Abstract
Objectives
We aim to describe our experiences and identify patients who may benefit from referral to a peripheral nerve surgeon for removal of contraceptive subdermal implants in which neurovascular injury may occur and describe a treatment pathway for optimal care.
Study Design
We reviewed the charts of 22 patients who were referred to the Division of Family Planning for difficult removal of etonogestrel contraceptive implants between January 1, 2014 and April, 1 2016. Of these, 5 were referred to a peripheral nerve surgeon due to pain or location of the implant. We evaluated and described these cases and from our findings, developed recommendations for care in a multidisciplinary team approach.
Results
Two patients reported pain, including one with four previous failed removal attempts. In the two patients with pain, the implants were adherent to a sensory nerve. In another, the implant was within the biceps muscle and difficult to locate. In all cases, ultrasound imaging, general anesthesia, and a wide exposure allowed for safe removal and good outcomes. Our multidisciplinary care approach has elucidated important referral and technical considerations that improve patient care and safety.
Conclusion
When necessary, multidisciplinary care with a Family Planning expert and possibly a peripheral nerve surgeon may be beneficial in safely removing etonogestrel contraceptive implants that would be difficult or risky to remove in an ambulatory setting.
Keywords: etonogestrel contraceptive implant, nerve injury, multidisciplinary care, Implanon, difficult implant removal
1. Introduction
The use of long-acting, reversible subdermal contraceptive implants increased by 50% between 2009 and 2012 [1]. The only etonogestrel contraceptive implant available in the US is Nexplanon® (Merck Inc., Whitehouse Station, NJ), which replaced its predecessor, Implanon® (Merck Inc., Whitehouse Station, NJ). Both are single-rod implants are 4 cm long and placed subdermally along the medial upper arm [2, 3]. The implant is inserted using a simple applicator in an ambulatory clinic, and practitioners usually perform removal in an office setting as well. Practitioners in the US and abroad are required to complete insertion and removal training to prevent complications, but very rarely, serious adverse events have occurred such as migration or embolization of implants. The manufacturer estimates that intravascular placement has occurred in just over one patient per million Nexplanon® implants sold[4]. While serious adverse events related to insertion and removal are exceedingly rare, [5–7], prior reports have described cases that required specialized surgical expertise to remove all varieties of implants from the upper extremity while minimizing additional risks to the patient [8–18]. It is not always easy to identify someone qualified to treat these types of cases, but Family Planning specialists who may be found at some academic medical centers should be the first point of referral to triage such patients.
Depending on the Family Planning specialist’s findings, select cases may benefit from partnership with physicians with additional upper extremity surgical proficiency. A peripheral nerve surgeon has training, knowledge and interest in the treatment of traumatic and compressive disorders of the peripheral nerves. Usually they have completed a residency in plastic or orthopedic surgery with subsequent subspecialty fellowship in hand and upper extremity surgery. They are uniquely qualified to remove implants that are directly adjacent to neurovascular structures or deep within the musculature.
Few reports have stressed the importance or illustrated the anatomy of neurovascular structures running superficial to the deep fascia. Here, we discuss five cases that, despite evaluation by a family planning specialist with experience in difficult removals (author DLE), ultimately required surgical extraction of the implant by a peripheral nerve surgeon (author IKF). The purpose of this report is to educate practitioners about the complex anatomy of the upper arm and help them guide patients through a referral pathway to a peripheral nerve specialist when needed.
2. Materials and Methods
After obtaining IRB approval, we reviewed the charts of 22 patients referred to Family Planning specialists in the Department of Obstetrics and Gynecology for difficult etonogestrel implant removal between January 1, 2014 and April, 1 2016, based on their log of such referrals. Reasons for referral included failed attempt by referring provider, nonpalpable implant, concern for deep location and neuropathic pain. By using Current Procedural Terminology codes 11976 (Removal, implantable contraceptive) and 11982 (Removal, non-biodegradable drug delivery implant) to query the Plastic and Reconstructive Surgery billing database, we identified all cases that were subsequently referred to for care by peripheral nerve surgeons. We then performed review of the electronic medical record of these five identified patients to extract relevant data including age, BMI, insertion site and date, type of implant, reason for removal, prior removal attempts, examination of the extremity and answers from a pain questionnaire, preoperative imaging, operative data, and postoperative complications.
