Abstract
Background
Assisted reproductive technology (ART) has been associated with birth defects, but the contributions of multiple births and underlying subfertility remain unclear. We evaluated the effects of subfertility and mediation by multiple births on associations between ART and nonchromosomal birth defects.
Methods
We identified a retrospective cohort of Massachusetts live births and stillbirths from 2004–2010 among ART-exposed, ART-unexposed subfertile, and fertile mothers using linked information from fertility clinics, vital records, hospital discharges, and birth defects surveillance. Log-binomial regression was used to estimate prevalence ratios and 95% confidence intervals. Mediation analyses were performed to deconstruct the ART-birth defects association into the direct effect of ART, the indirect effect of multiple births, and the effect of ART-multiples interaction.
Results
Of 17,829 ART-exposed births, 355 had a birth defect, compared with 162 of 9431 births to subfertile mothers and 6183 of 445,080 births to fertile mothers. The adjusted prevalence ratio was 1.5 (95% confidence interval, 1.3–1.6) for ART and 1.3 (1.1–1.5) in subfertile compared with fertile deliveries. We observed elevated rates of several birth defects with ART, including tetralogy of Fallot and hypospadias. Subfertility and multiple births affect these associations, with multiple births explaining 36% of the relative effect of ART on nonchromosomal birth defects.
Conclusion
Although the risk of birth defects with ART is small, a substantial portion of the relative effect is mediated through multiple births, with subfertility contributing an important role. Future research is needed to determine the impact of newer techniques, such as single embryo transfer, on these risks.
Keywords: Assisted Reproductive Technology, birth defects, mediation, multiple births, subfertility, infertility
Introduction
The use of assisted reproductive technology (ART) procedures, in which both sperm and egg are manipulated outside the body, has increased steadily, with an estimated 1.6% of 2013 US births conceived using ART (Sunderam, et al., 2015). The results of several studies and meta-analyses have suggested that ART is associated with an increased risk of birth defects, although the magnitude of these associations and the spectrum of defects involved remain unclear (Hansen, et al., 2013, Kallen, et al., 2010, Qin, et al., 2015, Wen, et al., 2012, Yin, et al., 2013). ART has been associated with several specific cardiac birth defects, including septal heart defects and tetralogy of Fallot, as well as a number of non-cardiac defects, including cleft lip with or without cleft palate, hypospadias, neural tube defects, and esophageal, anorectal and large intestinal atresias (Benedum, et al., 2016, Boulet, et al., 2016, Davies, et al., 2012, Funke, et al., 2010, Reefhuis et al., 2009, Tararbit, et al., 2013).
Hypothesized mechanisms to explain the observed associations between ART and birth defects include underlying subfertility, ovulation induction medications, and micromanipulation involved in ART procedures, as well as increases in multiple gestations (Bhattacharya and Kamath, 2014, Qin et al., 2015, Wijers, et al., 2015, Yin, et al., 2013). Despite high rates of multiple births among ART users and evidence that multiple births have an increased risk of birth defects even among spontaneous conceptions (Parazzini, et al., 2015), few studies have assessed the indirect (mediation) effect of multiple births on observed associations between ART and birth defects.
Recent advances in epidemiology have led to improved methods for evaluating and quantifying the contribution of potential mediator variables on the causal pathway between an exposure variable and an outcome variable (Figure 1). These newer methods allow for assessment of potential exposure-mediator variable interaction (Valeri and VanderWeele, 2013). Although mediation analysis offers opportunities for identifying mechanisms through which exposures operate, few studies have applied these methods to research on birth defects (Benedum et al., 2016, Tararbit et al., 2014).
Figure 1.
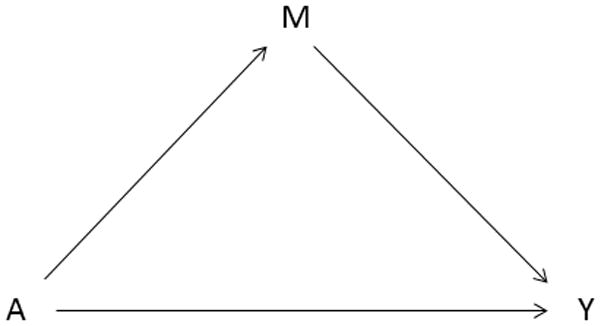
Classic mediation model described in Baron and Kenny, 1986, where A represents the exposure (assisted reproductive technology), M represents the mediator (multiple births), and Y represents the outcome (birth defects).
Many studies have separately assessed the effect of ART on birth defects among singletons and multiples, but to date only one study has used formal mediation methods to quantify the degree to which plurality acts as a mediator on the pathway between ART and birth defects, reporting that multiple births explained 23.6% of the association between ART use and tetralogy of Fallot without associated chromosomal abnormality (Tararbit, et al., 2014). However, this study only examined a single defect and did not address the possibility of interaction between ART and multiple births.
Massachusetts has one of the highest rates of ART procedures in the United States, with an estimated 2.9% of 2013 live births in the state conceived with ART (Sunderam, et al., 2015). In this study, we estimated the prevalence of nonchromosomal birth defects among Massachusetts deliveries to mothers who used ART compared with fertile mothers and evaluated potential mediation of the association between ART and birth defects by multiple births. In addition, we examined the prevalence of birth defects among deliveries to subfertile mothers who did not use ART compared with fertile mothers and among deliveries to ART-exposed mothers compared with subfertile mothers.
This study aims to better quantify the association between ART use and birth defects and to evaluate the contributions of underlying subfertility and mediation by multiple births on observed associations.
