SUMMARY
Background
The serum biomarkers, elevated 7αC4 (C4) and decreased FGF19, have been proposed as screening tests for bile acid diarrhoea.
Aim
To analyse prevalence, specificity and reproducibility of fasting C4 and FGF19 in identifying bile acid diarrhoea in patients with irritable bowel syndrome with predominant diarrhoea or functional diarrhoea (summarized as IBS-D).
Methods
We prospectively studied fasting serum C4 and FGF19 in 101 IBS-D patients; we reviewed data from 37 of the 101 patients with prior fasting serum C4 and FGF19 and from 30 of the 101 patients with prior faecal bile acids per 48 hours. We compared results with normal values [C4 ≥52.5ng/mL (n=184), FGF-19 ≤61.7pg/mL (n=50)]. We used Spearman correlation and Bland Altman plots to appraise reproducibility.
Results
Among the 101 patients, there was a negative correlation between serum C4 and FGF19 (Rs=−0.342, p=0.0005). Bile acid diarrhoea was diagnosed in 10 patients based on elevated serum C4 levels [mean 23.5±23.1 (SD) ng/mL] and 21 patients based on decreased FGF19 levels (121.6±84.2pg/mL). With replicate tests in patients with stable IBS-D, 78% of C4 and 70% of FGF19 measurements remained concordant, with 3% and 11% respectively consistently positive for bile acid diarrhoea in the 101 patients. Compared to 48h faecal bile acids, specificity for C4 and FGF19 was 83% and 78%, respectively. Bland Altman plots demonstrated greater reliability of C4 than FGF19.
Conclusions
Among 101 patents with IBS-D, fasting FGF19 and C4 levels had good specificity and negative predictive value, suggesting utility as screening tests to exclude bile acid diarrhoea.
Keywords: bile acid diarrhoea, serum biomarkers, 7αC4 (C4), FGF19, screening tests, irritable bowel syndrome
INTRODUCTION
Bile acid diarrhoea is a putative pathophysiology of 10–30% of patients with chronic diarrhoea or diarrhoea-predominant irritable bowel syndrome (IBS-D) and of ~1% of the entire population(1–3). Bile acids are detergent molecules produced by the liver and released into the GI tract in response to a meal to assist with fat emulsification and absorption. Approximately 95% of bile acids are reabsorbed in the terminal ileum(3). When excess bile acids reach the colon and the associated bacteria, they interact with the G-protein coupled bile acid receptor 1 (GPBAR1 or TGR5), resulting in increased colonic motility, transit, visceral sensation, fluid secretion and mucosal permeability(4).
Studies have demonstrated that there is a limited role of increased bile acid synthesis from the liver in the aetiology of bile acid diarrhoea(3). Bile acid diarrhoea is characterised into 3 major categories, based on underlying pathophysiology: type 1 – ileal disease, indicating poor bile acid reabsorption in the ileum; type 2 – idiopathic, seen in IBS-D; or type 3 – secondary causes of bile acid diarrhoea, such as small intestinal bacterial overgrowth, cholecystectomy, and chronic pancreatitis(5). Treatment with bile acid sequestrants (such as colesevelam, cholestyramine, and colestipol) has demonstrated an 80–96% response in patients with moderate to severe bile acid diarrhoea (selenium-75-labeled homocholic acid conjugated taurine [75SeHCAT] retention <10%)(2,6). Although the main effect of bile acid sequestrants is to bind bile acids, these medications are nonspecific in their binding properties and they can potentially affect other mechanisms associated with diarrhea. For example, bile acid sequestrants bind Clostridium difficile toxin (7, 8) and decrease C. difficile spore colony formation in mice (9), change virulence of vibrio (10), promote eubiosis (11), and treat bacterial infection in mammals (12). Therefore, although bile acid binders are utilized in clinical practice when bile acid diarrhoea is suspected and there are no easily available diagnostic tests, a positive response does not necessarily confirm the diagnosis.
The gold standard test used to diagnose bile acid diarrhoea is the 75SeHCAT retention test. However, this test requires ingestion of a radio-labeleded bile acid derivative; whole-body gamma camera scans at baseline and after 7 days, and is unavailable in the United States. Therefore, several screening tests have been evaluated including the 24- and 48-hour stool faecal bile acid test; the 14C-glycoholate breath and stool test; and two serum biomarkers, 7α-hydroxy-4-cholesten-3-one (C4) and fibroblast growth factor 19 (FGF19). 14C-glycoholate breath and stool test is currently not used due to radiation exposure, varying normal values, and difficulties in interpretation in the presence of small bowel bacterial overgrowth(3, 13). C4 is a metabolic intermediate in the rate limiting step for the synthesis of BAs from hepatic cholesterol and is significantly correlated with faecal loss of bile acids(8). It is validated as a method to detect bile acid diarrhoea when compared to the 75SeHCAT retention test(11). FGF19 is a hormone released by ileal enterocytes after stimulation of nuclear farnesoid X receptors, typically by absorbed bile acids, and FGF19 provides negative feedback for bile acid synthesis in hepatocytes.(3, 14)
With a cut-off value of <10% retention of tracer 75SeHCAT at 7 days to identify patients with bile acid diarrhoea, 75SeHCAT retention has the highest average diagnostic yield of 30.8%, followed by 48‐hour faecal bile acid excretion (25.5%), FGF19 (24.8%), and C4 (17.1%) among patients with functional diarrhoea or IBS-D(15). Unfortunately, the 48-hour faecal bile acid test requires cumbersome 48‐hour stool collection and a 4-day, 100-gram fat/day intake. Therefore, the serum biomarkers, C4 and FGF19, may be the preferable screening tests for bile acid diarrhoea, and the utility of each has been evaluated in comparison with both 75SeHCAT and faecal bile acids(14, 16–18). Currently, the serum biomarkers are not being used in the clinical setting, likely due to lack of availability of the tests. However, there is potential value in having valid screening tests for bile acid diarrhoea in patients with apparent IBS-D or functional diarrhoea, both in diagnostic practice and in characterization of patients for eligibility to participate in clinical trials. Before proposing these as alternative screening tests for bile acid diarrhoea, it is important to understand the performance characteristics of the serum markers, C4 and FGF19.
