis, HDL-C was found to be inversely related to male gander, BMI and TG, and positively correlated to LDL-C, LDL-PS and oxHDL.
Table 2.
Correlations of HDL-cholesterol or oxidized HDL (outcome variables) with other variables (predictive variables)
| Outcome variable | For HDL-cholesterol | For oxidized HDL | ||
|---|---|---|---|---|
|
|
||||
| Predictive variable | r (P-value) | β (P-value) | r (P-value) | β (P-value) |
| Age, years | 0.12 (0.15) | 0.06 (0.37) | 0.23 (< 0.01)** | 0.09 (0.26) |
| Gender, male | −0.25 (< 0.01)** | −0.21 (< 0.01)** | −0.28 (< 0.01)** | −0.15 (0.05) |
| Body mass index, kg/m2 | −0.43 (< 0.01)** | −0.31 (< 0.01)** | −0.16 (0.04)* | −0.05 (0.61) |
| Mean blood pressure, mmHg | −0.19 (0.02)* | 0.11 (0.14) | 0.06 (0.47) | 0.11 (0.284) |
| Plasma glucose, mmol/l | 0.07 (0.41) | −0.01 (0.93) | 0.26 (< 0.01)** | 0.19 (0.01)* |
| LDL-cholesterol, mmol/l | 0.04 (0.64) | 0.16 (< 0.01)** | −0.02 (0.86) | 0.02 (0.75) |
| Triglyceride, mmol/l | −0.50 (< 0.01)** | −0.25 (< 0.01)** | −0.20 (0.02)* | −0.02 (0.85) |
| LDL particle size, nm | 0.49 (< 0.01)** | 0.32 (< 0.01)** | −0.17 (0.04)* | 0.03 (0.75) |
| HDL-cholesterol, mmol/l | − | − | 0.46 (< 0.01)** | 0.38 (< 0.01)** |
| Oxidized HDL, U/ml | 0.46 (< 0.01)** | 0.24 (< 0.01)** | − | − |
HDL: high-density lipoprotein, LDL: low-density lipoprotein.
r: simple correlation test (Pearson’s test); β: multiple linear regression analysis for HDL-cholesterol or oxidized HDL.
The values of triglyceride and oxidized HDL were log-transformed because of their skewed distributions.
Significance level: * P < 0.05, ** P < 0.01.
A simple linear correlation analysis for oxHDL showed that it was significantly and inversely correlated with male gander, BMI, TG or LDL-PS, whereas it was positively correlated with age, PG and HDL-C. After multiple linear regression analysis, oxHDL was still found to be positively correlated with PG and HDL-C.
Furthermore, the ratio of oxHDL/HDL-C was found to be positively correlated with PG (β = 0.19, P = 0.02) by multiple regression analysis. Although no other statistically significant correlation between the ratio of oxHDL/HDL-C and the other atherosclerotic risk variables were observed (data not shown), there was a trend for an inverse relationship mildly between the ratio of oxHDL/HDL-C and LDL-PS (β = –0.12, P = 0.20).
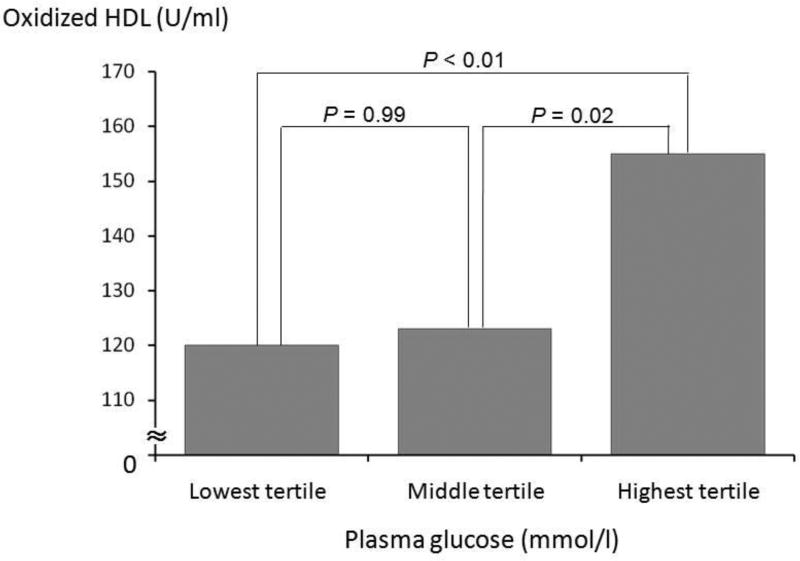
As shown in Figure 1, oxHDL levels showed a stepwise increase with increasing PG when subjects were grouped into tertiles (P < 0.01). A significant difference in the oxHDL levels was seen between the lowest PG tertile group (n = 49, PG: 4.83 ± 0.20 mmol/l) and the highest PG tertile group (n = 52, 6.24 ± 0.44 mmol/l: P < 0.01). There was also a significant difference in the oxHDL levels between the middle PG tertile group (n = 54, PG: 5.33 ± 0.13 mmol/l) and the highest PG tertile (P = 0.02).
