Abstract
The loss of dopaminergic neurons in the substantia nigra pars compacta (SNpc) and the accumulation of protein inclusions (Lewy bodies) are the pathological hallmarks of Parkinson’s disease (PD). PD is triggered by genetic alterations, environmental/occupational exposures and aging. However, the exact molecular mechanisms linking these PD risk factors to neuronal dysfunction are still unclear. Alterations in redox homeostasis and bioenergetics (energy failure) are thought to be central components of neurodegeneration that contribute to the impairment of important homeostatic process in dopaminergic cells such as protein quality control mechanisms, neurotransmitter release/metabolism, axonal transport of vesicles and cell survival. Importantly, both bioenergetics and redox homeostasis are coupled to neuro-glial central carbon metabolism. We and others have recently established a link between the alterations in central carbon metabolism induced by PD risk factors, redox homeostasis and bioenergetics and their contribution to the survival/death of dopaminergic cells. In this review, we focus on the link between metabolic dysfunction, energy failure and redox imbalance in PD, making an emphasis in the contribution of central carbon (glucose) metabolism. The evidence summarized here strongly supports the consideration of PD as a disorder of cell metabolism.
Keywords: neurodegeneration, glycolysis, glucose, TCA cycle, oxidative stress, bioenergetics, mitochondria
1. Introduction
Oxidative stress and energy failure associated with mitochondrial dysfunction are cardinal hallmarks of neuronal cell death associated with neurodegenerative disorders [71, 215, 257, 348]. Mitochondrial derived reactive oxygen species (ROS) contribute to neuronal cell death in neurodegeneration. However, the lack of success in the clinic of antioxidant-based therapies suggests that other contributors associated with mitochondrial dysfunction should also play an important role [1, 156, 225, 300]. Cellular bioenergetics is directly coupled with central carbon metabolism, and in the brain, glucose is the primary energy substrate. Glucose metabolism has been shown to be altered in different neurodegenerative disorders. For example, in Alzheimer’s disease a disruption in glucose uptake and metabolism is found [59, 145, 341]. Hyperglycemia and amyloid β promote excitotoxicity in neuronal cells [7]. Other studies have also revealed that glucose metabolism is decreased in the cortex and basal ganglia of Huntington’s disease patients [69]. Interestingly, an increase in the levels of glucose transporters was found to correlate with a decreased age of onset in Huntington’s disease [327]. While cancer and diabetes/obesity are considered metabolic disorders clearly associated with a dysfunction in glucose metabolism, less is understood about the role of central carbon metabolism in neurodegeneration. Recently, we and others have revealed links between alterations in glucose metabolism and alterations in cellular bioenergetics, redox homeostasis and cell death progression induced by PD-related risk factors. In this review, we present an integrated overview of these results to evidence how alterations in central carbon metabolism have a central role in determining changes in cellular bioenergetics and redox homeostasis in PD. These findings are posed to make a significant contribution to our understanding of PD pathogenesis, and make a case for considering PD a metabolic disorder.
2. Brain Metabolism, Bioenergetics and Redox Balance
2.1. Energy Metabolism in the Brain
The brain can be considered the most complex organ in the human body. The regulation and maintenance of brain function requires high amounts of energy. The brain accounts for ~20% of total body energy consumption (25% of the glucose and 20% of the oxygen consumption), and brain energy levels are more resistant to starvation compared to other organs [279]. Glucose is considered the obligatory energy substrate in the adult brain requiring its continuous delivery across the blood brain barrier (BBB) (Figure 1.1); however, alternate energy sources can fuel brain function under energetic stress situations. For example, lactate transport across the BBB can provide ~8%–10% of the brain’s energy requirements and has been estimated to supply ~20%–25% of energy at the expense of glucose consumption during energy demanding activities. Importantly the extracellular levels of lactate (0.5–1.5 mM) are similar to those of glucose, suggesting that an important pool of lactate is always available when required for consumption [209]. Ketone bodies derived from fatty acid metabolism in the liver can also cross the BBB [125].
Figure 1. Redox homeostasis and metabolic coupling between neurons and glia.
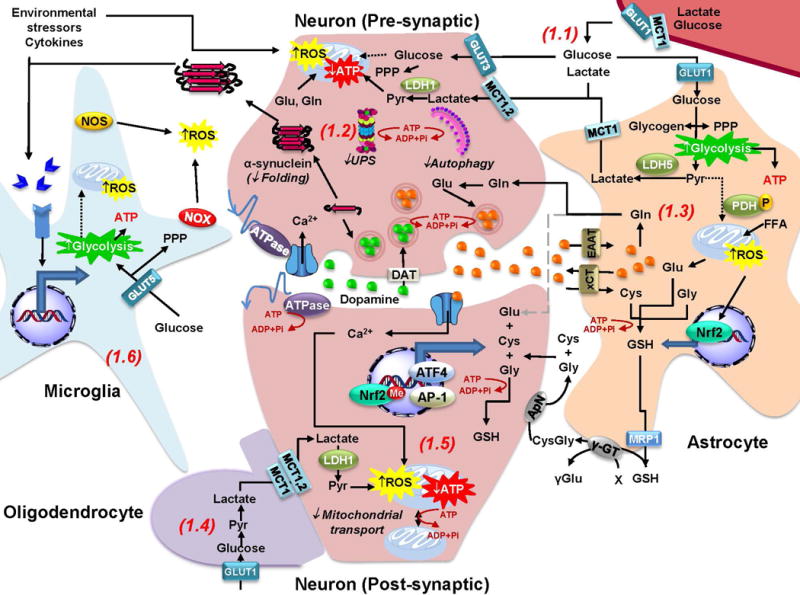
Redox and metabolic homeostasis is carried out by a complex interaction between neurons, glia and the extracellular microenvironment. 1.1. Glucose and lactate from blood circulation cross the blood brain barrier (BBB) through the GLUT1 (glucose transporter 1) and MCT1 (monocarboxylate transporter 1) transporters. Lactate is mainly taken up by neurons, while glucose is thought to be preferentially consumed by glial cells. 1.2 Neuronal ATP generation is dependent on oxidative phosphorylation, while glucose metabolism is primarily directed towards the PPP to generate NADPH required for antioxidant defense. Energy failure in PD is expected to impair a number of ATP-dependent processes that include: a) protein quality control mechanisms, including protein folding and protein aggregate (α-synuclein) degradation via the UPS and autophagy; b) transport of mitochondria across the axon and dendritic terminals (See 1.5); c) dopamine and glutamate capture into vesicles; and d) maintenance of ionic gradients and plasma membrane potential during synaptic transmission. 1.3. Glycolysis in astrocytes exceeds energy demands and thus lactate is shuttled as an energy substrate to neurons, which have a limited ability to upregulate glycolysis in response to mitochondrial dysfunction as it occurs in PD. Glucose flux to the TCA cycle is reduced in astrocytes due to phosphorylation of PDH. Astrocytes are able to store glucose in the form of glycogen. Glutamate (Glu) uptake from the inter-synaptic space via EAATs prevents excytotoxicity and facilitates neurotransmitter recycling via the synthesis of glutamine (Gln) that is also shuttled to neurons. In addition, Glu exchange for Cystine (that is reduced to Cysteine [Cys] inside the cell) via xCT (or Xc-), maintains Cys supply for de novo GSH synthesis. Furthermore, Nrf2 activation in response to oxidative stress promotes GSH synthesis, which is also released to provide precursors for its de novo synthesis in neurons (See 1.5). Astrocytes also have the capacity to use oxidize FFA to fuel mitochondria but its functional relevance is unclear. 1.4. Oligodendrocytes also shuttle lactate as energy fuel to myelinated axons. 1.5. In neurons, Nrf2 is repressed by methylation. Nevertheless, upregulation of antioxidant defenses is dependent on neuronal activity and the activation of ATF4 and AP-1 transcription factors. 1.6. Environmental toxicants, α-synuclein oligomers, and cytokines activate microglia and induce oxidative stress. Importantly the proinflammatory M1 phenotype of activated microglia has been reported to be associated to a switch in their metabolism from oxidative phosphorylation to glycolysis that also enhances carbon flux to the PPP.
All brain regions are considered to be metabolically active with heterogeneity among structures. Remarkably, metabolic activity of the brain is constant over time. Brain metabolic activity consists of the oxidation of glucose to carbon dioxide and water (oxygen consumption), which results in the production of energy in the form of ATP. Importantly both oxygen consumption and glucose metabolism do not necessarily parallel each other. Glucose is delivered to brain cells through the blood and changes in brain glucose consumption are accompanied with vascular flow modifications. An increase in blood flow of oxygen and glucose parallels an increase in brain activity. However, only glucose consumption (glycolysis) increases proportionately, while oxygen consumption (oxidative phosphorylation) does not, despite its increased supply [231, 267]. Interestingly, only 10% of glucose yields lactate. In addition to the generation of energy, glucose metabolism in the brain is also important for the synthesis of carbohydrates for glycoproteins and glycolipids, amino acids, one-carbon donors for methylation, and neurotransmitter precursors [231].
2.2. Cellular Composition of the Brain
The brain tissue is made up of about 100 billion neurons and trillions of glia cells, and its bioenergetics and redox homeostasis is an integrated process between these different cell populations. Neurons are highly polarized cells with subcellular domains that serve different functions: the cell body (or soma), branching dendrites (signal receivers), and the axon that conducts nerve signals (Figure 1.2 and 1.5). Neuronal types and distribution are highly specialized across to the different brain regions. Specialization in both cytoarchitecture and neurotransmitter communication differentiates the function of neuronal populations [31, 304].
Glial cells make up 90 percent of the brain’s cells and regulate a number of physiological processes required for proper neuronal survival and function. Three types of glial cells exist in the CNS: oligodendrocytes, astrocytes, and microglia (Figure 1.3, 1.4 and 1.6). Oligodendrocytes are responsible for nerve fiber myelination, which provides axons with an “insulating coat” that enhances nerve impulse conduction. Oligodendrocytes have several processes and form several internodal segments of myelin on axons separated by gaps (Ranvier nodes) (Figure 1.4) [28, 299]. Astrocytes are small cells with processes arranged radially and a considerable molecular, structural, and functional diversity at the regional level. Astrocyte processes cover the external surface of capillaries in blood vessels (perivascular feet), the initial segment of most axons, and the bare segments of axons at the Ranvier nodes. Astrocytes processes cover a defined territory forming highly organized domains interconnected via gap junctions. They regulate neurotransmitter levels in the synaptic cleft, provide neurons with energetic and antioxidant precursors (Figure 1.3), play an important role in the migration of immature neurons, tissue repair, and regulate blood flow and inflammatory processes by release of signaling mediators [301]. Microglial cells are resident macrophages distributed throughout the CNS that are embryologically unrelated to the other glial cells [44]. As innate immune cells, they are activated by infection, tissue injury (ischemia or trauma), or neurodegenerative processes. Upon activation, microglia cells retract their processes and migrate to the site of the lesion, where they proliferate and become antigen presenting cells. Microglia phagocyte degenerating cells and also act as sources of immunoregulatory and neuromodulatory factors such as cytokines, chemokines, neurotrophic factors. Microglia can be activated by cell-surface receptors for endotoxin, cytokines, chemokines, misfolded proteins, serum factors and ATP (Figure 1.6). While mild activation is a key adaptive immune response, continuous activation or overactivation of microglia is thought to contribute to neurodegeneration [97, 122, 123].
The BBB refers to a barrier of capillaries that restrict the exchange of solutes between the blood and the brain extracellular fluid. Endothelial cells that form BBB capillaries are connected by gap junctions and regulate the exchange of metabolites and signaling molecules while preventing the entrance of deleterious molecules or agents to the brain (Figure 1.1) [261, 304].
2.3. Metabolic Specialization in Brain Cells
Neurons are high energy consumers observing high rates of oxidative metabolism compared to glial cells. Neurons utilize most of their energy at the synapse to maintain and restore ionic gradients, and for the uptake and recycle of neurotransmitters (>80%). Interestingly, neurons are thought to metabolize glucose primarily via the pentose phosphate pathway (PPP) to provide reducing equivalents required to maintain antioxidant defenses via the production of nicotinamide adenine dinucleotide phosphate (NADPH) (Figure 1.2 and 2.5) [39]. NADPH is also required for antioxidant defense in glial cells, but it can actually mediate ROS generation by NADPH-oxidases (NOXs) (Figure 3.1b). NADPH is a reducing agent as well, required for the synthesis of lipids and nucleic acids and for the metabolism of neurotransmitters and aldehydes. Other mechanisms of generating NADPH exist, which include folate metabolism, NADP-malate dehydrogenase (malic enzyme), isocitrate dehydrogenase (IDH1 and 2) (Figure 2.6), glutamate dehydrogenase and nicotinamide nucleotide transhydrogenase (Nnt) (Figure 2.7). However, their contribution to NAPDH metabolism in neurons or glial cells has not been well defined [95]. In isolated brain mitochondria Nnt has been reported to be important in the removal of peroxides [202, 349].
Figure 2. Interaction of Parkinson disease-related genes and risk factors with bioenergetics and central carbon metabolism.
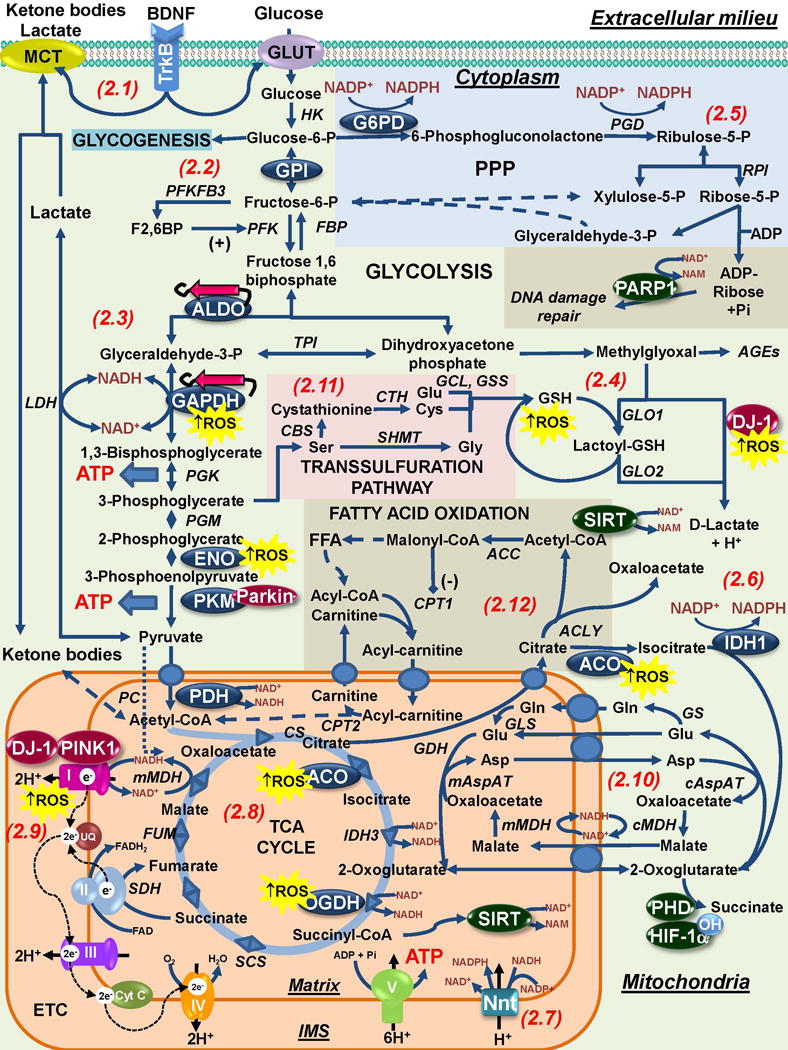
2.1. BDNF released by glial cells (and activation of the tropomyosin receptor kinase B [TrkB]) has protective effects in dopaminergic cells that can potentially be associated with the regulation of neuronal bioenergetics. 2.2 In astrocytes, energy can be stored as glycogen or it can be metabolized via glycolysis to meet energy demands. Glucose metabolism is also directed to lactate production that can be shuttled to neurons. In neurons PFKFB3 is constantly degraded and thus, they depend primarily in oxidative phosphorylation to meet their energy demands. GPI has been demonstrated to exert a protective effect against α-synuclein toxicity via glycolysis. 2.3. Downstream the glycolytic pathway a number of enzymes have been shown to be altered / regulated in PD and by PD-related risk factors. Aldolase (ALDO), GAPDH and enolase (ENO) are found aggregated and oxidized in PD. Amyloid-like α-synuclein fibrils are expected to interact with and likely inhibit metastable glycolytic enzymes such as ALDO. Other groups have reported on the role of Parkin inhibiting PKM activity by ubiquitination. 2.4. Spontaneous generation of methylglyoxal is thought to account for 0.1–0.4% of glycolytic flux. Accumulation of AGEs and dopamine-related methylglyoxal derivatives is linked to PD. DJ-1 is a cofactor-independent GLO III system that detoxifies methylglyoxal while also generating D-lactate that can contribute to the maintenance of mitochondrial function. DJ-1 is a redox-sensitive protein whose protective effects against PD-related insults can be impaired by oxidative stress. 2.5 In neurons, glucose is primarily metabolized via the PPP to generate NADPH that provides reducing equivalents for antioxidant defense. Astrocytes have higher levels of NADPH and G6PD, and in glial cells, NADPH also has a pro-oxidant role by providing reducing equivalents for the generation of ROS by NOX and NOS. In addition, the ribose-5-P from the PPP is used for the generation ADP-ribose that is used during DNA-damage repair as a substrate for PARP-1 mediated ADP-ribosylation. NADPH is also regenerated by IDH1 in cytosol (2.6) and in the mitochondria by Nnt (2.7), and both systems have been reported to protect against PD-related insults MPP+ and paraquat. 2.8. Alterations in the TCA cycle have also been found associated with PD. In the mitochondria, pyruvate decarboxylation by PDH is a necessary step for the generation of acetyl-CoA and Mn (manganese), a PD-risk factor has been reported to inhibit its activity. Aconitase (ACO) inactivation by oxidative stress is a biomarker of oxidative damage induced by PD-related insults or PINK1 mutants, while a decrease in OGDH is found in PD. 2.9. A decrease in Complex I activity is found in PD. In addition Complex I has been reported to be targeted by environmental toxicants acting as Complex I inhibitors or inducing oxidative stress. PD-related genes DJ-1 and PINK1 regulate Complex I activity via direct interaction or phosphorylation of its subunits. 2.10. The mitochondrial shuttles enable electrons and precursors transport across the inner membrane. Glutamate is metabolized to glutamine by GS an enzyme exclusively present in the astrocytes. Glutamine is shuttled from astrocytes to neurons where it is metabolized back to glutamate by GLS. A number of metabolites act as substrates for the activity of signaling proteins (highlighted in green circles). Prolyl hydroxylase (PDH)-dependent hydroxylation of HIF-1α requires 2-oxoglutarate. 2.11. One-carbon metabolism of serine to glycine and cysteine (via the transsulfuration pathway) contribute to the formation of GSH. 2.12. Neurons very poorly metabolize FFA to obtain energy due the high O2 demand and resultant generation of superoxide anion (O2•−) from β-oxidation. However, 20% of total adult brain energy comes from FFA oxidation, mostly in astrocytes, but its functional relevance is unclear. Enzymes highlighted in blue circles are those within central carbon metabolism found to be modulated by oxidative stress and PD-related risk factors. PD-related genes are highlighted in red circles. Abbreviations and enzyme commission (EC) numbers for enzymes involved in central carbon metabolism: ACC, Acetyl-CoA carboxylase [EC: 6.4.1.2]; ACLY, ATP-citrate synthase [EC:2.3.3.8]; ACO, Aconitase [EC:4.2.1.3]; ALDO (A/B), Fructose-bisphosphate aldolase [EC:4.1.2.13]; cAspAT, Aspartate aminotransferase, cytoplasmic [EC:2.6.1.1 2.6.1.3]; mAspAT, Aspartate aminotransferase, mitochondrial [EC:2.6.1.1 2.6.1.7]; AR, aldose reductase [1.1.1.21]; CBS, Cystathionine beta-synthase [EC: 4.2.1.22]; CPT1, carnitine O-palmitoyltransferase 1 (CPT1 or 2 (CPT2) [EC: 2.3.1.21]; CS, Citrate synthase [EC:2.3.3.1]; CTH, Cystathionine gamma-lyase [EC: 4.4.1.1]; ENO, Enolase [EC:4.2.1.11]; FBP, fructose-1,6-bisphosphatase I [EC:3.1.3.11]; FUM, Fumarate hydratase [EC:4.2.1.2]; G3PP; Glucose-3-phosphate permease [EC: ]; G6PD, Glucose-6-phosphate 1-dehydrogenase [EC:1.1.1.49]; GAPDH, Glyceraldehyde 3-phosphate dehydrogenase [EC:1.2.1.12]; GCL, Glutamate cysteine ligase [EC: 6.3.2.2]; GDH, Glutamate dehydrogenase, [EC: 1.4.1.3]; GLO1, Glyoxalase 1 or Lactoylglutathione lyase [EC:4.4.1.5]; GLO2, Glyoxalase 2 or Hydroxyacylglutathione hydrolase [EC 3.1.2.6]; GLS, Glutaminase [EC: 3.5.1.2]; GPI, Glucose-6-phosphate isomerase [EC:5.3.1.9]; GS, Glutamine synthetase [EC:6.3.1.2]; GSS, glutathione synthetase [EC: 6.3.2.3]; HK, hexokinase [EC:2.7.1.1]; IDH1, Isocitrate dehydrogenase 1 (NADP+), soluble [EC:1.1.1.42]; IDH3, isocitrate dehydrogenase 3 (NAD+); LDH, L-lactate dehydrogenase [EC:1.1.1.27]; MDH, Malate dehydrogenase [EC:1.1.1.37]; OGDH, 2-Oxoglutarate dehydrogenase, [EC:1.2.4.2]; SCS, Succinyl-CoA synthetase [EC:6.2.1.4 6.2.1.5]; PC, Pyruvate carboxylase [EC:6.4.1.1]; PGD, 6-phosphogluconate dehydrogenase [EC:1.1.1.44]; PDH, Pyruvate dehydrogenase [EC:1.2.4.1]; PGK, phosphoglycerate kinase [EC:2.7.2.3]; PGM, Phosphoglucomutase [EC:5.4.2.2]; PFK, 6-phosphofructokinase 1 [EC:2.7.1.11]; PFKFB3, 6-phosphofucto-2-kinase/fructose-2,6-biphosphatase 3 [EC:3.1.3.46]; PKM, Pyruvate Kinase [EC:2.7.1.40]; RPI, Ribose 5-phosphate isomerase A [EC:5.3.1.6]; SDH, Succinate dehydrogenase [EC:1.3.5.1]; SHMT, Serine hydroxymethyltransferase [EC 2.1.2.1]; TPI, triose-phosphate isomerase [EC:5.3.1.1].
Figure 3. Bioenergetic requirements of antioxidant systems.