The aims of our study were to prospectively evaluate the prevalence of abnormal results on repeat testing of fasting serum C4 and FGF19 in detection of bile acid diarrhoea in patients with IBS-D or chronic diarrhoea, and to assess the reproducibility and performance characteristics of these serum biomarkers.
METHODS
Participants and Study Design
This was a single-center, prospective study. Participants were recruited from an ~950 patient population base at Mayo Clinic of those with IBS-D and chronic diarrhoea based on Rome III criteria. Participants completed the validated bowel disease questionnaires (including assessment by stool daily diary for 8 days of stool frequency and stool consistency using the Bristol Stool Form Scale) and the Hospital Anxiety and Depression Scale(19, 20).
Exclusion criteria included: (1) use of drugs or agents that alter GI transit and analgesia (opioids, anticholinergics, tricyclic antidepressants, and serotonin–norepinephrine reuptake inhibitors); (2) pregnant females; and (3) chronic liver disease or history of elevated AST or ALT; (4) secondary causes of bile acid diarrhoea, such as coeliac disease, microscopic colitis, terminal ileum resection,. Cholecystectomy was not an exclusion criterion.
This study was approved by Mayo Clinic Institutional Review Board on 2/12/2015 (IRB #15–000014). The 101 enrolled subjects signed written, informed consent.
Participants provided a fasting blood sample before 9:00 a.m. (66%) or 10:00 a.m. (90%) for the measurements of C4 and FGF19. We had prior measurements of C4 and FGF19 in 37 of the same participants performed at least 5 years previously, and the recorded values were used for analysis of replicate samples from the same patients (Figure 1).
Figure 1.
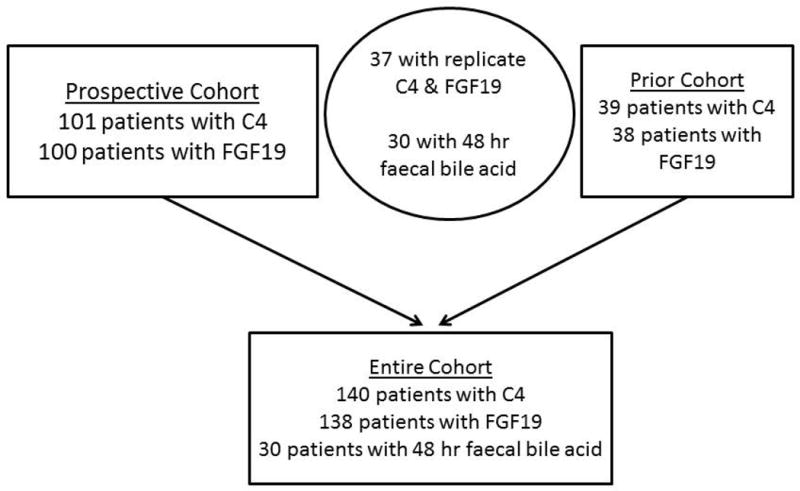
Patient distribution in the prospective and prior cohort
Serum 7α-hydroxy-4-cholesten-3-one (C4)
The C4 assay performed in our center [adapted from Galman et al.(21)] is based on HPLC/tandem mass spectrometry(22). We utilised C4 ≥52.5ng/mL to diagnose bile acid diarrhoea, based on 95th percentile seen in 184 healthy adult (110 female, 74 male) volunteers(23).
Serum Fibroblast Growth Factor 19 (FGF19)
Serum FGF19 was measured by ELISA (FGF19 Quantikine Enzyme-Linked Immunosorbent Assay Kit, R&D Systems, Minneapolis, MN), as in previous studies(24). We used FGF19 ≤61.7pg/mL to diagnose bile acid diarrhoea, based on 5th percentile seen in 50 healthy adult (39 female, 11 male) volunteers(14, 16).
Comparison of Serum C4 and FGF19 and Prior Measurements of Faecal Bile Acids
Among the 37 patients with replicate C4 and FGF19 samples, there were recorded data on 48‐hour faecal bile acid excretion from 30 participants (Figure 1). The faecal bile acid assay and method of measurement were described in previous studies(25). Patients were defined as having bile acid diarrhoea, based on a faecal bile acid cut off ≥2619μmol/48h. We compared bile acid diarrhoea diagnosis with the individual serum biomarkers(14).
Statistical Analysis
We used Spearman correlation between the prospectively collected C4 and FGF19 data and the replicate data. Pattni et al. utilised an index equation to adjust for BMI and age for serum FGF19 values(11). Therefore, we analysed BMI and age as covariates for the fasting serum biomarkers with a linear model using SAS/STAT® software. Bland-Altman plots were used to assess the variability among the replicate samples to appraise the performance characteristics of the tests. To help understand any changes in bowel function in the patients with replicate serum biomarkers, we performed analysis using 2-tailed, paired t test to compare bowel movements and stool consistency in those patients appraised at least 5 years apart. By comparison to the 48‐hour faecal bile acid excretion, we calculated specificity, sensitivity, negative predictive value, and positive predictive value for both serum biomarkers. Additionally, we determined the sensitivities and specificities of C4 and FGF19 in combination, assuming both serum tests would be performed together rather than sequentially.
RESULTS
Demographics
Table 1 shows the participant demographics. Of the 101 participants, 84 patients had IBS-D, and 17 were classified as functional diarrhoea (FD). Participants were predominantly (84%) female, overweight (average BMI, 28.7kg/m2), and aged 42.2±12.9 (SD) years. Of note, 14 patients had previously undergone cholecystectomy.
Table 1.
