Abstract
Background
Although only a small proportion of thin melanomas result in lymph node metastasis, the abundance of these lesions results in a relatively large absolute number of patients with a diagnosis of nodal metastases, determined by either sentinel lymph node (SLN) biopsy or clinical nodal recurrence (CNR).
Methods
Independent cohorts with thin melanoma and either SLN metastasis or CNR were identified at two melanoma referral centers. At both centers, SLN metastasis patients were included. At center 1, the CNR cohort included patients with initial negative clinical nodal evaluation followed by CNR. At center 2, the CNR cohort was restricted to those presenting in the era before the use of SLN biopsy. Uni- and multivariable analyses of melanoma-specific survival (MSS) were performed.
Results
At center 1, 427 CNR patients were compared with 91 SLN+ patients. The 5- and 10-year survival rates in the SLN group were respectively 88 and 84 % compared with 72 and 49 % in the CNR group (p < 0.0001). The multivariate analysis showed age older than 50 years (hazard ratio [HR] 1.5; 95 % confidence interval [CI] 1.2–1.9), present ulceration (HR 1.9; 95 % CI 1.2–2.9), unknown ulceration (HR 1.6; 95 % CI 1.3–2.1), truncal site (HR 1.6; 95 % CI 1.2–2.2), and CNR (HR 3.3; 95 % CI 1.8–6.0) to be associated significantly with decreased MSS (p < 0.01 for each). The center 2 cohort demonstrated remarkably similar findings, with a 5-year MSS of 88 % in the SLN (n = 29) group and 76 % in the CNR group (n = 39, p = 0.09).
Conclusion
Patients with nodal metastases from thin melanomas have a substantial risk of melanoma death. This risk is lower among patients whose disease is discovered by SLN biopsy rather than CNR.
The incidence of melanoma has increased dramatically in recent decades, and much of that increase has occurred among patients with thin primary tumors. These lesions are generally low risk with good to excellent long-term melanoma-specific survival (MSS).1,2 However, it is well known that a minority of these patients (< 5 %) will have tumor spread to regional lymph nodes, and the 10-year melanoma-specific and overall survival rates for this cohort range from approximately 4.5–8 %.3–7
Although the group of patients with nodal metastases represents a relatively small proportion of the population, the abundance of thin melanomas (nearly 70 % of newly diagnosed lesions) results in a substantial absolute number of patients who fall into the nodal metastasis group.5 This group is difficult to study, and as a result, recommendations for treatment of thin melanoma have been challenging.6–8
Our two melanoma referral centers have large prospectively maintained clinical databases and were early users of the sentinel lymph node (SLN) biopsy technique. This technique not only allows identification of a relatively large number of SLN-positive patients, but also provides a follow-up period long enough to identify patients with nodal metastases who did not undergo SLN biopsy but rather had nodal disease discovered through CNR, which can present after a long interval in patients with thin melanomas.
We analyzed these groups of nodal metastasis patients to compare features of patients with metastases discovered through SLN biopsy with those of CNR patients. We also compared the outcomes of those groups. Because the analysis was retrospective, we examined data from the two participating centers separately in an effort to determine the reproducibility of the findings.
For thin melanomas, the frequency of nodal involvement is relatively low.9,10 As a result, it would be very difficult to demonstrate a survival advantage in a prospective randomized trial. Nodal surgery would not be expected to carry therapeutic value if pathologically nonmalignant lymph nodes are removed, so any benefit that might be present for those patients who harbor lymph node metastases would be diluted by the vast majority of patients who did not have such disease. Conducting a trial of sufficient size to identify a statistically significant survival difference is not practical. Moreover, the moderate morbidity of complete lymph node dissection (LND) would be difficult to justify, even from the standpoint of regional control of disease, for a population in which 95 % of the patients have lymph nodes uninvolved with metastatic disease.11
Although the best criteria for selecting patients with thin melanoma for SLN biopsy remains an area of active research, several studies have confirmed the prognostic significance of SLN metastases, even for these generally low-risk lesions.11–18 Similar to patients with thick melanomas, for whom SLN biopsy can be performed with minimal morbidity and appears to carry important, independent prognostic information, patients with thin melanoma can undergo SLN biopsy with similar low procedural risks and potential of prognostic value for selected patients.19–22 The prognosis for patients with thin melanomas and nodal metastases detected by SLN biopsy compared with the prognosis for those with this condition detected clinically is less well defined.
