SUMMARY
The global epidemic of obesity and its associated chronic diseases is largely attributed to an imbalance between caloric intake and energy expenditure. While physical exercise remains the best solution, the development of muscle-targeted “exercise mimetics” may soon provide a pharmaceutical alternative to battle an increasingly sedentary life style. At the same time, these advances are fueling a raging debate on their escalating use as performance enhancing drugs in high profile competitions such as the Olympics.
The physically active hunter-gatherer existence of ancient man with its intermittent food intake forged genomic adaptations reflecting physiologic and metabolic elements of a fit lifestyle. As predatory primates, humans also evolved endurance running as a way to track down and weaken prey by forced exhaustion (Bramble and Lieberman, 2004). Like most predatory animals, humans are able to accommodate cycles of feast and famine, yet in modern times a progressively more sedentary lifestyle allows us to acquire energy (i.e. food) without the need to expend energy. As a result, in absence of daily exercise, the excess calories not burned are turned into fat, taking our species down the road of a global epidemic of obesity and obesity-associated diseases (Chakravarthy and Booth, 2004). Collectively, the USA alone is about 5 billion pounds overweight (calculated from data in the 2015 United States health report, (CDC, 2015)). Add a conservative 5 billion pounds for the rest of the world and we find ourselves heading into a non-reversible medical catastrophe. Ironically, the solution to this global crisis is rather simple: physical exercise. Paradoxically, as the obesity epidemic expands, the most gifted athletes are turning to performance enhancing drugs as a means to gain a competitive edge. Because of the local, national and global visibility of sports, it is not uncommon for individuals, coaches, and even governments to support sophisticated doping programs that more frequently turn into doping scandals. Witness the recent IOC ruling banning over 100 Russian athletes from competing in the Rio Olympics (IOC, 2016).
In regards to health, exercise is widely accepted to prevent or mitigate metabolic diseases such as obesity, type 2 diabetes, metabolic syndrome, and cardiovascular diseases and in some cases even outperforming available drugs (Pedersen and Saltin, 2015) (Knowler et al., 2002). Although the exact molecular mechanism(s) underlying the beneficial effects of exercise are not fully understood (Neufer et al., 2015), it is generally believed that a cascade of events in skeletal muscle initiates the process. During physical exercise, skeletal muscle burns significant amounts of carbohydrates and lipids, contributing to over 90% of total energy expenditure in active states; this is key to achieve caloric balance. Exercise has also been shown to positively impact diseases that lack apparent connection with energy metabolism in muscle, including cancer, neurodegenerative diseases, and mental disorders (Warburton et al., 2006) (Neufer et al., 2015). Muscle-secreted factors (myokines) facilitate crosstalk with other tissues including the brain, liver, gut and adipose depots, and likely play a role in the health of these tissues and their associated diseases (Pedersen and Febbraio, 2012).
Despite these well-established health benefits of exercise, participation in physical exercise is disappointingly low in patients as well as in the general population (Sherwood and Jeffery, 2000) (Carlson et al., 2010). In reality, it is difficult to mandate an entire community, country or the world to eat less and exercise more. Therefore, pharmacologic intervention, if even possible, must be considered as a potential therapeutic weapon. Accordingly, the chemistry of exercise mimetics was developed out of the science of exercise. Our view is that mitochondrial remodeling and bioenergetics are key components of exercise-induced muscle adaptation and play a major role in determining the metabolic gains of exercise. Therefore, we define exercise mimetics as pharmacologic compounds that are able to produce the benefits of fitness, such as mitochondrial remodeling effects including increased mitochondrial oxidative phosphorylation (OXPHOS) and fatty acid metabolism (Narkar et al., 2008). As proof of concept, a handful of prototype muscle-targeted exercise mimetics have been developed, offering exercise-mimicking benefits such as lower blood glucose levels, reduced inflammation and increased endurance. Notably, two of the most commonly known exercise mimetics, GW501516 and AICAR, have become widely used by endurance athletes resulting in their listing as banned substrates by the World Anti-Doping Agency.
How do they work? Are they safe? And what are their therapeutic societal implications?
Exercise-induced muscle remodeling
Skeletal muscle is broadly composed of two different types of myofibers, which based on their metabolic properties are known as oxidative and glycolytic fibers. Oxidative fibers are richer in mitochondria compared to their glycolytic counterparts, have a higher myoglobin content (the intramuscular oxygen carrier) and are more densely vascularized, thus their deep red appearance. They also contain more lipid metabolizing enzymes and are highly capable of utilizing both glucose and fatty acids as energy sources (Schiaffino and Reggiani, 2011).
One important feature of skeletal muscle is that it is highly adaptive to external demand, the best known of which is exercise. Exercise can significantly remodel glycolytic fibers to become more oxidative: that is to produce more myoglobin, increase lipid metabolism and enhance mitochondrial biogenesis and angiogenesis (Holloszy, 1967) (Hawley et al., 2014). Indeed, this adaptive potential is how training works. However, because the benefits are adaptive they are also transient and recede in the absence of continued training, as demonstrated in a “wheel-lock” study in which rats showed acute increases in lipid synthesis and fat mass upon cessation of 21 days of voluntary wheel running (Kump and Booth, 2005). This underpins the dilemma of the obesity epidemic whose hallmark is self re-enforcing sedentary behavior. In the past two decades, significant progress has been made in understanding the molecular mechanisms of adaptation, which has made it possible to identify several promising targets for “exercise mimetic” therapies (Fan et al., 2013).
