Abstract
Among the many areas being revolutionized by the recent introduction of culture independent microbial identification techniques is investigation of the relationship between close contact with large animals, antibiotics, breast feeding, mode of birth, and other exposures during infancy as related to a reduced risk of asthma and allergic disease. These exposures were originally clustered under the “Hygiene Hypothesis” which has morphed into the “Microbiota Hypothesis”. This review begins by summarizing epidemiologic studies suggesting that the common feature of these allergy risk-related exposures is their influence on the founding and early development of a child's gut microbiota. Next studies using culture independent techniques are presented showing that the microbiota of children who have experienced the exposures of interest have altered gut microbiota. Finally, selected mouse and human studies are presented which begin to corroborate the protective exposures identified in epidemiologic studies by identifying mechanisms through which microbes can alter immune development and function. These microbially driven immune alterations demonstrate that microbial exposures in many cases could alter the risk of subsequent allergic disease and asthma. Hopefully a better understanding of how microbes influence allergic disease will lead to safe and effective methods for reducing the prevalence of all forms of allergic disease.
Introduction
Our understanding of the interactions between microbes and humans is undergoing a massive explosion as the ability to study microbes by culture-independent techniques continues to expand rapidly. Among the many fascinating interactions being illuminated are those related to the lower risk of allergic disease and asthma in children highly exposed to animals during the first year of life. This area of epidemiologic investigation was initially thought of under the rubric of the “Hygiene Hypothesis” but more recently the “Microbiota Hypothesis” appears to be more appropriate terminology.1 Accumulating evidence strongly suggests that much of the apparent animal-related allergy-protective effect, as well as effects from other putative preventive and risk factors, are through their ability to alter the gut microbiota of children during the first year of life. This review will focus on the relationships between microbes and the risk or resistance to allergic disease and asthma. We will briefly explore the epidemiologic development of this area, the factors influencing the composition of the human microbiome during the first year and some of the mechanisms that appear to shift or prevent allergic immune responses from becoming established.
Asthma and other allergic diseases, such as allergic rhinitis and food allergy, have increasingly become common chronic illnesses in developed countries over the past forty years.2 Even though these illnesses infrequently cause death, they do produce very high levels of morbidity and result in large economic costs. Based on the National Health Interview Survey, the Centers for Disease Control estimated that from 2001 to 2013 the prevalence of asthma increased among children 0-17 years of age in the United States by 0.06% per year reaching 8.91 (S.E. 0.05)%.3 According to a 2011 report from the American Lung Association, asthma remains the most common chronic health disorder in childhood, with 8.5 million children aged 5-17 in the United States having been diagnosed with asthma in 2009 (prevalence estimate of 161.3/1000 (95%CI 150.7-171.9).4 Health expenditures for children with asthma are three times higher than for children without asthma.5 Therefore today's new parents are faced with the risk that their newborn will be the one out of 10-11 children in the United States diagnosed with asthma. If the parents are African American, the risk nearly doubles to approximately 1 in 5 children.4 Consequently newborns today have a high risk of requiring daily medications for years, having to endure numerous visits to doctors and hospitals, missing an average of 9.2 days of school each year,6 causing parents to miss work, and having a substantially lower quality of life.7 This poor outlook does not even take into account the costs of asthma to the medical care system (estimated at $56 billion annually from 2002 to 2007).8 The rapid change in the prevalence of allergic disease and asthma has occurred too rapidly to be caused by genetic evolution; therefore, focus has shifted to possible environmental changes that could be causally related.
The Evolution of the Hygiene Hypothes is and Allergic Disorders
Strachan proposed an atopy-related “Hygiene Hypothesis” in 1989,9 suggesting that as living and hygiene standards improved and families became smaller there were fewer infections within families; and that this reduced number of infections led to a greater risk of allergy. The Hygiene Hypothesis was later under-girded by a biologically plausible mechanism--the concept of post-natal immune deviation. This theory holds that, at birth, the human immune system has a more prominent Th2 (allergy promoting) than Th1 (allergy retarding) response profile and that exposures to infectious agents in early life produce a gradual shift toward greater Th1 responses and a “normal immune response balance”.10-13 Immune deviation as the primary underlying mechanism has been challenged by “counter-regulatory” or “gate-keeper” hypotheses.14-18 Proponents noted that over the last half of the 20th century, as the prevalence of many infectious diseases declined precipitously, immune related diseases caused by both Th1 (e.g. diabetes type 1, Crohn's disease) and Th2 (e.g. asthma, allergies) mechanisms increased in an apparent reciprocal relationship.15 Thus the increase in immune-mediated disease could be caused by a lack of infections where that lack results in a less well developed immune regulatory response characterized, for example, by lower levels of the immuno-suppressive cytokine interleukin (IL)10. The fact that IL10 can be up-regulated by viral, bacterial and parasitic infections provides a possible explanation for the relatively low prevalence of allergic disease in countries with high levels of endemic parasitism where serum IgE levels are frequently elevated.19 The concept of counter-regulation fits with observations that high levels of parasite infection are associated with strong Th2 immune responses to parasites but low levels of allergic disease.14,16
Rook expanded upon this hypothesis.20 He suggested that if an environmental change was related to the increase of allergic (and autoimmune) disease it should satisfy two criteria: 1) It must be something that has been present throughout the evolution of the mammalian immune system, and 2) It must be something that has been progressively depleted from the environment of developed countries.20 Rook noted that saprophytic environmental organisms, such as soil or gut bacteria, were better candidates than the putative changing exposure to viral respiratory infections. His criteria support an “altered microbiota” mechanism leading to a “Hygiene-Microbiota Hypothesis”. This theory was initially supported by evidence from early studies using culture-based methods that the microbes making up the normal intestinal microbiota, which have presumably always been part of mammalian life, are different in children with and without atopy as well as between children growing up in developed affluent countries, where the risk of allergic disease is higher, than in children growing up in less well developed countries, with lower risks of allergic disease.21-24
Supporting this theory from an ecological perspective are the dramatic changes related to bacterial exposure that characterized the 20th century as atopic diseases were on the rise. While Strachan reported that number of siblings and particularly older siblings in a household was inversely related to hay fever prevalence,9 US Census data reveal that the average number of persons per household dropped from 4.11 in 1930 to 2.57 in 2004, with 10.9% of homes having 7 or more persons compared to 1.2% respectively.25 Considering other “hygiene factors”, in 1940, nearly half of American homes lacked complete plumbing, a condition of over 70% of homes in some states. That number fell to 1% by 1990.26 Other population changes occurred over the same period. The percentage of the US population living on farms was 12.2% in 1950 but declined to 2.6% by 1990. The poor quality of municipal water treatment in the US was addressed with the US Safe Drinking Water Act which became effective December 16, 1974 and was amended in 1986, 1996, 2005, 2011 and 2015 to broaden the coverage and to address specific concerns such as lead free plumbing and algal toxins.27 A major result of these regulatory changes was greater water purity (fewer bacteria) in nearly all municipal water systems. During this same time interval, there have been many changes to food growth and processing. One example is the use of antibiotics to enhance weight gain of animals (poultry, cattle, hogs) for food consumption. Current estimates suggest that 80% of the antibiotics used in the US are given to food animals for non-therapeutic purposes (i.e., more rapid growth).28
Early Epidemiologic Studies Suggesting Effects of Gut Microbes on Asthma and Allergy
Early ecological and cross-sectional studies provided evidence to support the importance of early exposure to microbial products in the development of atopic disease.29-31 A study of two ethnically identical and geographically contiguous populations, Finnish and Russian Karelians, found that atopy was less common in children with antibodies to enteroviruses, which are found more commonly in the low SES group (Russian Karelians)32 and in those drinking water with a higher microbial content (Russian Karelians).33 A causal link in these studies was suggested by a highly significant inverse correlation between the prevalence of childhood atopy defined by prick skin tests and the number of microorganisms in the drinking water. Several studies have also shown that allergic disease and asthma are much less common in children who drink unpasteurized cow milk, a source of viable microorganisms, especially during the first year of life.34 A study comparing the responses of human cord blood T cells (CD4+CD25-CD127+) from children in modern Australia to those whose mothers live traditional lives in Papua New Guinea found enhanced and more rapid proliferation, in an autologous APC-dependent culture system, in the Australian infants because of differences in the activity of the neonatal APCs and not differences in T-cell activity.35 The findings of this study suggest that APCs from an environment with greater levels of bacterial exposure (Papua New Guinea) may be less responsive to many environmental stimuli.
