Abstract
Despite emerging evidence suggesting a biological basis to our social tiles, our understanding of the neural processes which link two minds is unknown. We implemented a novel approach, which included connectome similarity analysis using resting state intrinsic networks of parent-child dyads as well as daily diaries measured across 14 days. Intrinsic resting-state networks for both parents and their adolescent child were identified using independent component analysis (ICA). Results indicate that parents and children who had more similar RSN connectome also had more similar day-to-day emotional synchrony. Furthermore, dyadic RSN connectome similarity was associated with children’s emotional competence, suggesting that being neurally in-tune with their parents confers emotional benefits. We provide the first evidence that dyadic RSN similarity is associated with emotional synchrony in what is often our first and most essential social bond, the parent-child relationship.
Keywords: parent-child dyad, emotional synchrony, resting-state fMRI, functional connectome similarity, independent component analysis (ICA)
INTRODUCTION
The human mind is continuously coupled to those around us, and this shared social synchrony influences the way we perceive, respond to, and thrive in a complex social world (Wheatley et al., 2012). As far back as 384 BC, Aristotle described human nature as inherently social (Saunders, 1995 trans.), and scientists today describe humans as wired to connect with others (Lieberman, 2013; Schilbach et al., 2013; Wheatley et al., 2012; Wiltermuth and Heath, 2009). Indeed, social synchrony appears in infants as young as one-day-old (Singer, 2006), suggesting that humans are biologically prepared to connect with others (Feldman, 2007b), and is found across species including mice (Langford et al., 2006), suggesting that synchrony is evolutionarily conserved. Despite emerging evidence suggesting a biological basis to our social tiles, our understanding of the neural processes which link two minds is unknown.
Although social synchrony is found across many forms of human relationships, synchrony occurs most with similar or close others, a phenomenon found in humans and mice (Langford et al., 2006). From an evolutionary perspective of parenting in mammals, parents and their child are wired to connect, which promotes survival (De Waal, 2007). Social synchrony in mother-child dyads includes the coordination of ongoing exchanges of sensory, hormonal, and physiological stimuli between parent and child and ranges from the initial consolidation of biological rhythms during pregnancy to the emergence of complex social and emotional exchanges between parent and child throughout development (Feldman, 2007a, b; Rosenblatt, 1965; Schneirla, 1946). Given the protracted dependence of humans on their parents, coordinated social synchrony may be co-opted into childhood and adolescence, ultimately providing the foundation for youth to develop socioemotional competence. Indeed, dyadic synchrony influences emotion regulation and adjustment (Barber et al., 2001) such that coherence of emotional states between parents and children (e.g., shared affect) provides critical inputs for youths’ social and emotional well-being (Feldman, 2007a, b; Feldman et al., 1999).
Significant work has begun to examine the biological underpinnings of parent-child dyadic synchrony, such that physiological arousal (e.g., heart rate)(Feldman et al., 2011) and hormonal levels (e.g., cortisol levels)(Papp et al., 2009) are frequently in-synch between parents and their child. For instance, during free-play, mothers’ and infants’ heart rates become synchronized, and during stressful events, infants who engage in more synchronous interactions with their mothers show better autonomic regulation (Feldman, 2007b). Despite these exciting advances in the field of dyadic synchrony, we know relatively little about how two minds are coupled. If indeed, the human mind is wired to connect, then an exciting new research direction is to test whether parents and their children show similar patterns of neural connectivity.
In the current study, we examined how neural connectivity patterns are shared between parents and their child. We implemented novel statistical and methodological techniques to examine how similarity between parent-child intrinsic resting-state network (RSN) connectivity is associated with day-to-day emotional synchrony. Moving beyond the individual, resting state functional magnetic resonance imaging (rs-fMRI) was administered for both parents and children. Rs-fMRI provides an ideal method for examining the neural connectome, as it assesses the strength of multiple intrinsic functional neural networks, networks active and synchronized when the brain is at rest independent of stimulus-induced brain activity usually driven by experimental demands (Cole et al., 2010; Uddin et al., 2010). Intrinsic resting-state networks for both parents and their adolescent child were identified using independent component analysis (ICA). Parent-child dyads also completed daily diaries, in which they indicated their daily mood each evening for two weeks. From these daily diaries, we were able to capture parent-child emotional synchrony, or the extent to which their mood fluctuated together day-by-day. We examined how dyadic connectome similarity is associated with the quality of daily emotional synchrony and whether the association between brain similarity and daily emotional synchrony confers benefits to adolescents’ emotional competence. We hypothesized that greater similarity of intrinsic functional connectome in parent-child dyad would increase daily emotional synchrony and be linked to adolescents’ emotional well-being.
METHODS
Participants
As part of a larger study, we recruited 76 participants (37 adolescent children and 39 primary caregivers). All participants provided informed consent/assent, and no participants reported any mental health problems (e.g., current clinical diagnose or pharmacological intervention for a mental illness). Among all participants, 31 parent-child dyads (n = 62) successfully completed the dyadic resting state scan (parent Mage = 43.06 years, range = 33 – 57, 12.90% father; child Mage = 14.80 years, range = 13 – 17, 48.39% female). All parent-child dyads were biologically related and provided written informed consent/assent. No participants were excluded due to excessive motion (i.e., mean framewise displacement, FD > 0.5 mm) or reported any mental health problems (e.g., current clinical diagnose or pharmacological intervention for a mental illness).
