Abstract
Background:
Clopidogrel is by far the most prescribed platelet adenosine diphosphate (ADP) antagonist in Puerto Rico despite the advent of newer agents (prasugrel and ticagrelor). Given the paucity of data on clopidogrel responsiveness in Hispanics, we sought to determine the association between clinical characteristics and platelet reactivity in Puerto Rican patients on clopidogrel therapy.
Study population:
A total of 100 Puerto Rican patients on clopidogrel therapy were enrolled and allocated into two groups: Group I, without high on-treatment platelet reactivity (HTPR); and Group II, with HTPR. Platelet function was measured ex vivo using the VerifyNow® P2Y12 assay.
Results:
The cohort was comprised of Hispanic patients with coronary artery disease (57%), peripheral artery disease (32%), carotid artery stenosis (7%), cerebral artery aneurysm (2%), and stroke (2%). Mean platelet reactivity was 200 ± 61 P2Y12 reaction units (PRUs) (range: 8–324), and 35% of patients had HTPR (PRUs ⩾ 230). Multivariable logistic regression analysis determined that diabetes mellitus (DM) [odds ratio (OR) = 3.27; 95% confidence interval (CI): 1.20–8.96], use of proton-pump inhibitors (PPIs) (OR = 3.60; 95% CI: 1.09–11.82), and calcium channel blockers (CCBs) (OR = 3.10; 95% CI: 1.09–8.83) were independent predictors of HTPR (p < 0.05) after adjusting for other clinical variables.
Conclusions:
In a sample of 100 Puerto Rican Hispanic patients on clopidogrel, 35% had HTPR. Furthermore, DM, PPIs and CCBs predicted HTPR. Clinical outcome data are needed to identify appropriate PRU thresholds for risk prediction in the Puerto Rican population.
Keywords: platelet reactivity, clopidogrel, Hispanics, Puerto Rico
Introduction
Platelet adenosine diphosphate (ADP) receptor antagonists are the standard of care for prevention of recurrent atherothrombotic events. Despite the advent of newer agents (i.e. prasugrel, ticagrelor), clopidogrel remains the most prescribed ADP receptor antagonist used by up to 40 million patients worldwide. 1 However, significant variability in clinical response and platelet inhibition has been observed among individuals, leading to failure in reducing adverse cardiovascular outcomes in some patients. 2 Several clinical and genetic factors have been suggested as possible determinants for clopidogrel response variability.3–6 Yet, current evidence is inconsistent about whether clinical factors are independent predictors of high on-treatment platelet reactivity (HTPR). 7
Data on antiplatelet drug response in Hispanics, who are often under-represented in clinical studies are very limited, 8 underscoring the need for studies designed specifically for this high-risk patient population. Moreover, prior studies have demonstrated that Hispanics have a proinflammatory risk status that may contribute to their increased propensity to develop atherosclerotic disease,9,10 as well as a higher prevalence of cardiovascular risk factors, recurrence rate of thrombotic events and worse cardiovascular outcomes when compared to non-Hispanic Whites.11–13 In addition, clopidogrel is preferred among ADP receptor blockers in Puerto Rico, largely because of its availability as a generic drug and lower cost.
Since more emphasis is necessary on the relevance in a resource-poor setting of determining clopidogrel responsiveness and given that clinical predictors of impaired response to clopidogrel are not currently known in Caribbean Hispanics, we sought to determine potential predictors of high platelet reactivity in a small sample of Puerto Rican patients on clopidogrel therapy.
Methods
Study design and ethics
This was a multicenter cross-sectional study of Puerto Rican patients receiving antiplatelet therapy recruited from January to February 2017. The study was approved by the Institutional Review Board (Protocol No. A4070416) and it was conducted in accordance with the Declaration of Helsinki in compliance with Good Clinical Practice. Verbal and written informed consent was obtained from all participants included in the study.
