Abstract
Introduction
Despite recent advances in cancer therapy, the overall 5-year survival rate of patients with lung cancer remains low. The aim of our study was to search for novel markers for early diagnosis in patients with lung cancer.
Methods
Complementary DNA microarray analysis was performed in primary lung adenocarcinomas and cell lines to search for differentially expressed genes, followed by in vivo and in vitro tumorigenic assays to characterize the oncogenic potential of the candidate genes. Gene body methylation was analyzed by 450K methylation array, bisulfite sequencing, and quantitative methylation-specific polymerase chain reaction assays. In silico analysis of The Cancer Genome Atlas data set was also performed.
Results
Inositol-trisphosphate 3-kinase A gene (ITPKA), a kinase with limited tissue distribution, was identified as a potential oncogene. We showed that ITPKA expression is up-regulated in many forms of cancers, including lung and breast cancers, and that overexpressed ITPKA contributes to tumorigenesis. We also demonstrated that ITPKA expression is regulated by epigenetic DNA methylation of ITPKA gene body through modulation of the binding of SP1 transcription factor to the ITPKA promoter. ITPKA gene body displayed low or absent levels of methylation in most normal tissue but was significantly methylated in malignant tumors. In lung cancer, ITPKA gene body methylation first appeared at the in situ carcinoma stage and progressively increased after invasion.
Conclusions
ITPKA is a potential oncogene that it is overexpressed in most tumors, and its overexpression promotes tumorigenesis. ITPKA gene body methylation regulates its expression and thus serves as a novel and potential biomarker for early cancer detection.
Keywords: ITPKA, Oncogene, Gene body methylation, SP1, Biomarker
Introduction
Lung cancer is the leading cause of cancer mortality and accounts for 1.6 million deaths annually in the world.1 Despite recent advances in cancer therapy, the overall 5-year survival rate of patients with lung cancer remains low. There is an urgent need for the development of novel markers for early diagnosis and therapy.
Lung cancers, like other epithelial cancers, are frequently preceded by a series of preneoplastic and preinvasive changes (multistage pathogenesis) resulting from the accumulation of genetic and epigenetic and abnormalities. 2 Aberrant DNA methylation is one of the epigenetic abnormalities commonly observed in cancers. Inmammals, DNA methylation, a process in which methyl groups are added to DNA, is catalyzed by three major DNA methyltransferases (DNMTs), namely DNMT1, DNMT3A, and DNMT3B. DNA methylation in the promoter region is one of the major mechanisms leading to the inactivation of tumor suppressor genes in human cancers.3–6 Emerging evidence has indicated that DNA methylation in the gene body region also plays an important role in tumorigenesis by promoting oncogene expression,7–9 and gene body methylation may hence serve as a therapeutic target for cancer treatment.10
Inositol-trisphosphate 3-kinase A (ITPKA) regulates inositol phosphate metabolism and calcium signaling by phosphorylating the second messenger, inositol 1,4,5-trisphosphate (Ins-1,4,5-P3), to inositol-1,3,4,5-tetrakisphosphate (Ins-1,3,4,5-P4).11 ITPKA, which was previously known as a neuron-specific F-actin bundling protein, has recently been shown to be associated with increased metastatic potential.12 However, the role and regulation of the inositol-trisphosphate 3-kinase A gene (ITPKA) in cancers remains largely unknown. In this study, we systematically examined the expression of ITPKA expression in lung and other cancers and demonstrated the oncogenic activity of overexpressed ITPKA. We also showed that ITPKA overexpression is regulated by its gene body methylation, and that ITPKA body methylation serves as a potential marker for early detection of many cancers, including cancers of the lung and breast.
Materials and Methods
The following items are described in Supplementary Digital Content 1: cell lines; reagents; constructs; cell transfection; gene knockdown; quantitative polymerase chain reaction (qPCR); assays of cell proliferation, colony formation, migration, and invasion; luciferase reporter assay; and in vivo xenograft tumor formation assay.
Human Samples
All specimens were obtained after approval from the respective institutional review boards and informed consent from all participating subjects. Details are provided in the Supplementary Digital Content 1.
Genomic DNA Extraction and Measurement of Methylation Level
Details of genomic DNA extraction and measurement of methylation level by bisulfite sequencing, quantitative methylation-specific polymerase chain reaction (qMSP), and 450K methylation array are described in Supplementary Digital Content 1.
Chromatin-Immunoprecipitation Assay
DNA-binding affinity of SP1 transcription factor was measured by the MAGnify Chromatin Immunoprecipitation System (Invitrogen, Eugene, OR) according to the manufacturer’s instructions. The immunoprecipitated DNA was amplified by PCR using SP1-specific primers (Supplementary Table 3 in Supplementary Digital Content 2).
Statistical Analyses
Details of the statistical analyses used in this article are described in Supplementary Digital Content 1.
Results
ITPKA Is a Potential Oncogene That Is Up-regulated in Lung Cancer and Other Cancers
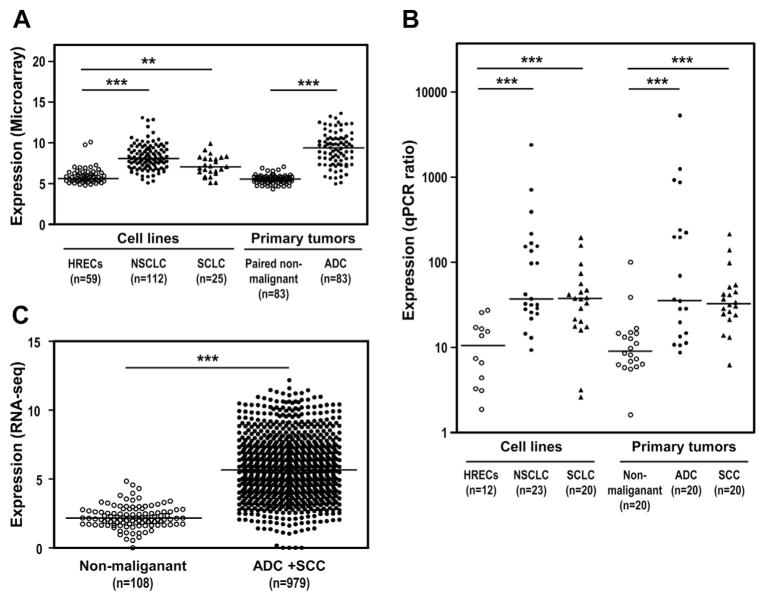
To identify potential oncogenes that are involved in the pathogenic development of lung cancer, complementary DNA microarray analysis was performed to search for differentially expressed genes in 83 primary lung adenocarcinomas (ADCs) and their paired adjacent nonmalignant lung tissues. Supplementary Table 1 in Supplementary Digital Content 3 lists the top 20 up-regulated genes in lung ADCs. Many of them have been previously reported to be dysregulated in various types of cancer, and some of them have been well studied.13–20 However, the role of ITPKA in cancer remains poorly understood. The expression of ITPKA was up-regulated in primary lung cancers and lung cancer cell lines as compared with in the corresponding nonmalignant tissues and immortalized human respiratory epithelial cells (HRECs)21 by complementary DNA microarray analysis (Fig. 1A) and the real-time qPCR method (Fig. 1B). To increase the sample size, we examined ITPKA expression by analyzing the next-generation RNA sequencing data of 979 lung cancer samples and 108 corresponding nonmalignant lung samples in The Cancer Genome Atlas data set (TCGA)22 and showed that ITPKA was expressed at significantly higher levels in the lung cancers than in the nonmalignant lung samples (Fig. 1C). In addition to lung cancer, ITPKA was also overexpressed in many other cancers (Table 1).
