Abstract
Objective
Evaluating electronic cigarettes (ECIGs) in the clinical laboratory is critical to understanding their effects. However, laboratory evaluation of ECIGs can be challenging, as they are a novel, varied, and evolving class of products. The objective of this paper is to describe some methodological challenges to the clinical laboratory evaluation of ECIGs.
Methods
The authors gathered information about challenges involved in the laboratory evaluation of ECIGs. Challenges were categorized and solutions provided when possible.
Results
Methods used to study combustible cigarettes may need to be adapted to account for ECIG novelty and differences within the class. Challenges to ECIG evaluation can include issues related to 1) identification of ECIG devices and liquids, 2) determination of short -term ECIG abstinence, 3) measurement of use behavior, and 4) assessment of dependence. These challenges are discussed, and some suggestions to inform ECIG evaluation using clinical laboratory methods are provided.
Conclusions
Awareness of challenges and developing, validating, and reporting methods used to address them aids interpretation of results and replication efforts, thus enhancing the rigor of science used to protect public health through appropriate, empirically-based, ECIG regulation.
Keywords: electronic cigarette, electronic nicotine delivery system, clinical laboratory, methods, evaluation
INTRODUCTION
In recent years the use of electronic cigarettes (ECIGs, also electronic nicotine delivery systems, or ENDS) has been increasing rapidly in adolescents and adults.1–3 The effects of ECIG use are beginning to be evaluated, and a complete understanding of them will require a variety of methods, including in vitro and in vivo non -human animal studies, clinical trials, qualitative work, and quantitative surveys. Used together, these methods can help reveal the influence of product design and user behavior on the long-term impact of ECIG use on individual and public health (for reviews of such work, see 4–7).
The clinical laboratory is one setting in which many questions about ECIGs can be answered, as has been done for medication development (eg, 8–10), other drugs of abuse11–13 as well as many other tobacco products (eg, 14–16). For example, studies conducted in the clinical laboratory showed that cigarette smokers change their puffing behavior (puff topography) when they switch from “full-flavor” to “light” cigarettes, and thus are exposed to similar levels of toxicants (eg, 17,18). Indeed, epidemiological studies have confirmed that the health benefits of switching to “light” cigarettes are minimal at best (eg, 19–22). Similarly, clinical laboratory-based studies have shown that a single episode of tobacco smoking using a waterpipe delivers significantly higher levels of CO than the smoking of a single cigarette23,24, as well as that smokers’ use of some smokeless tobacco products reduces exposure to toxicants such as CO and the tobacco specific nitrosamine NNK25 but fails to suppress withdrawal adequately. 26 Such findings from the clinical laboratory have direct implications for increasing understanding of the individual and public health burden of tobacco product use.
Of course, cigarettes, waterpipe, and smokeless tobacco differ on a variety of characteristics – ingredient types and amounts, design, packaging, method of use – and thus require different methods to evaluate their effects. Relative to other tobacco products, for example, mass market cigarettes are more homogenous and generally are more standardized with respect to packaging, size, shape, and nicotine content. Consequently, identification of product, quantification of cigarette use patterns, and other issues relevant to clinical laboratory research are relatively straightforward, increasing the validity of cross-study comparisons and aiding study replication. Also convenient for evaluating the effects of cigarettes, as well as other combusted tobacco products (eg, waterpipe, cigars), is that measurement of recent product use is possible via analysis of exhaled air carbon monoxide (CO). In contrast, non-combusted products that do not produce CO require the measurement of other biological fluids for verification of abstinence (eg, 27,28). Each tobacco product presents methodological challenges for the evaluation of its effects in the clinical laboratory.
Clinical laboratory researchers now face similar challenges for evaluating the effects of ECIGs, a relatively new and fast-evolving product. Currently available clinical laboratory methods must be adapted, and new methods must be developed and validated, to provide the rigorous science that will inform regulation designed to protect individual and public health. The purpose of this paper is to describe some methodological challenges to the clinical laboratory evaluation of ECIGs, including: 1) identification of ECIG devices and liquids, 2) determination of short-term ECIG abstinence, 3) measurement of ECIG use behavior, and 4) assessment of ECIG dependence. Where possible, solutions to these problems are also discussed.
ECIG Devices and Liquids
ECIGs are a class of products that use an electrically-powered heating element to aerosolize a liquid that contains solvents such as propylene glycol (PG) and vegetable glycerin (VG), flavorants, and, usually, nicotine. There are at least 466 distinct ECIG brands and over 7,700 distinct liquid flavors that contain nicotine in concentrations ranging from 0 to 36 mg/ml or higher.4,29 The vast number of combinations and permutations of these brands and liquids presents a challenge for systematic ECIG evaluation.
ECIG devices
ECIG systems can be “closed”, in which case the user cannot add any liquid (eg, cartridges are pre-filled by the manufacturer), or “open”, in which case the user adds liquid to the device as desired (eg, cartridges or reservoirs called “tanks” are filled by the user and are often reusable). Thus, the liquid ingredients and the amount of liquid available for use per ECIG episode is determined at least partially by which system is chosen. ECIGs may also vary in terms of device power (measured in Watts), which is a function of electromagnetic force (E, measured in Volts) provided by the battery, and resistance (R, measured, in Ohms) of the heating element (power is equal to E2/R). Thus device power increases as voltage increases and resistance decreases, and increased power results in increased yield of nicotine and other toxicants.30,31 In clinical laboratory investigations of ECIG effects, decisions regarding which device configurations to use likely will influence study outcome and may depend on study goals. Choices may differ if the goal is, for example, to generalize results to users of a commonly purchased ECIG type/brand, to study specific ECIG use behaviors (eg, dripping of liquid directly onto heater coil; 32–34), or to ensure delivery of nicotine reliably (eg, 35). Also important for consideration is whether the device chosen mirrors that used by the study participants, when those participants have previous experience with an ECIG. Whatever device configuration is used in clinical laboratory work, accurate measurement and reporting of voltage and resistance as well as any other relevant characteristics (eg, number of heating elements) is essential.
ECIG liquid
ECIG liquid can be purchased in pre-filled cartridges or in bottles of various volumes and nicotine concentrations via specialty shops and internet vendors. Unfortunately, however, product labeling may not reflect actual liquid content. For example, objective testing confirms variability in nicotine content across samples of products with the same labeled concentration.36–40 Samples of one brand of liquid labeled as 0 mg nicotine were found to contain from 0.07 to 21.8 mg nicotine per cartridge, while those labeled as 24 mg nicotine actually contained 0.09 to 20.6 mg nicotine per cartridge.40 Thus, while some product labels may be accurate41, others are not (eg, approximately 20% in difference in labeled versus actual nicotine concentration38). Such discrepancies between product labeling and liquid content necessitate that researchers confirm the nicotine concentration of liquid products purchased for use in clinical laboratory studies. In these situations, researchers are faced not only with the additional costs and time lost for independent testing, but also the determination of an acceptable margin of error for a given study. Another approach is to for researchers to make liquids themselves, thus affording complete control over all ingredients. This control may be important, as nicotine yield may be affected by other liquid ingredients. For instance, increased levels of PG, relative to VG, may result in increased nicotine yield.31 Therefore, whether liquids are purchased from independent vendors or made in-house, verifying and reporting concentrations of each constituent is important.
Researchers must also make choices concerning the flavor of liquid used. Nearly 20% of adult ECIG users report use of flavored ECIG liquid.42 Liquid flavor preference may differ as a function of cigarette smoking status, such that former smokers (ie, exclusive ECIG users) may be more likely to prefer fruit/sweet flavors while current smokers (ie, dual ECIG-cigarette users) may prefer tobacco flavors (43 but see 44) Users may also mix multiple flavors to create a unique taste profile45 or switch between flavors often. 43 Flavor switching may occur be cause users perceive a blunting of the flavor with long term use,43 or what has been referred to as “vaper’s tongue”.46 For these reasons, the flavor(s) of liquid used in a clinical laboratory study is likely to affect outcomes such as subjective experience, choice behavior, and/or patterns of use.
Summary
Researchers should be mindful of the combination of device and liquid features chosen for study, recognizing their influence on study outcomes and the potential limitations of generalizability of study findings. Equally important is the accurate reporting of such features (eg, actual nicotine concentration of the liquid) and the ECIG-related characteristics of the sample (eg, previous experience with specific types of ECIG devices/liquids).
