Abstract
Background
In the present study, we aimed to investigate the prognostic value of epithelial membrane protein 1 (EMP-1) gene in patients diagnosed with laryngeal carcinoma (LC).
Material/Methods
Patients who were pathologically diagnosed with LC were enrolled in the present study. The expression levels of EMP-1 in tumor tissues and corresponding normal tissues collected from the LC patients were detected by semi-reverse transcriptase polymerase chain reaction (semi-RT-PCR). Chi-square analysis was used to evaluate the relationship between EMP-1 expression level and clinical characteristics. Survival analysis for the study population was analyzed by Kaplan-Meier method with log rank test. Additionally, Cox regression model was applied to evaluate the prognostic value of EMP-1 in LC patients.
Results
106 LC patients, including 55 men and 51 women, were enrolled in the present study. Semi-RT-PCR demonstrated that the expression level of EMP-1 was decreased in tumor tissues, compared with adjacent normal tissues (p<0.001). Moreover, the level was significantly associated with lymph node metastasis, histological grade, and clinical stage (p<0.05 for all). In addition, low levels of EMP-1 was significantly correlated with poor survival rate (log rank test, p=0.020). Cox regression analysis indicated that EMP-1 was an independent marker for LC prognosis (HR=2.755, 95% CI=1.123–6.760, p=0.027).
Conclusions
The abnormal expression of EMP-1 may be associated with progression of LC and the gene may act as a prognostic marker for LC.
MeSH Keywords: Genes, abl; Laryngeal Cartilages; Prognosis
Background
Laryngeal carcinoma (LC) is one of the most common head-neck malignancies in the world [1]. LC includes various malignant tumors occurring in larynx and its major type is laryngeal squamous cell carcinoma [2]. Recently, the morbidity of LC has increased in developing countries, probably due to the abuse of alcohol and tobacco [3]. The treatments for LC include surgery, radiotherapy, and chemotherapy or a combination [4]. However, due to the resistance to radiotherapy and chemotherapy, surgery has become the main treatment [5]. Despite the advance in surgical techniques, the quality-of-life (QoL) in patients with LC seriously declines after open surgery, including QoL issues such as pain, speech disorders, and dry mouth) [6]. The survival rate of LC patients is unsatisfactory due to metastasis and recurrence [2,7]. Therefore, it is urgent to explore novel biomarkers related to the tumorigenesis of LC for early detection and prognosis.
Epithelial membrane protein 1 (EMP-1) gene belongs to the peripheral myelin protein (PMP)-22 family, also named as CL-40, tumor-associated membrane protein (TMP), and B4B [8,9]. It has been reported that EMP-1 plays an important role in various cellular processes. In the study of Sun et al., over-expression of EMP-1 in a gastric cancer cell line was shown to inhibit cell proliferation, migration and invasion, and induce cell apoptosis [10]. A study carried out by Wang et al. indicated that EMP-1 could promote the growth of human esophageal cancer cell lines and the gene might be associated with carcinogenesis of esophagus [11]. Lai et al. concluded that EMP-1 may be involved in the development and progress of non-small cell lung cancer by activating the PI3K/AKT pathway [12]. In conclusion, EMP-1 may act as oncogene or suppressor gene in different cancers. The effects of EMP-1 on LC have been rarely reported in previous studies. Therefore, in the study we investigated the expression patterns of EMP-1 in LC tissues and explored its clinical significance in cancer prognosis.
In the present study, patients who were pathologically diagnosed with LC were enrolled in the study, and tissue specimens were collected from them. The expression level of EMP-1 in tumor tissue and corresponding normal tissue were compared and the prognostic significance of the gene in LC was also evaluated. The current study may exploit a novel prognostic marker for LC.
Material and Methods
Study subjects
The present study was carried out in in The Army General Hospital of PLA. Patients who were pathologically diagnosed with laryngeal carcinoma (LC) and not receiving chemotherapy or radiotherapy were enrolled in the study. Tumor tissue and the corresponding non-cancerous tissue that was 10 mm from the laryngeal tumors were collected from the patients and kept in liquid nitrogen. All the tissue samples were confirmed by the histopathologist based on the most recent World Health Organization (WHO) criteria. The clinical information of the participants was available and informed consents were obtained from all the patients or their family. In addition, all of the patients received follow-up interviews and the total period for investigation was five years. The current study was approved by the Ethics Committee of our hospital.