Studies have examined specific bacterial species in stool samples and demonstrated their abundance correlated with atopy and asthma development.21-23,36-38 A study of 1 year olds in Estonia (low allergy prevalence) and Sweden (high allergy prevalence) revealed that more Estonian children possessed Lactobacilli and Eubacteria in their stool compared to Swedish children, who were more likely to be colonized by Clostridium difficile.24 A follow-up study of Estonian and Swedish children who were sampled over the first year of life and followed to two years of age demonstrated that infants who developed allergy consistently exhibited lower levels of Bifidobacteria colonization.21 Species-specific Q-PCR analysis of the feces of 957 one-month-old infants in the KOALA cohort demonstrated that high abundance of Escherichia coli or C. difficile was associated with the development of eczema or atopy, respectively.22 A 2011 study from Copenhagen demonstrated that bacterial diversity in stool samples at age one and 12 months was inversely associated with atopy, eosinophils, and allergic rhinitis at age six years.39 A 2015 report from the Canadian CHILD cohort found that 22 children classified at age one year as “at high risk for asthma” demonstrated a reduction in four bacterial genera in feces samples collected at the age of three months.40 A recent study from Finland showed that early use of macrolide antibiotics in children was associated with long term (>2years post macrolide use) changes in gut microbes and with increased risk of asthma and weight gain.41 Thus, epidemiologic evidence suggests that early events in gut microbial exposure and colonization may play key roles in development of disease at sites remote from the GI tract, and that gut microbiomes influence allergy and atopic disease.1,42 Definitive results await the accomplishment of multiple birth cohort studies with large sample sizes, long term follow up, and allergic outcomes that have been defined using carefully constructed and specific phenotypes.43-45
Direct evidence demonstrating the influence of the gut microbiome on allergic sensitization and asthma has been provided by several recent studies. A study of BALB/c mice found that gavage feeding of L. reuteri increased the numbers of CD4+CD25+ T cells in mediastinal nodes and inhibited the allergic inflammation provoked by inhalational challenge with ovalbumin in sensitized mice.46 A study of 20 children selected from a randomized controlled trial of probiotics found that children who later developed IgE-associated eczema had significant differences in stool microbes at one week of age, notably a lower abundance of Ruminococcacea, and that the abundance of Ruminococcus was inversely associated with TLR2 induced IL-6 and TNF-α. The abundance of Proteobacteria at one week was also inversely associated with TLR4 induced TNF-α.47
Two reports suggest that adults with asthma and allergies display different fecal microbiota when compared to health controls.48,49 The findings from these two cross-sectional studies raise the interesting question of whether the differences found in adults are a cause or an effect of allergies and asthma. As with adult GI bacterial consortia, inter-personal differences in GI microbial communities are evident in infants, particularly in rate and stability of communities colonizing neonates.50 External influences (delivery mode, feeding patterns, antibiotics, older siblings) are hypothesized to play a role in shaping this community.36,51
One theory encompassed by the evolution of the Hygiene Hypothesis to the Microbiota Hypothesis is the premise that when children live in close proximity to household pets or farm animals they are exposed to home microbiome compositions more similar to early 20th century environments countering the trend of greater “home hygiene”. This home microbiome may directly or indirectly impact immune development and maturation of the infant and subsequently development of clinically important outcomes such as allergic asthma.52 Several studies have indicated that the presence of cats and especially dogs influences the home microbiome.53-57 Other recent reports have suggested that animal exposure is associated with an altered gut microbiome in children.58-60 Further a systemic effect has been shown in murine studies, in the hypothesized direction, of ingested dog-associated dust on airway pathology, cytokine production and cecal microbiota.61 A plausible mechanism explaining the protective effects of household pets against allergy is emerging: pets generate environments containing microbial profiles that affect the child's personal gut microbiome which alters early life immune development, with subsequent decreased risk of allergic outcomes.62-64
A critical link in this hypotheses is that children, including infants, ingest significant amounts of house dust allowing the bacterial present in the house dust access to the child's GI tract. This creates the possibility for ingested bacteria to influence the microbial composition of the child's GI tract. It has been shown that particles, fluids and microbes inhaled by infants are also swallowed,1 and infants have frequent hand-to-mouth activity, as has been shown in environmental toxicology studies.65 There is a robust literature estimating soil and dust ingestion by children related to hand to mouth activity in environmental toxicology. The US EPA has well established estimates of soil ingestion to promulgate regulations of toxic soil cleanup. EPA guidelines do not provide estimates for children less than 6 months of age but children 6 to 12 months of age are estimated to ingest 60 mg of dust and soil per day.66 A meta-analysis published in 2007 by Xue and colleagues summarized nine studies of hand to mouth activity in young children, representing observations of 429 children over more than 2,000 hours.65 The factors most closely related to hand-to-mouth activity in children were the location (indoors or outdoors) and the age of the child. Children had a higher frequency of hand-to-mouth contact when they were indoors and younger children had more hand to mouth contact than older children. The impact of transferring materials from dust by hand-to-mouth activity was shown in a study examining lead concentrations in house dust and soil samples from the child's yard in relationship to the child's blood lead level.67 Both soil and house dust lead levels were independently associated with blood lead levels. In another study blood lead levels were strongly correlated with hand-to-mouth activity (r=0.56, p=0.006) and with the frequency of placing non-food objects in the mouth (r=0.48, p=0.02).68 When the gut microbiomes of a small subsample of children participating in the Canadian CHILD study were examined in relationship to dust from their homes investigators found that 14 bacterial OTUs were significantly higher in matched pairs of dust and stool suggesting a relationship between home dust and gut microbes in these children.69 In these same 20 children, dog or cat ownership was associated with specific bacterial OTUs in house dust but there was not a clear difference in the stool microbes of the children. While no study to date specifically examines the relationship between hand-to-mouth activity and gut microbial composition in children, the extensive toxicology literature using trace elements clearly demonstrates that children and adults transfer measurable amounts of dust into their bodies via hand-to-mouth behavior.