Determination of Short -Term ECIG Abstinence
The evaluation of tobacco product effects (eg, abstinence symptom suppression, toxicant exposure) often requires that study participants abstain from nicotine/tobacco either in the short- or long-term. Short-term (eg, <12 hours) nicotine/tobacco abstinence is often used to assess product-related nicotine delivery and/or abstinence symptom suppression (eg, 47–50). Longer-term abstinence (eg, days or weeks) may be required as a negative control condition in studies designed to examine user toxicant exposure (eg, 14,26), the effects of nicotine after more than 12 hours abstinence (eg,51,52), or product cessation outcomes (eg, 53,54). Common measures of product abstinence include self-report and biochemical markers of exposure such as expired air carbon monoxide (CO) or nicotine or cotinine in body fluids.
Self-report
Self-report measures include items such as “Have you used any nicotine-containing products in the past [12 hours/24 hours/7 days]?” or similar (eg, 55–57), depending on the abstinence interval of interest. However, the validity of self-reported smoking status/smoking behavior is sometimes questionable (eg, 58,59), particularly when participation is a clinical laboratory study is contingent upon short-term abstinence. In such cases, some motivated participants may misrepresent their recent nicotine/tobacco use history. Moreover, given the ease of taking even only a few puffs from an ECIG, participants may be more likely to misremember recent ECIG use than cigarette use. Thus, to ensure that the required abstinence period is met, self-report alone may not be the ideal measure.
Expired air CO
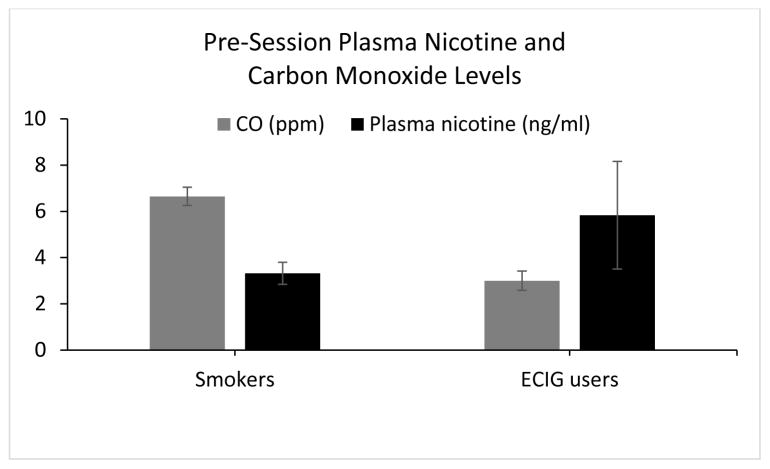
CO is produced when tobacco or other carbon-containing compounds are burned incompletely, so usually it is inhaled by users of combustible tobacco products. In humans, CO has a relatively short half-life of 2–3 hours60 and is therefore often used to verify short-term combustible tobacco abstinence.23,61–63 In contrast, ECIGs do not burn anything when operated as intended, so these products do not produce CO and do not deliver it to users (eg, 64). Thus, CO also is not a good index of recent ECIG use in individuals who use these products. For example, data from a study of ECIG-naïve cigarette smokers (N= 33) and experienced ECIG users(N = 25) who were instructed to abstain from all nicotine/tobacco demonstrates that, for cigarette smokers, low expired air CO concentration (Mean= 6.7 ppm; SD =2.3) was accompanied by low plasma nicotine concentration (Mean= 3.3 ng/ml; SD = 2.8). However, for the ECIG users, low CO concentrations (Mean = 3.0 ppm; SD= 2.1) were associated with plasma nicotine concentration that was significantly higher than the smokers (Mean =5.8 ng/ml; SD= 11.6), indicating that at least some ECIG users likely did not abstain for as long as the cigarette smokers did (See Figure 1; adapted from 65). In particular, of the 25 experienced ECIG users participating in this multi -session study, 8 had at least one baseline plasma nicotine concentration greater than 10 ng/ml, despite mean CO levels of 3.5 ppm (SD= 2.5) at baseline. Plasma nicotine concentrations of greater than 10 ng/ml are consistent with recent tobacco/ECIG use.64 Clearly, CO is not as informative a method for verifying pre-study abstinence in ECIG users as it is for users of combustible tobacco products. Indeed, combustible tobacco users may turn to ECIGs to help them comply with abstinence instructions, adding a level of complexity to studies of combustible products as well.
Figure 1.
Mean plasma nicotine and CO levels (± SEM) for 33 daily cigarette smokers and 25 daily ECIG users, after ~12 hours of self-reported tobacco/nicotine abstinence (includes cigarettes and ECIGs; methods fully described in 35 and 136 and presented in 65. Asterisk indicates a significant group difference for plasma nicotine level, using an independent samples t-test (p< .05).
Nicotine
Nicotine can be measured in body fluids and has a half -life of 1–2 hours (eg 66–69). However, nicotine measurement in body fluids involves analytical chemistry methods and therefore requires specific equipment and expertise that may not be available immediately to clinical laboratory researchers, introducing delays of days or weeks between sample collection and result reporting. Thus, while nicotine concentration is a useful tool for verifying short -term ECIG abstinence in theory, non-compliance may not be detected in practice until after a participant has completed a protocol. Depending on the study, data from a non-compliant participant may be unusable, thus decreasing power or requiring the expense and time associated with recruiting additional compliant participants to replace those found to be non-compliant.
Urine cotinine
Cotinine is a metabolite of nicotine, and has a half -life of approximately 16–19 hours.70 Thus, it offers a better index of long -term product abstinence than nicotine or CO. For example, urine cotinine concentration has been used to confirm nicotine abstinence over days (eg, 14,26) and weeks (eg, 71,72), and is recommended for use in the biochemical verification of tobacco abstinence in cessation studies.54 Importantly, cotinine can be measured in urine semi -quantitatively or quantitatively. Semi-quantitative measurement involves the use of a test strip that can be dipped into urine or saliva, providing an immediate estimate of cotinine concentration. However, over a 5-day period, this method can lead to mis-identification of abstinent tobacco users as non-abstinent if the test strips cross-react with the cotinine metabolite trans-3′-hydroxycotinine (eg, 73). Quantitative measurement of cotinine using analytical chemistry methods is more specific, but, like nicotine analysis, requires the associated equipment, expertise, and time.
Summary
Overall, determining short -term abstinence from ECIGs in a time-and cost -efficient manner remains a challenge. Because of this ongoing challenge, studies in which short-term ECIG abstinence is critical may require a variety of procedures to enhance compliance, including the use of boguspipeline methods (eg, 74–77, observing participants for several hours before testing to ensure abstinence), and, ultimately, plasma nicotine analysis to eliminate data from non-compliant participants. Long-term ECIG abstinence can be determined by measuring saliva/urine cotinine concentration quantitatively. For studies involving participants who use a combination of combustible and non-combustible tobacco products, a combination of measures will likely be required. The field would be aided immensely by a valid, reliable, specific, rapid, and cost-effective method for the quantitative assessment of nicotine and cotinine in body fluids.
Measurement of ECIG Use Behavior
Clinical laboratory research with tobacco users requires an accurate understanding of an individual’s tobacco product use history, broadly defined, to determine if potential study participants meet inclusion/exclusion criteria and characterize the study sample. Detailed use patterns (eg, puff topography measures) also are sometimes required to understand factors influencing self-administration of nicotine (and other toxicants). For tobacco cigarettes, instruments for measuring use behavior (eg, lifetime and current use; cigarettes/day; 3,78–80) and topography81–84 are well -established and similar instruments are becoming more standardized for other tobacco products, such as waterpipe.85,86 For ECIGs, characterizing broad use patterns is challenging, though topography measurement is evolving.
