Abstract
Both HIV infection and antiretroviral therapy (ART) are associated with lower bone mineral density (BMD) and increased fracture risk. The relative contributions of ART and untreated HIV to BMD loss are unclear, it is important to quantify the effect of ART on bone. We compared the effect of early ART initiation (CD4 >500 cells/μL) with deferred ART on change in BMD in the START Bone Mineral Density substudy, a randomised trial evaluating the effect of immediate ART initiation versus deferring ART (to CD4 <350 cells/μL). BMD was measured annually at the lumbar spine and hip by dual-energy X-ray absorptiometry (DXA). Percent change in BMD by treatment assignment (intent-to-treat analysis) was estimated using longitudinal mixed models and linear regression. Baseline and follow-up DXA scans were available for 399 (195 immediate, 204 deferred) participants (median age 32 years, 80% non-white, 26% women, median CD4 count 642 cells/μL). ART (most commonly including tenofovir and efavirenz) was used for 95% and 18% of follow-up in the immediate and deferred ART groups, respectively. Through 2.2 years mean follow-up, immediate ART resulted in greater BMD declines than deferred ART at the hip (−2.5% vs. −1.0%; difference −1.5%, 95% CI −2.2 to −0.8, p<0.001) and spine (−1.9% vs. −0.4%; difference −1.6%, 95% CI −2.2 to −1.0, p<0.001). BMD declines were greatest in the first year of ART. In the immediate ART group, spine BMD stabilized after year 1, while hip BMD declined progressively over 2 years. After year 1, BMD changes were similar in the immediate and deferred groups. No clinical, HIV-related or ART characteristic predicted greater BMD loss in either group. All HIV treatment guidelines now recommend ART initiation at HIV diagnosis, due to the reduced risk of serious clinical outcomes. Better understanding of the longer term consequences of the observed reductions in BMD is needed.
Keywords: Bone Mineral Density, HIV, Antiretroviral therapy, Clinical Trials, DXA
INTRODUCTION
Low bone mineral density (BMD), osteoporosis and fractures are more common in HIV-infected adults than in HIV-negative controls (1, 2). Uncontrolled studies have found that initiation of antiretroviral therapy (ART) is followed by a 2–6% reduction in BMD, mainly over the first 1–2 years. Most studies report stabilisation thereafter, although follow-up was generally less than 3 years (1, 3). Antiretroviral guidelines now recommend immediate ART initiation regardless of CD4 count, in large part due to the results of the INSIGHT Strategic Timing of Antiretroviral Therapy (START) trial, which reported a 57% reduction in serious AIDS and non-AIDS related morbidity and mortality. (4, 5)
Bone loss in HIV-infected adults is multifactorial. Greater bone loss has been reported with use of tenofovir disoproxil fumarate (TDF) and ritonavir-boosted protease inhibitors (PIs) in the ART regimen (3, 6). However, some bone loss occurs with all ART (7), probably due to increased bone catabolism following suppression of HIV viral load and immune reconstitution (8, 9). Untreated HIV is also associated with lower BMD, possibly due to greater prevalence of conventional risk factors, or to HIV infection of osteoblasts or increased bone metabolism (10, 11). Cross-sectional studies, however, have not reported consistent relationships between BMD and duration of HIV infection, viral load, or CD4 count (12, 13).
The relative contributions of ART and untreated HIV infection to BMD loss are unclear and it is important to quantify the bone effects of ART to fully determine its risk-benefit profile. There are no prospective, randomised trial data describing the effect of ART versus no ART on bone. We report the results of the Bone Mineral Density substudy of START, a randomized trial comparing the effects of immediate versus deferred ART on hip and spine BMD. We hypothesized that immediate ART would result in greater BMD loss than deferred ART.
MATERIALS and METHODS
Study Design and Participants
The START study randomized 4685 HIV-positive, ART-naïve adults with high CD4 counts (>500 cells/μL) to one of two strategies of ART initiation, either immediate ART initiation or deferred ART (initiation when CD4 count fell below 350 cells/μL or HIV disease progression) (14). ART regimens were not protocol-specified, but the initial regimen was selected by investigators pre- randomisation. At 33 clinical sites in 11 countries, all eligible START participants were offered BMD substudy co-enrolment. Eligibility criteria were broad, and excluded only those receiving treatment for low BMD (calcium, vitamin D and hormone replacement therapy were permitted) or for whom valid BMD scans could not be obtained. The substudy was approved by the institutional review board at each participating clinical site, and was performed in compliance with the principles of the Declaration of Helsinki and local regulatory requirements. All participants provided written, informed consent prior to enrolment.
BMD at the hip and lumbar spine (L1–L4) was measured at baseline (within 120 days before randomisation) and annually by dual-energy x-ray absorptiometry (DXA). Each of the 16 radiology centres used either a Hologic (Hologic Inc., Bedford, MA, United States; n=290 participants) or GE Lunar (GE Healthcare, Madison, WI, United States; n=134) scanner. All DXA images were obtained using a standardized protocol, and read centrally at the study’s DXA quality assurance (QA) centre (University of California, San Francisco, CA, United States). Procedures to ensure quality and standardization of BMD measurements have been described (15). In brief, equipment and radiology technicians at each radiology centre were certified by the DXA QA centre; the quality of each scan submitted was evaluated immediately at the QA centre, and unacceptable scans were repeated. All scans were standardised for longitudinal and cross-sectional consistency by the DXA QA centre, using two types of phantom scans: (1) phantoms provided by the DXA equipment manufacturers were scanned prior to each participant scan and at least three times a week; and (2) a set of three cross-calibration phantoms were scanned at each radiology centre. BMD measures obtained on GE Lunar equipment were standardised to Hologic measures using validated linear transformation equations (16, 17). T-scores and Z-scores were calculated from longitudinally and cross-sectionally adjusted BMD readings. T-scores were calculated relative to peak bone mass in young white women (18). Z-scores were calculated relative to U.S. reference populations matched by age, sex and race/ethnicity (19). Low BMD (below the expected range for age) was defined by a BMD Z-score (spine, hip, femoral neck) ≤ −2, consistent with recommendations for young populations by the U.S. National Osteoporosis Foundation and the International Society for Clinical Densitometry. (20, 21) WHO classifies BMD T-scores ≤ −2.5 as osteoporosis.(22)
Fractures and osteoporosis treatment were recorded at baseline and annually for all START participants. Use of vitamin D and calcium supplements was recorded at baseline and annually in the BMD substudy participants.
Study Outcomes
The co-primary outcomes were changes from baseline in total hip BMD and lumbar spine BMD. The primary objective was to compare changes in hip and spine BMD through follow-up between the immediate and deferred ART groups by intent-to-treat. Pre-specified secondary objectives included change in femoral neck BMD, incidence of osteoporosis or low BMD, rates of BMD loss upon ART initiation in the immediate ART group and among participants in the deferred group prior to ART start (untreated HIV), and evaluation of clinical parameters associated with rates of BMD change.
Statistical analyses
The sample size of 400 participants was estimated to detect between-group differences of 1.0% and 1.2% in mean percent change in total hip and spine BMD, respectively, from baseline through follow-up, with 80% power at a 5% significance level, assuming standard deviations of 3.8% and 4.8%, respectively (3, 23).
All changes in BMD from baseline were expressed as percent of baseline BMD. Follow-up was censored at each participant’s last DXA scan prior to May 27, 2015 (when the Data and Safety Monitoring Board recommended all START participants be offered ART)(14). Unless noted otherwise, all treatment group comparisons were by intent-to-treat. The primary analysis was the intent-to-treat comparison between the immediate and deferred ART groups for percent change in BMD using longitudinal mixed models, adjusted for baseline BMD and visit. Groups were compared for changes in BMD to each year of follow-up using ANCOVA models adjusted for baseline BMD. Rates of BMD change were estimated within each group using unadjusted, longitudinal mixed models with the subject-specific annual percent change in BMD as response variable; to compare the groups, models were adjusted for baseline BMD and visit. The groups were compared for incidence of low BMD and T-scores ≤−2.5 using unadjusted Cox regression models. To assess the effect of ART versus strictly untreated HIV, we compared the immediate group (excluding participants who did not start ART within the first year) versus the deferred group censored at ART start. Subgroup analyses for the co-primary outcomes were performed to determine whether the treatment effect differed across baseline characteristics; we considered only subgroups that included at least 20 participants pooled across the two treatment arms. Homogeneity of treatment effect was assessed by testing for interaction between the subgroup variable and treatment group indicator in longitudinal mixed models adjusted for baseline BMD and visit. Associations of baseline factors with changes in BMD following ART initiation in the immediate ART group were estimated in longitudinal mixed models. To evaluate the effect of time-updated ART use, the annual percent change in BMD was used as response in longitudinal mixed models, and the subject-specific proportion of follow-up time that specific drugs (or any ART) were used during each year were included as time-updated predictors in the models, along with baseline predictors.
