Abstract
Genotoxicity potential is a critical component of any comprehensive toxicological profile. Compounds that induce DNA or chromosomal damage often activate p53, a transcription factor essential to cell cycle regulation. Thus, within the U.S. Tox21 Program, we screened a library of ~10,000 (~8,300 unique) environmental compounds and drugs for activation of the p53-signaling pathway using a quantitative high-throughput screening assay employing HCT-116 cells (p53+/+) containing a stably integrated β-lactamase reporter gene under control of the p53 response element (p53RE). Cells were exposed (-S9) for 16 h at 15 concentrations (generally 1.2 nM to 92 μM) three times, independently. Excluding compounds that failed analytical chemistry analysis or were suspected of inducing assay interference, 365 (4.7%) of 7,849 unique compounds were concluded to activate p53. As part of an in-depth characterization of our results, we first compared them with results from traditional in vitro genotoxicity assays (bacterial mutation, chromosomal aberration); ~15% of known, direct-acting genotoxicants in our library activated the p53RE. Mining the Comparative Toxicogenomics Database revealed that these p53 actives were significantly associated with increased expression of p53 downstream genes involved in DNA damage responses. Furthermore, 53 chemical substructures associated with genotoxicity were enriched in certain classes of p53 actives, e.g., anthracyclines (antineoplastics) and vinca alkaloids (tubulin disruptors). Interestingly, the tubulin disruptors manifested unusual non-monotonic concentration response curves suggesting activity through a unique p53 regulatory mechanism. Through analysis of our results, we aim to define a role for this assay as one component of a comprehensive toxicological characterization of large compound libraries.
Keywords: HTS, genotoxicity, DNA damage response, mutagenicity, p53 response element
Introduction
The National Toxicology Program’s (NTP) 2004 Roadmap and Vision Statements are directed at establishing a new toxicology testing approach for the 21st century. This new approach is needed to keep pace with the increasing number of chemicals in commerce with insufficient toxicological data by transitioning toxicology from a predominantly observational science at the level of disease-specific models to a predominantly predictive science focused on a broad variety of target-specific, mechanism-based, biological observations (NTP, 2016). This led to the establishment in 2008 of the U.S. Tox21 Program, a collaborative effort initially among three government agencies that shared this vision: the NTP at the National Institute of Environmental Health Sciences (NIEHS), the National Center for Computational Toxicology (NCCT) within the Environmental Protection Agency (EPA), and the National Human Genome Research Institute’s NIH Chemical Genomics Center (NCGC), now part of the NIH Center for Advancing Translational Sciences (NCATS). In 2010, the Food and Drug Administration (FDA) formally joined the Tox21 consortium. To address the need for additional toxicity data on thousands of untested compounds, the Tox21 partners created a large and varied chemical library consisting of approximately 10,000 environmental, industrial, and pharmaceutical compounds (~8300 unique compounds) (see http://www2.epa.gov/chemical-research/toxicity-forecaster-toxcasttm-data for details on the compounds) for screening in quantitative high-throughput screening (qHTS) assays that would provide broad coverage of potential toxicological activities. The focus of the qHTS assays is on nuclear receptor and stress response signaling pathways (Tice et al. 2013).
Among the stress response pathways of interest to Tox21 are those induced in response to DNA damage. Thus, included in the Tox21 qHTS testing strategy are several assays capable of providing information on the ability of compounds to interact directly or indirectly with DNA. These include assays that monitor activation of p53 (Sohn et al. 2002; Levine and Oren 2009), post-translational modification of ATAD5 (ATPase family AAA domain-containing protein 5) (Fox et al. 2012), and induction of γH2AX (Nikolova et al. 2014), as well as a differential cytotoxicity assay that evaluates proliferation in two chicken DT40 isogenic cell lines knocked out for specific DNA repair genes compared to the wild-type DNA repair competent cell line (Ji et al. 2009; Yamamoto et al. 2011; Nishihara et al. 2015). An analysis of the aggregate responses among the five assays has been initiated. Here, we focus on characterizing the results obtained with the Tox21 10K compound library in the p53RE qHTS assay, which was the first of the five assays to be completed.
Because p53 activation is often up-regulated in cells subjected to physiological stress and particularly that which involves DNA damage, assays that measure increases in nuclear p53 levels generated through increased production or stabilization of the usually transient p53 protein were of interest as a means of providing simple and rapid identification of potential genotoxicants. The multiple, complex, and critical roles of the p53 protein in tumor suppression, initiation of apoptosis and senescence, and cell cycle check point regulation, as well as other stress-induced signaling pathways, have been well documented and reviewed (Sohn et al., 2002; Levine et al., 2006; Levine and Oren, 2009). Under basal conditions, p53 is expressed at low levels and contributes to maintenance of cellular homeostasis. Cellular stress signals prompt post-translational modifications of p53 that allow it to escape suppressive regulation by MDM2 (an E3 ubiquitin ligase that targets p53 for proteasomal destruction) and engage various responses through transcriptional activities. The diversity of post-translational modifications of p53, the amount of p53 expression, the variation in p53 response element sequences, and the effects of co-activators all contribute to the discrete regulation of p53 in response to specific events.