Although how exercise training promotes muscle remodeling remains elusive, it appears in part to involve stress-induced adaptive improvements, a process known as “hormesis”. In addition to the well-known mechanical damage-induced muscle repair and growth, physical exercise causes a mild energetic stress in the muscle by its consumption of ATP and other energy substrates such as NADH. Exercise thus results in an increase in the signaling molecules AMP and NAD+ that in turn activate the metabolic sensors AMPK (AMP-activated protein kinase) and SIRT1 (Sirtuin 1). AMPK and SIRT1 respectively phosphorylate and deacetylate target proteins and promote oxidative remodeling in muscle (Jager et al., 2007; Lagouge et al., 2006). Another type of exercise-induced stressor is free radicals (such as reactive oxygen species or ROS) produced by muscle contraction, which act as important signaling molecules and play a critical role in regulating muscle remodeling. Thus, if the production of free radicals during exercise is neutralized by antioxidants, the health benefits of exercise are greatly diminished (Ristow et al., 2009).
No matter what the initial signaling mechanism, exercise-induced oxidative muscle remodeling ultimately tracks to the remodeling of a network of genes involved in mitochondrial biogenesis, antioxidant defense, angiogenesis, and fatty acid oxidation, and the repression of glycolytic genes.
Nuclear Receptors and the genomic control of exercise
One of the best-known genomic regulators of exercise-induced muscle remodeling is PCG1α, first identified as an activator of the nuclear receptor PPARγ the master transcription factor for adipose tissue and fat remodeling (Puigserver et al., 1998) and later found to interact with many nuclear receptors including all ERRs and PPARs. PGC1α is commonly expressed in high energy-demanding tissues such as heart, muscle, and brown adipose tissue (Wu et al., 1999). Exercise-induced activation of either AMPK or SIRT1 in muscle is known to activate PGC1α where it drives oxidative fiber remodeling by activating pro-oxidative transcription factors (Lagouge et al., 2006; Narkar et al., 2008). While exercise also promotes the expression of PGC1α, muscle mitochondrial biogenesis occurs in the absence of PGC1α to a similar level as in the wild-type controls (Rowe et al., 2012), suggesting that its function can be compensated by the closely related PGC1β. Thus, skeletal muscle only displays severe energetic and mitochondrial defects when both PGC1α and PGC1β are deleted (Zechner et al., 2010).
While PCG1α/β are important, other critical co-factors contributing to exercise-induced transcriptional regulation include the transcriptional co-repressors RIP140 (receptor-interacting protein 140) and NCOR1 (nuclear receptor co-repressor 1) (Fan et al., 2013). RIP140 is preferentially expressed in glycolytic myofibers and its ablation increases mitochondrial biogenesis, myoglobin content, and fatty acid oxidation (Seth et al., 2007). While exercise does not affect RIP140 expression, it induces its translocation from the nucleus to the cytoplasm, thereby decreasing its repression of target genes. The expression of NCOR1 is similar in both oxidative and glycolytic myofibers but is significantly decreased during exercise. Similar to RIP140, the ablation of muscle NCOR1 results in the oxidative remodeling of glycolytic myofibers (Yamamoto et al., 2011). As RIP140 and NCOR1 repress similar sets of genes that are activated by PGC1α, they may act in concert with PGC1α in regulating the expression of oxidative/glycolytic genes during exercise.
The three transcriptional regulators described above are all co-factors which lack DNA-binding domains, thus to regulate target gene expression they must be recruited to key transcription factors that activate a ready-to-be-induced gene network. As summarized in a previous review (Mouchiroud et al., 2014), a number of key transcription factors have been identified that contribute to exercise-induced oxidative remodeling, including the nuclear receptors PPARδ (peroxisome proliferator-activated receptor δ), ERRα (estrogen-related receptor α), and ERRγ (Fan and Evans, 2015). PPARδ is known to play an important role in regulating fatty acid metabolism. Its overexpression in skeletal muscle is sufficient to promote the oxidative remodeling of glycolytic myofibers with the induction of not only fatty acid oxidation but also mitochondrial biogenesis. The superior endurance performance of these mice lead to their description as “marathon mice” (Wang et al., 2004). In an allied fashion both ERRα and ERRγ directly control many aspects of mitochondrial energy metabolism, including mitochondrial biogenesis, oxidative phosphorylation, fatty acid oxidation, and the TCA cycle (Dufour et al., 2007). The overexpression of ERRγ induces similar oxidative remodeling and endurance enhancement as seen in the PPARδ transgenic mice. On top of that, it also increases blood vessel development in glycolytic muscles (Narkar et al., 2011). PGC1α, RIP140, and NCOR1 interact with all three nuclear receptors and cooperatively control their transcriptional activities. The fact that PPARδ, ERRα, and ERRγ regulate similar sets of exercise-induced oxidative genes strongly suggests that they are key factors in mediating exercise-induced transcriptional changes.
