Abstract
Antibody (Ab) crosslinking of HLA I molecules on the surface of endothelial cells triggers proliferative and pro-survival intracellular signaling, which is implicated in the process of chronic allograft rejection, also known as transplant vasculopathy. The purpose of this study was to investigate the role of mammalian target of rapamycin (mTOR) in HLA I antibody-induced signaling cascades. Everolimus provides a tool to establish how the mTOR signal network regulates HLA I-mediated migration, proliferation, and survival. We found that everolimus inhibits mTORC1 by disassociating Raptor from mTOR, thereby preventing class I-induced phosphorylation of mTOR, p70S6K, S6RP, and 4E-BP1, and resultant class I-stimulated cell migration and proliferation. Furthermore, we found that everolimus inhibits class I-mediated mTORC2 activation (1) by disassociating Rictor and Sin1 from mTOR; (2) by preventing class I-stimulated Akt phosphorylation; and (3) by preventing class I-mediated ERK phosphorylation. These results suggest that everolimus is more effective than sirolimus at antagonizing both mTORC1 and mTORC2, the latter of which is critical in endothelial cell functional changes leading to transplant vasculopathy in solid organ transplantation after HLA I crosslinking. Our findings point to a potential therapeutic effect of everolimus in prevention of chronic antibody-mediated rejection.
Keywords: Everolimus, mTOR, Endothelial cells, HLA, Signal transduction, Transplantation
INTRODUCTION
Antibody mediated rejection (AMR) is caused by the development of donor-specific antibodies (DSA) against polymorphic HLA molecules expressed by the transplanted organ. DSA is directly associated with acute and chronic rejection and late graft failure (1–3). AMR is a sequential process leading to arterial and parenchymal damage, and ultimately transplant vasculopathy (TV) and graft dysfunction (1, 4–5).
Although donor-specific anti-HLA Ab are linked to TV, the mechanisms of the pathologic effect of Ab binding to the graft endothelium have only recently been explored. Studies by our group and others have shown that ligation of HLA I molecules on the surface of endothelial cells (EC) by murine monoclonal and human monoclonal and polyclonal Ab triggers diverse biological functional changes (6–16). Ligation of HLA I molecules induces RhoA, Src, focal adhesion kinase (FAK) and paxillin activation, leading to assembly of focal adhesions and stress fiber formation (13, 17–18). Engagement of HLA I molecules by Ab stimulates activation of the phosphoinositide-3 kinase (PI3K)/Akt pathway (11, 17), which regulates anti-apoptotic signaling through Bcl-2 and Bcl-xL in EC (11). HLA I crosslinking also triggers activation of the mTORC1 target S6 ribosomal protein (S6RP), which promotes cellular proliferation and protein synthesis (14, 19).
mTOR is a serine-threonine kinase that plays a central role in the regulation of cell proliferation and of targets that control translation and protein synthesis (20–21). mTOR activation is initiated through PI3K and Akt, which inactivate tuberous sclerosis complex 1 and 2 (TSC1/TSC2) (22–23). mTOR forms two molecular complexes with distinct functional capacities. Complex 1 (mTORC1), containing mTOR, regulatory associated protein of TOR (Raptor), and GβL. mTORC1 activates p70 ribosomal S6 kinase (S6K) and eukaryotic initiation factor 4E (eIF4E) binding protein 1 (4E-BP1) (20, 24), leading to increased ribosomal biosynthesis and translation of cell cycle mRNA. A second mTOR complex, mTORC2, contains mTOR, rapamycin insensitive companion of TOR (Rictor), and GβL. Stress-activated protein kinase-interacting protein 1 (Sin1) also associates with mTORC2, maintains complex integrity and facilitates Akt phosphorylation at Ser473 (25–26). mTORC2 was recently described to regulate the cytoskeleton through Rho GTPases (27–28).
The pharmacological inhibitors sirolimus (rapamycin) and its analog everolimus are effective mTOR antagonists and are U.S. FDA-approved immunosuppressive agents for solid organ transplant (29). Everolimus and sirolimus both bind to FKBP12 and inhibit complex formation between mTOR, raptor and GβL (mTORC1) (19–20, 30), preventing downstream cell metabolism, growth, and proliferation (31). Although mTORC2 was initially described to be insensitive to rapamycin, recent studies suggest that prolonged exposure prevents the assembly of mTORC2, blocks Akt Ser473 phosphorylation, and induces EC apoptosis (32–33).
The aim of this study was to elucidate the effect of everolimus on HLA I-mediated activation of the mTOR signaling network. We show that everolimus effectively inhibited HLA I-mediated activation of mTORC1 and mTORC2 signaling pathways and blocked HLA I-induced proliferation and migration. Notably, everolimus more effectively inhibited endothelial functional changes compared with sirolimus. Our results suggest that everolimus prevents HLA I-stimulated functional changes by antagonizing both mTORC1 and mTORC2, as well as downstream MAP kinase pathways.
MATERIALS AND METHODS
Antibodies and chemicals
Everolimus (RAD001) was synthesized at Novartis Pharma AG (Basel, Switzerland) for biomedical research. Rapamycin (sirolimus) was purchased from Sigma. The purity of both sirolimus and everolimus was >97% by HPLC. U0126 was from Calbiochem. Stock solutions were reconstituted in dimethylsulfoxide (DMSO), and working solutions were diluted in Medium M199 (Mediatech, Inc) containing 0.2% fetal bovine serum (FBS) (Hyclone). Anti-HLA I monoclonal antibody W6/32 (mouse IgG2a), recognizing a monomorphic epitope on HLA I molecules, was purified from cultured supernatants of the hybridoma HB-95 (ATCC). The mouse IgG isotype control, protein A-agarose, and mitomycin C were purchased from Sigma. Polyclonal Ab against phospho-mTOR (Ser2448), phospho-S6K (Thr389), phospho-S6K (Thr421/Ser424), phospho-S6RP (Ser235/236), phospho-4E-BP1 (Thr37/46), phospho-Akt (Ser473), mTOR, S6K, S6RP, 4E-BP1, Akt, extracellular signal-regulated kinase (ERK), Raptor and rabbit anti-α-Actin mAb; mouse mAb against ERK (Thr202/Tyr204), mTOR, and ERK were purchased from Cell Signaling Technology (Beverly, MA). Anti-Rictor, Raptor, and Sin1 mAb were from Millipore/Upstate. Anti-Rictor (A300-458A and A300-459A), and anti-Sin1 (A300-910A) rabbit Ab were from Bethyl Laboratory (Montgomery, TX). The goat anti-rabbit horseradish peroxidase (HRP) and goat anti-mouse HRP Ab were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Vybrant™ CFDA-SE Cell Tracer Kit was purchased from Invitrogen.
Cell culture
Primary human aortic endothelial cells (HAEC) were isolated from the aortic rings of deceased donor hearts (CAR or CAS), or obtained from Clonetics (lot number: EC5555) and cultured as described previously (12, 17, 34). Cells from passage 3 to 8 were used at a confluence of 80%. Prior to use in experiments, cells were grown for 16hr in medium M199 containing 0.2% FBS.
Preparation of Cell Lysates and Western Blot
HAEC were seeded in 35 mm dishes coated with 0.1% gelatin. Quiescent cells were pre-treated with or without sirolimus or everolimus, then treated with mAb W6/32, or mouse isotype IgG as control, and cell lysates for Western Blot were prepared as described previously (12). The phosphorylated bands were scanned and quantified using the ImageJ software.
mTOR complex formation by co-immunoprecipitation
HAEC were seeded in duplicate 100 mm dishes for each experimental condition. Quiescent sub-confluent HAEC were pre-treated with or without sirolimus or everolimus, then treated with anti-HLA I mAb W6/32, or control mouse IgG, and cell lysates for co-immunoprecipitation and Western blot were prepared as described previously (12, 19).
Cell proliferation assays by CFSE labeling and BrdU incorporation
HAEC were grown in 35 mm culture dishes to 70% confluence and starved in medium M199 without FBS for 6hr. Quiescent HAEC were labeled with 2µM CFSE, pre-treated with or without sirolimus or everolimus, and then stimulated with anti-HLA I mAb W6/32 for 48hr in M199 with 2% FBS. Cells were detached and proliferation was measured by flow cytometry and analyzed as described previously (19–20). This method is comparable to measurement of proliferation by flow cytometric measurement of BrdU incorporation (BD Pharmingen BrdU Kits), which we performed as described (17, 35). Cells were stimulated as above in the presence of BrdU at 10µM, fixed and permeabilized, incubated with FITC-anti-BrdU.
Cell migration assay by wound healing
In vitro wound healing assay was performed as previously described (35). Briefly, confluent HAEC were pretreated with 10µg/ml mitomycin C for 2hr to inhibit cell proliferation. A scratch wound was created with a sterile pipette tip, and detached cells were rinsed off. Wounded cells were pretreated with or without everolimus, followed by anti-HLA I mAb W6/32 in M199 with 2% FBS for 16hr. The cells were fixed, stained with Wright-Giemsa (Sigma-Aldrich) and wound closure was monitored by microscopy. EC incubated in complete medium served as the positive control, normalized to 100% wound closure.
Cell migration assay by transwell insert system
The migration of HAEC was measured in a transwell insert system (8.0µm pore size, Corning). HAEC were grown in 24-well plate coated with 0.1% gelatin up to 90% confluence. Quiescent cells pretreated with or without everolimus or sirolimus were trypsinized, resuspended, and 30,000 cells were seeded on the upper chamber of insert and stimulated with 1µgmL anti-HLA I mAb W6/32 or 10ng/mL positive control VEGF. After 16hr, cells on the upper surface of the membrane were removed, and migrated cells were fixed with methanol and stained with crystal violet. Three fields per insert were photographed with 10x objective lens, and counted.