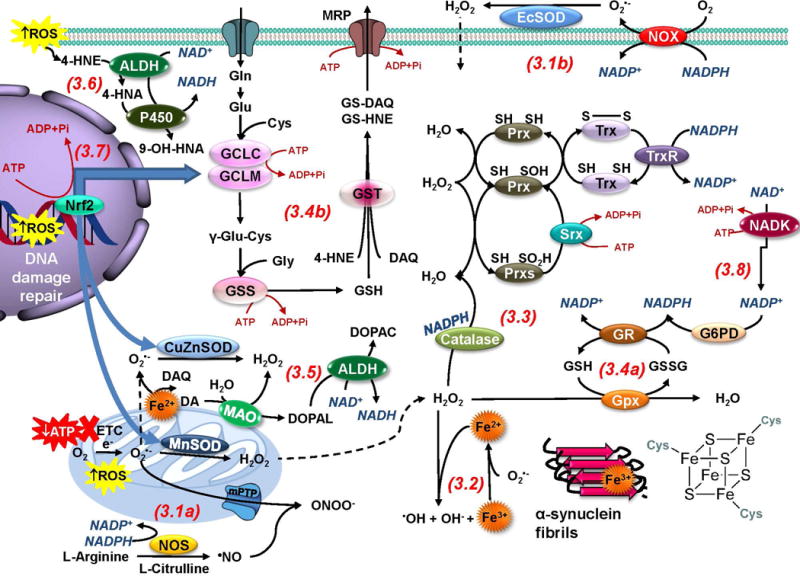
Energy failure and ROS are the main consequences associated with mitochondrial dysfunction in PD. 3.1a. Electron leakage from the mitochondria leads to one electron reduction of O2 to O2•− that can be dismutated by SODs to H2O2.•NO can outcompete SODs reacting with O2•− to generate −ONOO. 3.1b. In the plasma membrane O2•− can be generated by NOXs. 3.2. In the presence of metals such as Fe, H2O2 and O2•− generate •OH through the Fenton/Haber-Weiss reaction. In PD, α-synuclein, neuromelanin and Fe-S clusters are important pools of Fe. 3.3. Catalase and Prxs catalyze H2O2 degradation. The Trx/TrxR system supplies reducing equivalents for most Prxs. Prx hyperoxidation is reversed by Srx. 3.4a. Gpxs detoxifies peroxides using GSH which is reduced back from GSSG by GR using NADPH. 3.4b. GSH also detoxifies electrophiles (DAQ and 4-HNE) via the action of GSTs, and these adducts can be transported outside of the cell to become eliminated by the activity of MRP proteins. 3.5. In the cytosol, dopamine (DA) becomes auto-oxidized in the presence of Fe generating DAQs and ROS. DA metabolism by MAO also generates ROS and DOPAL, which is further metabolized by ALDHs. 3.6. ALDHs also detoxify 4-HNE into 4-hydroxy-2-nonenoic acid (HNA) that is metabolized by cytochrome P450 enzymes (P450). 3.7. Oxidative stress triggers antioxidant response elements (AREs)-regulated gene transcription by Nrf2, which promotes GCLC/GCLM, CuZnSOD and MnSOD transcription. 3.8. A number of antioxidant systems and ROS generating enzymes utilize reducing equivalents from NADPH (in blue letters) that is regenerated by enzymes such as G6PD, and synthesized de novo via the phosphorylation of NAD+ by NAD-kinase (NADK). Thus, antioxidant systems are tightly coupled to NAD+ and central carbon metabolism. In addition, many antioxidant enzymes and stress responses (DNA-damage repair and the transcription of antioxidant enzymes by Nrf2) require energy consumption (ATP, highlighted in dark red) demonstrating that redox homeostasis is tightly coupled to bioenergetics and cell metabolism.
Neurons lack 6-phosphofructose-2-kinase/fructose-2,6-bisphosphatase-3 (PFKFB3) due to its continuous degradation by the ubiquitin-proteasome (UPS) pathway [9]. PFKFB3 is involved in the biogenesis and degradation of fructose-2,6-bisphosphate (F2,6BP), a glycolytic activator (Figure 2.2). In astrocytes, PFKFB3 is activated by AMPK (adenosine monophosphate-activated protein kinase) and promotes glycolysis [131]. Because neurons have a limited capacity to upregulate glycolysis in response to stress, they depend on the shuttle of lactate from astrocytes and oligodendrocytes as fuel for mitochondrial OXPHOS (oxidative phosphorylation) (Figure 1.3 and 1.4) [32, 102, 183, 237]. Neuronal function ex vivo can be maintained by lactate in the absence of glucose [96]. To date, the primary neuronal energy substrate (glucose vs lactate) and the relative contributions of glycolysis and OXPHOS to neuronal bioenergetics (ATP) are still under debate [85, 142, 258, 290]. However, the fact that mice with a decreased expression of the neuron-specific glucose transporter GLUT3 do not observe alterations in glucose utilization, while astrocytes-specific GLUT1 deficiency results in a severe neurological phenotype supports the idea of the preferential consumption of glucose by astrocytes and the astrocytes-neuron lactate shuttle hypothesis (Figure 1.1) [308, 331].
Even though glial cells and neurons have the same oxidative capacity, glial cells are highly glycolytic and a large portion of glucose metabolism is directed to lactate production and its release to the extracellular space. Astrocyte glucose consumption does not match their energy expenditure, which only accounts for 5–15% of the glucose consumed by the brain. Recent studies have demonstrated that the activity of pyruvate dehydrogenase (PDH), which determines the entry of pyruvate into the tricarboxylic acid (TCA or Krebs) cycle (Figure 1.3), is reduced by its phosphorylation in astrocytes [360]. Glucose storage within brain cells is found in astrocytes in the form of glycogen, which can also be broken down to lactate, but some evidence exist for the generation of glucose via glucogenesis [231]. In addition, glutamate can be used as a carbon source for the TCA cycle in both neurons and astrocytes, while acetate is preferentially used by astrocytes [32, 150, 208:, 306]. The creatine / phosphocreatine / creatine kinase system has also been proposed to contribute to the regeneration of ATP in brain [30]. Ketone bodies (3-β-hydroxybutyrate [3BHM], acetoacetate, and acetone) and free fatty acids (and lactate) can be transported across the BBB during starvation and are thought to provide two thirds of the total energy required for the brain. Neurons very poorly metabolize hydrogen-rich free fatty acids (FFA) to obtain energy (Figure 2.12), which has been related to the high O2 demand and resultant generation of superoxide anion (O2•−) from β-oxidation [287]. However, 20 % of total adult brain energy comes from FFA oxidation, mostly in astrocytes. Astrocytes can oxidize FFA (Figure 1.3) and ketone bodies, while neurons and oligodendrocytes can only use ketone bodies. β-oxidation of fatty acids by astrocytes provides ketone bodies for neurons as well [138, 287].
Astrocytes are important players in the regulation of neuronal excitability by the removal of released neurotransmitters. The excitatory amino acid glutamate (Glu) is taken up by the Na+-dependent excitatory amino acid transporter-1 (EAAT1 or GLAST) and EAAT2 (GLT-1) and converted to glutamine (Gln) by the astrocyte-specific enzyme glutamine synthetase (GS) (Figure 1.3 and 2.10). Glutamine is exported and transferred to neurons (glutamate-glutamine cycle) to regenerate glutamine via glutaminase (GLS) activity (Figure 1.2 and 2.10). Glutamate can also be utilized for de novo synthesis of the antioxidant glutathione (GSH) (Figure 1.3, 1.5, 2.11 and 3.4b) [32, 288]. In general, neurons have limited defense mechanisms against ROS when compared to astrocytes (Figure 1.2 and 1.3). Astrocytes contain higher levels of endogenous antioxidants and antioxidant systems being more resistant to oxidative stress, which is explained by the activation of the antioxidant response via the nuclear factor erythroid-2-related factor 2 (Nrf2) transcription factor (Figure 1.3 and 3.7) [293]. While several reports demonstrate that Nrf2 signaling can be induced by oxidative stress in neurons [120], it has been recently suggested that neuronal Nrf2 is epigenetically silenced [33], and that induction of the Nrf2 pathway does not seem to restore their antioxidant protection (Figure 1.5) [152]. Astrocytes also have higher levels of NADPH and G6PD (glucose-6-phosphate dehydrogenase) [105]. Interestingly, antioxidant genes in neurons are transcriptionally regulated independent of Nrf2 by synaptic activity through the activating transcription factor 4 (ATF4) and the activator protein 1 (AP-1) (Figure 1.5) [29, 189]. Both neurons and astrocytes can synthesize GSH, but neurons depend on the supply of GSH precursors (Figure 1.3 and 1.5). GSH is released from astrocytes via the ATP-binding cassette transporters subfamily C member 1 transporter (ABCC1, or multidrug-resistance-associated protein 1 [MRP1]) [133]. Extracellular GSH is then degraded by the γ-glutamyl transpeptidase (γGT) to produce l-cysteine-l-glycine (CysGly), which is cleaved further by the neuronal aminopeptidase N (ApN) into the amino acids glycine and cysteine that are taken up by neurons for GSH synthesis (Figure 1.5) [19, 32]. Thus, both the glutamate-glutamine cycle and the GSH export are also involved in the regulation of the neuronal redox environment by astrocytes.
During inflammation, changes in central carbon metabolism have been recently demonstrated to contribute to the activation and survival of astrocytes and microglia. Activation of astrocytes increases the generation of ROS, but themselves are largely resistant to these high ROS levels. Interestingly, glucose deprivation impairs GSH metabolism in astrocytes and their survival upon activation [65]. Inflammation also alters mitochondrial dynamics by favoring mitochondrial fission over fusion in astrocytes via autophagy [238]. Upon injury, exposure to pro-inflammatory signals, or chemicals/xenobiotics microglia become polarized to a pro-inflammatory M1 phenotype triggering the production of pro-inflammatory cytokines and the upregulation of ROS production. The M1 phenotype of microglia is considered an adaptive immune response that was recently reported to be paralleled by a switch in their metabolism from oxidative phosphorylation to glycolysis that also enhances carbon flux to the PPP (Figure 1.6) [110, 249, 329]. An alternative activation state defined as M2 (with different subclasses) is characterized by the release of anti-inflammatory factors to re-establish cell homeostasis. Interestingly, M2 upon activation of microglia with interleukin (IL)-4/IL13, no changes in mitochondrial oxygen consumption or lactate production were evidenced by extracellular flux analysis [249]. A comparative study demonstrated that astrocytes are more resistant to oxidative damage than microglia or oligodendrocytes [135].
3. Parkinson’s Disease
PD is the second most common neurodegenerative disorder affecting ~6 million people worldwide. PD is characterized by the progressive and selective loss of the dopaminergic (A9) neurons in the SNpc that conveys motor dysfunction. Although neuronal cell death is a cardinal feature of PD, the mechanisms and pathways involved remain unclear, mainly because in the majority of cases the cause of PD is unknown. The major risk factor identified for PD is aging as its prevalence and incidence increases exponentially from ages 65 to 90. A fraction of PD occurrence is also directly related to mutations in genes such as those encoding α-synuclein (SNCA), DJ-1 (PARK7), PTEN-induced putative kinase 1 (PINK1), leucine rich repeat kinase 2 (LRRK2) and Parkin (PARK2), while other genetic modifications only increase the risk of developing this condition. Over 90% of PD occurs in a sporadic (idiopathic) form from which only 5% of those cases are linked to de novo genetic alterations (SNCA and LRRK2) [165]. Thus, PD occurs most commonly without a clearly defined genetic basis. While the etiology of PD has not been clearly established, epidemiological data suggests that exposure to environmental / occupational agents might play a key role in this disorder [112]. Thus, it is now thought that PD arises as a syndrome from the convergence of genetic susceptibility, environmental exposures, and aging. At the molecular level, PD risk factors have been shown to trigger neurodegeneration by mitochondrial dysfunction, oxidative stress, and abnormal protein accumulation [188, 347], but the exact mechanisms are still unclear.
In addition to neurons, the function of other brain cells is altered in PD, which is also reported to contribute to the degeneration of dopaminergic neurons. Since astrocytes contribute to both energy and antioxidant homeostasis in neurons it is likely that dopaminergic neurons are more susceptible to oxidative stress and energy failure due to the low number of astrocytes in the SNpc compared to other brain areas. [228]. Neurotrophic factors released by astrocytes such as the mesencephalic astrocyte-derived neurotrophic factor (MANF), the glial cell line-derived neurotrophic factor (GDNF), and the brain-derived neurotrophic factor (BDNF) regulate neuronal bioenergetics (Figure 2.1), and are also thought to contribute to the survival of dopaminergic neurons [213]. While astrocytes normally serve a neuroprotective role, reactive astrocytes are found in PD brains and are considered to contribute to neuroinflammatory processes [57, 330].
The density of microglial cells is higher in the SN compared to other brain regions. Increased levels of activated microglia and pro-inflammatory cytokines (IL-1β, tumor necrosis-factor α [TNFα], IL-2 and IL-6) have been found in the SNpc of elderly individuals. Furthermore, activation of microglia in PD brains has been proposed to be triggered by aggregates / fibrils of α-synuclein, environmental toxicants, infectious events, and ATP and/or signaling proteins released from neurons (Figure 1.6) [163, 200, 274, 359]. PD-related genes PARK7, PINK1 and LRRK2 have also been reported to modulate mitochondrial dysfunction, oxidative stress and the inflammatory response of glial cells [63, 234, 239, 240, 280]. Besides microglia, infiltrating cells such as T-lymphocytes, and neutrophiles have been reported to contribute to inflammation in neurodegenerative disorders including PD [115, 147, 276]. For example, pathogenic T-cell populations potentiate microglial activation [41, 275]. Importantly, peripheral cells such as lymphocytes and neutrophiles from PD patients observe mitochondrial dysfunction and increased oxidative stress [18, 55, 107, 263, 264, 326], but whether ROS generation from infiltrating cells contributes to PD has not been explored. A disruption in the BBB has also been linked to aging and PD, which might involve dysfunction in both endothelial and astrocytes [45, 117]. Breakdown of the BBB leads to a dysregulation in ionic transport and xenobiotic/toxin clearance, and the initiation of an inflammatory response that contributes to neuronal dysfunction and neurodegeneration [247].
3.1. Mitochondrial Dysfunction in Parkinson’s Disease
3.1.1. Genetics and Complex I Dysfunction
Mitochondrial dysfunction is a common trait for many human disorders including neurodegeneration. The central role of mitochondrial dysfunction was recognized very early in the PD field. A decrease in the activity of the mitochondrial electron transport chain (ETC), primarily Complex I, is found in the SNpc of patients with PD (Figure 2.9 and 3.1a) [129, 160, 283, 285]. Mitochondrial DNA (mtDNA) mutations have also been discovered in rare families exhibiting parkinsonism. Somatic (acquired) point mutations and deletions in mtDNA also accumulate with age in the SNpc, but no specific mutations responsible for mitochondrial dysfunction in PD have been identified [70]. Mutations in polymerase gamma (POLG) are linked to mitochondrial defects in Complex I and IV acquired in PD [271]. Accordingly, a high frequency of mitochondrial Complex I mutations was found in PD patients [298]. Gene variations in the mitochondrial transcription factor A (TFAM) that controls mtDNA have also been associated with PD [10].
Mitochondrial dysfunction in PD has been linked to oxidative stress, as electron leakage from its metabolism is the primary source for ROS formation (Figure 3.1a). PD-associated genes have been shown to alter mitochondrial function and homeostasis leading to increased ROS formation [317]. Accumulation of α-synuclein has been suggested to trigger mitochondrial oxidative stress [83]. DJ-1, PINK1 and Parkin deficiency or mutations impair mitochondrial respiration and render cells more susceptible to oxidative stress and mitochondrial dysfunction (Figure 2.9 and 4) [4, 13, 149, 236, 254, 310]. Phosphorylation of the Complex I subunit NdufA10 by PINK1 is necessary for ubiquinone reduction (Figure 2.9) [235]. Gain-of-function of LRRK2 mutations disturb mitochondrial bioenergetics and mitochondrial dynamics leading to increased ROS formation [246, 256]. In the mitochondria, O2•− is generated in the matrix by electron leakage from Complex I [118], and in both the matrix and the inner membrane space (IMS) by electron leakage from Complex III (Figure 3.1a) [58]. In vitro and in vivo, dopaminergic neurons are highly sensitive to Complex I inhibitors whose toxicity is ascribed, at least partially, to the generation of ROS [27, 173, 216, 278, 343]. Other environmental agents linked to PD or dopaminergic toxins have also been shown to primarily mediate dopaminergic cell death via mitochondrial dysfunction [50, 111, 278].
Figure 4. Mitochondrial dynamics and biogenesis are coupled to oxidative stress and energy failure.
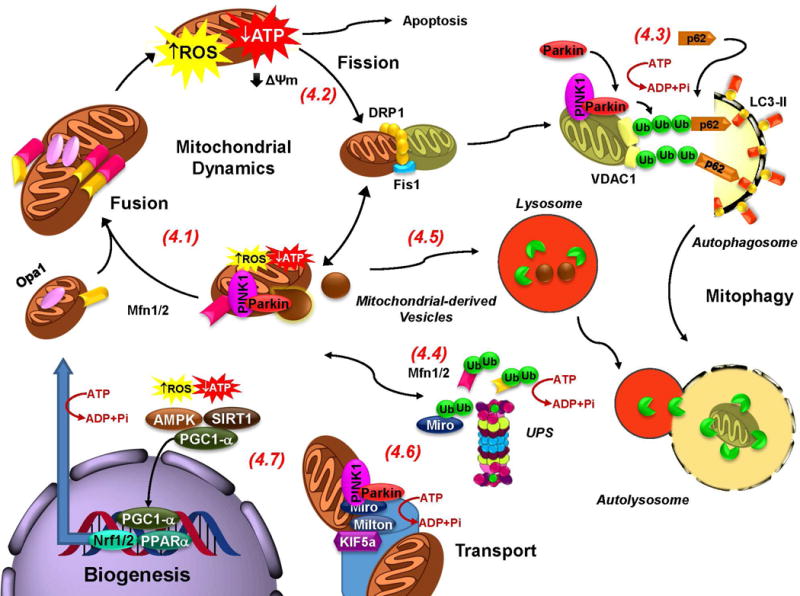
4.1. Mitochondrial fusion rescues “moderately” dysfunctional mitochondria by enabling their “damaged” content to be mixed between neighboring mitochondria. Fusion is impaired by the ubiquitination of Mfns by PINK1 and Parkin (See 4.4). 4.2. Fission, transforms damaged mitochondria into a form suitable for engulfment by mitophagy, and it has been suggested to facilitate transport of mitochondria into terminals (See 4.6). 4.3. Parkin translocates to the mitochondria and interacts with PINK1 in response to a loss in membrane potential (ΔΨm) induced by energy failure or oxidative damage. VDAC1 is polyubiquitinated and recruits p62, which in turn interacts with LC3 at the autophagosomal membrane. Finally, engulfed damaged mitochondria are degraded after the autophagosome fuses with the lysosome and lysosomal hydrolases are released within the fused autolysosome. 4.5. It has also been proposed that Parkin and PINK1 induce the formation of mitochondrial derived vesicles that translocate damaged mitochondrial proteins into lysosomes. 4.6. Transportation of mitochondria to axonal and dendritic terminations is essential to meet energy demands associated with synaptic transmission. PINK1 and Parkin mediate phosphorylation, ubiquitination and degradation of Miro leading to the detachment of mitochondria from microtubules. 4.7. Oxidative stress and energy failure activate PGC-1α to stimulate mitochondrial biogenesis. Energy failure in PD is expected to impair transport of mitochondria into terminals, and the mitochondrial quality control mechanisms mitophagy and UPS. 4.4. The proteasome has also been proposed to translocate to the mitochondria and mediate the degradation of damaged proteins. (ATP consumption is highlighted in red).
3.1.2. Bioenergetics
While mitochondrial dysfunction in PD is thought to mediate the increased generation of ROS and subsequent oxidative damage, another major consequence is energy failure linked to the inability of neurons to compensate their lack of capacity to generate ATP. Very few studies have aimed at understanding and distinguishing between the contribution of both oxidative stress and energy failure in dopaminergic cell death associated with mitochondrial dysfunction. A number of studies have shown that antioxidants protect dopaminergic cells from the toxicity of Complex I inhibitors [26, 159, 167, 191, 266]. However, we and others have clearly demonstrated that dopaminergic cell death induced by Complex I inhibition cannot be directly linked to the generation of ROS or completely prevented (if at all) by antioxidants [47, 49, 100, 171, 178, 217, 241, 278, 282, 355]. This might explain the failure of antioxidants (particularly mitochondria targeted antioxidants) in PD therapy [1, 300], despite their reported success in preclinical models [108, 302]. Furthermore, some studies suggest that impaired mitochondrial bioenergetics might be the main cause for neuronal cell death induced by Complex I inhibitors rotenone and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP, or its active analog 1-methyl-4-phenylpyridinium [MPP+]) [27, 86, 343]. Supplementation of cells with Coenzyme Q10 (ubiquinone), an electron carrier in the ETC, protects against the toxicity of Complex I inhibitors that block the electron flow from NADH dehydrogenase to Coenzyme Q [2]. Similarly, directly delivery of electrons from NADH to cytochrome c using methylene blue attenuates dopaminergic neurodegeneration induced by rotenone [345]. Importantly, because a 50% inhibition of Complex I is required to cause significant ATP depletion, and Complex I activity is only reduced by 25–30% in PD, ATP depletion might as well not be the single causative event for dopaminergic neurodegeneration [77, 284].
Dopaminergic SNpc neurons are particularly susceptible to energy failure since they consume a significant amount of energy during their pacemaking activity to maintain a basal dopamine (DA) tone in innervated regions (striatum). This level of activity and energy consumption also leads to increased levels of basal oxidative stress [121]. In addition, their extended (and probably unmyelinated) axon, and the number of striatal synapses established are characteristics expected to exert a high energy demand on the maintenance of plasma membrane potential (ionic gradients), protein/organelle (mitochondria) traffic and homeostasis [218, 253, 262]. Importantly, the intrinsic bioenergetic capacity between dopaminergic and non-dopaminergic presynaptic synaptosomes do not differ [66], suggesting that other factors besides energy dysfunction contribute to the loss of dopaminergic cells. Energy failure in dopaminergic cells would be expected to siphon energy from other important ATP-dependent homeostatic processes in an attempt to maintain their excitability (including active uptake of neurotransmitters into vesicles) and function. These processes include: 1) protein quality control mechanisms (autophagy and the UPS) whose failure leads to the accumulation of misfolded /oxidized protein aggregates (Figure 1.2) [73]; 2) transport of mitochondria to pre-synaptic and post-synaptic sites (kinesin motors require ATP) (Figure 4.6) [68]; and 3) mitochondrial biogenesis and dynamics (fusion, fission and mitophagy) (Figure 1.2 and 4)[221]. In addition, energy depletion is expected to impair the ATP-dependent proton pump that drives vesicular accumulation of DA augmenting its cytosolic levels and oxidative stress as a consequence (Figure 1.2) [17, 203, 204]. Glutamatergic afferents in the SNpc suggest that energy impairment in astrocytes and neurons might also contribute to dopaminergic cell death due to an impaired uptake of extracellular glutamate that leads to excitotoxic cell death, but this hypothesis requires further experimental support [12, 224, 343].
While neurons have a limited capability to upregulate glycolysis, an increase in glycolysis counteracts energy failure and dopaminergic cell death induced by mitochondrial dysfunction.[54, 136, 174]. Alternative energy sources have also been demonstrated to protect against PD neurodegeneration. Under energy deprivation, ADP phosphorylation by creatine kinase (CK) to ATP requires phosphocreatine for the donation of the phosphoryl group. The phosphocreatine pool is maintained / regenerated from creatine and ATP under normal conditions by both the cytosolic and the mitochondrial forms of creatine kinase. Creatine supplementation has been shown to protect against MPTP toxicity [16, 219]. However, in clinical trials creatine supplementation does not slow down the progression of PD [161]. Conversely, the administration of ketone bodies (D-beta-hydroxybutyrate) bypasses the Complex I blockade by MPTP and reduce dopaminergic neurodegeneration by entering the mitochondrial ETC directly at Complex II. [314]. Accordingly, a ketogenic diet has been reported to exert protective effects in PD [323].
3.1.3. Mitochondrial dynamics
Mitochondrial dysfunction in PD has also been linked to the indirect alteration of mitochondrial dynamics (fusion and fission, turnover, biogenesis, and transport) that is involved in the regulation of mitochondrial health, mass and subcellular location in response to stress (Figure 4) [94, 322]. We will next discuss these processes in the context of how they regulate both bioenergetics and redox homeostasis in PD.