RNA extraction and relative expression of EMP-1
Total RNA samples were extracted from the collected tissues specimens using TRIzol (Invitrogen, CA, USA) according to the instructions of the manufacturer. The concentration and quality of the RNA samples were detected by UV absorbance (A260/A280) and 1% agarose gel electrophoresis, respectively.
Semi-reverse transcriptase polymerase chain reaction (semi-RT-PCR) was used to evaluate the relative expression of EMP-1 in the collected tissue specimens. Then cDNA was obtained from the total RNA using PrimeScript II 1st strand cDNA synthesis kit (TaKaRa, Dalian, China). Semi-RT-PCR was used to detect EMP-1 mRNA expression level in tissue specimens and GAPDH acted as internal control. The PCR solution was carried out in a total of 25 μL reaction volume. The amplification systems included 12.5 μL Master Mix (2×), 0.5 μL corresponding primers, 3 μL cDNA samples, and 8.5 μL deionized water. The PCR reaction system was as follows: 94°C for five minutes; followed by 25 cycles: 94°C for 30 seconds, 55°C for 30 seconds, and 72°C for 30 seconds; additional extension for five minutes at 72°C. The sequences of the primers used were as follows: GAPDH forward-5′-CGCTCTCTGCTCCTCCTGTTC-3′, and reverse-5′-ATCCGTTGACTCCGACCTTCAC-3′; EMP-1 forward-5′-GGATCAGGGCTCCTAGGCTCA-3′ and reverse-5′-GGTGGCTTGCCCTCAACATT-3′. A 1.5% agarose gel was applied to detected the PCR products and the concentration of the target PCR products was analyzed by UV light and Gel-Pro analyzer software version 3.1 (Media cybernetics, Inc., Silver Spring, MD, USA), and compared to the internal control.
In addition, Western blotting was used to detect the protein level of EMP-1 in the current study following the method used in a previous report [13]. Briefly, the lysates from fresh tissues were first separated with the SDS-polyacrylamide gel electrophoresis and then transferred to the PVDF membrane. After blocking with 5% BSA, the membranes were incubated with a primary antibody against EMP-1 (1: 1,000, Abcam) and β-actin (1: 1,000, Beyotime) at 4°C overnight. Then the HRP-conjugated secondary antibody was applied to develop the blots. The results were detected by chemiluminescence detection system (Amersham Life Science, Piscataway, NJ, USA).
Statistical analysis
Statistical analysis was performed with SPSS 18.0 software. The relative expression of EMP-1 in tumor tissues and corresponding adjacent normal tissues were evaluated by student’s t-test and shown as mean ±SD. The association between the clinical characteristics of the patients and their relative expression of EMP-1 was evaluated by chi-square analysis. The survival analysis was analyzed by Kaplan-Meier with log rank test. Cox regression analysis model was used to investigate the prognostic value of EMP-1. For all analysis, a p value less than 0.05 was considered statistically significant.
Results
Demographic characteristics of the study subjects
In all, 106 patients who were pathologically diagnosed with LC were enrolled in the present study. The study participants included 55 men and 51 women. The clinical characteristics of the study participants are listed in the Table 1.
Table 1.
Demographic characteristics of the study population.