Fracture incidence rates were estimated in the parent START population, and groups were compared using a Cox proportional hazards model. Analyses were performed with SAS version 9.3 (SAS Institute, Cary, North Carolina, United States) and R version 3 (24).
RESULTS
Participant Characteristics
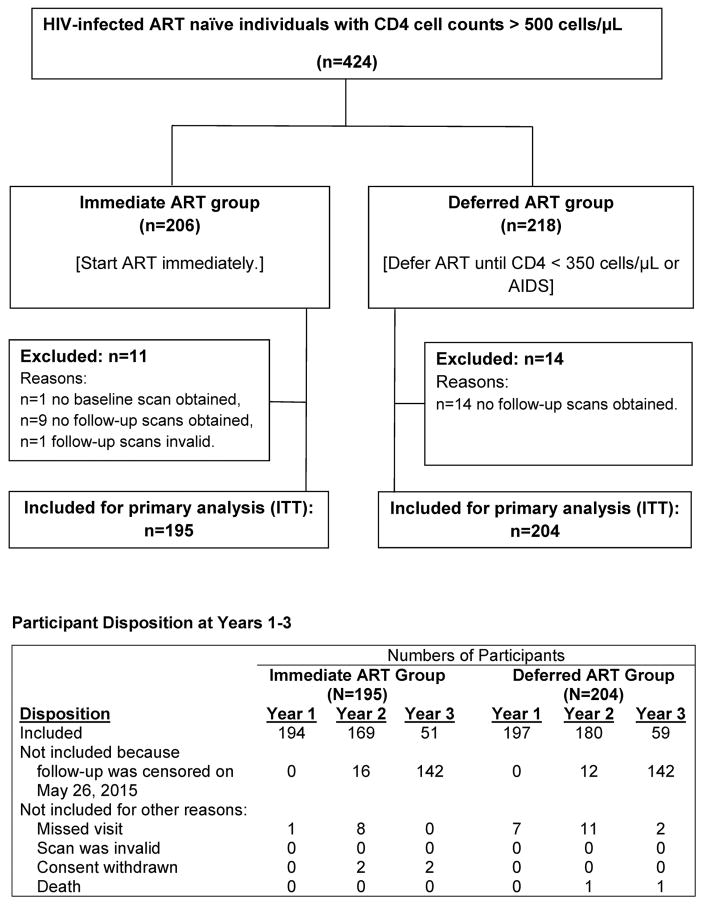
The BMD substudy co-enrolled 424 START participants between June 2011 and June 2013. Baseline characteristics of the substudy population have been reported (15); 25 participants (5.9%) did not have analysable baseline or follow-up scans and were excluded from analysis (Figure 1). Table 1 shows the baseline characteristics of the 399 analysed participants (195 in the immediate group, 204 in the deferred ART groups). The racially-diverse population had a median age of 32 years; 26.1% were women; median time since HIV diagnosis was 0.7 years. Forty-five participants (11.3%) had low BMD relative to their age group (Z-score ≤ −2) at the spine, total hip or femoral neck.
Figure 1.
Study design, CONSORT diagram, and participant disposition at each follow-up visit.
Of the 399 participants included in the primary analysis, 349 (87.5%) had year 2 BMD data, 110 (27.6%) had year 3 data. For 284 of the 289 participants without year 3 data, the year 3 visit was due after the common censoring date, May 26, 2015.
Table 1.
Baseline characteristics
| Total (N=399) Median [IQR] or N (%) |
Immediate ART group
(N=195) Median or % |
Deferred ART group
(N=204) Median or % |
|
|---|---|---|---|
| Demographics | |||
| Age (years) | 32 [26, 41] | 32 | 33 |
| Sex | |||
| Male | 295 (73.9) | 73.3 | 74.5 |
| Premenopausal female | 91 (22.8) | 24.1 | 21.6 |
| Postmenopausal female | 13 (3.3) | 2.6 | 3.9 |
| Race | |||
| Asian | 126 (31.6) | 30.8 | 32.4 |
| Black | 74 (18.5) | 16.9 | 20.1 |
| Latino/Hispanic | 97 (24.3) | 25.6 | 23.0 |
| White | 80 (20.1) | 19.5 | 20.6 |
| Other | 22 (5.5) | 7.2 | 3.9 |
| Clinical factors | |||
| Previous fracture (any)a | 31 (7.8) | 8.7 | 6.9 |
| Previous fragility fracture a | 17 (4.3) | 4.1 | 4.4 |
| BMI (kg/m2) | 23.9 [21.4, 27.3] | 24.1 | 23.8 |
| Current smoker | 77 (19.3) | 16.9 | 21.6 |
| Alcohol useb | 16 (4.0) | 4.6 | 3.4 |
| Current medication use | |||
| Corticosteroids | 1 (0.3) | 0.0 | 0.5 |
| Vitamin D | 22 (5.5) | 4.1 | 6.9 |
| Calcium supplements | 19 (4.8) | 4.1 | 5.4 |
| Hormone replacement therapy | 1 (0.3) | 0.0 | 0.5 |
| HIV History | |||
| Known HIV duration (years) | 0.7 [0.3, 2.8] | 0.6 | 0.9 |
| ART, pre-specified prior to randomization | |||
| Tenofovir DF | 334 (83.7) | 83.1 | 84.3 |
| Efavirenz | 333 (83.5) | 82.6 | 84.3 |
| Protease inhibitor | 42 (10.5) | 11.8 | 9.3 |
| Laboratory Results | |||
| CD4 count (cells/μL) | 642 [579, 738] | 644 | 640 |
| HIV viral load (copies/mL) | 14940 [3399, 53293] | 20257 | 12332 |
| eGFR (mL/min/1.73 m2)d | 114.0 [98.9, 122.4] | 114.2 | 113.4 |
| Bone Mineral Density | |||
| Spine | |||
| BMD (g/cm2) | 1.01 [0.94, 1.11] | 1.02 | 1.01 |
| T-score e | −0.31 [−1.00, 0.59] | −0.24 | −0.33 |
| Z-score f | −0.66 [−1.33, 0.25] | −0.63 | −0.69 |
| Total Hip | |||
| BMD (g/cm2) | 0.96 [0.87, 1.04] | 0.94 | 0.97 |
| T-score e | 0.14 [−0.61, 0.84] | 0.02 | 0.24 |
| Z-score f | −0.37 [−0.94, 0.18] | −0.47 | −0.31 |
| Femoral Neck | |||
| BMD (g/cm2) | 0.84 [0.76, 0.93] | 0.83 | 0.85 |
| T-score e | −0.06 [−0.84, 0.77] | −0.16 | 0.04 |
| Z-score f | −0.44 [−1.05, 0.19] | −0.53 | −0.39 |
| Low BMD relative to age groupg | 45 (11.3) | 13.3 | 9.3 |
| T-score ≤−2.5 at the spine, hip or femoral neck | 8 (2.0) | 3.1 | 1.0 |
Fractures after age of 18. Fragility fracture defined as fracture occurring following fall from standing height or equivalent.
Consumed alcohol four to seven days a week with at least 2 drinks per day.
Current recreational drug use, at least once in the past month.
eGFR was calculated using the CKD-EPI formula.
BMD T-scores were standardized relative to young adult Caucasian women.
BMD Z-scores were standardized relative to age, gender and race/ethnicity (black/white/hispanic) matched reference populations. White reference populations are used for all other races/ethnicities.
BMD Z-score ≤ −2 at the lumbar spine, hip or femoral neck.
