Abstract
Dynamic changes of adipose tissue leukocytes, including adipose tissue macrophage (ATM) and adipose tissue dendritic cells (ATDC) contribute to obesity-induced inflammation and metabolic disease. However, clear discrimination between ATDC and ATM in adipose tissue has limited progress in the field of immunometabolism. In this study, we utilize CD64 to distinguish ATM and ATDC and investigated the temporal and functional changes in these myeloid populations during obesity. Flow cytometry and immunostaining demonstrated that the definition of ATM as F4/80+CD11b+ cells overlaps with other leukocytes and that CD45+CD64+ is specific for ATM. The expression of core DC genes were enriched in CD11c+CD64− cells (ATDC), while core macrophage genes were enriched in CD45+CD64+ cells (ATM). CD11c+CD64− ATDC expressed MHCII and co-stimulatory receptors and had similar capacity to stimulate CD4+ T cell proliferation as ATM. ATDC were predominantly CD11b+ conventional DCs and made up the bulk of CD11c+ cells in adipose tissue with moderate high fat diet exposure. Mixed chimeric experiments with Ccr2−/− mice demonstrated that high-fat diet (HFD) induced ATM accumulation from monocytes was dependent on CCR2; while ATDC accumulation was less CCR2-dependent. ATDC accumulation during obesity was attenuated in Ccr7−/− mice and was associated with decreased adipose tissue inflammation and insulin resistance. CD45+CD64+ ATM and CD45+CD64−CD11c+ ATDC were identified in human obese adipose tissue and ATDC were increased in subcutaneous adipose tissue compared to omental. These results support a revised strategy for unambiguous delineation of ATM and ATDC and suggests that ATDC are independent contributors to adipose tissue inflammation during obesity.
Introduction
Obesity-induced inflammation is a potent contributing factor to the development of type 2 diabetes and metabolic dysfunction associated with insulin resistance. Metabolic inflammation (“metainflammation”) is driven largely by the induction of inflammation in obese adipose tissue and is regulated by a network of adipose tissue leukocytes (1, 2). The inflammatory components in adipose tissue include both innate and adaptive immune cells that are resident in adipose tissue in the lean state and undergo both qualitative and quantitative changes with obesity. Myeloid cells were among the first leukocytes identified as being deranged in obese adipose tissue and in the circulation (3). Obese children as young as three years of age have increased circulating neutrophils indicating an early influence of obesity on myeloid cell regulation (4). Associations between classical monocytes (Ly6chi in mice and CD14+CD16+ in humans), adipose tissue macrophage content, and insulin resistance suggest a mechanistic contribution of myeloid cells to the development of metabolic disease (5, 6). Furthermore, obesity-induced activation of neutrophils and macrophages in adipose tissue are required for insulin resistance in obese mice and correlate with metabolic disease in humans (7, 8).
Adipose tissue macrophages (ATM) are a dominant innate immune cell in adipose tissue and can comprise up to 40% of the non-adipocyte stromal vascular fraction (SVF) in obese adipose tissue (9–11). Murine ATM have been primarily defined as F4/80+CD11b+ and with obesity F4/80+CD11b+CD11c+ ATM accumulate (9, 12). The importance of CD11c+ ATM has been emphasized in numerous studies in mice and humans (6, 12, 13). Obesity induces CD11c expression in macrophages and circulating monocytes to enhance their trafficking into adipose tissue (14). Ablation of CD11c+ cells attenuates adipose tissue inflammation and improves glucose tolerance without changes in body weight (15, 16). CD11c+ ATM have been described as having a metabolically active phenotype with lysosomal activation and characteristics of classically activated M1 macrophages (10, 11). In addition to classical innate immune cytokine production, ATM express high levels of MHC class II and are active antigen presenting cells that shape the expansion of conventional Th1 CD4+ T cells, induction of effector/memory T cells, and the attenuation of regulatory T cells (Tregs) (16–18).
The induction of CD11c+ cells in adipose tissue suggests the possibility that adipose tissue dendritic cells (ATDC) may play a role in obesity-associated inflammation. Adipose tissue CD1c expression correlates with HOMA-IR in clinical studies suggesting an association between ATDC expansion and insulin resistance (19). However, since CD11b and F4/80 expression overlaps between macrophages and DCs, it has been difficulty to clarify the contribution of ATDC to adipose tissue inflammation. In mouse obesity models, ATDC have been defined as CD11b+CD11c+, CD11c+F4/80lo, CD11c+, or CD11c+F4/80neg-lo cells depending on the study (19–24). Based on these definitions, conventional (cDC) and plasmacytoid (pDC; B220+) ATDC have been reported, but how these overlap with the previously defined CD11c+ and CD11c− ATM has not been clarified. Ex vivo, ATDC can induce a Th17 profile in T cells that may explain the associations between Th17 cytokines and insulin resistance (19). Perforin+ ATDC can contribute to the regulation of adipose tissue expansion by controlling adipose tissue T cell clonal expansion (23). GM-CSF dependent DCs contribute to adipose tissue expansion in lean animals (24). CD64− ATDC in perinodal adipose tissue play a role in antigen capture and presentation to lymph nodes, but how this interfaces with metabolic inflammation in obesity is unclear (22).
Almost all studies of murine ATM have utilized F4/80 as the primary marker despite its known expression on non-macrophage leukocytes such as DCs and eosinophils. The Immunologic Genome Consortium identified markers including CD64 and MerTK that improve the specificity of tissue macrophage phenotyping in several tissues including adipose tissue (25). The use of CD64 has been useful in untangling macrophage and DC identity and diversity in the gut (26, 27). Therefore, we used CD64 to revisited the F4/80 ATM phenotyping strategy in hopes of improving our understanding of the dynamics and functions of ATM and ATDC in obesity. We support the use of CD64 as a highly specific ATM marker and that CD64−CD11c+ staining defines a pure population of bona-fide myeloid ATDC present in lean and obese states in mice and humans. We distinguish CD11c+ ATM and CD11c+ ATDC induced by obesity with distinct gene expression profiles and lipid storage capacity. Using competitive reconstitution experiments, CD11c+ ATM accumulation was found to be CCR2 dependent while ATDC were only partially CCR2 dependent. With moderate high fat diet feeding, ATDC are the dominant CD11c+ cell population induced in adipose tissue and Ccr7−/− mice with decreased ATDC content were protected from insulin resistance. This work increases the resolution by which adipose tissue myeloid cells can be identified and suggests that ATDC are an independent contributor to obesity-induced adipose tissue inflammation.
Materials and Methods
Animal studies
CD45.1 and CD45.2 C57BL/6J mice, CC chemokine receptor (CCR) 7−/− (B6.129P2(C)-CCR7tm1Rfor/J), and OT-II (B6.Cg-Tg(TcraTcrb)425Cbn/J) mice were obtained from Jackson Laboratories. Ccr2−/− and Csf2−/− mice were kindly provided by Dr. Beth Moore and Dr. John Osterholzer at the University of Michigan, respectively. Male mice were ad libitum fed a normal diet (ND, 4.5% fat, LabDiet) or fed a high-fat diet (HFD, 60% fat, Research Diets) ad libitum beginning at 6 weeks of age. All animal experiments were approved by the University Committee on Use and Care of Animals at the University of Michigan and conducted in compliance with the Institute of Laboratory Animal Research Guide for the Care and Use of Laboratory Animals.
Bone marrow transplantation
Competitive bone marrow transplants were performed as described (28). Briefly, bone marrow cells were isolated from donor groups (CD45.1+ CCR2+/+ and CD45.2+ CCR2−/−) and mixed in a 1:1 ratio. Six week old CD45.1 mice were lethally irradiated (900 Rad) and intravenously injected six hours after irradiation with 10 × 106 cells of the mixed bone marrow donor cells re-suspended in 150 μl of PBS. Bone marrow reconstitution efficiency was evaluated by examining peripheral blood leukocyte CD45.1+:CD45.2+ ratios at 6 weeks after transplantation, and recipient animals were fed either a ND or HFD for 15 weeks.
Human adipose tissue
Human omental and subcutaneous adipose tissues were collected intraoperatively from patients undergoing bariatric surgery at the University of Michigan and the Ann Arbor VA Hospital. All human use protocols were approved by the University of Michigan and Ann Arbor VA Hospital Institutional Review Boards.
