Abstract
In an evaluation of carbon nanotubes (CNTs) for the IARC Monograph 111, the Mechanisms Subgroup was tasked with assessing the strength of evidence on the potential carcinogenicity of CNTs in humans. The mechanistic evidence was considered to be not strong enough to alter the evaluations based on the animal data. In this paper, we provide an extended, in-depth examination of the in vivo and in vitro experimental studies according to current hypotheses on the carcinogenicity of inhaled particles and fibers. We cite additional studies of CNTs that were not available at the time of the IARC meeting in October 2014, and extend our evaluation to include carbon nanofibers (CNFs). Finally, we identify key data gaps and suggest research needs to reduce uncertainty. The focus of this review is on the cancer risk to workers exposed to airborne CNT or CNF during the production and use of these materials. The findings of this review, in general, affirm those of the original evaluation on the inadequate or limited evidence of carcinogenicity for most types of CNTs and CNFs at this time, and possible carcinogenicity of one type of CNT (MWCNT-7). The key evidence gaps to be filled by research include: investigation of possible associations between in vitro and early-stage in vivo events that may be predictive of lung cancer or mesothelioma, and systematic analysis of dose–response relationships across materials, including evaluation of the influence of physico-chemical properties and experimental factors on the observation of nonmalignant and malignant endpoints.
Keywords: Cancer mechanisms, carbon nanofibers, carbon nanotubes, cell proliferation, fibrosis, genotoxicity, inflammation, lung cancer, mesothelioma, particle retention, pulmonary, translocation
Introduction
Scope and objectives
In October 2014, the International Agency for Research on Cancer (IARC) convened a monograph meeting of international experts on the carcinogenicity of three fiber or fiber-like materials, including fluoro-edenite, silicon carbide whiskers, and carbon nanotubes (CNTs) (Grosse et al. 2014). The monograph expert group included subgroups in epidemiology, animal studies, and mechanisms. Of the three substances evaluated, CNTs were the most diverse and heterogeneous group of materials, had the most extensive scientific literature, and yet also had the most uncertainty regarding the available evidence for specific types of CNTs. The Mechanisms Subgroup was tasked with examining the extensive mechanistic data and identifying key data gaps. CNT heterogeneity and data gaps for most types of CNTs resulted in a high degree of uncertainty with regard to assessing the potential carcinogenicity of the various types of CNTs to which people (especially workers) could potentially be exposed.
The purpose and scope of this critical review paper are to further examine the available evidence, including the additional studies on CNTs that were published after the IARC Monograph 111 meeting and the published studies on carbon nanofibers (CNF) (CNF was not evaluated in the IARC Monograph 111). In addition, some key areas of evidence such as the mechanisms of cell proliferation were examined in greater depth. A diversity of expert judgments was expressed within the subgroup, as summarized in the IARC 111 monograph (in press) and in this paper with regard to interpreting the strength of the mechanistic evidence on the potential carcinogenicity of CNTs. Agreement was generally achieved on the key areas of evidence needed to evaluate the potential carcinogenicity of CNTs and CNFs, which are based on current hypotheses on the carcinogenicity of inhaled particles and fibers, as well as missing information concerning that evidence. This follow-on paper also examines whether the additional data evaluated since the monograph meeting provide any new insights on the physico-chemical and other factors that may be associated with the potential cancer risk of occupational exposure to airborne CNTs and/or CNFs. This review includes those studies that provide information on the doses and responses to CNTs or CNFs in rodent lungs, pleura, or peritoneum, as well as in vitro studies in human or rodent cells at relevant experimental conditions. Consideration is given to the dose–response relationships in the animal studies compared to the estimated equivalent pulmonary or pleural doses of CNTs or CNFs in humans with potential occupational airborne exposures.
The objective of this follow-on review (as in the original review for the IARC Monograph 111 meeting on CNTs) is to critically evaluate the available evidence on the key steps in the development of cancer in the lungs or mesothelium associated with exposure to CNTs or CNFs. Studies were examined for the availability of relevant data across the various types of CNTs and CNFs and for consistency or differences in the results on cancer or precursor events. Data gaps in the key biological events are identified, as well as the research needs to strengthen the evidence for making decisions about the potential carcinogenicity of specific CNTs or CNFs or categories of materials.
Worker exposures and lung responses
Workers in facilities that produce or use CNTs and/or CNFs have the potential for inhalation exposure when these particles become airborne and enter the workers’ breathing zone. Workplace airborne exposure concentration measurements have been reported in several studies of single-walled or multi-walled CNTs (SWCNT, MWCNT, respectively) (Maynard et al. 2004; Han et al. 2008; Bello et al. 2008, 2009, 2010; Tsai et al. 2009; Johnson et al. 2010; Lee et al. 2010; Cena & Peters 2011; Dahm et al. 2011, 2012) and CNFs (Methner et al. 2007, 2012; Evans et al. 2010; Birch et al. 2011; Dahm et al. 2015). For a complete review of the CNT occupational exposure studies, the reader is referred to the IARC monograph (in press). Pulmonary health effects studies in workers are extremely limited. No studies of health effects in workers exposed to CNTs or CNFs were available at the time of the Monograph 111 meeting. Since then, a few studies have reported biomonitoring endpoints associated with exposure to MWCNTs (Lee et al. 2015; Fatkhutdinova et al. 2016; Shvedova et al. 2016).
Lee et al. (2015) examined nine manufacturing workers and four office workers at a large-scale manufacturing facility which produced MWCNT using a continuous thermal chemical vapor deposition (CVD) process. Noninvasive exhaled breath condensate (EBC) was used to monitor the potential effects of MWNCT exposures on inflammatory and oxidative stress in the respiratory tract. Particles in the inhalable, thoracic and alveolar sizes were all measured in the workplace air. Particle sizes ranged from approximately 8 to 300 nm in diameter, with the peak diameter at ~100–200 nm; lengths were not reported. Workers’ personal exposures, measured as the inhalable mass concentrations of elemental carbon, were 6.2–9.3 μg/m3. The respirable conentrations were estimated to be 1.6–2.3 mg/m3, assuming from other studies that the inhalable particle concentration included 25% respirable particles. No significant differences were reported in the age, gender, smoking status, or working duration of manufacturing or office workers. The pulmonary function tests and hematology and blood biochemistry values in both office and manufacturing workers were reported to be in the normal ranges. Some of the EBC biomarkers of oxidative stress were significantly higher in the total manufacturing workers (i.e., malondialde-hyde (MDA), 4-hydroxy-2-hexanal (4-HHE), and n-hexanal). Nonsignificant increases in blood molybdenum (Mo: used as a catalyst in MWCNT manufacturing) were also measured in the total manufacturing workers, and blood Mo was positively correlated with the EBC oxidative markers MDA and n-hexanol.
Fatkhutdinova et al. (2016) reported significantly elevated pro-fibrotic inflammatory mediators, including IL-1β, IL-4, IL-10, and TNF-α, in sputum and serum samples in workers exposed to MWCNT (diameter 8–15 nm; length ≥2 μm; Ni and Co catalyst <5%) compared to unexposed control groups at a company in Tambov, Russia. In this small, cross-sectional study, workers in the exposed group (n = 10) had potential exposures to MWCNT for more than one year, while the unexposed control group (n = 12) worked at the same facility. The exposure concentrations to respirable elemental carbon were 0.7–2.8 μg/m3 and inhalable fractions were 3.5–17.1 μg/m3 (8-h time-weighted average concentration) across occupations. Of the 22 workers, 18 were male and 4 female aged 19–63 years. Six of the 22 workers were current smokers. MWCNTs caused significant increases in IL-1β, IL6, TNF-α, inflammatory cytokines and KL-6, a serological biomarker for interstitial lung disease in collected sputum samples. Limited statistical analysis of the effects of age (years), sex (M/F), and smoking (Y/N) on the association between MWCNT exposure and inflammatory mediators was performed in generalized linear models with one each of the individual variables and the main effect variable of MWCNT exposure; and these models showed that only KL-6 in sputum was significantly elevated. Analyses of inflammatory cytokines in blood resulted in overall null results with the exception of a possible elevation of TGF-β1 in young workers (<30 years).
Shvedova et al. (2016) studied changes in global non-coding RNA (ncRNA), including long ncRNA and micro RNA and messenger RNA (mRNA) expression profiles in blood of workers exposed to MWCNTs at the same facility as reported by Fatkhutdinova et al. (2016). Eight MWCNT exposed workers and seven nonexposed controls were studied. Airborne elemental carbon concentrations in worker breathing zones were 0.54–6.11 μg/m3 (respirable) or 0.71–29.6 μg/m3 (inhalable). Exposed workers were aged 18–60 years with ~6–24 months exposure to MWCNT, while unexposed workers were aged 20–30 years. A number of changes in mRNA and ncRNA expression profiles that have been associated with pulmonary (inflammation, fibrosis), cardiovascular, or carcinogenic outcomes were observed in the MWCNT exposed workers. No evaluation was performed on the role of age, sex, smoking, or other factors on the changes in these expression profiles. These early studies suggest that measuring biomarkers in worker populations may provide further information on possible early-stage lung effects from CNT exposure. Other epidemiology studies on workers exposed to CNT or other nanomaterial are ongoing in the USA, Netherlands/Belgium, France, and Australia (Liou et al. 2015).
Worker populations have had fairly low exposure duration (e.g., compared to a full working lifetime of 45 years) due to the relatively recent start of global CNT and CNF production (in the past 10 or so years). Due to uncertainty about the potential for adverse lung effects, including cancer, an evaluation of all the relevant evidence including mechanistic data is needed to examine the extent to which the various types of CNTs and CNFs may be carcinogenic. At this time, due to the limited human data, experimental studies in rodents and cells provide the available data to assess the state of the evidence on the potential carcinogenicity of the various types of CNTs and CNFs. Such evaluations are important to identify the current state of the science and the key data gaps, for example, for consideration in developing evidence-based occupational health guidance.
Rodent cancer data on CNTs
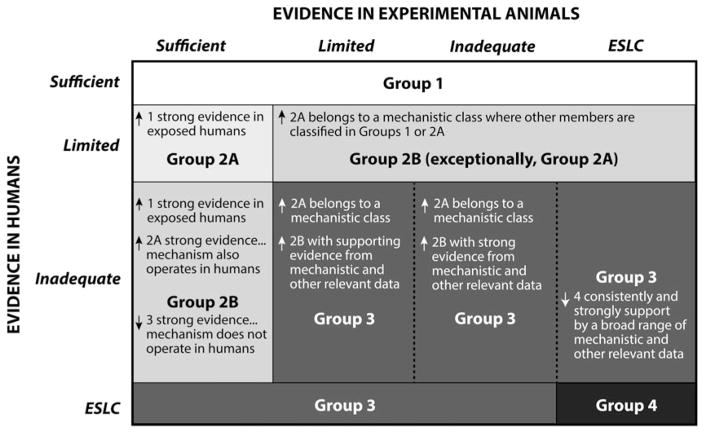
Due to inadequate evidence in humans, the animal data on carcinogenicity of specific CNTs provided the evidence basis for the cancer classifications (Grosse et al. 2014; IARC, in press). The evidence considered in IARC cancer evaluations (IARC 2006) is summarized in Figure 1. Mechanistic evidence, if sufficiently strong, can support the modification (up or down) of the default classification based on the human and/or animal evidence (IARC 2006) (Figure 2). Mechanistic evidence can include “preneoplastic lesions, tumor pathology, genetic and related effects, structure–activity relationships, metabolism and toxicokinetics, physicochemical parameters and analogous biological agents” (IARC 2006). No mechanistic evidence in humans exposed to CNTs was available for IARC monograph 111 evaluation, and only limited data have been published subsequently (Section “Worker Exposures and Lung Responses”). Animal studies that provide mechanistic data on the potential carcinogenicity of CNTs and CNFs include sub-chronic or chronic studies in rats and mice, with exposure routes by inhalation, intratracheal instillation (including intratracheal spraying), oropharyngeal aspiration, intrapleural injection, or intraperitoneal (IP) injection (discussed in Sections “Deposition, Clearance, and Retention Kinetics Relevant to the Potential Carcinogenicity of CNTs and CNFs”; “Indirect Genotoxicity of CNTs and CNFs: Rodent Studies”; “Genotoxicity”; “Role of Physico-Chemical Properties of CNTs or CNFs on Genotoxic or Carcinogenic Effects”).
Figure 1.
Evidence considered in IARC two-tier cancer evaluation process (IARC 2006).
Source: IARC Monograph Program; IARC (2006); Cogliano (2011). [Copyright permission from IARC. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Volume 111. Some Nanomaterials and Some Fibres. IARC, Lyon (in press)].
Figure 2.
Role of mechanistic evidence in IARC cancer hazard classifications: possible modulation of default classification group based on human and animal evidence (IARC 2006). ESLC: Evidence suggesting lack of carcinogenicity.
Source: IARC Monograph Program; IARC (2006); Cogliano et al. (2008). [Copyright permission from John Wiley and Sons Inc. for Environmental and Molecular Mutagenesis].
A total of 11 cancer studies of various types of MWCNTs and SWCNTs in rats or mice were available for evaluation at the monograph meeting (Tables 1 and 2). Sufficient evidence of cancer in animals (two or more adequate studies) was available for one type of CNT (MWCNT-7), and limited evidence in animals (one adequate study) was available for each of two other types of MWCNT with similar dimensions as MWCNT-7 (Grosse et al. 2014; IARC, in press). The evidence on the carcinogenicity of MWCNT-7 includes the increased incidence of peritoneal mesothelioma in rats following IP injection (Nagai et al. 2011) or intrascrotal injection (Sakamoto et al. 2009), along with evidence on tumor promotion and increased incidences of bronchiolo-alveolar and adenocarcinoma in mice at 17 weeks after a three-week inhalation exposure (Sargent et al. 2014) and mesothelioma in genetically modified (p53±) mice after IP injection (Takagi et al. 2008, 2012). Two other types of MWCNT (with similar dimensions to MWCNT-7) were associated with increased incidence of mesothelioma in rats in one experiment each (Nagai et al. 2011). The mechanistic evidence on carcinogenicity, as discussed in this paper, was considered by a majority of the working group to be not strong enough to support modification of the classifications based on the animal evidence. The uncertainty in chronic endpoints, inconsistent evidence across the various types of CNTs, major gaps in the evidence in animals, and lack of information from exposed humans precluded the use of mechanistic data to classify specific CNTs as to their carcinogenicity or to generalize to other CNTs. Thus, the IARC overall evaluations were determined by the animal evidence available at the time. MWCNT-7 was classified as “possibly carcinogenic to humans (Group 2B)”; and SWCNT and all other MWCNT (excluding MWCNT-7) were considered “not classifiable as to their carcinogenicity to humans (Group 3)” (Grosse et al. 2014; IARC, in press).
Table 1.
Studies of mesothelioma in rodents administered MWCNT or SWCNT or other substances.
| Type of CNT, vehicle control, or comparison material | CNT length (μm) | CNT diameter (μm) | CNT catalyst, metal content, treatment | Dose – Mass (mg) | Dose – Fiber number | Mortality (%) during study; mean survival (days) | Mesothelioma proportion (%) | Reference; species, strain, gender; study duration |
|---|---|---|---|---|---|---|---|---|
|
Intraperitoneal
injection (IP), single
| ||||||||
| Vehicle control | na | na | na | 0 | 0 | 2 (739) | 1/50 (2) | Rittinghausen et al. 2014; rat, Wistar, male; 24 mo.§,‡‡‡ |
| MWCNT A (low) | 2.72 ± 2.29† | 0.085 ± 1.60† | Fe | 0.2 | 0.48×109 | 98 (213) | 49/50 (98) | |
| MWCNT A (high) | 8.57 ± 1.51‡ | 1.0 | 2.39×109 | 90 (194) | 45/50 (90) | |||
| MWCNT B (low) | 2.13 ± 2.46† | 0.062 ± 1.71† | Fe | 0.6 | 0.96×109 | 92 (294) | 46/50 (92) | |
| MWCNT B (high) | 9.30 ± 1.63‡ | 3.0 | 4.80×109 | 86 (207) | 45/50 (90) | |||
| MWCNT C (low) | 4.18 ± 2.41† | 0.040 ± 1.57† | Fe | 0.08 | 0.87×109 | 84 (415) | 42/50 (84) | |
| MWCNT C (high) | 10.24 ± 1.64‡ | 0.4 | 4.36×109 | 94 (265) | 47/50 (94) | |||
| MWCNT D (low) | 2.53 ± 2.02† | 0.037 ± 1.45† | Co | 0.05 | 1.51×109 | 40 (666) | 20/50 (40) | |
| MWCNT D (high) | 7.91 ± 1.40‡ | 0.25 | 7.54×109 | 70 (585) | 35/50 (70) | |||
| Amosite asbestos (long) | 6.22 ± 3.12† 13.95 ± 2.10‡ |
3.94 ± 1.83† | na | 1.4 | 0.14×109 | 66 (623) | 33/50 (66) | |
|
IP (half of the total
mass dose was delivered two times in one week)
| ||||||||
| Control | na | na | na | 0 | 0 | 1*** (nr) | 0/23 (0) | Nagai et al. 2011, 2013; rat, F344/Brown Norway F1 hybrid, male & female; 12 mo. |
| MWCNT: NT50a††† (-agg) | Fe, Cu | 2¶¶ | ~3 × 107¶¶ | 40*** | 12/15 (80)* | |||
| NT50a††† (low) | 5.29 ± 0.12 | 0.050 ± 0.0006 | 1 | nr | 70*** | 13/13(100)* | ||
| NT50a††† (high) | 10 | nr | >72*** | 43/43 (100)* | ||||
| NT50b (high) | 4.6 ± 0.1 | 0.052 ± 0.0007 | Fe | 10 | nr | >54*** | 6/6 (100)* | |
| NT145 (low) | 4.6 ± 0.08 | 0.14 ± 0.0016 | none | 1 | ~3 × 107¶¶ | 1*** | 5/29 (17)* | |
| NT145 (high) | 10 | nr | >59*** | 28/30 (93)* | ||||
| NTtngl | nd§§ | nd§§ | Fe | 10 | nr | >60*** | 0/15 (0)¶ | |
|
Intraperitoneal
injection (IP), single
| ||||||||
| Vehicle control (Takagi et al. 2008) | na | na | na | 0 | 0 | 0 | 0/10 (0) | Takagi et al. 2008, 2012; mouse, C57Bl/6, P53+/−, male; 25 to 52 wks |
| Fullerene | nr | nr | nr | 3 | nr | 0 | 0/10 (0) | |
| MWCNT-7 | 1–19 (2 median); 27.5% >5 μm | 0.070–0.170 (0.090 median) | Fe | 0.003 | 1×106|| | 20 (nr) | 5/20 (25)* | |
| 0.03 | 1×107 | 100 | 17/20 (85)* | |||||
| 0.3 | 1×108 | 90 | 19/20 (95)* | |||||
| 3 | 1×109 | 90 | 14/16 (88)*|| | |||||
| Crocidolite (UICC) | 3 | 1×1010 | 50 | 14/18 (78)* | ||||
|
Intraperitoneal
injection (IP), single
| ||||||||
| Vehicle control | na | na | na | 0 | nr f | 60 (nr)†† | 1/26 (3.8) | Muller et al. 2009; rat, Wistar, male; 24 mo. |
| MWCNT (+)** (low) | ~0.7 | 0.011 (±0.0039) | AL, Fe, Co | 2 | 40 | 2/50 (4) | ||
| MWCNT (+) (high) | 20 | 50 | 0/50 (0) | |||||
| MWCNT (−) (high) | 20 | 60 | 3/50 (6) | |||||
| Crocidolite | 2 | 70 | 9/26 (35) | |||||
|
Intraperitoneal
implantation of capsule
| ||||||||
| Crystalline ZnO‡‡ | nr | nr | 0 (all rats survived to end of study) | 0/6 (0) | Varga and Szendi 2010; rat, F344, (nr); 12 mo. | |||
| SWCNT | 4–15 | <0.002 | 90% purity | 10 | 0/6 (0) | |||
| MWCNT | 1–2 | 0.01–0.03 | 98% purity | 10 | 0/6 (0) | |||
|
Intrascrotal
injection, single
| ||||||||
| Vehicle control | na | na | na | 0 | na | 0 | 0/5 (0) | Sakamoto et al. 2009; rat, F344, male |
| MWCNT-7 | 1–4 (2 peak) | 0.070–0.110 (0.090 peak) | Fe (~0.3%) | 0.24 | 8.52×107 | 86 (nr) | 6/7* (86) | |
| Crocidolite | 0.1–5 (1.1–1.2 peak) | 0.03–0.4 (0.11–0.20 peak) | Fe (26–29%) | 0.47 | 1.38×109 | 0 | 0/10 (0) | |
Source: Created for this paper. Some information is the same as in Tables 3.1–3.6 of Monograph 111 (IARC, in press); some additional information is added (e.g., mortality percent); and a new study is added‡‡‡. na: not applicable; nd: not determined; nr: not reported.
Statistically significant (p <0.05).
All fiber sizes.
WHO fiber length (>5 μm length, <3 μm diameter; ≥3:1 length:width ratio).
Fibers >20 μm (% of WHO fibers): 3.81, 9.35, 11.77, 2.13, and 28.55, respectively, for MWCNT A, B, C, and D, and amosite asbestos.
NTtngl: a second group of 6 rats followed for 3 years post-exposure had 0/6 mesothelioma incidence.
Post-exposure duration of 25 wks (vs. 52 wks for other dose groups).
MWCNT(+) denotes material with structural defects; MWCNT(−) denotes material without defects. The authors noted that nanotube numbers were not obtained because agglomeration made counting individual nanotubes unreliable. The authors further noted that the proportion of individual nanotubes >5 μm in length was estimated to be “extremely low.”
No significant difference in survival among groups (p = 0.16).
No vehicle control reported.
Manufacturer reported length 3 μm and diameter 0.015 μm (Table S1, Nagai et al. 2011). All structure sizes in Table 2A for Nagai et al. (2011) are author-reported.
Fiber concentration of NT145 or NT50a(-agg) was ~15 × 103 fibers/μl (Figure 6B of Nagai et al. 2011). Since 2 ml total was injected (i.e., 1 ml, two times in one wk), this would be equal to ~30 × 106 fibers injected of NT145 (at 0.5 mg/ml for 1 mg total) and NTa(-agg) (at 1 mg/ml for 2 mg total).
Calculated from Figure 6(E) of Nagai et al. (2011) for control, NT50a(-agg), NT50a (1 mg) and NT145 (1 mg) based on survival at 350d post-injection. Calculated from Table S2 in Nagai et al. (2011) for the 10 mg dose groups of NT50a, NT50b, NT145, and NTtngl, which is based on survival within several days (~half of the rats in these groups died within several days); the survival is not reported for the 10 mg group at 350 d post-exposure, but it is assumed that the mortality % is greater than that at several days post-injection.
NTa is the same as MWCNT-7.
Study published after the IARC monograph 111 meeting.
Table 2.
Studies of lung cancer in rodents administered multi-walled or single-walled carbon nanotubes (MWCNT or SWCNT), by route of exposure.
| Type of CNT, vehicle control, or asbestos | CNT length (μm) | CNT diameter (μm) | CNT catalyst, metal content | Dose – Mass (mg) | Dose – Fiber number | Mortality (%) during study; age at death | Tumor proportion‡ | Reference; species, strain, gender; study duration |
|---|---|---|---|---|---|---|---|---|
|
Inhalation, 5
mg/m3 of MWCNT, 5 hr/d, 15 d (initiation-promotion
study) - Bronchiolo-alveolar adenoma or carcinoma
| ||||||||
| Air | na | na | na | na | na | 5 (11.2 mo)* | 13/56 (23) | Sargent et al. 2014; mouse, B6C3F1, male; 17 mo. PE |
| MCA | 8 (12.3 mo) | 28/54 (52) | ||||||
| MWCNT-7 | 4.5 | 0.049 | Fe | nr | nr | 11 (10.6 mo) | 13/49 (27) | |
| MCA +MWCNT-7 | 24 (11.2 mo) | 38/42† (90) | ||||||
|
Intratracheal
instillation, single – Lung adenomas and
adenocarcinoma
| ||||||||
| Control, saline | na | na | na | na | na | nr | 0/3 | Yu et al. 2013; mouse, C57BL/6, male; 6 mo. |
| MWCNT, as produced | 7.71 | 0.0135 | 0.1 | nr | 1–3/3‡ | |||
| MWCNT, acid-treated | 0.567 | 0.0075 | 0.1 | nr | 1/3 | |||
|
Intratracheal
instillation, single – No tumors
| ||||||||
| SWCNT | Individual tubes: 1.2 (max.
length) Aggregates: 0.32 |
Individual tubes: 0.003
(±0.0011) Aggregates: 0.012 (±0.0065) |
Fe, Ni, Cr, Al, Mn (0.05% total metal content) | Control (saline), 0.04, 0.2, 1, 2 mg/kg BW | Control; 1.8×1012; 8.8×1012; 4.4×1013; 8.8×1013 particles/kg BW | 0 (all mice survived to end of study) | 0/6, 0/6, 0/6, 0/6, 0/6 | Kobayashi et al. 2011; rat, Crl: CD (SD), male; up to 6 mo. PE |
|
Subcutaneous
injection, single – Various types of tumors
| ||||||||
| Control | 10 (mean) | 0.1 (mean) | >99.9% carbon | Control (saline); | nr | 0 | 0/10 | Takanashi et al. 2012; mouse, rasH2 (C57BL/6); male; 26 wk PE |
| Carbon black | ~75 mg/kg | 1 (22 wk) | 1/10 adenoma | |||||
| MWCNT | ~75 mg/kg | 0 | 0/10 | |||||
| MNU§ | ~75 mg/kg | 4 (12–21 wk) | 10/10 (various organs) | |||||
Source: Created for this paper. Some information is the same as in Tables 3.1–3.6 of Monograph 111 (IARC, in press), and some additional information is added (e.g., mortality percent). na: not applicable; nr: not reported.
Estimated from Figure 7 in Sargent et al. (2014). Mean age of death among animals euthanized early (no significant difference).
Statistically significant (p <0.05).
Proportion of animals with tumors, except in Yu et al. (2013), which reported the tumor incidence (2 adenomas and 1 adenocarinoma in the “MWCNT, as produced” group; and 1 adenoma in the “MWCNT, acid-treated” group) but did not report the the number of animals with tumors.
N-methyl-N-nitrosourea (MNU) and high-density polyethylene34.
An additional study (Rittinghausen et al. 2014) that investigated four additional types of MWCNTs, all of which were carcinogenic in rats by IP injection, was published after the IARC monograph meeting. A lack of adequate studies on the carcinogenicity of other types of CNTs, including SWCNTs, or CNFs remains a significant data gap. Summaries of the published cancer studies of MWCNTs and SWCNTs in rats or mice are provided in Tables 1 and 2. No studies of carcinogenicity of CNFs were available at the time of the IARC evaluation or this review.
Most of the studies of CNT carcinogenicity in rodents use an IP injection route of exposure (Tables 1 and 2). The IP studies provide relevant qualitative information on the cancer hazard potential, as determined by international expert groups, including IARC. No chronic inhalation rodent bioassays are available (to date) for any CNTs or CNFs. One study of short-term (3-wk) inhalation exposure to MWCNT-7, followed by chronic post-exposure (17 mo.), has been performed in mice (Sargent et al. 2014). At the time of the IARC Monograph 111 meeting, all of the studies that showed an increased incidence of mesothelioma were IP studies in rats. A recent article (Suzui et al. 2016) reported mesothelioma in rats exposed to MWCNT-N by trans-tracheal intrapulmonary spraying (see Section “Overview of Mechanisms”).
For more information on the IARC evaluation of the rodent cancer studies, the reader is referred to IARC (in press) and Grosse et al. (2014). The focus of this review is on the mechanistic evidence and key data gaps in assessing the carcinogenicity of CNTs and CNFs in humans.
Substances and endpoints evaluated
Data that are especially useful in a cancer hazard assessment of CNTs and CNFs include: the physico-chemical characteristics of the particles, and the cellular responses in major steps in the carcinogenic process, particularly from in vivo (e.g., rodent) studies. Most of the in vivo and in vitro data available for the IARC evaluation and for this review were on MWCNTs and SWCNTs, with studies on CNF also evaluated in this review. The descriptions of the physico-chemical characteristics of these materials varied widely across study, making it difficult to assess the role of specific characteristics on the potential carcinogenicity. When such data were reported, the properties that related to toxicity (both cancer and noncancer endpoints) included the differences in the CNT wall number, diameter and length; form and entanglement, degree of and stability of agglomeration; purity (metal content); framework defects, and/or post-production treatment (functionalization). Studies of genotoxicity and sustained cell proliferation provide clues to the cellular responses to carcinogenic agents. The disruption of the cell cycle control mechanisms results in sustained proliferation that does not resolve after the stimulus is removed. Chronically sustained proliferative signaling that persists after the stimulus disappears is a hallmark of cancer (Hanahan & Weinberg 2011; Engstrom et al. 2015; Smith et al. 2016). Normal cells differ fundamentally from tumor cells by their “inherent capacity for unrestrained proliferation” (Engstrom et al. 2015). Normal cell proliferation is under tight control and ceases if the stimulus disappears or if cells are exposed to growth inhibitory signals (Hanahan & Weinberg 2011; Engstrom et al. 2015). These responses can be investigated with different cell types including target respiratory cells, as discussed in this review. In vitro studies considered were those using human lung epithelial or mesothelial cells and examining genotoxicity endpoints.
Gentoxicity and cell proliferation/hyperplasia endpoints were reported in some rodent studies of CNTs or CNFs (Tables 4 and 6), which could indicate either direct or indirect (secondary) carcinogenic mechanisms (Section “Hypotheses on Mechanisms Related to Genotoxicity and Carcinogenicity of Inhaled Particles or Fibers”). Due to the complex nature of tumor development, understanding tumor biology requires studying both the individual specialized tumor cell types and the “tumor microenvironment,” which develops during the multistep tumorigenesis process (Hanahan & Weinberg 2011). Early development of a “tumor microenvironment” could involve the formation of a pro-inflammatory cellular milieu (e.g., due to oxidative stress and chronic inflammation from intrinsic or extrinsic stressors). In this review, these early endpoints are examined in rodents and cells exposed to CNTs or CNFs.
Table 4.
Summary of in vivo data on genotoxicity and gene expression endpoints in lung tissue.*
| Endpoint | MWCNT study and tube length | SWCNT study and tube length |
|---|---|---|
| DNA oxidation products | − Cao et al. (2014): 0.7–3, 0.4–4 μm | − Vesterdal et al. (2014b): 1 μm |
| − Pothmann et al. (2015): 1.1 μm | + Folkmann et al. (2009): 1 μm (oral exposure) | |
| DNA breaks (SB) | + (2% Fe) Kim et al. (2012): 20 μm | + Jacobsen et al. (2009): 1 μm (BALF cells) |
| + Poulsen et al. (2015): 4.1 μm | − Vesterdal et al. (2014b): 1 μm | |
| + Kato et al. (2013): 2 μm | − Naya et al. (2012): 4.4 μm | |
| + Cao et al. (2014): 0.7–3, 0.7.4 μm | ||
| + Poulsen et al. (2015): 0.85 μm | ||
| + (2% Fe) Kim et al. (2014): 0.33 μm | ||
| − Ema et al. (2013b): 2.7 μm | ||
| − Pothmann et al. (2015): 1.1 μm | ||
| Micronuclei | + Muller et al. (2008b): 0.7 μm | + Shvedova et al. (2014): 1–3 μm (inhalation) |
| Mutations | + Kato et al. (2013): 2 μm (gpt locus) | + Shvedova et al. (2008): 1 μm (inhalation) |
| − Shvedova et al. (2008): 1 μm (aspiration) | ||
| Gene expression | + Snyder-Talkington et al. (2013a) Egfr (downregul), Junb (upregul) | + Park et al. (2011a) p53 protein upregulation |
| + Guo et al. (2012) Bcl3 (slightly upregul), Egfr (downregul) | + Park et al. (2011b) p53 protein upregulation, purified sample | |
| + Huang et al. (2014) Cdkn1 (upregul) | + Park et al. (2013) p53 protein upregulation | |
| + Poulsen et al. (2013) Bcl3 (upregul), Aurka (upregul), Myb (downregul) |
Source: Adapted from Tables 4.4.2 and 4.4.3 of IARC monograph 111 (IARC, in press), which was originally developed by authors on this paper. This current table has been restructured and includes only the results in lung issue (e.g., excludes information from IP or GI routes of exposure).
Bcl3: B-cell cll/lymphoma 3; Cdkn1a: cyclin-dependent kinase inhibitor 1a (P21, Cip1); Egfr: epidermal growth factor receptor; Junb: Jun-B proto-oncogene; Myb: V-Myb avian myeloblastosis viral oncogene homolog.
Levels of DNA damage, mutations, chromosome damage and cellular transformation are increased (+) or unaltered (−) in exposed cells compared to unexposed controls. Gene expressions include oncogenes, tumor suppressor genes, and genes involved in DNA repair and cell cycle regulation. (+) means a differential expression between control (untreated) and treated cells.
Table 6.
Overall summary of studies of lung and pleural responses to specific types of carbon nanotube or nanofiber (CNT or CNF) in animals.
Source: Created for this paper. An earlier draft of this table was developed by the Mechanisms subgroup members and used in some of its deliberations, but the earlier table was not included in the monograph.
Key:
 Positive study (i.e., adverse
effect observed on specified endpoint by type of CNT or CNF for the doses
and assays used)
Positive study (i.e., adverse
effect observed on specified endpoint by type of CNT or CNF for the doses
and assays used)
 Negative study (i.e., no
adverse effect on specified endpoint by type of CNT or CNF for the doses and
assays used)
Negative study (i.e., no
adverse effect on specified endpoint by type of CNT or CNF for the doses and
assays used)
 Lack of data
Lack of data
Abbreviations: (G): ground CNT; nr: not reported
Approximate mid-point, if not stated otherwise; values were reported differently across publications.
Hazard evaluations, including the IARC cancer hazard evaluations, are qualitative in nature, and typically do not take into account the quantitative nature of the dose–response relationships in the animal and/or human studies. Criteria for causality including strength, consistency, specificity, and other criteria (Hill 1965) are considered in these weight of evidence evaluations (IARC 2006). Observation of an association between the dose of a substance and the adverse response contributes to the evidence of a causal relationship (IARC 2006). Dose is also considered in a hazard review in the context of assessing whether multiple mechanisms could be involved in tumor development, including at different dose levels (IARC 2006). Qualitatively, dose is taken into account in evaluating the extent to which the materials in the experimental studies are similar to those to which humans could be exposed (IARC 2006). The quantitative target tissue dose might also be considered in extrapolating the hazard evidence from animals to humans and in interpreting in vitro findings (IARC 2006).
Initial literature searches for the Monographs are provided by IARC, and meeting participants are expected to supplement the initial searches with additional relevant studies (IARC 2006). Following the Monograph 111 meeting, the authors of this review identified additional relevant studies on CNF and any newly published studies on CNT that provide evidence on key mechanistic endpoints where data are especially limited (e.g., cancer, in vivo genotoxicity, and cell proliferation) and any subchronic or chronic studies, especially by inhalation. These studies were located through regular literature searching (using Pubmed, Embase, Toxline, Web of Science, and/or Google Scholar) and regular review of professional journals. Additional references are also cited that discussed the general mechanisms on the carcinogenicity of inhaled particles and fibers. Study outcome was not a criterion for selection; i.e., studies with both positive and negative responses to CNTs or CNFs were considered for relevancy and adequacy of experimental design. The IARC (2006) guidelines were followed in this review; these guidelines do not provide specific experimental design criteria for mechanistic studies, but do require that any study limitations are clearly outlined. In IARC cancer evaluations, possible mechanisms are identified and “a representative selection of key data from humans and experimental systems is summarized” (IARC 2006). The studies that were added to this review since the IARC Monograph 111 meeting are indicated in the respective sections and tables and summarized in the Discussion (Section “Overview of Mechanisms”). Only a few new in vivo studies were added; a larger number of in vitro studies were added. Only reports that have been published or accepted for publication in openly available scientific literature are reviewed (IARC 2006).
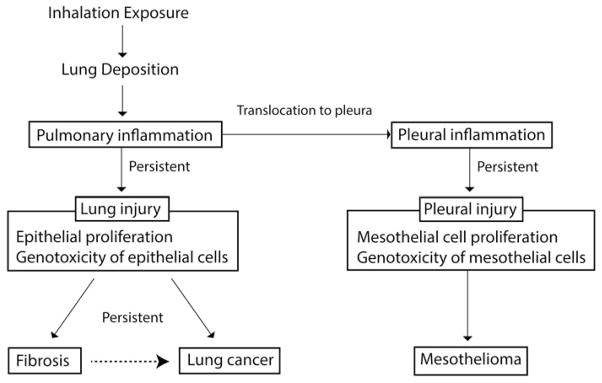
In the future, new studies that fill the data gaps identified in this paper in key mechanistic areas (Section “Research Needs and Recommendations”) (Table 6) will be especially useful in futher evaluations of the evidence on the potential carcinogenicity of CNTs and CNFs. The carcinogenic potential of inhalation exposure to CNTs and CNFs includes several key biological events in common with other inhaled particles or fibers, as shown in Figures 3 and 4, and summarized in Table 6. These key events begin with airborne exposure to particulate substances in the breathing zone (e.g., workers).
Figure 3.
Key events in cancer pathways: Indirect genotoxicity of particles or fibers via persistent inflammation.
Source: Adapted from a figure developed by Y Morimoto and N Kobayashi for IARC Monograph 111. [Copyright permission from IARC. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Volume 111. Some Nanomaterials and Some Fibres. IARC, Lyon (in press)].
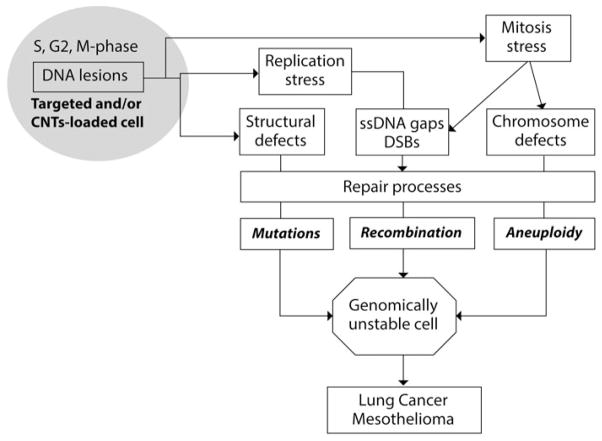
Figure 4.
Mechanisms of genomic instability generated by fibres: Cancer arises from genomic instability (GIN), and the genotoxic effects of CNTs are consistent with an ability to generate GIN. Inhaled CNTs induce a local inflammation associated with the production of cytokines, growth factors (GFs), and reactive oxygen species (ROS) (see chapters on inflammation and Figure 4), which can induce genomic insult and stimulate cell growth. Otherwise, fibres can be internalised by many cell types, resulting in a physical insult due to fibre load. In these “targeted and/or fibre-loaded” cells, the lesions in DNA produce defects in DNA structure. DNA breakage is generated by replication stress, and mitosis stress generates both DNA breaks and chromosome defects. Various repair mechanisms and cell cycle checkpoints are then activated to control genome integrity. Unrepaired or error-prone repair processes can entail mutations, chromosomal rearrangements and variations in chromosome number or morphology, which are the causes of genomic instability (GIN). Selection and amplification of genomically unstable cells can progress to lung cancer and mesothelioma.
Source: Adapted from a figure developed by M-C Jaurand for IARC Monograph 111 (IARC, in press). [Copyright permission from IARC. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Volume 111. Some Nanomaterials and Some Fibres. IARC, Lyon (in press)].
Deposition, clearance, and retention kinetics relevant to potential carcinogenicity of CNTs and CNFs
Airborne exposure
The first step to evaluate workers’ potential cancer risk from CNTs and/or CNFs is to assess their possible routes of exposure. The respiratory tract is the target organ of inhaled particles (nonfibrous or fibrous), including CNTs or CNFs, and cancers can develop in the respiratory tract regions where particles or fibers deposit or translocate, especially the tissues and cells in the bronchial, pulmonary, and pleural regions (ICRP 1994) (Figure 5). Airborne CNTs and/or CNFs (e.g., in the workplace) could be inhaled and deposited in the respiratory tract, where the possible respiratory hazard depends on the dose. Airborne exposures to CNTs and/or CNFs in the work-place have been reported in facilities manufacturing or using CNTs and/or CNF in several countries, including the U.S.A. and S. Korea (Section “Worker Exposures and Lung Responses”). The airborne particle size distribution depends on the physical form of the material and the energy applied to it in a given process or task. In experimental studies of CNTs and CNFs, the particle size distribution delivered to the animal or cell can vary with the material preparation (e.g., milling, grinding, sieving, sonication), the suspension media (air or liquid), and/or particle aerosolization techniques. A comprehensive summary of the exposure generation methods used in rodent studies of CNTs and CNFs can be found in Oberdorster et al. (2015).
Figure 5.
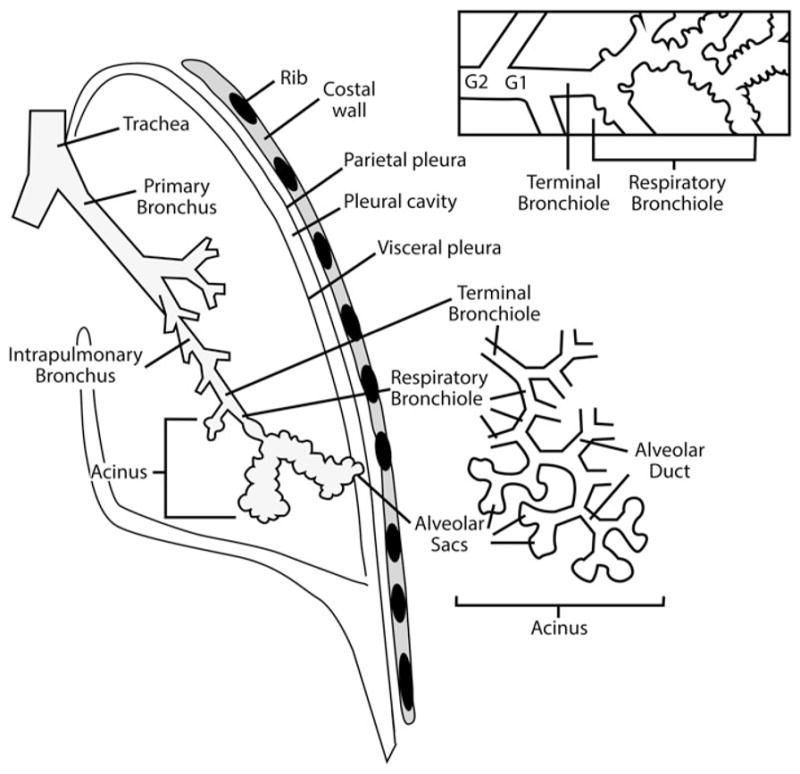
Schematic of the tracheobronchial and alveolar airway path to the pleura for the human lung. G1 and G2 signify the last two conducting airway generations prior to reaching the designated terminal bronchiole opening into respiratory bronchioles and alveolar ducts.
Source: Figure prepared by M-C Jaurand for this article. [Tracheobronchial and alveolar pathway is reprinted from Comparative Biology of the Normal Lung, 2nd ed., Plopper CG and Hyde DM, Epithelial cells of the bronchiole, pp. 83–92, 2015, with permission from Elsevier; while thoracic and pleural region is adapted from Sureka et al. (2013), with use permitted by the Indian Journal of Radiology].
Inhalation and deposition
Airborne particulate can potentially enter the respiratory tract through breathing and then be either exhaled or deposited in one of the regions of the respiratory tract (Figure 5). The probability that particles are inhaled and deposited in the respiratory tract depends on several factors including the particle’s aerodynamic (or thermodynamic) diameter (ICRP 1994; Maynard & Kuempel 2005; Kulkarni & Baron 2011). Shape and orientation are additional factors that can influence the deposition probability of nonspherical particles such as fibers (Schulz et al. 2000). “Inhalable” particles are defined as those capable of entering the nose or mouth and depositing anywhere in the respiratory tract; e.g., particles with aerodynamic diameter of 100 μm have an approximately 50% probability of being inhaled and deposited. “Thoracic” particles are those capable of reaching the thoracic region and depositing in the lung airways; e.g., 10 μm diameter particles have an ~50% probability of depositing in that region. “Respirable” particles are those capable of reaching and depositing in the pulmonary (alveolar) region of the lungs; e.g., 4 μm diameter particles have an ~50% probability of depositing in the gas-exchange region. These definitions are based on mathematical modeling and aerosol measurement devices that have been developed to replicate the particle deposition efficiencies (as aerosol mass fractions) in the human respiratory tract (ICRP 1994; ISO 1995; ACGIH 2015).
Nanoparticles (<100 nm diameter) have an estimated deposition efficiency of up to 99% in the human respiratory tract, including up to 60% in the alveolar region (ICRP 1994; Maynard & Kuempel 2005). The inhalable and respirable particle mass fractions in rodents consist of smaller particle sizes than those in humans due to the differences in the size and geometry of the respiratory tract across species (Miller 2000). Airborne CNTs and CNFs are often agglomerated (Birch et al. 2011; Dahm et al. 2012, 2015; Chen et al. 2012); and as such their deposition efficiency in the rodent or human respiratory tract has been assumed to approximate that of spherical particles and estimated using spherical particle dosimetry models (e.g., Multiple-path Particle Dosimetry Model, MPPD) and data on CNT or CNF aerodynamic diameter and density (ARA 2009; NIOSH 2013).
CNT and CNF agglomerates can be less than unit density (1 g/ml) (Ma-Hock et al. 2009; Pauluhn 2010; NIOSH 2013), which results in a lower pulmonary deposition fraction, as estimated in the MPPD model (ARA 2009; NIOSH 2013). Airborne mass samples of CNT and CNF in the workplace are reported to consist mostly of agglomerates of CNT and CNF in the thoracic and inhalable size fractions, as well as a smaller mass fraction of respirable CNT and CNF; the observed size fractions can also depend on the specific material and process (Birch et al. 2011; Dahm et al. 2015).
