Abstract
The release of membrane-bound vesicles from cells has been increasingly recognized as a mechanism for intercellular communication. Extracellular vesicles (EVs) are also produced by virus-infected cells and are thought to be involved in intercellular communication between infected and uninfected cells. Viruses, in particular oncogenic viruses and viruses that establish chronic infections, have been shown to modulate the production and content of EVs. Viral microRNAs, protein and even entire virions can be incorporated into EVs, which can impact immune recognition of viruses or modulate neighboring cells. In this Review, we will discuss the roles that EVs have during virus infection to either promote or restrict viral infection in target cells. We will also discuss our current understanding of the molecular mechanisms that underlie these effects, the potential consequences for the infected host, and possible future diagnostic applications.
Table of Contents blurb
The release of membrane-bound vesicles from cells has been increasingly recognized as a mechanism for intercellular communication. In this Review, Raab-Traub and Dittmer discuss the roles that extracellular vesicles have during virus infection.
Introduction
Extracellular vesicles (EVs) are secreted from healthy, malignant and virus-infected cells. EVs are either released directly from the plasma membrane or during fusion between multivesicular bodies (MVB) and the plasma membrane1,2. EVs released from the MVB are termed exosomes. Similar to EVs, viruses can be released through multiple pathways including the plasma membrane and/or via the MVB route (reviewed in 3). For example, some retroviruses such as human immunodeficiency virus type 1 (HIV-1) assemble at the inner leaflet of the plasma membrane whereas other retroviruses, such as Mason-Pfizer monkey virus (MPMV) assemble in the cytoplasm first before trafficking to the cell surface (reviewed in 4). Some viruses are non-enveloped and do not require an envelope for infectivity, but nevertheless can be incorporated into EVs. For example, hepatitis A virus (HAV) was recently shown to be secreted within EVs that can potentially transmit to uninfected cells within an infected individual5,6. Other enteroviruses may package up to ~20 particles within a single membrane vesicle and bud without destroying the cell 7. Autophagy, which is usually thought of as a regulated mechanism to provide nutrients through digestion of intracellular organelles, is utilized in different ways in the egress of multiple viruses. Not only do some enteroviruses exit infected cells in packages wrapped into autophagic membranes, autophagic membranes form part of the envelope for herpesviruses, para and orthomyxoviruses 8. Lipidated LC3, an essential mark of autophagosomes, has been detected in extracellular microvesicles containing coxsackie virus. Additionally, the exosome marker flotillin-1 was also found in these vesicles suggesting that picornaviruses utilize autophagy related EV release as one pathway for their exocytosis 9
EVs are thought to have an important role in virus infection and a number of interactions between viral components and cellular components that are required for the biogenesis of EVs have been reported. Therefore, a critical comparison of virus particles with EVs may lead to a greater understanding of both viral life cycles and the function of EVs.
Due to their small size and similar biochemical composition, viruses and EVs can have similar biophysical properties. The term exosome is used if EVs are ≤100 nm in diameter and originate from the MVB, microvesicle if the diameter is 100–1,000 nm or apoptotic body if the diameter is >1,000 nm. Similarly, viruses range in diameter from 30 nm for poliovirus, 120–140 nm for herpesviruses and 200–300 nm for poxviruses (reviewed in 10). This similarity in biophysical properties increases the difficulty in obtaining pure populations of EVs that are not contaminated with viruses and vice versa, which makes it difficult to determine the precise composition of EVs and virions (Box 1). The identification and characterization of virion-associated proteins has been the subject of intense study over many years11, whereas the identification of proteins that are associated with EVs has been more recent. Recent studies have identified some key components of EVs that can be used as markers to identify and assess the purity EVs (Table 1); however, it is important to recognize that not all EVs carry all of these markers12. In the context of virus infection, viral RNAs and proteins have been found in EVs13–16, which could be the result of selective incorporation of specific RNAs and these proteins or alternatively, reflect the total intracellular constituents. Further work is required to determine the precise composition of EVs. The development of mass spectrometers with enhanced specificities and sensitivities compared to existing instruments will undoubtedly advance our understanding of the composition of EVs. Further work is also required to optimize the purification of EVs and therefore careful consideration is required when attributing functional phenotypes to EVs in the context of virus infection.
Box 1. Differentiating EVs from virions.
The purity of any preparation of EVs is determined by measuring light-scatter and Brownian motion and by electron microscopy (Figure 2). More recently, flow cytometry-based methods have been introduced, but the small size of EVs makes it difficult to detect EVs using conventional instruments. EVs can be isolated by size-exclusion chromatography, differential ultracentrifugation, density flotation, crowding agents, flow-cytometry or affinity purification (Table 2). Each method has specific advantages158. Size-exclusion chromatography is the best method for preserving the structure of EVs159–161, though it does not separate virions from EVs11,151,162. The use of crowding agents, such as PEG3000 followed by precipitation is the fasted way to isolate and concentrate EVs for subsequent applications, however, this approach also enriches soluble proteins and contaminants that are not part of EVs. When profiling miRNAs in EVs it is important to consider that are Ago-associated miRNAs are present in serum163. These Ago-miRNA complexes also co-purify with EVs when only crowding agents are used, however, this problem can be circumvented by using affinity-based purification methods. Affinity purification using magnetic beads enables the high-throughput purification of EVs on robot platforms and it is able separate EVs from viruses 13. For example, EVs from B cell lymphomas are enriched for B cell surface antigens, including CD81 and CD63, which can be used to affinity purify EVs using antibodies that bind these proteins 164. However, this approach will exclude populations of EVs which do not have the particular surface marker used for affinity purification, but which may nevertheless contribute biological functions of EVs12.
Table 2.
| Method | Mechanism | Input volume | Virion co-purification |
|---|---|---|---|
| Differential ultracentrifugation | Density and size | 35 ml | Yes |
| ExoQuick (SBI Biotech Inc.) | Precipitation | 250 μl | Yes |
| Total EV (Invitrogen Inc.) | Precipitation | 250 μl | Yes |
| PEG-2000 | Precipitation | 250 μl – 250 ml | Yes |
| CD63 magnetic beads | Bead-based surface marker | 1 – 35 ml | No |
| Composite magnetic beads | Bead-based surface markers (n=5 markers) | 100 μl – 1ml | No |
| Size exclusion chromatography | Size-bases isolation | 100 μl – 35 ml | Yes |
| Density flotation (for example, Iodixanol) | Density | 35 ml | Yes |
Table 1.
Prominent EV markers and EV associated viral proteins.
| Location | Protein* [Au:* OK? above refers to protein?] | Structural Class | Function |
|---|---|---|---|
| Surface exposed on EVs | CD9 | Tetraspanin | Cell adhesion |
| CD63 | Tetraspanin | Cell signaling | |
| CD81 | Tetraspanin | Cell signaling, proliferation marker | |
| MHC-I | Histocompatibility antigen, class I | Antigen presentation | |
| MHC-II | Histocompatibility antigen, class II | Antigen presentation | |
| CD86 | Type I membrane protein, IgG superfamily | CTLA-4 Counter-Receptor B7.2 | |
| FLOT1 | Integral membrane component of caveolae | Scaffolding protein for vesicle formation | |
| ANXA5 | calcium-dependent phospholipid binding protein (Annexin) | Phospholipid binding | |
| Internal to EVs | HSP70 | heat shock protein | Mediates folding |
| HSP90 | heat shock protein | Mediates folding | |
| ALIX | PDCD6-Interacting Protein | ESCRT pathway | |
| TSG101 | - | ESCRT pathway, tumor suppressor | |
| Virus | EBV LMP1 | Membrane protein | Cell signaling/CD40 analog |
| EBV LMP2a | Membrane protein | Cell signaling | |
| EBV gp350 | Membrane/virion protein | Receptor binding | |
| HIV Nef | Membrane protein | CD4, MHC-I downregulation | |
| HSV-1 gB | Membrane/virion protein | Receptor binding | |
| Vaccina virus glycoproteins | Membrane/virion protein | Receptor binding | |
| HCV (whole virus) | virus (flavivirus), enveloped | infection | |
| HAV (whole virus) | Virus (picornavirus), non-enveloped | infection | |
| Poliovirus, Coxsackievirus,, Rhinovirus | Virus (picornavirus), non-enveloped | infection |
Based on information in 10,166 and www.exocarta.org. Note that not all markers are present in all EVs as demonstrated by comprehensive MS/MS analyses12
Research into understanding the role of EVs in viral infections is driven to a large extent by commercial interests in biomarker development. For example, miR-122 that is incorporated into EVs during acute liver injury could be used as a biomarker to determine the extent of damage to the liver 17–19. The miR-122 is the most abundant miRNA in liver cells and perhaps reflecting this abundance it is incorporated into EVs. The correlation between the levels of miR-122 and alanine aminotransferase, an enzyme that is also released when the liver is damaged, has been established in the clinic. Therefore, miR-122 could be used as an alternative, more specific biomarker to alanine aminotransferase in the clinic. Notably, miR-122 is also required for HCV replication and therefore it is an attractive drug target for the development of new therapies against HCV 20–22. The success of such therapies could be determined by minimally invasive profiling of EVs and plasma miR-122 levels since miR-122 would add information about liver cell status beyond viral load. The use of highly multiplexed assays that are able to detect multiple miRNAs and viruses, next-generation sequencing and mass spectrometry will help drive the research of EVs for diagnostic applications
Studying EVs in the context of virus infection has been crucial in demonstrating the potential contribution of EVs to viral pathogenesis 23, as EVs from virus-infected cells often transfer viral components to uninfected cells, for example, Epstein-Barr virus (EBV) LMP1 protein and viral miRNAs16,24. This intercellular transfer of viral cargo occurs in the absence of cell-to-cell fusion, cellular synapses or membrane nanotubes25–27 and represents the existence of a host transfer mechanism that occurs in the absence of virus spread. Roles for intercellular transport by EVs have been described, for example, in mediating cross presentation for T cells28–30 and in mediating synaptic transmissions31. Many of the vesicles that are used during these processes share biogenesis features and fusion mechanisms that are similar to EV and viruses. In the context of cross-priming, however, the vesicles tend to stay in the immediate microenvironment, such as the lymphnodes, and are not found circulating systemically in body fluids.
In this Review we will focus on the identified molecular and biological properties of EVs released from virally infected cells and consider how the virally modified EVs may either facilitate viral infection or promote resistance to immune recognition by antibodies or inhibition of innate immunity activation within recipient cells. In particular we focus on human viral infections with HIV, HAV, and HCV, and the two herpesviruses EBV and Kaposi Sarcoma-associated herpesviruses (KSHV). It is in the context of these chronic, persistent, and latent infections that EVs have been most thoroughly explored.
Biological roles for EVs in viral infections
Oncogenic viruses and viruses that are able to establish long-term persistent infections have been shown to alter the content of EVs, which has been hypothesized to facilitate infection and contribute to persistence and pathogenesis. Persistent or chronic infections are characterized by low levels of viral replication and circulating virus particles (~101–104 particles/ml) 3. By contrast, during latent infections viruses cannot be detected in circulation. Latency has a defining role in herpesvirus and lentivirus infections. In the case of latent herpesvirus infections, viral miRNAs can be detected within EVs at times when conventional viral load assays are negative13,16,24. In the case of HIV-1 the viral protein Nef has been found in EVs that circulate in infected individuals32–34. Systemic circulation of viral proteins in EVs enables these viruses to modulate host cells without exposing viral proteins or virions to the immune system. By contrast, rapidly replicating viruses, such as Ebola virus, influenza A virus or Zika virus accumulate to high titers (106–1011 particles/ml) in blood within days of primary infection. For these viruses, the viral titer is similar to the number of circulating EVs (1010–1012 particles/ml) 35. Despite these high viral titers, it is possible that EVs that carry viral proteins or nucleic acids could modulate host cells, for example by determining their permissiveness to infection.
The biological function of EVs in the context of viral infections can affect viral infection in two opposing ways (reviewed in 23,36–38). On the one hand, EVs can either modulate recipient cells by promoting viral replication or, on the other hand, EVs can restrict viral replication through triggering host immune responses.
Properties of EVs
Biogenesis
EVs are small membrane-bound carriers of intracellular cargo that are derived from MVBs or from the plasma membrane (Figure 1). Unlike virus particles, the membranes of EVs do not enclose a structured core, such as a capsid. Specific properties of EVs define and distinguish EVs from other types of microvesicles10. The assembly of EVs is an active, energy dependent and regulated process39,40. This process has been shown to specifically require sphingomyelinase41 and components of the endosomal sorting complex required for transport (ESCRT) machinery. The content of EVs is determined by the protein and RNA composition of the cells from which they are derived. The composition of EVs frequently reflects the relative abundance of contents within the EV-producing cell. Thus EVs that are derived from virus-infected cells contain highly expressed viral miRNAs13,16,24. In addition, several recent studies have provided evidence for differential loading, where the relative abundance of the EV miRNAs is distinct from that within the producing cell16,24,42–45.