Materials and Methods
DATA SOURCES
We identified a retrospective cohort of live birth and stillbirth deliveries among Massachusetts residents between September 2004 and December 2010 from the Massachusetts Outcomes Study of Assisted Reproductive Technology (MOSART) database. MOSART contains ART cycle information from the Society for Assisted Reproductive Technology Clinic Outcome Reporting System (SART CORS) linked with vital records, hospital discharge, and birth defects surveillance data from the Pregnancy to Early Life Longitudinal (PELL) database. SART CORS, PELL, and the resulting MOSART linkage have been described previously (Kotelchuck, et al., 2014). Briefly, SART CORS includes data reported from ART clinics across the United States and contains information on specific ART therapies, infertility history, and treatment parameters, with data validated annually. All ART clinics operating in Massachusetts during the study period reported cycle data to SART CORS. The population-based PELL data system contains linked information on Massachusetts mothers and infants, including birth and fetal death records, hospital utilization data, and birth defects data.
The birth defects data in the PELL database comes from the Massachusetts Birth Defects Monitoring Program (BDMP), which conducts population-based active surveillance of structural birth defects among Massachusetts residents diagnosed through 1 year of age via multiple sources, including delivery and specialty care hospitals, birthing centers, and vital records. The medical records for all potential cases undergo standardized review by trained abstractors, and identified birth defects are coded using the International Classification of Diseases, Ninth Revision, Clinical Modification, modified British Pediatric Association (ICD-9-CM/BPA) system. All cases receive clinical review, with complex cases evaluated by a clinical geneticist (A.E.L.) for accurate classification.
Human Subjects approval for this study was obtained from the Massachusetts Department of Public Health and the Dartmouth Committee for the Protection of Human Subjects.
PREDICTOR VARIABLES, OUTCOME VARIABLES AND COVARIATES
Live births and stillbirths in MOSART were classified as either 1) ART-exposed, 2) ART unexposed but subfertile, or 3) ART unexposed fertile (Declercq, et al., 2014). The ART-exposed group includes deliveries for which the index pregnancy matched to a cycle in the SART CORS database, indicating ART use. The subfertile group includes deliveries to mothers with no indication of ART use for the index pregnancy, but with one of the following indicators of subfertility: fertility treatment recorded on the current birth or fetal death certificate or on a delivery record for the same mother in the previous 5 years, infertility noted at hospital admission or discharge (including observational stays and emergency room visits), and/or a history of prior ART documented in SART CORS during the study period. The fertile group includes deliveries with no indication of ART use for the index pregnancy and no indication of subfertility documented in PELL or SART CORS.
Outcome variables include birth defects ascertained by the BDMP, excluding those cases with a Mendelian gene syndrome or chromosomal defect. BDMP surveillance includes structural birth defects with ICD-9-CM codes 740.00–759.99, along with selected codes outside this range, with some defects requiring additional criteria for inclusion (e.g., postnatal confirmation or surgical treatment). A detailed description of the defects included in this study, along with the corresponding ICD-9-CM/BPA codes is provided in Supplemental Table 1.
Information on covariates was obtained from vital records. Covariates were initially selected a priori based on the literature (Boulet, et al., 2016, Declercq, et al., 2014, Reefhuis, et al., 2009, Tararbit, et al, 2014). Potential confounders included maternal age, race/ethnicity, education level, parity, pre-pregnancy cigarette smoking, pre-pregnancy diabetes, pre-pregnancy hypertension, paternal age, year of birth and insurance status at delivery (determined from a combination of vital records and hospital discharge information). We assessed potential confounding of the exposure-outcome, mediator-outcome, and exposure-mediator associations. Maternal age (<35 years, ≥35 years) was considered as potential effect measure modifier in this study. Plurality information was obtained from vital records using the method described by Lazar, et al., 2006. This variable was categorized as singleton or multiple and was evaluated both as a potential effect modifier and as a potential mediator of the ART and birth defects association.
STATISTICAL ANALYSES
Results are presented overall, for cardiac and non-cardiac birth defects, for birth defects grouped by body system, and for specific birth defects with 11 or more ART-exposed cases. Prevalence rates for hypospadias were calculated only among males and were limited to more severe cases (second or third degree).
Distributions of covariates were evaluated by fertility status (ART-exposed, subfertile, and fertile). The birth defect prevalence rates (per 10,000 live births) were calculated separately for each exposure group. Log-binomial regression models were used to calculate prevalence ratios and 95% confidence intervals (CI) comparing the ART group to the fertile group for each birth defect category and for specific defects with sufficient numbers of ART-exposed cases. Primary analyses compared the prevalence of birth defects among deliveries to ART-exposed and fertile women, while secondary analyses compared subfertile to fertile women and ART-exposed women to subfertile women.
Maternal age was included in all adjusted analyses. To identify the set of additional confounders to be included in the fully adjusted models, covariates were added to the age-adjusted models when they exhibited a change in the age-adjusted PR of at least 10%. Fully adjusted models include maternal age (<35, ≥35 years), insurance status (private, non-private), and race (white, nonwhite).
Additive interaction of ART with multiple births and with maternal age was explored via stratification and calculation of the relative excess risk due to interaction (Andersson, et al., 2005). Prevalence rates for stratified analyses and analyses within subgroups are presented for defects and categories with 6 or more ART-exposed cases. Given that some mothers contribute more than one delivery (i.e., a multiple delivery or more than one singleton delivery within the study period), generalized estimating equation methods with an exchangeable correlation matrix were used to separately evaluate the potential effect of clustering among deliveries to the same mother and among siblings within the same delivery.
The effect of subfertility on birth defects was evaluated by comparing prevalence rates among the non-ART subfertile group relative to the fertile group for defects and defect groups with sufficient numbers of exposed cases and by directly comparing the ART-exposed group to the subfertile group. Subgroup analyses examined the effect of limiting the ART-exposed group to those who had specific procedures, including intracytoplasmic sperm injection (ICSI), frozen cycles, and assisted hatching.