The p53 signaling pathway is one of the most complex signaling pathways in cells, influencing the transcription of approximately 275 genes (Menendez et al. 2013). Once activated in response to DNA damage, p53 can elicit transcriptional programs to engage cell cycle arrest, enhance DNA repair, initiate senescence, or induce apoptosis (Clewell et al., 2014). In addition to DNA damage, p53 is activated in response to other cellular stressors, including hypoxia, oncogene activation, oxidative damage, osmotic shock, spindle damage, small molecule inhibitors of MDM2/p53 interaction, and ribonucleotide depletion (Riley et al., 2008). Thus, p53 integrates information from numerous stress-induced signaling pathways in order to regulate transcriptional programs crucial for maintaining cellular integrity. Therefore, although activation of p53 is suggestive of DNA damage, the versatility of the p53 response confounds the interpretation of p53-activating chemicals as genotoxicants only. This limitation underscores the need for orthogonal assays to help characterize the potential genotoxicity of compounds that influence p53 signaling.
To determine the usefulness of screening for p53 activation as part of a comprehensive toxicity profile approach, we screened the Tox21 compound library in a commercially available reporter gene assay. The Invitrogen CellSensor® p53RE-bla HCT-116 assay (https://tools.thermofisher.com/content/sfs/manuals/cellsensor_p53HCT116_man.pdf) was optimized for quantitative assessment of compound activity over a broad concentration range in a 1536-well robotic platform. In this system, the gene for β-lactamase is under the transcriptional control of a p53 response element (RE) that is similar to the RE for p53 induction of p21 (CDKN1A), a key G1/S cell cycle checkpoint protein. Hence, chemical-dependent induction and activation of endogenous p53 is monitored by induction of β-lactamase. To inform data interpretation, cell viability was monitored in the same well by measuring ATP levels. To evaluate the performance of the p53RE assay and elucidate the role for the assay in prediction of genotoxicity for chemicals of unknown potential, results of the qHTS screening were assessed in relation to chemical specific data on the enhanced expression of various downstream genes in the p53 signaling cascade, the mechanisms of action (MOA), and results obtained from traditional genetic toxicity assays or in silico bacterial mutagenicity predictions.
Materials and Methods
Tox21 10K compound library
The Tox21 compound library currently consists of ~13,100 compounds (~9,000 unique, the remainder present in multiple copies) procured from commercial sources by the EPA, NIEHS/NTP, and NCATS. The library consists of a large variety of chemicals, including pesticides, industrial chemicals, natural food products, and drugs. The latter category includes failed drugs that did not make it to market, drugs that are no longer marketed, and drugs that are marketed currently. The list of unique compound substances, including chemical names and Chemical Abstracts Service Registry Numbers (CASRNs), as well as curated chemical structures and auto-generated structure identifiers (formula, systematic names, SMILES, desalted SMILES, InChI) can be downloaded from the EPA DSSTox website (https://www3.epa.gov/research/COMPTOX/toxcast_chemical_info.html). In the study reported here, 10,496 substances (8,306 unique) were available at the time for screening. Each substance was prepared as a stock solution (generally at 20 mM) in dimethyl sulfoxide (DMSO) and serially diluted in 1536-well microplates to yield 15 concentrations generally ranging from 1.2 nM to 92 μM (final concentrations in the wells). Eighty-eight duplicate compounds were intentionally included on each of the screening plates to evaluate technical variability across plates and runs. The Tox21 compound library stock solutions have been undergoing chemical analysis to assess compound sample identity, purity, concentration, and stability under conditions of use. The resulting data are available at https://tripod.nih.gov/tox21/samples. Chemical analysis is still ongoing for 21% (2232) of the 10,496 substances screened in the p53 assay. For the substances with completed chemical analysis, about 8% (648/8264) have a suboptimal purity rating (purity codes F [incorrect MW], Fnc [no sample detected], and Fc [very low concentration, <5% of expected value]). Substances with suboptimal purity ratings were excluded in the evaluation of p53 activation. In total, 9,848 (including the 2,232 without chemical analysis data) substances (7,849 unique chemicals) were evaluated for activity in the p53 assay.
Tox21 p53RE-bla assay
Cell culture
The CellSensor® p53RE-bla HCT-116 cell line (Invitrogen, Carlsbad, CA, USA), stably expresses a β-lactamase reporter gene under control of the p53 response element. P53RE-bla cells were cultured in McCoy’s 5A Medium supplemented with 10% dialyzed fetal bovine serum (FBS), 5 μg/mL blasticidin, and 100 U/mL penicillin and 100 μg/mL streptomycin (all acquired from Invitrogen). The cells were maintained at 37ºC under a humidified atmosphere and 5% CO2 (see Table 1 for more details).
Table I.
Protocol for the p53RE qHTS assay
| Step parameter | Value | Description |
|---|---|---|
| Plate cells1 | 5 μL | 4,000 p53RE-bla HCT-116 cells/well |
| Incubation time2 | 6 h at 37 ºC | Cells adhere and acclimate |
| Tox21 compound library addition3 | 23 nL | 92 μM to 0.59 nM titration series |
| Positive/vehicle control compound addition4 | 23 nL | Mitomycin C, 2 columns: 0.7 nM – 23 μM; 11.5 μM Nutlin-3, 2 columns: 1.4 nM – 46 μM; 23 μM Tetraoctylammonium bromide, partial column: 92 μM (cytotoxicity positive control) DMSO: 1 column |
| Incubation2 | 16 h at 37 °C | Induce p53 reporter element |
| Reagent5 | 1 μL | Beta lactamase detection mix |
| Incubation | 2 h, room temp. | Cells load and cleave substrate |
| Assay readout6 | Ex = 405/8 nm | EnVision™ plate reader |
1536-well plates, single tip dispensing of 4000 cells per well into all wells
Incubated at 37 ± 1°C under humidified atmosphere and 5% CO2
Pintool transfer of library to columns 5–48.