Figure 1.
Inositol-triphosphate 3-kinase A gene (ITPKA) is up-regulated in lung cancer cell lines and primary tumors. (A) ITPKA expression was determined by microarray analysis of 59 immortalized nonmalignant human respiratory epithelial cells (HRECs) versus 112 non–small cell lung cancer (NSCLC) and 25 small cell lung cancer (SCLC) cell lines and of 83 paired adenocarcinomas (ADCs) and corresponding nonmalignant lung tissues. (B) ITPKA expression determined by quantitative polymerase chain reaction (qPCR) analysis of 12 HRECs, 23 NSCLC and 20 SCLC cell lines, 20 nonmalignant lung tissues, and 20 primary and 20 squamous cell carcinoma (SCC) samples. (C) ITPKA expression determined by analyzing RNA sequencing data of 108 nonmalignant samples and 979 ADCs and SCCs available in The Cancer Genome Atlas data sets. The horizontal line represents the median value of expression level, and p value was also included.
Table 1.
ITPKA Expression and Gene Body Methylation in Cancers
| Cancer Type | ITPKA Expression (RNA-seq) | ITPKA Body Methylation (β-Value) | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Ratio (T/N) | Tumor | Nonmalignant | p Value (T vs. N) | Z-score (T-N) | Tumor | Nonmalignant | p Value (T vs. N) | |
| Breast invasive carcinoma | 4.25 | 3.40 (n = 161) | 0.80 (n = 112) | 1.03E-49 | 0.35 | 0.48 (n = 743) | 0.13 (n = 97) | 3.79E-53 |
|
| ||||||||
| Lung squamous cell carcinoma | 2.55 | 5.23 (n = 490) | 2.05 (n = 50) | 3.80E-38 | 0.34 | 0.46 (n = 360) | 0.12 (n = 43) | 1.32E-32 |
|
| ||||||||
| Lung adenocarcinoma | 2.96 | 6.46 (n = 489) | 2.18 (n = 58) | 5.98E-30 | 0.29 | 0.42 (n = 465) | 0.13 (n = 32) | 4.86E-23 |
|
| ||||||||
| Kidney renal clear cell carcinoma | 3.25 | 3.84 (n = 531) | 1.18 (n = 72) | 1.84E-26 | 0.06 | 0.13 (n = 324) | 0.07 (n = 160) | 3.62E-24 |
|
| ||||||||
| Thyroid carcinoma | 1.68 | 3.63 (n = 497) | 2.16 (n = 59) | 2.67E-26 | 0.04 | 0.11 (n = 506) | 0.07 (n = 56) | 1.06E-07 |
|
| ||||||||
| Liver hepatocellular carcinoma | 1.54 | 7.15 (n = 268) | 4.65 (n = 50) | 1.56E-13 | 0.04 | 0.61 (n = 259) | 0.57 (n = 36) | 5.93E-03 |
|
| ||||||||
| Head and neck squamous cell carcinoma | 1.52 | 5.13 (n = 496) | 3.37 (n = 43) | 1.83E-13 | 0.24 | 0.36 (n = 527) | 0.12 (n = 50) | 4.68E-18 |
|
| ||||||||
| Kidney renal cell papillary carcinoma | 2.61 | 2.87 (n = 240) | 1.10 (n = 32) | 4.83E-10 | 0.00 | 0.08 (n = 225) | 0.08 (n = 45) | 2.05E-03 |
|
| ||||||||
| Bladder urothelial carcinoma | 1.25 | 3.81 (n = 309) | 3.05 (n = 19) | 8.55E-03 | 0.17 | 0.25 (n = 373) | 0.08 (n = 20) | 2.66E-05 |
|
| ||||||||
| Uterine corpus endometrial carcinoma | 1.09 | 3.93 (n = 169) | 3.62 (n = 24) | 1.41E-01 | 0.12 | 0.23 (n = 438) | 0.11 (n = 46) | 1.97E-06 |
|
| ||||||||
| Colon adenocarcinoma | 0.79 | 7.45 (n = 273) | 9.46 (n = 41) | 1.14E-17 | 0.07 | 0.61 (n = 302) | 0.54 (n = 38) | 5.13E-06 |
|
| ||||||||
| Prostate adenocarcinoma | 0.75 | 2.58 (n = 419) | 3.46 (n = 51) | 6.94E-09 | 0.17 | 0.41 (n = 428) | 0.24 (n = 50) | 8.11E-11 |
Note: All data are derived from The Cancer Genome Atlas data sets. Ranking by p value.
ITPKA, inositol-trisphosphate 3-kinase A gene; RNA-seq, RNA sequencing, T, tumor; N, nonmalignant.
ITPKA Promotes Oncogenic Transformation and Tumorigenesis
We next investigated the role of ITPKA in cancer development. The NSCLC cell lines NCI-H2009 and NCI-H1299, which expressed endogenous ITPKA at modest levels (Supplementary Fig. 1 in Supplementary Digital Content 3), were subjected to gain-of-function studies by exogenous expression of ITPKA (Fig. 2A [left]). As shown, overexpression of ITPKA enhanced cell proliferation (Fig. 2B [left]), colony formation (Fig. 2C [left]), and in vivo xenograft tumor growth in NSG mice (Fig. 2D [top]). Reciprocal experiments were performed by depleting the expression of endogenous ITPKA in NCI-H2228 cells, which expressed endogenous ITPKA at a relatively high level, as well as in NCI-H2009 and NCI-H1299, by the small interfering RNA oligomers method (Fig. 2A [middle]). Knockdown of ITPKA expression suppressed cell proliferation (Fig. 2B [middle]) and colony formation (Fig. 2C [middle]). When ITPKA expression was stably knocked down using the lentivirus-based short hairpin RNA approach, similar results were obtained (Fig. 2B and C[right]). In addition, stable knockdown of ITPKA suppressed xenograft tumor growth in NSG mice (Fig. 2D [bottom]). Taken together, these results indicate that ITPKA expression promotes tumor formation.
Figure 2.