Procedures
Adolescent children and their primary caregiver completed a brain scan during which resting state was acquired. Children and their parent also completed daily checklists for 14 days. Participants either completed the checklists by accessing a secure website or by using pencil and paper. For those completing with paper/pencil, we monitored completion of the checklists by providing participants with fourteen manila envelopes and an electronic time stamper. The time stamper is a small, hand-held device that imprints the current date and time and is programmed with a security code so that the correct date and time cannot be altered. Participants were instructed to place their completed checklists into a sealed envelope each night and to stamp the seal of the envelope with the time stamper. For those completing the surveys on the secure website, an email with the link to each daily survey was sent separately to the parent and child, and the time and date of completion were monitored via the website. In addition to the daily diaries, parents and children completed a questionnaire, which included adolescents’ emotional competence, as well as several measures as part of the larger study and published elsewhere (e.g., Lee & Telzer, 2016).
Questionnaires
Daily emotional synchrony
Children and their parents each completed daily checklists for two weeks (a total 14 daily measures). Each night before going to bed, participants responded to three questions about their positive mood (e.g., “joyful” “calm” “happy”) and 10 questions about their negative mood (e.g., “sad” “hopeless” “discouraged” “uneasy”) using a five-point scale (1 = “Not at all” to 5 = “Extremely”). From these 14 daily measures, we first calculated the daily concordance between adolescents’ and parents’ mood. The mood concordance for each dyad was estimated by predicting children’s daily mood from parents’ daily mood that day (positive and negative coherence, respectively). Given the nested nature of the data, we used Hierarchical Linear Modeling (HLM) which was designed to analyze nested data of the type that were collected for this study (i.e., daily level data nested within individuals) as follows:
Mood on a particular day (i) for a particular child (j) was modeled as a function of the average mood of the children across days (b0j) and the parent’s mood that day (b1j). Separate models were run for positive and negative mood, and the empirical Bayes estimate for each dyad over the 14 days was extracted from each of the statistical models. The empirical Bayes estimate is an optimally weighted average that combines the dyad’s average slope and “shrinks” it towards the mean slope of the group (Diez, 2002) for each mood category such that higher values indicate higher concordance between parent and child for a given mood. Finally, we calculated an emotional synchrony index between parents and children by subtracting the concordance score of negative mood from the positive mood concordance score such that higher values represent more synchronized daily emotion between parents and their child toward positive mood and away from negative mood. On average, parent’s daily mood did not predict children’s daily mood (B = 0.14, SE = .01, p=.10). However, there was significant variability in parent-child emotional synchrony (M = −0.10, SD = 0.66, range = −1.25 – 0.66), indicating that some families are desycnronous and others are highly synchronized. Three adolescent children did not complete the daily checklists.
Child’s emotional competence
Emotional competence was measured using the Toronto Alexithymia Scale (Bagby et al., 1994). Using a five-point scale (1 = “strongly disagree” to 5 = “strongly agree”), adolescent children responded to 20 items examining (1) difficulty in identifying feelings (e.g., “When I am upset, I don’t know if I am sad, frightened, or angry”); (2) difficulty in describing feelings (e.g., “It is difficult for me to find the right words for my feelings”), and (3) external-oriented thinking (e.g., “I prefer to just let things happen rather than to understand why they turned out that way”). The 20 items were summed and reverse scored (Telzer et al., 2014), such that higher scores indicate greater emotional competence. The scale’s internal consistency was α = .72. We failed to get responses from four adolescent children.
Resting-state fMRI (rs-fMRI)
Data acquisition, preprocessing
Participants completed a 6-minute resting state scan, during which they were instructed to view a black screen with a white fixation cross. All imaging data were collected using a 3T-Siemens Trio MRI scanner with a 32-channel matrix coil. High-resolution structural images (T1-MPRAGE) were acquired first (repetition time or TR = 1.9 s, echo time or TE = 2.3 ms, matrix size = 256 X 256, field of view or FOV = 230 mm, flip angle or FA = 90°, 1 mm isotropic voxel). The resting-state data were acquired from a gradient-echo echo-planar image sequence. The resting-state scan parameters for 15 dyads were 180 volumes, 38 slices with 0.3 mm-slice gap, TR = 2 s, matrix = 92 X 92, FOV = 230 mm, FA = 90°, voxel size 2.5 X 2.5 X 3.0 mm3, and 6 min duration, and the other 16 dyads were 120 volumes; 36 slices with no inter-slice gap, TR = 3 s, matrix = 64 X 64, FOV = 220 mm, FA = 90°, voxel size 3.5 X 3.5 X 4.0 mm3 and 6 min duration. Data preprocessing were performed using FMRIB’s Software Library (FSL; www.fmrib.ox.ac.uk/fsl), including skull stripping of structural images with BET, motion correction with MCFLIRT, smoothing with full-width half-maximum 6 mm, masking of non-brain voxels; 128 s high-pass, voxel-wise demeaning and normalization into 2mm-MNI-standard via individual T1-weighted anatomical image with FLIRT. Noise signals were identified individually and removed using MELODIC ICA and an automated signal classification toolbox (an average of 4.7 components (13.4 %) were removed from each participant; mean FD = 0.03 mm, range = 0.01 – 0.10 mm)(Tohka et al., 2008). Although there are several strategies suggested to rigorously correct for motion-related noise in resting state data, such as spike regression with 24-type of motion parameters (Lemieux et al., 2007; Satterthwaite et al., 2013) and individual high-motion contaminated volume scrubbing (Power et al., 2012), several considerations of these strategies also exist such that they can introduce overfitting by the use of a large set of nuisance regressors, linear assumption about motion, and negative influences in the autocorrelation structure of data (see Pruim et al., 2015b). Therefore, we applied ICA denoising approach for the current analysis given the recent evidence that ICA denoising can effectively enhance the fidelity of data quality in terms of motion control (Birn et al., 2008; Pruim et al., 2015a; Starck et al., 2013). It has been well demonstrated that RSNs estimated by ICA are less prone to artifactual effects from noise such as physiological signal, global signal fluctuation and motion due to the ability of ICA to account for the existence of such noise effects within additional non-RSN components (Boubela et al., 2013; Cole et al., 2010) with robustness of RSN identification in rs-fMRI (Poldrack et al., 2011). Furthermore, a recent study demonstrates that ICA-based denoising can diminish potential differences due to multi-center/protocol data collection in rs-fMRI (Feis et al., 2015; Paolini et al., 2015).
