Abstract
Objective
While tobacco smoking is a well-known risk factor for head and neck squamous cell carcinoma (HNSCC), the molecular mechanisms underlying tobacco-induced HNSCC remain unclear. This study sought to comprehensively identify microRNA (miRNA) alterations and evaluate their clinical relevance in smoking-induced HNSCC pathogenesis and progression.
Materials and methods
Using small RNA-sequencing data and clinical data from 145 HNSCC patients, we performed a series of differential expression and correlation analyses to identify a panel of tobacco-dysregulated miRNAs associated with key clinical characteristics in HNSCC. We then examined the expression patterns of these miRNAs in normal epithelial cell lines following exposure to cigarette smoke extract.
Results
Our analyses revealed distinct panels of miRNAs to be dysregulated with smoking status and associated with additional clinical features, including tumor stage, metastasis, anatomic site, and patient survival. The differential expression of key miRNAs, including miR-101, miR-181b, miR-486, and miR-1301, was verified in cigarette-treated epithelial cell lines, suggesting their potential roles in the early development of smoking-related HNSCCs.
Conclusion
Specific alterations in miRNA expression may be traced to tobacco use and are associated with important HNSCC clinical characteristics. Future studies of these miRNAs may be valuable for furthering the understanding and targeted treatment of smoking-associated HNSCC.
Keywords: Smoking, Head and Neck Neoplasms, RNA, Untranslated, MicroRNAs
Introduction
Head and neck squamous cell carcinoma (HNSCC) affects more than 650,000 people annually, with 5-year mortality rates exceeding 50% [1, 2]. Because HNSCCs are linked to several distinct risk factors, including tobacco use, alcohol consumption, and HPV infection [3], and arise in a number of different sites within the head and neck region, they collectively exhibit significant heterogeneity at the molecular and genetic level and disparate response to treatment and clinical outcome.
Tobacco is the predominant etiological agent of HNSCC, with previous studies showing that 68% of HNSCC patients have previous history of smoking [4]. However, despite strong clinical evidence implicating smoking in HNSCC, the full spectrum of molecular mechanisms underlying tobacco-induced HNSCC onset and progression have not been fully elucidated [4, 5]. Currently, it is widely accepted that tobacco smoke promotes HNSCC pathogenesis via the genotoxic effects of its constituents, including nitrosamines and polycyclic hydrocarbons [6, 7]. These carcinogens bind covalently to DNA, resulting in the formation of DNA adducts, which, if unrepaired, can activate oncogenes such as Kras and inactivate tumor suppressors such as TP53 [8, 9]. Additional studies suggest that tobacco smoke epigenetically interferes with cellular function, such as through hypermethylation of p16INK4a and E-cadherin (CDH1)[10].
In contrast, considerably less characterized are the specific effects of tobacco smoke on the expression and function of microRNAs (miRNAs), a class of small non-coding RNA molecules involved in RNA silencing and post-transcriptional gene regulation that have been increasingly viewed as key players in cancer development and progression [11]. Recent studies have heavily implicated miRNA dysregulation in HNSCC, suggesting miRNAs circulating in the blood, serum, or plasma may potentially serve as promising biomarkers for oral cancer [12]. Past findings indicate that differences in tumor etiology, clinical phenotype, and patient prognosis may be associated with the expression patterns of specific miRNAs [13-15]. Our previous investigation of non-coding RNAs in HNSCC, for instance, confirmed that miRNAs including miR-196, miR-21, and miR-31 are consistently upregulated in HNSCC tumors, while other miRNAs, including miR-375, the miR-29 family, miR-204, and miR-99, are widely downregulated in HNSCC, irrespective of etiology [15]. Yet in an additional study, we found that miR-30a and miR-934 were upregulated only specifically in alcohol-associated HNSCC [16]. These results suggest that a distinct panel of miRNAs may also be regulated by tobacco smoke and mediate previously unknown functions in the pathogenesis of smoking-induced HNSCC.
In this study, we aimed to comprehensively identify and analyze the expression patterns of miRNAs that are specifically regulated by tobacco smoke in the context of HNSCC pathogenesis and progression. Utilizing small RNA-sequencing data and clinical data from 145 HNSCC patients in the TCGA database, we performed differential expression and correlation analyses to identify smoking-dysregulated miRNAs that are also associated with clinical characteristics such as patient survival, clinical stage, tumor site, and metastasis. Finally, we verified the dysregulation of these smoking-associated miRNAs in normal epithelial cell lines treated with cigarette smoke extract, to further establish their potential involvement in the early stages of smoking-induced HNSCC pathogenesis.