Inositol-triphosphate 3-kinase A gene (ITPKA) promotes tumorigenesis. (A) Western blot analysis of non–small cell lung cancer cells stably overexpressing ITPKA (left), and cells in which the expression of endogenous ITPKA was knocked down using small interfering RNA (siRNA) oligomers (oligo) (middle) and lentivirus-based short hairpin RNA (shRNA) approach (right). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) served as the loading control. (B) Cell proliferation rate was determined using cell counting kit-8 reagent. (C) Colony formation assay was performed by plating and maintaining cells for 12 days, and colony numbers were scored after crystal violet staining. (D) Cells were subcutaneously injected into NSG mice (n = 6 per group), and tumor volumes were monitored. HA, hemagglutinin.
ITPKA Expression Is Modulated by Its Gene Body Methylation
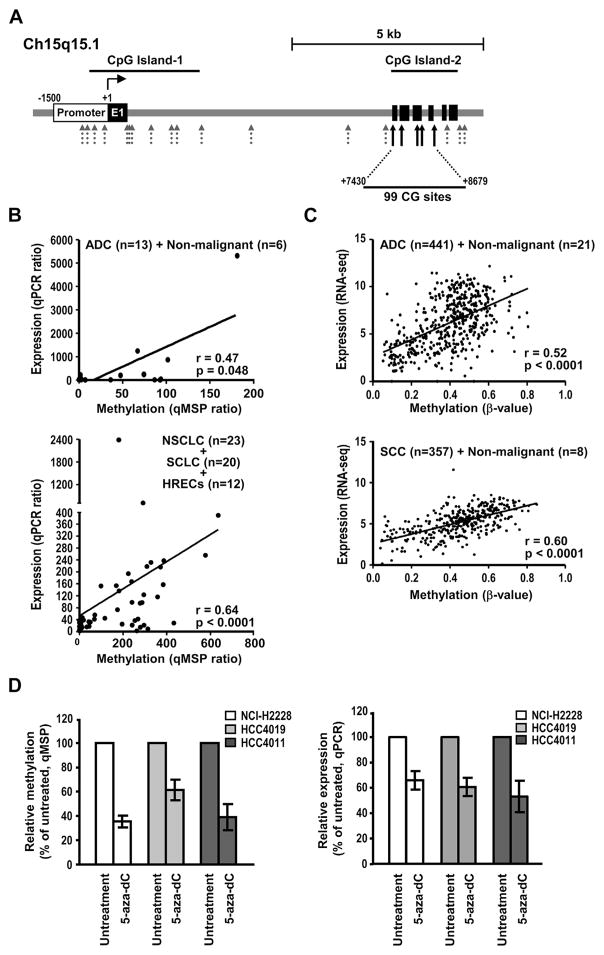
To explore the mechanisms leading to ITPKA overexpression in lung cancer, we examined the roles of gene mutations and DNA amplification. Only three mutations (c.665T>G; p.L222R, c.341T>C; p.L114P, and c.557A>T; p.Y186F) were identified in 698 patients with lung cancer in the TCGA lung cancer data sets. Similarly, sporadic low-level gains (approximately three copies) of ITPKA allele were detected in lung cancer cell lines (N = 103) by using single nucleotide polymorphism (SNP) array analysis (Illumina Human 1M-Duo DNA, Illumina, San Diego, CA) and in primary lung cancer specimens (N = 451) derived from the TCGA database. These results suggest that gene mutations and DNA copy number alterations play only a minor role in the regulation of ITPKA expression. We next examined the possible involvement of epigenetic alterations in DNA methylation and found aberrant methylation in ITPKA gene body. As illustrated in Figure 3A, two short stretches of DNA regions containing high frequency of CG sequence (CpG) islands were identified in the ITPKA gene by using two algorithms, Methyl Primer Express (Invitrogen) and the University of California at Santa Cruz Genome Browser.23 CpG island 1 spans from the promoter region to intron 1, and CpG island 2 is located in the gene body region from exon 2 to intron 5. We determined the methylation patterns of these two CpG islands by performing 450K methylation array in 158 lung cancer cell lines and 28 immortalized HRECs. As shown in Supplementary Table 2 in Supplementary Digital Content 3, eight probes were mapped to CpG island 1: five of them displayed very low levels of methylation signals across all cell lines, and the remaining three displayed the same levels of methylation signals in the malignant and nonmalignant samples. By contrast, the six probes encompassing CpG island 2 showed significantly higher methylation intensity in the lung cancer cell lines. We further examined this result in primary lung cancers in the TCGA lung cancer data set. Five of the six probes (cg09299055, cg08680048, cg03927133, cg11789612, and cg03177551) located in the CpG island 2 were consistently methylated at significantly elevated levels in primary lung cancer samples (Supplementary Table 2 in Supplementary Digital Content 3). Because the sixth probe, cg27501645, was hypermethylated in both the malignant and nonmalignant tissues, we excluded this probe when analyzing the methylation score of CpG island 2 region in subsequent analyses. These five probes in the CpG island 2 (cg09299055, cg08680048, cg03927133, cg11789612, and cg03177551) span a region of 1250 base pairs (from the downstream of ITPKA promoter nt 7430 to nt 8679), which contains a total of 99 CpG sites in the ITPKA gene body (Fig. 3A). To establish the link between ITPKA expression and its gene body methylation, ITPKA expression by the qPCR method and its gene body methylation using specific qMSP analysis were examined in 13 primary ADCs and six adjacent nontumorous tissues and in lung cancer cell lines, including 23 NSCLCs, 20 small cell lung cancers, and 12 HRECs. As shown in Figure 3B, a trend of positive correlation between ITPKA expression and body methylation was observed in both primary lung samples and cell lines. We further examined ITPKA expression and its gene body methylation status in 441 ADCs and 21 nonmalignant tissue samples, as well as in 357 squamous cell carcinomas (SCCs) and eight nonmalignant tissues that were available in the TCGA data set. Consistent results were obtained in ADC and SCC, with Pearson correlation coefficients of 0.53 and 0.60, respectively (Fig. 3C). In addition, a similar finding in breast cancer (n = 824) was also observed (Supplementary Fig. 2A in Supplementary Digital Content 3). Combined, these results indicate that ITPKA expression is positively correlated with its gene body methylation. To further determine whether ITPKA expression is regulated by its gene body methylation, 5-aza-2′-deoxycytidine (5-aza-dC), a DNA methyltransferase inhibitor, was used to decrease the methylation levels of ITPKA gene body. Transketolase-like 1 gene (TKTL1)24 was included as a control (Supplementary Fig. 3 in Supplementary Digital Content 3). The NSCLC cell lines NCI-H2228, HCC4011, and HCC4019, which expressed the endogenous ITPKA at high levels (Supplementary Fig. 1 in Supplementary Digital Content 3), were cultured in the presence or absence of 5-aza-dC and showed significantly decreased ITPKA gene body methylation after 5-aza-dC treatment (Fig. 3D [top]). Most importantly, a concomitant decrease in ITPKA expression after 5-aza-dC treatment was also observed (Fig. 3D [bottom]), suggesting that reduced ITPKA expression results from the decreased methylation of ITPKA gene body.
Figure 3.
