Abstract
Objectives
To compare a mechanical heterogeneity index derived from ultrasound vibration elastography with physical findings before and after dry-needling treatment of spontaneously painful active myofascial trigger points in the upper trapezius muscle.
Methods
Forty-eight patients with chronic myofascial pain enrolled in a prospective interventional trial of 3 weekly dry-needling treatments for active myofascial trigger points. Trigger points were evaluated at baseline and at treatment completion using palpation, the pressure-pain threshold, and the mechanical heterogeneity index. Thirty patients were reevaluated at 8 weeks. Trigger points that “responded” changed to tissue that was no longer spontaneously painful, with or without the presence of a palpable nodule. Trigger points that “resolved” changed to tissue without a palpable nodule. The mechanical heterogeneity index was defined as the proportion of the upper trapezius muscle that appeared mechanically stiffer on elastography. Statistical significance for comparisons was determined at P < .05.
Results
Following 3 dry needle treatments, the mechanical heterogeneity index decreased significantly for the 38 myofascial trigger points (79% of 48) that responded to treatment. Among these, the baseline mechanical heterogeneity index was significantly lower for the 13 trigger points (27% of 38) that resolved, but the decrease after 3 dry needle treatments did not reach significance. The pressure-pain threshold improved significantly for both groups. At 8 weeks, the mechanical heterogeneity index decreased significantly for the 22 trigger points (73% of 30) that responded and for the 10 (45% of 22) that resolved. The pressure-pain threshold improvement was significant for trigger points that responded but did not reach significance for resolved trigger points.
Conclusions
The mechanical heterogeneity index identifies changes in muscle tissue properties that correlate with changes in the myofascial trigger point status after dry needling.
Keywords: dry needling, musculoskeletal ultrasound, myofascial pain syndrome, myofascial trigger points, ultrasound elastography
Myofascial pain syndrome is a common public health problem.1 It accounts for 95% of people with chronic pain disorders2 and approximately 15% of medical visits.3,4 Myofascial pain syndrome has generated controversy in part because there has been disagreement about criteria for diagnosis, and the etiology and pathophysiologic characteristics of the syndrome are still uncertain. In this study, we used the definitions of myofascial pain syndrome and myofascial trigger points given by Simons et al.3,4 Myofascial pain syndrome is a nonarticular musculoskeletal disorder characterized by the presence of a palpable discrete painful nodule within a taut band of skeletal muscle. A trigger point is active if the nodule is spontaneously painful and latent if the nodule is painful only when elicited by a mechanical stimulus. The myofascial trigger point is an objective physical finding that can reliably be identified and used for diagnosis of myofascial pain syndrome, and it is a target of a number of interventions for alleviating pain associated with the syndrome.3–6 Dry needling is widely used in clinical practice and is considered a standard for reducing pain associated with active myofascial trigger points.6 However, its effect on the trigger point is not known. Although dry needling has been shown to be effective in reducing myofascial pain,7 its mechanism(s) of action and effects have not been demonstrated, in part because of a lack of objective measures of pain and the trigger point status. Pain measurements are based on a patient’s self-report and are sensitive to a patient’s interpretation and pain threshold. The status of the trigger point can only be evaluated through physical examination by a trained examiner. Therefore, there is a need to develop objective, repeatable, and reliable measures for assessing the effect of dry needling therapy on the morphologic characteristics and mechanical heterogeneity of tissue with myofascial trigger points that correspond to the physical findings and pain. The purpose of this study was to determine whether imaging can provide an objective and reproducible measure of a change in the status of myofascial trigger points as determined by physical examination.
Ultrasound elastography is emerging as a valuable modality for characterizing mechanical properties of muscle. In recent years, shear wave elastography and strain imaging have become commercially available on some premium ultrasound systems. However, most practitioners do not have access to these relatively expensive systems. In our previous work, we used a vibration elastographic method that uses existing equipment with conventional color Doppler imaging capability to characterize muscle tissue properties. We have shown that myofascial trigger points can be visualized and characterized by real-time B-mode imaging as well as vibration elastography.8–11 We have also developed a standard evaluation approach that distinguishes people with trigger points and myofascial pain from those without pain.12
This study investigated whether vibration elastography is a valid and reproducible technique for objectively documenting changes in tissue properties after dry needling of myofascial trigger points. To the best of our knowledge, a study using image-based data to determine whether changes in the physical findings associated with treatment of myofascial trigger points correlate with elastographic physical properties has not been reported previously.
Materials and Methods
Study Population and Clinical Examination
The study was approved by the Chesapeake Institutional Review Board. Informed consent was obtained from all participants. All physical examinations were performed by experienced physicians (L.H.G. and J.P.S.), who each had more than 20 years of experience in evaluating and treating myofascial pain syndrome. For all patients included in this study, pain was present without provocation, and on physical examination, there was at least 1 palpable nodule in the upper trapezius muscle whose palpation reproduced the patients’ spontaneous pain symptoms. Radiation of pain to the head, neck, or face on trigger point palpation was acceptable but not required for inclusion. Entry exclusions included chronic fatigue syndrome, other chronic pain conditions (eg, fibromyalgia and radiculopathy), head or neck surgery, narcotic pain prescriptions, recent medication changes (<6 weeks), and current use of acupuncture.
All eligible patients received treatment consisting of 3 successive dry-needling treatments 1 week apart. The area of the upper trapezius muscle, approximately midway between the cervical vertebrae and the acromion process, was the focus of the examination, since myofascial trigger points are predominantly observed in this area, as described in previous publications.9,11,13 This area was palpated on each side and trigger points were identified. Trigger points were classified as follows: active trigger points were spontaneously painful, and latent trigger points were not spontaneously painful but became painful on palpation. Each patient had at least 1 active trigger point but could have had more than 1 trigger point or latent trigger points. In this study, only 1 active trigger point was selected for treatment for each patient. If there was more than 1 active trigger point, only the most symptomatic one was treated with dry needling because we wanted to observe an isolated effect of perturbation on a single active myofascial trigger point; treatment of multiple active trigger points could have introduced confounding cumulative effects.