Measuring broad use patterns
The measurement of broad ECIG use patterns has presented several challenges to extant monitoring systems (see Table 1 for a listing of common frequency/consumption measures). First, the terminology used to describe and identify ECIGs is not yet established and appears to be shifting with marketing/industry influence.29 Evolving terms to describe these products include “vape pens”, “e-hookahs”, and “vaporizers”.87 The variability in device characteristics (eg, voltage, resistance), which even some users have difficulty distinguishing between88, and their influence on nicotine delivery adds additional complexity. Second, there is no standardized ECIG measurement unit that is analogous to a single cigarette. For example, some ECIG users may use their devices constantly throughout the day89, and/or may use multiple device types, making less meaningful the concept of a “single ECIG use episode” and complicating items assessing daily or weekly use. It is possible that broad categories of ECIG use behavior may exist. In-depth assessment of a large group of users may be necessary to quantify and describe these patterns. Third, to measure the intensity of ECIG consumption accurately, an important concern is whether a potential participant uses an open or closed system: with an open system the unit of measure may include ml used/day, and for a closed system the unit of measure may include number of disposable ECIGs or prefilled cartridges used (see Table 1).90–92 Liquid nicotine concentration is also relevant to intensity of use, at least inasmuch as nicotine dependence is a criterion for inclusion into a study. Obtaining this information may be difficult given the variability in manufacturer labeling, accuracy of that labeling, lack of knowledge on the part of the ECIG user, and the ability of ECIG users to prepare and mix their own liquid.89 The ease of purchase and preparation of ECIG liquid base constituents (propylene glycol, vegetable glycerin, nicotine base) can make this determination more difficult for some participants and perhaps those who mix their own liquids may need to be excluded from some studies.
Table 1.
Common ECIG Frequency/Consumption Measures
| Measure | Representative empirical reports |
|---|---|
| Frequency of daily ECIG use | |
| Number of puffing episodes | 119 |
| Number of puffs | 44,131–133 |
| Number of hours for one refill/cartridge (hours) | 131,133 |
| Intensity (amount) of daily ECIG use | |
| Number of refills per day | 131,133 |
| mL of liquid per day | 44,113,120,134 |
| cartridges per day | 134 |
| ECIG device and liquid characteristics | |
| Preferred type/brand | 92,105,119,133–135 |
| Preferred battery brand | 135 |
| Preferred cartridge/liquid nicotine concentration/strength | 92,119,133,134 |
| Preferred cartridge/liquid brand | 92 |
| Preferred cartridge/liquid flavor | 92,105,133 |
Measuring ECIG puff topography
Another methodological obstacle faced by researchers interested in studying ECIGs concerns the measurement of puff topography. Puff topography is the detailed examination of puffing behaviors, including puff number, duration, volume, inter-puff-interval (IPI), and flow rate.93 Puff topography measurement is critical to understanding the effects of tobacco products because nicotine intake, and the intake of other harmful smoke or aerosol constituents, is determined in part by how a product is used.94,95 Historically, topography measurement has been assessed in laboratory settings using observational methods and mouthpiece-based computerized devices, both of which are reliable and valid approaches for cigarette smokers.93,96,97 Both may present challenges to researchers interested in measuring topography in ECIG users.
Observational measurement of puff topography typically consists of trained scorers measuring variables from smoking bouts previously video-recorded in the laboratory. Unfortunately, this method of topography measurement presents challenges for the study of ECIG use that are similar to those encountered in studies of cigarette smoking: it is labor intensive and does not allow measurement of some critical variables (eg, puff volume; see 93). Moreover, operational definitions developed for measurement of combustible cigarette topography may not be adapted easily for measurement of ECIG topography. For cigarettes, distinct cues can be used to determine reliably the start and stop of a puff, such as the red glow at the burning cigarette end that becomes more pronounced upon inhalation and/or the contact of a user’s lips with the mouth-end of their cigarette.93,98 For ECIGs, however, characterizing the start and stop of ECIG puffs is made difficult by the wide variability in design features. For instance, some closed system ECIGs include a light-emitting diode (LED) on the non-mouth end to simulate the glow of a cigarette tip, while others have no LED or one that glows whenever a button is pressed to activate the battery.99 Indeed, LED activation in some instances does not represent puff onset.100,101 For example, users may activate their device (therefore turning on the LED) before or after placing it in their mouth, or may leave their device in their mouth while not puffing actively.100 Some users also clearly inhale from their ECIG before LED activation, presumably in “cigalike” devices that require user inhalation to activate.101 Thus, neither the glow of a LED nor users’ lip contact with their ECIG appear to be as well-correlated with puff onset/offset as these cues are with the onset/offset of a puff from a tobacco cigarette.
Mouthpiece-based computerized devices measure tobacco cigarette user puff topography with the aid of specialized mouthpiece capable of detecting flow-induced pressure changes that occur during inhalation; the cigarette must be placed in the mouthpiece which is designed to hold it. Stationary and portable versions of these computerized topography devices are available to researchers93 and the portable version has been used to examine ECIG topography. 37,102–104 This approach has presented several methodological challenges for characterizing ECIG puffing behavior. First, one commonly used device stops recording data after 43 puffs have been measured, and while this cutoff far exceeds the average number of puffs taken from a single tobacco cigarette, may not exceed the number of puffs taken during a single ECIG use episode, resulting in incomplete data capture for some participants.102,104 Second, when long puff durations are taken with existing portable topography devices, ECIG liquid may be drawn into the mouthpiece and alter device sensitivity.102 Third, measuring ECIG topography with a mouthpiece-based device requires that the ECIG fit firmly into the mouthpiece. Many ECIG models do not meet this requirement, limiting the types of ECIGs for which topography can be measured. Last, design parameters of existing mouthpiece-based devices may not provide adequate sensitivity for measuring ECIG topography.105 Typically, existing topography devices detect flow rate values at or above 15 ml/sec106, well below average values usually observed in cigarette smokers.104,105 However, experienced ECIG users puff with lower flow rates than cigarette smokers, sometimes close to 15 ml/sec.102,105,107 Thus, commercially available topography recording devices may not have design parameters sensitive enough to measure ECIG puff topography accurately.105
To address concerns associated with using existing computerized devices to measure ECIG topography, researchers have created mouthpiece-based topography devices designed specifically for ECIGs.105,108 For example, devices have been designed without puff number recording limitations108 and with sufficient sensitivity for recording puffs with low flow rates. 105 Yet these instruments share some limitations with those they are intended to replace. ECIG aerosols can condense inside the mouthpiece and affect topography measurement and mouthpiece shape limits the types of ECIGs that can be studied (as in 105). Future research may benefit from topography measurement instrumentation that does not require a specialized mouthpiece, permitting participants to use their preferred device without modification and likely resulting in more naturalistic ECIG topography assessments.
Summary
Measuring ECIG use behavior accurately and reliably is critical for clinical laboratory researchers so that they can screen potential participants, report participant use history, and study factors that influence user toxicant exposure. Future work in this area offers opportunities for testing and development of measures for clinical laboratory use that assess broad patterns of ECIG consumption with validity and reliability. Qualitative data may be useful for addressing product terminology (as in 88), as with other tobacco products (eg, cigars; 109). Prospective methods are needed for characterizing those ECIG use patterns that differ between device categories or types of users. Ecological momentary assessment110 would be particularly informative, as this technique would allow individuals to record their ECIG use in real-time and in their natural environment. Of course, each of these measurement methods has limitations (eg, underreporting of ECIG use for some ecological momentary assessment methods111), though their combined use should elucidate users’ actual patterns of consumption. There is an ongoing need for instrumentation that allows topography measurement across all ECIG devices in order to characterize user behavior and toxicant exposure more completely and, potentially, to relate these outcomes to dependence and other health-related outcomes.