Tenofovir DF, Tenofovir disoproxil fumarate; NSAID, nonsteroidal anti-inflammatory drug; eGFR, estimated glomerular filtration rate.
Participants were followed for a mean of 2.2 years. In the immediate group, 95% of participants started ART within 8 weeks of randomization. In the deferred group, 14.7%, 27.9%, and 44.6% had started ART by Months 12, 24, and 36, respectively. ART was used for 95% and 18% of cumulative follow-up in the immediate and deferred ART groups, respectively. In the immediate ART group, initial ART contained TDF for 82.8% of participants, efavirenz (EFV) for 78.1%, and a PI for 13.0%. No tenofovir alafenamide was used. Almost all participants on ART had plasma HIV viral load ≤200 copies/mL.(14) Two deferred arm participants commenced treatment for osteoporosis after Month 12.
Changes in BMD
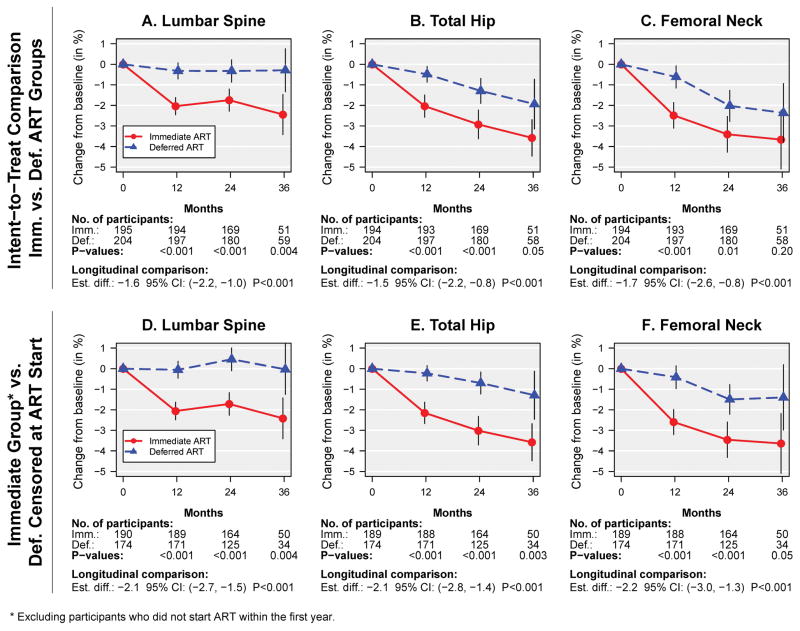
The mean percent changes in BMD in the immediate and deferred ART groups and the estimated overall mean treatment differences were: −1.9% vs −0.4% (difference −1.6% [95% CI −2.2 to −1.0]) at the lumbar spine; −2.5% vs −1.0% (difference −1.5% [95% CI −2.2 to −0.8]) at the total hip; and −3.0% vs −1.4% (difference −1.7% [95% CI −2.6 to −0.8]) at the femoral neck (Figure 2A–C, Table 2; all p<0.001). Treatment groups differed significantly in BMD decline from baseline to Months 12, 24 and 36 for spine and total hip BMD. In the deferred ART group, when censoring follow-up at ART initiation, BMD changed by 0.1%, −0.3% and −0.6% per year at the spine, hip and femoral neck, respectively (Figure 2D–F, Supplemental Table 1). The differences between the immediate versus deferred groups were evident at Year 1, and remained about constant afterwards. Rates of BMD decline were steeper in the immediate ART group at all 3 skeletal sites. In the immediate ART group, spine BMD declined sharply during the first year (by 2.0%), and remained stable afterward. At the total hip and femoral neck, BMD declined during Year 1 by 2.0% and 2.5%, respectively, and from Year 1 to Year 2 by 1.0% and 1.0%, respectively (Table 2, Figure 2B–C, Supplemental Table 1). The apparent continued decline in hip BMD after Year 2 in Figure 2 is due to a cohort effect; hip BMD remains about constant after Year 2 when restricting the analysis to participants with 3 years of follow-up (Table 2, Supplemental Figure 1B–C). After Year 1, rates of BMD decline were similar in the immediate and deferred ART groups (Table 2).
Figure 2.
Mean percent change (95% CIs) in BMD by treatment group. Panels A–C show intent-to-treat comparisons. In panels D–F follow-up in the deferred group is censored at ART start, and participants in the immediate group who did not start ART within the first year are excluded. (A and D) Lumbar spine (L1–L4) BMD, (B and E) Total hip BMD, (C and F) Femoral neck BMD.
Table 2.
Percent change in BMD at the spine (L1–L4), hip and femoral neck, compared between the immediate and deferred ART groups by intent-to-treat.
| Spine (L1–L4) | Immediate ART Group | Deferred ART | Immediate - Deferred ART Groups | |||
|---|---|---|---|---|---|---|
| N | Mean (95% CI) | N | Mean (95% CI) | Estimated Difference (95% CI) | P | |
| Baseline to Year 1 | 194 | −2.04 (−2.46, −1.62) | 197 | −0.32 (−0.71, 0.07) | −1.72 (−2.29, −1.14) | <0.001 |
| Baseline to Year 2 | 169 | −1.74 (−2.28, −1.21) | 180 | −0.33 (−0.87, 0.22) | −1.41 (−2.18, −0.65) | <0.001 |
| Baseline to Year 3 | 51 | −2.45 (−3.41, −1.48) | 59 | −0.29 (−1.33, 0.75) | −2.16 (−3.60, −0.71) | <0.01 |
|
| ||||||
| Overall | 195 | −1.92 (−2.34, −1.50) | 204 | −0.35 (−0.76, 0.06) | −1.57 (−2.16, −0.98) | <0.001 |
|
| ||||||
| Change from Year 1 to Year 2 (%) | 168 | 0.29 (−0.16, 0.74) | 173 | −0.10 (−0.54, 0.35) | 0.39 (−0.25, 1.03) | 0.23 |
| Change from Year 2 to Year 3 (%) | 50 | 0.25 (−0.54, 1.03) | 59 | −0.49 (−1.21, 0.23) | 0.78 (−0.31, 1.86) | 0.16 |
|
| ||||||
| Rate of change after Year 1 (% per year) | 0.24 (−0.11, 0.59) | −0.17 (−0.51, 0.16) | 0.42 (−0.07, 0.90) | 0.10 | ||
|
| ||||||
| Total Hip | ||||||
|
| ||||||
| Baseline to Year 1 | 193 | −2.03 (−2.57, −1.50) | 197 | −0.49 (−0.87, −0.11) | −1.54 (−2.21, −0.88) | <0.001 |
| Baseline to Year 2 | 169 | −2.93 (−3.62, −2.23) | 180 | −1.30 (−1.90, −0.69) | −1.58 (−2.50, −0.65) | <0.001 |
| Baseline to Year 3 | 51 | −3.58 (−4.46, −2.70) | 58 | −1.94 (−3.14, −0.73) | −1.57 (−3.09, −0.04) | 0.05 |
|
| ||||||
| Overall | 194 | −2.53 (−3.03, −2.02) | 204 | −0.99 (−1.48, −0.49) | −1.53 (−2.24, −0.82) | <0.001 |
|
| ||||||
| Change from Year 1 to Year 2 (%) | 168 | −0.97 (−1.45, −0.49) | 173 | −0.81 (−1.28, −0.34) | −0.11 (−0.79, 0.57) | 0.75 |
| Change from Year 2 to Year 3 (%) | 50 | 0.03 (−0.64, 0.69) | 58 | −0.96 (−1.58, −0.34) | 1.03 (0.11, 1.95) | 0.03 |
|
| ||||||
| Rate of change after Year 1 (% per year) | −0.74 (−1.12, −0.36) | −0.84 (−1.20, −0.47) | 0.15 (−0.38, 0.68) | 0.58 | ||
|
| ||||||
| Femoral Neck | ||||||
|
| ||||||
| Baseline to Year 1 | 193 | −2.49 (−3.11, −1.86) | 197 | −0.62 (−1.15, −0.08) | −1.96 (−2.78, −1.14) | <0.001 |
| Baseline to Year 2 | 169 | −3.41 (−4.27, −2.54) | 180 | −2.02 (−2.78, −1.27) | −1.46 (−2.61, −0.32) | 0.01 |
| Baseline to Year 3 | 51 | −3.66 (−5.08, −2.25) | 58 | −2.36 (−3.78, −0.95) | −1.32 (−3.34, 0.69) | 0.20 |
|
| ||||||
| Overall | 194 | −3.02 (−3.64, −2.40) | 204 | −1.44 (−2.05, −0.83) | −1.70 (−2.57, −0.83) | <0.001 |
|
| ||||||
| Change from Year 1 to Year 2 (%) | 168 | −1.00 (−1.63, −0.37) | 173 | −1.46 (−2.08, −0.84) | 0.46 (−0.42, 1.35) | 0.30 |
| Change from Year 2 to Year 3 (%) | 50 | −0.32 (−1.37, 0.74) | 58 | −0.58 (−1.56, 0.40) | 0.25 (−1.21, 1.71) | 0.74 |
|
| ||||||
| Rate of change after Year 1 (% per year) | −0.87 (−1.37, −0.38) | −1.23 (−1.70, −0.75) | 0.36 (−0.33, 1.06) | 0.30 | ||
Follow-up was censored at the start of osteoporosis therapy for 2 participants (both in the deferred group, month 24). Mean changes in BMD from baseline to each year were calculated without adjustments, differences at each year were estimated in regression models adjusted for baseline BMD, and overall changes in BMD were estimated and compared using longitudinal mixed models adjusted for both baseline BMD and visit. Rates of changes in spine BMD after year 1 were estimated in longitudinal mixed models using annual BMD changes as response.