Statistical analysis
Each experiment was repeated three times. Data are presented as mean ± SEM. Differences in protein phosphorylation, cell proliferation, or cell migration were calculated using student’s t test (two-sided) or one way analysis of variance (ANOVA) with Fisher’s least significant difference (LSD). p < 0.05 was considered significant.
RESULTS
Everolimus inhibits HLA I-stimulated cell proliferation more potently than sirolimus
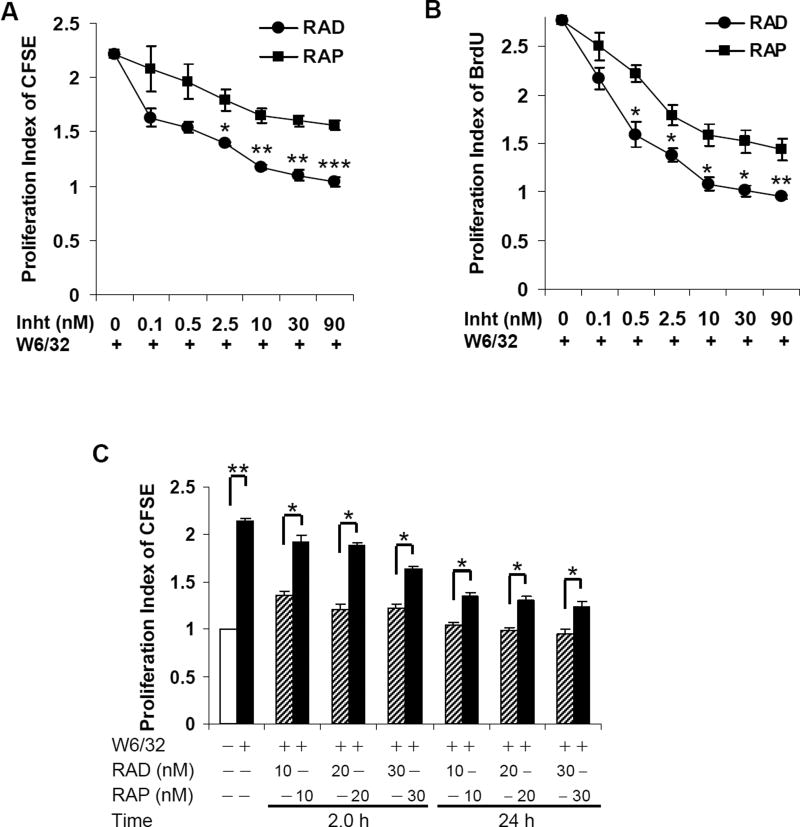
To examine the impact of everolimus and sirolimus on class I-mediated endothelial cell proliferation, HAEC were pretreated for 24hr with increasing concentrations of these allosteric mTORC inhibitors at concentrations ranging from 0.1–90 nM (consistent with previously published studies (19, 36–38)) followed by stimulation with mAb W6/32 (1.0 µg/ml). Ligation of HLA I on HAEC with W6/32 induced a significant increase in cell proliferation (scored by either CFSE or BrdU incorporation) compared with untreated control (Fig. 1A and B). Pretreatment of HAEC with everolimus for 24hr inhibited HLA I-mediated cell proliferation in a dose-dependent manner (68%, 2.5nM; 86%, 10nM; 92%, 30nM) with maximal inhibition at 10nM. In comparison, sirolimus inhibited proliferation to a significantly lesser extent than everolimus (34.7%, 2.5nM; 46%, 10nM; 50%, 30nM) (Fig. 1A and B) with maximum inhibition at 10nM. Similar results were obtained when EC were pretreated with sirolimus or everolimus for only 2hr (Fig. 1C). Our results indicate that everolimus more potently inhibits HLA I Ab-induced cellular proliferation compared to sirolimus.
Fig. 1. Everolimus inhibits HLA I-stimulated cell proliferation more potent than sirolimus.
Quiescent EC were pretreated with different concentrations of everolimus or sirolimus for 24hr and then stimulated with 1.0µg/ml anti-HLA I mAb for 48hr. Proliferation was determined by A, CFSE dilution or B, BrdU incorporation. For CFSE dilution, EC proliferation was measured by flow cytometry and analyzed by Modfit LT software. Cell proliferation was calculated using the Proliferation Wizard Model. The proliferation index (PI) is the sum of the cells in all generations divided by the computed number of original parent cells present at the start of the experiment. For BrdU incorporation, proliferation index is presented as fold increase in the percent of cells positive for BrdU normalized to untreated control. C EC were pretreated with 10, 20, or 30nM of everolimus or sirolimus for 2 or 24hr, and proliferation in response to HLA I mAb was measured by CFSE dilution. *p<0.05, **p<0.01, and ***p<0.001 were analyzed by one way ANOVA with Fisher’s LSD. Data represent at least three independent experiments. HAEC used in these experiments include CAR, CAS, and 5555.
Effect of Everolimus on HLA I-mediated signal transduction
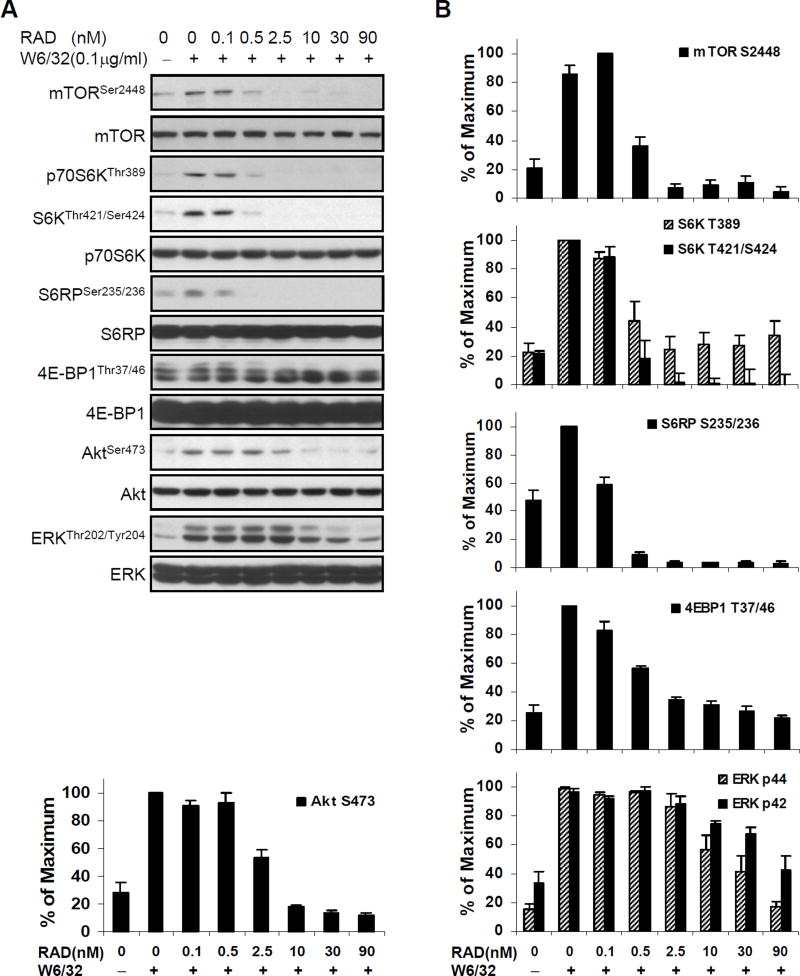
To examine the impact of everolimus on class I-mediated signaling, we established the kinetics and concentration of HLA antibody for optimal class I-mediated activation of the mTORC1 and mTORC2 pathways in primary cultures of human aortic EC (Supplemental Figure 1). The duration (10min) and dose of anti-class I mAb W6/32 (0.1µg/ml) yielding optimal mTOR and ERK phosphorylation and endothelial cell proliferation were used for all signaling experiments (12–13, 17, 35). Quiescent HAEC were next pretreated with everolimus (RAD) for 24hr using the same range of doses that yielded potent inhibition of HAEC proliferation (Fig. 1A and 1B) and then stimulated with anti-HLA I mAb W6/32 (Fig. 2). Pretreatment of HAEC with all doses of everolimus strongly inhibited HLA I Ab-stimulated phosphorylation of mTOR, p70 S6K, S6RP, and 4E-BP1 (Fig. 2A). Fifty percent inhibition of mTOR, S6K, S6RP, and 4E-BP1 phosphorylation was observed between 0.1 and 0.5nM of everolimus, with complete inhibition by 2.5nM. In contrast, a higher dose of everolimus, between 2.5 and 10nM, was required to achieve 50% inhibition of Akt and ERK phosphorylation, with complete inhibition at 10nM (Fig. 2A, 2B).
Fig. 2. Everolimus pharmacokinetics in HLA I-induced activation of mTOR signal pathway, Akt and ERK in EC.
A, Quiescent EC were pretreated with various doses of everolimus for 24hr and stimulated with 0.1µg/ml of anti-HLA I mAb W6/32 for 10 min. Cells were lysed and proteins in the pre-cleared cell lysates were separated by SDS-PAGE followed by immunoblotting with anti-phospho-mTOR Ser2448, anti-phospho-p70S6K Thr389, Thr421/Ser424, anti-phospho-S6RP Ser235/236, anti-phospho-4E-BP1 Thr37/46, anti-phospho-Akt Ser473 or ERK Thr202/Tyr204 Abs. The membrane was reprobed with anti-mTOR, anti-S6K, anti-S6RP, anti-4E-BP1, anti-Akt, or anti-ERK total Abs to confirm equal loading of proteins. B, Phosphorylated protein bands shown in A were quantified by densitometry scan analysis and results are expressed as the mean ± SEM percentage of maximal increase in phosphorylation above control values. Data are representative of three independent experiments. HAEC used in these experiments include CAR, CAS, and 5555.