The primary function of the respiratory tract in humans and rodents is gas exchange (oxygen uptake and carbon dioxide release), which takes place in the pulmonary (alveolar) region across the thin epithelial and endothelial cell layers in humans (Figure 5) and rodents. Although airborne inhalable and respirable CNT and CNF particles have been measured in the work-place (Dahm et al. 2012, 2015), no studies are currently available on the deposition, clearance, and retention of CNTs or CNFs in the respiratory tract or translocation to other organs in humans. On the other hand, there are a number of rodent studies of how MWCNTs are deposited, cleared, and retained in the lung, as well as translocated to the liver, kidney, spleen, heart, and brain (as discussed below and in more detail in the IARC monograph 111 [IARC, in press]). The fraction of the inhaled MWCNTs that deposits in the rodent pulmonary region is estimated at 1–4% in mice (Shvedova et al. 2008; Mercer et al. 2013a) and 5–20% in rats (Pauluhn 2010; Oyabu et al. 2011). Human pulmonary deposition fractions for MWCNTs or SWCNTs (of same aerodynamic diameter as those studied in rodents) were estimated to be 8–10% in workers (NIOSH 2013), based on the airborne particle size according to the MPPD model (ARA 2009). The degree of dispersion and disaggregation of MWCNT (Baytubes®) influenced the airborne particle sizes and therefore the pulmonary deposition fractions (Pauluhn & Rosenbruch 2015). The mass median aerodynamic diameter (MMAD) was 2.6 or 0.79 for dry- or wet-dispersed material, respectively, and the estimated pulmonary deposition fraction was 3.1% or 8.2%, respectively, using MPPD 2.11 (Pauluhn & Rosenbruch 2015). Thus, the smaller the particle size (more dispersed), the greater the pulmonary deposition.
Differences in airway branching patterns (bipodial or tripodial in humans vs. monopodial in rats) can result in differences in particle deposition patterns in the tracheobronchial region and the downstream alveolar region (Pinkerton et al. 1997). Particle deposition is dictated by physical mechanisms (impaction, interception, sedimentation, diffusion) that depend on the structure of the respiratory tract region and the particle size, shape, and orientation in the airstream (Miller 2000; Schulz et al. 2000). Fiber deposition depends on diameter because fibers tend to align with the airstream, which enables long fibers (>10 μm in length) to reach the alveolar region (Kulkarni & Baron 2011). Fiber length comes into play as it influences the interception with the airway, and enhanced fiber deposition at airway bifurcations has been observed in studies of asbestos (Schulz et al. 2000). The airborne characteristics of particles and the differences in respiratory tract structure and physiology have been taken into account in respiratory tract dosimetry models in rodents and humans, which allows for the reasonably accurate prediction of the deposited dose of particles in the respiratory tract (e.g., ICRP 1994; ARA 2009), including CNTs and CNFs with similar aerodynamic characteristics. However, clearance of CNTs and CNFs is not known in humans, and limited information is available in rodents (Section “Clearance and Retention”).
Although CNTs and CNFs have not been measured in the human respiratory tract, these particles have been measured in human respiratory tract replicas from the nasal airways to the fourth airway generation (Su & Cheng 2014, 2015) following aerosolization using a medical nebulizer (after 24 hr of ultrasonication). Su and Cheng used both stacked cup CNTs (SCCNTs) (Shenzhen Nanotech Co., Shenzhen, China) and SWCNTs (SWeNT®, Southwest NanoTechnologies, Norman, OK, USA). The SCCNTs were produced to >95% purity by a chemical vapor deposition process and had a 10–20 nm diameter and a 5–15 μm length before aerosolization. The SWCNTs had a 0.93 ± 0.27 nm diameter (>90% carbon by weight), with a length to diameter aspect ratio of >1000. Size-classified diameters of the aerosol were 51, 101, and 215 nm. The morphology of the size-classified SCCNTs included “curved, rope-like, circular loop, and bird nestlike” single structures or aggregates. The size classified SWCNTs showed “twisted rubber band and open cage-like structures of very thin nanotubes.” Both the SCCNTs and SWCNTs had physical dimensions that were generally much larger than their aerodynamic diameters (Su & Cheng 2014, 2015). The total deposition fraction in the human airway replica (which included nasal-pharyngeal, tracheal, and four generations of airways) was approximately 7–19% for SCCNTs and 12–18% for SWCNTs Su and Cheng (2015). This implies that more than 80% of the structures with diameters in the range studied could penetrate beyond the fourth airway generation to the lower airways and alveoli and possibly deposit.
Clearance and retention
Some studies in rats and mice show reduced clearance of MWCNTs following inhalation exposure at lower lung particle doses (as mass or volume) than observed with other inhaled poorly-soluble respirable particles (Mercer et al. 2013a; Pauluhn 2010), while others have shown normal clearance rates in rats at the doses administered (Oyabu et al. 2011). A reduction in the normal lung clearance rate is important because it would result in an increased lung retention rate and greater lung burden for a given duration of exposure. Oyabu et al. (2011) reported a lung retention half time of approximately 50 days in rats exposed to 0.37 ± 0.18 mg/m3 of an aerosol of short MWCNTs (1.1 μm geometric mean length, almost all <10 μm; 2.7 GSD) and Triton X-100 by whole-body inhalation (6 h/d, 5 d/wk for 4 wk). Pauluhn (2010) reported reduced pulmonary clearance rates compared to those in rats at non-overload conditions, i.e., normal retention half-times (t1/2) of ~60 d in rats (Snipes 1989) compared to 151, 350, 318, or 375 d in Wistar rats following subchronic inhalation to 0.1, 0.4, 1.5, or 6 mg/m3 of MWCNTs (Baytubes®) of relatively short lengths (median ~200–1000 nm); however, the retention half-time was reported to be unreliable at 0.1 mg/m3 due to the lung dose measurements being at the limit of detection and to the potential binding of soluble cobalt (used to estimate the MWCNT lung burden) in the lung tissues. Subsequently, the estimated retention t1/2 was revised to 84–105 d in rats exposed to 0.4 mg/m3 MWCNTs (Baytubes®) (Pauluhn & Rosenbruch 2015).
The pulmonary clearance rate of MWCNTs (Baytubes®) was shown to depend on the degree of dispersion and disaggregation (Pauluhn & Rosenbruch 2015). The retention t1/2 was 46 d for wet- or dry-dispersed MWCNT, respectively. Thus, the smaller MWCNT (MMAD = 0.79 μm) was cleared from the lungs faster than the larger MWCNT (MMAD = 2.6 μm). The reason for the faster clearance from the lungs of the more highly dispersed MWCNT was suggested to be through the lymphatic system into the pleura, as seen by the “sustained black discoloration of the visceral surfaces of the lung and lung-associated lymph nodes” (Pauluhn & Rosenbruch 2015). Consistent with this observation is the greater septal thickening seen in rats exposed to the smaller airborne MWCNT particles. Thus, the shorter retention t1/2 of the smaller MWCNT from the lungs does not take into account the whole body retention including in the lymph nodes.
A study of well-dispersed MWCNT (geometric mean diameter and length: 48 nm and 2.5 μm, respectively; Nikkisco Co. Ltd., Tokyo, Japan) administered to rats by IT instillation (0.20 or 0.55 mg doses) showed significant pulmonary retention at 364 days post-instillation (Shinohara et al. 2016). Approximately 30% of the MWCNT appeared to be cleared within 24 h of administration, while the lung burden of MWCNT did change significantly one year later. MWCNT were observed inside alveolar macrophages, but were not detected by mass in the liver or brain at one year post-instillation (Shinohara et al. 2016).
The rodent lung overloading mechanism has been well-studied for many types of poorly-soluble particles (recently reviewed in Pauluhn 2014a; Morfeld et al. 2015; Borm et al. 2015). At sufficiently high doses, the rodent (rat and mouse) alveolar macrophages become overloaded with engulfed particles, resulting in impaired pulmonary clearance and increased particle build-up and retention in the lungs (Bolton et al. 1983; Morrow 1988; Elder et al. 2005). The effects of overloading involve a sequence of events including persistent pulmonary inflammation, fibrosis, and tumorigenesis (Oberdorster 1995). The degree of rat lung overloading has been associated with the total mass or volume of retained particles (Muhle et al. 1990; Bellmann et al. 1991). Reduced alveolar-macrophage mediated clearance was “regularly seen at particulate burdens above approximately 1–3 mg in the rat lung” (Oberdorster 1995), although nanoscale or highly toxic materials were associated with impaired pulmonary clearance at a lower mass or volumetric particle dose than for micro-scale poorly-soluble low toxicity particles (Bellmann et al. 1991; Oberdorster et al. 1994). The studies on overloading of lung clearance in rodents were performed with particles like carbon black or TiO2, which have different characteristics than CNTs. For example, a recent paper illustrates that carbon black and TiO2 trigger toxicogenomic responses in mouse lungs that are different from those of CNTs (Nikota et al. 2016). In addition, some CNTs may undergo biodegradation (Section “Solubility/Degradation in Body or Cellular Fluids”) which could impact their clearance.
For nanoscale particles, some evidence suggests that the impairment of pulmonary clearance may be due to a different mechanism than macrophage volumetric overloading, such as altered alveolar macrophage function (phagocytosis or chemotaxis) (Renwick et al. 2001, 2004) and/or greater ability to enter the lung interstitium (Oberdorster et al. 1994). Particle surface area has been shown to better describe the decreased clearance and pulmonary responses to nanoscale compared to microscale particles (Tran et al. 2000). Although environmental exposures may not result in lung burdens equivalent to those in overloaded rats, occupational exposures in dusty environments such as coal mining have resulted in human lung burdens exceeding 10 mg/g lung, and pulmonary clearance was very slow or not measureable in those miners (Freedman & Robinson 1988). Human long-term retention of respirable particles apparently involves the sequestration of some portion of the dust, even at low exposures, below overloading in rats (Kuempel et al. 2001; Gregoratto et al. 2010). Humans tend to retain a greater proportion of particles in the alveolar interstitium, while rats retain a greater proportion of particles in the alveolar spaces (Nikula et al. 2001).
The mechanistic pathways operating at lower (non-overloading) doses may differ from those operating at higher doses if the defenses of the cell or organism are overwhelmed (McClellan 1997; Oberdorster et al. 2005a). Dose rate can influence the occurrence and severity of acute or sub/chronic effects in rats exposed to CNTs or other particles (Pauluhn 2014b; Baisch et al. 2014). In two studies of the same MWCNT, rats showed similar dose–response relationships for pulmonary septal thickening at 90 days following a one-day (6-h) inhalation exposure (Ellinger-Ziegelbauer & Pauluhn 2009) and after 13-weeks of inhalation exposure (Pauluhn 2010), based on estimated deposited lung dose (NIOSH 2013). However, the collagen observed in rats (by Sirius red staining), at 90 days following exposure to MWCNT at 241 mg/m3 for 6-h, was interpreted to be due to chronic alveolitis rather than to interstitial fibrosis (Ellinger-Ziegelbauer & Pauluhn 2009). In contrast, in rats exposed to MWCNT at lower concentrations for 13 weeks, “increased interstitial collagen staining (Sirius red)” was reported at the 1.6 or 6 mg/m3 doses, and “focal areas of increased collagen staining were adjacent to sites of increased particle deposition and inflammatory infiltrates” in rats exposed at 0.4 mg/m3 or higher doses (Pauluhn 2010).
Particles (including CNTs or CNFs) that are not cleared from the lungs can move into the lung interstitial tissue (either alone or inside macrophages). Particle retention in the interstitium increases the risk of fibrosis for poorly-soluble particles including CNTs (NIOSH 2013). Rodent studies have shown that both MWCNTs and SWCNTs can enter the lung interstium, and SWCNTs appear to do so to a greater extent as individual structures rather than being transported by alveolar macrophages (Mercer et al. 2008, 2010, 2011). Some studies have reported the translocation of MWCNT from the lungs to the lung-associated tissues and to systemic organs (discussed below); however investigation of translocation of SWCNT in vivo remains a research need.
In an inhalation study of mice (male C57BL/6) exposed to 5 mg/m3 of MWCNTs (MWCNT-7; mean length of 4.3 μm [Chen et al. 2012]) for 12 d (5 h/d, 3 wks), the lung burden measured on day 1 post-exposure was 28.1 μg (1321 × 109; fiber number estimate based on 47 million MWCNT fibers/μg [conversion reported in Chen et al. (2012)] (Mercer et al. 2013a,b). Of this lung burden, 84% was found in the alveolar (pulmonary) region of the lungs, and 16% was in the airways. The same group (Porter et al. 2010, 2013) observed a similar distribution of MWCNTs in two previous studies of MWCNTs in mice exposed by pharyngeal aspiration or acute inhalation, respectively. In the inhalation study (Mercer et al. 2013a), of the MWCNTs that deposited in the alveolar region, 56% was observed in alveolar macrophages, 5.7% was in the alveolar airways, and 20% was in the alveolar tissue at day 1 post-exposure (Mercer et al. 2013a). The distribution of MWCNTs in the lungs shifted over time from alveolar macrophages to the alveolar tissue (5.8–9.5 μg on day 1 and 168 post-exposure, respectively). Thus, the alveolar interstitial lung burden increased as MWCNTs in the alveoli were cleared (Mercer et al. 2013a). At 336 days post-exposure, 65% of the initial MWCNT lung burden (28.1 μg, measured 1 day-post-inhalation exposure) was retained in the lungs (18.2 μg MWCNT), most of which was retained in the alveolar region (96%, including 4.8% in subpleural tissue); 4% (0.77 μg) was retained in the airways (Mercer et al. 2013a). The number of larger or agglomerated MWCNT structures (>4 fibers/MWCNT) decreased over time – from 53 to 25% of the lung burden on 1 or 168 days post-exposure, respectively (Mercer et al. 2013a). The number of structures with 2, 3, or 4 fibers also decreased significantly. However, the percentage of single fibers in the MWCNT lung burden did not change significantly from 1 to 168 days post-exposure. Thus, the MWCNTs were decreasing in size, resulting in a relatively constant number of single MWCNTs in the lungs over time.
Inhaled MWCNTs were also observed in the pleural tissues at 1-day post-exposure (Mercer et al. 2013a). Approximately 1.2% (0.34 μg) of the MWCNT lung burden was observed as single fibers in the pleural compartment (including the subpleura and visceral pleura) at 1-day post-exposure. In a study of the same type of MWCNT carried out in mice (male C57BL/6J) exposed to MWCNT-7 (49 nm in diameter; 3.9 μm in length) by pharyngeal aspiration (10, 20, 40 and 80 μg MWCNT or vehicle), 18% of the MWCNTs were observed in airways, 81% in the alveolar region, and 0.6% in the subpleural tissue on d 1 post-exposure (80 μg dose) (Mercer et al. 2010). At 56 days after pharyngeal aspiration of mice, 8% of the total MWCNTs in the lungs were observed in the alveolar interstitial tissue; the subpleural tissue, “the region consisting of mesothelial cells of the visceral pleura and immediately adjacent Interstitium,” contained 1.6% of the total lung burden (Mercer et al. 2011). No MWCNTs were found in the airways of mice at 7, 28, and 56 days post-aspiration exposure (Mercer et al. 2011).
Pleural clearance of MWCNT has been shown to depend on the length of structures. Clearance was reduced in mice administered longer MWCNT (mean length 13 μm) by IP injection, compared to the same mass dose (5 μg/mouse) of shorter MWCNT (0.5–5 μm lengths) (Murphy et al. 2011; Donaldson et al. 2013). The inflammatory and fibrotic responses were also related to the length of MWCNT, including at 6 weeks post-exposure following pharyngeal aspiration into the lungs (Murphy et al. 2013). Clearance and retention studies of CNT and CNF following long-term inhalation exposure have not been reported.
Cell uptake and interaction
Airborne CNTs or CNFs deposited in the respiratory tract may enter cells by various mechanisms, such as passive internalization (diffusion or penetration of cell membrane) or active internalization (phagocytosis or other types of endocytosis) (Kunzmann et al. 2011; Ye et al. 2013). The mechanisms of cell uptake depend on the surface properties of the CNTs, the cell type encountered and the cell’s activation state. SWCNTs are poorly recognized by alveolar macrophages, and uptake is low (10% in murine alveolar macrophages) (Shvedova et al. 2005), increasing the likelihood of SWCNTs becoming interstitialized. In another study, 90% of dispersed SWCNT structures were observed in the lung interstitium of mice (Mercer et al. 2008).
MWCNTs are reportedly more effectively taken up by macrophages than SWCNTs (Mercer et al. 2010, 2011; Treumann et al. 2013), suggesting an increased likelihood of being cleared from the pulmonary region to the tracheobronchial region (via macrophage movement to the mucociliary escalator) and cleared from the lungs by cough or expectoration. Alveolar macrophage uptake is significantly increased by carboxylic acid functionalized (F) MWCNTs compared to original (O) or purified (P) MWCNTs (Silva et al. 2014). Dimensions of these MWCNTs were 20–30 nm diameter and 10–30 μm length. The residual metal catalysts contents were: O-MWCNT (4.49% Ni, 0.76% Fe); P-MWCNT (1.8% Ni, 0.08% Fe); and F-MWCNTs (non-detectable levels of Ni or Fe). The differences in these MWCNTs also influenced their location and their structural forms within the alveolar macrophages. In one experiment, rats were exposed by single (6-h) inhalation to O-, P-, or F-MWCNT at ~30 mg/m3 (by nebulization); aerodynamic diameters were MMAD (GSD) of 3.7 (2.5), 4.8 (2.0), and 3.3 (3.1) for O-, P-, and F-MWCNT, respectively. On day 1 after exposure, O-MWCNT and P-MWCNT were observed inside the phagolysosomes of the macrophages, and F-MWCNT was seen in the cytosol and protruding from the cell membrane. On day 21, the O- and P-MWCNT were no longer inside the phagolysosomes, but were observed in the cytosol as larger focal agglomerates; F-MWCNTs remained in the cytosol as smaller, dispersed aggregates. The acidic functional groups on the F-MWCNT increased the hydrophilicity, which has been “generally linked to easier clearance from the body” along with some evidence of reduced toxicity (Silva et al. 2014). The F-MWCNT were considered to reduce toxicity by preventing uptake into phagolysosomes and subsequent NLRP3 inflammasome activation (Silva et al. 2014).
In mice exposed to MWCNT-7 by pharyngeal aspiration, MWCNT penetrations were observed at day 1 post-exposure Mercer et al. (2010) to be most frequently in alveolar macrophages, followed by alveolar type II epithelial cells (which make up approximately 2% of normal epithelial surface), and less frequently in alveolar interstitial cells (where they were typically observed as fibers passing through adjacent epithelial cells). The investigators found that MWCNTs inside cells were not confined to phagolysosomes and extended from the cell surface through the nuclei and other organelles. In the airways, the MWCNTs were observed in the mucous layer above airway epithelial cells and in airway macrophages in the cilia-mucous lining; penetrations by MWCNTs in the airways were rare. At the 20 μg dose, a total of 15 million MWCNT penetrations were observed in the 11 million alveolar type I epithelial cells in mouse lungs (Mercer et al. 2010).
As observed for asbestos and other fibers, rigid MWCNTs that exceed the length of alveolar macrophages can pierce the macrophage membrane and release reactive oxygen species (ROS) in the lungs, causing “frustrated phagocytosis” (Donaldson et al. 2013). The threshold fiber length resulting in pulmonary inflammation (14 μm) in mice after aspiration exposure to silver nanowires was found to be greater than the threshold length causing pleural inflammation (5 μm) (Schinwald et al. 2012), which is consistent with observations with asbestos (Davis et al. 1986; Donaldson et al. 2010). Differences in threshold fiber length have been attributed to different clearance mechanisms in the lung and the pleural space, i.e., macrophage-mediated clearance of particles or fibers to the mucociliary escalator in the airways or through the pleural stomata, respectively (Schinwald et al. 2012). The size of the parietal pleural stomata, through which the pleural fluid flows, was reported to range from 0.8–10 μm in mammalian species from mice to humans, respectively (Schinwald et al. 2012).
Translocation from the lungs to other organs
Several studies have reported translocation of MWCNTs from the lungs of rodents to the systemic circulation and to other organs. MWCNTs translocated from the lungs of mice were found in blood samples (Ingle et al. 2013). MWCNTs of two sizes (60–80 nm or 90–150 nm diameter) were observed as black pigments in liver tissue 1-day post-intratracheal instillation, and dose-dependent toxicity and necrosis were observed in the liver and kidney (Reddy et al. 2010). MWCNTs seen by transmission electron microscopy were located in alveolar macrophages in the subpleural region two weeks after inhalation exposure of 30 mg/m3 in mice (Ryman-Rasmussen et al. 2009). MWCNTs administered to rats by intrapulmonary spraying directly penetrated the pleural cavity from the lungs through the visceral pleura, which had visceral pleural cell proliferation at the end of the 9-day exposure (Xu et al. 2012). After a 90-day inhalation exposure in rats, MWCNTs (CM-100; diameter ~10–15 nm, length ~20 μm) were detected in the pleura at 28 days post-exposure (Kim et al. 2014). As discussed in Broaddus et al. (2011), the anatomy of the visceral pleural differs in rodents and humans, which may impact the translocation of particles from the lungs to the pleural space.
After inhalation exposure to 5 mg/m3 of MWCNTs (MWCNT-7) in mice (5 h/d, for 12 d, during 3 wk), most of the MWCNTs that translocated from the lungs were found in the tra-cheobronchial lymph nodes (1.08% on post-exposure day 1 and 7.34% on post-exposure day 336, as a percentage of the post-exposure day 1 lung burden) (Mercer et al. 2013b). The next highest extrapulmonary tissue burdens of MWCNTs were reported in the liver (0.0028% and 0.027% on post-exposure days 1 and 336, respectively) and kidneys (0.0010% and 0.0052% on post-exposure days 1 and 336, respectively). Lower amounts of MWCNTs were detected in the heart, brain, chest wall, and diaphragm (with higher amounts at post-exposure days 1 and 336 than in all tissues except the chest wall). In the lung, 54% of the MWCNT burden was agglomerated, while only single MWCNT structures (average length 6.9 μm) were observed in the liver, kidney, heart, brain, chest wall, and diaphragm (Mercer et al. 2013b). The MWCNT tissue concentration in extrapulmonary organs increased after the 3-wk inhalation exposure, from 1 to 7% of the lung burden (by mass) on post-exposure days 1 or 336 (Mercer et al. 2013b).
In another study of mice, 14C-radiolabeled MWCNTs (also administered by pharyngeal aspiration) were detected in the spleen and liver at 1 day post-exposure; the percentage of the administered dose in the spleen and liver increased from 0.1% to 1% at 6 and 12 months post-exposure, respectively, while the lung dose decreased to 20% and 10% of the administered dose at 6 and 12 months post-exposure, respectively (Czarny et al. 2014). To date, there are no reports of in vivo translocation studies of other types of CNTs, including SWCNTs, or CNFs.
Hypotheses on the mechanistic events related to genotoxicity and carcinogenicity of inhaled particles or fibers
Carcinogenicity is a multistep process that occurs at the cellular level, and genetic damage can occur at several steps in the pathway. Initiation is an irreversible first step in which mutation(s) become permanently integrated into the DNA. The initiated cell can remain quiescent or can undergo autonomous proliferation and clonal expansion by not responding to control signals for normal growth, or undergo senescence (Collado & Serrano 2005; Kilbey et al. 2008). Promoting agents (chemical, physiological, or physical stresses) can also result in cell proliferation, which can be sustained as long as the stimulus remains.
The hallmarks of cancer pathways include the following key biological events: sustained proliferative signaling, evasion of growth suppression, activation of invasion and metastasis, enabled replicative immortality, induction of angiogenesis; and resistance to cell death (Hanahan & Weinberg 2000; Hanahan & Weinberg 2011; Engstrom et al. 2015). Genomic instability and inflammation underlie these hallmarks (Hanahan & Weinberg 2011; Engstrom et al. 2015). Reprograming of energy metabolism and evading immune destruction are considered enabling characteristics (Hanahan & Weinberg 2011). Particle-induced persistent inflammation and inflammatory factors, which may be released in the tumor microenvironment by neighboring cells, can indirectly participate in the neoplastic process (Table 7).
Table 7.
Key Characteristics of Dusts and Fibres Evaluated for Carcinogenicity by IARC, including Carbon Nanotubes and Nanofibers.
| Characteristic | Example of relevant evidence | Carbon black† | Asbestos fibers‡ | Carbon nanotubes and nanofibers§ |
|---|---|---|---|---|
| 1. Is Electrophilic or Can Be Metabolically Activated | Compound or metabolite with electrophilic structure, forms DNA and protein adducts | nr | nr | nr |
| 2. Is Genotoxic | DNA damage, gene mutations, chromosomal alterations | +/− | + | + |
| 3. Alters DNA Repair or Causes Genomic Instability | Alterations of DNA replication or repair | nr | nr | nr |
| 4. Induces Epigenetic Alterations | DNA methylation, histone modification, micro RNA expression | nr | nr | nr |
| 5. Induces Oxidative Stress | Cell-derived oxygen radicals, oxidative damage to macromolecules, redox imbalance | + | + | +/− |
| 6. Induces Chronic Inflammation | Elevated inflammatory cells, altered production of cytokines and chemokines | + | + | +/− |
| 7. Is Immunosuppressive | Decrease immunosurveillance, immune system dysfunction | nr | nr | nr |
| 8. Modulates Receptor-Mediated Effects | Receptor activation or modulation of endogenous ligands | nr | nr | nr |
| 9. Causes Immortalization | Inhibition of senescence | nr | nr | nr |
| 10. Causes Sustained Cell Proliferation, Cell Death, or Altered Nutrient Supply | Changes in growth factors, energetics and signaling pathways related to cell cycle control, angiogenesis | nr* | +* | −/+* |
Source: Created for this paper. Derived from information in Smith et al. (2016) and applied to the authors’ understanding of the scientific literature for CNT and CNF, as well as for the comparison materials of ultrafine carbon black and asbestos.
Table 7 is modified from Smith et al. (2016) and applied to specific particles (nonfibrous and fibrous), Key characteristics of carcinogens as a basis for organizing data on mechanisms of carcinogenesis, IARC (in press).
Definitions: “nr” not reported; “− “studies that examined these characteristics reported no relationship with exposure to the material; “+” studies that have reported a relationship between these characteristics and exposure to the material.
It is recognized that exposure to a sufficient dose of biopersistent materials such as asbestos can cause chronic inflammation, resulting in disruption of local tissue homeostasis and altered cell signaling that involves persistent cell proliferation (Mossman et al., 2013; Smith et al., 2016). In addition, pathways that are initiated by proto-oncogenes in pre-neoplastic and neoplastic cells can also recruit inflammatory cells, resulting in accelerated tumor promotion and progression (Grivennikov et al. 2010; Smith et al. 2016). Thus, characteristics 6 and 10 can be inter-related, and it is not currently feasible to distinguish the specific mechanisms involved. For CNTs and CNFs, persistent cell proliferation has been observed in a number of studies (Section “Weight of Mechanistic Evidence and Key Data Gaps”; Table 6), and angiogenesis has also been observed (Section “Other indicative effects: Gene expression and cell transformation”). However, most of these published studies reporting cell proliferation have not critically examined the mechanisms of sustained proliferative signaling to the extent that has been reported in published studies with asbestos fibers.
Carbon black was most recently evaluated by IARC as Group 2B (IARC, vol. 93, 2010). Carbon black represents a class of poorly-soluble particles with low toxicity that impairs alveolar macrophage clearance at sufficiently high doses in rodents leading to persistent inflammation. Carbon black comes in different particle sizes and purities (some samples have high content of PAHs); evidence of genotoxicity reported in Jacobsen et al. (2007, 2011); Kyjovska et al. (2015); in vitro evidence of proliferative signaling in epithelial cells exposed to ultrafine carbon black reported in Tamaoki et al. (2004); Weissenberg et al. (2010).
Asbestos and erionite fibres were most recently evaluated by IARC as Group 1 (IARC, vol.100C, 2012) supported by established evidence for induction of genotoxicity, oxygen radical-induced injury, oxidative stress, and chronic inflammation; evidence of persistent cell proliferation mechanism described in Adamson et al. (1993); Adamson (1997); Adamson and Bakowska (2001); Heintz et al. (2010); Mossman et al. (2013).
Carbon nanotubes were recently evaluated by IARC and one type of MWCNT was classified as Group 2B (IARC, vol. 111, in press). This paper reviews the available experimental evidence for induction of genotoxicity, oxidative stress, and chronic inflammation following exposure to diverse types of carbon nanotubes. Significant data gaps and conflicting results for the various SWCNT and MWCNT samples reported in the literature; see Table 6; evidence for persistent cell proliferation for MWCNT-7 in Sargent et al. (2014).
Simplistically, carcinogenic agents including particles can be broadly grouped as either directly or indirectly genotoxic (Velazquez et al. 1996; Schins & Knaapen 2007). Genotoxic agents can cause permanent changes in cellular DNA by direct interaction (or via metabolic activation), whereas nongenotoxic agents do not interact with DNA in a biologically significant way but act indirectly through other pathways such as persistent inflammation and cell proliferation (Velazquez et al. 1996). Relatively few chemicals are complete carcinogens, i.e., capable of enabling all of the hallmarks on their own (Engstrom et al. 2015; Smith et al. 2016). Yet, exposure to individual or multiple agents can activate the hallmark mechanisms of cancer and disrupt normal cell function, thereby enabling the cancer pathways (Engstrom et al. 2015). For example, agents that activate the Ras oncogene or inhibit the tumor suppressor p53 gene act on the cell cycle and cell proliferation (Engstrom et al. 2015).
Persistent inflammation, oxidative stress, epithelial or mesothelial injury, cell proliferation, and genotoxicity are considered to be key events on the pathway(s) to the development of lung cancer and mesothelioma from exposure to poorly-soluble particles or fibers, including CNTs and CNFs (Figures 3 and 4) (Table 7). Cell proliferation and hyperplasia resulting from sustained inflammatory response and apoptosis (programmed cell death) have been reported for asbestos (Buder-Hoffmann et al. 2001; Heintz et al. 2010; Smith et al. 2016). Sustained inflammatory response can be triggered by repeated exposures and/or biopersistent substances at sufficient doses, as observed for CNTs and CNFs in some animal studies (Tables 3 and 6). Cell proliferation alone was not predictive of carcinogenesis for chemicals (Melnick et al. 1993).
Table 3.
Studies on pulmonary and pleural inflammatory and fibrotic effects of carbon nanotubes or nanofibers (CNT or CNF) in experimental animals.
| Exposure route | CNT or CNF type | Characterization | Experimental animals | Concentration/dose | Recovery period | Results | Reference |
|---|---|---|---|---|---|---|---|
| Inhalation | MWCNT | Length 200–300 nm, Surface area 253 m2/g, Impurities Co 0.53% | Male Wistar rats |
11, 248 mg/m3, 6 hours | 90 days | Persistent inflammation and fibrosis (248 mg/m3) | Ellinger-Ziegelbauer & Pauluhn (2009) |
| Inhalation | MWCNT | Diameter 94.1–98 nm, Length 5.53–6.19 μm, Surface area 24–28 m2/g, MMAD: 1.4–1.6 μm, Impurities >99.6–99.8% purity | Male & female F344 rats |
0, 0.2, 1, 5 mg/m3, 13 weeks, 5 days/week, 6 h/d | 0 day | Increase in lung weights, BALF inflammatory
parameters. Granulomatous changes, focal fibrosis of the alveolar wall, inflammatory infiltration in the visceral pleural and subpleural areas was observed at 0.2 mg/m3 (male) and 1 mg/m3 (female) |
Kasai et al. (2015) |
| Inhalation | MWCNT | Diameter 5–15 nm, Length 0.1–10 μm, Surface area 250–300 m2/g, MMAD 0.5–1.3 μm, Impurities 10% metal oxide | Male & female Wistar rats |
2, 8, 32 mg/m3, 5 days, 6 h/d | 3 days | Increase in BALF total cell counts, protein content, enzyme activities | Ma-Hock et al. (2009) |
| 0.1, 0.5, 2.5 mg/m3 13 wk, 6h/d | 0 day | Granulomatous Inflammation: 0.1 mg/m3(minimal), 0.5 mg/m3(slight), 2.5 mg/m3(moderate) | |||||
| Inhalation | MWCNT (mixture of MWCNTs and graphitic nanofibres) | Diameter 10–20 nm, Length 5–15 μm, Surface area 100 m2/g, MMAD 700–1000 nm/1800 nm, Impurities 0.5% Ni and Fe | Male C57BL/6 mice |
0.3, 1, 5.3 mg/m3, 7 and 14 days, 6 h/d | 0 day | No local pulmonary effects. Non-monotonic systemic immune suppression | Mitchell et al. (2007) |
| 0.3, 1 mg/m3, 14 days, 6 h/d | 0 day | Systemic immune suppression, not due to systemic uptake of MWCNT, but due to release of immune suppressing signals from the lung | Mitchell et al. (2009) | ||||
| Inhalation | MWCNT | Diameter 44 nm, Surface area 69 m2/g, Impurities Fe 0.0005% | Male Wistar rats |
0.37 mg/m3 (>70% individual), 4 weeks | 3 months | No inflammation No fibrosis |
Morimoto et al. (2012b) |
| Inhalation | MWCNT (Baytubes®) | Length 200–300 nm, Surface area: 253 m2/g, Impurities Co 0.46–0.53% | Male & female Wistar rats |
0.1, 0.45, 1.62, 5.98 mg/m3, 13 weeks | 6 months | No inflammation (0.1
mg/m3) Transient inflammation (0.45 mg/m3) Persistent inflammation (1.62 mg/m3) Persistent inflammation (5.98 mg/m3) Fibrosis, interstitial collagen staining (1.62 & 5.98 mg/m3) |
Pauluhn (2010) |
| Inhalation | MWCNT | Diameter 12.1 nm, Length 1.07 ± 1.1 μm, Surface area 225.6 m2/g Impurities 3.0% Al and 2.7% Fe (batch in 90d study) | Male & female Wistar rats |
0.06, 0.28, 4.84 mg/m3, 90 days | 90 days | No inflammation (0.0.06
mg/m3) Transient inflammation (0.28 mg/m3) Persistent inflammation (4.84 mg/m3) Minimal or slight interstitial collagen deposition (4.84 mg/m3) |
Pothmann et al. (2015) |
| Inhalation | MWCNT | Diameter 10–30 nm, Length 0.5–40 μm, Surface area 40–300 m2/g | Male C57BL/6 mice |
1, 30 mg/m3, 6 hours | 14 weeks | Subpleural fibrosis (transient) | Ryman-Rasmussen et al. (2009) |
| Inhalation | MWCNT | Diameter 5–10 nm, Length 10–30 μm, Surface area 168, 182, 224 m2/g | Male SD rats |
70 mg/m3, 6 hours | 21 days | No inflammation | Silva et al. (2014) |
| Inhalation | MWCNT: CM-95, CM-100 | CM-95: Diameter: 10–15 nm; Length: ~20 μm (2.57 μm ave); Surface area: 201 m2/g (BET); Purity: >95 wt% (iron, Mo, & Al2O3 impurities <2–3 wt% each) | |||||
| CM-100: Diameter: 10–15 nm; Length: ~200 μm (330 μm ave); Surface area: 225 m2/g (BET); Purity: >95 wt% (iron, Co, & Al2O3 impurities <2–4 wt% each) | Male & female F344 rats | 0, 0.2, 0.5, 1.0 mg/m3, 6 h/d, 5 d/wk, 28 | 0, 28 (male only), 90 days | Genotoxicity (comet assay) was significant in all exposed groups compared to controls. DNA damage was retained at 90-d post-exposure in male rats at 1 mg/m3 and in female rats at 0.5 & 1 mg/m3. Inflammatory cytokine levels in BALF were not elevated. The short-length MWCNT were persistent at 90 d post-exposure. | Kim et al. (2014) | ||
| Inhalation | SWCNT | Diameter 3 nm, Surface area 1064 m2/g, Impurities 0.03% | Male Wistar rats |
0.03,0.13 mg/m3, 4 weeks, 6h/d | 3 months | No inflammation, No fibrosis | Morimoto et al. (2012a) |
| Inhalation | CNF | Diameter 158 nm, Length 5.8 μm, Surface area 13.8 m2/g, Impurities carbon >99.5% | Male & female SD rats |
0.54, 2.5, 25 mg/m3, 13 weeks | 90 days | Persistent inflammation (25 mg/m3); thickening of interstitial walls; slight hypertrophy/hyperplasia | DeLorme et al. (2012) |
| Intratracheal instillation | MWCNT | Diameter 88 nm, Length 5 μm, Fe 0.44% | Male F344 rats |
40, 160 μg/rat | 91 days | Persistent inflammation and fibrosis | Aiso et al. (2010) |
| Intratracheal instillation | MWCNT | Diameter 10–30 nm, Length 0.3–50 μm, BET 109 m2/g | Male SD rats |
4 mg/kg | 21days | Slight fibrosis | Cesta et al.(2010) |
| Intratracheal instillation | MWCNT | Diameter 20–50 nm, Length 0.5–2 μm, Surface area 280 m2/g, Impurities >95% purity | Male SD rats |
1, 10, 100 μg/rat | 6 months | No inflammation, apoptosis of macrophages having phagocytosed MWCNTs (elimination) | Elgrabli et al. (2008) |
| Intratracheal aspiration | MWCNT | Diameter 31 nm, Length 20 μm, Surface area 50 m2/g, Impurity 3.5 wt% | Female C57BI mice |
20, 40 μg/mouse | 7 days | Transient inflammation | Han et al.(2010) |
| Intratracheal instillation | MWCNT | Diameter 60 nm, Length 1.5 μm, Surface area 23.0 m2/g, Impurities: 99.79% carbon (7–8% carbon soot), | Male SD rats |
0.04, 0.2, 1 mg/kg | 6 months | Increase in BALF neutrophils, eosinophils,
LDH, and TP levels increased BALF cytokine levels not changed Transient inflammation Minimal granuloma (1 mg/kg) |
Kobayashi et al. (2010) |
| Intratracheal instillation | MWCNT | Diameter 44 nm, Surface area 69 m2/g, Fe 0.0005% | Male Wistar rats |
0.66, 3.3 mg/kg | 6 months | Transient inflammation (0.66 mg/kg), Persistent inflammation (3.3 mg/kg) | Morimoto et al. (2012b) |
| Intratracheal instillation | MWCNT | CNT: length 5.9 μm Ground CNT: length 0.7 μm |
Female SD rats |
0.5, 2, 5 mg/rat | 60 days | Inflammation (until 15
days) Granuloma |
Muller et al. (2005) |
| Intratracheal instillation | MWCNT | Diameter 11–170 nm, Length 5–9 μm, Surface area 12.83 m2/g, Impurities >90% carbon | Male ICR mice |
5, 20, 50 mg/kg | 14 days | Increase in immune cells. Increase in proinflammatory cytokines (IL-1, TNF-α, IL-6, IL-4, IL-5, IL-10, IL-12, IFN-γ) and IgE. Distribution of B-cells in spleen |
Park et al. (2009) |
| Intratracheal instillation | MWCNT | Long, straight and bended tubes (SEM
micrographs) Slightly skewed unimodal size distribution with a peak size at ca. 5000 nm (DLS, dynamic light scattering) |
Female C57BL/6 mice |
18, 54, or 162 μg/ mouse (BALF) plus 2 or 6 μg/mouse (microarrays) | 24 hours | Increased number of inflammatory cells in BALF
in all treated groups (Neutrophils and eosinophils enhanced/control;
macrophages reduced Activation of acute phase and inflammation response in mouse lungs |
Poulsen et al. (2013) |
| Intratracheal instillation | MWCNT | Diameter 5–10 nm, Length 10–30 μm, Surface area 168, 182, 224 m2/g | Male SD rats |
0, 10, 50, 200 μg/rat | 21 days | Transient inflammation | Silva et al. (2014) |
| Intratracheal instillation (Intrapulmonary spraying) | MWCNT | MWCNT-N: Median and average lengths 3.02 and
3.64 ± 2.26 μm MWCNTM: Median and average lengths 4.47 and 5.11 ± 2.91 μm |
Male Fisher 344 rats |
250 μg/rat, five times over a 9-day period. Total: 1.25 mg/rat. | End of exposure | Frequent deposition of MWCNTs in mediastinal
lymph nodes and only a few penetrated MWCNTs through visceral
pleura Suggest that translocation route of MWCNT from lung into pleural cavity is lymphatic flow |
Xu et al. (2012) |
| Intratracheal instillation (Intrapulmonary spraying) | MWCNT | “L” sample Diameter 150 nm, Length 8 μm (Mean: 7.33 ± 6.69 μm, Median: 7.38 μm) |
F344 rats | 125 μg, 13 times over 24 weeks. Total: 1.25 mg/rat | End of exposure | Deposition in the parietal pleura; MWCNTs in
the pleural lavage Inflammation in the pleural cavity Thickened fibrotic areas, both parietal and visceral pleura Mesothelial proliferation, both parietal and visceral pleura Inflammation in the lung, lower than with “S” MWCNT in mediastinum, mesentery, lymph nodes |
Xu et al. (2014) |
| “S” sample Diameter 15 nm, Length 3 μm, Cotton-like |
F344 rats | 125 μg, 13 times over 24 weeks. Total: 1.25 mg/rat | End of exposure | MWCNT not found in the parietal
pleura Inflammation in the pleural cavity Thickened visceral pleura No mesothelial proliferation Inflammation in the lung, higher than with “L” MWCNT in mediastinum, mesentery, lymph nodes |
|||
| Intratracheal instillation | SWCNT | Diameter 1–2 nm, Length several μm (No exact characterization) | Male ICR mice |
0.5 mg/kg | 14 days | Release of cytokines (NF-κB) | Chou et al. (2008) |
| Intratracheal instillation | SWCNT | Diameter 44 nm, Surface area 877.7 m2/g | Male Wistar rats |
0.2, 0.4 mg/rat | 754 days | Granuloma (365 days) No granuloma (754 days) |
Fujita et al. (2015) |
| Intratracheal instillation | SWCNT | Diameter 12 nm, Length 0.32 μm, Surface area 1064 m2/g, Impurity 0.05% total metal | Male Wistar rats |
0.04, 0.2, 1, 2 mg/kg | 6 months (3 months: 2 mg/kg) | Increase in BALF neutrophils, macrophages,
lymphocytes, eosinophils, LDH, protein, and IL-1β,
IL-6. Transient inflammation (0.04 mg/kg), Persistent inflammation (1 and 2 mg/kg) Minimal granuloma (1 mg/kg) |
Kobayashi et al. (2011) |
| Intratracheal instillation | SWCNT | Diameter 1.8 nm, Surface area 878 m2/g | Male Wistar rats |
0.66, 1.32 mg/kg | 6 months | Persistent inflammation Minimal granuloma |
Morimoto et al. (2012c) |
| Intratracheal instillation | SWCNT | Nominal diameter 1.4 nm, Length >1 μm, Agglomerated rope ~30 nm | Male CD rats |
1, 5 mg/kg | 3 months | Transient inflammation | Warheit et al. (2004) |
| Intranasal instillation | Purified DWCNT (80% DWCNT, 20% SWCNT) | Diameter 1.2–3.2 nm, Length 1–10 μm (bundles up to 100 μm) | Male Swiss mice |
1.5 mg/kg | 48 hours | Local and systemic inflammation. No increase in TNF-α. Decrease in local oxidative stress |
Crouzier et al. (2010) |
| Pharyngeal aspiration | MWCNT | Diameter 49 nm, Length 3.86
μm, Impurity 0.78% total metal |
Male C57BL/6J mice |
10, 20, 40, 80 μg/mouse | 56 days | Increase in BAL PMNs, LDH,
albumin. Persistent inflammation Progressive fibrosis (80μg) |
Porter et al.(2010) Mercer et al. (2011) |
| Pharyngeal aspiration | SWCNT | Diameter 1–4 nm, Length 1–3 μm, Surface area 1040 m2/g; | Female C57BL/6 mice |
40 μg/mouse | 28 days | Persistent inflammation and granuloma | Murray et al. (2012) |
| Pharyngeal aspiration | Purified SWCNT | Diameter 1–4 nm, Surface area: 1040 m2/g, Impurities: 0.23% Fe | Female C57BL/6 mice |
0, 10, 20, 40 μg/mouse (0, 0.5, 1, 2 mg/kg) | 60 days | Inflammation (TNF-α and IL-1β
increased). Persistent inflammation and fibrosis |
Shvedova et al. (2005) |
| Pharyngeal aspiration | Purified SWCNT | Diameter 1–4 nm, Surface area: 1040 m2/g, Impurities: 0.23% Fe | Female C57 BL/6 mice |
0, 40 μg/mouse (0, 1.9 mg/kg) | 28 days | Robust, acute inflammation (PMNs, TNF-α, IL-6, LDH increased). | Shvedova et al. (2007) |
| Pharyngeal aspiration | SWCNT | Diameter 0.8–1.2 nm, Length 100–1000 nm, Surface area: 508 m2/g, Impurities: 17.7% Fe | Female C57 BL/6 mice |
0, 5, 10, 20 μg/mouse (0, 0.25, 0.5, 1 mg/kg) | 28 days | Inflammation (TNF-α, IL-6 and TGF-β increased) GSH depletion, lipid peroxidation, oxidised proteins | Shvedova et al. (2008) |
| Pharyngeal aspiration | DWCNT | Diameter 1–2 nm, Length <5 μm | Male C57BL/6 mice |
1, 10, 40 μg/mouse | 56 days | Persistent alveolitis(10 and 40μg) Interstitial fibrosis (10 and 40 μg) | Sager et al.(2013) |
| Pharyngeal aspiration | CNF | Diameter 80–160 nm, Length 5–30 μm, Surface area: 35–45 m2/g | Female C57BL/6 mice | 0, 120 μg/mouse | 1, 7, 28 days | Oxidative stress, alveolar connective tissue thickening (interstitial fibrosis) | Murray et al. (2012) |
Source: Adapted from, Tables 4.4.2 and 4.4.3 in the IARC monograph 111 (IARC, in press), which were originally developed by authors on this paper. Those tables were combined and re-structured in this current table; some of the study information has been revised; and additional studies have been added: Kim et al., 2014; Murray et al., 2012; Pothmann et al. 2015; Poulsen et al., 2013; Silva et al., 2014; Xu et al., 2012, 2014.