An ever increasing literature implicates the human GI microbiome as critical in health and disease.70 The developing GI microbial community over the first year of life is crucial to maturation of immune function.71-75 Associations have been demonstrated between the presence and abundance of specific microbial species in the GI tract during the first 6 months of life and subsequent development of allergic disease.36,76 This suggests early patterns of GI colonization predispose the child to risk for allergic disease later in life.1 After weaning is completed (typically by 1 year) the bacterial community is thought to resemble adult GI populations,50 a dense, relatively stable consortium in which Bacteroidetes and Firmicutes represent the two most dominant phyla.50,77,78 Several studies have demonstrated that the most dramatic developmental changes in the lower gastrointestinal microbiome occur over the first year of life as the assemblage progresses through periods of increasing bacterial burden, anaerobiosis and a shift to fermentative metabolism.50,79 However, at the age of six months, concurrent with introduction of solid foods, or likely more importantly, the cessation of breastfeeding,80 the GI microbiota, at least at the phylum level, begins to stabilize and resemble that of an adult microbial community.79
While this review focuses on the infant gut microbiota and specifically bacteria as central in the causal pathway to pediatric allergic disorders, other infant microbiota sites and microorganisms likely play an important role. In 2007 Bisgaard and colleagues reported that colonization of the hypopharyngeal region of one month old infants by Streptococcus penumoniae, Haemophilus influenzae or Moraxella catarrhalis but not Staphylococcus aureus were significantly associated with persistent wheeze, acute exacerbation of wheeze, and hospitalization for wheeze by age five years in a birth cohort of 312 Danish children.81 Colonization was independent of sex, maternal antibiotics, breast-feeding, gestational age and older children in the home. A similar study followed nasopharyngeal bacteria in 234 children prospectively during the first year of life. The investigators found that there was a progression of bacteria that tended to dominate this site and that early, asymptomatic colonization with Streptococcus was a strong predictor of asthma.82 While culture independent techniques are being used to examine the microbiomes of the lungs most studies have examined cystic fibrosis and other inflammatory lung diseases with few published studies of the lung microbiome as a risk factor for asthma.83 There does appear to be a relationship between the lung microbiota and asthma severity, but this may partially be due to treatments such as inhaled corticosteroids.84-86 A recent report from the Denmark group found that one putative risk factor for asthma, caesarian section (C-section), discussed below, impacts the neonate's microbiota of the gut but not the airways, providing evidence that the early gut microbiota is perhaps the more fundamental link on the atopic disease causal pathway.87
There are other microbes besides bacteria that could contribute to the relationship between microbial exposure and allergic risk. Exposures to fungi or fungal products could be associated with an increased risk of allergic disease.88-90 Our finding of an inverse relationship between the diversity of bacteria and fungi in house dusts would mean that the microbiomes of homes with animals would have more bacterial species and fewer fungal species leading to a lower risk of allergic disease.91 The study of Ege et al. also found an inverse relationship between certain fungi and asthma.63 It is also clear that viruses play a role in the etiology of pediatric asthma.92
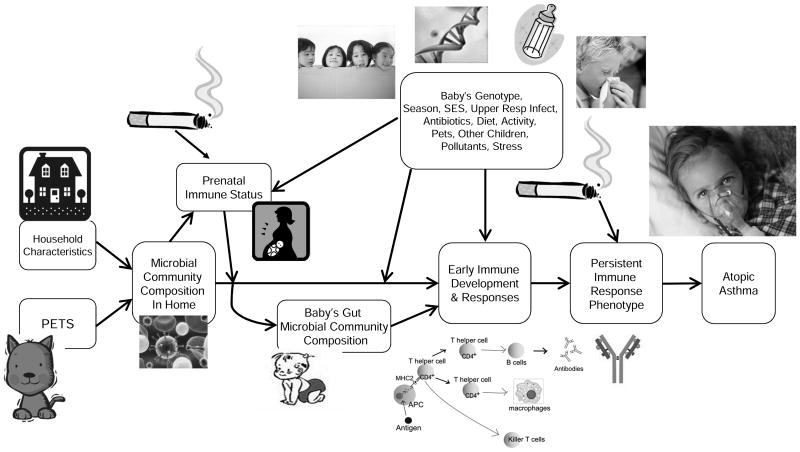
Figure 1 portrays a posited causal pathway with the infant gut microbiome as the central focus. We will now explore links on this proposed chain of causation.
Figure 1. Causal Pathway Relating the Environment, Infant Gut Microbiome and Pediatric Allergy and Atopic Asthma.
Prenatal Microbial Immune Effects
Early studies on the Microbial Hypothesis were based on the assumption that the uterine environment of the fetus is sterile and insulated from the effects of maternal microbes. Contemporary studies are challenging this assumption.93-95 A fascinating study in mice utilized a genetically engineered Escherichia coli (HA107).96 This E. coli strain can be given orally to germ-free mice but it does not persist in the intestine so pregnant dams become germ-free again before delivery. Gestation-only colonization of pregnant dams with HA107 altered the number of early postnatal intestinal innate leukocytes in wild-type C57BL/6 mice pups. The increase in intestinal innate lymphoid cell (ILC) proportions and total numbers compared with controls persisted and reached a maximum in 14-21 day old pups and the increase persisted even after weaning.
Human studies have also shown that there is a microbiome of the placenta even in healthy, term, pregnancies.97 Interestingly the placental microbiome is more similar to the oral microbiome than to the vaginal or gut microbiomes.97 The placental microbiome varies with maternal gestational weight gain and the microbial alterations associated with gestational weight gain were associated with decreased folate biosynthesis and butanoate metabolism.98 Interestingly, in a non-human primate model a high-fat maternal or postnatal diet altered the structure of the offspring's intestinal microbiome. The altered offspring gut microbiome could only partially be corrected by a postnatal low fat diet after weaning.99 While none of these areas have been well explored in relationship to allergies and asthma, all of the findings suggest that the maternal microbiome begins to influence the child's microbiome and subsequent immune development during in utero development. These preliminary findings also suggest that studies should be conducted to learn whether it is possible to safely alter the microbiome of reproductive age women in the hope of reducing allergic disease in their children.
Birth: The Explosive Increase of the Infant Microbiome
Birth is associated with an onslaught of exposure to a wide variety of microbes. A growing number of studies have shown major differences in the early microbiomes of children delivered vaginally compared to those delivered by C-section, and that effects may differ by whether the surgery was elective versus emergency.51,64,87,100-105 Not surprisingly, the microbiomes of vaginally delivered infants show the strong influence of the mother's vaginal and gut microbiotas while the microbiomes of children delivered by C-section are dominated by microbes common to human skin.64 A study of the birth mode-dependent association between pre-pregnancy maternal weight and neonatal gut microbiome suggested that maternal weight-related microbes are passed to infants only during vaginal delivery.106 Microbes on the skin during infancy appear to be essential for developing long-lasting tolerance to bacteria that are normally skin commensals. In a mouse model, either blocking the influx of T cells into the skin or not having the bacteria on the skin as the T cell influx occurred resulted in skin inflammation from ski n contact with the bacteria later in life.107 A separate mouse study demonstrated that the lung microbiome was more closely related to the mother's vaginal microbiome than to the mouse's own gut microbiome.108
The alterations of the infant microbiome related to delivery by C-section appear to have health related consequences. Bentley and colleagues used linked medical and birth records to examine the relationship between mode of birth, breast-feeding and hospitalization for acute gastroenteritis in 893,360 singleton live births of at least 33 weeks of gestation in Australia.109 After controlling for other variables, C-section was associated with a 20% increased risk of hospital admission for acute gastroenteritis. The combination of preterm, C-section birth, and formula-only feeding increased acute gastroenteritis risk by 62-78%. A meta-analysis of 20 studies involving 9,938 cases found that after adjusting for other variables, birth by C-section significantly increased the risk of Type 1 diabetes (OR 1.23, 95% CI 1.15-1.32).110,111 A recent register-based birth cohort study from Scandinavia studied risks of immune function related diseases, discriminating between elective versus emergency cesarean section in reference to vaginal delivery.101 Elective surgeries were associated with higher risks of lower respiratory tract infections, juvenile idiopathic arthritis and asthma.