| Characteristics | Case (n) | EMP-1 high expression group (n=64) | EMP-1 low expression group (n=42) | χ2 | P |
|---|---|---|---|---|---|
| Gender | 0.508 | 0.476 | |||
| Male | 55 | 35 | 20 | ||
| Female | 51 | 29 | 22 | ||
| Age (year) | 0.512 | 0.474 | |||
| >60 | 65 | 41 | 24 | ||
| ≤60 | 41 | 23 | 18 | ||
| Tumor size (cm) | 0.006 | 0.940 | |||
| >2 | 50 | 30 | 20 | ||
| ≤2 | 56 | 34 | 22 | ||
| Smoking history | 2.299 | 0.129 | |||
| Yes | 56 | 30 | 26 | ||
| No | 50 | 34 | 16 | ||
| Lymph node metastasis | 8.582 | 0.003 | |||
| Yes | 40 | 17 | 23 | ||
| No | 66 | 47 | 19 | ||
| Histological grade | 5.679 | 0.017 | |||
| G1/G2 | 53 | 38 | 15 | ||
| G3 | 55 | 26 | 27 | ||
| Clinical stage | 8.206 | 0.004 | |||
| I/II | 51 | 38 | 13 | ||
| III/IV | 55 | 26 | 29 |
EMP-1 expression level and its correlation with clinical characteristics
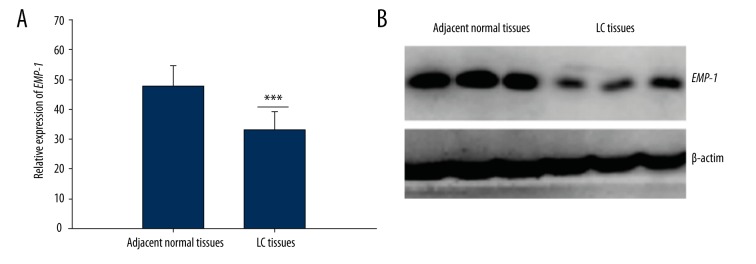
Semi-RT-PCR analysis indicated that the mRNA expression level of EMP-1 in LC tissues was significantly lower than that in the adjacent normal tissues (p<0.001) (Figure 1A). Moreover, the results of Western blotting showed that compared with the control tissues, the protein level of EMP-1 was significantly downregulated, which was in accordance with the mRNA level. β-actin was used as the reference (Figure 1B).
Figure 1.
Relative expression of EMP-1 in tumor tissues and adjacent normal tissues. (A) The expression level of EMP-1 was lower in LC tissues than in the corresponding normal tissues detected by semi-RT-PCR. ***: indicated p<0.001. (B) EMP-1 expression in protein levels measured by Western blotting was significant decreased in LC tissue specimens compared with the matched noncancerous tissue samples. β-actin was used as the reference.
The eligible patients were divided into a high expression group (n=64) and a low expression group (n=42), according to the average level of EMP-1. Chi-square analysis was applied to evaluate the relationship between EMP-1 level and clinical characteristics based on the study population. Analysis results indicated that the EMP-1 level was significantly correlated with lymph node metastasis (p=0.003), histological grade (p=0.017), and clinical stage (p=0.004). The expression level of EMP-1 was not related to age, gender, smoking history, or tumor size in patients with LC (p>0.05 for all) (Table 1).
Survival analysis
Overall survival of the study patients was evaluated by Kaplan-Meier method with log rank test. Analysis results suggested that patients with decreased level of EMP-1 had a lower survival rate than those in the high expression group (44.57 versus 53.69 months, log rank test, p=0.020) (Figure 2).
Figure 2.
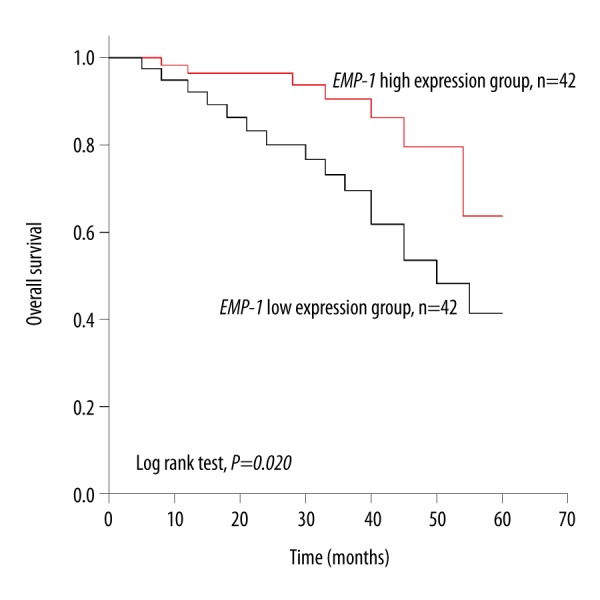
Overall survival analysis for LC patients according to EMP-1 expression. Kaplan-Meier method with log rank test was applied to evaluate overall survival for LC patients. Analysis results indicated that patients with high level of EMP-1 had a higher survival rate than those with low level (log rank test, p=0.020).
Prognostic analysis
Cox regression model was applied to calculate the prognostic significance of the factors in LC patients. Univariate analysis demonstrated that EMP-1 level was remarkably correlated with prognosis of patients with LC (HR=2.755, 95% CI=1.123–7.670, p=0.027). In addition, multivariate analysis indicated that EMP-1 was an independent marker for LC prognosis (HR=2.755, 95% CI=1.123–7.670, p=0.027) (Table 2).