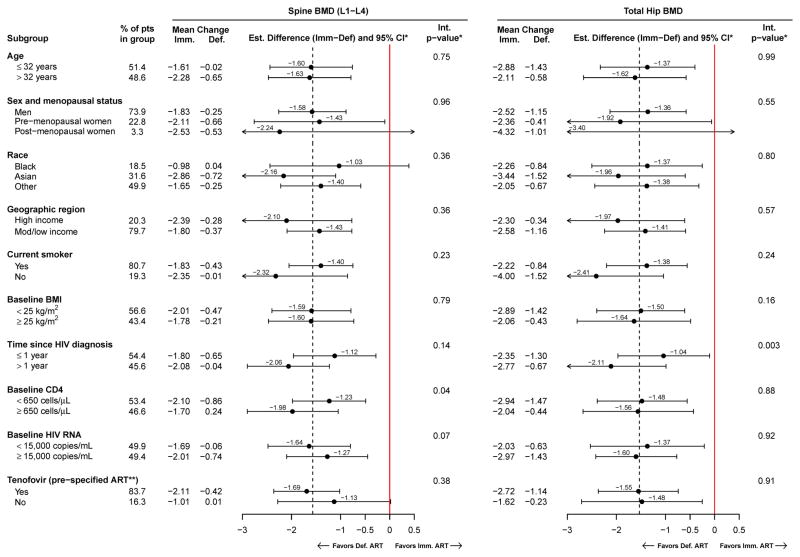
In all subgroups, spine and hip BMD declined more in the immediate ART group (Figure 3). In addition to the subgroups shown in Figure 3, subgroups were analysed by baseline BMD, season of enrolment, mode of HIV infection, CD8 count, CD4:CD8 ratio, calcium or vitamin D supplements, recreational drug use, eGFR, and pre-specified PI use. Treatment differences were homogenous across all subgroups, except for two cases. For spine BMD, the treatment effect was stronger among those with higher baseline CD4 count (p=0.04). For hip BMD, the treatment effect was stronger in those diagnosed with HIV more than one year prior (p=0.003). Notably, there was no evidence for larger differences between the immediate and deferred ART groups in the TDF or PI subgroups; however, power to detect heterogeneity across TDF and PI subgroups was low because of the low sample size in the non-TDF and the PI subgroup categories. Results were similar when restricting follow-up to the first 2 years, except that there was no evidence for a differential treatment effect by baseline CD4 counts (Supplemental Figure 2).
Figure 3.
Subgroup analyses: Mean percent change from baseline, and treatment differences (immediate minus deferred ART groups) in spine (L1–L4) and total hip BMD are estimated within subgroups, with 95% confidence intervals. P-values are for tests of heterogeneity of the treatment difference across subgroups.
* Estimated in a longitudinal mixed model, adjusted for visit and baseline BMD. The interaction p-value for heterogeneity across subgroups was calculated using continuous variables for age, BMI, time since HIV diagnosis, CD4 count, and log10 HIV RNA levels.
** In the immediate ART group, a tenofovir-containing ART regimen was selected prior to randomization (“pre-specified”) for 162 participants. Of those who were pre-specified tenofovir and had the corresponding follow-up scans, 155 (96.3%).138 (97.2%), and 45 (97.8%) used tenofovir at years 1, 2, and 3, respectively.
Predictors of BMD decline
No clinical, demographic, or HIV-related factor was consistently associated with BMD change across both spine and hip in either treatment group (Table 3). In the immediate ART group, being Asian (Table 3), and using PIs in the initial regimen (Table 4) was associated with steeper BMD decline at the spine. At the hip, TDF use was associated with steeper BMD decline (Table 4), and recreational drug use with less decline (Table 3). In a time-updated analysis, no single drug nor drug class was associated with steeper BMD declines (Supplemental Table 2).
Table 3.
Factors associated with change in BMD in the immediate ART group (N=190). Participants who did not start ART within the first year were excluded.
| Factor | N (%) in subgroup | Spine (L1–L4) | Total Hip | Femoral Neck | |||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| Est. Coefficient (95% CI) | P+ | Est. Coefficient (95% CI) | P+ | Est. Coefficient (95% CI) | P+ | ||
| Age (per 10 years) | −0.10 (−0.69, 0.50) | 0.75 | 0.56 (−0.19, 1.31) | 0.14 | 0.88 (−0.08, 1.85) | 0.07 | |
|
| |||||||
| Sex and menopausal status | 0.43 | 0.49 | 0.41 | ||||
| Male | 138 (72.6%) | Ref. | -- | Ref. | -- | Ref. | -- |
| Pre-menopausal female | 47 (24.7%) | −0.85 (−2.55, 0.84) | 0.32 | 1.12 (−1.05, 3.29) | 0.31 | −0.51 (−3.10, 2.07) | 0.69 |
| Post-menopausal female | 5 (2.6%) | −2.01 (−5.35, 1.34) | 0.24 | −0.36 (−4.65, 3.94) | 0.87 | −3.46 (−8.58, 1.66) | 0.18 |
|
| |||||||
| Race | 0.03 | 0.81 | 0.27 | ||||
| Black | 33 (17.4%) | 0.60 (−0.97, 2.18) | 0.45 | 0.35 (−1.65, 2.34) | 0.73 | 1.80 (−0.65, 4.25) | 0.15 |
| Asian | 58 (30.5%) | −1.31 (−2.47, −0.15) | 0.03 | −0.33 (−1.79, 1.14) | 0.66 | −0.32 (−2.10, 1.45) | 0.72 |
| White/other | 99 (52.1%) | Ref. | -- | Ref. | -- | Ref. | -- |
|
| |||||||
| Location of enrollment | |||||||
| Low or medium income (vs high income) | 153 (80.5%) | 0.84 (−0.43, 2.11) | 0.19 | 0.22 (−1.38, 1.82) | 0.78 | 0.32 (−1.62, 2.27) | 0.74 |
|
| |||||||
| Season of enrollment | |||||||
| Summer/Autumn (vs Spring/Winter) | 92 (48.4%) | 0.21 (−0.66, 1.07) | 0.59 | −0.04 (−1.12, 1.04) | 0.94 | 0.06 (−1.25, 1.37) | 0.93 |
|
| |||||||
| Current smoker | 33 (17.4%) | −0.22 (−1.36, 0.92) | 0.70 | −1.38 (−2.82, 0.07) | 0.06 | −0.82 (−2.58, 0.94) | 0.36 |
|
| |||||||
| Recreational drug use | 21 (11.1%) | −0.68 (−2.09, 0.72) | 0.34 | 2.18 (0.39, 3.97) | 0.02 | 0.62 (−1.54, 2.79) | 0.57 |
|
| |||||||
| BMI (per kg/m2) | −0.02 (−0.13, 0.09) | 0.70 | 0.01 (−0.13, 0.15) | 0.89 | 0.04 (−0.13, 0.21) | 0.62 | |
|
| |||||||
| eGFR (per 1 mL/min/1.73m2) | 0.02 (−0.01, 0.06) | 0.21 | 0.02 (−0.03, 0.06) | 0.47 | 0.02 (−0.03, 0.07) | 0.46 | |
|
| |||||||
| Mode of HIV infection | 0.83 | 0.25 | 0.22 | ||||
| Opposite sex contact | 57 (30.0%) | Ref. | -- | Ref. | -- | Ref. | -- |
| Same sex contact | 118 (62.1%) | −0.04 (−1.66, 1.59) | 0.96 | 1.48 (−0.56, 3.53) | 0.15 | 0.98 (−1.48, 3.45) | 0.43 |
| Other | 15 (7.9%) | 0.54 (−1.36, 2.44) | 0.57 | −0.40 (−2.79, 1.99) | 0.74 | −1.79 (−4.70, 1.12) | 0.23 |
|
| |||||||
| Time since HIV diagnosis (per year) | 0.07 (−0.10, 0.25) | 0.39 | −0.19 (−0.41, 0.02) | 0.08 | −0.15 (−0.41, 0.11) | 0.27 | |
|
| |||||||
| CD4 count (per 100 cells/μL) | 0.07 (−0.22, 0.36) | 0.62 | 0.22 (−0.15, 0.58) | 0.25 | 0.12 (−0.32, 0.57) | 0.58 | |
|
| |||||||
| CD8 count (per 100 cells/μL) | −0.06 (−0.15, 0.03) | 0.18 | 0.03 (−0.08, 0.14) | 0.61 | −0.03 (−0.16, 0.10) | 0.64 | |
|
| |||||||
| HIV viral load (per log10) | 0.14 (−0.39, 0.68) | 0.60 | −0.57 (−1.25, 0.11) | 0.10 | −0.25 (−1.07, 0.57) | 0.55 | |
|
| |||||||
| Calcium or vitamin D use | 9 (4.7%) | −0.03 (−2.07, 2.01) | 0.98 | −0.44 (−3.03, 2.15) | 0.74 | −1.83 (−4.97, 1.30) | 0.25 |