METHODS
The John Wayne Cancer Institute (JWCI) and the University of Pennsylvania’s Pigmented Lesion Group (Penn) have prospectively maintained melanoma clinical databases for several decades. These databases were queried for patients who had a diagnosis of melanomas with a thickness of 1 mm or more but had no clinical evidence of lymph node metastases.
For this analysis, we identified patients with lymph node metastases detected either by SLN biopsy or at the time of clinical nodal recurrence (CNR). The SLN biopsy technique and pathologic assessment were performed as previously described.3,23 Completion LND was routinely recommended for patients with a positive SLN, and completion LND was performed for the majority (69 % JWCI, 83 % Penn). For the inguinal sites, this typically entailed a superficial groin dissection. For patients with CNR at inguinal sites, therapeutic lymphadenectomy was performed with either superficial or radical groin dissection depending on the extent of disease noted clinically and radiographically and on surgeon discretion. The study received approval from the institutional review boards (IRBs) of both institutions.
The characteristics of the two cohorts were examined including demographic characteristics (sex, age) and primary tumor characteristics (Breslow thickness, Clark level, ulceration, body site). The number of the Penn patients with available information regarding mitotic rate, tumor-infiltrating lymphocytes (TIL), regression, lymphovascular invasion (LVI), microsatellites, and T stage also was sufficient for analysis. Patients with false-negative SLN biopsies were excluded from analyses but were considered as a group separately.
The decision for SLN biopsy was ultimately left to the discretion of the surgeon and likely varied over time as more robust data became available regarding predictors of SLN positivity in thin melanoma. In addition to thickness, factors such as vertical growth phase, mitogenicity, ulceration, younger age, lymphovascular invasion, elevated Clark level (4 or 5), and positive deep margin may have contributed to decision making, particularly earlier in the experience.
Characteristics of the SLN and CNR groups were compared. Survival time was measured from the date of definitive treatment for the primary melanoma in both the SLN and CNR groups.
The JWCI cohort was subjected to uni- and multivariate survival analyses using Kaplan–Meier plots, log-rank testing, and Cox proportional hazard models. Unknown ulceration and Clark level groups were excluded from these analyses. Using variables identified as significant in the multivariate analysis, adjusted survival curves were plotted to examine the independent effect of early nodal treatment. To address issues related to bias in the SLN and CNR groups, pairs of patients with SLN metastases or CNR were matched using the significant prognostic variables, and survival analyses of these matched groups were performed.
The demographic and pathologic characteristics of the SLN-positive and CNR patients in the Penn cohort also were compared. Survival analyses of these patients were performed to determine whether they were congruent with the JWCI outcomes.
RESULTS
Patient Characteristics and Demographics
The demographic and pathologic characteristics of the JWCI and Penn cohorts are provided in Tables 1 and 2, respectively. The JWCI SLN-positive group was older than the CNR group (mean age, 45 years for CNR vs 48.9 years) and had a higher proportion of Clark levels 4 and 5 lesions. In addition, more patients in the CNR group had unknown ulceration status, whereas the rate of ulceration among patients with known ulceration status was similar between the two groups. Breslow thickness was similar between the groups, although when thickness was analyzed as a continuous variable, the SLN-positive group had slightly thicker lesions (0.77 vs. 0.73 mm; p = 0.11). The median time to the development of nodal recurrence in the CNR group was 34.2 months.
TABLE 1.
Clinicopathologic characteristics of John Wayne Cancer Institute (JWCI) patients
| Characteristic | SLN-positive (n = 91) % (n) |
Clinical recurrence (n = 426) % (n) |
p Value |
|---|---|---|---|
| Sex | |||
| Female | 44 (40) | 38 (160) | 0.255 |
| Male | 56 (51) | 62 (266) | |
| Age (years) | |||
| ≤50 | 47 (43) | 67 (285) | <0.001 |
| >50 | 53 (48) | 33 (141) | |
| Primary site | |||
| Axial | 69 (63) | 67 (286) | 0.699 |
| Extremity | 31 (28) | 33 (140) | |
| Breslow (mm) | |||
| ≤0.75 | 44 (40) | 47 (202) | 0.548 |
| >0.75 | 56 (51) | 53 (224) | |
| Clark level | |||
| 1–3 | 55 (50) | 78 (334) | <0.001 |
| 4–5 | 40 (36) | 15 (63) | |
| Unknown | 5 (5) | 7 (29) | |
| Ulceration | |||
| Absent | 84 (76) | 64 (274) | <0.001 |
| Present | 10 (9) | 7 (29) | |
| Unknown | 7 (6) | 29 (123) |
SLN sentinel lymph node
TABLE 2.