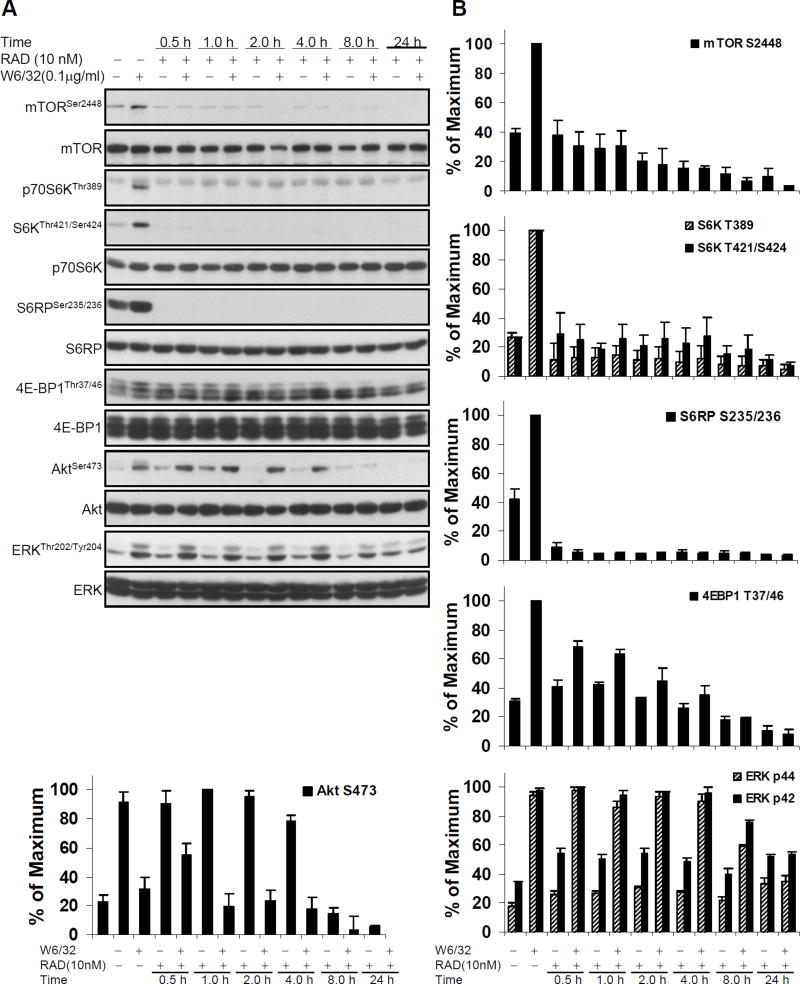
To determine the kinetics of everolimus on HLA I-stimulated signaling, HAEC were treated with W6/32 with or without pretreatment with 10nM everolimus for different time periods. As shown in Fig. 3A and 3B, pretreatment of HAEC with everolimus for 30min was sufficient to block phosphorylation of mTOR, p70S6K, and S6RP. Partial inhibition of 4E-BP1 phosphorylation was observed at 2hr, with complete inhibition at 8hr. A longer preincubation with everolimus, for 8hr, was required to block HLA I-induced Akt and ERK phosphorylation, with complete inhibition at 24hr (Fig. 3A and B).
Fig. 3. Everolimus time kinetics study in HLA I-induced activation of mTOR signal pathway, Akt and ERK in EC.
A, Quiescent EC were pretreated with 10nM everolimus for various time points and were stimulated with anti-HLA I mAb W6/32 for 10 min. Cells were lysed and proteins in the pre-cleared cell lysates were separated by SDS-PAGE followed by immunoblotting with anti-phospho-mTOR Ser2448, anti-phospho-p70S6K Thr389, Thr421/Ser424, anti-phospho-S6RP Ser235/236, anti-phospho-4E-BP1 Thr37/46, anti-phospho-Akt Ser473 or ERK Thr202/Tyr204 Abs. The membrane was reprobed with anti-mTOR, anti-S6K, anti-S6RP, anti-4E-BP1, anti-Akt, or anti-ERK total Abs to confirm equal loading of proteins. B, Phosphorylated protein bands shown in A were quantified by densitometry scan analysis and results are expressed as the mean ± SEM percentage of maximal increase in phosphorylation above control values. Data are representative of three independent experiments. HAEC used in these experiments include CAR, CAS, and 5555.
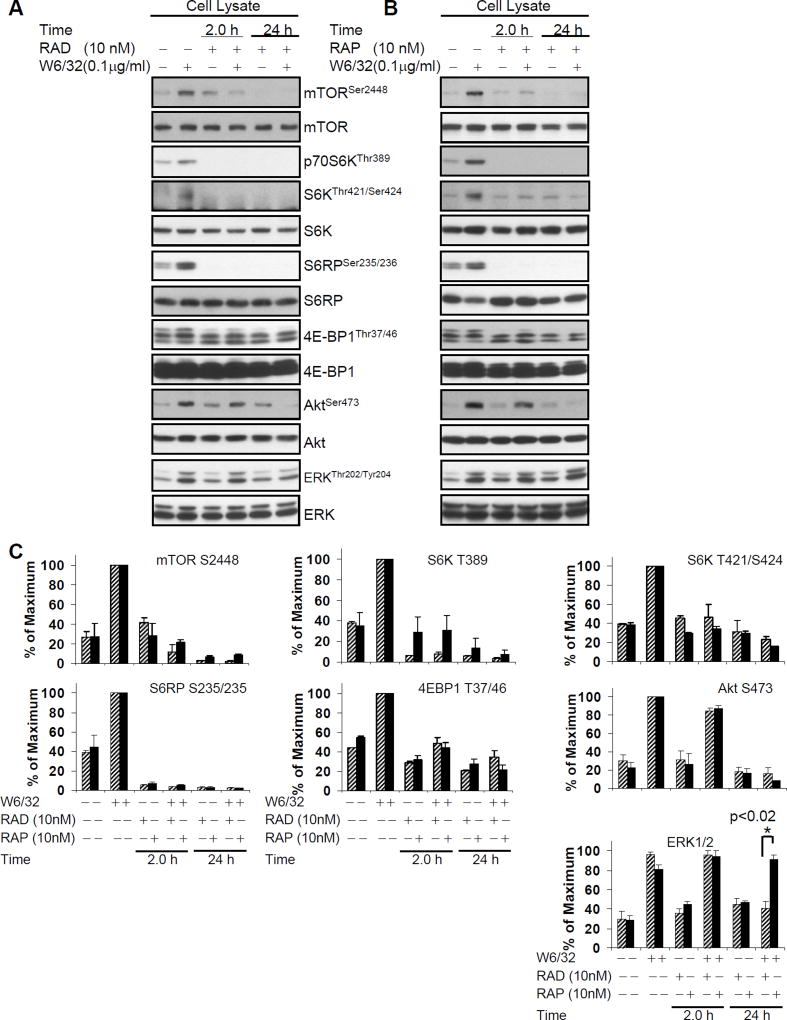
We next sought to compare the effects of everolimus with its parent drug sirolimus on HLA I Ab-stimulated activation of the mTOR signaling network. Both drugs were used at a concentration of 10nM which was determined to maximally inhibit HLA I Ab-induced HAEC proliferation (Fig. 1). 2hr pretreatment of HAEC with either sirolimus or everolimus strongly inhibited HLA I Ab-stimulated phosphorylation of mTOR, and associated mTORC1 targets p70S6K, S6RP, and 4E-BP1 in HAEC (Fig. 4A, B and C). While pretreatment of EC with sirolimus or everolimus for 2hr did not inhibit phosphorylation of Akt at Ser473 (Fig. 4A, B and C), 24hr pretreatment completely inhibited class I-induced phosphorylation of Akt (Fig. 4A, B and C), consistent with previous reports that mTORC2 phosphorylates Akt at this site.
Fig. 4. Everolimus inhibits HLA I-induced phosphorylation of mTOR signal pathway, Akt, and ERK in EC.
Quiescent EC were pretreated with A 10nM of everolimus or B 10nM of sirolimus for 2 or 24hr and stimulated with anti-HLA I mAb W6/32 for 10 min. Cells were lysed and proteins in the pre-cleared cell lysates were separated by SDS-PAGE followed by immunoblotting with anti-phospho-mTOR Ser2448, anti-phospho-p70S6K Thr389, Thr421/Ser424, anti-phospho-S6RP Ser235/236, anti-phospho-4E-BP1 Thr37/46, anti-phospho-Akt Ser473 or ERK Thr202/Tyr204 Abs. The membrane was reprobed with anti-mTOR, anti-S6K, anti-S6RP, anti-4E-BP1, anti-Akt, or anti-ERK total Abs to confirm equal loading of proteins. C, Phosphorylated protein bands shown in A and B were quantified by densitometry scan analysis and results are expressed as the mean ± SEM percentage of maximal increase in phosphorylation above control values. Hatched bar is everolimus treated, black bar is sirolimus treated. Differences in ERK phosphorylation were analyzed by student’s t test, *p<0.05. Data are representative of three independent experiments. HAEC used in these experiments include CAR, CAS, and 5555.