The mechanism of cell proliferation was examined in more depth in this review since it is a hallmark of cancer observed for other poorly-soluble particles and fibers, and relatively few such data are available to date for CNTs and CNFs. For example, a study showing focal adenomatous hyperplasia in alveolar epithelial tissues in mice inhaling MWCNT-7 (Sargent et al. 2014) is examined in greater detail (in Section “Epithelial cell proliferation and hyperplasia–MWCNT”) since that is the only study to date to report lesions that resemble the human preneoplastic lesion of atypical adenomatous hyperplasia (AAH) (Brambilla et al. 2001), although the incidence of spontaneous lung tumor in the control group is high (>20%). Smith et al. (2016) describe three scenarios involving alterations in cellular replication and/or cell-cycle control that are related to carcinogenesis – i.e., sustained replication, unrepaired DNA damage, and escape from normal cell-cycle control (Characteristic 10 of Table 7).
In general, it is recognized that different agents could act at different stages in the carcinogenic process, and that more than one mechanism may be involved (IARC 2006). Attention is given to key gaps in the data and to the relevance of the mechanisms to humans, especially when data are derived from experimental systems, as for CNTs and CNFs. In addition, consideration is given to current understanding and data gaps in the mechanisms of particle- and fiber-induced carcinogenesis in rodents and humans.
In vivo (rodent) effects associated with indirect genotoxicity
Biological events that can contribute to carcinogenicity without direct interaction of the toxicant with DNA include persistent inflammation, oxidative stress, and deregulation of pathways involved in stress response signaling, apoptosis, and cell proliferation. In this review and the IARC monograph (in press), in vivo studies in rodents were considered the most relevant assays currently available to assess these endpoints. In order to evaluate the strength of the evidence regarding the potential carcinogenicity of CNTs, we examined the available evidence on the key steps in the hypothesized cancer pathways, as shown in Figures 3 and 4. Inhaled particles and fibers deposited in the lung can induce transient or persistent pulmonary inflammation and fibrosis. Deposited particles that are not cleared can be translocated towards the pleura. When particles and fibers are retained in the lung, persistent inflammatory responses may occur. As a result, persistent lung injury and inflammation can trigger secondary genotoxicity due to oxidative stress and frustrated phagocytosis of long, rigid fibers or nanotubes. Cell proliferation is triggered in response to lung cell injury as well as by cytokines and growth factors released from inflammatory cells (Colotta et al. 2009; Guerard et al. 2015). Persistent inflammation, oxidative stress, and epithelial or mesothelial injury and cell proliferation are considered to play important roles in the development of lung cancer and mesothelioma. The role of fibrosis in carcinogenesis has been debated, and in a recent IARC review, the proposed mechanism for carcinogenicity of asbestos fibers considered fibrosis as a separate pathological response to pulmonary inflammatory events (Figure 4.2 in vol. 100C of IARC 2012). Studies on pulmonary inflammation, fibrosis and injury that investigated persistent responses in the lungs and pleura following inhalation or instillation of CNTs or CNFs were selected for evaluation. A summary of these studies is provided in Table 3, and the key events are discussed further below.
Inhalation of poorly-soluble particles or fibers into the lung is associated with persistent inflammation, oxidative stress, fibrosis, and cancer in rodents (Borm et al. 2004; Madl & Pinkerton 2009; Mossman et al. 2011, 2013). In the absence of regulatory exposure limits specific to CNTs or CNFs, these materials are by default grouped in the class of poorly-soluble respirable particles by regulatory agencies; moreover, CNTs and CNFs are high-aspect ratio nanomaterials similar to asbestos fibers (Section “Evidence on possible steps in pathway(s) to lung cancer”), and concern about potential carcinogenicity relates to the fibrous structure as well as biopersistence of CNTs and CNFs. Acute and persistent release of proinflammatory mediators from lung target cells have been used as a biomarker for inflammation induced by exposure to CNTs, as well as asbestos fibers (IARC 2012; Boyles et al. 2014). Studies on pulmonary inflammatory effects of CNTs in experimental animals are summarized in Table 3.
Inflammation
Three mechanisms have been proposed for the release of proinflammatory mediators from lung macrophages following uptake of CNTs and other high-aspect ratio nanomaterials: (1) frustrated phagocytosis elicited by long, high-aspect ratio nanomaterials (Johnston et al. 2010); (2) activation of the NLRP3 inflammasome leading to release of two key cytokines, IL-1β and IL-18 (Hamilton et al. 2009; Palomaki et al. 2011; Biswas et al. 2011); and (3) release of alarmins, including IL-1α, IL-33, and high mobility group box protein (HMGB1) following cell necrosis (Chan et al. 2012; Jessop & Holian 2014; Rabolli et al. 2014). These proinflammatory mediators trigger continued recruitment of inflammatory cells and persistent inflammation (Grivennikov et al. 2010). Elevated neutrophils in BALF (bronchioalveolar lavage fluid) have been measured in workers with respiratory impairment who had exposure to asbestos, coal, or silica (Rom 1991), in coal miners with pneumoconiosis (Vallyathan et al. 2000), and in patients with acute silicosis (Goodman et al. 1992; Lapp & Castranova 1993). Constitutive upregulation of intracellular signaling pathways, e.g., NF-κB, STAT-3, and AP-1, amplifies release of additional proinflammatory mediators, including chemokines, prostaglandins, and heat shock proteins (Laskin et al. 2011; Kundu & Surh 2012; DiDonato et al. 2012; Fan et al. 2013). The combination of persistent recruitment and activation of inflammatory cells, ongoing tissue injury, and impaired tissue regeneration has been viewed as a tumor-promoting environment (Kuriashy et al. 2011; Trinchieri 2012). Persistent inflammation has been viewed as the “seventh hallmark of cancer” and has been linked to the development of genetic instability (Colotta et al. 2009; Hanahan & Weinberg 2011). Persistent release of reactive oxygen and nitrogen species is associated with DNA and chromosomal damage, impaired DNA repair, and aberrant methylation and gene silencing via epigenetic alterations (O’Hagan et al. 2011; Baylin 2012; Kidane et al. 2014).
Recent studies using genetically-engineered mice that are deficient in key proinflammatory mediators or their receptors or in components of the NLRP3 inflammasome provide evidence for a mechanistic link between the generation of ROS and acute inflammation following exposure to asbestos fibers (Dostert et al. 2008) or CNTs (Girtsman et al. 2014; Sun et al. 2015). However, acute inflammatory responses assessed 24 h post-exposure may not be sustained at later time points depending on the physico-chemical properties of the CNTs, dose, and route of delivery (Silva et al. 2014). Mice genetically deficient for the IL-1 receptor were found to have an initial inflammatory response to CNTs that was blocked, while the response 28 days post-exposure was elevated (Girtsman et al. 2014). It is anticipated that biopersistent CNTs would be associated with persistent inflammation. Only a few studies have quantitated CNT clearance or retention (Mercer et al. 2013a,b; Silva et al. 2014); in one of the studies, persistent inflammation (on day 21 post-exposure) was not induced by somewhat biopersistent MWCNTs (i.e., at least 10% of the ~200 μg instilled MWCNT mass in male Sprague-Dawley rats was still present in the caudal lobes at day 21; the primary length of the MWCNT was 10–30 μm in dry bulk form, but the hydrodynamic size was generally <1 μm in length; as-produced MWCNT was more inflammogenic than the purified or carboxylic acid functionalized forms) (Silva et al. 2014).
A causal association between inflammasome activation, acute inflammation, and induction of malignant mesothelioma by asbestos fibers was not confirmed in NLRP3-deficient mice (Chow et al. 2012) despite the postulated links between persistent inflammation and chronic lung diseases associated with inhalation of crystalline silica or asbestos fibers (Chaput et al. 2013; De Nardo et al. 2014). Inflammasome activation may have different pathological outcomes depending on the initiating agent, stage in tumor development and progression, and the target tissue (Kolb et al. 2014). While there is experimental evidence linking persistent inflammation with genetic and epigenetic alterations in asbestos-induced lung cancer and malignant mesothelioma (Mossman et al. 2011; Broaddus et al. 2011; Mossman et al. 2013), there are no chronic studies linking persistent inflammation, genetic and epigenetic alterations, and cancer following exposure to CNTs.
Fibrosis
Lung granulomas accompanied by fibrosis are observed in most, but not all, studies of rodents exposed to CNTs (Landsiedel et al. 2014) (Table 3). In humans, granulomas are induced in response to biopersistent stimuli, such as aspirated foreign materials and fungal or mycobacterial infections. Sarcoidosis is a systemic disease of unknown etiology that can also induce lung granulomas (Mukhopadhyay et al. 2012). Granulomas may become fibrotic and calcified and may be confused with lung cancer on radiographic images; however, granulomas are not neoplastic (Borczuk 2012). There is limited evidence for a causal association between sarcoidosis and the development of lung cancer (Artinian and Kvale 2004; Bonifazi et al. 2015; Chopra & Judson 2015).
A potential causal association between pulmonary fibrosis and lung cancer is controversial. Interstitial pulmonary fibrosis in humans encompasses a range of diseases that may be idiopathic or secondary to dust inhalation, cigarette smoking, or exposure to radiation or drugs (Raghu et al. 2004; Katzenstein et al. 2010; Vassallo 2012). These diseases are characterized by repeated episodes of lung epithelial injury with impaired repair and attempted healing by fibrosis. Human epidemiological studies linking interstitial pulmonary fibrosis and lung cancer have shown different results depending on the patient population and geographic location (Sharma & Lamb 2003). Humans with lung diseases characterized by chronic inflammation and dysplasia (which includes idiopathic pulmonary fibrosis and diffuse interstitial fibrosis associated with pneumoconiosis, not related to asbestos exposure) are reported to have an increased risk of lung cancer in a cohort of 563 patients without asbestos exposure (Katabami et al. 2000).
In studies of other poorly-soluble particles or fibers, lung fibrosis is induced following inhalation of crystalline silica or asbestos fibers in animals and humans (Mossman et al. 2011; Leung et al. 2012) and may be linked to the development of lung cancer (IARC 2002; Laskin et al. 2011; IARC 2012). Some types of CNTs have also been shown to induce fibrosis in rodents following intratracheal instillation, pharyngeal aspiration, or inhalation (Bonner 2010 and summarized in Table 3). An in vitro study of well-dispersed SWCNTs suggested that individual CNT structures can mimic lung basement membrane substrate, enhancing fibroblast proliferation and collagen production (Wang et al. 2010). Macrophages and other resident inflammatory cells in the lung release profibrotic mediators may contribute to granuloma formation (Huizar et al. 2011), airway hyper-reactivity (Beamer et al. 2013), and impaired pulmonary and cardiovascular function (Wang et al. 2011b; Katwa et al. 2012). These pathological endpoints are associated with recruitment of innate immune cells, mast cell activation, and alternative macrophage activation associated with release of IL-13, IL-33, and osteopontin following exposure to CNTs in mice (Huizar et al. 2011; Katwa et al. 2012; Beamer et al. 2013; Wang et al. 2014b). The possible mechanistic links between these immunological responses associated with exposure to CNTs and development of cancer are unknown. However, in general during tumor development, macrophages in the tumor microenvironment shift their phenotype from an M1 proinflammatory phenotype to an M2 profibrotic phenotype (Sica & Mantovani 2012) with production of IL-10, an anti-inflammatory cytokine, arginase, and TGF-β, which contribute to fibrosis as well as a local immunosuppressive microenvironment (Hanahan & Coussens 2012; Multhoff et al. 2012). In vitro exposure to CNTs has been shown to induce co-expression of macrophage M1 proinflammatory and M2 profibrotic markers (Sanchez et al. 2011; Meng et al. 2015). It has been postulated that IL-33 release from lung epithelial cells polarizes macrophages toward an M2 profibrotic phenotype (Kurowska-Stolarska et al. 2009). The contributions of acute inflammation (i.e., resolves after the end of exposure) versus chronic inflammatory or immune response (i.e., persists after the end of exposure, or with repeated exposures) to the development of fibrosis and other chronic pathological endpoints, including cancer have not been systematically investigated following exposure to CNTs.
In a recent review article, Vietti et al. (2016) discuss the key events involved in the lung fibrotic reaction induced by CNTs. These events can include the activation of fibroblasts indirectly through the release of pro-inflammatory and pro-fibrotic mediators by inflammatory cells, induction of oxidative stress, activation of inflammasome or NF-kB (Nuclear Factor Of Kappa Light Polypeptide Gene Enhancer In B-Cells), or the direct induction of fibroblast proliferation, differentiation, and collagen production via signaling by ERK 1/2 (Mitogen-Activated Protein Kinases 1 and 2) or Smad (a family of proteins, named for their similarity to the Drosophila gene Mothers Against Decapentaplegic, Mad).
Pleural inflammation, fibrosis, and cancer associated with asbestos exposure
Inhalation of asbestos or erionite fibers can induce both non-neoplastic and neoplastic pleural reactions (Broaddus et al. 2011). Pleural effusions, fibrotic parietal pleural plaques, and diffuse visceral pleural fibrosis occur in humans with variable latent periods following initial exposure (Chapman et al. 2003). In contrast to these nonneoplastic reactions, diffuse pleural malignant mesothelioma occurs less frequently and often requires a prolonged latent period (e.g., 30–40 years in humans). A causal relationship between these inflammatory and fibrotic pleural reactions and the development of asbestos-related malignant mesothelioma has not been established, although bilateral calcified pleural plaques are a biomarker for asbestos exposure (Nishimura & Broaddus 1998). However, a statistically significant association was observed between mesothelioma and pleural plaques in asbestos-exposed male subjects. The presence of pleural plaques may be an independent risk factor for pleural mesothelioma (Pairon et al. 2013).
A recent study in humans revealed potential molecular links between pleural inflammation and hyperplasia with tumorigenesis mechanisms in pleura (Ramírez-Salazar et al. 2014). This study examined micro-RNA expression in a small number of mesothelioma cases (n = 5) compared to an equal number of cases with pleural fibrosis and chronic inflammation or atypical mesothelial hyperplasia. The targets of four down-regulated miRNAs in MPM (mir-181a-5p, miR-101–3p, miR-145–5p and miR-212–3p), one in PP (mir-101–3p) and one in HP (mir-494) were significantly enriched in “pathways in cancer”. The patients with mesothelial hyperplasia had a diagnosis of atypical mesothelial hyperplasia, which is considered by some pathologists to be a precursor lesion to malignant mesothelioma (it has some molecular changes characteristic of mesothelioma). However, no images were provided to confirm this diagnosis. Further studies are needed to determine the links between early changes and mesothelioma development.
The specific physico-chemical characteristics to predict fiber-induced cancer are not known, although the number of longer and thinner structures has been strongly associated with cancer in humans and in animals. In workers with exposures to airborne chrysotile asbestos during textile manufacturing, the fiber dimensions that best predicted lung cancer were structures of >10 μm in length and <0.25 μm in diameter; however, other fiber dimensions were also significantly associated with lung cancer and with asbestosis in those workers (which could have been due, in part, to correlations in the fiber size categories across cumulative exposures) (Stayner et al. 2008). Similar findings were reported in earlier animal studies. In rats administered amphibole asbestos or other fibrous minerals (by pleural implantation, in 72 experiments) Stanton et al. (1981) reported that the best predictor of pleural sarcoma was the number of structures of >8 μm in length and <0.25 μm in diameter; however, a highly siginificant relationship was also found for fibers of >4 μm in length and <1.5 μm in diameter (Stanton et al. 1981). The authors noted that fibers of other dimensions could not be determined to be noncarcinogenic due to correlations in the number of particles in various size categories. In another study in rats (by inhalation or intraperitoneal injection), significantly elevated pulmonary tumors and pleural mesothelioma were associated with exposure to long amosite (30% fibers >5 μm) compared to rats exposed to short amosite (1% fibers >5 μm) (Davis et al. 1986). These authors suggested that although shorter (<5 μm) fibers could translocate to the pleura, they were less carcinogenic than the longer fibers (~8–15 μm), and that the longest fibers (>25 μm) were unlikely to reach the pleura (Davis et al. 1986). Other fiber dimensions and aspect ratios have been proposed by investigators (e.g., Pott et al. 1987; Lippmann 1990; Berman et al. 1995; Quinn et al. 2000).
The mechanism of carcinogenesis of biopersistent particles (both nonfibrous and fibrous) involves persistent inflammation and lung injury, which can trigger secondary genotoxicity due to oxidative stress. For long, rigid structures including nanostructures (e.g., asbestos fibers or MWCNT-7), incomplete uptake by macrophages and/or frustrated phagocytosis are hypothesized to be important drivers of persistent inflammation and lung or mesothelial cell injury (Nagai & Toyokuni 2012; Donaldson et al. 2013). The translocation of fibers or nanotubes to the pleura is also considered to be important in the development of mesothelioma. Direct instillation of carbon nanotubes into the peritoneal space of mice has been shown to induce similar acute inflammatory responses as asbestos fibers, although the intensity of this response depends on the dimensions and agglomeration state of the sample used (Poland et al. 2008). The causal association between these acute inflammatory responses and development of malignant mesothelioma following exposure to carbon nanotubes has not been critically investigated in rodents due to the difficulty in imaging this anatomic compartment and the long latent period before tumors develop (Broaddus et al. 2011).
In vitro (cellular) responses associated with direct genotoxicity
Measurement of genotoxicity
DNA damage, chromosomal alterations, and cell cycle or centrosome disruption in vivo (rodents) or in vitro (human or rodent cells) are well-established markers of direct genotoxicity in humans and animals and are therefore considered to be the most relevant precursor endpoints to assess the potential carcinogenicity of CNTs and CNFs. IARC emphasizes that biomarkers that measure irreversible effects (e.g., chromosome aberrations and mutations) should be given higher weight than reversible effects (e.g., DNA damage measured by the comet assay) in the assessment of whether or not a specific mechanism of genotoxicity is operating in humans (IARC 2006). However, tests for irreversible effects are typically not suited for high through-put screening of many types of nanomaterials because they are laborious and there is an element of further exploration of test results (e.g., assessment of mutagenic spectrum after a positive test result on mutant frequency). One can expect that the development of genomic approaches to DNA repair and mutagenesis will be highly beneficial for finding relevant biomarkers (Wyrick & Roberts 2015). A correlation between aneuploidy in vitro and mesothelioma in vivo was found for asbestos and other fibers of dimensions reported by Stanton (length >8 um, diameter ≤0.25 um) in cultured rat pleural mesothelial cells and in rats by intrapleural injection, suggesting that chromosomal mis-segregation (resulting in aneuploidy) is a critical step in mesothelioma development (Yegles et al. 1995). Simple assays for DNA damage (e.g., comet assay) can be used to compare many types of nanomaterials within the same experimental setting of cell cultures and experimental animal models. The same is true for measurements of gene expression patterns where it principally should be possible to compare transcription profiles between benchmark carcinogenic CNTs (e.g., MWCNT-7) and other types of CNTs in attempt to bridge pathologic information and hazards.
The measurement of direct genotoxicity includes a number of endpoints, such as DNA strand breaks, including double strand breaks, and oxidatively damaged DNA base products. Measurement of oxidatively damaged DNA has mainly encompassed 8-oxo-7,8-dihydro-2′-deoxyguanosine (8-oxodG) as determined by chromatographic techniques, antibody-based methods or comet assay modified with repair enzymes to detect oxidatively damaged DNA. This lesion is mutagenic in mammalian cells and gives rise predominantly to G to T transversions (Moriya 1993). Oxidatively damaged DNA (measured by the modified comet assay) has been observed in human bronchial epithelial, lung and mesothelial cells (Jacobsen et al. 2008; Pacurari et al. 2008; Lindberg et al. 2009, 2013), human colon carcinoma tissue (HT29) cells (Pelka et al. 2013), fibroblasts (Kisin et al. 2007, 2011; Yang et al. 2009), phytohemagglutinin-stimulated lymphocytes (Kim & Yu 2014), macrophages (Migliore et al. 2010; Di Giorgio et al. 2011), and human hepatocytes (Alarifi et al. 2014; Vesterdal et al. 2014a). In principle, oxidatively generated DNA lesions can be repaired and, therefore, may not give rise to permanent genomic changes, such as cytogenetic lesions and mutations.
Misrepaired or unrepaired DNA double strand breaks and mitotic defects induce structural and numerical chromosome abnormalities, respectively. DNA damage at the chromosome level is measured by micronucleus assay, which may differentiate clastogenic (fragments without centromeres) and aneugenic (entire chromosomes with centromeres) effects, and by numerical changes in fluorescent in situ hybridization of inter-phase cells and chromosomal aberrations in mitotic cells. Micronuclei are chromosome fragments resulting from chromosome breakage and/or mitotic spindle damage, which are observed in interphase cells (Kisin et al. 2011).
The alkaline comet assay has been particularly popular for studying direct genotoxicity by exposure to CNTs as well as other types of engineered nanomaterials and particulate matter from combustion-derived air pollution (Møller et al. 2015a). The comet assay measures DNA migration in an agarose gel by electrophoresis at either neutral or alkaline pH. DNA double strand breaks are detected by the neutral comet assay, whereas the alkaline comet assay (pH above 13) detects DNA strand breaks and lesions that are converted to DNA strand breaks by high pH conditions (so-called “alkaline labile sites”). The alkaline comet assay detects DNA damage; however, the damage is referred to as DNA strand breaks in this review to distinguish this type of damage from other lesions that are detected by modified versions of the assay. One such modified version of the comet assay uses a digestion step with DNA repair enzymes from bacterial or human cells. The enzymes encompass formamidopyrimidine DNA glysolase (FPG), endo-nuclease III (ENDOIII) or human oxoguanine DNA glycosylase (hOGG1). The FPG enzyme cleaves DNA at ring-opened formamidopyrimidine lesions, including 2,6-diamino-4-hydroxy-5-formamidopyrimidine (FapyGua) and 4,6-diamino-5-formamidopyrimidine (FapyAde). ENDOIII lesions encompass oxidized pyrimidines, such as uracil glycol, thymine glycol, 5-hydroxycytosine and 5-hydroxyuracil. hOGG1 can detect 8-oxo-7,8-dihydroguanine (8-oxoGua) or 8-oxodG. Results from these enzyme-modified comet assays are reported either as total sites (DNA strand breaks plus extra breaks generated by the enzyme) or enzyme-sensitive sites (breaks generated by the enzyme minus basal level of DNA strand breaks).
Concordance of approximately 80% has been reported between exposure to animal carcinogens and positive outcome in terms of elevated levels of DNA strand breaks (as assessed by the comet assay in cultured cells and animal tissues) (Anderson et al. 1998; Sasaki et al. 2000; Møller 2005). The alkaline version of the comet assay, for measurement of DNA strand breaks, complements the micronucleus assay as standard genotoxicity tests (Rothfuss et al. 2010). Indeed, a recently published critical review of studies on various types of engineered nanomaterials cites the concordance between comet assay results and micronuclei at approximately 80% (Karlsson et al. 2015).
The finding of genotoxicity by the comet and micronucleus assays suggests that genetic and related effects could occur in mammals. Comet assays indicates the occurrence of DNA breakage, either single or double strand, depending on the analytical method used. The detection of single strand breaks (SSB) depends on the location of the lesion on untranscribed (global genome Nucleotide Excision Repair, NER) or transcribed (transcription coupled-NER) regions of DNA (Hanawalt & Spivak 2008; Scharer 2013). Double strand breaks (DSB) can generate errors in DNA following repair by non-homologous end-joining of breaks and may be a source of mutations (Malkova & Haber 2012; Guirouilh-Barbat et al. 2014). A micronucleus is formed during anaphase in mitosis. It contains a whole or fragment of chromosome. This is indicative of defective chromosome segregation. In the next mitoses, a daughter cell may lack a whole chromosome or part of a chromosome. These mitotic defects contribute to chromosomal instability.
There are several methods to investigate mutations. Bacterial assays (Ames tests) are well known for studying mutations induced by chemicals and are done by determining the rate of histidine + auxotrophs revertants grown on glucose-minimal salts agar plates. However, the applicability of bacterial assays for studying mutation caused by particles is limited due to lack of particle uptake by bacteria (Jaurand et al. 2009). Mutation tests are available for studying eukaryotic cells. With mammalian cells, mutation is determined via the hypoxanthine-guanine phosphoribosyltransferase (HGPRT) locus; the HGPRT gene plays a role in the purine salvage pathway for DNA synthesis. After treatment with a tested agent, mammalian cells are cultured in a medium containing 6-thio-guanine, which is metabolized to a toxic compound that kills the cells. However, if the HGPRT gene is mutated, the cells remain viable. Transgenic mice and cells from transgenic mice, such as guanine phosphoribosyltransferase (gpt) delta mice and Big Blue® mouse and Big Blue® rat models, are used to test mutagenicity. The mutations detected are principally base pair substitution, frameshift, and small insertions/deletions. The gpt delta mice carry the gpt gene of E. Coli and approximately 80 copies of phage lambda EG10 DNA. The lacI Big Blue® uses a lambda shuttle vector containing the bacterial lacI gene, which encodes the repressor protein of the lacZ gene. These assays are used to determine the number and type of mutations in target genes in different tissues (Environmental Health Criteria 233. Transgenic Animal mutagenicity assays. 2006. www.who.int/ipcs/publications/ehc/ehc233.pdf; Transgenic Rodent Gene Mutation Assays (OECD) by G. R. Douglas. http://www.oecd.org/chemicalsafety/testing/46161373.pdf). Detection of mutations in mammalian cells and tissues can be also made directly by DNA sequencing analysis in specific genes, especially oncogenes. Transgenic rodent mutation assays allow the determination of mutations in different organs. There is no target tissue restriction since the mutations can be determined in tissues where the particles translocate, or even if an effect at a distant site occurs. The advantages (availability of different modes of administration and analysis of numerous tissues, assay focusing on gene mutations, correspondence with transgenic in vitro assays) and disadvantages (limited sensitivity to clastogens, spontaneous mutant frequency and cost) have been recently summarized (Lambert et. al. 2005). WHO considers that a positive study is predictive of carcinogenicity, and a negative result in a properly conducted transgenic mutation assay demonstrates that the agent is not a gene mutagen (WHO 2006).
Genotoxicity is an important endpoint related to potential carcinogenicity. In vivo genotoxicity data on CNTs and CNFs were limited at the time of the IARC Monograph 111 meeting, and some targeted literature searches were subsequently performed for this review. A full systematic literature search was not performed; however, the following two regular, targeted literature searches were performed. One search strategy used PubMed, limited to 5 years, with the keywords carbon nanotube (toxicity OR microarray). Publications on genotoxicity or gene expression were selected. Another search strategy used PubMed, EMBASE and Web of Science, with an approach similar to that described in Møller & Loft (2010). The search terms for materials (carbon nanotubes, SWCNT, MWCNT) were combined with those for endpoints (DNA damage, genotoxicity, strand breaks, oxidative DNA damage, micronuclei, micronucleus assay, comet assay, FPG, ENDOIII, 8-oxodG, 8-OHdG, 8-oxoguanine, chromosome aberrations, mutations, Ames test). Publications were selected that reported results in mammalian species (the exception being Ames test or mutations in E. coli). A larger number of in vitro genotoxicity studies have been published, and the number is growing; similar literature search strategies were performed to update the available information in this area. Both positive and negative studies were evaluated for inclusion in this review. A few of the selected studies were excluded (as described in Tables S-1–S-4 in the online supplemental material).
Inclusion criteria for DNA damage endpoints
Only studies that have used characterized materials have been included in the review. Information on dimensions, specific surface area and purity was considered necessary. The abstracted information in the tables is primarily fiber characteristics in dry form as supplied from the supplier. This inclusion criterion is pragmatic, as a very strict inclusion criterion would limit the number of studies in the review without promoting a better comparison of effects between studies.
The level of DNA damage in the comet assay is obtained by measuring the extent of DNA migration in a single nucleus in an agarose gel using fluorescence microscopy. It is standard practice in the comet assay to calculate the mean or median from 50 to 100 comets per gel and regard this as the experimental unit for statistical analysis (Møller & Loft 2014). However, a frequent flaw in comet assay studies has been to include all comets in the statistical analysis, sometimes even without independent replicates on different days, which gives rise to remarkably high statistical power (Møller et al. 2015a). This issue applies to all versions of the comet assay, although it seems most often to be observed in studies using the alkaline version for detection of strand breaks. The inclusion criterion for comet assay endpoints in the present critical review is from studies that report results from independent replication, typically on three different days in cultured cells or from different animals. The statistical analysis is based on the cell culture (not individual comets) or animals.
Another issue related to assessment of DNA damage by the comet assay is a lack of efficient blinding of samples before the inspection of slides in a microscope. It is sometimes suspected that the comet assay is a subjective technique for determination of DNA damage because it relies on the investigator finding the comets in the gel. Indeed, there is inter-investigator variation in the scoring of comets by the comet assay, but a formal assessment of this type of process has shown that individual investigators display a remarkable consistency in scoring over time (Forchhammer et al. 2008). Thus, subjectivity related to selecting comets is not a problem if it is the same investigator who analyzes all blinded samples in a study. Still, it is not possible to assess whether or not samples have been properly blinded before analysis in the comet assay; a lack of clarity about blinding does not per se indicate that the samples have not been blinded before analysis. It should also be noted that a formal assessment of the effect of blinding in the comet assay in an inter-laboratory validation trial with 12 laboratories did not indicate that investigators were biased when they knew the content of the samples (Forchhammer et al. 2012). Still, it should be stressed that the recent OECD guidelines for the in vivo comet assay specifically states that “all slides for analysis, including those of positive and negative controls, should be independently coded and scored “blinded” so the scorer is unaware of the treatment condition” (OECD 2014). The same applies to in vitro studies, although it should be acknowledged that efficient coding of samples can be a problem for high concentrations of certain types of nanomaterials because there can be residual particles left in the DNA or agarose gel despite repeated washings of the cells after the exposure in culture dishes. The complete blinding of samples can be a challenge in vivo as well since particles may be visible in tissues or BALF.
The inclusion criterion for studies on 8-oxodG by chromatographic or antibody based techniques is that levels of DNA lesions are less than 5 lesions/106 dG. The European Committee on Oxidative DNA Damage recommends that studies with higher baseline levels of 8-oxodG than 5 lesions/106 dG in unexposed cells or animals should be interpreted with caution because of risk of flawed methodology (ESCODD 2003). This was clearly demonstrated by the fact that much higher values of 8-oxodG are obtained by ELISA techniques as compared to chromatographic assays (Barregard et al. 2013). Studies on particulate matter from air pollution or engineered nanomaterials with non-optimal assays for detection of oxidatively damaged DNA in animal tissues more often show increased genotoxicity than studies with optimal assays (Møller et al. 2013). In addition, studies with non-optimal detection of oxidatively damaged DNA report a larger effect size than studies with optimal assays (Møller et al. 2015b). Publication bias is a likely explanation for the fact that studies with positive test results on oxidatively damaged DNA are more likely to be reported in the literature because these assays are typically easy to perform and can be purchased as commercially kits. The consequence of using a poor method of 8-oxodG measurement and flawed statistical analysis can perhaps be appreciated by the fact that two papers on oxidative stress and genotoxicity in titanium dioxide exposed mice were retracted by the editor of Particle and Fiber Toxicology (see Pubmed identification number (PMID) 26169674 and 26169780). Only studies that report levels of oxidatively damaged DNA as lesions/106 dG or have reported DNA damage levels in units that can be converted to lesions/106 dG are included in the review. In addition, only studies that investigated genotoxicity in lung tissue are included in the review.
The Supplementary material (Tables S-1 and S-3) provides information about studies that examine effects in tissues other than the lung. Studies that assessed genotoxicity in cultured cells are provided in Supplementary Tables S-2 and S-4. Some of the genotoxicity endpoints evaluated in IARC Monograph 111 are not included in this review paper, for the reasons discussed above. Genotoxicity endpoints from new studies were evaluated for inclusion in this review paper using the same criteria. In general, there is approximately 50% overlap between the genotoxicity endpoints included in the IARC Monograph 111 (IARC, in press) and in this review paper, for both in vitro and in vivo results.
Indirect genotoxicity of CNTs and CNFs: rodent studies
Evidence on possible steps in pathway(s) to mesothelioma
Overall evidence
For some MWCNTs, evidence is available on the translocation of MWCNTs from lungs to the pleural cavity, inflammation and fibrosis in the pleural cavity, and proliferation of mesothelial cells. These studies, which are summarized below, provide the current state of evidence to evaluate the potential for exposure of MWCNTs in the respiratory tract to cause possible precursor events related to the development of malignant mesothelioma.
There are no studies to date on the translocation of SWCNTs from the lungs into the pleural cavity, inflammation in the pleural cavity, fibrosis in the pleural cavity, or proliferation of mesothelial cells in experimental animals. No studies were found that had investigated these endpoints with SWCNT. No studies of these endpoints were found for CNFs.
Translocation to pleura – MWCNT
Translocation of MWCNTs from the lungs to the pleural cavity was reported in several inhalation and intratracheal instillation studies. Mercer et al. (2010) performed a pharyngeal aspiration study in mice exposed to MWCNT-7 and showed that MWCNTs had penetrated the intrapleural space from the lungs 56 days post-exposure (80 μg dose). The translocation was confirmed by morphological observation (Porter et al. 2010). MWCNTs were observed in rat pleural cavity lavage after administration of two types of MWCNTs – MWCNT-M (same as MWCNT-7); and MWCNT-N – at a total dose of 1.25 mg/rat by intrapulmonary spraying (Xu et al. 2012). The investigators found frequent deposition of MWCNTs in mediastinal lymph nodes but only a few MWCNTs penetrated through the visceral pleura, suggesting that translocation of MWCNTs from the lung into the pleural cavity occurs via lymphatic flow. However, the mechanism of translocation is not well known. Fibers could also reach the pleura via capillaries, and parietal lymphatic drainage may concentrate the fibers in areas of the parietal pleura (Miserocchi et al. 2008). Yet, in the Xu et al. (2012) study, no MWCNTs were observed in the parietal pleura. In another study, inhaled MWCNTs were located in the subpleural area 1 day after inhalation (Ryman-Rasmussen et al. 2009). Those researchers proposed that activated CNT-containing macrophages travel via pleural lymphatic drainage and stimulate mononuclear cell recruitment, thus increasing focal aggregates (Ryman-Rasmussen et al. 2009).
When F344 rats were exposed by transtracheal spraying to a larger sized needle-like MWCNT (MWCNT-L) and a smaller sized MWCNT (MWCNT-S) that forms cotton-like aggregates over a period of 24 weeks, the MWCNT-L, but not the MWCNT-S, translocated into the pleural cavity, deposited in the parietal pleura, and was found in pleural lavage (Xu et al. 2014).
Pleural cavity inflammation – MWCNT
Inflammation following inhalation or intratracheal instillation of MWCNTs has been shown by some studies (Ryman-Rasmussen et al. 2009; Xu et al. 2012; Xu et al. 2014). Acute pleural mononuclear cell aggregates were found following inhalation of 30 mg/m3 of MWCNTs (6 hr in 1 day; examined up to 14 wk post-exposure); however, cell aggregates returned to control levels 6 weeks post-exposure (Ryman-Rasmussen et al. 2009). In a study of two types of MWCNTs (MWCNT-7 and MWCNT-N) administered using intrapulmonary spraying at a total dose of 1.25 mg/rat, the proportion of macrophages increased, while the proportion of neutrophils and lymphocytes decreased, in the pleural cavity lavage fluid compared to the vehicle control (Xu et al. 2012); a no treatment control was not included in that study. In a more recent study, rats were exposed to MWCNT-L and MWCNT-S over 24 weeks (total dose: 1.625 mg/rat; 0.125 mg/dose once every 2 weeks, total 13 times). MWCNT-L induced stronger inflammatory reactions than MWCNT-S, including increased inflammatory cell number and cytokine / chemokine levels in the pleural cavity lavage (Xu et al. 2014). Inflammation in the pleural cavity to either MWCNT-S or MWCNT-L was significantly greater than that in the vehicle or no treatment controls (Xu et al. 2014). To date, a limited number of studies have been published on chronic pleural inflammation of MWCNT administered to the lungs (by inhalation, intratracheal instillation, or pharyngeal aspiration) (i.e., Xu et al. 2014 only is published at this time).
Pleural cavity fibrosis – MWCNT
Pleural fibrosis following CNT exposure has been described by Ryman-Rasmussen et al. (2009) and Xu et al. (2012). Following an inhalation exposure of 30 mg/m3 of MWCNTs, subpleural fibrosis disappeared at 14 weeks post-exposure (Ryman-Rasmussen et al. 2009). Xu et al. (2012) examined fibrotic changes by Azan-Mallory’s staining on lung tissue from rats exposed to two types of MWCNTs by intrapulmonary spraying. Subpleural collagenous fibrosis was found under mesothelial cell proliferation. However, pleural and alveolar fibrosis could not be readily distinguished. In the study by Xu et al. (2014) using intrapulmonary spraying, MWCNT-L, but not MWCNT-S, induced fibrosis as assessed by a significant increase in parietal and visceral thickening as compared to controls.
Mesothelial cell proliferation – MWCNT
We have judged the proliferation of mesothelial cells as a marker of pleural injury. Xu et al. (2012) showed that MWCNTs induce visceral mesothelial cell proliferation by proliferating cell nuclear antigen (PCNA) immunostaining after acute exposure (five intratracheal doses over 9 days), accompanied with elevated pleural inflammation and fibrosis. MWCNTs and crocidolite each induced PCNA at approximately 10-fold that of the vehicle control. Patchy parietal mesothelial proliferation lesions were found in rats treated with MWCNT-L, but not MWCNT-S (Xu et al. 2014). However, limited data are available to date on chronic or persistent pleural injury following exposure to MWCNTs through inhalation.
Evidence on possible steps in pathway(s) to lung cancer
Overall evidence
A number of studies of various types of MWCNT, several studies of SWCNT, and a few studies of double wall CNT (DWCNT) or CNF have shown pulmonary inflammatory and fibrotic responses associated with exposure to these materials by various routes of exposure to the respiratory tract (Table 3). The observed endpoints include increase in proinflammatory cytokines, granulomas and granulomatous inflammation, persistent inflammation, and fibrosis. In some studies, these endpoints were not observed or were not persistent at post-exposure time points (Table 3). MWCNTs of various types are the most studied of these materials to date. The studies of SWCNT tend to show persistent inflammation and fibrosis, while the findings in the MWCNT studies were more mixed (Table 3). The influence of the various material-specific vs. experimental factors on these response endpoints has not been assessed, including the contribution of the dose and duration of exposure, the physical-chemical characteristics of the materials, and the experimental design factors including species and route of exposure.
Pulmonary inflammation
MWCNT
There are many studies in which investigators observed pulmonary inflammation following inhalation and intratracheal instillation of MWCNTs in rats or mice. Duration of exposure and post-recovery period for the inhalation exposure are indicated in Table 3.
Persistent inflammation was found in three 13-week inhalation studies of two MWCNTs and one CNF in rats. Ma-Hock et al. (2009) and Pauluhn (2010) detected inflammation in the lungs of both female and male rats. High concentrations of CNFs were found to induce inflammation (DeLorme et al. 2012), while persistent or moderate inflammation was found to occur with exposure to minimum concentrations of 2.5 mg/m3 (Ma-Hock et al. 2009) or 1.5 mg/m3 (Pauluhn 2010) MWCNTs and 25 mg/m3 (DeLorme et al. 2012) CNFs.
Two 4-week inhalation studies of Wistar rats exposed to MWCNTs resulted in no evidence of persistent inflammation (Morimoto et al. 2012b; Kim et al. 2014). The maximum concentrations tested, which did not induce significant inflammation, were 0.37 mg/m3 (Morimoto et al. 2012b) and 0.96 mg/m3 (Kim et al. 2014). The lung burdens and retention kinetics were not reported in those studies. An acute (6-h) inhalation study of MWCNTs in rats provided evidence of persistent inflammation at the concentration of 241 mg/m3 at 3 months post-exposure (Ellinger-Ziegelbauer & Pauluhn 2009); the deposited pulmonary dose at the end of the 6-h exposure can be estimated at approximately 840 μg (as described in NIOSH 2013, Section A.2.2, assuming a ventilation rate of 0.21 L/min and 0.046 alveolar deposition fraction), which is in the range of the minimum mass particle dose associated with overloading of pulmonary clearance in rats (Section “Clearance and Retention”) and would be a higher volumetric dose given the less than unit density of the MWCNT Baytubes® (Pauluhn 2010). Another acute (6-h) inhalation study of three types of MWCNTs in rats at 70 mg/m3 showed no inflammation on day 21 post-exposure; the estimated deposited dose was reported as 380 μg for each of the MWCNT materials inhaled, “as produced”, purified, or carboxylic acid functionalized MWCNT in dispersion media (assuming a ventilation rate = 0.15 L/min, and 0.1 alveolar and tracheobronchial deposition fraction) (Silva et al. 2014). In the same study (Silva et al. 2014), 200 μg of each MWCNT materials administered by intratracheal instillation resulted in inflammation on day 1 but resolved by day 21 post-exposure. The administered and estimated doses in Silva et al. (2014) were below the minimum rat overloading doses on a mass basis (Section “Clearance and Retention”); density was not reported, which is needed to estimate the equivalent volumetric doses.
There are many intratracheal instillation or pharyngeal aspiration studies of MWCNTs that report the development of persistent inflammation in the lungs of rats and mice (e.g., Han et al. 2010; Aiso et al. 2010; Cesta et al. 2010; Porter et al. 2010; Kobayashi et al. 2011; Morimoto et al. 2012b; Murray et al. 2012; Sager et al. 2013). On the other hand, several intratracheal instillation studies of MWCNTs showed only transient inflammation in the lungs of rats (Kobayashi et al. 2010; Silva et al. 2014). In a study by Xu et al. (2014), rats were exposed to MWCNT-L and MWCNT-S over 24 weeks (total dose: 1.625 mg/rat; 0.125 mg/dose once every 2 weeks, total 13 times), and in contrast to the pleura, MWCNT-S induced stronger inflammation in the lung than MWCNT-L.
Exposure to MWCNTs has been found to induce expression of a variety of cytokines that trigger inflammation. When pulmonary and systemic immune responses induced by intratracheal instillation of MWCNTs were investigated in mice (Park et al. 2009), the total number of immune cells in BALF were significantly increased in treated groups (5, 20, and 50 mg/kg doses of MWCNTs), and the distribution of neutrophils was elevated 1 day after instillation. Pro-inflammatory cytokines (IL-1, TNF-α, IL-6, IL-4, IL-5, IL-10, IL-12, and IFN-γ) were also increased in a dose-dependent manner in BALF. The highest levels of most cytokines occurred 1 day after instillation and thenceforward decreased. Th2-type cytokines (IL-4, IL-5, and IL-10) were elevated in mice exposed to MWCNTs compared to Th1-type cytokines (IL-12 and IFN-γ). Mice that were intranasally instilled with 1.5 mg/kg of double walled carbon nanotubes (DWCNTs) displayed lung inflammation and a decrease in oxidative perturbations, which were investigated using electron spin resonance and spin trapping experiments (Crouzier et al. 2010). Poulsen et al. (2013) exposed C57BL/6 mice to a single intratracheal instillation of 18, 54, or 162 μg of MWCNT-7/mouse. BALF and lung tissue samples were collected 24 h post-exposure. The total number of inflammatory cells in BALF was significantly increased in all treated groups, and while the numbers of neutrophils and eosinophils were elevated, the number of macrophages was reduced. DNA microarrays, confirmed by gene-specific RT-qPCR (employed to study mechanistic responses), showed a dose-dependent inflammatory response was observed in BALF from the mouse lungs, even at the lowest dose of 2 μg/mouse.
There are reports of transient pulmonary inflammation that are not accompanied by increased cytokines in BALF. When biological responses to a single intratracheal instillation of MWCNTs were assessed in rats (Kobayashi et al. 2010), transient pulmonary inflammation was observed in the lungs of rats exposed to 1 mg/kg of MWCNTs. However, BALF cytokine levels did not significantly change at any time point.
SWCNT
A four-week inhalation study of Wistar rats exposed to SWCNTs revealed no evidence of persistent inflammation in the lung (Morimoto et al. 2012a). On the other hand, intratracheal instillation and pharyngeal aspiration studies demonstrated that exposure to SWCNTs did cause the development of persistent inflammation in the lung (Lam et al. 2004; Shvedova et al. 2005; Morimoto et al. 2012c). One intratracheal instillation study reported that rats exposed to SWCNTs had transient inflammatory responses in the lung (Warheit et al. 2004).
SWCNTs have been found associated with altered expression of a variety of cytokines, including proinflammatory cytokines, with the advent of inflammation. Pulmonary and systemic responses were assessed in rats after intratracheal instillation of highly pure, well-dispersed, and well-characterized SWCNTs (Kobayashi et al. 2011). The number of BALF inflammatory cells increased in a dose-dependent manner. Significant increases in IL-1β and IL-6 levels were observed at several time points. However, only small differences were observed for IL-1α, IL-2, IL-4, IL-10, GM-CSF, INF-γ, or TNF-α between SWCNT-exposed groups and controls at any of the time points. Shvedova et al. (2005) demonstrated that pharyngeal aspiration of SWCNTs elicited acute inflammation with early onset and progressive fibrosis and granulomas. An early neutrophil accumulation, followed by lymphocyte and macrophage influx, was accompanied by early elevation of proinflammatory cytokines (TNF-α, IL-1β) and then by fibrogenic transforming growth factor, TGF-β1. Mice exposed by inhalation to the more dispersed, smaller SWCNT developed an approximately four-fold greater pulmonary inflammation, interstitial collagen deposition, and fibrosis, when compared with mice exposed by pharyngeal aspiration to the less dispersed, larger SWCNT. However, the effects of particle size and dispersion could not be entirely determined because of the different routes of exposure and iron content, which was 0.2% Fe in the purified SWCNT administered by pharyngeal aspiration (Shvedova et al. 2005) and 17.7% Fe in the unpurified SWCNT used in the inhalation study (Shvedova et al. 2008).