3. Results
Of the 22 patients referred for difficult implant removal, 17 had successful removal completed by the Family Planning specialists, some requiring ultrasound localization and/or guidance. Without further attempts at removal, the specialists referred the five other patients to a peripheral nerve surgeon due to either pain or location of implant being deep within the muscle or in too close proximity to neurovascular structures.
Summary data for these 5 patients are shown in Table 1. Patient 1 and Patient 5 both complained of neuropathic pain. Patient 1 developed pain and presented for removal. Patient 5 underwent a removal attempt under local anesthesia and developed subsequent changes in sensation and neuropathic pain. Given the pain experienced by patients 1 and 5, electrodiagnostic testing was performed before surgery due to concern for a nerve injury. Patient 3 had had a palpable implant after placement, but when she presented for removal on device expiration it had become nonpalpable. Patient 2 and Patient 4 had implants that were never palpable on examination.
Table 1.
Cases 1–5. Patient demographics, pertinent history and physical examination data, surgical
| Patient 1* (Figure 1) | Patient 2 (Figure 2) | Patient 3 (Figure 3) | Patient 4 (Figure 4) | Patient 5 (Figure 5) | |
|---|---|---|---|---|---|
| Age (years) | 36 | 19 | 48 | 23 | 25 |
| BMI (kg/m2) | 18.3 | 31.2 | 20.5 | 21.9 | 19.8 |
| Time from placement(years) | 4 | 3 | 4 | 4 | 3 |
| Reason for removal | Neuropathic pain | Migration of implant | Expired implant | Expired implant | Expired implant |
| Prior removal attempts | 4 | 1 | 0 | 0 | 1 |
| Reason for referral to peripheral nerve surgeon | Neuropathic pain and failed removal | Device subfascial on imaging | Device within biceps muscle | Device subfascial on imaging | Failed removal and subsequent pain |
| Pain | Yes | No | No | No | Yes |
| Strength | Diminished | Normal | Normal | Normal | Normal |
| Sensation | Normal | Normal | Normal | Normal | Abnormal |
| Exam | 3 transverse scars over implant site | Healing transverse scar over implant site | - | - | 2 healing incisions at implant site |
| Implant palpable | No | No | No | No | Yes |
| Implant location | Deep to bicipital fascia; adherent to brachial neurovascular bundle | Deep to bicipital fascia; adherent to sensory nerve branch | Within bicep muscle, parallel to muscle fibers | Adjacent to ulnar nerve and brachial artery | Deep to triceps fascia, adherent to sensory nerve |
| Outcome | Implant removed, pain resolved | Implant removed, no issues | Implant removed, no issues | Implant removed, no issues | Implant removed, pain resolved |
| findings, and outcomes. | |||||
Distal compression of the median and ulnar nerve at the level of the wrist was noted on exam of the patient and a carpal tunnel and Guyon’s canal release were completed at the time of device removal. This may have been entirely unrelated to the device.
Each of these patients was taken to the operating room and the implant was removed under general anesthesia by a peripheral nerve specialist (Figures 1–5). This allowed for wide exposure of the implant, intraoperative ultrasound for localization of the implant, stimulation of the nerves, adequate hemostasis and sufficient visualization of both ends of the implant prior to handling and extracting the device. Postoperatively, we performed and documented the upper extremity neurovascular examination. Therapy for scar management, desensitization, range of motion, and strengthening were ordered when necessary for each patient. Furthermore, the two patients with preoperative pain were prescribed gabapentin to assist with reduction of neuropathic pain. All recovered satisfactorily with no complications.
