Abstract
The mysteriously diverse phenotypes in mice lacking the p53 homolog p73 are recently unified by new analysis showing p73 is required for formation of multiciliated epithelia. p73 directly activates FoxJ1, the central transcriptional driver for multiciliation, and induces a host of genes critical for ciliogenesis.
Keywords: Primary cilia, p73, p63, p53, differentiation, multiciliated epithelia
Nearly 20 years ago it was recognized that the p53 tumor suppressor is a member of a multi-protein family, including the related transcription factors p63 and p73 [1]. When knockout mice deficient for these proteins were made, however, they revealed prominent roles for these proteins in development rather than in cancer. p63 knockout mice displayed a failure of epithelia to stratify properly, leading to aberrant development of the epidermis and its appendages, as well as abnormal limb development. In contrast, the phenotypes observed upon p73 ablation – hydrocephalus, rhinitis, otitis, propensity for infections and inflammation, gastrointestinal hemorrhaging, hippocampal dysgenesis, and pheromone sensing defects – were seemingly disparate, without a clear cellular or molecular basis [2,3]. Through renewed analysis of p73 null phenotypes, a recent study in Cell Reports from Marshall et al. present a unifying mechanism for these diverse phenotypes [4]. Using a combination of mouse genetics, histological analysis, and genomics, the authors find that p73 drives multiciliogenesis, both through transcriptional activation of a master ciliogenesis transcription factor FoxJ1 and through regulation of a myriad of genes central to ciliogenesis.
Cilia are microtubule-based and apically-positioned organelles with key roles in signaling and various effector functions, including epithelial morphogenesis. Defects in cilia structure or function have recently emerged as etiological mechanisms underpinning diverse human diseases [5]. While many eukaryotic cells present one or two cilia, some cells and unicellular organisms show extensive multiciliation [6]. In vertebrates, multiciliated cells (MCCs) are specialized post-mitotic cells that may present dozens to over one hundred motile cilia. The multiciliated phenotype is driven by a post-mitotic endocycle where centrioles continue to duplicate via local activation cycles of master centriolar regulators Plk4 and Myb to form basal bodies (Figure 1). The basal bodies migrate to the apical surface of the post-mitotic cell to initiate multiple cilia (see [7]). To initiate centriolar duplication and to increase expression of key components of cilia, a specific transcription factor, FoxJ1 stimulates expression of dozens of genes that encode ciliary proteins. Other transcription factors, including those of the Rfx family, cooperate with FoxJ1 to enhance specific populations of ciliation in different tissues, including specialized regions of the brain.
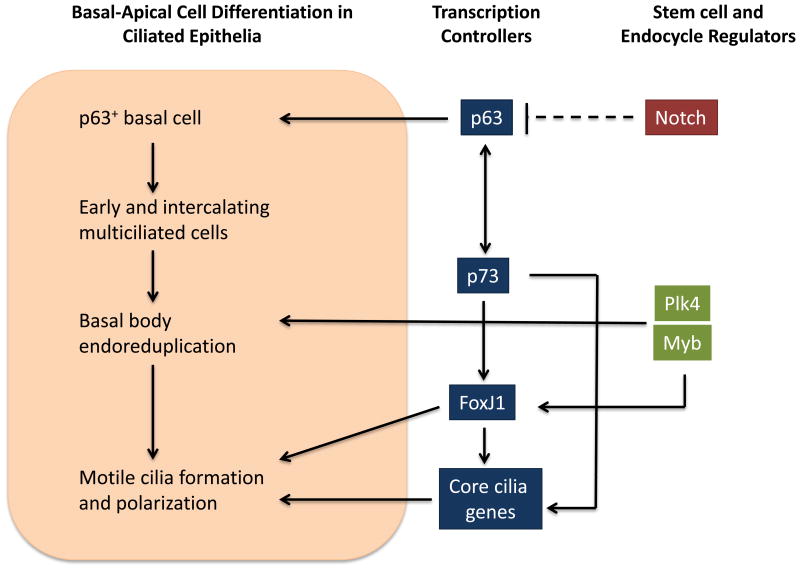
Figure 1. A Basal-Apical Pathway for p73-Dependent Activation of FoxJ1, a Central Regulator of Transcription in Multiciliated Cells.
Populations of epithelial cell precursors proceeding from basal to apical ciliated cells are shown (orange box). The key transcriptional regulators p63 and p73 function in the basal and apical compartments, and are potentially cross-regulated. The p73 transcription factor can bind to 3 sites on the FoxJ1 gene plus 105 additional ciliary genes. FoxJ1 is the central transcriptional controller of multiciliated cells. The progression from p63 basal activity to p73 apical activity, with the outcome of activating a general regulator of multiciliated, appears to function in known multiciliated tissues.
Once formed, cilia beat in a synchronous and polarized pattern to organize the flow of mucus, cerebrospinal fluid or oocytes. In primary ciliary dyskinesis, a human genetic condition often caused by mutations in dynein subunits, ciliary beating is disorganized, and incoherent beating leads to defects in flow and consequently to altered mucus clearance and susceptibility to infections. In brain ependyma, a failure of ciliary beating results in defects in cerebro-spinal fluid flow, causing hydrocephalus and hippocampal dysgenesis.