Group ICA
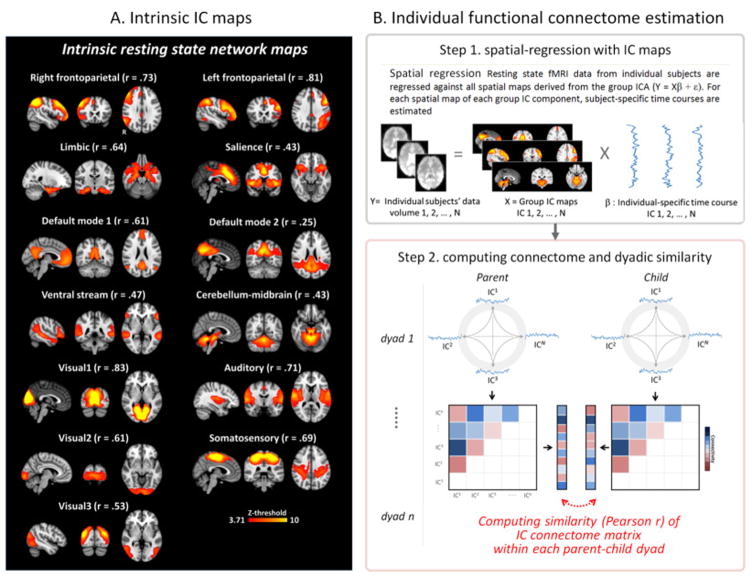
In order to compare children and their parents and obtain commonly shared resting-state networks (RSNs), we first pooled our data for all possible adolescent children and parents (n = 76). Then, temporal concatenate group ICA was applied with probabilistic principal component analysis (PCA) where the number of dimensions was estimated using the Laplace approximation to the Bayesian evidence of the model order (Beckmann et al., 2009; Beckmann and Smith, 2004; Minka, 2000), yielding 19 network spatial maps. To distinguish group-level brain networks from artefactual components (e.g., residual head movement and physiological noise) and to identify canonical RSNs, all network maps were spatially cross-correlated with canonical RSN templates (i.e., template-matching procedure) acquired from previous resting-state studies (Laird et al., 2011; Shirer et al., 2012; Smith et al., 2009). We finally identified 13 intrinsic RSN components (cross-validation threshold r > 0.25; Figure 1A); two default mode networks (DMN1 and DMN2), right and left frontoparietal network (RFPN and LFPN), salience network (SN), limbic network (LN), ventral stream network (VSN), three visual networks (VN1, VN2 and VN3), somatosensory network (SMN), auditory network (AN) and cerebellum-midbrain network (CMN). The six remaining group level components were considered artifactual (e.g., physiological) due to predominant activation in white matter, ventricles, vasculature, or head movement. To ensure that these networks were consistently shared across participants regardless of their age group (parents and children), we additionally ran the same group ICA analysis for each age group separately and found these RSNs exist in both groups consistently. These group level maps are all available in a public repository of human brain imaging (http://neurovault.org/).
Figure 1.
(A) Group-level intrinsic resting state network (RSN) maps used in the current analysis. These 13 network maps were identified and adopted from a paper published previously on the larger sample in which the current participant sample was included(Lee and Telzer, 2016). Each “r” indicates spatially cross-correlation coefficient value with canonical RSN templates acquired from previous studies (Laird et al., 2011; Shirer et al., 2012; Smith et al., 2009; see also Clewett et al., 2014). (B) Schematic of analytical approach to characterize individual RSN connectome and calculate the connectome similarity for each parent-child dyad. In step 1, individual-specific time courses were estimated based on the RSN maps from Fig. 1A using spatial regression approach(Filippini et al., 2009). In step 2, the estimated individual time-courses for each RSN were then were correlated to create a functional resting-state connectome matrix. In this phase, correlation values (Pearson-r) between all possible pairs of time-courses among 13 RSNs were calculated. Finally, the connectome matrix values for each individual was Fisher-Z transformed, vectorized and correlated between each pair of parent-child dyads to calculate connectome similarity. Note that only the upper half of the connectome matrix excluding the diagonal is shown in the figure.