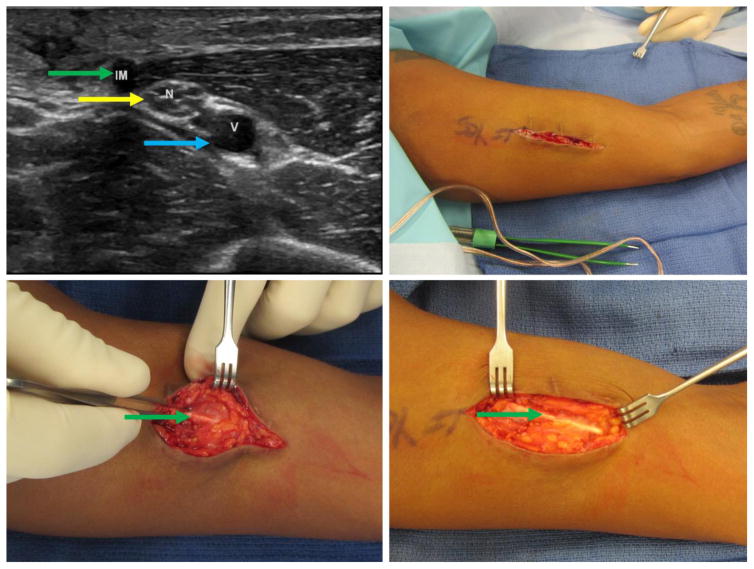
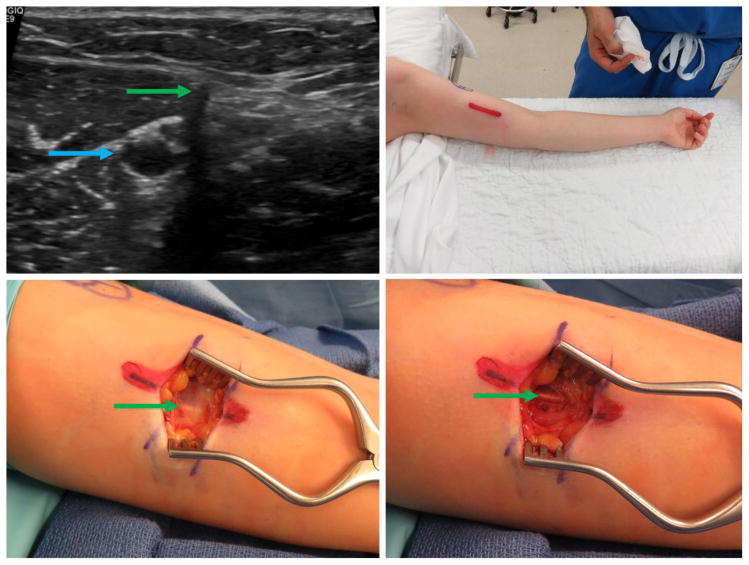
Figure 1.
Patient 1 – 36-year-old (BMI 18.3), multiple extraction attempts and significant pain. Clockwise from top left: a) Ultrasound image with proximity to neurovascular structures; note nerve fascicles directly abutting the implant in cross-section. b) Initial incision; note transverse scars (black arrows) from prior extraction attempts. c) Wide exposure of the implant with significant scarring. d) Removal of the implant. Green arrow marks the implant, yellow marks nerve, blue marks blood vessel. (Printed with permission ©2016 nervesurgery.wustl.edu)
Figure 5.
Patient 5. 25-year-old patient (BMI 19.8), expired device and pain after failed removal attempt. a) Ultrasound imaging with transverse view of implant (green arrow) deep to tricep fascia in close proximity to cutaneous nerve and vein. b) Preoperative marking and positioning, note posterior implant location. c) Triceps fascia with visible implant. d) Implant deep to triceps fascia with thick encapsulation. (Printed with permission ©2016 nervesurgery.wustl.edu)
4. Discussion
Despite the rarity of neurovascular complications associated with the etonogestrel contraceptive implant, there are a few reports of median and ulnar nerve injury associated with device extraction, particularly during challenging removals [19–21]. To minimize such risks, the manufacturer addresses the importance of avoiding the bicipital groove and ensuring the tip of the inserter is visible throughout the insertion procedure to avoid deep insertion and recommends placement at least 8cm above the medial epicondyle [3, 22, 23]. However, because of the anatomic complexity and superficiality of sensory nerves and vessels, avoiding the bicipital groove does not eliminate risks [24–27]. Thus, when the patient experiences subdermal implant-associated pain or change in sensation or is at risk of neurovascular damage on removal (characterized by location of implant deep to muscle fascia or proximity of the implant to a neurovascular structure on physical exam or imaging), referring the patient to a family planning specialist with experience managing difficult implant removals [28] and subsequently to a peripheral nerve surgeon may optimize outcomes.