The dry-needling intervention is described in detail elsewhere.14 The selected active trigger point was prepared by wiping the area with an alcohol pad, and a 32-gauge acupuncture needle within its plastic guide tube was placed over the trigger point. A tapping motion was used to advance the needle, and then the guide tube was removed. Stellate movement was attempted around the nodule in an effort to elicit a small muscle twitch. This response was achieved in 70% of patients at the first treatment, 66% at the second, and 50% at the third. Needling was stopped once the twitch was elicited. If no twitch was elicited, needling was stopped after 2 or 3 stellate movements.
All evaluations (physical examination, pain pressure threshold, and ultrasound imaging) were performed at baseline and after the last treatment (third week) on both left and right upper trapezius muscles in each patient. In a subset of 30 patients, all evaluations were repeated 8 weeks after baseline. Trigger points that changed from active to latent or palpably normal tissue after treatment were defined as “responded.” Trigger points that changed from active to palpably normal tissue after treatment were defined as “resolved.” Therefore, the resolved trigger points were a subset of the responded category. Some active trigger points changed to latent trigger points; thus, they responded but did not resolve. Active trigger points that did not respond were the ones that stayed active. Response and resolution relate to 2 different concepts. In the group that responded to treatment, the upper trapezius muscle was no longer spontaneously painful after treatment. However, there may have been latent trigger points that were painful on palpation. Therefore, the response was determined from the perspective of pain symptoms and not tissue properties. In the second group, the tissue properties changed so that the upper trapezius muscle at the treated site was palpably normal without nodules, and there was no spontaneous pain. The pressure-pain threshold was measured with a pressure algometer (Commander; Tech Medical, Salt Lake City, UT). Patients were asked to identify the moment at which they felt a qualitative shift from pressure to pain when the examiner pressed the algometer into the upper trapezius muscle. When the shift occurred, the pressure (in pounds per square in) was recorded for each site.
Vibration Elastography
Two sites for each patient were scanned by B-mode ultrasound imaging and vibration elastography using an SonixRP ultrasound scanner (Ultrasonix Corporation, Vancouver, British Columbia, Canada) to assess the size and tissue characteristics of the nodule. Vibration elastography was performed by using color Doppler variance imaging of the upper trapezius while it was vibrated with an external massager (NC70209; North Coast Medical, Inc, Gilroy, CA) at a frequency of about 100 Hz. Previous work has shown that ultrasound can be used to visualize myofascial trigger points. They are observed as hypoechoic regions in the grayscale image (B-mode) and appear as regions with a color deficit in the color Doppler variance image during vibration elastography9 (Figures 1 and 2). The latter phenomenon is explained by the fact that when the upper trapezius muscle is vibrated, palpably stiff nodules vibrate with lower amplitude.8 Methods for quantifying the trigger point size from these elastograms have been described previously.9 Briefly, the color variance mode was used to visualize the vibration amplitude. All machine settings were preset to fixed values for all patients: the imaging frequency was 10 MHz; color gain was 50%; B-mode gain was 54%; pulse repetition frequency was 0.2 kHz; wall filter setting was 36 Hz; ensemble size was 10; imaging depth was 25 mm; and frame rate was 17 Hz. These settings were optimized on the basis of our previous studies.9 With these settings, the vibration amplitude images were saturated in normal upper trapezius muscle, and a color void was observed in the region of the myofascial trigger point, as shown in Figure 1.
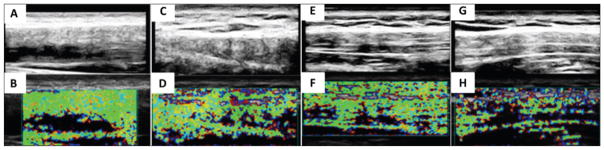
Figure 1.
Examples of heterogeneous soft tissue properties in the upper trapezius muscles of 4 patients with active myofascial trigger points. The top row shows the B-mode images enhanced with histogram equalization to enhance contrast, and the bottom row shows the corresponding elastograms. On physical examination, the tissue was described in A and B as ropey, in C and D as having a chain of nodules, in E and F as lumpy, and in G and H as inhomogeneous.
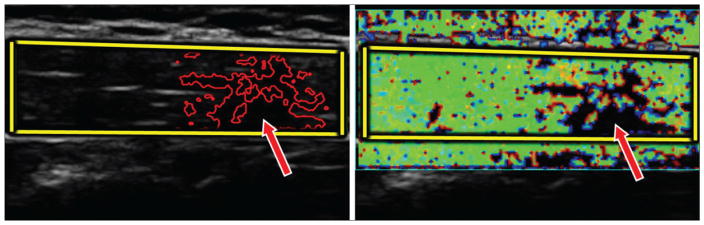
Figure 2.
Grayscale image (left) and color Doppler image (right) of an upper trapezius muscle with an active myofascial trigger point (arrows). The fascia borders (bright lines in the grayscale image) were used as guidelines to mark the upper trapezius muscle manually (yellow borders). Vibration elastography allows measurement of the mechanical heterogeneity index from the color Doppler variance image.
In this larger study, we examined the trigger point within the context of its surrounding tissue. On palpation, muscle tissue with myofascial trigger points may be described as ropey, as having a chain of nodules, as lumpy, and as inhomogeneous. These subjective differences can be documented by elastograms of the tissue, as shown in Figure 1. Therefore, we report findings of an area of mechanical tissue heterogeneity, which we define as the area of muscle that vibrates with a lower amplitude relative to the surrounding tissue and is the region devoid of color in the vibration elastogram. We defined the ratio of the area of the stiffer region to the area of the upper trapezius muscle visible in the 2-dimensional image as the “mechanical heterogeneity index.” This parameter is a new quantity developed specifically for our study. The motivation for this quantity is the observation that not all active myofascial trigger points are isolated discrete nodules, but rather, the soft tissue neighborhood of the muscle surrounding the trigger point is affected in terms of the mechanical properties. The mechanical heterogeneity index therefore is a measure of the proportion of the upper trapezius muscle that is adjacent to the active trigger point and has heterogeneity of stiffness that is different from that of normal muscle, which usually is more homogeneous. A decrease in the mechanical heterogeneity index would indicate that the muscle becomes similar to palpably normal muscle. For example, palpably normal upper trapezius muscle should ideally have a mechanical heterogeneity index of 0, and an index of 0.5 indicates that half of the upper trapezius muscle is involved. To determine the reproducibility of this measure, we performed a repeated measures study, as described in the “Statistical Analysis” section.