Pintool transfer of controls to columns 1–4; 2 columns with mitomycin C, 1 column with DMSO only, several wells in column 3 with tetraoctyl ammonium bromide (this column divided between MMC and TAB).
LiveBLAzer™ B/G FRET substrate (Invitrogen, CA)
460/25 and 530/20 nm emission filters and 405/8 excitation filter.
qHTS of multiplexed p53 β-lactamase reporter gene and cell viability assays
The p53RE-bla cells, suspended in OPTI-MEM medium containing 0.5% dialyzed FBS, were dispensed at 4,000 cells/5 μL/well in 1536-well black wall/clear bottom plates using a Thermo Scientific Multidrop Combi (Thermo Fisher Scientific Inc., Waltham, MA, USA). After the assay plates had been incubated in a 37ºC/5% CO2 incubator for 6 hours, 23 nL of each test compound dissolved in DMSO, the positive controls dissolved in DMSO, or DMSO alone was transferred to the assay plate by a pin tool (Kalypsys, San Diego, CA, USA) resulting in a 217-fold dilution. The final compound concentration in the 5 μL assay volume generally ranged from 1.2 nM to 92 μM in 15 concentrations (concentration spacing ~ 0.35 log10 unit, 2.2 fold). The assay was conducted on three separate occasions, resulting in ~37,500 15-point concentration response curves each for p53 activation and for cell viability. The plate format for the positive controls was as follows: Column 1, concentration-response titration of mitomycin C (Calbiochem, San Diego, CA, USA), a known genotoxic compound, from 0.7 nM to 23 μM in duplicates (16 concentrations; concentration spacing ~ 2 fold); Column 2, concentration-response titration of nutlin-3 (Calbiochem, San Diego, CA, USA), the non-genotoxic positive control recommended by Promega for this assay, from 1.4 nM to 46 μM in duplicates (16 concentrations,; concentration spacing ~ 2 fold); Column 3, row 1–14 for 11.5 μM mitomycin C; row 15–28 for nutlin-3; row 29–32 for 92 μM tetraoctyl ammonium bromide, the positive control for cell viability. Column 4 wells were reserved for DMSO only. The plates were incubated at 37 °C for 16 h; this incubation duration was recommended by Invitrogen for the p53 assay. Next, 1 μL of LiveBLAzer™ B/G FRET substrate (Invitrogen) was added using a Flying Reagent Dispenser (FRD, Aurora Discovery, Carlsbad, CA, USA), the plates were incubated at room temperature for 2 h, and fluorescence intensity at 460 and 530 nm emission was measured at 405 nm excitation by an EnVision™ plate reader (Perkin Elmer, Shelton, CT, USA). Subsequently, for the cell viability readout, 4 μL/well of CellTiter-Glo® reagent (Promega, Madison, WI, USA) was added into the assay plates using a FRD (Aurora Discovery). After 30 min incubation at 37 ºC, the luminescence intensity in the plates was measured using a ViewLux plate reader (PerkinElmer). Data were expressed as the ratio of 460nm/530nm emissions for the p53RE-bla assay.
Tox21 qHTS data analysis
The raw plate reads for each titration point were first normalized relative to the positive control compound (mitomycin C, 100%; tetraoctylammonium bromide, −100% [cell viability]) and DMSO-only wells (0%) as follows: % Activity = [(Vcompound − VDMSO)/(Vpos − VDMSO)] × 100, where Vcompound denotes the compound well values, Vpos denotes the median value of the positive control wells, and VDMSO denotes the median values of the DMSO-only wells. The % Activity was rescaled so that the baseline value was 0%. The data set was then corrected using the DMSO-only compound plates at the beginning and end of the compound plate stack by applying an NCATS in-house pattern correction algorithm. The normalized concentration-response data at each run (three runs in total) were applied to a qHTS noise filtering algorithm, Curvep (Hsieh et al. 2015; Sedykh et al. 2011) with assay noise level derived from the response variation in the 88 technical replicates (Behl et al. 2015). In the p53 assay, 10% and 25% were used in the noise filtering for the ratio data (the ratio of reporter gene channel relative fluorescence units (RFU) and background channel relative fluorescence units (RFU)) and cell viability data, respectively. Four activity parameters, including weighted area-under-curve (wAUC, total activity), point-of-departure (POD, concentration at which the response is equivalent to the noise threshold), EC50 (half maximal effect concentration), and Emax (maximal response), were reported on each curve. Curves with wAUC > 0 are considered as having significant responses; curves with wAUC = 0 are considered as having no response. The POD values from the no-response curves were set as the highest tested concentration. Substances with > 50% of curves with significant responses (i.e., 2 of 3 concentration response curves) were considered to be active. Additionally, p53 actives that might be due to assay interference, including auto-fluorescence/quenching, were flagged (Hsieh et al. 2015). The flagged actives are labeled as inconclusive. The data for substances were further summarized for each chemical (i.e., CASRN) after excluding data from the substances with suboptimal purity. Thus, actives are defined as those chemicals that have significant responses for >50% of the non-flagged substances, and the reported average activity values were based on the non-flagged active substances only.
Tox21 p53 activity data vs other data
Several cross-database analyses were conducted to investigate the relationship between the results in our p53RE assay and other relevant information for the compounds, including chemical-gene interactions using the Comparative Toxicogenomics Database, mechanisms of action using data from DrugMatrix and DrugBank, traditional genotoxicity test data from three routinely used assays, and in silico mutagenicity Quantitative Structure-Activity Relationship (QSAR) models. Details of these methods are described in the following sections.