Breast Milk and the Developing Infant Gut Microbiome
Analogous to the thinking that infants were in a sterile in utero environment, it has long been assumed that human breast milk is sterile. However, recent studies have shown that breast milk has a unique microbiome as well as various nutrients and other substances that strongly influence the growth of microbes.112-115 The primary microbes in human milk are Proteobacteria and Firmicutes. Pre- or pro-biotic compounds (such as oligosaccharides) also stimulate and help shape the growth of certain bacteria along with various immunoglobulins (IgG, IgM and secretory IgA).116,117 The full role of microbes in human breast milk has not been elucidated but one study found that the microbial composition of breast milk was independent of preterm or term birth, C-section or vaginal birth, and infant sex.113 However, another study found that breast milk microbial composition at one month post-partum varied by mode of deliver.118 Other studies have shown that intrapartum antibiotic prophylaxis for Group B Streptococcus alter the infant's gut microbiome and that these changes are independent of breast-feeding in the first weeks of life.119,120 An issue with these studies is that tests for the significance of antibiotic exposure were in comparison to the infants of mothers who did not have detectable Group B Streptococcus. The reason that this comparison group is a concern is a third study which found that the gut microbiomes of infants born to mothers who were culture positive for Group B Streptococcus differed even after adjusting for intrapartum antibiotics, maternal race, and antifungal use.121 A study of the effects of milk oligosaccharides in severely malnourished infants showed that these milk components were essential to restoring normal growth beyond supplementation with appropriate calories, presumably through the effects of oligosaccharides on microbial growth and metabolism.114
Impact of Microbes on the Development of Early Immune Responses
Immune development, including the capacity to synthesize the key Th1 cytokine, IFNΥ, appears to be shaped by microbial exposure.122 Microbes are thought to influence the process of immune maturation through repeated stimulation via pathogen-associated molecular patterns (PAMPs) that are recognized by pattern recognition receptors such as toll-like receptors (TLRs) found on cells of the innate immune system.13,123 Simultaneous TLR stimulation with multiple ligands, mimicking exposure in microbe-rich environments, appears to induce neonatal dendritic cells to mature, primarily through the production of theTh1-polarizing cytokine, IL12.124 This suggests that environments with high microbial content may induce early life T cell responses toward the Th1 phenotype, and boost baseline airway Treg activity.125,15,126
Little is known of the precise mechanisms through which microbes alter the developing human immune system but mouse models suggest multiple possibilities. Among the many microbial products with the potential to alter immune function are short-chain fatty acids (SCFAs) frequently produced by microbes during fermentation of dietary fiber.127 SCFAs can bind to G-protein coupled receptor 43 (GPR43) and strongly affect inflammatory responses.128 The necessity for GPR43 stimulation in the resolution of inflammation was demonstrated in mice deficient for the Gpr43 gene, which could not resolve inflammation in models of asthma, arthritis and colitis.129 A very recent study examined alterations in gastrointestinal microbiota derived short-chain fatty acids (SCFA) in a mouse model of graft-versus-host disease following allogeneic bone marrow transplantation.130 In this study, an unbiased analysis identified an alteration in only one SCFA, butyrate as associated with disease. Supplementation of the mice with any of 17 rationally selected strains of high butyrate producing Clostridia significantly reduced the graft-versus-host disease. Another mouse study found that Bacteroides fragilis, a common human commensal organism, produces polysaccharide A (PAS), which suppresses inflammation by down-regulation of IL-17. 131 Monocolonization of germ-free mice with Bacteroides fragilis increased the conversion of CD4+T cells into Foxp3+ Treg cell capable of producing the anti-inflammatory cytokine IL-10. Further examination showed that the conversion of cells into Treg cells was produced by PSA during intestinal inflammation. This same bacteria species was found to be inversely associated with TLR4 mRNA expression among a population of 64 Swedish infants, and correspondingly, the down-regulation of PBMC response to LPS, with lower production of the inflammatory cytokines and chemokines IL-6, IL-8, IL-17 and CCL4.132
Conclusions
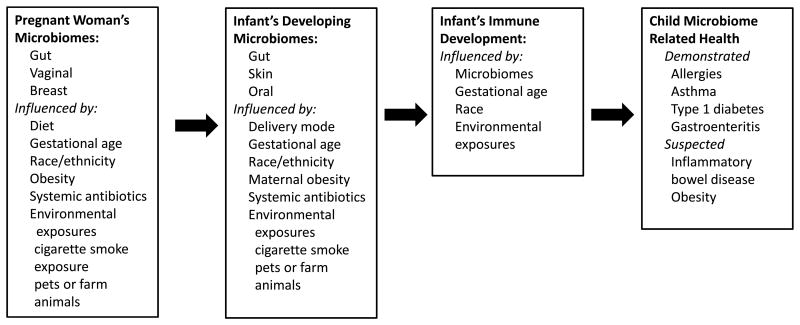
We are only beginning to investigate the myriad interactions between microbes and humans. Many different types of investigations collectively support the hypothesis that exposures to microbes in the first months of life have strong effects on the developing human immune system, as summarized in Figure 2. Some of these effects may reduce or increase the risks of allergic disorders and asthma. While much more needs to be learned, it appears that it may be possible to reduce substantially the prevalence of allergic diseases and asthma by altering the microbiomes of children. Adequate alterations of gut microbiomes in children may depend on earlier alterations of the microbiomes of women during or perhaps even before pregnancy.
Figure 2. Variables Related to the Sequential Passage of Microbes Impacting Immune Development and Health in Children.