Materials and Methods
miRNA-sequencing datasets and clinical data
Level 3-normalized miRNA expression datasets for 145 HNSCC patients were obtained on 12 April 2016 from The Cancer Genome Atlas (TCGA) (https://tcga-data.nci.nih.gov/tcga). The TCGA barcodes for all patients whose datasets were used in this study are provided in Supplementary Table 1. Patient clinical data, including smoking status, were downloaded from the TCGA Data Portal on 20 March 2015 (https://tcga-data.nci.nih.gov/tcga/findArchives.htm).
miRNA differential expression analyses
HPV(-) patients were separated into two cohorts based on reported smoking history: HNSCC current smokers and normal lifelong nonsmokers. miRNA read counts were extracted from the TCGA Level 3 gene expression files. The read count tables were imported into edgeR v3.0 (http://www.bioconductor.org/packages/release/bioc/html/edgeR.html) [15], and lowly expressed miRNAs (counts-per-million < 1 in more than one-half of samples) were filtered from the analysis. Following TMM (trimmed mean of M values) normalization, pairwise designs were applied to identify significantly differentially expressed miRNAs between the two cohorts. Candidate miRNAs were filtered based on cutoffs for fold change magnitude (≥ 4 between two cohorts) and false discovery rate (FDR) ≤ 10–4. CircosPlot software was used to produce a circular representation of the genome-wide expression of candidate miRNAs [17].
Association of miRNA expression with clinical covariates and patient survival
Candidate miRNAs dysregulated between HPV(−) Smoking HNSCC and HPV(−) Nonsmoking Normal cohorts were evaluated for clinical significance. Employing the Kruskal-Wallis test, we investigated miRNA association with anatomic neoplasm subdivision, clinical and pathologic stages, lymphovascular and perineural invasion, and pathological nodal extracapsular spread, using clinical data and miRNA expression values (cpm) from HPV(−) smoking HNSCC patients. In clinical stage analyses, patients with Stage I and II were classified as “Early Stage” and patients with Stage III and IV were classified as “Late Stage.” Patients with no available information for a given characteristic were filtered from analyses involving that variable.
Survival analyses were performed using the Cox proportional hazards model, with miRNA expression in HNSCC tumors designated as a binary variable based on expression above or below the median. Because HPV status has been shown to profoundly influence molecular signatures and clinical outcomes in HNSCC, we limited our cohort to HPV(−) HNSCC Smokers to minimize confounding variables. We first performed univariate Kaplan-Meier analysis and univariate Cox regression analysis to identify candidates significantly associated with patient outcome (p < 0.05), and then performed multivariate Cox analysis to evaluate whether correlations were independent of clinical variables such as race, age, clinical stage, and status of lymphovascular invasion.
Cell culture
HaCaT, a spontaneously transformed immortal keratinocyte cell line derived from human skin, was a generous gift from Dr. Victor Nizet at the Center for Immunity, Infection, and Inflammation of the UC San Diego School of Medicine. The cells were cultured in DMEM supplemented with 10% fetal bovine serum, 2% penicillin/streptomycin, and 2% L-glutamate (GIBCO) and maintained at 37°C in a humidified 5% CO2/95% air atmosphere.
Normal, early passage, oral epithelial cell line OKF4 (derived from the floor of the mouth) was a generous gift from the Rheinwald Lab at Harvard Medical School. The cells were cultured in keratinocyte serum-free media (Life Technologies) supplemented with EGF, bovine pituitary extract, 2% L-glutamine, 2% penicillin/streptomycin, and CaCl2 and maintained at 37°C in a humidified 5% CO2/95% air atmosphere.
Cigarette treatment
The cigarette-treated media was made using Marlboro Red filter cigarettes, which were determined by the Federal Trade Commission in a 2000 report to contain 1.2 mg of nicotine per cigarette. Cigarette vapor was pulled through media using negative pressure, and the resulting extract was filter-sterilized with a 0.2 μm pore-size filter before treating cell cultures. One-time 24-hour treatment was performed with 0.1% cigarette extract.
Quantification of miRNA expression by qRT-PCR
Total RNA was isolated (Fisher Scientific) from cultured cells following treatment with cigarette. cDNA was synthesized using the QuantiMiR™ RT kit (System Biosciences) as per the manufacturer's instructions. Real-time PCR reaction mixes were created using FastStart Universal SYBR Green Master Mix (Roche Diagnostics), and run on a StepOnePlus™ Real-Time PCR System (Applied Biosystems) using the following program: 50°C for 2 min, 95°C for 10 min, 95°C for 30 s, and 60°C for 1 min, for 40 cycles. Experiments were analyzed using the ddCt method. U6 primers and a Universal Reverse Primer were used from the QuantiMiR™ RT kit, and custom primers (Integrated DNA Technologies) were ordered using the sequences listed in Supplementary Table 3.