We used a mediation approach to assess the potential impact of multiple births on the causal pathway between ART and birth defects. Mediation analysis was performed on overall birth defects, cardiac, and non-cardiac defects, as well as specific defects that showed a significant association with ART and had at least 6 ART-exposed multiples. Using the method described by VanderWeele, 2013, we deconstructed the excess relative risk of ART exposure on birth defects (i.e., the portion of the relative risk greater than 1.0) into three components 1) the direct effect of ART, 2) the indirect effect through multiple births, and 3) the effect of interaction between ART and multiple births. The proportion of the relative effect of ART due to mediation by multiple births was calculated using the method described by Valeri and VanderWeele, 2013. Parameter estimates from logistic regression models were used to compute these effects with and without exposure-mediator interaction. The computed odds ratios (ORs) provide reasonable approximations of the corresponding prevalence ratios, given the rarity of the outcomes of interest (birth defects). All analyses were performed using SAS version 9.3, SAS Institute, Cary, NC.
Results
MATERNAL AND INFANT CHARACTERISTICS
The distributions of characteristics by ART-exposure/fertility status are presented in Table 1. Compared to fertile mothers, ART-exposed mothers were more likely to be 35 years of age or older, white, to have a college degree, and to have private insurance. ART-exposed mothers were less likely to have a history of cigarette smoking before the index pregnancy, but had slightly higher rates of pre-pregnancy diabetes and hypertension. There was a greater frequency of older fathers in the ART-exposed group. With the exception of the frequency of multiples and primiparity, which were higher among ART users, the distributions of characteristics among subfertile mothers were similar to those of the ART-exposed mothers. Among ART users in our study, 38.8% used ICSI, 12.4% used thawed embryos, and 24.8% involved assisted hatching.
TABLE 1.
Characteristics of Live Births and Stillbirths by Maternal Fertility Exposure Status
| Characteristics | ARTa Exposed n=17,829 |
Subfertile n=9431 |
Fertile N=445,080 |
|||
|---|---|---|---|---|---|---|
|
| ||||||
| n | % | n | % | n | % | |
| Mother | ||||||
| Age (years) | ||||||
| <25 | 58 | 0.3 | 152 | 1.7 | 104,129 | 23.4 |
| 25–29 | 1496 | 8.4 | 1005 | 10.7 | 112,192 | 25.2 |
| 30–34 | 5821 | 32.6 | 3309 | 35.1 | 135,705 | 30.5 |
| 35–39 | 6898 | 38.7 | 3614 | 38.3 | 76,844 | 17.3 |
| ≥ 40 | 3555 | 19.9 | 1351 | 14.3 | 16,200 | 3.6 |
| Race/Ethnicity | ||||||
| White, Non-Hispanic | 15,159 | 85.0 | 7958 | 84.4 | 294,482 | 66.2 |
| Black, Non-Hispanic | 582 | 3.3 | 315 | 3.3 | 40,495 | 9.1 |
| Hispanic | 632 | 3.6 | 403 | 4.3 | 66,162 | 14.9 |
| Asian/Pacific Islander | 1248 | 7.0 | 642 | 6.8 | 33,942 | 7.6 |
| Other | 184 | 1.0 | 107 | 1.1 | 9586 | 2.2 |
| Education | ||||||
| High school or less | 1636 | 9.2 | 1117 | 11.9 | 167,654 | 37.8 |
| Some college/Associate | 2832 | 15.9 | 1638 | 17.4 | 95,265 | 21.5 |
| Bachelor degree or higher | 13,314 | 74.9 | 6659 | 70.7 | 180,277 | 40.7 |
| Insurance status at delivery | ||||||
| Private | 16,910 | 94.8 | 8505 | 90.2 | 251,868 | 56.6 |
| Public | 586 | 3.3 | 764 | 8.1 | 186,505 | 41.9 |
| Self-pay | 283 | 1.6 | 134 | 1.4 | 4145 | 0.9 |
| Free care | 50 | 0.3 | 26 | 0.3 | 2544 | 0.6 |
| Pre-pregnancy smoking | 593 | 3.3 | 431 | 4.6 | 64,114 | 14.4 |
| Pre-pregnancy diabetes | 370 | 2.1 | 163 | 1.7 | 5477 | 1.2 |
| Pre-pregnancy hypertension | 552 | 3.1 | 247 | 2.6 | 7530 | 1.7 |
| Father | ||||||
| Ageb (years) | ||||||
| <35 | 5396 | 30.8 | 3223 | 35.0 | 257,932 | 63.8 |
| 35–39 | 6409 | 36.6 | 3465 | 37.6 | 93,047 | 23.0 |
| 40–44 | 3702 | 21.1 | 1772 | 19.2 | 37,550 | 9.3 |
| 45–49 | 1367 | 7.8 | 551 | 6.0 | 11,453 | 2.8 |
| ≥ 50 | 646 | 3.7 | 208 | 2.3 | 4,134 | 1.0 |
| Pregnancy | ||||||
| Parity | ||||||
| No previous births | 8852 | 49.8 | 3393 | 36.2 | 200,265 | 45.2 |
| 1 previous birth | 6586 | 37.1 | 3807 | 40.6 | 151,799 | 34.2 |
| 2 or more previous births | 2333 | 13.1 | 2177 | 23.2 | 91,315 | 20.6 |
| Plurality | ||||||
| Singleton | 10,230 | 57.4 | 7812 | 82.8 | 432,653 | 97.2 |
| Twins | 7185 | 40.3 | 1380 | 14.6 | 12,191 | 2.7 |
| Triplets or more | 414 | 2.3 | 239 | 2.5 | 236 | 0.1 |
| Birth year | ||||||
| 2004–2006 | 6325 | 35.5 | 3511 | 37.2 | 166,598 | 37.4 |
| 2007–2008 | 5461 | 30.6 | 3004 | 31.8 | 143,403 | 32.2 |
| 2009–2010 | 6043 | 33.9 | 2916 | 30.9 | 135,079 | 30.4 |
Percentages may not add to 100% due to rounding.
Assisted reproductive technology.
Missing values for paternal age: ART-exposed: 464 (2.6%); Subfertile: 257 (2.7%); ART-unexposed, fertile: 42,471 (9.5%).