Inositol-triphosphate 3-kinase A gene (ITPKA) expression is regulated by its gene body methylation. (A) Schematic representation of organization of the ITPKA gene and the relative positions of methylation probes on chromosome 15q15.1. The open and filled boxes represent ITPKA promoter and exons, respectively. The arrows under the ITPKA gene indicate the probe positions of 450K methylation array, and the black solid arrows represent the ones that were used for the prediction of ITPKA gene body methylation. (B and C) The correlation of ITPKA expression and gene body methylation in primary lung cancer samples and lung cancer cell lines. In (B), ITPKA expression was determined by quantitative polymerase chain reaction (qPCR) analysis, and methylation was measured by quantitative methylation-specific polymerase chain reaction (qMSP) in 13 adenocarcinoma (ADCs) and six nonmalignant lung tissues (top), and in 23 non–small cell lung cancers (NSCLCs), 20 small cell lung cancers (SCLCs) and 12 human respiratory epithelial cells (HRECs) (bottom). In (C), ITPKA expression was determined by RNA-sequencing and methylation was measured by 450K methylation array in 462 ADCs and 365 squamous cell carcinomas (SCCs). Methylation levels were assessed as the average of the β-values of the five probes cg09299055, cg08680048, cg03927133, cg11789612, and cg03177551. Pearson’s correlation coefficient was included. (D) Cells were cultured in the absence and presence of 5-aza-dC, and ITPKA gene body methylation and gene expression were measured. Gene body methylation status was determined by qMSP analysis and ITPKA expression was determined by the qPCR method. Data are shown as mean plus or minus SD. seq, sequence; CpG island, short stretch of DNA containing high frequency of CG sequence; CG, CG sequence.
ITPKA Body Methylation Modulates SP1-Mediated ITPKA Transcription in a DNMT3B-Dependent Manner
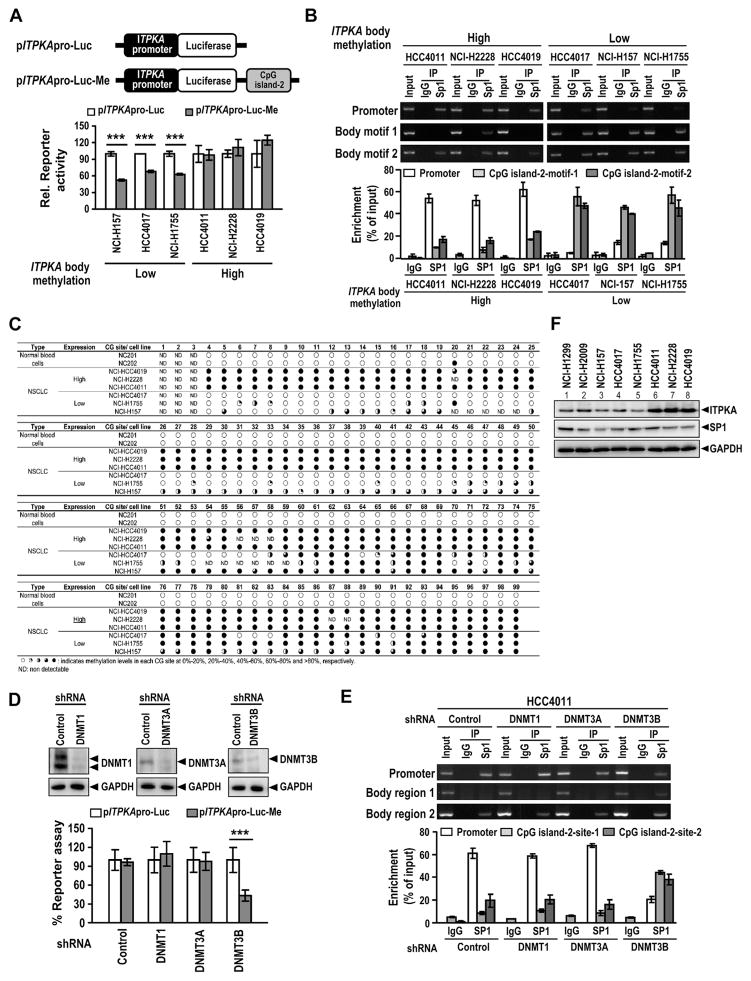
We next explored whether ITPKA gene body methylation directly regulates its transcription. Luciferase reporter assay was performed to measure ITPKA promoter activity in NSCLC cell lines with high ITPKA body methylation compared with in three cell lines that were specified to display low ITPKA body methylation. An ITPKA promoter–driving luciferase reporter construct, pITPKApro-Luc, as well as a pITPKApro-Luc-Me construct in which the DNA fragment of CpG island 2 was inserted downstream of luciferase of pITPKApro-Luc, were used. As shown in Figure 4A, the presence of the CpG island 2 region significantly suppressed ITPKA promoter activity in NCI-H157, HCC4017, and NCI-H1755 cells that displayed low ITPKA body methylation, but it had no effect on ITPKA promoter activity in cell lines that displayed high levels of ITPKA body methylation, suggesting that the methylation status of ITPKA gene body may regulate the promoter activity. To examine whether ITPKA body methylation may regulate its gene expression by modulating the binding of transcription factors, we searched for transcription factor binding motifs present in both ITPKA promoter and CpG island 2 regions. Using the Softberry NSITE Program (Softberry, Inc., Mt. Kisco, NY), we identified putative binding motifs for SP1 in the promoter (nts −89 to −140) as well as CpG island 2 (motif 1 nts 220–247 and motif 2 nts 739–935) regions. SP1, a well-known transcription activator that regulates the transcription of TATA-less promoters,25 has been reported to bind to the ITPKA promoter and drive gene expression.26 To examine whether ITPKA gene body may regulate its promoter activity through sequestration of SP1 binding to the promoter, chromatin immunoprecipitation assay was utilized to examine binding of SP1 to the promoter region and to the motifs 1 and 2 within CpG island 2. The bar graph in Figure 4B shows the quantitation of the bound DNA by qPCR analysis. SP1 binding to the ITPKA promoter was readily detected in cells displaying high ITPKA gene body methylation, in which the binding of SP1 to ITPKA gene body CpG island 2 region was relatively weak (in particular, the motif 1). In contrast, SP1 binding to the CpG island 2 was abundantly detected in cells displaying low ITPKA body methylation, in which SP1 binding to the promoter region was relatively weak. We also examined the SP1 protein levels in these cells to rule out the possibility that SP1-promoter binding ability may result from SP1 protein levels (Fig. 4F). All these data suggest that low methylated CpG island 2 in the gene body may sequester SP1 from binding to the promoter, whereas hypermethylated CpG island 2 fails to attract SP1 and renders promoter to drive SP1-mediated ITPKA transcription in cells displaying high ITPKA body methylation. Accordingly, we provided an explanation that the relative lower ITPKA promoter activity in pITPKApro-Luc-Me as compared with that in pITPKApro-Luc in the cell lines displaying low ITPKA gene body methylation results from the sequestration of SP1 by the unmethylated gene body CpG island. In contrast, the pITPKA-pro-Luc and pITPKApro-Luc-Me constructs yielded similar promoter activity in the cell lines displaying high levels of gene body methylation because the gene body CpG island is methylated in the pITPKApro-Luc-Me construct and failed to sequester SP1. Using the direct bisulfite sequencing analysis, we further confirmed that the 99 CpG sites in the CpG island 2 of the ITPKA body region were methylated at low levels in the HCC4017, NCI-H157, and NCI-H1755 cells (Fig. 4C) and the unmethylated CpG sites sequestered SP1 from binding to the promoter (Fig. 4B), yielding low ITPKA expression (Fig. 4A). By contrast, these 99 CpG sites were fully methylated in HCC4011, NCI-H2228, and NCI-H4019 cells (Fig. 4C), allowing for SP1 binding to the promoter as detected (Fig. 4B) and leading to high ITPKA expression (Fig. 4A). These results suggest that the methylation status of the ITPKA gene body may regulate SP1-promoter binding and influence ITPKA expression. To further substantiate that methylation of ITPKA gene body may regulate ITPKA expression, we searched for DNMTs that are responsible for the methylation of ITPKA gene body. Using a lentivirus-based knockdown approach, we showed that depletion of endogenous DNMT3B, but not DNMT1 or DNMT3A, significantly decreased ITPKA promoter activity in the high-ITPKA–expressing HCC4011 cells (Fig. 4D) and was accompanied by the shifting of SP1 binding from the promoter to the CpG island 2 regions (Fig. 4E), suggesting that DNMT3B is responsible for the methylation of ITPKA gene body in cells displaying high ITPKA gene body methylation. Taken together, our data supported the notion that methylation status of ITPKA gene body surrounding the SP1-DNA sites may regulate ITPKA expression through modulation of the binding of SP1 to ITPKA promoter.