Clinicopathologic characteristics of Pennsylvania’s Pigmented Lesion Group (Penn) patients
| Characteristic | SLN-positive (n = 29) % (n) |
Clinical recurrence (n = 36) % (n) |
p value |
|---|---|---|---|
| Sex | |||
| Male | 59 (17) | 58 (21) | 0.82 |
| Female | 41 (12) | 42 (15) | |
| Age (years) | |||
| ≤50 | 62 (18) | 75 (27) | 0.26 |
| >50 | 38 (11) | 25 (9) | |
| Primary site | |||
| Axial | 48 (14) | 72 (26) | 0.07 |
| Extremity | 52 (15) | 28 (10) | |
| Breslow (mm) | |||
| 0.01–0.75 | 28 (8) | 53 (19) | 0.047 |
| ≥0.76 | 72 (21) | 47 (17) | |
| Clark level | |||
| 2–3 | 24 (7) | 75 (27) | <0.001 |
| 4–5 | 72 (21) | 25 (9) | |
| Unknown | 3 (1) | 0 (0) | |
| Ulceration | |||
| Absent | 79 (23) | 83 (30) | 0.009 |
| Present | 0 (0) | 14 (5) | |
| Unknown | 21 (6) | 3 (1) | |
| Mitoses | |||
| Absent | 7 (2) | 25 (9) | 0.029 |
| Present | 83 (24) | 75 (27) | |
| Unknown | 10 (3) | 0 (0) | |
| TIL | |||
| Absent | 24 (7) | 53 (19) | 0.016 |
| Present | 66 (19) | 47 (17) | |
| Unknown | 10 (3) | 0 (0) | |
| Regression | |||
| Absent | 72 (21) | 69 (25) | 0.57 |
| Present | 14 (4) | 22 (8) | |
| Unknown | 14 (4) | 8 (3) | |
| LVI | |||
| Absent | 69 (20) | 94 (34) | 0.013 |
| Present | 7 (2) | 0 (0) | |
| Unknown | 24 (7) | 6 (2) | |
| Microsatellites | |||
| Absent | 86 (25) | 97 (35) | 0.227 |
| Present | 7 (2) | 0 (0) | |
| Unknown | 7 (2) | 3 (1) | |
| Tumor stage | |||
| T1a | 7 (2) | 22 (8) | 0.045 |
| T1b | 83 (24) | 78 (28) | |
| Unknown | 10 (3) | 0 (0) |
SLN sentinel lymph node, TIL tumor-infiltrating lymphocytes, LVI lymphovascular invasion
The Penn cohort had a greater proportion of Clark levels 4 and 5 primary tumors and a greater proportion of mitogenic primary tumors in the SLN group than in the CNR group. In contrast to the JWCI patients, there was a higher proportion of “unknown” ulceration patients and a lower proportion of “present” ulceration patients in the SLN category than in the CNR group. The SLN-positive patients more frequently had tumors with a Breslow thickness of 0.76 mm or more (p = 0.047). The SLN-positive patients also differed from the CNR patients with regard to LVI and TIL. The overall rate of SLN positivity in the Penn cohort was 3.7 %. The median time to the development of CNR was 3.7 years.
Prognostic Factors for Survival
For the JWCI cohort, older age, axial anatomic site, ulceration, and presentation status of nodal metastasis (CNR vs. SLN biopsy) all were associated with decreased MSS (Table 3) in the univariate analysis. These factors all remained significantly associated with MSS (Table 3) in the multivariate analysis. With unknown ulceration status excluded, the same variables remained significant in the multivariate analysis (data not shown).
TABLE 3.