ASSOCIATION BETWEEN ART AND NONCHROMOSOMAL BIRTH DEFECTS
Birth defect prevalence estimates and age-adjusted prevalence ratios comparing rates of birth defects in the subfertile group and the ART-exposed group relative to fertile deliveries are shown in Table 2. Of 17,829 infants born to mothers who used ART, 355 had a nonchromosomal birth defect, for a prevalence rate of 199.1 per 10,000 live births, compared to 6183 of 445,080 infants born to fertile mothers, for a prevalence rate of 138.9 per 10,000 live births. The age-adjusted prevalence ratio for ART exposure was 1.4 (95% confidence interval, 1.3–1.6). Among 9431 infants born to subfertile mothers, 162 had a birth defect, resulting in a birth defect prevalence rate among subfertile mothers of 171.8 per 10,000 live births, less than the rate among ART mothers but greater than that among non-ART, fertile mothers. The age-adjusted prevalence ratio for subfertile compared with fertile mothers was 1.2 (95% confidence interval, 1.1–1.4), not significantly different than for ART-exposed mothers. ART-exposure was associated with increases in the age-adjusted prevalence rates of cardiac and non-cardiac defects overall, conotruncal/aortic arch defects, including tetralogy of Fallot, atrial and ventricular septal defects, gastrointestinal defects, genitourinary defects, including hypospadias, and musculoskeletal defects. Subfertility without ART was associated with increases in the age-adjusted prevalence ratios for birth defects overall and for orofacial defects (Table 2).
TABLE 2.
Prevalence Rates and Age-Adjusted Prevalence Ratios for Nonchromosomal Birth Defects by Maternal Fertility Exposure Status
| Birth defect | Prevalence Rate per 10,000 Live Births | Subfertile Prevalence Ratiob (95% CI) |
ARTa Exposed Prevalence Ratiob (95% CI) |
||
|---|---|---|---|---|---|
|
| |||||
| ARTa Exposed n=17,829 |
Subfertile n=9,431 |
Fertile n=445,080 |
|||
| Overall c,d | 199.1 | 171.8 | 138.9 | 1.2 (1.1–1.4) | 1.4 (1.3–1.6) |
| Cardiac Defectd | 63.9 | 47.7 | 38.6 | 1.2 (0.9–1.6) | 1.6 (1.3–1.9) |
| Conotruncal/Aortic arch | 12.9 | 6.4 | 6.6 | 1.0 (0.4–2.2) | 1.9 (1.3–3.0) |
| Tetralogy of Fallot | 9.5 | - | 3.0 | - | 3.2 (1.9–5.5) |
| Left-Sided obstruction | 6.2 | - | 6.2 | - | 0.9 (0.5–1.7) |
| Right-Sided obstruction | 9.5 | 9.5 | 6.9 | 1.3 (0.7–2.9) | 1.3 (0.8–2.1) |
| Pulmonary valve stenosis | 8.4 | 8.5 | 5.6 | 1.4 (0.7–2.9) | 1.4 (0.8–2.4) |
| Septal heart defects | 35.9 | 26.5 | 20.5 | 1.2 (0.8–1.8) | 1.7 (1.3–2.2) |
| Atrial septal defecte | 24.1 | 14.8 | 14.0 | 1.0 (0.6–1.7) | 1.6 (1.2–2.2) |
| Ventricular septal defectf | 14.6 | 11.7 | 8.0 | 1.4 (0.8–2.6) | 1.8 (1.2–2.7) |
| Non-cardiac Defect | 145.8 | 133.6 | 109.1 | 1.2 (1.0–1.5) | 1.4 (1.2–1.5) |
| Central nervous system | 11.2 | - | 12.0 | - | 1.0 (0.6–1.6) |
| Respiratory defects | 6.2 | - | 3.3 | - | 1.8 (1.0–3.4) |
| Orofacial | 17.4 | 25.4 | 15.7 | 1.6 (1.1–2.4) | 1.1 (0.8–1.6) |
| Cleft lip with/without cleft palate | 10.1 | 9.5 | 7.0 | 1.4 (0.7–2.7) | 1.4 (0.9–2.4) |
| Gastrointestinal | 20.8 | 17.0 | 14.1 | 1.2 (0.7–2.0) | 1.5 (1.1–2.1) |
| Genitourinary | 47.1 | 44.5 | 32.9 | 1.3 (0.9–1.8) | 1.3 (1.1–1.7) |
| Hypospadias, 2nd or 3rd degreeg | 45.8 | 25.1 | 23.8 | 1.0 (0.6–1.8) | 1.8 (1.3–2.5) |
| Obstructive genitourinary defect | 15.7 | 14.8 | 12.0 | 1.2 (0.7–2.1) | 1.3 (0.9–1.9) |
| Musculoskeletal | 54.4 | 40.3 | 41.1 | 1.0 (0.8–1.4) | 1.4 (1.2–1.8) |
| Club foot | 14.0 | 9.5 | 11.5 | 0.9 (0.5–1.8) | 1.4 (0.9–2.1) |
| Craniosynostosis | 7.9 | 7.4 | 4.0 | 1.8 (0.8–3.7) | 1.9 (1.1–3.3) |
| Polydactyly/Syndactyly | 14.0 | 19.1 | 12.9 | 1.6 (1.0–2.5) | 1.2 (0.8–1.8) |
95% CI: 95% confidence interval. Prevalence rates reported per 10,000 live births. Prevalence rates not calculated for case counts less than 6.
Assisted reproductive technology.
Adjusted for maternal age (<35 years, ≥35 years). Fertile group is referent.
Any defect collected as part of surveillance by the Massachusetts Birth Defects Monitoring Program as described in Supplemental Table 1. Excludes cases with a Mendelian gene syndrome or chromosomal defect.
Excludes patent ductus arteriosus.
Does not include primum type.
Does not include canal type or isolated muscular type.
Prevalence per 10,000 male live births.
Analyses stratified by plurality are shown in Table 3. Compared to multiples born to fertile mothers, singletons born to fertile mothers had a lower prevalence of all birth defects examined except polydactyly/syndactyly. ART exposure was associated with significantly elevated prevalence ratio estimates among singletons for birth defects overall and for conotruncal/aortic arch defects, including tetralogy of Fallot. The increased relative risk of tetralogy of Fallot with ART was confined to singletons. There was little evidence of additive interaction of the ART and birth defects association by age, with similar associations observed for younger and older mothers (Supplemental Table 2).