Mitochondria are dynamic organelles that undergo continuous events of biogenesis, remodeling and turnover. Fusion and fission are opposing processes working in concert to maintain the shape, size, number of mitochondria and their physiological function. Fusion enables content to be mixed between neighboring mitochondria and has been proposed to rescue “moderately” dysfunctional mitochondria. Fusion is mediated by the initial oligomerization of dynamin GTPases mitofusins 1 and 2 (Mfns) at the outer membrane to tether adjacent mitochondria together, and the subsequent fusion of the inner membranes by the optic atrophy GTPase Opa1 (Figure 4.1). Fission represents a quality control mechanism to transform damaged elongated mitochondria into a form suitable for engulfment by mitochondrial autophagy or mitophagy, and has also been proposed to modulate mitochondrial axonal transport. Fission requires recruitment of the GTPase Drp1 (dynamin-related protein 1) via mitochondrial surface receptors (mitochondrial fission 1 protein [Fis1], mitochondrial fission factor [Mff] and mitochondrial dynamics proteins 49 and 51 [MiD49, MiD51]) for the assembly of the fission machinery subsequently leading to membrane scission (Figure 4.2) [259]. Complex I inhibition promotes Drp1-mediated fission and mitophagy. Conversely, inhibition of fission protects against dopaminergic cell death induced by Complex I inhibitors [322]. Importantly, recent evidence indicates that alterations in mitochondrial dynamics by PINK1 deficiency are a result of impaired bioenergetics [325]. Mitophagy has been shown to be regulated by PINK1 and the translocation of Parkin from the cytoplasm to “defective” mitochondria (Figure 4.3). Fusion arrest by polyubiquitination and proteasomal degradation of mitofusins (Mfn1 and Mfn2) by Parkin is required for mitophagy (Figure 4.4). On the other hand, polyubiquitination of the voltage-dependent anion channel 1 (VDAC1) by Parkin recruits the ubiquitin binding protein p62 that also interacts with the autophagosomal protein LC3/GABARAP (Figure 4.3). Loss of the E3-ubiquitin ligase Parkin and PINK1 has been shown to promote Drp1-dependent fission, loss of mitochondrial membrane potential, and energy failure [207]. Contradictory results exist regarding the role of Parkin on promoting proteasomal degradation of the fission promoting proteins Drp1 and Fis1, but if corroborated, they would suggest a complex scenario where Parkin can regulate both mitochondrial fusion and fission events depending on the degree of mitochondrial stress [94]. Importantly, recent reports have demonstrated that only prolonged (chronic) mitochondrial depolarization induces Parkin translocation or mitophagy in neurons (Figure 4.2) [180, 321]. Furthermore, mitophagy induced by dysfunction in mitochondrial respiration is independent from Parkin in dopaminergic neurons [305]. These results suggest that disruption in mitophagy is not the primary cause for PD linked to Parkin mutations. Supportive of this hypothesis is the observation that Parkin and PINK1 have also been demonstrated to mediate the formation of mitochondria-derived vesicles for the degradation of oxidized and damaged proteins via the lysosome (Figure 4.5) [223].
Other PD related genes also affect mitochondrial dynamics. α-synuclein has also been shown to impair mitochondrial fusion, which seems to precede mitochondrial dysfunction [242], while the A53T mutant was reported to stimulate mitophagy [67]. Loss of DJ-1 function promotes mitochondrial fission and mitophagy, but mitochondrial alterations are prevented by PINK1 and parkin [141, 313]. A pathogenic increase in the activity of LRRK2 also promotes fission [333]. Activation of AMPK by starvation promotes mitophagy, but its role in PD has not been clearly elucidated [92].
Mitochondria are actively transported into axonal and dedritic terminations due to the high energy requirements for synaptic neurotransmission (Figure 4.6). Notably, dopaminergic axons have reduced mitochondrial numbers compared to other synaptic terminals. Complex I inhibitors and PD-related genes impair mitochondrial transport, which leads to energy depletion in synaptic terminals and dopaminergic axons that already have reduced mitochondrial numbers compared to other terminals [162, 190]. PINK1 and Parkin also regulate mitochondrial movement via phosphorylation, ubiquitination and degradation of Miro that disrupts its interaction with the adapter protein Milton and the kinesin motor protein KIF5a. The loss of this interaction between Miro and Milton/KIF5a leads to the detachment of mitochondria from microtubules (Figure 4.6) [199, 332]. Finally, Parkin has also been shown to induce the proteasomal degradation of the parkin-interacting substrate (PARIS), a transcriptional repressor of the peroxisome proliferator-activated receptor gamma-co-activator 1-alpha (PGC-1α), which stimulates mitochondrial biogenesis (Figure 4.7) [294].
Similar to neurons, alterations in mitochondrial function and quality control are also expected to occur in glial cells affected by PD risk factors. However, other than its involvement in the activation of inflammatory processes and increased ROS formation (mentioned in the next section), the pathological consequences that glial mitochondrial dysfunction has in PD is unclear. Astrocytes have the same oxidative capacity as neurons, but their energy requirements are met through glycolysis, and they are resistant to mitochondrial toxins. The physiological importance of mitochondrial oxidative metabolism in glial cells is still obscure. Astrocytes derived from Parkin knockout and transgenic mutant α-synuclein exhibit mitochondrial functional and morphological defects, which translate into the impairment of their neurotrophic effects in neuronal differentiation [286]. DJ-1 knockout in astrocytes alters mitochondrial function and impairs neuroprotection against Complex I inhibition [175]. There is no evidence that glial cells degenerate (or die) in PD. Interestingly, impaired astrocyte proliferation (astrogliosis) has been reported in PD brains and this phenomenon correlates inversely with α-synuclein accumulation [315]. Accordingly, PINK1 knockout astrocytes exhibit defective proliferative signals, which were linked to alterations mitochondrial metabolism [63]. Recently, damaged mitochondria have been reported to be transferred from neuronal axons for their turnover in astrocytes [78], and conversely, astrocytes have been shown to transfer mitochondria to promote neuronal survival [127]. Thus, it is plausible that when transferred, defective mitochondria from astrocytes can have deleterious effects in neuronal function during PD.
3.2. Oxidative Stress and Alterations in Redox Metabolism
3.2.1. Generation of Reactive Oxygen Species and Oxidative Damage
In addition to the role of mitochondria as the primary source for O2•−, other important alterations in redox balance contribute to dopaminergic dysfunction. Several other organelles are important sources for ROS as well, and under certain circumstances, might actually play a bigger role than mitochondria in ROS generation [42]. ROS are produced in the endoplasmic reticulum (ER) as a consequence of the activity of oxidoreductases that catalyze disulfide bond formation in nascent proteins. Protein disulfide isomerase (PDI) accepts electrons from cysteine thiol residues leading to the formation of protein disulfide bonds. ERO1 (ER oxidoreduction) transfers electrons from PDI to O2, which results in the production of H2O2 (hydrogen peroxide) [311]. ER stress and the activation of the unfolded protein response (UPR) have been reported in PD; however, its causative role in oxidative damage has not been established [230].
Another important source of O2•− is the NOX family of enzymes that catalyze the production of O2•− from O2 and NADPH (Figure 3.1b) [139]. Distinct isoforms of NOX enzymes have been reported in different brain regions, but their activation appears to be primarily restricted to glial cells as a response to environmental exposures or inflammatory agents [103]. NOX-derived ROS from glial cells has been shown to contribute to oxidative damage and dopaminergic neurodegeneration (Figure 1.6) [139, 309]. Nitric oxide (•NO) is generated from L-arginine by nitric oxide synthases (NOS) which also requires reducing equivalents from NADPH [233]. When O2•− reacts with •NO it generates peroxynitrite (ONOO−) (Figure 3.1a) [82]. Inducible NOS (iNOS) is also activated by mitochondrial toxins and inflammatory events in microglia at the substantia nigra to promote neurodegeneration (Figure 1.6) [20, 194]. Neuronal NOS (nNOS) also contributes to dopaminergic cell death induced by MPTP [124, 265]. In addition, NOS uncoupling by the PD-related pesticide paraquat has also been recognized as an important source for O2•− [206, 212].
O2•− can be dismutated in via enzymatic (via O2•− dismutases [SODs]) or non-enzymatic reactions to H2O2 (Figure 3.1a and 3.1b). SOD activity was found to be increased in post-mortem PD brains [51, 281]. Metals such as iron (Fe) can also promote ROS formation via the Fenton/Haber-Weiss reaction that catalyzes the formation of hydroxyl radical (•OH) by their reaction with H2O2 (Figure 3.2). In the brain, Fe is most abundant in SNpc dopaminergic neurons. Increased Fe deposition and increased free Fe concentrations have been found in the SNpc of PD brains, which may lead to increased generation of •OH [295]. In PD, Fe is deposited abundantly in the neuromelanin (NM) granules of SNpc dopaminergic neurons [295]. Fe deposition in PD might be related to alterations in Fe transport and storage systems [90, 134, 154, 177]. •OH formation by Fe has also been proposed to involve α-synuclein (Figure 3.2) [61, 214, 251, 320]. Because of their high Fe content, lysosomes are another important potential source of ROS [170], and lysosomal breakdown has also been linked to PD pathogenesis [81]. Finally, disruption of Fe-Sulfur (S) clusters by oxidative stress induced by paraquat or PINK1 mutants has also been proposed to enhance oxidative damage (Figure 3.2) [46, 93].
Oxidative stress in PD is also associated with the pro-oxidant properties of dopamine. Mutant α-synuclein down-regulates the vesicular monoamine transporter (VMAT2) [203, 204]. In the cytosol, dopamine is either metabolized by monoamine oxidase (MAO) to generate H2O2, or auto-oxidized in the presence of Fe generating O2•−, H2O2 and dopamine-quinone species (DAQ) (Figure 3.5) [3]. Dopaminergic cell death induced by mitochondrial toxins has been proposed to depend on dopamine oxidation [158, 197]. DAQ byproducts from DA oxidation have been reported to react with protein cysteine thiols [126], and with glutathione (GSH) to form adducts (GS-DAQ) by the activity of GSH-S-transferases (GSTs) [75, 291]. GSTs are also involved in the detoxification of GSH adducts with byproducts of lipid peroxidation such as 4-hydroxynonenal (4-HNE) which has been shown to generate toxic protein adducts in PD (Figure 3.4b) [352].
3.2.2. Antioxidant Defenses
Oxidative stress is counteracted by cellular antioxidant mechanisms. A number of scavenging mechanisms exist against H2O2. Catalase mediates the decomposition of H2O2 and is primarily localized in the peroxisomes (Figure 3.3). The activity of catalase was reported to be reduced in the SN and putamen of PD brains [11]. Peroxiredoxins (Prxs) are thiol peroxidases primarily seen as regulators of peroxide signaling [74]. Except for Prx6, which depends on the reducing power of GSH, thioredoxins (Trx) provide the reducing equivalents required for the peroxide scavenging activity of Prxs. The Trx redox system itself depends on thiol-disulfide exchange reactions at the active site, and Trx reductase (TrxR) transfers reducing equivalents from NADPH to reduce Trxs (Figure 3.3). Overexpression of Prx1, Prx2 and Prx4 protects against 6-hydroxydopamine (6-OHDA)-induced dopaminergic cell death [137, 184], whereas silencing mitochondrial Prx3 and Prx5 increases sensitivity to MPP+ [80]. The Trx/TrxR system also protects against MPP+- 6-OHDA- and paraquat-induced toxicity in dopaminergic cells [21, 23, 201, 346].
A decrease in the levels of GSH is one of the earliest biochemical alterations associated with PD as demonstrated by the observation that GSH loss occurs in incidental Lewy body disease, which is considered an asymptomatic precursor to PD [146, 260, 296]. Accordingly, inducible depletion of GSH promotes nigrostriatal degeneration in mouse [62]. GSH peroxidases (GPx) and Prx6 use the reducing power of GSH to hydrolyze peroxides generating GSH disulfide (GSSG) as a byproduct (Figure 3.4a). Mice deficient in GPx1 exhibit an increased sensitivity to MPTP toxicity [166], while its overexpression protects against MPP+- and 6-OHDA-induced toxicity in vitro and in vivo [35, 157, 277, 312]. GSH-dependent Prx6 was recently shown to exacerbate dopaminergic neurodegeneration induced by MPTP, but this effect was linked to its calcium-independent phospholipase A2 activity and increased 4-HNE levels [354]. GSH reductase (GR), which recycles GSSG back to GSH, requires NADPH as the electron donor reluctant, and the pentose phosphate pathway enzyme glucose-6-phosphate dehydrogenase (G6PD), is indispensable for the regeneration of NADPH from NADP+ (Figure 2.5 and 3.4a). Downregulation of PPP enzymes and a failure to increase the antioxidant reserve capacity are early events in the pathogenesis of sporadic PD [89]. Transgenic mice overexpressing G6PD in dopaminergic cells are resistant to MPTP-induced toxicity [227]. Impairment in the PPP leads to the depletion of NADPH and dysfunction in the GSH/Grx, Prx/Trx/TrxR and catalase systems, as well as the decrease in purine synthesis via ribose 5-phoshate [349]. We have recently demonstrated that the PD-related pesticide paraquat hijacks the NADPH from the PPP for its redox cycling, promoting oxidative stress and a toxic synergism when combined with α-synuclein overexpression [14, 186]. NOX and NO-mediated formation of ROS and reactive nitrogen species is also expected to be directly associated with NADPH levels. In contrast G6PD and IDH1 exert a protective effect against the toxicity of MPTP/MPP+ [227, 344]. These observations might explain why there is no generalized association between changes in G6PD activity and PD [104].
Glial cells also regulate antioxidant defenses in neuronal cells. Recently, the role of astrocytes in the protection of neurons against oxidative stress was elegantly demonstrated. Conditional depletion of astrocytes was observed to promote neuronal injury by oxidative stress [289]. Accordingly, transcriptional regulation of antioxidant systems via the nuclear factor (erythroid-derived 2)-like 2 (Nrf-2) in astrocytes prevents dopaminergic cell death induced by MPTP [56].
3.2.3. Bioenergetics and Redox Balance
Alterations in the bioenergetics of dopaminergic cells also have an impact on antioxidant systems. Energy is required for a number of antioxidant systems to work. GSH (l-γ-glutamyl-L-cysteinyl-glycine) synthesis is initiated by generation of γ-glutamylcysteine from glutamate and cysteine via the glutamate-cysteine ligase (GCL), and the subsequent addition of glycine by the activity of GSH synthetase (GSS) (Figure 1.3 and 3.4b) [101, 226, 297]. Both reactions requiring ATP consumption. The transsulfuration pathway (Figure 2.11) contributes to the generation of cysteine, the rate limiting substrate for GSH synthesis, from one carbon metabolism of serine → cystathionine → cysteine. Interestingly, high levels of homocysteine, a co-substrate with serine for cystathionine formation, have been found in PD brains [36]. Both glycine and glutamate are also byproducts of central carbon metabolism (glycolysis or TCA cycle, respectively) (Figure 2.10 and 2.11). However, the contribution of their biosynthetic pathways to redox balance in neuro-glial cells is unclear. Efflux of GSH-adducts (including those of DA and 4-HNE) is ATP-dependent as well. Thus, GSH-dependent antioxidant systems and detoxification pathways are expected to be impaired by energy failure associated with mitochondrial dysfunction (Figure 3.4b) [72]. The reduction of hyperoxidized Prxs (sulfenylated, SO2H) by sulfiredoxin (Srx) relies on the initial formation of a sulfinic phosphoryl ester (Cys-SPO2PO32−) between the Srx and Prx that requires ATP consumption (Figure 3.3) [38]. ATP is also required for the synthesis of NADP+ via NAD+ kinase (Figure 3.8). Transcriptional regulation of antioxidant defenses (SOD1 [CuZnSOD], SOD2 [MnSOD], the modifier and catalycic GCL subunits [GCLM and GCLC], and GSTs) via Nrf2 and conversely, the ubiquitination and degradation of the Nrf2-kelch-like ECH-associated protein 1 (Keap1) cytosolic complex also require energy (Figure 3.7) [155]. ATP depletion is also anticipated to impair oxidative DNA-damage repair systems (Figure 3.7) [76, 119, 307, 336]. GCLM knockout was shown to increase dopaminergic neurodegeneration induced by paraquat [192] and recently, it was also reported that GSH depletion in GCLM knockout astrocytes induces a depletion in glycogen levels demonstrating a link between redox balance and bioenergetics/central carbon metabolism [176].
3.3. Alterations in Central Carbon Metabolism
3.3.1. Glucose Metabolism and Glycolysis
In addition to the energy failure linked to mitochondrial dysfunction, alterations in central carbon metabolism have also been reported to occur in PD. A decrease in glucose metabolism and abnormal elevated levels of lactate / pyruvate have been observed in PD patients [5, 91, 130, 255]. Disruption of glycolysis in astrocytes and oligodendrocytes triggers axon damage and neurodegeneration [182, 328]. Interestingly, glucose deprivation promotes α-synuclein aggregation (Figure 1.2) [34]. In contrast, lactate has been reported to exert an opposite effect on α-synuclein [151]. We and others have demonstrated that glycolysis is upregulated in response to mitochondrial dysfunction, and that ATP generation via glycolysis exerts a protective role against Complex I inhibition [14, 22, 52–54, 220, 337, 351], As mentioned before, failure of neuronal cells to upregulate this pathway seems to make them rather sensitive to mitochondrial dysfunction [132]. Interestingly, dietary restriction and administration of 2-deoxy-d-glucose exerted protective effects against MPTP toxicity [87]. We have recently found that paraquat inhibits glucose metabolism and upregulates carbon flux via the PPP to increase its redox cycling by hijacking reducing equivalents from NADPH [186]. This effect was paralleled by an impairment of NADPH-dependent antioxidant systems leading to GSH depletion and Prx hyperoxidation [278]. Inhibition of glycolysis by paraquat, was likely associated with the inhibition of phosphofructokinase (PFK) by citrate whose accumulation is linked to the inhibition of aconitase by PQ-derived ROS (Figure 2.2 and 2.8) [46, 106, 186, 243].
PD-related genes SNCA (α-synuclein), PARK2 (Parkin), PINK1 and PARK7 (DJ-1) have been reported to indirectly regulate glycolysis by modulation of different signaling proteins including p53, the hypoxia-inducible factor 1-alpha (HIF-1α) (Figure 2.10) and the adenosine monophosphate-activated protein kinase (AMPK) [272, 273, 292, 356]. Alterations in cellular energy are tightly monitored by the master regulator of metabolism AMPK [48]. We have recently demonstrated that AMPK signaling protects against paraquat-induced dopaminergic cell death and that this effect is independent from glucose metabolism and likely linked to the regulation of mitochondrial bioenergetics (Figure 4.7) [14]. Accordingly, a protective role for AMPK against mitochondrial dysfunction and toxicity induced by Parkin- LRRK2-mutations, α-synuclein and MPTP/MPP+ has also been previously reported [64, 88, 244]. In contrast, other reports have shown that AMPK mediates dopaminergic cell death induced by rotenone and 6-OHDA [164, 342]. Contradictory results have also been reported regarding the role of AMPK in α-synuclein aggregation and toxicity [88, 151]. AMPK is a central regulator of cellular metabolism via modulation of a myriad of processes. Thus, differences between the roles of AMPK in dopaminergic cell death induced by different PD-related insults might be related to distinct processes being regulated by AMPK, or to the diverse metabolic cues involved in cell death or survival.
Glycolysis has been recently demonstrated to be directly regulated by PD-genes. Glycolysis generates methylglyoxal (MGO) as a byproduct from the metabolism of glyceraldehyde 3-phosphate and dihydroxyacetone phosphate (DHAP) (Figure 2.4). MGO is a potent glycative agent that readily reacts with lipids, nucleic acids and protein lysines / arginines to form advanced glycation end products (AGEs). MGO also induces mitochondrial dysfunction and is detoxified by the glyoxalase system through the sequential activity of glyoxalase-1 (GLO1) and GLO2 using GSH as a co-factor (Figure 2.4) [8, 79, 303]. An increase in AGEs has been reported in PD [324]. MGO is actually a precursor for 1-acetyl-6, 7- dihydroxyl-1, 2, 3, 4-tetrahydroisoquinoline (ADTIQ), a dopamine (DA)-derived tetrahydroisoquinoline (TIQ) toxin that is also found in PD brain tissue [339]. GLO1 converts the intermediary hemithioacetal (spontaneous reaction of GSH with MGO) to lactoyl-GSH, which is then metabolized to D-lactate by GLO2 that also recycles GSH (Figure 2.4). The activity of GLOs is higher in astrocytes than neurons [8], and is inhibited by glutathionylation (mixed disulfide bond formation between a protein cysteine and GSH) [37]. MGO formation seems to occur primarily in astrocytes as neurons have low rates of glycolysis, are very sensitive to MGO and GLO1 inhibition, and when upregulated, the glyoxylase system fails to protect them against MGO [8]. These observations actually correlate with the higher expression of DJ-1 in astrocytes when compared to neurons [25], and the discovery that DJ-1 is a cofactor-independent GLO III system (Figure 2.4) [179]. In addition to contributing to the detoxification of MGO, DJ-1 has been proposed to metabolize MGO and glyoxal (a byproduct from fatty acid peroxidation) to generate D-lactate and glycolate, respectively, and that these metabolites contribute to the maintenance of mitochondrial function [316]. Parkin was also recently shown to regulate glucose metabolism via ubiquitination of pyruvate kinase M1 and PKM2 that results in a decrease in their enzymatic activity (Figure 2.3) [198].
An increased oxidation (4-HNE adduct formation) of the glycolytic enzymes glyceraldehydes 3-phosphate dehydrogenase (GAPDH), enolase 1 and aldolase A was found in the frontal cortex from patients with PD, and dementia with Lewy bodies (DLB) [114]. These glycolytic enzymes have been reported to be metastable and thus, prone to interact with and potentially become sequestered by amyloid-like structures such as α-synuclein fibrils (Figure 2.3) [248, 340]. GAPDH has been shown to directly regulate α-synuclein aggregation and apoptotic neuronal cell death, which appears to be independent from its role in glycolysis. Glucose phosphate isomerase 1 (GPI-1) was recently demonstrated to exert a protective effect against proteotoxic stress induced by α-synuclein in dopaminergic neurons and this effect was shown to be linked to glycolysis (Figure 2.2) [168]. We have observed that the mechanism by which α-synuclein enhances metabolic dysfunction induced by environmental toxicants is linked to an impairment of glycolysis [14]. Accordingly, other reports have reported a possible interaction between α-synuclein and glycolytic enzymes such as aldolase [153, 222]. Conversely, it has been previously reported that α-synuclein knockdown increases glycative stress [169].
3.3.2. Tricarboxylic Acid Cycle
A dysfunction in the tricarboxylic acid (TCA or Krebs) cycle has been reported in PD brains [5, 109]. In particular, a decrease in the activity of the 2-oxoglutarate (α-ketoglutarate) dehydrogenase (OGDH or KGDH) is found in PD brains (Figure 2.8) [109]. Oxidative inactivation of aconitase has been shown to be induced by Complex I inhibitors, paraquat and PINK1 mutations [46, 93, 157, 193, 229]. It has been demonstrated that inactivation of aconitase by oxidative stress can be compensated by OGDH, which allows glutamate to fuel the TCA cycle. This likely occurs because aconitase is more sensitive to oxidative damage than OGDH [318]. In contrast, oxidative stress induced by MAO was reported to preferentially target Complex I and OGDH (Figure 2.8) [210].
3.3.3. Nicotinamide Adenine Dinucleotide Metabolism
Nicotinamide adenine dinucleotide (NAD+) participates in many biological reactions involved in energy metabolism, DNA repair and transcription. NAD+ is required for the oxidation of glyceraldehyde 3-phosphate to 1,3-biphosphoglycerate by glyceraldehyde 3-phosphate dehydrogenase (GAPDH) in glycolysis (Figure 2.3). In the TCA cycle, NAD+ is a substrate for: 1) the oxidative decarboxylation of isocitrate to oxoglutarate (α-ketoglutarate) by IDH3; 2) pyruvate / oxoglutarate decarboxilation to thiamine phyrophosphate by pyruvate / oxoglutarate dehydrogenase (PDH / OGDH) that is required for the formation of acetyl / succinyl coenzyme A (CoA); and 3) the oxidation of malate to oxalacetate (Figure 2.8). NAD+ is also used to transfer reducing equivalents from NADH to NADP+ regenerating NADPH by NNT (Figure 2.7). The resultant reduced NADH is reoxidized in the cytosol by either the activity of lactate dehydrogenase (LDH) (Figure 2.3) or glycerol 3-phosphate dehydrogenase (GPDH), and by Complex I in the mitochondria (Figure 2.9). NAD+ is also consumed by poly (ADP-ribose) polymerase-1 (PARP-1) during DNA-damage repair (Figure 2.5). Contradictory reports exist regarding the role of PARP-1 genetic variants in PD [40, 140], but a number of studies have suggested that PARP-1 activation upon PD-related insults leads to energy depletion associated to the consumption of ATP by the NAD+ salvage pathway, and subsequent neuronal cell loss with necrotic characteristics (Parthanatos) [143, 144, 164, 181, 185, 195, 211, 252, 338, 350]. Parthanatos might be a secondary or complementary cell death mechanism to apoptosis in PD [334]. NAD+ siphoning by PARP-1 can also have an impact on glycolysis, but this possibility has not been explored. In contrast, it has been demonstrated that PARP-1 activity has a protective role in neuronal cell death under mild and chronic oxidative stress conditions due to its role in oxidative-DNA damage repair [84]. Accordingly, we have observed that inhibition of PARP-1 enhances dopaminergic cell death induced by paraquat, its synergism with α-synuclein, and 6-OHDA toxicity as well (unpublished results). Ribose 5-phosphate from the PPP is also used for ADP-ribose synthesis (Figure 2.5). Thus a link between carbon flux through the PPP and DNA damage repair by PARP-1 in PD-related oxidative damage is also plausible.