Analysis for dyadic resting-state intrinsic functional connectome similarity
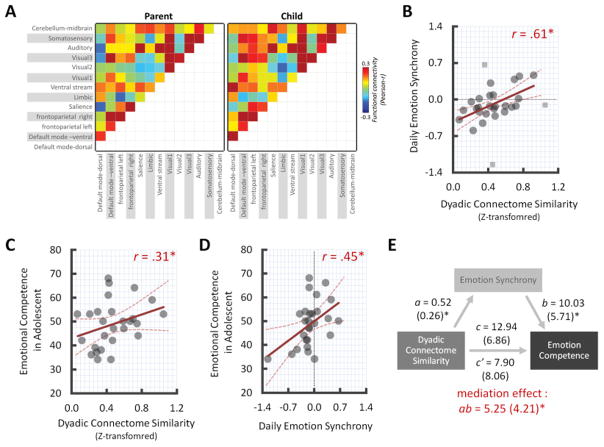
Using the group level RSN maps yielded by group ICA, we estimated specific time-courses of each RSN for each participant (parent-child dyad; n = 31) using the spatial regression approach of the dual-regression method (Filippini et al., 2009). In the spatial regression, we performed linear model estimation with the full set of group-level ICA network maps against the separate individual datasets and estimated subject-specific temporal dynamics for each RSN map while controlling for the influence of other network dynamics including noise components (Figure 1B.Step1). The estimated individual time-courses for each RSN were then used to create an ICA-based functional network connectivity matrix (i.e., connectome) by correlating (Pearson-r) all possible pairs of time-courses from 13 RSNs as shown in Figure 1B.Step 2. Finally, the connectivity matrix for each individual was Fisher-Z transformed, vectorized and correlated between each pair of parent-child dyads to calculate RSN connectome similarity (see Figure 2A for the averaged functional RSN connectome in both parent and child groups). Note that the large-scale group-level network maps were used for individual-specific estimations to calculate functional connectivity in each individual rather than group-level comparison (e.g., parents vs. children). The neural similarity varied across parent-child dyads (M similarity = 0.47, SD = 0.23, range = 0.07 – 1.05).
Figure 2.
(A) The averaged RSN connectomes for each parent and child groups. Scatter plots between the degree of dyadic RSN connectome similarity and (B) daily emotional synchrony score (C) emotional competence score (D) between of emotional competence score and daily emotional synchrony score and (E) mediation path. Note that Fisher’s z-transformed connectome similarity values were used for statistical analyses. The Colored-dash line indicates 95% confidence interval of regression lines. The gray dotted-line indicates zero point at each axis. *p < 0.05 at 95% CI after bootstrapping resampling (n=50,000). The squares were bivariate outliers identified and deweighted by robust-method but note that all significant effects remained significant when the outlieres were included (correlation r =0.34, p < 0.05, CI=[0.07, 0.65]; mediation effect ab=5.47, p < 0.05, CI=[0.05 14.50].
RESULTS
Relationship between RSN connectome similarity, daily-emotional synchrony and children’s emotional competence
To examine how dyadic RSN connectome similarity, daily emotional synchrony, and children’s emotional competence are related, we performed Pearson correlation analyses using robust method with non-parametric bootstrapping resampling (n = 50,000) at 95% confidence level (Pernet et al., 2012). We first examined how RSN connectome similarity influences daily emotional synchrony between parents and their child, and found that there is a significant positive correlation, r (28) = 0.61, p < .05, 95% CI = [0.30, 0.80], suggesting that parent-child dyads who showed higher RSN connectome similarity also showed greater emotional synchrony (Figure 2B). Next, we ran correlation analyses linking RSN connectome similarity and children’s emotional competence. Children who have more similar RSN connectome with their parents reported better emotional competence, r (27) = 0.31, p < .05, 95% CI = [0.04, 0.58] (Figure 2C). Finally, we observed a significant positive relationship between daily emotional synchrony and children’s emotional competence, r (27) = 0.45, p < .05, 95% CI = [0.11, 0.70] (Figure 2D), suggesting that higher emotional synchrony is related to enhanced emotional competence in adolescents. Together, these results indicate that parent-child dyads who show more similar brain connectomes exhibit more daily-emotional synchrony, each of which is associated with enhanced emotional competence.
Mediation between RSN connectome similarity, daily-emotional synchrony and children’s emotional competence
Given that the pairwise relationships between RSN connectome similarity, daily emotional synchrony, and adolescents’ emotional competence were statistically meaningful, we conducted mediation analyses focusing on whether dyadic connectome similarity (i.e., independent variable) was associated with adolescents’ emotional competence (i.e., outcome) through daily-emotional synchrony (i.e., mediator) (model 1). The magnitude and the significance were calculated based on a robust approach and bias-corrected confidence interval with bootstrapping resampling (n = 5000) using the mediation toolbox (Wager et al., 2008). Results indicate that daily-emotional synchrony significantly mediated the link between neural connectome similarity and emotional competence, B = 5.34 (SE = 4.22), p < 0.05, 95% CI = [0.21, 15.16]. That is, parent-child dyads with more similar brain connectomes exhibit better emotional competence via positively biased mood-synchronization (Figure 2E).
In addition, we tested the possibility that daily emotional synchrony leads to enhanced emotional competence through shaping similar brain connectomes, assuming that synchronized daily emotions influence emotional competence in adolescents by utilizing the same brain connectome. In this model, emotional synchrony was used as the independent variable to predict emotional competence via dyadic brain similarity (model 2). We did not find a significant indirect effect of emotional synchrony via brain similarity on emotional competence (95% CI = [−2.68, 8.90]). Collectively, these mediation analyses indicate that dyadic brain similarity plays an important role in children’s emotional competence by contributing to more synchronized emotional mood fluctuations between parents and their children.