TABLE 3.
Prevalence Rates and Age-Adjusted Prevalence Ratios for Nonchromosomal Birth Defects among Women Using Assisted Reproductive Technology and Fertile Women Stratified by Plurality
| Birth Defect | Singletons | Multiples | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| ARTa Exposed Rate n=10,230 |
ARTa Unexposed, Fertile Rate n=432,653 |
Prevalence Ratiob (95% CI) |
ARTa Exposed Rate n=7,599 |
ARTa Unexposed, Fertile Rate n=12,427 |
Prevalence Ratiob (95% CI) |
|
| Overall c,d | 174.0 | 137.0 | 1.3 (1.1–1.5) | 232.9 | 205.2 | 1.2 (1.0–1.4) |
| Cardiac Defect d | 54.7 | 37.6 | 1.4 (1.0–1.8) | 76.3 | 74.8 | 1.0 (0.7–1.5) |
| Conotruncal/Aortic Arch | 12.7 | 6.4 | 2.0 (1.1–3.5) | 13.2 | 12.1 | 1.0 (0.4–2.4) |
| Tetralogy of Fallot | 8.8 | 2.8 | 3.3 (1.6–6.6) | 10.5 | 8.0 | 1.1 (0.4–3.0) |
| Left-sided obstruction | - | 6.1 | - | 9.2 | 8.9 | 1.1 (0.4–3.0) |
| Right-sided obstruction | 8.8 | 6.7 | 1.2 (0.6–2.4) | 10.5 | 13.7 | 0.8 (0.3–1.9) |
| Pulmonary valve stenosis | 7.8 | 5.4 | 1.3 (0.6–2.6) | 9.2 | 11.3 | 0.9 (0.4–2.3) |
| Septal heart defects | 27.4 | 19.8 | 1.3 (0.9–1.9) | 47.4 | 45.1 | 1.1 (0.7–1.7) |
| Atrial septal defecte | 16.6 | 13.6 | 1.2 (0.7–1.9) | 34.2 | 27.4 | 1.2 (0.7–2.0) |
| Ventricular septal defectf | 13.7 | 7.7 | 1.7 (1.0–2.9) | 15.8 | 17.7 | 1.1 (0.5–2.2) |
| Non-cardiac Defect | 130.0 | 108.2 | 1.2 (1.0–1.5) | 167.1 | 140.8 | 1.2 (1.0–1.5) |
| Central nervous system | 7.8 | 11.8 | 0.7 (0.3–1.4) | 15.8 | 19.3 | 1.0 (0.5–2.0) |
| Respiratory defects | 5.9 | 3.3 | 1.7 (0.8–4.0) | - | - | - |
| Orofacial | 18.6 | 15.6 | 1.2 (0.8–1.9) | 15.8 | 17.7 | 0.9 (0.4–1.8) |
| Cleft Lip with/without cleft palate | 8.8 | 6.9 | 1.3 (0.7–2.5) | 11.8 | 10.5 | 1.0 (0.4–2.5) |
| Gastrointestinal | 19.6 | 13.8 | 1.4 (0.9–2.2) | 22.4 | 24.1 | 1.0 (0.5–1.9) |
| Genitourinary | 40.1 | 32.5 | 1.2 (0.8–1.6) | 56.6 | 46.7 | 1.2 (0.8–1.7) |
| Hypospadias, 2nd or 3rd degreeg | 38.1 | 23.3 | 1.5 (1.0–2.4) | 56.0 | 41.2 | 1.2 (0.7–2.2) |
| Obstructive genitourinary defect | 12.7 | 11.9 | 1.0 (0.6–1.8) | 19.7 | 17.7 | 1.1 (0.6–2.2) |
| Musculoskeletal | 43.0 | 40.7 | 1.2 (0.8–1.6) | 69.7 | 54.7 | 1.3 (0.9–2.0) |
| Club foot | 9.8 | 11.4 | 1.0 (0.5–1.8) | 19.7 | 16.1 | 1.3 (0.6–2.6) |
| Craniosynostosis | 8.8 | 4.0 | 2.1 (1.0–4.1) | - | - | - |
| Polydactyly/Syndactyly | 8.8 | 12.9 | 0.7 (0.4–1.4) | 21.1 | 12.9 | 1.9 (0.9–3.8) |
95% CI: 95% confidence interval. Prevalence rates reported per 10,000 live births. Prevalence estimates not calculated for case counts less than 6.
Assisted reproductive technology.
Adjusted for maternal age (<35 years, ≥35 years). Fertile group is referent.
Any defect collected as part of surveillance by the Massachusetts Birth Defects Monitoring Program as described in Supplemental Table 1. Excludes cases with a Mendelian gene syndrome or chromosomal defect.
Excludes patent ductus arteriosus.
Does not include primum type.
Does not include canal type or isolated muscular type.
Prevalence per 10,000 male live births.
MEDIATION BY MULTIPLE BIRTHS
Mediation analyses are summarized in Table 4. Fully-adjusted models, which include maternal age, race/ethnicity, and insurance type, are presented with and without ART-multiples interaction. The proportion of the total relative effect due to multiple births was calculated based on the direct effect of ART on birth defects and the indirect effect through multiple births. For overall nonchromosomal birth defects accounting for ART-multiples interaction, the adjusted OR was 1.5 for the total effect, 1.3 for the direct effect of ART, and 1.1 for the indirect effect of multiple births. Based on these results, 36% of the total effect of ART-exposure on nonchromosomal birth defects is mediated by multiple births. The degree of mediation varied by defect, with multiple births accounting for 38.6% of the relative association between ART and hypospadias, but only 10.5% of the relative association between ART and tetralogy of Fallot.
TABLE 4.