In B-mode images, the upper trapezius muscle can be easily identified and its size determined by manual marking (Figures 2 and 3). The mechanical heterogeneity index is measured by accounting for nonvibrating regions within the marked upper trapezius muscle and approximately 5 mm away from the 2 vertical margins of the image.
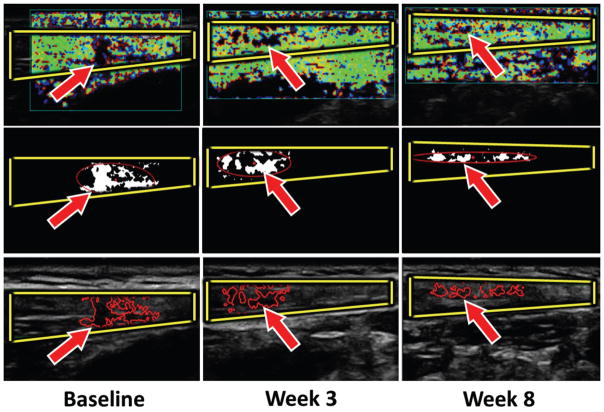
Figure 3.
Myofascial trigger point (arrows) size evolution. Top row, Color Doppler variance images. Middle row, Binary images of the mechanical heterogeneity region. Bottom row, Yellow borders indicate B-mode images of the upper trapezius muscle, and red borders indicate regions with a lower vibration amplitude (red line).
All image-processing methods were implemented in MATLAB (The MathWorks, Inc, Natick, MA). The measurements were repeated twice at each location to quantify reproducibility.
Statistical Analysis
For the analysis reported in this article, the main outcome measures were the changes in the trigger point status (from active to either latent or no palpable nodule) from baseline to 3 weeks after treatment, pain reduction as assessed by the pressure-pain threshold score, and the reduction in the mechanical heterogeneity index measured by postprocessing of the vibration elastograms. Secondary outcome measures were the changes in the same measures from baseline to follow-up (8 weeks).
Repeatability of the cross-sectional areas of the upper trapezius muscle and myofascial trigger point and the mechanical heterogeneity index was assessed by Bland-Altmanplots in MATLAB. The minimum detectable change in the trigger point area and mechanical heterogeneity index was calculated with a 95% confidence interval (CI). The minimum detectable change (MDC) is defined as , where SD is the average standard deviation, and R is the correlation coefficient of 2 repeated sets of measurements.
A normality test showed that the mechanical heterogeneity index was log-normally distributed, and tests were performed on the logarithm of this ratio. As described previously, data were grouped into “all treated,” “responded,” “did not respond,” “responded but did not resolve,” and “resolved” categories. Changes from baseline in the logarithm of the mechanical heterogeneity index and the pressure-pain threshold scores at weeks 3 and 8 were analyzed by paired t tests for all of these groups. The mechanical heterogeneity index for the groups are reported as geometric means with 95% CIs. These analyses were conducted with Microsoft Excel 2010 software (Microsoft Corporation, Redmond, WA).
Analysis of covariance models were conducted at weeks 3 and 8 to determine whether trigger point resolution was related to the change in the mechanical heterogeneity index, adjusted for the baseline value, age, and exercise status. SAS software (SAS Institute Inc, Cary, NC) was used for all regression models. Statistical significance for comparisons was determined at P < .05, without adjustment for multiple testing.
Results
Patient Demographics
Forty-eight patients were included in the study (18 male and 30 female). Their mean age ± SD was 35 ± 13 years. All patients had at least 1 active myofascial trigger point. Twenty-three patients (11 male and 12 female; mean age, 35 ± 12 years) had bilateral active trigger points. In all patients, only the most symptomatic active trigger point was treated. The study as originally designed to include measurements only at baseline and at treatment completion (3 weeks), with clinical follow-up at 8 weeks. At a later date, we decided to include all measurements at the 8-week follow-up as a secondary measure. As a result, follow-up imaging at 8 weeks was not performed on the first 14 patients. Four patients were lost to follow-up. Therefore, 30 patients (13 male and 17 female; mean age, 37 ± 12 years) were examined 8 weeks after the baseline visit.
Repeatability and Minimum Detectable Change in the Mechanical Heterogeneity Index
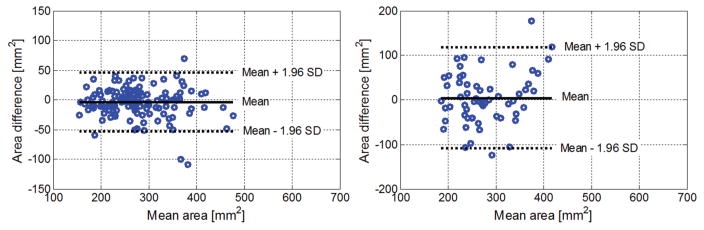
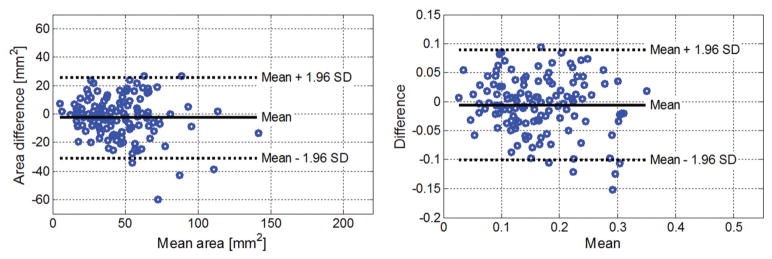
Measurements of the upper trapezius areas were repeatable between 2 consecutive measurements as well as between measurements performed at baseline and follow-up visits with a 95% CI (Figure 4). Both myofascial trigger point size and mechanical heterogeneity index were repeatable between 2 consecutive measurements (Figure 5). Based on the Bland-Altman plot, the minimum detectable change was estimated for the mechanical heterogeneity index. We found that a change of 21% from the baseline mechanical heterogeneity index or an absolute difference of 0.034 in the index could be detected with a 95% CI.
Figure 4.
Bland-Altman plots of the cross-sectional areas of the upper trapezius muscle. Left, Repeatability of the upper trapezius area between 2 consecutive measurements. Right, Repeatability of the mean upper trapezius area between measurements performed at baseline and follow-up visits.
Figure 5.
Bland-Altman plots of the mechanical heterogeneity area. Left, Myofascial trigger point area. Right, Mechanical heterogeneity index.