As nuclear receptors have ligand-binding domains their transcriptional activities can be regulated by endogenous and synthetic ligands. Although endogenous ligands for ERRα and ERRγ remain elusive, PPARδ is known to be activated by a variety of fatty acid metabolites, many of which are produced during exercise from elevated lipolysis in fat tissues. Therefore, in addition to the transcriptional co-factors such as PGC1α, energy demand via exercise may also directly regulate the production of PPARδ ligands completing the circuit needed to release co-repressors and promote PGC1α activation.
Exercise mimetics
Our increased understanding of the molecular mechanisms of exercise-induced muscle remodeling has spurred development of drugs that stimulate exercise-mimicking effects, even in sedentary animals. These natural or synthetic compounds mimic exercise by activating some of the key regulators described above and induce genes involved in the metabolic remodeling of skeletal muscle. However, from a societal point of view, exercise mimetics have opened up a new doping dilemma. Some of these molecules and their mechanisms are described below.
AMPK activators
Activators of AMPK, the master metabolic sensor that directly responds to exercise-induced energetic stress, are currently some of the most effective exercise mimetics. Recognition of this fact led to the 2012 arrest of a sports doctor (and 9 others) of the Spanish cycling team in connection with an international network supplying the synthetic AMPK activator AICAR as a ‘next generation’ performance enhancing drug (MacMichael, 2012). AICAR has been long known for its acute effects in inducing muscle glucose uptake and fatty acid oxidation (Merrill et al., 1997). Moreover, similar to exercise, treatment with AICAR directly activates muscle AMPK, initiating a molecular cascade that induces mitochondrial biogenesis as well as fatty acid oxidation in sedentary mice (Winder et al., 2000). As a result, these mice display exercise-mimicking physiological changes including protection against diet-induced obesity, improvement of insulin sensitivity, and endurance enhancement (Narkar et al., 2008). The AMPK-activating property of AICAR is attributed to its intracellular conversion into ZMP, an endogenous metabolite that allosterically activates AMPK. Compound 14 (Cpd14), a synthetic small molecule that elevates the intracellular level of ZMP and activates AMPK has been recently developed. Similar to AICAR, treatment of obese mice with Cpd14 reduces weight gain and improves glucose tolerance, further confirming the health benefits of AMPK activation (Asby et al., 2015). In the liver, metformin, the first-line medication used to treat type-2-diabetes, activates AMPK by its direct and mild inhibition of mitochondrial complex I which reduces cellular ATP production (Foretz et al., 2014). A new complex I inhibitor R419 has recently been identified which activates AMPK much more potently than metformin, including robust induction in the muscle (Marcinko et al., 2015). Notably, R419 induces exercise-mimicking beneficial effects in the skeletal muscle of mice, including improved insulin sensitivity and endurance performance.
PPARδ ligands and the Olympic scandal
In 2015, the Russian Race Walking team included the reigning Olympic 20 kilometer champion Elena Lashmanova was suspended for testing positive for GW501516, a synthetic PPARδ ligand (Press, 2014). Clearly, GW501516 has embedded itself as part of the Olympic scandal, but how does it work? Firstly, while PPARδ can respond to multiple inputs, GW501516 alone is sufficient to powerfully induce fatty acid oxidation and energy expenditure (Narkar et al., 2008). This is probably why it is a favorite for endurance athletes. Secondly, when AICAR is co-administered with GW501516 in mice, a synergistic boost in mitochondrial biogenesis, oxidative metabolism and genes linked to these processes is observed (Narkar et al., 2008). Unfortunately though for those doping athletes, GW501516 is a very stable drug with a long half-life and thus easy to detect in blood or urine samples. Furthermore, its clinical development was suspended due to poor pharmacokinetics, inappropriate drug clearance from muscle and liver, unusual potency, systemic accumulation and non-specific activation of PPARα. However, studies in mice suggest a safe drug is possible (Wang et al., 2004) and new generations of PPARδ ligands are currently being advanced that are designed to enhance the key metabolic networks in a safe and healthful fashion.
SIRT1 activators
In addition to the widely-known AICAR and GW501516, resveratrol a natural compound found in the skins of red grapes, has also been shown to exert exercise-mimicking effects in muscle (Lagouge et al., 2006). While originally described as a direct SIRT1 activator, further studies showed that resveratrol acts through AMPK and indirectly activates SIRT1 by elevating the level of its substrate NAD+ (Canto et al., 2009; Canto et al., 2010). Nevertheless, resveratrol treatment activates muscle SIRT1 to increase PGC1α activity, leading to the triple activation of ERRα, ERRγ and PPARδ. This combined genomic boost triggers the collective induction of genes controlling mitochondrial biogenesis, fatty acid transport and oxidative metabolism. As a result, resveratrol though relatively weak, is still able to promote exercise-mimicking benefits such as protection against diet-induced obesity (Lagouge et al., 2006). Recently, the exercise-mimicking effects of SIRT1 activation have been further confirmed by animal studies using other NAD+-boosting compounds, including a natural NAD+ precursor nicotinamide riboside (NR) and PARP1 inhibitors, which enhance mitochondrial oxidative metabolism and protect against diet-induced metabolic abnormalities (Canto et al., 2012) (Pirinen et al., 2014).