Immunofluorescence microscopy
Immunofluorescence staining was performed as described (29) using the following antibodies: anti-CD64 (X54-5/7.1), anti-F480 (A3-1), anti-Mgl1 (MP23), anti-CD11c (N418), anti-Mac2 (M3/38), and anti-Caveolin (2297). Images were collected using an Olympus Fluoview 100 laser scanning confocal microscope.
SVF isolation and flow cytometry analysis
Stromal vascular fraction cells (SVF) were isolated as described (30). Cells were incubated in Fc Block for 10 min on ice and stained with indicated antibodies for 30 min at 4°C. Antibodies for flow cytometry are provided in Supplementary Table 1. Live/dead fixable violet staining kits (Life Technology) and BODIPY (Life Technology) were used according to the manufacturers’ instruction. Cells were analyzed on a FACSCanto II Flow Cytometer (BD Biosciences) using FlowJo 10.6 software (Treestar). For fluorescence-activated cell sorting (FACS), SVF were suspended in RPMI/2%HI-FBS and purified by FACSAria III (BD Biosceinces). Fluorescence minus one (FMO) controls were used to confirm SiglecF, Ly6G, and CD11c expression in SVF analyses in mice and humans in at least three independent experiments.
Microarray and real-time RT-PCR
Male C57BL/6 mice fed normal diet or HFD for 20 weeks were used for gene expression analysis in macrophages and dendritic cells. Cells were identified as M1 macrophages (CD45+CD11c+CD64+), M2 macrophages (CD45+CD11c−CD64+) or DC (CD45+CD11c+CD64−) and sorted using a BD FACSAria III cell sorter. Microarray analyses were carried out as previously described (31). RNA was extracted using QIAzol (Qiagen) and amplified and hybridized on the Affymetrix Mouse Gene 2.1 ST array. RNA quality was assessed on Agilent Bioanalyzer Picochip. After quality control assessments probe sets with unadjusted p-value of 0.05 or less were identified. Differences in gene expression were identified and analysis was performed through the University of Michigan Microarray Core using affy, limman, and affy PLM packages of bioconductor implemented in the R statistical environment. The data discussed in this publication have been deposited in NCBI’s Gene Expression Omnibus and are accessible through GEO Series accession number GSE85846 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE85846).
For real-time PCR, RNA from tissues and cells was prepared using RNeasy Midi Kits (QIAGEN), and cDNA was generated using high-capacity cDNA reverse transcription kits (Applied Biosystems). Real-time RT-PCR analyses were done in duplicate on the ABI PRISM 7900 Sequence Detector TaqMan System with the SYBR Green PCR kit as instructed by the manufacturer (Applied Biosystems). Primers are shown in Supplementary Table 2. Arbp or 18S was used as a housekeeping gene/internal standard for normalization. Relative expression was determined using standard 2−ΔΔCt method.
CFSE dilution assay
Antigen-specific T cell activation assay was performed as previously described (18). Briefly, FACS-sorted cells were grown in 90-well U bottom plates, then pulsed with 100 μg/ml whole OVA (ThermoScientific). CD4+ T cells were isolated from the spleen of OT-II mice using CD4+ T cell negative selection kites (Miltenyi Biotec). CD4+ T cells were labeled with 2 μmol/L carboxy fluorescein succinimidyl ester (CFSE) and added to antigen-pulsed cells at a 1:1 ratio. After 5 days, T cells were stained for flow cytometry, and CFSE dilution was examined in viable CD3+CD4+ lymphocytes.
Metabolic evaluation
Body weights were measured weekly. Blood glucose and plasma insulin were measured by glucometer and ELISA kit (Crystal Chem), respectively.
Statistical Analysis
Data are expressed as means ± SEM. Statistical differences were assessed using a 2-tailed t test or analysis of variance (with Tukey’s posttest analysis), using GraphPad Prism software. A P value of less than .05 was considered statistically significant.
Results
CD64 distinguishes ATM from eosinophils, neutrophils, and ATDC in lean and obese mice
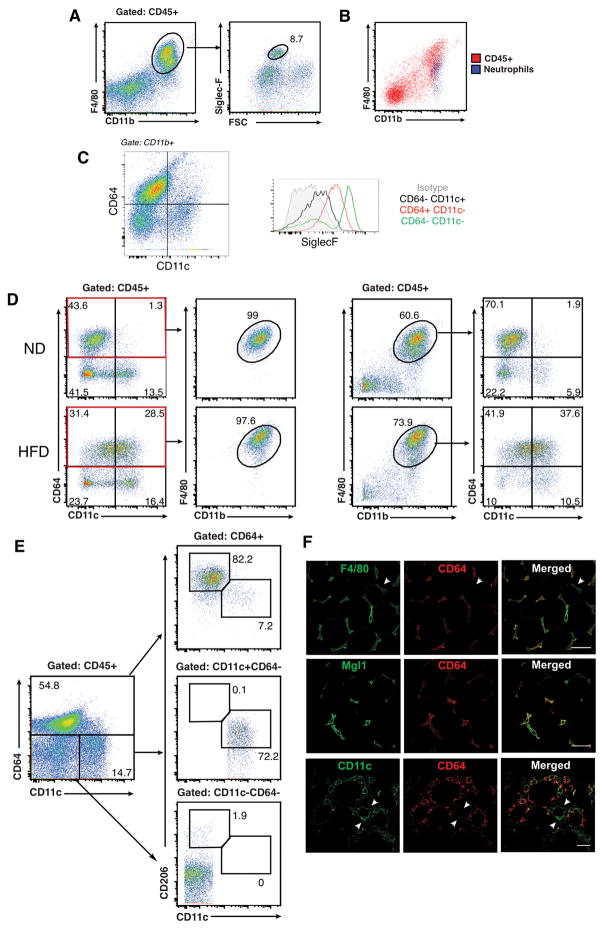
Our group and others have defined ATM as CD45+F4/80+CD11b+ cells in the SVF despite the potential for non-macrophage F4/80+ cell contamination. Co-staining with markers for eosinophils (Siglec-F) and neutrophils (Ly6G) demonstrated that these cells are present within the CD45+F4/80+CD11b+ fraction in both lean and obese mice (Figure 1A–B). We examined the utility of CD64 as a more specific marker of ATM in lean and obese (HFD fed for 10–12 weeks) mice. Adipose tissue eosinophils were CD64− in both lean and obese mice (Figure 1C) with CD64+ macrophages showing intermediate SiglecF expression. Using CD64 and CD11c, lean mice demonstrated prominent populations of CD64+CD11c− (putative ATM) and CD64−CD11c+ cells (putative ATDC) with rare CD64+CD11c+ cells (Figure 1D). In obese mice, there was an increase in CD64+CD11c+ (CD11c+ ATM) while distinct CD64+CD11c− and CD64−CD11c+ cells were still identifiable. All CD45+CD64+ cells expressed CD11b and F4/80, indicating that CD64 is capable of labeling cells that have been previously identified as CD45+F4/80+CD11b+ATM. However, >20% of CD45+F4/80+CD11b+ cells in both lean and obese adipose tissue were CD64− further supporting the presence of non-macrophage F4/80+ cells.
Figure 1. CD64 is a specific adipose tissue macrophage (ATM) marker in lean and obese mice.
Analysis of SVF from eWAT in C57BL/6 mice were fed with ND of HFD for 20 weeks. (A) Flow cytometry analysis of eosinophils (siglec-F+ and high side scatter) on CD11b+F480+ cells after gating CD45+ SVFs from lean mice. (B) Overlap between F480+ CD45+ SVF cells (red) and neutrophils (blue) from obese mice. (C) Analysis of Siglec-F expression in CD64 stratified SVF cells. (D) Comparison of CD64 and F4/80 staining in SVF from ND and HFD fed mice. (E) Flow analysis of CD11c and CD206 expression in CD64+ (top), CD11c+CD64−(middle) and CD11c−CD64− (bottom) SVF cells from lean mice. (F) Immunofluorescence analysis of CD64+ (red) cells in eWAT from HFD-fed mice. Scale bar = 100 μm. Data are representative of at least three independent experiments with three mice per group.
F4/80+CD11b+ ATM in lean mice have high expression of markers for alternatively activated macrophages such as CD206 and CD301/Mgl1 (32). In lean mice, of the CD64+ population, 82% of the were CD206+ with rare CD64−CD11c−CD206+ cells identified (Figure 1E). The ability of CD64 to identify ATM was further confirmed with immunofluorecence microscopy. There was co-localization of F4/80 and CD64 staining in lean mice, however F4/80+ cells were also identified that did not stain for CD64. Colocalization between Mgl1 and CD64 staining was prominent in lean adipose tissue (Figure 1F). In obese mice, where CD11c+ cells accumulate, colocalization between CD11c and CD64 was observed. However, distinct populations of CD11c+ cells were identified that did not stain positive for CD64 in obese adipose tissue. Mac2 and CD64 staining showed significant overlap especially in crown-like structures (CLS) where dense myeloid cell accumulation is known to occur.