Results
Identification of smoking-dysregulated miRNAs
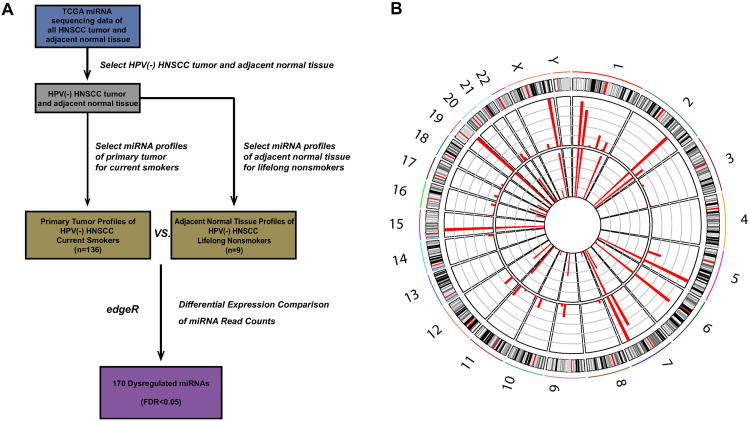
In order to identify miRNAs specifically dysregulated in smoking-related HNSCC, we examined 145 RNA-seq datasets from TCGA with available clinical data (dataset IDs in Supplementary Table 1). We aimed to observe miRNAs purely involved in smoking-related HNSCC pathogenesis by attempting to eliminate two potentially confounding factors. First, only current smokers as opposed to former smokers were used in the HNSCC smoking cohort, as former smokers are thought to possess decreased cancer risk and thereby distinct HNSCC molecular profiles based on time since smoking cessation[18]. Second, only HPV(-) patients were used in our analysis, as HPV(+) disease has been suggested to display a unique set of molecular alterations and clinical profiles compared to HNSCCs of other etiologies[19]. Using negative binomial-based differential expression testing, we compared miRNA expression between HNSCC smoking and normal lifelong nonsmoking cohorts, identifying 170 dysregulated miRNAs (FDR<0.05; Fig 1; Supplementary Table 2).
Fig. 1.
(A) Schematic illustrating the analysis approach used to identify smoking-dysregulated miRNA candidates (p<0.05, FDR < 0.05). (B) Smoking status and miRNA expression patterns. Comparison of miRNA expression between the adjacent normal tissue of HPV(-) lifelong nonsmokers (average of 9 cases, inside circle) and primary tumor of HPV(-) HNSCC current smokers (average of 136 cases, outside circle).
Clinical significance of smoking-dysregulated miRNAs in HNSCC
Recent studies suggest that even HNSCCs of the same etiology can become highly heterogeneous tumors that differ in location, pathogenesis, and treatment [6]. Therefore, we assessed the clinical and functional potential of all 170 smoking-specific miRNAs in the context of HNSCC clinical features to better understand their role in modulating tumor phenotype and clinical outcome in HNSCC. Analyses were performed using only data from HPV(-) HNSCC smokers in order to minimize the confounding influence of HPV infection on miRNA expression patterns. Specifically, we evaluated miRNA expression relative to four clinical variables in HNSCC: anatomic site, tumor stage, tumor metastasis, and patient survival.
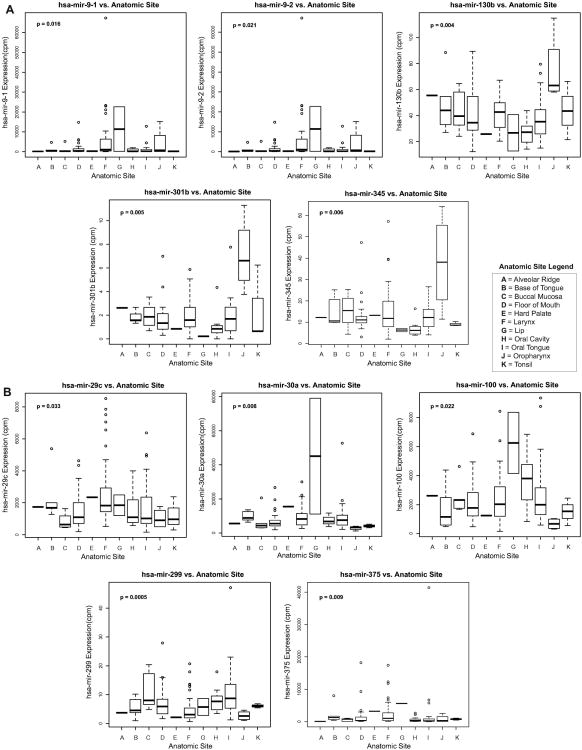
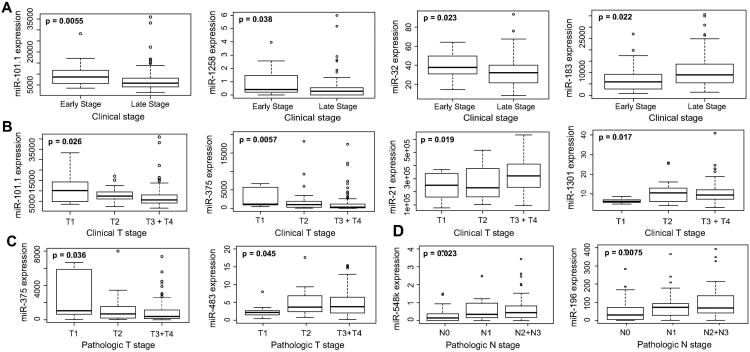
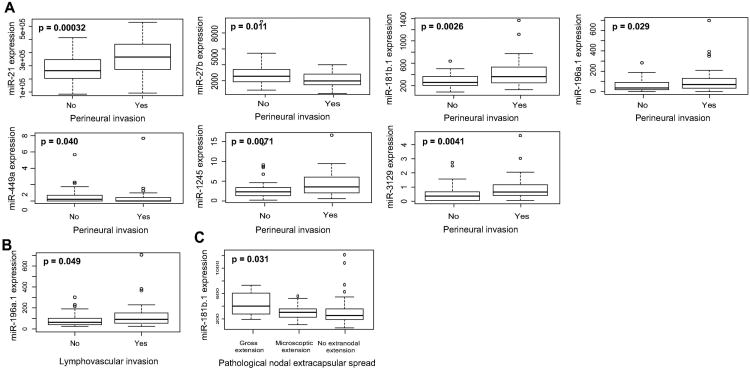