Figure 4.
Inositol-triphosphate 3-kinase A gene (ITPKA) gene body methylation regulates its promoter activity through modulation of SP1-DNA binding. (A) Schematic representations of pITPKApro-Luc and pITPKApro-Luc-Me reporter constructs and relative promoter activity in non–small cell lung cancer (NSCLC) cells. (B) Chromatin-immunoprecipitation assay of SP1-DNA binding activity in NSCLC cells. SP1 binding to the promoter and CpG island 2 motif1 and motif 2 was measured. The quantitation of SP1-DNA binding activity by using quantitative polymerase chain reaction analysis was also shown in relation to the amount of input. (C) Bisulfite sequencing analysis of the methylation status of the individual 99 CG sites within ITPKA gene body methylation CpG island 2 region in normal blood cells and NSCLC cell lines expressing high and low amounts of ITPKA. The open and quarter-filled, half-filled, three-quarters–filled, and fully filled circles indicated the methylation level of each CpG site was within 0% to 20%, 20% to 40%, 40% to 60%, 60% to 80%, and 100%, respectively. (D) Reporter assay of ITPKA promoter activity in NSCLC HCC4011 cells upon knocking down endogenous DNMT1, DNMT3A or DNMT3B. Western blot analysis of DNMTs knockdown efficiency was also shown. (E) Chromatin-immunoprecipitation assay of SP1-DNA binding activity in NSCLC cells HCC4011 cells upon knocking down the endogenous DNA methyltransferases (DNMTs) DNMT1, DNMT3A or DNMT3B. The quantitation of DNA binding activity by quantitative polymerase chain reaction analysis was also included. (F) Western blot analysis of ITPKA and SP1 expression in NSCLC cell lines. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) served as the loading control. shRNA, short hairpin RNA; IgG, immunoglobulin G.
ITPKA Gene Body Methylation May Serve as a Common Biomarker for Early Cancer Detection
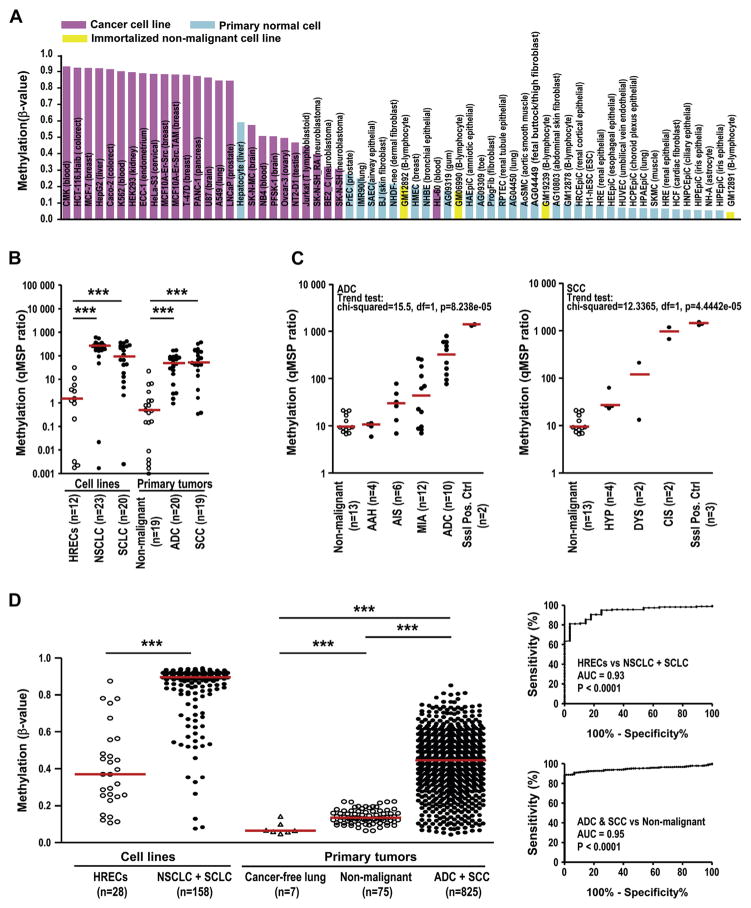
Because ITPKA expression promotes tumorigenesis and its expression is regulated by gene body methylation, we next investigated whether ITPKA gene body methylation may serve as a DNA-based marker for malignant transformation. We first analyzed the status of ITPKA gene body methylation in 469 nontumorous samples derived from 24 different tissues of cancer-free donors in the National Center for Biotechnology Information Gene Expression Omnibus data sets (for GSE60654)27 and showed that most of the nontumorous tissues displayed low or undetectable levels of ITPKA gene body methylation (β-values < 0.2) (see Supplementary Fig. 4 in Supplementary Digital Content 3). These data were consistent with our findings that the 99 CpG sites in the ITPKA gene body were hardly methylated in the peripheral blood samples (noncancerous) of healthy individuals (Fig. 4C). We next analyzed the methylation status in immortalized and cancer cell lines by examining 450K methylation array data in the Encyclopedia of DNA Elements database.28 As shown in Figure 5A, the ITPKA gene body was hypomethylated in the immortalized cell lines (yellow bars) and nonmalignant primary cells (light blue bars) with a methylation score of approximately or lower than 0.2 and was hypermethylated in cancer-derived cell lines (pink bars), with the exception of the leukemic cell line HL-60 with a mean β-value of 0.7. In support of this, we also demonstrated that ITPKA gene body methylation levels were significantly higher in lung cancer cell lines and primary tumors than that in the immortalized lung cell lines and nonmalignant lung tissues by the specific qMSP method (Fig. 5B). Furthermore, we examined the status of ITPKA gene body methylation in the multistage pathogenic development of lung cancer29 by the qMSP method. For lung adenocarcinoma, formalin-fixed, paraffin-embedded sections representative of nonmalignant lung, atypical adenomatous hyperplasia, adenocarcinoma in situ, minimally invasive adenocarcinoma, and invasive ADC were examined (Fig. 5C). All nonmalignant lung and atypical adenomatous hyperplasia samples were negative for methylation (we set the baseline of ITPKA body methylation ratio as 21.8, which is the highest value for nonmalignant tissues). In contrast, adenocarcinoma in situ, minimally invasive adenocarcinoma, and ADC showed significantly increased ITPKA methylation. For SCCs, 13 nonmalignant lung samples and six premalignant lesions, including four hyperplasia and two dysplasia samples, showed minimal to moderate ITPKA methylation, whereas high levels of methylation were present in the SCC in situ specimens. These data showed that ITPKA gene body methylation was initiated at an early stage and progressively increased during multistage pathogenesis of both adenocarcinoma and SCC by the trend test.