Measurement of ECIG Dependence
ECIG nicotine delivery is a function of device design, liquid constituents, and user behavior112 and, while there is considerable variability, at least some ECIGs deliver physiologically active doses of nicotine under some conditions.105,113 Because nicotine is a dependence-producing, psychoactive drug, those ECIGs that deliver nicotine effectively may support nicotine dependence. As with product use history, clinical laboratory researchers may need to measure dependence to ensure that potential participants meet study inclusion/exclusion criteria, to describe groups of participants, and/or to study the factors that are influenced by or that influence dependence level. While there are numerous scales for measuring dependence in cigarette smokers and SLT users114–117, development of a valid and reliable instrument that can be used to measure dependence in ECIG users has begun only recently.118–120 This ongoing development effort is complicated by all of the issues related to devices, liquids, and ECIG use patterns discussed above. That is, nicotine delivery profile varies widely as a function of device power and liquid constituents, and ECIG use episodes cannot be characterized easily and likely differ considerably across individuals. In addition, self-report items commonly used to measure dependence in cigarette smokers may not be adapted easily for use in measuring ECIG dependence. For example, some items assess smokers’ difficulty with abstaining from cigarettes in places where smoking is prohibited (eg, 116). ECIG use is sometimes permitted in locations where cigarettes are not121, and, more importantly, ECIG users have developed techniques to use these products covertly122,123, thus rendering an item assessing difficulty abstaining from ECIG use in certain settings potentially meaningless (although self-reports of this covert use behavior may themselves be indicative of dependence). All of these issues may be related to the observation that ECIG users’ scores on existing dependence instruments are lower than those for cigarette smokers118–120. Of course, some ECIGs, particularly those that deliver nicotine inefficiently (eg, 124) simply may be less likely to produce/maintain dependence (see 118). Exploring dependence in ECIG users empirically likely will require a valid and reliable instrument that is specific to this population.125
DISCUSSION
Some ways of addressing the issues raised here are outlined below, as well as summarized in Table 2. With regard to device characteristics and liquid constituents, the ever-changing ECIG landscape presents an ongoing issue that requires independent measurement and thorough reporting of all relevant variables, to the extent possible. At the least, published work should specify that battery voltage, heater resistance, heating element number/design, and liquid nicotine concentration were verified. Studies that require participants to meet short-term ECIG abstinence criterion may need to include an observation period of several hours’ duration to ensure that criterion is met. Otherwise, biochemical verification after the fact (ie, plasma nicotine concentration below some pre-defined criterion) may be the only option, though it is costly and often not timely. Measurement of use patterns and assessment of dependence likely requires development of ECIG-specific instruments, and this development effort is one in which clinical laboratory researchers have much to contribute. For example, laboratory studies of ad libitum use behavior over an extended period can inform definitions of ECIG use episodes and help understand use patterns generally. Clinical laboratory studies can reveal the extent to which several hours of observed and verified ECIG abstinence reveals hallmarks of dependence such as compulsion to use an ECIG, aversive symptoms suppressed by subsequent ECIG use, evidence of tolerance, and preoccupation with use (eg, 126). Such work will be important for understanding ECIG effects, and likely will also aid design of clinical trials and epidemiological studies.
Table 2.
Potential Solutions to the Methodological Challenges of Clinical Laboratory Electronic Cigarette Evaluation
| Challenge | Potential solutions | ||
|---|---|---|---|
| Device and liquid variability | Measure and report device characteristics (eg, battery voltage, heater resistance). | Report liquid characteristics (eg, flavor additives, PG: VG ratio). | Verify liquid nicotine concentration. Consider implications of flavor choice, if it is restricted. |
| Abstinence verification | Use a combination of bogus pipeline procedures and biological fluid assays. | Observe participant for several hours before beginning testing. | Develop reliable, valid, specific, sensitive, quick, and cost-effective methods of measuring recent nicotine exposure. |
| Use behavior measurement | Operationalize product terms and user behavior patterns. | Measure device and liquid characteristics used by respondents. | Report topography device specifications (eg, flow rate threshold to determine puff onset/offset; puff number cutoffs) |
| Dependence measurement | Consider behaviors specific to ECIG use. | Measure device and liquid characteristics used by respondents. | Develop reliable and valid ECIG dependence measurement instrument. |
Clinical laboratory evaluation of ECIGs and their effects is necessary but presents many challenges only some of which are detailed here: space constraints do not allow an elaboration of the difficulties in recruiting exclusive ECIG users (many ECIG users also report concurrent cigarette use127), challenges in providing study participants with their own brand/flavor of ECIG liquid when many users consume several flavors within a single day, and the possibility that drugs other than nicotine may be found in ECIG liquids.128,129 The rapid evolution of products and use behaviors and the lack of regulatory control over ECIGs in many countries suggests that these and other issues will continue to remain an important feature of clinical laboratory studies of ECIG effects.
IMPLICATIONS FOR TOBACCO REGULATION
The effects of electronic cigarette (ECIG) use are beginning to be evaluated, and a complete understanding of them will require a variety of methods, including in vitro and in vivo non-human animal studies, clinical trials, qualitative work, and quantitative surveys. Used together, these methods can help reveal the influence of product design and user behavior on the long-term impact of ECIG use on individual and public health. The clinical laboratory is one setting in which many of the questions about ECIGs can be answered, and clinical laboratory researchers face challenges evaluating the effects of ECIGs, as these are a relatively new and fast-evolving product group. Currently available clinical laboratory methods must be adapted, and new methods must be developed and validated, to provide the rigorous science that will inform regulation designed to protect individual and public health. These future regulations of ECIGs may involve restrictions on devices, liquids, and flavors, and thus, researchers must use methods that clearly elucidate the effects of each ECIG component. Indeed, the May, 2016 announcement by the U.S. Food and Drug Administration (FDA) that ECIGs and their components are to be regulated by the FDA, and that some products may be required to undergo premarket review, highlights the need for addressing the challenges discussed here.130 In general, awareness of challenges and developing, validating, and reporting methods used to address them aids interpretation of results and replication efforts, thus enhancing the rigor of science used to protect public health through appropriate, empirically-based, ECIG regulation.
Acknowledgments
This work was supported by the National Institute on Drug Abuse of the National Institutes of Health under Award Number P50DA036105 and the Center for Tobacco Products of the U.S. Food and Drug Administration. The content is solely the responsibility of the authors and does not necessarily represent the views of the NIH or the FDA.
Footnotes
Human Subjects Statement
All studies included in this analysis were approved by the Institutional Review Board of Virginia Commonwealth University.
Conflict of InterestStatement
All authors of this article declare they have no conflicts of interest.
Contributor Information
Melissa D. Blank, Assistant Professor, West Virginia University, Morgantown, WV.
Alison B. Breland, Assistant Professor, Virginia Commonwealth University, Center for the Study of Tobacco Products, Richmond, VA.
Caroline O. Cobb, Assistant Professor, Virginia Commonwealth University, Center for the Study of Tobacco Products, Richmond, VA.
Tory Spindle, Virginia Commonwealth University, Center for the Study of Tobacco Products, Richmond, VA.
Carolina Ramôa, Post-Doctoral Fellow, Virginia Commonwealth University, Center for the Study of Tobacco Products, Richmond, VA.
Thomas Eissenberg, Thomas Eissenberg, Professor, Virginia Commonwealth University, Center for the Study of Tobacco Products, Richmond, VA.