Fibrosis and granulomas
MWCNT
Pulmonary fibrosis, as well as induction of lung granulomas, has been observed following inhalation and intratracheal instillation of MWCNTs in rats and mice. Studies of Wistar rats exposed to two different types of MWCNTs for 13 weeks via inhalation provide evidence of granulomatous inflammation and fibrosis (Ma-Hock et al. 2009; Pauluhn 2010). Fibrotic responses in rat lung were found following 0.4 mg/m3 MWCNT exposure by both sexes (Pauluhn 2010). Six-hour inhalation studies of MWCNTs (Ellinger-Ziegelbauer & Pauluhn 2009; Ryman-Rasmussen et al. 2009) also provide evidence of persistent fibrosis in male rats and mice. In contrast, in a 4-week inhalation study, Wistar rats exposed to MWCNTs showed no evidence of lung fibrosis (Morimoto et al. 2012b). The maximum MWCNT concentration that did not induce fibrosis was 0.37 mg/m3.
A number of intratracheal instillation studies using rats or mice reveal that following exposure to MWCNTs, animals develop persistent or progressive fibrosis in the lung (Muller et al. 2005; Aiso et al. 2010; Cesta et al. 2010; Porter et al. 2010; Mercer et al. 2011, 2013a; Murray et al. 2012; Sager et al. 2013). On the other hand, some intratracheal instillation studies of rats exposed to MWCNTs report only transient or minimum fibrosis in the lung (Kobayashi et al. 2010; Morimoto et al. 2012b). In the above-mentioned studies (Kobayashi et al. 2010; Morimoto et al. 2012b), MWCNT length was relatively short but within an appropriate range of airborne MWCNTs that may be found in a work environment (Han et al. 2008).
SWCNT
Some intratracheal instillation, pharyngeal aspiration, and inhalation studies show that exposure to SWCNTs causes persistent or progressive fibrosis in the lungs in mice (Lam et al. 2004; Shvedova et al. 2005, 2008). In those studies, SWCNTs containing certain metals (26% nickel and 5% yttrium) (Lam et al. 2004) or higher metal content (17.7% vs. 0.2% iron) (Shvedova et al. 2005, 2008); or 27% vs. 2% iron (Lam et al. 2004) were more fibrogenic than SWCNTs with the lower metal content. In addition, the SWCNT containing nickel and yttrium resulted in high mortality; 5 of 9 mice died 4–7 days after intratracheal instillation of the 0.5 mg dose. Yet, SWCNT with either low or high metal content was associated with early onset and persistent pulmonary fibrosis, following exposure by either IT instillation, pharyngeal aspiration, or inhalation (Lam et al. 2004; Shvedova et al. 2005; 2008).
Other studies did not observe transient or minimum lung fibrosis in rats exposed to SWCNTs by intratracheal instillation (Morimoto et al. 2012c; Fujita et al. 2015). In the Fujita et al. (2015) study, granuloma formation in the lungs did not disappear until 2-years post-exposure. SWCNTs used in the study were relatively short compared to those in other SWCNT studies.
Epithelial cell proliferation and hyperplasia
MWCNT
Some studies provide evidence of proliferation of bronchiolar and alveolar epithelial cells following exposure to MWCNTs or CNFs by inhalation or instillation. In a 13-week inhalation study of CNFs in rats, cell proliferation in the terminal bronchiole, alveolar duct, and subpleural region of the respiratory tract was induced in the lungs of male and female rats; however, proliferation was no longer significantly elevated in males at 3 months post-exposure nor in females except in the subpleural tissue (DeLorme et al. 2012). An intratracheal instillation study of MWCNTs revealed that pristine (a.k.a. as produced) or functionalized MWCNT exposure induced proliferation of alveolar and bronchiolar epithelial cells and macrophages at 16 days post-exposure (Roda et al. 2011). Another intratracheal instillation study reported that MWCNT exposure did not induce hypertrophy of bronchial and alveolar epithelial cells up to 6 months post-exposure (Kobayashi et al. 2010). An aspiration exposure of B6C3F1 mice to MWCNT-7 demonstrated bronchiolo-alveolar hyperplasia and cellular atypia that were present at 2 days after exposure to 80 μg of MWCNT-7, and the hyperplasia persisted at 56 days post-exposure (Porter et al. 2010). When mice were exposed to MWCNT-7 (inhalation of 10 mg/m3, 5 h/d), bronchiolo-alveolar hyperplasia was observed at all time points examined (Porter et al. 2013). An additional study reported bronchiolo-alveolar hyperplasia 28 days after C567BL/6 mice were exposed by aspiration or inhalation of SWCNT (Shvedova et al 2008).
In a cancer promotion study, mice were exposed to MWCNT-7 (MCA- or MCA+) by inhalation (5 mg/m3, 5 hr/d, for 15 d; 17 mo post-exposure) with or without pretreatment with cancer initiator 3-methylcholanthrene (MCA) by IP administration. The initial MWCNT lung burden was 31.2 ± 0.9 μg/lung. Focal adenomatous alveolar epithelial hyperplasia was observed at 17 months post-exposure in the terminal bronchiole/alveolar duct region (Sargent et al. 2014). In humans, atypical adenomatous hyperplasia (AAH) is the form of primary bronchoalveolar hyperplasia considered to be preneoplastic (Foley et al. 1991; Malkinson 1991; Brambilla et al. 2001; Ress et al. 2003; Sargent et al. 2014; Pandiri 2015). For this reason, Sargent et al. (2014) separated hyperplasia into general hyperplasia and focal adenomatous hyperplasia, which resembles human AAH and which was “characterized by an increase in the number of crowded alveolar epithelial cells that outlined contiguous alveolar septa in discrete, generally random locations” (Sargent et al. 2014).
The incidence of focal adenomatous alveolar hyperplastic lesions (all severity levels) was 12, 15, 28, or 62%, respectively, for the air, MCA, MWCNT, and MCA + MWCNT exposed mice; and the incidence of those lesions of marked severity was 2, 2, 5, or 27%, respectively, at 17 months following a 3 week inhalation exposure to 5 mg/m3 MWCNT (Sargent et al. 2014). The incidence of carcinoma (bronchiolo-alveolar and/or adenocarcinoma) was 13, 22, 14, or 62%, respectively, for the same exposure groups (or, 23, 52, 27, 90% for all tumors, including adenomas). Thus, the focal adenomatous alveolar hyperplasia incidence was two-fold higher than the cancer incidence in the mice exposed to MWCNT only. The mechanism of carcinogenesis induced by MCA is gene mutation (Maddox et al. 2008), which differs from that expected for CNTs. MWCNT-7 does not appear to be a strong mutagen, but induces chromosomal aberrations (aneugens and clastogens); it also stimulates cell growth in vivo, and stimulates proliferation of MCA-mutated cells.
In a subsequent study of the same groups of mice studied in Sargent et al. (2014), Snyder-Talkington et al. (2016) observed increased expression of total mRNA and miRNA in the blood of the mice 17 months after exposure to MWCNT-7. In mice that developed pathological changes in the lungs – including hyperplasia, fibrosis, bronchiolo-alveolar adenoma, and bronchiolo-alveolar carcinoma – after the MCA/corn oil administration followed by MWCNT/air inhalation, numerous mRNAs and miRNAs in the blood were significantly up- or down-regulated (Snyder-Talkington et al. 2016). For a given pathology, the expression profile was different between the exposure groups, suggesting a specific response to the different exposures. Moreover, in comparisons of mRNA and miRNA expression in mice with bronchiolo-alveolar adenoma versus bronchiolo-alveolar carcinoma, each exposure group showed different profiles, indicating different regulation of the transition from adenoma to adenocarcinoma. Pathway analyses (Ingenuity Pathway) allowed determination of the top five canonical pathways associated with this transition from adenoma to adenocarcinoma: (1) initiation and progression of inflammation, (2) loss of epithelial features with mesenchymal transition, (3) neuronal cell survival after injury, (4) cell growth and transformation, and (5) hematopoiesis. In the MWCNT exposed group, the observed pathways were: wound repair, fibrosis and tumorigenesis, cell growth, proliferation and invasion, and cell apoptosis owing to stress to the endoplasmic reticulum. In the MCA + MWCNT exposed group, the observed pathways were: lipid metabolism and glycolysis, inflammation, cell proliferation, invasion and tumor immune evasion, and leukocyte migration (Snyder-Talkington et al. 2016).
A study of two types of MWCNT – “long” and “short/tangled” – found type II hypertrophy but not hyperplasia in mice exposed to the “long” (but not “short/tangled”) MWCNT (Muhlfeld et al. 2012). The “long” MWCNT (from Mitsui & Co. Ltd., Japan, but apparently different than MWCNT-7 based on reported mean length) was produced by catalytic chemical vapor synthesis using the floating reaction method, and the “short/tangled” MWCNT (from NanoLab, Inc., MA, USA) was produced by catalytic vapor discharge with a ceramic oxide (alumino-silicate) catalytic support that was removed post-synthesis by acid treatment. “Long” MWCNT was 13 μm in length and 40–50 nm diameter, while “short/tangled” MWCNT was 1–5 μm in length and 15 nm diameter (as reported by the manufacturers) (Muhlfeld et al. 2012). The dose was 10 μg/mouse (female C57BL/6) exposed by pharyngeal aspiration and examined 28 days post-treatment. The lack of observed alveolar epithelial type II hyperplasia or AHH in the Muhlfeld et al. (2012) study is not necessarily unexpected given the quantitative differences in the mouse lung doses used by Sargent et al. (2014) and Muhlfeld et al. (2012), with an initial lung dose of 31 vs. 10 μg and a post-exposure duration of 17 mo. vs. 1 mo., respectively. The two studies also differed by MWCNT materials and mouse strain and gender.
SWCNT
One intratracheal instillation study of SWCNTs in rats showed that exposure to SWCNTs did not induce the proliferation of lung parenchymal cells (assessed by 5-bromo-2-deoxyuridine (BrdU) incorporation into dividing cells’ DNA) (Warheit et al. 2004).
Genotoxicity
In vivo studies in rodents
No studies have been conducted yet that investigate genotoxic endpoints in humans with exposure to CNTs or CNFs. Therefore, observations from studies of animal tissue and cultured cells (Section “In Vitro Studies in Cultured Cells”) presently provide the most relevant information with regard to mechanistic evidence of carcinogenicity. A summary of in vivo data on genotoxicity and gene expression endpoints in lung tissue is provided in Table 4.
DNA damage in lung tissue following exposure to MWCNTs or SWCNTs
Fifteen studies were found that assessed the levels of DNA damage in rodent pulmonary tissue after exposure to CNTs (Table S-1). Rats exposed to 330 nm long MWCNTs (0.17–0.96 mg/m3, 6 h/days, 5 d/wk) by nose-only inhalation for 28 days had increased levels of DNA strand breaks in lung tissue (2.4-fold) at the end of exposure, which had decreased at 90 days post-exposure (1.4-fold compared to the control group) (Kim et al. 2014). In a similar study by the same authors, rats exposed to 2.6 μm long MWCNTs (0.16–0.94 mg/m3, 6 hr/d, 5 d) using whole-body inhalation exposure had increased levels of DNA strand breaks in lung tissue immediately after the exposure (1.5-fold) and 1-month post-exposure (1.3-fold) (Kim et al. 2012). Inhalation of MWCNTs (1.1 μm long) for 90 days did not affect the level of DNA strand breaks or DNA lesions measured by the hOGG1-modified comet assay in lung tissue of rats (Pothmann et al. 2015). Kato et al. (2013) found that a single intratracheal instillation of MWCNT-7 in mice (50 or 200 μg/animal) caused increased DNA strand breaks in lung tissue 3 h post-exposure. Intratracheal instillation of 0.7–3.0 μm long MWCNTs in mice (25.6 μg/wk, 5 wk) was associated with elevated DNA strand breaks in lung tissue, whereas there were unaltered levels of FPG-sensitive sites in the same tissue (Cao et al. 2014). In contrast to these studies, Ema et al. (2013b) found no difference in DNA strand breaks in lung tissue from rats after intratracheal instillation (0.2 or 1 mg/kg body weight, or 0.04 or 0.2 mg/kg body weight, once a wk, 5 wk) of MWCNTs with a length of 2.7 μm. Intratracheal instillation of MWCNTs (“large” and “small” sample; 3 doses; 24 h; 3 and 28 d post-exposure) resulted in DNA strand breaks (comet assay) in the lung. Small MWCNTs significantly enhanced DNA breakage at 54 and 162 μg/mouse on post-exposure day 3, and large MWCNT significantly enhanced DNA breakage at all doses but only after one day (Poulsen et al. 2015).
Following intratracheal instillation (0.2 or 1 mg/kg body weight, or 0.04 or 0.2 mg/kg body weight once a wk, 5 wk) of 4.4. μm long SWCNTs in rats, DNA strand breaks in lung tissue were unchanged (Naya et al. 2012). Intratracheal instillation of SWCNTs with a length of less than 1 μm (0.5 mg/kg bodyweight at 26 and 2 h before sacrifice, total dose = 1 mg/kg) did not increase the level of DNA strand breaks and FPG-sensitive sites (Vesterdal et al. 2014b). However, intratracheal instillation of the same type of SWCNTs in mice (54 μg/animal) increased DNA strand breaks in cells collected from BALF three hours post-exposure (Jacobsen et al. 2009). The latter study is limited by the fact that cell composition in the BALF differed in exposed and control animals. Therefore, it is unknown whether the observed increase in DNA strand breaks was due to different cell compositions (with different basal levels of DNA strand breaks) or SWCNT exposure.
Genotoxicity studies are informative of the ability of CNT to cause DNA base oxidation. No evidence of oxidatively damaged DNA (i.e., FPG-sensitive sites) was found in a study of atherosclerosis-prone mice following pulmonary exposure to MWCNTs and SWCNTs, but the administered doses were low (maximal dose of 1 mg/kg after two intratracheal instillations (Vesterdal et al. 2014b) and 25.6 μg/mouse per week (Cao et al. 2014). Gastrointestinal exposure by gavage of this SWCNT material in either saline suspension or corn oil (0.064 and 0.64 mg/kg bodyweight) was associated with increased levels of 8-oxodG in lung and liver tissue from rats, whereas the same doses did not affect the level of 8-oxodG in colon mucosa cells (Folkmann et al. 2009), suggesting a genotoxic mechanism arising as a consequence of oxidative stress, although it is impossible to distinguish between direct and indirect genotoxic mechanisms.
These studies show that increased levels of DNA strand breaks were found in lung tissue following exposure by intratracheal instillation or inhalation of MWCNTs, including MWCNT-7 (Kim et al. 2012, 2014; Kato et al. 2013; Cao et al. 2014; Poulsen et al. 2015). However, one study had negative results (Ema et al. 2013b), and two studies did not report increased DNA strand breaks in lung tissue after intratracheal instillation of SWCNTs (Naya et al. 2012; Vesterdal et al., 2014b), whereas one study showed increased levels of DNA strand breaks in cells from the BALF (Jacobsen et al. 2009). In summary, there is evidence suggesting that pulmonary exposure to different types of MWCNTs and SWCNTs is associated with increased levels of DNA damage in lung tissue. There does not appear to be a straightforward relationship between the fiber length and level of genotoxicity. In general, the MWCNTs and SWCNTs studied contained ~2–5% iron and had lengths of a few microns.
Chromosomal alterations, micronuclei and mutations in lung tissue following exposure to MWCNTs or SWCNTs
Table S-3 lists studies that assessed levels of micronuclei, chromosomal damage, and mutations in animal tissues after exposure to CNTs. It has been shown that intratracheal instillation of 0.7 μm long MWCNTs (0.5–2 mg/rat) increases micronuclei frequency in type II pneumocytes at 3 days post-exposure (Muller et al. 2008a). Intratracheal instillation of MWCNT-7 in mice (0.2 μg/animal, once a wk, 4 wk) enhanced gpt mutation frequencies in the lung, whereas there were no effects after 1 or 2 instillations (Kato et al. 2013). The predominant type of mutation was G:C to C:G transversions, which may be caused by oxidation of DNA bases. The investigators reported increased levels of oxidatively generated DNA lesions in lung tissue, but the baseline levels of 8-oxodG in the control group was approximately 4.8 lesions/106 nucleotides, corresponding to 22 lesions/106 dG. This result suggests methodological problems related to spurious oxidation of DNA.
C57BL/6 mice that inhaled 5 mg/m3 of SWCNTs for 4 days (5 h/d) had an increased frequency of pulmonary mutation of the K-ras proto-oncogene (Shvedova et al. 2008). The same researchers demonstrated increased levels of K-ras mutations 1-year post-exposure in the lung tissue of mice that were exposed to SWCNTs via inhalation (5 μg/animal, 5 mg/m3, 5 h/d, 4 d) or pharyngeal aspiration (40 μg/animal) (Shvedova et al. 2014).
The strongest evidence of mutagenesis comes from animal studies showing increased levels of gpt mutations in lung tissue after intratracheal exposure to MWCNT-7 (Kato et al. 2013) and K-ras mutations after inhalation exposure to SWCNTs (Shvedova et al. 2008, 2014). MWCNT (Nanolab, CVD produced, acid-washed; 1 μm length; 15 nm diameter) has been shown to be a strong anuegen and clastogen (Siegrist et al. 2014). Increased centromere positive micronuclei suggest that CNF is also an aneugen (Kisin et al. 2011). Aneuploidy is an early event in the progression of many types of cancers (Pitot & Dragan 1993; Yegles et al. 1995; Sargent et al. 1996; Duesberg et al. 2011; Cortez et al. 2016).
In vitro genotoxicity in cultured lung cells
DNA damage
A substantial number of studies show positive associations between exposure to CNTs and genotoxicity in terms of DNA strand breaks. The studies indicate a similar ability of MWCNTs and SWCNTs to generate genotoxicity, and there appears to be no fiber characteristic uniquely associated with potency for DNA damage. The in vitro data on genotoxicity and gene expression of cellular transformation endpoints are provided in Table 5.
Table 5.
Summary of in vitro data on genotoxicity, gene expression of cellular transformation endpoints.
Source: Adapted, from Table 3 in Sections 4–6 of IARC monograph 111 (IARC, in press), which was originally developed by authors on this paper. This current table has been restructured and includes some different presentations of the data, such as indicating which material was tested for cancer in animals.
Abbreviations: Bcl2: B-cell cll/lymphoma 2; Bcl3: B-cell cll/lymphoma 3; Cdkn1a: cyclin-dependent kinase inhibitor 1a (P21, Cip1); Cdkn2a: cyclin-dependent kinase inhibitor 2a; Cdkn2c: cyclin-dependent kinase inhibitor 2c (P18, Inhibits CDK4); Egfr: epidermal growth factor receptor; Junb: Jun-B proto-oncogene; Myb: V-Myb avian myeloblastosis viral oncogene homolog; pRb: retinoblastoma protein; Trp53: tumor protein p53.
Notes: Levels of DNA damage, mutations, chromosome damage and cellular transformation are increased (+) or unaltered (−) in exposed cells compared to unexposed controls. Gene expressions include oncogenes, tumor suppressor genes, and genes involved in DNA repair and cell cycle regulation. (+) means a differential expression between control (untreated) and treated cells. Bold text refers to observation on materials that have been tested for carcinogenicity in animal models.
Contains a mixed material with more than 50% SWCNTs and 40% other nanotubes.
MWCNTs defined as “long” (0.1–20 μm) was genotoxic, whereas a “short” type (1–5 μm) was not genotoxic.
Included 15 different materials, only MWCNT-7 and OECD materials MN400 and MN402 have been highlighted.
Same effect of pristine and amide-functionalized SWCNTs.
Three materials did not generate micronuclei (369 nm, 726 nm, 3.4 μm).
Heating (2400 °C) of the ground sample abolished genotoxicity. Samples that were heated and subsequently ground increased the formation of MN.
Both pristine and functionalized forms.
Only material with 1–3 μm in length.
Includes both “long” (approximately 10 μm) and “short” (150 nm) types of fibers.
Also increased level of DNA strand breaks and FPG-sensitive sites after exposure COOH-functionalized MWCNTs.
Also increased mutation frequency of COOH-functionalized SWCNTs. No effect of COOH-functionalized MWCNTs.
No effect of COOH-functionalized CNTs.
Unaltered micronuclei frequency of COOH-functionalized MWCNTs.
MWCNTs
Table S-2 lists studies that assess levels of DNA damage in cell culture after exposure to CNTs. In contrast to animal data, there is ample evidence of genotoxicity in cultured cells following exposure to MWCNTs. Of the studies on pristine (as produced CNTs), 14 were positive for DNA damage (Karlsson et al. 2008; Barillet et al. 2010; Migliore et al. 2010; Di Giorgio et al. 2011; Ghosh et al. 2011; Cavallo et al. 2012; Kermanizadeh et al. 2012, 2013; Aldeiri et al. 2013; Lindberg et al. 2013; Darne et al. 2014; Ursini et al. 2014; Visalli et al. 2015; Kim et al. 2016) and 4 studies showed unaltered levels of DNA strand breaks for some types of MWCNTs (Thurnherr et al. 2011; Aldeiri et al. 2013; Darne et al. 2014; Jackson et al. 2015). In a large study with 15 different MWCNTs, DNA strand break induction was determined in FE1-Muta™ mouse lung epithelial cells (5 concentrations, 12.5–200 μg/mL for 24 hr) (Jackson et al. 2015). Samples were grouped by physical characteristics (thin, thick, short: groups I, II, III, respectively), and each set included pristine, hydroxylated, and carboxylated MWCNTs (Jackson et al. 2015). MWCNTs were weakly genotoxic, including MWCNT-7 (referred to as NRCWE-006 in Jackson et al. 2015). Collectively, a significant dose-dependent increase in strand break levels was observed. However, when samples were analyzed individually, DNA strand breaks were significantly increased only following exposure to a COOH-functionalized material (Jackson et al. 2015). MWCNTs in Group II (thick, 50–80 nm; length 10–20 μm) showed significantly higher DNA strand breaks than MWCNTs in Group I (thin, 13–18 nm; length 1–12 μm). Moreover, OH-functionalized MWCNTs from Groups I–III together resulted in lower levels of DNA strand breaks than the pristine and COOH forms (Jackson et al. 2015). A principal component analysis did not show statistically significant associations when all of the physico-chemical characteristics of the MWCNTs and the biological effects were included in the analysis. MWCNTs in Group II (which induced DNA strand breaks) had a large diameter and were associated with increased levels of NiO.
However, MWCNT-7 and a number of other types of MWCNTs did not alter levels of DNA strand breaks in lung epithelial cells (Jackson et al. 2015). Thurnherr et al. (2011) found no alterations in the levels of DNA strand breaks in human alveolar basal epithelial adenocarcinomic (A549) cells after exposure to MWCNTs with a length of 2–5 μm and only 0.4% impurities (Thurnherr et al. 2011). No alterations in the levels DNA strand breaks were reported in V79 lung fibroblasts from Chinese hamsters using the same samples as used in SHE cells (Darne et al. 2014). Darne et al. (2014) reported levels of strand breaks resulting from exposure to three samples of double walled carbon nanotubes (DWCNTs) in SHE cells and V79 fibroblasts; only one sample produced strand breaks in SHE cells.
Presently, reliable measurements of oxidatively damaged DNA base products in cultured cells have only been assessed by the modified comet assay with use of repair enzymes. Exposure to MWCNT was found to increase ENDOIII- and FPG-sensitive sites in rat RAW 264.7 macrophages (Migliore et al. 2010) and A549 cells (Visalli et al. 2015). However, unaltered levels of FPG-sensitive sites were found in A549 and BEAS-2B cells following exposure to MWCNTs (Karlsson et al. 2008; Cavallo et al. 2012; Ursini et al. 2014). Another group of investigators showed ambiguous results regarding the level of total sites after FPG treatment in human hepatoblastoma and renal cell lines (Kermanizadeh et al. 2012, 2013) and in SHE cells and V79 fibroblasts (Darne et al. 2014).
The same type of MWCNT, NM400 and NM402, were used in a European study of Organization for Economic Co-operation and Development (OECD) materials in animals and cultured cells; an overall null effect was found for oxidatively damaged DNA (Kermanizadeh et al. 2012, 2013; Cao et al. 2014). Thus, presently there is weak evidence of a genotoxic mechanism of CNTs that generates oxidatively damaged DNA lesions.
With use of the neutral comet assay that detects double strand breaks, results have indicated unaltered levels of double strand breaks in A549 cells after exposure to MWCNTs (Ju et al. 2014). Similarly, neither “short” (0.1–5 μm), nor “long” (0.1–20 μm) MWCNTs increased γH2AX immunostaining in rat kidney epithelial cells (Barillet et al. 2010). MWCNTs did not alter γH2AX immunostaining in Chinese hamster V79 cells (Mrakovcic et al. 2015). Other studies have assessed the presence of double strand breaks in cells via γH2AX immunostaining. Increased immunostaining for γH2AX in human endothelial cells was found following exposure to short MWCNTs (less than 1 μm in length) (Guo et al. 2011). Another study showed that MWCNTs increased the formation of γH2AX in human lymphocytes (Cveticanin et al. 2010).
These findings show that a number of studies have reported that exposure to various types of MWCNTs increase the levels of DNA strand breaks in human bronchial (BEAS-2B) and lung epithelial (A549) cells (Karlsson et al. 2008; Cavallo et al. 2012; Ursini et al. 2014), lymphocytes (Ghosh et al. 2011), murine macrophages (Migliore et al. 2010; Di Giorgio et al. 2011), human and rat renal epithelial cells (Barillet et al. 2010; Kermanizadeh et al. 2013), human hepatocytes (Kermanizadeh et al. 2012), and Syrian hamster embryo cells (SHE) (Darne et al. 2014). MWCNT-7 was found to cause a 10-fold increase in macrophage DNA strand breaks following 24 h of exposure, whereas a treatment that decreased the iron content rendered MWCNT-7 non-genotoxic (Aldieri et al. 2013).
SWCNTs
Studies of SWCNTs predominantly show an association between exposure and increased levels of DNA strand breaks in cultured cells (Kisin et al. 2007, 2011; Pacurari et al. 2008; Yang et al. 2009; Lindberg et al. 2009, 2013; Migliore et al. 2010; Cicchetti et al. 2011; Di Giorgio et al. 2011; Pelka et al. 2013; Alarifi et al. 2014; Kim & Yu 2014; Vesterdal et al. 2014a), whereas a few studies have shown null effect (Jacobsen et al. 2008; Darne et al. 2014; Bayat et al. 2015). Although these studies clearly show that exposure to CNTs is related with increased levels of DNA strand breaks, an assessment of relevant fiber characteristics responsible for this effect remains inconclusive. This is exemplified in Table 5 (and Tables S-2), where the individual studies in Table 5 are sorted with respect to the fiber length of the material in dry form (typically reported by the supplier). It should be emphasized that some studies have attempted to characterize the material in the suspension vehicle, but unfortunately this does not offer enough information to allow comparison of gentoxicity across studies.
FE1-MML mouse lung epithelial and human hepatoblastoma HepG2 cells exposed to SWCNTs were found to have increased levels of FPG-sensitive sites (Jacobsen et al. 2008; Vesterdal et al. 2014a). There were also increased levels of ENDOIII- and FPG-sensitive sites in rat RAW 264.7 macrophages after exposure to SWCNTs (Migliore et al. 2010). However, SWCNT exposure did not increase the level of extra FPG sites in human colon carcinoma cells (Pelka et al. 2013).
Studies of oxidatively generated DNA damage – essentially measured by the comet assay as FPG-sensitive sites – in cultured human cells indicate genotoxicity after SWCNT exposure (Jacobsen et al. 2008; Migliore et al. 2010; Vesterdal et al. 2014a). Identical SWCNTs were used in three in vivo studies (Jacobsen et al. 2009; Folkmann et al. 2009; Vesterdal et al. 2014a, 2014b). This material was used by a number of other laboratories as part of a large European Sixth Framework Program (FP6) project on the risk of nanomaterials called Particle Risk (Johnston et al. 2013).
Exposure to SWCNTs has been associated with increased levels of double strand breaks (measured by γH2AX immunostaining) in human lymphocytes (Cveticanin et al. 2010) and A549 cells (Mrakovcic et al. 2015). Another study showed unaltered levels of γH2AX immunostaining in human mesothelial cells, although the levels were regarded to be nominally increased (approximately 1.2-fold) (Pacurari et al. 2008).
Chromosomal alterations, micronuclei, and mutations in cultured cells exposed to MWCNTs and SWCNTs
Micronuclei formation in cultured cells exposed to MWCNTs and SWCNTs
Chromosomal damage and alterations in chromosome number have been found in exposure studies of cultured primary human lymphocytes and SWCNTs (Catalán et al. 2012), bronchial epithelial cell line BEAS-2B and MWCNTs (Siegrist et al. 2014), and murine macrophages (RAW 264.7) with both MWCNTs and SWCNTs (Di Giorgio et al. 2011) (Table 5). In addition, 13 out of 18 studies report increased micronuclei frequency in human cell lines after exposure to MWCNTs, including three independent studies of MWCNT-7 (Muller et al. 2008a, 2008b; Asakura et al. 2010; Cveticanin et al. 2010; Migliore et al. 2010; Di Giorgio et al. 2011; Srivastava et al. 2011; Kato et al. 2013; Wu et al. 2013; Darne et al. 2014; Tavares et al. 2014; Visalli et al. 2015; Kim et al. 2016); five studies showed null effects (Szendi & Varga 2008; Thurnherr et al. 2011; Lindberg et al. 2013; Ponti et al. 2013; Mrakovcic et al. 2015). Eight out of 13 studies report increased micronuclei frequency in human cell lines after exposure to either SWCNTs or MWCNTs (Cveticanin et al. 2010; Migliore et al. 2010; Cicchetti et al. 2011; Di Giorgio et al. 2011; Kisin et al. 2011; Manshian et al. 2013; Darne et al. 2014; Kim & Yu 2014; Kim et al. 2016); five studies showed null effects (Kisin et al. 2007; Lindberg et al. 2009; Lindberg et al. 2013; Pelka et al. 2013; Mrakovcic et al. 2015). Null effect studies used CNTs similar to the CNTs that caused micronuclei formation with respect to diameter, length, specific surface area and purity, although it should be emphasized that many publications contain insufficient information on fiber characteristics.
Table S-4 lists studies that assess chromosomal alterations, micronuclei and mutations in cell cultures after exposure to CNTs. Conflicting results are reported with regard to micronuclei induction in cultured cells after CNT exposure. There appears to be no difference between the distribution of studies showing increased formation of micronuclei and null effect with regard to the use of the cytokinesis-block micronucleus protocol or other protocols to score micronuclei. Therefore, specific assay protocols are not highlighted in descriptions of in vitro cell culture findings.
Human lymphocytes exposed to a panel of MWCNTs showed statistical differences in micronuclei frequency at all tested concentrations after exposure to MWCNTs with short fiber length (394 nm); two human lymphocyte samples generated micronuclei when exposed to 1–2 low concentrations (including MWCNT-7 and NM402), and three samples did not generate micronuclei (Tavares et al. 2014). The investigators concluded that tube diameter and length, surface area and transition metal content could not explain the observed results (Tavares et al. 2014).
MWCNT-7 has been shown to increase micronuclei frequency in hamster lung fibroblasts (Asakura et al. 2010) and human immortalized lung cancer epithelial cells (A549) (Kato et al. 2013), and human lymphocytes (Tavares et al. 2014). Increased frequency of micronuclei was observed in A549 cells and immortalized bronchial epithelial cells (BEAS-2B) that were exposed to MWCNTs (Srivastava et al. 2011; Wu et al. 2013; Visalli et al. 2015). Exposure to MWCNTs has been associated with increased frequency of micronuclei in lymphocytes (1–5 μm long, Cveticanin et al. 2010), murine macrophage cell lines (RAW 264.7) (0.5–50 μm, Di Giorgio et al. 2011) and human breast cancer epithelial cells (MCF-7) (700 nm, Muller et al. 2008a). Another study showed increased micronuclei in BEAS-2B and immortalized human lymphoblastoid TK ± cells (MCL-5) cells after exposure to MWCNTs with short (400–800 nm), medium (1–3 μm) and long (5–30 μm) fiber length (Manshian et al. 2013). A sample of MWCNTs with relatively short fiber length (0.7 μm) and low transition metal content (0.48% Fe and 0.49% Co) was used to study the impact of MWCNT structural defects and metals on the formation of micronuclei in rat lung epithelial cell lines (RLE cells) (Muller et al. 2008b). Other short samples of MWCNTs produced a significant increase in micronuclei frequency in SHE cells and V79 fibroblasts (Darne et al. 2014). MWCNTs with structural defects increased micronuclei frequency, whereas heated and ground MWCNTs were not as genotoxic in regards to micronuclei formation (Muller et al. 2008b). A number of studies have shown unaltered levels of micronuclei after MWCNT exposure in lung epithelial A549 cells (2–5 μm, Thurnherr et al. 2011; 0.5–2 μm, Mrakovcic et al. 2015), BEAS-2B (1–5 μm, Lindberg et al. 2013), Chinese hamster V79 fibroblasts (0.5–2 μm, Mrakovcic et al. 2015), and murine fibroblasts (1.5 μm, Ponti et al. 2013). Cultured lymphocytes exposed to 1–2 μm long MWCNTs were observed to have no change in micronuclei formation and sister chromatid exchange (Szendi & Varga 2008).
Exposure to 20 μm long SWCNTs has been associated with increased frequency of micronuclei in phytohemagglutinin-stimulated human lymphocytes (Kim & Yu 2014). Increased levels of micronuclei formation were also observed in human lymphocytes (1–5 μm, Cveticanin et al. 2010), gingival fibroblasts (760 nm, Cicchetti et al. 2011), immortalized murine macrophages (RAW 264.7) (2–5 μm and 0.5–100 μm, Migliore et al. 2010; Di Giorgio et al. 2011), and V79 fibroblasts (>1 μm in length, Darne et al. 2014). Elevated levels of micronuclei were observed in hamster lung fibroblasts following exposure to SWCNTs 1–3 μm in length, crocidolite asbestos, or CNF (Kisin et al. 2011). An earlier study with the same SWCNT material resulted in a trend for increased micronuclei in hamster lung fibroblasts (Kisin et al. 2007). Likewise, a study using short SWCNTs (0.5–2 μm) showed increased micronuclei formation in Chinese hamster V79 fibroblasts (Mrakovcic et al. 2015). However, a number of studies have not found increased formation of micronuclei following exposure to SWCNTs in hamster lung fibroblasts (0.5 μm, Pelka et al. 2013), BEAS-2B (1–5 μm or 0.5–100 μm, Lindberg et al. 2009, 2013), and SHE cells (>1 μm in length, Darne et al. 2014). CNF induced micronuclei formation (primarily aneugenic) in primary human small airway epithelial cells (SAEC) (Kisin et al. 2011).
In summary, cell culture studies document the ability of MWCNT, SWCNT, and CNF to increase the frequency of micronuclei in proliferating cells. However, these effects may differ substantially between studies, possibly originating from differences in cell types, characteristics of CNTs, dispersion protocols and assay conditions.
Alterations in chromosome morphology and number, chromosomal aberrations in cells exposed to MWCNTs and SWCNTs
Table S-4 lists in vitro investigations in which primary cells as well as established cell lines were exposed to CNTs. Exposure to SWCNTs has been shown to be associated with aneuploidy in primary or immortalized human airway epithelial cells (Sargent et al. 2009, 2012). This mechanism is considered to result from physical interaction and interference between CNTs and the mitotic apparatus or fragmentation of the centrosome and is considered to be relevant for in vivo (airway) exposure in humans. As determined by analysis of chromosome number by fluorescence in situ hybridization (FISH), primary human small airway epithelial (SAEC) or immortalized BEAS-2B cells were found to have errors in chromosome number after exposure to 1 μm long MWCNTs (Siegrist et al. 2014). Three-dimensional reconstructions of 0.1 μm optical sections showed MWCNTs integrated with microtubules, DNA, and within the centrosome structure (Siegrist et al. 2014). Chromosome breakage and translocations between chromosomes as well as aneuploidy were observed in an immortalized mouse macrophage cell line (RAW 264.7 cells) following exposure to 0.5–100 μm long MWCNTs (Di Giorgio et al. 2011). There was an 8–34-fold increase in polyploidy in Chinese Hamster lung cells treated with 5 μm long MWCNT-7 (Asakura et al. 2010). Using phyto-hemagglutinin-stimulated human lymphocytes, Catalán et al. (2012) demonstrated that 1–2 μm long MWCNTs increased chromosome and chromatid breakage. In another investigation of immortalized Chinese hamster ovary cells, no aneuploidy was observed following exposure to short (150 nm) or long (10 μm) MWCNTs (Kim et al. 2011). Short MWCNTs (0.2–1.0 μm) were also found associated with unaltered levels of chromosome aberrations in Chinese hamster lung fibroblasts cells (Wirnitzer et al. 2009).
Chromosome breakage and translocations between chromosomes as well as aneuploidy were observed in an immortalized mouse macrophage cell line (RAW 264.7 cells) following exposure to 2–5 μm long SWCNTs (Di Giorgio et al. 2011). The modal number of the macrophage cell line karyotype was 40 chromosomes; however, the number of chromosomes per cell after exposure to either SWCNTs or MWCNTs had a mean number of 20–60 chromosomes/cell with no distinct modal number, indicating a high degree of aneuploidy in the original cell line (Di Giorgio et al. 2011). About 1–5 μm long SWCNTs were found to increase chromosome and chromatid breakage in phytohemagglutinin-stimulated human lymphocytes (Catalán et al. 2012). Cultured primary human lung epithelial or BEAS-2B cells had errors in chromosome number after exposure to 1 μm long SWCNTs, as determined by analysis of chromosomes number by FISH (Sargent et al. 2009, 2012). Mitotic disruption associated with SWCNT-treatment resulted in a G2/M block in the cell cycle, which was mechanistically different from MWCNT treated cells that had a block in G1/S (Sargent et al. 2009, 2012; Siegrist et al. 2014). When mammalian cells are exposed to agents that cause a block in S-phase, the DNA is repaired by homologous recombination. Further analysis by confocal microscopy and transmission electron microscopy demonstrated fragmented centrosomes following exposure to either SWCNTs or MWCNTs (Sargent et al. 2009; 2012; Siegrist et al. 2014). These investigations document the ability of CNTs to disrupt the mitotic spindle and fragment the centrosome. In acellular conditions, intercalation with DNA, as well as formation of a microtubule/carbon nanotube-hybrid, leads to physical interference with the mitotic apparatus and/or fragmentation of the centrosome in response to both SWCNTs and MWCNTs (approximately 1 μm) (Li et al. 2006; Dinu et al. 2009). These interactions lead to aneuploidy in the daughter cells of exposed immortalized and primary human lung epithelial cells. However, some studies demonstrate unaltered levels of chromosome aberrations in immortalized Chinese hamster ovary cells after exposure to SWCNTs with a length of 1.2 μm (Naya et al. 2011), 20 μm (Kim et al. 2015) or a material where the length was not reported (Ema et al. 2013a).
In summary, in vitro investigations of immortalized and primary cells document the ability of CNTs to increase the frequency of chromosome damage and aneuploidy in proliferating cells. Similar to the results from studies of micronuclei frequency, these effects may differ substantially between studies, possibly originating from differences in cell types, CNT characteristics, dispersion protocols and assay conditions. The data demonstrating chromosome damage and errors in chromosomes following in vitro exposure with either SWCNTs or MWCNTs suggest an altered integrity of the mitotic spindle, which causes a block in the cell cycle of cultured cells.
Mutations
An unaltered mutation frequency was found in the hypoxanthine-guanine phosphoribosyltransferase (HGPRT) gene after Chinese hamster lung cells were exposed to MWCNT-7 (Asakura et al. 2010). Mutations in the HGPRT gene were increased in BEAS-2B cells after exposure to SWCNTs with a length of 1–5 μm, whereas shorter (0.4–0.8 μm) and longer (5–30 μm) SWCNTs were not associated with mutagenicity (Manshian et al. 2013). Exposure to SWCNTs (0.5–2 μm) was associated with increased mutation frequency in the HGPRT gene in Chinese hamster V79 cells, whereas MWCNTs (0.5–2 μm) did not induce mutations (Mrakovcic et al. 2015). Long-term exposure (24 days) of FE1-Muta™Mouse lung epithelial cells to SWCNTs (less than 1 μm in length) showed no increase in mutation frequency in the cII locus (Jacobsen et al. 2008).
The mutagenic effect of CNTs has been evaluated with bacterial test systems using Salmonella typhimurium strains TA97, TA98, TA100, TA102, TA1535, TA1537, TA1538, YG1024, YG1029, and Escherichia coli strain WP2uvrA in the presence and absence of the metabolic activation system S9. These studies do not show mutagenicity after exposure to MWCNTs (Di Sotto et al. 2009; Wirnitzer et al. 2009; Kim et al., 2011; Taylor et al., 2014) or SWCNTs (Kisin et al. 2007; Naya et al. 2011; Ema et al. 2012, Kim et al. 2015). Thus, there is little evidence to suggest that exposure to MWCNTs or SWCNTs is associated with a strong mutagenic potential in mammalian cells or bacteria.
To summarize, mutagenesis in mammalian cells has been found negative, including one study of MWCNT-7 (Asakura et al. 2010) and two studies of SWCNTs (Jacobsen et al. 2008; Manshian et al. 2013). Lack of mutagenetic activity in terms of frameshift and base pair substitutions has been shown following bacterial exposure to MWCNTs (Di Sotto et al. 2009; Wirnitzer et al. 2009; Kim et al. 2011; Taylor et al. 2014) and SWCNTs (Kisin et al. 2007; Ema et al. 2013a; Naya et al. 2011; Kim et al. 2015). The discrepancy between increased mutagenicity in animal models and lack of such an effect in cultured cells suggests that in vitro studies may not be a reliable experimental model of in vivo mutagenicity. Moreover, bacterial systems are not appropriate to evaluate the mutagenic potency of particles, and the large majority of mutation studies were carried out with prokaryotes.
Other indicative effects: gene expression and cell transformation
Genotoxic mechanisms of both MWCNTs and SWCNTs are supported by observations of modulated expression of genes encoding proteins involved in DNA repair, apoptosis and cell cycle control in animal models (eight studies [Snyder-Talkington et al. (2013a), Guo et al. (2012), Huang et al. (2014), Poulsen et al. (2013), Park et al. (2011a, 2011b, 2013)]) and human cell culture (six studies [Ravichandran et al. (2010), Srivastava et al. (2011), Kim (2012), Sarkar et al. (2007), Wang et al. (2011a), Pelka et al. (2013)]).
Guo et al. (2012) determined a set of 35 genes, constituting a MWCNT signature in mice exposed to MWCNT-7. Among these genes, several encode proteins involved in lung cancer development and progression (Guo et al., 2012). Gene expression studies in mice exposed by intratracheal instillation to MWCNT-7 revealed upregulation of genes involved in proliferation. At low dose (18 μg/mouse), there was an upregulation of genes associated with promoting cell cycle transitions and/or mitosis entry: Ccna2 (cyclin A2), Ccne (cyclin E) and Cdca3 (cell division cycle associated 3), in comparison with control mice (Poulsen et al. 2013). There was an upregulation, in a dose-dependent manner, of Myc (V-myc avian myelocytomatosis viral oncogene homolog) in the lungs of exposed mice in comparison with control mice (Poulsen et al. 2013). MWCNT-7 administered to the mouse lung epithelial cell line FE1 resulted in differential gene expression compared to controls, including genes stimulating cell proliferation; upregulation of fos-like antigen 1 (Fosl1), (jun-protooncogene (Jun) and Myc and ER stress response, DNA-damage-Inducible transcript 3 (Ddit3/Gadd153), with potential oncogenic functions. Several genes play a role in the control of cell cycle progression as check points or repair systems (RAD1 checkpoint DNA exonuclease, Rad1; N-myc downstream regulated gene 1, Ndrg1; centromere protein 5, CenpV and RAD9 homolog A (S. Pombe), Rad9). A down regulation of growth-arrest-specific 1 (Gas1) involved in growth suppression and blockage of entry in S phase was also observed (Poulsen et al. 2013). These findings show that MWCNT-7 exposure engages cells to control their DNA integrity and growth, and that cell growth may be activated by the overexpression of genes that activate growth and the underexpression of genes which normally exert a negative control. Snyder-Talkington et al. (2013b) reported alterations of genes associated to cellular growth and proliferation in telomerase immortalized-human small airway epithelial cells (SAEC) after in vitro exposure to MWCNT-7. Several of these genes, were upregulated such as V-Akt murine thymoma viral oncogene homolog 1 (AKT1), which is activated by growth factors; vascular endothelial growth factor A (VEGFA); smoothened, frizzled class receptor (SMO) and Sonic Hedgehog (SHH), involved in hedgehog signaling and carcinogenesis; as well as downregulation of B-cell CLL/lymphoma 2 (BCL2), an apoptosis inhibitor. The expression of transcription factors CAMP responsive element binding protein 3 (CREB3) and E2F transcription factor 4 (E2F4), which regulate cell proliferation, were also enhanced. This may suggest a growth advantage of MWCNTs-exposed SAEC. Moreover, these genes have a role in lung adenocarcinoma (Snyder-Talkington et al. (2013b). Early passage immortalized cells are used in these studies to analyze genetic changes in a cell population in response to a toxic agent; these results are reliable because early passage immortalized cells have a stable genotype. This can be verified by assurance of the passage of the cells as well as genetic analysis of the untreated cells. As an example, studies of immortalized cells have been used in many investigations in liver and skin carcinogenesis to examine changes in mutations and gene expression that predict stage specific changes in primary liver and skin cancer (Sargent et al 1996).
In two studies in which primary and early passage immortalized cells and primary SAEC cells were exposed in vitro to 10–15 nm MWCNTs (Siegrist et al 2014) or to 1–4 nm SWCNTs (Sargent et al. 2012), increased colony formation was observed at the lowest dose of 0.024 μg/cm2 CNT. Clonal growth was considered to indicate increased cell proliferation. The lowest in vitro dose in Siegrist et al. (2014) was equivalent (on a cell surface area basis) to an in vivo dose of 10 μg in mice, which was estimated to be equivalent to 34 years of exposure of a human to the USA National Institute for Occupational Safety and Health recommended exposure limit (NIOSH REL) of 1 μg/m3 for CNT or CNF.