Using the Kruskal-Wallis test (p<0.05), we identified 45 miRNAs to exhibit variable expression in HPV(-) smoking-associated HNSCCs of different anatomical sites. Notably, of these 45 miRNAs, we identified 17 out of 26 upregulated miRNAs (including miR-9-1, miR-9-2, miR-103b, miR-310b, and miR-345) to exhibit significantly elevated expression in oropharyngeal tumors while all 19 downregulated miRNAs (including miR-29c, miR-30a, miR-100, miR-299, and miR-375) exhibited significantly lowered expression in oropharyngeal tumors, suggesting the potential of these miRNAs to specifically play a role in modulating malignancies of the oropharynx (Fig 2). Our analysis also revealed several miRNAs to associate significantly with clinical and pathologic stage, with downregulated miRNAs miR-101-1 and miR-375 displaying significantly reduced expression in multiple measures of higher stage (Kruskal-Wallis, p<0.05; Fig 3). Among a panel of miRNAs correlating with metastatic variables, upregulated transcripts miR-181b-1 and miR-196a-1 exhibited significantly elevated expression in tumors exhibiting perineural invasion, lymphovascular invasion, and/or nodal extracapsular spread, suggesting their potential role in mediating HNSCC metastasis (Kruskal-Wallis, p<0.05; Fig 4).
Fig. 2.
Boxplots indicating significant variation of (A) smoking-upregulated miRNA expression and (B) smoking-downregulated miRNA expression with anatomic tumor sites (Kruskal-Wallis, p < 0.05).
Fig. 3.
Boxplots associating expression of smoking-dysregulated miRNAs to (A) clinical stages, (B) clinical T stage, (C) pathologic T stage and (D) pathologic N stage (Kruskal-Wallis, p < 0.05). In clinical stage analyses, patients with Stage I and II were classified as “Early Stage” and patients with Stage III and IV were classified as “Late Stage.”
Fig. 4.
Boxplots correlating expression of smoking-dysregulated miRNAs to (A) perineural invasion (B) lymphocascular invasion and (C) pathological nodal extracapsular spread (Kruskal-Wallis, p < 0.05).
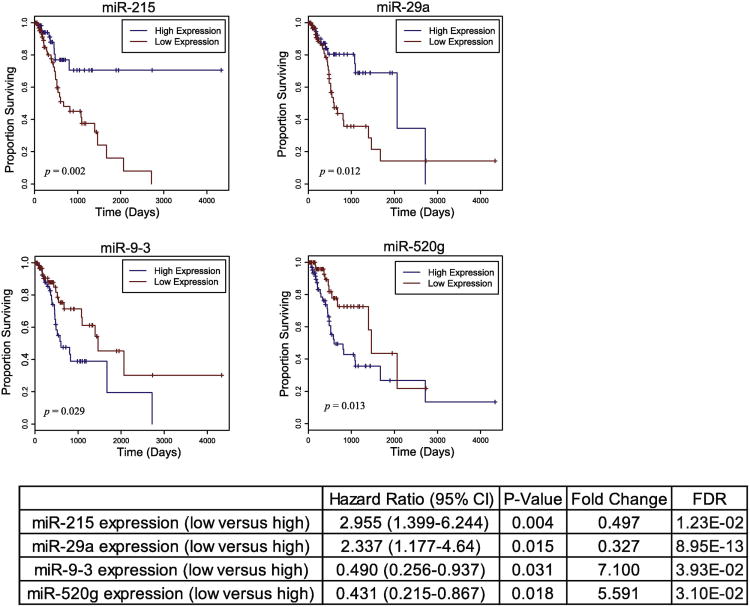
We then examined all 170 candidate miRNAs for association between expression level and overall survival among HPV(-) HNSCC smoking patients with available data (n=140) using univariate Cox regression analysis. Modeling miRNA expression as a binary variable (high/low), we found that relative high expression of two upregulated miRNAs (miR-9-3 and miR-520g) and relative low expression of two downregulated miRNAs (miR-215 and miR-29a) significantly associated with poor patient survival (p<0.05, Fig 5). Multivariate Cox regression analyses were further performed on these genes to ensure that their association with patient outcome was independent of prognostic factors such as race, age, clinical stage, and status of lymphovascular invasion (Supplementary Table 3).
Fig. 5.
Kaplan-Meier curves depicting survival outcomes based on relative high and low expression of candidate miRNAs dysregulated by smoking (p<0.05). Hazard ratio with 95% confidence interval are presented for each survival correlation.