Everolimus inhibits HLA I-stimulated phosphorylation of ERK Thr202/Tyr204 more potently than sirolimus in HAEC
We previously reported the novel findings that siRNA knockdown of Rictor but not Raptor blocked HLA I-stimulated phosphorylation of ERK in HAEC, demonstrating that class I-mediated ERK activation is dependent on mTORC2 (38). Pretreatment of HAEC with either everolimus or sirolimus for 2hr did not inhibit HLA I-induced phosphorylation of ERK at Thr202/Tyr204 (Fig. 4A, B and C), whereas long-term (8hr and 24hr) treatment with 10nM everolimus efficiently blocked phosphorylation of ERK (Fig. 3A and 4A). However, pretreatment with 10nM sirolimus for 24hr had no effect on HLA I-induced phosphorylation of ERK (Fig. 4B). Our data indicate that long-term exposure to everolimus, but not sirolimus, inhibits ERK activation after HLA I crosslinking (Fig. 4C).
Everolimus inhibits HLA I-triggered mTORC1 and mTORC2 formation
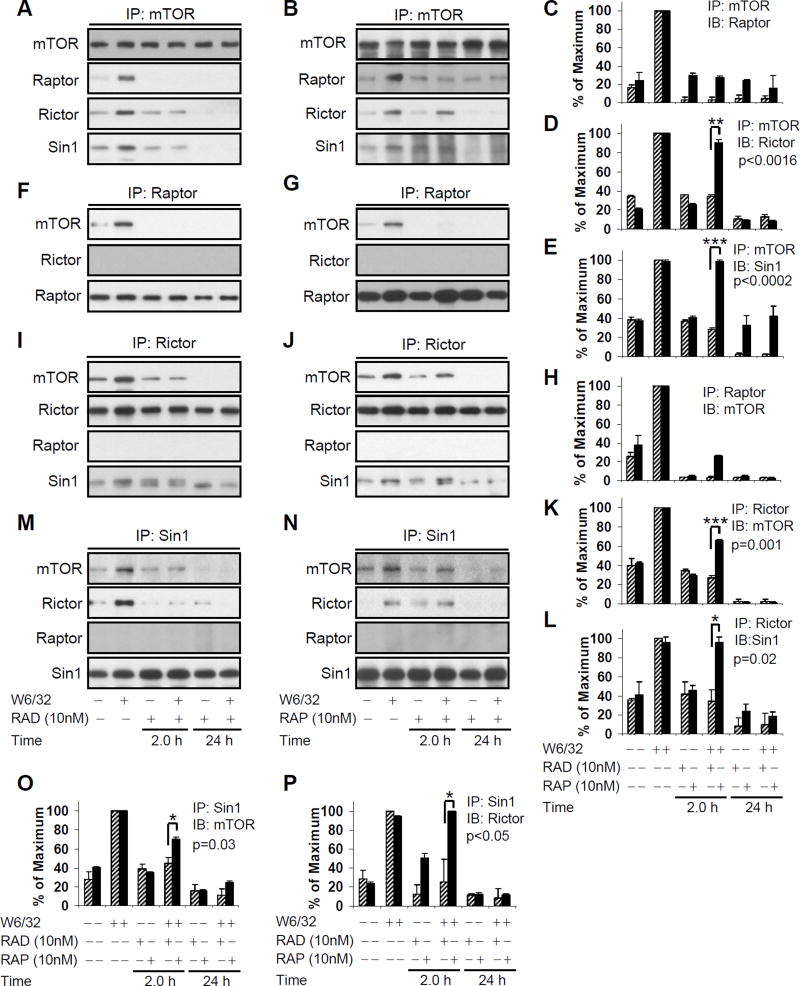
We next determined the effect of everolimus and sirolimus on HLA I-mediated mTORC1 formation. HAEC were pretreated with 10nM everolimus or sirolimus for 2 or 24hr, followed by anti-class I Ab W6/32, and mTOR immunoprecipitated lysates were analyzed by immunoblotting for Raptor. Pretreatment of EC with everolimus or sirolimus for 2 and 24hr prevented mTOR binding to Raptor (Fig. 5A, B and C). We confirmed this observation by immunoprecipitating cell lysates with anti-Raptor Ab, and immunoblotting with anti-mTOR Ab (Fig. 5F, G, and H). Our data indicate that everolimus and sirolimus inhibit HLA I Ab-mediated mTORC1 formation by dissociating the mTOR-Raptor complex.
Fig. 5. Everolimus inhibited HLA I-mediated mTORC1 and mTORC2 formation in EC.
Quiescent EC were pretreated with A, F, I, M 10nM of everolimus or B, G, J, N 10nM of sirolimus for 2 or 24hr, and were stimulated with 0.1µg/ml anti-HLA I mAb for 10 min. Cells were lysed. The pre-cleared lysates were immunoprecipitated A and B with anti-mTOR Ab followed by immunoblotting with anti-Raptor, anti-Rictor, or anti-Sin1 Abs. The membrane was reprobed with anti-mTOR Ab to confirm equal loading of proteins; F and G The pre-cleared lysates were immunoprecipitated with anti-Raptor Ab followed by immunoblotting with anti-mTOR, or anti-Rictor Abs. The membrane was reprobed with anti-Raptor Ab to confirm equal loading of proteins. I and J the pre-cleared lysates were immunoprecipitated with anti-Rictor Ab followed by immunoblotting with anti-mTOR, anti-Raptor, or anti-Sin1 Abs. The membrane was reprobed with anti-Rictor Ab to confirm equal loading of proteins. M and N the pre-cleared lysates were immunoprecipitated with anti-Sin1 Ab followed by immunoblotting with anti-mTOR, anti-Rictor, or anti-Raptor. The membrane was reprobed with anti-Sin1 Ab to confirm equal loading of proteins. C, D, E, H, K, L, O, P Protein bands in the immuno complexes shown in A, B, F, G, I, J, M, N were quantified by densitometry analysis and results are expressed as the mean ± SEM percentage of maximal increase in protein expression above control values. *p<0.05, **p<0.01, and ***p<0.001 were analyzed by one way ANOVA with Fisher’s LSD. Data represent at least three independent experiments. HAEC used in these experiments include CAR, CAS, and 5555.
While it has been reported that everolimus and sirolimus mainly target mTORC1, the literature suggests that longer exposure to sirolimus may inhibit the assembly of mTORC2 (33). Sin1 is a recently identified component of mTORC2 which is essential for its function (39). To determine the effect of everolimus on HLA I-mediated mTORC2 formation, the cytoplasmic extracts of HLA Ab-stimulated EC were analyzed for mTOR-Rictor, mTOR-Sin1, and Rictor-Sin1 molecular association by coimmunoprecipitation. Pretreatment of HAEC with everolimus for 2hr significantly decreased mTOR binding to Rictor and Sin1 after HLA I crosslinking (Fig. 5A and D), while sirolimus failed to prevent mTOR binding to Rictor and Sin1 (Fig. 5B, D, E, K, L). Long-term exposure (24hr) to both everolimus and sirolimus completely blocked mTOR complex formation with Rictor (Fig. 5A, B, D) and Sin1 (Figure 5A, B, E). We confirmed these observations by reverse coimmunoprecipitations (Fig. 5I, J, K, L, M, N, O and P). Our results show that ligation of HLA I on EC with Ab triggers mTOR/Rictor/Sin1 association to form mTORC2. Moreover, while short-term pretreatment with sirolimus prevents mTORC1 but not mTORC2 formation after HLA I crosslinking, everolimus potently and rapidly inhibits both mTORC1 and mTORC2 in endothelial cells.
Everolimus inhibits HLA I-induced cell migration more potently than sirolimus
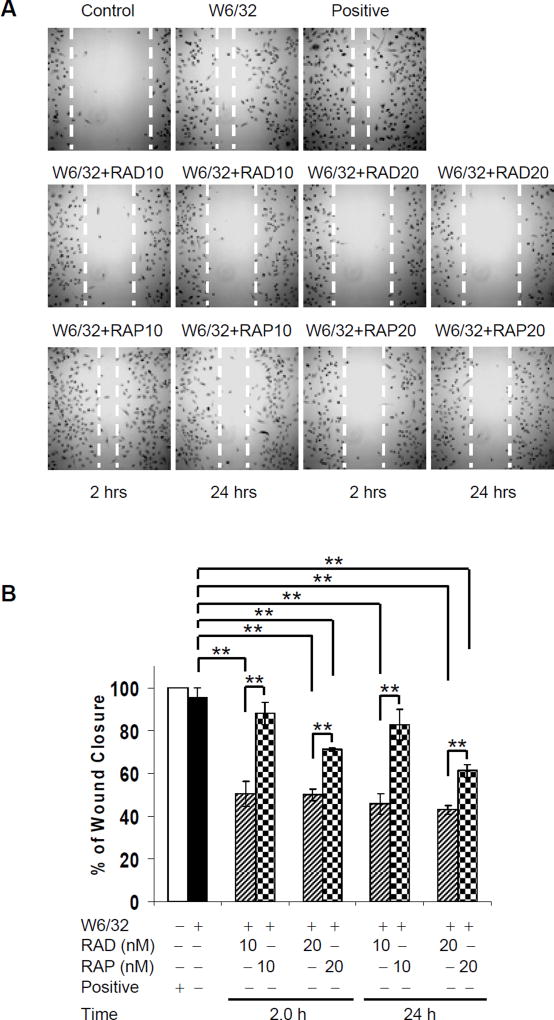
mTORC2 regulates cytoskeletal changes and cell migration through downstream Rho GTPases (27). Given that everolimus effectively inhibited mTORC2 formation, we next determined the effect of mTOR inhibitors on HLA I-stimulated cell migration using a scratch wound assay. As shown in Fig. 6A, HLA I ligation stimulated strong re-endothelialization. Everolimus (10nM for 2hr) significantly inhibited HLA I-induced wound healing by 47.14%, while sirolimus had a weaker inhibitory effect (7.70% inhibition). HLA I-induced cell migration was inhibited by 24hr pretreatment by 52.07% with everolimus, and by 13.40% with sirolimus. Pretreatment of EC with a higher dose, 20nM, of everolimus further reduced class I-induced cell migration (2hr 47.68%; 24hr 54.98%), to a greater extent than sirolimus (2hr 25.40%; 24hr 35.87%). In each condition the inhibitory effect of everolimus on endothelial migration in response to HLA I antibodies was stronger than that of sirolimus (Fig. 6A and B).