Gene expression profiling supports distinguishing ATM and ATDC based on CD64
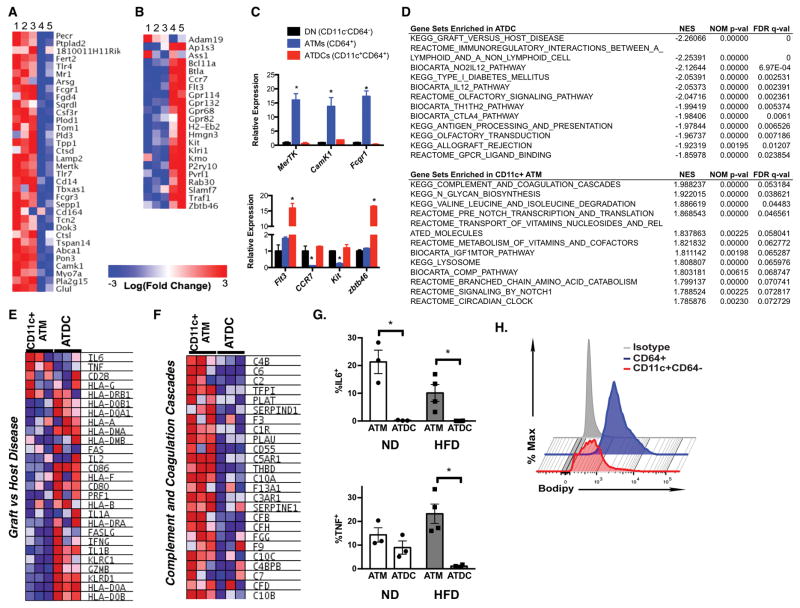
These initial studies suggest that ATM are more stringently defined as CD45+CD64+CD11c+/− cells and may permit the delineation of CD45+CD64−CD11c+ ATDC. To validate this, microarray analysis was performed on RNA from FACS sorted CD45+CD64+CD11c−, CD45+CD64+CD11c+, and CD45+CD64−CD11c+ cells from lean and obese mice. Due to their low numbers, CD64+CD11c+ in lean mice were not included in the analysis. When compared against the ImmGen core set of macrophage specific genes (25), CD64+ cells were significantly enriched for macrophage genes compared to CD64− cells both in lean and obese mice (Figure 2A). In contrast, expression of the core DC cell genes were highly enriched in CD64− cells compared to CD64+ cells in both lean and obese conditions (Figure 2B). Independent samples of FACS sorted CD64+ and CD64− cells from adipose tissue confirmed these differences (Figure 2C). Core macrophages genes such as Mertk and Camk1 were highly expressed in CD64+ cells while DC genes Flt3 and Zbtb46 were enriched in CD64−CD11c+ cells.
Figure 2. Gene expression profiling of ATM and ATDCs.
Microarray analysis heat map of ImmGen (A) macrophage core signature genes and (B) dendritic cell core signature genes from adipose tissue myeloid cells. 1, lean ATM CD11c−CD64+; 2, obese ATM CD11c−CD64+; 3, obese ATM CD11c+CD64+; 4, lean ATDC CD11c+CD64−; 5, obese ATDC CD11c+CD64−. (C) qPCR analysis of macrophage specific genes (upper panel) and DC specific genes (lower panel) in CD11c−CD64−(DN), CD64+(ATM), and CD11c+CD64− (ATDC) cells from eWAT of obese mice. (D) GSEA pathways enriched in ATDC and CD11c+ ATMs. Heat maps shown for differentially expresssed genes for (E) Graft_versus_host disease and (F) Complement_and_Coagulation_Cascade. (G) Intracellular cytokine staining for ATM and ATDCs from ND and HFD fed mice EWAT. (H) Flow cytometry analysis of intracellular lipid content in CD64+ ATM and CD11c+CD64− ATDC from obese mice. Data are from 2 independent experiments with three mice per group. * p<0.05.
Gene Set Enrichment Analysis (GSEA) was used to identify uniquely enriched networks in ATDC and CD11c+ ATMs in obese mice (Figure 2D). The top category for ATDC was enrichment for genes involved in Graft vs Host Disease and interactions between antigen presenting cells and lymphoid cells. Examining this set showed that ATDC are enriched for the expression of cytokines genes such as Il2, Il1a, Ifng, and Il1b, but not for Il6 and Tnfa (Figure 2E–F). We confirmed this by intracellular cytokine staining that showed that higher number of IL-6+ and TNFα+ ATMs compared to ATDC in lean and obese mice (Figure 2G). For CD11c+ ATMs, Complement and Coagulation Cascade genes had the highest enrichment score along with genes involved in Glycan Biosynthesis and Lysosomes. A lipid-laden phenotype with activated lysosomes is associated with obesity and seen in obese animals and human ATM (33, 34). When ATM and ATDC from HFD fed mice were examined for lipid accumulation by BODIPY staining, ATM had significantly higher BODIPY staining compared to ATDC (Figure 2H).
ATDC are predominantly CD11b+ conventional DCs
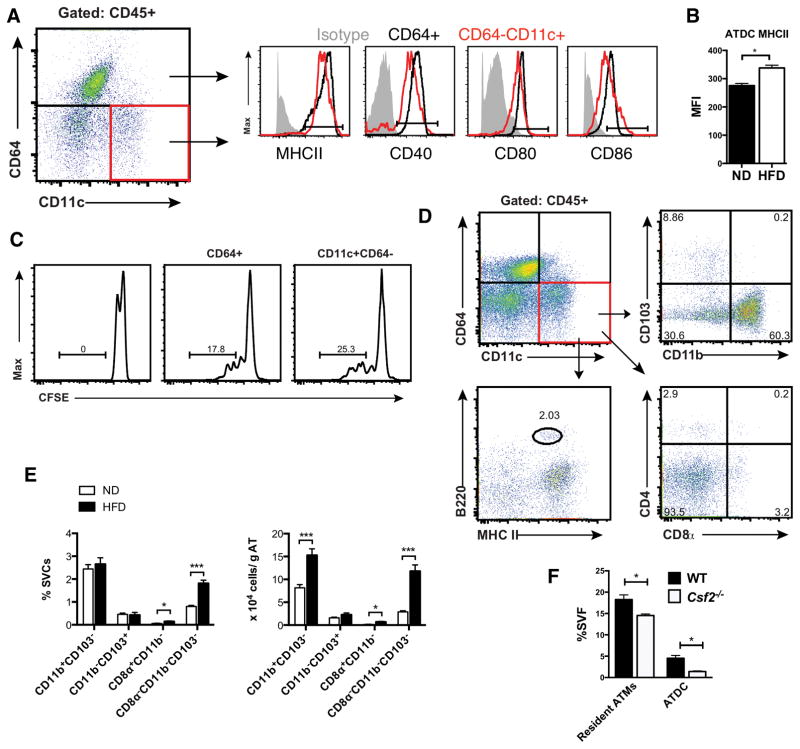
The surface marker profile of CD45+CD64−CD11c+ ATDC was examined to further evaluate the phenotypes of ATDC (Figure 3A). Both ATM and ATDC from lean mice express MHCII, CD40, CD80, and CD86. All of these APC markers were expressed at higher levels in ATM compared to ATDC, which suggests an immature phenotype of ATDC in lean mice. With obesity, MHCII expression ATDC was significantly increased (Figure 3B). We have previously shown that ATM are potent functional APCs. In vitro T cell stimulation assays were performed to compare ATM and ATDC APC function (Figure 3C). CFSE labeled CD4+ T cells from OT-II mice were incubated with ATM and ATDC after loading with OVA (100 μg/ml). Both ATM and ATDC were able to stimulate T cell proliferation to a similar extent.
Figure 3. Myeloid DCs predominate in adipose tissue.
(A) Flow cytometry analysis of MHCII and costimulatory molecules in CD64+ ATM (blue) and CD11c+CD64− ATDC (red) from eWAT. (B) MHCII expression in ATDC in ND and HFD mice. (C) OTII- CD4+ T cell proliferation after incubation with OVA pulsed CD64+ ATM (middle) and CD11c+CD64− ATDC (right). One representative experiment from three independent replicates is shown. (D) ATDC subsets based on CD103, CD11b, CD4, CD8α, B220 and MHCII. (E) Quantitation of ATDC subsets in eWAT from ND and HFD fed C57 mice. (F) Quantitation of ATM and ATDC in lean WT and Csf2−/− mice *, p<0.05; ***, p<0.001.