Figure 1.
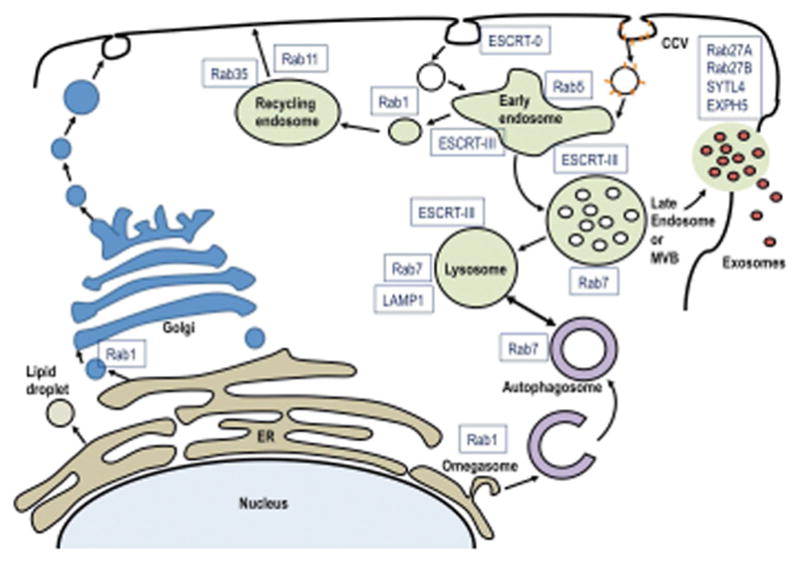
Protein complexes in the EV maturation pathway that are modulated during viral infection. Shown are the principal egress pathways of a cell starting with the nucleus. The late endosome or MVB sorts contents from the early endosome into either the lysosome or EVs for egress. The early endosome is the first step in vesicle uptake and recycling. The individual molecules identified here have demonstrated functions in EVs or virus maturation.
The budding of EVs into MVBs requires the ESCRT proteins tumor susceptibility gene 101 protein (TSG101) and Alix (also known as programmed cell death 6-interacting protein), which are also known markers of EVs2. TSG101 and Alix are also required for ESCRT-dependent budding of enveloped viruses from the plasma membrane, such as HSV-146, therefore it is difficult to determine whether these are specific EVs as thus they do not conclusively establish EV originating from MVBs or the plasma membrane using these proteins as markers. Other membrane proteins that localize to lipid rafts in the plasma membrane, including tetraspanins such as CD63 and CD81, are also enriched in EVs. This process involves palmitoylation 47 which has been shown to be required for the incorporation of the EBV protein LMP1. LMP1 is enriched in lipid rafts and in EVs48. Depending on the experimental approach and cell line, other proteins such as Sortillin (SORT1), Synthenin-1 (SDCBP), or Syndecan-1 (SDC1) have also been found to have a role in the biogenesis of EVs49–51.
The Rab family of small GTPases regulates multiple steps in the trafficking of vesicles to distinct endocytic compartments and they also function in docking of the MVB to the plasma membrane (Figure 1). For example, Ras-related protein Rab-11A and Rab35 function during the recycling and sorting endosomes, whereas Rab27A and Rab27B are essential for the secretion of EVs52. It is likely that some viruses modulate this complex process to facilitate viral entry, trafficking, and egress. Rab6 and Rab7 also affect the flux between lysosomes and autophagosomes and could contribute to determining the content of EVs53–55. Multiple viruses affect the expression of ESCRT and Rab GTPases to promote viral entry and egress and are therefore likely to modulate the content of EVs56.
Additionally, it is known that members of the Rab family of GTPases also interact with members of the Ral family of GTPases and function during intracellular trafficking. It has been shown in Caenorhabditis elegans that Ral1 (the homologue of human RALA and RALB) regulates both MVB biogenesis and the secretion of EVs57. Activated Ral1 associates with syntaxin 5 (SYX5), a soluble N-ethylmalemide-sensitive fusion protein attachment protein receptor (SNARE) complex protein, at the plasma membrane and is required for the secretion of EVs. Interestingly, SYX5, is also required for the release of human cytomegalovirus (HCMV) and syntaxin-4, another SNARE complex protein has been found to regulate HCV release58,59. Additional SNARE complex proteins have also been shown to interact with herpesvirus glycoproteins to promote release60. These examples demonstrate that viruses co-opt the cellular vesicular transport system during egress and therefore, they are also likely to modulate the content and secretion of EVs through similar interactions.
EV Uptake
Viruses use cell surface receptors to initiate fusion with the plasma membrane and are known to use specific receptors to target specific cell types. This receptor-specificity determines their cellular tropism and is a distinguishing feature of EVs, which have the ability to enter a greater variety of cell types than viruses. Using fluorescent dyes that are incorporated into EVs, it was demonstrated that membranes of EVs can fuse with cell membranes16,24,61,62. Most cell types that have been tested can fuse with EVs and therefore EVs can be used to deliver cargo to a wide variety of cell types. This process is analogous to cationic lipid-mediated transfection approaches. In some cases, EVs use specific receptors for entry12,63 and therefore the fusion of EVs with cellular membranes can be specific. For example, EVs from EBV-infected cells that express the viral glycoprotein gp350, or EVs that are engineered to express gp350, specifically target B cells that express the viral entry receptor CD21 and can block EBV infection of naïve B cells61,64.
Heparin is involved in the initiation of host cell entry for many viruses, including retroviruses and herpesviruses65. It is a glycosaminoglycan that binds almost all viral envelopes. Thus, exogenous heparin or heparin beads can inhibit virion attachment through competitive binding. Cell surface-bound heparin is thought to concentrate virions prior to specific receptor and co-receptor engagement. It is also thought to have a role in the entry of EVs66, though the fusion of EVs with the plasma membrane can occur at heparin concentrations that block herpes simplex virus 1 (HSV-1) entry. This provides an experimental tool to separate virion effects from EV phenotypes, as virus entry, even of non-infectious or defective HSV-1 particles, can be blocked by heparin. It suggests that EVs use different mechanisms for entry to cells compared to most viruses.
Similarly, annexin A5 (and potentially other annexin family members) mediates the fusion of EVs to the plasma membrane through binding to phosphatidyl-serine67. Importantly, Annexin A5 does not antagonize virus entry and thus can also be used to distinguish EV-mediated phenotypes from virus particle or soluble molecule-mediated phenotypes. Although the biogenesis of virions and EVs are similar, EVs and virions likely use different mechanisms to enter cells.
Integrins and integrin-binding matrix proteins, many of which contain a signature motif of arginine, glycine, and aspartic acid (RGD), have an important role in virus entry and their potential role in the entry of EVs is also beginning to be understood 68,69. They are thought to act as attachment factors or co-receptors that synergize with the primary receptor for virus entry. Both EBV and KSHV utilize integrins as a co-receptor for viral entry70. RGD peptides and anti-integrin antibodies can interfere with the attachment of virions and EVs to cells68,70 and affect signaling through the integrin homodimer and heterodimer and thus have pleotropic effects on cell physiology. For example, the anti-integrin antibody Etaracizumab is in clinical trials as anti-angiogenesis agent in non-virus associated cancers, because blocking integrin signaling can induce cell death.
Viruses have a strict requirement for cell-type specific receptors and co-receptors. These engage specific envelope glycoproteins and force large molecular rearrangements to expose the components of the viral fusion complex. Hence, hyper immune serum that was raised against virion components has clinical utility in blocking infection, for example, in treating patients that have been exposed to Ebola virus71. By contrast, entry of EVs is more promiscuous than viruses and clathrin-dependent, caveolae-dependent, macropinocytosis 56,62, phagocytosis, and lipid raft-mediated uptake72 have all been shown to contribute to the entry of EVs (reviewed in 63).
EV-meditated functions
Much of what we know about the physiological function of systemically circulating EVs derives from studies that have analyzed their contribution to cancer metastasis 73–76. It was found that EVs released from tumor cells can modulate cells in the surrounding microenvironment and drive distant metastasis by modulating stromal cell growth, cell migration, induce growth factor secretion, and vascular permeability. A supportive microenvironment, for instance by providing growth factors and extracellular matrix attachment opportunities and increased endothelial cell permeability, are also essential for systemic virus spread. In the following sections we will review how EVs contribute to viral infection
HCV
HCV is a member of the Flaviviridae. It is distinct from the arbovirus members of this virus family as it is transmitted by blood-to-blood contact or through sexual intercourse, rather than by an insect vector. HCV virions are enveloped and are smaller than EVs (~50 nm in diameter in contrast to ~ 100 nm of EVs)77. EVs isolated from HCV-infected human hepatoma cell lines have been shown to contain HCV78,79. Some of the subgenomic HCV RNA co-localizes with CD81 and CD63 (markers of EVs). The HCV E2 protein was also found to co-localize with CD81 in EVs and EVs were found to transmit viral RNA to uninfected cells79–81. CD81 is the dominant HCV co-receptor82–84, therefore it is likely that HCV virions are incorporated into EVs by their interaction with CD81. HCV RNA that is transmitted by EVs induces an innate interferon (IFN)-alpha response in neighboring dendritic cells (DCs). This is in contrast to natural HCV infection, which also delivers viral RNA to cells, but down-regulates TLR and RLR signaling through the action of the viral NS3/4 protease. Therefore by specifically transmitting viral RNA, but not viral proteins, which antagonize innate immunity, EVs may provide a protective function for uninfected cells in the immediate microenvironment during infection. Systemically circulating EVs that contain HCV virions may have a pro-viral role, as HCV has been shown to spread in the presence of neutralizing antibodies78,85. This spread may be facilitated by the masking of viral proteins in the EVs (Figure 2, panel A). Such a strategy of host evasion in which persistent viruses, such as HCV and more prominently HAV (see below), to escape the evolutionary selection pressure of neutralizing antibodies by being incorporated in EVs may be widespread. The carriage of HCV virions in EVs also suggests that HCV could enter a range of different cell types, in addition to hepatocytes, through EV-mediated fusion in which infection would not be dependent on the expression of a specific viral receptor.
Figure 2.
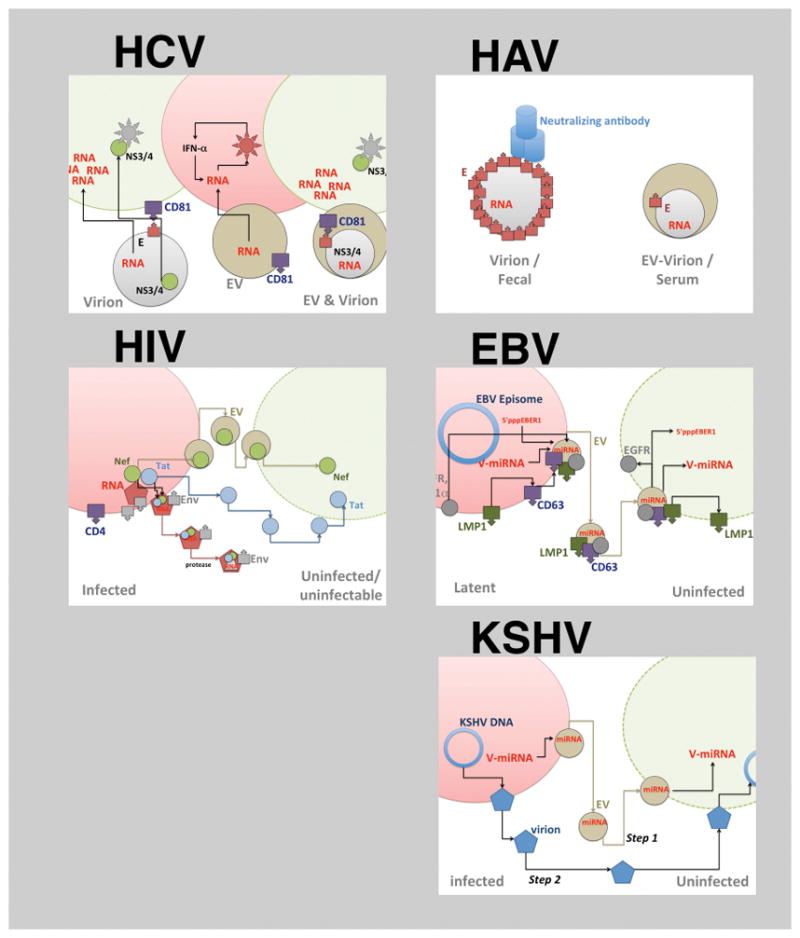
Modes of virus-EV interaction. The different panels representing different viruses (HCV, HAV, HIV, EBV and KSHV). For each virus a virion-dependent transfer and the EVs dependent transfer step is shown. Only in the case of HCV and HAV have entire virions been identified within EVs. For the other viruses, only individual RNAs or proteins, but never the whole genome is seen in EVs. In case of EBV many different moieties are transmitted via EVs from infected cells: cellular proteins, viral proteins cellular miRNAs, viral miRNAs as well as other small viral RNAs and RNA breakdown products. In case of KSHV (E) the cartoon exemplifies a temporal model, whereby viral miRNAs transmitted by EVs precede infection by a viral particle (Step 1) and thus may prepare the recipient cells for infection (Step 2)
HAV
HAV, a non-enveloped picornavirus, is the cause of acute enterically transmitted hepatitis and replicates efficiently in the liver. HAV rapidly replicates in susceptible cells and viral particles are released from these cells, however, no cytopathic effect has been observed. RNA-containing proteinaceous particles (density of 1.22 – 1.28 g/ml) represent the major species of HAV in feces 6. HAV can be neutralized by antibodies that are elicited by the current HAV vaccines. It has been shown that HAV can be released from cells in host-derived membranes at a density of 1.06–1.10 g/ml5. These EVs that contain HAV are infectious and circulate in the blood of infected humans, whereas non-enveloped virus has only been found in feces (Figure 2, panel B)6. Coxsackie B virus and Enterovirus 71 (EV71), two other picornaviruses, have also been found in EVs7,86,87.