References
- 1.Arrazola RA, Kuiper NM, Dube SR. Patterns of current use of tobacco products among u.S. High school students for 2000–2012--findings from the national youth tobacco survey. J Adolesc Health. 2014;54(1):54–60. e59. doi: 10.1016/j.jadohealth.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.King BA, Patel R, Nguyen KH, et al. Trends in awareness and use of electronic cigarettes among us adults, 2010–2013. Nicotine Tob Res. 2015;17(2):219–227. doi: 10.1093/ntr/ntu191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singh T, Arrazola RA, Corey CG, et al. Tobacco use among middle and high school students -united states, 2011–2015. MMWR Morb Mortal Wkly Rep. 2016;65(14):361–367. doi: 10.15585/mmwr.mm6514a1. [DOI] [PubMed] [Google Scholar]
- 4.Breland A, Soule E, Lopez A, et al. Electronic cigarettes: What are they and what do they do? Ann NY Acad Sci. 2016 doi: 10.1111/nyas.12977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hajek P, Etter JF, Benowitz N, et al. Electronic cigarettes: Review of use, content, safety, effects on smokers and potential for harm and benefit. Addiction. 2014;109(11):1801–1810. doi: 10.1111/add.12659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lopez AA, Eissenberg T. Science and the evolving electronic cigarette. Prev Med. 2015;80:101–106. doi: 10.1016/j.ypmed.2015.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pisinger C, Dossing M. A systematic review of health effects of electronic cigarettes. Prev Med. 2014;69:248–260. doi: 10.1016/j.ypmed.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 8.Walsh SL, Eissenberg T. The clinical pharmacology of buprenorphine: Extrapolating from the laboratory to the clinic. Drug Alcohol Depend. 2003;70(2 Suppl):S13–27. doi: 10.1016/s0376-8716(03)00056-5. [DOI] [PubMed] [Google Scholar]
- 9.Comer SD, Ashworth JB, Foltin RW, et al. The role of human drug self-administration procedures in the development of medications. Drug Alcohol Depend. 2008;96(1–2):1–15. doi: 10.1016/j.drugalcdep.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cooper ZD, Foltin RW, Hart CL, et al. A human laboratory study investigating the effects of quetiapine on marijuana withdrawal and relapse in daily marijuana smokers. Addict Biol. 2013;18(6):993–1002. doi: 10.1111/j.1369-1600.2012.00461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walsh SL, Donny EC, Nuzzo PA, et al. Cocaine abuse versus cocaine dependence: Cocaine self-administration and pharmacodynamic response in the human laboratory. Drug Alcohol Depend. 2010;106(1):28–37. doi: 10.1016/j.drugalcdep.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donny EC, Brasser SM, Bigelow GE, et al. Methadone doses of 100 mg or greater are more effective than lower doses at suppressing heroin self-administration in opioid-dependent volunteers. Addiction. 2005;100(10):1496–1509. doi: 10.1111/j.1360-0443.2005.01232.x. [DOI] [PubMed] [Google Scholar]
- 13.Hart CL, Ilan AB, Gevins A, et al. Neurophysiological and cognitive effects of smoked marijuana in frequent users. Pharmacol Biochem Behav. 2010;96(3):333–341. doi: 10.1016/j.pbb.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Breland AB, Kleykamp BA, Eissenberg T. Clinical laboratory evaluation of potential reduced exposure products for smokers. Nicotine Tob Res. 2006;8(6):727–738. doi: 10.1080/14622200600789585. [DOI] [PubMed] [Google Scholar]
- 15.Malson JL, Lee EM, Moolchan ET, et al. Nicotine delivery from smoking bidis and an additive-free cigarette. Nicotine Tob Res. 2002;4(4):485–490. doi: 10.1080/1462220021000018498. [DOI] [PubMed] [Google Scholar]
- 16.Fant RV, Henningfield JE, Nelson RA, et al. Pharmacokinetics and pharmacodynamics of moist snuff in humans. Tob Control. 1999;8(4):387–392. doi: 10.1136/tc.8.4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baldinger B, Hasenfratz M, Battig K. Switching to ultralow nicotine cigarettes: Effects of different tar yields and blocking of olfactory cues. Pharmacol Biochem Behav. 1995;50(2):233–239. doi: 10.1016/0091-3057(94)00302-y. [DOI] [PubMed] [Google Scholar]
- 18.Zacny JP, Stitzer ML. Cigarette brand-switching: Effects on smoke exposure and smoking behavior. J Pharmacol Exp Ther. 1988;246(2):619–627. [PubMed] [Google Scholar]
- 19.Mannino DM, Ford E, Giovino GA, et al. Lung cancer mortality rates in birth cohorts in the united states from 1960 to 1994. Lung Cancer. 2001;31(2–3):91–99. doi: 10.1016/s0169-5002(00)00170-7. [DOI] [PubMed] [Google Scholar]
- 20.Burns DM, Major JM, Shanks TG, et al. Smoking lower-yield cigarettes and disease risk. Bethesda, MD: U.S. Department of Health and Human Services. Public Health Service. National Institutes of Health, National Cancer Institute; 2001. [Google Scholar]
- 21.Burns DM, Shanks TG, Choi W, et al. The american cancer society cancer prevention study i: 12-year followup of 1 million men and women. Bethesda, MD: National Cancer Institute1996; [Google Scholar]
- 22.Thun MJ, Day-Lally C, Myers DG, et al. Trends in tobacco smoking and mortality from cigarette use in cancer prevention studies i (1959 through 1965) and ii (1982-through 1988) Bethesda, MD: U.S. Department of Health and Human Services. Public Health Service. National Institutes of Health, National Cancer Institute; 1997. [Google Scholar]
- 23.Cobb CO, Shihadeh A, Weaver MF, et al. Waterpipe tobacco smoking and cigarette smoking: A direct comparison of toxicant exposure and subjective effects. Nicotine Tob Res. 2011;13(2):78–87. doi: 10.1093/ntr/ntq212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jacob P, 3rd, Abu Raddaha AH, Dempsey D, et al. Comparison of nicotine and carcinogen exposure with water pipe and cigarette smoking. Cancer Epidemiol Biomarkers Prev. 2013;22(5):765–772. doi: 10.1158/1055-9965.EPI-12-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mendoza-Baumgart MI, Tulunay OE, Hecht SS, et al. Pilot study on lower nitrosamine smokeless tobacco products compared with medicinal nicotine. Nicotine Tob Res. 2007;9(12):1309–1323. doi: 10.1080/14622200701704228. [DOI] [PubMed] [Google Scholar]
- 26.Blank MD, Eissenberg T. Evaluating oral noncombustible potential-reduced exposure products for smokers. Nicotine Tob Res. 2010;12(4):336–343. doi: 10.1093/ntr/ntq003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bauman KE, Koch GG, Bryan ES, et al. On the measurement of tobacco use by adolescents. Validity of self-reports of smokeless tobacco use and validity of cotinine as an indicator of cigarette smoking. Am J Epidemiol. 1989;130(2):327–337. doi: 10.1093/oxfordjournals.aje.a115339. [DOI] [PubMed] [Google Scholar]
- 28.Jain R, Jhanjee S, Jain V, et al. Biochemical validation of self-reported smokeless tobacco abstinence among smokeless tobacco users: Results from a clinical trial of varenicline in india. J Psychoactive Drugs. 2015;47(4):331–335. doi: 10.1080/02791072.2015.1073412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu SH, Sun JY, Bonnevie E, et al. Four hundred and sixty brands of e-cigarettes and counting: Implications for product regulation. Tob Control. 2014;23(Suppl 3):iii3–9. doi: 10.1136/tobaccocontrol-2014-051670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Talih S, Balhas Z, Eissenberg T, et al. Effects of user puff topography, device voltage, and liquid nicotine concentration on electronic cigarette nicotine yield: Measurements and model predictions. Nicotine Tob Res. 2015;17(2):150–157. doi: 10.1093/ntr/ntu174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kosmider L, Sobczak A, Knysak J, et al. Effects of solvent and battery output voltage on nicotine levels released from eletronic cigarette. Paper presented at: 20th Annual Meeting of the Society for Research on Nicotine and Tobacco; 2014; Philadelphia, PA. Availableat: http://www.srnt.org/page/Past. (Author Contact: Andrzej Sobczak, Ph.D.; Institute of Occupational Medicine and Environmental Health; Poland; andsobcz@poczta.onet.pl) [Google Scholar]
- 32.McQueen A, Tower S, Sumner W. Interviews with “vapers”: Implications for future research with electronic cigarettes. Nicotine Tob Res. 2011;13(9):860–867. doi: 10.1093/ntr/ntr088. [DOI] [PubMed] [Google Scholar]