Demographics, psychosomatic scores and quantitative data on bile acid diarrhoea serum markers (BSFS: Bristol Stool Form Scale; HAD: Hospital Anxiety and Depression Scale)
| Mean ± SD | IBS-D/Chronic diarrhoea |
|---|---|
| N | 101 |
| IBS-D/functional diarrhoea | 84 / 17 |
| Age (yrs) | 42.2 ± 12.9 |
| Gender (F/M) | 85/16 |
| BMI, kg/m2 | 28.7 ± 7.4 |
| Bowel movements/day | 2.2 ± 1.2 |
| Bowel consistency (BSFS) | 4.7 ± 1.0 |
| Anxiety score (HAD) | 4.5 ± 2.9 |
| Depression score (HAD) | 1.6 ± 1.7 |
| Abdominal surgery (# of patients) | |
| Hysterectomy | 13 |
| Cholecystectomy | 14 |
| Appendectomy | 10 |
| Fasting serum C4 (ng/mL) | 23.5 ± 23.1 |
| Number with C4 ≥52.5ng/mL | 10/101 (10%) |
| Fasting serum FGF19 (pg/mL) | 121.6 ± 84.22 |
| Number with FGF19 ≤61.7pg/mL | 21/100 (21%) |
Bowel Function in Patients with Replicate Serum Measurements
In the first cohort of 37 patients, bowel frequency and stool consistency were respectively 1.9±0.6 bowel movements (BM)/day and 5.0±0.8 stool consistency/BM on the Bristol Stool Form Scale. The patients in the prospective cohort had 2.2±0.9 BM/day and 5.0±1.0 stool consistency/BM on the Bristol Stool Form Scale.
The intra-individual comparisons using paired t test demonstrated no differences in bowel frequency (p=0.41) or stool consistency (p=0.92) in the patients with replicate serum C4 and FGF19 measurements, indicating that the clinical status of their main symptom (diarrhoea) had not changed significantly between the two serum collections.
Relationship of Fasting Serum C4 and FGF19 Data
Figure 2 demonstrates the expected significant negative correlation (Rs=−0.342, p=0.0005) between the prospectively gathered FGF19 and C4 data in 100 patients. When the cholecystectomy patients were excluded from the analysis, the data continued to demonstrate a significant negative correlation between C4 and FGF19 (Rs = −0.0.28, p=0.009). Figure 2 also shows that the same overall inverse correlation exists in patients with prior cholecystectomy, but with the small numbers (n=14), this was not statistically significant.
Figure 2.
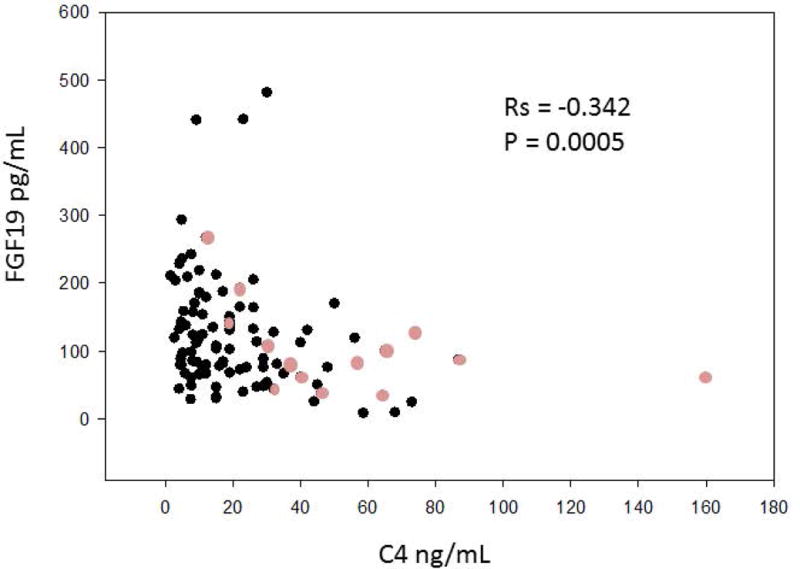
Negative Spearman correlation between prospectively collected fasting serum FGF19 and C4. The lighter circles represent patients who have undergone cholecystectomy.
Abnormal Fasting Serum C4 and FGF19 Measurements in Two Patient Cohorts
Prospective cohort
There was no significant difference when evaluating BMI and age as covariates for the fasting serum biomarkers. Using C4 and FGF19 cutoffs that were either above or below, respectively, the reference values of a healthy population, positive results for bile acid diarrhoea in the prospectively collected samples were 10% (10/101) and 21% (21/100), respectively. The results were concordant between the two tests in 74% (74/100) of participants: 5% were positive and 69% negative for bile acid diarrhoea by both tests.
In order to compare (see below) the serum biomarkers with the current gold standard in the USA, which is total faecal bile acid excretion, we included data from another 39 patients who had serum C4 and FGF19, as well as total 48-hour faecal bile acid excretion.
Repeat Testing of Fasting Serum C4 and FGF19 Measurements
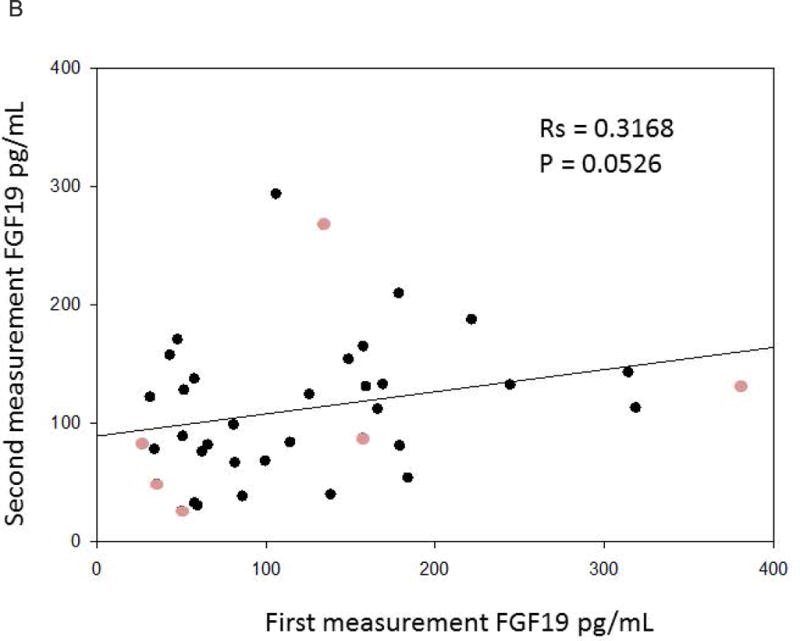
Figures 3a and 3b represent the Spearman correlations between the replicate samples of the serum biomarkers in patients with no significant changes in clinical evaluation of bowel functions. Statistically significant correlations were observed for the replicate C4 values, while borderline significance was observed between the repeat FGF19 measurements (p=0.0526).