Patient population and data collection
A total of 100 patients, males and females, of Hispanic Puerto Rican descent on clopidogrel therapy for any diagnosis, were consecutively recruited from all geographic regions of the island. Patient information was gathered from the medical record by a single physician. The study included Puerto Rican Hispanics >21 years old who were receiving 75 mg/day maintenance dose of clopidogrel for at least 7 consecutive days. Patients taking any oral anticoagulant or who were recently treated with glycoprotein IIb/IIIa inhibitors were excluded. Other exclusion criteria were Hematocrit (Hct) ⩽ 25%, platelet count < 100,000/mm3, Blood Urea Nitrogen (BUN)/creatinine > 30/1.5 mg/dl or active hepatic disease. The study cohort was divided into two groups based on P2Y12 reaction units (PRUs) cutoff values previously reported in the GRAVITAS study: 14 Group I (65 patients) without HTPR (PRUs < 230) and Group II (35 patients) with HTPR (PRUs ⩾ 230).
Platelet function testing
An initial 2 ml blood sample was collected from each participant and saved for other laboratory tests as part of the preadmission process. A second tube containing 3.2% sodium citrate was then collected with 2 ml blood for platelet function testing. The blood was collected from a peripheral vein and platelet reactivity was assessed within 4 h of blood sampling. Platelet function was measured ex vivo using the United Stated Food and Drug Administration (US FDA)-approved point-of-care VerifyNow P2Y12 analyzer following manufacturer instructions (Accumetrics, Inc. San Diego, CA, USA).
Statistical analysis
Continuous variables were compared using the two-tailed Student’s t-test, and categorical data were assessed using either Chi-square or Fisher’s exact tests as appropriate. Spearman or Pearson correlation tests were used to determine the association between all measurements. A multiple logistic regression was performed to determine predictors of HTPR and a multiple linear regression was used to describe the contribution of clinical characteristics towards PRU values. Receiver operating characteristic (ROC) analysis was used for the evaluation of predictive models. Statistical analyses were performed using SAS software version 9.4 (SAS Institute, Cary, NC, US.), and p-values <0.05 were considered statistically significant.
Results
Study population
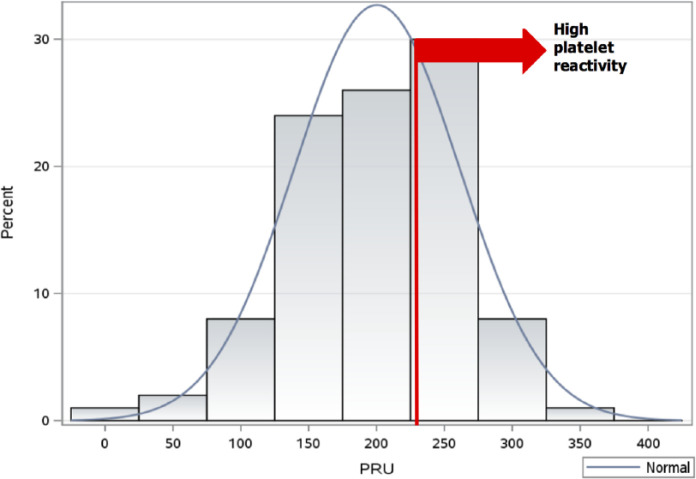
The study cohort (n = 100) consisted of patients with coronary artery disease (CAD; 57%), peripheral artery disease (PAD; 32%), carotid artery stenosis (7%), cerebral artery aneurysm (2%), and stroke (2%) on clopidogrel therapy for secondary prevention of thromboembolic events. All patients were on 75 mg/day maintenance dose of clopidogrel for more than 7 days. Among all enrolled patients, the mean platelet reactivity was 200 ± 61 PRUs (range: 8–325) and 35 had HTPR (PRUs ⩾ 230). Figure 1 illustrate the wide distribution of platelet reactivity in the studied population. Moreover, patient baseline characteristics are depicted in Table 1. The non-HTPR and HTPR groups significantly differed in their history of diabetes mellitus (DM), use of proton-pump inhibitors (PPIs), and calcium channel blockers (CCBs) (p < 0.05). No patient reported being on morphine or amiodarone.
Figure 1.
Distribution of platelet reactivity as measured by P2Y12 reaction units (PRUs).
Table 1.