Acetyl-CoA, succinyl-CoA and malonyl-CoA are used as substrates for post-translational Lys acylation (acetylation, succinylation and malonylation). Sirtuins (SIRT) deacetylase proteins by transferring the acetyl group using NAD+ as a co-substrate (Figure 2.6 and 2.7). Sirtuins regulate gene transcription by histone deacetylation, but proteins other than histones are also targeted by sirtuins [250]. Deacetylation of mitochondrial proteins within the ETC, the TCA cycle, and pyruvate metabolism by SIRT3 regulates metabolic function (Figure 2.8) [128, 269]. SIRT3 also regulates mitochondrial dynamics and biogenesis in response to oxidative stress via the forkhead transcription factor FOXO3 [319]. Knockout of mitochondrial SIRT3 increases the acetylation of multiple Complex I components and reduces its activity and energy production [6]. Similarly, IDH2 and MnSOD activity is enhanced by deacetylation via SIRT3 [60, 353]. NAD+ consumption by SIRT1 is contended by PARP-1 activity demonstrating the metabolic competitiveness for NAD+ consumption [24]. SIRT5 has NAD+-dependent desuccinylation and demalonylation activity and also regulates glycolysis, mitochondrial metabolism, ATP synthesis and respiration [43, 245, 268]. SIRT3 exerts a protective effect against rotenone induced toxicity in vitro, but other reliable evidence regarding the role of sirtuins in PD does not currently exist [357].
NAD+ consumption by PARPs and sirtuins generates nicotinamide (NAM), which is recycled back to NAD+ by the salvage pathway. First, nicotinamide phosphoribosyltransferase (NAMPT) catalyzes the condensation of nicotinamide with 5-phosphoribosyl-1-pyrophosphate to generate nicotinamide mononucleotide (NMN). Then, nicotinamide mononucleotide adenylyltransferase (NMNAT) catalyzes the condensation of NMN or nicotinic acid mononucleotide (NaMN), the precursor of NAD+ de novo synthesis, to form NAD+ or nicotinic acid mononucleotide (NaAD), respectively, while consuming ATP. NaAD requires further amidation to generate NAD+ [116]. Nicotinamide and NMN supplementation restores ATP, mitochondrial function and protects against 6-OHDA- MPP+- or rotenone-induced toxicity [148, 205, 361]. We have observed that while PARP-1 activity protects against paraquat toxicity in dopaminergic cells, targeted overexpression of NAMPT to the cytosol or mitochondria fails to do so (unpublished data), but the effect of NAD+ precursors has not been evaluated yet.
NAD+ is also a substrate for aldehyde dehydrogenases (ALDH). Cytosolic ALDH1A1 has been reported to be specifically located in dopaminergic neurons in the SNpc and to be involved in the metabolism of 3,4-dihydroxyphenylacetaldehyde (DOPAL), a toxic oxidative by product from dopamine deamination, to 3,4-dihydroxyphenylacetic acid (DOPAC) (Figure 3.5). ALDH1A1 and the mitochondrial ALDH2 are also involved in the metabolism of 4-HNE (Figure 3.6) [358]. Lipid peroxidation byproducts and mitochondrial / environmental toxins inhibit ALDH activity [98, 99, 187, 270]. Importantly, the inhibition of ALDH activity by rotenone is directly associated with decreased NAD+ availability [113, 187, 232]. ALDH1A1 is downregulated in PD brains and ALDH1A1 deficiency is linked to an increase in α-synuclein induced toxicity [196]. Interestingly, inihibition or downregulation of ALDH activity potentiates the toxicity of rotenone but not MPP+ [172, 196]. Age-dependent loss of dopaminergic neurons in the substantia nigra is observed in mouse lacking both ALDH1A1 and ALDH2, but not ALDH1A1 alone [15, 335].
4. Conclusions and Perspectives
Mitochondrial dysfunction has been well recognized and established as a causative mediator of PD pathogenesis. Mitochondrial dysfunction has also been highlighted as the main reason behind energy failure and oxidative stress in dopaminergic cells leading to their progressive degeneration. However, up till now, therapeutic approaches aimed at either preventing (mitochondrial) ROS formation and oxidative damage, or supplementing energy “fuel” to the brain have for the most part, failed to stop or ameliorate PD progression. We and others have demonstrated that dopaminergic cell death associated with PD-risk factors (genes and environmental exposures) is mediated by a dysfunction in both bioenergetics and redox homeostasis coupled to alterations in central carbon metabolism. In this review, we have aimed to provide an integrated view between bioenergetics, redox homeostasis, and central carbon metabolism and their potential role in dopaminergic degeneration (and glial dysfunction) in PD. Taken together these findings strengthen the idea that PD is a complex metabolic disorder that cannot be simplistically and independently explained by either oxidative stress or energy failure.
Highlights.
Mitochondrial dysfunction in PD triggers oxidative stress and energy failure
We provide an integrated view of bioenergetics, redox and central carbon metabolism in PD
Clinical trials demonstrate that PD cannot be explained simply by oxidative stress and energy failure
These findings strengthen the idea that PD is a complex metabolic disorder
Acknowledgments
This work was supported by the National Institutes of Health Grants P20RR17675 Centers of Biomedical Research Excellence (COBRE), the Scientist Development Grant of the American Heart Association (12SDG12090015, R.F.), and the Office of Research of the University of Nebraska-Lincoln. M.S.J. contributed to this work as part of an internship done at R.F. laboratory while doing her M.S. in Genetics and Cell Biology studies at Claude Bernard-Lyon 1 University. We apologize to those colleagues whose work was not cited due to space restrictions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Effects of tocopherol and deprenyl on the progression of disability in early Parkinson’s disease. N Engl J Med. 1993;328:176–183. doi: 10.1056/NEJM199301213280305. [DOI] [PubMed] [Google Scholar]
- 2.Abdin AA, Hamouda HE. Mechanism of the neuroprotective role of coenzyme Q10 with or without L-dopa in rotenone-induced parkinsonism. Neuropharmacology. 2008;55:1340–1346. doi: 10.1016/j.neuropharm.2008.08.033. [DOI] [PubMed] [Google Scholar]
- 3.Abou-Sleiman PM, Muqit MM, Wood NW. Expanding insights of mitochondrial dysfunction in Parkinson’s disease. Nat Rev Neurosci. 2006;7:207–219. doi: 10.1038/nrn1868. [DOI] [PubMed] [Google Scholar]
- 4.Abramov AY, Gegg M, Grunewald A, Wood NW, Klein C, Schapira AH. Bioenergetic consequences of PINK1 mutations in Parkinson disease. PLoS One. 2011;6:e25622. doi: 10.1371/journal.pone.0025622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahmed SS, Santosh W, Kumar S, Christlet HT. Metabolic profiling of Parkinson’s disease: evidence of biomarker from gene expression analysis and rapid neural network detection. J Biomed Sci. 2009;16:63. doi: 10.1186/1423-0127-16-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahn BH, Kim HS, Song S, Lee IH, Liu J, Vassilopoulos A, Deng CX, Finkel T. A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis. Proc Natl Acad Sci U S A. 2008;105:14447–14452. doi: 10.1073/pnas.0803790105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akhtar MW, Sanz-Blasco S, Dolatabadi N, Parker J, Chon K, Lee MS, Soussou W, McKercher SR, Ambasudhan R, Nakamura T, Lipton SA. Elevated glucose and oligomeric beta-amyloid disrupt synapses via a common pathway of aberrant protein S-nitrosylation. Nat Commun. 2016;7:10242. doi: 10.1038/ncomms10242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allaman I, Belanger M, Magistretti PJ. Methylglyoxal, the dark side of glycolysis. Front Neurosci. 2015;9:23. doi: 10.3389/fnins.2015.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Almeida A, Moncada S, Bolanos JP. Nitric oxide switches on glycolysis through the AMP protein kinase and 6-phosphofructo-2-kinase pathway. Nat Cell Biol. 2004;6:45–51. doi: 10.1038/ncb1080. [DOI] [PubMed] [Google Scholar]
- 10.Alvarez V, Corao AI, Sanchez-Ferrero E, De Mena L, Alonso-Montes C, Huerta C, Blazquez M, Ribacoba R, Guisasola LM, Salvador C, Garcia-Castro M, Coto E. Mitochondrial transcription factor A (TFAM) gene variation in Parkinson’s disease. Neurosci Lett. 2008;432:79–82. doi: 10.1016/j.neulet.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 11.Ambani LM, Van Woert MH, Murphy S. Brain peroxidase and catalase in Parkinson disease. Arch Neurol. 1975;32:114–118. doi: 10.1001/archneur.1975.00490440064010. [DOI] [PubMed] [Google Scholar]
- 12.Ambrosi G, Cerri S, Blandini F. A further update on the role of excitotoxicity in the pathogenesis of Parkinson’s disease. J Neural Transm (Vienna) 2014;121:849–859. doi: 10.1007/s00702-013-1149-z. [DOI] [PubMed] [Google Scholar]
- 13.Amo T, Saiki S, Sawayama T, Sato S, Hattori N. Detailed analysis of mitochondrial respiratory chain defects caused by loss of PINK1. Neurosci Lett. 2014;580:37–40. doi: 10.1016/j.neulet.2014.07.045. [DOI] [PubMed] [Google Scholar]
- 14.Anandhan A, Lei S, Levytskyy R, Pappa A, Panayiotidis MI, Cerny RL, Khalimonchuk O, Powers R, Franco R. Glucose Metabolism and AMPK Signaling Regulate Dopaminergic Cell Death Induced by Gene (alpha-Synuclein)-Environment (Paraquat) Interactions. Mol Neurobiol. 2016 doi: 10.1007/s12035-016-9906-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anderson DW, Schray RC, Duester G, Schneider JS. Functional significance of aldehyde dehydrogenase ALDH1A1 to the nigrostriatal dopamine system. Brain Res. 2011;1408:81–87. doi: 10.1016/j.brainres.2011.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andres RH, Ducray AD, Perez-Bouza A, Schlattner U, Huber AW, Krebs SH, Seiler RW, Wallimann T, Widmer HR. Creatine supplementation improves dopaminergic cell survival and protects against MPP+ toxicity in an organotypic tissue culture system. Cell Transplant. 2005;14:537–550. doi: 10.3727/000000005783982756. [DOI] [PubMed] [Google Scholar]
- 17.Anne C, Gasnier B. Vesicular neurotransmitter transporters: mechanistic aspects. Curr Top Membr. 2014;73:149–174. doi: 10.1016/B978-0-12-800223-0.00003-7. [DOI] [PubMed] [Google Scholar]
- 18.Annesley SJ, Lay ST, De Piazza SW, Sanislav O, Hammersley E, Allan CY, Francione LM, Bui MQ, Chen ZP, Ngoei KR, Tassone F, Kemp BE, Storey E, Evans A, Loesch DZ, Fisher PR. Immortalized Parkinson’s disease lymphocytes have enhanced mitochondrial respiratory activity. Dis Model Mech. 2016;9:1295–1305. doi: 10.1242/dmm.025684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aoyama K, Watabe M, Nakaki T. Regulation of neuronal glutathione synthesis. J Pharmacol Sci. 2008;108:227–238. doi: 10.1254/jphs.08r01cr. [DOI] [PubMed] [Google Scholar]
- 20.Arimoto T, Bing G. Up-regulation of inducible nitric oxide synthase in the substantia nigra by lipopolysaccharide causes microglial activation and neurodegeneration. Neurobiol Dis. 2003;12:35–45. doi: 10.1016/s0969-9961(02)00017-7. [DOI] [PubMed] [Google Scholar]
- 21.Arodin L, Miranda-Vizuete A, Swoboda P, Fernandes AP. Protective effects of the thioredoxin and glutaredoxin systems in dopamine-induced cell death. Free Radic Biol Med. 2014;73:328–336. doi: 10.1016/j.freeradbiomed.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 22.Badisa RB, Darling-Reed SF, Soliman KF. The protective role of D-glucose against 1-methyl-4-phenylpyridinium ion (MPP+): induced mitochondrial dysfunction in C6 astroglial cells. Neurochem Res. 2010;35:1413–1421. doi: 10.1007/s11064-010-0200-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bai J, Nakamura H, Hattori I, Tanito M, Yodoi J. Thioredoxin suppresses 1-methyl-4-phenylpyridinium-induced neurotoxicity in rat PC12 cells. Neurosci Lett. 2002;321:81–84. doi: 10.1016/s0304-3940(02)00058-7. [DOI] [PubMed] [Google Scholar]
- 24.Bai P, Canto C, Oudart H, Brunyanszki A, Cen Y, Thomas C, Yamamoto H, Huber A, Kiss B, Houtkooper RH, Schoonjans K, Schreiber V, Sauve AA, Menissier-de Murcia J, Auwerx J. PARP-1 inhibition increases mitochondrial metabolism through SIRT1 activation. Cell Metab. 2011;13:461–468. doi: 10.1016/j.cmet.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bandopadhyay R, Kingsbury AE, Cookson MR, Reid AR, Evans IM, Hope AD, Pittman AM, Lashley T, Canet-Aviles R, Miller DW, McLendon C, Strand C, Leonard AJ, Abou-Sleiman PM, Healy DG, Ariga H, Wood NW, de Silva R, Revesz T, Hardy JA, Lees AJ. The expression of DJ-1 (PARK7) in normal human CNS and idiopathic Parkinson’s disease. Brain. 2004;127:420–430. doi: 10.1093/brain/awh054. [DOI] [PubMed] [Google Scholar]
- 26.Barkats M, Horellou P, Colin P, Millecamps S, Faucon-Biguet N, Mallet J. 1-methyl-4-phenylpyridinium neurotoxicity is attenuated by adenoviral gene transfer of human Cu/Zn superoxide dismutase. J Neurosci Res. 2006;83:233–242. doi: 10.1002/jnr.20696. [DOI] [PubMed] [Google Scholar]
- 27.Bates TE, Heales SJ, Davies SE, Boakye P, Clark JB. Effects of 1-methyl-4-phenylpyridinium on isolated rat brain mitochondria: evidence for a primary involvement of energy depletion. J Neurochem. 1994;63:640–648. doi: 10.1046/j.1471-4159.1994.63020640.x. [DOI] [PubMed] [Google Scholar]
- 28.Baumann N, Pham-Dinh D. Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol Rev. 2001;81:871–927. doi: 10.1152/physrev.2001.81.2.871. [DOI] [PubMed] [Google Scholar]
- 29.Baxter PS, Hardingham GE. Adaptive regulation of the brain’s antioxidant defences by neurons and astrocytes. Free Radic Biol Med. 2016 doi: 10.1016/j.freeradbiomed.2016.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beard E, Braissant O. Synthesis and transport of creatine in the CNS: importance for cerebral functions. J Neurochem. 2010;115:297–313. doi: 10.1111/j.1471-4159.2010.06935.x. [DOI] [PubMed] [Google Scholar]
- 31.Becker WM, Kleinsmith LJ, Hardin J. The world of the cell. 4th. Benjamin/Cummings; San Francisco: 2000. [Google Scholar]
- 32.Belanger M, Allaman I, Magistretti PJ. Brain energy metabolism: focus on astrocyte-neuron metabolic cooperation. Cell Metab. 2011;14:724–738. doi: 10.1016/j.cmet.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 33.Bell KF, Al-Mubarak B, Martel MA, McKay S, Wheelan N, Hasel P, Markus NM, Baxter P, Deighton RF, Serio A, Bilican B, Chowdhry S, Meakin PJ, Ashford ML, Wyllie DJ, Scannevin RH, Chandran S, Hayes JD, Hardingham GE. Neuronal development is promoted by weakened intrinsic antioxidant defences due to epigenetic repression of Nrf2. Nat Commun. 2015;6:7066. doi: 10.1038/ncomms8066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bellucci A, Collo G, Sarnico I, Battistin L, Missale C, Spano P. Alpha-synuclein aggregation and cell death triggered by energy deprivation and dopamine overload are counteracted by D2/D3 receptor activation. J Neurochem. 2008;106:560–577. doi: 10.1111/j.1471-4159.2008.05406.x. [DOI] [PubMed] [Google Scholar]
- 35.Bensadoun JC, Mirochnitchenko O, Inouye M, Aebischer P, Zurn AD. Attenuation of 6-OHDA-induced neurotoxicity in glutathione peroxidase transgenic mice. Eur J Neurosci. 1998;10:3231–3236. doi: 10.1046/j.1460-9568.1998.00345.x. [DOI] [PubMed] [Google Scholar]
- 36.Bhattacharjee N, Borah A. Oxidative stress and mitochondrial dysfunction are the underlying events of dopaminergic neurodegeneration in homocysteine rat model of Parkinson’s disease. Neurochem Int. 2016;101:48–55. doi: 10.1016/j.neuint.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 37.Birkenmeier G, Stegemann C, Hoffmann R, Gunther R, Huse K, Birkemeyer C. Posttranslational modification of human glyoxalase 1 indicates redox-dependent regulation. PLoS One. 2010;5:e10399. doi: 10.1371/journal.pone.0010399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Biteau B, Labarre J, Toledano MB. ATP-dependent reduction of cysteine-sulphinic acid by S. cerevisiae sulphiredoxin. Nature. 2003;425:980–984. doi: 10.1038/nature02075. [DOI] [PubMed] [Google Scholar]
- 39.Brekke EM, Walls AB, Schousboe A, Waagepetersen HS, Sonnewald U. Quantitative importance of the pentose phosphate pathway determined by incorporation of 13C from [2–13C]-and [3–13C]glucose into TCA cycle intermediates and neurotransmitter amino acids in functionally intact neurons. J Cereb Blood Flow Metab. 2012;32:1788–1799. doi: 10.1038/jcbfm.2012.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brighina L, Riva C, Bertola F, Fermi S, Saracchi E, Piolti R, Goldwurm S, Pezzoli G, Ferrarese C. Association analysis of PARP1 polymorphisms with Parkinson’s disease. Parkinsonism Relat Disord. 2011;17:701–704. doi: 10.1016/j.parkreldis.2011.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brochard V, Combadiere B, Prigent A, Laouar Y, Perrin A, Beray-Berthat V, Bonduelle O, Alvarez-Fischer D, Callebert J, Launay JM, Duyckaerts C, Flavell RA, Hirsch EC, Hunot S. Infiltration of CD4+ lymphocytes into the brain contributes to neurodegeneration in a mouse model of Parkinson disease. J Clin Invest. 2009;119:182–192. doi: 10.1172/JCI36470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brown GC, Borutaite V. There is no evidence that mitochondria are the main source of reactive oxygen species in mammalian cells. Mitochondrion. 2012;12:1–4. doi: 10.1016/j.mito.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 43.Buler M, Aatsinki SM, Izzi V, Uusimaa J, Hakkola J. SIRT5 is under the control of PGC-1alpha and AMPK and is involved in regulation of mitochondrial energy metabolism. FASEB J. 2014;28:3225–3237. doi: 10.1096/fj.13-245241. [DOI] [PubMed] [Google Scholar]
- 44.Byrne JH, Roberts JL. From molecules to networks : an introduction to cellular and molecular neuroscience. 2nd. Academic Press/Elsevier; Amsterdam; Boston: 2009. [Google Scholar]
- 45.Cabezas R, Avila M, Gonzalez J, El-Bacha RS, Baez E, Garcia-Segura LM, Jurado Coronel JC, Capani F, Cardona-Gomez GP, Barreto GE. Astrocytic modulation of blood brain barrier: perspectives on Parkinson’s disease. Front Cell Neurosci. 2014;8:211. doi: 10.3389/fncel.2014.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cantu D, Schaack J, Patel M. Oxidative inactivation of mitochondrial aconitase results in iron and H2O2-mediated neurotoxicity in rat primary mesencephalic cultures. PLoS One. 2009;4:e7095. doi: 10.1371/journal.pone.0007095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cappelletti G, Surrey T, Maci R. The parkinsonism producing neurotoxin MPP+ affects microtubule dynamics by acting as a destabilising factor. FEBS Lett. 2005;579:4781–4786. doi: 10.1016/j.febslet.2005.07.058. [DOI] [PubMed] [Google Scholar]
- 48.Cardaci S, Filomeni G, Ciriolo MR. Redox implications of AMPK-mediated signal transduction beyond energetic clues. J Cell Sci. 2012;125:2115–2125. doi: 10.1242/jcs.095216. [DOI] [PubMed] [Google Scholar]
- 49.Cartelli D, Ronchi C, Maggioni MG, Rodighiero S, Giavini E, Cappelletti G. Microtubule dysfunction precedes transport impairment and mitochondria damage in MPP+ -induced neurodegeneration. J Neurochem. 2010;115:247–258. doi: 10.1111/j.1471-4159.2010.06924.x. [DOI] [PubMed] [Google Scholar]
- 50.Castello PR, Drechsel DA, Patel M. Mitochondria are a major source of paraquat-induced reactive oxygen species production in the brain. J Biol Chem. 2007;282:14186–14193. doi: 10.1074/jbc.M700827200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ceballos I, Lafon M, Javoy-Agid F, Hirsch E, Nicole A, Sinet PM, Agid Y. Superoxide dismutase and Parkinson’s disease. Lancet. 1990;335:1035–1036. doi: 10.1016/0140-6736(90)91099-v. [DOI] [PubMed] [Google Scholar]
- 52.Chalmers-Redman RM, Fraser AD, Carlile GW, Pong A, Tatton WG. Glucose protection from MPP+-induced apoptosis depends on mitochondrial membrane potential and ATP synthase. Biochem Biophys Res Commun. 1999;257:440–447. doi: 10.1006/bbrc.1999.0487. [DOI] [PubMed] [Google Scholar]
- 53.Chan P, Langston JW, Irwin I, DeLanney LE, Di Monte DA. 2-deoxyglucose enhances 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced ATP loss in the mouse brain. J Neurochem. 1993;61:610–616. doi: 10.1111/j.1471-4159.1993.tb02165.x. [DOI] [PubMed] [Google Scholar]
- 54.Chaudhuri AD, Kabaria S, Choi DC, Mouradian MM, Junn E. MicroRNA-7 Promotes Glycolysis to Protect against 1-Methyl-4-phenylpyridinium-induced Cell Death. J Biol Chem. 2015;290:12425–12434. doi: 10.1074/jbc.M114.625962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen CM, Liu JL, Wu YR, Chen YC, Cheng HS, Cheng ML, Chiu DT. Increased oxidative damage in peripheral blood correlates with severity of Parkinson’s disease. Neurobiol Dis. 2009;33:429–435. doi: 10.1016/j.nbd.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 56.Chen PC, Vargas MR, Pani AK, Smeyne RJ, Johnson DA, Kan YW, Johnson JA. Nrf2-mediated neuroprotection in the MPTP mouse model of Parkinson’s disease: Critical role for the astrocyte. Proc Natl Acad Sci U S A. 2009;106:2933–2938. doi: 10.1073/pnas.0813361106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen PS, Peng GS, Li G, Yang S, Wu X, Wang CC, Wilson B, Lu RB, Gean PW, Chuang DM, Hong JS. Valproate protects dopaminergic neurons in midbrain neuron/glia cultures by stimulating the release of neurotrophic factors from astrocytes. Mol Psychiatry. 2006;11:1116–1125. doi: 10.1038/sj.mp.4001893. [DOI] [PubMed] [Google Scholar]