Chemical-gene interactions
To investigate the association between compounds active in the p53RE assay and downstream genes in the p53 signaling pathway, we used the p53 pathway defined by the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway ID: hsa04115, http://www.genome.jp/kegg-bin/show_pathway?hsa04115 and the Comparative Toxicogenomics Database (CTD) (access date: Aug. 8th 2016; Davis et al., 2015). Sixteen genes immediately downstream of p53 were identified in the KEGG p53 signaling pathway. The 16 genes (Gene ID is from Entrez: Gene, https://www.ncbi.nlm.nih.gov/gene) included CDKN1A (Gene ID: 1026), SFN (Gene ID: 2810), RPRM (Gene ID: 56475), GADD45A (Gene ID: 1647), GTSE1 (Gene ID: 51512), FAS (Gene ID: 355), PIDD1 (Gene ID: 55367), BAX (Gene ID: 581), EI24 (Gene ID: 9538), SHISA5 (Gene ID: 51246), IGFBP3 (Gene ID: 3486), SERPINE1 (Gene ID: 5054), DDB2 (Gene ID: 1643), PTEN (Gene ID: 5728), STEAP3 (Gene ID: 55240), and MDM2 (Gene ID: 4193). TP53 (Gene ID: 7157) was also included for comparison. These genes were applied as input to the CTD. This database consists of extensive manually curated information about chemical–gene/protein interactions as well as chemical–disease and gene–disease relationships. We focused on the data related to chemical-gene interactions from either humans, rats, or mice. In addition, since CTD provides controlled vocabulary for the interactions, we parsed the interactions with the phrase “CHEMICAL results in increased expression of GENE).” Under the universe of Tox21 chemicals registered in CTD, Fisher’s exact test with Bonferroni correction (α < 0.05) was applied to identify the genes where chemicals result in increased expression and also tend to be active in the Tox21qHTS p53RE assay.
Mechanisms of action
Chemical annotations retrieved from two databases were compiled and an interface (Tox21 Enricher, http://hurlab.med.und.edu/tox21enricher/tox21enricher.cgi, access date: Aug. 10, 2016) was constructed to help perform the chemical enrichment analysis for the Tox21 chemical library (i.e., to identify the annotations that are over-represented by the query chemicals). We focused on the chemical annotations from DrugMatrix (https://ntp.niehs.nih.gov/toxfx/register_DM.php) and DrugBank (http://www.drugbank.ca/), both of which contain mechanism of action and classification data for drugs. Fisher’s exact test with Bonferroni correction (α < 0.05) was applied to identify the chemical annotations that are over-represented by the qHTS p53RE actives.
Traditional and predicted genotoxicity data
Bacterial mutagenicity (Ames; BM) data for Tox21 compounds were retrieved from the Leadscope® SAR Genetox Database (http://www.leadscope.com/product_info.php?products_id=77). We focused primarily on in vitro genotoxicity in Salmonella typhimurium and Escherichia coli strains in the absence of S9 metabolic activation (field names in Leadscope database: TA100-ma-absent, TA98-ma-absent, TA1535-ma-absent, TA1537-ma-absent, E-coliWP2-ma-absent). In addition to these data sets, genotoxicity data from S. typhimurium strain TA102 without S9 and in vitro mammalian cell chromosome aberration (CA) data without S9 were obtained through Leadscope customer support. Since there are no data fields in the Leadscope database related to the in vitro micronucleus assay without S9, in vivo rodent erythrocyte micronucleus data (field name in database: in vivo Micronucleus Rodent) were included for comparison. To partially account for confounding by metabolic activation in in vivo micronucleus test data, positive compounds that appeared to require metabolic activation for mutagenicity based on either BM data or in vitro CA data were excluded. For BM data, a summary call was created using available strain data without S9. A compound was assigned a positive call if it was positive in E. coli WP2 or any one of these Salmonella strains: TA100, TA98, TA1535, TA1537, TA102; a negative call required that the compound be negative and tested in at least TA100 and TA98 plus either E. coli WP2 or TA102 (these two strains are considered to be analogous in terms of mutagenic chemical detection). In total, three data sets were created for comparison with qHTS p53 data: bacterial mutagenicity without S9 (BM), in vitro chromosome aberration induction without S9 (CA), and in vivo micronucleus (MN).
In addition to the experimental genotoxicity data obtained from Leadscope, in silico mutagenicity QSAR models were used to predict BM. The Tox21 compounds were screened against two types of QSAR models (Salmonella model or E. coli/Salmonella TA102 model) provided by either Leadscope, Inc. (Salmonella Mut model and E Coli - Sal 102 A-T Mut model, http://www.leadscope.com/) or MultiCASE, Inc. (GT1_A7B model and GT1_AT_ECOLI model, http://www.multicase.com). Compounds predicted to be positive within the applicability domain in either model were assigned a positive call; compounds predicted to be negative within the applicability domain in both models were considered negative.
For each of the five endpoints (three traditional genotoxicity endpoints plus two in silico BM endpoints), a two-by-two contingency table was constructed with true positive (TP, active in p53 and positive in the endpoint), false positive (FP, active in p53 but negative in the endpoint), false negative (FN, inactive in p53 but positive in the endpoint), and true negative (TN, inactive in p53 and negative in the endpoint). The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV), were calculated. The bootstrap statistics (R boot package, number of bootstrap replicates = 2000) of 95% confidence interval (percentile method) for each of the parameters were also calculated.