Uni- and multivariable analysis of factors associated with melanoma-specific survival in the John Wayne Cancer Institute (JWCI) cohort
| Characteristic | Univariable analysis | Multivariable analysisa | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| HR | 95 % CI | p value | HR | 95 % CI | p value | |
| Sex | ||||||
| Female | Ref. | – | – | – | – | – |
| Male | 1.06 | (0.83–1.35) | 0.651 | – | – | – |
| Age (years) | ||||||
| ≤50 | Ref. | – | – | – | – | – |
| >50 | 1.35 | (1.06–1.73) | 0.016 | 1.42 | (1.11–1.81) | 0.005 |
| Primary site | ||||||
| Extremity | Ref. | – | – | Ref. | – | – |
| Axial | 1.40 | (1.08–1.81) | 0.012 | 1.42 | (1.09–1.85) | 0.009 |
| Breslow (mm) | ||||||
| ≤0.75 | Ref. | – | – | – | – | – |
| >0.75 | 1.24 | (0.98–1.57) | 0.077 | – | – | – |
| Clark level | ||||||
| 1–3 | Ref. | – | – | – | – | – |
| 4–5 | 1.08 | (0.80–1.47) | 0.62 | – | – | – |
| Unknown | 0.78 | (0.46–1.32) | 0.35 | – | – | – |
| Ulceration | ||||||
| Absent | Ref. | – | – | Ref. | – | – |
| Present | 1.79 | (1.15–2.80) | 0.011 | 1.92 | (1.22–3.01) | 0.005 |
| Unknown | 1.89 | (1.46–2.44) | <0.001 | 1.64 | (1.27–2.12) | <0.001 |
| Nodal evaluation | ||||||
| SLN | Ref. | – | – | Ref. | – | – |
| CNR | 3.42 | (1.91–6.11) | <0.001 | 3.29 | (1.83–5.93) | <0.001 |
HR hazard ratio, CI confidence interval, SLN sentinel lymph node, CNR clinical nodal recurrence
Multivariable analysis was performed using both continuous and categorical variables. The model using categorical variables is shown
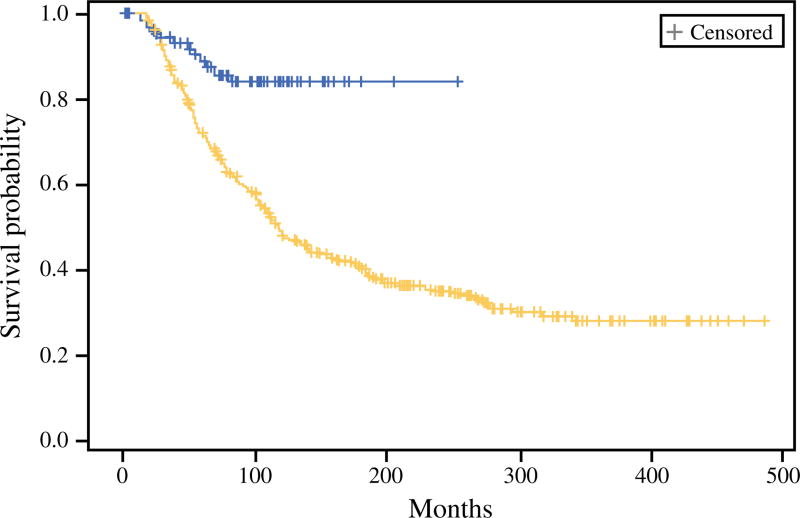
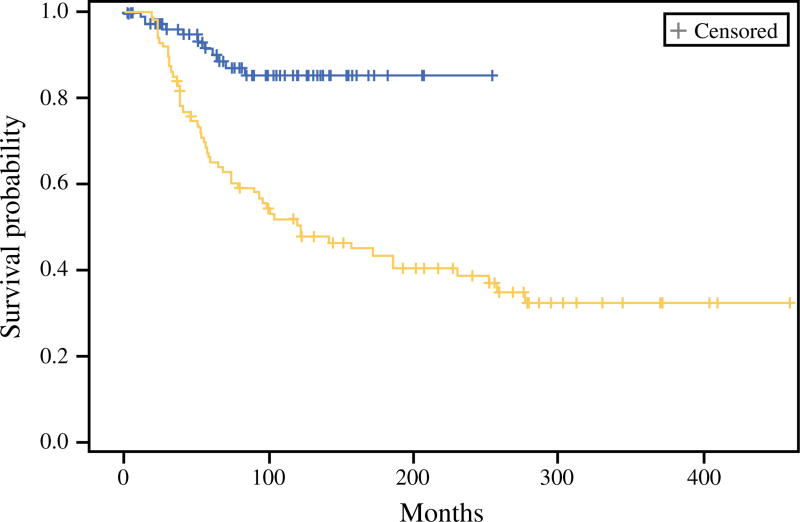
The MSS rates for the SLN-positive patients compared with the CNR patients were respectively 88 and 72 % at 5-years and 84 and 49 % at 10 years (p < 0.0001, log-rank) (Fig. 1). The median follow-up period for the surviving patients was 87.4 months. After matching using covariates identified as significant by multivariate analysis (age, ulceration status, and anatomic site) in 88 patient pairs, nodal disease identified by SLN biopsy was associated with significantly improved survival compared with CNR disease (Fig. 2).