|
| |||||||
| Baseline BMD | 1.52 (−1.98, 5.01) | 0.39 | 0.83 (−3.94, 5.60) | 0.73 | −4.92 (−10.8, 0.91) | 0.10 | |
P-value for association of the factor with percent change in BMD, estimated in longitudinal mixed models, adjusted for all factors listed and visit.
eGFR, estimated glomerular filtration rate.
Table 4.
Association of specific antiretroviral drugs in the first ART regimen with percent change in BMD, estimated within the immediate ART group (N=190). Participants who did not start ART within the first year were excluded.
| Specific Drug in First ART Regimen | N (%) | Spine (L1–L4) | Total Hip | Femoral Neck | |||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| Est. (95% CI) | P+ | Est. (95% CI) | Pl+ | Est. (95% CI) | P+ | ||
| EFV | 148 (77.9%) | 0.26 (−0.89, 1.42) | 0.65 | −0.08 (−1.57, 1.41) | 0.92 | 0.13 (−1.67, 1.94) | 0.88 |
| TDF | 157 (82.6%) | −0.75 (−2.09, 0.58) | 0.27 | −1.71 (−3.38, −0.03) | 0.05 | −1.88 (−3.90, 0.15) | 0.07 |
| PI | 25 (13.2%) | −1.79 (−3.05, −0.53) | <0.01 | −0.70 (−2.37, 0.97) | 0.41 | −1.13 (−3.14, 0.89) | 0.27 |
| NNRTI | 156 (82.1%) | 0.65 (−0.53, 1.83) | 0.28 | 0.31 (−1.22, 1.83) | 0.69 | 0.35 (−1.50, 2.20) | 0.71 |
P-value for association between use of the drug in the first ART regimen and percent change in BMD, estimated separately for each drug in longitudinal mixed models which also included age, gender, race, location and season of enrollment, smoking status, alcohol use, drug use, diabetes, BMI, hepatitis B, hepatitis C, previous fracture, eGFR, mode of HIV infection, time since HIV diagnosis, baseline CD4 and CD8, CD4:CD8 ratio, HIV RNA, calcium or vitamin D supplements, baseline BMD and visit. Associations were estimated in separate longitudinal models for each drug. There was no adjustment for multiple comparisons.
EFV=efavirenz; eGFR=estimated glomerular filtration rate; NNRTI=non-nucleoside reverse transcriptase inhibitor; PI=protease inhibitor; TDF=tenofovir disoproxil fumarate.
In the deferred ART group, only low baseline CD4 cell counts were independently associated with steeper spine BMD decline, by −0.34% per 100 cells/μL lower (Table 5). At the femoral neck, lower baseline BMI, calcium and vitamin D use, and higher baseline BMD were independently associated with steeper BMD loss (Table 5).
Table 5.
Factors associated with change in BMD in the deferred ART group, with follow-up censored at ART initiation (N=175).
| Factor | N (%) | Spine (L1–L4) | Total Hip | Femoral Neck | |||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| Est. (95% CI) | P+ | Est. (95% CI) | P+ | Est. (95% CI) | P+ | ||
| Age (per 10 years) | −0.53 (−1.11, 0.04) | 0.07 | 0.24 (−0.32, 0.80) | 0.39 | 0.39 (−0.40, 1.19) | 0.33 | |
|
| |||||||
| Sex and menopausal status | 0.41 | 0.29 | 0.39 | ||||
| Male | 127 (72.6%) | Ref. | -- | Ref. | -- | Ref. | -- |
| Pre-menopausal female | 40 (22.9%) | −0.94 (−2.36, 0.48) | 0.19 | 0.64 (−0.75, 2.04) | 0.36 | −0.12 (−2.07, 1.84) | 0.91 |
| Post-menopausal female | 8 (4.6%) | −0.88 (−3.19, 1.43) | 0.45 | −0.89 (−3.05, 1.27) | 0.42 | −2.05 (−5.16, 1.06) | 0.19 |
|
| |||||||
| Race | 0.56 | 0.44 | 0.76 | ||||
| Black | 34 (19.4%) | 0.58 (−0.65, 1.80) | 0.35 | −0.53 (−1.71, 0.65) | 0.38 | 0.66 (−1.09, 2.41) | 0.46 |
| Asian | 59 (33.7%) | −0.07 (−1.17, 1.03) | 0.90 | 0.27 (−0.77, 1.31) | 0.61 | 0.26 (−1.22, 1.75) | 0.73 |
| White/other | 82 (46.9%) | Ref. | -- | Ref. | -- | Ref. | -- |
|
| |||||||
| Location of enrollment | |||||||
| Low or medium income (vs high income) | 139 (79.4%) | 0.13 (−1.07, 1.32) | 0.83 | 0.02 (−1.12, 1.16) | 0.98 | −0.07 (−1.67, 1.53) | 0.93 |
|
| |||||||
| Season of enrollment | |||||||
| Summer/autumn (vs Spring/Winter) | 80 (45.7%) | −0.04 (−0.86, 0.77) | 0.91 | −0.01 (−0.78, 0.76) | 0.98 | −0.20 (−1.30, 0.90) | 0.72 |
|
| |||||||
| Current smoker | 39 (22.3%) | 0.19 (−0.85, 1.23) | 0.72 | −0.63 (−1.62, 0.36) | 0.21 | −0.63 (−2.03, 0.78) | 0.38 |
|
| |||||||
| Recreational drug use | 20 (11.4%) | 0.04 (−1.24, 1.32) | 0.95 | 0.63 (−0.58, 1.83) | 0.31 | −0.44 (−2.16, 1.28) | 0.61 |
|
| |||||||
| BMI (per kg/m2) | −0.01 (−0.12, 0.10) | 0.87 | 0.05 (−0.06, 0.16) | 0.36 | 0.15 (0.00, 0.30) | 0.04 | |
|
| |||||||
| eGFR (per 1 mL/min/1.73m2) | 0.00 (−0.02, 0.03) | 0.84 | 0.01 (−0.02, 0.03) | 0.45 | −0.00 (−0.04, 0.03) | 0.80 | |
|
| |||||||
| Mode of HIV infection | 0.39 | 0.21 | 0.70 | ||||
| Opposite sex contact | 66 (37.7%) | Ref. | -- | Ref. | -- | Ref. | -- |
| Same sex contact | 98 (56.0%) | −0.47 (−1.83, 0.89) | 0.50 | 0.76 (−0.57, 2.08) | 0.26 | 0.58 (−1.28, 2.44) | 0.54 |
| Other | 11 (6.3%) | −1.26 (−3.11, 0.58) | 0.18 | 1.50 (−0.26, 3.25) | 0.09 | −0.42 (−2.91, 2.07) | 0.74 |
|
| |||||||
| Time since HIV diagnosis (per year) | 0.07 (−0.05, 0.19) | 0.25 | 0.06 (−0.05, 0.18) | 0.29 | −0.09 (−0.26, 0.07) | 0.25 | |
|
| |||||||
| CD4 count (per 100 cells/μL) | 0.34 (0.04, 0.64) | 0.03 | 0.19 (−0.09, 0.48) | 0.18 | 0.39 (−0.01, 0.80) | 0.06 | |
|
| |||||||
| CD8 count (per 100 cells/μL) | −0.08 (−0.16, 0.01) | 0.09 | −0.03 (−0.12, 0.05) | 0.42 | −0.05 (−0.17, 0.07) | 0.41 | |
|
| |||||||
| HIV viral load (per log10) | −0.22 (−0.74, 0.30) | 0.40 | −0.09 (−0.59, 0.40) | 0.71 | 0.25 (−0.44, 0.95) | 0.47 | |
|
| |||||||
| Calcium or vitamin D use | 13 (7.4%) | −0.20 (−1.84, 1.44) | 0.81 | −1.00 (−2.53, 0.53) | 0.20 | −2.62 (−4.80, −0.44) | 0.02 |
|
| |||||||
| Baseline BMD | 1.33 (−2.18, 4.84) | 0.45 | 2.03 (−1.66, 5.72) | 0.28 | −6.57 (−11.6, −1.53) | 0.01 | |
P-value for association of the factor with percent change in BMD, estimated in longitudinal mixed models, adjusted for all factors listed and visit.