Effect of Mediation by Multiple Births on Associations between Assisted Reproductive Technology (ART) and Nonchromosomal Birth Defects
| Effect | Birth Defects Overalla ART-Multiples Interaction |
Cardiac Defects ART-Multiples Interaction |
Tetralogy of Fallot ART-Multiples Interaction |
Non-Cardiac Defects ART-Multiples Interaction |
Hypospadiasb ART-Multiples Interaction |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | |
|
| ||||||||||
| aORc (95% CI) |
aORc (95% CI) |
aORc (95% CI) |
aORc (95% CI) |
aORc (95% CI) |
aORc (95% CI) |
aORc (95% CI) |
aORc (95% CI) |
aORc (95% CI) |
aORc (95% CI) |
|
| Direct effect | 1.2 (1.1–1.4) |
1.3 (1.1–1.5) |
1.2 (1.0–1.5) |
1.4 (1.1–1.8) |
2.4 (1.3–4.4) |
3.2 (1.6–6.3) |
1.2 (1.1–1.4) |
1.3 (1.1–1.5) |
1.3 (0.9–1.9) |
1.4 (0.9–2.2) |
| Indirect effect | 1.2 (1.1–1.2) |
1.1 (1.0–1.2) |
1.3 (1.2–1.4) |
1.2 (1.0–1.4) |
1.4 (1.0–2.0) |
1.1 (0.7–1.6) |
1.1 (1.0–1.2) |
1.1 (1.0–1.2) |
1.2 (1.1–1.5) |
1.2 (0.9–1.6) |
| Total effect | 1.5 (1.3–1.6) |
1.5 (1.3–1.6) |
1.6 (1.3–2.0) |
1.6 (1.3–2.0) |
3.4 (2.0–5.8) |
3.4 (2.0–5.8) |
1.4 (1.2–1.6) |
1.4 (1.2–1.6) |
1.7 (1.2–2.3) |
1.7 (1.2–2.3) |
| % Mediatedd | 47.5 | 36.0 | 63.2 | 36.3 | 42.7 | 10.5 | 36.2 | 32.6 | 50.2 | 38.6 |
aOR: Adjusted odds ratio; 95% CI: 95% confidence interval.
Any defect collected as part of surveillance by the Massachusetts Birth Defects Monitoring Program as described in Supplemental Table 1. Excludes cases with a Mendelian gene syndrome or chromosomal defect.
Among males.
Adjusted for maternal age (<35, ≥35 years), race (white, nonwhite), and type of insurance (private, nonprivate).
Calculation of percent mediated by multiple births is done according to the method described by VanderWeele, 2013. Since the outcome is rare, odds ratios approximate relative risk (RR) in this formula, which in turn approximate the prevalence ratios described in this study.
Assessment of the effect of clustering among deliveries to the same mother in the dataset, such as for siblings in a multiple delivery or for another delivery to the same mother during the study period, showed no meaningful differences; thus results are presented without taking clustering into account.
EFFECT OF SUBFERTILITY
The rate of birth defects in the subfertile group was not significantly different than that observed in the presence of ART-exposure, with substantial overlap of confidence intervals for both these estimates (Table 2). The fully adjusted prevalence ratio for nonchromosomal birth defects among subfertile mothers without ART compared to fertile mothers was 1.3 (95% confidence interval, 1.1–1.5). The adjusted prevalence ratio for ART-exposed births relative to births to subfertile mothers was 1.2 [1.0–1.4].
EFFECT OF ART PROCEDURE
Subanalyses limited to those who used specific ART procedures compared to the fertile group showed slightly higher age-adjusted prevalence ratio estimates for cardiac defects and hypospadias with assisted hatching and similar estimates with frozen cycles (Supplemental Table 3). The use of ICSI resulted in slightly lower prevalence ratio estimates for birth defects overall, as well as for cardiac and non-cardiac birth defects, including hypospadias.
Discussion
In this retrospective cohort study, we observed modest increases in the overall prevalence of nonchromosomal birth defects among deliveries to those who used ART compared with fertile deliveries. We observed significant associations with ART for specific defects and groups of defects, including tetralogy of Fallot and hypospadias. The adjusted prevalence ratio of 1.5 for birth defects with ART that we observed is similar to the relative risk reported in a meta-analysis by Wen et al., 2012 and in another meta-analysis among studies considered high quality (Qin, et al., 2015). Consistent with previous studies (Reefhuis et al., 2009, Boulet et al., 2016), we observed a greater effect of ART among singletons for several defects, likely because the baseline prevalence rates of most birth defects examined were lower for singleton births than for multiples. The association of ART and TOF was confined to singletons in our study, although the number of ART-exposed TOF cases was small.
This study demonstrates the use of formal mediation methods to clarify the role of multiple births in observed associations between ART and birth defects. Using these methods and including ART-multiples interaction showed that approximately 36% of the relative association of ART with nonchromosomal birth defects is mediated by multiple births. The degree of mediation varies by defect, with mediation by multiples accounting for 39% of the relative association with hypospadias, but only 10% of the association with tetralogy of Fallot. A recent study found a greater proportion (23.6%) of the effect of ART on nonchromosomal tetralogy of Fallot to be mediated by multiple births (Tararbit, et al., 2014). However, that study did not address possible interaction between ART and multiples. Our results showed a similar total effect of ART on tetralogy of Fallot (adjusted odds ratio 3.4, 95% confidence interval 2.0–5.8) compared to what was observed in that study (adjusted odds ratio 3.7 [2.0–7.0]).
Consistent with several previous reports (Funke, et al., 2010, Hansen, et al., 2012) we found an increase in hypospadias with ART use. Our mediation findings suggest that 39% of the relative association between ART and hypospadias is due to multiple births.
It has been proposed that the association between ART and birth defects could result in part from underlying infertility (Davies et al., 2012, and Zhu et al., 2006). In our study, we observed no significant associations when we compared the ART-exposed group to a subfertile referent group, suggesting that underlying subfertility could explain a substantial portion of the effect of ART on birth defects.