Neoplastic-like transformation – as indicated by anchorage-independent cell growth, increased cell invasiveness, apoptosis resistance, increased cell motility, and angiogenesis – was found after prolonged exposure of human mesothelial cells to SWCNTs (Lohcharoenkal et al. 2014). These traits are associated with autonomous cell proliferation. Also associated with the cell transformation was the up-regulation of H-Ras and activation of ERK1/2, a downstream effector of Ras that plays a pivotal role in the regulation of cell proliferation cells.
Role of physico-chemical properties associated with genotoxic or carcinogenic effects
Comparison of properties among CNTs, CNFs, and asbestos
Considerable uncertainty remains in understanding the role of physico-chemical properties in the toxicity of CNTs and CNFs. Physico-chemical characteristics that can contribute to differences in pulmonary responses include the tube or fiber dimensions (length and width), structural defects, metal contaminants, post-synthesis treatments, and surface functionalization of the materials prepared and tested. CNTs and CNFs can vary in their physicochemical properties depending on the method of preparation and the application (e.g., industrial vs. biomedical). Most toxic particles, including silica and asbestos, are not single toxic entities, but rather materials for which toxicity varies by source and modifications at the particle surface. This is even more the case for CNTs. Although CNTs and CNFs have variable dimensions, most CNTs and CNFs fall within the World Health Organization definition of fibers. Because of their high aspect ratios and high durability, which suggests substantial biopersistence, CNTs have been compared to asbestos; and similarities in biological responses to some types of CNTs and asbestos have been reported in both in vitro and in vivo studies (e.g., Donaldson et al. 2011). CNFs and CNTs have similar basic physico-chemical properties (Fubini, et al. 2011, Table 2) and limited studies have shown qualitatively similar pulmonary effects (DeLorme et al. 2012; Murray et al. 2012).
In addition to the properties of aspect ratio and durability, surface reactivity can modulate the toxicity of CNTs (e.g., Sanchez et al. 2009; Jaurand et al. 2009, Fubini et al. 2011). The surface chemistry of CNTs and asbestos in their native forms can be “quite different” (Fubini et al. 2011). One major difference is that unfunctionalized CNTs are highly hydrophobic, while asbestos is highly hydrophilic in all its forms. The surface charge of unfunctionalized CNTs is very low to negative at physiological pH, while asbestos has a high surface charge (i.e., negative for amphiboles, and positive for chrysotile). The types and amounts of bioavailable metals in unpurified CNTs can be highly variable, including metallic and/or ionic moieties (e.g., Co, Ni, Fe, agglomerated within the tube as residual catalyst). By comparison, asbestos typically occurs only with Fe ions present (i.e., mostly Fe2+/3+ in amphiboles, as stoichiometric complements that are regularly organized in the crystalline structure, and a few isolated Fe2+ions that are substituted for magnesium ions in all asbestos forms) (Fubini & Otero-Aréan 1999; Fubini et al. 2011). Agglomeration in aqueous media is much more pronounced for hydrophobic CNTs compared to asbestos, which can split longitudinally into fibrils. Enzymatic degradation has been reported for specific preparations of CNTs (with carboxyl functionalities at the surface obtained by oxidation) (Section “Solubility/Degradation in Body or Cellular Fluids”), while similar effects have not been reported for asbestos fibers, likely reflecting the difference between a carbon and a silica framework.
The variability among CNTs and CNFs in physico-chemical properties is the result of multiple factors, including the following: (1) wall number, e.g., preparation as SWCNT or MWCNT, which results in different diameters and flexibility; (2) presence of various metals, which may have been used as catalysts and then remain within the carbon framework in trace or substantial amounts and may be fully or partially accessible by fluid molecules; (3) small or large number of framework defects, depending on the mechanical and thermal procedure to which CNTs have been submitted; (4) length and shape [straight, curled or entangled]; (5) “functionalization,” meaning that organic chains are linked to the carbon framework, a process usually done by oxidation to make CNTs more hydrophilic and easily dispersed in aqueous media, or to give them the potential to carry drugs, peptides or other groups of interest; and (6) formation of different types of protein corona in suspension vehicles (Shannahan et al. 2013).
A recent quantitative analysis of data across studies was used to examine the contribution of physico-chemical characteristics of CNTs on the dose–response relationship for pulmonary inflammation (Gernand & Casman 2014). Classification and regression tree methods were used since these methods are better able to handle statistical challenges in meta-analyses, such as missing data. Of note, classification and regression tree techniques are developed for prediction purposes, and causal interpretations have to be done with reservation. The results showed that the most important characteristics contributing to pulmonary toxicity were metallic impurity (positive association with cobalt and mixed effect of other metals), CNT length (negatively correlated with most toxicity indicators), CNT diameter (positively associated with toxicity), aggregate size (positive or negative correlations depending on the toxicity endpoint), and specific surface area (negatively associated with toxicity indicators). These results are somewhat unexpected based on evidence from the fiber and nanoscale particle paradigms. However, it must be mentioned that variables considered by the authors were endpoints measured in BALF extracted from the lungs of animals exposed by inhalation or instillation: increase of PMNs, macrophages, LDH, and total protein, which represent only some of the potential biological responses to CNTs. Closer examination of these associations may reveal reasons for the findings, such as that a given mass dose of shorter CNTs contains a higher concentration of reactive free ends. In addition, extension of such databases to examine pre-cancer endpoints could provide useful insights on the role of physico-chemical properties, assuming the data across studies are sufficient for meta-analysis.
Few studies have purposely modified or prepared sets of CNTs for in vitro or in vivo testing in order to associate toxicity to particular physico-chemical properties (see, for instance, Muller et al. 2008b; Roda et al. 2011; Aldieri et al. 2013; Bonner et al. 2013; Hamilton et al. 2013; Ponti et al. 2013; Sager et al. 2014). In most cases, the CNTs tested are based on the availability of materials as prepared for a given purpose or application (see for instance Li et al., 2013; Jackson et al., 2015). CNTs are used in a wide range of applications including in the areas of medicine (imaging, drug delivery), environment, and electrochemistry. Because of their hydrophobicity, pristine CNTs must be functionalized for certain applications. The large number of potential applications of CNTs and CNFs may result in a potentially wide variety of CNTs or CNFs that are produced for those purposes, resulting in potential differences in the biokinetics and toxicities of those materials. Thus, on the one hand, the responses to CNTs and CNFs could be expected to be similar to asbestos, while on the other hand, the potential differences in the surface properties of these materials (e.g., agglomeration, scavenging, ion release, charge) may imply different molecular mechanims. The overall surface activity would be a combination of these properties.
At this time, it is not possible to define the specific characteristics that account for the reported differences in toxic responses to various types of CNTs and CNFs. Differences in the physico-chemical characteristics of the materials as prepared and tested (e.g., dimensions, structure, defects, functionalization, contaminants) clearly play a role, but the key parameters and their relative importance have not been definitively determined. A number of studies discussed below provide information on the properties that were shown to modulate the toxicity of CNTs.
Form, size, length and thickness
Long, rigid CNTs were observed to induce more inflammation in the abdominal cavity than tangled ones (Poland et al. 2008; Nagai et al. 2011). CNTs over 4 μm long were reported to be pathogenic to the pleura of mice, and a threshold length value (4–5 μm) was proposed by Schinwald et al. (2012) for inducing acute inflammation in a mouse model. The acute pleural inflammation was attributed to the longer fiber lengths regardless of the material composition (i.e., silver or nickel nanowires or amosite asbestos) (Schinwald et al. 2012). A similar size-dependent inflammatory response was observed in mice administered MWCNTs or Ni nanowires by intrapleural injection (5 μg/mouse) (Murphy et al. 2011). In the same study, pleural clearance of long fiber structures (13 μm mean length) was reduced in mice administered MWCNT, compared to the same mass dose of MWCNT consisting of shorter structures (0.5–5 μm lengths); and the mice administered the long MWCNT developed inflammation and fibrosis of the parietal pleura compared to “no or modest resolving inflammation, with no parietal pleural pathological features” in mice administered the shorter MWCNTs at the same mass dose (Murphy et al. 2011; Donaldson et al. 2013). In a recent two-year study of rats, four CNTs differing in shape caused mesotheliomas, but the appearance of the tumors occurred much earlier with long and straight CNTs than curled CNTs (Rittinghausen et al. 2014). This result is similar to asbestos, but a strict analogy between asbestos fibers and CNTs is questionable, considering their differences in chemical composition and structure (Fubini et al. 2010, 2011). Pleural inflammation and fibrosis are induced only by long (>10 μm) CNTs after IP (Kolosnjaj-Tabi et al. 2010) or intrapleural injection (Murphy et al. 2011). The adverse effects of exposure to long, rigid (>10 μm) CNTs have been related to their physical interaction with cells, resulting in incomplete internalization, frustrated phagocytosis, and length-limited clearance from pleura through lymphatic stomata. Manshian et al. (2013) found that SWCNTs significantly increased the micronucleus frequency in BEAS-2B and lymphoblastoid (MCL-5) cells at sub-cytotoxic concentrations, with potency markedly decreasing with length, but only the shortest SWCNTs being mutagenic in mammalian cells.
No mesothelioma was found in six rats 12 months after IP injection of 10 mg tangled MWCNT (dimensions not reported), although significant mesothelioma was reported in rats (n = 6–30) administered 1 or 10 mg of MWCNTs with diameters of 50 to 140 nm and lengths of approximately 5 μm (Nagai et al. 2011, 2013). Although the Nagai et al. (2011, 2013) studies provide some data suggesting a lower carcinogenic potency of tangled carbon nanotubes, the IARC monograph 111 Working Group concluded that the investigations had an insufficient number of animals to show a negative result (IARC, in press).
Thinner (diameter ≈ 50 nm) MWCNTs appear to be significantly more active than thicker (diameter ≈ 150 nm) or tangled (diameter ≈ 2–20 nm) CNTs in causing mesothelial toxicity and mesothelioma in rats, but thin and thick MWCNTs were found to affect macrophages similarly (Nagai et al. 2011). In contrast, thin MWCNTs (diameter 9.4 nm) were more toxic in vivo (rats, lung) and in vitro (murine alveolar macrophages) than thicker MWCNTs (diameter ≈ 70 nm) (Fenoglio et al. 2012). Thinner CNTs were also found to be more cytotoxic to EAhy926, A549, HepG2, DMBM-2 V79 and TK-6 cells than thicker CNTs (Frohlich et al. 2013). However, long and thick CNTs, but not short and thin MWCNTs, were shown to cause inflammation in mice (Yamashita et al. 2010). Longer SWCNTs (>10 μm) induced granuloma formation, while shorter SWCNTs (<300 nm) were excreted from the body in mice (Kolosnjaj-Tabi et al. 2010).
Qualitatively, these animal IP studies suggest that the carcinogenic potency (e.g., proportion of animals with tumors at a given dose) of CNTs appears to follow the fiber paradigm, with greater cancer potency associated with longer, biopersistent structures. However, CNT and CNF materials typically consist of a distribution of sizes, and specific dimensions of CNT structures associated with the carcinogencity of CNTs could not be determined in the IARC monograph (IARC, in press). It should be noted that definitive sizes of asbestos and other fibers that are carcinogenic has also not been determined, although long fibers are more carcinogenic that short fibers, when using a cutoff at 5 μm (Boulanger et al 2014). Regulatory standards for asbestos are typically based on fiber counts of structures >5 μm in length with a 3:1 (length:width) aspect ratio, as measured by phase contrast microscopy (in which structures greater than approximately 250 nm are visible). Research is needed to determine which length limits, if any, would be applicable to CNTs.
Defects
An ideal CNT is formed only by hexagonal rings of sp2 hybridized carbons. CNTs currently produced are far from perfect as the graphene layers contain various degrees of defects that either arise directly from CNT synthesis or may be introduced post synthesis. Typical defects are non-hexagonal rings, atom vacancies, sp3 hybridized carbon, incomplete bonding, and/or oxygenated groups (Ebbesen & Takada 1995; Charlier 2002; Galano et al. 2010). In a study of the same MWCNT specimen, with or without defects, studied by Raman spectroscopy, micronuclei formation in cultured cells and inflammation and fibrogenicity in vivo correlated with the presence of broken C-C bonding generated by grinding (Muller et al. 2008a). Unaltered levels of micronuclei frequency were observed when all defects were eliminated by thermal treatment (Muller et al. 2008a).
Surface functionalization
The effect of surface functionalization is controversial because both lower and higher toxicity have been reported when functionalized and unfunctionalized CNTs were compared. This may arise from two contrasting factors: (1) an improved dispersibility, which increases “the dose” by allowing direct contact of single CNTs with cells and tissues, and (2) some surface functionalities, e.g., carboxyls, that reduce adverse effects. The latter case concerns effects that depend on the chemical nature of the functionality employed. Surface oxygenated functionalities increase CNT toxicity in some models (Bottini et al. 2006; Vittorio et al. 2009; Pietroiusti et al. 2011). In contrast, Cheng et al. (2008) reported that purified PEGylated (polyethylen glycol) SWCNTs, albeit reversibly internalized and translocated into the nucleus, were non-genotoxic in mammalian cells in terms of cell cycle distribution and mitosis after 5 days of continuous exposure. Anionic functionalization (COOH and PEG) decreased pulmonary fibrogenic potential compared to prepared MWCNTs, whereas strong cationic functionalization (polyethyleneimine) induced more pulmonary fibrosis. Neutral (NH2) and weakly cationic (SW-NH2) functionalized CNTs have similar fibrogenic potential compared to as-produced CNTs. The mechanism of these effects involves differences in cellular uptake of MWCNTs, lysosomal damage, and cathepsin B release associated with NLRP3 inflammasome activation (Li et al. 2013).
Hamilton et al. (2013) examined alveolar macrophages from C57BL/6 mice exposed to four distinct MWCNTs: (1) poorly water soluble raw, (2) “as-received,” (3) purified, highly water soluble –COOH-terminated raw and (4) purified. While simple purification reduced cellular toxicity and inflammasome activation only slightly compared to raw MWCNTs, functionalization of MWCNTs with –COOH groups dramatically reduced both outcomes. All particles were taken up by alveolar macrophages; however, purified MWCNTs were taken up in large vacuoles or phagolysomes and did not appear to be free in the cytoplasm. In contrast, the two functionalized MWCNTs appeared not to be in large vacuoles but more evenly distributed in smaller phagolysosomal structures or free in the cytoplasm. The results confirm that MWCNTs activate the NLRP3 inflammasome through a process involving phagolysosomal permeabilization, release of cathepsin B, and activation of caspase-1. Sager et al. (2014) investigated the same set of MWCNT in vivo. Unmodified and surface functionalized with -COOH MWCNTs were instilled intratracheally into C57BL/6 mice. Biomarkers for pulmonary inflammation included cytokines (IL-1b, IL-18, IL-33), profibrotic mediators, inflammatory cells (neutrophils), lysosomal release of cathepsin B, and markers of injury (albumin and lactate desydrogenase). The results showed that surface modification by the addition of the -COOH group to the MWCNT significantly reduced bioactivity and pathogenicity. Difference in bioactivity correlated with the activation of the NLRP3 inflammasome.
Solubility/degradation in body fluids
In vivo
The graphitic structure of CNTs makes them highly insoluble, yet several studies report that the carbon structure may be attacked and biodegraded in the lung by endogenous oxidants, predominantly peroxidases (e.g., myeloperoxidase [MPO] or eosinophil peroxidase [EPO]) in biological simulation fluids or in vivo (MPO, Kagan et al. 2010, EPO, Shvedova et al. 2012). Using MPO-deficient mice, Shvedova et al. (2012) showed that MPO contributes to the pulmonary oxidative biodegradation of SWCNTs in vivo.
In vitro
Two different routes of attack and degradation of CNTs by endogenous oxidants have been reported. The first route is via peroxidation: several peroxidases, such as MPO (Kagan et al. 2010) and eosinophil peroxidase (EPO; Shvedova et al. 2012), can degrade chemically cut, short CNTs. Andon et al. (2013) reported SWCNT degradation upon incubation with human EPO, lactoperoxidase, and hydrogen peroxide. Biodegradation of the SWCNTs was higher in the presence of sodium bromide, but neither EPO alone nor hydrogen peroxide alone caused the degradation of nanotubes. The second route is via nonenzymatic degradation, which can occur when CNTs are in contact with simulated phagolysosomal fluid (Liu et al. 2010; Stern et al. 2012; Elgrabli et al. 2015). Surface functionalization directly influences the degree to which CNTs can be biodegraded (Liu et al. 2010, Bianco et al., 2011). The rate of degradation is associated with both the extent of surface functional groups and type of CNTs. MWCNTs are more resistant to degradation than SWCNTs and, thus, take longer to degrade. However, workers may be less likely to be exposed to functionalized or cut CNTs than to the raw or pristine CNTs that potentially have longer retention half-lives.
Radical production in cells and cell-free systems
MWCNTs and SWCNTs have the ability to quench free radicals (Fenoglio et al. 2006, 2008; Galano 2010). In vitro, CNTs retard the oxidation of polystyrene, polyethylene, polypropylene, and polyvinylidene fluoride due to their strong radical accepting ability, which may interrupt chain propagation, leading to antioxidant effects in polymeric material (Watts et al. 2003). Lucente-Schultz and coworkers demonstrated that pristine SWCNTs are powerful antioxidants in a cell-free system (Lucente-Schultz et al. 2009). Various modified CNTs exhibit different chemical composition and structure at defective sites. Ultraviolet-visible and Raman spectra revealed that the efficiency of hydroxyl or 2,2-diphenyl-1-picrylhydrazyl to scavenge radicals increased with increasing number of defective sites on modified CNTs (Shieh & Wang 2014). In the absence of defects, the quenching potential is lost (Fenoglio et al. 2008; Muller et al. 2008b).
Discussion of review findings
Overview of mechanisms
CNTs and CNFs share some common attributes with poorly-soluble particles, which have been shown to induce persistent inflammation and lung cancer in rodents (IARC 1997; Borm et al. 2004). Many types of CNTs and CNFs also have fiber characteristics, and some fibers (e.g., asbestos) have been associated with elevated risk of lung cancer and mesothelioma in humans and animals (IARC 2012). Rodent studies of intraperitoneal injection of certain MWCNTs have shown elevated mesothelioma, and MWCNT-7 has been identified as a promoter of lung cancer in mice (Section “Rodent Cancer Data on CNTs”). The translocation of fibers or nanotubes to the pleura is considered an important factor in the development of mesothelioma following inhalation exposure (Sections “Translocation from the Lungs to Other Organs” and “In Vivo (Rodent) Effects Associated with Indirect Genotoxicity”) (Figure 3).
The mechanism for particle and fiber-induced lung cancer involves the deposition and retention of particles in the lungs, which at sufficient doses can trigger the persistent influx of neutrophils, generation of reactive oxygen and nitrogen species, cell injury, cell proliferation, fibrosis, DNA oxidation damage, mutation and/or chromosomal alterations, and eventually cancer (Knaapen et al. 2006; Schins & Knaapen 2007; Baan et al. 2007; IARC 2010) (Figures 3 and 4). Fibrous structures (fibers or tubes) of sufficient length and rigidity can cause an elevated inflammatory response due to frustrated phagocytosis (Sections “Cell Uptake and Interaction” “In Vivo (Rodent) Effects Associated with Indirect Genotoxicity”; “Form, Size, Length and Thickness”). The mechanistic evidence is based primarily on studies in rodents, especially rats. The exact physico-chemical characteristics to predict particle- and fiber-induced cancers are not known, and multiple factors could be involved. Some evidence in humans and rodents on asbestos and other mineral fibers has shown that the longer and thinner structures (e.g., >5–10 μm in length and <0.25 μm in diameter) are strongly associated with lung cancer or mesothelioma; however, other fiber dimensions are also significantly associated with cancer (Section “In vivo (rodent) effects associated with indirect genotoxicity – Pleural inflammation, fibrosis, and cancer associated with asbestos exposure”).
Inflammation-mediated cancer is considered to be an indirect (secondary) cancer mechanism. In addition, asbestos fibers, MWCNT, and SWCNT have been shown to physically disrupt cells and cellular structures, resulting in DNA damage and chromosomal alterations in dividing cells (Section “Genotoxicity”). Genetic instability (due to DNA mutations or chromosomal alterations) and the selection and amplification of genomically unstable cells are considered to be key events in the development of lung cancer or mesothelioma from exposure to biopersistent fibers of various types, and potentially to CNTs and CNFs (Table 7) (Figure 4). However, the available evidence is too limited to link the key events in the hypothesized cancer pathway across the various types of CNTs and CNFs.
A summary of the available evidence – and gaps – for key events in possible carcinogenic pathways is shown in Table 6. Reducing uncertainty in the relationships between these events and carcinogenesis and validating the predictive endpoints would permit assessment of a larger set of substances than is currently available. Such evidence might be used in future IARC evaluations, in which mechanistic evidence can substitute for conventional cancer bioassays when there is less than sufficient evidence in experimental animals or in epidemiological studies (Figure 2) (IARC 2006; Cogliano et al. 2008). Mechanistic data are likely to become increasingly available, especially for new materials such as CNTs and CNFs; thus, gaining a better understanding of these mechanisms will improve the evidence basis in the future. In order to develop such mechanistic evidence, further research and validation is needed to link the cancer-related (precursor) responses in the in vitro studies and the short-term in vivo studies to the cancer findings in vivo, with regard to the dose across experimental systems and to the physico-chemical properties of the materials.
The goals of this critical review were to summarize and further examine the mechanistic evidence and data gaps on the potential carcinogenicity of CNTs and CNFs. We extended our evaluation from the original IARC evaluation to include published studies on CNFs, and additional published studies in key areas of evidence with limited data including studies of pulmonary responses in workers and experimental animal studies on genotoxicity and cell proliferation as follows (see also Section “Substances and Endpoints Evaluated”). Recently published biomonitoring studies in workers exposed to MWCNT are discussed in Section “Worker Exposures and Lung Responses” (Fatkhutdinova et al. 2016; Shvedova et al. 2016). One two-year rodent cancer bioassay of four types of MWCNT was published after the IARC Monograph 111 meeting and added to this review (Rittinghausen et al. 2014) (Table 1). A recent article (Suzui et al. 2016) showing mesothelioma in rats exposed to MWCNT-N by the pulmonary route is briefly discussed at the end of this section. One new subchronic inhalation study in rats of one type of MWCNT was published after the monograph meeting and added to this review (Pothmann et al. 2015). A recent study of pulmonary deposition and retention kinetics of MWCNT based on in aggregated vs. dispersed aerosolized MWCNT is included (Pauluhn & Rosenbruch 2015). Two rodent studies of CNF were added (Murray et al. 2012; DeLorme et al. 2012). One acute inhalation study in rats of three variations of a MWCNT was added (Silva et al. 2014). Several studies are cited that assessed in vivo genotoxicity, gene and miRNA expression and cell transformation endpoints, DNA damage, and micronucleus frequency in rodent lung issues, which included: Shvedova et al. 2008, 2014; Kato et al. 2013; Snyder-Talkington et al. 2013a, 2016; Vesterdal et al. 2014b; Poulsen et al. 2015; (Table 4). Some studies were excluded following examination (as described in Tables S-1 and S-3). A number of in vitro geno-toxicity studies in human lung or mesothelial cells were added to this review (Tables S-2 and S-4) (as described in Section “In vitro (cellular) responses associated with direct genotoxicity–Measurement of genotoxicity”). Further discussion in this review on key events and mechanisms of carcino-genesis provides additional relevant references since the monograph meeting. Quantitative estimates of total deposited doses in rodent studies and workers are compared (Sections S-1–S-3 in online supplementary material). Finally, the currently available evidence and data gaps (Tables 6 and 7) are described in this critical review.
The scientific literature is moving rapidly on the potential carcinogenicity of CNTs. Illustrating this point, a recent article (Suzui et al. 2016) reported increased incidences of malignant mesothelioma (6/38) and bronchiolo-alveolar lung tumors (adenomas and carcinomas) (14/38) in rats (F344/Crj male) following trans-tracheal intrapulmonary spraying (TIPS) of three different fractions of MWCNT-N (NIKKISO Co., Ltd) compared to 0/28 of either mesothelioma or lung tumor response in the controls (Suzui et al. 2016). The average lengths of the MWCNTs were 4.2 μm before filtration and 2.6 μm in the flow-through fraction. The total dose was 1 mg/rat, administered during the initial two weeks of the experiment; and rats were observed up to 109 weeks. All malignant mesotheliomas were seen in the pericardial pleural cavity. This is apparently the second published study showing carcinogenicity of MWCNT-N in rats, which could provide adequate evidence to revise the IARC (2014) finding on that material. It is not our purpose in this review paper to reevaluate the evidence and findings of the IARC Monograph working group, although we cite this recent article as it may be a key development in the carcinogenicity literature of MWCNTs.
Evaluation of evidence at key steps in carcinogenesis
Inhalation, deposition, and retention
Data are on airborne exposures to CNTs and CNFs measured in the workers’ breathing zone (Section “Worker Exposures and Lung Responses”) indicate that these substances are inhalable and that some fraction of the inhaled mass would deposit in the respiratory tract, including the pulmonary (gas-exchange) region (Section “Inhalation and Deposition”). In rodents, reduced clearance has been reported in some but not all studies of CNTs, and may depend on the dose and duration as well as the particle size (Section “Clearance and Retention”). Chronic exposure studies are lacking for all types of CNTs and CNFs, and the long-term retention of CNTs or CNFs in the lung and pleura tissues has not been measured in animals.
Studies with quantitative data on the kinetics and fate of CNTs in the respiratory tract and other organs provide information that can help to reduce the uncertainty in extrapolating animal study findings to humans. Such data are available for some MWCNTs (Pauluhn 2010; Mercer et al. 2010, 2011, 2013b), but not for SWCNTs or CNFs.
No studies are available in humans on the inhalation, deposition, retention, or translocation of CNTs or CNFs. Nonetheless, uncertainty is low that airborne exposure to CNT and/or CNF could result in the inhalation and deposition of these substances in the respiratory tract given the established aerosol deposition models in human and rodent respiratory tracts. Uncertainty is also relative low in the quantitative estimation of the deposited mass dose, given exposure, since the aerosol deposition models for in animals and humans are expected to apply to CNTs and CNFs with similar aerosol characteristics. Uncertainty is moderate/high on the CNT/CNF dose that is retained in the lungs or translocated to the pleura in humans because – although the currently available data on the short-term/subchronic clearance, retention, and translocation of CNT in rodents are expected to be useful in estimating those processes in humans – such models have not yet been developed/validated, and data on the long-term kinetics are still lacking in animals or humans.
Migration to the pleura
Evidence from studies in rodents that have observed MWCNTs in pleural tissues following inhalation exposure (Ryman-Rasmussen et al. 2009; Xu et al. 2012; Mercer et al. 2013b) (Section “Translocation from the Lungs to Other Organs”), and from studies of other types of fibers (e.g., asbestos and man-made fibers) in pleural tissues in animals and humans following inhalation exposure, suggest that humans could receive pleural tissue doses of MWCNTs (e.g., from airborne exposure in the workplace). However, the uncertainty is moderate/high on the quantitative doses of CNTs that would reach the pleura at specific exposure scenarios. Section S-3 provides an example of using available data to estimate human-equivalent airborne exposures associated with pleural tissue doses associated with mesothelioma in rats. These estimates suggest that the IP doses in the rat studies were not exceptionally high compared to those estimated for repeated exposures over a working lifetime. The dose rate is clearly different, however, and the role of dose rate on the pleural response to CNTs is not known.
Although IP injection studies are widely used for cancer hazard evaluations (Pott et al. 1991; JRC 1999; SCOEL 2012), and the findings from IP studies are used by IARC and others in carcinogen classifications, including for MWCNTs (Grosse et al. 2014; IARC, in press), IP study results are typically not used in quantitative assessments (e.g., potency or risk) because of the nonphysiological routes of exposure resulting in higher dose rates in the animal IP studies compared to equivalent pleural tissue doses in humans over a working lifetime (Section S-3). The limited amount of quantitative data on the translocation of CNTs from the lungs to the pleura (e.g., Mercer et al. 2013b) could be useful in developing models to estimate the pleural tissue dose of CNTs, e.g., from occupational exposure, and the risk of mesothelioma. Further data analyzes would also be useful to explore possible correlations in the cancer findings from in vivo studies (including IP) and possible precursor events in short-term in vivo or in vitro studies (e.g., Yegles et al. 1995, as shown for asbestos and other mineral fibers).
Lung inflammation and fibrosis
Acute exposure to CNTs (MWCNTs, SWCNTs, DWCNTs) is associated with transient inflammation that resolves over time, although CNTs may persist in the tissues. Long-term exposure to CNTs induces a sustained inflammatory response associated with granuloma formation, fibrosis, and subpleural thickening. Acute or persistent pulmonary inflammation, pulmonary granuloma or fibrosis, and other effects were observed in most of the studies with MWCNTs, SWCNTs, and other CNTs. Some of the pulmonary responses might be due to the overload or bolus effects of CNT. Regardless of the number of walls or extent of purification, statistically significant dose–response relationships were observed for these pulmonary endpoints. Inhalation exposures to other samples of short (<2 μm) MWCNTs or SWCNTs did not induce pulmonary inflammation in rats, and intratracheal instillation of the same MWCNTs induced reversible mild pulmonary fibrosis. (See Sections “Pleural cavity inflammation – MWCNT”; “Pleural cavity fibrosis – MWCNT”; “Pulmonary inflammation”; “Fibrosis and granulomas” and Table 3 for references). The uncertainties in using this evidence to evaluate the potential carcinogenicity of CNTs and CNFs pertain to: (1) fibrosis as a precursor event in carcinogenesis (Section “In Vivo (Rodent) Effects Associated with Indirect Genotoxicity” and “Fibrosis”); and (2) persistence of the inflammatory response across the various types of CNTs and CNFs with repeated or chronic exposures (Section “In vivo (rodent) effects associated with indirect genotoxicity–Inflammation”).
Cell proliferation, hyperplasia, and cell signaling
Very few studies have critically examined cell proliferation and hyperplasia in rodent lungs exposed to CNTs. Cell injury, which can lead to cell proliferation, and hyperplasia (which is due to increased cell proliferation) may occur in the early stages of carcinogenesis, but these responses are potentially reversible if the stimulus (e.g., CNTs) is removed. However, repeated exposures to biopersistent materials, such as CNTs, with repeated occupational exposure, has the potential for continual stimulation of cell proliferation. Fibro-proliferative effects that can occur from biopersistent particles include septal lung fibrosis, hyperplasia, cell injury and turnover, and ultimately lung cancer (Donaldson et al. 2011; DeLorme et al. 2012). Some MWCNTs have been shown to persist in the lungs following inhalation exposure in rodents (Pauluhn 2010; Mercer et al. 2013a). Lung epithelial cell proliferation was observed in two studies in mice exposed to SWCNTs by pharyngeal aspiration or short-term inhalation, and in rats exposed to MWCNT or to CNF by subchronic inhalation. (Sections “Mesothelial cell proliferation –MWCNT” and “Epithelial cell proliferation and hyperplasia” and Table 3).
The finding of significantly increased focal adenomatous alveolar hyperplastic lesions in mice (male, B6C3F1) following inhalation of MWCNT-7 (Sargent et al. 2014) provides evidence of pre-neoplastic changes similar to those observed in humans that have the potential to progress to bronchiolo-alveolar carcinoma or adenocarcinoma (Brambilla et al. 2001; Pandiri 2015). Presently, the mechanism of the cell proliferation is not known. Knowledge of mutations in target genes, and regulation of cell cycle and associated pathways would be necessary to understand the cell proliferation mechanism. The significantly increased incidence in focal adenomatous alveolar hyperplasia among mice exposed to only MWCNT-7 indicates the capability of MWCNT-7 to induce cell proliferation and pre-neoplastic lesions (Sargent et al. 2014).
Evidence of altered gene expression and activation of cell cycle signaling pathways was recently reported for SWCNTs in an in vitro study (Chen et al. 2015). The gene expression profile was examined in human bronchial lung epithelial cells (BEAS-2B) continuously exposed to low doses (0.02 μg/cm2) of SWCNTs for 6 months (Chen et al 2015). The authors found an increased expression of RAS family genes, and activation of WNT signaling pathway. The RAS family of genes encodes proteins involved in cellular signal transduction, which result in cell growth and division. RAS are common oncogenes, mutated in many cancers (Regad 2015). The WNT signaling pathway is activated in many cancers and regulates proliferation and metabolism (Sherwood 2015).
Genotoxicity in vivo and in vitro (epithelial or mesothelial cells)
SWCNTs and MWCNTs induce genetic lesions in experimental animals and cultured human and animal cells and have similar genetic injury end-points (Section “Genotoxicity”; Tables 4 and 5). Positive results were reported in human primary and immortalized lung and mesothelial cells in short-term assays in vitro. DNA strand breaks, oxidized DNA bases, mutations, micronucleus formation, and numerical and structural chromosome abnormalities have been reported. In vitro studies report increased micronucleus frequency and increased levels of sister chromatid exchange in cells exposed to MWCNT-7 (Kato et al. 2013) and other types of CNTs (Table S-4).
SWCNTs and MWCNTs interact with and perturb the cellular mitotic apparatus, including microtubules and centrosomes, in human lung epithelial cells. K-ras point mutations have been reported in lung tissue of mice 1 and 28 days and 1 year following a 4-day inhalation exposure to one type of SWCNT; at 1 year post-exposure, karyotypical changes were shown by micronuclei and multinucleated cells in type II pneumocytes. SWCNT was genotoxic in mice after inhalation exposure (5 mg/m3, 5 h/d for 4 days) to short (1–3 μm) CNTs (K-ras mutations 28 days and 1 year post exposure; micronuclei 1 year post-exposure) (Shvedova et al. 2008, 2014).
Consistent evidence indicates that SWCNTs and MWCNTs are genotoxic in vitro for the relevant human target cells in the lungs and pleura. In vitro studies that utilize the target tissue cell type (e.g., human epithelial type II cells) avoid some of the uncertainty inherent in inter-species extrapolation. The number of in vivo studies of genotoxity of CNTS or CNFs is currently limited, and future research is needed to investigate possible correlations in cancer-related responses in vivo in rodents and in vitro in human lung and pleural cells (e.g., as shown for asbestos and other mineral fibers; Yegles et al. 1995). Such studies could substantially increase the evidence basis on which to evaluate the potential carcinogenicity across the various types of CNTs and CNFs. To date, experimental studies are too limited to predict genotoxicity of CNTs based on specific physico-chemical properties.
Cancer
Although cancer studies are available for several types of MWCNTs (Section “Rodent Cancer Data on CNTs”), cancer studies are lacking for SWCNTs, CNFs, and many other types of MWCNTs. A limited number of studies of precursor steps (persistent inflammation, genotoxicity) are available for only a few types of MWCNTs. Even for the highly studied MWCNT-7, gaps in knowledge of the key events are apparent (e.g., no animal genotoxicity data for the lung).
Dose–response data are lacking for the development of lung cancer or mesothelioma, and so the shape of the dose–response relationships (including at low doses) is not known from inhalation studies. Dose–response relationships were reported in several IP injection studies. However, the IARC classifications are based on hazard and do not consider dose (unless the mechanisms at the doses in the experimental animal studies are not likely to operate in humans). Most of the rodent cancer studies of MWCNs were based on IP injection to deliver the dose directly to the peritoneum (vs. pleura). An inhalation study in mice showed that MWCNT-7 is a cancer promoter (of adenocarcinoma) in mice following a single IP injection of MCA (Sargent et al. 2014) (Table 3). Inadequate number of animals in some dose groups or controls reduced the statistical validity of results in some of those studies (Tables 2 and 3).
Data are lacking on the dose of MWCNTs that would reach the pleura with chronic inhalation exposure at relatively low concentrations, such as have been measured in the workplace. As mentioned in the Section “Migration to the pleura”, rough estimates of the working lifetime equivalent airborne concentration of MWCNTs that would result in a human-equivalent pleural dose suggest that the IP doses are not unreasonably high (Table S-7). For example, at the lowest IP doses in rats (0.05 mg) and mice (0.003 mg) – which were associated with significant mesothelioma incidence (Table 1) –the equivalent working lifetime airborne exposure estimates were 18 and 13 μg/m3, respectively (Table S-7). These rough estimates are based on the estimated working lifetime total deposited lung dose of CNT (assuming no clearance) and the estimated fraction of that dose that translocates from the lungs to the pleura (Section S-3).
Weight of mechanistic evidence and key data gaps
Although a considerable body of experimental data on CNTs and CNFs exists, significant data gaps remain in the key steps related to the hypothesized carcinogenic mechanisms of specific types of CNTs and CNFs. These gaps are due to the heterogeneity of CNTs and CNFs, inadequate systematic evaluation, and limited chronic studies. Some mechanistic evidence is available for in vivo end-points related to mesothelioma for a few MWCNTs but is lacking for SWCNTs and other CNTs and CNFs. For in vivo end-points related to lung cancer, mechanistic evidence is available for some MWCNTs and SWCNTs (Table 6) but not for other types of CNTs or CNFs.
The most mechanistic evidence available is for MWCNTs of various types. Several studies have demonstrated epithelial cell proliferation and persistent pulmonary inflammation following MWCNT exposure by rodents (Ma-Hock et al. 2009; Pauluhn 2010; Aiso et al. 2010; Porter et al. 2010; Kobayashi et al. 2011; Murray et al. 2012; Sager et al. 2013; Xu et al. 2014; Sargent et al. 2014). Persistent pulmonary fibrosis was shown in several rodent studies of MWCNTs (Muller et al. 2005; Aiso et al. 2010; Cesta et al. 2010; Mercer et al. 2011, 2013a; Pauluhn 2010; Murray et al. 2012; Porter et al. 2010; Sager et al. 2013). Sargent et al. (2014) found fibrosis in male B6C3F1 mice at 17 months following inhalation of MWCNT-7 (with or without MCA). Pleural penetration of two types of MWCNTs was shown by Ryman-Rasmusssen et al. (2009) and Mercer et al. (2013b), and Xu et al. (2012, 2014) found mesothelial cell proliferation following MWCNT exposure. On the contrary, several studies have demonstrated transient or no pulmonary imflammation, fibrosis and proliferation following MWCNT exposure (Kobayashi et al. 2010; Morimoto et al. 2012a, 2012b; Silva et al. 2014). Evaluation of the factors that contribute to the differences in pulmonary responses to various types of CNTs and CNFs is a critical research need, including the role of dose and duration, physical-chemical properties, species/strain/gender, and other experimental factors.
The diversity of CNTs and CNFs precludes a systematic analysis of individual materials with wide variability in the physico-chemical properties of the materials tested, including size of the individual and agglomerated structures. Inconsistencies are found in the available data for the various types of CNTs. For example, although long (>5 μm) fibers are typically associated with persistent inflammation (considered to be a key event in particle and fiber carcinogenicity), short MWCNT (<1 μm) cause DNA damage in the absence of persistent inflammation. Differences in experimental procedures (e.g., affecting CNT dispersion or agglomeration) can also influence results. Although CNTs are typically hydrophobic and tend to agglomerate, these structures have been effectively dispersed in a solution that mimics the components of the alveolar lining fluid (although at lower concentrations) (Porter et al. 2008). Well-dispersed SWCNTs or MWCNTs caused an enhanced pulmonary interstitial fibrotic response in mice compared to mice administered poorly-dispersed materials (Mercer et al. 2008; Shvedova et al. 2008). However, agglomerated CNTs have also been shown to cause pulmonary and pleural effects. For example, visceral pleural thickening was observed in rats following subchronic inhalation at 1.5 or 6 mg/m3 of agglomerated MWCNT (MMAD of ~3 μm) (Pauluhn 2010).
Studies of CNTs and CNFs in the workplace have reported that the airborne structures are generally agglomerated and short (less than approximately 5 μm in length) (Han et al. 2008; Birch et al. 2011; Dahm et al. 2012; Dahm et al. 2015), although individual structures were occasionally observed (Dahm et al. 2015). CNFs share some physico-chemical properties with MWCNTs; however, the different arrangements of the graphitic building blocks suggest differences in reactivity (e.g., due to the mechanisms of defect generation or retention of metallic impurities). Further study is needed to provide systematic evaluation of CNTs and CNFs that differ in defined physico-chemical properties in experimental systems in order to delineate the role of specific properties.
Standardized study designs and protocols would reduce variability due to experimental factors and facilitate comparative analyses across CNT and CNF materials. For example, subchronic inhalation study guidelines have been established by OECD (2009), and minimal data and dosimetric considerations have been discussed in a recent review (Oberdorster et al. 2015). A core set of experimental assays and endpoints on a representative set of CNTs and CNFs would provide a set of reference materials for comparative analyses to a wider variety of materials and assays. Guidance is needed to promote experimental design and data reporting that are useful for hazard and risk assessments. At the same time, flexibility to develop and incorporate new methods or approaches must be retained, for example, with regard to developing and validating high throughput systems. In addition to standard experimental designs, standardized criteria for interpreting the mechanistic evidence would facilitate cancer hazard evaluations. For example, the IARC (2006) guidance could be extended to include more specific criteria on evaluation of mechanistic evidence of carcinogencity. Such guidance would be especially useful given the increasing trend toward alternative testing strategies and reduction in chronic bioassays. In addition, dose–response data sufficient to develop and test predictive models are needed to provide the evidence basis for reliably interpreting the mechanistic data in the absence of chronic bioassay data.
Although recognizing certain data limitations, some coauthors consider that the collective evidence is sufficiently strong to consider all types of CNTs to be potentially carcinogenic to humans (as discussed in IARC Monograph 111) (IARC, in press). Occupational exposure studies have shown that airborne CNTs are inhalable and respirable; studies in human respiratory tract replicas, and in rodent studies, show that CNTs and CNFs can be deposited and retained in the respiratory tract; some studies in rodents have shown that MWCNT can translocate to the pleura. Persistent pulmonary inflammation and fibrosis have been shown in some (but not all) studies in rodents exposed to MWCNT or SWCNT by inhalation or other routes. Pleural inflammation and injury have been shown in a few studies of MWCNTs in rodents. Many types of CNTs have been shown to induce primary or direct genotoxicity –including DNA and chromosomal damage and interference with mitosis – in a large number of in vitro studies and in the few in vivo studies that have examined these mechanisms. Several types of genetic damage (different mechanistic end points) have been demonstrated in independent experiments in numerous mammalian cells types (including human lung and mesothelial cells). If such DNA and chromosomal damage is not correctly repaired, additional genetic and epigenetic changes may accumulate leading to mutations in cell cycle regulatory genes, oncogenes, and tumor suppressor genes and development of cancer (Figure 4). The weight of evidence in the different steps leading to cancer is not necessarily the same, but genomic damage is mandatory in the mechanistic process. Each type of CNT has not been tested for each step leading to cancer, except for genotoxicity. However, globally, each step has been demonstrated to occur among the various types of CNTs. For MWCNTs, each step is completed, by one or another MWCNTs subtype, and as documented by several independent studies (Muller et al. 2008a; Ryman-Rasmussen et al. 2009; Aiso et al. 2010; Porter et al. 2010, 2013; Mercer et al. 2011; Treumann et al. 2013; Sargent et al. 2014; Kasai et al. 2015). New reported studies extend these results to additional types of CNTs, including an IP study showing carcinogenicity of four samples of MWCNTs (Rittinghausen et al. 2014), and a recent article that reports malignant and nonmalignant lung tumors and mesothelioma in rats exposed by trans-tracheal intrapulmonary spraying to three different fractions of MWCNT-N (Suzui et al. 2016).
The majority of the IARC monograph 111 Working Group considered that the lack of coherent evidence among the various CNTs precluded the prediction of carcinogenicity for specific CNTs based on mechanistic evidence alone (Grosse et al. 2014; IARC, in press). Thus, the IARC classification was based on the animal cancer studies available at the time (Section “Rodent Cancer Data on CNTs”). The relevance of the animal-based mechanisms to humans is an important criterion in evaluation of the mechanistic evidence (IARC 2006). For CNTs, the majority of the Working Group considered that the mechanistic evidence in animals is relevant to humans, including the potential for the deposition and retention of airborne CNTs in the lung, translocation beyond the lung, and the development of inflammation, lung and pleural injury, fibrosis, and genotoxicity (Grosse et al. 2014; IARC, in press). However, a substantial gap in the current data is the lack of relevant mechanistic information from exposed humans (Grosse et al. 2014; IARC, in press). Ongoing epidemiology studies in humans may provide data to evaluate whether particular mechanisms are likely to be operative in humans. IARC (2006) considers that the strongest mechanistic evidence would “derive from data on humans or biological specimens obtained from exposed humans.” The diversity of MWCNTs, SWCNTs, DWCNTs, and CNFs used in the workplace, and the generally limited number of exposed subjects in these environments, make these studies and data synthesis challenging.
Research needs and recommendations
One of the main objectives of this review was to identify the significant data gaps in the mechanistic evidence in evaluating the potential carcinogencity of CNTs and CNFs, and to suggest research to fill those gaps. This evaluation builds on our work during the IARC monograph 111 meeting (IARC, in press). The practical use of this information is to guide improvements in the evidence basis for future evaluations of the various types of these materials. Given the large variety of CNTs and CNFs, it is unlikely that the standard two-year bio-assay will be performed for most of these materials, and increasing reliance will be placed on mechanistic data (IARC 2006; Cogliano et al. 2008). Consideration of the biological mechanisms related to dose and dose rate may require further investigation to better assess the cancer risk in humans (McClellan 1997; Oberdorster et al. 2005a).
The technological capability to modify CNTs or CNFs to have specific structural types (wall number), dimensions, and functional groups introduces the possibility to “engineer” materials that are safer (less biopersistent, less biolocially reactive) compared to other materials that may be used for similar applications. The physico-chemical characteristics which influence the safety or harm of these materials is an important question, which is not fully understood for inhaled particles in general, nor specifically for CNTs or CNFs.