Two hypotheses have been made to explain importance of EV-encapsulated picornaviruses as a biological mechanism rather than a side product of cellular inefficiencies6. Firstly, the envelopment of HAV by host membranes may expand the tropism of the viruses as now EV surface proteins rather than the viral surface proteins engage the target cells. This may provide an additional route for HAV to spread within the liver and systemically to distant organs, such as the spleen, and lymph nodes, which function to filter and survey systemic fluids 3. Second, the acquisition of a host membrane by HAV demonstrates that some non-enveloped viruses can acquire an envelope that is devoid of viral transmembrane proteins and thus provides evidence for a second, alternative egress pathway. This suggests that the egress that is mediated by EVs would be distinct from the normal egress route. The acquisition of a host-derived membrane could also affect antibody recognition of HAV capsid proteins and may allow persistence and spread in the presence of neutralizing antibodies.
HIV
HIV is a human retrovirus that contains an RNA genome and acquires its envelope from the cellular plasma membrane, where it buds from areas that are enriched in the viral Gag protein. After a month-long period of acute replication (105–107 viral particles/ml), HIV establishes a latent infection, which slowly progresses to acquired immune deficiency syndrome (AIDS) over a period of years in untreated individuals as the virus depletes CD4+ T cells. It is believed that during viral latency (≤ 5 * 101 viral particles/ml in plasma), HIV directly and indirectly modulates the immune system, leading to chronic pathology. Many of these indirect effects on the immune system are observed even in patients on anti-retroviral therapy. Various hypotheses for this phenomenon have been proposed, including a role for EVs. In addition, soluble viral proteins, such as HIV Tat as well as secondary events to immune compromise, such as extended LPS translocation may contribute to this phenotype 88,89 (reviewed in 36). HIV assembly and the biogenesis of EVs have many similarities90–92. These similarities impair the biochemical and physical separation of EVs away from virions, which include mature, immature, and even defective particles. The molecular pathogenesis of HIV suggests potential EV-mediated effects on neighboring cells (Figure 2, panel C), because HIV kills more abortively infected or uninfected T cells than infected cells 93–96. HIV infected cells may also release EVs with trapped HIV virions94–97.
More importantly, HIV encodes a number of accessory proteins, such as Vif98, which interfere with the cellular antiviral response. These pro-viral proteins may also be carried by EVs to prime neighboring cells to promote infection, in what is called the ‘Trojan Horse’ hypothesis 23,36. Conversely, cellular anti-viral factors that are carried via EVs could facilitate an antiviral response in neighboring cells99. Additionally, EVs packaged with viral proteins may function to introduce viral proteins and would activate B cells and T cells through endosomal presentation of proteins, a process termed cross priming, rather than endogenous synthesis and presentation within MHC molecules. Thus these immune cells would become specifically activated without actual viral infection 100,101.
It is known that HIV infection changes the repertoire of miRNAs within infected cells102–104 and considering that miRNAs can be packaged into EVs13, the EVs from HIV-infected cells may contain distinct miRNAs compared to EVs from uninfected cells. In the context of AIDS-defining malignancies, such as Kaposi’s sarcoma, EVs isolated from HIV-infected patients have distinct miRNAs profiles prior to and concurrent with lesion development13,105. Initially, HIV was thought to encode miRNAs106, but these observations have since been questioned107,108. It is becoming clear that miRNAs with Drosha-dependent hairpin-looped precursors are not the only small RNAs that can be transcribed from RNA virus (including retroviruses) genomes109–111 and some of these RNA species have been found in EVs112. The potential roles of miRNAs (as defined by their biogenesis and structural composition) or other small RNAs that do not fit the classical definition of miRNAs to EV function is a new area of investigation that is likely to reveal new properties that affect viral infection and pathogenesis.107,108,113.
EVs from HIV-infected cells contain the viral protein Nef 32–34. In addition, soluble HIV Tat protein circulates in interstitial spaces, blood, and mucosal barriers and can perform biological activities, such as promoting angiogenesis and endothelial cell reprogramming89,114–116. Soluble Tat has a fusogenic peptide sequence that enables its efficient uptake into cells117,118. Since the HIV Tat protein would not need to be incorporated into EVs to enter cells, the biological relevance of the incorporation of Tat into EVs remains unclear. HIV Nef associates with membranes and with members of the vesicular trafficking system, such as Alix and others 119,120. Nef can be incorporated into EVs and may modulate the contents of EVs including miRNAs32–34,121; however, others have contested these findings as the level of Nef in EVs is at the limit of detection122. In sum, HIV provides an example of how latent viruses may use EVs to maintain a susceptible host environment over long periods of time during which no virions are detectable.
EBV
EBV, a gamma herpesvirus, was the first human tumor virus to be identified and a major human pathogen3. EVs containing viral proteins were first shown to be produced from B cells that were infected with EBV. The major EBV oncoprotein, latent membrane protein 1 (LMP1), was identified in EVs secreted from EBV infected cell lines123. LMP1 is required for B lymphocyte transformation (reviewed in 124). The incorporation of this protein into EVs has not only been demonstrated in B cells and epithelial cells that were cultured in vitro but has also been detected in exosomes in the serum of patients with EBV-associated tumors and in the serum from mice carrying nasopharyngeal carcinomas (NPCs)16,125. The interaction of LMP1 with the tetraspannin CD63 may contribute to the selective incorporation of LMP1 into EVs48,126 as does selective palmitoylation of LMP147. Additionally, LMP1 is known to localize to lipid rafts within membranes and lipid rafts are present within the membranes of EVs 127. It is possible that the presence of LMP1 in lipid rafts may contribute to enrichment of LMP1 within MVBs and subsequently enriched within exosomes.
EVs secreted from B cells containing LMP1 inhibit T cell proliferation and NK cell cytotoxicity123,128. EVs that are secreted from EBV-infected NPC cells also contain galectin-9, which is thought to contribute to these immunosuppressive effects128–130. It has long been known that EBV-infected NPC tumors are infiltrated with T cells that are apparently nonfunctional as they do not kill tumor cells or impair tumor growth. This lack of activity may reflect the abundant secretion of EVs during EBV infection, thus representing another viral immune evasion strategy.
Importantly, LMP1-containing EVs have been shown to deliver activated signaling proteins into uninfected cells131. This potentially important feature of EVs was revealed in studies that showed that the epidermal growth factor receptor (EGFR), which is highly induced by LMP1, was also abundant in LMP1-containing EVs16. LMP1 has also been shown to increase PI3CA levels within lipid rafts and within EVs. The delivery of LMP1 and EGFR and PI3CA through EVs induced growth stimulating signaling pathways in recipient cells, including the activation of the PI3kinase target, Akt1, and ERK1131. An early study revealed increased incorporation of fibroblast growth factor 2 (FGF2) from LMP1 expressing cells into EVs which could potentially affect the tumor environment through the direct growth stimulation of infected cells or supporting stromal cells132. Additionally, it has been demonstrated that LMP1 activates HIF1-alpha, which is also transferred by EVs into recipient cells and can activate HIF1-alpha targets133. HIF1-alpha is the major transcriptional regulator in hypoxic conditions that are characteristic of many tumors and could promote survival of tumor cells in an anoxic environment. An important target of HIF1-alpha is the vascular endothelial growth factor, which induces angiogenesis. Thus, through the transfer of EVs, EBV can affect the growth of neighboring cells. It is also known that within tumors that are caused by EBV infection, not all cells express LMP1. Therefore the secretion and uptake of LMP1 into cells that do not express LMP1 could affect the growth of additional tumor cells. This may be particularly important in the pathogenesis of NPC where not all cells express detectable levels of LMP1.
LMP1 may also modulate the selective sorting of proteins into the exosomal pathway, suggesting that EBV manipulates these pathways for intercellular communication. The possibility of LMP1-mediated specific effects on the content of EVs was revealed from quantitative proteomics and 2-dimensional gel analysis of EVs that were purified from B-cell lines that were uninfected, infected with EBV, KSHV, or with both viruses131. Analysis of LMP1-positive versus LMP1-negative cell lines revealed 217 protein spots with significantly different expression (P < 0.05). Principal component analysis to identify the distinguishing features among the EVs from these different cells lines revealed that LMP1 was a major determinant for the variance between samples. This strongly suggested that LMP1 had an effect on the exosomal protein content and provided additional evidence for specific viral effects on this process.
Spectral counting analysis also indicated that both KSHV and EBV had distinct effects on the content of EVs and that these effects reflected cellular changes that occur in infected cells131. Gene ontology pathway analyses of proteins that were identified in EVs that were derived from infected cells predicted that EVs from EBV and KSHV infected cells likely modulate cell death and survival, ribosome function, and protein synthesis. Analyses of the content of EVs from infected cells also indicated that KSHV EVs could affect cellular metabolism and that EVs from EBV infected cells could activate cellular signaling mediated by integrins, actin, interferon, and NF-kB through the transfer of critical regulatory proteins in these pathways.
An additional, novel finding was that EBV-encoded miRNAs were also detected in EVs that were secreted from EBV infected cells and that these viral miRNAs could then be transferred to uninfected recipient cells16,24. Importantly, the viral miRNAs were shown to specifically decrease previously identified viral miRNA targets, thus providing evidence of functional delivery of miRNAs through EVs24. This transfer also likely occurs in vivo as uninfected B cells that were isolated from patients with NPC contain viral miRNAs. Interestingly, EVs from NPCs have distinct patterns of EBV miRNA abundance compared to the intracellular levels in the producing cells16. This observation supports the hypothesis that there is selective sorting of specific miRNAs into EVs.
In addition to viral proteins and miRNAs, 5′pppEBER1, a small non-coding viral RNA, has also been found in EVs that are secreted from EBV infected cells134. EBER1 is the most abundant viral RNA in infected cells and 5′pppEBER1 enhances the immune function of dendritic cells. This unusual finding may indicate that EVs contribute to autoimmune diseases, such as lupus, that have been linked to EBV infection3. Overall, these findings suggest that EBV modulates EVs to specifically secrete viral and cellular proteins and miRNAs that likely contribute to intercellular communication and affect the function of uninfected cells. Modulating the content of EVs could be important for affecting the tumor environment by inducing cell growth, promoting angiogenesis, and inhibiting immune cell function, and also for potentiating metastasis.