Acknowledgments
Funding sources: US National Institutes of Health NIAID (P01AI089473)
Footnotes
The authors have no conflict of interest. The authors alone are responsible for the content and writing of this paper. All of the authors have read the journal's authorship agreement.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Penders J, Stobberingh EE, van den Brandt PA, Thijs C. The role of the intestinal microbiota in the development of atopic disorders. Allergy. 2007;62(11):1223–36. doi: 10.1111/j.1398-9995.2007.01462.x. [DOI] [PubMed] [Google Scholar]
- 2.Platts-Mills TA. The allergy epidemics: 1870-2010. J Allergy Clin Immunol. 2015;136(1):3–13. doi: 10.1016/j.jaci.2015.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akinbami LJ, Simon AE, Schoendorf KC. Trends in allergy prevalence among children aged 0-17 years by asthma status, United States, 2001-2013. J Asthma. 2016;53(4):356–62. doi: 10.3109/02770903.2015.1126848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Epidemiology and Statistics Unit ALA. Trends in Asthma Morbidity and Mortality. 2011 [Google Scholar]
- 5.Bryant-Stephens T. Asthma disparities in urban environments. J Allergy Clin Immunol. 2009;123(6):1199–206. doi: 10.1016/j.jaci.2009.04.030. [DOI] [PubMed] [Google Scholar]
- 6.Moonie SA, Sterling DA, Figgs L, Castro M. Asthma Status and Severity Affects Missed School Days. Journal of School Health. 2006;76:18–24. doi: 10.1111/j.1746-1561.2006.00062.x. [DOI] [PubMed] [Google Scholar]
- 7.Clarke SA, Calam R. The effectiveness of psychosocial interventions designed to improve health-related quality of life (HRQOL) amongst asthmatic children and their families: a systematic review. QualLife Res. 2011 doi: 10.1007/s11136-011-9996-2. [DOI] [PubMed] [Google Scholar]
- 8.Barnett SB, Nurmagambetov TA. Costs of asthma in the United States: 2002-2007. J Allergy Clin Immunol. 2011;127(1):145–52. doi: 10.1016/j.jaci.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 9.Strachan DP. Hay fever, hygiene, and household size. BMJ. 1989;299(6710):1259–60. doi: 10.1136/bmj.299.6710.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holt PG. Postnatal maturation of immune competence during infancy and childhood. Pediatr Allergy Immunol. 1995;6:59–70. doi: 10.1111/j.1399-3038.1995.tb00261.x. [DOI] [PubMed] [Google Scholar]
- 11.Schaub B, Lauener R, von ME. The many faces of the hygiene hypothesis. J Allergy Clin Immunol. 2006;117(5):969–77. doi: 10.1016/j.jaci.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 12.Bloomfield SF, Stanwell-Smith R, Crevel RW, Pickup J. Too clean, or not too clean: the hygiene hypothesis and home hygiene. Clin ExpAllergy. 2006;36(4):402–25. doi: 10.1111/j.1365-2222.2006.02463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Romagnani S. Coming back to a missing immune deviation as the main explanatory mechanism for the hygiene hypothesis. J Allergy Clin Immunol. 2007;119(6):1511–3. doi: 10.1016/j.jaci.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 14.Yazdanbakhsh M, Kremsner PG, van Ree R. Allergy, parasites, and the Hygiene Hypothesis. Science. 2002;296:490–4. doi: 10.1126/science.296.5567.490. [DOI] [PubMed] [Google Scholar]
- 15.Bach JF. The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med. 2002;347(12):911–20. doi: 10.1056/NEJMra020100. [DOI] [PubMed] [Google Scholar]
- 16.Gale EA. A missing link in the hygiene hypothesis? Diabetologia. 2002;45(4):588–94. doi: 10.1007/s00125-002-0801-1. [DOI] [PubMed] [Google Scholar]
- 17.Romagnani S. The increased prevalence of allergy and the hygiene hypothesis: missing immune deviation, reduced immune suppression, or both? Immunology. 2004;112(3):352–63. doi: 10.1111/j.1365-2567.2004.01925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Halonen M, Lohman IC, Stern DA, et al. Th1/Th2 patterns and balance in cytokine production in the parents and infants of a large birth cohort. J Immunol. 2009;182(5):3285–93. doi: 10.4049/jimmunol.0711996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walsh KP, Brady MT, Finlay CM, Boon L, Mills KH. Infection with a helminth parasite attenuates autoimmunity through TGF-beta-mediated suppression of Th17 and Th1 responses. J Immunol. 2009;183(3):1577–86. doi: 10.4049/jimmunol.0803803. [DOI] [PubMed] [Google Scholar]
- 20.Rook GA. Clean living increases more than just atopic disease. Immunol Today. 2000;21(5):249–50. doi: 10.1016/s0167-5699(00)01630-3. [DOI] [PubMed] [Google Scholar]
- 21.Bjorksten B, Sepp E, Julge K, Voor T, Mikelsaar M. Allergy development and the intestinal microflora during the first year of life. J Allergy Clin Immunol. 2001;108(4):516–20. doi: 10.1067/mai.2001.118130. [DOI] [PubMed] [Google Scholar]
- 22.Penders J, Thijs C, van den Brandt PA, et al. Gut microbiota composition and development of atopic manifestations in infancy: the KOALA Birth Cohort Study. Gut. 2007;56(5):661–7. doi: 10.1136/gut.2006.100164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kalliomaki M, Kirjavainen P, Eerola E, Kero P, Salminen S, Isolauri E. Distinct patterns of neonatal gut microflora in infants in whom atopy was and was not developing. J Allergy Clin Immunol. 2001;107(1):129–34. doi: 10.1067/mai.2001.111237. [DOI] [PubMed] [Google Scholar]
- 24.Sepp E, Julge K, Vasar M, Naaber P, Bjorksten B, Mikelsaar M. Intestinal microflora of Estonian and Swedish infants. Acta Paediatr. 1997;86(9):956–61. doi: 10.1111/j.1651-2227.1997.tb15178.x. [DOI] [PubMed] [Google Scholar]
- 25.Bureau UC. U. S. Households by Size. 2007:1790–2006. [Google Scholar]
- 26.Bureau UC. Historical Census of Housing Tables. Plumbing Facilities. 2004 [Google Scholar]
- 27.https://www.epa.gov/sdwa/title-xiv-public-health-service-act-safety-public-water-systems-safe-drinking-water-act. (accessed 05/11/2016
- 28.https://louise.house.gov/media-center/press-releases/congresswoman-louise-slaughter-reacts-proposed-rule-fda-antibiotic-use. (accessed 05/11/2016
- 29.Crane J, O'Donnell TV, Prior IA, Waite DA. The relationships between atopy, bronchial hyperresponsiveness, and a family history of asthma: a cross-sectional study of migrant Tokelauan children in New Zealand. J Allergy Clin Immunol. 1989;84(5 Pt 1):768–72. doi: 10.1016/0091-6749(89)90307-2. [DOI] [PubMed] [Google Scholar]
- 30.vM E, Fritzsch C, Weiland SK, Rolla G, Magnussen H. Prevalence of asthma and allergic disordes among children in United Germany. BMJ. 1992;305:1395–9. doi: 10.1136/bmj.305.6866.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bjorksten B. Risk factors in early childhood for the development of atopic diseases. Allergy. 1994;49:400–7. doi: 10.1111/j.1398-9995.1994.tb00831.x. [DOI] [PubMed] [Google Scholar]
- 32.Seiskari T, Kondrashova A, Viskari H, et al. Allergic sensitization and microbial load--a comparison between Finland and Russian Karelia. Clin ExpImmunol. 2007;148(1):47–52. doi: 10.1111/j.1365-2249.2007.03333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.von Hertzen L, Laatikainen T, Pitkanen T, et al. Microbial content of drinking water in Finnish and Russian Karelia - implications for atopy prevalence. Allergy. 2007;62(3):288–92. doi: 10.1111/j.1398-9995.2006.01281.x. [DOI] [PubMed] [Google Scholar]
- 34.Braun-Fahrlander C, von ME. Can farm milk consumption prevent allergic diseases? Clin Exp Allergy. 2011;41(1):29–35. doi: 10.1111/j.1365-2222.2010.03665.x. [DOI] [PubMed] [Google Scholar]