The following research needs and recommendations were developed following the IARC monograph 111 meeting, during the development of this critical review paper. These recommendations focus on specific areas of experimental investigation or data analysis which would help to improve the evidence basis and reduce uncertainty in future evaluations of the carcinogencity across the range of the various types of CNTs and CNFs:
Clarify the role of cellular studies in an evaluation of the mechanistic evidence on carcinogenicity
Despite the large number of cellular studies that showed genotoxicity of CNTs according to standard tests (Table 5), these data did not carry much weight in the original evaluation (IARC, in press). The lack of evidence in vivo for many types of CNTs on several steps in the pathway (Table 6), including the lack of chronic inhalation studies, was the basis for a majority finding that the evidence was not strong enough to support modifying the carcinogen classification for any specific CNT or group of CNTs based on the animal data. Other Mechanisms Subgroup experts considered that the positive genotoxicity findings in cultured cells for a wide variety of CNTs, in conjunction with animal evidence on genotoxicity and cell proliferation for some CNTs, provided strong mechanistic evidence for the potential human carcinogenicity of the broader category of CNTs. Supporting that view are the findings of elevated DNA strand breaks (assessed by comet assay in cultured cells or animal tissues), which have been shown to be a reliable predictor of animal carcinogens generally (i.e., ~80% concordance); a high level of concordance was also reported between the findings of comet assays and micronucleus assays for various types of nanomaterials (Section “Measurement of genotoxicity”). Evidence of increased DNA strand breaks, micronuclei, and other measures of genotoxicity and gene expression was found in most, but not all, of the in vivo and in vitro studies of CNTs and CNFs to date (Table 4 and 5).
Although some guidance is available on evaluating the results of various tests for genetic and related effects “in view of the relevance of gene mutation and chromosomal aberration/aneuploidy to carcinogenesis” (Vainio et al. 1992; McGregor et al. 1999) (IARC 2006), the role of in vitro assays in the weight-of-evidence in the mechanistic data has not been well-defined, including for cultured cells from animals or humans corresponding to the target tissues for cancer (e.g., bronchiolar or alveolar epithelial cells or pleural mesothelial cells) in the absence of human data for comparison. Moreover, the studies cited in the IARC (2006) guidance on the types of assays to be considered in an evaluation of the mechanistic evidence (Montesano et al. 1986; Vainio et al. 1992; McGregor et al. 1999) pre-date some of the more recent advances in experimental assays. For example, the comet assay and micronucleus assay to measure DNA damage (Section “Measurement of genotoxicity”) are now standard genotoxicity tests for which OECD guidelines have been developed. An updated assessment of the role of these assays in mechanistic evaluations may be useful, especially given the increasing reliance on mechanistic data that is anticipated in many evaluations of potential carcinogens (IARC 2006; Cogliano et al. 2008).
Also needed is an examination of the predictivity of the more recently developed cell transformation assays (e.g., Wang et al. 2014a) on the potential carcinogenicity of various types of CNTs and CNFs. Linking these experimental assays to biomarkers measured in epidemiological studies of workers with exposures to CNTs or CNFs would strengthen the mechanistic evidence for evaluation, although such analyses will require sufficient data and the development and validation of predictive models.
Recent technological advances in experimental systems have a high potential for providing insights into the mechanisms of carcinogenicity. “Omics” assays have high output for determining the cell responses to xenobiotics and for investigating the molecular characteristics of the resulting pathologies, including the progression from the preneoplastic to neoplastic state. Pathway analyses allow investigations to focus on specific physio-pathological responses. For example, genomic approaches have been developed to study DNA repair and mutagenesis (Wyrick & Roberts 2015). In silico comparison using data obtained in human neoplasms, especially lung cancers and mesothelioma, provide information on general mechanisms of carcinogenicity and on animal species specificities. Comparison of data between several types of CNTs or CNFs could also be done. Genomic studies can be performed, not only on cultured cells, but also on normal and pathological tissues. The comparison between both types of data is of high interest to validate the in vitro systems to screen a large number of CNTs and CNFs. These assays, although in their infancy in the toxicological field, can be anticipated to be available in future evaluations.
Evaluate the strength of evidence associated with preneoplastic events
To strengthen understanding of the linkages between early changes and cancer, further study is needed on the early biological events that are potentially predictive of lung cancer or mesothelioma development following exposure to CNTs or CNFs, e.g., screening assays based on experimental assays in cellular systems. If such assay data are shown to correlate well with in vivo responses, then such dose–response data, in combination with validated dosimetry models that predict tissue dose, could be used to predict lung cancer and mesothelioma risk depending on the exposure and the physico-chemical properties of the material.
A key research need is to determine the relationship between pre-neoplastic lesions, such as AAH, and carcinoma following subchronic or chronic inhalation exposure to various types of CNT or CNF. Such studies are needed to improve the mechanistic evidence for assessing the potential carcinogenicity of CNT and CNF, since it is not feasible to perform chronic rodent bioassays of each material. The focal adenomatous hyperplasia observed in alveolar epithelial tissues in mice exposed to MWCNT-7 (Sargent et al. 2014) is considered to resemble the preneoplastic lesion AAH in humans (Section “Epithelial cell proliferation and hyperplasia—MWCNT”) (Brambilla et al. 2001; Pandiri 2015). It would be useful to investigate any influence of the physico-chemical properties of the material on the dose–response relationships for pre-neoplastic lesions. It would also be useful to further examine the characteristics of the hyperplastic lesions in animals with the characteristics of preneoplastic lesions (e.g., AAH) in humans (Section “Epithelial cell proliferation and hyperplasia—MWCNT”) (Brambilla et al. 2001, Pandiri 2015).
Investigate the dose–response relationships for precursor events and cancer in the respiratory tract
Currently available toxicological data (in vitro and in vivo) could be utilized to develop meta-data sets to examine possible correlations between various early events in cellular systems and in animals that may be predictive of lung cancer or mesothelioma. These meta-analyses could be updated as new information becomes available. In vitro and in vivo biomarkers and endpoints on the pathway(s) to cancer development could be examined for their relationship to genotoxic and carcinogenic events in vivo (Sections “Hypotheses on Mechanisms Related to Genotoxicity and Carcinogenicity of Inhaled Particles or Fibers”; “Indirect Genotoxicity of CNTs and CNFs: Rodent Studies”; “Genotoxicity”). Comparable biomarker data from ongoing epidemiology studies in workers could be added to these evaluations when such data become available.
Ideally, information on the dose–response relationships for cancer, and associated precursor events, would include data from a chronic inhalation study on a selected set of CNTs or CNFs in rats and mice, such as from the US NTP chronic bioassay protocol. Earlier time points for examination of short-term and subchronic responses would provide data for comparison to chronic endpoints in the same study. Such a study would be technically and economically challenging to achieve, including technological changes in the generation and measurement of CNTs aerosols (e.g., as discussed in Chen et al. 2012). The potential for adverse effects in other tissues and organs beyond the respiratory tract should also be investigated in a chronic bioassay.
Determine the physico-chemical and other properties that influence the dose–response relationships
A systematic investigation of a set of CNTs and CNFs that vary by specific physico-chemical properties would provide highly useful data for examining quantitative structure-activity relationships in conjunction with dose–response analyses. The influence of other experimental factors would also need to be evaluated, including the route of exposure, the duration of exposure and post-exposure, the rodent species/strain/gender, and other experimental study conditions. A subset of materials which are relatively well-studied in vivo should be included as control or reference/benchmark materials. MWCNT-7 and ultrafine carbon black are relatively well-studied materials that has been examined across various assays and experimental systems. The selected set of assays and endpoints would be those shown with other materials (e.g., poorly-soluble particles or fibers) to be relevant to cancer pathway events in vivo (Section “Hypotheses on Mechanisms Related to Genotoxicity and Carcinogenicity of Inhaled Particles or Fibers”). A tiered testing and benchmark approach (as described in Oberdorster et al. 2005b; Kuempel et al. 2012; Nel et al. 2013) would improve the efficiency of the experimental system for development of quantitative dose–response data for comparative studies across a set of materials and assays.
Quantitative analyses of dose–response relationships for malignant and related non-malignant endpoints, with consideration of the various dose metrics (mass, volume, surface area, number) and physico-chemical properties that could modify the dose–response relationships, would provide key evidence for predicting the cancer risk of exposure to specific CNT or CNF materials. The establishment of these relationships could strengthen the evidence basis to evaluate other CNTs or CNFs that have not been studied in animals. Such quantitative analyses could provide estimates of relative potency and support the development of occupational health guidance. However, due to the varying nature of these structures, and the variety of experimental designs, it remains a challenge to obtain a large enough database needed for valid statistical inference. Experimental factors need to be accounted for in any meta-analysis in order to reliably characterize the dose–response relationships for CNTs and CNFs from different studies. Experimental factors include the animal species/strain and gender, route of exposure, number of dose groups, animals per dose group, material preparation procedures, dose levels, and dose rates. Few studies to date provide sufficient dose–response data for quantitative comparisons among CNTs or CNFs. Greater emphasis on providing quantitative dose and response in a core set of validated assays, as well as a basic set of physico-chemical descriptors, would go a long way to providing useable data for meta-analysis.
Develop validated dosimetry models for CNTs and CNFs in the rodent and human respiratory tract
Current deposition models for inhaled particles or fibers in the respiratory tract may provide reasonable estimates of the deposition fractions of CNTs or CNFs based on the aerosol particle size. Verification that current deposition models can predict deposition of airborne CNTs and CNFs would reduce uncertainty in using those models to predict deposited dose.
In contrast to deposition, the clearance and retention kinetics are more uncertain for CNTs and CNFs; and the inhaled particle or fiber models of clearance retention, and translocation need to be evaluated and possibly revised or extended to estimate the retention and clearance kinetics of CNTs and CNFs. Although some studies are available on the disposition of inhaled MWCNTs, data are lacking on the disposition of inhaled SWCNTs or CNFs, including clearance, retention, and/or translocation. Data available for several types of MWCNTs indicate that MWCNTs can rapidly migrate from the lungs to the pleura, as well as to extrapulmonary organs. Agglomerated CNTs remain in the lungs, while single CNTs are found in other tissues. Currently available clearance and retention models developed from data of inhaled fibers could be evaluated for fit to the limited kinetics data for MWCNT in rodents. Further data on clearance, retention, and translocation of CNTs or CNFs with repeated exposures are needed to understand the fate of inhaled CNTs and CNFs over time. The ultimate goal of a validated dosimetry model (e.g., developed from rodent data and extrapolated to humans) is to estimate the pulmonary and pleural dose of CNTs and CNFs, e.g., in workers, in order to estimate the risk of adverse effects given exposure.
Expand the current biomonitoring studies in workers
Further studies in humans on exposure, dose, and biomarker response are needed to strengthen the mechanistic data regarding the potential carcinogenicity of CNTs and CNFs. The strength of the evidence that any carcinogenic effect observed is due to a particular mechanism is evaluated in the IARC evaluation using terms such as “weak”, “moderate,” or “strong” (IARC 2006), prior to assessing whether that particular mechanism is likely to be operative in humans. The most relevant evidence that a particular mechanism could operate in humans will be from data in exposed humans, or from biological specimens from exposed humans, that show “the agent in question has caused changes in exposed humans that are on the causal pathway to carcinogenesis” (IARC 2006). It is also relevant to note that many types of CNTs in develpment may not have resulted in much exposure to humans, and thus such mechanistic data may not be available for the evaluations (IARC 2006). This situation would also offer an opportunity to better understand the carcinogenic hazard potential before significant exposure to humans occurs (i.e., get ahead of the curve in protecting the health of workers and the general population).
Conclusions of review and next steps
This review provides an updated, somewhat broader, and more in-depth review of the literature assessed for the IARC Monograph 111 evaluation of the evidence for the carcinogenicity of CNTs (Grosse et al. 2014; IARC, in press). The subsequent studies strengthen the data basis that was evaluated in the IARC Monograph 111 regarding the carcinogenicity evidence for certain types of MWCNTs (Rittinghausen et al. 2014), yet significant data gaps remain for other types of CNTs and CNFs. Certain aspects of DNA damage have been documented in lung tissue and cultured cells, especially comet assay endpoints, after exposure to a range of CNTs. However there is a paucity of studies on some of the endpoints in the pathway to lung cancer, including mutations and chromosomal damage. Also, genotoxic effects to mesothelial cells in vivo following inhalation exposure remain to be investigated. Most notably is of course the absence of data from exposed humans.
MWCNT-7 is the most studied type of CNT, including data on lung and pleural early effects as well as cancer studies. In the absence of those data for other types of CNTs, it is unclear to what extent the data on MWCNT-7 can be extended to other types of CNTs. Some studies have shown greater inflammatory responses for longer CNTs compared to shorter (or tangled) CNTs (Poland et al. 2008; Nagai et al. 2013; Xu et al. 2014). A thinner diameter was also associated with higher tumor induction (Nagai et al. 2013). Heterogeneity in the types of CNTs and CNFs (by structure, dimensions, functionalization, contaminants) studied in the various steps on the hypothesized carcinogenic pathways; limitations in the animal cancer studies (few CNT types and no CNF evaluted, inadequate experimental design); and key data gaps in hypothesized cancer pathways were the main sources of uncertainty in the evidence available on the potential carcinogenicity of CNTs and CNFs.
Systematic, targeted research is needed to reduce uncertainty evidence about the potential carcinogenicity of various types of CNTs or CNFs and to develop predictive models based on physico-chemical properties. The application of a consistent study design across a range of well-characterized CNTs differentiated from each other by a single physico-chemical feature would facilitate the pooling and comparison of data from multiple studies. These types of comparative studies are vital to make connections between new and existing data and build on current knowledge of inhaled particles and fibers.
Supplementary Material
Acknowledgments
The authors, IARC Mechanisms Subgroup members, gratefully acknowledge the IARC Secretariat and Monograph 111 Working Group members for their scientific review and input on the mechanistic evidence sections that we drafted for the monograph. This manuscript is based on, and updates, the material that we originally prepared for the carbon nanotubes section of the monograph, and it is also informed by the review comments and discussions before and during the monograph meeting. We would especially like to thank the IARC/WHO Secretariat members Dr. Yann Grosse, Dr. Kathryn Guyton, and Dr. Kurt Straif for their insightful review comments on an earlier draft of this paper. Special thanks go to the IARC staff for their administrative support during the monograph meeting, and to Ms Suzette Smiley-Jewell, University of California, Davis, and Ms Ellen Galloway, NIOSH, for editorial assistance on this article. We thank Ms Vanessa B. Williams and Ms Nicole M. Romero, NIOSH, for preparing the final version of the figures. Finally, we would like to thank the four anonymous peer reviewers for their critical review comments which were helpful in improving the final version of this paper.
Footnotes
Declaration of interest
The affiliation of the authors is as shown on the cover sheet. The authors were all originally selected by IARC to participate in the review of the carcinogenic hazard of carbon nanotubes held in October 2014, a review they participated in as independent scientists. As noted in the article, that review will be published by IARC as Monograph 111 [now published, May 19, 2017; available from: http://monographs.iarc.fr/]. This paper was prepared by the authors as an independent endeavor. This review, the interpretations, the conclusions drawn, and the recommendations made are exclusively those of the authors and are not necessarily those of their employers or IARC. None of the authors have appeared in either legal or regulatory proceedings related to the contents of the paper.
The findings and conclusions in this article are those of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health.
Supplemental material for this article is available online here.
References
- ACGIH. Appendix C: particle size-selective sampling criteria for airborne particulate matter. Cincinnati, OH: American Conference of Governmental Industrial Hygienists; 2015. 2015 TLVs® and BEIs® R based on the documentation of the threshold limit values for chemical substances and physical agents and biological exposure indices. [Google Scholar]
- Adamson YR. Early mesothelial cell proliferation after asbestos exposure: in vivo and in vitro studies. Environ Health Perspect. 1997;105:1205–1208. doi: 10.1289/ehp.97105s51205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamson IYR, Bakowska J. KGF and HGF are growth factors for mesothelial cells in pleural lavage fluid after intratracheal asbestos. Exp Lung Res. 2001;27:605–616. doi: 10.1080/019021401753181854. [DOI] [PubMed] [Google Scholar]
- Adamson IYR, Bakowska J, Bowden DH. Mesothelial cell proliferation after instillation of long or short asbestos fibers into mouse lung. Am J Pathol. 1993;142:1209–1216. [PMC free article] [PubMed] [Google Scholar]
- Aiso S, Yamazaki K, Umeda Y, Asakura M, Kasai T, Takaya M, Toya T, Koda S, Nagano K, Arito H, et al. Pulmonary toxicity of intratracheally instilled multiwall carbon nanotubes in male Fischer 344 rats. Ind Health. 2010;48:783–795. doi: 10.2486/indhealth.ms1129. [DOI] [PubMed] [Google Scholar]
- Alarifi S, Ali D, Verma A, Almajhdi FN, Al-Qahtani AA. Single-walled carbon nanotubes induce cytotoxicity and DNA damage via reactive oxygen species in human hepatocarcinoma cells. In Vitro Cell Dev Biol Anim. 2014;50:714–722. doi: 10.1007/s11626-014-9760-3. [DOI] [PubMed] [Google Scholar]
- Aldieri E, Fenoglio I, Cesano F, Gazzano E, Gulino G, Scarano D, Attanasio A, Mazzucco G, Ghigo D, Fubini B. The role of iron impurities in the toxic effects exerted by short multiwalled carbon nanotubes (MWCNT) in murine alveolar macrophages. J Toxicol Environ Health A. 2013;76:1056–1071. doi: 10.1080/15287394.2013.834855. [DOI] [PubMed] [Google Scholar]
- Anderson D, Yu T-W, McGregor DB. Comet assay responses as indicators of carcinogen exposure. Mutagenesis. 1998;13:539–555. doi: 10.1093/mutage/13.6.539. [DOI] [PubMed] [Google Scholar]
- Andon FT, Kapralov AA, Yanamala N, Feng W, Baygan A, Chambers BJ, Hultenby K, Ye F, Toprak MS, Brandner BD, et al. Biodegradation of single-walled carbon nanotubes by eosinophil peroxidase. Small. 2013;9:2721–2729. doi: 10.1002/smll.201202508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ARA. Multiple-path particle dosimetry (MPPD 2.1) model. Raleigh (NC): Applied Research Associates, Inc; 2009. [Google Scholar]
- Artinian V, Kvale PA. Cancer and interstitial lung disease. Curr Opin Pulm Med. 2004;10:425–434. doi: 10.1097/00063198-200409000-00017. [DOI] [PubMed] [Google Scholar]
- Asakura M, Sasaki T, Sugiyama T, Takaya M, Koda S, Nagano K, Arito H, Fukushima S. Genotoxicity and cytotoxicity of multi-wall carbon nanotubes in cultured Chinese hamster lung cells in comparison with chrysotile A fibers. J Occup Health. 2010;52:155–166. doi: 10.1539/joh.l9150. [DOI] [PubMed] [Google Scholar]
- Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F, Cogliano V WHO International Agency for Research on Cancer Monograph Working Group. Carcinogenicity of carbon black, titanium dioxide, and talc. Lancet Oncol. 2006;7(4):295–296. doi: 10.1016/s1470-2045(06)70651-9. [Erratum in: Lancet Oncol. 2006. 7(5):365] [DOI] [PubMed] [Google Scholar]
- Baisch BL, Corson NM, Wade-Mercer P, Gelein R, Kennell AJ, Oberdorster G, Elder A. Equivalent titanium dioxide nanoparticle deposition by intratracheal instillation and whole body inhalation: the effect of dose rate on acute respiratory tract inflammation. Part Fibre Toxicol. 2014;11:5. doi: 10.1186/1743-8977-11-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barillet S, Simon-Deckers A, Herlin-Boime N, Mayne-L’Hermite M, Reynaud C, Cassio D, Gouget B, Carriere M. Toxicological consequences of TiO2, SiC nanoparticles and multi-walled carbon nanotubes exposure in several mammalian cell types: an in vitro study. J Nanopart Res. 2010;12:61–73. [Google Scholar]
- Barregard L, Møller P, Henriksen T, Mistry V, Koppen G, Rossner P, Sram R, Weimann A, Poulsen H, Nataf R, et al. Human and methodological sources of variability in the measurement of urinary 8-oxo-7,8-dihydro-2’-deoxyguanosine. Antioxid Redox Signal. 2013;18:2377–2391. doi: 10.1089/ars.2012.4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayat N, Lopes VR, Scholermann J, Jensen LD, Cristobal S. Vascular toxicity of ultra-small TiO2 nanoparticles and single walled carbon nanotubes in vitro and in vivo. Biomaterials. 2015;63:1–13. doi: 10.1016/j.biomaterials.2015.05.044. [DOI] [PubMed] [Google Scholar]
- Baylin SB. The cancer epigenome: its origins, contributions to tumorigenesis, and translational implications. Proc Am Thorac Soc. 2012;9:64–65. doi: 10.1513/pats.201201-001MS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beamer CA, Girtsman TA, Seaver BP, Finsaas KJ, Migliaccio CT, Perry VK, Rottman JB, Smith DE, Holian A. IL-33 mediates multi-walled carbon nanotube (MWCNT)-induced airway hyper-activity via the mobilization of innate helper cells in the lung. Nanotoxicol. 2013;7:1070–1081. doi: 10.3109/17435390.2012.702230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellmann B, Muhle H, Creutzenberg O, Dasenbrook C, Kilpper R, MacKenzie JC, Morrow P, Mermelstein R. Lung clearance and retention of toner, utilizing a tracer technique, during chronic inhalation exposure in rats. Fundam Appl Toxicol. 1991;17:300–313. doi: 10.1016/0272-0590(91)90220-x. [DOI] [PubMed] [Google Scholar]
- Bello D, Hart JA, Ahn K, Hallock M, Yamamoto N, Garcia EJ, Ellenbecker MJ, Wardle BL. Particle exposure levels during CVD growth and subsequent handling of vertically-aligned carbon nanotube films. Carbon. 2008;46:974–981. [Google Scholar]
- Bello D, Wardle BL, Yamamoto N, de Villora RG, Garcia EJ, Hart AJ, Ahn K, Ellenbecker MJ, Hallock M. Exposure to nanoscale particles and fibers during machining of hybrid advanced composites containing carbon nanotubes. J Nanopart Res. 2009;11:231–249. [Google Scholar]
- Bello D, Wardle BL, Zhang J, Yamamoto N, Santeufemio C, Hallock M, Virji MA. Characterization of exposures to nanoscale particles and fibers during solid core drilling of hybrid carbon nanotube advanced composites. Int J Occup Environ Health. 2010;16:434–450. doi: 10.1179/107735210799159996. [DOI] [PubMed] [Google Scholar]
- Berman DW, Crump KS, Chatfield EJ, Davis JM, Jones AD. The sizes, shapes, and mineralogy of asbestos structures that induce lung tumors or mesothelioma in AF/HAN rats following inhalation. Risk Anal. 1995;15:181–195. doi: 10.1111/j.1539-6924.1995.tb00312.x. [DOI] [PubMed] [Google Scholar]
- Bianco A, Kostarelos K, Prato M. Making carbon nanotubes biocompatible and biodegradable. Chem Commun (Camb) 2011;47:10182–10188. doi: 10.1039/c1cc13011k. [DOI] [PubMed] [Google Scholar]
- Birch ME, Ku B-K, Evans DE, Ruda-Eberenz T. Exposure and emissions monitoring during carbon nanofiber production-Part I: elemental carbon and iron-soot aerosols. Ann Occup Hyg. 2011;55:1016–1036. doi: 10.1093/annhyg/mer073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas R, Bunderson-Schelvan M, Holian A. Potential role of the inflammasome-derived inflammatory cytokines in pulmonary fibrosis. Pulm Med. 2011;2011:105707–8. doi: 10.1155/2011/105707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton RE, Vincent HJ, Jones AD, Addison J, Beckett ST. An overload hypothesis for pulmonary clearance of UICC amosite fibres inhaled by rats. Br J Ind Med. 1983;40:264–272. doi: 10.1136/oem.40.3.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifazi M, Bravi F, Gasparini S, La Vecchia C, Gabrielli A, Wells AU, Renzoni EA. Sarcoidosis and cancer risk: systematic review and meta-analysis of observational studies. Chest. 2015;147:778–791. doi: 10.1378/chest.14-1475. [DOI] [PubMed] [Google Scholar]
- Bonner JC. Nanoparticles as a potential cause of pleural and interstitial lung disease. Proc Am Thorac Soc. 2010;7:138–141. doi: 10.1513/pats.200907-061RM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner JC, Silva RM, Taylor AJ, Brown JM, Hilderbrand SC, Castranova V, Porter D, Elder A, Oberdorster G, Harkema JR, et al. Interlaboratory evaluation of rodent pulmonary responses to engineered nanomaterials: the NIEHS nano GO consortium. Environ Health Perspec. 2013;121:676–682. doi: 10.1289/ehp.1205693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borczuk AC. Neoplastic and nonneoplastic benign mass lesions of the lung. Arch Pathol Lab Med. 2012;136:1227–1233. doi: 10.5858/arpa.2012-0233-RA. [DOI] [PubMed] [Google Scholar]
- Borm P, Cassee FR, Oberdorster G. Lung particle overload: old school -new insights? Part Fibre Toxicol. 2015;12:10. doi: 10.1186/s12989-015-0086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borm PJ, Schins RP, Albrecht C. Inhaled particles and lung cancer, part B: paradigms and risk assessment. Int J Cancer. 2004;110:3–14. doi: 10.1002/ijc.20064. [DOI] [PubMed] [Google Scholar]
- Bottini M, Bruckner S, Nika K, Bottini N, Bellucci S, Magrini A, Bergamaschi A, Mustelin T. Multi-walled carbon nanotubes induce T lymphocyte apoptosis. Toxicol Lett. 2006;160:121–126. doi: 10.1016/j.toxlet.2005.06.020. [DOI] [PubMed] [Google Scholar]
- Boulanger G, Andujar P, Pairon JC, Billon-Galland MA, Dion C, Dumortier P, Brochard P, Sobaszek A, Bartsch P, Paris C, Jaurand MC. Quantification of short and long asbestos fibers to assess asbestos exposure: a review of fiber size toxicity. Environ Health. 2014;13:59. doi: 10.1186/1476-069X-13-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyles MSP, Stoehr LC, Schlinkert P, Himly M, Duschl A. The significance and insignificance of carbon nanotube-induced inflammation. Fibers. 2014;2:45–74. [Google Scholar]
- Brambilla B, Travis WD, Colby TV, Corrinz B, Shimosato Y. The new World Health Organization classification of lung tumours. Series Highlights in Lung Cancer. Eur Respir J. 2001;18:1059–1068. doi: 10.1183/09031936.01.00275301. [DOI] [PubMed] [Google Scholar]
- Broaddus VC, Everitt JI, Black B, Kane AB. Non-neoplastic and neoplastic pleural endpoints following fiber exposure. J Toxicol Environ Health B Crit Rev. 2011;14:153–178. doi: 10.1080/10937404.2011.556049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buder-Hoffmann S, Palmer C, Vacek P, Taatjes D, Mossman B. Different accumulation of activated extracellular signal-regulated kinases (ERK 1/2) and role in cell-cycle alterations by epidermal growth factor, hydrogen peroxide, or asbestos in pulmonary epithelial cells. Am J Respir Cell Mol Biol. 2001;24:405–413. doi: 10.1165/ajrcmb.24.4.4290. [DOI] [PubMed] [Google Scholar]
- Cao Y, Jacobsen NR, Danielsen PH, Lenz AG, Stoeger T, Loft S, Wallin H, Roursgaard M, Mikkelsen L, Møller P. Vascular effects of multi-walled carbon nanotubes in dyslipidemic ApoE−/− mice and cultured endothelial cells. Toxicol Sci. 2014;138:104–116. doi: 10.1093/toxsci/kft328. [DOI] [PubMed] [Google Scholar]
- Catalán J, Jarventaus H, Vippola M, Savolainen K, Norppa H. Induction of chromosomal aberrations by carbon nanotubes and titanium dioxide nanoparticles in human lymphocytes in vitro. Nanotoxicology. 2012;6:825–836. doi: 10.3109/17435390.2011.625130. [DOI] [PubMed] [Google Scholar]
- Cavallo D, Fanizza C, Ursini CL, Casciardi S, Paba E, Ciervo A, Fresegna AM, Maiello R, Marcelloni AM, Buresti G, et al. Multi-walled carbon nanotubes induce cytotoxicity and genotoxicity in human lung epithelial cells. J Appl Toxicol. 2012;32:454–464. doi: 10.1002/jat.2711. [DOI] [PubMed] [Google Scholar]
- Cena LG, Peters TM. Characterization and control of airborne particles emitted during production of epoxy/carbon nanotube nanocomposites. J Occup Environ Hyg. 2011;8:86–92. doi: 10.1080/15459624.2011.545943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesta MF, Ryman-Rasmussen JP, Wallace DG, Masinde T, Hurlburt G, Taylor AJ, Bonner JC. Bacterial lipopolysaccharide enhances PDGF signaling and pulmonary fibrosis in rats exposed to carbon nanotubes. Am J Respir Cell Mol Biol. 2010;43:142–151. doi: 10.1165/rcmb.2009-0113OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan JK, Roth J, Oppenheim JJ, Tracey KJ, Vogl T, Feldmann M, Horwood N, Nanchahal J. Alarmins: awaiting a clinical response. J Clin Invest. 2012;122:2711–2719. doi: 10.1172/JCI62423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman SJ, Cookson WOC, Musk AW, Lee YC. Benign asbestos pleural diseases. Curr Opin Pulm Med. 2003;9:266–271. doi: 10.1097/00063198-200307000-00004. [DOI] [PubMed] [Google Scholar]
- Chaput C, Sander LE, Suttorp N, Opitz B. NOD-like receptors in lung diseases. Front Immunol. 2013;4:393. doi: 10.3389/fimmu.2013.00393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlier JC. Defects in carbon nanotubes. Acc Chem Res. 2002;35:1063–1069. doi: 10.1021/ar010166k. [DOI] [PubMed] [Google Scholar]
- Chen BT, Schwegler-Berry D, McKinney W, Stone S, Cumpston JL, Friend S, Porter DW, Castranova V, Frazer DG. Multi-walled carbon nanotubes: sampling criteria and aerosol characterization. Inhal Toxicol. 2012;24:798–820. doi: 10.3109/08958378.2012.720741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Stueckle TA, Luanpitpong S, Rojanasakul Y, Lu Y, Wang L. Gene expression profile of human lung epithelial cells chronically exposed to single-walled carbon nanotubes. Nanoscale Res Lett. 2015;10:12. doi: 10.1186/s11671-014-0707-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, Fernando KA, Veca LM, Sun YP, Lamond AI, Lam YW, Cheng SH. Reversible accumulation of PEGylated single-walled carbon nanotubes in the mammalian nucleus. ACS Nano. 2008;2:2085–2094. doi: 10.1021/nn800461u. [DOI] [PubMed] [Google Scholar]
- Chopra A, Judson MA. How are cancer and connective tissue diseases related to sarcoidosis? Curr Opin Pulm Med. 2015;21:517–524. doi: 10.1097/MCP.0000000000000186. [DOI] [PubMed] [Google Scholar]
- Chou CC, Hsiao HY, Hong QS, Chen CH, Peng YW, Chen HW, Yang PC. Single-walled carbon nanotubes can induce pulmonary injury in mouse model. Nano Lett. 2008;8:437–445. doi: 10.1021/nl0723634. [DOI] [PubMed] [Google Scholar]
- Chow MT, Tschopp J, Moller A, Smyth MJ. NLRP3 promotes inflammation-induced skin cancer but is dispensable for asbestos-induced mesothelioma. Immunol Cell Biol. 2012;90:983–986. doi: 10.1038/icb.2012.46. [DOI] [PubMed] [Google Scholar]
- Cicchetti R, Divizia M, Valentini F, Argentin G. Effects of single-wall carbon nanotubes in human cells of the oral cavity: geno-cytotoxic risk. Toxicol In Vitro. 2011;25:1811–1819. doi: 10.1016/j.tiv.2011.09.017. [DOI] [PubMed] [Google Scholar]
- Cogliano VJ, Baan RA, Straif K, Grosse Y, Secretan B, El Ghissassi F. Use of mechanistic data in IARC evaluations. Environ Mol Mutagen. 2008;49:100–109. doi: 10.1002/em.20370. [DOI] [PubMed] [Google Scholar]
- Cogliano V. A comparison of carcinogen classification systems. Presentation to NIOSH; Sept. 6, 2011.2011. [Google Scholar]
- Collado M, Serrano M. The senescent side of tumor suppression. Cell Cycle. 2005;4:1722–1724. doi: 10.4161/cc.4.12.2260. [DOI] [PubMed] [Google Scholar]
- Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30:1073–1081. doi: 10.1093/carcin/bgp127. [DOI] [PubMed] [Google Scholar]
- Cortez BA, Rezende-Teixeira P, Redick S, Doxsey S, Machado-Santelli GM. Multipolar mitosis and aneuploidy after chrysotile treatment: a consequence of abscission failure and cytokinesis regression. Oncotarget. 2016;7:8979–8992. doi: 10.18632/oncotarget.6924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouzier D, Follot S, Gentilhomme E, Flahaut E, Arnaud R, Dabouis V, Castellarin C, Debouzy JC. Carbon nanotubes induce inflammation but decrease the production of reactive oxygen species in lung. Toxicology. 2010;272:39–45. doi: 10.1016/j.tox.2010.04.001. [DOI] [PubMed] [Google Scholar]
- Cveticanin J, Joksic G, Leskovac A, Petrovic S, Sobot AV, Neskovic O. Using carbon nanotubes to induce micronuclei and double strand breaks of the DNA in human cells. Nanotechnology. 2010;21:015102. doi: 10.1088/0957-4484/21/1/015102. [DOI] [PubMed] [Google Scholar]
- Czarny B, Georgin D, Berthon F, Plastow G, Pinault M, Patriarche G, Thuleau A, L’Hermite MM, Taran F, Dive V. Carbon nanotube translocation to distant organs after pulmonary exposure: insights from in situ (14)C-radiolabeling and tissue radioimaging. ACS Nano. 2014;8:5715–5724. doi: 10.1021/nn500475u. [DOI] [PubMed] [Google Scholar]
- Dahm MM, Evans DE, Schubauer-Berigan MK, Birch ME, Fernback JE. Occupational exposure assessment in carbon nanotube and nanofiber primary and secondary manufacturers. Ann Occup Hyg. 2012;56:542–556. doi: 10.1093/annhyg/mer110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahm MM, Yencken MS, Schubauer-Berigan MK. Exposure control strategies in the carbonaceous nanomaterial industry. J Occup Environ Med. 2011;53:S68–S73. doi: 10.1097/JOM.0b013e31821b1d3b. [DOI] [PubMed] [Google Scholar]
- Dahm MM, Schubauer-Berigan MK, Evans DE, Birch ME, Fernback JE, Deddens JA. Carbon nanotube and nanofiber exposure assessments: an analysis of 14 site visits. Ann Occup Hyg. 2015;59:705–723. doi: 10.1093/annhyg/mev020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darne C, Terzetti F, Coulais C, Fontana C, Binet S, Gate L, Guichard Y. Cytotoxicity and genotoxicity of panel of single- and multiwalled carbon nanotubes: in vitro effects on normal Syrian hamster embryo and immortalized v79 hamster lung cells. J Toxicol. 2014;2014:872195. doi: 10.1155/2014/872195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JM, Addison J, Bolton RE, Donaldson K, Jones AD, Smith T. The pathogenicity of long versus short fibre samples of amosite asbestos administered to rats by inhalation and intraperitoneal injection. Br J Exp Pathol. 1986;67:415–430. [PMC free article] [PubMed] [Google Scholar]
- De Nardo D, De Nardo CM, Latz E. New insights into mechanisms controlling the NLRP3 inflammasome and its role in lung disease. Am J Pathol. 2014;184:42–54. doi: 10.1016/j.ajpath.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLorme MP, Muro Y, Arai T, Banas DA, Frame SR, Reed KL, Warheit DB. Ninety-day inhalation toxicity study with a vapor grown carbon nanofiber in rats. Toxicol Sci. 2012;128:449–460. doi: 10.1093/toxsci/kfs172. [DOI] [PubMed] [Google Scholar]
- DiDonato JA, Mercurio F, Karin M. NF-κB and the link between inflammation and cancer. Immunol Rev. 2012;246:379–400. doi: 10.1111/j.1600-065X.2012.01099.x. [DOI] [PubMed] [Google Scholar]
- Di Giorgio ML, Di BS, Ragnelli AM, Aimola P, Santucci S, Poma A. Effects of single and multi walled carbon nanotubes on macrophages: cyto and genotoxicity and electron microscopy. Mutat Res. 2011;722:20–31. doi: 10.1016/j.mrgentox.2011.02.008. [DOI] [PubMed] [Google Scholar]
- Dinu CZ, Bale SS, Zhu G, Dordick JS. Tubulin encapsulation of carbon nanotubes into functional hybrid assemblies. Small. 2009;5:310–315. doi: 10.1002/smll.200801434. [DOI] [PubMed] [Google Scholar]
- Di Sotto A, Chiaretti M, Carru GA, Bellucci S, Mazzanti G. Multi-walled carbon nanotubes: lack of mutagenic activity in the bacterial reverse mutation assay. Toxicol Lett. 2009;184:192–197. doi: 10.1016/j.toxlet.2008.11.007. [DOI] [PubMed] [Google Scholar]
- Donaldson K, Murphy FA, Duffin R, Poland CA. Asbestos, carbon nanotubes and the pleural mesothelium: a review of the hypothesis regarding the role of long fibre retention in the parietal pleura, inflammation and mesothelioma. Part Fibre Toxicol. 2010;7:5. doi: 10.1186/1743-8977-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson K, Murphy F, Schinwald A, Duffin R, Poland CA. Identifying the pulmonary hazard of high aspect ratio nanoparticles to enable their safety-by-design. Nanomedicine (Lond) 2011;6:143–156. doi: 10.2217/nnm.10.139. [DOI] [PubMed] [Google Scholar]
- Donaldson K, Poland CA, Murphy FA, MacFarlane M, Chernova T, Schinwald A. Pulmonary toxicity of carbon nanotubes and asbestos – similarities and differences. Adv Drug Deliv Rev. 2013;65:2078–2086. doi: 10.1016/j.addr.2013.07.014. [DOI] [PubMed] [Google Scholar]
- Dostert C, Pétrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320:674–677. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesberg P, Mandrioli D, McCormack A, Nicholson JM. Is carcinogenesis a form of speciation? Cell Cycle. 2011;10:2100–2114. doi: 10.4161/cc.10.13.16352. [DOI] [PubMed] [Google Scholar]
- Ebbesen TW, Takada T. Topological and sp3 defect structures in nanotubes. Carbon. 1995;33:973–978. [Google Scholar]
- Elder A, Gelein R, Finkelstein JN, Driscoll KE, Harkema J, Oberdorster G. Effects of subchronically inhaled carbon black in three species. I. Retention kinetics, lung inflammation, and histopathology. Toxicol Sci. 2005;88:614–629. doi: 10.1093/toxsci/kfi327. [DOI] [PubMed] [Google Scholar]
- Elgrabli D, Abella-Gallart S, Robidel F, Rogerieux F, Boczkowski J, Lacroix G. Induction of apoptosis and absence of inflammation in rat lung after intratracheal instillation of multiwalled carbon nanotubes. Toxicology. 2008;253:131–136. doi: 10.1016/j.tox.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Elgrabli D, Dachraoui W, Menard-Moyon C, Liu XJ, Begin D, Begin-Colin S, Bianco A, Gazeau F, Alloyeau D. Carbon nanotube degradation in macrophages: live nanoscale monitoring and understanding of biological pathway. ACS Nano. 2015;9:10113–10124. doi: 10.1021/acsnano.5b03708. [DOI] [PubMed] [Google Scholar]
- Ellinger-Ziegelbauer H, Pauluhn J. Pulmonary toxicity of multi-walled carbon nanotubes (Baytubes®) relative to alpha-quartz following a single 6h inhalation exposure of rats and a 3 months post-exposure period. Toxicology. 2009;266:16–29. doi: 10.1016/j.tox.2009.10.007. [DOI] [PubMed] [Google Scholar]
- Ema M, Imamura T, Suzuki H, Kobayashi N, Naya M, Nakanishi J. Evaluation of genotoxicity of multi-walled carbon nanotubes in a battery of in vitro and in vivo assays. Regul Toxicol Pharmacol. 2012;63:188–195. doi: 10.1016/j.yrtph.2012.03.014. [DOI] [PubMed] [Google Scholar]
- Ema M, Imamura T, Suzuk H, Kobayashi N, Naya M, Nakanishi J. Genotoxicity evaluation for single-walled carbon nanotubes in a battery of in vitro and in vivo assays. J Appl Toxicol. 2013a;33:933–939. doi: 10.1002/jat.2772. [DOI] [PubMed] [Google Scholar]
- Ema M, Masumori S, Kobayashi N, Naya M, Endoh S, Maru J, Hosoi M, Uno F, Nakajima M, Hayashi M, Nakanishi J. In vivo comet assay of multi-walled carbon nanotubes using lung cells of rats intratracheally instilled. J Appl Toxicol. 2013b;33:1053–1060. doi: 10.1002/jat.2810. [DOI] [PubMed] [Google Scholar]
- Engstrom W, Darbre P, Eriksson S, Gulliver L, Hultman T, Karamouzis MV, Klaunig JE, Mehta R, Moorwood K, Sanderson T, et al. The potential for chemical mixtures from the environment to enable the cancer hallmark of sustained proliferative signalling. Carcinogenesis. 2015;36:S38–S60. doi: 10.1093/carcin/bgv030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ESCODD (European Standards Committee on Oxidative DNA Damage) Measurement of DNA oxidation in human cells by chromatographic and enzymic methods. Free Radic Biol Med. 2003;34:1089–1099. doi: 10.1016/s0891-5849(03)00041-8. [DOI] [PubMed] [Google Scholar]
- Evans DE, Ku B-K, Birch ME, Dunn KH. Aerosol monitoring during carbon nanofiber production: mobile direct-reading sampling. Ann Occup Hyg. 2010;54:514–531. doi: 10.1093/annhyg/meq015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Mao R, Yang J. NF-κB and STAT3 signaling pathways collaboratively link inflammation to cancer. Protein Cell. 2013;4:176–185. doi: 10.1007/s13238-013-2084-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatkhutdinova LM, Khaliullin TO, Vasil’yeva OL, Zalyalov RR, Mustafin IG, Kisin ER, Birch ME, Yanamala N, Shvedova AA. Fibrosis biomarkers in workers exposed to MWCNTs. Toxicol Appl Pharmacol. 2016;299:125–131. doi: 10.1016/j.taap.2016.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenoglio I, Aldieri E, Gazzano E, Cesano F, Colonna M, Scarano D, Mazzucco G, Attanasio A, Yakoub Y, Lison D, et al. Thickness of multiwalled carbon nanotubes affects their lung toxicity. Chem Res Toxicol. 2012;25:74–82. doi: 10.1021/tx200255h. [DOI] [PubMed] [Google Scholar]
- Fenoglio I, Greco G, Tomatis M, Muller J, Raymundo-Piñero E, Béguin F, Fonseca A, Nagy JB, Lison D, Fubini B. Structural defects play a major role in the acute lung toxicity of multiwall carbon nanotubes: physicochemical aspects. Chem Res Toxicol. 2008;21:1690–1697. doi: 10.1021/tx800100s. [DOI] [PubMed] [Google Scholar]
- Fenoglio I, Tomatis M, Lison D, Muller J, Fonseca A, Nagy JB, Fubini B. Reactivity of carbon nanotubes: free radical generation or scavenging activity? Free Radic Biol Med. 2006;40:1227–1233. doi: 10.1016/j.freeradbiomed.2005.11.010. [DOI] [PubMed] [Google Scholar]
- Foley JF, Anderson MW, Stoner GD, Gaul BW, Hardisty JF, Maronpot RR. Proliferative lesions of the mouse lung: progression studies in strain a mice. Exp Lung Res. 1991;17:157–168. doi: 10.3109/01902149109064408. [DOI] [PubMed] [Google Scholar]
- Folkmann JK, Risom L, Jacobsen NR, Wallin H, Loft S, Møller P. Oxidatively damaged DNA in rats exposed by oral gavage to C60 fullerenes and single-walled carbon nanotubes. Environ Health Perspect. 2009;117:703–708. doi: 10.1289/ehp.11922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forchhammer L, Brauner EV, Folkmann JK, Danielsen PH, Nielsen C, Jensen A, Loft S, Friis G, Møller P. Variation in assessment of oxidatively damaged DNA in mononuclear blood cells by the comet assay with visual scoring. Mutagenesis. 2008;23:223–231. doi: 10.1093/mutage/gen006. [DOI] [PubMed] [Google Scholar]
- Forchhammer L, Ersson C, Loft S, Moller L, Godschalk RW, van Schooten FJ, Jones GD, Higgins JA, Cooke M, Mistry V, et al. Inter-laboratory variation in DNA damage using a standard comet assay protocol. Mutagenesis. 2012;27:665–672. doi: 10.1093/mutage/ges032. [DOI] [PubMed] [Google Scholar]