Figure 5.
Inositol-triphosphate 3-kinase A gene (ITPKA) gene body methylation serves as a potential diagnostic biomarker for early cancer detection. (A) Methylation levels of ITPKA gene body in cancer cell lines (pink), immortalized nonmalignant cell lines (yellow) and primary normal cells (light blue), respectively. Methylation levels were assessed as the average of the β-values of five probes (cg09299055, cg08680048, cg03927133, cg11789612 and cg03177551). (B and C) Quantitative methylation-specific polymerase chain reaction (qMSP) analysis of ITPKA gene body methylation in lung cancer cell lines and primary lung cancers (B) and in formalin-fixed, paraffin-embedded sections from patients with cancer (C). The median values are indicated by red horizontal lines. Methyltransferase SssI–treated cells were included as positive controls for qMSP analysis. (D) Methylation levels of ITPKA gene body detected by 450K methylation array in lung cancer cell lines and primary lung cancers. The receiver operating characteristic curve analysis is shown, and the area under the curve (AUC) score and p value are also included. HREC, human respiratory epithelial cell; NSCLC, non–small cell lung cancer; SCLC, small cell lung cancer; ADC, adenocarcinoma; SCC, squamous cell cancer; AAH, atypical adenomatous hyperplasia; AIS, adenocarcinoma in situ; MIA, minimally invasive adenocarcinoma; HYP, hyperplasia; DYS, dysplasia; CIS, carcinoma in situ.
To further test the diagnostic sensitivity of ITPKA gene body methylation for detection of lung cancers, a larger number of lung cancer cell lines and the TCGA lung cancer data set were analyzed. As shown in Fig. 5D, cancer-derived cell lines (n = 158) showed significantly increased methylation score as compared with immortalized HREC lines (n = 28), and the under the receiver operating characteristic curve was 0.93. Furthermore, the methylation levels in primary ADC and SCC (n=825) were statistically higher than those in the adjacent nonmalignant (n=75) and cancer-free lung tissues (n=7), with an AUC of 0.95. In addition, cancer-free lungs (data from National Center for Biotechnology Information Gene Expression Omnibus data sets for GSE30654) showed considerably lower methylation intensities than those in nonmalignant lung tissues adjacent to invasive carcinomas, emphasizing the possible use of ITPKA gene body methylation as an early diagnostic marker for lung cancers. Similarly, ITPKA gene body methylation can distinguish breast cancers (n = 743) from the nonmalignant breast tissues (n = 97), with an AUC value of 0.93 (Supplementary Fig. 2B in Supplementary Digital Content 3). Other than lung and breast cancers, increased ITPKA gene body methylation was also observed in many other types of tumor samples (Table 1), suggesting the potential use of ITPKA gene body methylation as a marker to differentiate malignant from nonmalignant tissues.
Discussion
In this study, we demonstrated that ITPKA is upregulated in lung, breast, and many other types of cancer and that overexpressed ITPKA promotes malignant transformation in vitro and in vivo. Furthermore, we showed that ITPKA expression is tightly regulated by its gene body methylation. In various tumors, epigenetic modification through DNA methylation of gene promoter regions has been shown to silence numerous tumor suppressor genes.3–6 Although promoter hypermethylation serves as a repressive epigenetic mark that down-regulates gene expression, gene body methylation, which is more prevalent than promoter hypermethylation in the genome, may be associated with increased gene expression.7 Nevertheless, the role of gene body methylation and its action in regulating gene expression are poorly understood. It has been suggested that gene body methylation may repress spurious intragenic transcription and therefore allows for efficient transcriptional elongation.25 However, most of the regions of gene body methylation in the genome are associated with nontranscription initiation sites.30 In this study, we showed that gene body methylation may influence gene expression through the modulation of the binding of transcription factor(s). Methylation within transcription factor binding sites has been shown to retard the binding of transcription factor and thus alleviate gene expression.31,32 In several studies, hypermethylation of the SP1-DNA binding site has been proved to block the binding of SP1.33–35 We identified two SP1-DNA binding motifs in the ITPKA gene body CpG island 2 region and showed that these two SP1-DNA binding sites in the gene body may serve as decoys to recruit and sequester SP1 from binding to the promoter. After the action of DNMT3B, the fully methylated body region is turned refractory to SP1 binding, releasing SP1 for binding to the promoter to drive gene expression. Bisulfate sequencing analysis showed that the SP1 binding motifs in the gene body, located at 13–16 CpG sites and 81–84 CpG sites within the 99 CpG sites in the CpG island 2 region, were hypermethylated in high-ITPKA–expressing cells and hypomethylated in low-ITPKA–expressing cells (Fig. 4C), suggesting that the methylation levels within SP1-DNA binding site in the ITPKA gene body is indeed correlated with its gene expression. However, the SP1 binding motif 1 displayed a more significant difference in methylation levels between the high- and low-ITPKA–expressing cell lines (Fig. 4C), suggesting that SP1-binding motif 1 may play a more important role in body methylation-regulated expression of ITPKA. These data warrant further studies to explore the regulation of gene expression by gene body methylation and the identification of oncogenes other than ITPKA that are regulated by similar mechanism.