Table 2.
Prognosis analysis for laryngeal carcinoma patients by cox regression analysis.
| Characteristics | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95%CI | P | HR | 95%CI | P | |
| EMP-1 level (low vs. high) | 2.755 | 1.123–7.670 | 0.027 | 2.755 | 1.123–7.670 | 0.027 |
| Gender (Male vs. Female) | 0.940 | 0.412–2.149 | 0.884 | – | – | – |
| Age (>60 vs. ≤60) | 1.053 | 0.457–2.424 | 0.904 | – | – | – |
| Smoking history (Yes vs. no) | 1.388 | 0.580–3.321 | 0.462 | – | – | – |
| Tumor size (>2 vs. ≤2) | 1.383 | 0.606–3.157 | 0.441 | – | – | – |
| Lymph node metastasis (yes vs. no) | 1.917 | 0.845–4.350 | 0.119 | – | – | – |
| Histological stage (G3 vs. G1/G2) | 1.192 | 0.522–2.718 | 0.677 | – | – | – |
| Clinical stage (III/IV vs. I/II) | 2.168 | 0.890–5.283 | 0.089 | – | – | – |
‘–’ – Indicated no available data.
Discussion
LC is a common malignancy which can severely impact the quality-of-life of patients [14]. The mechanism of the LC is poorly understood. Various factors may contribute to the occurrence of LC, including environmental and genetic factors. Smoking and alcohol are the common reasons for the occurrence and recurrence of LC [15,16]. A case-control study based on the French population indicated that asbestos exposure combined with tobacco smoking and alcohol drinking accounting for a major of LC case [17]. In addition, numerous genes are involved in the occurrence and progression of LC. For example, cyclin D1 (CCND1), the key factor during the control of cell cycle, has been found to be involved in the development and prognosis of larynx carcinoma in a study by Rydzanicz et al. [18]. The study conducted by Marco et al. demonstrated that the aberrant expression of epidermal growth factor receptor (EGFR) was closely correlated with LC progression [19]. In our present study, the expression patterns of EMP-1 in LC were investigated, which may be considered as a potential prognostic marker for LC.
Tumor tissues and corresponding normal tissues were collected from the LC patients and EMP-1 expression levels in the tissue specimens were detected by semi-RT-PCR. Results demonstrated that compared with adjacent normal tissues, LC tissues exhibited a decreased expression level of EMP-1. Moreover, the decreased level of EMP-1 was significantly associated with positive lymph node metastasis, high histological grade, and clinical stage. The results may suggest that EMP-1 acts as a suppressor gene in LC progression. This conclusion was in accordance with previous studies. Liu et al. found that the mRNA levels of EMP-1 were significantly downregulated in LC cells, compared with the matched primary normal epithelial cells [20]. However, in their study the expression level of EMP-1 was not associated with stage and differentiation of LC, which was a notable difference from our study. A study based on oral squamous cell carcinoma patients demonstrated that decreased levels of EMP-1 were significantly correlated to clinical stage and lymph node metastasis [21]. There is not an agreement on this issue, and a study including more participants is needed.
In our current study, we evaluated the overall survival of the participants according to their expression of EMP-1. Results indicated that the survival rate was higher in the high expression group than in the low expression group. A literature search found that low expression of EMP-1 was significantly associated with poor overall survival in patients with gastric cancer, colorectal carcinoma, nasopharyngeal cancer, and breast cancer [10,22–24]. These results indicated that EMP-1 may be a potential predictor for outcomes of patients with cancers.
Prognostic studies are important for cancer treatment. In the previous studies, various biomarkers were shown to predict outcomes for LC patients. In the study of Li et al., the prognostic value of microRNA-101 in laryngeal squamous cell carcinoma was evaluated and the low level of microRNA-101 was related to poor prognosis [25]. A study by Han et al. demonstrated that the growth-regulated oncogene α (GROα) was a prognostic marker for patients with laryngeal squamous cell carcinoma [26]. The clinical significance of HS1-associated protein X-1 (HAX-1) in LC has been evaluated by You et al. Results indicated that HAX-1 was significantly associated with clinical characteristics of LC patients; moreover, HAX-1 was shown to be an independent prognostic marker for LC [27]. In our present study, we investigated the prognostic value of EMP-1 in LC patients. Cox regression analysis suggested that EMP-1 may be a potential indicator for LC prognosis. Although a large number of factors were shown to act as prognostic biomarkers for LC, few of them are applied to clinical practice. Therefore, a great deal of research is needed to investigate the clinical application of EMP-1.