Figure 3.
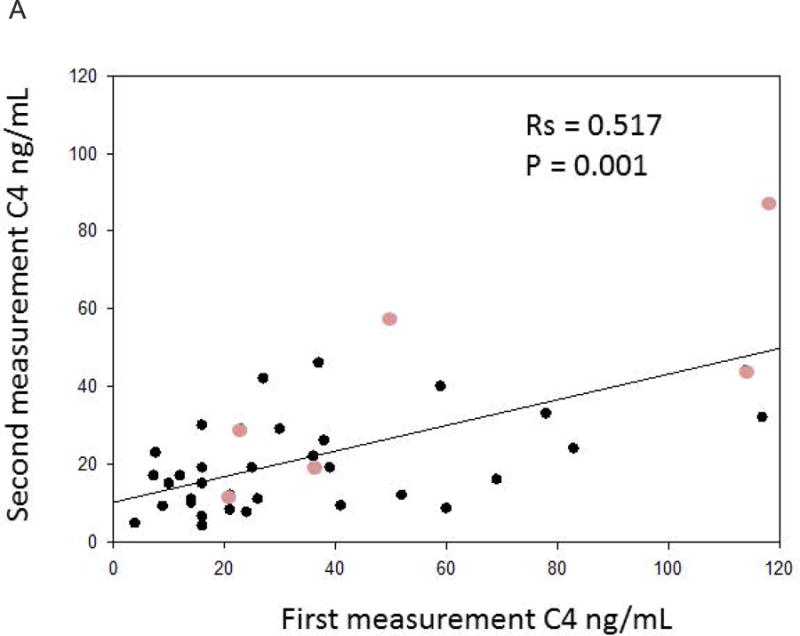
a. Positive Spearman correlation between replicate C4 samples over time. The lighter circles represent patients who have undergone cholecystectomy.
b. Positive Spearman correlation between replicate FGF19 samples over time. The lighter circles represent patients who have undergone cholecystectomy.
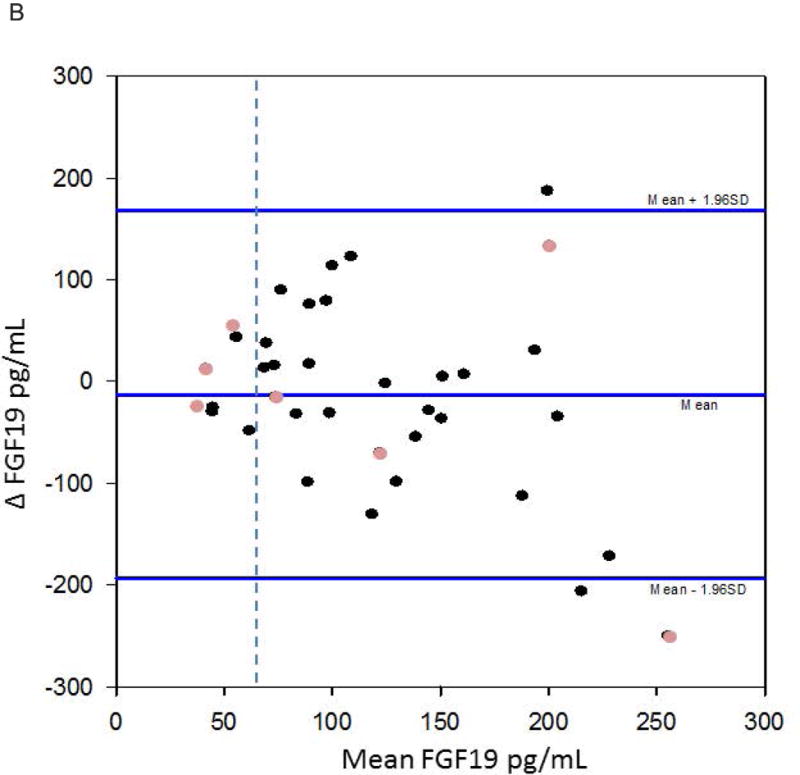
Figures 4a and 4b show the Bland Altman plots for the replicate samples. C4 demonstrates greater variability above the cutoff value (52.5ng/mL) to diagnose bile acid diarrhoea. In contrast, the abnormal values of serum FGF19 appear to be more consistent.
Figure 4.
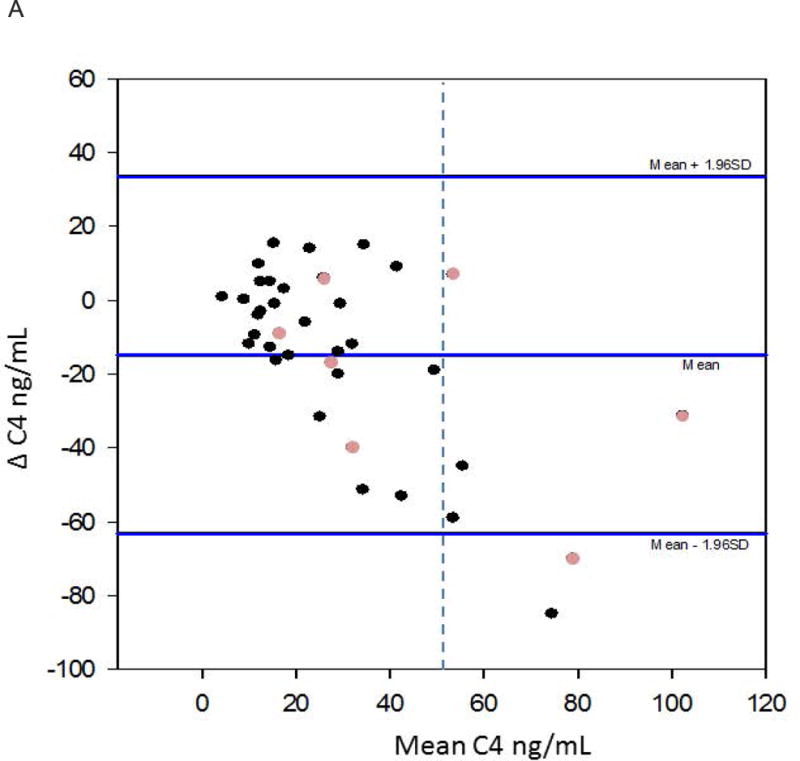
a. Bland-Altman for replicate C4 values. The dashed vertical line depicts C4=52.5ng/mL. This graph depicts greater variability in the higher C4 values; of note, they are diagnostic for bile acid diarrhoea. The lighter circles represent patients who have undergone cholecystectomy.
b. Bland-Altman for replicate FGF19 values. The dashed vertical line demonstrates FGF19=61.7pg/mL. There was less variability compared to the C4 Bland-Altman plot. The lighter circles represent patients who have undergone cholecystectomy.