Baseline clinical characteristics of the study patients according to on-treatment platelet reactivity.
| Characteristics* | Non-HTPR (n = 65) |
HTPR (n = 35) |
All patients (n = 100) |
p-value |
|---|---|---|---|---|
| Age (years) | 67 ± 11 | 71 ± 12 | 69 ± 11 | 0.19 |
| Males | 35 (54) | 15 (43) | 50 | 0.30 |
| BMI (kg/m2) | 27 ± 5 | 30 ± 7 | 28 ± 6 | 0.14 |
| Medical history | ||||
| Hypertension | 56 (86) | 34 (97) | 90 | 0.16 |
| Diabetes mellitus | 31 (48) | 26 (74) | 57 | 0.01 |
| Dyslipidemia | 54 (83) | 30 (86) | 84 | 0.73 |
| Active smoker | 13 (20) | 2 (6) | 15 | 0.07 |
| Main cardiovascular diagnosis | ||||
| Coronary artery disease | 39 (60) | 18 (51) | 57 | 0.46 |
| Peripheral artery disease | 18 (27) | 14 (40) | 32 | |
| Carotid stenosis | 6 (9) | 1 (3) | 7 | |
| Stroke | 1 (2) | 1 (3) | 2 | |
| Cerebral aneurysm | 1 (2) | 1 (3) | 2 | |
| Concomitant therapy | ||||
| Aspirin | 41 (63) | 21 (60) | 62 | 0.76 |
| Proton-pump inhibitors | 8 (12) | 11 (32) | 19 | 0.02 |
| Statins | 48 (74) | 28 (80) | 76 | 0.49 |
| Calcium channel blockers | 10 (15) | 15 (43) | 25 | <0.01 |
Values are mean ± SD or n (%).
BMI, Body mass index; HTPR, high on-treatment platelet reactivity.
Correlation between clinical characteristics and HTPR
Significant univariate correlations were observed between HTPR and DM, as well as use of PPIs and CCBs (p < 0.05). No other clinical variables were associated with HTPR. A total of five clinical characteristics (age, DM, active smoking, PPIs, CCBs) previously reported to affect the pharmacokinetics and pharmacodynamics of clopidogrel among non-Hispanics were included in a multivariable logistic regression analysis. Only history of DM, use of PPIs and CCBs were independently correlated with HTPR [odds ratio (OR) = 3.27, 95% confidence interval (CI): 1.20–8.96; OR = 3.60, 95% CI: 1.09–11.82; OR = 3.10, 95% CI: 1.09–8.83; respectively] after adjusting for all other clinical variables (Table 2). Additionally, 28% of the total variation in PRUs was explained by these five clinical factors (R2 = 0.28, p < 0.01).
Table 2.
Stepwise logistic regression analysis to determine the best predictor of high on-treatment platelet reactivity.
| Clinical variables | OR | 95% CI | p-value | OR adjusted | 95% CI | p-value |
|---|---|---|---|---|---|---|
| Age | 1.02 | 0.99–1.07 | 0.19 | 1.02 | 0.97–1.06 | 0.49 |
| Diabetes mellitus | 3.17 | 1.29–7.80 | 0.01 | 3.27 | 1.20–8.96 | 0.02 |
| Active smoker | 0.24 | 0.05–1.14 | 0.07 | 0.24 | 0.04–1.37 | 0.11 |
| Proton-pump inhibitors | 3.27 | 1.17–9.13 | 0.02 | 3.60 | 1.09–11.82 | 0.03 |
| Calcium channel blockers | 4.13 | 1.60–10.66 | <0.01 | 3.10 | 1.09–8.83 | 0.03 |
CI, confidence interval; OR, odds ratio.
Development of an HTPR predictive model
Several predictive models to estimate the log odds of HTPR were obtained using both simple and multivariate logistic regression analysis. The best fit model was selected based on the area under the curve (AUC) obtained by a receiver operating characteristic (ROC) analysis and it is represented as follows:
As noticed, the proposed model included all the variables independently associated with HTPR (DM, PPIs and CCBs), as they all together showed the higher AUC (0.7319, 95% CI: 0.6359–0.8278) when compared with the nested simple models (Figure 2).
Figure 2.