Figure 1.
Oxidized high-density lipoprotein (HDL) levels according to the tertiles of fasting plasma glucose levels.
The lowest/middle threshold level of glucose: 5.11 mmol/l, the middle/highest threshold level of plasma glucose: 5.67 mmol/l. The oxHDL levels were shown as geometric means.
The comparison was analyzed by one-way ANOVA with multiple comparison tests.
In addition, we estimated the relative proportion of oxidation of HDL particles compared to total HDL with our immunoprecipitation method in 3 subjects. The levels of oxHDL-containing oxidized apoA-I and total apoA-I on HDL were as follows: subject 1, 33 ng/ml to 70944 ng/ml (0.05%); subject 2, 33 ng/ml to 67592 ng/ml (0.05%); subject 3, 24 ng/ml to 85760 ng/ml (0.03%). From these results, the amount of oxidized HDL compared to total HDL was relatively small, less than 0.1% of the total HDL.
4. Discussion
Several findings concerning the relationship of HDL-C and oxHDL with the other atherosclerotic risk variables was revealed in this study of non-diabetic dyslipidemic subjects. After adjusting for age, gender and the other atherosclerotic risk variables, HDL-C was found to be inversely related to BMI and TG and positively associated with LDL-C and LDL-PS. The inverse correlation between HDL-C and BMI [29] or TG [30,31] is well documented, and is most likely due to increased catabolism TG-enriched HDL after lipolysis by hepatic lipase [32]. Similarly, HDL-C and LDL-C are often found to be positively correlated [31], possibly because of an increase of CETP-mediated transfer of cholelsteryl esters from HDL to LDL [33]. A positive correlation between HDL-C and LDL-PS (a measure of small dense LDL) is also consistent with an earlier study [34] and may be linked to TG-related metabolism [35–37]. Increased TG enrichment on both HDL and LDL particles leads to increase lipolysis and the generation of smaller dense LDL and lower HDL-C levels [34–37].
A novel major finding from this study is that oxHDL showed an independent, significant and positive correlation with PG and HDL-C even in non-diabetic dyslipidemic subjects. This suggests that PG even in the normal range can lead to the oxidative modification of HDL, which would be expected to render it less functional [3] and therefore may be associated with CVD events [38]. Dyslipidemia, in itself, is considered a pro-oxidative condition [39], so there can be potentially a synergistic interaction between elevated PG and dyslipidemia, leading to HDL oxidation. The positive relationship observed between oxHDL and HDL-C may simply be due to the increase in the level of substrate native HDL particles that can undergo modification by oxidation. If so, the ratio of oxHDL/HDL-C, as has been previously described [22], may be the best way to assess the oxidative state of subjects. Although PG was not found to be related to HDL-C it was positively correlatd to oxHDL, so the ratio of oxHDL/HDL-C was found in this study to be correlated with high PG. Finally, LDL-PS was found to be a statistically non-significant but inverse correlation was found to the oxHDL/HDL-C ratio. Because the small dense LDL is associated with the oxidative milieu [35–37], similar oxidative stress-related factors that relate to its generation may also be acting on HDL.