- 33.Talih S, Balhas Z, Salman R, et al. “Direct dripping”: A high-temperature, high-formaldehyde emission electronic cigarette use method. Nicotine Tob Res. 2016;18(4):453–459. doi: 10.1093/ntr/ntv080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang L, Rudy SF, Cheng JM, et al. Electronic cigarettes: Incorporating human factors engineering into risk assessments. Tob Control. 2014;23(Suppl 2):ii47–53. doi: 10.1136/tobaccocontrol-2013-051479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lopez AA, Hiler MM, Soule EK, et al. Effects of electronic cigarette liquid nicotine concentration on plasma nicotine and puff topography in tobacco cigarette smokers: A preliminary report. Nicotine Tob Res. 2015 doi: 10.1093/ntr/ntv182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davis B, Dang M, Kim J, et al. Nicotine concentrations in electronic cigarette refill and do-it-yourself fluids. Nicotine Tob Res. 2015;17(2):134–141. doi: 10.1093/ntr/ntu080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goniewicz ML, Kuma T, Gawron M, et al. Nicotine levels in electronic cigarettes. Nicotine Tob Res. 2013;15(1):158–166. doi: 10.1093/ntr/nts103. [DOI] [PubMed] [Google Scholar]
- 38.Goniewicz ML, Gupta R, Lee YH, et al. Nicotine levels in electronic cigarette refill solutions: A comparative analysis of products from the u.S., korea, and poland. Int J Drug Policy. 2015;26(6):583–588. doi: 10.1016/j.drugpo.2015.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Han S, Chen H, Zhang X, et al. Levels of selected groups of compounds in refill solutions for electronic cigarettes. Nicotine Tob Res. 2015 doi: 10.1093/ntr/ntv189. [DOI] [PubMed] [Google Scholar]
- 40.Trehy ML, Ye W, Hadwiger ME, et al. Analysis of electronic cigarette cartridges, refill solutions, and smoke for nicotine and nicotine related impurities. Journal of Liquid Chromatography & Related Technologies. 2011;34:1444–1458. [Google Scholar]
- 41.Etter JF, Zather E, Svensson S. Analysis of refill liquids for electronic cigarettes. Addiction. 2013;108(9):1671–1679. doi: 10.1111/add.12235. [DOI] [PubMed] [Google Scholar]
- 42.Villanti AC, Richardson A, Vallone DM, et al. Flavored tobacco product use among u.S. Young adults. Am J Prev Med. 2013;44(4):388–391. doi: 10.1016/j.amepre.2012.11.031. [DOI] [PubMed] [Google Scholar]
- 43.Farsalinos KE, Romagna G, Tsiapras D, et al. Impact of flavour variability on electronic cigarette use experience: An internet survey. Int J Environ Res Public Health. 2013;10(12):7272–7282. doi: 10.3390/ijerph10127272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dawkins L, Turner J, Roberts A, et al. ‘Vaping’ profiles and preferences: An online survey of electronic cigarette users. Addiction. 2013;108(6):1115–1125. doi: 10.1111/add.12150. [DOI] [PubMed] [Google Scholar]
- 45.Wang L, Zhan Y, Li Q, et al. An examination of electronic cigarette content on social media: Analysis of e-cigarette flavor content on reddit. Int J Environ Res Public Health. 2015;12(11):14916–14935. doi: 10.3390/ijerph121114916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. [Accessed June 2, 2016];The dreaded vaper’s tongue: Dealing with olfactory fatigue. 2014 Jan 22; http://onvaping.com/the-dreaded-vapers-tongue-dealing-with-olfactory-fatigue/
- 47.Blank MD, Cobb CO, Eissenberg T, et al. Acute effects of “hyping” a black&mild cigarillo. Nicotine Tob Res. 2016;18(4):460–469. doi: 10.1093/ntr/ntv063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bidwell LC, Leventhal AM, Tidey JW, et al. Effects of abstinence in adolescent tobacco smokers: Withdrawal symptoms, urge, affect, and cue reactivity. Nicotine Tob Res. 2013;15(2):457–464. doi: 10.1093/ntr/nts155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kotlyar M, Mendoza-Baumgart MI, Li ZZ, et al. Nicotine pharmacokinetics and subjective effects of three potential reduced exposure products, moist snuff and nicotine lozenge. Tob Control. 2007;16(2):138–142. doi: 10.1136/tc.2006.018440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Robinson CD, Pickworth WB, Heishman SJ, et al. The acute tobacco withdrawal syndrome among black smokers. Psychol Addict Behav. 2014;28(1):173–181. doi: 10.1037/a0031950. [DOI] [PubMed] [Google Scholar]
- 51.Allen AM, Lunos S, Heishman SJ, et al. Subjective response to nicotine by menstrual phase. Addict Behav. 2015;43:50–53. doi: 10.1016/j.addbeh.2014.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tidey JW, Colby SM, Xavier EM. Effects of smoking abstinence on cigarette craving, nicotine withdrawal, and nicotine reinforcement in smokers with and without schizophrenia. Nicotine Tob Res. 2014;16(3):326–334. doi: 10.1093/ntr/ntt152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cropsey KL, Trent LR, Clark CB, et al. How low should you go? Determining the optimal cutoff for exhaled carbon monoxide to confirm smoking abstinence when using cotinine as reference. Nicotine Tob Res. 2014;16(10):1348–1355. doi: 10.1093/ntr/ntu085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.SRNT Subcommittee on Biochemical Verification. Biochemical verification of tobacco use and cessation. Nicotine Tob Res. 2002;4(2):149–159. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- 55.Bernstein SL, Rosner J, Toll B. Concordance between timeline follow -back and single question assessment of self-reported smoking in a clinical trial. Subst Abus. 2016 doi: 10.1080/08897077.2016.1154494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Perkins KA, Lerman C, Karelitz JL, et al. Sensitivity and specificity of a procedure for early human screening of novel smoking cessation medications. Addiction. 2013;108(11):1962–1968. doi: 10.1111/add.12273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schuck K, Bricker JB, Otten R, et al. Effectiveness of proactive quitline counselling for smoking parents recruited through primary schools: Results of a randomized controlled trial. Addiction. 2014;109(5):830–841. doi: 10.1111/add.12485. [DOI] [PubMed] [Google Scholar]
- 58.Gerritsen M, Berndt N, Lechner L, et al. Self-reporting of smoking cessation in cardiac patients: How reliable is it and is reliability associated with patient characteristics? J Addict Med. 2015;9(4):308–316. doi: 10.1097/ADM.0000000000000137. [DOI] [PubMed] [Google Scholar]
- 59.Stein LA, Colby SM, O’Leary TA, et al. Response distortion in adolescents who smoke: A pilot study. J Drug Educ. 2002;32(4):271–286. doi: 10.2190/GL7E-B8MV-P9NH-KCVV. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marrone GF, Paulpillai M, Evans RJ, et al. Breath carbon monoxide and semiquantitative saliva cotinine as biomarkers for smoking. Hum Psychopharmacol. 2010;25(1):80–83. doi: 10.1002/hup.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stevens KR, Munoz LR. Cigarette smoking: Evidence to guide measurement. Res Nurs Health. 2004;27(4):281–292. doi: 10.1002/nur.20024. [DOI] [PubMed] [Google Scholar]
- 62.Blank MD, Nasim A, Hart A, Jr, et al. Acute effects of cigarillo smoking. Nicotine Tob Res. 2011;13(9):874–879. doi: 10.1093/ntr/ntr070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Breland AB, Buchhalter AR, Evans SE, et al. Evaluating acute effects of potential reduced-exposure products for smokers: Clinical laboratory methodology. Nicotine Tob Res. 2002;4(Suppl 2):S131–140. doi: 10.1080/1462220021000032780. [DOI] [PubMed] [Google Scholar]
- 64.Vansickel AR, Cobb CO, Weaver MF, et al. A clinical laboratory model for evaluating the acute effects of electronic “cigarettes”: Nicotine delivery profile and cardiovascular and subjective effects. Cancer Epidemiol Biomarkers Prev. 2010;19(8):1945–1953. doi: 10.1158/1055-9965.EPI-10-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Breland AB, Cobb CO, Blank MD, et al. Methodological challenges in the clinical laboratory evaluation of electronic cigarettes; 22nd annual meeting of the Society for Research on Nicotine and Tobacco; 2016; Chicago, IL. Available at http://www.srnt.org/?page=Past (Author contact: Alison Breland, Ph.D.; Virginia Commonwealth University; Richmond, VA; abbrelan@vcu.edu). [Google Scholar]
- 66.Jacob N, Golmard JL, Berlin I. Relationships between nicotine and cotinine concentrations in maternal milk and saliva. Acta Paediatr. 2015;104(8):e360–366. doi: 10.1111/apa.13031. [DOI] [PubMed] [Google Scholar]
- 67.Naidong W, Shou W, Chen YL, et al. Novel liquid chromatographic-tandem mass spectrometric methods using silica columns and aqueous-organic mobile phases for quantitative analysis of polar ionic analytes in biological fluids. J Chromatogr B Biomed Sci Appl. 2001;754(2):387–399. doi: 10.1016/s0378-4347(01)00021-4. [DOI] [PubMed] [Google Scholar]
- 68.Liachenko N, Boulamery A, Simon N. Nicotine and metabolites determination in human plasma by ultra performance liquid chromatography-tandem mass spectrometry: A simple approach for solving contamination problem and clinical application. Fundam Clin Pharmacol. 2015;29(5):499–509. doi: 10.1111/fcp.12132. [DOI] [PubMed] [Google Scholar]