REV-ERBα ligands
Other in vivo tested exercise mimetics include SR9009 and SR9011, synthetic ligands of REV-ERBα a nuclear receptor that plays a critical role in regulating circadian rhythm and energy metabolism. When activated by these compounds, REV-ERBα recruits co-repressors such as SMRT and NCOR1 and suppresses the expression of its target genes. Most metabolic genes in muscle are circadian and thus the relative amplitude of these circuits are REV-ERB dependent (Zhao et al., 2016). In the muscle, REV-ERBα is selectively expressed in oxidative myofibers and is further induced by exercise. Its overexpression in muscle increases mitochondrial biogenesis and fatty acid metabolism, while its ablation results in the reverse effects. REV-ERBα does not directly regulate mitochondrial genes but rather may induce their expression indirectly through the AMPK-SIRT1-PGC1α pathway (Canto et al., 2010), though the mechanism remains unclear. This action makes REV-ERBα an ideal candidate for the development of exercise mimetics. Indeed, in vivo SR9009 or SR9011 treatment mimics exercise by inducing mitochondrial biogenesis as well as fatty acid oxidation, and improves endurance performance (Woldt et al., 2013).
ERRγ ligands
ERRγ is another promising target for exercise mimetics because of its direct regulation of mitochondrial oxidative genes. Treatment of primary muscle cells with the synthetic ERRγ agonist GSK4716 robustly upregulates genes involved in mitochondrial biogenesis, fatty acid oxidation, and the TCA cycle, consistent with known ERRγ target genes and suggesting a direct effect (Dufour et al., 2007; Narkar et al., 2011; Rangwala et al., 2010). However, the in vivo exercise-mimicking effect of ERRγ agonism remains to be determined.
Influence on health
The exercise mimetics described above offer hope for combating the global epidemic of obesity and obesity-associated diseases. Although the mechanism is not fully understood, exercise mimetics in general induce energy expenditure without any change in activity, which allows calorie burning without physical exercise. The elevated energy expenditure is typically linked to the induction of uncoupling proteins UCP2 and UCP3, which could dissipate mitochondrial membrane potential into heat and induce energy expenditure without physical activity (Clapham et al., 2000). Secondly, they remodel the skeletal muscle to more efficiently and preferentially use fatty acid oxidation for energy production (Wang et al., 2004) (Narkar et al., 2008) (Canto et al., 2009). Fat is a more efficient means to store energy compared to carbohydrate, and represents an evolutionary adaptation that helped our ancestors survive the daily and seasonal feast-famine cycles. It also worked to power up running endurance which, in turn, became the evolutionary foundation of our nascent predatory behavior. However, in modern times, continuous food exposure and the sedentary lifestyle of modern civilization have eroded the need to survive starvation or run long distances to track down prey. As a consequence, the trait of “permanent” adiposity has become a defining feature of modern man. Therefore, the tremendous and unexpected potential of exercise mimetics to efficiently promote fat burning represents a true medical opportunity. Indeed, this ‘class’ of drugs shares the ability to protect against obesity and promote insulin sensitivity, simultaneously addressing two major health problems of a technologically advanced society.
A collateral benefit of exercise mimetics is the possible induction of muscle-secreted factors that are released into the circulation during and after exercise. Myokine production has been proposed to be at least part of the mechanism of how exercise exerts its systemic effects in tissues other than muscle. By extension, it has been shown that AICAR improves cognition and motor coordination, while both AICAR and GW501516 promote hippocampal neurogenesis and spatial memory without crossing the blood-brain barrier, suggesting their effects in the brain may indeed be mediated by secreted factors from muscle (Kobilo et al., 2011) (Kobilo et al., 2014). If druggable, such exercise-induced secreted factors offer additional targets for the development of yet a new class of exercise mimetic.
However it is noteworthy that most of the above-mentioned exercise mimetics have only been tested in animal models and it remains to be investigated whether their mitochondrial remodeling effects and health benefits translate into man. In addition, severe side effects of some of the first-generation exercise mimetics have prevented their further clinical development. Many AMPK activators directly inhibit mitochondrial ATP production, resulting in energy deficits which, in absence of clear dosing studies, could cause lactic acidosis or adversely affect tissues with high energetic demand such as the heart (Pernicova and Korbonits, 2014). Related to this concern, phenformin, an AMPK activator with a higher potency than metformin, was withdrawn by the FDA in the 1970s due to a very rare but fatal side effect of lactic acidosis (Luft et al., 1978). Increased cardiac risk and gastrointestinal disturbance are also major concerns for recurrent use of AMPK activators (Boussageon et al., 2012). GW501516, a potent and stable PPARδ agonist, showed promising effects in dyslipidemia in both animal models and human clinical trials but was later abandoned (Sahebkar et al., 2014). However, the next generation of synthetic exercise mimetics are being developed and provide great hope for therapeutic applications, such as Cpd19, which activates AMPK without inhibiting mitochondrial energy production (Asby et al., 2015), and new PPARδ agonists that are milder and more selective (Sahebkar et al., 2014). In addition, although the mechanism is not clear, natural products including the anti-oxidant (−)-epicatechin (Nogueira et al., 2011) and urolithin A (Ryu et al., 2016), a promoter of mitochondrial remodeling, have also demonstrated potential as a new class of low toxic exercise mimetics.