- 35.Lisciandro JG, Prescott SL, Nadal-Sims MG, et al. Neonatal antigen-presenting cells are functionally more quiescent in children born under traditional compared with modern environmental conditions. J Allergy Clin Immunol. 2012;130(5):1167–74.e10. doi: 10.1016/j.jaci.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 36.Adlerberth I. Factors influencing the establishment of the intestinal microbiota in infancy. Nestle Nutr Workshop Ser Pediatr Program. 2008;62:13–29. doi: 10.1159/000146245. discussion -33. [DOI] [PubMed] [Google Scholar]
- 37.Oddy WH, Holt PG, Sly PD, et al. Association between breast feeding and asthma in 6 year old children: findings of a prospective birth cohort study. BMJ. 1999;319(7213):815–9. doi: 10.1136/bmj.319.7213.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kalliomaki M, Salminen S, Poussa T, Arvilommi H, Isolauri E. Probiotics and prevention of atopic disease: 4-year follow-up of a randomised placebo-controlled trial. Lancet. 2003;361(9372):1869–71. doi: 10.1016/S0140-6736(03)13490-3. [DOI] [PubMed] [Google Scholar]
- 39.Bisgaard H, Li N, Bonnelykke K, et al. Reduced diversity of the intestinal microbiota during infancy is associated with increased risk of allergic disease at school age. J Allergy Clin Immunol. 2011;128(3):646–52 e1-5. doi: 10.1016/j.jaci.2011.04.060. [DOI] [PubMed] [Google Scholar]
- 40.Arrieta MC, Stiemsma LT, Dimitriu PA, et al. Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci Transl Med. 2015;7(307):307ra152. doi: 10.1126/scitranslmed.aab2271. [DOI] [PubMed] [Google Scholar]
- 41.Korpela K, Salonen A, Virta LJ, et al. Intestinal microbiome is related to lifetime antibiotic use in Finnish pre-school children. Nat Commun. 2016;7:10410. doi: 10.1038/ncomms10410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McLoughlin RM, Mills KH. Influence of gastrointestinal commensal bacteria on the immune responses that mediate allergy and asthma. J Allergy Clin Immunol. 2011;127(5):1097–107. doi: 10.1016/j.jaci.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 43.Bridgman SL, Kozyrskyj AL, Scott JA, Becker AB, Azad MB. Gut microbiota and allergic disease in children. Ann Allergy Asthma Immunol. 2016;116(2):99–105. doi: 10.1016/j.anai.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 44.Prince BT, Mandel MJ, Nadeau K, Singh AM. Gut Microbiome and the Development of Food Allergy and Allergic Disease. Pediatr Clin North Am. 2015;62(6):1479–92. doi: 10.1016/j.pcl.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Simonyte Sjödin K, Vidman L, Rydén P, West CE. Emerging evidence of the role of gut microbiota in the development of allergic diseases. Curr Opin Allergy Clin Immunol. 2016 doi: 10.1097/ACI.0000000000000277. [DOI] [PubMed] [Google Scholar]
- 46.Karimi K, Inman MD, Bienenstock J, Forsythe P. Lactobacillus reuteri-induced regulatory T cells protect against an allergic airway response in mice. Am J Respir Crit Care Med. 2009;179(3):186–93. doi: 10.1164/rccm.200806-951OC. [DOI] [PubMed] [Google Scholar]
- 47.West CE, Rydén P, Lundin D, Engstrand L, Tulic MK, Prescott SL. Gut microbiome and innate immune response patterns in IgE-associated eczema. Clin Exp Allergy. 2015;45(9):1419–29. doi: 10.1111/cea.12566. [DOI] [PubMed] [Google Scholar]
- 48.Hevia A, Milani C, López P, et al. Allergic Patients with Long-Term Asthma Display Low Levels of Bifidobacterium adolescentis. PLoS One. 2016;11(2):e0147809. doi: 10.1371/journal.pone.0147809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hua X, Goedert JJ, Pu A, Yu G, Shi J. Allergy associations with the adult fecal microbiota: Analysis of the American Gut Project. EBioMedicine. 2016;3:172–9. doi: 10.1016/j.ebiom.2015.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Palmer C, Bik EM, DiGiulio DB, Relman DA, Brown PO. Development of the human infant intestinal microbiota. PLoS Biol. 2007;5(7):e177. doi: 10.1371/journal.pbio.0050177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Penders J, Thijs C, Vink C, et al. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics. 2006;118(2):511–21. doi: 10.1542/peds.2005-2824. [DOI] [PubMed] [Google Scholar]
- 52.Fall T, Lundholm C, Örtqvist AK, et al. Early Exposure to Dogs and Farm Animals and the Risk of Childhood Asthma. JAMA Pediatr. 2015;169(11):e153219. doi: 10.1001/jamapediatrics.2015.3219. [DOI] [PubMed] [Google Scholar]
- 53.Fujimura KE, Johnson CC, Ownby DR, et al. Man's best friend? The effect of pet ownership on house dust microbial communities. J Allergy Clin Immunol. 2010;126(2):410–2. doi: 10.1016/j.jaci.2010.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kettleson EM, Adhikari A, Vesper S, Coombs K, Indugula R, Reponen T. Key determinants of the fungal and bacterial microbiomes in homes. Environ Res. 2015;138:130–5. doi: 10.1016/j.envres.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maier RM, Palmer MW, Andersen GL, et al. Environmental determinants of and impact on childhood asthma by the bacterial community in household dust. Appl Environ Microbiol. 2010;76(8):2663–7. doi: 10.1128/AEM.01665-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dunn RR, Fierer N, Henley JB, Leff JW, Menninger HL. Home life: factors structuring the bacterial diversity found within and between homes. PLoS One. 2013;8(5):e64133. doi: 10.1371/journal.pone.0064133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Konya T, Koster B, Maughan H, et al. Associations between bacterial communities of house dust and infant gut. Environ Res. 2014;131:25–30. doi: 10.1016/j.envres.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 58.Azad MB, Konya T, Maughan H, et al. Infant gut microbiota and the hygiene hypothesis of allergic disease: impact of household pets and siblings on microbiota composition and diversity. Allergy Asthma Clin Immunol. 2013;9(1):15. doi: 10.1186/1710-1492-9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nermes M, Niinivirta K, Nylund L, et al. Perinatal pet exposure, faecal microbiota, and wheezy bronchitis: is there a connection? ISRN Allergy. 2013;2013:827934. doi: 10.1155/2013/827934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nermes M, Endo A, Aarnio J, Salminen S, Isolauri E. Furry pets modulate gut microbiota composition in infants at risk for allergic disease. J Allergy Clin Immunol. 2015;136(6):1688–90.e1. doi: 10.1016/j.jaci.2015.07.029. [DOI] [PubMed] [Google Scholar]
- 61.Fujimura KE, Demoor T, Rauch M, et al. House dust exposure mediates gut microbiome Lactobacillus enrichment and airway immune defense against allergens and virus infection. Proc Natl Acad Sci U S A. 2014;111(2):805–10. doi: 10.1073/pnas.1310750111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.von Hertzen L, Haahtela T. Disconnection of man and the soil: reason for the asthma and atopy epidemic? J Allergy Clin Immunol. 2006;117(2):334–44. doi: 10.1016/j.jaci.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 63.Ege MJ, Mayer M, Normand AC, et al. Exposure to environmental microorganisms and childhood asthma 1. N Engl J Med. 2011;364(8):701–9. doi: 10.1056/NEJMoa1007302. [DOI] [PubMed] [Google Scholar]
- 64.Dominguez-Bello MG, Costello EK, Contreras M, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci U S A. 2010;107(26):11971–5. doi: 10.1073/pnas.1002601107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xue J, Zartarian V, Moya J, et al. A meta-analysis of children's hand-to-mouth frequency data for estimating nondietary ingestion exposure. Risk Anal. 2007;27(2):411–20. doi: 10.1111/j.1539-6924.2007.00893.x. [DOI] [PubMed] [Google Scholar]
- 66.Agency UEP. Child-Specific Exposure Factors Handbook (Final Report) 2008. 2008 [Google Scholar]
- 67.Lanphear BP, Matte TD, Rogers J, et al. The contribution of lead-contaminated house dust and residential soil to children's blood lead levelsA pooled analysis of 12 epidemiologic studies. Environ Res. 1998;79(1):51–68. doi: 10.1006/enrs.1998.3859. [DOI] [PubMed] [Google Scholar]