A number of studies have suggested that birth defects may be associated with specific types of ART treatment, such as ICSI and assisted hatching (Yin, 2013; Hansen, 2012). We observed marginally greater prevalence ratios with assisted hatching (Supplemental Table 3). Our study did not show an increased effect with ICSI, a finding which is consistent with a ten year review that noted an increased risk of chromosomal abnormalities but no increased risk of major malformations with ICSI compared with IVF (Devroey and Van Steirteghem, 2004).
Several studies suggest that the risk of birth defects with ART is decreasing over time (Hansen, et al., 2012, Kallen et al., 2010). This may be a result of changes in the population using ART as well as improvements in ART methods, including the trend toward transferring fewer embryos (Bhattacharya and Kamath, 2014). Our finding that a substantial component of the relative association between ART and birth defects is mediated by multiple births is consistent with this view.
Strengths of this study include ascertainment of birth defects using an active, population based surveillance program, with each case clinically reviewed, and ART exposure information provided by ART clinics through the SART CORS database. Our analyses were limited to birth defects without a known genetic etiology, to ensure that we were evaluating only those cases that might have resulted from ART treatment and where mediation by multiples might play a role. In addition, our study was able to evaluate birth defect rates in a group of subfertile women and to exclude these women from our referent group for analyses of associations with ART, providing a more homogenous reference population. Massachusetts has high rates of insurance coverage. As a result, our population of ART users may be more diverse than that of other states. While several previous studies have evaluated singletons and multiples separately, the use of mediation analysis accounting for ART-multiples interaction allowed us to quantify the contribution of plurality to the ART-birth defects association.
Our study has several limitations. Small numbers of cases among ART-exposed and subfertile mothers precluded the evaluation of certain specific defects. Also, our study was limited to live births and stillbirths; birth defects among deliveries with other pregnancy outcomes (e.g., terminations of pregnancy) were not included. This may partly explain why the overall birth defect prevalence rates we observed (2% in the ART group, 1.4% in the fertile group) are low compared with some other studies (Hansen et al., 2012, Kallen et al., 2010). Our exclusion of Mendelian gene defects as well as chromosomal defects and the additional criteria required by our surveillance program for certain birth defects also likely contributed to our lower observed prevalence rates. Pregnancy terminations for birth defects may be less likely if only one twin has a birth defect (Hansen, et al., 2012) and thus may inflate birth defect rates among multiples when only live births and stillbirths are examined. However, none of the associations we observed occurred among birth defect categories with high rates of elective terminations (e.g., central nervous system defects), and this is therefore unlikely to have affected our results.
There is also the possibility for misclassification of fertility status. Women who had a known prior ART pregnancy or a diagnosis of infertility were excluded from the fertile group. However, some subfertile women may still have been included in the fertile group. Those subfertile women who had non-ART fertility treatments (e.g., intrauterine insemination or fertility medication), time to conception over one year, or ART prior to the study period but without an infertility diagnosis recorded would have been inadvertently misclassified as fertile. Infertility treatment is known to be underreported on the birth certificate (Luke et al., 2016); however our use of several sources of infertility treatment information would be expected to improve our identification of women who had such treatment. The subfertile cohort was designed to err on the side of misclassifying some subfertile women as fertile, rather than risk including fertile women in the subfertile group (Declercq et al., 2014). Because of the large size of the fertile group relative to the prevalence of subfertility, misclassification of subfertile women as fertile is unlikely to meaningfully bias the results. We also cannot rule out the possibility of misclassification of covariates, including plurality, based on available vital records information. Although we adjusted for several potential confounders, we were unable to evaluate the effects of obesity or time to pregnancy; nor were we able to assess the zygosity of twins.
Our mediation analysis was limited to two types of specific defects (tetralogy of Fallot and hypospadias) where there was a significant overall association with ART and a sufficient number of ART-exposed cases, even among multiples. In addition, small numbers only allowed limited evaluation of specific ART procedures.
As this study is limited to Massachusetts residents with in-state deliveries, our findings may not be generalizable to other populations, especially given the high rates of insurance coverage for ART in this state, which may affect the demographic and clinical characteristics of our ART-exposed population. There could also be regional differences in the use of various ART procedures, including single embryo transfer.
Our results show modest associations between ART use and prevalence of nonchromosomal birth defects, including hypospadias and tetralogy of Fallot. Mediation analysis shows that multiple births explain a substantial portion of the relative association between ART and birth defects. Underlying subfertility also appears to play an important role. Future research is needed to determine the degree to which newer ART procedures, such as single embryo transfer, can further minimize the risk of birth defects among ART conceived pregnancies.
Supplementary Material
Acknowledgments
This study was supported in part through the National Institutes of Health Grant R01 HD067270-06 and the Centers for Disease Control and Prevention, Grant 1U01DD001037. Dominique Heinke was supported in part by pre-doctoral training grants from the National Institutes of Health (T32HD060454) and the U.S. Department of Health and Human Services (T03MC07648). We thank the Massachusetts Outcomes Study of Assisted Reproductive Technology staff and the Pregnancy to Early Life Longitudinal staff for providing data and assistance for this project. The findings and conclusions in this report are those of the authors and do not necessarily represent the official positions of the Massachusetts Department of Public Health. Barbara Luke is a research consultant for the Society for Assisted Reproductive Technology.
Footnotes
The other authors have no conflicts of interest or financial disclosures relevant to this manuscript.