DISCUSSION
Children and their parents are in sync. They frequently share their ideas, feelings, and behaviors. Such dyadic synchrony confers emotional benefits and provides the foundation for youth to adapt to an increasingly complex social environment. Despite the importance of the interpersonal connection between parents and their child, our understanding of how this shared process between individuals is represented at the neural systems level remains unknown. No prior study has scanned parent–child dyads and related similarities in their resting state connectome to interpersonal processes. We provide the first empirical evidence unpacking how the brain’s functional organization is shared between individuals and influences emotional synchrony, ultimately conferring benefits for youths’ development.
The brain connectome is a rich index to evaluate how the large-scale brain architecture within an organism is connected (Barch et al., 2013; Sporns et al., 2005). The connectome built on rs-fMRI provides an index of the individual’s unique brain fingerprint (Gabard-Durnam et al., 2016; Pizoli et al., 2011; Van Dijk et al., 2012; Zuo et al., 2012). While each intrinsic RSN corresponds to a different functionality (Cole et al., 2010; Laird et al., 2011; Smith et al., 2009; Uddin et al., 2010), patterns of how all network-specific temporal dynamics are functionally coupled (i.e., functional connectivity across intrinsic networks) leads to individual variability in terms of mind and behavior (Finn et al., 2015; Smith et al., 2013; Van Dijk et al., 2012). Evidence suggests that this functional connectome is modified and tuned gradually by individuals’ accumulating socio-emotional experiences (Gabard-Durnam et al., 2016), suggesting that the brain’s functional connectome is flexible and functionally plastic. Thus, we propose that children’s neural connectome is a psychological representation at the neural systems level, resulting from shared experiences with their primary caregiver. Children, therefore, exhibit a more tuned functional connectome to their parents resulting in more shared emotional experiences between them, and ultimately conferring more optimal emotional adjustment. These findings are consistent with recent theoretical work suggesting that more similar neural states allow individuals to connect and be attuned to their environment in a more harmonious way (Wheatley et al., 2012). Importantly, we also found that the degree of neural similarity in parent-child dyads promotes youths’ psychological adjustment, consistent with previous evidence showing that dyadic similarity and synchrony is a key factor in promoting youths’ positive adjustment throughout development (Barber et al., 2001; Boyum and Parke, 1995; Carson and Parke, 1996; Feldman, 2007b; Feng et al., 2007; Harrist and Waugh, 2002; Lindsey et al., 2008). Our findings indicate that children and parents’ emotional connection occurs at the neural systems level, and highlights the brain’s functional plasticity (i.e., tuned functional architecture of brain) derived from interpersonal experiences in supporting youths’ positive adjustment.
While we propose that experience tunes the neural functional architecture shared by parents and their child, it is also possible that genes play an important role in shared neural processes. Although previous studies have shown that parent-child dyads are more likely to be synchronized at the psychological and behavioral level (Eisenberg et al., 1998; Harrist and Waugh, 2002; Morris et al., 2007), evidence also indicates that the quality of their relationships matters significantly for this shared dyadic process (Peterson and Rollins, 1987; Siegel, 1999 ; Smetana et al., 2006). Indeed, the observed neural similarity in the current study varies across parent-child dyads (M similarity = 0.47, SD = 0.23, range = 0.07 – 1.05). In addition, there was significant variability in daily emotional synchrony (M emotion synchrony index = −0.10, SD = 0.66, range = −1.25 – 0.66). That is, not all parent-child dyads are necessarily in-tuned at the neural systems level or emotional level. Therefore, genetic similarity likely does not explain the observed effects in the current study. Future research should examine how experience shapes neural similarity patterns in parent-child dyads. Moreover, to unpack genetic effects, future research should examine neural similarity in genetically unrelated dyads (e.g., adopted children) and other important relationships (e.g., married couples). Although our findings contribute to our understanding of the neural underpinnings of dyadic synchrony, our data could not examine developmental trajectories of this interpersonal brain tuning process. As noted above, the formation of functional connectome can be fluid according to accumulating experiences. Thus, future research focusing on longitudinal changes in brain similarity will shed light on how our brains adapt in a complex social environment and on what basis interpersonal neural similarity occurs.
In the current study, we examined neural concordance between parents and their child by focusing on the large-scale functional connectome using ICA. We were interested in examining large-scale brain concordance (i.e., connectome similarity) across the entire brain in parent-child dyads without focusing on a specific network-based-hypothesis. ICA is a data-driven approach that focuses more on defining networks of covariant activity instead of investigating a specific functionality of each sub-intrinsic network (e.g., default-mode network). ICA identifies each intrinsic network by estimating the temporal coherences with consideration of spatial independence of the signal regardless of possible structural differences in boundary or size of core regions in each intrinsic network between adolescent children and adults. Therefore, we adopted the ICA to evaluate the brain’s functional system because it focuses more on the functionality represented by time-domain coherence and spatial independence rather than predetermined structural location (e.g., seed-based). Future research can further specify functional connectome similarity by focusing on a specific functionality of a certain network and link it to a specific psychosocial behavior. For example, given the major role of the right-frontoparietal network (RFPN) in cognitive control (e.g., Fair et al., 2007), it is possible to evaluate how dyadic similarity of the RFPN-centered-connectome could index the maturity of self-control. Indeed, we have found that connectivity between the RFPN and limbic system predicts more optimal self-control and later substance use in adolescents (Lee and Telzer, 2016). In future research, our approach could be extended to examine specific networks (e.g., RFPN) as they relate to parental influence on neural development and behavior such as self-control.