Although only a relatively small percentage (less than 0.1%) of total HDL was found to be oxidatively modified with our assay, its positive correlation with PG suggests that it may serve as a marker for a pro-atherogenic state and/or dysfunctional HDL. Several plausible explanations for the mechanism(s) underlying the PG-oxHDL correlation are considered. Qualitative modifications of HDL are well described with glucose exposure [40]. For instance, a direct association has been observed between the PG level and apoA-I glycation [41,42], and this modification of HDL promotes atherosclerotic processes that are often accompanied with oxidative stress [43–45]. In addition, HDL particles from diabetic subjects have a significant reduction in their antioxidative properties, which further implicates a role of glucose in HDL oxidation [46]. Oxidative stress conditions are also known to lead to decreased insulin sensitivity and increased PG levels [47].
There are several limitations to the present study. First, the sample size was relatively small, but was sufficient to find many of the previously described relationships between atherosclerotic risk factors, thus the statistical significant findings related to oxHDL are likely robust. The study population was restricted to dyslipidemic but non-diabetic and clinically CVD-free subjects. The findings, therefore, may not be generalizable to other populations, although these types of subjects are commonly seen in cardiology clinics. The study was a cross-sectional design, and therefore, cannot completely be used to establish casual relationships.
In conclusion, the present study showed an independent positive association between the levels of fasting PG and serum oxHDL, but not HDL-C, among non-diabetic and dyslipidemic subjects. This suggests that high PG levels, even in the non-diabetic states, may lead to the oxidative modification of HDL, irrespective of HDL-cholesterol levels. These results also suggest that the oxHDL assay may be useful to assess the pathophysiology of glucose and lipoprotein metabolism in patients at risk for CVD. Future studies with larger and various populations, examining a possible link of oxHDL with evidence of atherosclerosis will be needed, as well as future prospective studies with clinical CVD endpoints, to fully assess the clinical utility of oxHDL as a CVD biomarker.
Acknowledgments
Study was partly supported by research funds from the intramural National Heart, Lung, and Blood Institute from the NIH.
Footnotes
Conflict of interest statement: All authors declare that they have no conflict of interest with regard to this work.
References
- 1.Deaton C, Froelicher ES, Wu LH, Ho C, Shishani K, Jaarsma T. The global burden of cardiovascular disease. Eur J Cardiovasc Nurs. 2011;10:S5–13. doi: 10.1016/S1474-5151(11)00111-3. [DOI] [PubMed] [Google Scholar]
- 2.Davidson MH. Focusing on high-density lipoprotein for coronary heart disease risk reduction. Cardiol Clin. 2011;29:105–22. doi: 10.1016/j.ccl.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 3.Navab M, Reddy ST, Van Lenten BJ, Fogelman AM. HDL and cardiovascular disease: atherogenic and atheroprotective mechanisms. Nat Rev Cardiol. 2011;8:222–32. doi: 10.1038/nrcardio.2010.222. [DOI] [PubMed] [Google Scholar]
- 4.Nakajima T, Origuchi N, Matsunaga T, Kawai S, Hokari S, Nakamura H, et al. Localization of oxidized HDL in atheromatous plaques and oxidized HDL binding sites on human aortic endothelial cells. Ann Clin Biochem. 2000;37:179–86. doi: 10.1258/0004563001899186. [DOI] [PubMed] [Google Scholar]