In our experience in these five cases, currently described techniques were inadequate for implant removal. Although such techniques include use of intraoperative ultrasound to guide dissection [11] and dissection with a mosquito clamp, they do not include direct visualization of the entire implant before extraction. One may reduce the risk of nerve injury by using a wide operative exposure and avoiding grasping the implant with a surgical instrument until it has been identified in its entirety & separated from adjoining tissues as described in our results.
4.1 Anatomy of the upper extremity
When evaluating a patient for etonogestrel contraceptive implant removal, we consider the proximity of the brachial artery, ulnar and median nerves, and superficial structures including the medial antebrachial cutaneous (MABC) nerve, other sensory nerve branches, and basilic vein. The ulnar and median nerves and brachial artery run within the deep fascia and become more superficial within the bicipital groove as they run distally. The MABC nerve, which provides forearm sensation, has anterior and posterior branches lying deep in the bicipital groove until 14 cm proximal to the medial epicondyle, where it pierces the fascia and lies within the subcutaneous fat—in the vicinity of where the implants are inserted (Appendix 1). Damage during placement or removal of contraceptive implants could lead to loss of sensation, neuroma formation, and chronic neuropathic pain.
4.2 Initial evaluation of patient with nonpalpable implant
If the implant is not easily palpable or is in an unexpected location, we do not attempt removal until the implant is localized with imaging [3]. Our standard history includes a review of prior attempts at removal and any upper extremity pain or functional deficit. Examination should include attempted implant palpation, documentation of the scar location and size, and basic sensory and motor testing of the upper extremity. We document distal pulses and existing neurovascular deficits during the first visit with all patients. The Family Planning expert should then use clinical judgement to proceed with removal or referral. With any abnormal findings (neuropathic pain, change in distal sensory/motor function), or perineural/perivascular and/or subfascial implant location on imaging, we suggest direct referral to a peripheral nerve or hand surgery specialist. Electrodiagnostic testing may be ordered prior to surgery depending on clinical concern for a nerve injury.
4.3 Minimizing Risk
If the patient desires another form of contraception, that plan should be established before implant removal [3]. It is important that the correct ultrasound probe and a clinician with experience in implant localization, such as the referring Family Planning expert, are available in the operating room, [11, 17] as we used intraoperative ultrasound after positioning and/or incision to locate the implant in all cases. Because the implants are located in the upper arm, a tourniquet is not used (Figure 2). We avoid local anesthetic and paralytics as they preclude intraoperative nerve stimulation. Having an ultrasound with the correct probes in the room to confirm the location and reinforce markings is ideal.
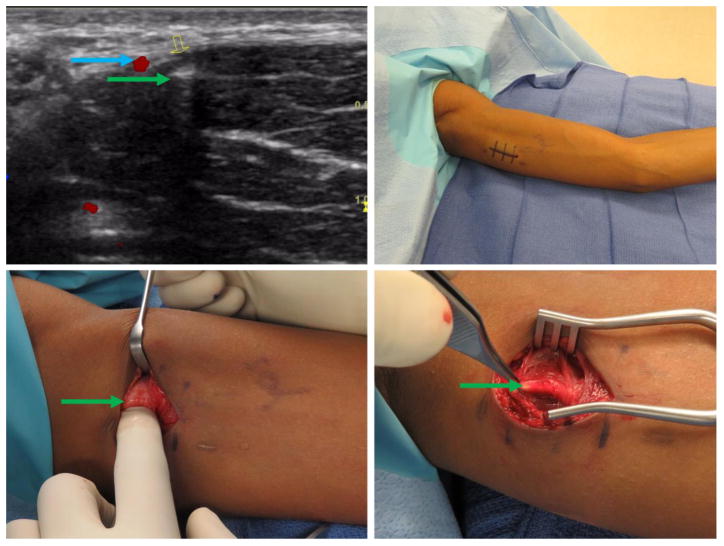
Figure 2.