The current findings suggest that static-but-attuned brain architecture has a fundamental role in conferring the benefits of shared and synchronized emotional experiences to some of the most important people in our social network. The most novel features of the current study are the use of dyadic brain scans combined with the daily diaries to link brain concordance to individuals’ interpersonal emotional experiences. Most importantly, we examined the brain’s functional connectome similarity between individuals instead of focusing on a single brain. To our knowledge, this is the first empirical evidence comparing the brain’s functional connectome pattern similarity across two individuals. Consequently, our study expands the scope and scale of brain connectome research beyond a single brain to understand the neural underpinnings of human mind-to-mind coupling. Furthermore, we adopted a diary method to monitor how affect changes day-by-day, a method that captures real life experience as it is lived (Bolger et al., 2003) and does not rely on retrospective accounts. Because we administered this diary measurement to both parents and their child, we could assess how real-life emotional experiences fluctuate together (i.e., dyadic emotional synchrony). This interdisciplinary technique of linking dyadic neural responses to interpersonal emotional experiences provides us with a robust and integrated understanding of brain-behavior associations. This novel approach significantly contributes to brain science by understanding how the human brain and mind are wired interpersonally.
HIGHLIGHTS.
Examined a link between neural and behavioral concordance in parent-child dyads
Parent-child dyads with more neural concordance show higher behavioral concordance
Dyadic concordance enhances children’s emotional competence
Being in-tune with parents confers developmental benefits for youth
Acknowledgments
This work was supported by the National Institute of Health (1R01DA039923).
Footnotes
Tae-Ho Lee and Michelle Miernicki contributed equally to this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bagby RM, Parker JD, Taylor GJ. The twenty-item Toronto Alexithymia Scale—I. Item selection and cross-validation of the factor structure. Journal of psychosomatic research. 1994;38:23–32. doi: 10.1016/0022-3999(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Barber JG, Bolitho F, Bertrand L. Parent-child synchrony and adolescent adjustment. Child and Adolescent Social Work Journal. 2001;18:51–64. [Google Scholar]
- Barch DM, Burgess GC, Harms MP, Petersen SE, Schlaggar BL, Corbetta M, Glasser MF, Curtiss S, Dixit S, Feldt C. Function in the human connectome: task-fMRI and individual differences in behavior. Neuroimage. 2013;80:169–189. doi: 10.1016/j.neuroimage.2013.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann C, Mackay C, Filippini N, Smith S. Group comparison of resting-state FMRI data using multi-subject ICA and dual regression. Neuroimage. 2009;47:S148. [Google Scholar]
- Beckmann C, Smith S. Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Trans Med Imaging. 2004;23:137–152. doi: 10.1109/TMI.2003.822821. [DOI] [PubMed] [Google Scholar]
- Birn RM, Murphy K, Bandettini PA. The effect of respiration variations on independent component analysis results of resting state functional connectivity. Human brain mapping. 2008;29:740–750. doi: 10.1002/hbm.20577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger N, Davis A, Rafaeli E. Diary methods: Capturing life as it is lived. Annu Rev Psychol. 2003;54:579–616. doi: 10.1146/annurev.psych.54.101601.145030. [DOI] [PubMed] [Google Scholar]
- Boubela RN, Kalcher K, Huf W, Kronnerwetter C, Filzmoser P, Moser E. Beyond noise: using temporal ICA to extract meaningful information from high-frequency fMRI signal fluctuations during rest. Frontiers in Human Neuroscience. 2013;7:168. doi: 10.3389/fnhum.2013.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyum LA, Parke RD. The role of family emotional expressiveness in the development of children’s social competence. Journal of Marriage and the Family. 1995:593–608. [Google Scholar]
- Carson JL, Parke RD. Reciprocal negative affect in parent-child interactions and children’s peer competency. Child Development. 1996:2217–2226. [PubMed] [Google Scholar]
- Cole D, Smith S, Beckmann C. Advances and pitfalls in the analysis and interpretation of resting-state FMRI data. Frontiers in systems neuroscience. 2010;4:8. doi: 10.3389/fnsys.2010.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Waal FB. The ‘Russian doll’ model of empathy and imitation. On being moved: From mirror neurons to empathy. 2007:35–48. [Google Scholar]
- Diez R. A glossary for multilevel analysis. Journal of epidemiology and community health. 2002;56:588. doi: 10.1136/jech.56.8.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg N, Cumberland A, Spinrad TL. Parental socialization of emotion. Psychological inquiry. 1998;9:241–273. doi: 10.1207/s15327965pli0904_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Dosenbach NU, Church JA, Cohen AL, Brahmbhatt S, Miezin FM, Barch DM, Raichle ME, Petersen SE, Schlaggar BL. Development of distinct control networks through segregation and integration. Proceedings of the National Academy of Sciences. 2007;104:13507–13512. doi: 10.1073/pnas.0705843104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feis RA, Smith SM, Filippini N, Douaud G, Dopper EG, Heise V, Trachtenberg AJ, van Swieten JC, van Buchem MA, Rombouts SA. ICA-based artifact removal diminishes scan site differences in multi-center resting-state fMRI. Frontiers in neuroscience. 2015:9. doi: 10.3389/fnins.2015.00395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman R. Parent–infant synchrony and the construction of shared timing; physiological precursors, developmental outcomes, and risk conditions. Journal of Child Psychology and Psychiatry. 2007a;48:329–354. doi: 10.1111/j.1469-7610.2006.01701.x. [DOI] [PubMed] [Google Scholar]