Transcription factors SP1 and RE1 silencing transcription factor (REST)/NRSF have been previously reported to bind to ITPKA promoter, with SP1 positively and REST/NRSF negatively regulating ITPKA expression. 31 In this study, we showed that SP1-mediated ITPKA expression is modulated by its gene body methylation, suggesting the involvement of epigenetic regulation. We also explored whether the levels of SP1 and REST may contribute to the deregulated expression of ITPKA upon malignant transformation by assessing the correlation between ITPKA expression and the expression of SP1 and REST expression using microarray analysis of lung cancer cell lines, including 113 NSCLCs, 29 small cell lung cancers, and 59 HRECs. The Pearson correlation coefficients of ITPKA with SP1 and with REST were 0.01 and −0.20 (data not shown), respectively, suggesting that the expression level of SP1 and REST plays a minor role in the regulation of ITPKA expression. In Figure 3C, we examined the correlation of ITPKA gene body methylation and its expression in primary lung cancer tissues. Although the Spearman correlation coefficients in ADC and SCC were not fully satisfied (r = 0.52 and 0.6), a trend of positive correlation was observed between gene expression and body methylation. We suspected that the infiltration of normal cells in the tumor tissues as well as other factors that are involved in the regulation of ITPKA expression may dilute the correlation.
Through genomewide analysis of DNA methylation patterns, we have previously demonstrated that human secretin gene (SCT) promoter is also frequently hypermethylated in lung cancer.36 In the lung, SCT is expressed at an undetectable level in normal cells as well as in malignant cells regardless of its promoter methylation status. Although the functional implications of SCT promoter methylation remains to be defined, our study validated SCT promoter methylation as a biomarker for lung cancer.36 In this study, we showed that the CpG island 2 in ITPKA gene body is highly methylated in lung cancer and ITPKA gene body methylation serves as a marker for early detection of lung cancer. Furthermore, we showed that ITPKA gene body methylation promotes its expression, facilitating the development of malignant phenotypes. Thus, in contrast to SCT, ITPKA methylation is associated with gene expression which facilitates oncogenesis. Because ITPKA is overexpressed in multiple types of cancers and drives tumorigenesis, ITPKA may serve as a potential therapeutic target.
Under physiological conditions, ITPKA is highly expressed in neurons during brain development and in the testis.37 Because ITPKA expression is regulated by its gene body methylation, normal brains consistently display ITPKA body methylation at high levels as shown in Supplementary Figure 4 in Supplementary Digital Content 3. Of interest, we also noted placenta displays high levels of ITPKA body methylation. Placental tissues include cytotrophoblast and syncytiotrophoblast, and the extravillous trophoblast cells in the placenta have been demonstrated to migrate, invade, and remodel the maternal decidua and develop a vascular supply in a manner similar to cancers.38 In addition, many tumor suppressor genes and oncogenes are thought to play important roles in normal placental development, and the epigenetic program of the placenta shows some similarities to those of cancer cells.39,40 These findings support the concept that the placenta may be regarded as a self-limited malignancy. This concept further supports that ITPKA body methylation is significantly increased in malignant tumors and contributes to tumorigenesis.
In summary, the highly specific and sensitive patterns of ITPKA expression and body methylation in a wide range of tumor types suggest that deregulation of ITPKA plays an important role in the pathogenesis of lung and other cancers. Most importantly, ITPKA body methylation, which is absent in nonmalignant lungs, appears at the premalignant stage and progressively increases with cancer development, further emphasizing its potential application on early cancer detection.
Supplementary Material
Acknowledgments
This work was supported by generous grants from the Canary Foundation; the Early Detection Research Network (U01CA086402), National Cancer Institute, National Institutes of Health, Bethesda, Maryland; and the University of Texas SPORE in Lung Cancer PO (P50CA70907), National Cancer Institute, National Institutes of Health Bethesda, Maryland. The work was also supported in part by funding from Academic Sinica, Taiwan, Republic of China (to Dr. Chen) and a post-doctoral fellowship to Dr. Wang. The authors thank Drs. Ignacio Wistua (for specimen collection), John Minna (for intellectual contributions and resources), and Kai Song (for bioinformatics analysis).
Footnotes
Disclosure: The authors declare no conflict of interest.
Note: To access the supplementary material accompanying this article, visit the online version of the Journal of Thoracic Oncology at www.jto.org and at http://dx.doi.org/10.1016/j.jtho.2016.05.010.
References
- 1.World Health Organization. [Accessed June 14, 2016];Cancer. http://www.who.int/mediacentre/factsheets/fs297/en/
- 2.Macaluso M, Paggi MG, Giordano A. Genetic and epigenetic alterations as hallmarks of the intricate road to cancer. Oncogene. 2003;22:6472–6478. doi: 10.1038/sj.onc.1206955. [DOI] [PubMed] [Google Scholar]
- 3.Herman JG, Merlo A, Mao L, et al. Inactivation of the CDKN2/p16/MTS1 gene is frequently associated with aberrant DNA methylation in all common human cancers. Cancer Res. 1995;55:4525–4530. [PubMed] [Google Scholar]
- 4.Herman JG, Jen J, Merlo A, et al. Hypermethylation-associated inactivation indicates a tumor suppressor role for p15INK4B. Cancer Res. 1996;56:722–727. [PubMed] [Google Scholar]
- 5.Sanchez-Cespedes M, Esteller M, Wu L, et al. Gene promoter hypermethylation in tumors and serum of head and neck cancer patients. Cancer Res. 2000;60:892–895. [PubMed] [Google Scholar]
- 6.Kane MF, Loda M, Gaida GM, et al. Methylation of the hMLH1 promoter correlates with lack of expression of hMLH1 in sporadic colon tumors and mismatch repair-defective human tumor cell lines. Cancer Res. 1997;57:808–811. [PubMed] [Google Scholar]
- 7.Jones PA. Functions of DNA methylation: islands, start sites, gene bodies and beyond. Nat Rev Genet. 2012;13:484–492. doi: 10.1038/nrg3230. [DOI] [PubMed] [Google Scholar]