- 68.K S, S PD, V CM, B HJ. Relationships of video assessments of touching and mouthing behaviors during outdoor play in urban residential yards to parental perceptions of child behaviors and blood lead levels. J Expo Sci Environ Epidemiol. 2007;17:47–57. doi: 10.1038/sj.jes.7500519. [DOI] [PubMed] [Google Scholar]
- 69.Konya T, Koster B, Maughan H, et al. Associations between bacterial communities of house dust and infant gut. Environ Res. 2014;131:25–30. doi: 10.1016/j.envres.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 70.Fujimura KE, Slusher NA, Cabana MD, Lynch SV. Role of the gut microbiota in defining human health. Expert Rev Anti Infect Ther. 2010;8(4):435–54. doi: 10.1586/eri.10.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cash HL, Whitham CV, Behrendt CL, Hooper LV. Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science. 2006;313(5790):1126–30. doi: 10.1126/science.1127119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rakoff-Nahoum S, Medzhitov R. Innate immune recognition of the indigenous microbial flora. MucosalImmunol. 2008;1(Suppl 1):S10–S4. doi: 10.1038/mi.2008.49. [DOI] [PubMed] [Google Scholar]
- 73.Chung H, Kasper DL. Microbiota-stimulated immune mechanisms to maintain gut homeostasis. Curr Opin Immunol. 2010;22(4):455–60. doi: 10.1016/j.coi.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 74.Salzman NH. Microbiota-immune system interaction: an uneasy alliance. Curr Opin Microbiol. 2011;14(1):99–105. doi: 10.1016/j.mib.2010.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kaplan JL, Shi HN, Walker WA. The role of microbes in developmental immunologic programming. Pediatr Res. 2011;69(6):465–72. doi: 10.1203/PDR.0b013e318217638a. [DOI] [PubMed] [Google Scholar]
- 76.Copperstock M. Human-intestinal Microflora in Health and Disease. New York City: Academic Press; 1983. [Google Scholar]
- 77.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444(7122):1022–3. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 78.Ley RE, Backhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci USA. 2005;102(31):11070–5. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Koenig JE, Spor A, Scalfone N, et al. Succession of microbial consortia in the developing infant gut microbiome. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4578–85. doi: 10.1073/pnas.1000081107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Backhed F, Roswall J, Peng Y, et al. Dynamics and Stabilization of the Human Gut Microbiome during the First Year of Life. Cell Host Microbe. 2015;17(5):690–703. doi: 10.1016/j.chom.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 81.Bisgaard H, Hermansen MN, Buchvald F, et al. Childhood asthma after bacterial colonization of the airway in neonates. N Engl J Med. 2007;357(15):1487–95. doi: 10.1056/NEJMoa052632. [DOI] [PubMed] [Google Scholar]
- 82.Teo SM, Mok D, Pham K, et al. The infant nasopharyngeal microbiome impacts severity of lower respiratory infection and risk of asthma development. Cell Host Microbe. 2015;17(5):704–15. doi: 10.1016/j.chom.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Martin C, Burgel PR, Lepage P, et al. Host-microbe interactions in distal airways: relevance to chronic airway diseases. Eur Respir Rev. 2015;24(135):78–91. doi: 10.1183/09059180.00011614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang Q, Cox M, Liang Z, et al. Airway Microbiota in Severe Asthma and Relationship to Asthma Severity and Phenotypes. PLoS One. 2016;11(4):e0152724. doi: 10.1371/journal.pone.0152724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Denner DR, Sangwan N, Becker JB, et al. Corticosteroid therapy and airflow obstruction influence the bronchial microbiome, which is distinct from that of bronchoalveolar lavage in asthmatic airways. J Allergy Clin Immunol. 2016;137(5):1398–405 e3. doi: 10.1016/j.jaci.2015.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Huang YJ, Nariya S, Harris JM, et al. The airway microbiome in patients with severe asthma: Associations with disease features and severity. J Allergy Clin Immunol. 2015;136(4):874–84. doi: 10.1016/j.jaci.2015.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Stokholm J, Thorsen J, Chawes BL, et al. Cesarean section changes neonatal gut colonization. J Allergy Clin Immunol. 2016 doi: 10.1016/j.jaci.2016.01.028. [DOI] [PubMed] [Google Scholar]
- 88.Bush RK, Portnoy JM, Saxon A, Terr AI, Wood RA. The medical effects of mold exposure. J Allergy Clin Immunol. 2006;117(2):326–33. doi: 10.1016/j.jaci.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 89.Portnoy JM, Barnes CS, Kennedy K. Importance of mold allergy in asthma. Curr Allergy Asthma Rep. 2008;8(1):71–8. doi: 10.1007/s11882-008-0013-y. [DOI] [PubMed] [Google Scholar]
- 90.Kauffman HF, van der Heide S. Exposure, sensitization, and mechanisms of fungus-induced asthma. Curr Allergy Asthma Rep. 2003;3(5):430–7. doi: 10.1007/s11882-003-0080-z. [DOI] [PubMed] [Google Scholar]
- 91.Fujimura KE, Johnson CC, Ownby DR, et al. Man's best friend? The effect of pet ownership on house dust microbial communities. J Allergy Clin Immunol. 2010;126(2):410–2. 2 e1–3. doi: 10.1016/j.jaci.2010.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jackson DJ, Gern JE, Lemanske RF. The contributions of allergic sensitization and respiratory pathogens to asthma inception. J Allergy Clin Immunol. 2016;137(3):659–65. doi: 10.1016/j.jaci.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mshvildadze M, Neu J, Shuster J, Theriaque D, Li N, Mai V. Intestinal microbial ecology in premature infants assessed with non-culture-based techniques. J Pediatr. 2010;156(1):20–5. doi: 10.1016/j.jpeds.2009.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Collado MC, Rautava S, Aakko J, Isolauri E, Salminen S. Human gut colonisation may be initiated in utero by distinct microbial communities in the placenta and amniotic fluid. Sci Rep. 2016;6:23129. doi: 10.1038/srep23129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mor G, Kwon JY. Trophoblast-microbiome interaction: a new paradigm on immune regulation. Am J Obstet Gynecol. 2015;213(4 Suppl):S131–7. doi: 10.1016/j.ajog.2015.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gomez de Aguero M, Ganal-Vonarburg SC, Fuhrer T, et al. The maternal microbiota drives early postnatal innate immune development. Science. 2016;351(6279):1296–302. doi: 10.1126/science.aad2571. [DOI] [PubMed] [Google Scholar]
- 97.Aagaard K, Ma J, Antony KM, Ganu R, Petrosino J, Versalovic J. The placenta harbors a unique microbiome. Sci Transl Med. 2014;6(237):237ra65. doi: 10.1126/scitranslmed.3008599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Antony KM, Ma J, Mitchell KB, Racusin DA, Versalovic J, Aagaard K. The preterm placental microbiome varies in association with excess maternal gestational weight gain. Am J Obstet Gynecol. 2015;212(5):653.e1–16. doi: 10.1016/j.ajog.2014.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ma J, Prince AL, Bader D, et al. High-fat maternal diet during pregnancy persistently alters the offspring microbiome in a primate model. Nat Commun. 2014;5:3889. doi: 10.1038/ncomms4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jakobsson HE, Abrahamsson TR, Jenmalm MC, et al. Decreased gut microbiota diversity, delayed Bacteroidetes colonisation and reduced Th1 responses in infants delivered by caesarean section. Gut. 2014;63(4):559–66. doi: 10.1136/gutjnl-2012-303249. [DOI] [PubMed] [Google Scholar]
- 101.Kristensen K, Henriksen L. Cesarean section and disease associated with immune function. J Allergy Clin Immunol. 2016;137(2):587–90. doi: 10.1016/j.jaci.2015.07.040. [DOI] [PubMed] [Google Scholar]