Patient 2 – 19-year-old (BMI 31.2), migration of implant and prior failed extraction. Clockwise from top left: a) Ultrasound imaging with implant in cross-section directly adjacent to neurovascular structure. b) Pre-operative marking and positioning of the extremity. c) Implant exposure; implant directly adjacent to superficial sensory nerve. d) Note similarities in color and size of the implant and sensory nerve. Green arrow marks the implant, yellow marks nerve, blue marks blood vessel. (Printed with permission ©2016 nervesurgery.wustl.edu)
In cases of peripheral nerve symptoms, we counsel patients to expect a 4–5 cm incision along the axis of the implant. Smaller incisions have been described [13, 15, 20] but may not allow visualization of the complete implant. A fibrous sheath often encases the implant which can make it virtually indistinguishable from subcutaneous nerves (Figure 2) so we do not handle the device with forceps or grasping instruments until we have confirmed its identity.
Any distal loss of sensory and motor function associated with insertion or removal of an etonogestrel contraceptive implant is treated as a suspected transection injury and managed within days, if possible. A number of treatment options are available to minimize chronic pain and disability (e.g., nerve repair, nerve grafting, distal nerve transfer), but only if intervention occurs in a timely fashion) [29].
4.4 Further recommendations
The best way to optimize outcomes is to ensure proper subdermal implant insertion. Implants are to be placed in the between the dermis and subdermal tissue under local anesthesia, and should remain palpable throughout the lifespan of the device[30]. If the implant is placed at the proper depth, critical neurovascular structures should be avoided by inserting the implant at least 8cm proximal to the medial epicondyle[25]. While previous literature shows a migration rate as high as 39% of patients[31], a recently published study of 4294 practitioners, showed migration in only 0.26% of cases[5]. Migration is typically no more than 2cm from the initial insertion site. While the focus is often on avoiding too deep placement; superficial placement can also cause problems. Placement of the implant between the epidermis and dermis risks implant exposure and continuous painful stimulus to the sensory receptors and nerves lying in this layer.
Placing the etonogestrel contraceptive implant in a less anatomically complex location than the upper arm may further improve the safety of contraceptive implants. For example, Wechselberger et al. [27] recommended placing implants in the median supraumbilical region as this location is inconspicuous and contains essentially no neurovascular structures.
Alternatively, a change in device design may be more appropriate. Both etonogestrel contraceptive implants are off-white and resemble the appearance of nerves and vessels in the upper extremity, particularly when encased in scar tissue. Changing the color of the device to turquoise or green (such as the placebo used during FDA-required manufacturer training programs) could avoid confusion and assist with recognition of the device upon extraction. However, this would require reformulation and retesting of the device and would be financially burdensome for the rare event that the implant is directly adjacent to a peripheral nerve.
Finally, as etonogestrel contraceptive implants gain popularity, continued education and multidisciplinary care is key. Given the simple design of the applicator [32] and low-risk nature of insertion, training is thorough and mandatory and does include neurovascular anatomy. However it does not address neurovascular complications and their management. Given the rarity of such adverse events related to the contraceptive implant it is crucial that patients experiencing them are referred to clinicians with expertise such as a Family Planning expert and when necessary they may need to collaborate with others. If there is a suspected nerve injury or attempts at ultrasound-guided extraction by a Family Planning expert fail, early referral and partnership with a peripheral nerve surgeon may successfully optimize our patient experiences.