- 5.Thorne RF, Mhaidat NM, Ralston KJ, Burns GF. CD36 is a receptor for oxidized high density lipoprotein: implications for the development of atherosclerosis. FEBS Lett. 2007;581:1227–32. doi: 10.1016/j.febslet.2007.02.043. [DOI] [PubMed] [Google Scholar]
- 6.Witztum JL, Steinberg D. The oxidative modification hypothesis of atherosclerosis: does it hold for humans? Trends Cardiovasc Med. 2001;11:93–102. doi: 10.1016/s1050-1738(01)00111-6. [DOI] [PubMed] [Google Scholar]
- 7.Matsunaga T, Koyama I, Hokari S, Komoda T. Detection of oxidized high-density lipoprotein. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;781:331–43. doi: 10.1016/s1570-0232(02)00556-1. [DOI] [PubMed] [Google Scholar]
- 8.Bowry VW, Stanley KK, Stocker R. High density lipoprotein is the major carrier of lipid hydroperoxides in human blood plasma from fasting donors. Proc Natl Acad Sci U S A. 1992;89:10316–20. doi: 10.1073/pnas.89.21.10316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shao B, Oda MN, Vaisar T, Oram JF, Heinecke JW. Pathways for oxidation of high-density lipoprotein in human cardiovascular disease. Curr Opin Mol Ther. 2006;8:198–205. [PubMed] [Google Scholar]
- 10.Pankhurst G, Wang XL, Wilcken DE, Baernthaler G, Panzenböck U, Raftery M, et al. Characterization of specifically oxidized apolipoproteins in mildly oxidized high density lipoprotein. J Lipid Res. 2003;44:349–55. doi: 10.1194/jlr.M200256-JLR200. [DOI] [PubMed] [Google Scholar]
- 11.Zheng L, Nukuna B, Brennan ML, Sun M, Goormastic M, Settle M, et al. Apolipoprotein A-I is a selective target for myeloperoxidase-catalyzed oxidation and functional impairment in subjects with cardiovascular disease. J Clin Invest. 2004;114:529–41. doi: 10.1172/JCI21109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.von Eckardstein A, Walter M, Holz H, Benninghoven A, Assmann G. Site-specific methionine sulfoxide formation is the structural basis of chromatographic heterogeneity of apolipoproteins A-I, C-II, and C-III. J Lipid Res. 1991;32:1465–76. [PubMed] [Google Scholar]
- 13.Francis GA. High density lipoprotein oxidation: in vitro susceptibility and potential in vivo consequences. Biochim Biophys Acta. 2000;1483:217–35. doi: 10.1016/s1388-1981(99)00181-x. [DOI] [PubMed] [Google Scholar]
- 14.Nakano T, Nagata A. Oxidative susceptibility of apolipoprotein AI in serum. Clin Chim Acta. 2005;362:119–24. doi: 10.1016/j.cccn.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 15.Ohmura H, Watanabe Y, Hatsumi C, Sato H, Daida H, Mokuno H, et al. Possible role of high susceptibility of high-density lipoprotein to lipid peroxidative modification and oxidized high-density lipoprotein in genesis of coronary artery spasm. Atherosclerosis. 1999;142:179–84. doi: 10.1016/s0021-9150(98)00235-4. [DOI] [PubMed] [Google Scholar]
- 16.Chen NG, Azhar S, Abbasi F, Carantoni M, Reaven GM. The relationship between plasma glucose and insulin responses to oral glucose, LDL oxidation, and soluble intercellular adhesion molecule-1 in healthy volunteers. Atherosclerosis. 2000;152:203–8. doi: 10.1016/s0021-9150(99)00460-8. [DOI] [PubMed] [Google Scholar]
- 17.Younis N, Charlton-Menys V, Sharma R, Soran H, Durrington PN. Glycation of LDL in non-diabetic people: Small dense LDL is preferentially glycated both in vivo and in vitro. Atherosclerosis. 2009;202:162–8. doi: 10.1016/j.atherosclerosis.2008.04.036. [DOI] [PubMed] [Google Scholar]
- 18.Jenkins AJ, Rowley KG, Lyons TJ, Best JD, Hill MA, Klein RL. Lipoproteins and diabetic microvascular complications. Curr Pharm Des. 2004;10:3395–418. doi: 10.2174/1381612043383188. [DOI] [PubMed] [Google Scholar]