- Feldman R. Parent–infant synchrony biological foundations and developmental outcomes. Current directions in psychological science. 2007b;16:340–345. [Google Scholar]
- Feldman R, Greenbaum CW, Yirmiya N. Mother–infant affect synchrony as an antecedent of the emergence of self-control. Developmental Psychology. 1999;35:223. doi: 10.1037//0012-1649.35.1.223. [DOI] [PubMed] [Google Scholar]
- Feldman R, Magori-Cohen R, Galili G, Singer M, Louzoun Y. Mother and infant coordinate heart rhythms through episodes of interaction synchrony. Infant Behavior and Development. 2011;34:569–577. doi: 10.1016/j.infbeh.2011.06.008. [DOI] [PubMed] [Google Scholar]
- Feng X, Shaw DS, Skuban EM, Lane T. Emotional exchange in mother-child dyads: stability, mutual influence, and associations with maternal depression and child problem behavior. Journal of Family Psychology. 2007;21:714. doi: 10.1037/0893-3200.21.4.714. [DOI] [PubMed] [Google Scholar]
- Filippini N, MacIntosh B, Hough M, Goodwin G, Frisoni G, Smith S, Matthews P, Beckmann C, Mackay C. Distinct patterns of brain activity in young carriers of the APOE-ε4 allele. Proceedings of the National Academy of Sciences. 2009;106:7209–7214. doi: 10.1073/pnas.0811879106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn ES, Shen X, Scheinost D, Rosenberg MD, Huang J, Chun MM, Papademetris X, Constable RT. Functional connectome fingerprinting: identifying individuals using patterns of brain connectivity. Nature neuroscience. 2015 doi: 10.1038/nn.4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabard-Durnam L, Gee D, Goff B, Flannery J, Telzer E, Humphreys K, Lumian D, Fareri D, Caldera C, Tottenham N. Stimulus-elicited connectivity influences resting-state connectivity years later in human development: a prospective study. Journal of Neuroscience. 2016;36:4771–4784. doi: 10.1523/JNEUROSCI.0598-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrist AW, Waugh RM. Dyadic synchrony: Its structure and function in children’s development. Developmental review. 2002;22:555–592. [Google Scholar]
- Laird A, Fox P, Eickhoff S, Turner J, Ray K, McKay D, Glahn D, Beckmann C, Smith S, Fox P. Behavioral interpretations of intrinsic connectivity networks. Journal of cognitive neuroscience. 2011;23:4022–4037. doi: 10.1162/jocn_a_00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langford DJ, Crager SE, Shehzad Z, Smith SB, Sotocinal SG, Levenstadt JS, Chanda ML, Levitin DJ, Mogil JS. Social modulation of pain as evidence for empathy in mice. Science. 2006;312:1967–1970. doi: 10.1126/science.1128322. [DOI] [PubMed] [Google Scholar]
- Lee TH, Telzer EH. Negative functional coupling between the right frontoparietal and limbic resting state networks predicts increased self-control and later substance use onset in adolescence. Developmental cognitive neuroscience. 2016;20:35–42. doi: 10.1016/j.dcn.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemieux L, Salek-Haddadi A, Lund TE, Laufs H, Carmichael D. Modelling large motion events in fMRI studies of patients with epilepsy. Magnetic resonance imaging. 2007;25:894–901. doi: 10.1016/j.mri.2007.03.009. [DOI] [PubMed] [Google Scholar]
- Lieberman MD. Social: Why our brains are wired to connect. OUP; Oxford: 2013. [Google Scholar]
- Lindsey EW, Colwell MJ, Frabutt JM, Chambers JC, MacKinnon-Lewis C. Mother-child dyadic synchrony in European American and African American families during early adolescence: Relations with self-esteem and prosocial behavior. Merrill-Palmer Quarterly. 2008;54:289–315. [Google Scholar]
- Minka TP. Automatic choice of dimensionality for PCA. NIPS. 2000:598–604. [Google Scholar]
- Morris AS, Silk JS, Steinberg L, Myers SS, Robinson LR. The role of the family context in the development of emotion regulation. Social development. 2007;16:361–388. doi: 10.1111/j.1467-9507.2007.00389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolini M, Keeser D, Ingrisch M, Werner N, Kindermann N, Reiser M, Blautzik J. Resting-state networks in healthy adult subjects: a comparison between a 32-element and an 8-element phased array head coil at 3.0 Tesla. Acta Radiologica. 2015;56:605–613. doi: 10.1177/0284185114567703. [DOI] [PubMed] [Google Scholar]
- Papp LM, Pendry P, Adam EK. Mother-adolescent physiological synchrony in naturalistic settings: within-family cortisol associations and moderators. Journal of Family Psychology. 2009;23:882. doi: 10.1037/a0017147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernet CR, Wilcox R, Rousselet GA. Robust correlation analyses: false positive and power validation using a new open source Matlab toolbox. Frontiers in psychology. 2012:3. doi: 10.3389/fpsyg.2012.00606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson GW, Rollins BC. Handbook of marriage and the family. Springer; 1987. Parent-child socialization; pp. 471–507. [Google Scholar]