- 58.Chen Q, Vazquez EJ, Moghaddas S, Hoppel CL, Lesnefsky EJ. Production of reactive oxygen species by mitochondria: central role of complex III. J Biol Chem. 2003;278:36027–36031. doi: 10.1074/jbc.M304854200. [DOI] [PubMed] [Google Scholar]
- 59.Chen Z, Zhong C. Decoding Alzheimer’s disease from perturbed cerebral glucose metabolism: implications for diagnostic and therapeutic strategies. Prog Neurobiol. 2013;108:21–43. doi: 10.1016/j.pneurobio.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 60.Cheng A, Yang Y, Zhou Y, Maharana C, Lu D, Peng W, Liu Y, Wan R, Marosi K, Misiak M, Bohr VA, Mattson MP. Mitochondrial SIRT3 Mediates Adaptive Responses of Neurons to Exercise and Metabolic and Excitatory Challenges. Cell Metab. 2016;23:128–142. doi: 10.1016/j.cmet.2015.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chew KC, Ang ET, Tai YK, Tsang F, Lo SQ, Ong E, Ong WY, Shen HM, Lim KL, Dawson VL, Dawson TM, Soong TW. Enhanced autophagy from chronic toxicity of iron and mutant A53T alpha-synuclein: implications for neuronal cell death in Parkinson disease. J Biol Chem. 2011;286:33380–33389. doi: 10.1074/jbc.M111.268409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chinta SJ, Kumar MJ, Hsu M, Rajagopalan S, Kaur D, Rane A, Nicholls DG, Choi J, Andersen JK. Inducible alterations of glutathione levels in adult dopaminergic midbrain neurons result in nigrostriatal degeneration. J Neurosci. 2007;27:13997–14006. doi: 10.1523/JNEUROSCI.3885-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Choi I, Kim J, Jeong HK, Kim B, Jou I, Park SM, Chen L, Kang UJ, Zhuang X, Joe EH. PINK1 deficiency attenuates astrocyte proliferation through mitochondrial dysfunction, reduced AKT and increased p38 MAPK activation, and downregulation of EGFR. Glia. 2013;61:800–812. doi: 10.1002/glia.22475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Choi JS, Park C, Jeong JW. AMP-activated protein kinase is activated in Parkinson’s disease models mediated by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Biochem Biophys Res Commun. 2010;391:147–151. doi: 10.1016/j.bbrc.2009.11.022. [DOI] [PubMed] [Google Scholar]
- 65.Choi JW, Shin CY, Yoo BK, Choi MS, Lee WJ, Han BH, Kim WK, Kim HC, Ko KH. Glucose deprivation increases hydrogen peroxide level in immunostimulated rat primary astrocytes. J Neurosci Res. 2004;75:722–731. doi: 10.1002/jnr.20009. [DOI] [PubMed] [Google Scholar]
- 66.Choi SW, Gerencser AA, Lee DW, Rajagopalan S, Nicholls DG, Andersen JK, Brand MD. Intrinsic bioenergetic properties and stress sensitivity of dopaminergic synaptosomes. J Neurosci. 2011;31:4524–4534. doi: 10.1523/JNEUROSCI.5817-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Choubey V, Safiulina D, Vaarmann A, Cagalinec M, Wareski P, Kuum M, Zharkovsky A, Kaasik A. Mutant A53T alpha-synuclein induces neuronal death by increasing mitochondrial autophagy. J Biol Chem. 2011;286:10814–10824. doi: 10.1074/jbc.M110.132514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chu Y, Morfini GA, Langhamer LB, He Y, Brady ST, Kordower JH. Alterations in axonal transport motor proteins in sporadic and experimental Parkinson’s disease. Brain. 2012;135:2058–2073. doi: 10.1093/brain/aws133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ciarmiello A, Cannella M, Lastoria S, Simonelli M, Frati L, Rubinsztein DC, Squitieri F. Brain White-Matter Volume Loss and Glucose Hypometabolism Precede the Clinical Symptoms of Huntington’s Disease. Journal of Nuclear Medicine. 2006;47:215–222. [PubMed] [Google Scholar]
- 70.Clark J, Dai Y, Simon DK. Do somatic mitochondrial DNA mutations contribute to Parkinson’s disease? Parkinsons Dis. 2011;2011:659694. doi: 10.4061/2011/659694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cobb CA, Cole MP. Oxidative and nitrative stress in neurodegeneration. Neurobiol Dis. 2015;84:4–21. doi: 10.1016/j.nbd.2015.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cole SP. Targeting multidrug resistance protein 1 (MRP1, ABCC1): past, present, and future. Annu Rev Pharmacol Toxicol. 2014;54:95–117. doi: 10.1146/annurev-pharmtox-011613-135959. [DOI] [PubMed] [Google Scholar]
- 73.Cook C, Stetler C, Petrucelli L. Disruption of protein quality control in Parkinson’s disease. Cold Spring Harb Perspect Med. 2012;2:a009423. doi: 10.1101/cshperspect.a009423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cox AG, Winterbourn CC, Hampton MB. Mitochondrial peroxiredoxin involvement in antioxidant defence and redox signalling. Biochem J. 2010;425:313–325. doi: 10.1042/BJ20091541. [DOI] [PubMed] [Google Scholar]
- 75.Dagnino-Subiabre A, Cassels BK, Baez S, Johansson AS, Mannervik B, Segura-Aguilar J. Glutathione transferase M2-2 catalyzes conjugation of dopamine and dopa o-quinones. Biochem Biophys Res Commun. 2000;274:32–36. doi: 10.1006/bbrc.2000.3087. [DOI] [PubMed] [Google Scholar]
- 76.Dahan-Grobgeld E, Livneh Z, Maretzek AF, Polak-Charcon S, Eichenbaum Z, Degani H. Reversible induction of ATP synthesis by DNA damage and repair in Escherichia coli. In vivo NMR studies. J Biol Chem. 1998;273:30232–30238. doi: 10.1074/jbc.273.46.30232. [DOI] [PubMed] [Google Scholar]
- 77.Davey GP, Clark JB. Threshold effects and control of oxidative phosphorylation in nonsynaptic rat brain mitochondria. J Neurochem. 1996;66:1617–1624. doi: 10.1046/j.1471-4159.1996.66041617.x. [DOI] [PubMed] [Google Scholar]
- 78.Davis CH, Kim KY, Bushong EA, Mills EA, Boassa D, Shih T, Kinebuchi M, Phan S, Zhou Y, Bihlmeyer NA, Nguyen JV, Jin Y, Ellisman MH, Marsh-Armstrong N. Transcellular degradation of axonal mitochondria. Proc Natl Acad Sci U S A. 2014;111:9633–9638. doi: 10.1073/pnas.1404651111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.de Arriba SG, Stuchbury G, Yarin J, Burnell J, Loske C, Munch G. Methylglyoxal impairs glucose metabolism and leads to energy depletion in neuronal cells—protection by carbonyl scavengers. Neurobiol Aging. 2007;28:1044–1050. doi: 10.1016/j.neurobiolaging.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 80.De Simoni S, Goemaere J, Knoops B. Silencing of peroxiredoxin 3 and peroxiredoxin 5 reveals the role of mitochondrial peroxiredoxins in the protection of human neuroblastoma SH-SY5Y cells toward MPP+ Neurosci Lett. 2008;433:219–224. doi: 10.1016/j.neulet.2007.12.068. [DOI] [PubMed] [Google Scholar]
- 81.Dehay B, Martinez-Vicente M, Caldwell GA, Caldwell KA, Yue Z, Cookson MR, Klein C, Vila M, Bezard E. Lysosomal impairment in Parkinson’s disease. Mov Disord. 2013;28:725–732. doi: 10.1002/mds.25462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Denicola A, Souza JM, Radi R. Diffusion of peroxynitrite across erythrocyte membranes. Proc Natl Acad Sci U S A. 1998;95:3566–3571. doi: 10.1073/pnas.95.7.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Devi L, Raghavendran V, Prabhu BM, Avadhani NG, Anandatheerthavarada HK. Mitochondrial import and accumulation of alpha-synuclein impair complex I in human dopaminergic neuronal cultures and Parkinson disease brain. J Biol Chem. 2008;283:9089–9100. doi: 10.1074/jbc.M710012200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Diaz-Hernandez JI, Moncada S, Bolanos JP, Almeida A. Poly(ADP-ribose) polymerase-1 protects neurons against apoptosis induced by oxidative stress. Cell Death Differ. 2007;14:1211–1221. doi: 10.1038/sj.cdd.4402117. [DOI] [PubMed] [Google Scholar]
- 85.Dienel GA. Brain lactate metabolism: the discoveries and the controversies. J Cereb Blood Flow Metab. 2012;32:1107–1138. doi: 10.1038/jcbfm.2011.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dranka BP, Zielonka J, Kanthasamy AG, Kalyanaraman B. Alterations in bioenergetic function induced by Parkinson’s disease mimetic compounds: lack of correlation with superoxide generation. J Neurochem. 2012 doi: 10.1111/j.1471-4159.2012.07836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Duan W, Mattson MP. Dietary restriction and 2-deoxyglucose administration improve behavioral outcome and reduce degeneration of dopaminergic neurons in models of Parkinson’s disease. J Neurosci Res. 1999;57:195–206. doi: 10.1002/(SICI)1097-4547(19990715)57:2<195::AID-JNR5>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 88.Dulovic M, Jovanovic M, Xilouri M, Stefanis L, Harhaji-Trajkovic L, Kravic-Stevovic T, Paunovic V, Ardah MT, El-Agnaf OM, Kostic V, Markovic I, Trajkovic V. The protective role of AMP-activated protein kinase in alpha-synuclein neurotoxicity in vitro. Neurobiol Dis. 2014;63:1–11. doi: 10.1016/j.nbd.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 89.Dunn L, Allen GF, Mamais A, Ling H, Li A, Duberley KE, Hargreaves IP, Pope S, Holton JL, Lees A, Heales SJ, Bandopadhyay R. Dysregulation of glucose metabolism is an early event in sporadic Parkinson’s disease. Neurobiol Aging. 2014;35:1111–1115. doi: 10.1016/j.neurobiolaging.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dusek P, Jankovic J, Le W. Iron dysregulation in movement disorders. Neurobiol Dis. 2012;46:1–18. doi: 10.1016/j.nbd.2011.12.054. [DOI] [PubMed] [Google Scholar]
- 91.Eberling JL, Richardson BC, Reed BR, Wolfe N, Jagust WJ. Cortical glucose metabolism in Parkinson’s disease without dementia. Neurobiol Aging. 1994;15:329–335. doi: 10.1016/0197-4580(94)90028-0. [DOI] [PubMed] [Google Scholar]
- 92.Egan DF, Shackelford DB, Mihaylova MM, Gelino S, Kohnz RA, Mair W, Vasquez DS, Joshi A, Gwinn DM, Taylor R, Asara JM, Fitzpatrick J, Dillin A, Viollet B, Kundu M, Hansen M, Shaw RJ. Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science. 2011;331:456–461. doi: 10.1126/science.1196371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Esposito G, Vos M, Vilain S, Swerts J, De Sousa Valadas J, Van Meensel S, Schaap O, Verstreken P. Aconitase causes iron toxicity in Drosophila pink1 mutants. PLoS Genet. 2013;9:e1003478. doi: 10.1371/journal.pgen.1003478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Exner N, Lutz AK, Haass C, Winklhofer KF. Mitochondrial dysfunction in Parkinson’s disease: molecular mechanisms and pathophysiological consequences. EMBO J. 2012;31:3038–3062. doi: 10.1038/emboj.2012.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fan J, Ye J, Kamphorst JJ, Shlomi T, Thompson CB, Rabinowitz JD. Quantitative flux analysis reveals folate-dependent NADPH production. Nature. 2014;510:298–302. doi: 10.1038/nature13236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fernandez-Fernandez S, Almeida A, Bolanos JP. Antioxidant and bioenergetic coupling between neurons and astrocytes. Biochem J. 2012;443:3–11. doi: 10.1042/BJ20111943. [DOI] [PubMed] [Google Scholar]
- 97.Finsen B, Owens T. Innate immune responses in central nervous system inflammation. FEBS Lett. 2011;585:3806–3812. doi: 10.1016/j.febslet.2011.05.030. [DOI] [PubMed] [Google Scholar]
- 98.Fitzmaurice AG, Rhodes SL, Lulla A, Murphy NP, Lam HA, O’Donnell KC, Barnhill L, Casida JE, Cockburn M, Sagasti A, Stahl MC, Maidment NT, Ritz B, Bronstein JM. Aldehyde dehydrogenase inhibition as a pathogenic mechanism in Parkinson disease. Proc Natl Acad Sci U S A. 2013;110:636–641. doi: 10.1073/pnas.1220399110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Florang VR, Rees JN, Brogden NK, Anderson DG, Hurley TD, Doorn JA. Inhibition of the oxidative metabolism of 3,4-dihydroxyphenylacetaldehyde, a reactive intermediate of dopamine metabolism, by 4-hydroxy-2-nonenal. Neurotoxicology. 2007;28:76–82. doi: 10.1016/j.neuro.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 100.Fonck C, Baudry M. Toxic effects of MPP(+) and MPTP in PC12 cells independent of reactive oxygen species formation. Brain Res. 2001;905:199–206. doi: 10.1016/s0006-8993(01)02551-3. [DOI] [PubMed] [Google Scholar]
- 101.Franco R, Cidlowski JA. Glutathione Efflux and Cell Death. Antioxid Redox Signal. 2012 doi: 10.1089/ars.2012.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Funfschilling U, Supplie LM, Mahad D, Boretius S, Saab AS, Edgar J, Brinkmann BG, Kassmann CM, Tzvetanova ID, Mobius W, Diaz F, Meijer D, Suter U, Hamprecht B, Sereda MW, Moraes CT, Frahm J, Goebbels S, Nave KA. Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature. 2012;485:517–521. doi: 10.1038/nature11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gao HM, Zhang F, Zhou H, Kam W, Wilson B, Hong JS. Neuroinflammation and alpha-synuclein dysfunction potentiate each other, driving chronic progression of neurodegeneration in a mouse model of Parkinson’s disease. Environ Health Perspect. 2011;119:807–814. doi: 10.1289/ehp.1003013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gao L, Mir P, Diaz-Corrales FJ, Mejias R, Carrillo F, Vime PJ, Diaz-Martin J, Palomino A, Carballo M, Pintado E, Lucas M, Lopez-Barneo J. Glucose-6-phosphate dehydrogenase activity in Parkinson’s disease. J Neurol. 2008;255:1850–1851. doi: 10.1007/s00415-008-0937-0. [DOI] [PubMed] [Google Scholar]
- 105.Garcia-Nogales P, Almeida A, Bolanos JP. Peroxynitrite protects neurons against nitric oxide-mediated apoptosis. A key role for glucose-6-phosphate dehydrogenase activity in neuroprotection. J Biol Chem. 2003;278:864–874. doi: 10.1074/jbc.M206835200. [DOI] [PubMed] [Google Scholar]
- 106.Gardner PR, Fridovich I. Superoxide sensitivity of the Escherichia coli aconitase. J Biol Chem. 1991;266:19328–19333. [PubMed] [Google Scholar]
- 107.Gatto EM, Carreras MC, Pargament GA, Riobo NA, Reides C, Repetto M, Fernandez Pardal MM, Llesuy S, Poderoso JJ. Neutrophil function, nitric oxide, and blood oxidative stress in Parkinson’s disease. Mov Disord. 1996;11:261–267. doi: 10.1002/mds.870110308. [DOI] [PubMed] [Google Scholar]
- 108.Ghosh A, Chandran K, Kalivendi SV, Joseph J, Antholine WE, Hillard CJ, Kanthasamy A, Kalyanaraman B. Neuroprotection by a mitochondria-targeted drug in a Parkinson’s disease model. Free Radic Biol Med. 2010;49:1674–1684. doi: 10.1016/j.freeradbiomed.2010.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gibson GE, Kingsbury AE, Xu H, Lindsay JG, Daniel S, Foster OJ, Lees AJ, Blass JP. Deficits in a tricarboxylic acid cycle enzyme in brains from patients with Parkinson’s disease. Neurochem Int. 2003;43:129–135. doi: 10.1016/s0197-0186(02)00225-5. [DOI] [PubMed] [Google Scholar]
- 110.Gimeno-Bayon J, Lopez-Lopez A, Rodriguez MJ, Mahy N. Glucose pathways adaptation supports acquisition of activated microglia phenotype. J Neurosci Res. 2014;92:723–731. doi: 10.1002/jnr.23356. [DOI] [PubMed] [Google Scholar]
- 111.Glinka Y, Tipton KF, Youdim MB. Mechanism of inhibition of mitochondrial respiratory complex I by 6-hydroxydopamine and its prevention by desferrioxamine. Eur J Pharmacol. 1998;351:121–129. doi: 10.1016/s0014-2999(98)00279-9. [DOI] [PubMed] [Google Scholar]
- 112.Goldman SM. Environmental toxins and Parkinson’s disease. Annu Rev Pharmacol Toxicol. 2014;54:141–164. doi: 10.1146/annurev-pharmtox-011613-135937. [DOI] [PubMed] [Google Scholar]
- 113.Goldstein DS, Sullivan P, Cooney A, Jinsmaa Y, Kopin IJ, Sharabi Y. Rotenone decreases intracellular aldehyde dehydrogenase activity: implications for the pathogenesis of Parkinson’s disease. J Neurochem. 2015;133:14–25. doi: 10.1111/jnc.13042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gomez A, Ferrer I. Increased oxidation of certain glycolysis and energy metabolism enzymes in the frontal cortex in Lewy body diseases. J Neurosci Res. 2009;87:1002–1013. doi: 10.1002/jnr.21904. [DOI] [PubMed] [Google Scholar]
- 115.Gonzalez H, Pacheco R. T-cell-mediated regulation of neuroinflammation involved in neurodegenerative diseases. J Neuroinflammation. 2014;11:201. doi: 10.1186/s12974-014-0201-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gossmann TI, Ziegler M, Puntervoll P, de Figueiredo LF, Schuster S, Heiland I. NAD(+) biosynthesis and salvage–a phylogenetic perspective. FEBS J. 2012;279:3355–3363. doi: 10.1111/j.1742-4658.2012.08559.x. [DOI] [PubMed] [Google Scholar]
- 117.Gray MT, Woulfe JM. Striatal blood-brain barrier permeability in Parkinson’s disease. J Cereb Blood Flow Metab. 2015;35:747–750. doi: 10.1038/jcbfm.2015.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Grivennikova VG, Vinogradov AD. Generation of superoxide by the mitochondrial Complex I. Biochim Biophys Acta. 2006;1757:553–561. doi: 10.1016/j.bbabio.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 119.Guzder SN, Sung P, Prakash L, Prakash S. Yeast Rad7-Rad16 complex, specific for the nucleotide excision repair of the nontranscribed DNA strand, is an ATP-dependent DNA damage sensor. J Biol Chem. 1997;272:21665–21668. doi: 10.1074/jbc.272.35.21665. [DOI] [PubMed] [Google Scholar]
- 120.Guzman-Beltran S, Espada S, Orozco-Ibarra M, Pedraza-Chaverri J, Cuadrado A. Nordihydroguaiaretic acid activates the antioxidant pathway Nrf2/HO-1 and protects cerebellar granule neurons against oxidative stress. Neurosci Lett. 2008;447:167–171. doi: 10.1016/j.neulet.2008.09.079. [DOI] [PubMed] [Google Scholar]
- 121.Guzman JN, Sanchez-Padilla J, Wokosin D, Kondapalli J, Ilijic E, Schumacker PT, Surmeier DJ. Oxidant stress evoked by pacemaking in dopaminergic neurons is attenuated by DJ-1. Nature. 2010;468:696–700. doi: 10.1038/nature09536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hanisch UK. Proteins in microglial activation–inputs and outputs by subsets. Curr Protein Pept Sci. 2013;14:3–15. doi: 10.2174/1389203711314010003. [DOI] [PubMed] [Google Scholar]
- 123.Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10:1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- 124.Hantraye P, Brouillet E, Ferrante R, Palfi S, Dolan R, Matthews RT, Beal MF. Inhibition of neuronal nitric oxide synthase prevents MPTP-induced parkinsonism in baboons. Nat Med. 1996;2:1017–1021. doi: 10.1038/nm0996-1017. [DOI] [PubMed] [Google Scholar]
- 125.Hasselbalch SG, Knudsen GM, Jakobsen J, Hageman LP, Holm S, Paulson OB. Blood-brain barrier permeability of glucose and ketone bodies during short-term starvation in humans. Am J Physiol. 1995;268:E1161–1166. doi: 10.1152/ajpendo.1995.268.6.E1161. [DOI] [PubMed] [Google Scholar]
- 126.Hastings TG, Lewis DA, Zigmond MJ. Role of oxidation in the neurotoxic effects of intrastriatal dopamine injections. Proc Natl Acad Sci U S A. 1996;93:1956–1961. doi: 10.1073/pnas.93.5.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hayakawa K, Esposito E, Wang X, Terasaki Y, Liu Y, Xing C, Ji X, Lo EH. Transfer of mitochondria from astrocytes to neurons after stroke. Nature. 2016;535:551–555. doi: 10.1038/nature18928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hebert AS, Dittenhafer-Reed KE, Yu W, Bailey DJ, Selen ES, Boersma MD, Carson JJ, Tonelli M, Balloon AJ, Higbee AJ, Westphall MS, Pagliarini DJ, Prolla TA, Assadi-Porter F, Roy S, Denu JM, Coon JJ. Calorie restriction and SIRT3 trigger global reprogramming of the mitochondrial protein acetylome. Mol Cell. 2013;49:186–199. doi: 10.1016/j.molcel.2012.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Henchcliffe C, Beal MF. Mitochondrial biology and oxidative stress in Parkinson disease pathogenesis. Nat Clin Pract Neurol. 2008;4:600–609. doi: 10.1038/ncpneuro0924. [DOI] [PubMed] [Google Scholar]
- 130.Henchcliffe C, Shungu DC, Mao X, Huang C, Nirenberg MJ, Jenkins BG, Beal MF. Multinuclear magnetic resonance spectroscopy for in vivo assessment of mitochondrial dysfunction in Parkinson’s disease. Ann N Y Acad Sci. 2008;1147:206–220. doi: 10.1196/annals.1427.037. [DOI] [PubMed] [Google Scholar]
- 131.Herrero-Mendez A, Almeida A, Fernandez E, Maestre C, Moncada S, Bolanos JP. The bioenergetic and antioxidant status of neurons is controlled by continuous degradation of a key glycolytic enzyme by APC/C-Cdh1. Nat Cell Biol. 2009;11:747–752. doi: 10.1038/ncb1881. [DOI] [PubMed] [Google Scholar]
- 132.Herrero-Mendez A, Almeida A, Fernandez E, Maestre C, Moncada S, Bolanos JP. The bioenergetic and antioxidant status of neurons is controlled by continuous degradation of a key glycolytic enzyme by APC/C-Cdh1. Nat Cell Biol. 2009;11:747–752. doi: 10.1038/ncb1881. [DOI] [PubMed] [Google Scholar]
- 133.Hirrlinger J, Dringen R. Multidrug resistance protein 1-mediated export of glutathione and glutathione disulfide from brain astrocytes. Methods Enzymol. 2005;400:395–409. doi: 10.1016/S0076-6879(05)00023-6. [DOI] [PubMed] [Google Scholar]
- 134.Hochstrasser H, Tomiuk J, Walter U, Behnke S, Spiegel J, Kruger R, Becker G, Riess O, Berg D. Functional relevance of ceruloplasmin mutations in Parkinson’s disease. FASEB J. 2005;19:1851–1853. doi: 10.1096/fj.04-3486fje. [DOI] [PubMed] [Google Scholar]
- 135.Hollensworth SB, Shen C, Sim JE, Spitz DR, Wilson GL, LeDoux SP. Glial cell type-specific responses to menadione-induced oxidative stress. Free Radic Biol Med. 2000;28:1161–1174. doi: 10.1016/s0891-5849(00)00214-8. [DOI] [PubMed] [Google Scholar]