KSHV
KSHV causes Kaposi’s sarcoma and a variety of hyperplastic and neoplastic B cell disorders, such as primary effusion lymphoma (PEL). PEL usually present with liquid effusions without tumor masses in serous body cavities. Cell-free, primary PEL fluid is highly enriched in tumor-derived EVs13. Primary KSHV infection is asymptomatic in the healthy host and results in lifelong latency. In rare cases, immune reconstitution inflammatory syndrome follows, which is associated with severe disease flares and clinical symptoms consistent with infection and inflammation. Common to all Kaposi’s sarcoma pathology is neo-angiogenesis and the infiltration of the environment around the tumor cells with uninfected, non-transformed host cells, such as endothelial cells and macrophages. After hemangioma, Kaposi sarcoma is the most angiogenic cancer and therefore the study of KSHV can be useful in understanding the interaction between EVs and endothelial cells. KSHV angiogensis is driven by paracrine effectors such as soluble cytokines and the growth factors vascular endothelial growth factor 1 (VEGF-1) and platelet derived growth factors PDGF (1–4) 135. Initially, the paracrine drivers of Kaposi’s sarcoma were thought to be only soluble cytokines (for example, vascular endothelial growth factor and platelet derived growth factor or IL6). More recently EVs have been shown to mediate some of these phenotypes such endothelial cell remodeling, migration and proliferation independent of IL-6 13. PEL and Kaposi’s sarcoma tumor cells release large quantities of EVs, which drive endothelial cell proliferation and invasion in the presence of neutralizing antibodies to IL-6 13.
KSHV encodes multiple viral miRNAs136,137 and these viral miRNAs constitute up to 50% of all miRNAs in infected cells138. Viral miRNAs and mRNAs have been detected in virion preparations of almost all herpesviruses14,15,139. Viral miRNAs are also readily detected in Kaposi’s sarcoma-tumor derived EVs140. The levels of viral miRNAs in EVs are 10–100-fold higher than what has been reported for virion-associated miRNAs. Owing to the similar biophysical characteristics of virions and EVs, it is unclear if reports of virion-associated RNAs reflect contamination by EVs.
Several lines of evidence have attributed phenotypes that had been attributed to miRNAs incorporated into virons to miRNAs incorporated into EVs. These include the biochemical separation of EVs by positive selection on anti-CD63 beads (a marker of EVs) (Figure 4), as well as genetic approaches, such as the detection of KSHV miRNA-containing EVs in KSHV-microRNA transgenic mice, which cannot produce virus 13. This suggests that during KSHV latency viral miRNAs can be incorporated into EVs via the same host cellular pathways that load host miRNAs into EVs. Similar to KSHV virions that contain a large proportion of the total pool of intracellular miRNAs, a large proportion of KSHV miRNAs is also incorporated into systemically circulating EVs13. The identity and abundance of miRNAs in EVs thus represents a snapshot of their cellular origin. A more active model hypothesizes that viral miRNAs and proteins are specifically and differentially loaded into EVs16,24,42–45.
Figure 4.
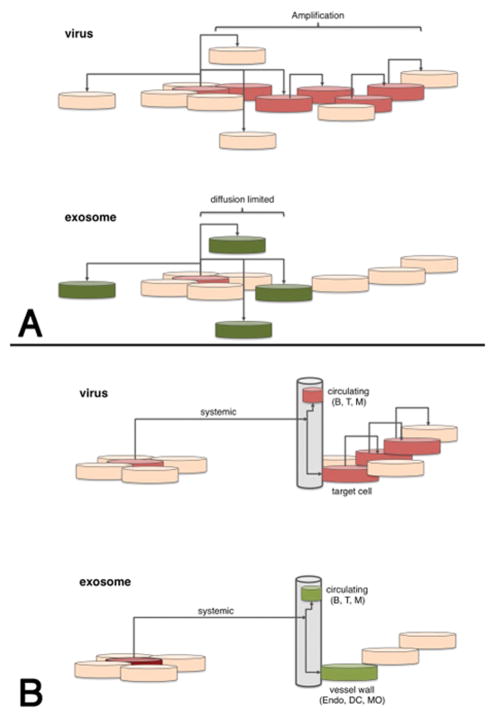
Conceptual difference between (A) viruses and (B) EVs. Cells are indicated as disks. Spread is indicated by black connecting arrows. Red indicates amplification of input material (virus), green indicates delivery (EV) without subsequent amplification. The upper two panels refer to local spread, i.e. within a tissue or microenvironment and the lower panels refer to systemic spread via blood, lymph or interstitial fluids. Upon virus infection the signal (i.e. virus derived miRNAs and proteins) is amplified in each permissive cell and over time the number of altered cells increases. Upon EVs absorption no amplification takes place and any phenotype caused by EVs is diffusion-limited. Only cells directly exposed to EVs in the circulation, which are mostly endothelial cells, can be modulated by EVs.
KSHV infection changes many aspects of the physiology of the infected cell, including lipid metabolism and presumably vesicle biogenesis. The protein composition of EVs and their secretion is modulated during viral reactivation from PEL cell lines16, though KSHV proteins have not been detected in EVs131. The replication and maturation of KSHV is much slower compared to other viral infections3. In culture models of KSHV, the number of infectious virions is approximately 105 copies/ml, compared to 107 copies/ml for EBV or 109 copies/ml for flaviviruses141. In Kaposi’s sarcoma patients, the viral titer ranges from 103–105 copies/ml, whereas EBV or HCMV titers exceed 106 copies/ml in patients and viral titers during hepatitis virus infections range from 105–109copies/ml. We speculate that for KSHV, preventing the accumulation highly immunogenic virions, and instead using host EVs to distribute viral miRNAs to neighboring cells represents a novel strategy to persist in the host (Figure 2, panel E). In this model, RNAs in EVs offer an evolutionary advantage for virus spread by priming neighboring cells for infection. KSHV infects and replicates primarily in endothelial cells. These need to be attracted and re-programmed to migrate towards the initially infected cell. PEL derived EVs that are secreted by PELs confer this property onto uninfected endothelial cells in culture through the transfer of viral miRNAs or even and protein coding RNAs 142,143. In sum, KSHV represents another example, by which the virus modulates the host local environment by releasing EVs through EV reprogramming. Interfering with EV the release of EVs during KSHV infection may therefore have the potential to limit virus spread or virus pathology.
Alphaherpesviruses and betaherpesviruses
Alphaherpesviruses, such as herpes simplex virus type-1 (HSV-1), and betaherpesviruses, such as HCMV, also influence the biogenesis pathway of EVs and use these pathways for egress144–146. Similar to the biogenesis of EVs, HCMV virion maturation is dependent on the ESCRT machinery and they contain cellular markers that are associated with EVs147,148. Some of the observed systemic and biological phenotypes that are associated with HSV-1 and HCMV infection, may be result of massive re-programming of MHC-I and MHC-II trafficking from the secretory pathway. HCMV replicates in a variety of cell types including endothelial cells that line the blood and lymphatic vasculature and can cause graft rejection in organ transplantation. EVs that are released from HCMV-infected endothelial cells can exacerbate allogeneic graft rejection 149.
More recently, it was shown that the immune sensor stimulator of interferon genes protein (STING) is incorporated into EVs that are secreted from HSV-1 infected cells and that the STING ligand cyclic GMP-AMP synthase (cGAMP) is present in EVs that are released from cells that are infected with murine cytomegalovirus150,151. The cGAMP nucleotide triggers the recognition of foreign molecules via STING and also augments RIG-I and TLR signaling, leading to a strong interferon response. It is possible that introducing cGAMP into cells via EVs may trigger an antiviral response in neighboring cells152. This represents perhaps the most direct demonstration that cellular EVs can play an anti-viral and pro-host role.
HSV-1 viral miRNAs miR-H28 and miR-H29 are also incorporated into EVs. These have been shown to have a pro-viral role in infection and facilitate infection in neighboring cells153, perhaps by weakening innate immune defenses. Further studies are needed to fully understand this observation, but it seems counterintuitive that the same infected cell would secrete pro-viral and antiviral EVs at the same time. More likely, these distinct effects identified in cultured cell lines represent different steps in natural infection perhaps by altering initial infection of different cell types, enhancing or limiting systemic spread throughout the host, or by altering viral virulence to facilitate persistence or establishment of latency. In sum, herpesviruses, by virtue of their large genomes (encoding > 100 proteins, as well as miRNAs and lncRNAs 154) modulate EV biogenesis and EV function through multiple independent mechanisms.
Summary and outlook
In this Review, we have discussed the multiple ways in which different viruses manipulate EVs for their benefit in order to increase their persistence, pathogenesis, and transmission. In recent years EVs have emerged as specific carriers of cellular and viral components including miRNAs, proteins, and viral genomes. This can happen during active viral replication or during viral latency. The majority of experiments that have been performed explore how EVs can deliver cytoplasmic content from one cell to the cells in the surrounding environment in the absence of cell-cell fusion. However, the role of EVs can also be more far-reaching than the local environment in which they are released into (Figure 4); the presence of EVs in blood and the lymphatic system suggests that EVs are able to transmit cargo over long distances.
The role of EVs in viral pathogenesis is similar to the role of EVs in cancer metastasis, where they are known to prime distant sites (soil) for reception of metastatic cells (seed) (See Box). EVs and their effects on recipient cells are mediated by individual EVs and the recipient cells do not produce and amplify these EVs. This is in contrast to virally infected cell which can produce thousands of viruses from a single infected cell (Figure 1) However, during chronic or latent viral infection a single infected cell may actually release EVs orders of magnitude greater than infectious viral particles, therefore the utilization of EVs to prime and enhance systemic viral infection is likely.
Experimental data that supports a role for EVs in priming the innate immune response has been reported. Cargo in EVs can elicit a TLR-dependent immune response in mice that have tumors155 or prime neighboring cells, including DCs, to respond to viral infection by priming the adaptive response or by releasing interferon 134,156. During this scenario, EVs may have evolved a role in the protection from pathogens, perhaps through the activation of innate immune responses in neighboring cells. A role for EVs in adaptive immunity has already been established, in which EVs (or some specialized membrane encapsulated vesicles) can efficiently cross-prime innate immunity and adaptive immune memory responses 27,28,30,101,157.
Importantly, it is likely that the basic research in EV biogenesis and fusion will be enhanced by previous, similar studies that characterized virion egress and entry. Many steps in the biogenesis of EVs are also used by viruses for entry and egress, for example, the ESCRT family of proteins. Further study of viral effects on the pathways that involve EVs is likely to identify the critical regulators of endosomal and exosomal trafficking in host cell physiology as well as new mechanisms that modulate the infection of a complex organism through enhancing and inhibitory effects on the infecting virus and resultant viability of the infected host cell. The further study of EV biology provides a rich area to enhance our understanding of the complexities of viral redirection of cellular processes and the determination of how viral effects on EV production and content contribute to both viral pathogenesis and persistence.
Figure 3.
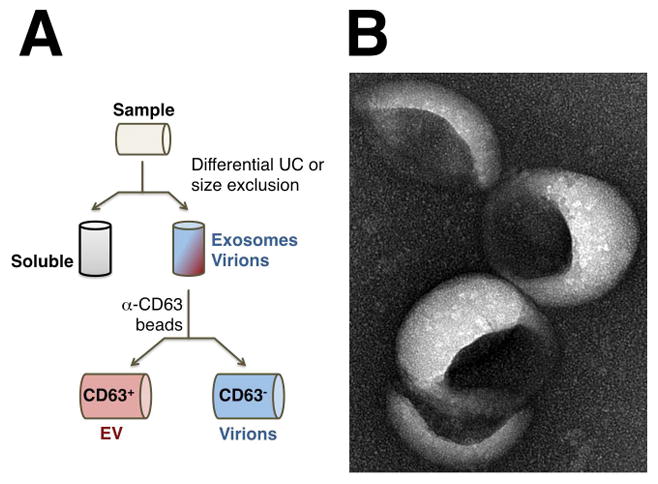
A. Separation of EVs from virions by positive selection. First, particles are concentrated on the basis of biophysical properties such as density, size and/or weight. Most virions co-purify with EVs under these conditions. Next, EVs are positively selected using anti-EV surface antibodies and virions, which do not contain these markers, are in the column flow-through. The reverse experiment (not shown) is possible as well, namely were virions are selected positively and EVs are present in the flow-through. P, pellet after filtration and differential ultracentrifugation (UC); S, supernatant, E, pellet of EVs and virions. B. Electron micrograph (EM) of EV. Shown is a surface EM picture of tumor cell line derived EVs after isolation and silver staining (courtesy of P.Chugh, S.Ozgur and J.Griffith).
Box 2. EVs in cancer.