- Freedman AP, Robinson SE. Noninvasive magnetopneumographic studies of lung dust retention and clearance in coal miners. In: Frantz RL, Ramani RV, editors. Respirable dust in the mineral industries: health effects, characterization, and control. University Park (PA): The Pennsylvania State University; 1988. pp. 181–186. [Google Scholar]
- Frohlich E, Meindl C, Hofler A, Leitinger G, Roblegg E. Combination of small size and carboxyl functionalisation causes cytotoxicity of short carbon nanotubes. Nanotoxicology. 2013;7:1211–1224. doi: 10.3109/17435390.2012.729274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fubini B, Otero-Aréan C. Chemical aspects of the toxicity of inhaled mineral dusts. Chem Soc Rev. 1999;28:373–381. [Google Scholar]
- Fubini B, Ghiazza M, Fenoglio I. Physico-chemical features of engineered nanoparticles relevant to their toxicity. Nanotoxicology. 2010;4:347–363. doi: 10.3109/17435390.2010.509519. [DOI] [PubMed] [Google Scholar]
- Fubini B, Fenoglio I, Tomatis M, Turci F. Effect of chemical composition and state of the surface on the toxic response to high aspect ratio nanomaterials. Nanomedicine (Lond) 2011;6:899–920. doi: 10.2217/nnm.11.80. [DOI] [PubMed] [Google Scholar]
- Fujita K, Fukuda M, Fukui H, Horie M, Endoh S, Uchida K, Shichiri M, Morimoto Y, Ogami A, Iwahashi H. Intratracheal instillation of single-wall carbon nanotubes in the rat lung induces time-dependent changes in gene expression. Nanotoxicology. 2015;9:290–301. doi: 10.3109/17435390.2014.921737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galano A. Carbon nanotubes: promising agents against free radicals. Nanoscale. 2010;2:373–380. doi: 10.1039/b9nr00364a. [DOI] [PubMed] [Google Scholar]
- Galano A, Francisco-Marquez M, Martinez A. Influence of point defects on the free-radical scavenging capability of single-walled carbon nanotubes. J Phys Chem C. 2010;114:8302–8308. [Google Scholar]
- Gernand JM, Casman EA. A meta-analysis of carbon nanotube pulmonary toxicity studies-how physical dimensions and impurities affect the toxicity of carbon nanotubes. Risk Anal. 2014;34:583–597. doi: 10.1111/risa.12109. [DOI] [PubMed] [Google Scholar]
- Ghosh M, Chakraborty A, Bandyopadhyay M, Mukherjee A. Multi-walled carbon nanotubes (MWCNT): induction of DNA damage in plant and mammalian cells. J Hazard Mater. 2011;197:327–336. doi: 10.1016/j.jhazmat.2011.09.090. [DOI] [PubMed] [Google Scholar]
- Girtsman TA, Beamer CA, Wu N, Buford M, Holian A. IL-1R signalling is critical for regulation of multi-walled carbon nanotubes-induced acute lung inflammation in C57Bl/6 mice. Nanotoxicology. 2014;8:17–27. doi: 10.3109/17435390.2012.744110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman GB, Kaplan PD, Stachura I, Castranova V, Pailes WH, Lapp NL. Acute silicosis responding to corticosteroid therapy. Chest. 1992;101:366–370. doi: 10.1378/chest.101.2.366. [DOI] [PubMed] [Google Scholar]
- Gregoratto D, Bailey MR, Marsh JW. Modelling particle retention in the alveolar-interstitial region of the human lungs. J Radiol Prot. 2010;30:491–512. doi: 10.1088/0952-4746/30/3/005. [DOI] [PubMed] [Google Scholar]
- Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosse Y, Loomis D, Guyton KZ, Lauby-Secretan B, El Ghissassi F, Bouvard V, Benbrahim-Tallaa L, Guha N, Scoccianti C, et al. International Agency for Research on Cancer Monograph Working Group. Carcinogenicity of fluoro-edenite, silicon carbide fibres and whiskers, and carbon nanotubes. Lancet Oncol. 2014;15:1427–1428. doi: 10.1016/S1470-2045(14)71109-X. [DOI] [PubMed] [Google Scholar]
- Guerard M, Baum M, Bitsch A, Eisenbrand G, Elhajouji A, Epe B, Habermeyer M, Kaina B, Martus HJ, Pfuhler S, et al. Assessment of mechanisms driving non-linear dose–response relationships in genotoxicity testing. Mutat Res Rev Mutat Res. 2015;763:181–201. doi: 10.1016/j.mrrev.2014.11.001. [DOI] [PubMed] [Google Scholar]
- Guirouilh-Barbat J, Lambert S, Bertrand P, Lopez BS. Is homologous recombination really an error-free process? Front Genet. 2014;5:175. doi: 10.3389/fgene.2014.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo NL, Wan YW, Denvir J, Porter DW, Pacurari M, Wolfarth MG, Castranova V, Qian Y. Multiwalled carbon nanotube-induced gene signatures in the mouse lung: potential predictive value for human lung cancer risk and prognosis. J Toxicol Environ Health Part A. 2012;75:1129–1153. doi: 10.1080/15287394.2012.699852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo YY, Zhang J, Zheng YF, Yang J, Zhu XQ. Cytotoxic and genotoxic effects of multi-wall carbon nanotubes on human umbilical vein endothelial cells in vitro. Mutat Res. 2011;721:184–191. doi: 10.1016/j.mrgentox.2011.01.014. [DOI] [PubMed] [Google Scholar]
- Hamilton RF, Wu N, Porter Buford M, Wolfarth M, Holian A. Particle length-dependent titanium dioxide nanomaterials toxicity and bioactivity. Part Fibre Toxicol. 2009;6:35. doi: 10.1186/1743-8977-6-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton RF, Jr, Xiang C, Li M, Ka I, Yang F, Ma D, Porter DW, Wu N, Holian A. Purification and sidewall functionalization of multi-walled carbon nanotubes and resulting bioactivity in two macrophage models. Inhal Toxicol. 2013;25:199–210. doi: 10.3109/08958378.2013.775197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SG, Andrews R, Gairola CG. Acute pulmonary response of mice to multi-wall carbon nanotubes. Inhal Toxicol. 2010;22:340–347. doi: 10.3109/08958370903359984. [DOI] [PubMed] [Google Scholar]
- Han JH, Lee EJ, Lee JH, So KP, Lee YH, Bae GN, Lee SB, Ji JH, Cho MH, Yu IJ. Monitoring multiwalled carbon nanotube exposure in carbon nanotube research facility. Inhal Toxicol. 2008;20:741–749. doi: 10.1080/08958370801942238. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21:309–322. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg W. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Hanawalt PC, Spivak G. Transcription-coupled DNA repair: two decades of progress and surprises. Nat Rev Mol Cell Biol. 2008;9:958–970. doi: 10.1038/nrm2549. [DOI] [PubMed] [Google Scholar]
- Heintz NH, Janssen-Heininger YM, Mossman BT. Asbestos, lung cancers, and mesotheliomas: from molecular approaches to targeting tumor survival pathways. Am J Respir Cell Mol Biol. 2010;42:133–139. doi: 10.1165/rcmb.2009-0206TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill AB. The environment and disease: association or causation? Proc R Soc Med. 1965;58:295–300. doi: 10.1177/003591576505800503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Zhang F, Sun X, Choi KY, Niu G, Zhang G, Guo J, Lee S, Chen X. The genotype-dependent influence of functionalized multiwalled carbon nanotubes on fetal development. Biomaterials. 2014;35:856–865. doi: 10.1016/j.biomaterials.2013.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizar I, Malur A, Midgette YA, Kukoly C, Chen P, Ke PC, Podila R, Rao AM, Wingard CJ, Dobbs L, et al. Novel murine model of chronic granulomatous lung inflammation elicited by carbon nanotubes. Am J Respir Cell Mol Biol. 2011;45:858–866. doi: 10.1165/rcmb.2010-0401OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IARC [Internet] Silica, some silicates, coal dust and para-aramid fibrils. Vol. 68. Lyon, France: International Agency for Research on Cancer; 1997. IARC monographs on the evaluation of carcinogenic risks to humans. Available from: http://monographs.iarc.fr/ [PMC free article] [PubMed] [Google Scholar]
- IARC [Internet] IARC monographs on the evaluation of carcinogenic risks to human: Man-made vitreous fibres. Vol. 81. Lyon, France: International Agency for Research on Cancer; 2002. Available from: http://monographs.iarc.fr/ [PMC free article] [PubMed] [Google Scholar]
- IARC [Internet] Preamble to the IARC Monographs. Lyon, France: International Agency for Research on Cancer; 2006. Available from: http://monographs.iarc.fr/ [Google Scholar]
- IARC [Internet] IARC monographs on the evaluation of carcinogenic risks to humans: Carbon black, titanium dioxide, and talc. Vol. 93. Lyon, France: International Agency for Research on Cancer; 2010. Available from: http://monographs.iarc.fr. [PMC free article] [PubMed] [Google Scholar]
- IARC [Internet] A review of human carcinogens. 100C. Lyon, France: International Agency for Research on Cancer; 2012. IARC monographs: Arsenic, metals, fibres, and dusts. Available from: http://monographs.iarc.fr/ [Google Scholar]
- IARC [in press] Some nanomaterials and some fibres. Vol. 111. Lyon: IARC; IARC monographs on the evaluation of carcinogenic risks to humans. [Google Scholar]
- ICRP. International commission on radiological protection. Tarrytown (NY): Elsevier Science Ltd; 1994. Human respiratory tract model for radiological protection. [PubMed] [Google Scholar]
- Ingle T, Dervishi E, Biris AR, Mustafa T, Buchanan RA, Biris AS. Raman spectroscopy analysis and mapping the biodistribution of inhaled carbon nanotubes in the lungs and blood of mice. J Appl Toxicol. 2013;33:1044–1052. doi: 10.1002/jat.2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISO. Air Quality – particle size fraction definitions for health-related sampling, ISO 7708. Geneva: International Standards Organisation; 1995. [Google Scholar]
- Jackson P, Kling K, Jensen KA, Clausen PA, Madsen AM, Wallin H, Vogel U. Characterization of genotoxic response to 15 multiwalled carbon nanotubes with variable physicochemical properties including surface functionalizations in the FE1-Muta(TM) mouse lung epithelial cell line. Environ Mol Mutagen. 2015;56:183–203. doi: 10.1002/em.21922. [DOI] [PubMed] [Google Scholar]
- Jacobsen NR, Møller P, Jensen JA, Vogel U, Ladefoged O, Loft S, Wallin H. Lung inflammation and genotoxicity following pulmonary exposure to nanoparticles in ApoE-/- mice. Part Fibre Toxicol. 2009;6:2. doi: 10.1186/1743-8977-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen NR, Pojana G, White P, Møller P, Cohn CA, Korsholm KS, Vogel U, Marcomini A, Loft S, Wallin H. Genotoxicity, cytotoxicity and reactive oxygen species induced by single-walled carbon nanotubes and C60 fullerenes in the FE1-MutaTMMouse lung epithelial cells. Environ Mol Mutagen. 2008;49:476–487. doi: 10.1002/em.20406. [DOI] [PubMed] [Google Scholar]
- Jacobsen NR, Saber AT, White P, Møller P, Pojana G, Vogel U, Loft S, Gingerich J, Soper L, Douglas GR, Wallin H. Increased mutant frequency by carbon black, but not quartz, in the lacZ and cII transgenes of muta mouse lung epithelial cells. Environ Mol Mutagen. 2007;48:451–461. doi: 10.1002/em.20300. [DOI] [PubMed] [Google Scholar]
- Jacobsen NR, White PA, Gingerich J, Møller P, Saber AT, Douglas GR, Vogel U, Wallin H. Mutation spectrum in FE1-MUTA(TM) mouse lung epithelial cells exposed to nanoparticulate carbon black. Environ Mol Mutagen. 2011;52:331–337. doi: 10.1002/em.20629. [DOI] [PubMed] [Google Scholar]
- Jaurand MC, Renier A, Daubriac J. Mesothelioma: do asbestos and carbon nanotubes pose the same health risk? Part Fibre Toxicol. 2009;6:16. doi: 10.1186/1743-8977-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessop F, Holian A. Extracellular HMGB1 regulates multi-walled carbon nanotube-induced inflammation in vivo. Nanotoxicol. 2014;1:1–8. doi: 10.3109/17435390.2014.933904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston HJ, Hutchison GR, Christensen FM, Peters S, Hankin S, Aschberger K, Stone V. A critical review of the biological mechanisms underlying the in vivo and in vitro toxicity of carbon nanotubes: the contribution of physico-chemical characteristics. Nanotoxicology. 2010;4:207–246. doi: 10.3109/17435390903569639. [DOI] [PubMed] [Google Scholar]
- Johnson DR, Methner MM, Kennedy AJ, Steevens JA. Potential for occupational exposure to engineered carbon-based nanomaterials in environmental laboratory studies. Environ Health Perspect. 2010;118:49–54. doi: 10.1289/ehp.0901076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston H, Pojana G, Zuin S, Jacobsen NR, Møller P, Loft S, Semmler-Behnke M, McGuiness C, Balharry D, Marcomini A, et al. Engineered nanomaterial risk. Lessons learnt from completed nanotoxicology studies: potential solutions to current and future challenges. Crit Rev Toxicol. 2013;43:1–20. doi: 10.3109/10408444.2012.738187. [DOI] [PubMed] [Google Scholar]
- JRC. European Commission Joint Research Centre: II3. Carcinogenicity of synthetic mineral fibres after intraperitoneal injection in rats. In: Bernstein DM, Sintes JMR, editors. Methods for the determination of the hazardous properties for human health of man made mineral fibres (MMMF) Ispra (Italy): European Commission Joint Research Centre, Institute for Health and Consumer Protection; 1999. ECB/TM/18(97) rev. JRC EUR 18748 EN. [Google Scholar]
- Ju L, Zhang G, Zhang X, Jia Z, Gao X, Jiang Y, Yan C, Duerksen-Hughes PJ, Chen FF, Li H, et al. Proteomic analysis of cellular response induced by multi-walled carbon nanotubes exposure in a549 cells. PLoS One. 2014;9: e84974. doi: 10.1371/journal.pone.0084974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan VE, Konduru NV, Feng W, Allen BL, Conroy J, Volkov Y, Vlasova II, Belikova NA, Yanamala N, Kapralov A, et al. Carbon nanotubes degraded by neutrophil myeloperoxidase induce less pulmonary inflammation. Nat Nanotechnol. 2010;5:354–359. doi: 10.1038/nnano.2010.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson HL, Cronholm P, Gustafsson J, Moller L. Copper oxide nanoparticles are highly toxic: a comparison between metal oxide nanoparticles and carbon nanotubes. Chem Res Toxicol. 2008;21:1726–1732. doi: 10.1021/tx800064j. [DOI] [PubMed] [Google Scholar]
- Karlsson HL, Di Bucchianico S, Collins AR, Dusinska M. Can the comet assay be used reliably to detect nanoparticle-induced genotoxicity? Environ Mol Mutagen. 2015;56:82–96. doi: 10.1002/em.21933. [DOI] [PubMed] [Google Scholar]
- Kasai T, Umeda Y, Ohnishi M, Kondo H, Takeuchi T, Aiso S, Nishizawa T, Matsumoto M, Fukushima S. Thirteen-week study of toxicity of fiber-like multi-walled carbon nanotubes with whole-body inhalation exposure in rats. Nanotoxicology. 2015;9:413–422. doi: 10.3109/17435390.2014.933903. [DOI] [PubMed] [Google Scholar]
- Katabami M, Dosaka-Akita H, Honma K, Saitoh Y, Kimura K, Uchida Y, Mikami H, Ohsaki Y, Kawakami Y, Kikuchi K. Pneumoconiosis-related lung cancers: preferential occurrence from diffuse interstitial fibrosistype pneumoconiosis. Am J Respir Crit Care Med. 2000;162:295–300. doi: 10.1164/ajrccm.162.1.9906138. [DOI] [PubMed] [Google Scholar]
- Kato T, Totsuka Y, Ishino K, Matsumoto Y, Tada Y, Nakae D, Goto S, Masuda S, Ogo S, Kawanishi M, et al. Genotoxicity of multi-walled carbon nanotubes in both in vitro and in vivo assay systems. Nanotoxicology. 2013;7:452–461. doi: 10.3109/17435390.2012.674571. [DOI] [PubMed] [Google Scholar]
- Katwa P, Wang X, Urankar RN, Podila R, Hilderbrand SC, Fick RB, Rao AM, Ke PC, Wingard CJ, Brown JM. A carbon nanotube toxicity paradigm driven by mast cells and the IL-33/ST2 axis. Small. 2012;8:2904–2912. doi: 10.1002/smll.201200873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzenstein AL, Mukhopadhyay S, Zanardi C, Dexter E. Clinically occult interstitial fibrosis in smokers: classification and significance of a surprisingly common finding in lobectomy specimens. Hum Pathol. 2010;41:316–325. doi: 10.1016/j.humpath.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Kermanizadeh A, Gaiser BK, Hutchison GR, Stone V. An in vitro liver model—assessing oxidative stress and genotoxicity following exposure of hepatocytes to a panel of engineered nanomaterials. Part Fibre Toxicol. 2012;9:28. doi: 10.1186/1743-8977-9-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kermanizadeh A, Vranic S, Boland S, Moreau K, Baeza-Squiban A, Gaiser BK, Andrzejczuk LA, Stone V. An in vitro assessment of panel of engineered nanomaterials using a human renal cell line: cytotoxicity, pro-inflammatory response, oxidative stress and genotoxicity. BMC Nephrol. 2013;14:96. doi: 10.1186/1471-2369-14-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidane D, Chae WJ, Czochor J, Eckert KA, Glazer PM, Bothwell AL, Sweasy JB. Interplay between DNA repair and inflammation, and the link to cancer. Crit Rev Biochem Mol Biol. 2014;49:116–139. doi: 10.3109/10409238.2013.875514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilbey A, Terry A, Cameron ER, Neil JC. Oncogene-induced senescence: an essential role for Runx. Cell Cycle. 2008;7:2333–2340. doi: 10.4161/cc.6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JS, Lee K, Lee YH, Cho HS, Kim KH, Choi KH, Lee SH, Song KS, Kang CS, Yu IJ. Aspect ratio has no effect on genotoxicity of multi-wall carbon nanotubes. Arch Toxicol. 2011;85:775–786. doi: 10.1007/s00204-010-0574-0. [DOI] [PubMed] [Google Scholar]
- Kim JS, Sung JH, Song KS, Lee JH, Kim SM, Lee GH, Ahn KH, Lee JS, Shin JH, Park JD, Yu IJ. Persistent DNA damage measured by comet assay of Sprague Dawley rat lung cells after five days of inhalation exposure and 1 month post-exposure to dispersed multi-wall carbon nanotubes (MWCNTs) generated by new MWCNT aerosol generation system. Toxicol Sci. 2012;128:439–448. doi: 10.1093/toxsci/kfs161. [DOI] [PubMed] [Google Scholar]
- Kim JS, Sung JH, Choi BG, Ryu HY, Song KS, Shin JH, Lee JS, Hwang JH, Lee JH, Lee GH, et al. In vivo genotoxicity evaluation of lung cells from Fischer 344 rats following 28 days of inhalation exposure to MWCNTs, plus 28 days and 90 days post-exposure. Inhal Toxicol. 2014;26:222–234. doi: 10.3109/08958378.2013.878006. [DOI] [PubMed] [Google Scholar]
- Kim JS, Song KS, Yu IJ. Evaluation of in vitro and in vivo genotoxicity of single-walled carbon nanotubes. Toxicol Ind Health. 2015;31:747–757. doi: 10.1177/0748233713483201. [DOI] [PubMed] [Google Scholar]
- Kim JS, Song KS, Yu IJ. Multiwall carbon nanotube-induced DNA damage and cytotoxicity in male human peripheral blood lymphocytes. Int J Toxicol. 2016;35:27–37. doi: 10.1177/1091581815598749. [DOI] [PubMed] [Google Scholar]
- Kim JS, Yu IJ. Single-wall carbon nanotubes (SWCNT) induce cytotoxicity and genotoxicity produced by reactive oxygen species (ROS) generation in phytohemagglutinin (PHA)-stimulated male human peripheral blood lymphocytes. J Toxicol Environ Health A. 2014;77:1141–1153. doi: 10.1080/15287394.2014.917062. [DOI] [PubMed] [Google Scholar]
- Kisin ER, Murray AR, Keane MJ, Shi XC, Schwegler-Berry D, Gorelik O, Arepalli S, Castranova V, Wallace WE, Kagan VE, Shvedova AA. Single-walled carbon nanotubes: geno- and cytotoxic effects in lung fibroblast V79 cells. J Toxicol Environ Health A. 2007;70:2071–2079. doi: 10.1080/15287390701601251. [DOI] [PubMed] [Google Scholar]
- Kisin ER, Murray AR, Sargent L, Lowry D, Chirila M, Siegrist KJ, Schwegler-Berry D, Leonard S, Castranova V, Fadeel B, et al. Genotoxicity of carbon nanofibers: are they potentially more or less dangerous than carbon nanotubes or asbestos? Toxicol Appl Pharmacol. 2011;252:1–10. doi: 10.1016/j.taap.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knaapen AM, Gungor N, Schins RP, Borm PJ, Van Schooten FJ. Neutrophils and respiratory tract DNA damage and mutagenesis: a review. Mutagenesis. 2006;21:225–236. doi: 10.1093/mutage/gel032. [DOI] [PubMed] [Google Scholar]
- Kobayashi N, Naya M, Ema M, Endoh S, Maru J, Mizuno K, Nakanishi J. Biological response and morphological assessment of individually dispersed multi-wall carbon nanotubes in the lung after intratracheal instillation in rats. Toxicology. 2010;276:143–153. doi: 10.1016/j.tox.2010.07.021. [DOI] [PubMed] [Google Scholar]
- Kobayashi N, Naya M, Mizuno K, Yamamoto K, Ema M, Nakanishi J. Pulmonary and systemic responses of highly pure and well-dispersed single-wall carbon nanotubes after intratracheal instillation in rats. Inhal Toxicol. 2011;23:814–828. doi: 10.3109/08958378.2011.614968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb R, Liu G-H, Janowski AM, Sutterwala FS, Zhang W. Inflammasomes in cancer: a double-edged sword. Protein Cell. 2014;5:12–20. doi: 10.1007/s13238-013-0001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolosnjaj-Tabi J, Hartman KB, Boudjemaa Ananta JS, Morgant G, Szwarc H, Wilson LJ, Moussa F. In vivo behavior of large doses of ultrashort and full-length single-walled carbon nanotubes after oral and intraperitoneal administration to Swiss mice. ACS Nano. 2010;4:1481–1492. doi: 10.1021/nn901573w. [DOI] [PubMed] [Google Scholar]
- Kuempel ED, O’Flaherty EJ, Stayner LT, Smith RJ, Green FHY, Vallyathan V. A biomathematical model of particle clearance and retention in the lungs of coal miners. Regul Toxicol Pharmacol. 2001;34:69–87. doi: 10.1006/rtph.2001.1479. [DOI] [PubMed] [Google Scholar]
- Kuempel ED, Geraci CL, Schulte PA. Risk assessment and risk management of nanomaterials in the workplace: translating research to practice. Ann Occup Hyg. 2012;56:491–505. doi: 10.1093/annhyg/mes040. [DOI] [PubMed] [Google Scholar]
- Kulkarni P, Baron P. Introduction to aerosol characterization in aerosol measurements: principles, applications, and techniques. New York: John Wiley & Sons, Inc; 2011. [Google Scholar]
- Kundu JK, Surh Y-J. Emerging avenues linking inflammation and cancer. Free Radic Biol Med. 2012;52:2013–2037. doi: 10.1016/j.freeradbiomed.2012.02.035. [DOI] [PubMed] [Google Scholar]
- Kunzmann A, Andersson B, Thurnherr T, Krug H, Scheynius A, Fadeel B. Toxicology of engineered nanomaterials: focus on biocompatibility, biodistribution and biodegradation. Biochim Biophys Acta. 2011;1810:361–373. doi: 10.1016/j.bbagen.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Kuriashy A, Karin M, Grivennikov SI. Tumor promotion via injury-and death-induced inflammation. Immunity. 2011;35:467–477. doi: 10.1016/j.immuni.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurowska-Stolarska M, Stolarski B, Kewin P, Murphy G, Corrigan CJ, Ying S, Pitman N, Mirchandani A, Rana B, Van Rooijen N, et al. IL-33 amplifies the polarization of alternatively activated macrophages that contribute to airway inflammation. J Immunol. 2009;183:6469–6477. doi: 10.4049/jimmunol.0901575. [DOI] [PubMed] [Google Scholar]
- Kyjovska ZO, Jacobsen NR, Saber AT, Bengtson S, Jackson P, Wallin H, Vogel U. DNA damage following pulmonary exposure by instillation to low doses of carbon black (Printex 90) nanoparticles in mice. Environ Mol Mutagen. 2015;56:41–49. doi: 10.1002/em.21888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam CW, James JT, McCluskey R, Hunter RL. Pulmonary toxicity of single-wall carbon nanotubes in mice 7 and 90 days after intratracheal instillation. Toxicol Sci. 2004;77:126–134. doi: 10.1093/toxsci/kfg243. [DOI] [PubMed] [Google Scholar]
- Lambert IB, Singer TM, Boucher SE, Douglas GR. Detailed review of transgenic rodent mutation assays. Mutat Res. 2005;590:1–280. doi: 10.1016/j.mrrev.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Landsiedel R, Sauer UG, Ma-Hock L, Schnekenburger J, Wiemann M. Pulmonary toxicity of nanomaterials: a critical comparison of published in vitro assays and in vivo inhalation or instillation studies. Nanomedicine (Lond) 2014;9:2557–2585. doi: 10.2217/nnm.14.149. [DOI] [PubMed] [Google Scholar]
- Lapp NL, Castranova V. How silicosis and coal workers’ pneumoconiosis develop-a cellular assessment. State Art Rev Occup Med. 1993;8:35–56. [PubMed] [Google Scholar]
- Laskin DL, Sunil VR, Gardner CR, Laskin JD. Macrophages and tissue injury: agents of defense or destruction? Annu Rev Pharmacol Toxicol. 2011;51:267–288. doi: 10.1146/annurev.pharmtox.010909.105812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JS, Choi JC, Shin JH, Lee JH, Lee Y, Park SY, Baek JE, Park JD, Ahn K, Yu IJ. Health surveillance study of workers who manufacture multi-walled carbon nanotubes. Nanotoxicology. 2015;9:802–811. doi: 10.3109/17435390.2014.978404. [DOI] [PubMed] [Google Scholar]
- Lee JH, Lee SB, Bae GN, Jeon KS, Yoon JU, Ji JH, Sung JH, Lee BG, Lee JH, Yang JS, et al. Exposure assessment of carbon nanotube manufacturing workplaces. Inhal Toxicol. 2010;22:369–381. doi: 10.3109/08958370903367359. [DOI] [PubMed] [Google Scholar]
- Leung CC, Yu ITS, Chen W. Silicosis. Lancet. 2012;379:2008–2018. doi: 10.1016/S0140-6736(12)60235-9. [DOI] [PubMed] [Google Scholar]
- Li X, Peng Y, Qu X. Carbon nanotubes selective destabilization of duplex and triplex DNA and inducing B-A transition in solution. Nucleic Acids Res. 2006;34:3670–3676. doi: 10.1093/nar/gkl513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Wang X, Ji Z, Sun B, Zhang H, Chang CH, Lin S, Meng H, Liao YP, Wang M, et al. Surface charge and cellular processing of covalently functionalized multiwall carbon nanotubes determine pulmonary toxicity. ACS Nano. 2013;7:2352–2368. doi: 10.1021/nn305567s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg HK, Falck GC, Singh R, Suhonen S, Jarventaus H, Vanhala E, Catalán J, Farmer PB, Savolainen KM, Norppa H. Genotoxicity of short single-wall and multi-wall carbon nanotubes in human bronchial epithelial and mesothelial cells in vitro. Toxicology. 2013;313:24–37. doi: 10.1016/j.tox.2012.12.008. [DOI] [PubMed] [Google Scholar]
- Lindberg HK, Falck GC, Suhonen S, Vippola M, Vanhala E, Catalán J, Savolainen K, Norppa H. Genotoxicity of nanomaterials: DNA damage and micronuclei induced by carbon nanotubes and graphite nanofibres in human bronchial epithelial cells in vitro. Toxicol Lett. 2009;186:166–173. doi: 10.1016/j.toxlet.2008.11.019. [DOI] [PubMed] [Google Scholar]
- Liou SH, Tsai CS, Pelclova D, Schubauer-Berigan MK, Schulte PA. Assessing the first wave of epidemiological studies of nanomaterial workers. J Nanopart Res. 2015;17:413. doi: 10.1007/s11051-015-3219-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippmann M. Effects of fiber characteristics on lung deposition, retention and disease. Environ Health Perspect. 1990;88:311–317. doi: 10.1289/ehp.9088311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Hurt RH, Kane AB. Biodurability of single-walled carbon nanotubes depends on surface functionalization. Carbon N Y. 2010;48:1961–1969. doi: 10.1016/j.carbon.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohcharoenkal W, Wang L, Stueckle TA, Park J, Tse W, Dinu C-Z, Rojanasakul Y. Role of H-Ras/ERK signaling in carbon nanotube-induced neoplastic-like transformation of human mesothelial cells. Front Physiol. 2014;5:222. doi: 10.3389/fphys.2014.00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucente-Schultz RM, Moore VC, Leonard AD, Price BK, Kosynkin DV, Lu M, Partha R, Conyers JL, Tour JM. Antioxidant single-walled carbon nanotubes. J Am Chem Soc. 2009;131:3934–3941. doi: 10.1021/ja805721p. [DOI] [PubMed] [Google Scholar]
- Ma-Hock L, Treumann S, Strauss V, Brill S, Luizi F, Mertler M, Wiench K, Gamer AO, Ravenzwaay B, Landsiedel R. Inhalation toxicity of multiwall carbon nanotubes in rats exposed for 3 months. Toxicol Sci. 2009;112:468–481. doi: 10.1093/toxsci/kfp146. [DOI] [PubMed] [Google Scholar]
- Maddox C, Wang B, Kirby PA, Wang K, Ludewig G. Mutagenicity of 3-methylcholanthrene, pcb3, and 4-oh-pcb3 in the lung of transgenic bigblue rats. Environ Toxicol Pharmacol. 2008;25:260–266. doi: 10.1016/j.etap.2007.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madl AK, Pinkerton KE. Health effects of inhaled engineered and incidental nanoparticles. Crit Rev Toxicol. 2009;39:629–658. doi: 10.1080/10408440903133788. [DOI] [PubMed] [Google Scholar]
- Malkinson AM. Genetic-studies on lung-tumor susceptibility and histogenesis in mice. Environ Health Perspect. 1991;93:149–159. doi: 10.1289/ehp.9193149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkova A, Haber JE. Mutations arising during repair of chromosome breaks. Annu Rev Genet. 2012;46:455–473. doi: 10.1146/annurev-genet-110711-155547. [DOI] [PubMed] [Google Scholar]
- Manshian BB, Jenkins GJS, Williams PM, Wright C, Barron AR, Brown AP, Hondow N, Dunstan PR, Rickman R, Brady K, Doak SH. Single-walled carbon nanotubes: differential genotoxic potential associated with physico-chemical properties. Nanotoxicology. 2013;7:144–156. doi: 10.3109/17435390.2011.647928. [DOI] [PubMed] [Google Scholar]
- Maynard AD, Baron PA, Foley M, Shvedova AA, Kisin ER, Castranova V. Exposure to carbon nanotube material: aerosol release during the handling of unrefined single walled carbon nanotube material. J Toxicol Environ Health Part A. 2004;67:87–107. doi: 10.1080/15287390490253688. [DOI] [PubMed] [Google Scholar]
- Maynard AD, Kuempel E. Airborne nanostructured particles and occupational health. J Nanoparticle Res. 2005;7:587–614. [Google Scholar]
- McClellan RO. Use of mechanistic data in assessing human risks from exposure to particles. Environ Health Perspect. 1997;105:1363–1372. doi: 10.1289/ehp.97105s51363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor DB, Rice JM, Venitt S, editors. IARC Scientific Publication No 146. Lyon, France: International Agency for Research on Cancer; 1999. The use of short- and medium-term tests for carcinogens and data on genetic effects in carcinogenic hazard evaluation. [PubMed] [Google Scholar]
- Melnick RL, Huff J, Barrett JC, Maronpot RR, Lucier G, Portier CJ. Cell proliferation and chemical carcinogenesis: symposium overivew. Environ Health Perspect. 1993;101:3–8. doi: 10.1289/ehp.93101s53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng J, Li X, Wang C, Guo H, Liu J, Xu H. Carbon nanotubes activate macrophages into a M1/M2 mixed status: recruiting naive macrophages and supporting angiogenesis. ACS Appl Mater Interfaces. 2015;7:3180–3188. doi: 10.1021/am507649n. [DOI] [PubMed] [Google Scholar]
- Mercer RR, Hubbs AF, Scabilloni JF, Wang L, Battelli LA, Schwegler-Berry D, Castranova V, Porter DW. Distribution and persistence of pleural penetrations by multi-walled carbon nanotubes. Part Fibre Toxicol. 2010;7:1–11. doi: 10.1186/1743-8977-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer RR, Hubbs AF, Scabilloni JF, Wang L, Battelli LA, Friend S, Castranova V, Porter DW. Pulmonary fibrotic response to aspiration of multi-walled carbon nanotubes. Part Fibre Toxicol. 2011;8:21. doi: 10.1186/1743-8977-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer RR, Scabilloni JF, Hubbs AF, Battelli LA, McKinney W, Friend S, Wolfarth MG, Andrew M, Castranova V, Porter DW. Distribution and fibrotic response following inhalation exposure to multi-walled carbon nanotubes. Part Fibre Toxicol. 2013a;10:33. doi: 10.1186/1743-8977-10-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer RR, Scabilloni JF, Hubbs AF, Wang L, Battelli LA, McKinney W, Castranova V, Porter DW. Extrapulmonary transport of MWCNT following inhalation exposure. Part Fibre Toxicol. 2013b;10:38. doi: 10.1186/1743-8977-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer R, Scabilloni J, Wang L, Kisin E, Murray AD, Shvedova AA, Castranova AV. Alteration of deposition patterns and pulmonary response as a result of improved dispersion of aspirated single-walled carbon nanotubes in a mouse model. Am J Physiol Lung Cell Mol Physiol. 2008;294:L87–L97. doi: 10.1152/ajplung.00186.2007. [DOI] [PubMed] [Google Scholar]
- Methner MM, Birch ME, Evans DE, Ku B-K, Crouch KG, Hoover MD. Case study: identification and characterization of potential sources of worker exposure to carbon nanofibers during polymer composite laboratory operations. J Occup Environ Hyg. 2007;4:D125–D130. doi: 10.1080/15459620701683871. [DOI] [PubMed] [Google Scholar]
- Methner M, Crawford C, Geraci C. Evaluation of the potential airborne release of carbon nanofibers during the preparation, grinding, and cutting of epoxy-based nanocomposite material. J Occup Environ Hyg. 2012;9:308–318. doi: 10.1080/15459624.2012.670790. [DOI] [PubMed] [Google Scholar]
- Migliore L, Saracino D, Bonelli A, Colognato R, D’Errico MR, Magrini A, Bergamaschi A, Bergamaschi E. Carbon nanotubes induce oxidative DNA damage in RAW 264.7 cells. Environ Mol Mutagen. 2010;51:294–303. doi: 10.1002/em.20545. [DOI] [PubMed] [Google Scholar]
- Miller FJ. Dosimetry of particles in laboratory animals and humans in relationship to issues surrounding lung overload and human health. risk assessment: a critical review. Inhal Toxicol. 2000;12:19–57. doi: 10.1080/089583700196536. [DOI] [PubMed] [Google Scholar]
- Miserocchi G, Sancini G, Mantegazza F, Chiappino G. Translocation pathways for inhaled asbestos fibers. Environ Health. 2008;7:4. doi: 10.1186/1476-069X-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell LA, Gao J, Wal RV, Gigliotti A, Burchiel SW, McDonald JD. Pulmonary and systemic immune response to inhaled multiwalled carbon nanotubes. Toxicol Sci. 2007;100:203–214. doi: 10.1093/toxsci/kfm196. [DOI] [PubMed] [Google Scholar]
- Mitchell LA, Lauer FT, Burchiel SW, McDonald JD. Mechanisms for how inhaled multiwalled carbon nanotubes suppress systemic immune function in mice. Nat Nanotechnol. 2009;4:451–456. doi: 10.1038/nnano.2009.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Møller P. Genotoxicity of environmental agents assessed by the alkaline comet assay. Basic Clin Pharmacol Toxicol. 2005;96:1–42. [PubMed] [Google Scholar]
- Møller P, Danielsen PH, Jantzen K, Roursgaard M, Loft S. Oxidatively damaged DNA in animals exposed to particles. Crit Rev Toxicol. 2013;43:96–118. doi: 10.3109/10408444.2012.756456. [DOI] [PubMed] [Google Scholar]
- Møller P, Hemmingsen JG, Jensen DM, Danielsen PH, Karottki DG, Jantzen K, Roursgaard M, Cao Y, Kermanizadeh H, Klingberg H, et al. Applications of the comet assay in particle toxicology: air pollution and engineered nanomaterials exposure. Mutagenesis. 2015a;30:67–83. doi: 10.1093/mutage/geu035. [DOI] [PubMed] [Google Scholar]
- Møller P, Jensen DM, Christophersen DV, Kermanizadeh A, Jacobsen NR, Hemmingsen JG, Danielsen PH, Karottki DG, Roursgaard M, Cao Y, et al. Measurement of oxidative damage to DNA in nanomaterial exposed cells and animals. Environ Mol Mutagen. 2015b;56:97–110. doi: 10.1002/em.21899. [DOI] [PubMed] [Google Scholar]
- Møller P, Loft S. Oxidative damage to DNA and lipids as biomarkers of exposure to air pollution. Environ Health Perspect. 2010;118:1126–1136. doi: 10.1289/ehp.0901725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Møller P, Loft S. Statistical analysis of comet assay results. Front Genet. 2014;5:292. doi: 10.3389/fgene.2014.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montesano R, Bartsch H, Vainio H, Wilbourn J, Yamasaki H, editors. IARC Scientific Publication No 83. Lyon, France: International Agency for Research on Cancer; 1986. Long-term and short-term assays for carcinogenesis - a critical appraisal. [Google Scholar]
- Morfeld P, Bruch J, Ngiewich Y, Chaudhuri I, Muranko H, Myerson R, McCunney R. Translation toxicology in setting occupational exposure limits for dusts and hazard classification – a critical evaluation of a recent approach to translate dust overload findings from rats to humans. Part Fibre Toxicol. 2015;12:3. doi: 10.1186/s12989-015-0079-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto Y, Hirohashi M, Kobayashi N, Ogami A, Horie M, Oyabu T, Myojo T, Hashiba M, Mizuguchi Y, Kambara T, et al. Pulmonary toxicity of well-dispersed single-wall carbon nanotubes after inhalation. Nanotoxicology. 2012a;6:766–775. doi: 10.3109/17435390.2011.620719. [DOI] [PubMed] [Google Scholar]
- Morimoto Y, Hirohashi M, Ogami A, Oyabu T, Myojo T, Todoroki M, Yamamoto M, Hashiba M, Mizuguchi Y, Lee BW, et al. Pulmonary toxicity of well-dispersed multi-wall carbon nanotubes following inhalation and intratracheal instillation. Nanotoxicology. 2012b;6:587–599. doi: 10.3109/17435390.2011.594912. [DOI] [PubMed] [Google Scholar]
- Morimoto Y, Hirohashi M, Horie M, Ogami A, Oyabu T, Myojo T, Hashiba M, Mizuguchi Y, Kambara T, Lee BW, et al. Pulmonary toxicity of well-dispersed single-wall carbon nanotubes following intratracheal instillation. J Nano Res. 2012c;18–19:9–25. [Google Scholar]
- Moriya M. Single-stranded shuttle phagemid for mutagenesis studies in mammalian cells: 8-oxoguanine in DNA induces targeted G.C-T.A transversions in simian kidney cells. Proc Natl Acad Sci USA. 1993;90:1122–1126. doi: 10.1073/pnas.90.3.1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow PE. Possible mechanisms to explain dust overloading of the lungs. Fundam Appl Toxicol. 1988;10:369–384. doi: 10.1016/0272-0590(88)90284-9. [DOI] [PubMed] [Google Scholar]
- Mossman BT, Morton L, Hesterberg TW, Kelsey KT, Barchowsky A, Bonner JC. Pulmonary endpoints (lung carcinomas and asbestosis) following inhalation exposure to asbestos. J Toxicol Environ Health B Crit Rev. 2011;14:76–121. doi: 10.1080/10937404.2011.556047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossman BT, Shukla A, Heintz NH, Verschraegen CF, Thomas A, Hassan R. New insights into understanding the mechanisms, pathogenesis and management of malignant mesotheliomas. Am J Pathol. 2013;182:1065–1077. doi: 10.1016/j.ajpath.2012.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrakovcic M, Meindl C, Leitinger G, Roblegg E, Frohlich E. Carboxylated short single-walled carbon nanotubes but not plain and multi-walled short carbon nanotubes show in vitro genotoxicity. Toxicol Sci. 2015;144:114–127. doi: 10.1093/toxsci/kfu260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhle H, Bellmann B, Creutzenberg O, Heinrich U, Ketkar M, Mermelstein R. Dust overloading of lungs after exposure of rats to particles of low solubility: comparative studies. J Aerosol Science. 1990;21:374–377. [Google Scholar]
- Muhlfeld C, Poland CA, Duffin R, Brandenberger C, Murphy FA, Rothen-Rutishauser B, Gehr P, Donaldson K. Differential effects of long and short carbon nanotubes on the gas-exchange region of the mouse lung. Nanotoxicology. 2012;6:867–879. doi: 10.3109/17435390.2011.626533. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay S, Farver CF, Vaszar LT, Dempsey OJ, Popper HH, Mani H, Capelozzi VL, Fukuoka J, Kerr KM, Zeren EH, et al. Causes of pulmonary granulomas: a retrospective study of 500 cases from seven countries. J Clin Pathol. 2012;65:51–57. doi: 10.1136/jclinpath-2011-200336. [DOI] [PubMed] [Google Scholar]
- Muller J, Decordier I, Hoet PH, Lombaert N, Thomassen L, Huaux F, Lison D, Kirsch-Volders M. Clastogenic and aneugenic effects of multi-wall carbon nanotubes in epithelial cells. Carcinogenesis. 2008a;29:427–433. doi: 10.1093/carcin/bgm243. [DOI] [PubMed] [Google Scholar]
- Muller J, Delos M, Panin N, Rabolli V, Huaux F, Lison D. Absence of carcinogenic response to multiwall carbon nanotubes in a 2-year bio-assay in the peritoneal cavity of the rat. Toxicol Sci. 2009;110:442–448. doi: 10.1093/toxsci/kfp100. Available from: http://dx.doi.org/10.1093/toxsci/kfp100. [DOI] [PubMed] [Google Scholar]
- Muller J, Huaux F, Fonseca A, Nagy JB, Moreau N, Delos M, Raymundo-Pinero E, Beguin F, Kirsch-Volders M, Fenoglio I, et al. Structural defects play a major role in the acute lung toxicity of multiwall carbon nanotubes: toxicological aspects. Chem Res Toxicol. 2008b;21:1698–1705. doi: 10.1021/tx800101p. [DOI] [PubMed] [Google Scholar]
- Muller J, Huaux F, Moreau N, Misson P, Heilier JF, Delos M, Arras M, Fonseca A, Nagy JB, Lison D. Respiratory toxicity of multiwall carbon nanotubes. Toxicol Appl Pharmacol. 2005;207:221–231. doi: 10.1016/j.taap.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Multhoff G, Molls M, Radons J. Chronic inflammation in cancer development. Front Immunol. 2012;2:98–17. doi: 10.3389/fimmu.2011.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy FA, Poland CA, Duffin R, Al-Jamal KT, Ali-Boucetta H, Nunes A, Byrne F, Prina-Mello A, Volkov Y, Li S, et al. Length-dependent retention of carbon nanotubes in the pleural space of mice initiates sustained inflammation and progressive fibrosis on the parietal pleura. Am J Pathol. 2011;178:2587–2600. doi: 10.1016/j.ajpath.2011.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy FA, Poland CA, Duffin R, Donaldson K. Length-dependent pleural inflammation and parietal pleural responses after deposition of carbon nanotubes in the pulmonary airspaces of mice. Nanotoxicology. 2013;7:1157–1167. doi: 10.3109/17435390.2012.713527. [DOI] [PubMed] [Google Scholar]
- Murray AR, Kisin ER, Tkach AV, Yanamala N, Mercer R, Young SH, Fadeel B, Kagan VE, Shvedova AA. Factoring-in agglomeration of carbon nanotubes and nanofibers for better prediction of their toxicity versus asbestos. Part Fibre Toxicol. 2012;9:10. doi: 10.1186/1743-8977-9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai H, Okazaki Y, Chew SH, Misawa N, Miyata Y, Shinohara H, Toyokuni S. Intraperitoneal administration of tangled multiwalled carbon nanotubes of 15 nm in diameter does not induce mesothelial carcinogenesis in rats. Pathol Int. 2013;63:457–462. doi: 10.1111/pin.12093. [DOI] [PubMed] [Google Scholar]
- Nagai H, Okazaki Y, Chew SH, Misawa N, Yamashita Y, Akatsuka S, Ishihara T, Yamashita K, Yoshikawa Y, Yasui H, et al. Diameter and rigidity of multiwalled carbon nanotubes are critical factors in mesothelial injury and carcinogenesis. Proc Natl Acad Sci USA. 2011;108:E1330–E1338. doi: 10.1073/pnas.1110013108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai H, Toyokuni S. Differences and similarities between carbon nanotubes and asbestos fibers during mesothelial carcinogenesis: shedding light on fiber entry mechanism. Cancer Sci. 2012;103:1378–1390. doi: 10.1111/j.1349-7006.2012.02326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naya M, Kobayashi N, Endoh S, Maru J, Honda K, Ema M, Tanaka J, Fukumuro M, Hasegawa K, Nakajima M, et al. In vivo genotoxicity study of single-wall carbon nanotubes using comet assay following intratracheal instillation in rats. Regul Toxicol Pharmacol. 2012;64:124–129. doi: 10.1016/j.yrtph.2012.05.020. [DOI] [PubMed] [Google Scholar]
- Naya M, Kobayashi N, Mizuno K, Matsumoto K, Ema M, Nakanishi J. Evaluation of the genotoxic potential of single-wall carbon nanotubes by using a battery of in vitro and in vivo genotoxicity assays. Regul Toxicol Pharmacol. 2011;61:192–198. doi: 10.1016/j.yrtph.2011.07.008. [DOI] [PubMed] [Google Scholar]
- Nel AE, Nasser E, Godwin H, Avery D, Bahadori T, Bergeson L, Beryt E, Bonner JC, Boverhof D, Carter J, et al. A multi-stakeholder perspective on the use of alternative test strategies for nanomaterial safety assessment. ACS Nano. 2013;7:6422–6433. doi: 10.1021/nn4037927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikota J, Williams A, Yauk CL, Wallin H, Vogel U, Halappanavar S. Meta-analysis of transcriptomic responses as a means to identify pulmonary disease outcomes for engineered nanomaterials. Part Fibre Toxicol. 2016;13:25. doi: 10.1186/s12989-016-0137-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikula KJ, Vallyathan V, Green FH, Hahn FF. Influence of exposure concentration or dose on the distribution of particulate material in rat and human lungs. Environ Health Perspect. 2001;109:311–318. doi: 10.1289/ehp.01109311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIOSH. Current Intelligence Bulletin 65: Occupational Exposure to Carbon Nanotubes and Nanofibers. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health; Cincinnati, Ohio: 2013. DHHS (NIOSH) Publication Number 2013–14. [Google Scholar]
- Nishimura SL, Broaddus VC. Asbestos-induced pleural disease. Clin Chest Med. 1998;19:311–329. doi: 10.1016/s0272-5231(05)70079-4. [DOI] [PubMed] [Google Scholar]
- Oberdorster G. Lung particle overload: implications for occupational exposures to particles. Regulatory Toxicol Pharmacol. 1995;27:123–135. doi: 10.1006/rtph.1995.1017. [DOI] [PubMed] [Google Scholar]
- Oberdorster G, Castranova V, Asgharian B, Sayre P. Inhalation exposure to carbon nanotubes (CNT) and carbon nanofibers (CNF): methodology and dosimetry. J Toxicol Environ Health B Crit Rev. 2015;18:121–212. doi: 10.1080/10937404.2015.1051611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberdorster G, Ferin J, Soderholm S, Gelein R, Cox C, Baggs R, Morrow PE. Increased pulmonary toxicity of inhaled ultrafine particles: due to lung overload alone? Ann Occup Hyg. 1994;38:295–302. [Google Scholar]
- Oberdorster G, Maynard A, Donaldson K, Castranova V, Fitzpatrick J, Ausman K, Carter J, Karn B, Kreyling W, et al. ILSI Research Foundation/Risk Science Institute Nanomaterial Toxicity Screening Working Group. Principles for characterizing the potential human health effects from exposure to nanomaterials: elements of a screening strategy. Report of the International Life Sciences Institute Research Foundation/Risk Science Institute Nanomaterial Toxicity Screening Working Group. Part Fibre Toxicol. 2005b;2:8. doi: 10.1186/1743-8977-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberdorster G, Oberdorster E, Oberdorster J. Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ Health Perspect. 2005a;113:823–839. doi: 10.1289/ehp.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OECD [Internet] OECD Guideline for the Testing of Chemicals: Subchronic Inhalation Toxicity: 90-Day Study. OECD/PCDE TG. 2009:413. Adopted: 7 September 2009. Available from: http://www.oecd-ilibrary.org/environment/test-no-413-subchronic-inhalation-toxicity-90-day-study_9789264070806-en.