Additional markers were used to identify ATDC subpopulations (Figure 3D). The majority of ATDC in lean mice were CD11b+ (>60%). Rare populations of CD103+, B220+, CD4+, and CD8+ ATDC were also identified in lean mice suggesting that the majority of ATDC are of the conventional CD11b+ type. In obese mice, CD11b+ myeloid cDCs remained the dominant ATDC type and the quantity of CD11b+ cATDC normalized to adipose tissue weight was increased by two-fold compared to lean mice (Figure 3E). There was a small, but significant increase in CD8a+CD11b− ATDC with HFD as well as a significant increase in ATDC that were negative for CD103, CD8 or CD11b. Consistent with the prominence of myeloid-derived cATDC (CD11b+ DC), ATDC content was decreased in Csf2−/− mice (Figure 3F).
ATDC are dominant CD11c+ population with moderate high fat diet exposure
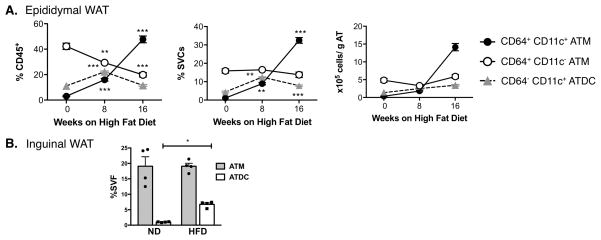
We next performed time course studies to examine the kinetics of accumulation of ATM (CD11c+ and CD11c−) and ATDC in gonadal/epididymal adipose tissue (eWAT) (Figure 4A). Mice were examined after 8 and 16 weeks of HFD feeding. 16 weeks of HFD induced a significant increase in body weight associated with eWAT hypertrophy and the development of fasting hyperglycemia (Data not shown). In lean mice, CD11c− ATM were the dominant myeloid cell population as a percentage of all CD45+ leukocytes and as a percentage of SVF. The majority of CD11c+ cells in lean mice were ATDC and not ATM. With eight weeks of HFD, both CD11c+ ATM and CD11c+ ATDC were increased to numbers that were similar to CD11c− ATM. At this time point, ATDC were still the dominant CD11c+ cell in adipose tissue. 16 weeks of HFD exposure led to a substantial increase in CD11c+ ATM and the maintenance of a prominent population of ATDC. In subcutaneous/inguinal adipose tissue, ATM content was not significantly increased with HFD feeding (Figure 4B). However, there was a significant induction of ATDC in inguinal adipose tissue similar to what is seen in eWAT.
Figure 4. Time course of ATM and ATDC accumulation in adipose tissue with HFD induced obesity.
C57BL/6 male mice were fed high-fat diet (HFD) for various time periods (0, 8, and 16 weeks). (A) eWAT SVFs quantified for CD11c+ ATM, CD11c− ATM and ATDC by flow cytometry normalized for adipose tissue mass. (B) Quantitation of ATM and ATDC in inguinal WAT (iWAT) at 16 weeks of HFD. **, p<0.05; ***, p<0.001.
Differential dependence of CD11c+ ATM and ATDC on CCR2 during diet-induced obesity
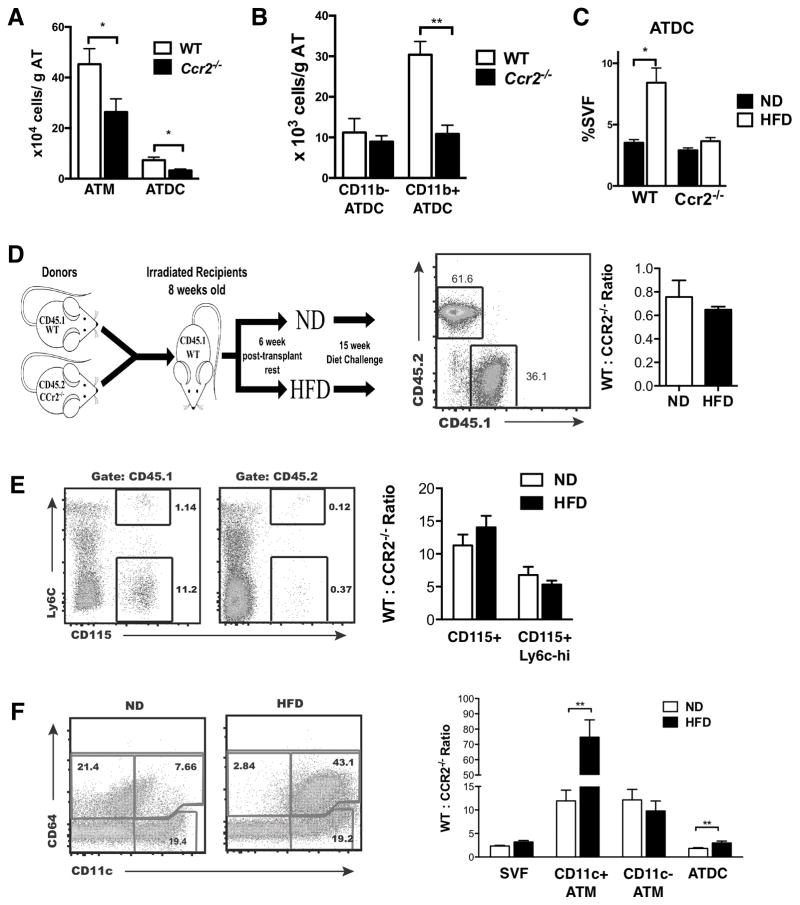
Monocyte recruitment has been shown to be a primary mechanism by which CD11c+ ATM accumulate in adipose tissue in mice (32, 35). Since our results suggest that the prior definition of F4/80+CD11b+CD11c+ ATM is contaminated with ATDC and other leukocytes, we revisited ATM and ATDC accumulation in Ccr2−/− mice using CD64. Ccr2−/− mice had significantly reduced ATM and ATDC compared to WT (Figure 5A). Specifically, CD11b+ ATDC were decreased in Ccr2−/− mice, but there were no differences in CD11b− ATDC (Figure 5B). With 2 weeks of HFD, we observed an increase in ATDC in WT, but not in Ccr2−/− mice indicating that CCR2 is required for ATDC accumulation with short term HFD exposure (Figure 5C).
Figure 5. CCR2 is required for obesity-induced CD11c+ ATM and ATDC migration into adipose tissue.
(A) Quantitation of CD11c+ ATM, CD11c− ATM and ATDC in eWAT from WT and Ccr2−/− mice. (B) Quantitation of CD11b+ and CD11b− ATDC subsets in eWAT from WT and Ccr2−/− mice. (C) ATM and ATDC content in WT and Ccr2−/− mice after 2 weeks of ND or HFD feeding. (D) Diagram of mixed chimera experiment design. Ccr2−/− (CD45.2) and WT (CD45.1) BM mixed in a 1:1 ratio prior to injection in irradiated recipients. Chimerism analysis of blood leukocytes shown. (E) Frequency and ratio of CD45.1 (WT) and CD45.2 (Ccr2−/−) in blood monocytes from lean and obese (15 week HFD) chimeric mice. (F) Frequency and CD45.1: CD45.2 ratio in CD11c+ ATM, CD11c− ATM and ATDC in eWAT. **, p<0.01 vs ND.
Since Ccr2−/− mice have fewer circulating monocytes (35), it is difficult to discern whether ATM and ATDC accumulation relies on Ccr2 dependent signals for monocyte trafficking or is dependent on the size of the monocyte pool. To evaluate this, we used a mixed chimera model where lethally irradiated recipients were reconstituted with a 1:1 mixture of bone marrow cells from WT (CD45.1) and Ccr2−/− (CD45.2) donor mice (Figure 5D). After reconstitution, the chimeras were fed ND or HFD for 16 weeks. Blood chimerism analysis was performed to evaluate the ratio of WT:Ccr2−/− cells (CD45.1/CD45.2) relative to the BM input ratio of 1. Analysis of total peripheral blood leukocytes demonstrated a slight bias towards reconstitution with cells from Ccr2−/− donors with the ratio of WT:Ccr2−/− cells (CD45.1/CD45.2) less than 1 in both ND and HFD conditions. However, analysis of circulating CD115+ monocytes demonstrated a significant increase in monocytes derived from WT compared to Ccr2−/− mice with a WT:Ccr2−/− ratio of 11.3 ± 1.6 (Figure 5E). Circulating WT derived cells dominated over Ccr2−/− cells in both Ly-6chi and Ly-6clo monocyte subsets. This is consistent with the requirement of CCR2 to regulate monocyte exit from the bone marrow compartment into the circulation.