In vitro validation of smoking-induced miRNA dysregulation
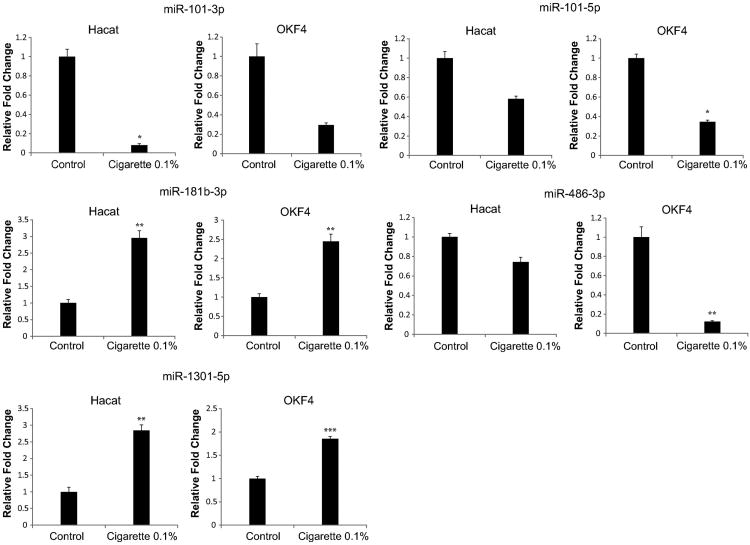
In order to verify the association of our most clinically relevant miRNAs with smoking, we assessed their expression levels in the normal human epithelial cell lines HaCaT and OKF4 before and after exposure to cigarette smoke extract. Normal epithelial cells were used in order to evaluate whether smoking directly promotes dysregulation of the miRNAs as an early event in malignant transformation. In order to remain consistent with our analysis of miRNA dysregulation in current smokers and evaluate the immediate dysregulation of miRNAs after cigarette exposure, we performed a 24-hour treatment on our cells with 0.1mM cigarette smoke extract. Even with short-term exposure, we found miR-101-1, which associated with clinical stage, to be downregulated in both HaCaT and OKF4, consistent with the TCGA analysis. Similarly, we found miR-1301, which associated with anatomic site, miR-181b-1, which associated with metastasis, and miR-486 to exhibit upregulation in both cell lines, verifying the expected direction of dysregulation from TCGA data (Fig 6).
Fig 6.
qRT-PCR verifies that 0.1% cigarette treatment dysregulates miR-101-3p, miR-101-5p, miR-181b-3p, miR-486-3p, and miR-1301-5p in HaCaT and OKF4. All bar graphs are presented with mean and error bars representing standard deviations. *p< 0.05, **p<0.01, ***p<0.001, Student's t-test.
Discussion
To the best of our knowledge, we are the first to globally profile miRNA alterations in smoking-specific HNSCC. Previous profiles of miRNA expression in HNSCC suggest that head and neck malignancies display heavily etiology-dependent molecular landscapes [16, 20, 21]. Furthermore, studies on smoking-induced miRNA dysregulation in lung and rectal carcinomas suggest that smoking status plays a significant role in producing etiology-specific miRNA alterations, and therefore may do so in HNSCC as well [22, 23].
Using RNA-seq data of 145 patients from TCGA, we identified 170 miRNAs to be differentially expressed between HNSCC Current Smokers vs. Normal Lifelong Nonsmokers. Next, in order to further assess the roles of our miRNAs in promoting varied phenotypes of smoking-specific HNSCC, we associated expression of all 170 miRNAs with HNSCC clinical characteristics. Our studies revealed distinct sets of miRNAs to be significantly implicated in progression of different clinical features, including tumor stage, metastasis, anatomic site, and patient survival.
Our analysis indicated smoking-downregulated miRNAs miR-101-1 and miR-375 in particular to display significant decrease of expression with higher tumor stage. This is especially important, considering that HNSCC of early clinical stages (stages I and II) are known to display 5-year survival rates between 70-90%, relatively independent of tumor sublocation, while HNSCCs of late clinical stages (stages III and IV) are limited to a 50% complete response rate, with significantly varied survival based on tumor histology and sublocation. Previously, miR-101 has been recognized for widespread downregulation in multiple cancers including breast, gastric, thyroid, and head and neck malignancies, as well as for its ability to inhibit cell proliferation, migration, and invasion [24-26]. In HNSCC in particular, miR-101 loss is thought to induce these phenotypes by upregulating histone methyltransferase EZH2, which in turn downregulates rap1GAP, a critical tumor suppressor downregulated in many aggressive cancers [27]. Similarly, among induction of various cancer phenotypes, low miR-375 expression has been found to associate with higher clinical stage in pancreatic cancer and poor outcome and increased metastasis in HNSCC, potentially by directly upregulating the oncogene AEG-1/MTDH which interferes with signaling pathways including PI3K/Akt, NF-κB, Wnt/β-catenin and MAPK [28-31]. Our findings therefore support the putative role of miR-101-1 and miR-375 in suppressing progression of HNSCC and modulating stage-associated phenotypes.