Approximately 20%-30% of all cancers are associated with viral infections and many features of cancer are also features of viral infection, such as dissemination (metastasis in cancer), uncontrolled DNA replication and metabolic perturbation (for example, glycolysis and nucleotide biogenesis). Hence, insights that are gained from studying the physiological phenotypes of EVs in the context of cancer and metastasis could also be relevant to understanding the role of EVs in viral persistence165. EVs have been extensively studied in cancer research as they are released in high levels from tumor cells73–76. The contents of EVs that are released from tumor cells mirror the contents within the producing cells and therefore, these EVs are useful biomarkers. Therefore, the identification and characterization of EVs within body fluids from ‘liquid biopsies’ provides a noninvasive diagnostic and prognostic indicator for cancer development and progression13,131. The identification of proteins such as p53, epidermal growth factor (EGF), and fibroblastic growth factor (FGF) in EVs suggests a contributing role for EVs in oncogenesis. EVs are able to transfer proteins from malignant cells to neighboring or to distant cells, which can promote cancer growth through potential effects on the microenvironment, inhibition of the anti-cancer immune responses, and induction of angiogenesis. Interestingly, EVs have also been shown to facilitate metastasis by inducing epithelial to mesenchymal transition (EMT) and other changes within the target microenvironment73–75.
Key points.
The considerable biochemical and physical similarity between viruses and exosomes complicates exosome purification. Both exosomes and viruses utilize the host vesicle trafficking machinery and therefore contain multiple proteins enriched in extracellular vesicles and viruses.
Viruses have specific receptors and therefore usually have a more restricted repertoire of cells that permit viral entry. In contrast, exosome uptake is almost universal and can occur through several cellular endocytosis mechanisms in addition to direct fusion, a property that enables systemic delivery of exosome content.
The differences in viral vs. exosome receptor usage can be used to separate and purify exosomes. This enables the identification of specific exosome-mediated effects and provides a mechanism to test novel compounds to inhibit exosome delivery and function.
Viruses that establish chronic, persistent infections within the infected host likely utilize exosomes to enhance establishment and maintenance of infection. The exosomes produced from virus-infected cells may also restrict virus infection perhaps to enable continued host viability and persistent viral infection.
Exosomes are also abundantly produced by malignant cells and have been shown to facilitate tumorigenesis and metastasis through effects on the tumor environment. Similarly, tumor viruses such as KSHV and EBV manipulate exosome content to enhance viral transformation and maintenance of latency.
The non-enveloped small RNA viruses including picornaviruses and Hepatitis A virus may acquire a membrane envelope through exosome secretion. This would inhibit recognition of viral proteins by the immune system and facilitate spread within the host.
Acknowledgments
Work in the authors’ laboratories is supported by public health service grantsDA040394, CA019014, and the University Cancer Research Fund (UCRF). We would like to thank our colleagues R. McNamara, P. Chugh, and B. Damania, for insightful discussions.
Glossary
- Capsid
Proteins that encapsulate viral genomes. Capsids are rigid, highly structured and are similar to crystals with a defined symmetry. The size, shape and symmetry of the capsid can be determined by electron microscopy and is sometimes used to classify viruses into taxa.
- Endosomal sorting complex required for transport (ESCRT) machinery
A multi-protein complex that is involved in membrane vesicle biogenesis. Viruses use the ESCRT machinery to assemble virions and bud
- Nanotubes
Membranous protrusions that connect adjacent cells over extended distances (up to 100 μM) and can transfer cellular components and viruses.
- Cross-priming
Transfer of antigens from one cell to another, often to a professional antigen presenting cell, that does not make the antigen; the phrase was originally coined to explain counter-intuitive aspects of T cell responses.
- Latent infection
Long-term presence of viral genomes (DNA or RNA) in a cell without evidence of virion production, for example, HIV integrated into the host genome or herpesvirus plasmids located in the nucleus. To differentiate latent from abortive or non-productive infection events, reactivation and subsequent release of virion particles is required for latent infection. Viral genomic material (RNA) that is transmitted by EVs can only lead to abortive infection.
- Paracrine
Affecting the physiology of neighboring cells without cell-to-cell contact, typically by cytokines or growth factors. If the growth factors act on the same cell, that they originate from the process is called autocrine loop.
- Persistent infection or chronic infection
Long-term presence of viral genomic information (DNA or RNA) in a cell or person with evidence of virus production and circulating infectious particles, but at a much lower level than observed during initial (primary) infection.
- Autophagy
Autophagy is a regulated mechanism to provide nutrients through digestion of intracellular organelles, is utilized in different ways in the egress of multiple viruses.
- Biomarker
Protein, mRNA or other small molecule that can be measured and that is associated with disease outcome either independent of treatment (prognostic) or in relation to treatment (predictive).
- Multivesicular bodies (MVB)
Are structures below the plasma membrane that serve as the central hub for sorting of molecules into other specialized compartments, as well as in and out of the cell.
- Sphingomyelinase (EC 3.1.4.12)
Enzyme involved in the synthesized of sphingomyelin.
- endosomal sorting complex required for transport (ESCRT)
A structure below the plasma membrane that serves to remodel the plasma membrane for the purpose for exporting macromolecules and membrane proteins.
- RGD motif
The tripeptide Arg-Gly-Asp (RGD) is recognized by many integrins either as part of a short peptide (blocking peptide) or as repeat region in extracellular matrix proteins.
- Hyperimmune serum
Serum obtained from one or many infected, convalescent animals that contains high levels of blocking antibodies to the target virus.
- Cytopathic effect
Measure of infectious virus on monolayer cells.
- Trojan Horse hypothesis
Initially introduced by Gould23 this hypothesis posits (a) an evolutionary relationship between retrovirus particle biogenesis and EV biogenesis and (b) the utility of this relationship as an alternative means to transfer viruses, or virus proteins and RNAs to neighboring cells independently of virion maturation and thereby unseen by the host immune response.
- Interstitial spaces
Small, narrow spaces between tissues, which are typically filled with interstitial liquid.
- Angiogenesis
Process of vessel growth into an organ carrying either blood or lymph, involving the migration, growth, and differentiation of endothelial cells
- Principal component analysis
Statistical method to uncover relationships defined by 10–1000 or more correlated variables, which identifies those factors that contribute to variability. Typically the first 3–5 principal components are composed of those variables which have the greatest explanatory power.
- Hierarchical cluster analysis
Statistical method to visualize relationships among variables and samples. Samples that on the basis of multiple variables are most similar are arranged together.
- Spectral counting
Method that determines the relative presence or absence of a peptide in a pair of samples analyzed by mass spectroscopy methods
- Gene ontology
Fixed vocabulary of cellular processes and molecules that defines concepts/classes used to describe gene function, and relationships between these concepts.
- Primary effusion lymphoma
Diffuse large B cell lymphoma that is caused by Kaposi’s sarcoma associated herpesvirus.
- Crowding agents
Chemicals such as polyethylene glycol or acetone that change the availability of free solvent for proteins and other macromolecular structures with the result of causing aggregation.
Biographies
Dr. Raab-Traub received her Ph.D. from the University of Chicago and post-doctoral training at the University of North Carolina. She has studied contribution of the Epstein Barr Virus to the development of malignancy with particular focus on nasopharyngeal carcinoma. She identified the viral genes expressed in cancer and the properties of the viral oncogenes and miRNAs.
Dr. Dittmer obtained his PhD from Princeton University, followed by postdoctoral studies in virology at Stanford University and the University of California San Francisco. His research is focused on AIDS-defining cancers, particularly Kaposi Sarcoma and the role of EVs in viral pathogenesis. He directs the UNC Lineberger Vironomics core.
Footnotes
Conflict of interest
D.P.D. has received early access and beta testing reagents related to EVs and miRNA diagnostics from JSR Micro, Life Sciences Inc., and Aclea Inc. This did not influence the opinions expressed in this review.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200:373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kowal J, Tkach M, Thery C. Biogenesis and secretion of exosomes. Curr Opin Cell Biol. 2014;29:116–125. doi: 10.1016/j.ceb.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 3.Knipe DM, Howley PM. Fields virology. 6. Wolters Kluwer/Lippincott Williams & Wilkins Health; 2013. [Google Scholar]
- 4.Freed EO. HIV-1 assembly, release and maturation. Nat Rev Microbiol. 2015;13:484–496. doi: 10.1038/nrmicro3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feng Z, et al. A pathogenic picornavirus acquires an envelope by hijacking cellular membranes. Nature. 2013;496:367–371. doi: 10.1038/nature12029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hirai-Yuki A, Hensley L, Whitmire JK, Lemon SM. Biliary Secretion of Quasi-Enveloped Human Hepatitis A Virus. MBio. 2016;7 doi: 10.1128/mBio.01998-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen YH, et al. Phosphatidylserine vesicles enable efficient en bloc transmission of enteroviruses. Cell. 2015;160:619–630. doi: 10.1016/j.cell.2015.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Munz C. The Autophagic Machinery in Viral Exocytosis. Front Microbiol. 2017;8:269. doi: 10.3389/fmicb.2017.00269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robinson SM, et al. Coxsackievirus B exits the host cell in shed microvesicles displaying autophagosomal markers. PLoS pathogens. 2014;10:e1004045. doi: 10.1371/journal.ppat.1004045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meckes DG, Jr, Raab-Traub N. Microvesicles and viral infection. J Virol. 2011;85:12844–12854. doi: 10.1128/JVI.05853-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spear PG, Roizman B. Proteins specified by herpes simplex virus. V. Purification and structural proteins of the herpesvirion. J Virol. 1972;9:143–159. doi: 10.1128/jvi.9.1.143-159.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kowal J, et al. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc Natl Acad Sci U S A. 2016;113:E968–977. doi: 10.1073/pnas.1521230113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chugh PE, et al. Systemically circulating viral and tumor-derived microRNAs in KSHV-associated malignancies. PLoS pathogens. 2013;9:e1003484. doi: 10.1371/journal.ppat.1003484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cliffe AR, Nash AA, Dutia BM. Selective uptake of small RNA molecules in the virion of murine gammaherpesvirus 68. J Virol. 2009;83:2321–2326. doi: 10.1128/JVI.02303-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amen MA, Griffiths A. Packaging of Non-Coding RNAs into Herpesvirus Virions: Comparisons to Coding RNAs. Frontiers in genetics. 2011;2:81. doi: 10.3389/fgene.2011.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meckes DG, Jr, et al. Human tumor virus utilizes exosomes for intercellular communication. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:20370–20375. doi: 10.1073/pnas.1014194107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bihrer V, et al. Serum miR-122 as a Biomarker of Necroinflammation in Patients With Chronic Hepatitis C Virus Infection. Am J Gastroenterol. 2011 doi: 10.1038/ajg.2011.161. [DOI] [PubMed] [Google Scholar]
- 18.Xu J, et al. Circulating microRNAs, miR-21, miR-122, and miR-223, in patients with hepatocellular carcinoma or chronic hepatitis. Mol Carcinog. 2011;50:136–142. doi: 10.1002/mc.20712. [DOI] [PubMed] [Google Scholar]
- 19.Laterza OF, et al. Plasma MicroRNAs as sensitive and specific biomarkers of tissue injury. Clin Chem. 2009;55:1977–1983. doi: 10.1373/clinchem.2009.131797. [DOI] [PubMed] [Google Scholar]