Structure activity relationship (SAR)
Leadscope structural fingerprints were generated for the Tox21 chemicals. For each of the fingerprints where at least 50% of the chemicals having the fingerprint were active in the p53RE assay, Fisher’s exact test with Bonferroni correction was applied to identify those structural fingerprints that are enriched with actives in p53RE assay (α < 0.05). Significance of the enrichment value is closely tied to the number of chemicals having the fingerprint (i.e., fingerprints with only 2 active compounds in p53RE assay would not be significant due to insufficient data).
Data availability
The concentration-response data can be downloaded and visualized in https://tripod.nih.gov/tox21/assays/ and https://ntp.niehs.nih.gov/sandbox/tox21-curve-visualization/. The activity data used in this study is available in the Supporting Information File.
Results
qHTS assay performance
The Tox21 10K library was screened three times against the p53RE assay, which was multiplexed with the CellTiterGlo® cell viability assay in each well. Nutlin-3 and mitomycin C, the positive controls in the p53RE assay, produced an EC50 value of 3.1 ± 1.3 μM (mean ± standard deviation (SD)) and 1.8 ± 1.3 μM (mean ± SD), respectively. The p53RE assay worked well in qHTS format as evaluated by average signal-to-background (S/B) ratios of 3.3, average coefficients of variation (CV) of 6.01, and an average Z′ factor of 0.60, after normalization to the mitomycin C positive control. The cell viability assay also produced good performance with average S/B ratios of 136, average CV values of 8.3, and an average Z′ factor of 0.76. For the triplicate data, the signal reproducibility is also high. The median pairwise Pearson’s correlation coefficient between runs is 0.97 and 0.97 for the ratio and viability data, respectively; the median pairwise Cohen’s kappa (i.e., active/inactive concordance) between runs is 0.85 and 0.84 for the ratio and viability data, respectively. For the 88 technical duplicates, the pooled SD of the POD value is 0.17 and 0.19 log10 unit (< 2 fold) for the ratio and viability data, respectively. The results demonstrate the high signal reliability of the two assays.
Identification of actives in the p53 assay
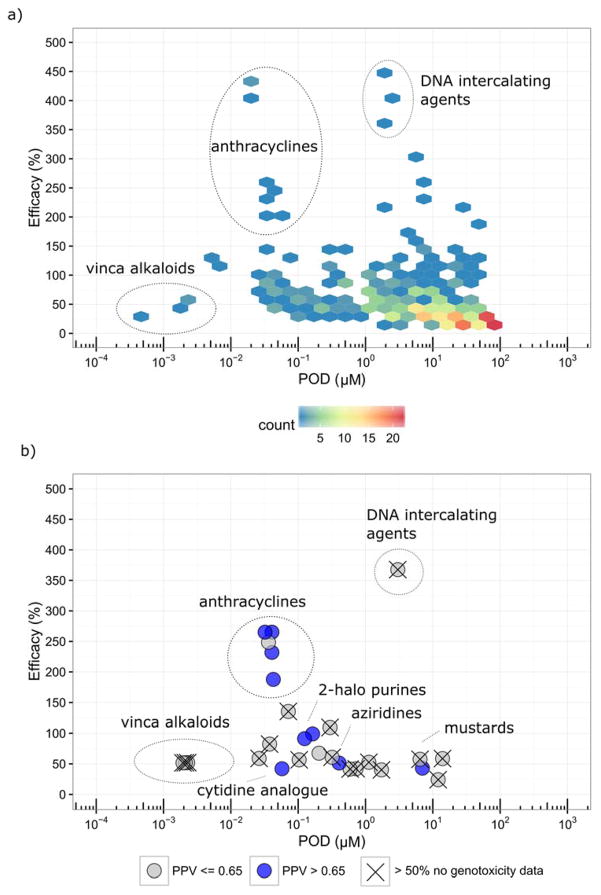
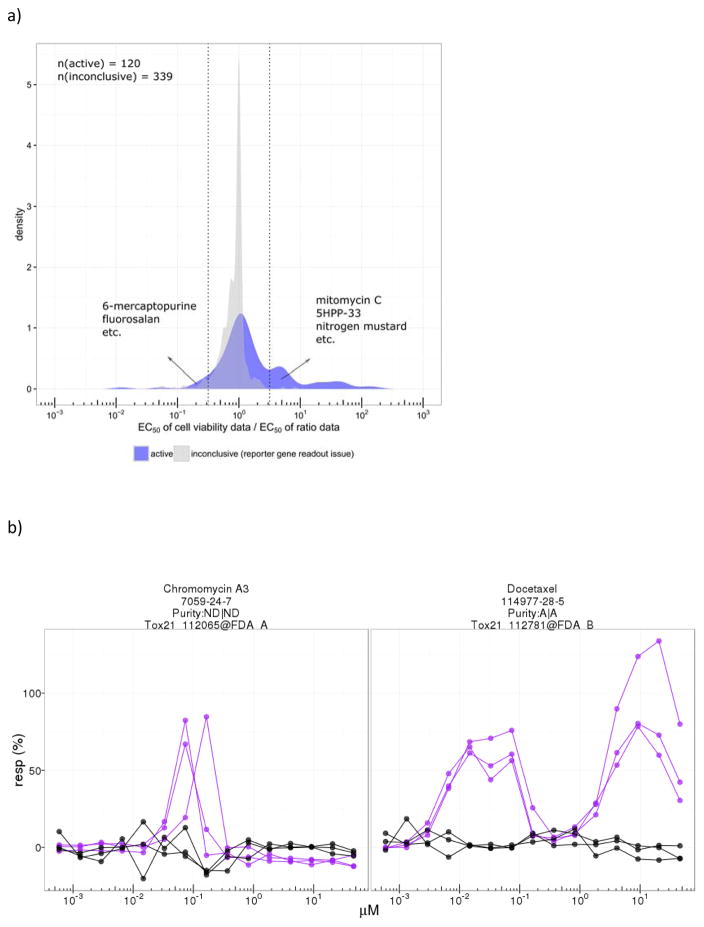
Of the total number of chemicals tested in the p53 assay, there were 7,849 unique chemicals with acceptable or unknown QC information (457 unique chemicals were found upon chemical analysis to have suboptimal purity, and these were excluded). Of the 7,849 chemicals, 365 chemicals (4.7%) were classified as active, 6,807 (86.7%) as inactive, and 677 (8.6%) as inconclusive. About 33% of the actives (120/365) also decreased cell viability within the concentration range tested (< 100 μM, typically). The most highly active chemicals included pharmaceuticals such as anthracyclines and tubulin inhibitors. The distribution of actives based on POD (potency) and Emax (efficacy) is shown in Figure 1a. Most of the actives (~93%; 340/365) have POD values > 0.01 μM and have Emax responses < 150% of the positive control, mitomycin C. Outliers having POD < 0.01 μM and Emax < 150% are all vinca alkaloids. A second class of outliers, compounds with Emax > 150%, are anticancer agents including a number of anthracyclines. Of the 677 chemicals classified as inconclusive, 66 were flagged for having signals possibly confounded by compound fluorescence, 170 were flagged for having weak and/or noisy signals making interpretation of the response unclear, and 441 were flagged for having non-increasing signals in the reporter gene readout for p53 activity, (Channel 2, 460 nm emission) despite a significant increase in the ratio data (Channel 2/Channel 1, 460/530 emissions). In these cases, the increase in the emissions ratio was driven by a decrease in the Channel 1 signal in the absence of an increase in the Channel 2 signal (reporter gene), suggesting