Change From Baseline After Treatment at the 3-Week Follow-up
At 3 weeks, 38 myofascial trigger points (79% of 48) responded, and of these, 13 (27% of 38) resolved. The main outcome measures at the 3-week follow-up are summarized in Table 1. For all treated trigger points, the mechanical heterogeneity index decreased significantly (P = .008) and the pressure-pain threshold increased significantly (P < .001) from baseline. The mechanical heterogeneity index decreased significantly and the pressure-pain threshold increased significantly from baseline in the responded (ie, active trigger point to latent trigger point or palpably normal tissue) group and responded but did not resolve (ie, active trigger point to latent trigger point) subgroup. In the resolved subgroup (ie, active trigger point to palpably normal tissue), the pressure-pain threshold increased significantly, but the mechanical heterogeneity index reduction was not significant. The reason for this finding was that the baseline mechanical heterogeneity index for this subgroup was significantly lower than those for the other subgroups, and although the index decreased further at 3 weeks, it did not reach significance for the small number of patients (n = 13).
Table 1.
Changes in the Mechanical Heterogeneity Index and Pressure-Pain Threshold for Active Myofascial Trigger Points From Baseline to the 3-Week Follow-up After a 3-Week Course of Dry Needling
| MTrP Status at 3 wk | Age, y | n | Male | Female | Variable | Baseline Mean (95% CI) | 3-wk Follow-up Mean (95% CI) | P |
|---|---|---|---|---|---|---|---|---|
| All treated | 35 ± 13 | 48 | 18 | 30 | MHI | 0.16 (0.14–0.18) | 0.13 (0.11–0.15) | .008 |
| PPT | 7.20 (6.27–8.13) | 9.00 (8.01–9.99) | <.001 | |||||
| Responded (active MTrP to latent MTrP or normal) | 37 ± 14 | 38 | 14 | 24 | MHI | 0.17 (0.15–0.19) | 0.13 (0.11–0.15) | .010 |
| PPT | 7.30 (6.31–8.29) | 9.10 (7.96–10.24) | <.001 | |||||
| Did not respond | 33 ± 11 | 10 | 4 | 6 | MHI | 0.14 (0.08–0.24) | 0.12 (0.07–0.19) | .42 |
| PPT | 6.90 (4.28–9.32) | 7.80 (5.82–9.78) | .27 | |||||
| Responded but did not resolve (active MTrP to latent MTrP) | 37 ± 14 | 25 | 9 | 16 | MHI | 0.19 (0.17–0.21) | 0.14 (0.12–0.17) | .007 |
| PPT | 6.80 (5.94–7.66) | 8.80 (7.47–10.13) | .008 | |||||
| Resolved (active MTrP to normal) | 35 ± 11 | 13 | 5 | 8 | MHI | 0.13 (0.1–0.16) | 0.11 (0.09–0.15) | .51 |
| PPT | 8.30 (5.96–10.64) | 10.10 (8.09–12.11) | .02 |
Data are presented as mean ± SD where applicable. MHI indicates mechanical heterogeneity index; MTrP, myofascial trigger point, and PPT, pressure-pain threshold.
Change From Baseline at the 8-Week Follow-up
At 8 weeks, 22 myofascial trigger points (73% of 30) responded, and of these, 10 (45% of 22) resolved. The outcome measures at the 8-week follow-up are summarized in Table 2. For all treated trigger points, the mechanical heterogeneity index decreased significantly (P < .001) and the pressure-pain threshold increased significantly (P = .036) from baseline. For trigger points in the responded group, the mechanical heterogeneity index decreased significantly and the pressure-pain threshold increased significantly from baseline. For the trigger points in the resolved subgroup, the mechanical heterogeneity index decreased significantly but the pressure-pain threshold change from baseline did not reach significance, in part because of the small sample size in this subgroup (n = 10). The mechanical heterogeneity index and pressure-pain threshold showed similar trends in the responded but did not resolve subgroup.
Table 2.
Changes in the Mechanical Heterogeneity Index and Pressure-Pain Threshold for Active Myofascial Trigger Points From Baseline to the 8-Week Follow-up After a 3-Week Course of Dry Needling
| MTrP Status at 8 wk | Age, y | n | Male | Female | Variable | Baseline Mean (95% CI) | 8-wk Follow-up Mean (95% CI) | P |
|---|---|---|---|---|---|---|---|---|
| All treated | 37 ± 12 | 30 | 13 | 17 | MHI | 0.16 (0.14–0.19) | 0.11 (0.09–0.13) | <.001 |
| PPT | 7.30 (6.05–8.55) | 8.60 (7.35–9.85) | .036 | |||||
| Responded (active MTrP to latent MTrP or normal) | 37 ± 12 | 22 | 11 | 11 | MHI | 0.16 (0.13–0.19) | 0.10 (0.09–0.12) | <.001 |
| PPT | 7.3 (6.26–8.34) | 8.90 (7.81–9.99) | .027 | |||||
| Did not respond | 38 ± 14 | 8 | 2 | 6 | MHI | 0.18 (0.14–0.23) | 0.12 (0.09–0.17) | .093 |
| PPT | 7.30 (3.35–11.25) | 7.50 (3.83–11.17) | .84 | |||||
| Responded but did not resolve (active MTrP to latent MTrP) | 33 ± 12 | 12 | 6 | 6 | MHI | 0.17 (0.14–0.21) | 0.13 (0.10–0.16) | .006 |
| PPT | 6.70 (5.68–7.72) | 8.00 (6.59–9.41) | .15 | |||||
| Resolved (active MTrP to normal) | 41 ± 11 | 10 | 5 | 5 | MHI | 0.14 (0.11–0.18) | 0.08 (0.06–0.10) | <.001 |
| PPT | 7.9 (5.98–9.82) | 10 (8.45–11.55) | .11 |
Notations are as in Table 1.
Change From Baseline in the Subgroup With a Detectable Change in the Mechanical Heterogeneity Index
At the 3-week follow-up, 33 treated myofascial trigger points (69% of 48) had a change in the mechanical heterogeneity index that was greater than the 95% CI for detectability (ie, change >21% based on the reproducibility study). The main outcome measures are summarized in Table 3. Of the 38 trigger points that responded, 26 (68% of 38) had a detectable change in the mechanical heterogeneity index, and of the 13 trigger points that resolved, 6 (46% of 13) had a detectable change. Those 6 upper trapezius muscles with a detectable change had a mechanical heterogeneity index value at baseline of 0.15 ± 0.07, whereas the 7 muscles with no detectable change had a significantly lower value at baseline of 0.12 ± 0.05.