- Pizoli CE, Shah MN, Snyder AZ, Shimony JS, Limbrick DD, Raichle ME, Schlaggar BL, Smyth MD. Resting-state activity in development and maintenance of normal brain function. Proceedings of the National Academy of Sciences. 2011;108:11638–11643. doi: 10.1073/pnas.1109144108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack RA, Mumford JA, Nichols TE. Handbook of functional MRI data analysis. Cambridge University Press; 2011. [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruim R, Mennes M, Buitelaar J, Beckmann C. Evaluation of ICA-AROMA and alternative strategies for motion artifact removal in resting state fMRI. Neuroimage. 2015a;112:278–287. doi: 10.1016/j.neuroimage.2015.02.063. [DOI] [PubMed] [Google Scholar]
- Pruim R, Mennes M, van Rooij D, Llera A, Buitelaar J, Beckmann C. ICA-AROMA: a robust ICA-based strategy for removing motion artifacts from fMRI data. Neuroimage. 2015b;112:267–277. doi: 10.1016/j.neuroimage.2015.02.064. [DOI] [PubMed] [Google Scholar]
- Rosenblatt JS. The basis of synchrony in the behavioral interaction between the mother and her offspring in the laboratory rat. Determinants of Infant Behavior. 1965;3:3–41. [Google Scholar]
- Satterthwaite TD, Elliott MA, Gerraty RT, Ruparel K, Loughead J, Calkins ME, Eickhoff SB, Hakonarson H, Gur RC, Gur RE. An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. Neuroimage. 2013;64:240–256. doi: 10.1016/j.neuroimage.2012.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders TJ. Aristotle: Politics: books I and II. Clarendon Press; 1995. [Google Scholar]
- Schilbach L, Timmermans B, Reddy V, Costall A, Bente G, Schlicht T, Vogeley K. Toward a second-person neuroscience. Behavioral and Brain Sciences. 2013;36:393–414. doi: 10.1017/S0140525X12000660. [DOI] [PubMed] [Google Scholar]
- Schneirla TC. Problems in the bio-psychology of social organization. The Journal of Abnormal and Social Psychology. 1946;41:385. doi: 10.1037/h0055210. [DOI] [PubMed] [Google Scholar]
- Shirer W, Ryali S, Rykhlevskaia E, Menon V, Greicius M. Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cerebral cortex. 2012;22:158–165. doi: 10.1093/cercor/bhr099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel DJ. The developing mind. Guilford Press; New York: 1999. [Google Scholar]
- Singer T. The neuronal basis and ontogeny of empathy and mind reading: review of literature and implications for future research. Neuroscience & Biobehavioral Reviews. 2006;30:855–863. doi: 10.1016/j.neubiorev.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Smetana JG, Metzger A, Gettman DC, Campione-Barr N. Disclosure and secrecy in adolescent–parent relationships. Child Development. 2006;77:201–217. doi: 10.1111/j.1467-8624.2006.00865.x. [DOI] [PubMed] [Google Scholar]
- Smith S, Fox P, Miller K, Glahn D, Fox P, Mackay C, Filippini N, Watkins K, Toro R, Laird A, Beckmann C. Correspondence of the brain’s functional architecture during activation and rest. Proceedings of the National Academy of Sciences. 2009;106:13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S, Vidaurre D, Beckmann C, Glasser M, Jenkinson M, Miller K, Nichols T, Robinson E, Salimi-Khorshidi G, Woolrich M. Functional connectomics from resting-state fMRI. Trends in Cognitive Sciences. 2013;17:666–682. doi: 10.1016/j.tics.2013.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporns O, Tononi G, Kötter R. The human connectome: a structural description of the human brain. PLoS comput biol. 2005;1:e42. doi: 10.1371/journal.pcbi.0010042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starck T, Nikkinen J, Rahko J, Remes J, Hurtig T, Haapsamo H, Jussila K, Kuusikko-Gauffin S, Mattila M-L, Jansson-Verkasalo E. Resting state fMRI reveals a default mode dissociation between retrosplenial and medial prefrontal subnetworks in ASD despite motion scrubbing. 2013 doi: 10.3389/fnhum.2013.00802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer EH, Qu Y, Goldenberg D, Fuligni AJ, Galván A, Lieberman MD. Adolescents’ emotional competence is associated with parents’ neural sensitivity to emotions. Frontiers in Human Neuroscience. 2014:8. doi: 10.3389/fnhum.2014.00558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohka J, Foerde K, Aron AR, Tom SM, Toga AW, Poldrack RA. Automatic independent component labeling for artifact removal in fMRI. Neuroimage. 2008;39:1227–1245. doi: 10.1016/j.neuroimage.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Supekar K, Menon V. Typical and atypical development of functional human brain networks: insights from resting-state FMRI. Frontiers in systems neuroscience. 2010;4:21. doi: 10.3389/fnsys.2010.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk KR, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage. 2012;59:431–438. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008;59:1037–1050. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheatley T, Kang O, Parkinson C, Looser CE. From mind perception to mental connection: Synchrony as a mechanism for social understanding. Soc Personal Psychol Compass. 2012;6:589–606. [Google Scholar]
- Wiltermuth SS, Heath C. Synchrony and cooperation. Psychological Science. 2009;20:1–5. doi: 10.1111/j.1467-9280.2008.02253.x. [DOI] [PubMed] [Google Scholar]
- Zuo XN, Ehmke R, Mennes M, Imperati D, Castellanos FX, Sporns O, Milham MP. Network centrality in the human functional connectome. Cerebral cortex. 2012;22:1862–1875. doi: 10.1093/cercor/bhr269. [DOI] [PubMed] [Google Scholar]