that cytotoxicity was responsible for the increase in the emissions ratio. By comparing these results with those from the multiplexed cell viability assay that measured ATP levels, a majority of these chemicals (~77%, 339/441) were, in fact, determined to be cytotoxic within the concentration range tested, indicating that chemicals in this group (441) are causing a reduction in cell viability in the absence of activation of the p53RE. A further investigation into these compounds revealed that most showed similar potencies between the cell viability readout and the ratio data (Figure 4a.) Classes of chemicals in this group include, e.g., organotins and organometallics.
Figure 1.
Figure 1a. Distribution of potency and efficacy values for compounds active in the p53RE assay.
1b. Distribution of potency and efficacy values for structural fingerprints that are enriched with compounds active in the p53RE assay. POD, point of departure; PPV, positive predictive value.
Figure 4.
Figure 4a. Distribution of the fold change of the EC50 values for the cell viability data and the EC50 values for the p53RE ratio data. Dotted vertical lines indicate 3.2-fold difference between ratio data and cytotoxicity, where cytotoxicity occurs at higher concentrations (right side) or lower concentrations (left side) than the p53RE response.
4b. Examples of dose response curves of known (docetaxel) and potential (chromomycin A3) tubulin inhibitors. Purple: ratio data; black: cell viability data. Plots generated by https://ntp.niehs.nih.gov/sandbox/tox21-curve-visualization/
Associations of p53RE actives with other activities
Several different types of analyses were conducted to investigate the relationship between the results in our p53RE assay and other available information for the compounds to gain insight into the sensitivity and the specificity of the p53RE assay for detecting known genotoxicants in the Tox21 library, with a goal of understanding the best use of this assay in a comprehensive battery of screening assays. These additional analyses included chemical-gene interactions using the Comparative Toxicogenomics Database (CTD), mechanisms of action (MOA) using data from DrugMatrix and DrugBank, traditional genotoxicity test data from three routine assays, and in silico mutagenicity Quantitative Structure-Activity Relationship (QSAR) models. Results of these analyses are described in the following sections.
Chemical-gene interaction data
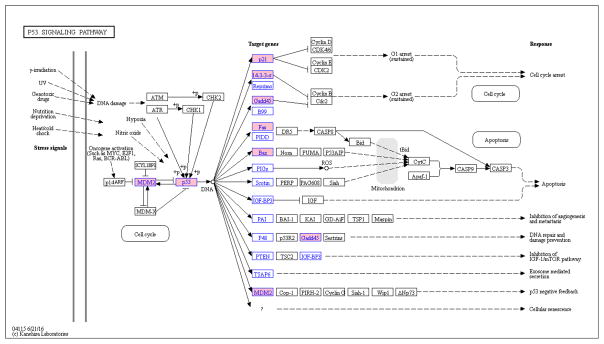
In total, there are 3,784 Tox21 chemicals (including 208 that are active in p53RE assay) registered in the CTD. For the 17 p53-related genes queried in CTD, the median number of Tox21 chemical-gene interactions (CHEMICAL results in increased expression of GENE) is 36, with the BAX gene having the greatest number of Tox21 chemicals (202) linked to it and the STEAP3 gene having the least (7). The number of chemical-gene interactions represents the number of unique publications (based on PubMedID) reporting the specific interaction. An association analysis was performed to identify the genes where linked Tox21 chemicals tended to be active in the p53RE assay (Table 2). In total, 7 out of the 17 p53-related genes in CTD were considered to have a significant association with chemicals that were active in the p53RE assay. The significance of the association with CDKN1A is the highest, followed by MDM2 and TP53. The insignificant associations tended to have fewer chemical-gene interactions (below the median number) except for SERPINE1 and IGFBP3. The results are plotted onto the KEGG pathway for p53 signaling (Figure 2). Looking at the KEGG pathway map, we see that compounds active in the p53RE assay are associated with genes controlling events relating to cell cycle arrest, apoptosis, and the negative feedback loop in the p53 pathway, but no significant associations were seen between p53 actives and genes involved in inhibition of the IGF-1 pathway and metastasis/angiogenesis.