- 20.Lanford RE, et al. Therapeutic silencing of microRNA-122 in primates with chronic hepatitis C virus infection. Science. 2010;327:198–201. doi: 10.1126/science.1178178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jopling CL, Yi M, Lancaster AM, Lemon SM, Sarnow P. Modulation of hepatitis C virus RNA abundance by a liver-specific MicroRNA. Science. 2005;309:1577–1581. doi: 10.1126/science.1113329. [DOI] [PubMed] [Google Scholar]
- 22.Masaki T, et al. miR-122 stimulates hepatitis C virus RNA synthesis by altering the balance of viral RNAs engaged in replication versus translation. Cell Host Microbe. 2015;17:217–228. doi: 10.1016/j.chom.2014.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gould SJ, Booth AM, Hildreth JE. The Trojan exosome hypothesis. Proc Natl Acad Sci U S A. 2003;100:10592–10597. doi: 10.1073/pnas.1831413100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pegtel DM, et al. Functional delivery of viral miRNAs via exosomes. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:6328–6333. doi: 10.1073/pnas.0914843107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sowinski S, et al. Membrane nanotubes physically connect T cells over long distances presenting a novel route for HIV-1 transmission. Nat Cell Biol. 2008;10:211–219. doi: 10.1038/ncb1682. [DOI] [PubMed] [Google Scholar]
- 26.Karlikow M, et al. Drosophila cells use nanotube-like structures to transfer dsRNA and RNAi machinery between cells. Sci Rep. 2016;6:27085. doi: 10.1038/srep27085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu W, et al. HIV-1 evades virus-specific IgG2 and IgA responses by targeting systemic and intestinal B cells via long-range intercellular conduits. Nat Immunol. 2009;10:1008–1017. doi: 10.1038/ni.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wolfers J, et al. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nature medicine. 2001;7:297–303. doi: 10.1038/85438. [DOI] [PubMed] [Google Scholar]
- 29.Arrode G, Boccaccio C, Abastado JP, Davrinche C. Cross-presentation of human cytomegalovirus pp65 (UL83) to CD8+ T cells is regulated by virus-induced, soluble-mediator-dependent maturation of dendritic cells. J Virol. 2002;76:142–150. doi: 10.1128/JVI.76.1.142-150.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Joffre OP, Segura E, Savina A, Amigorena S. Cross-presentation by dendritic cells. Nat Rev Immunol. 2012;12:557–569. doi: 10.1038/nri3254. [DOI] [PubMed] [Google Scholar]
- 31.Mori Y, et al. Human herpesvirus-6 induces MVB formation, and virus egress occurs by an exosomal release pathway. Traffic. 2008;9:1728–1742. doi: 10.1111/j.1600-0854.2008.00796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lenassi M, et al. HIV Nef is secreted in exosomes and triggers apoptosis in bystander CD4+ T cells. Traffic. 2010;11:110–122. doi: 10.1111/j.1600-0854.2009.01006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee JH, et al. HIV-Nef and ADAM17-Containing Plasma Extracellular Vesicles Induce and Correlate with Immune Pathogenesis in Chronic HIV Infection. EBioMedicine. 2016;6:103–113. doi: 10.1016/j.ebiom.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raymond AD, et al. HIV Type 1 Nef is released from infected cells in CD45(+) microvesicles and is present in the plasma of HIV-infected individuals. AIDS Res Hum Retroviruses. 2011;27:167–178. doi: 10.1089/aid.2009.0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Caby MP, Lankar D, Vincendeau-Scherrer C, Raposo G, Bonnerot C. Exosomal-like vesicles are present in human blood plasma. Int Immunol. 2005;17:879–887. doi: 10.1093/intimm/dxh267. [DOI] [PubMed] [Google Scholar]
- 36.Nolte-’t Hoen E, Cremer T, Gallo RC, Margolis LB. Extracellular vesicles and viruses: Are they close relatives? Proc Natl Acad Sci U S A. 2016;113:9155–9161. doi: 10.1073/pnas.1605146113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meckes DG., Jr Exosomal communication goes viral. J Virol. 2015;89:5200–5203. doi: 10.1128/JVI.02470-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Longatti A. The Dual Role of Exosomes in Hepatitis A and C Virus Transmission and Viral Immune Activation. Viruses. 2015;7:6707–6715. doi: 10.3390/v7122967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hergenreider E, et al. Atheroprotective communication between endothelial cells and smooth muscle cells through miRNAs. Nature cell biology. 2012;14:249–256. doi: 10.1038/ncb2441. [DOI] [PubMed] [Google Scholar]
- 40.Gibbings DJ, Ciaudo C, Erhardt M, Voinnet O. Multivesicular bodies associate with components of miRNA effector complexes and modulate miRNA activity. Nature cell biology. 2009;11:1143–1149. doi: 10.1038/ncb1929. [DOI] [PubMed] [Google Scholar]
- 41.Kosaka N, et al. Secretory mechanisms and intercellular transfer of microRNAs in living cells. J Biol Chem. 2010;285:17442–17452. doi: 10.1074/jbc.M110.107821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mathivanan S, Fahner CJ, Reid GE, Simpson RJ. ExoCarta 2012: database of exosomal proteins, RNA and lipids. Nucleic Acids Res. 2012;40:D1241–1244. doi: 10.1093/nar/gkr828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mittelbrunn M, et al. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat Commun. 2011;2:282. doi: 10.1038/ncomms1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Koppers-Lalic D, et al. Nontemplated nucleotide additions distinguish the small RNA composition in cells from exosomes. Cell Rep. 2014;8:1649–1658. doi: 10.1016/j.celrep.2014.08.027. [DOI] [PubMed] [Google Scholar]
- 45.Lunavat TR, et al. Small RNA deep sequencing discriminates subsets of extracellular vesicles released by melanoma cells--Evidence of unique microRNA cargos. RNA Biol. 2015;12:810–823. doi: 10.1080/15476286.2015.1056975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kharkwal H, Smith CG, Wilson DW. Herpes Simplex Virus Capsid Localization to ESCRT-VPS4 Complexes in the Presence and Absence of the Large Tegument Protein UL36p. J Virol. 2016;90:7257–7267. doi: 10.1128/JVI.00857-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Verweij FJ, et al. Exosomal sorting of the viral oncoprotein LMP1 is restrained by TRAF2 association at signalling endosomes. J Extracell Vesicles. 2015;4:26334. doi: 10.3402/jev.v4.26334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hurwitz SN, et al. CD63 Regulates Epstein-Barr Virus LMP1 Exosomal Packaging, Enhancement of Vesicle Production, and Noncanonical NF-kappaB Signaling. J Virol. 2017;91 doi: 10.1128/JVI.02251-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wilson CM, et al. Sortilin mediates the release and transfer of exosomes in concert with two tyrosine kinase receptors. J Cell Sci. 2014;127:3983–3997. doi: 10.1242/jcs.149336. [DOI] [PubMed] [Google Scholar]
- 50.Roucourt B, Meeussen S, Bao J, Zimmermann P, David G. Heparanase activates the syndecan-syntenin-ALIX exosome pathway. Cell Res. 2015;25:412–428. doi: 10.1038/cr.2015.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ghossoub R, et al. Syntenin-ALIX exosome biogenesis and budding into multivesicular bodies are controlled by ARF6 and PLD2. Nat Commun. 2014;5:3477. doi: 10.1038/ncomms4477. [DOI] [PubMed] [Google Scholar]
- 52.Ostrowski M, et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol. 2010;12:19–30. doi: 10.1038/ncb2000. sup pp 11–13. [DOI] [PubMed] [Google Scholar]
- 53.Fotheringham JA, Raab-Traub N. Epstein-Barr virus latent membrane protein 2 induces autophagy to promote abnormal acinus formation. J Virol. 2015;89:6940–6944. doi: 10.1128/JVI.03371-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Inoue J, et al. HBV secretion is regulated through the activation of endocytic and autophagic compartments mediated by Rab7 stimulation. J Cell Sci. 2015;128:1696–1706. doi: 10.1242/jcs.158097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Johns HL, Gonzalez-Lopez C, Sayers CL, Hollinshead M, Elliott G. Rab6 dependent post-Golgi trafficking of HSV1 envelope proteins to sites of virus envelopment. Traffic. 2014;15:157–178. doi: 10.1111/tra.12134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Veettil MV, et al. ESCRT-0 Component Hrs Promotes Macropinocytosis of Kaposi’s Sarcoma-Associated Herpesvirus in Human Dermal Microvascular Endothelial Cells. J Virol. 2016;90:3860–3872. doi: 10.1128/JVI.02704-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hyenne V, et al. RAL-1 controls multivesicular body biogenesis and exosome secretion. J Cell Biol. 2015;211:27–37. doi: 10.1083/jcb.201504136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cruz L, et al. Potent Inhibition of Human Cytomegalovirus by Modulation of Cellular SNARE Syntaxin 5. J Virol. 2017;91 doi: 10.1128/JVI.01637-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Elgner F, et al. Characterization of alpha-taxilin as a novel factor controlling the release of hepatitis C virus. Biochem J. 2016;473:145–155. doi: 10.1042/BJ20150717. [DOI] [PubMed] [Google Scholar]
- 60.Kawabata A, Serada S, Naka T, Mori Y. Human herpesvirus 6 gM/gN complex interacts with v-SNARE in infected cells. J Gen Virol. 2014;95:2769–2777. doi: 10.1099/vir.0.069336-0. [DOI] [PubMed] [Google Scholar]
- 61.Vallhov H, et al. Exosomes containing glycoprotein 350 released by EBV-transformed B cells selectively target B cells through CD21 and block EBV infection in vitro. J Immunol. 2011;186:73–82. doi: 10.4049/jimmunol.1001145. [DOI] [PubMed] [Google Scholar]
- 62.Fitzner D, et al. Selective transfer of exosomes from oligodendrocytes to microglia by macropinocytosis. J Cell Sci. 2011;124:447–458. doi: 10.1242/jcs.074088. [DOI] [PubMed] [Google Scholar]
- 63.van Dongen HM, Masoumi N, Witwer KW, Pegtel DM. Extracellular Vesicles Exploit Viral Entry Routes for Cargo Delivery. Microbiol Mol Biol Rev. 2016;80:369–386. doi: 10.1128/MMBR.00063-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ruiss R, Jochum S, Mocikat R, Hammerschmidt W, Zeidler R. EBV-gp350 confers B-cell tropism to tailored exosomes and is a neo-antigen in normal and malignant B cells--a new option for the treatment of B-CLL. PLoS One. 2011;6:e25294. doi: 10.1371/journal.pone.0025294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shukla D, Spear PG. Herpesviruses and heparan sulfate: an intimate relationship in aid of viral entry. J Clin Invest. 2001;108:503–510. doi: 10.1172/JCI13799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Christianson HC, Svensson KJ, van Kuppevelt TH, Li JP, Belting M. Cancer cell exosomes depend on cell-surface heparan sulfate proteoglycans for their internalization and functional activity. Proc Natl Acad Sci U S A. 2013;110:17380–17385. doi: 10.1073/pnas.1304266110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Koumangoye RB, Sakwe AM, Goodwin JS, Patel T, Ochieng J. Detachment of breast tumor cells induces rapid secretion of exosomes which subsequently mediate cellular adhesion and spreading. PLoS One. 2011;6:e24234. doi: 10.1371/journal.pone.0024234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sung BH, Ketova T, Hoshino D, Zijlstra A, Weaver AM. Directional cell movement through tissues is controlled by exosome secretion. Nat Commun. 2015;6:7164. doi: 10.1038/ncomms8164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lee HD, Koo BH, Kim YH, Jeon OH, Kim DS. Exosome release of ADAM15 and the functional implications of human macrophage-derived ADAM15 exosomes. FASEB J. 2012;26:3084–3095. doi: 10.1096/fj.11-201681. [DOI] [PubMed] [Google Scholar]
- 70.Akula SM, Pramod NP, Wang FZ, Chandran B. Integrin alpha3beta1 (CD 49c/29) is a cellular receptor for Kaposi’s sarcoma-associated herpesvirus (KSHV/HHV-8) entry into the target cells. Cell. 2002;108:407–419. doi: 10.1016/s0092-8674(02)00628-1. [DOI] [PubMed] [Google Scholar]
- 71.Zheng X, et al. Treatment with hyperimmune equine immunoglobulin or immunoglobulin fragments completely protects rodents from Ebola virus infection. Sci Rep. 2016;6:24179. doi: 10.1038/srep24179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.de Gassart A, Geminard C, Fevrier B, Raposo G, Vidal M. Lipid raft-associated protein sorting in exosomes. Blood. 2003;102:4336–4344. doi: 10.1182/blood-2003-03-0871. [DOI] [PubMed] [Google Scholar]
- 73.Peinado H, et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nature medicine. 2012;18:883–891. doi: 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ono M, et al. Exosomes from bone marrow mesenchymal stem cells contain a microRNA that promotes dormancy in metastatic breast cancer cells. Science signaling. 2014;7:ra63. doi: 10.1126/scisignal.2005231. [DOI] [PubMed] [Google Scholar]