eGFR = estimated glomerular filtration rate,
Incidence of Low BMD and fractures
Among participants without low BMD at baseline (Z-score > −2), 22 participants in the immediate ART group developed low BMD at the spine, hip or femoral neck (Z-score ≤ −2) (rate 6.2 per 100 person-years), compared with 6 (rate 1.4 per 100 person-years) in the deferred ART group, HR=4.7 (95% CI 1.9 to 11.7, p<0.001). For 4 participants (rate 1.0 per 100 person-years) in the immediate ART group, the T-score at the spine, hip or femoral neck newly declined to ≤ −2.5, compared with 6 (rate 1.3 per 100 person-years) in the deferred group, HR=0.78 (95% CI 0.22 to 2.76, p=0.70).
Rates of fracture and of fragility fracture were similar between groups in the parent START population. In the immediate ART group, 66 participants (rate of 0.92 per 100 person-years) experienced a new fracture, compared with 61 (rate 0.83) in the deferred group, HR=1.09 (95% CI 0.77 to 1.55, p=0.62). Of these, 17 and 27 fractures occurred with minimal trauma in the immediate and deferred groups, respectively, at rates of 0.23 and 0.37 per 100 person-years, HR=0.63 (95% CI 0.35 to 1.16, p=0.14).
DISCUSSION
In this diverse population of adults with HIV infection and near-normal CD4 counts, immediate initiation of ART resulted in significantly greater reductions in BMD at the spine and total hip, compared with deferred ART. During the first year, participants in the immediate ART group lost 2.0% of BMD at the lumbar spine and total hip. This 2.0% BMD decline is less than that reported in earlier studies of ART initiation (2–6% over 2 years) (3, 25), but similar to recent studies (0.9–3.7% declines in BMD over 1–2 years) (26–29). The 2% loss of BMD is similar to the BMD loss seen with administration of oral glucocorticoids (0.8–3.0%) (30); a BMD loss of 0.5% to 0.7% is associated with a fracture risk 1.5 to 2.4-fold greater than in non-glucocorticoid users (31). Whether similar BMD declines in ART-treated HIV-infected patients will translate into increased risk of fractures is unknown.
After the first 12 months of ART, BMD remained stable at the spine, but continued to decline through the second year at the hip, albeit at a lower rate (by 0.9%). In the deferred ART group, spine BMD remained stable, while hip BMD declined at a rate of 0.3%, and 0.6% at the femoral neck prior to initiation of ART. In the general population, the annual decline in BMD at the hip and the lumbar spine for premenopausal women and men less than 50 years is 0.15% to 0.4% (19, 32). Our study population had a median age of 32 years, so stability of spine BMD is to be expected. With no ART, the annual 0.3–0.6% BMD decline at the hip suggests a role for untreated HIV. However, the magnitude of the overall steeper BMD decline in the immediate versus deferred ART group suggests that ART is a greater contributor to BMD loss than HIV itself.
The difference in percent change in BMD from baseline between those who started ART in the immediate group, and the deferred group prior to ART start developed in year 1, and remained about constant thereafter, as participants in the deferred ART group gradually initiated ART. At the spine, the BMD loss was largely restricted to the first year of ART. At the hip, BMD continued to decline through the second year of ART use (see Figure 2 and supplemental Figure 1, immediate ART group). Whether ART use causes ongoing BMD loss beyond the first year or two is uncertain, however. Stabilization of BMD after the first 2 years of ART has been demonstrated in clinical trials (usually less than 3 years duration) and several cohort studies (33, 34). In other studies, BMD continues to decline beyond the first year of ART, by approximately 1% per year (23, 25, 35). We have previously observed ongoing BMD decline with ART in the SMART study, where hip BMD declined by 0.8% per year for up to 4 years of follow-up in participants who were ART-experienced at study entry, and continued using ART (23, 36). Continued decline in BMD has been confirmed by others, where the rate of BMD decline was steeper during the 2 years after ART initiation compared with HIV negative people of similar age; while the rate of BMD loss slowed after 2 years, spine BMD loss remained significantly greater compared with the HIV negative group through 7.5 years of follow-up (37). Switching ART for virological failure has also been shown to be associated with another significant fall in BMD of 2% before stabilization again (38). Given that ART use is lifelong in those with HIV, continued decline of BMD at rates greater than those observed in the general population, if that should be the case, may result in adverse outcomes in the aging HIV population.
There was no difference in fracture rates between immediate and deferred ART groups in the 4684 participants in the parent START study. Whether ART increases fracture risk is unclear, with contradictory results reported from multiple studies. The effect of ART is likely small in women under the age of 50 years and men under 60 years (39). The young age of our study population may have contributed to the low rate of fractures and lack of treatment group difference (14).
There was no independent association between baseline CD4 count and change in BMD in the immediate ART group. In contrast, lower CD4 count was a significant predictor of greater BMD loss at both the spine and the hip in the deferred ART group (prior to any ART). A lower CD4 count and higher HIV viral load have been associated with lower bone mass in cross-sectional studies, suggesting a role for HIV infection or the immunological response to HIV in bone loss (7, 40). Lower pre-ART CD4 counts were associated with greater bone loss following ART initiation in combined ACTG studies, but only in those with CD4 counts <50 cells/μL (41). The observed effect of ART was consistent across demographic, HIV-related and traditional risk factors for BMD loss. Greater bone loss at the spine after ART initiation in Asians compared with white race participants has not been described previously, and this observation may be spurious. Most studies evaluating change in BMD after ART report greater bone loss with TDF and/or ritonavir-boosted PI-containing ART (3, 27–29). In START, ART type was not mandated, and most participants received TDF and EFV. Although we found that TDF use was associated with greater loss of BMD at the hip and PI use was associated with greater BMD decline at the spine, our study was underpowered to separate the effects of TDF from those of other ART drugs.