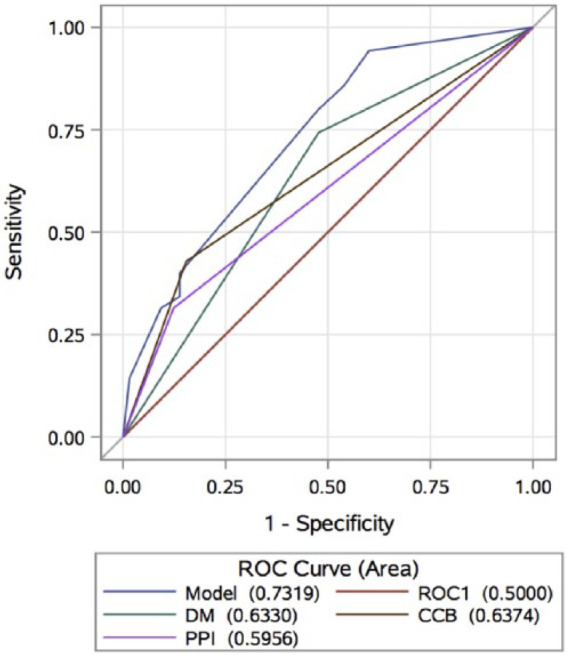
Receiver operating characteristic curves for HTPR predictive model diagnostics.
CCB, calcium channel blocker; DM, diabetes mellitus; HTPR, high on-treatment platelet reactivity; PPI, proton-pump inhibitors; ROC, receiver operating characteristic.
Discussion
The paucity of studies exploring the association between clinical factors and HTPR in non-White populations prompted our study on the clinical variables of on-treatment platelet reactivity, which identified a novel association between selected clinical variables and HTPR in a heterogeneous cohort of Puerto Rican Hispanics on clopidogrel therapy.
Similar to other studied populations, history of DM, the use of PPIs and CCBs were independent predictors of HTPR.6,15,16 However, other clinical characteristics such as current smoking status and use of statins were not significantly associated with HTPR in our study cohort. Clinical variables accounted for ~28% of the observed variation in platelet responsiveness. This predicted value is higher than the addition of both clinical and pharmacogenetic factors, previously reported by Larsen and collaborators. 17 The effect of this interaction may be more prominent in Puerto Ricans as they are disproportionately affected by DM and other cardiovascular risk factors when compared with other ethnic groups. 18
High platelet reactivity is an objective measurement of poor clopidogrel responsiveness that has been associated in several studies with an increased risk for thrombotic events.19–21 However, due to a lack of consensus on a standard PRU threshold value to define HTPR, comparisons of reported HTPR prevalence rates are difficult to make and are at high risk for bias. Recent systematic reviews have estimated the prevalence of HTPR to be between 16–50% among patients treated with clopidogrel.21,22 However, proposed HTPR cutoff values are imprecise, and are likely highly dependent on the unique clinical and pharmacogenetic characteristics of each specific studied population. 23 Using the VerifyNow P2Y12 assay, a PRU cutoff value between 230–240 has been reported as prognostic for subsequent major adverse cardiovascular events (MACEs).14,24
We also developed a fitting model to predict HTPR in Puerto Rican Hispanics on 75 mg/day maintenance dose of clopidogrel for at least 7 consecutive days. This model may be useful to target those patients with a high likelihood of having poor response to clopidogrel and consequently, facilitate an early therapy optimization to prevent further adverse cardiovascular events. However, this model, although novel for this population, may benefit from additional clinical, genetic or epigenetic predictors.
One of the limitations of this study is its relatively small sample size, which may decrease its power. However, our intent was to make a preliminary exploration about possible trends in the association between clinical variables and platelet reactivity using VerifyNow point-of-care testing in Puerto Rico. To our knowledge, this is the first time that such a relationship has been studied in the Caribbean. In addition, the cohort does not currently include patients with acute coronary syndromes, so our results may not be generalizable to this specific indication. However, many of our patients were scheduled to undergo percutaneous coronary intervention (PCI) or other vascular stenting procedures shortly after being enrolled in our study. Although most reported studies are focused on the influence of HTPR on MACEs in PCI patients after a loading dose of clopidogrel, the impact of reaching an adequate platelet reactivity value or switching antiplatelet therapy based on clopidogrel responsiveness several days before this procedure remains unclear. Also, despite all patients self-reporting their adherence to clopidogrel, it is possible that some of the enrolled patients may not have been completely adherent. Finally, this study did not include CYP2C19 or other candidate genes reported to influence clopidogrel responsiveness; however, pharmacogenetic analyses are currently underway, which will form the basis of a subsequent manuscript on this important and under-represented population.