Fig. 6. Everolimus inhibits HLA I-mediated cell migration more potent than sirolimus.
A HAEC were grown in 35 mm culture dishes coated with 0.1% gelatin up to confluence. Quiescent cells were pretreated with 10 µg/ml of mitomycin C for 2hr to inhibit cell proliferation before being assayed for their ability to migrate. A scratch wound was created with a sterile 200-µl pipette tip. Wounded cells were pretreated with 10 or 20nM of everolimus or sirolimus at 10nM or 20nM for 2 or 24hr, and then were stimulated with 1.0 µg/ml of anti-HLA I mAb W6/32 for 24hr. EC were incubated in complete medium as positive control. Representative microscopy fields are shown. B, The distance between two edges was measured; migration rate was analyzed by calculating the distance of two front edges of class I-stimulated EC divided by the distance of two edges of control EC. C, HAEC were grown in 24-well plate coated with 0.1% gelatin up to 90% confluence. Quiescent endothelial cells were pretreated with or without everolimus or sirolimus for 2 or 24hr. Migration of HAEC was measured in a transwell insert system. HAEC pretreated with or without mTOR inhibitors were added to the upper chamber of insert and stimulated with mAb W6/32. HAEC treated with VEGF at 10ng/mL served as positive controls. After incubation for 16hr at 37°C, the cells on the upper surface of the membrane were removed with a cotton swab, and the migrated cells were fixed with methanol, stained with crystal violet, and three middle fields per insert were photographed with 10 × objective lens, and counted. Fluorescence 10x microscopy images are presented. D, The bar graph shows the mean ± SEM number of migrated cells. *p<0.05, **p<0.01, and ***p<0.001 were analyzed by one way ANOVA with Fisher’s LSD. Data represent at least three independent experiments. HAEC used in these experiments include CAR, CAS, and 5555.
We next studied the effect of everolimus on endothelial migration using a transwell assay. As shown in Fig. 6C, HLA I ligation with Ab stimulated a 3.18-fold increase in transwell migration over the isotype mIgG control. Pretreatment with everolimus (10nM and 20nM) for 2hr or 24hr significantly inhibited HLA I-induced cell migration by 80–97%; in contrast, sirolimus had a weaker inhibitory effect, reducing migration by only 30.17% (10nM, 2hr) to 69.68% (20nM, 24hr). In each condition the inhibitory effect of everolimus on endothelial migration in response to HLA I antibodies was stronger than that of sirolimus (Fig. 6C and D).
Combination of sirolimus and MEK inhibitor U0126 abolishes HLA I-stimulated cell proliferation
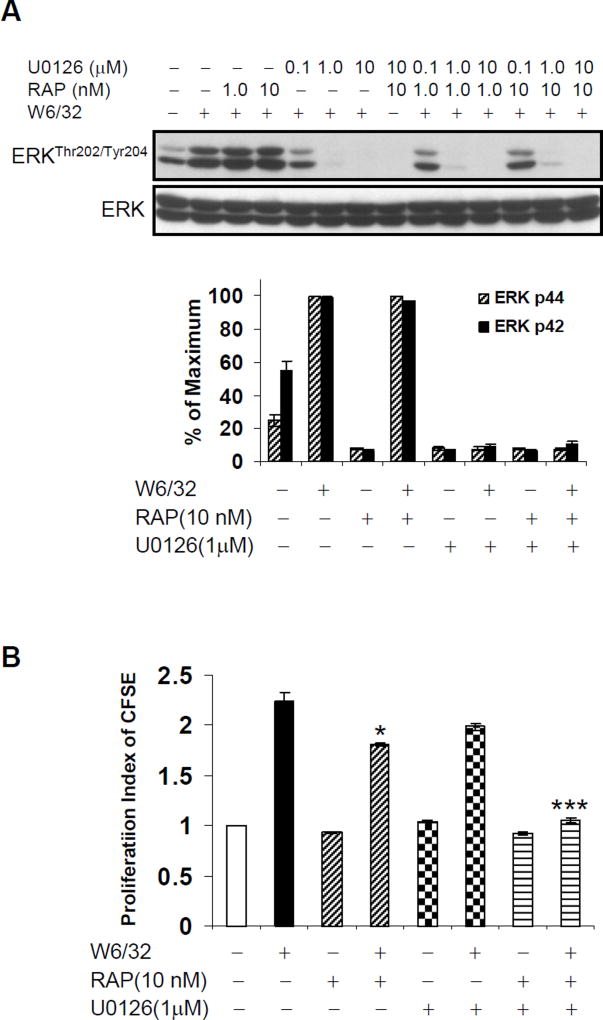
We previously demonstrated that HLA I Ab-triggered cell growth was dependent on ERK, which becomes activated downstream of FGFR signaling (38, 40–41). Everolimus (Fig. 3A and 4A) but not sirolimus (Fig. 4B) prevented HLA I antibody-induced ERK1/2 phosphorylation. Since sirolimus alone, at the dose of 10 nM, could not completely inhibit HLA I-mediated cell proliferation (Fig. 1), we postulated that signaling pathways regulating HLA I-induced cell proliferation include additional signaling pathways, such as MAP kinase. Therefore, we sought to completely block HLA I-induced proliferative signaling through combinatorial inhibition of mTOR with sirolimus and ERK with MEK inhibitor U0126. HAEC were pretreated with sirolimus and/or U0126, and HLA I mAb-induced ERK phosphorylation was assessed. Sirolimus alone could not inhibit HLA I-induced phosphorylation of ERK, while MEK inhibitor U0126 abolished ERK activation as expected (Fig. 7A).
Fig. 7. Combinatorial blockade of ERK and mTOR blocks HLA I antibody-mediated cell proliferation.
A Quiescent EC were pretreated with 10nM of sirolimus for 24hr or/and 1.0 µM of U0126 for 30 min, and then stimulated with anti-HLA I mAb for 10 min. Cells were lysed and proteins in the pre-cleared cell lysates were separated by SDS-PAGE followed by immunoblotting with anti-ERK Thr202/Tyr204 Abs. The membrane was reprobed with anti-ERK total Abs to confirm equal loading of proteins. Phosphorylated protein bands were quantified by densitometry scan analysis and results are expressed as the mean ± SEM percentage of maximal increase in phosphorylation above control values. B EC were pretreated with 10nM of sirolimus for 24hr or/and 1.0 µM of U0126 for 30 min, and then stimulated with 1.0µg/ml anti-HLA I mAb for 48hr. Proliferation was measured by CFSE dilution. *p<0.05, and ***p<0.001 versus no inhibitor were analyzed by one way ANOVA with Fisher’s LSD. Data represent at least three independent experiments. HAEC used in these experiments include CAR, CAS, and 5555.
We next investigated whether combinatorial blockade of ERK and mTOR was sufficient to abolish HLA I antibody-mediated cell proliferation. EC were pretreated with sirolimus with or without U0126, and then stimulated with anti-HLA I mAb W6/32 for 48hr. As above, pretreatment of HAEC with sirolimus alone slightly inhibited HLA I-induced proliferation (Fig. 7B). ERK inhibitor alone, at the dose of 1.0 µM, did not significantly inhibit HLA I-stimulated cell proliferation. Notably, combined treatment with sirolimus and U0126 reduced class I-induced proliferation to background levels (Fig. 7B).
These results demonstrate an additive effect of sirolimus with ERK inhibition, and suggest that inhibition of both mTOR and MAP kinase pathways is needed to completely block HLA I-stimulated ERK phosphorylation and cell proliferation. As everolimus alone was sufficient to abolish HLA I Ab-mediated proliferation, we postulate that everolimus may act by inhibiting both mTOR and MAP kinase pathways.
DISCUSSION
We show that mTORC1 and mTORC2 play critical roles in anti-HLA I Ab-induced proliferative and migratory signaling in endothelial cells. We observed that both everolimus and sirolimus inhibit HLA I-induced mTORC1 formation. For the first time, we demonstrate that ligation of HLA I with Ab stimulates molecular association of Sin1, mTOR and Rictor, which could be blocked by everolimus. Importantly, we demonstrate that everolimus inhibits HLA I-mediated phosphorylation of ERK and mTORC2, and more potently inhibits HLA I Ab-induced functional changes in endothelial cells compared with sirolimus.
Everolimus has the same mechanism of action as sirolimus, but lacks O-alkylation at C40. Despite the lower affinity of everolimus for FKBP12, we were surprised to find that everolimus is a more potent inhibitor of HLA I antibody-induced signaling and functional changes compared with sirolimus, which is not in agreement with previous studies (42). While the IC50 of everolimus is 3-fold higher than sirolimus (37, 42), it has shown comparable activity in vivo and increased oral bioavailability and stability (43–44) compared with sirolimus (37). The concentrations we used in in vitro experiments were based on previous published work by Novartis (37) and by our group (19) and were comparable to target clinical trough levels, which range from 16–24ng/mL in plasma for sirolimus, equivalent to 13.1–21.8nM, and 3–8ng/mL, or 3.13–8.35nM for everolimus (45–47). Notably, the recommended levels of everolimus are lower than for sirolimus, consistent with our findings that everolimus more potently inhibits mTOR and related signaling.