FIG. 1.
Melanoma-specific survival among sentinel lymph node (SLN)-positive patients compared with clinical nodal recurrence (CNR) at John Wayne Cancer Institute (JWCI). The survival of the SLN-positive patients (blue) was 88 % at 5 years and 84 % at 10 years. The survival of the CNR (yellow) patients was 72 % at 5 years and 49 % at 10 years
FIG. 2.
Melanoma-specific survival in a matched cohort of sentinel lymph node (SLN)-positive (blue) and clinical nodal recurrence (CNR) patients (yellow) at John Wayne Cancer Institute (JWCI) after matching for age, anatomic site, and ulceration status
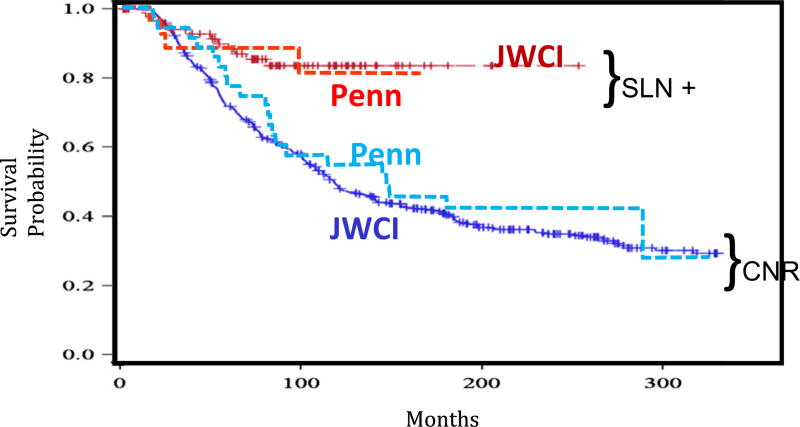
Among the factors also available in the JWCI cohort, two factors in the Penn cohort also were significantly associated with decreased MSS, namely, male sex (hazard ratio [HR] 4.5; 95 % confidence interval [CI] 1.5–13.2) and axial site (HR 3.6; 95 % CI 1.2–10.3). Given the small sample size, a multivariate analysis was not performed for this cohort. The estimated HR suggested better survival in the SLN-positive group than in the CNR group of a magnitude similar to that of the JWCI cohort (HR 2.3; 95 % CI 0.9–6.2; p = 0.10). The median follow-up period was 6.3 years in the SLN group and 11.7 years in the CNR group. The survival curves were similar in the two independent center cohorts (Fig. 3).
FIG. 3.
Melanoma-specific survival among sentinel lymph node (SLN)-positive patients compared with clinical nodal recurrence (CNR) at John Wayne Cancer Institute (JWCI) and Pennsylvania’s Pigmented Lesion Group (Penn). The SLN-positive patients at JWCI (solid red) and Penn (dashed red). The CNR patients at JWCI (solid blue) and Penn (dashed blue)
False-Negative SLN Biopsy Patients
During the follow-up period, 17 patients in the JWCI cohort and 5 patients in the Penn cohort experienced regional nodal recurrence after SLN biopsy in that nodal basin (false-negative SLN biopsy patients). The 5-year MSS rates in the respective centers were 66.7 and 60 %. When false-negative patients were included in the SLN biopsy group of the JWCI patients, MSS differed significantly between the SLN and CNR groups (p < 0.0001).
DISCUSSION
Nodal metastasis occurs in patients with thin melanomas, as does death from melanoma. Although this is well known and well described, the relative infrequency of nodal spread in this group makes the phenomenon difficult to study. For example, a randomized trial examining the therapeutic effect of SLN biopsy in this population would be impractical due to the need for thousands of subjects to achieve acceptable statistical power, and such a trial is very unlikely ever to be conducted.