Influence on performance enhancement and competitive sports
Of course the most widely reported use of exercise mimetics is for performance enhancement, especially in endurance sports such as long distance and intermediate distance running, race walking, cycling, long distance swimming, cross-country skiing, and all types of "-athlons". While almost all of the compounds discussed above increase endurance when administered in mice, the exact mechanism remains elusive. It is generally believed that increased mitochondrial biogenesis plays a major role by enhancing the energetic capacity in the muscle, though other factors are likely to come into play. For example, the positive effect of ERRγ on skeletal muscle angiogenesis and the increased delivery of oxygen and nutrients similar to that of trained muscle would be a great benefit (Narkar et al., 2011). Moreover, although often overlooked in the literature, carbohydrate depletion is a major limitation for endurance capacity in both humans and rodents. Many marathon runners or cyclists experience a condition termed “hitting the wall”, which is a state of complete exhaustion of carbohydrate storage in the body, mainly glycogen. In addition to their effects on mitochondrial biogenesis, most of the exercise mimetics described above also increase fatty acid oxidation, thereby shifting the energy source from carbohydrates to fatty acids during exercise, and sparing the vital carbohydrates to extend endurance exercise. However, it remains unclear how reciprocal metabolic regulation occurs and to what extent such a metabolic shift contributes to exercise mimetic-induced endurance.
Although most of the described exercise mimetics have only been tested in animal models, the molecular pathways such as mitochondrial biogenesis and oxidative metabolism are clearly conserved in people. While the goal in building and exploring this class of molecules was based on molecular science, the unsanctioned transition to performance-enhancing drugs may be inevitable. Thus, both AICAR and GW501516 continue to enjoy widespread used by endurance athletes to improve their performance. Since these drugs are not technically available for human use, either pirated compounds or government sponsored pharmaceutical production would be needed. At the same time these drugs can be obtained from multiple sites on the internet. Not surprisingly, one year after the 2008 Narkar Cell paper, the World Anti-Doping Agency added AICAR and GW501516 to its banned substances list under the category of metabolic modulators and a detection method was quickly developed. The reality that numerous athletes have tested positive for GW501516 and been suspended from competitions, sadly, confirms the presumptive potential benefit of exercise mimetics in people. Interestingly, resveratrol, a natural (though weak) compound that has been shown to improve endurance performance in animals, has not appeared in the prohibited list, probably due to its presence in food, the difficulty in obtaining the massive amounts need to boost performance and the lack of reported benefits in people.
Concluding remarks
While nothing can fully replace the physical and mental benefits of exercise, exercise mimetics have the potential to offer at least some of the benefits of exercise in people lacking or unable to achieve adequate physical exercise. The abilities of these drugs to enhance energy expenditure and fatty acid oxidation alone may be a big first step in combating the global epidemic of obesity and obesity-associated diseases. Collectively, the molecular dissection of the mechanisms of how exercise mimetics exert their effects has revealed a complex interconnective networks as depicted in Figure 1. Although most of these potential therapeutics are still in pre-clinical stages with some even showing severe side effects, we are optimistic that exercise mimetics will work in humans, perhaps in the not so distant future. Hopefully, the next generation of improved compounds with minimal side effects will soon be available. But until then, let’s keep on running.
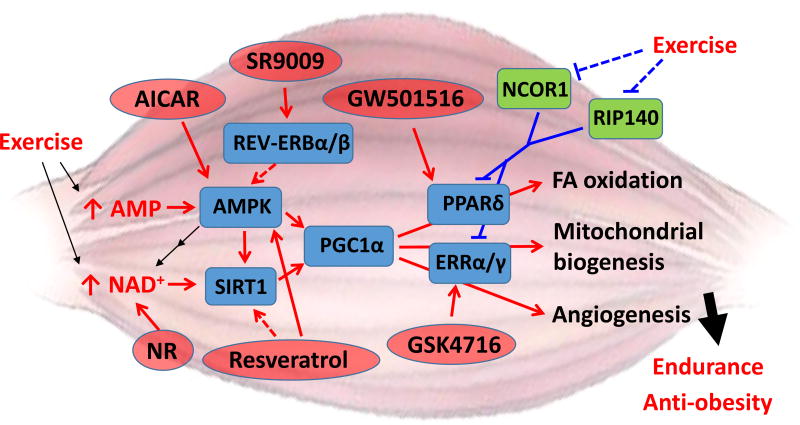
Figure 1. Exercise and exercise mimetics induced muscle remodeling.