- OECD [Internet] OECD Guideline for the Testing of Chemicals: In Vivo Mammalian Alkaline Comet Assay. OECD/OCDE TG. 2014:489. Adopted: 26 September 2014. Available from: http://www.oecd-ilibrary.org/environment/test-no-489-in-vivo-mammalian-alkaline-comet-assay_9789264224179-en.
- O’Hagan HM, Wang W, Sen S, Destefano Shields C, Lee SS, Zhang YW, Clements EG, Cai Y, Van Neste L, Easwaran H, et al. Oxidative damage targets complexes containing DNA methyltransferases, SIRT1, and polycomb members to promoter CpG Islands. Cancer Cell. 2011;20:606–619. doi: 10.1016/j.ccr.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyabu T, Myojo T, Morimoto Y, Ogami A, Hirohashi M, Yamamoto M, Todoroki M, Mizuguchi Y, Hashiba M, Lee BW, et al. Biopersistence of inhaled MWCNT in rat lungs in a 4-week well-characterized exposure. Inhal Toxicol. 2011;23:784–791. doi: 10.3109/08958378.2011.608096. [DOI] [PubMed] [Google Scholar]
- Pacurari M, Yin XJ, Zhao J, Ding M, Leonard SS, Schwegler-Berry D, Ducatman BS, Sbarra D, Hoover MD, Castranova V, Vallyathan V. Raw single-wall carbon nanotubes induce oxidative stress and activate MAPKs, AP-1, NF-kappaB, and Akt in normal and malignant human mesothelial cells. Environ Health Perspect. 2008;116:1211–1217. doi: 10.1289/ehp.10924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pairon JC, Laurent F, Rinaldo M, Clin B, Andujar P, Ameille J, Brochard P, Chammings S, Ferretti G, Galateau-Sallé F, et al. Pleural plaques and the risk of pleural mesothelioma. J Natl Cancer Inst. 2013;105:293–301. doi: 10.1093/jnci/djs513. [DOI] [PubMed] [Google Scholar]
- Palomaki J, Valimaki E, Sund J, Vippola M, Clausen PA, Jensen KA, Savolainen K, Matikainen S, Alenius H. Long, needle-like carbon nanotubes and asbestos activate the NLRP3 inflammasome through a similar mechanism. ACS Nano. 2011;5:6861–6870. doi: 10.1021/nn200595c. [DOI] [PubMed] [Google Scholar]
- Pandiri A. Comparative pathobiology of environmentally induced lung cancers in humans and rodents. Toxicol Pathol. 2015;43:107–114. doi: 10.1177/0192623314556516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park EJ, Cho WS, Jeong J, Yi J, Choi K, Park K. Pro-inflammatory and potential allergic responses resulting from B cell activation in mice treated with multi-walled carbon nanotubes by intratracheal instillation. Toxicology. 2009;259:113–121. doi: 10.1016/j.tox.2009.02.009. [DOI] [PubMed] [Google Scholar]
- Park EJ, Roh J, Kim SN, Kang MS, Han YA, Kim Y, Hong JT, Choi K. A single intratracheal instillation of single-walled carbon nanotubes induced early lung fibrosis and subchronic tissue damage in mice. Arch Toxicol. 2011a;85:1121–1131. doi: 10.1007/s00204-011-0655-8. [DOI] [PubMed] [Google Scholar]
- Park EJ, Roh J, Kim SN, Kang MS, Lee BS, Kim Y, Choi S. Biological toxicity and inflammatory response of semi-single-walled carbon nanotubes. PLoS One. 2011b;6:e25892. doi: 10.1371/journal.pone.0025892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park EJ, Roh J, Kim SN, Kim Y, Han SB, Hong JT. CCR5 plays an important role in resolving an inflammatory response to single-walled carbon nanotubes. J Appl Toxicol. 2013;33:845–853. doi: 10.1002/jat.2744. [DOI] [PubMed] [Google Scholar]
- Pauluhn J. Subchronic 13-week inhalation exposure of rats to multi-walled carbon nanotubes: toxic effects are determined by density of agglomerate structures, not fibrillar structures. Toxicol Sci. 2010;113:226–242. doi: 10.1093/toxsci/kfp247. [DOI] [PubMed] [Google Scholar]
- Pauluhn J. Derivation of occupational exposure levels (OELs) of low-toxicity isometric biopersistent particles: How can the kinetic lung overload paradigm be used for improved inhalation toxicity study design and OEL-derivation? Part Fibre Toxicol. 2014a;11:72. doi: 10.1186/s12989-014-0072-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauluhn J. The metrics of MWCNT-induced pulmonary inflammation are dependent on the selected testing regimen. Regul Toxicol Pharmacol. 2014b;68:343–352. doi: 10.1016/j.yrtph.2014.01.010. [DOI] [PubMed] [Google Scholar]
- Pauluhn J, Rosenbruch M. Lung burdens and kinetics of multi-walled carbon nanotubes (Baytubes) are highly dependent on the disaggregation of aerosolized MWCNT. Nanotoxicology. 2015;9:242–252. doi: 10.3109/17435390.2014.918204. [DOI] [PubMed] [Google Scholar]
- Pelka J, Gehrke H, Rechel A, Kappes M, Hennrich F, Hartinger CG, Marko D. DNA damaging properties of single walled carbon nanotubes in human colon carcinoma cells. Nanotoxicology. 2013;7:2–20. doi: 10.3109/17435390.2011.626536. [DOI] [PubMed] [Google Scholar]
- Pietroiusti A, Massimiani M, Fenoglio I, Colonna M, Valentini F, Palleschi G, Camaioni A, Magrini A, Siracusa G, Bergamaschi A, et al. Low doses of pristine and oxidized single-wall carbon nanotubes affect mammalian embryonic development. ACS Nano. 2011;5:4624–4633. doi: 10.1021/nn200372g. [DOI] [PubMed] [Google Scholar]
- Pinkerton KE, Plopper CG, Hyde DM, Harkema JR, Tyler WS, Morgan KT, St George JA, Kay M, Mariassy A. Structure and function of the respiratory tract. In: Massaro EJ, editor. Handbook of human toxicology. Chapter 11. New York (NY): CRC Press; 1997. pp. 469–491. [Google Scholar]
- Pitot HC, Dragan YP. Stage of tumor progression, progressor agents, and human risk. Proc Soc Exp Biol Med. 1993;202:37–43. doi: 10.3181/00379727-202-43511f. [DOI] [PubMed] [Google Scholar]
- Plopper CG, Hyde DM. Epithelial cells of the bronchiole. In: Parent RA, editor. Comparative biology of the normal lung. 2. Chapter 7. San Diego (CA): Academic Press; 2015. pp. 83–92. [Google Scholar]
- Poland CA, Duffin R, Kinloch I, Maynard A, Wallace WA, Seaton A, Stone V, Brown S, Macnee W, Donaldson K. Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study. Nat Nanotechnol. 2008;3:423–428. doi: 10.1038/nnano.2008.111. [DOI] [PubMed] [Google Scholar]
- Ponti J, Broggi F, Mariani V, De ML, Colognato R, Marmorato P, Gioria S, Gilliland D, Pascual GC, Meschini S, et al. Morphological transformation induced by multiwall carbon nanotubes on Balb/3T3 cell model as an in vitro end point of carcinogenic potential. Nanotoxicology. 2013;7:221–233. doi: 10.3109/17435390.2011.652681. [DOI] [PubMed] [Google Scholar]
- Porter DW, Hubbs AF, Chen BT, McKinney W, Mercer RR, Wolfarth MG, Battelli L, Wu N, Sriram K, Leonard S, et al. Acute pulmonary dose–responses to inhaled multi-walled carbon nanotubes. Nanotoxicology. 2013;7:1179–1194. doi: 10.3109/17435390.2012.719649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter DW, Hubbs AF, Mercer RR, Wu N, Wolfarth MG, Sriram K, Leonard SS, Battelli L, Schwegler-Berry D, Friend S, et al. Mouse pulmonary dose-and time course-responses induced by exposure to multi-walled carbon nanotubes. Toxicology. 2010;269:136–147. doi: 10.1016/j.tox.2009.10.017. [DOI] [PubMed] [Google Scholar]
- Porter DW, Sriram K, Wolfarth M, Jefferson A, Schwegler-Berry D, Andrew M, Castranova V. A biocompatible medium for nanoparticle dispersion. Nanotoxicol. 2008;2:144–154. [Google Scholar]
- Pothmann D, Simar S, Schuler D, Dony E, Gaering S, Le Net JL, Okazaki Y, Chabagno JM, Bessibes C, Beausoleil J, et al. Lung inflammation and lack of genotoxicity in the comet and micronucleus assays of industrial multiwalled carbon nanotubes Graphistrength((c)) C100 after a 90-day nose-only inhalation exposure of rats. Part Fibre Toxicol. 2015;12:21. doi: 10.1186/s12989-015-0096-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pott F, Roller M, Rippe RM, German P-G, Bellmann B. Tumours by the intraperitoneal and intrapleural routes and their significance for the classification of mineral fibres. In: Brown RC, Hoskins JA, Johnson NF, editors. Mechanisms in fibre carcinogenesis. New York: Plenum Press; 1991. pp. 547–565. [Google Scholar]
- Pott F, Ziem U, Reiffer F, Huth F, Ernst H, Mohr U. Carcinogenicity studies of fibres, metal compounds and some other dusts in rats. Exp Path. 1987;32:129–152. doi: 10.1016/s0232-1513(87)80044-0. [DOI] [PubMed] [Google Scholar]
- Poulsen SS, Jacobsen NR, Labib S, Wu D, Husain M, Williams A, Bogelund JP, Andersen O, Kobler C, Molhave K, et al. Transcriptomic analysis reveals novel mechanistic insight into murine biological responses to multi-walled carbon nanotubes in lungs and cultured lung epithelial cells. PLoS One. 2013;8:e80452. doi: 10.1371/journal.pone.0080452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulsen SS, Saber AT, Williams A, Andersen O, Kobler C, Atluri R, Pozzebon ME, Mucelli SP, Simion M, Rickerby D, et al. MWCNTs of different physicochemical properties cause similar inflammatory responses, but differences in transcriptional and histological markers of fibrosis in mouse lungs. Toxicol Appl Pharmacol. 2015;284:16–32. doi: 10.1016/j.taap.2014.12.011. [DOI] [PubMed] [Google Scholar]
- Quinn MM, Smith TJ, Eisen EA, Wegman DH, Ellenbecker MJ. Implications of different fiber measures for epidemiologic studies of man-made vitreous fibers. Am J Ind Med. 2000;38:132–139. doi: 10.1002/1097-0274(200008)38:2<132::aid-ajim3>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Rabolli V, Badissi AA, Devosse R, Uwambayinema F, Yakoub Y, Palmai-Pallag M, Lebrun A, De Gussem V, Couillin I, Ryffel B, et al. The alarmin IL-1α is a master cytokine in acute lung inflammation induced by silica micro- and nanoparticles. Part Fibre Toxicol. 2014;11:69. doi: 10.1186/s12989-014-0069-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghu G, Nyberg F, Morgan G. The epidemiology of interstitial lung disease and its association with lung cancer. Br J Cancer. 2004;91:S3–S10. doi: 10.1038/sj.bjc.6602061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez-Salazar EG, Salinas-Silva LC, Vázquez-Manríquez ME, Gayosso-Gómez LV, Negrete-Garcia MC, Ramírez-Rodriguez SL, Chávez R, Zenteno E, Santillán P, Kelly-García J, Ortiz-Quintero B. Analysis of microRNA expression signatures in malignant pleural mesothelioma, pleural inflammation, and atypical mesothelial hyperplasia reveals common predictive tumorigenesis-related targets. Exper Molec Path. 2014;97:375–385. doi: 10.1016/j.yexmp.2014.09.016. [DOI] [PubMed] [Google Scholar]
- Ravichandran P, Baluchamy S, Sadanandan B, Gopikrishnan R, Biradar S, Ramesh V, Hall JC, Ramesh GT. Multiwalled carbon nanotubes activate NF-kappaB and AP-1 signaling pathways to induce apoptosis in rat lung epithelial cells. Apoptosis. 2010;15:1507–1516. doi: 10.1007/s10495-010-0532-6. [DOI] [PubMed] [Google Scholar]
- Reddy ARN, Krishna DR, Reddy YN, Himabindu V. Translocation and extra pulmonary toxicities of multi wall carbon nanotubes in rats. Toxicol Mech Methods. 2010;20:267–272. doi: 10.3109/15376516.2010.484077. [DOI] [PubMed] [Google Scholar]
- Regad T. Targeting RTK signaling pathways in cancer. Cancers. 2015;7:1758–1784. doi: 10.3390/cancers7030860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renwick LC, Brown D, Clouter A, Donaldson K. Increased inflammation and altered macrophage chemotactic responses caused by two ultrafine particles. Occup Environ Med. 2004;61:442–447. doi: 10.1136/oem.2003.008227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renwick LC, Donaldson K, Clouter A. Impairment of alveolar macrophage phagocytosis by ultrafine particles. Toxicol Applied Pharmacol. 2001;172:119–127. doi: 10.1006/taap.2001.9128. [DOI] [PubMed] [Google Scholar]
- Ress NB, Chou BJ, Renne RA, Dill JA, Miller RA, Roycroft JH, Hailey JR, Haseman JK, Bucher JR. Carcinogenicity of inhaled vanadium pentoxide in F344/N rats and B6C3F1 mice. Toxicol Sci. 2003;74:287–296. doi: 10.1093/toxsci/kfg136. [DOI] [PubMed] [Google Scholar]
- Rittinghausen S, Hackbarth A, Creutzenberg O, Ernst H, Heinrich U, Leonhardt A, Schaudien D. The carcinogenic effect of various multi-walled carbon nanotubes (MWCNTs) after intraperitoneal injection in rats. Part Fibre Toxicol. 2014;11:59. doi: 10.1186/s12989-014-0059-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roda E, Coccini T, Acerbi D, Barni S, Vaccarone R, Manzo L. Comparative pulmonary toxicity assessment of pristine and functionalized multi-walled carbon nanotubes intratracheally instilled in rats: morphohistochemical evaluations. Histol Histopathol. 2011;26:357–367. doi: 10.14670/HH-26.357. [DOI] [PubMed] [Google Scholar]
- Rom WL. Relationship of inflammatory cell cytokines to disease severity in individuals with occupational inorganic dust exposure. Am J Indus Med. 1991;19:15–27. doi: 10.1002/ajim.4700190104. [DOI] [PubMed] [Google Scholar]
- Rothfuss A, O’Donovan M, De BM, Brault D, Czich A, Custer L, Hamada S, Plappert-Helbig U, Hayashi M, Howe J, et al. Collaborative study on fifteen compounds in the rat-liver Comet assay integrated into 2-and 4-week repeat-dose studies. Mutat Res. 2010;702:40–69. doi: 10.1016/j.mrgentox.2010.07.006. [DOI] [PubMed] [Google Scholar]
- Ryman-Rasmussen JP, Cesta MF, Brody AR, Shipley-Phillips JK, Everitt JI, Tewksbury EW, Moss OR, Wong BA, Dodd DE, Andersen ME, Bonner JC. Inhaled carbon nanotubes reach the subpleural tissue in mice. Nat Nanotechnol. 2009;4:747–751. doi: 10.1038/nnano.2009.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sager TM, Wolfarth MW, Andrew M, Hubbs A, Friend S, Chen TH, Porter DW, Wu N, Yang F, Hamilton RF, Holian A. Effect of multi-walled carbon nanotube surface modification on bioactivity in the C57BL/6 mouse model. Nanotoxicology. 2014;8:317–327. doi: 10.3109/17435390.2013.779757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sager TM, Wolfarth MW, Battelli LA, Leonard SS, Andrew M, Steinbach T, Endo M, Tsuruoka S, Porter DW, Castranova V. Investigation of the pulmonary bioactivity of double-walled carbon nanotubes. J Toxicol Environ Health A. 2013;76:922–936. doi: 10.1080/15287394.2013.825571. [DOI] [PubMed] [Google Scholar]
- Sakamoto Y, Nakae D, Fukumori N, Tayama K, Maekawa A, Imai K, Hirose A, Nishimura T, Ohashi N, Ogata A. Induction of mesothelioma by a single intrascrotal administration of multi-wall carbon nanotube in intact male Fischer 344 rats. J Toxicol Sci. 2009;34:65–76. doi: 10.2131/jts.34.65. http://dx.doi.org/10.2131/jts.34.65. [DOI] [PubMed] [Google Scholar]
- Sanchez VC, Pietruska JR, Miselis NR, Hurt RH, Kane AB. Biopersistence and potential adverse health impacts of fibrous nanomaterials: what have we learned from asbestos? Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2009;1:511–529. doi: 10.1002/wnan.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez VC, Weston P, Yan A, Hurt RH, Kane AB. A 3-dimensional in vitro model of epithelioid granulomas induced by high aspect ratio nanomaterials. Part Fibre Toxicol. 2011;8:17. doi: 10.1186/1743-8977-8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent L, Dragan Y, Xu YH, Sattler G, Wiley J, Pitot HC. Karyotypic changes in a multistage model of chemical hepatocarcinogenesis in the rat. Cancer Res. 1996;56:2985–2991. [PubMed] [Google Scholar]
- Sargent LM, Hubbs AF, Young SH, Kashon ML, Dinu CZ, Salisbury JL, Benkovic SA, Lowry DT, Murray AR, Kisin ER, et al. Single-walled carbon nanotube-induced mitotic disruption. Mutat Res. 2012;745:28–37. doi: 10.1016/j.mrgentox.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent LM, Shvedova AA, Hubbs AF, Salisbury JL, Benkovic SA, Kashon ML, Lowry DT, Murray AR, Kisin ER, Friend S, et al. Induction of aneuploidy by single-walled carbon nanotubes. Environ Mol Mutagen. 2009;50:708–717. doi: 10.1002/em.20529. [DOI] [PubMed] [Google Scholar]
- Sargent LM, Porter DW, Staska LM, Hubbs AF, Lowry DT, Battelli L, Siegrist KJ, Kashon ML, Mercer RR, Bauer AK, et al. Promotion of lung adenocarcinoma following inhalation exposure to multi-walled carbon nanotubes. Part Fibre Toxicol. 2014;11:3. doi: 10.1186/1743-8977-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S, Sharma C, Yog R, Periakaruppan A, Jejelowo O, Thomas R, Barrera EV, Rice-Ficht AC, Wilson BL, Ramesh GT. Analysis of stress responsive genes induced by single-walled carbon nanotubes in BJ Foreskin cells. J Nanosci Nanotechnol. 2007;7:584–592. [PMC free article] [PubMed] [Google Scholar]
- Sasaki YF, Sekihashi K, Izumiyama F, Nishidate E, Saga A, Ishida K, Tsuda S. The comet assay with multiple mouse organs: comparison of comet assay results and carcinogenicity with 208 chemicals selected from the IARC monographs and U.S. NTP carcinogenicity database. Crit Rev Toxicol. 2000;30:629–799. doi: 10.1080/10408440008951123. [DOI] [PubMed] [Google Scholar]
- Scharer OD. Nucleotide excision repair in eukaryotes. Cold Spring Harb Perspect Biol. 2013;5:a012609. doi: 10.1101/cshperspect.a012609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schins RPF, Knaapen AM. Genotoxicity of poorly soluble particles. Inhal Toxicol. 2007;19:189–198. doi: 10.1080/08958370701496202. [DOI] [PubMed] [Google Scholar]
- Schinwald A, Murphy FA, Prina-Mello A, Poland CA, Byrne F, Glass JR, Dickerson JC, Schultz DA, Movia D, Jeffree CE, et al. The threshold length for fibre-induced acute pleural inflammation: shedding light on the early events in asbestos-induced mesothelioma. Toxicol Sci. 2012;128:461–470. doi: 10.1093/toxsci/kfs171. [DOI] [PubMed] [Google Scholar]
- Schulz H, Brand P, Heyder J. Particle deposition in the respiratory tract. In: Gehr P, Heyder J, editors. Particle-lung interactions. New York: Marcel Dekker Inc; 2000. pp. 229–290. [Google Scholar]
- SCOEL. Recommendation from the Scientific Committee on Occupational Exposure Limits for man-made mineral fibres (MMMF) with no indication for carcinogenicity and not specified elsewhere. SCOEL/SUM/88. 2012:1–17. [Google Scholar]
- Shannahan JH, Brown JM, Chen R, Ke PC, Lai X, Mitra S, Witzmann FA. Comparison of nanotube-protein corona composition in cell culture media. Small. 2013;9:2171–2181. doi: 10.1002/smll.201202243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma OP, Lamb C. Cancer in interstitial pulmonary fibrosis and sarcoidosis. Curr Opin Pulm Med. 2003;9:398–401. doi: 10.1097/00063198-200309000-00010. [DOI] [PubMed] [Google Scholar]
- Sherwood V. WNT signaling: an emerging mediator of cancer cell metabolism? Mol Cell Biol. 2015;35:2–10. doi: 10.1128/MCB.00992-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara N, Nakazato T, Ohkawa K, Tamura M, Kobayashi N, Morimoto Y, Oyabu T, Myojo T, Shimada M, Yamamoto K, et al. Long-term retention of pristine multi-walled carbon nanotubes in rat lungs after intratracheal instillation. J Appl Toxicol. 2016;36:501–509. doi: 10.1002/jat.3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shvedova AA, Kisin ER, Mercer R, Murray AR, Johnson VJ, Potapovich AI, Tyurina YY, Gorelik O, Arepalli S, Schwegler-Berry D, et al. Unusual inflammatory and fibrogenic pulmonary responses to single-walled carbon nanotubes in mice. Am J Physiol Lung Cell Mol Physiol. 2005;289:L698–L708. doi: 10.1152/ajplung.00084.2005. [DOI] [PubMed] [Google Scholar]
- Shvedova AA, Kapralov AA, Feng WH, Kisin ER, Murray AR, Mercer RR, St Croix CM, Lang MA, Watkins SC, Konduru NV, et al. Impaired clearance and enhanced pulmonary inflammatory/fibrotic response to carbon nanotubes in myeloperoxidase-deficient mice. PLoS One. 2012;7:e30923. doi: 10.1371/journal.pone.0030923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shvedova AA, Kisin ER, Murray AR, Gorelik O, Arepalli S, Castranova V, Young SH, Gao F, Tyurina YY, Oury TD, Kagan VE. Vitamin E deficiency enhances pulmonary inflammatory response and oxidative stress induced by single-walled carbon nanotubes in C57BL/6 mice. Toxicol Appl Pharmacol. 2007;221:339–348. doi: 10.1016/j.taap.2007.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shvedova AA, Kisin E, Murray AR, Johnson VJ, Gorelik O, Arepalli S, Hubbs AF, Mercer RR, Keohavong P, Sussman N, et al. Inhalation vs. aspiration of single-walled carbon nanotubes in C57BL/6 mice: inflammation, fibrosis, oxidative stress, and mutagenesis. Am J Physiol Lung Cell Mol Physiol. 2008;295:L552–L565. doi: 10.1152/ajplung.90287.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shvedova AA, Yanamala N, Kisin ER, Khailullin TO, Birch ME, Fatkhutdinova LM. Integrated Analysis of Dysregulated ncRNA and mRNA Expression Profiles in Humans Exposed to Carbon Nanotubes. PLoS One. 2016;11:e0150628. doi: 10.1371/journal.pone.0150628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shvedova AA, Yanamala N, Kisin ER, Tkach AV, Murray AR, Hubbs A, Chirila MM, Keohavong P, Sycheva LP, Kagan VE, Castranova V. Long-term effects of carbon containing engineered nanomaterials and asbestos in the lung: one year postexposure comparisons. Am J Physiol Lung Cell Mol Physiol. 2014;306:L170–L182. doi: 10.1152/ajplung.00167.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shieh Y-T, Wang W-W. Radical scavenging efficiencies of modified and microwave-treated multiwalled carbon nanotubes. Carbon. 2014;79:354–362. [Google Scholar]
- Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122:787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegrist KJ, Reynolds SH, Kashon ML, Lowry DT, Dong C, Hubbs AF, Young SH, Salisbury JL, Porter DW, Benkovic SA, et al. Genotoxicity of multi-walled carbon nanotubes at occupationally relevant doses. Part Fibre Toxicol. 2014;11:6. doi: 10.1186/1743-8977-11-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva RM, Doudrick K, Franzi LM, TeeSy C, Anderson DS, Wu Z, Mitra S, Vu V, Dutrow G, Evans JE, et al. Instillation versus inhalation of multiwalled carbon nanotubes: exposure-related health effects, clearance, and the role of particle characteristics. ACS Nano. 2014;8:8911–8931. doi: 10.1021/nn503887r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MT, Guyton KZ, Gibbons CF, Fritz JM, Portier C, Rusyn I, DeMarini DM, Caldwell JC, Kavlock R, Lambert P, et al. Key characteristics of carcinogens as a basis for organizing data on mechanisms of carcinogenesis. Environ Health Perspect. 2016;124:713–721. doi: 10.1289/ehp.1509912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snipes MB. Long-term retention and clearance of particles inhaled by mammalian species. Crit Rev Toxicol. 1989;20:175–211. doi: 10.3109/10408448909017909. [DOI] [PubMed] [Google Scholar]
- Snyder-Talkington BN, Dong C, Sargent LM, Porter DW, Staska LM, Hubbs AF, Raese R, McKinney W, Chen BT, Battelli L, et al. mRNAs and miRNAs in whole blood associated with lung hyperplasia, fibrosis, and bronchiolo-alveolar adenoma and adenocarcinoma after multi-walled carbon nanotube inhalation exposure in mice. J Appl Toxicol. 2016;36:161–174. doi: 10.1002/jat.3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder-Talkington BN, Dymacek J, Porter DW, Wolfarth MG, Mercer RR, Pacurari M, Denvir J, Castranova V, Qian Y, Guo NL. System-based identification of toxicity pathways associated with multi-walled carbon nanotube-induced pathological responses. Toxicol Appl Pharmacol. 2013a;272:476–489. doi: 10.1016/j.taap.2013.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder-Talkington BN, Pacurari M, Dong C, Leonard SS, Schwegler-Berry D, Castranova V, Qian Y, Guo NL. Systematic analysis of multi-walled carbon nanotube-induced cellular signaling and gene expression in human small airway epithelial cells. Toxicol Sci. 2013b;133:79–89. doi: 10.1093/toxsci/kft019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava RK, Pant AB, Kashyap MP, Kumar V, Lohani M, Jonas L, Rahman Q. Multi-walled carbon nanotubes induce oxidative stress and apoptosis in human lung cancer cell line-A549. Nanotoxicology. 2011;5:195–207. doi: 10.3109/17435390.2010.503944. [DOI] [PubMed] [Google Scholar]
- Stayner LT, Kuempel E, Gilbert S, Hein M, Dement J. An epidemiologic study of the role of chrysotile asbestos fiber dimensions in determining respiratory disease risk in exposed workers. Occup Environ Med. 2008;65:613–619. doi: 10.1136/oem.2007.035584. [DOI] [PubMed] [Google Scholar]
- Stanton MF, Layard M, Tegeris A, Miller E, May M, Morgan E, Smith A. Relation of particle dimension to carcinogenicity in amphibole asbestos and other fibrous minerals. J Natl Cancer Inst. 1981;67:965–975. [PubMed] [Google Scholar]
- Stern ST, Adiseshaiah PP, Crist RM. Autophagy and lysosomal dysfunction as emerging mechanisms of nanomaterial toxicity. Part Fibre Toxicol. 2012;9:20. doi: 10.1186/1743-8977-9-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su W-C, Cheng YS. Carbon nanotubes size classification, characterization and nasal airway deposition. Inhal Toxicol. 2014;26:843–852. doi: 10.3109/08958378.2014.960107. [DOI] [PubMed] [Google Scholar]
- Su W-C, Cheng YS. Estimation of carbon nanotubes deposition in a human respiratory tract replica. J Aerosol Sci. 2015;79:72–85. [Google Scholar]
- Sun B, Wang X, Ji Z, Wang M, Liao Y-P, Chang CH, Li R, Zhang H, Nel AE, Xia T. NADPH oxidase-dependent NLRP3 inflammasome activation and its important role in lung fibrosis by multiwalled carbon nanotubes. Small. 2015;11:2087–2097. doi: 10.1002/smll.201402859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sureka B, Thukral BB, Mittal MK, Mittal A, Sinha M. Radiological review of pleural tumors. Indian J Radiol Imaging. 2013;23:313–320. doi: 10.4103/0971-3026.125577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzui M, Futakuchi M, Fukamachi K, Numano T, Elgied MA, Takahashi S, Ohnishi M, Omori T, Tsuruoka S, Hirose A, et al. Multiwalled carbon nanotubes intratracheally instilled into the rat lung induce development of pleural malignant mesothelioma and lung tumors. Cancer Science. 2016 doi: 10.1111/cas.12954. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szendi K, Varga C. Lack of genotoxicity of carbon nanotubes in a pilot study. Anticancer Res. 2008;28:349–352. [PubMed] [Google Scholar]
- Takagi A, Hirose A, Futakuchi M, Tsuda H, Kanno J. Dose-dependent mesothelioma induction by intraperitoneal administration of multi-wall carbon nanotubes in p53 heterozygous mice. Cancer Sci. 2012;103:1440–1444. doi: 10.1111/j.1349-7006.2012.02318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi A, Hirose A, Nishimura T, Fukumori N, Ogata A, Ohashi N, Kitajima S, Kanno J. Induction of mesothelioma in p53+/− mouse by intra-peritoneal application of multi-wall carbon nanotube. J Toxicol Sci. 2008;33:105–116. doi: 10.2131/jts.33.105. [DOI] [PubMed] [Google Scholar]
- Takanashi S, Hara K, Aoki K, Usui Y, Shimizu M, Haniu H, Ogihara N, Ishigaki N, Nakamura K, Okamoto M, et al. Carcinogenicity evaluation for the application of carbon nanotubes as biomaterials in rasH2 mice. Sci Rep. 2012;2:498. doi: 10.1038/srep00498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaoki J, Isono K, Takeyama K, Tagaya E, Nakata J, Nagai A. Ultrafine carbon black particles stimulate proliferation of human airway epithelium via EGF receptor-mediated signaling pathway. Am J Physiol Lung Cell Mol Physiol. 2004;287:L1127–L1133. doi: 10.1152/ajplung.00241.2004. [DOI] [PubMed] [Google Scholar]
- Tavares AM, Louro H, Antunes S, Quarre S, Simar S, De Temmerman PJ, Verleysen E, Mast J, Jensen KA, Norppa H, et al. Genotoxicity evaluation of nanosized titanium dioxide, synthetic amorphous silica and multi-walled carbon nanotubes in human lymphocytes. Toxicol In Vitro. 2014;28:60–69. doi: 10.1016/j.tiv.2013.06.009. [DOI] [PubMed] [Google Scholar]
- Taylor AA, Aron GM, Beall GW, Dharmasiri N, Zhang Y, McLean RJ. Carbon and clay nanoparticles induce minimal stress responses in gram negative bacteria and eukaryotic fish cells. Environ Toxicol. 2014;29:961–968. doi: 10.1002/tox.21824. [DOI] [PubMed] [Google Scholar]
- Thurnherr T, Brandenberger C, Fischer K, Diener L, Manser P, Maeder-Althaus X, Kaiser JP, Krug HF, Rothen-Rutishauser B, Wick P. A comparison of acute and long-term effects of industrial multiwalled carbon nanotubes on human lung and immune cells in vitro. Toxicol Lett. 2011;200:176–186. doi: 10.1016/j.toxlet.2010.11.012. [DOI] [PubMed] [Google Scholar]
- Tran CL, Buchanan D, Cullen RT, Searl A, Jones AD, Donaldson K. Inhalation of poorly soluble particles. II. Influence of particle surface area on inflammation and clearance. Inhal Toxicol. 2000;12:1113–1126. doi: 10.1080/08958370050166796. [DOI] [PubMed] [Google Scholar]
- Trinchieri G. Cancer and inflammation: an old intuition with rapidly evolving new concepts. Ann Rev Immunol. 2012;30:677–706. doi: 10.1146/annurev-immunol-020711-075008. [DOI] [PubMed] [Google Scholar]
- Treumann S, Ma-Hock L, Groters S, Landsiedel R, van Ravenzwaay B. Additional histopathologic examination of the lungs from a 3-month inhalation toxicity study with multiwall carbon nanotubes in rats. Toxicol Sci. 2013;134:103–110. doi: 10.1093/toxsci/kft089. [DOI] [PubMed] [Google Scholar]
- Tsai S, Hofmann M, Hallock M, Ada E, Kong J, Ellenbecker M. Characterization and evaluation of nanoparticle release during the synthesis of single-walled and multiwalled carbon nanotubes by chemical vapor deposition. Environ Sci Technol. 2009;43:6017–6023. doi: 10.1021/es900486y. [DOI] [PubMed] [Google Scholar]
- Ursini CL, Cavallo D, Fresegna AM, Ciervo A, Maiello R, Buresti G, Casciardi S, Bellucci S, Iavicoli S. Differences in cytotoxic, genotoxic, and inflammatory response of bronchial and alveolar human lung epithelial cells to pristine and COOH-functionalized multiwalled carbon nanotubes. BioMed Res Intl. 2014;2014:359506. doi: 10.1155/2014/359506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallyathan V, Goins M, Lapp LN, Pack D, Leonard S, Shi X, Castranova V. Changes in bronchoalveolar lavage indices associated with radiographic classification in coal miners. Am J Respir Crit Care Med. 2000;162:958–965. doi: 10.1164/ajrccm.162.3.9909074. [DOI] [PubMed] [Google Scholar]
- Vankoningsloo S, Piret JP, Saout C, Noel F, Mejia J, Coquette A, Zouboulis CC, Delhalle J, Lucas S, Toussaint O. Pro-inflammatory effects of different MWCNTs dispersions in p16(INK4A)-deficient telomerase-expressing human keratinocytes but not in human SV-40 immortalized sebocytes. Nanotoxicology. 2012;6:77–93. doi: 10.3109/17435390.2011.558642. [DOI] [PubMed] [Google Scholar]
- Varga C, Szendi K. Carbon nanotubes induce granulomas but not mesotheliomas. In Vivo. 2010;24:153–156. [PubMed] [Google Scholar]
- Vassallo R. Diffuse lung diseases in cigarette smokers. Semin Respir Crit Care Med. 2012;33:533–542. doi: 10.1055/s-0032-1325162. [DOI] [PubMed] [Google Scholar]
- Velazquez SF, Schoeny R, Rice GE, Cogliano VJ. Cancer risk assessment: historical perspectives, current issues, and future directions. Drug Chem Toxicol. 1996;19:161–185. doi: 10.3109/01480549608998233. [DOI] [PubMed] [Google Scholar]
- Vesterdal LK, Danielsen PH, Folkmann JK, Jespersen LF, Aguilar-Pelaez K, Roursgaard M, Loft S, Møller P. Accumulation of lipids and oxidatively damaged DNA in hepatocytes exposed to particles. Toxicol Appl Pharmacol. 2014a;274:350–360. doi: 10.1016/j.taap.2013.10.001. [DOI] [PubMed] [Google Scholar]
- Vesterdal LK, Jantzen K, Sheykhzade M, Roursgaard M, Folkmann JK, Loft S, Møller P. Pulmonary exposure to particles from diesel exhaust, urban dust or single-walled carbon nanotubes and oxidatively damaged DNA and vascular function in apoE(−/−) mice. Nanotoxicology. 2014b;8:61–71. doi: 10.3109/17435390.2012.750385. [DOI] [PubMed] [Google Scholar]
- Vainio H, Magee P, McGregor D, McMichael A, editors. IARC Scientific Publication No 116. Lyon, France: International Agency for Research on Cancer; 1992. Mechanisms of carcinogenesis in risk identification. [Google Scholar]
- Vietti G, Lison D, van den Brule S. Mechanisms of lung fibrosis induced by carbon nanotubes: towards an adverse outcome pathway (AOP) Part Fibre Toxicol. 2016;13:11. doi: 10.1186/s12989-016-0123-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visalli G, Bertuccio MP, Iannazzo D, Piperno A, Pistone A, Di PA. Toxicological assessment of multi-walled carbon nanotubes on A549 human lung epithelial cells. Toxicol In Vitro. 2015;29:352–362. doi: 10.1016/j.tiv.2014.12.004. [DOI] [PubMed] [Google Scholar]
- Vittorio O, Raffa V, Cuschieri A. Influence of purity and surface oxidation on cytotoxicity of multiwalled carbon nanotubes with human neuroblastoma cells. Nanomedicine (Lond Print) 2009;5:424–431. doi: 10.1016/j.nano.2009.02.006. [DOI] [PubMed] [Google Scholar]
- Wang X, Katwa P, Podila R, Chen P, Ke PC, Rao AM, Walters DM, Wingard CJ, Brown JM. Multi-walled carbon nanotube instillation impairs pulmonary function in C57BL/6 mice. Part Fibre Toxicol. 2011b;8:24. doi: 10.1186/1743-8977-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Luanpitpong S, Castranova V, Tse W, Lu Y, Pongrakhananon V, Rojanasakul Y. Carbon nanotubes induce malignant transformation and tumorigenesis of human lung epithelial cells. Nano Lett. 2011a;11:2796–2803. doi: 10.1021/nl2011214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Mercer RR, Rojanasakul Y, Qiu A, Lu Y, Scabilloni JF, Wu N, Castranova V. Direct fibrogenic effects of dispersed single-walled carbon nanotubes on human lung fibroblasts. J Toxicol Environ Health Part A. 2010;73:410–422. doi: 10.1080/15287390903486550. [DOI] [PubMed] [Google Scholar]
- Wang X, Shannahan JH, Brown JM. IL-33 modulates chronic airway resistance changes induced by multi-walled carbon nanotubes. Inhal Toxicol. 2014b;26:240–249. doi: 10.3109/08958378.2014.880202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Stueckle TA, Mishra A, Derk R, Meighan T, Castranova V, Rojanasakul Y. Neoplastic-like transformation effect of single-walled and multi-walled carbon nanotubes compared to asbestos on human lung small airway epithelial cells. Nanotoxicology. 2014a;8:484–507. doi: 10.3109/17435390.2013.801089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Sun P, Bao Y, Dou B, Song D, Li Y. Vitamin E renders protection to PC12 cells against oxidative damage and apoptosis induced by single-walled carbon nanotubes. Toxicol In Vitro. 2012;26:32–41. doi: 10.1016/j.tiv.2011.10.004. [DOI] [PubMed] [Google Scholar]
- Warheit DB, Laurence BR, Reed KL, Roach DH, Reynolds GAM, Webb TR. Comparative pulmonary toxicity assessment of single-wall carbon nanotubes in rats. Toxicol Sci. 2004;77:117–125. doi: 10.1093/toxsci/kfg228. [DOI] [PubMed] [Google Scholar]
- Watts PCP, Fearon PK, Hsu WK, Billingham NC, Kroto HW, Walton DRM. Carbon nanotubes as polymer antioxidants. J Mater Chem. 2003;13:491–495. [Google Scholar]
- Weissenberg A, Sydlik U, Peuschel H, Schroeder P, Schneider M, Schins RP, Abel J, Unfried K. Reactive oxygen species as mediators of membrane-dependent signaling induced by ultrafine particles. Free Radic Biol Med. 2010;49:597–605. doi: 10.1016/j.freeradbiomed.2010.05.011. [DOI] [PubMed] [Google Scholar]
- WHO. Transgenic animal mutagenicity assays. Geneva: World Health Organization; 2006. [Google Scholar]
- Wirnitzer U, Herbold B, Voetz M, Ragot J. Studies on the in vitro genotoxicity of baytubes, agglomerates of engineered multi-walled carbon-nanotubes (MWCNT) Toxicol Lett. 2009;186:160–165. doi: 10.1016/j.toxlet.2008.11.024. [DOI] [PubMed] [Google Scholar]
- Wu P, Yuan SS, Ho CC, Hsieh WY, Hong QS, Yu SL, Chen W, Chen HY, Wang CD, Li KC, et al. Focal amplification of HOXD-harboring chromosome region is implicated in multiple-walled carbon nanotubes-induced carcinogenicity. Nano Lett. 2013;13:4632–4641. doi: 10.1021/nl401658c. [DOI] [PubMed] [Google Scholar]
- Wyrick JJ, Roberts SA. Genomic approaches to DNA repair and mutagenesis. DNA Repair (Amst) 2015;36:146–155. doi: 10.1016/j.dnarep.2015.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Alexander DB, Futakuchi M, Numano T, Fukamachi K, Suzui M, Omori T, Kanno J, Hirose A, Tsuda H. Size- and shape-dependent pleural translocation, deposition, fibrogenesis, and mesothelial proliferation by multiwalled carbon nanotubes. Cancer Sci. 2014;105:763–769. doi: 10.1111/cas.12437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Futakuchi M, Shimizu H, Alexander DB, Yanagihara K, Fukamachi K, Suzui M, Kanno J, Hirose A, Ogata A, et al. Multi-walled carbon nanotubes translocate into the pleural cavity and induce visceral mesothelial proliferation in rats. Cancer Sci. 2012;103:2045–2050. doi: 10.1111/cas.12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita K, Yoshioka Y, Higashisaka K, Morishita Y, Yoshida T, Fujimura M, Kayamuro H, Nabeshi H, Yamashita T, Nagano K, et al. Carbon nanotubes elicit DNA damage and inflammatory response relative to their size and shape. Inflammation. 2010;33:276–280. doi: 10.1007/s10753-010-9182-7. [DOI] [PubMed] [Google Scholar]
- Yang H, Liu C, Yang D, Zhang H, Xi Z. Comparative study of cytotoxicity, oxidative stress and genotoxicity induced by four typical nanomaterials: the role of particle size, shape and composition. J Appl Toxicol. 2009;29:69–78. doi: 10.1002/jat.1385. [DOI] [PubMed] [Google Scholar]
- Ye R, Wang S, Wang J, Luo Z, Peng Q, Cai X, Lin Y. Pharmacokinetics of CNT-based drug delivery systems. Curr Drug Metab. 2013;14:910–920. doi: 10.2174/138920021131400113. [DOI] [PubMed] [Google Scholar]
- Yegles M, Janson X, Dong HY, Renier A, Jaurand MC. Role of fibre characteristics on cytotoxicity and induction of anaphase/telophase aberrations in rat pleural mesothelial cells in vitro: correlations with in vivo animal findings. Carcinogenesis. 1995;16:2751–2758. doi: 10.1093/carcin/16.11.2751. [DOI] [PubMed] [Google Scholar]
- Yu KN, Kim JE, Seo HW, Chae C, Cho MH. Differential toxic responses between pristine and functionalized multiwall nanotubes involve induction of autophagy accumulation in murine lung. J Toxicol Environ Health A. 2013;76:1282–1292. doi: 10.1080/15287394.2013.850137. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Yan B. Cell cycle regulation by carboxylated multiwalled carbon nanotubes through p53-independent induction of p21 under the control of the BMP signaling pathway. Chem Res Toxicol. 2012;25:1212–1221. doi: 10.1021/tx300059m. [DOI] [PubMed] [Google Scholar]
- Zhu L, Chang DW, Dai L, Hong Y. DNA damage induced by multi-walled carbon nanotubes in mouse embryonic stem cells. Nano Lett. 2007;7:3592–3597. doi: 10.1021/nl071303v. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.