References
- Andersson T, Alfredsson L, Kallberg H, et al. Calculating measures of biological interaction. Eur J Epidemiol. 2005;20:575–579. doi: 10.1007/s10654-005-7835-x. [DOI] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. J Pers Soc Pyschol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Benedum CM, Yazdy MM, Parker SE, et al. Association of clomiphene and assisted reproductive technologies with the risk of neural tube defects. Am J Epidemiol. 2016;183:977–987. doi: 10.1093/aje/kwv322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya S, Kamath MS. Reducing multiple births in assisted reproduction technology. Best Pract Res Clin Obstet Gynaecol. 2014;28:191–199. doi: 10.1016/j.bpobgyn.2013.11.005. [DOI] [PubMed] [Google Scholar]
- Boulet SL, Kirby RS, Reefhuis J, et al. Assisted reproductive technology and birth defects among liveborn infants in Florida, Massachusetts, and Michigan, 2000–2010. JAMA Pediatr. 2016;170:e154934. doi: 10.1001/jamapediatrics.2015.4934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies MJ, Moore VM, Willson KJ, et al. Reproductive technologies and the risk of birth defects. N Engl J Med. 2012;366:1803–1813. doi: 10.1056/NEJMoa1008095. [DOI] [PubMed] [Google Scholar]
- Declercq ER, Belanoff C, Diop H, et al. Identifying women with indicators of subfertility in a statewide population database: operationalizing the missing link in assisted reproductive technology research. Fertil Steril. 2014;101:463–471. doi: 10.1016/j.fertnstert.2013.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devroey P, Van Steirteghem A. A review of ten years experience of ICSI. Hum Reprod Update. 2004;10:19–28. doi: 10.1093/humupd/dmh004. [DOI] [PubMed] [Google Scholar]
- Funke S, Flach E, Kiss I, et al. Male reproductive tract abnormalities: more common after assisted reproduction? Early Hum Dev. 2010;86:547–550. doi: 10.1016/j.earlhumdev.2010.06.015. [DOI] [PubMed] [Google Scholar]
- Hansen M, Kurinczuk JJ, de Klerk N, et al. Assisted reproductive technology and major birth defects in Western Australia. Obstet Gynecol. 2012;120:852–863. doi: 10.1097/AOG.0b013e318269c282. [DOI] [PubMed] [Google Scholar]
- Hansen M, Kurinczuk JJ, Milne E, et al. Assisted reproductive technology and birth defects: a systematic review and meta-analysis. Hum Reprod Update. 2013;19:330–353. doi: 10.1093/humupd/dmt006. [DOI] [PubMed] [Google Scholar]
- Kallen B, Finnstrom O, Lindam A, et al. Congenital malformations in infants born after in vitro fertilization in Sweden. Birth Defects Res A Clin Mol Teratol. 2010;88:137–143. doi: 10.1002/bdra.20645. [DOI] [PubMed] [Google Scholar]
- Kotelchuck M, Hoang L, Stern JE, et al. The MOSART database: linking the SART CORS clinical database to the population-based Massachusetts PELL reproductive public health data system. Matern Child Health J. 2014;18:2167–2178. doi: 10.1007/s10995-014-1465-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazar J, Kotelchuck M, Nannini A, Barger M. Identifying multiple gestation groups using state-level birth and fetal death certificate data. Matern Child Health J. 2006;10:225–228. doi: 10.1007/s10995-005-0043-1. [DOI] [PubMed] [Google Scholar]
- Luke B, Brown MB, Spector LG. Validation of infertility treatment and assisted reproductive technology use on the birth certificate in eight states. Am J Obstet Gynecol. 2016;215:126–127. doi: 10.1016/j.ajog.2016.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parazzini F, Cipriani S, Bulfoni G, et al. The risk of birth defects after assisted reproduction. J Assist Reprod Genet. 2015;32:379–385. doi: 10.1007/s10815-014-0398-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin J, Sheng X, Wang H, et al. Assisted reproductive technology and risk of congenital malformations: a meta-analysis based on cohort studies. Arch Gynecol Obstet. 2015;292:777–798. doi: 10.1007/s00404-015-3707-0. [DOI] [PubMed] [Google Scholar]
- Reefhuis J, Honein MA, Schieve LA, et al. Assisted reproductive technology and major structural birth defects in the United States. Hum Reprod. 2009;24:360–366. doi: 10.1093/humrep/den387. [DOI] [PubMed] [Google Scholar]
- Sunderam S, Kissin DM, Crawford SB, et al. Assisted reproductive technology surveillance - United States, 2013. MMWR Surveill Summ. 2015;64:1–25. doi: 10.15585/mmwr.ss6411a1. [DOI] [PubMed] [Google Scholar]
- Tararbit K, Lelong N, Houyel L, et al. Assessing the role of multiple pregnancies in the association between tetralogy of Fallot and assisted reproductive techniques: a path-analysis approach. Orphanet J Rare Dis. 2014;9:27. doi: 10.1186/1750-1172-9-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tararbit K, Lelong N, Thieulin AC, et al. The risk for four specific congenital heart defects associated with assisted reproductive techniques: a population-based evaluation. Hum Reprod. 2013;28:367–374. doi: 10.1093/humrep/des400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valeri L, VanderWeele TJ. Mediation analysis allowing for exposure-mediator interactions and causal interpretation: theoretical assumptions and implementation with SAS and SPSS macros. Psychol Methods. 2013;18:137–150. doi: 10.1037/a0031034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanderWeele TJ. A three-way decomposition of a total effect into direct, indirect, and interactive effects. Epidemiology. 2013;24:224–232. doi: 10.1097/EDE.0b013e318281a64e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen J, Jiang J, Ding C, et al. Birth defects in children conceived by in vitro fertilization and intracytoplasmic sperm injection: a meta-analysis. Fertil Steril. 2012;97:1331–1337. doi: 10.1016/j.fertnstert.2012.02.053. [DOI] [PubMed] [Google Scholar]
- Wijers CH, van Rooij IA, Rassouli R, et al. Parental subfertility, fertility treatment, and the risk of congenital anorectal malformations. Epidemiology. 2015;26:169–176. doi: 10.1097/EDE.0000000000000226. [DOI] [PubMed] [Google Scholar]
- Yin L, Hang F, Gu LJ, et al. Analysis of birth defects among children 3 years after conception through assisted reproductive technology in China. Birth Defects Res A Clin Mol Teratol. 2013;97:744–749. doi: 10.1002/bdra.23116. [DOI] [PubMed] [Google Scholar]
- Zhu JL, Basso O, Obel C, et al. Infertility, infertility treatment, and congenital malformations: Danish national birth cohort. BMJ. 2006;333:679. doi: 10.1136/bmj.38919.495718.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