We previously observed that siRNA inhibition of mTOR blocked HLA I-induced cellular proliferation (19). Therefore, we postulated that mTOR/p70 S6K/S6RP signaling networks play an important role in regulating cell migration and proliferation, and may be therapeutic targets in preventing chronic TAV. In this study, we found that everolimus abolished HLA I-stimulated cell proliferation and migration, while sirolimus was significantly less effective. mTOR inhibitors are emerging as part of promising renal-sparing calcineurin inhibitor (CNI)-free immunosuppressive regimens in solid organ transplantation. Recent reports demonstrated that conversion from CNI-based immunomodulation to an mTOR inhibitor-based regimen improved graft function and survival in renal allograft patients (48), and reduced neointimal thickening in cardiac allografts compared with standard immunosuppression (49).
Angiogenesis is a complex stepwise process resulting from endothelial activation, cellular proliferation and migration, and vascular remodeling with new vessel formation has been demonstrated in native atherosclerosis. Angiogenic changes, characterized by new microvessel formation under the hyperplastic neointima, occur in cardiac transplant recipients with TAV (50). The new vessels are thought to arise not from angiogenic sprouting but from a repair response in which the microvessels functionally support the neointimal lesions. Both everolimus and sirolimus have anti-angiogenic effects in vitro and in experimental models (51–58) and may prevent neointimal thickening in chronic allograft rejection. Endothelial cell migration is important for the angiogenic processes which support neointimal hyperplasia, but is also critical for wound healing. As we observed in this study, rapamycin inhibits wound closure and re-endothelialization responses in vitro (59–61). Conflicting studies have reported sirolimus-associated wound healing complications in transplant recipients at high doses (62–63), while other reports have not demonstrated sirolimus-associated wound healing complications (64–66). A recent report showed that everolimus could be used in renal-sparing immunosuppression without these adverse effects in liver transplant recipients (67). However, increased thrombotic complications were observed in renal transplant patients treated with everolimus (68). Our data suggest that mTOR inhibitors may block the pro-angiogenic changes that contribute to transplant vasculopathy, while at the same time increasing the risk of wound healing complications.
ERK1 and ERK2 are members of a family of serine/threonine kinases that play an important role in cell proliferation, differentiation, survival, and reorganization of the actin cytoskeleton (69). We postulated that the higher efficacy of everolimus against HLA I-mediated cellular proliferation and migration was due to its inhibition of ERK1/2 activation, a capacity sirolimus notably lacked. We have previously demonstrated a functional link between ERK and mTORC2, suggesting that inhibition of ERK by everolimus is not due to off-target effects but to effects on mTORC2. We reported that knockdown of Rictor but not Raptor by siRNA blocked HLA I-induced phosphorylation of ERK1/2 (38), and moreover that ERK proteins colocalize with mTOR and Rictor after HLA I crosslinking (18). Our data demonstrate that everolimus completely blocks HLA I-stimulated cell proliferation by inhibiting both mTOR and ERK1/2 signal transduction pathways, while combinatorial blockade of ERK1/2 and mTOR by sirolimus was required to abolish proliferation. MEK/ERK pathways are canonically activated downstream of Raf, a pathway which may be implicated in FGFR-mediated activation of ERK1/2 in endothelial cells (12). Raf activity can be negatively regulated by Akt in tumor cells, which may lead to a reduction in ERK1/2 activation depending upon the concentration and nature of ligand (70); and reviewed in (71) and (72). Overexpression of an active Raf1 mutant during development increases expression of ERK, leading to commitment of venous ECs to lymphatic fate, and excessive ERK activation leads to lymphatic abnormalities (73). Further investigation is warranted to determine whether the Ras/Raf pathway is activated in endothelial cells after stimulation with HLA I antibodies, or whether increased Akt activity may blunt ERK1/2 activation downstream of Raf.
Taken together, we show that everolimus rapidly inhibits formation of mTORC2 at a lower dose than sirolimus and effectively targets mTORC2-dependent signaling and ERK1/2 activation. Moreover, everolimus more potently prevents HLA I antibody-triggered cellular proliferation and migration. These results suggest that everolimus may be used at lower concentrations in the clinic with similar efficacy, which is consistent with the lower recommended trough levels for everolimus compared with sirolimus. These findings merit further evaluation of HLA I-dependent endothelial signaling to help identify other molecular targets in antibody-mediated chronic allograft rejection.
Supplementary Material
Acknowledgments
Funding Sources: This work was supported by the National Research Service Award Vascular Biology Training Grant 5T32HL069766-12 (to N.M.V.), the National Institute of Allergy and Infectious Diseases Grant RO1 AI 042819 and NIH U018I077821, the National Heart Lung and Blood Institute Grant RO1 HL 090995, and the Novartis Investigator Initiated Research Grant (to E.F.R).
Abbreviations
- Ab
Antibody
- AMR
antibody-mediated rejection
- DSA
donor specific antibodies
- 4E-BP1
initiation factor 4E binding protein 1
- mTOR
mammalian target of rapamycin
- mTORC1/2
mTOR complex 1/2
- PI3K
phosphoinositide 3-kinase
- Raptor
regulatory associated protein of TOR
- Rictor
rapamycin insensitive companion of TOR
- S6K
p70 ribosomal S6 kinase
- S6RP
S6 ribosomal protein
- Sin1
stress-activated protein kinase-interacting protein 1
- TAV
transplant associated vasculopathy
Footnotes
DISCLOSURE
The authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation. This study was supported by the Novartis Investigator Initiated Research Grant.
References
- 1.Cai J, Terasaki PI. Post-transplantation antibody monitoring and HLA antibody epitope identification. Curr Opin Immunol. 2008 Oct;20(5):602–6. doi: 10.1016/j.coi.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 2.Terasaki PI. Humoral theory of transplantation. Am J Transplant. 2003 Jun;3(6):665–73. doi: 10.1034/j.1600-6143.2003.00135.x. [DOI] [PubMed] [Google Scholar]
- 3.Terasaki PI, Cai J. Humoral theory of transplantation: further evidence. Curr Opin Immunol. 2005 Oct;17(5):541–5. doi: 10.1016/j.coi.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 4.Costa AN, Scolari MP, Iannelli S, Buscaroli A, D’Arcangelo GL, Brando B, et al. The presence of posttransplant HLA-specific IgG antibodies detected by enzyme-linked immunosorbent assay correlates with specific rejection pathologies. Transplantation. 1997;63(1):167–9. doi: 10.1097/00007890-199701150-00033. [DOI] [PubMed] [Google Scholar]
- 5.Soleimani B, Lechler RI, Hornick PI, George AJ. Role of alloantibodies in the pathogenesis of graft arteriosclerosis in cardiac transplantation. Am J Transplant. 2006 Aug;6(8):1781–5. doi: 10.1111/j.1600-6143.2006.01401.x. [DOI] [PubMed] [Google Scholar]
- 6.Bian H, Harris PE, Mulder A, Reed EF. Anti-HLA antibody ligation to HLA class I molecules expressed by endothelial cells stimulates tyrosine phosphorylation, inositol phosphate generation, and proliferation. Human Immunology. 1997;53(1):90–7. doi: 10.1016/S0198-8859(96)00272-8. [DOI] [PubMed] [Google Scholar]
- 7.Bian H, Harris PE, Reed EF. Ligation of HLA class I molecules on smooth muscle cells with anti-HLA antibodies induces tyrosine phosphorylation, fibroblast growth factor receptor expression and cell proliferation. International Immunology. 1998;10(9):1315–23. doi: 10.1093/intimm/10.9.1315. [DOI] [PubMed] [Google Scholar]
- 8.Bian H, Reed EF. Alloantibody-Mediated Class I Signal Transduction in Endothelial Cells and Smooth Muscle Cells: Enhancement by IFN-gamma and TNF-alpha. J Immunol. 1999;163(2):1010–8. [PubMed] [Google Scholar]
- 9.Coupel S, Leboeuf F, Boulday G, Soulillou JP, Charreau B. RhoA activation mediates phosphatidylinositol 3-kinase-dependent proliferation of human vascular endothelial cells: an alloimmune mechanism of chronic allograft nephropathy. J Am Soc Nephrol. 2004 Sep;15(9):2429–39. doi: 10.1097/01.ASN.0000138237.42675.45. [DOI] [PubMed] [Google Scholar]