Metastasis is one of the most important factors in HNSCC treatment, with past studies indicating even a single micrometastasis to a lymph node to produce a significant difference in HNSCC recurrence and survival [32]. Our correlations of our 170 smoking-dysregulated miRNAs to perineural and lymphovascular invasion, two primary routes of HNSCC metastasis, and extracapsular nodal spread revealed upregulation of miR-181b-1 and miR-196a-1 to significantly associate with multiple metastatic factors, suggesting their ability to modulate metastatic-specific phenotypes in smoking-related HNSCC. Past studies indicate miR-181b to be significantly upregulated during progression of oral leukoplakia to invasive carcinoma, as well as in oral squamous cell carcinoma patients with lymph node metastasis, substantiating our present findings [33, 34]. miR-196a has also been found to exhibit upregulation in head and neck, ovarian, gastric, pancreatic, and colorectal cancers, possibly through interaction with the HOX family of transcription factors, but the exact mechanisms of both miR-181b and miR-196a functionality in HNSCC remain to be studied [35-38].
The various anatomic locations at which HNSCCs can occur are a significant contributor to its heterogeneity. Our study highlights 10 smoking-associated miRNAs exhibiting differential expression based on tumor site. Additionally, we found that 65% of smoking-upregulated miRNAs and all smoking-downregulated miRNAs exhibit significantly higher or lower expression, respectively, in tumors of the oropharynx, suggesting that oropharyngeal carcinomas may be more significantly modulated by these miRNAs. A previous finding that smoking contributes to 42% of oropharyngeal cases worldwide, in contrast to 16% from alcohol usage, is further suggestive of a correlation between smoking-induced alterations and oropharyngeal malignancies [39].
Analysis of all smoking-dysregulated miRNAs with respect to patient survival revealed 4 miRNAs that significantly associated with patient outcome, including miR-9-3 and miR-29a, members of the miR-9 and miR-29 families, respectively, that had other members implicated in anatomic site and tumor stage. Notably, high expression of miR-9-3 associated with poor patient survival, but past evidence on the role of miR-9 is conflicting. While studies of breast and liver cancer suggest miR-9 to be upregulated in carcinomas and to promote cell motility by targeting CDH1, interestingly, head and neck cancer studies have revealed significant miR-9-3 methylation in oral carcinomas and decreased cell proliferation and invasion with miR-9 overexpression [40-42]. Our data, conflicting with previous studies on miR-9 involvement in HNSCC, suggests that various genetic and epigenetic factors must be further studied to explain the pathways of the miRNA's functionality in different malignancies as well as within HNSCC itself.
We found miR-101-1, miR-181b-1, miR-486, and miR-1301 to be consistently upregulated in normal epithelial cell lines treated with cigarette smoke extract, suggesting the potential of these miRNAs to participate in the early stages of HNSCC pathogenesis and progression. While our study presents a comprehensive overview of smoking-induced miRNA dysregulation in HNSCC, some phenomena still remain unexplained, most importantly a lack of more overlap in miRNAs involved in proliferation, metastasis, and survival. For example, in vitro and clinical studies have identified miR-375 to associate with metastasis and outcome, yet our data only revealed a correlation with stage [28]. Further studies integrating additional datasets outside of TCGA and more comprehensive clinical histories could be used to validate our present findings and provide more meaningful conclusions on etiology-specific phenomena. Overall, our findings provide further evidence on the massive heterogeneity of HNSCCs, even among cases of the same etiology, and suggest the necessity to further investigate the genetic and transcriptional mediators involved in pathogenesis and progression.
Supplementary Material
Highlights.
RNA-seq analysis implicated 170 microRNAs in smoking-induced HNSCC
Distinct panels of microRNAs correlated with tumor site, stage, and metastasis
4 microRNAs associated with patient survival
In vitro treatment with cigarette smoke extract verified microRNA dysregulation
Acknowledgments
Funding: This work was supported by funding from the National Institutes of Health, grant number DE023242 to W.M.O. and by Academic Senate grants from the University of California to W.M.O. and J.W.R.
Role of the Funding Source: Federally funded research to promote the understanding of head and neck cancer.