However, the sheer number of patients who will receive a diagnosis of thin melanoma make examination of this population quite important. If only 3 % of the patients with thin melanoma have nodal metastases, but approximately 70 % of newly diagnosed melanomas are T1, the United States would have more than 1500 patients with thin melanoma nodal metastases each year. It is therefore important to examine this group in detail to identify important prognostic factors and help guide treatment decisions.
Our study examined a large collective cohort of patients with thin melanomas and nodal metastases with the benefit of long-term follow-up assessment. Several significant prognostic factors were identified including patient age, primary tumor site, and ulceration status. The strongest factor, however, was the method for diagnosing the metastasis, with disease diagnosed by clinical recurrence showing a threefold greater risk for melanoma death than disease diagnosed by SLN biopsy. This raises the suggestion that early diagnosis of such metastases would have a beneficial effect on the clinical course of the patients. This is perhaps a noteworthy finding considering the limitations of this study, including variability in the sample sizes between the two institutions as well as in the pathologic variables available for analysis.
This suggestion is intuitive in many ways because patients with nodal metastases from thin melanomas may be the most likely to benefit from early removal of that disease because they are least likely to have concomitant distant dissemination of their melanoma, which would render nodal surgery moot.24 This also is insinuated by the results of the Multicenter Selective Lymphadenectomy Trial (MSLT-1), which demonstrated apparently diminishing survival advantages for patients undergoing SLN biopsy with increasing tumor thickness.25
The rationale for excluding patients with thin melanomas from earlier elective lymph node dissection trials was not lack of a biologic rationale, but rather lack of statistical power and the need to subject excessive numbers of patients to complete nodal dissection in order to potentially benefit a few. Sentinel node biopsy avoids the need for more morbid complete dissections and makes consideration of nodal evaluation in thin melanoma possible.
However, any suggestion of a therapeutic effect from early nodal treatment for these patients certainly cannot be proved by the current analysis. It clearly is possible that our nonrandomized analysis was biased by factors other than nodal management that resulted in the observed survival difference. Indeed, the two groups differed. Many of the differences likely resulted from selection of high-risk patients to undergo SLN biopsy, for example, increased Clark’s level, mitoses, and lymphovascular invasion in the SLN biopsy group. Other differences such as younger age in the SLN biopsy group may have resulted from less aggressive treatment for older patients or perhaps biology. We have attempted to account for such bias.
Other known and measured prognostic variables were included in a multivariable analysis, and the substantial impact on outcome was retained. Pairs of patients matched for known prognostic variables were identified, and the survival of those with early nodal surgery was substantially superior to the survival of patients with similar characteristics but managed with nodal observation. Finally two independent populations of patients were examined at two institutions, and almost identical outcomes were observed. It may be difficult to identify a more useful data set for examination of this question.
An alternative hypothesis is that the melanoma metastases seen in the SLN were not clinically significant and would not progress to recurrent disease if observed. This hypothesis, however, is not supported by comparisons of the rates of nodal involvement for thin melanoma diagnosed by SLN biopsy, which after control for tumor thickness are similar to those seen clinically with nodal observation. In addition, multiple large series reporting outcomes for patients with thin melanomas undergoing SLN biopsy show nodal recurrence rates lower than 1 % after negative SLN biopsy, again suggesting that the nodal disease identified by the procedure was real.
Our analysis should not be interpreted as an endorsement for routine SLN biopsy for all patients with a diagnosis of thin primary melanomas. It is clear that many patients, particularly those with very thin tumors, can have their regional nodes safely observed. Current National Comprehensive Cancer Network (NCCN) Guidelines recommend discussion and offering of SLN biopsy to patients with thin primary melanomas 0.76 to 1 mm in thickness with either ulceration or a mitotic rate of 1 or more per mm2. However, precise criteria for selecting patients with thin melanoma whose risk is high enough to justify SLN biopsy overall still remain controversial, with no features consistently supported in the literature. The current analysis does not settle that question, but we think it may increase the urgency for developing selection algorithms that will help to identify the subgroups of our patients who should or should not have the procedure.