In the supplementary figure (in Supplemental Material), there is an outline of how C4 and FGF19 changed over time. For C4, 29/37 (78%) samples remained stable, with 1/37 (3%) being consistently positive for bile acid diarrhoea. For FGF19, 26/37 (70%) values remained stable, with 4/37 (11%) being consistently reduced below the normal range (<61.7pg/mL) suggesting bile acid diarrhoea.
Comparison of Serum Biomarkers and Faecal Bile Acid Excretion
Table 2 demonstrates the comparisons of bile acid diarrhoea screen with serum biomarkers and 48‐hour faecal bile acids. C4 and FGF19 demonstrated high negative predictive value and specificity for both markers 79%/83% and 78%/78%, respectively. When combining C4 and FGF19 together, the sensitivity increased to 50% and the specificity was 65%. When we excluded patients with cholecystectomy, serum C4 showed 40% sensitivity, 85% specificity, 40% PPV, and 85% NPV to diagnose bile acid diarrhoea. For FGF19, exclusion of patients with prior cholecystectomy resulted in 20% sensitivity, 75% specificity, 17% PPV, and 79% NPV to diagnose bile acid diarrhoea.
Table 2.
Statistical comparison of the diagnostic value of fasting serum C4 and FGF19 as compared to 48hr fecal bile acids
| Bile acid biomarker | >2619μmol/48h (+ bile acid diarrhoea) |
<2619 μmol/48h (− bile acid diarrhoea) |
PPV | NPV |
|---|---|---|---|---|
| C4 | ||||
| ≥52.5 ng/mL (+ bile acid diarrhoea) | 2 | 4 | 33% | NA |
| ≤52.5 ng/mL (− bile acid diarrhoea) | 5 | 19 | NA | 79% |
| Sensitivity | 29% | NA | ||
| Specificity | NA | 83% | ||
| FGF19 | ||||
| ≤61.7 pg/mL (+ bile acid diarrhoea) | 2 | 5 | 29% | NA |
| >61.7 pg/mL (− bile acid diarrhoea) | 5 | 18 | NA | 78% |
| Sensitivity | 29% | NA | ||
| Specificity | NA | 78% |
PPV=positive predictive value; NPV=negative predictive value;
Replication Cohort
From our prior cohort, there were an additional 39 patients diagnosed with IBS-D with C4 and 38 patients with FGF19. Utilizing the new fasting serum biomarker cutoffs, the prevalence of bile acid diarrhoea was 23% based on C4 and 34% based on FGF19.
In the entire patient database of 140 patients, including patient samples collected since 2012, there were 138 and 140 patients with measurements of fasting FGF19 and C4, respectively, in patients with IBS-D. Abnormal results were identified in 14% with C4 and 5% with FGF-19.
DISCUSSION
In this study, we have analysed replicate fasting serum FGF19 and C4 values in patients with functional diarrhoea and IBS-D. The participant demographics were similar (including psychosomatic scores) to those participating in previous studies with IBS-D and chronic diarrhoea at our center(26). We have observed significant correlations in replicate serum C4 measurements, as well as high levels of specificity and negative predictive values relative to faecal bile acid measurements. Thus, our findings suggest that serum C4 and FGF19 are biomarkers that can be used as screening methods for bile acid diarrhoea, using the cut-offs ≥52.1ng/mL and ≤61.7pg/mL, respectively. The serum biomarkers provide a simple method to rule out bile acid diarrhoea in patients with IBS-D. Those who test positive on the serum tests, 75SeHCAT or 48h fecal bile acid measurement, will require additional confirmatory testing, as indicated in the proposed algorithm (Figure 5). According to this algorithm, if fasting serum FGF19 and C4 are negative, then the patient can receive available symptomatic therapies for the IBS-D. If either C4 or FGF19 test is positive, the patient with IBS-D should undergo available confirmatory testing with measurement of fecal bile acids over 48h or 75SeHCAT retention.
Figure 5.