Table 3.
Changes in the Mechanical Heterogeneity Index and Pressure-Pain Threshold From Baseline to the 3-week Follow-up in the Subset of Patients With Reliably Detectable Changes in the Mechanical Heterogeneity Index
| Detectable Change in MHI (>21%) | Age, y | n | % n | Male | Female | Variable | Baseline Mean (95% CI) | 3-wk Follow-up Mean (95% CI) | P |
|---|---|---|---|---|---|---|---|---|---|
| All treated | 37 ± 13 | 33 | 69 (33/48) | 11 | 22 | MHI | 0.17 (0.15–0.20) | 0.13 (0.11–0.15) | .013 |
| PPT | 6.80 (5.61–7.99) | 8.90 (7.60–10.20) | .0013 | ||||||
| Responded | 37 ± 13 | 26 | 68 (26/38) | 9 | 17 | MHI | 0.17 (0.15–0.20) | 0.12 (0.10–0.15) | .0071 |
| PPT | 7.10 (5.75–8.45) | 9.50 (8.04–10.96) | .0026 | ||||||
| Resolved | 32 ± 9 | 6 | 46 (6/13) | 2 | 4 | MHI | 0.14 (0.10–0.20) | 0.11 (0.07–0.18) | .50 |
| PPT | 8.30 (3.50–13.10) | 9.90 (5.82–13.98) | .20 |
Notations are as in Table 1.
At the 8-week follow-up, 21 treated trigger points (70% of 30), had a change in the mechanical heterogeneity index that was greater than the 95% CI for detectability. The main outcome measures are summarized in Table 4. Of the 22 myofascial trigger points that responded, 14 (64% of 22) had a detectable change in the mechanical heterogeneity index, and of the 10 trigger points that resolved, 8 (80% of 10) had a detectable change.
Table 4.
Changes in the Mechanical Heterogeneity Index and Pressure-Pain Threshold From Baseline to the 8-week Follow-up in the Subset of Patients With Reliably Detectable Changes in the Mechanical Heterogeneity Index
| Detectable Change in MHI (>21%) | Age, y | n | % n | Male | Female | Variable | Baseline Mean (95% CI) | 8-wk Follow-up Mean (95% CI) | P |
|---|---|---|---|---|---|---|---|---|---|
| All treated | 39 ± 13 | 21 | 70 (21/30) | 9 | 12 | MHI | 0.18 (0.16–0.20) | 0.14 (0.11–0.17) | .030 |
| PPT | 6.50 (5.39–7.61) | 9.10 (7.99–10.21) | .0014 | ||||||
| Responded | 39 ± 13 | 14 | 64 (14/22) | 7 | 7 | MHI | 0.18 (0.16–0.20) | 0.09 (0.08–0.11) | <.0001 |
| PPT | 6.90 (5.59–8.21) | 9.40 (8.04–10.76) | .022 | ||||||
| Resolved | 43 ± 12 | 8 | 80 (8/10) | 4 | 4 | MHI | 0.16 (0.14–0.19) | 0.08 (0.06–0.10) | .0002 |
| PPT | 7.60 (5.52–9.68) | 10.30 (8.57–12.03) | 0.10 |
Notations are as in Table 1.
Change From Baseline Adjusted for Baseline Values, Age, and Exercise Status
The soft tissue properties of the upper trapezius muscle are expected to be different with age and regular exercise. The baseline mechanical heterogeneity index is also expected to influence whether the tissue properties change after treatment. We therefore investigated the change in the logarithm of the mechanical heterogeneity index from baseline to 3 and 8 weeks in myofascial trigger points that resolved compared to those that did not, adjusted for these 3 factors. Age and exercise status were not significant in any of the models. The logarithm of the mechanical heterogeneity index at baseline was significant in the models (Table 5), indicating that the trigger points that resolved after 3 or 8 weeks were significantly smaller at baseline compared to those that did not resolve. At 8 weeks, the least square means for change in the logarithm of the mechanical heterogeneity index, adjusted for the baseline logarithm, age, and exercise status, was larger for trigger points that responded (−0.64) versus those that did not (−0.312), but the difference (−0.329) did not reach significance (P = .0509).
Table 5.
Regression Estimates With Change in the Mechanical Heterogeneity Index as the Response Variable From the 3- and 8-Week Follow-ups Adjusted for Age, Exercise Status, and Baseline Mechanical Heterogeneity Index
| Parameter | Change From Baseline to 3 wka
|
Change From Baseline to 8 wkb
|
||||||
|---|---|---|---|---|---|---|---|---|
| Estimate | SE | t | P | Estimate | SE | t | P | |
| Intercept | −1.422 | 0.321 | −4.43 | <.001 | −1.091 | 0.426 | −2.56 | .0169 |
| Log MHI baseline | −0.591 | 0.147 | −4.02 | .0002 | −0.496 | 0.196 | −2.54 | .0179 |
| Trigger point resolved | −0.011 | 0.168 | 0.07 | .948 | −0.329 | 0.160 | −2.05 | .0509 |
| Trigger point did not resolve | Baseline | NA | NA | NA | Baseline | NA | NA | NA |
| Age | 0.004 | 0.006 | 0.73 | .4693 | −0.004 | 0.006 | −0.62 | .5396 |
| Exercise status: no | −0.123 | 0.162 | −0.76 | .4509 | 0.034 | 0.157 | 0.22 | .8293 |
| Exercise status: yes | Baseline | NA | NA | NA | Baseline | NA | NA | NA |
MHI indicates mechanical heterogeneity index; and NA, not applicable.
Model F = 4.57; P = .0036; R2 = 0.30; n = 48.
Model F = 2.52; P = .0664; R2 = 0.29; n = 30.