- 75.Atay S, et al. Oncogenic KIT-containing exosomes increase gastrointestinal stromal tumor cell invasion. Proc Natl Acad Sci U S A. 2014;111:711–716. doi: 10.1073/pnas.1310501111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhou W, et al. Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer cell. 2014;25:501–515. doi: 10.1016/j.ccr.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Catanese MT, et al. Ultrastructural analysis of hepatitis C virus particles. Proc Natl Acad Sci U S A. 2013;110:9505–9510. doi: 10.1073/pnas.1307527110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xiao F, et al. Hepatitis C virus cell-cell transmission and resistance to direct-acting antiviral agents. PLoS pathogens. 2014;10:e1004128. doi: 10.1371/journal.ppat.1004128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ramakrishnaiah V, et al. Exosome-mediated transmission of hepatitis C virus between human hepatoma Huh7.5 cells. Proc Natl Acad Sci U S A. 2013;110:13109–13113. doi: 10.1073/pnas.1221899110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dreux M, et al. Short-range exosomal transfer of viral RNA from infected cells to plasmacytoid dendritic cells triggers innate immunity. Cell Host Microbe. 2012;12:558–570. doi: 10.1016/j.chom.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Masciopinto F, et al. Association of hepatitis C virus envelope proteins with exosomes. Eur J Immunol. 2004;34:2834–2842. doi: 10.1002/eji.200424887. [DOI] [PubMed] [Google Scholar]
- 82.Zhang J, et al. CD81 is required for hepatitis C virus glycoprotein-mediated viral infection. J Virol. 2004;78:1448–1455. doi: 10.1128/JVI.78.3.1448-1455.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.von Schaewen M, et al. Expanding the Host Range of Hepatitis C Virus through Viral Adaptation. MBio. 2016;7 doi: 10.1128/mBio.01915-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pileri P, et al. Binding of hepatitis C virus to CD81. Science. 1998;282:938–941. doi: 10.1126/science.282.5390.938. [DOI] [PubMed] [Google Scholar]
- 85.Brimacombe CL, et al. Neutralizing antibody-resistant hepatitis C virus cell-to-cell transmission. J Virol. 2011;85:596–605. doi: 10.1128/JVI.01592-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Inal JM, Jorfi S. Coxsackievirus B transmission and possible new roles for extracellular vesicles. Biochem Soc Trans. 2013;41:299–302. doi: 10.1042/BST20120272. [DOI] [PubMed] [Google Scholar]
- 87.Mao L, et al. Enterovirus 71 transmission by exosomes establishes a productive infection in human neuroblastoma cells. Virus Genes. 2016;52:189–194. doi: 10.1007/s11262-016-1292-3. [DOI] [PubMed] [Google Scholar]
- 88.Brenchley JM, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nature medicine. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 89.Johnson TP, et al. Induction of IL-17 and nonclassical T-cell activation by HIV-Tat protein. Proc Natl Acad Sci U S A. 2013;110:13588–13593. doi: 10.1073/pnas.1308673110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Izquierdo-Useros N, et al. HIV and mature dendritic cells: Trojan exosomes riding the Trojan horse? PLoS pathogens. 2010;6:e1000740. doi: 10.1371/journal.ppat.1000740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fang Y, et al. Higher-order oligomerization targets plasma membrane proteins and HIV gag to exosomes. PLoS biology. 2007;5:e158. doi: 10.1371/journal.pbio.0050158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nguyen DG, Booth A, Gould SJ, Hildreth JE. Evidence that HIV budding in primary macrophages occurs through the exosome release pathway. J Biol Chem. 2003;278:52347–52354. doi: 10.1074/jbc.M309009200. [DOI] [PubMed] [Google Scholar]
- 93.Doitsh G, et al. Abortive HIV infection mediates CD4 T cell depletion and inflammation in human lymphoid tissue. Cell. 2010;143:789–801. doi: 10.1016/j.cell.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kadiu I, Narayanasamy P, Dash PK, Zhang W, Gendelman HE. Biochemical and biologic characterization of exosomes and microvesicles as facilitators of HIV-1 infection in macrophages. J Immunol. 2012;189:744–754. doi: 10.4049/jimmunol.1102244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Izquierdo-Useros N, et al. Capture and transfer of HIV-1 particles by mature dendritic cells converges with the exosome-dissemination pathway. Blood. 2009;113:2732–2741. doi: 10.1182/blood-2008-05-158642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wiley RD, Gummuluru S. Immature dendritic cell-derived exosomes can mediate HIV-1 trans infection. Proc Natl Acad Sci U S A. 2006;103:738–743. doi: 10.1073/pnas.0507995103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li M, et al. Quantitative proteomic analysis of exosomes from HIV-1-infected lymphocytic cells. Proteomics. 2012;12:2203–2211. doi: 10.1002/pmic.201100376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sheehy AM, Gaddis NC, Choi JD, Malim MH. Isolation of a human gene that inhibits HIV-1 infection and is suppressed by the viral Vif protein. Nature. 2002;418:646–650. doi: 10.1038/nature00939. [DOI] [PubMed] [Google Scholar]
- 99.Tumne A, et al. Noncytotoxic suppression of human immunodeficiency virus type 1 transcription by exosomes secreted from CD8+ T cells. J Virol. 2009;83:4354–4364. doi: 10.1128/JVI.02629-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Andre F, et al. Exosomes as potent cell-free peptide-based vaccine. I. Dendritic cell-derived exosomes transfer functional MHC class I/peptide complexes to dendritic cells. J Immunol. 2004;172:2126–2136. doi: 10.4049/jimmunol.172.4.2126. [DOI] [PubMed] [Google Scholar]
- 101.Kovar M, et al. Direct stimulation of T cells by membrane vesicles from antigen-presenting cells. Proc Natl Acad Sci U S A. 2006;103:11671–11676. doi: 10.1073/pnas.0603466103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gaulke CA, et al. Intestinal epithelial barrier disruption through altered mucosal microRNA expression in human immunodeficiency virus and simian immunodeficiency virus infections. J Virol. 2014;88:6268–6280. doi: 10.1128/JVI.00097-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chang ST, et al. Next-generation sequencing of small RNAs from HIV-infected cells identifies phased microrna expression patterns and candidate novel microRNAs differentially expressed upon infection. MBio. 2013;4:e00549–00512. doi: 10.1128/mBio.00549-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Triboulet R, et al. Suppression of microRNA-silencing pathway by HIV-1 during virus replication. Science. 2007;315:1579–1582. doi: 10.1126/science.1136319. [DOI] [PubMed] [Google Scholar]
- 105.Thapa DR, et al. Serum microRNAs in HIV-infected individuals as pre-diagnosis biomarkers for AIDS-NHL. Journal of acquired immune deficiency syndromes. 2014;66:229–237. doi: 10.1097/QAI.0000000000000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Narayanan A, et al. Exosomes derived from HIV-1-infected cells contain trans-activation response element RNA. J Biol Chem. 2013;288:20014–20033. doi: 10.1074/jbc.M112.438895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Whisnant AW, et al. In-depth analysis of the interaction of HIV-1 with cellular microRNA biogenesis and effector mechanisms. MBio. 2013;4:e000193. doi: 10.1128/mBio.00193-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cullen BR, Cherry S, ten Oever BR. Is RNA interference a physiologically relevant innate antiviral immune response in mammals? Cell Host Microbe. 2013;14:374–378. doi: 10.1016/j.chom.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 109.Kincaid RP, Chen Y, Cox JE, Rethwilm A, Sullivan CS. Noncanonical microRNA (miRNA) biogenesis gives rise to retroviral mimics of lymphoproliferative and immunosuppressive host miRNAs. MBio. 2014;5:e00074. doi: 10.1128/mBio.00074-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Whisnant AW, et al. Identification of novel, highly expressed retroviral microRNAs in cells infected by bovine foamy virus. J Virol. 2014;88:4679–4686. doi: 10.1128/JVI.03587-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kincaid RP, Burke JM, Sullivan CS. RNA virus microRNA that mimics a B-cell oncomiR. Proc Natl Acad Sci U S A. 2012;109:3077–3082. doi: 10.1073/pnas.1116107109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nolte-’t Hoen EN, et al. Deep sequencing of RNA from immune cell-derived vesicles uncovers the selective incorporation of small non-coding RNA biotypes with potential regulatory functions. Nucleic Acids Res. 2012;40:9272–9285. doi: 10.1093/nar/gks658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pare JM, Sullivan CS. Distinct antiviral responses in pluripotent versus differentiated cells. PLoS pathogens. 2014;10:e1003865. doi: 10.1371/journal.ppat.1003865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sufiawati I, Tugizov SM. HIV-associated disruption of tight and adherens junctions of oral epithelial cells facilitates HSV-1 infection and spread. PLoS One. 2014;9:e88803. doi: 10.1371/journal.pone.0088803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ensoli B, et al. Synergy between basic fibroblast growth factor and HIV-1 Tat protein in induction of Kaposi’s sarcoma. Nature. 1994;371:674–680. doi: 10.1038/371674a0. [DOI] [PubMed] [Google Scholar]
- 116.Debaisieux S, Rayne F, Yezid H, Beaumelle B. The ins and outs of HIV-1 Tat. Traffic. 2012;13:355–363. doi: 10.1111/j.1600-0854.2011.01286.x. [DOI] [PubMed] [Google Scholar]
- 117.Wender PA, et al. The design, synthesis, and evaluation of molecules that enable or enhance cellular uptake: peptoid molecular transporters. Proc Natl Acad Sci U S A. 2000;97:13003-13008. doi: 10.1073/pnas.97.24.13003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Nagahara H, et al. Transduction of full-length TAT fusion proteins into mammalian cells: TAT-p27Kip1 induces cell migration. Nature medicine. 1998;4:1449–1452. doi: 10.1038/4042. [DOI] [PubMed] [Google Scholar]
- 119.Amorim NA, et al. Interaction of HIV-1 Nef protein with the host protein Alix promotes lysosomal targeting of CD4 receptor. J Biol Chem. 2014;289:27744–27756. doi: 10.1074/jbc.M114.560193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dikeakos JD, et al. An interdomain binding site on HIV-1 Nef interacts with PACS-1 and PACS-2 on endosomes to down-regulate MHC-I. Mol Biol Cell. 2012;23:2184–2197. doi: 10.1091/mbc.E11-11-0928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Aqil M, et al. The HIV Nef protein modulates cellular and exosomal miRNA profiles in human monocytic cells. J Extracell Vesicles. 2014;3 doi: 10.3402/jev.v3.23129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Luo X, Fan Y, Park IW, He JJ. Exosomes are unlikely involved in intercellular Nef transfer. PLoS One. 2015;10:e0124436. doi: 10.1371/journal.pone.0124436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Flanagan J, Middeldorp J, Sculley T. Localization of the Epstein-Barr virus protein LMP 1 to exosomes. J Gen Virol. 2003;84:1871–1879. doi: 10.1099/vir.0.18944-0. [DOI] [PubMed] [Google Scholar]
- 124.Raab-Traub N. In: Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis. Arvin A, et al., editors. 2007. [PubMed] [Google Scholar]
- 125.Houali K, et al. A new diagnostic marker for secreted Epstein-Barr virus encoded LMP1 and BARF1 oncoproteins in the serum and saliva of patients with nasopharyngeal carcinoma. Clin Cancer Res. 2007;13:4993–5000. doi: 10.1158/1078-0432.CCR-06-2945. [DOI] [PubMed] [Google Scholar]
- 126.Verweij FJ, et al. LMP1 association with CD63 in endosomes and secretion via exosomes limits constitutive NF-kappaB activation. EMBO J. 2011;30:2115–2129. doi: 10.1038/emboj.2011.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ardila-Osorio H, et al. TRAF interactions with raft-like buoyant complexes, better than TRAF rates of degradation, differentiate signaling by CD40 and EBV latent membrane protein 1. Int J Cancer. 2005;113:267–275. doi: 10.1002/ijc.20503. [DOI] [PubMed] [Google Scholar]
- 128.Dukers DF, et al. Direct immunosuppressive effects of EBV-encoded latent membrane protein 1. J Immunol. 2000;165:663–670. doi: 10.4049/jimmunol.165.2.663. [DOI] [PubMed] [Google Scholar]
- 129.Klibi J, et al. Blood diffusion and Th1-suppressive effects of galectin-9-containing exosomes released by Epstein-Barr virus-infected nasopharyngeal carcinoma cells. Blood. 2009;113:1957–1966. doi: 10.1182/blood-2008-02-142596. [DOI] [PubMed] [Google Scholar]
- 130.Keryer-Bibens C, et al. Exosomes released by EBV-infected nasopharyngeal carcinoma cells convey the viral latent membrane protein 1 and the immunomodulatory protein galectin 9. BMC Cancer. 2006;6:283. doi: 10.1186/1471-2407-6-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Meckes DG, Jr, et al. Modulation of B-cell exosome proteins by gamma herpesvirus infection. Proc Natl Acad Sci U S A. 2013;110:E2925–2933. doi: 10.1073/pnas.1303906110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ceccarelli S, et al. Epstein-Barr virus latent membrane protein 1 promotes concentration in multivesicular bodies of fibroblast growth factor 2 and its release through exosomes. Int J Cancer. 2007;121:1494–1506. doi: 10.1002/ijc.22844. [DOI] [PubMed] [Google Scholar]