ATM in eWAT were examined in the chimeras (Figure 5F). As expected CD64+ ATM from ND fed mice were primarily CD11c− while HFD induced CD11c+ ATM accumulation. In lean mice, the WT:Ccr2−/− ratio in both CD11c− and CD11c+ ATM was elevated (12.2 ± 2.2 and 11.9 ± 2.2, respectively) and was similar to what was observed in blood monocytes. This suggests that in a competitive reconstitution model both ATM subsets in lean mice may be dependent on circulating monocyte for reconstitution. In contrast, the WT:Ccr2−/− ratio of ATDC in lean mice was significantly lower (1.8 ± 0.16) than what was observed in either ATM or circulating monocytes. In obese mice, the WT:Ccr2−/− ratio of CD11c+ ATM was markedly increased (74.7 ± 11.7) compared to lean mice. This demonstrates that, in the obese environment, WT monocytes had a significant competitive advantage in trafficking to adipose tissue from the circulation compared to Ccr2−/− monocytes. In contrast, there was no change in the WT:Ccr2−/− ratio of CD11c− ATM suggesting that any accumulation of CD11c− ATM during obesity is CCR2 independent. While the WT:Ccr2−/− ratio of ATDC was lower than that of monocytes in lean mice, there was a statistically significant increase in the WT:Ccr2−/− ratio in ATDC (3.0 ± 0.4) in obese mice suggesting some ATDC are dependent on CCR2 for trafficking to adipose tissue with obesity and consistent with analysis of Ccr2−/− mice. However, the CCR2-dependent induced of ATDC during obesity was less efficient than that of CD11c+ ATM (~1.6 fold in ATDC vs ~6.3 fold in CD11c+ ATM). Overall this suggests that in chronic obesity, CCR2 signaling plays a critical role in regulating monocyte trafficking into obese adipose tissue to generate CD11c+ ATM and ATDC.
CCR7 is required for the HFD-induced ATDC accumulation and insulin resistance
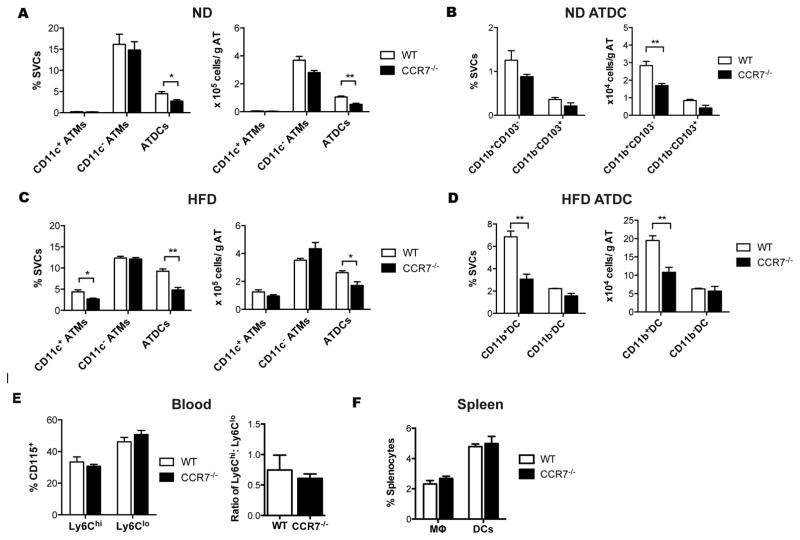
The microarray analysis identified Ccr7 as differentially expressed in ATDC compared to ATM. To evaluate whether CCR7 plays a role in the recruitment or maintenance of ATDC in adipose tissue, age-matched male WT and Ccr7−/− mice were fed ND or HFD ad libitum for 8 weeks. This feeding duration was chosen based on our observation that 8 weeks led to increased ATDC but minimal accumulation of CD11c+ ATM. In ND-fed mice, the quantity of CD11c+ and CD11c− ATM did not differ between genotypes. However, Ccr7−/− mice had significantly fewer ATDC compared to WT mice primarily due to a decrease in CD11b+ ATDC (Figure 6A–B). Eight-week HFD challenge induced CD11c+ ATM and ATDC accumulation in both genotypes, but HFD-induced ATDC accumulation was significantly impaired in Ccr7−/− mice when normalized to adipose tissue weight (Figure 6C). CD11b+ ATDC in Ccr7−/− adipose tissue were ~50% lower than WT adipose tissue while the quantity of CD11b− ATDC was similar between genotypes (Figure 6D). After 8-weeks of HFD, Ccr7−/− mice had normal numbers of circulating monocytes and similar levels of Ly-6Chi and Ly6Clo monocytes compared to WT (Figure 6E). Importantly, there was no difference in the accumulation of splenic macrophages and DCs between genotypes (Figure 6F). These data suggest that HFD induces ATDC accumulation via CCR7 dependent increases in CD11b+ ATDC.
Figure 6. CCR7 is required for ATDC accumulation during diet-induced obesity.
(A) Quantitation of ATM and ATDC in eWAT from chow fed WT and Ccr7−/− mice. (B) Quantitation of CD11b+CD103− and CD11b− CD103+ ATDC subsets in eWAT from WT and Ccr7−/− mice. (C) Quantitation of CD11c+ ATM, CD11c− ATM and ATDC in eWAT from WT and Ccr7−/− mice fed HFD for 8 weeks. (D) Quantitation of CD11b+ and CD11b− ATDC subsets in eWAT from WT and Ccr7−/− HFD fed mice. (E) Frequency of Ly6hi and Ly6lo blood monocytes from WT and Ccr7−/− mice. (F) Frequency of macrophages and dendritic cells in spleen from WT and Ccr7−/−. *, p<0.05; **,p<0.01.
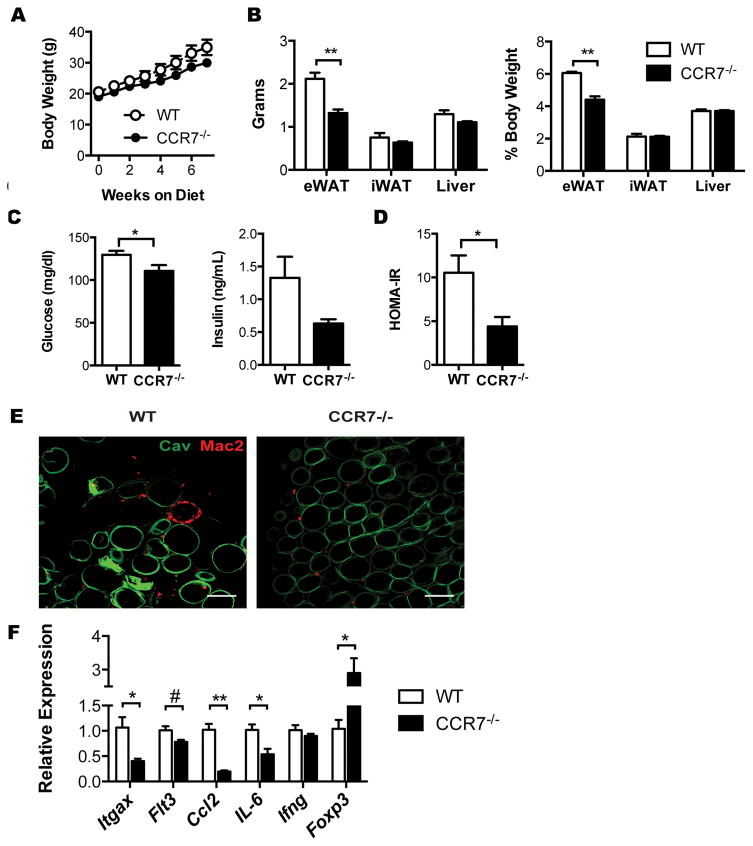
We next examined the impact of Ccr7 deficiency on HFD-induced insulin resistance and adipose tissue inflammation. Total body weight between the genotypes was not significantly different on HFD (Figure 7A). Ccr7−/− mice had lower eWAT weights (Figure 7B). Inguinal adipose tissue (iWAT) and liver weight were similar between genotypes. Compared to WT mice, Ccr7−/− mice had lower fasting glucose levels and fasting insulin levels resulting in an improvement in insulin sensitivity in Ccr7−/− mice based on the calculation of HOMA-IR (Figure 7C–D). Whole mount imaging of eWAT showed a significant accumulation of Mac2+ in CLS in WT eWAT, while CLSs were largely absent in Ccr7−/− eWAT (Figure 7E). Consistent with the flow cytometry data, gene expression of Itgax and Flt3 were decreased in Ccr7−/− eWAT. In addition, Ccl2 and IL-6 expression were decreased and Foxp3 expression was increased in Ccr7−/− eWAT (Figure 7F). Obese Ccr7−/− mice had an increase in adipose tissue CD4+ and CD8+ cells suggesting that a lack of T cells does not explain metabolic protection in these mice (data not shown). Overall, these data suggest that CCR7-dependent signals contribute to insulin resistance and adipose tissue inflammation by regulating ATDC content independent of ATM.