AMPK and SIRT1 are energetic sensors and transduce exercise-induced signals to the transcription co-activator PGC1α by post-translational modifications. In response to the elevated activity of PGC1α, PPARδ and ERRα/γ directly control the expression of downstream target genes involved in FA oxidation, mitochondrial biogenesis, and angiogenesis, which eventually lead to exercise-induced health benefits. Additionally, the co-repressors NCOR1 and RIP140 are suppressed by exercise through unknown mechanisms, which de-repress PPARδ and ERRα/γ and induce their target genes. A handful exercise mimetics are shown in red ovals and their direct targets labeled. Red and blue lines represent activation and repression events, respectively. Dash lines are used when the mechanism is not clear.
References
- Asby DJ, Cuda F, Beyaert M, Houghton FD, Cagampang FR, Tavassoli A. AMPK Activation via Modulation of De Novo Purine Biosynthesis with an Inhibitor of ATIC Homodimerization. Chemistry & biology. 2015;22:838–848. doi: 10.1016/j.chembiol.2015.06.008. [DOI] [PubMed] [Google Scholar]
- Boussageon R, Supper I, Bejan-Angoulvant T, Kellou N, Cucherat M, Boissel JP, Kassai B, Moreau A, Gueyffier F, Cornu C. Reappraisal of metformin efficacy in the treatment of type 2 diabetes: a meta-analysis of randomised controlled trials. PLoS medicine. 2012;9:e1001204. doi: 10.1371/journal.pmed.1001204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canto C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P, Auwerx J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458:1056–1060. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canto C, Houtkooper RH, Pirinen E, Youn DY, Oosterveer MH, Cen Y, Fernandez-Marcos PJ, Yamamoto H, Andreux PA, Cettour-Rose P, et al. The NAD(+) precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell metabolism. 2012;15:838–847. doi: 10.1016/j.cmet.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canto C, Jiang LQ, Deshmukh AS, Mataki C, Coste A, Lagouge M, Zierath JR, Auwerx J. Interdependence of AMPK and SIRT1 for metabolic adaptation to fasting and exercise in skeletal muscle. Cell metabolism. 2010;11:213–219. doi: 10.1016/j.cmet.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson SA, Fulton JE, Schoenborn CA, Loustalot F. Trend and prevalence estimates based on the 2008 Physical Activity Guidelines for Americans. American journal of preventive medicine. 2010;39:305–313. doi: 10.1016/j.amepre.2010.06.006. [DOI] [PubMed] [Google Scholar]
- CDC. Health, United States, 2015. 2015 http://wwwcdcgov/nchs/hus/
- Chakravarthy MV, Booth FW. Eating, exercise, and "thrifty" genotypes: connecting the dots toward an evolutionary understanding of modern chronic diseases. Journal of applied physiology. 2004;96:3–10. doi: 10.1152/japplphysiol.00757.2003. [DOI] [PubMed] [Google Scholar]
- Clapham JC, Arch JR, Chapman H, Haynes A, Lister C, Moore GB, Piercy V, Carter SA, Lehner I, Smith SA, et al. Mice overexpressing human uncoupling protein-3 in skeletal muscle are hyperphagic and lean. Nature. 2000;406:415–418. doi: 10.1038/35019082. [DOI] [PubMed] [Google Scholar]
- Dufour CR, Wilson BJ, Huss JM, Kelly DP, Alaynick WA, Downes M, Evans RM, Blanchette M, Giguere V. Genome-wide orchestration of cardiac functions by the orphan nuclear receptors ERRalpha and gamma. Cell metabolism. 2007;5:345–356. doi: 10.1016/j.cmet.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Fan W, Atkins AR, Yu RT, Downes M, Evans RM. Road to exercise mimetics: targeting nuclear receptors in skeletal muscle. Journal of molecular endocrinology. 2013;51:T87–T100. doi: 10.1530/JME-13-0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan W, Evans R. PPARs and ERRs: molecular mediators of mitochondrial metabolism. Current opinion in cell biology. 2015;33:49–54. doi: 10.1016/j.ceb.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foretz M, Guigas B, Bertrand L, Pollak M, Viollet B. Metformin: from mechanisms of action to therapies. Cell metabolism. 2014;20:953–966. doi: 10.1016/j.cmet.2014.09.018. [DOI] [PubMed] [Google Scholar]
- Hawley JA, Hargreaves M, Joyner MJ, Zierath JR. Integrative biology of exercise. Cell. 2014;159:738–749. doi: 10.1016/j.cell.2014.10.029. [DOI] [PubMed] [Google Scholar]
- Holloszy JO. Biochemical adaptations in muscle. Effects of exercise on mitochondrial oxygen uptake and respiratory enzyme activity in skeletal muscle. The Journal of biological chemistry. 1967;242:2278–2282. [PubMed] [Google Scholar]
- IOC. DECISION OF THE IOC EXECUTIVE BOARD CONCERNING THE PARTICIPATION OF RUSSIAN ATHLETES IN THE OLYMPIC GAMES RIO 2016. 2016 https://wwwolympicorg/news/decision-of-the-ioc-executive-board-concerning-the-participation-of-russian-athletes-in-the-olympic-games-rio-2016.