Footnotes
Abbreviations: HNSCC (head and neck squamous cell carcinoma), miRNA (microRNA), HPV (human papillomavirus), TCGA (The Cancer Genome Atlas)
Conflict of Interest Statement: The authors have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer JClin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Braakhuis BJ, Leemans CR, Brakenhoff RH. Expanding fields of genetically altered cells in head and neck squamous carcinogenesis. Semin Cancer Biol. 2005;15:113–20. doi: 10.1016/j.semcancer.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 3.Galbiatti AL, Padovani-Junior JA, Maniglia JV, Rodrigues CD, Pavarino EC, Goloni-Bertollo EM. Head and neck cancer: causes, prevention and treatment. Braz J Otorhinolaryngol. 2013;79:239–47. doi: 10.5935/1808-8694.20130041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hashibe M, Brennan P, Chuang SC, Boccia S, Castellsague X, Chen C, et al. Interaction between tobacco and alcohol use and the risk of head and neck cancer: pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. Cancer Epidemiol Biomarkers Prev. 2009;18:541–50. doi: 10.1158/1055-9965.EPI-08-0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Friemel J, Foraita R, Gunther K, Heibeck M, Gunther F, Pflueger M, et al. Pretreatment oral hygiene habits and survival of head and neck squamous cell carcinoma (HNSCC) patients. BMC Oral Health. 2016;16:33. doi: 10.1186/s12903-016-0185-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pai SI, Westra WH. Molecular pathology of head and neck cancer: implications for diagnosis, prognosis, and treatment. Annu Rev Pathol. 2009;4:49–70. doi: 10.1146/annurev.pathol.4.110807.092158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Phillips DH. DNA adducts in human tissues: biomarkers of exposure to carcinogens in tobacco smoke. Environ Health Perspect. 1996;104(3):453–8. doi: 10.1289/ehp.96104s3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brennan JA, Boyle JO, Koch WM, Goodman SN, Hruban RH, Eby YJ, et al. Association between cigarette smoking and mutation of the p53 gene in squamous-cell carcinoma of the head and neck. N Engl J Med. 1995;332:712–7. doi: 10.1056/NEJM199503163321104. [DOI] [PubMed] [Google Scholar]
- 9.Khariwala SS, Hatsukami D, Hecht SS. Tobacco carcinogen metabolites and DNA adducts as biomarkers in head and neck cancer: potential screening tools and prognostic indicators. Head Neck. 2012;34:441–7. doi: 10.1002/hed.21705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hasegawa M, Nelson HH, Peters E, Ringstrom E, Posner M, Kelsey KT. Patterns of gene promoter methylation in squamous cell cancer of the head and neck. Oncogene. 2002;21:4231–6. doi: 10.1038/sj.onc.1205528. [DOI] [PubMed] [Google Scholar]
- 11.Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, et al. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–8. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 12.Troiano G, Boldrup L, Ardito F, Gu X, Lo Muzio L, Nylander K. Circulating miRNAs from blood, plasma or serum as promising clinical biomarkers in oral squamous cell carcinoma: A systematic review of current findings. Oral Oncol. 2016;63:30–7. doi: 10.1016/j.oraloncology.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 13.Sethi N, Wright A, Wood H, Rabbitts P. MicroRNAs and head and neck cancer: reviewing the first decade of research. Eur J Cancer. 2014;50:2619–35. doi: 10.1016/j.ejca.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 14.Zhang P, Mirani N, Baisre A, Fernandes H. Molecular heterogeneity of head and neck squamous cell carcinoma defined by next-generation sequencing. Am J Pathol. 2014;184:1323–30. doi: 10.1016/j.ajpath.2014.01.028. [DOI] [PubMed] [Google Scholar]
- 15.Zou AE, Zheng H, Saad MA, Rahimy M, Ku J, Kuo SZ, et al. The non-coding landscape of head and neck squamous cell carcinoma. Oncotarget. 2016;7:51211–22. doi: 10.18632/oncotarget.9979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saad MA, Kuo SZ, Rahimy E, Zou AE, Korrapati A, Rahimy M, et al. Alcohol-dysregulated miR-30a and miR-934 in head and neck squamous cell carcinoma. Mol Cancer. 2015;14:181. doi: 10.1186/s12943-015-0452-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krzywinski M, Schein J, Birol I, Connors J, Gascoyne R, Horsman D, et al. Circos: an information aesthetic for comparative genomics. Genome Res. 2009;19:1639–45. doi: 10.1101/gr.092759.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.La Vecchia C, Franceschi S, Bosetti C, Levi F, Talamini R, Negri E. Time since stopping smoking and the risk of oral and pharyngeal cancers. J Natl Cancer Inst. 1999;91:726–8. doi: 10.1093/jnci/91.8.726a. [DOI] [PubMed] [Google Scholar]
- 19.Lewis A, Kang R, Levine A, Maghami E. The New Face of Head and Neck Cancer: The HPV Epidemic. Oncology (Williston Park) 2015;29:616–26. [PubMed] [Google Scholar]