Conclusions
Downregulated levels of EMP-1 in LC patients were significantly associated with positive lymph node metastasis, high histological grade, and clinical stage. Moreover, EMP-1 may be a novel indicator for LC prognosis.
Footnotes
Source of support: Departmental sources
References
- 1.Li P, Hu W, Zhu Y, Liu J, et al. Treatment and predictive factors in patients with recurrent laryngeal carcinoma: A retrospective study. Oncol Lett. 2015;10(5):3145–52. doi: 10.3892/ol.2015.3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu S, Sang M, Xu Y, et al. Expression of MAGE-A1, -A9, -A11 in laryngeal squamous cell carcinoma and their prognostic significance: a retrospective clinical study. Acta Otolaryngol. 2016;136:506–13. doi: 10.3109/00016489.2015.1126856. [DOI] [PubMed] [Google Scholar]
- 3.Koirala K. Epidemiological study of laryngeal carcinoma in Western Nepal. Asian Pac J Cancer Prev. 2015;16(15):6541–54. doi: 10.7314/apjcp.2015.16.15.6541. [DOI] [PubMed] [Google Scholar]
- 4.Maia D, de Carvalho AC, Horst MA, et al. Expression of miR-296-5p as predictive marker for radiotherapy resistance in early-stage laryngeal carcinoma. J Transl Med. 2015;13:262. doi: 10.1186/s12967-015-0621-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chera BS, Amdur RJ, Morris CG, et al. T1N0 to T2N0 squamous cell carcinoma of the glottic larynx treated with definitive radiotherapy. Int J Radiat Oncol Biol Phys. 2010;78(2):461–66. doi: 10.1016/j.ijrobp.2009.08.066. [DOI] [PubMed] [Google Scholar]
- 6.Luo J, Wu J, Lv K, et al. Analysis of postsurgical health-related quality of life and quality of voice of patients with laryngeal carcinoma. Medicine (Baltimore) 2016;95(1):e2363. doi: 10.1097/MD.0000000000002363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang R, Guo Y, Ma H, et al. Tumor necrosis factor superfamily member 13 is a novel biomarker for diagnosis and prognosis and promotes cancer cell proliferation in laryngeal squamous cell carcinoma. Tumour Biol. 2016;37(2):2635–45. doi: 10.1007/s13277-015-4016-8. [DOI] [PubMed] [Google Scholar]
- 8.Li ZY, Xiong SH, Hu M, Zhang CS. Knockdown epithelial membrane protein 1 suppresses human degenerative intervertebral disc-derived nucleus pulposus cell proliferation. Cartilage. 2011;2(3):300–6. doi: 10.1177/1947603510392022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li ZY, Xiong SH, Hu M, Zhang CS, et al. Epithelial membrane protein 1 inhibits human spinal chondrocyte differentiation. Anat Rec (Hoboken) 2011;294(6):1015–24. doi: 10.1002/ar.21395. [DOI] [PubMed] [Google Scholar]
- 10.Sun G, Zhao G, Lu Y, et al. Association of EMP1 with gastric carcinoma invasion, survival and prognosis. Int J Oncol. 2014;45(3):1091–98. doi: 10.3892/ijo.2014.2488. [DOI] [PubMed] [Google Scholar]
- 11.Wang HT, Liu ZH, Wang XQ, Wu M, et al. [Effect of EMP-1 gene on human esophageal cancer cell line]. Ai Zheng. 2002;21(3):229–32. [in Chnese] [PubMed] [Google Scholar]
- 12.Lai S, Wang G, Cao X, et al. EMP-1 promotes tumorigenesis of NSCLC through PI3K/AKT pathway. J Huazhong Univ Sci Technolog Med Sci. 2012;32(6):834–38. doi: 10.1007/s11596-012-1043-1. [DOI] [PubMed] [Google Scholar]
- 13.Ranganathan V, De PK. Western blot of proteins from Coomassie-stained polyacrylamide gels. Anal Biochem. 1996;234(1):102–4. doi: 10.1006/abio.1996.0057. [DOI] [PubMed] [Google Scholar]