- 136.Hong CT, Chau KY, Schapira AH. Meclizine-induced enhanced glycolysis is neuroprotective in Parkinson disease cell models. Sci Rep. 2016;6:25344. doi: 10.1038/srep25344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hu X, Weng Z, Chu CT, Zhang L, Cao G, Gao Y, Signore A, Zhu J, Hastings T, Greenamyre JT, Chen J. Peroxiredoxin-2 protects against 6-hydroxydopamine-induced dopaminergic neurodegeneration via attenuation of the apoptosis signal-regulating kinase (ASK1) signaling cascade. J Neurosci. 2011;31:247–261. doi: 10.1523/JNEUROSCI.4589-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Iglesias J, Morales L, Barreto GE. Metabolic and Inflammatory Adaptation of Reactive Astrocytes: Role of PPARs. Mol Neurobiol. 2016 doi: 10.1007/s12035-016-9833-2. [DOI] [PubMed] [Google Scholar]
- 139.Infanger DW, Sharma RV, Davisson RL. NADPH oxidases of the brain: distribution, regulation, and function. Antioxid Redox Signal. 2006;8:1583–1596. doi: 10.1089/ars.2006.8.1583. [DOI] [PubMed] [Google Scholar]
- 140.Infante J, Sanchez-Juan P, Mateo I, Rodriguez-Rodriguez E, Sanchez-Quintana C, Llorca J, Fontalba A, Terrazas J, Oterino A, Berciano J, Combarros O. Poly (ADP-ribose) polymerase-1 (PARP-1) genetic variants are protective against Parkinson’s disease. J Neurol Sci. 2007;256:68–70. doi: 10.1016/j.jns.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 141.Irrcher I, Aleyasin H, Seifert EL, Hewitt SJ, Chhabra S, Phillips M, Lutz AK, Rousseaux MW, Bevilacqua L, Jahani-Asl A, Callaghan S, MacLaurin JG, Winklhofer KF, Rizzu P, Rippstein P, Kim RH, Chen CX, Fon EA, Slack RS, Harper ME, McBride HM, Mak TW, Park DS. Loss of the Parkinson’s disease-linked gene DJ-1 perturbs mitochondrial dynamics. Hum Mol Genet. 2010;19:3734–3746. doi: 10.1093/hmg/ddq288. [DOI] [PubMed] [Google Scholar]
- 142.Ivanov AI, Malkov AE, Waseem T, Mukhtarov M, Buldakova S, Gubkina O, Zilberter M, Zilberter Y. Glycolysis and oxidative phosphorylation in neurons and astrocytes during network activity in hippocampal slices. J Cereb Blood Flow Metab. 2014;34:397–407. doi: 10.1038/jcbfm.2013.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Iwashita A, Mihara K, Yamazaki S, Matsuura S, Ishida J, Yamamoto H, Hattori K, Matsuoka N, Mutoh S. A new poly(ADP-ribose) polymerase inhibitor, FR261529 [2-(4-chlorophenyl)-5-quinoxalinecarboxamide], ameliorates methamphetamine-induced dopaminergic neurotoxicity in mice. J Pharmacol Exp Ther. 2004;310:1114–1124. doi: 10.1124/jpet.104.068932. [DOI] [PubMed] [Google Scholar]
- 144.Iwashita A, Yamazaki S, Mihara K, Hattori K, Yamamoto H, Ishida J, Matsuoka N, Mutoh S. Neuroprotective effects of a novel poly(ADP-ribose) polymerase-1 inhibitor, 2-[3-[4-(4-chlorophenyl)-1-piperazinyl] propyl]-4(3H)-quinazolinone (FR255595), in an in vitro model of cell death and in mouse 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson’s disease. J Pharmacol Exp Ther. 2004;309:1067–1078. doi: 10.1124/jpet.103.064642. [DOI] [PubMed] [Google Scholar]
- 145.Jagust WJ, Seab JP, Huesman RH, Valk PE, Mathis CA, Reed BR, Coxson PG, Budinger TF. Diminished Glucose Transport in Alzheimer’s Disease: Dynamic PET Studies. Journal of Cerebral Blood Flow & Metabolism. 1991;11:323–330. doi: 10.1038/jcbfm.1991.65. [DOI] [PubMed] [Google Scholar]
- 146.Jenner P. Altered mitochondrial function, iron metabolism and glutathione levels in Parkinson’s disease. Acta Neurol Scand Suppl. 1993;146:6–13. [PubMed] [Google Scholar]
- 147.Ji KA, Eu MY, Kang SH, Gwag BJ, Jou I, Joe EH. Differential neutrophil infiltration contributes to regional differences in brain inflammation in the substantia nigra pars compacta and cortex. Glia. 2008;56:1039–1047. doi: 10.1002/glia.20677. [DOI] [PubMed] [Google Scholar]
- 148.Jia H, Li X, Gao H, Feng Z, Zhao L, Jia X, Zhang H, Liu J. High doses of nicotinamide prevent oxidative mitochondrial dysfunction in a cellular model and improve motor deficit in a Drosophila model of Parkinson’s disease. J Neurosci Res. 2008;86:2083–2090. doi: 10.1002/jnr.21650. [DOI] [PubMed] [Google Scholar]
- 149.Jiang H, Ren Y, Zhao J, Feng J. Parkin protects human dopaminergic neuroblastoma cells against dopamine-induced apoptosis. Hum Mol Genet. 2004;13:1745–1754. doi: 10.1093/hmg/ddh180. [DOI] [PubMed] [Google Scholar]
- 150.Jiang L, Gulanski BI, De Feyter HM, Weinzimer SA, Pittman B, Guidone E, Koretski J, Harman S, Petrakis IL, Krystal JH, Mason GF. Increased brain uptake and oxidation of acetate in heavy drinkers. J Clin Invest. 2013;123:1605–1614. doi: 10.1172/JCI65153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Jiang P, Gan M, Ebrahim AS, Castanedes-Casey M, Dickson DW, Yen SH. Adenosine monophosphate-activated protein kinase overactivation leads to accumulation of alpha-synuclein oligomers and decrease of neurites. Neurobiol Aging. 2013;34:1504–1515. doi: 10.1016/j.neurobiolaging.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Jimenez-Blasco D, Santofimia-Castano P, Gonzalez A, Almeida A, Bolanos JP. Astrocyte NMDA receptors’ activity sustains neuronal survival through a Cdk5-Nrf2 pathway. Cell Death Differ. 2015;22:1877–1889. doi: 10.1038/cdd.2015.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Jin J, Li GJ, Davis J, Zhu D, Wang Y, Pan C, Zhang J. Identification of novel proteins associated with both alpha-synuclein and DJ-1. Mol Cell Proteomics. 2007;6:845–859. doi: 10.1074/mcp.M600182-MCP200. [DOI] [PubMed] [Google Scholar]
- 154.Jin L, Wang J, Zhao L, Jin H, Fei G, Zhang Y, Zeng M, Zhong C. Decreased serum ceruloplasmin levels characteristically aggravate nigral iron deposition in Parkinson’s disease. Brain. 2011;134:50–58. doi: 10.1093/brain/awq319. [DOI] [PubMed] [Google Scholar]
- 155.Johnson DA, Johnson JA. Nrf2—a therapeutic target for the treatment of neurodegenerative diseases. Free Radic Biol Med. 2015;88:253–267. doi: 10.1016/j.freeradbiomed.2015.07.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Johri A, Beal MF. Antioxidants in Huntington’s disease. Biochim Biophys Acta. 2012;1822:664–674. doi: 10.1016/j.bbadis.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kalivendi SV, Kotamraju S, Cunningham S, Shang T, Hillard CJ, Kalyanaraman B. 1-Methyl-4-phenylpyridinium (MPP+)-induced apoptosis and mitochondrial oxidant generation: role of transferrin-receptor-dependent iron and hydrogen peroxide. Biochem J. 2003;371:151–164. doi: 10.1042/BJ20021525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kang MJ, Gil SJ, Koh HC. Paraquat induces alternation of the dopamine catabolic pathways and glutathione levels in the substantia nigra of mice. Toxicol Lett. 2009;188:148–152. doi: 10.1016/j.toxlet.2009.03.026. [DOI] [PubMed] [Google Scholar]
- 159.Kaul S, Kanthasamy A, Kitazawa M, Anantharam V, Kanthasamy AG. Caspase-3 dependent proteolytic activation of protein kinase C delta mediates and regulates 1-methyl-4-phenylpyridinium (MPP+)-induced apoptotic cell death in dopaminergic cells: relevance to oxidative stress in dopaminergic degeneration. Eur J Neurosci. 2003;18:1387–1401. doi: 10.1046/j.1460-9568.2003.02864.x. [DOI] [PubMed] [Google Scholar]
- 160.Keeney PM, Xie J, Capaldi RA, Bennett JP., Jr Parkinson’s disease brain mitochondrial complex I has oxidatively damaged subunits and is functionally impaired and misassembled. J Neurosci. 2006;26:5256–5264. doi: 10.1523/JNEUROSCI.0984-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kieburtz K, Tilley BC, Elm JJ, Babcock D, Hauser R, Ross GW, Augustine AH, Augustine EU, Aminoff MJ, Bodis-Wollner IG, Boyd J, Cambi F, Chou K, Christine CW, Cines M, Dahodwala N, Derwent L, Dewey RB, Jr, Hawthorne K, Houghton DJ, Kamp C, Leehey M, Lew MF, Liang GS, Luo ST, Mari Z, Morgan JC, Parashos S, Perez A, Petrovitch H, Rajan S, Reichwein S, Roth JT, Schneider JS, Shannon KM, Simon DK, Simuni T, Singer C, Sudarsky L, Tanner CM, Umeh CC, Williams K, Wills AM. Effect of creatine monohydrate on clinical progression in patients with Parkinson disease: a randomized clinical trial. JAMA. 2015;313:584–593. doi: 10.1001/jama.2015.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kim-Han JS, Antenor-Dorsey JA, O’Malley KL. The parkinsonian mimetic, MPP+, specifically impairs mitochondrial transport in dopamine axons. J Neurosci. 2011;31:7212–7221. doi: 10.1523/JNEUROSCI.0711-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kim C, Ho DH, Suk JE, You S, Michael S, Kang J, Joong Lee S, Masliah E, Hwang D, Lee HJ, Lee SJ. Neuron-released oligomeric alpha-synuclein is an endogenous agonist of TLR2 for paracrine activation of microglia. Nat Commun. 2013;4:1562. doi: 10.1038/ncomms2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kim TW, Cho HM, Choi SY, Suguira Y, Hayasaka T, Setou M, Koh HC, Hwang EM, Park JY, Kang SJ, Kim HS, Kim H, Sun W. (ADP-ribose) polymerase 1 and AMP-activated protein kinase mediate progressive dopaminergic neuronal degeneration in a mouse model of Parkinson’s disease. Cell Death Dis. 2013;4:e919. doi: 10.1038/cddis.2013.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Klein C, Westenberger A. Genetics of Parkinson’s disease. Cold Spring Harb Perspect Med. 2012;2:a008888. doi: 10.1101/cshperspect.a008888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Klivenyi P, Andreassen OA, Ferrante RJ, Dedeoglu A, Mueller G, Lancelot E, Bogdanov M, Andersen JK, Jiang D, Beal MF. Mice deficient in cellular glutathione peroxidase show increased vulnerability to malonate, 3-nitropropionic acid, and 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine. J Neurosci. 2000;20:1–7. doi: 10.1523/JNEUROSCI.20-01-00001.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Klivenyi P, St Clair D, Wermer M, Yen HC, Oberley T, Yang L, Flint Beal M. Manganese superoxide dismutase overexpression attenuates MPTP toxicity. Neurobiol Dis. 1998;5:253–258. doi: 10.1006/nbdi.1998.0191. [DOI] [PubMed] [Google Scholar]
- 168.Knight AL, Yan X, Hamamichi S, Ajjuri RR, Mazzulli JR, Zhang MW, Daigle JG, Zhang S, Borom AR, Roberts LR, Lee SK, DeLeon SM, Viollet-Djelassi C, Krainc D, O’Donnell JM, Caldwell KA, Caldwell GA. The glycolytic enzyme, GPI, is a functionally conserved modifier of dopaminergic neurodegeneration in Parkinson’s models. Cell Metab. 2014;20:145–157. doi: 10.1016/j.cmet.2014.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kurz A, Rabbani N, Walter M, Bonin M, Thornalley P, Auburger G, Gispert S. Alpha-synuclein deficiency leads to increased glyoxalase I expression and glycation stress. Cell Mol Life Sci. 2011;68:721–733. doi: 10.1007/s00018-010-0483-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kurz T, Eaton JW, Brunk UT. Redox activity within the lysosomal compartment: implications for aging and apoptosis. Antioxid Redox Signal. 2010;13:511–523. doi: 10.1089/ars.2009.3005. [DOI] [PubMed] [Google Scholar]
- 171.Kweon GR, Marks JD, Krencik R, Leung EH, Schumacker PT, Hyland K, Kang UJ. Distinct mechanisms of neurodegeneration induced by chronic complex I inhibition in dopaminergic and non-dopaminergic cells. J Biol Chem. 2004;279:51783–51792. doi: 10.1074/jbc.M407336200. [DOI] [PubMed] [Google Scholar]
- 172.Lamensdorf I, Eisenhofer G, Harvey-White J, Nechustan A, Kirk K, Kopin IJ. 3,4-Dihydroxyphenylacetaldehyde potentiates the toxic effects of metabolic stress in PC12 cells. Brain Res. 2000;868:191–201. doi: 10.1016/s0006-8993(00)02309-x. [DOI] [PubMed] [Google Scholar]
- 173.Langston JW, Ballard P, Tetrud JW, Irwin I. Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science. 1983;219:979–980. doi: 10.1126/science.6823561. [DOI] [PubMed] [Google Scholar]
- 174.Lannuzel A, Michel PP, Hoglinger GU, Champy P, Jousset A, Medja F, Lombes A, Darios F, Gleye C, Laurens A, Hocquemiller R, Hirsch EC, Ruberg M. The mitochondrial complex I inhibitor annonacin is toxic to mesencephalic dopaminergic neurons by impairment of energy metabolism. Neuroscience. 2003;121:287–296. doi: 10.1016/s0306-4522(03)00441-x. [DOI] [PubMed] [Google Scholar]
- 175.Larsen NJ, Ambrosi G, Mullett SJ, Berman SB, Hinkle DA. DJ-1 knock-down impairs astrocyte mitochondrial function. Neuroscience. 2011;196:251–264. doi: 10.1016/j.neuroscience.2011.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Lavoie S, Allaman I, Petit JM, Do KQ, Magistretti PJ. Altered glycogen metabolism in cultured astrocytes from mice with chronic glutathione deficit; relevance for neuroenergetics in schizophrenia. PLoS One. 2011;6:e22875. doi: 10.1371/journal.pone.0022875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Lee DW, Andersen JK. Iron elevations in the aging Parkinsonian brain: a consequence of impaired iron homeostasis? J Neurochem. 2010;112:332–339. doi: 10.1111/j.1471-4159.2009.06470.x. [DOI] [PubMed] [Google Scholar]
- 178.Lee HS, Park CW, Kim YS. MPP(+) increases the vulnerability to oxidative stress rather than directly mediating oxidative damage in human neuroblastoma cells. Exp Neurol. 2000;165:164–171. doi: 10.1006/exnr.2000.7460. [DOI] [PubMed] [Google Scholar]
- 179.Lee JY, Song J, Kwon K, Jang S, Kim C, Baek K, Kim J, Park C. Human DJ-1 and its homologs are novel glyoxalases. Hum Mol Genet. 2012;21:3215–3225. doi: 10.1093/hmg/dds155. [DOI] [PubMed] [Google Scholar]
- 180.Lee S, Zhang C, Liu X. Role of glucose metabolism and ATP in maintaining PINK1 levels during Parkin-mediated mitochondrial damage responses. J Biol Chem. 2015;290:904–917. doi: 10.1074/jbc.M114.606798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Lee Y, Karuppagounder SS, Shin JH, Lee YI, Ko HS, Swing D, Jiang H, Kang SU, Lee BD, Kang HC, Kim D, Tessarollo L, Dawson VL, Dawson TM. Parthanatos mediates AIMP2-activated age-dependent dopaminergic neuronal loss. Nat Neurosci. 2013;16:1392–1400. doi: 10.1038/nn.3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Lee Y, Morrison BM, Li Y, Lengacher S, Farah MH, Hoffman PN, Liu Y, Tsingalia A, Jin L, Zhang PW, Pellerin L, Magistretti PJ, Rothstein JD. Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature. 2012 doi: 10.1038/nature11314. advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Lee Y, Morrison BM, Li Y, Lengacher S, Farah MH, Hoffman PN, Liu Y, Tsingalia A, Jin L, Zhang PW, Pellerin L, Magistretti PJ, Rothstein JD. Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature. 2012;487:443–448. doi: 10.1038/nature11314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Lee YM, Park SH, Shin DI, Hwang JY, Park B, Park YJ, Lee TH, Chae HZ, Jin BK, Oh TH, Oh YJ. Oxidative modification of peroxiredoxin is associated with drug-induced apoptotic signaling in experimental models of Parkinson disease. J Biol Chem. 2008;283:9986–9998. doi: 10.1074/jbc.M800426200. [DOI] [PubMed] [Google Scholar]
- 185.Lehmann S, Costa AC, Celardo I, Loh SH, Martins LM. Parp mutations protect against mitochondrial dysfunction and neurodegeneration in a PARKIN model of Parkinson’s disease. Cell Death Dis. 2016;7:e2166. doi: 10.1038/cddis.2016.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Lei S, Zavala-Flores L, Garcia-Garcia A, Nandakumar R, Huang Y, Madayiputhiya N, Stanton RC, Dodds ED, Powers R, Franco R. Alterations in energy/redox metabolism induced by mitochondrial and environmental toxins: a specific role for glucose-6-phosphate-dehydrogenase and the pentose phosphate pathway in paraquat toxicity. ACS Chem Biol. 2014;9:2032–2048. doi: 10.1021/cb400894a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Leiphon LJ, Picklo MJ., Sr Inhibition of aldehyde detoxification in CNS mitochondria by fungicides. Neurotoxicology. 2007;28:143–149. doi: 10.1016/j.neuro.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 188.Levy OA, Malagelada C, Greene LA. Cell death pathways in Parkinson’s disease: proximal triggers, distal effectors, and final steps. Apoptosis. 2009;14:478–500. doi: 10.1007/s10495-008-0309-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Lewerenz J, Maher P. Basal levels of eIF2alpha phosphorylation determine cellular antioxidant status by regulating ATF4 and xCT expression. J Biol Chem. 2009;284:1106–1115. doi: 10.1074/jbc.M807325200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Liang CL, Wang TT, Luby-Phelps K, German DC. Mitochondria mass is low in mouse substantia nigra dopamine neurons: implications for Parkinson’s disease. Exp Neurol. 2007;203:370–380. doi: 10.1016/j.expneurol.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 191.Liang LP, Huang J, Fulton R, Day BJ, Patel M. An orally active catalytic metalloporphyrin protects against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine neurotoxicity in vivo. J Neurosci. 2007;27:4326–4333. doi: 10.1523/JNEUROSCI.0019-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Liang LP, Kavanagh TJ, Patel M. Glutathione deficiency in Gclm null mice results in complex I inhibition and dopamine depletion following paraquat administration. Toxicol Sci. 2013;134:366–373. doi: 10.1093/toxsci/kft112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Liang LP, Patel M. Iron-sulfur enzyme mediated mitochondrial superoxide toxicity in experimental Parkinson’s disease. J Neurochem. 2004;90:1076–1084. doi: 10.1111/j.1471-4159.2004.02567.x. [DOI] [PubMed] [Google Scholar]
- 194.Liberatore GT, Jackson-Lewis V, Vukosavic S, Mandir AS, Vila M, McAuliffe WG, Dawson VL, Dawson TM, Przedborski S. Inducible nitric oxide synthase stimulates dopaminergic neurodegeneration in the MPTP model of Parkinson disease. Nat Med. 1999;5:1403–1409. doi: 10.1038/70978. [DOI] [PubMed] [Google Scholar]
- 195.Linsenbardt AJ, Breckenridge JM, Wilken GH, Macarthur H. Dopaminochrome induces caspase-independent apoptosis in the mesencephalic cell line, MN9D. J Neurochem. 2012;122:175–184. doi: 10.1111/j.1471-4159.2012.07756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Liu G, Yu J, Ding J, Xie C, Sun L, Rudenko I, Zheng W, Sastry N, Luo J, Rudow G, Troncoso JC, Cai H. Aldehyde dehydrogenase 1 defines and protects a nigrostriatal dopaminergic neuron subpopulation. J Clin Invest. 2014;124:3032–3046. doi: 10.1172/JCI72176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Liu HQ, Zhu XZ, Weng EQ. Intracellular dopamine oxidation mediates rotenone-induced apoptosis in PC12 cells. Acta Pharmacol Sin. 2005;26:17–26. doi: 10.1111/j.1745-7254.2005.00003.x. [DOI] [PubMed] [Google Scholar]
- 198.Liu K, Li F, Han H, Chen Y, Mao Z, Luo J, Zhao Y, Zheng B, Gu W, Zhao W. Parkin Regulates the Activity of Pyruvate Kinase M2. J Biol Chem. 2016;291:10307–10317. doi: 10.1074/jbc.M115.703066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Liu S, Sawada T, Lee S, Yu W, Silverio G, Alapatt P, Millan I, Shen A, Saxton W, Kanao T, Takahashi R, Hattori N, Imai Y, Lu B. Parkinson’s disease-associated kinase PINK1 regulates Miro protein level and axonal transport of mitochondria. PLoS Genet. 2012;8:e1002537. doi: 10.1371/journal.pgen.1002537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Long-Smith CM, Sullivan AM, Nolan YM. The influence of microglia on the pathogenesis of Parkinson’s disease. Prog Neurobiol. 2009;89:277–287. doi: 10.1016/j.pneurobio.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 201.Lopert P, Day BJ, Patel M. Thioredoxin reductase deficiency potentiates oxidative stress, mitochondrial dysfunction and cell death in dopaminergic cells. PLoS One. 2012;7:e50683. doi: 10.1371/journal.pone.0050683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Lopert P, Patel M. Nicotinamide nucleotide transhydrogenase (Nnt) links the substrate requirement in brain mitochondria for hydrogen peroxide removal to the thioredoxin/peroxiredoxin (Trx/Prx) system. J Biol Chem. 2014;289:15611–15620. doi: 10.1074/jbc.M113.533653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Lotharius J, Barg S, Wiekop P, Lundberg C, Raymon HK, Brundin P. Effect of mutant alpha-synuclein on dopamine homeostasis in a new human mesencephalic cell line. J Biol Chem. 2002;277:38884–38894. doi: 10.1074/jbc.M205518200. [DOI] [PubMed] [Google Scholar]
- 204.Lotharius J, Brundin P. Impaired dopamine storage resulting from alpha-synuclein mutations may contribute to the pathogenesis of Parkinson’s disease. Hum Mol Genet. 2002;11:2395–2407. doi: 10.1093/hmg/11.20.2395. [DOI] [PubMed] [Google Scholar]
- 205.Lu L, Tang L, Wei W, Hong Y, Chen H, Ying W, Chen S. Nicotinamide mononucleotide improves energy activity and survival rate in an in vitro model of Parkinson’s disease. Exp Ther Med. 2014;8:943–950. doi: 10.3892/etm.2014.1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Luo S, Lei H, Qin H, Xia Y. Molecular mechanisms of endothelial NO synthase uncoupling. Curr Pharm Des. 2014;20:3548–3553. doi: 10.2174/13816128113196660746. [DOI] [PubMed] [Google Scholar]
- 207.Lutz AK, Exner N, Fett ME, Schlehe JS, Kloos K, Lammermann K, Brunner B, Kurz-Drexler A, Vogel F, Reichert AS, Bouman L, Vogt-Weisenhorn D, Wurst W, Tatzelt J, Haass C, Winklhofer KF. Loss of parkin or PINK1 function increases Drp1-dependent mitochondrial fragmentation. J Biol Chem. 2009;284:22938–22951. doi: 10.1074/jbc.M109.035774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Magistretti PJ. Role of glutamate in neuron-glia metabolic coupling. Am J Clin Nutr. 2009;90:875S–880S. doi: 10.3945/ajcn.2009.27462CC. [DOI] [PubMed] [Google Scholar]