Detailing the complex spectrum of ciliary functions was crucial to understanding human or mouse genetic ciliopathies, as mammalian geneticists came to appreciate that the range of phenotypes reflected various roles of primary or multicilia in diverse tissues. For multiciliated cells, classic tissues include the inner ear, trachea and bronchioles for mucus flow, the oviduct for oocyte movement, and the ependymal cells to block ventricular hydrocephalus.
When p63 and p73 knockout mouse phenotypes were reported, the general bias was that these genes would reveal new aspects of tumor suppression following the theme for p53. Instead, p63 knockout mice display a clear defect in the formation of stratified epithelium and no tumor predisposition. The original papers on p73 knockout mice emphasized a host of varied phenotypes, rather than a tumor suppressor one [2,3]. Marshall et al. revisit the spectrum of phenotypes in p73 knockout mice, considering the hypothesis that these phenotypes represented loss of multiciliation in each of the affected epithelial compartments. Indeed, they show strong histological defects in ciliation in the oviduct, mucosal epithelium of the middle ear, sinus, bronchiole and trachea. Some phenotypes were associated with a loss of tissue homeostasis and apparent fibrosis, a defect seen in other ciliopathies. In contrast, TP53 and TP63 knockout mice did not show these defects. Thus, p73 promotes the differentiation of MCCs fundamental for proper epithelial function and is likely a key factor in forming the traditional histological population of ciliated epithelia.
Of note, the loss of p73 not only showed a strong quantitative loss of ciliated epithelial cells (37% versus 4% in trachea), but also a concomitant increase in the percentage of club cells, the bronchiolar exocrine cells that secrete protective factors for the lung (from 31% to 65%). Loss of TP73 thus causes a notable fate change. More subtle changes in mucin-producing and neuroendocrine cells were also reported.
Supporting the molecular picture, the p73 transcription factor can bind to 105 notable ciliary gene loci, including three sites in FoxJ1. Consistently, overexpression of TP73 induced FoxJ1 expression in cultured murine tracheal epithelial cells. Moreover, there is a population of p73 FoxJ1 double-positive cells in tracheal cultures, supporting the model that p73 works cell autonomously to induce FoxJ1. In addition, p73 null mice show a diminished tracheal basal cell compartment, which suggests that, beyond promoting MCC differentiation, p73 may help maintain progenitor populations. p63 and p73 are co-expressed in some basal cells, while p73 expression appears restricted to apical cells for MCC differentiation. Collectively, these findings highlight a critical role for both p73 and p63 in organizing proper epithelial organization in simple ciliated epithelia. It is known that p73 and p63 can heterodimerize and that each has an N-terminal transactivation domain (TA) and independent DN-terminal versions that lack a TA (see [8]). However, further work will be needed to clarify if these interactions help organize epithelia.
We know little about the overall regulatory mechanisms in epithelia that might organize ciliary signaling and epidermal stratification. In brain, singular primary cilia typically appear before multiciliated ependymal cells form [9]. In skin, there is evidence that primary cilia may cooperate with Notch signaling (for example, see [10]), but we do not know if specific ciliary receptors or ligands activate steps in ciliary signaling to regulate epithelial development. Many cilia signal through G-protein coupled receptors, so it would be interesting to consider how ciliary signals might regulate p73 and FoxJ1 transcription factors to coordinate cell-cell interactions in epithelia with transcriptional programs that enable changes in fate. p73 mice also show notable defects in ciliated tissues in the brain including sensory tissues like the vomeronasal gland. With a new view of how p73 contributes to epithelial development in mucus-producing cells, it will be of direct interest to generalize this thinking and further understand common mechanisms organizing apical ciliated cells in the brain.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yang A, et al. On the shoulders of giants: p63, p73 and the rise of p53. Trends Genet. 2002;18:90–95. doi: 10.1016/s0168-9525(02)02595-7. [DOI] [PubMed] [Google Scholar]
- 2.Yang A, et al. p73-deficient mice have neurological, pheromonal and inflammatory defects but lack spontaneous tumours. Nature. 2000;404:99–103. doi: 10.1038/35003607. [DOI] [PubMed] [Google Scholar]
- 3.Nemajerova A, et al. Targeted deletion of p73 in mice reveals its role in T cell development and lymphomagenesis. PLoS ONE. 2009;4:e7784. doi: 10.1371/journal.pone.0007784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marshall CB, et al. p73 is Required for Multiciliogenesis and Regulates the Foxj1-Associated Gene Network. Cell Rep. doi: 10.1016/j.celrep.2016.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hildebrandt F, et al. Ciliopathies. N Engl J Med. 2011;364:1533–1543. doi: 10.1056/NEJMra1010172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brooks ER, Wallingford JB. Multiciliated cells. Curr Biol. 2014;24:R973–82. doi: 10.1016/j.cub.2014.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jord AlA, et al. Centriole amplification by mother and daughter centrioles differs in multiciliated cells. Nature. 2014;516:104–107. doi: 10.1038/nature13770. [DOI] [PubMed] [Google Scholar]
- 8.Vargas L, Alvarez A. P73 and P63: The siblings that work together in neurodevelopment. Cell Cycle. 2015;14:3671–3672. doi: 10.1080/15384101.2015.1112615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Han YG, Alvarez-Buylla A. Role of primary cilia in brain development and cancer. Curr Opin Neurobiol. 2010;20:58–67. doi: 10.1016/j.conb.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ezratty EJ, et al. A role for the primary cilium in Notch signaling and epidermal differentiation during skin development. Cell. 2011;145:1129–1141. doi: 10.1016/j.cell.2011.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]