- 8.Renner M, Wolf T, Meyer H, et al. Integrative DNA methylation and gene expression analysis in high-grade soft tissue sarcomas. Genome Biol. 2013;14:r137. doi: 10.1186/gb-2013-14-12-r137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wagner JR, Busche S, Ge B, et al. The relationship between DNA methylation, genetic and expression inter-individual variation in untransformed human fibroblasts. Genome Biol. 2014;15:R37. doi: 10.1186/gb-2014-15-2-r37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang X, Han H, De Carvalho DD, et al. Gene body methylation can alter gene expression and is a therapeutic target in cancer. Cancer Cell. 2014;26:577–590. doi: 10.1016/j.ccr.2014.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shears SB. How versatile are inositol phosphate kinases? Biochem J. 2004;377:265–280. doi: 10.1042/BJ20031428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Windhorst S, Kalinina T, Schmid K, et al. Functional role of inositol-1,4,5-trisphosphate-3-kinase-A for motility of malignant transformed cells. Int J Cancer. 2011;129:1300–1309. doi: 10.1002/ijc.25782. [DOI] [PubMed] [Google Scholar]
- 13.Nakagawa K, Noguchi Y, Uenaka A, et al. XAGE-1 expression in non-small cell lung cancer and antibody response in patients. Clin Cancer Res. 2005;11:5496–5503. doi: 10.1158/1078-0432.CCR-05-0216. [DOI] [PubMed] [Google Scholar]
- 14.Hsin CH, Chen MK, Tang CH, et al. High level of plasma matrix metalloproteinase-11 is associated with clinicopathological characteristics in patients with oral squamous cell carcinoma. PLoS One. 2014;9:e113129. doi: 10.1371/journal.pone.0113129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawamura M, Endo C, Sakurada A, et al. The prognostic significance of eukaryotic elongation factor 1 alpha-2 in non-small cell lung cancer. Anticancer Res. 2014;34:651–658. [PubMed] [Google Scholar]
- 16.Gonzalez-Vallinas M, Vargas T, Moreno-Rubio J, et al. Clinical relevance of the differential expression of the glycosyltransferase gene GCNT3 in colon cancer. Eur J Cancer. 2015;51:1–8. doi: 10.1016/j.ejca.2014.10.021. [DOI] [PubMed] [Google Scholar]
- 17.Yoneda K, Iida H, Endo H, et al. Identification of cystatin SN as a novel tumor marker for colorectal cancer. Int J Oncol. 2009;35:33–40. [PubMed] [Google Scholar]
- 18.Liu L, Liao GQ, He P, et al. Detection of circulating cancer cells in lung cancer patients with a panel of marker genes. Biochem Biophys Res Commun. 2008;372:756–760. doi: 10.1016/j.bbrc.2008.05.101. [DOI] [PubMed] [Google Scholar]
- 19.Galvan JA, Garcia-Martinez J, Vazquez-Villa F, et al. Validation of COL11A1/procollagen 11A1 expression in TGF-beta1-activated immortalised human mesenchymal cells and in stromal cells of human colon adenocarcinoma. BMC Cancer. 2014;14:867. doi: 10.1186/1471-2407-14-867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hass HG, Jobst J, Scheurlen M, et al. Gene expression analysis for evaluation of potential biomarkers in hepatocellular carcinoma. Anticancer Res. 2015;35:2021–2028. [PubMed] [Google Scholar]
- 21.Ramirez RD, Sheridan S, Girard L, et al. Immortalization of human bronchial epithelial cells in the absence of viral oncoproteins. Cancer Res. 2004;64:9027–9034. doi: 10.1158/0008-5472.CAN-04-3703. [DOI] [PubMed] [Google Scholar]
- 22. [Accessed January 1, 2015];The Tumor Genome Atlas. http://cancergenome.nih.gov/
- 23.UCSC Genome Bioinformatics. [Accessed October 14, 2014];UCSC genome browser. https://genome.ucsc.edu/
- 24.Portela A, Esteller M. Epigenetic modifications and human disease. Nat Biotechnol. 2010;28:1057–1068. doi: 10.1038/nbt.1685. [DOI] [PubMed] [Google Scholar]
- 25.Wierstra I. Sp1: emerging roles—beyond constitutive activation of TATA-less housekeeping genes. Biochem Biophys Res Commun. 2008;372:1–13. doi: 10.1016/j.bbrc.2008.03.074. [DOI] [PubMed] [Google Scholar]
- 26.Chang L, Schwarzenbach H, Meyer-Staeckling S, et al. Expression regulation of the metastasis-promoting protein InsP3-kinase-A in tumor cells [e-pub ahead of print] [accessed June 13, 2016];Mol Cancer Res. doi: 10.1158/1541-7786.MCR-10-0556. http://dx.doi.org/10.1158/1541-7786. MCR-10-0556. [DOI] [PubMed]
- 27. [Accessed Janauary 1, 2014];National Center for Biotechnology Information Gene Expression Omnibus. GSE60654. http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE60654.
- 28. [Accessed January 14, 2014];ENCODE: Encyclopedia of DNA Elements at UCSC 2003–2012. http://genome.ucsc.edu/ENCODE.
- 29.Wistuba II, Gazdar AF. Lung cancer preneoplasia. Annu Rev Pathol. 2006;1:331–348. doi: 10.1146/annurev.pathol.1.110304.100103. [DOI] [PubMed] [Google Scholar]
- 30.Jjingo D, Conley AB, Yi SV, et al. On the presence and role of human gene-body DNA methylation. Oncotarget. 2012;3:462–474. doi: 10.18632/oncotarget.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perini G, Diolaiti D, Porro A, et al. In vivo transcriptional regulation of N-Myc target genes is controlled by E-box methylation. Proc Natl Acad Sci U S A. 2005;102:12117–12122. doi: 10.1073/pnas.0409097102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim J, Kollhoff A, Bergmann A, et al. Methylation-sensitive binding of transcription factor YY1 to an insulator sequence within the paternally expressed imprinted gene, Peg3. Hum Mol Genet. 2003;12:233–245. doi: 10.1093/hmg/ddg028. [DOI] [PubMed] [Google Scholar]
- 33.Zhang X, Yang R, Jia Y, et al. Hypermethylation of Sp1 binding site suppresses hypothalamic POMC in neonates and may contribute to metabolic disorders in adults: impact of maternal dietary CLAs. Diabetes. 2014;63:1475–1487. doi: 10.2337/db13-1221. [DOI] [PubMed] [Google Scholar]
- 34.Zhu WG, Srinivasan K, Dai Z, et al. Methylation of adjacent CpG sites affects Sp1/Sp3 binding and activity in the p21(Cip1) promoter. Mol Cell Biol. 2003;23:4056–4065. doi: 10.1128/MCB.23.12.4056-4065.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zelko IN, Mueller MR, Folz RJ. CpG methylation attenuates Sp1 and Sp3 binding to the human extracellular superoxide dismutase promoter and regulates its cell-specific expression. Free Radic Biol Med. 2010;48:895904. doi: 10.1016/j.freeradbiomed.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang YA, Ma X, Sathe A, et al. Validation of SCT methylation as a hallmark biomarker for lung cancers. J Thorac Oncol. 2016;11:346–360. doi: 10.1016/j.jtho.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vanweyenberg V, Communi D, D’Santos CS, et al. Tissue- and cell-specific expression of Ins(1,4,5)P3 3-kinase isoenzymes. Biochem J. 1995;306:429–435. doi: 10.1042/bj3060429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Novakovic B, Saffery R. Placental pseudo-malignancy from a DNA methylation perspective: unanswered questions and future directions. Front Genet. 2013;4:285. doi: 10.3389/fgene.2013.00285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Novakovic B, Saffery R. DNA methylation profiling highlights the unique nature of the human placental epigenome. Epigenomics. 2010;2:627–638. doi: 10.2217/epi.10.45. [DOI] [PubMed] [Google Scholar]
- 40.Schroeder DI, Blair JD, Lott P, et al. The human placenta methylome. Proc Natl Acad Sci U S A. 2013;110:6037–6042. doi: 10.1073/pnas.1215145110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.