- 209.Magistretti PJ, Allaman I. A cellular perspective on brain energy metabolism and functional imaging. Neuron. 2015;86:883–901. doi: 10.1016/j.neuron.2015.03.035. [DOI] [PubMed] [Google Scholar]
- 210.Mallajosyula JK, Chinta SJ, Rajagopalan S, Nicholls DG, Andersen JK. Metabolic control analysis in a cellular model of elevated MAO-B: relevance to Parkinson’s disease. Neurotox Res. 2009;16:186–193. doi: 10.1007/s12640-009-9032-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Mandir AS, Przedborski S, Jackson-Lewis V, Wang ZQ, Simbulan-Rosenthal CM, Smulson ME, Hoffman BE, Guastella DB, Dawson VL, Dawson TM. Poly(ADP-ribose) polymerase activation mediates 1-methyl-4-phenyl-1, 2,3,6-tetrahydropyridine (MPTP)-induced parkinsonism. Proc Natl Acad Sci U S A. 1999;96:5774–5779. doi: 10.1073/pnas.96.10.5774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Margolis AS, Porasuphatana S, Rosen GM. Role of paraquat in the uncoupling of nitric oxide synthase. Biochim Biophys Acta. 2000;1524:253–257. doi: 10.1016/s0304-4165(00)00167-7. [DOI] [PubMed] [Google Scholar]
- 213.Marosi K, Mattson MP. BDNF mediates adaptive brain and body responses to energetic challenges. Trends Endocrinol Metab. 2014;25:89–98. doi: 10.1016/j.tem.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Martin FL, Williamson SJ, Paleologou KE, Hewitt R, El-Agnaf OM, Allsop D. Fe(II)-induced DNA damage in alpha-synuclein-transfected human dopaminergic BE(2)-M17 neuroblastoma cells: detection by the Comet assay. J Neurochem. 2003;87:620–630. doi: 10.1046/j.1471-4159.2003.02013.x. [DOI] [PubMed] [Google Scholar]
- 215.Martin LJ. Biology of mitochondria in neurodegenerative diseases. Prog Mol Biol Transl Sci. 2012;107:355–415. doi: 10.1016/B978-0-12-385883-2.00005-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Martinez TN, Greenamyre JT. Toxin models of mitochondrial dysfunction in Parkinson’s disease. Antioxid Redox Signal. 2012;16:920–934. doi: 10.1089/ars.2011.4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Maruoka N, Murata T, Omata N, Takashima Y, Fujibayashi Y, Wada Y. Biphasic mechanism of the toxicity induced by 1-methyl-4-phenylpyridinium ion (MPP+) as revealed by dynamic changes in glucose metabolism in rat brain slices. Neurotoxicology. 2007;28:672–678. doi: 10.1016/j.neuro.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 218.Matsuda W, Furuta T, Nakamura KC, Hioki H, Fujiyama F, Arai R, Kaneko T. Single nigrostriatal dopaminergic neurons form widely spread and highly dense axonal arborizations in the neostriatum. J Neurosci. 2009;29:444–453. doi: 10.1523/JNEUROSCI.4029-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Matthews RT, Ferrante RJ, Klivenyi P, Yang L, Klein AM, Mueller G, Kaddurah-Daouk R, Beal MF. Creatine and cyclocreatine attenuate MPTP neurotoxicity. Exp Neurol. 1999;157:142–149. doi: 10.1006/exnr.1999.7049. [DOI] [PubMed] [Google Scholar]
- 220.Mazzio E, Soliman KF. D-(+)-glucose rescue against 1-methyl-4-phenylpyridinium toxicity through anaerobic glycolysis in neuroblastoma cells. Brain Res. 2003;962:48–60. doi: 10.1016/s0006-8993(02)03695-8. [DOI] [PubMed] [Google Scholar]
- 221.McCoy MK, Cookson MR. Mitochondrial quality control and dynamics in Parkinson’s disease. Antioxid Redox Signal. 2012;16:869–882. doi: 10.1089/ars.2011.4019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.McFarland MA, Ellis CE, Markey SP, Nussbaum RL. Proteomics analysis identifies phosphorylation-dependent alpha-synuclein protein interactions. Mol Cell Proteomics. 2008;7:2123–2137. doi: 10.1074/mcp.M800116-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.McLelland GL, Soubannier V, Chen CX, McBride HM, Fon EA. Parkin and PINK1 function in a vesicular trafficking pathway regulating mitochondrial quality control. EMBO J. 2014;33:282–295. doi: 10.1002/embj.201385902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.McNaught KS, Jenner P. Extracellular accumulation of nitric oxide, hydrogen peroxide, and glutamate in astrocytic cultures following glutathione depletion, complex I inhibition, and/or lipopolysaccharide-induced activation. Biochem Pharmacol. 2000;60:979–988. doi: 10.1016/s0006-2952(00)00415-9. [DOI] [PubMed] [Google Scholar]
- 225.Mecocci P, Polidori MC. Antioxidant clinical trials in mild cognitive impairment and Alzheimer’s disease. Biochim Biophys Acta. 2012;1822:631–638. doi: 10.1016/j.bbadis.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 226.Meister A. Glutathione biosynthesis and its inhibition. Methods Enzymol. 1995;252:26–30. doi: 10.1016/0076-6879(95)52005-8. [DOI] [PubMed] [Google Scholar]
- 227.Mejias R, Villadiego J, Pintado CO, Vime PJ, Gao L, Toledo-Aral JJ, Echevarria M, Lopez-Barneo J. Neuroprotection by transgenic expression of glucose-6-phosphate dehydrogenase in dopaminergic nigrostriatal neurons of mice. J Neurosci. 2006;26:4500–4508. doi: 10.1523/JNEUROSCI.0122-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Mena MA, Garcia de Yebenes J. Glial cells as players in parkinsonism: the “good,” the “bad,” and the “mysterious” glia. Neuroscientist. 2008;14:544–560. doi: 10.1177/1073858408322839. [DOI] [PubMed] [Google Scholar]
- 229.Mena NP, Bulteau AL, Salazar J, Hirsch EC, Nunez MT. Effect of mitochondrial complex I inhibition on Fe-S cluster protein activity. Biochem Biophys Res Commun. 2011;409:241–246. doi: 10.1016/j.bbrc.2011.04.137. [DOI] [PubMed] [Google Scholar]
- 230.Mercado G, Valdes P, Hetz C. An ERcentric view of Parkinson’s disease. Trends Mol Med. 2013;19:165–175. doi: 10.1016/j.molmed.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 231.Mergenthaler P, Lindauer U, Dienel GA, Meisel A. Sugar for the brain: the role of glucose in physiological and pathological brain function. Trends Neurosci. 2013;36:587–597. doi: 10.1016/j.tins.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Meyer MJ, Mosely DE, Amarnath V, Picklo MJ., Sr Metabolism of 4-hydroxy-trans-2-nonenal by central nervous system mitochondria is dependent on age and NAD+ availability. Chem Res Toxicol. 2004;17:1272–1279. doi: 10.1021/tx049843k. [DOI] [PubMed] [Google Scholar]
- 233.Miersch S, Espey MG, Chaube R, Akarca A, Tweten R, Ananvoranich S, Mutus B. Plasma membrane cholesterol content affects nitric oxide diffusion dynamics and signaling. J Biol Chem. 2008;283:18513–18521. doi: 10.1074/jbc.M800440200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Moehle MS, Webber PJ, Tse T, Sukar N, Standaert DG, DeSilva TM, Cowell RM, West AB. LRRK2 inhibition attenuates microglial inflammatory responses. J Neurosci. 2012;32:1602–1611. doi: 10.1523/JNEUROSCI.5601-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Morais VA, Haddad D, Craessaerts K, De Bock PJ, Swerts J, Vilain S, Aerts L, Overbergh L, Grunewald A, Seibler P, Klein C, Gevaert K, Verstreken P, De Strooper B. PINK1 loss-of-function mutations affect mitochondrial complex I activity via NdufA10 ubiquinone uncoupling. Science. 2014;344:203–207. doi: 10.1126/science.1249161. [DOI] [PubMed] [Google Scholar]
- 236.Morais VA, Verstreken P, Roethig A, Smet J, Snellinx A, Vanbrabant M, Haddad D, Frezza C, Mandemakers W, Vogt-Weisenhorn D, Van Coster R, Wurst W, Scorrano L, De Strooper B. Parkinson’s disease mutations in PINK1 result in decreased Complex I activity and deficient synaptic function. EMBO Mol Med. 2009;1:99–111. doi: 10.1002/emmm.200900006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Morrison BM, Lee Y, Rothstein JD. Oligodendroglia metabolically support axons and maintain structural integrity. Trends in cell biology. 2013;23 doi: 10.1016/j.tcb.2013.1007.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Motori E, Puyal J, Toni N, Ghanem A, Angeloni C, Malaguti M, Cantelli-Forti G, Berninger B, Conzelmann KK, Gotz M, Winklhofer KF, Hrelia S, Bergami M. Inflammation-induced alteration of astrocyte mitochondrial dynamics requires autophagy for mitochondrial network maintenance. Cell Metab. 2013;18:844–859. doi: 10.1016/j.cmet.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 239.Mullett SJ, Hinkle DA. DJ-1 knock-down in astrocytes impairs astrocyte-mediated neuroprotection against rotenone. Neurobiol Dis. 2009;33:28–36. doi: 10.1016/j.nbd.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Mullett SJ, Hinkle DA. DJ-1 deficiency in astrocytes selectively enhances mitochondrial Complex I inhibitor-induced neurotoxicity. J Neurochem. 2011;117:375–387. doi: 10.1111/j.1471-4159.2011.07175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Nakamura K, Bindokas VP, Marks JD, Wright DA, Frim DM, Miller RJ, Kang UJ. The selective toxicity of 1-methyl-4-phenylpyridinium to dopaminergic neurons: the role of mitochondrial complex I and reactive oxygen species revisited. Mol Pharmacol. 2000;58:271–278. doi: 10.1124/mol.58.2.271. [DOI] [PubMed] [Google Scholar]
- 242.Nakamura K, Nemani VM, Azarbal F, Skibinski G, Levy JM, Egami K, Munishkina L, Zhang J, Gardner B, Wakabayashi J, Sesaki H, Cheng Y, Finkbeiner S, Nussbaum RL, Masliah E, Edwards RH. Direct membrane association drives mitochondrial fission by the Parkinson disease-associated protein alpha-synuclein. J Biol Chem. 2011;286:20710–20726. doi: 10.1074/jbc.M110.213538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Newsholme EA, Sugden PH, Williams T. Effect of citrate on the activities of 6-phosphofructokinase from nervous and muscle tissues from different animals and its relationships to the regulation of glycolysis. Biochem J. 1977;166:123–129. doi: 10.1042/bj1660123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Ng CH, Guan MS, Koh C, Ouyang X, Yu F, Tan EK, O’Neill SP, Zhang X, Chung J, Lim KL. AMP kinase activation mitigates dopaminergic dysfunction and mitochondrial abnormalities in Drosophila models of Parkinson’s disease. J Neurosci. 2012;32:14311–14317. doi: 10.1523/JNEUROSCI.0499-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Nishida Y, Rardin MJ, Carrico C, He W, Sahu AK, Gut P, Najjar R, Fitch M, Hellerstein M, Gibson BW, Verdin E. SIRT5 Regulates both Cytosolic and Mitochondrial Protein Malonylation with Glycolysis as a Major Target. Mol Cell. 2015;59:321–332. doi: 10.1016/j.molcel.2015.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Niu J, Yu M, Wang C, Xu Z. Leucine-rich repeat kinase 2 disturbs mitochondrial dynamics via Dynamin-like protein. J Neurochem. 2012;122:650–658. doi: 10.1111/j.1471-4159.2012.07809.x. [DOI] [PubMed] [Google Scholar]
- 247.Obermeier B, Daneman R, Ransohoff RM. Development, maintenance and disruption of the blood-brain barrier. Nat Med. 2013;19:1584–1596. doi: 10.1038/nm.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Olzscha H, Schermann SM, Woerner AC, Pinkert S, Hecht MH, Tartaglia GG, Vendruscolo M, Hayer-Hartl M, Hartl FU, Vabulas RM. Amyloid-like aggregates sequester numerous metastable proteins with essential cellular functions. Cell. 2011;144:67–78. doi: 10.1016/j.cell.2010.11.050. [DOI] [PubMed] [Google Scholar]
- 249.Orihuela R, McPherson CA, Harry GJ. Microglial M1/M2 polarization and metabolic states. Br J Pharmacol. 2016;173:649–665. doi: 10.1111/bph.13139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Osborne B, Bentley NL, Montgomery MK, Turner N. The role of mitochondrial sirtuins in health and disease. Free Radic Biol Med. 2016 doi: 10.1016/j.freeradbiomed.2016.04.197. [DOI] [PubMed] [Google Scholar]
- 251.Ostrerova-Golts N, Petrucelli L, Hardy J, Lee JM, Farer M, Wolozin B. The A53T alpha-synuclein mutation increases iron-dependent aggregation and toxicity. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20:6048–6054. doi: 10.1523/JNEUROSCI.20-16-06048.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Outeiro TF, Grammatopoulos TN, Altmann S, Amore A, Standaert DG, Hyman BT, Kazantsev AG. Pharmacological inhibition of PARP-1 reduces alpha-synuclein- and MPP+-induced cytotoxicity in Parkinson’s disease in vitro models. Biochem Biophys Res Commun. 2007;357:596–602. doi: 10.1016/j.bbrc.2007.03.163. [DOI] [PubMed] [Google Scholar]
- 253.Pacelli C, Giguere N, Bourque MJ, Levesque M, Slack RS, Trudeau LE. Elevated Mitochondrial Bioenergetics and Axonal Arborization Size Are Key Contributors to the Vulnerability of Dopamine Neurons. Curr Biol. 2015;25:2349–2360. doi: 10.1016/j.cub.2015.07.050. [DOI] [PubMed] [Google Scholar]
- 254.Palacino JJ, Sagi D, Goldberg MS, Krauss S, Motz C, Wacker M, Klose J, Shen J. Mitochondrial dysfunction and oxidative damage in parkin-deficient mice. J Biol Chem. 2004;279:18614–18622. doi: 10.1074/jbc.M401135200. [DOI] [PubMed] [Google Scholar]
- 255.Palombo E, Porrino LJ, Bankiewicz KS, Crane AM, Sokoloff L, Kopin IJ. Local cerebral glucose utilization in monkeys with hemiparkinsonism induced by intracarotid infusion of the neurotoxin MPTP. J Neurosci. 1990;10:860–869. doi: 10.1523/JNEUROSCI.10-03-00860.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Papkovskaia TD, Chau KY, Inesta-Vaquera F, Papkovsky DB, Healy DG, Nishio K, Staddon J, Duchen MR, Hardy J, Schapira AH, Cooper JM. G2019S leucine-rich repeat kinase 2 causes uncoupling protein-mediated mitochondrial depolarization. Hum Mol Genet. 2012;21:4201–4213. doi: 10.1093/hmg/dds244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Pathak D, Berthet A, Nakamura K. Energy failure: does it contribute to neurodegeneration? Ann Neurol. 2013;74:506–516. doi: 10.1002/ana.24014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Pellerin L, Magistretti PJ. Sweet sixteen for ANLS. J Cereb Blood Flow Metab. 2012;32:1152–1166. doi: 10.1038/jcbfm.2011.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Perier C, Vila M. Mitochondrial biology and Parkinson’s disease. Cold Spring Harb Perspect Med. 2012;2:a009332. doi: 10.1101/cshperspect.a009332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Perry TL, Yong VW. Idiopathic Parkinson’s disease, progressive supranuclear palsy and glutathione metabolism in the substantia nigra of patients. Neurosci Lett. 1986;67:269–274. doi: 10.1016/0304-3940(86)90320-4. [DOI] [PubMed] [Google Scholar]
- 261.Peters A, Palay SL, Webster HD. The fine structure of the nervous system : neurons and their supporting cells. 3rd. Oxford University Press; New York: 1991. [Google Scholar]
- 262.Pissadaki EK, Bolam JP. The energy cost of action potential propagation in dopamine neurons: clues to susceptibility in Parkinson’s disease. Front Comput Neurosci. 2013;7:13. doi: 10.3389/fncom.2013.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Prigione A, Begni B, Galbussera A, Beretta S, Brighina L, Garofalo R, Andreoni S, Piolti R, Ferrarese C. Oxidative stress in peripheral blood mononuclear cells from patients with Parkinson’s disease: negative correlation with levodopa dosage. Neurobiol Dis. 2006;23:36–43. doi: 10.1016/j.nbd.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 264.Prigione A, Isaias IU, Galbussera A, Brighina L, Begni B, Andreoni S, Pezzoli G, Antonini A, Ferrarese C. Increased oxidative stress in lymphocytes from untreated Parkinson’s disease patients. Parkinsonism Relat Disord. 2009;15:327–328. doi: 10.1016/j.parkreldis.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 265.Przedborski S, Jackson-Lewis V, Yokoyama R, Shibata T, Dawson VL, Dawson TM. Role of neuronal nitric oxide in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced dopaminergic neurotoxicity. Proc Natl Acad Sci U S A. 1996;93:4565–4571. doi: 10.1073/pnas.93.10.4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Przedborski S, Kostic V, Jackson-Lewis V, Naini AB, Simonetti S, Fahn S, Carlson E, Epstein CJ, Cadet JL. Transgenic mice with increased Cu/Zn-superoxide dismutase activity are resistant to N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurotoxicity. J Neurosci. 1992;12:1658–1667. doi: 10.1523/JNEUROSCI.12-05-01658.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Raichle ME, Gusnard DA. Appraising the brain’s energy budget. Proc Natl Acad Sci U S A. 2002;99:10237–10239. doi: 10.1073/pnas.172399499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Rardin MJ, He W, Nishida Y, Newman JC, Carrico C, Danielson SR, Guo A, Gut P, Sahu AK, Li B, Uppala R, Fitch M, Riiff T, Zhu L, Zhou J, Mulhern D, Stevens RD, Ilkayeva OR, Newgard CB, Jacobson MP, Hellerstein M, Goetzman ES, Gibson BW, Verdin E. SIRT5 regulates the mitochondrial lysine succinylome and metabolic networks. Cell Metab. 2013;18:920–933. doi: 10.1016/j.cmet.2013.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Rardin MJ, Newman JC, Held JM, Cusack MP, Sorensen DJ, Li B, Schilling B, Mooney SD, Kahn CR, Verdin E, Gibson BW. Label-free quantitative proteomics of the lysine acetylome in mitochondria identifies substrates of SIRT3 in metabolic pathways. Proc Natl Acad Sci U S A. 2013;110:6601–6606. doi: 10.1073/pnas.1302961110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Rees JN, Florang VR, Anderson DG, Doorn JA. Lipid peroxidation products inhibit dopamine catabolism yielding aberrant levels of a reactive intermediate. Chem Res Toxicol. 2007;20:1536–1542. doi: 10.1021/tx700248y. [DOI] [PubMed] [Google Scholar]
- 271.Reeve A, Meagher M, Lax N, Simcox E, Hepplewhite P, Jaros E, Turnbull D. The impact of pathogenic mitochondrial DNA mutations on substantia nigra neurons. J Neurosci. 2013;33:10790–10801. doi: 10.1523/JNEUROSCI.3525-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Requejo-Aguilar R, Lopez-Fabuel I, Fernandez E, Martins LM, Almeida A, Bolanos JP. PINK1 deficiency sustains cell proliferation by reprogramming glucose metabolism through HIF1. Nat Commun. 2014;5:4514. doi: 10.1038/ncomms5514. [DOI] [PubMed] [Google Scholar]
- 273.Requejo-Aguilar R, Lopez-Fabuel I, Jimenez-Blasco D, Fernandez E, Almeida A, Bolanos JP. DJ1 represses glycolysis and cell proliferation by transcriptionally up-regulating Pink1. Biochem J. 2015;467:303–310. doi: 10.1042/BJ20141025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Reynolds AD, Glanzer JG, Kadiu I, Ricardo-Dukelow M, Chaudhuri A, Ciborowski P, Cerny R, Gelman B, Thomas MP, Mosley RL, Gendelman HE. Nitrated alpha-synuclein-activated microglial profiling for Parkinson’s disease. J Neurochem. 2008;104:1504–1525. doi: 10.1111/j.1471-4159.2007.05087.x. [DOI] [PubMed] [Google Scholar]
- 275.Reynolds AD, Stone DK, Hutter JA, Benner EJ, Mosley RL, Gendelman HE. Regulatory T cells attenuate Th17 cell-mediated nigrostriatal dopaminergic neurodegeneration in a model of Parkinson’s disease. J Immunol. 2010;184:2261–2271. doi: 10.4049/jimmunol.0901852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Rezai-Zadeh K, Gate D, Town T. CNS infiltration of peripheral immune cells: D-Day for neurodegenerative disease? J Neuroimmune Pharmacol. 2009;4:462–475. doi: 10.1007/s11481-009-9166-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Ridet JL, Bensadoun JC, Deglon N, Aebischer P, Zurn AD. Lentivirus-mediated expression of glutathione peroxidase: neuroprotection in murine models of Parkinson’s disease. Neurobiol Dis. 2006;21:29–34. doi: 10.1016/j.nbd.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 278.Rodriguez-Rocha H, Garcia-Garcia A, Pickett C, Li S, Jones J, Chen H, Webb B, Choi J, Zhou Y, Zimmerman MC, Franco R. Compartmentalized oxidative stress in dopaminergic cell death induced by pesticides and complex I inhibitors: distinct roles of superoxide anion and superoxide dismutases. Free Radic Biol Med. 2013;61:370–383. doi: 10.1016/j.freeradbiomed.2013.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Rolfe DF, Brown GC. Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiological Reviews. 1997;77:731–758. doi: 10.1152/physrev.1997.77.3.731. [DOI] [PubMed] [Google Scholar]
- 280.Russo I, Berti G, Plotegher N, Bernardo G, Filograna R, Bubacco L, Greggio E. Leucine-rich repeat kinase 2 positively regulates inflammation and down-regulates NF-kappaB p50 signaling in cultured microglia cells. J Neuroinflammation. 2015;12:230. doi: 10.1186/s12974-015-0449-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Saggu H, Cooksey J, Dexter D, Wells FR, Lees A, Jenner P, Marsden CD. A selective increase in particulate superoxide dismutase activity in parkinsonian substantia nigra. J Neurochem. 1989;53:692–697. doi: 10.1111/j.1471-4159.1989.tb11759.x. [DOI] [PubMed] [Google Scholar]
- 282.Sanchez-Ramos JR, Song S, Facca A, Basit A, Epstein CJ. Transgenic murine dopaminergic neurons expressing human Cu/Zn superoxide dismutase exhibit increased density in culture, but no resistance to methylphenylpyridinium-induced degeneration. J Neurochem. 1997;68:58–67. doi: 10.1046/j.1471-4159.1997.68010058.x. [DOI] [PubMed] [Google Scholar]
- 283.Schapira AH. Mitochondria in the aetiology and pathogenesis of Parkinson’s disease. Lancet Neurol. 2008;7:97–109. doi: 10.1016/S1474-4422(07)70327-7. [DOI] [PubMed] [Google Scholar]
- 284.Schapira AH, Cooper JM, Dexter D, Clark JB, Jenner P, Marsden CD. Mitochondrial complex I deficiency in Parkinson’s disease. J Neurochem. 1990;54:823–827. doi: 10.1111/j.1471-4159.1990.tb02325.x. [DOI] [PubMed] [Google Scholar]