- Jager S, Handschin C, St-Pierre J, Spiegelman BM. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:12017–12022. doi: 10.1073/pnas.0705070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM Diabetes Prevention Program Research, G. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. The New England journal of medicine. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobilo T, Guerrieri D, Zhang Y, Collica SC, Becker KG, van Praag H. AMPK agonist AICAR improves cognition and motor coordination in young and aged mice. Learning & memory. 2014;21:119–126. doi: 10.1101/lm.033332.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobilo T, Yuan C, van Praag H. Endurance factors improve hippocampal neurogenesis and spatial memory in mice. Learning & memory. 2011;18:103–107. doi: 10.1101/lm.2001611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kump DS, Booth FW. Sustained rise in triacylglycerol synthesis and increased epididymal fat mass when rats cease voluntary wheel running. The Journal of physiology. 2005;565:911–925. doi: 10.1113/jphysiol.2005.084525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Luft D, Schmulling RM, Eggstein M. Lactic acidosis in biguanide-treated diabetics: a review of 330 cases. Diabetologia. 1978;14:75–87. doi: 10.1007/BF01263444. [DOI] [PubMed] [Google Scholar]
- MacMichael S. Spanish police arrest ten as they break up 'next generation superdrug' doping ring in Operacion Skype. 2012 http://roadcc/content/news/55184-spanish-police-arrest-ten-they-break-next-generation-superdrug-doping-ring.
- Marcinko K, Bujak AL, Lally JS, Ford RJ, Wong TH, Smith BK, Kemp BE, Jenkins Y, Li W, Kinsella TM, et al. The AMPK activator R419 improves exercise capacity and skeletal muscle insulin sensitivity in obese mice. Molecular metabolism. 2015;4:643–651. doi: 10.1016/j.molmet.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill GF, Kurth EJ, Hardie DG, Winder WW. AICA riboside increases AMP-activated protein kinase, fatty acid oxidation, and glucose uptake in rat muscle. The American journal of physiology. 1997;273:E1107–1112. doi: 10.1152/ajpendo.1997.273.6.E1107. [DOI] [PubMed] [Google Scholar]
- Mouchiroud L, Eichner LJ, Shaw RJ, Auwerx J. Transcriptional coregulators: fine-tuning metabolism. Cell metabolism. 2014;20:26–40. doi: 10.1016/j.cmet.2014.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narkar VA, Downes M, Yu RT, Embler E, Wang YX, Banayo E, Mihaylova MM, Nelson MC, Zou Y, Juguilon H, et al. AMPK and PPARdelta agonists are exercise mimetics. Cell. 2008;134:405–415. doi: 10.1016/j.cell.2008.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narkar VA, Fan W, Downes M, Yu RT, Jonker JW, Alaynick WA, Banayo E, Karunasiri MS, Lorca S, Evans RM. Exercise and PGC-1alpha-independent synchronization of type I muscle metabolism and vasculature by ERRgamma. Cell metabolism. 2011;13:283–293. doi: 10.1016/j.cmet.2011.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufer PD, Bamman MM, Muoio DM, Bouchard C, Cooper DM, Goodpaster BH, Booth FW, Kohrt WM, Gerszten RE, Mattson MP, et al. Understanding the Cellular and Molecular Mechanisms of Physical Activity-Induced Health Benefits. Cell metabolism. 2015;22:4–11. doi: 10.1016/j.cmet.2015.05.011. [DOI] [PubMed] [Google Scholar]
- Nogueira L, Ramirez-Sanchez I, Perkins GA, Murphy A, Taub PR, Ceballos G, Villarreal FJ, Hogan MC, Malek MH. (−)-Epicatechin enhances fatigue resistance and oxidative capacity in mouse muscle. The Journal of physiology. 2011;589:4615–4631. doi: 10.1113/jphysiol.2011.209924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen BK, Febbraio MA. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nature reviews Endocrinology. 2012;8:457–465. doi: 10.1038/nrendo.2012.49. [DOI] [PubMed] [Google Scholar]
- Pedersen BK, Saltin B. Exercise as medicine - evidence for prescribing exercise as therapy in 26 different chronic diseases. Scandinavian journal of medicine & science in sports. 2015;25(Suppl 3):1–72. doi: 10.1111/sms.12581. [DOI] [PubMed] [Google Scholar]
- Pernicova I, Korbonits M. Metformin--mode of action and clinical implications for diabetes and cancer. Nature reviews Endocrinology. 2014;10:143–156. doi: 10.1038/nrendo.2013.256. [DOI] [PubMed] [Google Scholar]
- Pirinen E, Canto C, Jo YS, Morato L, Zhang H, Menzies KJ, Williams EG, Mouchiroud L, Moullan N, Hagberg C, et al. Pharmacological Inhibition of poly(ADP-ribose) polymerases improves fitness and mitochondrial function in skeletal muscle. Cell metabolism. 2014;19:1034–1041. doi: 10.1016/j.cmet.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Press A. Doping probe launched into Russian walkers. 2014 http://wwwespncom/espn/wire?section=trackandfield&id=11201932.