Figure 7. CCR7-deficient mice are protected from HFD-induced insulin resistance and adipose tissue inflammation.
(A) Body weights of WT and Ccr7−/− mice during 8 weeks HFD feeding. (B) Organ weights at the end of HFD exposure. (C) Fasting blood glucose and plasma insulin levels. (D) HOMA-IR in WT and Ccr7−/−mice fed and HFD for 8 weeks. (E) Immunofluorescence imaging of Mac2+ ATM (red) and caveolin+ adipocytes (green) in eWAT. Scale bar = 100μm. (F) Expression of inflammatory genes in eWAT from HFD-fed WT and Ccr7−/− mice. *, p<0.05; **,p<0.01; #=0.06.
CD64− ATDC are enriched in subcutaneous adipose tissue from obese humans
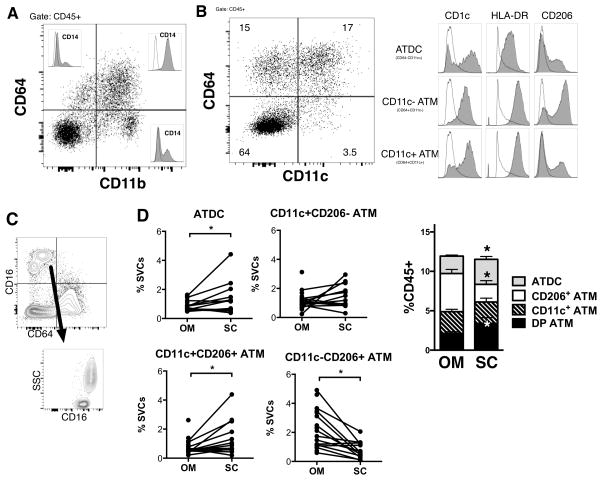
A range of markers has been used to quantify human ATM in published studies and many rely on CD14 as an ATM marker despite the demonstration of CD14+ DCs (36). We therefore wanted to evaluate the utility of CD64 in delineating ATM from ATDC in human samples. Multi-color flow cytometry was performed on SVF cells from subcutaneous (SC) and omental/visceral adipose tissue (OM) from obese subjects undergoing bariatric surgery. Using CD64, a major population of CD45+CD11b+CD64+ cells was identified with minor populations of CD45+CD11b+CD64− and CD45+CD11b−CD64+ cells (Figure 8A). CD45+CD11b+CD64+ stained uniformly positive for CD14+. However, a minor population of CD14+ cells was observed in the CD45+CD11b+CD64− and CD45+CD11b−CD64+ populations. Using CD64 and CD11c, we identified CD64+CD11c+, CD64+CD11c− and CD64−CD11c+ myeloid leukocyte populations (Figure 8B). All 3 populations expressed CD1c and HLA-DR. However, CD45+CD64−CD11c+ cells had the highest CD1c expression and moderate HLA-DR expression compared to the CD45+CD64+ population populations. CD206 was primarily expressed on CD64+CD11c− cells consistent with mouse studies suggesting this is the phenotype of resident ATM. CD64+ cells were CD16− in human adipose tissue and CD64−CD16+ cells were a mix of monocytes and neutrophils based on side scatter (Figure 8C). Overall this suggests that human ATDC can be defined as CD64−CD11c+ as in mice.
Figure 8. CD64− CD11c+ ATDC are enriched in subcutaneous adipose tissue in obese humans.
(A) SVFs were isolated from human omental (OM) and subcutaneous (SC) adipose tissue and analyzed by flow cytometry. Analysis of CD64 expressing cells and overlap with CD14 in OM. A representative plot of three independent experiments is shown. (B) Marker expression of CD64− CD11c+ ATDC, CD64+ CD11c− ATM and CD64+ CD11c+ ATM in OM. (C) Contour plot showing CD16 and CD64. (D) Frequency of ATDC, CD11c+CD206− ATM, CD11c+CD206+ ATM and CD11c−CD206+ ATM in paired OM and SC adipose tissue from obese patients. (n=10~14). *, p<0.05 vs OM
We next examined the proportion of ATDC (CD64−) and ATM (CD64+) subsets in paired samples from SC and OM depots (Figure 8D). CD11c+CD206+ ATM were enriched in SC compared to OM adipose tissue, while CD11c−CD206+ ATM were lower in SC. The frequency of ATDC was higher in SC compared to OM. Collectively, these results indicate that ATDC are enriched in SC adipose tissue in obese humans.
Discussion
There is considerable interest in the contribution of myeloid cells including neutrophils, monocytes, macrophages and dendritic cells to obesity-induced inflammation and metabolic disease pathogenesis. Since the adipose tissue leukocyte network is unique, the goal of this study was to clearly and definitively delineate ATM from ATDC and examine their regulation with obesity. By using CD64 as a specific macrophage marker, our primary findings suggest that defining ATM as F4/80+CD11b+ leukocytes does not fully exclude other leukocyte types. More importantly, the F4/80+CD11b+CD11c+ cell subset can be contaminated with a distinct ATDC population which comprises a significant population of the total myeloid network in adipose tissue. ATDC are primarily myeloid (CD11b+) cDCs and accumulate with obesity. However, ATDC accumulation appears to be regulated by different chemotactic cues compared to ATM. In particular ATDCs are CCR7-dependent and Ccr7−/− mice with lower ATDC content are protected from obesity-induced inflammation and insulin resistance.
ATM have been defined almost exclusively using the markers F4/80+CD11b+ by our group and others (8, 34, 37). Our experiments suggest that some studies may need to be re-evaluated and that changes in the F4/80+CD11b+CD11c+ ATM may be accounted for by changes in ATDC quantity. In addition, our experiments suggest that the use of CD11c as a single marker to define ATDC, which has been utilized by some groups, is inadequate given the clear identification of CD11c+ ATM particularly in obese mice (21). Care must also be taken with studies that utilize LysM-Cre recombinase as this may also target ATDC (38). Our studies agree with the results of the ImmGen Consortium supporting the specificity of CD64 as a marker of tissue macrophages (22, 25). In addition, we have extended their results to the study of obese mouse models and obese human adipose tissue and support the specificity of CD64 as a marker of ATM in both contexts.
Our observation that ATDC increase with early obesity and are the dominant CD11c+ cells in adipose tissue with moderate HFD may be important to the control of adaptive immunity. Eight weeks of HFD correlates with alterations in adaptive immunity including changes in CD8 and CD4 adipose tissue T cell populations (39–41). Ccr7−/− mice with fewer ATDCs showed metabolic improvement at eight weeks of HFD suggesting that ATDC signals may be important at the early stages of obesity. Lack of CD4 or CD8 T cells in adipose tissue did not explain this observation, although we cannot rule out other factors such as microbiome differences that may explain these differences. This suggests that ATDC and ATM independently contribute to directing adaptive immune responses induced by obesity since both populations are potent APC (15, 16). Ablation of CD11c+ cells improves glucose intolerance partially by deactivating conventional T cells in adipose tissue (16). Whether this is due to alterations in ATM, ATDC or both will have to be examined in future studies. We have previously shown that ATM are required for T cell activation using LysM-Cre mice, but the results from this study indicates that we can’t eliminate the possibility of ATDC contribution for this effect.