The main strengths of our study are the randomized design, the racially diverse study population, and the standardized acquisition and reading of the DXA scans. There are several limitations. First, follow-up is only 2 years for most participants. Longer follow-up of our participants is needed to clarify whether the early BMD loss with ART is sustained over time. Second, only 26% of participants were women, resulting in low power for detecting gender differences. Third, the study was not designed to identify effects of specific drugs. Fourth, our study was not powered to detect a moderate increase of fracture risk, since expected fracture rates would be very low in this young population. Finally, some of the factors identified as associated with change in BMD may be false positives; due to the high number of predictors, our results need to be interpreted with caution. If the 16 predictors were independent, the chance of observing one or more p-values ≤0.05 would be at least 56% for each of the BMD outcomes.
In summary, immediate initiation of ART at high CD4 cell counts compared with deferring ART results in accelerated bone loss at the spine and hip, over two years. All key ART guidelines now recommend ART initiation at HIV diagnosis regardless of CD4 cell count, due to the reduced risk of serious clinical outcomes relative to deferring ART. Although the START Bone Mineral Density substudy revealed an adverse effect of immediate ART, the overall benefits of ART for prevention of HIV transmission and adverse health outcomes prevail. It will be important to understand the longer term consequences of the observed reductions in BMD and whether these reductions continue or stabilise with longer therapy.
Supplementary Material
Acknowledgments
We thank all study participants, site co-ordinators and investigators, and staff at the radiology sites, the coordinating centres, and the UCSF QA Centre. (Supplementary appendix for INSIGHT START Bone Mineral Density substudy Credit Roster).
Funding Support: This work was supported by the National Institute of Allergy and Infectious Diseases, National Institute of Arthritis and Musculoskeletal and Skin Diseases, National Institutes of Health (Grants U01-AI068641, UM1-AI120197). Antiretroviral drugs were donated by AbbVie, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline/ViiV Healthcare, Janssen Scientific Affairs, and Merck. The content is solely that of the authors and does not represent the views of the National Institutes of Health, nor those of AbbVie, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline/ViiV Healthcare, Janssen Scientific Affairs, and Merck.
Footnotes
Clinical Trials Registration: NCT00867048
Author’s Roles: Study design: JH, BG, AVS, JS, KE, PA, AC. Study conduct and data collection: JH, AVS, JS, AA, SBF, SdW, SJ, ALR, MS, DW, AC. Data analysis and interpretation: JH, BG, MR (deceased), NEW, AVS, KE, AC. Manuscript drafting: JH, BG, AC. Manuscript revision, and approval of final version: all authors. BG takes responsibility for integrity of data analysis.
Disclosures
Potential Conflicts of Interest: JH’s institution received funding for her participation in Advisory Boards for Gilead Sciences, AbbVie and ViiV Healthcare. AC received research funding from Gilead Sciences, MSD, Pfizer and ViiV Healthcare; consultancy fees from Mayne Pharma, Gilead Sciences, MSD, and ViiV Healthcare; lecture and travel sponsorships from Bristol-Myers Squibb, Gilead Sciences, Janssen, MSD, and ViiV Healthcare; and has served on advisory boards for Gilead Sciences, MSD, and ViiV Healthcare. KE received travel support from Merck Sharp & Dohme for attendance at DMC meetings. AL received honoraria and salary from MSD and Janssen. AS has received a grant from Hologic Inc. BG, MR, NWE, AA, SBF, SdW, JS, PA, SP, DW, MS and JS declare no conflict of interest.
References
- 1.Brown TT, Qaqish RB. Antiretroviral therapy and the prevalence of osteopenia and osteoporosis: a meta-analytic review. AIDS. 2006;20(17):2165–74. doi: 10.1097/QAD.0b013e32801022eb. [DOI] [PubMed] [Google Scholar]
- 2.Triant VA, Brown TT, Lee H, Grinspoon SK. Fracture prevalence among human immunodeficiency virus (HIV)-infected versus non-HIV-infected patients in a large U.S. healthcare system. J Clin Endocrinol Metabol. 2008;93(9):3499–504. doi: 10.1210/jc.2008-0828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gallant JE, Staszewski S, Pozniak AL, DeJesus E, Suleiman JM, Miller MD, et al. Efficacy and safety of tenofovir DF vs stavudine in combination therapy in antiretroviral-naive patients: a 3-year randomized trial. JAMA. 2004;292(2):191–201. doi: 10.1001/jama.292.2.191. [DOI] [PubMed] [Google Scholar]
- 4.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services; 2016. [cited 2016 24th March 2016]. Available from: http://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf. [Google Scholar]
- 5.Gunthard HF, Saag MS, Benson CA, del Rio C, Eron JJ, Gallant JE, et al. Antiretroviral Drugs for Treatment and Prevention of HIV Infection in Adults: 2016 Recommendations of the International Antiviral Society-USA Panel. JAMA. 2016;316(2):191–210. doi: 10.1001/jama.2016.8900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoy J. Bone, fracture and frailty. Curr Opin HIV AIDS. 2011;6(4):309–14. doi: 10.1097/COH.0b013e3283478741. [DOI] [PubMed] [Google Scholar]
- 7.Brown TT, McComsey GA, King MS, Qaqish RB, Bernstein BM, da Silva BA. Loss of bone mineral density after antiretroviral therapy initiation, independent of antiretroviral regimen. JAIDS. 2009;51(5):554–61. doi: 10.1097/QAI.0b013e3181adce44. [DOI] [PubMed] [Google Scholar]
- 8.Grant PM, Kitch D, McComsey GA, Dube MP, Haubrich R, Huang J, et al. Low baseline CD4+ count is associated with greater bone mineral density loss after antiretroviral therapy initiation. Clin Infect Dis. 2013;57(10):1483–8. doi: 10.1093/cid/cit538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ofotokun I, Titanji K, Vikulina T, Roser-Page S, Yamaguchi M, Zayzafoon M, et al. Role of T-cell reconstitution in HIV-1 antiretroviral therapy-induced bone loss. Nature Comm. 2015;6:8282. doi: 10.1038/ncomms9282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cotter EJ, Malizia AP, Chew N, Powderly WG, Doran PP. HIV proteins regulate bone marker secretion and transcription factor activity in cultured human osteoblasts with consequent potential implications for osteoblast function and development. AIDS Res Human Retrovir. 2007;23(12):1521–30. doi: 10.1089/aid.2007.0112. [DOI] [PubMed] [Google Scholar]
- 11.Gibellini D, Borderi M, De Crignis E, Cicola R, Vescini F, Caudarella R, et al. RANKL/OPG/TRAIL plasma levels and bone mass loss evaluation in antiretroviral naive HIV-1-positive men. J Med Virol. 2007;79(10):1446–54. doi: 10.1002/jmv.20938. [DOI] [PubMed] [Google Scholar]
- 12.Brown TT, Chen Y, Currier JS, Ribaudo HJ, Rothenberg J, Dube MP, et al. Body composition, soluble markers of inflammation, and bone mineral density in antiretroviral therapy-naive HIV-1-infected individuals. JAIDS. 2013;63(3):323–30. doi: 10.1097/QAI.0b013e318295eb1d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamill MM, Ward KA, Pettifor JM, Norris SA, Prentice A. Bone mass, body composition and vitamin D status of ARV-naive, urban, black South African women with HIV infection, stratified by CD(4) count. Osteoporos Int. 2013;24(11):2855–61. doi: 10.1007/s00198-013-2373-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.INSIGHT Start Study Group. Lundgren JD, Babiker AG, Gordin F, Emery S, Grund B, et al. Initiation of Antiretroviral Therapy in Early Asymptomatic HIV Infection. N Engl J Med. 2015;373(9):795–807. doi: 10.1056/NEJMoa1506816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carr A, Grund B, Neuhaus J, Schwartz A, Bernardino JI, White D, et al. Prevalence of and risk factors for low bone mineral density in untreated HIV infection: a substudy of the INSIGHT Strategic Timing of AntiRetroviral Treatment (START) trial. HIV Medicine. 2015;16(Suppl 1):137–46. doi: 10.1111/hiv.12242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu Y, Fuerst T, Hui S, Genant HK. Standardization of bone mineral density at femoral neck, trochanter and Ward’s triangle. Osteoporos Int. 2001;12(6):438–44. doi: 10.1007/s001980170087. [DOI] [PubMed] [Google Scholar]