Conclusion
We identified specific clinical characteristics (DM, use of PPIs and CCBs) to be independently associated with HTPR (PRUs ⩾ 230) in a Hispanic Puerto Rican patient population treated with clopidogrel antiplatelet therapy. Further studies are warranted to determine if CYP2C19 or other pharmacogenetic determinants of clopidogrel responsiveness are relevant, as well as the role of platelet reactivity in guiding antiplatelet therapy and predicting future adverse cardiovascular events in the Puerto Rican population. Additionally, clinical outcomes data are needed to identify appropriate PRU thresholds for risk prediction in this population.
Acknowledgments
This publication was partially supported by the National Institute on Minority Health and Health Disparities of the National Institutes of Health (NIH) Award Numbers CCTRECD-R25MD007607, HiREC-S21MD001830 and the Research Minority Institutions (RCMI) award 8G12 MD007600. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. SAS was supported in part by the National Institute of General Medical Sciences of the NIH, through grant K23GM104401. JD is supported in part by the SC1 grant HL123911 from the National Heart, Lung and Blood Institute (NHLBI) and the MBRS SCORE Program of the National Institute of General Medical Sciences (NIGMS). Also, we would like to acknowledge Dr. Estela Estapé, the staff members of the Preadmission/Admission Department and Public Relationships Office at the Cardiovascular Center of Puerto Rico and the Caribbean, as well as the Endovascular Department of the University of Puerto Rico School of Medicine for their support during the development of this study.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Dagmar F. Hernandez-Suarez, ORCID ID: 0000-0003-1850-9078, University of Puerto Rico School of Medicine, Medical Sciences Building, PO Box 365067, San Juan, 00936-5067, Puerto Rico.
Stuart A. Scott, Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai, New York, NY, USA
Matthew I. Tomey, Cardiovascular Medicine Division, Icahn School of Medicine at Mount Sinai, New York, NY, USA
Kyle Melin, Department of Pharmacy Practice, University of Puerto Rico Medical Sciences Campus, San Juan, Puerto Rico, USA.
Angel Lopez-Candales, Cardiovascular Medicine Division, University of Puerto Rico School of Medicine, San Juan, Puerto Rico, USA.
Charlotte E. Buckley, University of Michigan College of Pharmacy, Ann Arbor, MI, USA
Jorge Duconge, Pharmaceutical Sciences Department, University of Puerto Rico Medical Sciences Campus, San Juan, Puerto Rico, USA.
References
- 1. Kitzmiller JP, Groen DK, Phelps MA, et al. Pharmacogenomic testing: relevance in medical practice: why drugs work in some patients but not in others. Cleve Clin J Med 2011; 78: 243–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Krishna V, Diamond GA, Kaul S. Do platelet function testing and genotyping improve outcome in patients treated with antithrombotic agents? The role of platelet reactivity and genotype testing in the prevention of atherothrombotic cardiovascular events remains unproven. Circulation 2012; 125: 1288. [DOI] [PubMed] [Google Scholar]
- 3. Simon T, Verstuyft C, Mary-Krause M, et al. Genetic determinants of response to clopidogrel and cardiovascular events. N Engl J Med 2009; 360: 363–375. [DOI] [PubMed] [Google Scholar]
- 4. Scott SA, Collet JP, Baber U, et al. Exome sequencing of extreme clopidogrel response phenotypes identifies B4GALT2 as a determinant of on-treatment platelet reactivity. Clinical pharmacology and therapeutics. Clin Pharmacol Ther 2016; 100: 287–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gagne JJ, Bykov K, Choudhry NK, et al. Effect of smoking on comparative efficacy of antiplatelet agents: systematic review, meta-analysis, and indirect comparison. BMJ 2013; 347: f5307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Angiolillo DJ, Jakubowski JA, Ferreiro JL, et al. Impaired responsiveness to the platelet P2Y12 receptor antagonist clopidogrel in patients with type 2 diabetes and coronary artery disease. J Am Coll Cardiol 2014; 64: 1005–1014. [DOI] [PubMed] [Google Scholar]