Our observation of protection from insulin resistance in Ccr7−/− mice agrees with recent reports and identifies loss of the ATDC population as a primary mechanism for this effect (42). CCR7 was identified in a module of signal transduction genes that were downregulated with weight loss after bariatric surgery that was disconnected from T cell signaling genes (43). The functional profile of ATDC in the setting of obesity is strikingly different than that of CD11c+ ATM. Unlike ATM, lysosomal and lipid storage are not induced in ATDC with obesity. Instead, pathways related to antigen presentation and cytokine signaling remain elevated in ATDC suggesting that they have an independent contribution to the adipose tissue immune activation environment.
The protective effect of Ccr2 deficiency in adipose tissue inflammation has been supported by several studies (35, 44, 45). However, separating the defects in circulating monocytes in Ccr2−/− mice from the defects in migration into peripheral tissues in this model is a challenge (46). Our competitive bone marrow experiments demonstrate that Ccr2+/+ monocytes have an advantage for recruitment from the circulation into adipose tissue compared to Ccr2−/− monocytes independent of effects on the quantity of circulating monocytes. This supports the regulation of CD11c+ ATM by CCR2-dependent mechanisms and suggests that some cATDC are recruited to adipose tissue by CCR2-dependent mechanisms. However, the CCR2-dependency was much lower for ATDC recruitment, compared to ATM, and suggests that ATDC are not likely monocyte derived. Thus ATDCs may not be monocyte derived, and may instead be dependent on pre-DC populations from the circulation.
Overall, our studies clarify the somewhat confusing literature on CD11c expression in adipose tissue myeloid cells. We propose that there are three primary myeloid cell populations in adipose tissue: resident CD45+CD64+CD11c− ATM, recruited CD45+CD64+CD11c+ ATM, and CD45+CD64−CD11c+ ATDC that are predominantly CD11b+ cATDC. Importantly, these observations extend to human adipose tissue and help inform future studies. ATDCs are not a minor population and can be as numerous as ATM subsets depending on the depot. The elevated levels of ATDC in human SC adipose tissue may explain the lower inflammatory capacity of this depot. Whether or not SC ATDC are similar in function as OM ATDC will be examined in future studies. An additional limitation is the lack of samples from lean individuals for comparison that is the topic of ongoing studies in our group. Clarifying the landscape of functional antigen presenting cells in adipose tissue may also advance our understanding of the mechanisms by which regulatory and conventional adaptive immunity is controlled in adipose tissue where resident T cells appear to have unique properties compared to other non-lymphoid tissues (17).
Supplementary Material
References
- 1.Mathis D. Immunological goings-on in visceral adipose tissue. Cell Metab. 2013;17:851–859. doi: 10.1016/j.cmet.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest. 2011;121:2111–2117. doi: 10.1172/JCI57132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lumeng CN. Innate immune activation in obesity. Mol Aspects Med. 2013;34:12–29. doi: 10.1016/j.mam.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Skinner AC, Steiner MJ, Henderson FW, Perrin EM. Multiple markers of inflammation and weight status: cross-sectional analyses throughout childhood. Pediatrics. 2010;125:e801–809. doi: 10.1542/peds.2009-2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poitou C, Dalmas E, Renovato M, Benhamo V, Hajduch F, Abdennour M, Kahn JF, Veyrie N, Rizkalla S, Fridman WH, Sautes-Fridman C, Clement K, Cremer I. CD14dimCD16+ and CD14+CD16+ monocytes in obesity and during weight loss: relationships with fat mass and subclinical atherosclerosis. Arterioscler Thromb Vac Biol. 2011;31:2322–2330. doi: 10.1161/ATVBAHA.111.230979. [DOI] [PubMed] [Google Scholar]
- 6.Wentworth JM, Naselli G, Brown WA, Doyle L, Phipson B, Smyth GK, Wabitsch M, O’Brien PE, Harrison LC. Pro-inflammatory CD11c+CD206+ adipose tissue macrophages are associated with insulin resistance in human obesity. Diabetes. 2010;59:1648–1656. doi: 10.2337/db09-0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Talukdar S, Oh da Y, Bandyopadhyay G, Li D, Xu J, McNelis J, Lu M, Li P, Yan Q, Zhu Y, Ofrecio J, Lin M, Brenner MB, Olefsky JM. Neutrophils mediate insulin resistance in mice fed a high-fat diet through secreted elastase. Nat Med. 2012;18:1407–1412. doi: 10.1038/nm.2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lumeng CN, DelProposto JB, Westcott DJ, Saltiel AR. Phenotypic switching of adipose tissue macrophages with obesity is generated by spatiotemporal differences in macrophage subtypes. Diabetes. 2008;57:3239–3246. doi: 10.2337/db08-0872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu X, Grijalva A, Skowronski A, van Eijk M, Serlie MJ, Ferrante AW., Jr Obesity activates a program of lysosomal-dependent lipid metabolism in adipose tissue macrophages independently of classic activation. Cell Metab. 2013;18:816–830. doi: 10.1016/j.cmet.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kratz M, Coats BR, Hisert KB, Hagman D, Mutskov V, Peris E, Schoenfelt KQ, Kuzma JN, Larson I, Billing PS, Landerholm RW, Crouthamel M, Gozal D, Hwang S, Singh PK, Becker L. Metabolic dysfunction drives a mechanistically distinct proinflammatory phenotype in adipose tissue macrophages. Cell Metab. 2014;20:614–625. doi: 10.1016/j.cmet.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu H, Perrard XD, Wang Q, Perrard JL, Polsani VR, Jones PH, Smith CW, Ballantyne CM. CD11c expression in adipose tissue and blood and its role in diet-induced obesity. Atertio Thromb Vasc Biol. 2010;30:186–192. doi: 10.1161/ATVBAHA.109.198044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tao T, Li S, Zhao A, Zhang Y, Liu W. Expression of the CD11c gene in subcutaneous adipose tissue is associated with cytokine level and insulin resistance in women with polycystic ovary syndrome. Eur J Endocrinol. 2012;167:705–713. doi: 10.1530/EJE-12-0340. [DOI] [PubMed] [Google Scholar]
- 14.Wu H, Gower RM, Wang H, Perrard XY, Ma R, Bullard DC, Burns AR, Paul A, Smith CW, Simon SI, Ballantyne CM. Functional role of CD11c+ monocytes in atherogenesis associated with hypercholesterolemia. Circulation. 2009;119:2708–2717. doi: 10.1161/CIRCULATIONAHA.108.823740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patsouris D, Li PP, Thapar D, Chapman J, Olefsky JM, Neels JG. Ablation of CD11c-positive cells normalizes insulin sensitivity in obese insulin resistant animals. Cell Metab. 2008;8:301–309. doi: 10.1016/j.cmet.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cho KW, Morris DL, DelProposto JL, Geletka L, Zamarron B, Martinez-Santibanez G, Meyer KA, Singer K, O’Rourke RW, Lumeng CN. An MHC II-dependent activation loop between adipose tissue macrophages and CD4+ T cells controls obesity-induced inflammation. Cell Reports. 2014;9:605–617. doi: 10.1016/j.celrep.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kolodin D, van Panhuys N, Li C, Magnuson AM, Cipolletta D, Miller CM, Wagers A, Germain RN, Benoist C, Mathis D. Antigen- and cytokine-driven accumulation of regulatory T cells in visceral adipose tissue of lean mice. Cell Metab. 2015;21:543–557. doi: 10.1016/j.cmet.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morris DL, Cho KW, Delproposto JL, Oatmen KE, Geletka LM, Martinez-Santibanez G, Singer K, Lumeng CN. Adipose tissue macrophages function as antigen-presenting cells and regulate adipose tissue CD4+ T cells in mice. Diabetes. 2013;62:2762–2772. doi: 10.2337/db12-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bertola A, Ciucci T, Rousseau D, Bourlier V, Duffaut C, Bonnafous S, Blin-Wakkach C, Anty R, Iannelli A, Gugenheim J, Tran A, Bouloumie A, Gual P, Wakkach A. Identification of adipose tissue dendritic cells correlated with obesity-associated insulin-resistance and inducing Th17 responses in mice and patients. Diabetes. 