- 285.Schapira AH, Cooper JM, Dexter D, Jenner P, Clark JB, Marsden CD. Mitochondrial complex I deficiency in Parkinson’s disease. Lancet. 1989;1:1269. doi: 10.1016/s0140-6736(89)92366-0. [DOI] [PubMed] [Google Scholar]
- 286.Schmidt S, Linnartz B, Mendritzki S, Sczepan T, Lubbert M, Stichel CC, Lubbert H. Genetic mouse models for Parkinson’s disease display severe pathology in glial cell mitochondria. Hum Mol Genet. 2011;20:1197–1211. doi: 10.1093/hmg/ddq564. [DOI] [PubMed] [Google Scholar]
- 287.Schonfeld P, Reiser G. Why does brain metabolism not favor burning of fatty acids to provide energy? Reflections on disadvantages of the use of free fatty acids as fuel for brain. J Cereb Blood Flow Metab. 2013;33:1493–1499. doi: 10.1038/jcbfm.2013.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Schousboe A, Sickmann HM, Walls AB, Bak LK, Waagepetersen HS. Functional importance of the astrocytic glycogen-shunt and glycolysis for maintenance of an intact intra/extracellular glutamate gradient. Neurotox Res. 2010;18:94–99. doi: 10.1007/s12640-010-9171-5. [DOI] [PubMed] [Google Scholar]
- 289.Schreiner B, Romanelli E, Liberski P, Ingold-Heppner B, Sobottka-Brillout B, Hartwig T, Chandrasekar V, Johannssen H, Zeilhofer HU, Aguzzi A, Heppner F, Kerschensteiner M, Becher B. Astrocyte Depletion Impairs Redox Homeostasis and Triggers Neuronal Loss in the Adult CNS. Cell Rep. 2015;12:1377–1384. doi: 10.1016/j.celrep.2015.07.051. [DOI] [PubMed] [Google Scholar]
- 290.Schurr A. Cerebral glycolysis: a century of persistent misunderstanding and misconception. Front Neurosci. 2014;8:360. doi: 10.3389/fnins.2014.00360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Segura-Aguilar J, Baez S, Widersten M, Welch CJ, Mannervik B. Human class Mu glutathione transferases, in particular isoenzyme M2-2, catalyze detoxication of the dopamine metabolite aminochrome. J Biol Chem. 1997;272:5727–5731. doi: 10.1074/jbc.272.9.5727. [DOI] [PubMed] [Google Scholar]
- 292.Shi SY, Lu SY, Sivasubramaniyam T, Revelo XS, Cai EP, Luk CT, Schroer SA, Patel P, Kim RH, Bombardier E, Quadrilatero J, Tupling AR, Mak TW, Winer DA, Woo M. DJ-1 links muscle ROS production with metabolic reprogramming and systemic energy homeostasis in mice. Nat Commun. 2015;6:7415. doi: 10.1038/ncomms8415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Shih AY, Johnson DA, Wong G, Kraft AD, Jiang L, Erb H, Johnson JA, Murphy TH. Coordinate regulation of glutathione biosynthesis and release by Nrf2-expressing glia potently protects neurons from oxidative stress. J Neurosci. 2003;23:3394–3406. doi: 10.1523/JNEUROSCI.23-08-03394.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Shin JH, Ko HS, Kang H, Lee Y, Lee YI, Pletinkova O, Troconso JC, Dawson VL, Dawson TM. PARIS (ZNF746) repression of PGC-1alpha contributes to neurodegeneration in Parkinson’s disease. Cell. 2011;144:689–702. doi: 10.1016/j.cell.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Sian-Hulsmann J, Mandel S, Youdim MB, Riederer P. The relevance of iron in the pathogenesis of Parkinson’s disease. J Neurochem. 2011;118:939–957. doi: 10.1111/j.1471-4159.2010.07132.x. [DOI] [PubMed] [Google Scholar]
- 296.Sian J, Dexter DT, Lees AJ, Daniel S, Agid Y, Javoy-Agid F, Jenner P, Marsden CD. Alterations in glutathione levels in Parkinson’s disease and other neurodegenerative disorders affecting basal ganglia. Ann Neurol. 1994;36:348–355. doi: 10.1002/ana.410360305. [DOI] [PubMed] [Google Scholar]
- 297.Sies H. Glutathione and its role in cellular functions. Free Radic Biol Med. 1999;27:916–921. doi: 10.1016/s0891-5849(99)00177-x. [DOI] [PubMed] [Google Scholar]
- 298.Smigrodzki R, Parks J, Parker WD. High frequency of mitochondrial complex I mutations in Parkinson’s disease and aging. Neurobiol Aging. 2004;25:1273–1281. doi: 10.1016/j.neurobiolaging.2004.02.020. [DOI] [PubMed] [Google Scholar]
- 299.Snell RS. Clinical neuroanatomy. 7th. Wolters Kluwer Health/Lippincott Williams & Wilkins; Philadelphia: 2010. [Google Scholar]
- 300.Snow BJ, Rolfe FL, Lockhart MM, Frampton CM, O’Sullivan JD, Fung V, Smith RA, Murphy MP, Taylor KM. A double-blind, placebo-controlled study to assess the mitochondria-targeted antioxidant MitoQ as a disease-modifying therapy in Parkinson’s disease. Mov Disord. 2010;25:1670–1674. doi: 10.1002/mds.23148. [DOI] [PubMed] [Google Scholar]
- 301.Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119:7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Solesio ME, Prime TA, Logan A, Murphy MP, Del Mar Arroyo-Jimenez M, Jordan J, Galindo MF. The mitochondria-targeted anti-oxidant MitoQ reduces aspects of mitochondrial fission in the 6-OHDA cell model of Parkinson’s disease. Biochim Biophys Acta. 2013;1832:174–182. doi: 10.1016/j.bbadis.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 303.Sousa Silva M, Gomes RA, Ferreira AE, Ponces Freire A, Cordeiro C. The glyoxalase pathway: the first hundred years… and beyond. Biochem J. 2013;453:1–15. doi: 10.1042/BJ20121743. [DOI] [PubMed] [Google Scholar]
- 304.Squire LR. Fundamental neuroscience. 3rd. Elsevier / Academic Press; Amsterdam; Boston: 2008. [Google Scholar]
- 305.Sterky FH, Lee S, Wibom R, Olson L, Larsson NG. Impaired mitochondrial transport and Parkin-independent degeneration of respiratory chain-deficient dopamine neurons in vivo. Proc Natl Acad Sci U S A. 2011;108:12937–12942. doi: 10.1073/pnas.1103295108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Stobart JL, Anderson CM. Multifunctional role of astrocytes as gatekeepers of neuronal energy supply. Front Cell Neurosci. 2013;7:38. doi: 10.3389/fncel.2013.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Storr SJ, Woolston CM, Zhang Y, Martin SG. Redox environment, free radical, and oxidative DNA damage. Antioxid Redox Signal. 2013;18:2399–2408. doi: 10.1089/ars.2012.4920. [DOI] [PubMed] [Google Scholar]
- 308.Stuart CA, Ross IR, Howell ME, McCurry MP, Wood TG, Ceci JD, Kennel SJ, Wall J. Brain glucose transporter (Glut3) haploinsufficiency does not impair mouse brain glucose uptake. Brain Res. 2011;1384:15–22. doi: 10.1016/j.brainres.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Taetzsch T, Block ML. Pesticides, microglial NOX2, and Parkinson’s disease. J Biochem Mol Toxicol. 2013;27:137–149. doi: 10.1002/jbt.21464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Taira T, Saito Y, Niki T, Iguchi-Ariga SM, Takahashi K, Ariga H. DJ-1 has a role in antioxidative stress to prevent cell death. EMBO Rep. 2004;5:213–218. doi: 10.1038/sj.embor.7400074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Tavender TJ, Bulleid NJ. Molecular mechanisms regulating oxidative activity of the Ero1 family in the endoplasmic reticulum. Antioxid Redox Signal. 2010;13:1177–1187. doi: 10.1089/ars.2010.3230. [DOI] [PubMed] [Google Scholar]
- 312.Thiruchelvam M, Prokopenko O, Cory-Slechta DA, Buckley B, Mirochnitchenko O. Overexpression of superoxide dismutase or glutathione peroxidase protects against the paraquat + maneb-induced Parkinson disease phenotype. J Biol Chem. 2005;280:22530–22539. doi: 10.1074/jbc.M500417200. [DOI] [PubMed] [Google Scholar]
- 313.Thomas KJ, McCoy MK, Blackinton J, Beilina A, van der Brug M, Sandebring A, Miller D, Maric D, Cedazo-Minguez A, Cookson MR. DJ-1 acts in parallel to the PINK1/parkin pathway to control mitochondrial function and autophagy. Hum Mol Genet. 2011;20:40–50. doi: 10.1093/hmg/ddq430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Tieu K, Perier C, Caspersen C, Teismann P, Wu DC, Yan SD, Naini A, Vila M, Jackson-Lewis V, Ramasamy R, Przedborski S. D-beta-hydroxybutyrate rescues mitochondrial respiration and mitigates features of Parkinson disease. J Clin Invest. 2003;112:892–901. doi: 10.1172/JCI18797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Tong J, Ang LC, Williams B, Furukawa Y, Fitzmaurice P, Guttman M, Boileau I, Hornykiewicz O, Kish SJ. Low levels of astroglial markers in Parkinson’s disease: relationship to alpha-synuclein accumulation. Neurobiol Dis. 2015;82:243–253. doi: 10.1016/j.nbd.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Toyoda Y, Erkut C, Pan-Montojo F, Boland S, Stewart MP, Muller DJ, Wurst W, Hyman AA, Kurzchalia TV. Products of the Parkinson’s disease-related glyoxalase DJ-1, D-lactate and glycolate, support mitochondrial membrane potential and neuronal survival. Biol Open. 2014;3:777–784. doi: 10.1242/bio.20149399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Trancikova A, Tsika E, Moore DJ. Mitochondrial dysfunction in genetic animal models of Parkinson’s disease. Antioxid Redox Signal. 2012;16:896–919. doi: 10.1089/ars.2011.4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Tretter L, Adam-Vizi V. Inhibition of Krebs cycle enzymes by hydrogen peroxide: A key role of [alpha]-ketoglutarate dehydrogenase in limiting NADH production under oxidative stress. J Neurosci. 2000;20:8972–8979. doi: 10.1523/JNEUROSCI.20-24-08972.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Tseng AH, Shieh SS, Wang DL. SIRT3 deacetylates FOXO3 to protect mitochondria against oxidative damage. Free Radic Biol Med. 2013;63:222–234. doi: 10.1016/j.freeradbiomed.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 320.Turnbull S, Tabner BJ, El-Agnaf OM, Moore S, Davies Y, Allsop D. alpha-Synuclein implicated in Parkinson’s disease catalyses the formation of hydrogen peroxide in vitro, Free. Radic Biol Med. 2001;30:1163–1170. doi: 10.1016/s0891-5849(01)00513-5. [DOI] [PubMed] [Google Scholar]
- 321.Van Laar VS, Arnold B, Cassady SJ, Chu CT, Burton EA, Berman SB. Bioenergetics of neurons inhibit the translocation response of Parkin following rapid mitochondrial depolarization. Hum Mol Genet. 2011;20:927–940. doi: 10.1093/hmg/ddq531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Van Laar VS, Berman SB. The interplay of neuronal mitochondrial dynamics and bioenergetics: implications for Parkinson’s disease. Neurobiol Dis. 2013;51:43–55. doi: 10.1016/j.nbd.2012.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Vanitallie TB, Nonas C, Di Rocco A, Boyar K, Hyams K, Heymsfield SB. Treatment of Parkinson disease with diet-induced hyperketonemia: a feasibility study. Neurology. 2005;64:728–730. doi: 10.1212/01.WNL.0000152046.11390.45. [DOI] [PubMed] [Google Scholar]
- 324.Vicente Miranda H, El-Agnaf OM, Outeiro TF. Glycation in Parkinson’s disease and Alzheimer’s disease. Mov Disord. 2016;31:782–790. doi: 10.1002/mds.26566. [DOI] [PubMed] [Google Scholar]
- 325.Vilain S, Esposito G, Haddad D, Schaap O, Dobreva MP, Vos M, Van Meensel S, Morais VA, De Strooper B, Verstreken P. The yeast complex I equivalent NADH dehydrogenase rescues pink1 mutants. PLoS Genet. 2012;8:e1002456. doi: 10.1371/journal.pgen.1002456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Vitte J, Michel BF, Bongrand P, Gastaut JL. Oxidative stress level in circulating neutrophils is linked to neurodegenerative diseases. J Clin Immunol. 2004;24:683–692. doi: 10.1007/s10875-004-6243-4. [DOI] [PubMed] [Google Scholar]
- 327.Vittori A, Breda C, Repici M, Orth M, Roos RA, Outeiro TF, Giorgini F, Hollox EJ. Copy-number variation of the neuronal glucose transporter gene SLC2A3 and age of onset in Huntington’s disease. Hum Mol Genet. 2014;23:3129–3137. doi: 10.1093/hmg/ddu022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Volkenhoff A, Weiler A, Letzel M, Stehling M, Klämbt C, Schirmeier S. Glial Glycolysis Is Essential for Neuronal Survival in <em>Drosophila</em>. Cell Metabolism. 22:437–447. doi: 10.1016/j.cmet.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 329.Voloboueva LA, Emery JF, Sun X, Giffard RG. Inflammatory response of microglial BV-2 cells includes a glycolytic shift and is modulated by mitochondrial glucose-regulated protein 75/mortalin. FEBS Lett. 2013;587:756–762. doi: 10.1016/j.febslet.2013.01.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Voutilainen MH, Back S, Porsti E, Toppinen L, Lindgren L, Lindholm P, Peranen J, Saarma M, Tuominen RK. Mesencephalic astrocyte-derived neurotrophic factor is neurorestorative in rat model of Parkinson’s disease. J Neurosci. 2009;29:9651–9659. doi: 10.1523/JNEUROSCI.0833-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Wang D, Pascual JM, Yang H, Engelstad K, Mao X, Cheng J, Yoo J, Noebels JL, De Vivo DC. A mouse model for Glut-1 haploinsufficiency. Hum Mol Genet. 2006;15:1169–1179. doi: 10.1093/hmg/ddl032. [DOI] [PubMed] [Google Scholar]
- 332.Wang X, Winter D, Ashrafi G, Schlehe J, Wong YL, Selkoe D, Rice S, Steen J, LaVoie MJ, Schwarz TL. PINK1 and Parkin target Miro for phosphorylation and degradation to arrest mitochondrial motility. Cell. 2011;147:893–906. doi: 10.1016/j.cell.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Wang X, Yan MH, Fujioka H, Liu J, Wilson-Delfosse A, Chen SG, Perry G, Casadesus G, Zhu X. LRRK2 regulates mitochondrial dynamics and function through direct interaction with DLP1. Hum Mol Genet. 2012;21:1931–1944. doi: 10.1093/hmg/dds003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Wani WY, Sunkaria A, Sharma DR, Kandimalla RJ, Kaushal A, Gerace E, Chiarugi A, Gill KD. Caspase inhibition augments Dichlorvos-induced dopaminergic neuronal cell death by increasing ROS production and PARP1 activation. Neuroscience. 2014;258:1–15. doi: 10.1016/j.neuroscience.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 335.Wey MC, Fernandez E, Martinez PA, Sullivan P, Goldstein DS, Strong R. Neurodegeneration and motor dysfunction in mice lacking cytosolic and mitochondrial aldehyde dehydrogenases: implications for Parkinson’s disease. PLoS One. 2012;7:e31522. doi: 10.1371/journal.pone.0031522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Wright RH, Lioutas A, Le Dily F, Soronellas D, Pohl A, Bonet J, Nacht AS, Samino S, Font-Mateu J, Vicent GP, Wierer M, Trabado MA, Schelhorn C, Carolis C, Macias MJ, Yanes O, Oliva B, Beato M. ADP-ribose-derived nuclear ATP synthesis by NUDIX5 is required for chromatin remodeling. Science. 2016;352:1221–1225. doi: 10.1126/science.aad9335. [DOI] [PubMed] [Google Scholar]
- 337.Wu EY, Langston JW, Di Monte DA. Toxicity of the 1-methyl-4-phenyl-2,3-dihydropyridinium and 1-methyl-4-phenylpyridinium species in primary cultures of mouse astrocytes. J Pharmacol Exp Ther. 1992;262:225–230. [PubMed] [Google Scholar]
- 338.Wu XL, Wang P, Liu YH, Xue YX. Effects of poly (ADP-ribose) polymerase inhibitor 3-aminobenzamide on blood-brain barrier and dopaminergic neurons of rats with lipopolysaccharide-induced Parkinson’s disease. J Mol Neurosci. 2014;53:1–9. doi: 10.1007/s12031-013-0175-5. [DOI] [PubMed] [Google Scholar]
- 339.Xie B, Lin F, Ullah K, Peng L, Ding W, Dai R, Qing H, Deng Y. A newly discovered neurotoxin ADTIQ associated with hyperglycemia and Parkinson’s disease. Biochem Biophys Res Commun. 2015;459:361–366. doi: 10.1016/j.bbrc.2015.02.069. [DOI] [PubMed] [Google Scholar]
- 340.Xu G, Stevens SM, Jr, Moore BD, McClung S, Borchelt DR. Cytosolic proteins lose solubility as amyloid deposits in a transgenic mouse model of Alzheimer-type amyloidosis. Hum Mol Genet. 2013;22:2765–2774. doi: 10.1093/hmg/ddt121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Xu J, Begley P, Church SJ, Patassini S, McHarg S, Kureishy N, Hollywood KA, Waldvogel HJ, Liu H, Zhang S, Lin W, Herholz K, Turner C, Synek BJ, Curtis MA, Rivers-Auty J, Lawrence CB, Kellett KA, Hooper NM, Vardy ER, Wu D, Unwin RD, Faull RL, Dowsey AW, Cooper GJ. Elevation of brain glucose and polyol-pathway intermediates with accompanying brain-copper deficiency in patients with Alzheimer’s disease: metabolic basis for dementia. Sci Rep. 2016;6:27524. doi: 10.1038/srep27524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Xu Y, Liu C, Chen S, Ye Y, Guo M, Ren Q, Liu L, Zhang H, Xu C, Zhou Q, Huang S, Chen L. Activation of AMPK and inactivation of Akt result in suppression of mTOR-mediated S6K1 and 4E-BP1 pathways leading to neuronal cell death in in vitro models of Parkinson’s disease. Cell Signal. 2014;26:1680–1689. doi: 10.1016/j.cellsig.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Yadava N, Nicholls DG. Spare respiratory capacity rather than oxidative stress regulates glutamate excitotoxicity after partial respiratory inhibition of mitochondrial complex I with rotenone. J Neurosci. 2007;27:7310–7317. doi: 10.1523/JNEUROSCI.0212-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Yang ES, Park JW. Knockdown of cytosolic NADP(+) -dependent isocitrate dehydrogenase enhances MPP(+) -induced oxidative injury in PC12 cells. BMB Rep. 2011;44:312–316. doi: 10.5483/BMBRep.2011.44.5.312. [DOI] [PubMed] [Google Scholar]
- 345.Yang SH, Li W, Sumien N, Forster M, Simpkins JW, Liu R. Alternative mitochondrial electron transfer for the treatment of neurodegenerative diseases and cancers: Methylene blue connects the dots. Prog Neurobiol. 2015 doi: 10.1016/j.pneurobio.2015.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Yang W, Tiffany-Castiglioni E, Koh HC, Son IH. Paraquat activates the IRE1/ASK1/JNK cascade associated with apoptosis in human neuroblastoma SH-SY5Y cells. Toxicol Lett. 2009;191:203–210. doi: 10.1016/j.toxlet.2009.08.024. [DOI] [PubMed] [Google Scholar]
- 347.Yao Z, Wood NW. Cell death pathways in Parkinson’s disease: role of mitochondria. Antioxid Redox Signal. 2009;11:2135–2149. doi: 10.1089/ars.2009.2624. [DOI] [PubMed] [Google Scholar]
- 348.Yin F, Boveris A, Cadenas E. Mitochondrial energy metabolism and redox signaling in brain aging and neurodegeneration. Antioxid Redox Signal. 2014;20:353–371. doi: 10.1089/ars.2012.4774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Ying W. NAD+/NADH and NADP+/NADPH in cellular functions and cell death: regulation and biological consequences. Antioxid Redox Signal. 2008;10:179–206. doi: 10.1089/ars.2007.1672. [DOI] [PubMed] [Google Scholar]
- 350.Yokoyama H, Kuroiwa H, Tsukada T, Uchida H, Kato H, Araki T. Poly(ADP-ribose)polymerase inhibitor can attenuate the neuronal death after 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurotoxicity in mice. J Neurosci Res. 2010;88:1522–1536. doi: 10.1002/jnr.22310. [DOI] [PubMed] [Google Scholar]
- 351.Yoon SY, Oh YJ. Glucose Levels in Culture Medium Determine Cell Death Mode in MPP(+)-treated Dopaminergic Neuronal Cells. Exp Neurobiol. 2015;24:197–205. doi: 10.5607/en.2015.24.3.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Yoritaka A, Hattori N, Uchida K, Tanaka M, Stadtman ER, Mizuno Y. Immunohistochemical detection of 4-hydroxynonenal protein adducts in Parkinson disease. Proc Natl Acad Sci U S A. 1996;93:2696–2701. doi: 10.1073/pnas.93.7.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Yu W, Dittenhafer-Reed KE, Denu JM. SIRT3 protein deacetylates isocitrate dehydrogenase 2 (IDH2) and regulates mitochondrial redox status. J Biol Chem. 2012;287:14078–14086. doi: 10.1074/jbc.M112.355206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Yun HM, Choi DY, Oh KW, Hong JT. PRDX6 Exacerbates Dopaminergic Neurodegeneration in a MPTP Mouse Model of Parkinson’s Disease. Mol Neurobiol. 2015;52:422–431. doi: 10.1007/s12035-014-8885-4. [DOI] [PubMed] [Google Scholar]
- 355.Zeevalk GD, Bernard LP. Energy status, ubiquitin proteasomal function, and oxidative stress during chronic and acute complex I inhibition with rotenone in mesencephalic cultures. Antioxid Redox Signal. 2005;7:662–672. doi: 10.1089/ars.2005.7.662. [DOI] [PubMed] [Google Scholar]
- 356.Zhang C, Lin M, Wu R, Wang X, Yang B, Levine AJ, Hu W, Feng Z. Parkin, a p53 target gene, mediates the role of p53 in glucose metabolism and the Warburg effect. Proc Natl Acad Sci U S A. 2011;108:16259–16264. doi: 10.1073/pnas.1113884108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Zhang JY, Deng YN, Zhang M, Su H, Qu QM. SIRT3 Acts as a Neuroprotective Agent in Rotenone-Induced Parkinson Cell Model. Neurochem Res. 2016;41:1761–1773. doi: 10.1007/s11064-016-1892-2. [DOI] [PubMed] [Google Scholar]
- 358.Zhang M, Shoeb M, Goswamy J, Liu P, Xiao TL, Hogan D, Campbell GA, Ansari NH. Overexpression of aldehyde dehydrogenase 1A1 reduces oxidation-induced toxicity in SH-SY5Y neuroblastoma cells. J Neurosci Res. 2010;88:686–694. doi: 10.1002/jnr.22230. [DOI] [PubMed] [Google Scholar]
- 359.Zhang W, Wang T, Pei Z, Miller DS, Wu X, Block ML, Wilson B, Zhou Y, Hong JS, Zhang J. Aggregated alpha-synuclein activates microglia: a process leading to disease progression in Parkinson’s disease. FASEB J. 2005;19:533–542. doi: 10.1096/fj.04-2751com. [DOI] [PubMed] [Google Scholar]
- 360.Zhang Y, Chen K, Sloan SA, Bennett ML, Scholze AR, O’Keeffe S, Phatnani HP, Guarnieri P, Caneda C, Ruderisch N, Deng S, Liddelow SA, Zhang C, Daneman R, Maniatis T, Barres BA, Wu JQ. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J Neurosci. 2014;34:11929–11947. doi: 10.1523/JNEUROSCI.1860-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Zou XD, Guo SQ, Hu ZW, Li WL. NAMPT protects against 6-hydroxydopamine-induced neurotoxicity in PC12 cells through modulating SIRT1 activity. Mol Med Rep. 2016;13:4058–4064. doi: 10.3892/mmr.2016.5034. [DOI] [PubMed] [Google Scholar]