- 69.Hukkanen J, Jacob P, 3rd, Benowitz NL. Metabolism and disposition kinetics of nicotine. Pharmacol Rev. 2005;57(1):79–115. doi: 10.1124/pr.57.1.3. [DOI] [PubMed] [Google Scholar]
- 70.Jarvis MJ, Russell MA, Benowitz NL, et al. Elimination of cotinine from body fluids: Implications for noninvasive measurement of tobacco smoke exposure. Am J Public Health. 1988;78(6):696–698. doi: 10.2105/ajph.78.6.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dinur-Klein L, Dannon P, Hadar A, et al. Smoking cessation induced by deep repetitive transcranial magnetic stimulation of the prefrontal and insular cortices: A prospective, randomized controlled trial. Biol Psychiatry. 2014;76(9):742–749. doi: 10.1016/j.biopsych.2014.05.020. [DOI] [PubMed] [Google Scholar]
- 72.David SP, Lancaster T, Stead LF, et al. Opioid antagonists for smoking cessation. Cochrane Database Syst Rev. 2013;6:CD003086. doi: 10.1002/14651858.CD003086. [DOI] [PubMed] [Google Scholar]
- 73.Acosta M, Buchhalter A, Breland A, et al. Urine cotinine as an index of smoking status in smokers during 96-hr abstinence: Comparison between gas chromatography/mass spectrometry and immunoassay test strips. Nicotine Tob Res. 2004;6(4):615–620. doi: 10.1080/14622200410001727867. [DOI] [PubMed] [Google Scholar]
- 74.Adams J, Parkinson L, Sanson-Fisher RW, et al. Enhancing self-report of adolescent smoking: The effects of bogus pipeline and anonymity. Addict Behav. 2008;33(10):1291–1296. doi: 10.1016/j.addbeh.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 75.Murray DM, O’Connell CM, Schmid LA, et al. The validity of smoking self-reports by adolescents: A reexamination of the bogus pipeline procedure. Addict Behav. 1987;12(1):7–15. doi: 10.1016/0306-4603(87)90003-7. [DOI] [PubMed] [Google Scholar]
- 76.Donny EC, Houtsmuller E, Stitzer ML. Smoking in the absence of nicotine: Behavioral, subjective and physiological effects over 11 days. Addiction. 2007;102(2):324–334. doi: 10.1111/j.1360-0443.2006.01670.x. [DOI] [PubMed] [Google Scholar]
- 77.Robinson LA, Vander Weg MW, Riedel BW, et al. “Start to stop”: Results of a randomised controlled trial of a smoking cessation programme for teens. Tob Control. 2003;12(Suppl 4):IV26–33. doi: 10.1136/tc.12.suppl_4.iv26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Delnevo CD, Bauer UE. Monitoring the tobacco use epidemic iii: The host: Data sources and methodological challenges. Prev Med. 2009;48(1 Suppl):S16–23. doi: 10.1016/j.ypmed.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 79.Jamal A, Homa DM, O’Connor E, et al. Current cigarette smoking among adults -united states, 2005–2014. MMWR Morb Mortal Wkly Rep. 2015;64(44):1233–1240. doi: 10.15585/mmwr.mm6444a2. [DOI] [PubMed] [Google Scholar]
- 80.Soulakova JN, Hartman AM, Liu B, et al. Reliability of adult self-reported smoking history: Data from the tobacco use supplement to the current population survey 2002–2003 cohort. Nicotine Tob Res. 2012;14(8):952–960. doi: 10.1093/ntr/ntr313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Eissenberg T, Adams C, Riggins EC, 3rd, et al. Smokers’ sex and the effects of tobacco cigarettes: Subject-rated and physiological measures. Nicotine Tob Res. 1999;1(4):317–324. doi: 10.1080/14622299050011441. [DOI] [PubMed] [Google Scholar]
- 82.Zacny JP, Stitzer ML, Yingling JE. Cigarette filter vent blocking: Effects on smoking topography and carbon monoxide exposure. Pharmacol Biochem Behav. 1986;25(6):1245–1252. doi: 10.1016/0091-3057(86)90119-x. [DOI] [PubMed] [Google Scholar]
- 83.Hatsukami D, Morgan SF, Pickens RW, et al. Smoking topography in a nonlaboratory environment. Int J Addict. 1987;22(8):719–725. doi: 10.3109/10826088709027453. [DOI] [PubMed] [Google Scholar]
- 84.Ossip-Klein DJ, Martin JE, Lomax BD, et al. Assessment of smoking topography generalization across laboratory, clinical, and naturalistic settings. Addict Behav. 1983;8(1):11–17. doi: 10.1016/0306-4603(83)90049-7. [DOI] [PubMed] [Google Scholar]
- 85.Brinkman MC, Kim H, Gordon SM, et al. Design and validation of a research-grade waterpipe equipped with puff topography analyzer. Nicotine Tob Res. 2016;18(5):785–793. doi: 10.1093/ntr/ntv180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Maziak W, Ben Taleb Z, Jawad M, et al. Expert Panel on Waterpipe Assessment in Epidemiological Studies. Consensus statement on assessment of waterpipe smoking in epidemiological studies. Tob Control. doi: 10.1136/tobaccocontrol-2016-052958. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chappell B. [Accessed 4/6/2016];Take it in: ‘Vape’ is the oxford dictionaries word of the year [Radio] 2014 http://www.npr.org/blogs/thetwo-way/2014/11/17/364802028/take-it-in-vape-is-the-oxford-dictionaries-word-of-the-year.
- 88.Hinds JT, 3rd, Loukas A, Chow S, et al. Using cognitive interviewing to better assess young adult e-cigarette use. Nicotine Tob Res. 2016 doi: 10.1093/ntr/ntw096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Farsalinos KE, Romagna G, Tsiapras D, et al. Characteristics, perceived side effects and benefits of electronic cigarette use: A worldwide survey of more than 19,000 consumers. Int J Environ Res Public Health. 2014;11(4):4356–4373. doi: 10.3390/ijerph110404356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kmietowicz Z. Market for e-cigarettes includes 466 brands and 7764 unique flavours. BMJ. 2014;348:g4016. doi: 10.1136/bmj.g4016. [DOI] [PubMed] [Google Scholar]
- 91.Dawkins L, Kimber C, Puwanesarasa Y, et al. First-versus second -generation electronic cigarettes: Predictors of choice and effects on urge to smoke and withdrawal symptoms. Addiction. 2014 doi: 10.1111/add.12807. [DOI] [PubMed] [Google Scholar]
- 92.Yingst JM, Veldheer S, Hrabovsky S, et al. Factors associated with electronic cigarette users’ device preferences and transition from first generation to advanced generation devices. Nicotine Tob Res. 2015;17(10):1242–1246. doi: 10.1093/ntr/ntv052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Blank MD, Disharoon S, Eissenberg T. Comparison of methods for measurement of smoking behavior: Mouthpiece-based computerized devices versus direct observation. Nicotine Tob Res. 2009;11(7):896–903. doi: 10.1093/ntr/ntp083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gust SW, Pickens RW. Does cigarette nicotine yield affect puff volume? Clin Pharmacol Ther. 1982;32(4):418–422. doi: 10.1038/clpt.1982.182. [DOI] [PubMed] [Google Scholar]
- 95.Herning RI, Jones RT, Benowitz NL, et al. How a cigarette is smoked determines blood nicotine levels. Clin Pharmacol Ther. 1983;33(1):84–90. doi: 10.1038/clpt.1983.12. [DOI] [PubMed] [Google Scholar]
- 96.Buchhalter AR, Eissenberg T. Preliminary evaluation of a novel smoking system: Effects on subjective and physiological measures and on smoking behavior. Nicotine Tob Res. 2000;2(1):39–43. doi: 10.1080/14622200050011286. [DOI] [PubMed] [Google Scholar]
- 97.Lichtenstein E, Antonuccio DO. Dimensions of smoking behavior. Addict Behav. 1981;6:365–376. [Google Scholar]
- 98.Frederiksen LW, Miller PM, Peterson GL. Topographical components of smoking behavior. Addict Behav. 1977;2(1):55–61. doi: 10.1016/0306-4603(77)90009-0. [DOI] [PubMed] [Google Scholar]
- 99.Breland AB, Spindle T, Weaver M, et al. Science and electronic cigarettes: Current data, future needs. J Addict Med. 2014;8(4):223–233. doi: 10.1097/ADM.0000000000000049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Farsalinos KE, Romagna G, Tsiapras D, et al. Evaluation of electronic cigarette use (vaping) topography and estimation of liquid consumption: Implications for research protocol standards definition and for public health authorities’ regulation. Int J Environ Res Public Health. 2013;10(6):2500–2514. doi: 10.3390/ijerph10062500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hua M, Yip H, Talbot P. Mining data on usage of electronic nicotine delivery systems (ends) from youtube videos. Tob Control. 2013;22(2):103–106. doi: 10.1136/tobaccocontrol-2011-050226. [DOI] [PubMed] [Google Scholar]