Supplementary Material
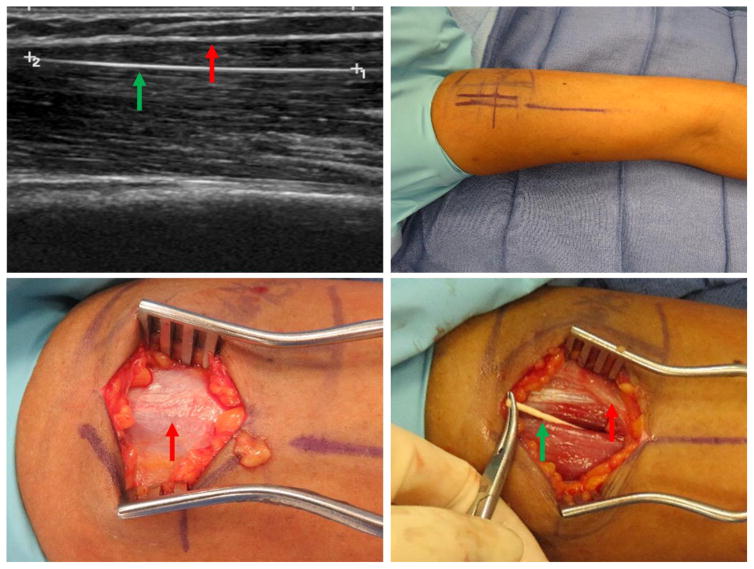
Figure 3.
Patient 3 – 48-year-old (BMI 20.5), expired device, intramuscular implant. Clockwise from top left: a) Ultrasound imaging with longitudinal view of implant deep to biceps fascia. Note parallel orientation with muscle fibers. b) Preoperative marking; note multiple marks due to change in implant location with any change in extremity position. c) Biceps fascia without observable implant or visible fascial defect. d) Implant extraction from within the biceps muscle body. Green arrow marks implant, red marks biceps fascia. (Printed with permission ©2016 nervesurgery.wustl.edu)
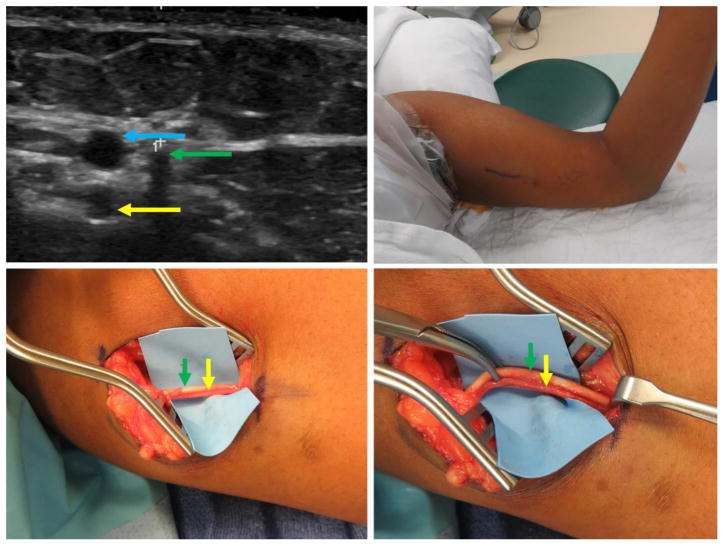
Figure 4.
Patient 4 – 23-year-old patient (BMI 21.9), expired device, subfascial implant. Clockwise from top left: a) Ultrasound imaging with transverse view of implant (green arrow) deep to biceps fascia in close proximity to the brachial neurovascular bundle (blue arrow). b) Preoperative marking and positioning. c) Biceps fascia without observable implant or visible fascial defect. d) Implant deep to biceps fascia. (Printed with permission ©2016 nervesurgery.wustl.edu)
Acknowledgments
Funding: This work was supported by T32CA190194 (PI: Colditz, funding for EO), the Foundation for Barnes-Jewish Hospital, and Siteman Cancer Center. The content is solely the responsibility of the authors and does not necessarily represent the official view of the NIH.