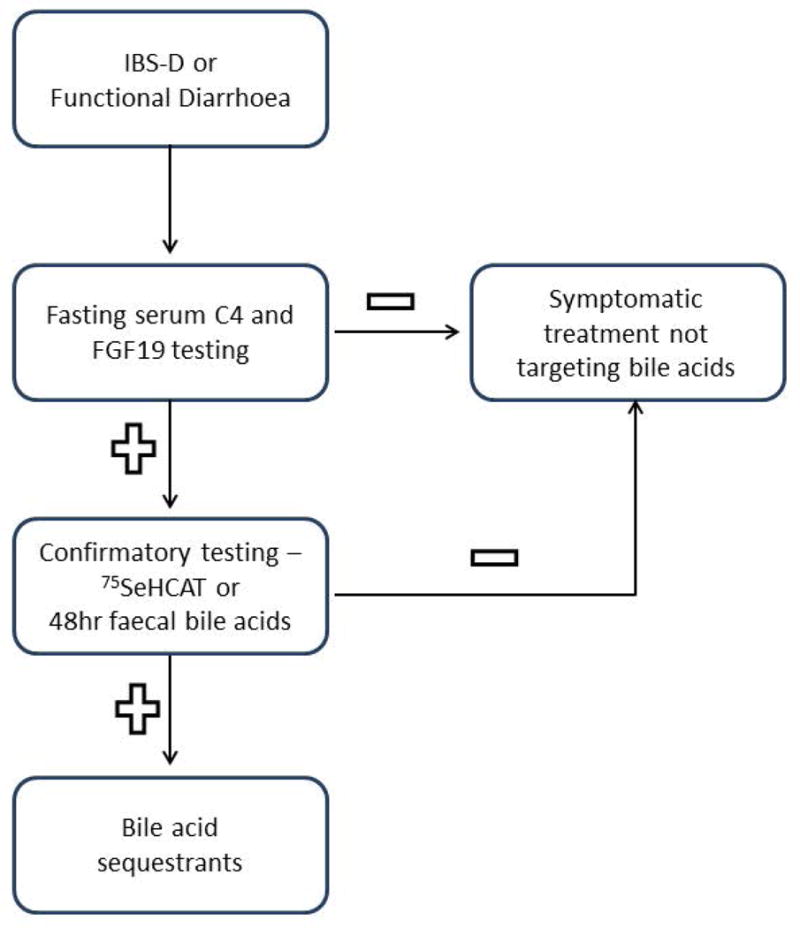
Proposed algorithm using fasting serum FGF19 and C4 in the diagnosis of bile acid diarrhoea in patients with IBS-D or functional diarrhoea
Consistent with previous reports in the literature, these serum biomarkers measured in the prospectively collected samples (~100 samples) diagnosed bile acid diarrhoea in 10% for C4 and 21% for FGF19; additionally, in the entire current database (~140 samples), bile acid diarrhoea was diagnosed in 14% and 25%, based on C4 and FGF19, respectively(3). The cutoff values over time for bile acid diarrhoea diagnosis have changed as more healthy volunteer data have been collected, relative to the values previously reported from our laboratory [e.g. C4 cutoff value of 47.1ng/mL(8,9)]. The new C4 cutoff based on our healthy volunteers (≥52.1ng/mL) was reproduced by Bajor et al.(21). Participants with 75SeHCAT retention ≤ 20% were treated on colestipol. Responders were defined by a decrease in symptoms and number of bowel movements by 50%. The responders were noted to have an average C4 value of 55ng/mL(27). Although the number of participants in our study was relatively small for the comparison to the gold standard of total faecal bile acid excretion over 48 hours, it demonstrated a high specificity and negative predictive value of fasting serum C4 and FGF19.
Our observations confirm those reported in the literature for studies of the individual biomarkers, serum C4 and FGF19. Sauter et al. demonstrated C4 sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of 90%, 79%, 73%, and 92%, respectively, for C4 ≥48ng/mL and 75SeHCAT <10%. Our data demonstrated a higher specificity (83%) with a higher cut-off of 52.5ng/mL. The higher positive predictive value observed by Sauter et al. is based on the increased prevalence of bile acid diarrhoea in that cohort of 17/24 (71%) patients with type 1 and type 3 bile acid diarrhoea(28). Pattni et al. described sensitivity and specificity of 58% and 84%, respectively, for FGF19 ≤145pg/mL as compared to 75SeHCAT retention values of <10%. Sensitivity increased to 68% when FGF19 values were combined with serum C4, BMI, and patient age(17). These differences in the observed sensitivity and specificity may be related to the differences in the diagnostic yield of 75SeHCAT compared to faecal bile acid measurements and different cutoff points used in different studies.
Our study also evaluated the concordance of these biomarkers on repeat testing in a patient population with no significant change in their bowel dysfunction. The measurements of C4 several years apart demonstrated a significant positive correlation. In addition, 78% of the C4 values were consistent over time and remained predominantly normal over time. Although the Bland-Altman plot for fasting serum C4 demonstrated variability in the higher values observed, these values were all above the cutoff line for bile acid diarrhoea; that is, the test results were abnormal, despite variability over time. More importantly, there was increased reliability (or less variability) within the normal range of C4 that excluded bile acid diarrhoea, indicating that participants whose values were normal remained normal overtime, thus, validating the utility of fasting C4 measurement as a screening test.
Fasting serum FGF19 showed a borderline significant correlation on repeat testing. Seventy percent of the FGF19 values remained consistent over time, with 59% and 11% remaining negative and positive over time, respectively. The Bland-Altman plot demonstrated more stable FGF19 values, with slightly greater variability with the higher absolute values. The abnormal values (≤61.7pg/mL, indicating bile acid diarrhoea) were also consistent and, therefore, reliable over time.
The strengths of our study include the appraisal of both serum markers for bile acid diarrhoea in the same cohort and comparison of results with earlier results from serum measurements and with faecal 48‐hour bile acid excretion. Additionally, based on the bowel diary data, the patients had no significant changes in their bowel dysfunction between the two times of testing. Given the diurnal variation in the serum FGF19 measurements(29) and the effect of meal ingestion on serum C4 measurements, an important precaution taken was to ensure that all measurements were performed during fasting and at approximately the same time of day, that is, 66% before 9:00 a.m. and 90% before 10:00 a.m. This is particularly relevant for FGF19 which shows significant variation, reaching peak levels approximately 3–4 hours after meals(29).
Limitations of our study include the need for further enriching the study cohort with more patients with bile acid diarrhoea. Three of the patients with positive results on the replicate C4 and FGF19 measurements had previously undergone cholecystectomy. However, it is worth noting that other patients had not done so, and there were equal numbers of post-cholecystectomy patients with normal or abnormal values of serum C4 and FGF19. From this observation, we also conclude that the serum biomarkers can be used to screen for bile acid diarrhoea among patients with post-cholecystectomy diarrhoea. An additional limitation is the absence of concurrent 48hour faecal bile acid measurement.
A concern in our study is the interpretation from the observation of discordant serum biomarkers. Five patients had similar interpretation of the C4 and FGF19 values on repeat testing, but the results were not consistent with the faecal bile acid data. Pattni et al.(17) suggested that combining the two serum biomarkers increased the yield for bile acid diarrhoea diagnosis. Our data combining C4 and FGF19 confirmed that recommendation, since there was improved sensitivity (50%) when considering C4 and FGF19 together (in parallel analysis). However, the specificity for diagnosis of bile acid diarrhoea was lower with the combination of C4 and FGF19, and this is demonstrated by the observation that 5 patients with positive combined serum C4 and FGF19 had negative faecal bile acid excretion results.