- 102.Behar RZ, Hua M, Talbot P. Puffing topography and nicotine intake of electronic cigarette users. PLoS One. 2015;10(2):e0117222. doi: 10.1371/journal.pone.0117222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lee YH, Gawron M, Goniewicz ML. Changes in puffing behavior among smokers who switched from tobacco to electronic cigarettes. Addict Behav. 2015;48:1–4. doi: 10.1016/j.addbeh.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Norton KJ, June KM, O’Connor RJ. Initial puffing behaviors and subjective responses differ between an electronic nicotine delivery system and traditional cigarettes. Tob Induc Dis. 2014;12(1):17. doi: 10.1186/1617-9625-12-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Spindle TR, Breland AB, Karaoghlanian NV, et al. Preliminary results of an examination of electronic cigarette user puff topography: The effect of a mouthpiece-based topography measurement device on plasma nicotine and subjective effects. Nicotine Tob Res. 2015;17(2):142–149. doi: 10.1093/ntr/ntu186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Stewart DW, Vinci C, Adams CE, et al. Smoking topography and outcome expectancies among individuals with schizotypy. Psychiatry Res. 2013;205(3):205–212. doi: 10.1016/j.psychres.2012.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Eissenberg T. Acute effects of electronic cigarettes in adults: Nicotine delivery and abuse liability. Paper presented at: 20th Annual Meeting of the Society for Research on Nicotine and Tobacco; 2014; Seattle, WA. [Google Scholar]
- 108.Robinson RJ, Hensel EC, Morabito PN, et al. Electronic cigarette topography in the natural environment. PLoS One. 2015;10(6):e0129296. doi: 10.1371/journal.pone.0129296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yerger V, Pearson C, Malone RE. When is a cigar not a cigar? African american youths’ understanding of “cigar” use. Am J Public Health. 2001;91(2):316–317. doi: 10.2105/ajph.91.2.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shiffman S. How many cigarettes did you smoke? Assessing cigarette consumption by global report, time-line follow-back, and ecological momentary assessment. Health Psychol. 2009;28(5):519–526. doi: 10.1037/a0015197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pearson J, Elmasry H, Das B, et al. Measuring real-time e-cigarette use: A comparison of ema and bluetooth-enabled device approaches. Poster presented at: 22nd Annual Meeting of the Society for Research on Nicotine and Tobacco; 2016; Chicago, IL. Available at: http://www.srnt.org/?page=Past (Author contact: Jennifer Pearson, Ph.D.; Schroeder Institute for Tobacco Research and Policy Studies at Truth Initiative; Washington, DC; jpearson@legacyforhealth.org) [Google Scholar]
- 112.Shihadeh A, Eissenberg T. Electronic cigarette effectiveness and abuse liability: Predicting and regulating nicotine flux. Nicotine Tob Res. 2015;17(2):158–162. doi: 10.1093/ntr/ntu175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Vansickel AR, Eissenberg T. Electronic cigarettes: Effective nicotine delivery after acute administration. Nicotine Tob Res. 2013;15(1):267–270. doi: 10.1093/ntr/ntr316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ebbert JO, Patten CA, Schroeder DR. The fagerstrom test for nicotine dependence-smokeless tobacco (ftnd-st) Addict Behav. 2006;31(9):1716–1721. doi: 10.1016/j.addbeh.2005.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Etter JF, Le Houezec J, Perneger TV. A self-administered questionnaire to measure dependence on cigarettes: The cigarette dependence scale. Neuropsychopharmacology. 2003;28(2):359–370. doi: 10.1038/sj.npp.1300030. [DOI] [PubMed] [Google Scholar]
- 116.Heatherton TF, Kozlowski LT, Frecker RC, et al. The fagerstrom test for nicotine dependence: A revision of the fagerstrom tolerance questionnaire. Br J Addict. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 117.Ebbert JO, Severson HH, Danaher BG, et al. A comparison of three smokeless tobacco dependence measures. Addict Behav. 2012;37(11):1271–1277. doi: 10.1016/j.addbeh.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Etter JF, Eissenberg T. Dependence levels in users of electronic cigarettes, nicotine gums and tobacco cigarettes. Drug Alcohol Depend. 2015;147:68–75. doi: 10.1016/j.drugalcdep.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Foulds J, Veldheer S, Yingst J, et al. Development of a questionnaire for assessing dependence on electronic cigarettes among a large sample of ex-smoking e-cigarette users. Nicotine Tob Res. 2015;17(2):186–192. doi: 10.1093/ntr/ntu204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Farsalinos KE, Romagna G, Tsiapras D, et al. Evaluating nicotine levels selection and patterns of electronic cigarette use in a group of “vapers” who had achieved complete substitution of smoking. Subst Abuse. 2013;7:139–146. doi: 10.4137/SART.S12756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sutfin EL, McCoy TP, Morrell HE, et al. Electronic cigarette use by college students. Drug Alcohol Depend. 2013;131(3):214–221. doi: 10.1016/j.drugalcdep.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bohrer J. Stealth. [Accessed May 23, 2015];VAPE Magazine. 2015 http://vapenewsmagazine.com/october-2014/stealth-vaping.
- 123.Mimms B. [Accessed May 23, 2015];Stealth vaping like a ninja: An animated guide. 2014 http://onvaping.com/stealth-vaping-like-a-ninja-an-animated-guide.
- 124.Yan XS, D’Ruiz C. Effects of using electronic cigarettes on nicotine delivery and cardiovascular function in comparison with regular cigarettes. Regul Toxicol Pharmacol. 2015;71(1):24–34. doi: 10.1016/j.yrtph.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 125.Fagerstrom K, Eissenberg T. Dependence on tobacco and nicotine products: A case for product-specific assessment. Nicotine Tob Res. 2012;14(11):1382–1390. doi: 10.1093/ntr/nts007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.World Health Organization. International statistical classification of diseases and related health problems, 10th revision (ICD-10) Geneva: WHO; 1992. [Google Scholar]
- 127.Pepper JK, Brewer NT. Electronic nicotine delivery system (electronic cigarette) awareness, use, reactions and beliefs: A systematic review. Tob Control. 2014;23(5):375–384. doi: 10.1136/tobaccocontrol-2013-051122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hadwiger ME, Trehy ML, Ye W, et al. Identification of amino-tadalafil and rimonabant in electronic cigarette products using high pressure liquid chromatography with diode array and tandem mass spectrometric detection. J Chromatogr A. 2010;1217(48):7547–7555. doi: 10.1016/j.chroma.2010.10.018. [DOI] [PubMed] [Google Scholar]
- 129.Giroud C, de Cesare M, Berthet A, et al. E-cigarettes: A review of new trends in cannabis use. Int J Environ Res Public Health. 2015;12(8):9988–10008. doi: 10.3390/ijerph120809988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Deeming tobacco products to be subject to the federal food, drug, and cosmetic act, as amended by the family smoking prevention and tobacco control act; restrictions on the sale and distribution of tobacco products and required warning statements for tobacco products. Department of Health and Human Services, Food and Drug Administration; 2016. [Accessed June 3, 2016]. https://www.federalregister.gov/articles/2016/05/10/2016-10685/deeming-tobacco-products-to-be-subject-to-the-federal-food-drug-and-cosmetic-act-as-amended-by-the. [PubMed] [Google Scholar]
- 131.Etter JF, Bullen C. A longitudinal study of electronic cigarette users. Addict Behav. 2014;39(2):491–494. doi: 10.1016/j.addbeh.2013.10.028. [DOI] [PubMed] [Google Scholar]
- 132.Etter JF, Bullen C. Electronic cigarette: Users profile, utilization, satisfaction and perceived efficacy. Addiction. 2011;106(11):2017–2028. doi: 10.1111/j.1360-0443.2011.03505.x. [DOI] [PubMed] [Google Scholar]
- 133.Etter JF, Bullen C. Saliva cotinine levels in users of electronic cigarettes. Eur Respir J. 2011;38(5):1219–1220. doi: 10.1183/09031936.00066011. [DOI] [PubMed] [Google Scholar]
- 134.Farsalinos KE, Romagna G, Voudris V. Factors associated with dual use of tobacco and electronic cigarettes: A case control study. Int J Drug Policy. 2015;26(6):595–600. doi: 10.1016/j.drugpo.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 135.Foulds J, Veldheer S, Berg A. Electronic cigarettes (e-cigs): Views of aficionados and clinical/public health perspectives. Int J Clin Pract. 2011;65(10):1037–1042. doi: 10.1111/j.1742-1241.2011.02751.x. [DOI] [PubMed] [Google Scholar]
- 136.Ramoa CP, Hiler MM, Spindle TR, et al. Electronic cigarette nicotine delivery can exceed that of combustible cigarettes: A preliminary report. Tob Control. 2016;25(e1):e6–9. doi: 10.1136/tobaccocontrol-2015-052447. [DOI] [PMC free article] [PubMed] [Google Scholar]