- 133.Aga M, et al. Exosomal HIF1alpha supports invasive potential of nasopharyngeal carcinoma-associated LMP1-positive exosomes. Oncogene. 2014;33:4613–4622. doi: 10.1038/onc.2014.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Baglio SR, et al. Sensing of latent EBV infection through exosomal transfer of 5′pppRNA. Proc Natl Acad Sci U S A. 2016;113:E587–596. doi: 10.1073/pnas.1518130113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Cavallin LE, Goldschmidt-Clermont P, Mesri EA. Molecular and Cellular Mechanisms of KSHV Oncogenesis of Kaposi’s Sarcoma Associated with HIV/AIDS. PLoS pathogens. 2014;10:e1004154. doi: 10.1371/journal.ppat.1004154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Cai X, et al. Kaposi’s sarcoma-associated herpesvirus expresses an array of viral microRNAs in latently infected cells. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:5570–5575. doi: 10.1073/pnas.0408192102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Samols MA, Hu J, Skalsky RL, Renne R. Cloning and identification of a microRNA cluster within the latency-associated region of Kaposi’s sarcoma-associated herpesvirus. J Virol. 2005;79:9301–9305. doi: 10.1128/JVI.79.14.9301-9305.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.O’Hara AJ, et al. Pre-micro RNA signatures delineate stages of endothelial cell transformation in Kaposi sarcoma. PLoS pathogens. 2009;5:e1000389. doi: 10.1371/journal.ppat.1000389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lin X, Li X, Liang D, Lan K. MicroRNAs and unusual small RNAs discovered in Kaposi’s sarcoma-associated herpesvirus virions. J Virol. 2012;86:12717–12730. doi: 10.1128/JVI.01473-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chugh P, Tamburro K, Dittmer DP. Profiling of pre-micro RNAs and microRNAs using quantitative real-time PCR (qPCR) arrays. J Vis Exp. 2010 doi: 10.3791/2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Myoung J, Ganem D. Generation of a doxycycline-inducible KSHV producer cell line of endothelial origin: maintenance of tight latency with efficient reactivation upon induction. Journal of virological methods. 2011;174:12–21. doi: 10.1016/j.jviromet.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Rainy N, Zayoud M, Kloog Y, Rechavi O, Goldstein I. Viral oncomiR spreading between B and T cells is employed by Kaposi’s sarcoma herpesvirus to induce non-cell-autonomous target gene regulation. Oncotarget. 2016 doi: 10.18632/oncotarget.9627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Singh VV, et al. Kaposi’s sarcoma-associated herpesvirus latency in endothelial and B cells activates gamma interferon-inducible protein 16-mediated inflammasomes. J Virol. 2013;87:4417–4431. doi: 10.1128/JVI.03282-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sullivan BM, Coscoy L. The U24 protein from human herpesvirus 6 and 7 affects endocytic recycling. J Virol. 2010;84:1265–1275. doi: 10.1128/JVI.01775-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Temme S, Eis-Hubinger AM, McLellan AD, Koch N. The herpes simplex virus-1 encoded glycoprotein B diverts HLA-DR into the exosome pathway. J Immunol. 2010;184:236–243. doi: 10.4049/jimmunol.0902192. [DOI] [PubMed] [Google Scholar]
- 146.Hogue IB, Scherer J, Enquist LW. Exocytosis of Alphaherpesvirus Virions, Light Particles, and Glycoproteins Uses Constitutive Secretory Mechanisms. MBio. 2016;7 doi: 10.1128/mBio.00820-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Tandon R, AuCoin DP, Mocarski ES. Human cytomegalovirus exploits ESCRT machinery in the process of virion maturation. J Virol. 2009;83:10797–10807. doi: 10.1128/JVI.01093-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Cepeda V, Esteban M, Fraile-Ramos A. Human cytomegalovirus final envelopment on membranes containing both trans-Golgi network and endosomal markers. Cell Microbiol. 2010;12:386–404. doi: 10.1111/j.1462-5822.2009.01405.x. [DOI] [PubMed] [Google Scholar]
- 149.Walker JD, Maier CL, Pober JS. Cytomegalovirus-infected human endothelial cells can stimulate allogeneic CD4+ memory T cells by releasing antigenic exosomes. J Immunol. 2009;182:1548–1559. doi: 10.4049/jimmunol.182.3.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kalamvoki M, Du T, Roizman B. Cells infected with herpes simplex virus 1 export to uninfected cells exosomes containing STING, viral mRNAs, and microRNAs. Proc Natl Acad Sci U S A. 2014;111:E4991–4996. doi: 10.1073/pnas.1419338111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kalamvoki M, Deschamps T. Extracellular vesicles during Herpes Simplex Virus type 1 infection: an inquire. Virol J. 2016;13:63. doi: 10.1186/s12985-016-0518-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Bridgeman A, et al. Viruses transfer the antiviral second messenger cGAMP between cells. Science. 2015;349:1228–1232. doi: 10.1126/science.aab3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Han Z, et al. miR-H28 and miR-H29 expressed late in productive infection are exported and restrict HSV-1 replication and spread in recipient cells. Proc Natl Acad Sci U S A. 2016;113:E894–901. doi: 10.1073/pnas.1525674113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Schifano JM, Corcoran K, Kelkar H, Dittmer DP. Expression of the Antisense-to-Latency Transcript Long Noncoding RNA in Kaposi’s Sarcoma-Associated Herpesvirus. J Virol. 2017;91 doi: 10.1128/JVI.01698-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Fabbri M, et al. MicroRNAs bind to Toll-like receptors to induce prometastatic inflammatory response. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:E2110–2116. doi: 10.1073/pnas.1209414109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Li J, et al. Exosomes mediate the cell-to-cell transmission of IFN-alpha-induced antiviral activity. Nat Immunol. 2013;14:793–803. doi: 10.1038/ni.2647. [DOI] [PubMed] [Google Scholar]
- 157.Robbins PD, Morelli AE. Regulation of immune responses by extracellular vesicles. Nat Rev Immunol. 2014;14:195–208. doi: 10.1038/nri3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Taylor DD, Shah S. Methods of isolating extracellular vesicles impact down-stream analyses of their cargoes. Methods. 2015;87:3–10. doi: 10.1016/j.ymeth.2015.02.019. [DOI] [PubMed] [Google Scholar]
- 159.de Menezes-Neto A, et al. Size-exclusion chromatography as a stand-alone methodology identifies novel markers in mass spectrometry analyses of plasma-derived vesicles from healthy individuals. J Extracell Vesicles. 2015;4:27378. doi: 10.3402/jev.v4.27378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hong CS, Funk S, Muller L, Boyiadzis M, Whiteside TL. Isolation of biologically active and morphologically intact exosomes from plasma of patients with cancer. J Extracell Vesicles. 2016;5:29289. doi: 10.3402/jev.v5.29289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Welton JL, Webber JP, Botos LA, Jones M, Clayton A. Ready-made chromatography columns for extracellular vesicle isolation from plasma. J Extracell Vesicles. 2015;4:27269. doi: 10.3402/jev.v4.27269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lock M, et al. Rapid, simple, and versatile manufacturing of recombinant adeno-associated viral vectors at scale. Hum Gene Ther. 2010;21:1259–1271. doi: 10.1089/hum.2010.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Arroyo JD, et al. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc Natl Acad Sci U S A. 2011;108:5003–5008. doi: 10.1073/pnas.1019055108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Oksvold MP, et al. Expression of B-cell surface antigens in subpopulations of exosomes released from B-cell lymphoma cells. Clinical therapeutics. 2014;36:847–862 e841. doi: 10.1016/j.clinthera.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 165.Zomer A, et al. In Vivo imaging reveals extracellular vesicle-mediated phenocopying of metastatic behavior. Cell. 2015;161:1046–1057. doi: 10.1016/j.cell.2015.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kalra H, et al. Vesiclepedia: a compendium for extracellular vesicles with continuous community annotation. PLoS biology. 2012;10:e1001450. doi: 10.1371/journal.pbio.1001450. [DOI] [PMC free article] [PubMed] [Google Scholar]
Highlighted references
- Kowal J, Tkach M, Thery C. Biogenesis and secretion of exosomes. Curr Opin Cell Biol. 2014;29:116–125. doi: 10.1016/j.ceb.2014.05.004. Comprehensive review of the contribution of disinct vesicle transport proteins in the formation and secretion of exosomes. [DOI] [PubMed] [Google Scholar]
- Feng Z, et al. A pathogenic picornavirus acquires an envelope by hijacking cellular membranes. Nature. 2013;496:367–371. doi: 10.1038/nature12029. Study to show that a nonenveloped virus can acquire EVs membranes and remain infectious after released using the endosomal sorting complex. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YH, et al. Phosphatidylserine vesicles enable efficient en bloc transmission of enteroviruses. Cell. 2015;160:619–630. doi: 10.1016/j.cell.2015.01.032. Demonstration of many viral particles inside large microvesicles. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chugh PE, et al. Systemically circulating viral and tumor-derived microRNAs in KSHV-associated malignancies. PLoS pathogens. 2013;9:e1003484. doi: 10.1371/journal.ppat.1003484. Study revealed secretion of host and viral miRNAs within EVs in patient sera and mouse models and showed that uptake of these exosomes modified cell behavior and induced cell migration and IL6 secretion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould SJ, Booth AM, Hildreth JE. The Trojan exosome hypothesis. Proc Natl Acad Sci U S A. 2003;100:10592–10597. doi: 10.1073/pnas.1831413100. Definition of the Trojan horse hypothesis to hide viruses and viral components inside EVs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meckes DG, Jr, et al. Human tumor virus utilizes exosomes for intercellular communication. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:20370–20375. doi: 10.1073/pnas.1014194107. First study to identify biologic properties of EVs produced by cells expressing the viral oncogene, latent membrane 1, and showed EVs uptake resulted in activation of the ERK and AKT signaling pathways. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao F, et al. Hepatitis C virus cell-cell transmission and resistance to direct-acting antiviral agents. PLoS pathogens. 2014;10:e1004128. doi: 10.1371/journal.ppat.1004128. Study shows direct cell-to-cell transfer of HCV that was resistant to antivirals but could be sensitized using entry inhibitors such as claudin antibody. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meckes DG, Jr, et al. Modulation of B-cell exosome proteins by gamma herpesvirus infection. Proc Natl Acad Sci U S A. 2013;110:E2925–2933. doi: 10.1073/pnas.1303906110. Comprehensive Proteomic analysis of EVs produced by EBV, KSHV, or dually EBV/KSHV B cell lines revealed that both EBV and KSHV EVs were predicted to modulate cell death and survival. EBV EVs activated cell signaling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfers J, et al. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nature medicine. 2001;7:297–303. doi: 10.1038/85438. Evidence for the role of EVs in antigenic crosspriming. [DOI] [PubMed] [Google Scholar]
- Pegtel DM, et al. Functional delivery of viral miRNAs via exosomes. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:6328–6333. doi: 10.1073/pnas.0914843107. Delivery of viral miRNAs into target cells through EVs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowal J, et al. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc Natl Acad Sci U S A. 2016;113:E968–977. doi: 10.1073/pnas.1521230113. Evidence for different populations of EVs circulating at the same time, with each population identified by different composition of surface markers. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathivanan S, Fahner CJ, Reid GE, Simpson RJ. ExoCarta 2012: database of exosomal proteins, RNA and lipids. Nucleic Acids Res. 2012;40:D1241–1244. doi: 10.1093/nar/gkr828. Online resource of proteins and RNAs reportedly associated with EVs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrowski M, et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol. 2010;12:19–30. doi: 10.1038/ncb2000. sup pp 11–13. Demonstration of the role of specific GPAses in the regulation of EVs. [DOI] [PubMed] [Google Scholar]
- Kalamvoki M, Du T, Roizman B. Cells infected with herpes simplex virus 1 export to uninfected cells exosomes containing STING, viral mRNAs, and microRNAs. Proc Natl Acad Sci U S A. 2014;111:E4991–4996. doi: 10.1073/pnas.1419338111. Demonstration that STING, an innate sensor, as well as viral mRNAs and host miRNAs are transferred from virally-infected to neighboring cells independent of infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridgeman A, et al. Viruses transfer the antiviral second messenger cGAMP between cells. Science. 2015;349:1228–1232. doi: 10.1126/science.aab3632. Demonstration that a small molecule involved in innate sensing is transferred by virions directly. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalra H, et al. Vesiclepedia: a compendium for extracellular vesicles with continuous community annotation. PLoS biology. 2012;10:e1001450. doi: 10.1371/journal.pbio.1001450. Online resource of proteins and RNAs reportedly associated with EVs. [DOI] [PMC free article] [PubMed] [Google Scholar]