- 10.Harris PE, Bian H, Reed EF. Induction of high affinity fibroblast growth factor receptor expression and proliferation in human endothelial cells by anti-HLA antibodies: a possible mechanism for transplant atherosclerosis. Journal of Immunology. 1997;159(11):5697–704. [PubMed] [Google Scholar]
- 11.Jin YP, Fishbein MC, Said JW, Jindra PT, Rajalingam R, Rozengurt E, et al. Anti-HLA class I antibody-mediated activation of the PI3K/Akt signaling pathway and induction of Bcl-2 and Bcl-xL expression in endothelial cells. Hum Immunol. 2004 Apr;65(4):291–302. doi: 10.1016/j.humimm.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 12.Jin YP, Singh RP, Du ZY, Rajasekaran AK, Rozengurt E, Reed EF. Ligation of HLA class I molecules on endothelial cells induces phosphorylation of Src, paxillin and focal adhesion kinase in an actin dependent manner. J Immunol. 2002;168(11):5415–23. doi: 10.4049/jimmunol.168.11.5415. [DOI] [PubMed] [Google Scholar]
- 13.Lepin EJ, Jin YP, Barwe SP, Rozengurt E, Reed EF. HLA class I signal transduction is dependent on Rho GTPase and ROK. Biochem Biophys Res Commun. 2004 Oct 8;323(1):213–7. doi: 10.1016/j.bbrc.2004.08.082. [DOI] [PubMed] [Google Scholar]
- 14.Lepin EJ, Zhang Q, Zhang X, Jindra PT, Hong LS, Ayele P, et al. Phosphorylated S6 ribosomal protein: a novel biomarker of antibody-mediated rejection in heart allografts. Am J Transplant. 2006 Jul;6(7):1560–71. doi: 10.1111/j.1600-6143.2006.01355.x. [DOI] [PubMed] [Google Scholar]
- 15.Narayanan K, Jaramillo A, Phelan DL, Mohanakumar T. Pre-exposure to sub-saturating concentrations of HLA class I antibodies confers resistance to endothelial cells against antibody complement-mediated lysis by regulating Bad through the phosphatidylinositol 3-kinase/Akt pathway. Eur J Immunol. 2004 Aug;34(8):2303–12. doi: 10.1002/eji.200324843. [DOI] [PubMed] [Google Scholar]
- 16.Nath N, Bian H, Reed EF, Chellappan SP. HLA Class I-Mediated Induction of Cell Proliferation Involves Cyclin E-Mediated Inactivation of Rb Function and Induction of E2F Activity. The Journal of Immunology. 1999;162:5351–8. [PubMed] [Google Scholar]
- 17.Jin YP, Korin Y, Zhang X, Jindra PT, Rozengurt E, Reed EF. RNA Interference Elucidates the Role of Focal Adhesion Kinase in HLA Class I-Mediated Focal Adhesion Complex Formation and Proliferation in Human Endothelial Cells. J Immunol. 2007 Jun 15;178(12):7911–22. doi: 10.4049/jimmunol.178.12.7911. [DOI] [PubMed] [Google Scholar]
- 18.Ziegler ME, Jin YP, Young SH, Rozengurt E, Reed EF. HLA class I-mediated stress fiber formation requires ERK1/2 activation in the absence of an increase in intracellular Ca2+ in human aortic endothelial cells. Am J Physiol Cell Physiol. 2012 Oct 15;303(8):C872–82. doi: 10.1152/ajpcell.00199.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jindra PT, Jin YP, Rozengurt E, Reed EF. HLA class I antibody-mediated endothelial cell proliferation via the mTOR pathway. J Immunol. 2008 Feb 15;180(4):2357–66. doi: 10.4049/jimmunol.180.4.2357. [DOI] [PubMed] [Google Scholar]
- 20.Kim DH, Sarbassov DD, Ali SM, King JE, Latek RR, Erdjument-Bromage H, et al. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell. 2002 Jul 26;110(2):163–75. doi: 10.1016/s0092-8674(02)00808-5. [DOI] [PubMed] [Google Scholar]
- 21.Nojima H, Tokunaga C, Eguchi S, Oshiro N, Hidayat S, Yoshino K, et al. The mammalian target of rapamycin (mTOR) partner, raptor, binds the mTOR substrates p70 S6 kinase and 4E-BP1 through their TOR signaling (TOS) motif. J Biol Chem. 2003 May 2;278(18):15461–4. doi: 10.1074/jbc.C200665200. [DOI] [PubMed] [Google Scholar]
- 22.Hay N. The Akt-mTOR tango and its relevance to cancer. Cancer Cell. 2005 Sep;8(3):179–83. doi: 10.1016/j.ccr.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 23.Sabatini DM. mTOR and cancer: insights into a complex relationship. Nat Rev Cancer. 2006 Sep;6(9):729–34. doi: 10.1038/nrc1974. [DOI] [PubMed] [Google Scholar]
- 24.Hara K, Maruki Y, Long X, Yoshino K, Oshiro N, Hidayat S, et al. Raptor, a binding partner of target of rapamycin (TOR), mediates TOR action. Cell. 2002 Jul 26;110(2):177–89. doi: 10.1016/s0092-8674(02)00833-4. [DOI] [PubMed] [Google Scholar]
- 25.Makino C, Sano Y, Shinagawa T, Millar JB, Ishii S. Sin1 binds to both ATF-2 and p38 and enhances ATF-2-dependent transcription in an SAPK signaling pathway. Genes Cells. 2006 Nov;11(11):1239–51. doi: 10.1111/j.1365-2443.2006.01016.x. [DOI] [PubMed] [Google Scholar]
- 26.Yang Q, Inoki K, Ikenoue T, Guan KL. Identification of Sin1 as an essential TORC2 component required for complex formation and kinase activity. Genes Dev. 2006 Oct 15;20(20):2820–32. doi: 10.1101/gad.1461206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sarbassov DD, Ali SM, Kim DH, Guertin DA, Latek RR, Erdjument-Bromage H, et al. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol. 2004 Jul 27;14(14):1296–302. doi: 10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 28.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005 Feb 18;307(5712):1098–101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 29.Gabardi S, Baroletti SA. Everolimus: a proliferation signal inhibitor with clinical applications in organ transplantation, oncology, and cardiology. Pharmacotherapy. 2010 Oct;30(10):1044–56. doi: 10.1592/phco.30.10.1044. [DOI] [PubMed] [Google Scholar]
- 30.Rosner M, Hengstschlager M. Cytoplasmic and nuclear distribution of the protein complexes mTORC1 and mTORC2: rapamycin triggers dephosphorylation and delocalization of the mTORC2 components rictor and sin1. Hum Mol Genet. 2008 Oct 1;17(19):2934–48. doi: 10.1093/hmg/ddn192. [DOI] [PubMed] [Google Scholar]
- 31.Majewski M, Korecka M, Joergensen J, Fields L, Kossev P, Schuler W, et al. Immunosuppressive TOR kinase inhibitor everolimus (RAD) suppresses growth of cells derived from posttransplant lymphoproliferative disorder at allograft-protecting doses. Transplantation. 2003 May 27;75(10):1710–7. doi: 10.1097/01.TP.0000063934.89714.19. [DOI] [PubMed] [Google Scholar]
- 32.Dormond O, Madsen JC, Briscoe DM. The effects of mTOR-Akt interactions on anti-apoptotic signaling in vascular endothelial cells. J Biol Chem. 2007 Aug 10;282(32):23679–86. doi: 10.1074/jbc.M700563200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sarbassov DD, Ali SM, Sengupta S, Sheen JH, Hsu PP, Bagley AF, et al. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell. 2006 Apr 21;22(2):159–68. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 34.Yeh M, Leitinger N, de Martin R, Onai N, Matsushima K, Vora DK, et al. Increased transcription of IL-8 in endothelial cells is differentially regulated by TNF-alpha and oxidized phospholipids. Arterioscler Thromb Vasc Biol. 2001 Oct;21(10):1585–91. doi: 10.1161/hq1001.097027. [DOI] [PubMed] [Google Scholar]
- 35.Zhang X, Rozengurt E, Reed EF. HLA class I molecules partner with integrin beta4 to stimulate endothelial cell proliferation and migration. Sci Signal. 2010;3(149):ra85. doi: 10.1126/scisignal.2001158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Edwards SR, Wandless TJ. The rapamycin-binding domain of the protein kinase mammalian target of rapamycin is a destabilizing domain. J Biol Chem. 2007 May 4;282(18):13395–401. doi: 10.1074/jbc.M700498200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schuler W, Sedrani R, Cottens S, Haberlin B, Schulz M, Schuurman HJ, et al. SDZ RAD, a new rapamycin derivative: pharmacological properties in vitro and in vivo. Transplantation. 1997 Jul 15;64(1):36–42. doi: 10.1097/00007890-199707150-00008. [DOI] [PubMed] [Google Scholar]
- 38.Jindra PT, Jin YP, Jacamo R, Rozengurt E, Reed EF. MHC class I and integrin ligation induce ERK activation via an mTORC2-dependent pathway. Biochem Biophys Res Commun. 2008 May 2;369(2):781–7. doi: 10.1016/j.bbrc.2008.02.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jacinto E, Facchinetti V, Liu D, Soto N, Wei S, Jung SY, et al. SIN1/MIP1 maintains rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell. 2006 Oct 6;127(1):125–37. doi: 10.1016/j.cell.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 40.Jin YP, Jindra PT, Gong KW, Lepin EJ, Reed EF. Anti-HLA class I antibodies activate endothelial cells and promote chronic rejection. Transplantation. 2005 Feb 15;79(3 Suppl):S19–21. doi: 10.1097/01.tp.0000153293.39132.44. [DOI] [PubMed] [Google Scholar]
- 41.Reed EF. Signal transduction via MHC class I molecules in endothelial and smooth muscle cells. Crit Rev Immunol. 2003;23(1–2):109–28. doi: 10.1615/critrevimmunol.v23.i12.60. [DOI] [PubMed] [Google Scholar]
- 42.Dunn C, Croom KF. Everolimus: a review of its use in renal and cardiac transplantation. Drugs. 2006;66(4):547–70. doi: 10.2165/00003495-200666040-00009. [DOI] [PubMed] [Google Scholar]