2012;61:2238–2247. doi: 10.2337/db11-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stefanovic-Racic M, Yang X, Turner MS, Mantell BS, Stolz DB, Sumpter TL, Sipula IJ, Dedousis N, Scott DK, Morel PA, Thomson AW, O’Doherty RM. Dendritic cells promote macrophage infiltration and comprise a substantial proportion of obesity-associated increases in CD11c+ cells in adipose tissue and liver. Diabetes. 2012;61:2330–2339. doi: 10.2337/db11-1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen Y, Tian J, Tian X, Tang X, Rui K, Tong J, Lu L, Xu H, Wang S. Adipose tissue dendritic cells enhances inflammation by prompting the generation of Th17 cells. PLoS One. 2014;9:e92450. doi: 10.1371/journal.pone.0092450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuan EL, Ivanov S, Bridenbaugh EA, Victora G, Wang W, Childs EW, Platt AM, Jakubzick CV, Mason RJ, Gashev AA, Nussenzweig M, Swartz MA, Dustin ML, Zawieja DC, Randolph GJ. Collecting lymphatic vessel permeability facilitates adipose tissue inflammation and distribution of antigen to lymph node-homing adipose tissue dendritic cells. J Immunol. 2015;194:5200–5210. doi: 10.4049/jimmunol.1500221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zlotnikov-Klionsky Y, Nathansohn-Levi B, Shezen E, Rosen C, Kagan S, Bar-On L, Jung S, Shifrut E, Reich-Zeliger S, Friedman N, Aharoni R, Arnon R, Yifa O, Aronovich A, Reisner Y. Perforin-Positive Dendritic Cells Exhibit an Immuno-regulatory Role in Metabolic Syndrome and Autoimmunity. Immunity. 2015;43:776–787. doi: 10.1016/j.immuni.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 24.Pamir N, Liu NC, Irwin A, Becker L, Peng Y, Ronsein GE, Bornfeldt KE, Duffield JS, Heinecke JW. Granulocyte/Macrophage Colony-stimulating Factor-dependent Dendritic Cells Restrain Lean Adipose Tissue Expansion. J Biol Chem. 2015;290:14656–14667. doi: 10.1074/jbc.M115.645820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gautier EL, Shay T, Miller J, Greter M, Jakubzick C, Ivanov S, Helft J, Chow A, Elpek KG, Gordonov S, Mazloom AR, Ma’ayan A, Chua WJ, Hansen TH, Turley SJ, Merad M, Randolph GJ. Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat Immunol. 2012;13:1118–1128. doi: 10.1038/ni.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tamoutounour S, Henri S, Lelouard H, de Bovis B, de Haar C, van der Woude CJ, Woltman AM, Reyal Y, Bonnet D, Sichien D, Bain CC, Mowat AM, Reis e Sousa C, Poulin LF, Malissen B, Guilliams M. CD64 distinguishes macrophages from dendritic cells in the gut and reveals the Th1-inducing role of mesenteric lymph node macrophages during colitis. Eur J Immunol. 2012;42:3150–3166. doi: 10.1002/eji.201242847. [DOI] [PubMed] [Google Scholar]
- 27.De Calisto J, Villablanca EJ, Mora JR. FcgammaRI (CD64): an identity card for intestinal macrophages. Eur J Immunol. 2012;42:3136–3140. doi: 10.1002/eji.201243061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singer K, DelProposto J, Morris DL, Zamarron B, Mergian T, Maley N, Cho KW, Geletka L, Subbaiah P, Muir L, Martinez-Santibanez G, Lumeng CN. Diet-induced obesity promotes myelopoiesis in hematopoietic stem cells. Molecular metabolism. 2014;3:664–675. doi: 10.1016/j.molmet.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martinez-Santibanez G, Cho KW, Lumeng CN. Imaging white adipose tissue with confocal microscopy. Methods Enzymol. 2014;537:17–30. doi: 10.1016/B978-0-12-411619-1.00002-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cho KW, Morris DL, Lumeng CN. Flow cytometry analyses of adipose tissue macrophages. Methods Enzymol. 2014;537:297–314. doi: 10.1016/B978-0-12-411619-1.00016-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lumeng CN, Deyoung SM, Bodzin JL, Saltiel AR. Increased inflammatory properties of adipose tissue macrophages recruited during diet-induced obesity. Diabetes. 2007;56:16–23. doi: 10.2337/db06-1076. [DOI] [PubMed] [Google Scholar]
- 32.Westcott DJ, Delproposto JB, Geletka LM, Wang T, Singer K, Saltiel AR, Lumeng CN. MGL1 promotes adipose tissue inflammation and insulin resistance by regulating 7/4hi monocytes in obesity. J Exp Med. 2009;206:3143–3156. doi: 10.1084/jem.20091333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shapiro H, Pecht T, Shaco-Levy R, Harman-Boehm I, Kirshtein B, Kuperman Y, Chen A, Bluher M, Shai I, Rudich A. Adipose tissue foam cells are present in human obesity. J Clin Endocrinol Metab. 2013;98:1173–1181. doi: 10.1210/jc.2012-2745. [DOI] [PubMed] [Google Scholar]
- 34.Kosteli A, Sugaru E, Haemmerle G, Martin JF, Lei J, Zechner R, Ferrante AW., Jr Weight loss and lipolysis promote a dynamic immune response in murine adipose tissue. J Clin Invest. 2010;120:3466–3479. doi: 10.1172/JCI42845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weisberg SP, Hunter D, Huber R, Lemieux J, Slaymaker S, Vaddi K, Charo I, Leibel RL, Ferrante AW. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest. 2005;116:115–124. doi: 10.1172/JCI24335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Collin M, McGovern N, Haniffa M. Human dendritic cell subsets. Immunology. 2013;140:22–30. doi: 10.1111/imm.12117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Orr JS, Puglisi MJ, Ellacott KL, Lumeng CN, Wasserman DH, Hasty AH. Toll-like receptor 4 deficiency promotes the alternative activation of adipose tissue macrophages. Diabetes. 2012;61:2718–2727. doi: 10.2337/db11-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jakubzick C, Bogunovic M, Bonito AJ, Kuan EL, Merad M, Randolph GJ. Lymph-migrating, tissue-derived dendritic cells are minor constituents within steady-state lymph nodes. J Exp Med. 2008;205:2839–2850. doi: 10.1084/jem.20081430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Winer S, Chan Y, Paltser G, Truong D, Tsui H, Bahrami J, Dorfman R, Wang Y, Zielenski J, Mastronardi F, Maezawa Y, Drucker D, Endleman E, Winer D, Dosch HM. Normalization of Obesity-Associated Insulin Resistance through Immunotherapy: CD4+ T Cells Control Glucose Homeostasis. Nat Med. 2009;15:921–929. doi: 10.1038/nm.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feuerer M, Herrero L, Cipolletta D, Naaz A, Wong J, Nayer A, Lee J, Goldfine AB, Benoist C, Shoelson S, Mathis D. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med. 2009;15:930–939. doi: 10.1038/nm.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, Otsu M, Hara K, Ueki K, Sugiura S, Yoshimura K, Kadowaki T, Nagai R. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15:914–920. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- 42.Sano T, Iwashita M, Nagayasu S, Yamashita A, Shinjo T, Hashikata A, Asano T, Kushiyama A, Ishimaru N, Takahama Y, Nishimura F. Protection from diet-induced obesity and insulin resistance in mice lacking CCL19-CCR7 signaling. Obesity (Silver Spring) 2015;23:1460–1471. doi: 10.1002/oby.21127. [DOI] [PubMed] [Google Scholar]
- 43.Poitou C, Perret C, Mathieu F, Truong V, Blum Y, Durand H, Alili R, Chelghoum N, Pelloux V, Aron-Wisnewsky J, Torcivia A, Bouillot JL, Parks BW, Ninio E, Clement K, Tiret L. Bariatric Surgery Induces Disruption in Inflammatory Signaling Pathways Mediated by Immune Cells in Adipose Tissue: A RNA-Seq Study. PLoS One. 2015;10:e0125718. doi: 10.1371/journal.pone.0125718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kang YS, Cha JJ, Hyun YY, Cha DR. Novel C-C chemokine receptor 2 antagonists in metabolic disease: a review of recent developments. Expert Opin Investig Drugs. 2011;20:745–756. doi: 10.1517/13543784.2011.575359. [DOI] [PubMed] [Google Scholar]
- 45.Obstfeld AE, Sugaru E, Thearle M, Francisco AM, Gayet C, Ginsberg HN, Ables EV, Ferrante AW., Jr C-C chemokine receptor 2 (CCR2) regulates the hepatic recruitment of myeloid cells that promote obesity-induced hepatic steatosis. Diabetes. 2010;59:916–925. doi: 10.2337/db09-1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tacke F, Alvarez D, Kaplan TJ, Jakubzick C, Spanbroek R, Llodra J, Garin A, Liu J, Mack M, van Rooijen N, Lira SA, Habenicht AJ, Randolph GJ. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J Clin Invest. 2007;117:185–194. doi: 10.1172/JCI28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.