- 14.Xiang Z, Li Q, Chang A, et al. Expression and significance of TWIST, a zinc finger transcription factor, in laryngeal carcinoma among Chinese population: A meta-analysis. Int J Clin Exp Med. 2015;8(10):18351–58. [PMC free article] [PubMed] [Google Scholar]
- 15.Bhattacharyya S, Mandal S, Banerjee S, et al. Cannabis smoke can be a major risk factor for early-age laryngeal cancer – a molecular signaling-based approach. Tumour Biol. 2015;36(8):6029–36. doi: 10.1007/s13277-015-3279-4. [DOI] [PubMed] [Google Scholar]
- 16.Liu C, Xu ZG. [Clinical analysis of relevant factors causing postoperative recurrence of laryngeal cancer after partial laryngectomy]. Zhonghua Zhong Liu Za Zhi. 2013;35(5):377–81. doi: 10.3760/cma.j.issn.0253-3766.2013.05.012. [in Chinese] [DOI] [PubMed] [Google Scholar]
- 17.Menvielle G, Fayossé A, Radoï L, et al. The joint effect of asbestos exposure, tobacco smoking and alcohol drinking on laryngeal cancer risk: evidence from the French population-based case-control study, ICARE. Occup Environ Med. 2016;73(1):28–33. doi: 10.1136/oemed-2015-102954. [DOI] [PubMed] [Google Scholar]
- 18.Rydzanicz M, Golusinski P, Mielcarek-Kuchta D, et al. Cyclin D1 gene (CCND1) polymorphism and the risk of squamous cell carcinoma of the larynx. Eur Arch Otorhinolaryngol. 2006;263(1):43–48. doi: 10.1007/s00405-005-0957-7. [DOI] [PubMed] [Google Scholar]
- 19.Lionello M, Lovato A, Staffieri A, et al. The EGFR-mTOR pathway and laryngeal cancer angiogenesis. Eur Arch Otorhinolaryngol. 2014;271(4):757–64. doi: 10.1007/s00405-013-2691-x. [DOI] [PubMed] [Google Scholar]
- 20.Liu YH, Tang PZ, Xu ZG, et al. [Differential expression of the epithelial membrane protein 1 of laryngeal carcinoma]. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2003;25(1):47–51. [in Chinese] [PubMed] [Google Scholar]
- 21.Zhang J, Cao W, Xu Q, Chen WT. The expression of EMP1 is downregulated in oral squamous cell carcinoma and possibly associated with tumour metastasis. J Clin Pathol. 2011;64(1):25–29. doi: 10.1136/jcp.2010.082404. [DOI] [PubMed] [Google Scholar]
- 22.Sun GG, Wang YD, Cui DW, et al. Epithelial membrane protein 1 negatively regulates cell growth and metastasis in colorectal carcinoma. World J Gastroenterol. 2014;20(14):4001–10. doi: 10.3748/wjg.v20.i14.4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun GG, Lu YF, Fu ZZ, et al. EMP1 inhibits nasopharyngeal cancer cell growth and metastasis through induction apoptosis and angiogenesis. Tumour Biol. 2014;35(4):3185–93. doi: 10.1007/s13277-013-1416-5. [DOI] [PubMed] [Google Scholar]
- 24.Sun GG, Wang YD, Lu YF, Hu WN. EMP1, a member of a new family of antiproliferative genes in breast carcinoma. Tumour Biol. 2014;35(4):3347–54. doi: 10.1007/s13277-013-1441-4. [DOI] [PubMed] [Google Scholar]
- 25.Li M, Tian L, Ren H, et al. MicroRNA-101 is a potential prognostic indicator of laryngeal squamous cell carcinoma and modulates CDK8. J Transl Med. 2015;13:271. doi: 10.1186/s12967-015-0626-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han L, Liu W, Chen Y, et al. GROalpha expression and its prognostic implications in laryngeal squamous cell carcinoma. Neoplasma. 2015;62(1):152–58. doi: 10.4149/neo_2015_020. [DOI] [PubMed] [Google Scholar]
- 27.You Y, Yao H, You B, et al. Clinical significance of HAX-1 expression in laryngeal carcinoma. Auris Nasus Larynx. 2015;42(4):299–304. doi: 10.1016/j.anl.2014.12.003. [DOI] [PubMed] [Google Scholar]