Table II.
Association analysis between chemical activity in the p53RE assay and chemical-induced increased expression of downstream genes in the p53 signaling pathway
| NCBI Gene Name | NCBI Gene ID | log10 (pvalue) | N_P | N | n_p | n |
|---|---|---|---|---|---|---|
| CDKN1A | 1026 | −10.7 | 208 | 3784 | 36 | 192 |
| MDM2 | 4193 | −8.7 | 208 | 3784 | 20 | 76 |
| TP53 | 7157 | −7.2 | 208 | 3784 | 27 | 158 |
| FAS | 355 | −6.1 | 208 | 3784 | 19 | 97 |
| GADD45A | 1647 | −5.6 | 208 | 3784 | 20 | 114 |
| BAX | 581 | −5.5 | 208 | 3784 | 28 | 202 |
| SFN | 2810 | −4.0 | 208 | 3784 | 9 | 36 |
| EI24 | 9538 | −2.4 | 208 | 3784 | 5 | 20 |
| GTSE1 | 51512 | −2.0 | 208 | 3784 | 4 | 16 |
| SHISA5 | 51246 | −1.8 | 208 | 3784 | 3 | 10 |
| SERPINE1 | 5054 | −1.8 | 208 | 3784 | 9 | 73 |
| DDB2 | 1643 | −1.7 | 208 | 3784 | 4 | 20 |
| PTEN | 5728 | −1.3 | 208 | 3784 | 4 | 25 |
| STEAP3 | 55240 | −1.3 | 208 | 3784 | 2 | 7 |
| PIDD1 | 55367 | −0.9 | 208 | 3784 | 2 | 12 |
| IGFBP3 | 3486 | −0.6 | 208 | 3784 | 4 | 46 |
| RPRM | 56475 | −0.3 | 208 | 3784 | 1 | 12 |
Bold text: significant association after Bonferroni correction; N_P: number of chemical-gene interactions (CHEMICAL results in increased expression of GENE); N: number of Tox21 chemical in Comparative Toxicogenomics Database (CTD); n_p: number of chemical-gene interactions (CHEMICAL results in increased expression of GENE) that the chemical is also active in p53RE assay; n: number of chemical-gene interactions (CHEMICAL results in increased expression).
Figure 2.
Association analysis between chemical activity in the p53RE assay and chemical-induced increased expression of downstream genes in the p53 signaling pathway. Blue text: chemical-gene data applied in the analysis; pink background: significant association between chemical activity in the p53RE assay and chemical-induced increased expression of the gene. Plot created by KEGG Mapper, http://www.kegg.jp/kegg/mapper.html
Mechanisms of action
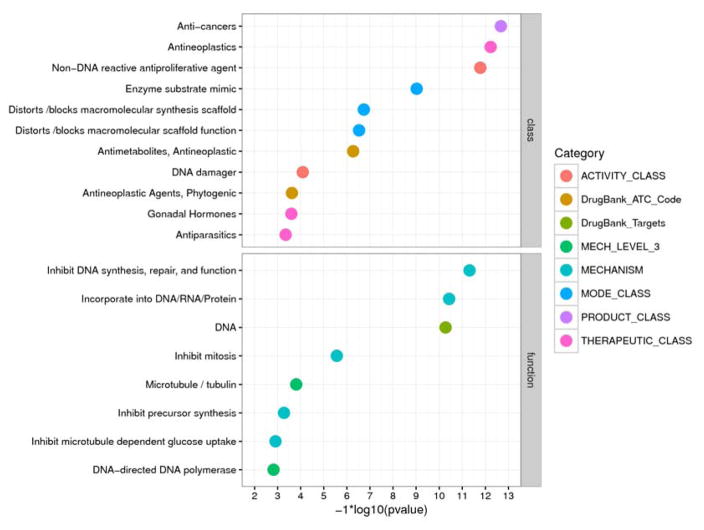
The 365 CAS Registry Numbers for the actives in the p53RE assay were used as input for the Tox21 Enricher (http://hurlab.med.und.edu/tox21enricher/). In total, there are 50 chemical annotations considered significant after Bonferroni correction. We particularly focused on the categories related to chemical classes (e.g., activity, mode of action, product, therapeutic) and mechanisms of action (e.g., molecular level targets). The results are ordered based on their significance (Figure 3). For the annotations related to functions, the most significant ones are all related to DNA interactions, followed by annotations related to microtubule inhibition (e.g., vincristine) and precursor synthesis inhibition (e.g., methotrexate). For annotations related to chemical classes, anticancer agents are among the most significant, followed by antinematodal agents (e.g., mebendazole). Interestingly, the annotation “gonadal hormones” is also associated with actives in the p53RE assay (e.g., 17alpha-ethinylestradiol, diethylstilbestrol). The associated annotations and the respective p53RE-active chemicals are presented as heat maps (Supporting Information, Figure 1). For the other annotations, see the Supporting Information File (“Enricher”).
Figure 3.
Association analysis between chemical activity in the p53RE assay and chemical annotations. Terms for chemical annotations are taken directly from the DrugMatrix and DrugBank databases; ATC: WHO drug classification system (ATC) identifiers. For additional descriptions of the categories, please refer to the Supporting Information Methods.