- 19.Soran H, Durrington PN. Susceptibility of LDL and its subfractions to glycation. Curr Opin Lipidol. 2011;22:254–61. doi: 10.1097/MOL.0b013e328348a43f. [DOI] [PubMed] [Google Scholar]
- 20.Ueda M, Hayase Y, Mashiba S. Establishment and evaluation of 2 monoclonal antibodies against oxidized apolipoprotein A-I (apoA-I) and its application to determine blood oxidized apoA-I levels. Clin Chim Acta. 2007;378:105–11. doi: 10.1016/j.cca.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 21.Honda H, Ueda M, Kojima S, Mashiba S, Suzuki H, Hosaka N, et al. Oxidized high-density lipoprotein is associated with protein-energy wasting in maintenance hemodialysis patients. Clin J Am Soc Nephrol. 2010;5:1021–8. doi: 10.2215/CJN.06110809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Honda H, Ueda M, Kojima S, Mashiba S, Michihata T, Takahashi K, et al. Oxidized high-density lipoprotein as a risk factor for cardiovascular events in prevalent hemodialysis patients. Atherosclerosis. 2012;220:493–501. doi: 10.1016/j.atherosclerosis.2011.10.038. [DOI] [PubMed] [Google Scholar]
- 23.Teramoto T, Sasaki J, Ueshima H, Egusa G, Kinoshita M, Shimamoto K, et al. Diagnostic criteria for dyslipidemia. Executive summary of Japan Atherosclerosis Society (JAS) guideline for diagnosis and prevention of atherosclerotic cardiovascular diseases for Japanese. J Atheroscler Thromb. 2007;14:155–8. doi: 10.5551/jat.e537. [DOI] [PubMed] [Google Scholar]
- 24.World Health Organization. Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia: report of a WHO/IDF consultation. World Health Organization; Geneva: 2006. [Google Scholar]
- 25.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2012;35:S64–71. doi: 10.2337/dc12-s064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsuzaki K, Kotani K, Fujiwara S, Sano Y, Matsuoka Y, Domichi M, et al. The Trp64Arg polymorphism of the beta3-adrenergic receptor gene is associated with increased small dense low-density lipoprotein in a rural Japanese population: the Mima study. Metabolism. 2007;56:1689–93. doi: 10.1016/j.metabol.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 27.Sakai J, Nohturfft A, Cheng D, Ho YK, Brown MS, Goldstein JL. Identification of complexes between the COOH-terminal domains of sterol regulatory element-binding proteins (SREBPs) and SREBP cleavage-activating protein. J Biol Chem. 1997;272:20213–21. doi: 10.1074/jbc.272.32.20213. [DOI] [PubMed] [Google Scholar]
- 28.Havel, R J, Eder HA, Bragdon JH. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955;34:1345–53. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kobayashi J, Murano S, Kawamura I, Nakamura F, Murase Y, Kawashiri MA, et al. The relationship of percent body fat by bioelectrical impedance analysis with blood pressure, and glucose and lipid parameters. J Atheroscler Thromb. 2006;13:221–6. doi: 10.5551/jat.13.221. [DOI] [PubMed] [Google Scholar]
- 30.Lipids and lipoproteins in symptomatic coronary heart disease. Distribution, intercorrelations, and significance for risk classification in 6,700 men and 1,500 women. The Bezafibrate Infarction Prevention (BIP) Study Group, Israel. Circulation. 1992;86:839–48. doi: 10.1161/01.cir.86.3.839. No author. [DOI] [PubMed] [Google Scholar]
- 31.McBride P. Triglycerides and risk for coronary artery disease. Curr Atheroscler Rep. 2008;10:386–90. doi: 10.1007/s11883-008-0060-9. [DOI] [PubMed] [Google Scholar]