Trends for Individual Patients
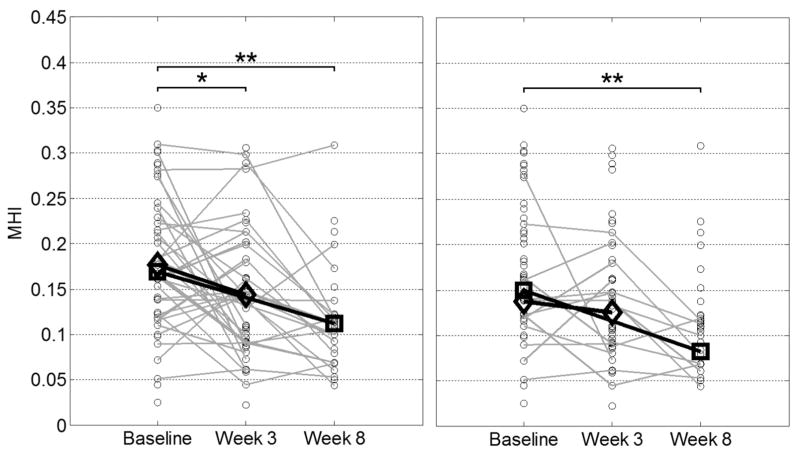
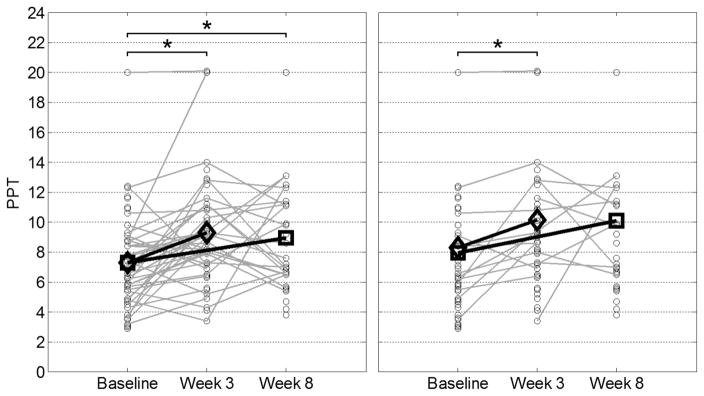
It should be noted that the pressure-pain threshold score and mechanical heterogeneity index did not improve monotonically over time. This finding means that of all myofascial trigger points that increased at 3 weeks, some decreased at 8 weeks and vice versa. Scatterplots of the data in Tables 1 and 2 are shown in Figures 6 and 7. These scatterplots indicate the trends in the overall groups, as well as for individual treatment sites for both the mechanical heterogeneity index and pressure-pain threshold. For trigger points with a baseline mechanical heterogeneity index of less than 0.14, the sensitivity and specificity for predicting trigger point resolution at 3 weeks were 69% and 80%, respectively.
Figure 6.
Changes in tissue status after dry needling at the 3- and 8-week follow-ups. Left, Open circles represent individual mechanical heterogeneity indices (MHI) of upper trapezius muscles with treated active myofascial trigger points at different visits; gray lines are drawn between individual active trigger points that responded at week 3 or 8; unconnected circles represent trigger points that did not respond; open diamonds represent mean mechanical heterogeneity indices of upper trapezius muscles with trigger points that responded at week 3; and open squares represent mean mechanical heterogeneity indices of upper trapezius muscles with trigger points that responded at week 8. Right, Open circles represent individual mechanical heterogeneity indices of upper trapezius muscles with treated active trigger points at different visits; gray lines are drawn between individual active trigger points that resolved at week 3 or 8; unconnected circles represent trigger points that did not resolve; open diamonds represent mean mechanical heterogeneity indices of upper trapezius muscles with trigger points that resolved at week 3; and open squares represent mean mechanical heterogeneity indices of upper trapezius muscles with trigger points that resolved at week 8. *P < .01; **P < .001.
Figure 7.
Changes in pressure-pain threshold (PPT) scores after dry needling at the 3- and 8-week follow-ups. Notations are as in Figure 6.
Discussion
Numerous studies have shown the benefit of dry needling for pain reduction in myofascial pain syndrome,7,15–22 and many consider the myofascial trigger point to be a necessary finding in this syndrome.3 However, to the best of our knowledge, no other study has used vibration elastography to quantify the mechanical heterogeneity of upper trapezius muscles with myofascial trigger points after dry needling. In a separate analysis, pain reduction was found to be significantly correlated to the change in the trigger point status from active to latent or normal.14 The objective of this study was to evaluate whether image-based measures can be used to document changes in the upper trapezius muscle and the status of the myofascial trigger point as observed by physical examination. The role of the dry-needling intervention was to perturb the system to alter the status of the trigger point and investigate whether the imaging measures are sensitive to this change. We anticipate that this investigation can answer an important question about the physical effect of a local mechanical perturbation induced by dry needling and how it affects the morphologic characteristics of stiffer regions in the upper trapezius muscle with active myofascial trigger points (Figure 3). However, our objective in this study was not to evaluate whether the dry-needling treatment is effective at the 3- or 8-week follow-up. The pressure-pain threshold was used as an additional objective measure of the tenderness of the upper trapezius muscle at the site of the trigger point.
Our data revealed that after a 3-week course of dry-needling treatment, the status of the myofascial trigger point changed at the treated sites as assessed on physical examination, and correspondingly, the mechanical heterogeneity index decreased significantly and the pressure-pain threshold score increased significantly at the treated sites. The trigger points that responded (ie, changed in status from active trigger points to latent trigger points or palpably normal tissue) had a significant reduction in the mechanical heterogeneity index at both the 3- and 8-week follow-ups and a significant improvement in the pressure-pain threshold score. However, not all of the treated trigger points responded. It can be observed from Figure 6 that there was a significant change in the mechanical heterogeneity index for the trigger points that responded at 3 weeks, and the downward trend continued to 8 weeks. A similar trend was observed for trigger points that resolved. In the latter subgroup, the differences at the 3-week follow-up were not significant but became significant at the 8-week follow-up. Therefore, the changes in the imaging measures can be used as objective documentation of the change in the status of the myofascial trigger point, especially at the 8-week follow-up. A decrease in the mechanical heterogeneity index of greater than 21% (ie, greater than the minimum detectable change) was observed in 68% of trigger points that responded and 80% of those that resolved at the 8-week follow-up.
For the trigger points that resolved (ie, changed from active trigger points to palpably normal tissue) the mechanical heterogeneity index at baseline was significantly lower (Table 5) than for the other subgroups. These results show that upper trapezius muscles that have active myofascial trigger points and a low mechanical heterogeneity index at baseline are more likely to completely resolve. For trigger points with a baseline mechanical heterogeneity index of less than 0.14, the sensitivity and specificity for predicting trigger point resolution at 3 weeks were 69% and 80%, respectively. Our results also show that for upper trapezius muscles with a low mechanical heterogeneity index at baseline, a longer follow-up period is required to detect significant tissue property changes on imaging (Figure 6).