- 102.Madan JC, Hoen AG, Lundgren SN, et al. Association of Cesarean Delivery and Formula Supplementation With the Intestinal Microbiome of 6-Week-Old Infants. JAMA Pediatr. 2016;170(3):212–9. doi: 10.1001/jamapediatrics.2015.3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sevelsted A, Stokholm J, Bisgaard H. Risk of Asthma from Cesarean Delivery Depends on Membrane Rupture. J Pediatr. 2016;171:38–42.e4. doi: 10.1016/j.jpeds.2015.12.066. [DOI] [PubMed] [Google Scholar]
- 104.Black M, Bhattacharya S, Philip S, Norman JE, McLernon DJ. Planned Repeat Cesarean Section at Term and Adverse Childhood Health Outcomes: A Record-Linkage Study. PLoS Med. 2016;13(3):e1001973. doi: 10.1371/journal.pmed.1001973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Almqvist C, Cnattingius S, Lichtenstein P, Lundholm C. The impact of birth mode of delivery on childhood asthma and allergic diseases--a sibling study. Clin Exp Allergy. 2012;42(9):1369–76. doi: 10.1111/j.1365-2222.2012.04021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mueller NT, Shin H, Pizoni A, et al. Birth mode-dependent association between pre-pregnancy maternal weight status and the neonatal intestinal microbiome. Sci Rep. 2016;6:23133. doi: 10.1038/srep23133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Scharschmidt TC, Vasquez KS, Truong HA, et al. A Wave of Regulatory T Cells into Neonatal Skin Mediates Tolerance to Commensal Microbes. Immunity. 2015;43(5):1011–21. doi: 10.1016/j.immuni.2015.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Barfod KK, Roggenbuck M, Hansen LH, et al. The murine lung microbiome in relation to the intestinal and vaginal bacterial communities. BMC Microbiol. 2013;13:303. doi: 10.1186/1471-2180-13-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bentley JP, Simpson JM, Bowen JR, Morris JM, Roberts CL, Nassar N. Gestational age, mode of birth and breastmilk feeding all influence acute early childhood gastroenteritis: a record-linkage cohort study. BMC Pediatr. 2016;16(1):55. doi: 10.1186/s12887-016-0591-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cardwell CR, Stene LC, Joner G, et al. Caesarean section is associated with an increased risk of childhood-onset type 1 diabetes mellitus: a meta-analysis of observational studies. Diabetologia. 2008;51(5):726–35. doi: 10.1007/s00125-008-0941-z. [DOI] [PubMed] [Google Scholar]
- 111.Paun A, Yau C, Danska JS. Immune recognition and response to the intestinal microbiome in type 1 diabetes. J Autoimmun. 2016 doi: 10.1016/j.jaut.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 112.Jeurink PV, van Bergenhenegouwen J, Jimenez E, et al. Human milk: a source of more life than we imagine. Benef Microbes. 2013;4(1):17–30. doi: 10.3920/BM2012.0040. [DOI] [PubMed] [Google Scholar]
- 113.Urbaniak C, Angelini M, Gloor GB, Reid G. Human milk microbiota profiles in relation to birthing method, gestation and infant gender. Microbiome. 2016;4:1. doi: 10.1186/s40168-015-0145-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Charbonneau MR, O'Donnell D, Blanton LV, et al. Sialylated Milk Oligosaccharides Promote Microbiota-Dependent Growth in Models of Infant Undernutrition. Cell. 2016;164(5):859–71. doi: 10.1016/j.cell.2016.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gomez-Gallego C, Garcia-Mantrana I, Salminen S, Collado MC. The human milk microbiome and factors influencing its composition and activity. Semin Fetal Neonatal Med. 2016 doi: 10.1016/j.siny.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 116.Vangay P, Ward T, Gerber JS, Knights D. Antibiotics, pediatric dysbiosis, and disease. Cell Host Microbe. 2015;17(5):553–64. doi: 10.1016/j.chom.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jeurink PV, van Esch BC, Rijnierse A, Garssen J, Knippels LM. Mechanisms underlying immune effects of dietary oligosaccharides. Am J Clin Nutr. 2013;98(2):572S–7S. doi: 10.3945/ajcn.112.038596. [DOI] [PubMed] [Google Scholar]
- 118.Cabrera-Rubio R, Mira-Pascual L, Mira A, Collado MC. Impact of mode of delivery on the milk microbiota composition of healthy women. J Dev Orig Health Dis. 2016;7(1):54–60. doi: 10.1017/S2040174415001397. [DOI] [PubMed] [Google Scholar]
- 119.Corvaglia L, Tonti G, Martini S, et al. Influence of Intrapartum Antibiotic Prophylaxis for Group B Streptococcus on Gut Microbiota in the First Month of Life. J Pediatr Gastroenterol Nutr. 2016;62(2):304–8. doi: 10.1097/MPG.0000000000000928. [DOI] [PubMed] [Google Scholar]
- 120.Azad MB, Konya T, Persaud RR, et al. Impact of maternal intrapartum antibiotics, method of birth and breastfeeding on gut microbiota during the first year of life: a prospective cohort study. BJOG. 2016;123(6):983–93. doi: 10.1111/1471-0528.13601. [DOI] [PubMed] [Google Scholar]
- 121.Cassidy-Bushrow AE, Sitarik A, Levin AM, et al. Maternal group B Streptococcus and the infant gut microbiota. J Dev Orig Health Dis. 2016;7(1):45–53. doi: 10.1017/S2040174415001361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Vuillermin PJ, Ponsonby AL, Saffery R, et al. Microbial exposure, interferon gamma gene demethylation in naive T-cells, and the risk of allergic disease. Allergy. 2009;64(3):348–53. doi: 10.1111/j.1398-9995.2009.01970.x. [DOI] [PubMed] [Google Scholar]
- 123.Horner AA. Toll-like receptor ligands and atopy: a coin with at least two sides. J Allergy Clin Immunol. 2006;117(5):1133–40. doi: 10.1016/j.jaci.2006.02.035. [DOI] [PubMed] [Google Scholar]
- 124.Krumbiegel D, Zepp F, Meyer CU. Combined Toll-like receptor agonists synergistically increase production of inflammatory cytokines in human neonatal dendritic cells. Hum Immunol. 2007;68(10):813–22. doi: 10.1016/j.humimm.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 125.Strickland DH, Judd S, Thomas JA, Larcombe AN, Sly PD, Holt PG. Boosting airway T-regulatory cells by gastrointestinal stimulation as a strategy for asthma control. Mucosal Immunol. 2011;4(1):43–52. doi: 10.1038/mi.2010.43. [DOI] [PubMed] [Google Scholar]
- 126.Liu AH. Innate microbial sensors and their relevance to allergy. J Allergy Clin Immunol. 2008;122(5):846–58. doi: 10.1016/j.jaci.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 127.Molloy J, Allen K, Collier F, Tang ML, Ward AC, Vuillermin P. The potential link between gut microbiota and IgE-mediated food allergy in early life. Int J Environ Res Public Health. 2013;10(12):7235–56. doi: 10.3390/ijerph10127235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Morrison DJ, Preston T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes. 2016;7(3):189–200. doi: 10.1080/19490976.2015.1134082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Maslowski KM, Vieira AT, Ng A, et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461(7268):1282–6. doi: 10.1038/nature08530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mathewson ND, Jenq R, Mathew AV, et al. Gut microbiome-derived metabolites modulate intestinal epithelial cell damage and mitigate graft-versus-host disease. Nat Immunol. 2016;17(5):505–13. doi: 10.1038/ni.3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Round JL, Mazmanian SK. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc Natl Acad Sci U S A. 2010;107(27):12204–9. doi: 10.1073/pnas.0909122107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sjogren YM, Tomicic S, Lundberg A, et al. Influence of early gut microbiota on the maturation of childhood mucosal and systemic immune responses. Clin Exp Allergy. 2009;39(12):1842–51. doi: 10.1111/j.1365-2222.2009.03326.x. [DOI] [PubMed] [Google Scholar]