- 32.Rashid S, Trinh DK, Uffelman KD, Cohn JS, Rader DJ, Lewis GF. Expression of human hepatic lipase in the rabbit model preferentially enhances the clearance of triglyceride-enriched versus native high-density lipoprotein apolipoprotein A-I. Circulation. 2003;107:3066–72. doi: 10.1161/01.CIR.0000070947.64595.47. [DOI] [PubMed] [Google Scholar]
- 33.Barter PJ, Brewer HB, Jr, Chapman MJ, Hennekens CH, Rader DJ, Tall AR. Cholesteryl ester transfer protein: a novel target for raising HDL and inhibiting atherosclerosis. Arterioscler Thromb Vasc Biol. 2003;23:160–7. doi: 10.1161/01.atv.0000054658.91146.64. [DOI] [PubMed] [Google Scholar]
- 34.Tsimihodimos V, Gazi I, Kostara C, Tselepis AD, Elisaf M. Plasma lipoproteins and triacylglycerol are predictors of small, dense LDL particles. Lipids. 2007;42:403–9. doi: 10.1007/s11745-007-3050-8. [DOI] [PubMed] [Google Scholar]
- 35.Packard CJ, Shepherd J. Lipoprotein heterogeneity and apolipoprotein B metabolism. Arterioscler Thromb Vasc Biol. 1997;17:3542–6. doi: 10.1161/01.atv.17.12.3542. [DOI] [PubMed] [Google Scholar]
- 36.Rizzo M, Berneis K. Low-density lipoprotein size and cardiovascular risk assessment. QJM. 2006;99:1–14. doi: 10.1093/qjmed/hci154. [DOI] [PubMed] [Google Scholar]
- 37.Berneis KK, Krauss RM. Metabolic origins and clinical significance of LDL heterogeneity. J Lipid Res. 2002;43:1363–79. doi: 10.1194/jlr.r200004-jlr200. [DOI] [PubMed] [Google Scholar]
- 38.Hanna-Moussa A, Gardner MJ, Kurukulasuriya LR, Sowers JR. Dysglycemia/prediabetes and cardiovascular risk factors. Rev Cardiovasc Med. 2009;10:202–8. doi: 10.3909/ricm0474. [DOI] [PubMed] [Google Scholar]
- 39.Maytin M, Leopold J, Loscalzo J. Oxidant stress in the vasculature. Curr Atheroscler Rep. 1999;1:156–64. doi: 10.1007/s11883-999-0012-z. [DOI] [PubMed] [Google Scholar]
- 40.Vergès B. Lipid modification in type 2 diabetes: the role of LDL and HDL. Fundam Clin Pharmacol. 2009;23:681–5. doi: 10.1111/j.1472-8206.2009.00739.x. [DOI] [PubMed] [Google Scholar]
- 41.Calvo C, Talussot C, Ponsin G, Berthézène F. Non enzymatic glycation of apolipoprotein A-I. Effects on its self-association and lipid binding properties. Biochem Biophys Res Commun. 1988;153:1060–7. doi: 10.1016/s0006-291x(88)81336-6. [DOI] [PubMed] [Google Scholar]
- 42.Calvo C, Ponsin G, Berthezene F. Characterization of the non enzymatic glycation of high density lipoprotein in diabetic patients. Diabete Metab. 1988;14:264–9. [PubMed] [Google Scholar]
- 43.Duell PB, Oram JF, Bierman EL. Nonenzymatic glycosylation of HDL and impaired HDL-receptor-mediated cholesterol efflux. Diabetes. 1991;40:377–84. doi: 10.2337/diab.40.3.377. [DOI] [PubMed] [Google Scholar]
- 44.Lyons TJ. Glycation and oxidation: a role in the pathogenesis of atherosclerosis. Am J Cardiol. 1993;71:26B–31B. doi: 10.1016/0002-9149(93)90142-y. [DOI] [PubMed] [Google Scholar]
- 45.Quintao EC 2000. Quintão EC, Medina WL, Passarelli M. Reverse cholesterol transport in diabetes mellitus. Diabetes Metab Res Rev. 2000;16:237–50. doi: 10.1002/1520-7560(200007/08)16:4<237::aid-dmrr127>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 46.Nobécourt E, Jacqueminet S, Hansel B, Chantepie S, Grimaldi A, Chapman MJ, et al. Defective antioxidative activity of small dense HDL3 particles in type 2 diabetes: relationship to elevated oxidative stress and hyperglycaemia. Diabetologia. 2005;48:529–38. doi: 10.1007/s00125-004-1655-5. [DOI] [PubMed] [Google Scholar]
- 47.Grattagliano I, Palmieri VO, Portincasa P, Moschetta A, Palasciano G. Oxidative stress-induced risk factors associated with the metabolic syndrome: a unifying hypothesis. J Nutr Biochem. 2008;19:491–504. doi: 10.1016/j.jnutbio.2007.06.011. [DOI] [PubMed] [Google Scholar]