- Rangwala SM, Wang X, Calvo JA, Lindsley L, Zhang Y, Deyneko G, Beaulieu V, Gao J, Turner G, Markovits J. Estrogen-related receptor gamma is a key regulator of muscle mitochondrial activity and oxidative capacity. The Journal of biological chemistry. 2010;285:22619–22629. doi: 10.1074/jbc.M110.125401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ristow M, Zarse K, Oberbach A, Kloting N, Birringer M, Kiehntopf M, Stumvoll M, Kahn CR, Bluher M. Antioxidants prevent health-promoting effects of physical exercise in humans. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:8665–8670. doi: 10.1073/pnas.0903485106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe GC, El-Khoury R, Patten IS, Rustin P, Arany Z. PGC-1alpha is dispensable for exercise-induced mitochondrial biogenesis in skeletal muscle. PloS one. 2012;7:e41817. doi: 10.1371/journal.pone.0041817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu D, Mouchiroud L, Andreux PA, Katsyuba E, Moullan N, Nicolet-Dit-Felix AA, Williams EG, Jha P, Lo Sasso G, Huzard D, et al. Urolithin A induces mitophagy and prolongs lifespan in C. elegans and increases muscle function in rodents. Nature medicine. 2016;22:879–888. doi: 10.1038/nm.4132. [DOI] [PubMed] [Google Scholar]
- Sahebkar A, Chew GT, Watts GF. New peroxisome proliferator-activated receptor agonists: potential treatments for atherogenic dyslipidemia and non-alcoholic fatty liver disease. Expert opinion on pharmacotherapy. 2014;15:493–503. doi: 10.1517/14656566.2014.876992. [DOI] [PubMed] [Google Scholar]
- Schiaffino S, Reggiani C. Fiber types in mammalian skeletal muscles. Physiological reviews. 2011;91:1447–1531. doi: 10.1152/physrev.00031.2010. [DOI] [PubMed] [Google Scholar]
- Seth A, Steel JH, Nichol D, Pocock V, Kumaran MK, Fritah A, Mobberley M, Ryder TA, Rowlerson A, Scott J, et al. The transcriptional corepressor RIP140 regulates oxidative metabolism in skeletal muscle. Cell metabolism. 2007;6:236–245. doi: 10.1016/j.cmet.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood NE, Jeffery RW. The behavioral determinants of exercise: implications for physical activity interventions. Annual review of nutrition. 2000;20:21–44. doi: 10.1146/annurev.nutr.20.1.21. [DOI] [PubMed] [Google Scholar]
- Wang YX, Zhang CL, Yu RT, Cho HK, Nelson MC, Bayuga-Ocampo CR, Ham J, Kang H, Evans RM. Regulation of muscle fiber type and running endurance by PPARdelta. PLoS biology. 2004;2:e294. doi: 10.1371/journal.pbio.0020294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburton DE, Nicol CW, Bredin SS. Health benefits of physical activity: the evidence. CMAJ : Canadian Medical Association journal = journal de l'Association medicale canadienne. 2006;174:801–809. doi: 10.1503/cmaj.051351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winder WW, Holmes BF, Rubink DS, Jensen EB, Chen M, Holloszy JO. Activation of AMP-activated protein kinase increases mitochondrial enzymes in skeletal muscle. Journal of applied physiology. 2000;88:2219–2226. doi: 10.1152/jappl.2000.88.6.2219. [DOI] [PubMed] [Google Scholar]
- Woldt E, Sebti Y, Solt LA, Duhem C, Lancel S, Eeckhoute J, Hesselink MK, Paquet C, Delhaye S, Shin Y, et al. Rev-erb-alpha modulates skeletal muscle oxidative capacity by regulating mitochondrial biogenesis and autophagy. Nature medicine. 2013;19:1039–1046. doi: 10.1038/nm.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98:115–124. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Williams EG, Mouchiroud L, Canto C, Fan W, Downes M, Heligon C, Barish GD, Desvergne B, Evans RM, et al. NCoR1 is a conserved physiological modulator of muscle mass and oxidative function. Cell. 2011;147:827–839. doi: 10.1016/j.cell.2011.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zechner C, Lai L, Zechner JF, Geng T, Yan Z, Rumsey JW, Collia D, Chen Z, Wozniak DF, Leone TC, et al. Total skeletal muscle PGC-1 deficiency uncouples mitochondrial derangements from fiber type determination and insulin sensitivity. Cell metabolism. 2010;12:633–642. doi: 10.1016/j.cmet.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Hirota T, Han X, Cho H, Chong LW, Lamia K, Liu S, Atkins AR, Banayo E, Liddle C, et al. Circadian Amplitude Regulation via FBXW7-Targeted REV-ERBalpha Degradation. Cell. 2016;165:1644–1657. doi: 10.1016/j.cell.2016.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]