Footnotes
Disclosures: Dr. Eisenberg is a certified Nexplanon® trainer and consultant for Merck & Co.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kavanaugh ML, Jerman J, Finer LB. Changes in Use of Long-Acting Reversible Contraceptive Methods Among U.S. Women, 2009–2012. Obstet Gynecol. 2015;126:917–27. doi: 10.1097/AOG.0000000000001094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Implanon [package insert] Whitehouse Station, NJ: Merck & Co, Inc; 2016. [Google Scholar]
- 3.Nexplanon [package insert] Whitehouse Station, NJ: Merck & Co, Inc; 2016. [Google Scholar]
- 4.Rowlands S, Mansour D, Walling M. Intravascular migration of contraceptive implants: two more cases. Contraception. 2017;95:211–4. doi: 10.1016/j.contraception.2016.07.015. [DOI] [PubMed] [Google Scholar]
- 5.Creinin MD, Kaunitz AM, Darney PD, et al. The US etonogestrel implant mandatory clinical training and active monitoring programs: 6-year experience. Contraception. 2017;95:205–10. doi: 10.1016/j.contraception.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 6.Blumenthal PD, Gemzell-Danielsson K, Marintcheva-Petrova M. Tolerability and clinical safety of Implanon. Eur J Contracept Reprod Health Care. 2008;13(Suppl 1):29–36. doi: 10.1080/13625180801960012. [DOI] [PubMed] [Google Scholar]
- 7.Darney P, Patel A, Rosen K, Shapiro LS, Kaunitz AM. Safety and efficacy of a single-rod etonogestrel implant (Implanon): results from 11 international clinical trials. Fertility and Sterility. 2009;91:1646–53. doi: 10.1016/j.fertnstert.2008.02.140. [DOI] [PubMed] [Google Scholar]
- 8.Biskamp C, Kauffman RP. Arm flexion during ultrasound assists localization of an intramuscular etonogestrel contraceptive implant. Contraception. 2016;93:273–5. doi: 10.1016/j.contraception.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 9.Bragg TWH, Jose RM, Bland JW, Matthews RN, Srivastava S. Implantable contraceptive devices: Primum non nocere. Journal of Family Planning and Reproductive Health Care. 2006;32:190–2. doi: 10.1783/147118906777888503. [DOI] [PubMed] [Google Scholar]
- 10.Chen MJ, Creinin MD. Removal of a Nonpalpable Etonogestrel Implant With Preprocedure Ultrasonography and Modified Vasectomy Clamp. Obstet Gynecol. 2015;126:935–8. doi: 10.1097/AOG.0000000000001082. [DOI] [PubMed] [Google Scholar]
- 11.James P, Trenery J. Ultrasound localisation and removal of non-palpable Implanon implants. Aust N Z J Obstet Gynaecol. 2006;46:225–8. doi: 10.1111/j.1479-828X.2006.00576.x. [DOI] [PubMed] [Google Scholar]
- 12.Mansour D, Fraser IS, Walling M, et al. Methods of accurate localisation of non-palpable subdermal contraceptive implants. J Fam Plann Reprod Health Care. 2008;34:9–12. doi: 10.1783/147118908783332285. [DOI] [PubMed] [Google Scholar]
- 13.Mansour D, Walling M, Glenn D, et al. Removal of non-palpable etonogestrel implants. J Fam Plann Reprod Health Care. 2008;34:89–91. [PubMed] [Google Scholar]
- 14.Merki-Feld GS, Brekenfeld C, Migge B, Keller PJ. Nonpalpable ultrasonographically not detectable Implanon rods can be localized by magnetic resonance imaging. Contraception. 2001;63:325–8. doi: 10.1016/s0010-7824(01)00209-8. [DOI] [PubMed] [Google Scholar]
- 15.Navani M, Robinson C. Clinical challenge with Implanon removal: a case report. J Fam Plann Reprod Health Care. 2005;31:161–2. doi: 10.1783/1471189053629437. [DOI] [PubMed] [Google Scholar]
- 16.Pymar HC, Creinin MD, Schwartz JL. “Pop-out” method of levonorgestrel implant removal. Contraception. 1999;59:383–7. doi: 10.1016/s0010-7824(99)00048-7. [DOI] [PubMed] [Google Scholar]