It is important to note that the definitions of active and latent myofascial trigger points are based on the physical examination. In these cases, the observed regions on the elastograms corresponded approximately to the palpable nodule, and we can say with reasonable certainty that the observed lesion on the elastogram included the palpable trigger point. However, even if no palpable nodule was found on physical examination, some regions with a color deficit on the elastograms frequently persisted. According to the clinical definition by Simons et al,3,4 these regions do not correspond to myofascial trigger points, since no palpable nodule was found on physical examination. However, residual lesions in the upper trapezius muscle may account for these imaging findings. It is possible that some nodules are too small to be identified by palpation and instead feel like heterogeneous tissue (ie, without discrete nodules). It is unknown how large a nodule has to be to be palpable. This factor is likely influenced by its stiffness and its depth.
Stecco et al23 suggested that myofascial pain syndrome might be associated with local alteration of the muscle viscosity that impairs intrafascicular gliding and creates internal stretch lesions. The occurrence of this phenomenon is hard to demonstrate because it does not create a macroscopic alteration of the morphologic characteristics of the fascia tissue. However, this phenomenon can be investigated in the future by using elastography.
In this study, we have shown that the mechanical heterogeneity index agreed with the myofascial trigger point status determined by palpation (ie, active, latent, or nonpalpable nodule) at all follow-ups. The pressure-pain threshold score agreed with physical findings at the 3-week follow-up, but not at 8 weeks. Figure 7 shows that there was an increasing trend in the pressure-pain threshold from baseline to the 3-week follow-up in both the responded and resolved categories, and these differences were significant. However, the increasing trend did not reach statistical significance at the 8-week follow-up for the resolved group, in part because of the small sample size in this subgroup (n = 10). In addition, the pressure-pain threshold and mechanical heterogeneity index are not directly correlated. Even though the pressure-pain threshold has been shown to be reliable in evaluating myofascial pain syndrome, it lacks sensitivity.24 Furthermore, the pressure-pain threshold may be measuring mechanoreceptor response rather than a sensory response; hence, the pain threshold may not reflect an improvement in sensory pain. The latter may result from both changes in the trigger point status and changes in the biochemical milieu, which we did not measure in this study.25
Study Limitations
There were several weaknesses in this study. The mechanical heterogeneity index is a repeatable measure, but the resolution of the elastographic technique needs to be improved. For example, further standardization of ultrasound transducer positioning and increasing the number of elastograms collected per site can lead to a reduction of the minimum detectable change in the mechanical heterogeneity index. The vibration elastography method that we used26 is easily translatable to existing clinical systems, but shear wave elastographic methods27 might yield more reproducible results with reduced variance.
The objective of this study was to investigate changes in the physical and mechanical properties of myofascial trigger points and surrounding soft tissue in the upper trapezius muscle before and after a perturbation of the active trigger point using dry needling. The results of these associations are not meant to establish the effectiveness of the dry-needling intervention for reducing pain. A trial establishing the effectiveness of dry needling active trigger points trial will require a randomized placebo-controlled study design. We did observe significant pain reductions in the treated patients at 3 weeks, although those results have been reported elsewhere.14 The goal of this study was to investigate the association between the status of a myofascial trigger point based on physical findings and imaging findings of mechanical heterogeneities at the same location before and after a perturbation using dry needling. A number of factors can influence why the nodule responds to treatment with or without resolution of the nodule, especially at the 8-week follow up. The specific mechanistic reasons for the change in the status of the nodule were not the focus of this study; therefore, we have not reported a number of additional measures that were collected as part of the study. However, we believe that the ability to objectively document the extent and changes of mechanical heterogeneities in the upper trapezius muscle using imaging in longitudinal studies of myofascial pain syndrome can be an important factor in facilitating the elucidation of underlying pathophysiologic mechanisms of myofascial trigger points as well as the therapeutic mechanisms of dry needling.
There were also limitations in our technique. We used 2-dimensional imaging and did not quantitatively ensure that the ultrasound probe was positioned at the same location for each follow-up visit. Myofascial trigger points are 3-dimensional, and the mechanical heterogeneity index, which is a ratio of cross-sectional areas, can only be considered a surrogate for the volumetric size of a palpable trigger point. In future studies, we plan to extend our methods to use 3-dimensional imaging. Nonetheless, we believe that the reported findings expand the understanding of myofascial trigger points and the effect of the dry needling on the mechanical properties of these nodules and surrounding tissue. Importantly, they provide an objective, reliable measure of changes in soft tissue properties in muscles with myofascial trigger points.
Conclusions
We have presented a new objective, quantitative measure, the mechanical heterogeneity index, which can be used for quantifying soft tissue properties of muscle based on vibration elastography in patients with myofascial trigger points. Our findings demonstrate that this measure provides objective and reproducible documentation of the extent of mechanical heterogeneities in the upper trapezius muscle and is sensitive to and correlates with changes in the status of the point as determined by physical examination. Myofascial trigger points in upper trapezius muscles that have lower heterogeneity at baseline were found to be more likely to resolve from active to palpably normal muscle. In our study population, for a baseline mechanical heterogeneity index of less than 0.14, we observed 69% sensitivity and 80% specificity for predicting trigger point resolution after a 3-week course of dry needling.
Acknowledgments
This work was supported by grant 1R01-AR057348 from the National Institutes of Arthritis and Musculoskeletal and Skin Diseases, National Institutes of Health, and was presented in part at the 2014 Ultrasonic Imaging and Tissue Characterization Symposium; June 9–11, 2014; Arlington, Virginia.