Acknowledgments
This study was supported by Grants P50-CA093372, P30-CA016520, P01-CA29605, and R01 CA189163 from the National Cancer Institute, the Dr. Miriam & Sheldon G. Adelson Medical Research Foundation (Boston, MA, USA), the Borstein Family Foundation, (Los Angeles, CA, USA), and the John Wayne Cancer Institute Auxiliary (Santa Monica, CA, USA). The content of this report is solely the responsibility of the authors and does not necessarily represent the official view of the National Cancer Institute or the National Institutes of Health.
Footnotes
Presented at the Society of Surgical Oncology Annual Cancer Symposium, March 2014.
CONFLICTS OF INTEREST The authors have no conflicts of interest to disclose regarding the contents of this study or its publication.
References
- 1.Cascinelli N, Morabito A, Santinami M, MacKie RM, Belli F. Immediate or delayed dissection of regional nodes in patients with melanoma of the trunk: a randomised trial. WHO Melanoma Programme. Lancet. 1998;351:793–6. doi: 10.1016/s0140-6736(97)08260-3. [DOI] [PubMed] [Google Scholar]
- 2.Balch CM, Soong S, Ross MI, Urist MM, Karakousis CP, Temple WJ, et al. Long-term results of a multi-institutional randomized trial comparing prognostic factors and surgical results for intermediate thickness melanomas (1.0 to 4.0 mm). Intergroup Melanoma Surgical Trial. Ann Surg Oncol. 2000;7:87–97. doi: 10.1007/s10434-000-0087-9. [DOI] [PubMed] [Google Scholar]
- 3.Gimotty PA, Guerry D, Ming ME, Elenitsas R, Xu X, Czerniecki B, et al. Thin primary cutaneous malignant melanoma: a prognostic tree for 10-year metastasis is more accurate than American Joint Committee on Cancer staging. J Clin Oncol. 2004;22:3668–76. doi: 10.1200/JCO.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 4.Balch CM, Gershenwald JE, Soong SJ, Thompson JF, Atkins MB, Byrd DR, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199–206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Howlader NNA, Krapcho M, Neyman N, Aminou R, Altekruse SF, Kosary CL, et al. SEER Cancer Statistics Review, 1975–2009 (Vintage 2009 Populations) National Cancer Institute; Bethesda, MD: 2011. http://seer.cancer.gov/csr/1975_2009_pops09/ [Google Scholar]
- 6.Wong SL, Balch CM, Hurley P, Agarwala SS, Akhurst TJ, Cochran A, et al. Sentinel lymph node biopsy for melanoma: American Society of Clinical Oncology and Society of Surgical Oncology joint clinical practice guideline. J Clin Oncol. 2012;30:2912–8. doi: 10.1200/JCO.2011.40.3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Plitas G, Ariyan CE. Controversies in the management of regional nodes in melanoma. J Natl Compr Cancer Netw. 2012;10:414–21. doi: 10.6004/jnccn.2012.0038. [DOI] [PubMed] [Google Scholar]
- 8.Ra JH, McMasters KM, Spitz FR. Should all melanoma patients undergo sentinel lymph node biopsy? Curr Opin Oncol. 2006;18:185–8. doi: 10.1097/01.cco.0000208793.30065.77. [DOI] [PubMed] [Google Scholar]
- 9.Karakousis GC, Gimotty PA, Botbyl JD, Kesmodel SB, Elder DE, Elenitsas R, et al. Predictors of regional nodal disease in patients with thin melanomas. Ann Surg Oncol. 2006;13:533–41. doi: 10.1245/ASO.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 10.Faries MB, Wanek LA, Elashoff D, Wright BE, Morton DL. Predictors of occult nodal metastasis in patients with thin melanoma. Arch Surg. 2010;145:137–42. doi: 10.1001/archsurg.2009.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Warycha MA, Zakrzewski J, Ni Q, Shapiro RL, Berman RS, Pavlick AC, Polsky D, et al. Meta-analysis of sentinel lymph node positivity in thin melanoma (≤1 mm) Cancer. 2009;115:869–79. doi: 10.1002/cncr.24044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karakousis GC, Gimotty PA, Czerniecki BJ, Elder DE, Elenitsas R, Ming ME, et al. Regional nodal metastatic disease is the strongest predictor of survival in patients with thin vertical growth phase melanomas: a case for SLN staging biopsy in these patients. Ann Surg Oncol. 2007;14:1596–603. doi: 10.1245/s10434-006-9319-y. [DOI] [PubMed] [Google Scholar]