- 7. Karathanos A, Geisler T. Monitoring aspirin and clopidogrel response: testing controversies and recommendations. Mol Diagn Ther 2013; 17: 123–137. [DOI] [PubMed] [Google Scholar]
- 8. Zhang T, Tsang W, Wijeysundera HC, Ko DT. Reporting and representation of ethnic minorities in cardiovascular trials: a systematic review. Am Heart J 2013; 166: 52–57. [DOI] [PubMed] [Google Scholar]
- 9. Lutsey PL, Cushman M, Steffen LM, et al. Plasma hemostatic factors and endothelial markers in four racial/ethnic groups: the MESA study. J Thromb Haemost 2006; 4: 2629–2635. [DOI] [PubMed] [Google Scholar]
- 10. Albert MA. Inflammatory biomarkers, race/ethnicity and cardiovascular disease. Nutr Rev 2007; 65: S234–S238. [DOI] [PubMed] [Google Scholar]
- 11. Derby CA, Wildman RP, McGinn AP, et al. Cardiovascular risk factor variation within a Hispanic cohort: SWAN, the Study of Women’s Health Across the Nation. Ethn Dis 2010; 20: 396–402. [PMC free article] [PubMed] [Google Scholar]
- 12. Simpson JR, Zahuranec DB, Lisabeth LD, et al. Mexican Americans with atrial fibrillation have more recurrent strokes than do non-Hispanic whites. Stroke 2010; 41: 2132–2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tang Y, Sampson B, Pack S, et al. Ethnic differences in out-of-hospital fatal pulmonary embolism. Circulation 2011; 123: 2219–2225. [DOI] [PubMed] [Google Scholar]
- 14. Price MJ, Berger PB, Teirstein PS, et al. Standard- vs high-dose clopidogrel based on platelet function testing after percutaneous coronary intervention: the GRAVITAS randomized trial. JAMA 2011; 305:1097–1105. [DOI] [PubMed] [Google Scholar]
- 15. Sherwood MW, Melloni C, Jones WS, et al. Individual proton-pump inhibitors and outcomes in patients with coronary artery disease on dual antiplatelet therapy: a systematic review. J Am Heart Assoc 2015; 4: e002245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gremmel T, Steiner S, Seidinger D, et al. Calcium-channel blockers decrease clopidogrel-mediated platelet inhibition. Heart 2010; 96: 186–189. [DOI] [PubMed] [Google Scholar]
- 17. Larsen PD, Johnston LR, Holley A, et al. Prevalence and significance of CYP2C19*2 and CYP2C19*17 alleles in a New Zealand acute coronary syndrome population. Intern Med J 2015; 45: 537–545. [DOI] [PubMed] [Google Scholar]
- 18. Pérez CM, Soto-Salgado M, Suárez E, et al. High prevalence of diabetes and prediabetes and their coexistence with cardiovascular risk factors in a Hispanic community. J Immigr Minor Health 2015; 17: 1002–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mega JL, Close SL, Wiviott SD, et al. Cytochrome P-450 polymorphisms and response to clopidogrel. N Engl J Med 2009; 360: 354–362. [DOI] [PubMed] [Google Scholar]
- 20. Bonello L, Tantry US, Marcucci R, et al. Consensus and future directions on the definition of high on-treatment platelet reactivity to adenosine diphosphate. J Am Coll Cardiol 2010; 56: 919–933. [DOI] [PubMed] [Google Scholar]
- 21. Fiolaki A, Katsanos AH, Kyritsis AP, et al. High on-treatment platelet reactivity to aspirin and clopidogrel in ischemic stroke: a systematic review and meta-analysis. J Neurol Sci 2017; 376: 112–116. [DOI] [PubMed] [Google Scholar]
- 22. Mallouk N, Labruyère C, Reny JL, et al. Prevalence of poor biological response to clopidogrel: a systematic review. Thromb Haemost 2012; 107: 494. [DOI] [PubMed] [Google Scholar]
- 23. Pendyala LK, Torguson R, Loh JP, et al. Racial disparity with on-treatment platelet reactivity in patients undergoing percutaneous coronary intervention. Am Heart J 2013; 166:266–272. [DOI] [PubMed] [Google Scholar]
- 24. Patti G, Nusca A, Mangiacapra F, et al. Point-of-care measurement of clopidogrel responsiveness predicts clinical outcome in patients undergoing percutaneous coronary intervention results of the ARMYDA-PRO (Antiplatelet therapy for Reduction of MYocardial Damage during Angioplasty-Platelet Reactivity Predicts Outcome) study. J Am Coll Cardiol 2008; 52: 1128–1133. [DOI] [PubMed] [Google Scholar]