Currently, we are unable to recommend just one blood test for bile acid diarrhoea diagnosis, but the serum C4 test appears to be most reliable as a screening test to exclude bile acid diarrhoea in view of the higher negative predictive value and specificity, and the more consistent values of normal C4 levels on replicate testing, as shown in the Bland-Altman plot.
Based on previous reports and our data, C4 has high specificity and reproducibility, particularly in patients with no evidence of bile acid diarrhoea. Combining both C4 and FGF19 can help increase the diagnostic yield for bile acid diarrhoea. We believe that these serum biomarkers are a good screening method for bile acid diarrhoea.
Without such a simple screening method, patients may choose to forgo the arduous 48‐hour faecal bile acid test and proceed with a therapeutic trial of bile acid sequestrants. However, these agents are nonspecific in their binding properties and are not a reliable test to diagnose bile acid diarrhoea (7–10) Additionally, the cost in the United States of full dose colesevelam (1.875g, b.i.d.) for 4 weeks is about 5-fold higher than the cost of measurements of serum C4 and FGF19. Since the serum biomarkers predict the response to bile acid sequestrants(30) and are cost-efficient compared to a 4‐week therapeutic trial, we believe they have a place as first-line tests and, if necessary, can be followed by confirmatory diagnostic tests such as total faecal bile acid excretion or, where available, 75SeHCAT retention test. Such a diagnostic approach would also decrease the risk of adverse effects from unnecessary treatment.
Future directions for this research include additional confirmatory testing of the diagnostic performance of fasting serum C4 and FGF19 with tests such as 75SeHCAT or 48‐hour faecal bile acid, based on availability, as well as conducting studies to understand why replicate C4 and FGF19 values change over time and if symptoms and severity change in accordance with the observed variation in the biomarker.
Supplementary Material
Acknowledgments
The authors thank Mrs. Cindy Stanislav for excellent secretarial assistance.
Financial support: Dr. Camilleri is funded by NIH grant R01-DK92179.
Abbreviations
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- BMI
body mass index
- ELISA
enzyme-linked immunosorbent assay
- HPLC
high performance liquid chromatography
- NPV
negative predictive value
- PPV
positive predictive value
- SD
standard deviation
Footnotes
DR MICHAEL CAMILLERI (Orcid ID : 0000-0001-6472-7514)
Guarantor of the article: Dr. Michael Camilleri takes responsibility for the integrity of this work as a whole, from inception to published article.
Authors’ contributions:
P. Vijayvargiya: research fellow and patient care, authorship of manuscript
M. Camilleri: principal investigator, patient care, authorship of manuscript
P. Carlson: lab measurements of serum FGF19
A. Lueke: lab measurements of serum C4 and faecal bile acids
J. O’Neill: study coordination, patient recruitment
D. Burton: database management
I. Busciglio: study coordination and database management
L. Donato: laboratory supervision, authorship of manuscript
All authors approved the final manuscript, including the authorship list.
STATEMENT OF INTERESTS
Competing interests: This manuscript is based on an abstract presented at Federation of Neurogastrointestinal Motility Societies in August 2015, and appears in Neurogastroenterol Motil 2015;28(Suppl. 1):9.
References
- 1.Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2012;10(7):712–21.e4. doi: 10.1016/j.cgh.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 2.Wedlake L, A’Hern R, Russell D, Thomas K, Walters JR, Andreyev HJ. Systematic review: the prevalence of idiopathic bile acid malabsorption as diagnosed by SeHCAT scanning in patients with diarrhoea-predominant irritable bowel syndrome. Alimentary pharmacology & therapeutics. 2009;30(7):707–17. doi: 10.1111/j.1365-2036.2009.04081.x. [DOI] [PubMed] [Google Scholar]
- 3.Camilleri M, Shin A, Busciglio I, Carlson P, Acosta A, Bharucha AE, et al. Validating biomarkers of treatable mechanisms in irritable bowel syndrome. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2014;26(12):1677–85. doi: 10.1111/nmo.12421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Camilleri M, Shin A, Busciglio I, Carlson P, Acosta A, Bharucha AE, et al. Genetic variation in GPBAR1 predisposes to quantitative changes in colonic transit and bile acid excretion. American journal of physiology Gastrointestinal and liver physiology. 2014;307(5):G508–16. doi: 10.1152/ajpgi.00178.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fromm H, Malavolti M. Bile acid-induced diarrhoea. Clinics in gastroenterology. 1986;15(3):567–82. [PubMed] [Google Scholar]
- 6.Wedlake L, Thomas K, Lalji A, Anagnostopoulos C, Andreyev HJ. Effectiveness and tolerability of colesevelam hydrochloride for bile-acid malabsorption in patients with cancer: a retrospective chart review and patient questionnaire. Clinical therapeutics. 2009;31(11):2549–58. doi: 10.1016/j.clinthera.2009.11.027. [DOI] [PubMed] [Google Scholar]
- 7.Halsey J. Current and future treatment modalities for Clostridium difficile-associated disease. American journal of health-system pharmacy : AJHP : official journal of the American Society of Health-System Pharmacists. 2008;65(8):705–15. doi: 10.2146/ajhp070077. [DOI] [PubMed] [Google Scholar]
- 8.Palace GP, Lazari P, Norton K. Analysis of the physicochemical interactions between Clostridium difficile toxins and cholestyramine using liquid chromatography with post-column derivatization. Biochimica et biophysica acta. 2001;1546(1):171–84. doi: 10.1016/s0167-4838(01)00138-8. [DOI] [PubMed] [Google Scholar]