- 17.Shulman LP, Gabriel H. Management and localization strategies for the nonpalpable Implanon rod. Contraception. 2006;73:325–30. doi: 10.1016/j.contraception.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 18.Walling M. How to remove impalpable Implanon implants. J Fam Plann Reprod Health Care. 2005;31:320–1. doi: 10.1783/147118905774480770. [DOI] [PubMed] [Google Scholar]
- 19.Gillies R, Scougall P, Nicklin S. Etonogestrel implants - case studies of median nerve injury following removal. Aust Fam Physician. 2011;40:799–800. [PubMed] [Google Scholar]
- 20.Vidin E, Garbin O, Rodriguez B, Favre R, Bettahar-Lebugle K. Removal of etonogestrel contraceptive implants in the operating theater: report on 28 cases. Contraception. 2007;76:35–9. doi: 10.1016/j.contraception.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 21.Belyea C, Ernat J, Gumboc R. Removal of a Contraceptive Implant From the Brachial Neurovascular Sheath. The Journal of Hand Surgery. 2017;42:e115–e7. doi: 10.1016/j.jhsa.2016.10.013. [DOI] [PubMed] [Google Scholar]
- 22.Rowlands S. Legal aspects of contraceptive implants. J Fam Plann Reprod Health Care. 2010;36:243–8. doi: 10.1783/147118910793048485. [DOI] [PubMed] [Google Scholar]
- 23.Searle S, O’Brien P, Rowlands S. Comment on ‘Inserting the etonogestrel contraceptive implant’. J Fam Plann Reprod Health Care. 2016;42:158–9. doi: 10.1136/jfprhc-2016-101448. [DOI] [PubMed] [Google Scholar]
- 24.Sarris I, Gobel F, Gainer M, Vardakas DG, Vogt MT, Sotereanos DG. Medial brachial and antebrachial cutaneous nerve injuries: effect on outcome in revision cubital tunnel surgery. J Reconstr Microsurg. 2002;18:665–70. doi: 10.1055/s-2002-36497. [DOI] [PubMed] [Google Scholar]
- 25.Brown M, Britton J. Neuropathy associated with etonogestrel implant insertion. Contraception. 2012;86:591–3. doi: 10.1016/j.contraception.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 26.Rowlands S. Nerve injuries related to etonogestrel implant. Contraception. 2013;88:431. doi: 10.1016/j.contraception.2012.10.025. [DOI] [PubMed] [Google Scholar]
- 27.Wechselberger G, Wolfram D, Pülzl P, Soelder E, Schoeller T. Nerve injury caused by removal of an implantable hormonal contraceptive. Am J Obstet Gyn. 2006;195:323–6. doi: 10.1016/j.ajog.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 28.Committee Opinion No 672: Clinical Challenges of Long-Acting Reversible Contraceptive Methods. Obstet Gynecol. 2016;128:e69–77. doi: 10.1097/AOG.0000000000001644. [DOI] [PubMed] [Google Scholar]
- 29.Fox IK, Mackinnon SE. Adult peripheral nerve disorders: nerve entrapment, repair, transfer, and brachial plexus disorders. Plast Reconstr Surg. 2011;127:105e–18e. doi: 10.1097/PRS.0b013e31820cf556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gillies R, Scougall P, Nicklin S. Etonogestrel implants Case studies of median nerve injury following removal. Australian Family Physician. 2011;40:799–800. [PubMed] [Google Scholar]
- 31.Ismail H, Mansour D, Singh M. Migration of Implanon. J Fam Plann Reprod Health Care. 2006;32:157–9. doi: 10.1783/147118906777888413. [DOI] [PubMed] [Google Scholar]
- 32.Mansour D. Nexplanon((R)): what Implanon((R)) did next. J Fam Plann Reprod Health Care. 2010;36:187–9. doi: 10.1783/147118910793048629. [DOI] [PubMed] [Google Scholar]
- 33.Susan E, Mackinnon M, Ida K, Fox M, Andrew Yee B, Lorna Kahn P, Huay-Zong Law M, Justin M, Brown M. Peripheral Nerve Surgery: A Resource for Surgeons. St. Louis, MO: 2016. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.