- 43.Kirchner GI, Meier-Wiedenbach I, Manns MP. Clinical pharmacokinetics of everolimus. Clin Pharmacokinet. 2004;43(2):83–95. doi: 10.2165/00003088-200443020-00002. [DOI] [PubMed] [Google Scholar]
- 44.Mahalati K, Kahan BD. Clinical pharmacokinetics of sirolimus. Clin Pharmacokinet. 2001;40(8):573–85. doi: 10.2165/00003088-200140080-00002. [DOI] [PubMed] [Google Scholar]
- 45.Backman L, Kreis H, Morales JM, Wilczek H, Taylor R, Burke JT. Sirolimus steady-state trough concentrations are not affected by bolus methylprednisolone therapy in renal allograft recipients. Br J Clin Pharmacol. 2002 Jul;54(1):65–8. doi: 10.1046/j.1365-2125.2002.01594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oberbauer R, Kreis H, Johnson RW, Mota A, Claesson K, Ruiz JC, et al. Long-term improvement in renal function with sirolimus after early cyclosporine withdrawal in renal transplant recipients: 2-year results of the Rapamune Maintenance Regimen Study. Transplantation. 2003 Jul 27;76(2):364–70. doi: 10.1097/01.TP.0000074360.62032.39. [DOI] [PubMed] [Google Scholar]
- 47.Schweiger M, Wasler A, Prenner G, Stiegler P, Stadlbauer V, Schwarz M, et al. Everolimus and reduced cyclosporine trough levels in maintenance heart transplant recipients. Transpl Immunol. 2006 Jun;16(1):46–51. doi: 10.1016/j.trim.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 48.Paoletti E, Ratto E, Bellino D, Marsano L, Cassottana P, Cannella G. Effect of early conversion from CNI to sirolimus on outcomes in kidney transplant recipients with allograft dysfunction. J Nephrol. 2012 Sep-Oct;25(5):709–18. doi: 10.5301/jn.5000044. [DOI] [PubMed] [Google Scholar]
- 49.Eisen HJ, Tuzcu EM, Dorent R, Kobashigawa J, Mancini D, Valantine-von Kaeppler HA, et al. Everolimus for the prevention of allograft rejection and vasculopathy in cardiac-transplant recipients. N Engl J Med. 2003 Aug 28;349(9):847–58. doi: 10.1056/NEJMoa022171. [DOI] [PubMed] [Google Scholar]
- 50.Atkinson C, Southwood M, Pitman R, Phillpotts C, Wallwork J, Goddard M. Angiogenesis occurs within the intimal proliferation that characterizes transplant coronary artery vasculopathy. J Heart Lung Transplant. 2005 May;24(5):551–8. doi: 10.1016/j.healun.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 51.Guba M, Koehl GE, Neppl E, Doenecke A, Steinbauer M, Schlitt HJ, et al. Dosing of rapamycin is critical to achieve an optimal antiangiogenic effect against cancer. Transpl Int. 2005 Jan;18(1):89–94. doi: 10.1111/j.1432-2277.2004.00026.x. [DOI] [PubMed] [Google Scholar]
- 52.Lane HA, Wood JM, McSheehy PM, Allegrini PR, Boulay A, Brueggen J, et al. mTOR inhibitor RAD001 (everolimus) has antiangiogenic/vascular properties distinct from a VEGFR tyrosine kinase inhibitor. Clin Cancer Res. 2009 Mar 1;15(5):1612–22. doi: 10.1158/1078-0432.CCR-08-2057. [DOI] [PubMed] [Google Scholar]
- 53.Mabuchi S, Altomare DA, Cheung M, Zhang L, Poulikakos PI, Hensley HH, et al. RAD001 inhibits human ovarian cancer cell proliferation, enhances cisplatin-induced apoptosis, and prolongs survival in an ovarian cancer model. Clin Cancer Res. 2007 Jul 15;13(14):4261–70. doi: 10.1158/1078-0432.CCR-06-2770. [DOI] [PubMed] [Google Scholar]
- 54.Mabuchi S, Altomare DA, Connolly DC, Klein-Szanto A, Litwin S, Hoelzle MK, et al. RAD001 (Everolimus) delays tumor onset and progression in a transgenic mouse model of ovarian cancer. Cancer Res. 2007 Mar 15;67(6):2408–13. doi: 10.1158/0008-5472.CAN-06-4490. [DOI] [PubMed] [Google Scholar]
- 55.Manegold PC, Paringer C, Kulka U, Krimmel K, Eichhorn ME, Wilkowski R, et al. Antiangiogenic therapy with mammalian target of rapamycin inhibitor RAD001 (Everolimus) increases radiosensitivity in solid cancer. Clin Cancer Res. 2008 Feb 1;14(3):892–900. doi: 10.1158/1078-0432.CCR-07-0955. [DOI] [PubMed] [Google Scholar]
- 56.Phung TL, Ziv K, Dabydeen D, Eyiah-Mensah G, Riveros M, Perruzzi C, et al. Pathological angiogenesis is induced by sustained Akt signaling and inhibited by rapamycin. Cancer Cell. 2006 Aug;10(2):159–70. doi: 10.1016/j.ccr.2006.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Semela D, Piguet AC, Kolev M, Schmitter K, Hlushchuk R, Djonov V, et al. Vascular remodeling and antitumoral effects of mTOR inhibition in a rat model of hepatocellular carcinoma. J Hepatol. 2007 May;46(5):840–8. doi: 10.1016/j.jhep.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 58.Shinohara ET, Cao C, Niermann K, Mu Y, Zeng F, Hallahan DE, et al. Enhanced radiation damage of tumor vasculature by mTOR inhibitors. Oncogene. 2005 Aug 18;24(35):5414–22. doi: 10.1038/sj.onc.1208715. [DOI] [PubMed] [Google Scholar]
- 59.Jin C, Zhao Y, Yu L, Xu S, Fu G. MicroRNA-21 mediates the rapamycin-induced suppression of endothelial proliferation and migration. FEBS Lett. 2013 Feb 14;587(4):378–85. doi: 10.1016/j.febslet.2012.12.021. [DOI] [PubMed] [Google Scholar]
- 60.Kwon YS, Hong HS, Kim JC, Shin JS, Son Y. Inhibitory effect of rapamycin on corneal neovascularization in vitro and in vivo. Invest Ophthalmol Vis Sci. 2005 Feb;46(2):454–60. doi: 10.1167/iovs.04-0753. [DOI] [PubMed] [Google Scholar]
- 61.Liu HT, Li F, Wang WY, Li XJ, Liu YM, Wang RA, et al. Rapamycin inhibits re-endothelialization after percutaneous coronary intervention by impeding the proliferation and migration of endothelial cells and inducing apoptosis of endothelial progenitor cells. Tex Heart Inst J. 2010;37(2):194–201. [PMC free article] [PubMed] [Google Scholar]
- 62.Dean PG, Lund WJ, Larson TS, Prieto M, Nyberg SL, Ishitani MB, et al. Wound-healing complications after kidney transplantation: a prospective, randomized comparison of sirolimus and tacrolimus. Transplantation. 2004 May 27;77(10):1555–61. doi: 10.1097/01.tp.0000123082.31092.53. [DOI] [PubMed] [Google Scholar]
- 63.Knight RJ, Villa M, Laskey R, Benavides C, Schoenberg L, Welsh M, et al. Risk factors for impaired wound healing in sirolimus-treated renal transplant recipients. Clin Transplant. 2007 Jul-Aug;21(4):460–5. doi: 10.1111/j.1399-0012.2007.00668.x. [DOI] [PubMed] [Google Scholar]
- 64.Flechner SM, Zhou L, Derweesh I, Mastroianni B, Savas K, Goldfarb D, et al. The impact of sirolimus, mycophenolate mofetil, cyclosporine, azathioprine, and steroids on wound healing in 513 kidney-transplant recipients. Transplantation. 2003 Dec 27;76(12):1729–34. doi: 10.1097/01.TP.0000093502.26208.42. [DOI] [PubMed] [Google Scholar]
- 65.Hulbert AL, Delahunty AJ, Rajab A, Forbes RC, Winters HA. The utilization of sirolimus and the impact on wound-healing complications in obese kidney transplant recipients. Clin Transplant. 2013 Jul-Aug;27(4):E521–7. doi: 10.1111/ctr.12183. [DOI] [PubMed] [Google Scholar]
- 66.Nashan B. Maximizing the clinical outcome with mTOR inhibitors in the renal transplant recipient: defining the role of calcineurin inhibitors. Transpl Int. 2004 Jul;17(6):279–85. doi: 10.1007/s00147-004-0716-5. [DOI] [PubMed] [Google Scholar]
- 67.Saliba F, De Simone P, Nevens F, De Carlis L, Metselaar HJ, Beckebaum S, et al. Renal function at two years in liver transplant patients receiving everolimus: results of a randomized, multicenter study. Am J Transplant. 2013 Jul;13(7):1734–45. doi: 10.1111/ajt.12280. [DOI] [PubMed] [Google Scholar]
- 68.Baas MC, Gerdes VE, Ten Berge IJ, Heutinck KM, Florquin S, Meijers JC, et al. Treatment with everolimus is associated with a procoagulant state. Thromb Res. 2013 Aug;132(2):307–11. doi: 10.1016/j.thromres.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 69.Chang F, Steelman LS, Lee JT, Shelton JG, Navolanic PM, Blalock WL, et al. Signal transduction mediated by the Ras/Raf/MEK/ERK pathway from cytokine receptors to transcription factors: potential targeting for therapeutic intervention. Leukemia. 2003 Jul;17(7):1263–93. doi: 10.1038/sj.leu.2402945. [DOI] [PubMed] [Google Scholar]
- 70.Deng Y, Larrivee B, Zhuang ZW, Atri D, Moraes F, Prahst C, et al. Endothelial RAF1/ERK activation regulates arterial morphogenesis. Blood. 2013 May 9;121(19):3988–96. S1–9. doi: 10.1182/blood-2012-12-474601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Moelling K, Schad K, Bosse M, Zimmermann S, Schweneker M. Regulation of Raf-Akt Cross-talk. J Biol Chem. 2002 Aug 23;277(34):31099–106. doi: 10.1074/jbc.M111974200. [DOI] [PubMed] [Google Scholar]
- 72.Deng Y, Simons M. Lymphatic fate determination: playing RAF with ERK. Cell Cycle. 2013 Apr 15;12(8):1157–8. doi: 10.4161/cc.24491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Deng Y, Atri D, Eichmann A, Simons M. Endothelial ERK signaling controls lymphatic fate specification. J Clin Invest. 2013 Mar 1;123(3):1202–15. doi: 10.1172/JCI63034. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.