- 9.Giel JL, Sorg JA, Sonenshein AL, Zhu J. Metabolism of bile salts in mice influences spore germination in Clostridium difficile. PloS one. 2010;5(1):e8740. doi: 10.1371/journal.pone.0008740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gotoh K, Kodama T, Hiyoshi H, Izutsu K, Park KS, Dryselius R, et al. Bile acid-induced virulence gene expression of Vibrio parahaemolyticus reveals a novel therapeutic potential for bile acid sequestrants. PloS one. 2010;5(10):e13365. doi: 10.1371/journal.pone.0013365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu A, Chen J, Wu P, Luo M, Zeng Y, Zheng H, et al. Cationic Polystyrene Resolves Nonalcoholic Steatohepatitis (NASH), Obesity, and Metabolic Disorders by Promoting Eubiosis of Gut Microbiota and Decreasing Endotoxemia. Diabetes. 2017 doi: 10.2337/db17-0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rankin KA, Alroy KA, Kudela RM, Oates SC, Murray MJ, Miller MA. Treatment of cyanobacterial (microcystin) toxicosis using oral cholestyramine: case report of a dog from Montana. Toxins. 2013;5(6):1051–63. doi: 10.3390/toxins5061051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vijayvargiya P, Camilleri M, Shin A, Saenger A. Methods for diagnosis of bile acid malabsorption in clinical practice. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2013;11(10):1232–9. doi: 10.1016/j.cgh.2013.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shin A, Camilleri M, Vijayvargiya P, Busciglio I, Burton D, Ryks M, et al. Bowel functions, fecal unconjugated primary and secondary bile acids, and colonic transit in patients with irritable bowel syndrome. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2013;11(10):1270–5.e1. doi: 10.1016/j.cgh.2013.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Valentin N, Camilleri M, Altayar O, Vijayvargiya P, Acosta A, Nelson AD, et al. Biomarkers for bile acid diarrhoea in functional bowel disorder with diarrhoea: a systematic review and meta-analysis. Gut. 2015 doi: 10.1136/gutjnl-2015-309889. [DOI] [PubMed] [Google Scholar]
- 16.Wong BS, Camilleri M, Carlson P, McKinzie S, Busciglio I, Bondar O, et al. Increased bile acid biosynthesis is associated with irritable bowel syndrome with diarrhea. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2012;10(9):1009–15.e3. doi: 10.1016/j.cgh.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pattni SS, Brydon WG, Dew T, Johnston IM, Nolan JD, Srinivas M, et al. Fibroblast growth factor 19 in patients with bile acid diarrhoea: a prospective comparison of FGF19 serum assay and SeHCAT retention. Alimentary pharmacology & therapeutics. 2013;38(8):967–76. doi: 10.1111/apt.12466. [DOI] [PubMed] [Google Scholar]
- 18.Brydon WG, Nyhlin H, Eastwood MA, Merrick MV. Serum 7 alpha-hydroxy-4-cholesten-3-one and selenohomocholyltaurine (SeHCAT) whole body retention in the assessment of bile acid induced diarrhoea. European journal of gastroenterology & hepatology. 1996;8(2):117–23. doi: 10.1097/00042737-199602000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta psychiatrica Scandinavica. 1983;67(6):361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 20.Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130(5):1480–91. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 21.Galman C, Arvidsson I, Angelin B, Rudling M. Monitoring hepatic cholesterol 7alpha-hydroxylase activity by assay of the stable bile acid intermediate 7alpha-hydroxy-4-cholesten-3-one in peripheral blood. Journal of lipid research. 2003;44(4):859–66. doi: 10.1194/jlr.D200043-JLR200. [DOI] [PubMed] [Google Scholar]
- 22.Camilleri M, Nadeau A, Tremaine WJ, Lamsam J, Burton D, Odunsi S, et al. Measurement of serum 7alpha-hydroxy-4-cholesten-3-one (or 7alphaC4), a surrogate test for bile acid malabsorption in health, ileal disease and irritable bowel syndrome using liquid chromatography-tandem mass spectrometry. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2009;21(7):734–e43. doi: 10.1111/j.1365-2982.2009.01288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stacy M, Kenyon AL, Meeusen Jeffrey W, Camilleri Michael, Donato Leslie J. Measurement of 7-alpha-hydroxy-cholestene-3-one in Serum Using LC-MS/MS for the Screening of Bile Acid Malabsorption in IBS-D. Manuscript in progress. 2017 [Google Scholar]
- 24.Walters JR, Tasleem AM, Omer OS, Brydon WG, Dew T, le Roux CW. A new mechanism for bile acid diarrhea: defective feedback inhibition of bile acid biosynthesis. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2009;7(11):1189–94. doi: 10.1016/j.cgh.2009.04.024. [DOI] [PubMed] [Google Scholar]
- 25.Tagliacozzi D, Mozzi AF, Casetta B, Bertucci P, Bernardini S, Di Ilio C, et al. Quantitative analysis of bile acids in human plasma by liquid chromatography-electrospray tandem mass spectrometry: a simple and rapid one-step method. Clinical chemistry and laboratory medicine. 2003;41(12):1633–41. doi: 10.1515/CCLM.2003.247. [DOI] [PubMed] [Google Scholar]
- 26.Vazquez-Roque MI, Camilleri M, Smyrk T, Murray JA, Marietta E, O’Neill J, et al. A controlled trial of gluten-free diet in patients with irritable bowel syndrome-diarrhea: effects on bowel frequency and intestinal function. Gastroenterology. 2013;144(5):903–11.e3. doi: 10.1053/j.gastro.2013.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bajor A, Törnblom H, Rudling M, Ung K-A, Simrén M. Increased colonic bile acid exposure: a relevant factor for symptoms and treatment in IBS. Gut. 2015;64(1):84–92. doi: 10.1136/gutjnl-2013-305965. [DOI] [PubMed] [Google Scholar]
- 28.Sauter GH, Munzing W, von Ritter C, Paumgartner G. Bile acid malabsorption as a cause of chronic diarrhea: diagnostic value of 7alpha-hydroxy-4-cholesten-3-one in serum. Digestive diseases and sciences. 1999;44(1):14–9. doi: 10.1023/a:1026681512303. [DOI] [PubMed] [Google Scholar]
- 29.Lundasen T, Galman C, Angelin B, Rudling M. Circulating intestinal fibroblast growth factor 19 has a pronounced diurnal variation and modulates hepatic bile acid synthesis in man. Journal of internal medicine. 2006;260(6):530–6. doi: 10.1111/j.1365-2796.2006.01731.x. [DOI] [PubMed] [Google Scholar]
- 30.Odunsi-Shiyanbade ST, Camilleri M, McKinzie S, Burton D, Carlson P, Busciglio IA, et al. Effects of chenodeoxycholate and a bile acid sequestrant, colesevelam, on intestinal transit and bowel function. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2010;8(2):159–65. doi: 10.1016/j.cgh.2009.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