- 17.Hui SL, Gao S, Zhou XH, Johnston CC, Jr, Lu Y, Gluer CC, et al. Universal standardization of bone density measurements: a method with optimal properties for calibration among several instruments. J Bone Miner Res. 1997;12(9):1463–70. doi: 10.1359/jbmr.1997.12.9.1463. [DOI] [PubMed] [Google Scholar]
- 18.Kanis JA, Melton LJ, 3rd, Christiansen C, Johnston CC, Khaltaev N. The diagnosis of osteoporosis. J Bone Min Res. 1994;9(8):1137–41. doi: 10.1002/jbmr.5650090802. [DOI] [PubMed] [Google Scholar]
- 19.Looker AC, Borrud LG, Hughes JP, Fan B, Shepherd JA, Melton LJ., 3rd Lumbar spine and proximal femur bone mineral density, bone mineral content, and bone area: United States, 2005–2008. Vital and health statistics Series 11, Data from the national health survey. 2012;(251):1–132. [PubMed] [Google Scholar]
- 20.Cosman F, de Beur SJ, LeBoff MS, Lewiecki EM, Tanner B, Randall S, et al. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Osteoporos Int. 2014;25(10):2359–81. doi: 10.1007/s00198-014-2794-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.International Society for Clinical Densitometry. Official Positions 2015 Adult & Pediatric 2015. 2017 Mar; Available from: https://iscd.app.box.com/v/op-iscd-2015-adult/
- 22.WHO Scientific Group on the Assessment of Osteoporosis at the Primary Health Care Level. WHO Scientific Group on the Assessment of Osteoporosis at the Primary Health Care Level Summary Meeting Report; Brussels, Belgium. 5–7 May 2004. [Google Scholar]
- 23.Grund B, Peng G, Gibert CL, Hoy JF, Isaksson RL, Shlay JC, et al. Continuous antiretroviral therapy decreases bone mineral density. AIDS. 2009;23(12):1519–29. doi: 10.1097/QAD.0b013e32832c1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2016. [cited 2016]. Available from: https://www.R-project.org/ [Google Scholar]
- 25.Brown TT, Hoy J, Borderi M, Guaraldi G, Renjifo B, Vescini F, et al. Recommendations for evaluation and management of bone disease in HIV. Clin Infect Dis. 2015;60(8):1242–51. doi: 10.1093/cid/civ010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sax PE, Wohl D, Yin MT, Post F, DeJesus E, Saag M, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate, coformulated with elvitegravir, cobicistat, and emtricitabine, for initial treatment of HIV-1 infection: two randomised, double-blind, phase 3, non-inferiority trials. Lancet. 2015;385(9987):2606–15. doi: 10.1016/S0140-6736(15)60616-X. [DOI] [PubMed] [Google Scholar]
- 27.Brown TT, Moser C, Currier JS, Ribaudo HJ, Rothenberg J, Kelesidis T, et al. Changes in Bone Mineral Density After Initiation of Antiretroviral Treatment With Tenofovir Disoproxil Fumarate/Emtricitabine Plus Atazanavir/Ritonavir, Darunavir/Ritonavir, or Raltegravir. J Infect Dis. 2015;212(8):1241–9. doi: 10.1093/infdis/jiv194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McComsey GA, Kitch D, Daar ES, Tierney C, Jahed NC, Tebas P, et al. Bone mineral density and fractures in antiretroviral-naive persons randomized to receive abacavir-lamivudine or tenofovir disoproxil fumarate-emtricitabine along with efavirenz or atazanavir-ritonavir: Aids Clinical Trials Group A5224s, a substudy of ACTG A5202. J Infect Dis. 2011;203(12):1791–801. doi: 10.1093/infdis/jir188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stellbrink HJ, Orkin C, Arribas JR, Compston J, Gerstoft J, Van Wijngaerden E, et al. Comparison of changes in bone density and turnover with abacavir-lamivudine versus tenofovir-emtricitabine in HIV-infected adults: 48-week results from the ASSERT study. Clin Infect Dis. 2010;51(8):963–72. doi: 10.1086/656417. [DOI] [PubMed] [Google Scholar]
- 30.Van Staa TP, Leufkens HG, Cooper C. The epidemiology of corticosteroid-induced osteoporosis: a meta-analysis. Osteoporos Int. 2002;13(10):777–87. doi: 10.1007/s001980200108. [DOI] [PubMed] [Google Scholar]
- 31.Van Staa TP, Leufkens HG, Abenhaim L, Zhang B, Cooper C. Use of oral corticosteroids and risk of fractures. J Bone Miner Res. 2000;15(6):993–1000. doi: 10.1359/jbmr.2000.15.6.993. [DOI] [PubMed] [Google Scholar]
- 32.Warming L, Hassager C, Christiansen C. Changes in bone mineral density with age in men and women: a longitudinal study. Osteoporos Int. 2002;13(2):105–12. doi: 10.1007/s001980200001. [DOI] [PubMed] [Google Scholar]
- 33.Bolland MJ, Wang TK, Grey A, Gamble GD, Reid IR. Stable bone density in HAART-treated individuals with HIV: a meta-analysis. J Clin Endocrinol Metabol. 2011;96(9):2721–31. doi: 10.1210/jc.2011-0591. [DOI] [PubMed] [Google Scholar]
- 34.Cassetti I, Madruga JV, Suleiman JM, Etzel A, Zhong L, Cheng AK, et al. The safety and efficacy of tenofovir DF in combination with lamivudine and efavirenz through 6 years in antiretroviral-naive HIV-1-infected patients. HIV Clin Trials. 2007;8(3):164–72. doi: 10.1310/hct0803-164. [DOI] [PubMed] [Google Scholar]
- 35.Bernardino JI, Mocroft A, Mallon PW, Wallet C, Gerstoft J, Russell C, et al. Bone mineral density and inflammatory and bone biomarkers after darunavir-ritonavir combined with either raltegravir or tenofovir-emtricitabine in antiretroviral-naive adults with HIV-1: a substudy of the NEAT001/ANRS143 randomised trial. Lancet HIV. 2015;2(11):e464–73. doi: 10.1016/S2352-3018(15)00181-2. [DOI] [PubMed] [Google Scholar]
- 36.Hoy J, Grund B, Roediger M, Ensrud KE, Brar I, Colebunders R, et al. Interruption or deferral of antiretroviral therapy reduces markers of bone turnover compared with continuous therapy: The SMART body composition substudy. J Bone Miner Res. 2013;28(6):1264–74. doi: 10.1002/jbmr.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grant PM, Kitch D, McComsey GA, Collier AC, Koletar SL, Erlandson KM, et al. Long-term Bone Mineral Density Changes in Antiretroviral-Treated HIV-Infected Individuals. J Infect Dis. 2016;214(4):607–11. doi: 10.1093/infdis/jiw204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haskelberg H, Mallon PW, Hoy J, Amin J, Moore C, Phanuphak P, et al. Bone mineral density over 96 weeks in adults failing first-line therapy randomized to raltegravir/lopinavir/ritonavir compared with standard second-line therapy. Journal of Acquired Immune Deficiency Syndromes: JAIDS. 2014;67(2):161–8. doi: 10.1097/QAI.0000000000000288. [DOI] [PubMed] [Google Scholar]
- 39.Hoy J, Young B. Do people with HIV infection have a higher risk of fracture compared with those without HIV infection? Curr Opin HIV AIDS. 2016;11(3):301–5. doi: 10.1097/COH.0000000000000249. [DOI] [PubMed] [Google Scholar]
- 40.Kooij KW, Wit FW, Bisschop PH, Schouten J, Stolte IG, Prins M, et al. Low bone mineral density in patients with well-suppressed HIV infection: association with body weight, smoking, and prior advanced HIV disease. J Infect Dis. 2015;211(4):539–48. doi: 10.1093/infdis/jiu499. [DOI] [PubMed] [Google Scholar]
- 41.Grant PM, Kitch D, McComsey GA, Dube MP, Haubrich R, Huang J, et al. Low baseline CD4+ count is associated with greater bone mineral density loss after antiretroviral therapy initiation. Clin Infect Dis. 2013;57(10):1483–8. doi: 10.1093/cid/cit538. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.