Abbreviations
- CI
confidence interval
References
- 1.Gerwin RD. Classification, epidemiology, and natural history of myofascial pain syndrome. Curr Pain Headache Rep. 2001;5:412–420. doi: 10.1007/s11916-001-0052-8. [DOI] [PubMed] [Google Scholar]
- 2.Fishbain DA, Goldberg M, Meagher BR, Steele R, Rosomoff H. Male and female chronic pain patients categorized by DSM-III psychiatric diagnostic criteria. Pain. 1986;26:181–197. doi: 10.1016/0304-3959(86)90074-6. [DOI] [PubMed] [Google Scholar]
- 3.Simons DG, Travell JG, Simons PT. Travell and Simons’ Myofascial Pain and Dysfunction: The Trigger Point Manual. Vol 1. Upper Half of Body. 2. Baltimore, MD: Williams & Wilkins; 1999. [Google Scholar]
- 4.Simons DG. Review of enigmatic MTrPs as a common cause of enigmatic musculoskeletal pain and dysfunction. J Electromyogr Kinesiol. 2004;14:95–107. doi: 10.1016/j.jelekin.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 5.Travell JG, Rinzler SH. The myofascial genesis of pain. Postgrad Med. 1952;11:452–434. doi: 10.1080/00325481.1952.11694280. [DOI] [PubMed] [Google Scholar]
- 6.Mense S, Simons DG. Muscle Pain: Understanding Its Nature, Diagnosis, and Treatment. Baltimore, MD: Lippincott Williams & Wilkins; 2001. [Google Scholar]
- 7.Tekin L, Akarsu S, Durmuş O, Cakar E, Dinçer U, Kiralp MZ. The effect of dry needling in the treatment of myofascial pain syndrome: a randomized double-blinded placebo-controlled trial. Clin Rheumatol. 2013;32:309–315. doi: 10.1007/s10067-012-2112-3. [DOI] [PubMed] [Google Scholar]
- 8.Sikdar S, Shah JP, Gebreab T, et al. Novel applications of ultrasound technology to visualize and characterize myofascial trigger points and surrounding soft tissue. Arch Phys Med Rehabil. 2009;90:1829–1838. doi: 10.1016/j.apmr.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ballyns JJ, Shah JP, Hammond J, Gebreab T, Gerber LH, Sikdar S. Objective sonographic measures for characterizing myofascial trigger points associated with cervical pain. J Ultrasound Med. 2011;30:1331–1340. doi: 10.7863/jum.2011.30.10.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ballyns JJ, Turo D, Otto P, et al. Office-based elastographic technique for quantifying mechanical properties of skeletal muscle. J Ultrasound Med. 2012;31:1209–1219. doi: 10.7863/jum.2012.31.8.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turo D, Otto P, Shah JP, et al. Ultrasonic characterization of the upper trapezius muscle in patients with chronic neck pain. Ultrason Imaging. 2013;35:173–187. doi: 10.1177/0161734612472408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gerber LH, Sikdar S, Armstrong K, et al. A systematic comparison between subjects with no pain and pain associated with active myofascial trigger points. PM R. 2013;5:931–938. doi: 10.1016/j.pmrj.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bennett R. Myofascial pain syndromes and their evaluation. Best Pract Res Clin Rheumatol. 2007;21:427–445. doi: 10.1016/j.berh.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 14.Gerber LH, Shah J, Rosenberger W, et al. Dry needling alters trigger points and reduces pain in subjects with chronic myofascial pain. PM R. 2015;7:711–718. doi: 10.1016/j.pmrj.2015.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lewit K. The needle effect in the relief of myofascial pain. Pain. 1979;6:83–90. doi: 10.1016/0304-3959(79)90142-8. [DOI] [PubMed] [Google Scholar]
- 16.Gunn CC, Milbrandt WE, Little AS, Mason KE. Dry needling of muscle motor points for chronic low-back pain: a randomized clinical trial with long-term follow-up. Spine (Phila Pa 1976) 1980;5:279–291. doi: 10.1097/00007632-198005000-00011. [DOI] [PubMed] [Google Scholar]
- 17.Baldry P. Superficial dry needling at myofascial trigger point sites. J Musculoskeletal Pain. 1995;3:117–126. [Google Scholar]
- 18.Cummings TM, White AR. Needling therapies in the management of myofascial trigger point pain: a systematic review. Arch Phys Med Rehabil. 2001;82:986–992. doi: 10.1053/apmr.2001.24023. [DOI] [PubMed] [Google Scholar]
- 19.Dommerholt J. Dry needling: peripheral and central considerations. J Man Manip Ther. 2011;19:223–227. doi: 10.1179/106698111X13129729552065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Furlan AD, van Tulder MW, Cherkin DC, et al. Acupuncture and dry-needling for low back pain. Cochrane Database Syst Rev. 2005;1:CD001351. doi: 10.1002/14651858.CD001351.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalichman L, Vulfsons A. Dry needling in the management of musculoskeletal pain. J Am Board Fam Med. 2010;23:640–646. doi: 10.3122/jabfm.2010.05.090296. [DOI] [PubMed] [Google Scholar]
- 22.Kietrys DM, Palombaro KM, Azzaretto E, et al. Effectiveness of dry needling for upper-quarter myofascial pain: a systematic review and meta-analysis. J Orthop Sports Phys Ther. 2013;43:620–634. doi: 10.2519/jospt.2013.4668. [DOI] [PubMed] [Google Scholar]
- 23.Stecco A, Gesi M, Stecco C, Stern R. Fascial components of the myofascial pain syndrome. Curr Pain Headache Rep. 2013;17:352. doi: 10.1007/s11916-013-0352-9. [DOI] [PubMed] [Google Scholar]
- 24.Park G, Kim CW, Park SB, Kim MJ, Jang SH. Reliability and usefulness of the pressure pain threshold measurement in patients with myofascial pain. Ann Rehabil Med. 2011;35:412–417. doi: 10.5535/arm.2011.35.3.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shah JP, Phillips TM, Danoff JV, Gerber LH. An in vivo microanalytical technique for measuring the local biochemical milieu of human skeletal muscle. J Appl Physiol. 2005;99:1977–1984. doi: 10.1152/japplphysiol.00419.2005. [DOI] [PubMed] [Google Scholar]
- 26.Parker KJ, Fu D, Graceswki SM, Yeung F, Levinson SF. Vibration sonoelastography and the detectability of lesions. Ultrasound Med Biol. 1998;24:1437–1447. doi: 10.1016/s0301-5629(98)00123-9. [DOI] [PubMed] [Google Scholar]
- 27.Eby SF, Song P, Chen S, Chen Q, Greenleaf JF, An KN. Validation of shear wave elastography in skeletal muscle. J Biomech. 2013;46:2381–2387. doi: 10.1016/j.jbiomech.2013.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]