- 20.Lajer CB, Garnaes E, Friis-Hansen L, Norrild B, Therkildsen MH, Glud M, et al. The role of miRNAs in human papilloma virus (HPV)-associated cancers: bridging between HPV-related head and neck cancer and cervical cancer. Br J Cancer. 2012;106:1526–34. doi: 10.1038/bjc.2012.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramdas L, Giri U, Ashorn CL, Coombes KR, El-Naggar A, Ang KK, et al. miRNA expression profiles in head and neck squamous cell carcinoma and adjacent normal tissue. Head Neck. 2009;31:642–54. doi: 10.1002/hed.21017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Russ R, Slack FJ. Cigarette-Smoke-Induced Dysregulation of MicroRNA Expression and Its Role in Lung Carcinogenesis. Pulm Med. 2012;2012:791234. doi: 10.1155/2012/791234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mullany LE, Herrick JS, Wolff RK, Stevens JR, Slattery ML. Association of cigarette smoking and microRNA expression in rectal cancer: Insight into tumor phenotype. Cancer Epidemiol. 2016;45:98–107. doi: 10.1016/j.canep.2016.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou X, Xia Y, Li L, Zhang G. MiR-101 inhibits cell growth and tumorigenesis of Helicobacter pylori related gastric cancer by repression of SOCS2. Cancer Biol Ther. 2015;16:160–9. doi: 10.4161/15384047.2014.987523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang C, Lu S, Jiang J, Jia X, Dong X, Bu P. Hsa-microRNA-101 suppresses migration and invasion by targeting Rac1 in thyroid cancer cells. Oncol Lett. 2014;8:1815–21. doi: 10.3892/ol.2014.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hao Y, Gu X, Zhao Y, Greene S, Sha W, Smoot DT, et al. Enforced expression of miR-101 inhibits prostate cancer cell growth by modulating the COX-2 pathway in vivo. Cancer Prev Res (Phila) 2011;4:1073–83. doi: 10.1158/1940-6207.CAPR-10-0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Banerjee R, Mani RS, Russo N, Scanlon CS, Tsodikov A, Jing X, et al. The tumor suppressor gene rap1GAP is silenced by miR-101-mediated EZH2 overexpression in invasive squamous cell carcinoma. Oncogene. 2011;30:4339–49. doi: 10.1038/onc.2011.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harris T, Jimenez L, Kawachi N, Fan JB, Chen J, Belbin T, et al. Low-level expression of miR-375 correlates with poor outcome and metastasis while altering the invasive properties of head and neck squamous cell carcinomas. Am J Pathol. 2012;180:917–28. doi: 10.1016/j.ajpath.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nohata N, Hanazawa T, Kikkawa N, Mutallip M, Sakurai D, Fujimura L, et al. Tumor suppressive microRNA-375 regulates oncogene AEG-1/MTDH in head and neck squamous cell carcinoma (HNSCC) J Hum Genet. 2011;56:595–601. doi: 10.1038/jhg.2011.66. [DOI] [PubMed] [Google Scholar]
- 30.Shi X, Wang X. The role of MTDH/AEG-1 in the progression of cancer. Int J Clin Exp Med. 2015;8:4795–807. [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou J, Song S, Cen J, Zhu D, Li D, Zhang Z. MicroRNA-375 is downregulated in pancreatic cancer and inhibits cell proliferation in vitro. Oncol Res. 2012;20:197–203. doi: 10.3727/096504013x13589503482734. [DOI] [PubMed] [Google Scholar]
- 32.Thakare E, Gawande M, Chaudhary M, Seralathan M, Kannan K. Detection of micrometastasis in lymph nodes of oral squamous cell carcinoma: A comparative study. J Oral Maxillofac Pathol. 2013;17:374–80. doi: 10.4103/0973-029X.125202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Irani S. miRNAs Signature in Head and Neck Squamous Cell Carcinoma Metastasis: A Literature Review. J Dent (Shiraz) 2016;17:71–83. [PMC free article] [PubMed] [Google Scholar]
- 34.Yang CC, Hung PS, Wang PW, Liu CJ, Chu TH, Cheng HW, et al. miR-181 as a putative biomarker for lymph-node metastasis of oral squamous cell carcinoma. J Oral Pathol Med. 2011;40:397–404. doi: 10.1111/j.1600-0714.2010.01003.x. [DOI] [PubMed] [Google Scholar]
- 35.Darda L, Hakami F, Morgan R, Murdoch C, Lambert DW, Hunter KD. The role of HOXB9 and miR-196a in head and neck squamous cell carcinoma. PLoS One. 2015;10:e0122285. doi: 10.1371/journal.pone.0122285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang B, Li SZ, Ma L, Liu HL, Liu J, Shao JJ. Expression and mechanism of action of miR-196a in epithelial ovarian cancer. Asian Pac J Trop Med. 2016;9:1105–10. doi: 10.1016/j.apjtm.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 37.Li HL, Xie SP, Yang YL, Cheng YX, Zhang Y, Wang J, et al. Clinical significance of upregulation of mir-196a-5p in gastric cancer and enriched KEGG pathway analysis of target genes. Asian Pac J Cancer Prev. 2015;16:1781–7. doi: 10.7314/apjcp.2015.16.5.1781. [DOI] [PubMed] [Google Scholar]
- 38.Huang F, Tang J, Zhuang X, Zhuang Y, Cheng W, Chen W, et al. MiR-196a promotes pancreatic cancer progression by targeting nuclear factor kappa-B-inhibitor alpha. PLoS One. 2014;9:e87897. doi: 10.1371/journal.pone.0087897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Danaei G, Vander Hoorn S, Lopez AD, Murray CJ, Ezzati M Comparative Risk Assessment collaborating g. Causes of cancer in the world: comparative risk assessment of nine behavioural and environmental risk factors. Lancet. 2005;366:1784–93. doi: 10.1016/S0140-6736(05)67725-2. [DOI] [PubMed] [Google Scholar]
- 40.Ma L, Young J, Prabhala H, Pan E, Mestdagh P, Muth D, et al. miR-9, a MYC/MYCN-activated microRNA, regulates E-cadherin and cancer metastasis. Nat Cell Biol. 2010;12:247–56. doi: 10.1038/ncb2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Minor J, Wang X, Zhang F, Song J, Jimeno A, Wang XJ, et al. Methylation of microRNA-9 is a specific and sensitive biomarker for oral and oropharyngeal squamous cell carcinomas. Oral Oncol. 2012;48:73–8. doi: 10.1016/j.oraloncology.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu J, Luo H, Liu X, Peng Y, Zhang B, Wang L, et al. miR-9 targets CXCR4 and functions as a potential tumor suppressor in nasopharyngeal carcinoma. Carcinogenesis. 2014;35:554–63. doi: 10.1093/carcin/bgt354. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.