- 13.Wright BE, Scheri RP, Ye X, Faries MB, Turner RR, Essner R, Morton DL. Importance of sentinel lymph node biopsy in patients with thin melanoma. Arch Surg. 2008;143:892–9. doi: 10.1001/archsurg.143.9.892. discussion 9–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ranieri JM, Wagner JD, Wenck S, Johnson CS, Coleman JJ., III The prognostic importance of sentinel lymph node biopsy in thin melanoma. Ann Surg Oncol. 2006;13:927–32. doi: 10.1245/ASO.2006.04.023. [DOI] [PubMed] [Google Scholar]
- 15.Bartlett EK, Gimotty PA, Sinnamon AJ, Wachtel H, Roses RE, Schuchter L, et al. Clark level risk stratifies patients with mitogenic thin melanomas for sentinel lymph node biopsy. Ann Surg Oncol. 2014;21:643–9. doi: 10.1245/s10434-013-3313-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Han D, Zager JS, Shyr Y, Chen H, Berry LD, Iyengar S, et al. Clinicopathologic predictors of sentinel lymph node metastasis in thin melanoma. J Clin Oncol. 2013;31:4387–93. doi: 10.1200/JCO.2013.50.1114. [DOI] [PubMed] [Google Scholar]
- 17.Murali R, Haydu LE, Quinn MJ, Saw RP, Shannon K, Spillane AJ, et al. Sentinel lymph node biopsy in patients with thin primary cutaneous melanoma. Ann Surg. 2012;255:128–33. doi: 10.1097/SLA.0b013e3182306c72. [DOI] [PubMed] [Google Scholar]
- 18.Wong SL, Brady MS, Busam KJ, Coit DG. Results of sentinel lymph node biopsy in patients with thin melanoma. Ann Surg Oncol. 2006;13:302–9. doi: 10.1245/ASO.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 19.Gajdos C, Griffith KA, Wong SL, Johnson TM, Chang AE, Cimmino VM, et al. Is there a benefit to sentinel lymph node biopsy in patients with T4 melanoma? Cancer. 2009;115:5752–60. doi: 10.1002/cncr.24660. [DOI] [PubMed] [Google Scholar]
- 20.Gershenwald JE, Mansfield PF, Lee JE, Ross MI. Role for lymphatic mapping and sentinel lymph node biopsy in patients with thick (≥4 mm) primary melanoma. Ann Surg Oncol. 2000;7:160–5. doi: 10.1007/s10434-000-0160-4. [DOI] [PubMed] [Google Scholar]
- 21.Thompson JF, Shaw HM. The prognosis of patients with thick primary melanomas: is regional lymph node status relevant, and does removing positive regional nodes influence outcome? Ann Surg Oncol. 2002;9:719–22. doi: 10.1007/BF02574492. [DOI] [PubMed] [Google Scholar]
- 22.Carlson GW, Murray DR, Hestley A, Staley CA, Lyles RH, Cohen C. Sentinel lymph node mapping for thick (≥4 mm) melanoma: should we be doing it? Ann Surg Oncol. 2003;10:408–15. doi: 10.1245/aso.2003.03.055. [DOI] [PubMed] [Google Scholar]
- 23.Morton DL, Thompson JF, Essner R, Elashoff R, Stern SL, Nieweg OE, et al. Validation of the accuracy of intraoperative lymphatic mapping and sentinel lymphadenectomy for early-stage melanoma: a multicenter trial. Multicenter Selective Lymphadenectomy Trial Group. Ann Surg. 1999;230:453–63. doi: 10.1097/00000658-199910000-00001. discussion 63–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Faries MB, Steen S, Ye X, Sim M, Morton DL. Late recurrence in melanoma: clinical implications of lost dormancy. J Am Coll Surg. 2013;217:27–34. doi: 10.1016/j.jamcollsurg.2013.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morton DL, Thompson JF, Cochran AJ, Mozzillo N, Nieweg OE, Roses DF, et al. Final trial report of sentinel-node biopsy versus nodal observation in melanoma. N Engl J Med. 2014;370:599–609. doi: 10.1056/NEJMoa1310460. [DOI] [PMC free article] [PubMed] [Google Scholar]