Traditional and predicted genotoxicity data
In an initial attempt to benchmark the performance of the p53RE assay, we compared the sensitivity of the assay for known genotoxicants in three well-known and accepted traditional genetic toxicity tests with large datasets in the Leadscope database (BM, in vitro CA, and in vivo MN). The sensitivity of the p53RE assay compared with these three traditional genotoxicity tests is < 20% (Table 3). Surprisingly, the highest sensitivity (17%) was seen for in vivo MN data, where some false negatives (positive in MN & inactive in p53RE) might have resulted from the lack of metabolic activation in the qHTS assay, although we attempted to restrict the MN dataset to compounds that did not require metabolic activation to produce a positive response in in vitro genotoxicity assays. The sensitivity of the BM and CA endpoints is about 12% and 11%, respectively. Overall, the average positive predictive value (PPV) is > 55% for the three endpoints; the highest PPV is 71%, based on the CA endpoint. To have a data set with more complete BM data for comparing with the p53RE assay data, two in silico BM endpoints were created from QSAR models provided by either Leadscope or CASE Ultra. Although these models include chemicals that require metabolic activation, the calculated sensitivity of the p53RE assay using these models is roughly in the same range as that generated with traditional experimental data (~11%), but with a lower PPV (55%).
Table III.
Comparison of p53RE qHTS assay activity calls and QSAR model predictions for bacterial mutagenicity or traditional genotoxicity assay results
| QSAR | Traditional genotoxicity assays | ||||
|---|---|---|---|---|---|
| BM (CASE Ultra QSAR) | BM (Leadscope QSAR) | BM (no S9) | CA (no S9) | MN (adjusted) | |
| Sensitivity | 0.11 [0.09 – 0.12] | 0.11 [0.09 – 0.13] | 0.12 [0.09 – 0.15] | 0.11 [0.08 – 0.15] | 0.17 [0.1 – 0.24] |
| Specificity | 0.97 [0.96 – 0.97] | 0.97 [0.96 – 0.98] | 0.96 [0.94 – 0.97] | 0.96 [0.94 – 0.98] | 0.96 [0.93 – 0.98] |
| PPV1 | 0.48 [0.42 – 0.53] | 0.53 [0.46 – 0.59] | 0.68 [0.58 – 0.78] | 0.71 [0.57 – 0.83] | 0.55 [0.39 – 0.71] |
| NPV2 | 0.78 [0.77 – 0.79] | 0.78 [0.76 – 0.79] | 0.6 [0.57 – 0.63] | 0.55 [0.51 – 0.59] | 0.79 [0.75 – 0.82] |
| True positive | 136 | 121 | 60 | 36 | 21 |
| False positive | 149 | 108 | 28 | 15 | 17 |
| False negative | 1147 | 997 | 430 | 289 | 100 |
| True negative | 4153 | 3465 | 632 | 356 | 377 |
Positive predictive value
Negative predictive value
True positive: chemicals active in both the p53RE assay and the specific traditional genotoxicity assay
False positive: chemicals active in the p53RE assay but inactive in the specific traditional genotoxicity assay
False negative: chemicals inactive in the p53RE assay but active in the specific traditional genotoxicity assay
True negative: chemicals inactive in both the p53RE assay and the specific traditional genotoxicity assay
Structure activity relationships
In total, there are 2,398 (out of 7,770 in Tox21 chemical space) structural fingerprints with at least one p53RE active chemical. To focus on the structural fingerprints that are more relevant to p53 activity, we filtered out the structural fingerprints with fewer than 50% p53RE assay active chemicals. Fisher’s exact test with Bonferroni correction was applied to the remaining fingerprints (117) to identify the fingerprints that are over-represented with p53 activity (53). The minimum number of chemicals allowed in a fingerprint was 3. The median POD and Emax values for the actives in each fingerprint were calculated. For each of the fingerprints, we also calculated the PPV (# of genotoxic/# of p53 actives) based on the aggregated genotoxicity data, that is, a chemical was considered genotoxic if it was positive in any of the three traditional endpoints (BM, CM, and MN). We recognized that low PPVs may be due to insufficient traditional genotoxicity data. We therefore indicated the fingerprints that had > 50% of the chemicals with no confirmed call for any of the three genotoxicity endpoints (i.e., data-poor fingerprints). The 53 structural fingerprints were further collapsed if they had the same median POD and Emax values, and the data from the fingerprint with the maximum PPV are reported and plotted (Figure 1b and Supporting Information, Table 1). The fingerprints that are the most efficacious (median Emax > 100%) include the anthracycline (e.g., daunomycin) and camptothecin (e.g., topotecan) classes of chemotherapeutic agents. The anthracycline-related fingerprints are data-rich and have high PPVs. Other data-rich fingerprints also tend to have high PPVs, for example, 2-halo purines (e.g., cladribine), cytidine analogues (e.g., cytarabine), and podophyllotoxin derivatives (e.g., etoposide). Although some fingerprints are labeled as data-poor, they are known to be associated with compounds likely to be genotoxic, for example, anticancer agents (e.g., chromomycin A3, bleomycin), nucleoside analogues (e.g., 6-azacytidine), aziridines (e.g., thiotepa), and mustards (e.g., nitrogen mustard). Anti-tubulins (e.g., nocodazole, vincristine) are also found to be enriched in some fingerprints (2-amino benzimidazoles, vinca alkaloids), but these tend to be data-poor in the database.
In addition, we did an association analysis based on 106 Leadscope mutagen structural alerts (Supporting Information, Table 2) that include at least one Tox21 chemical. Thirteen alerts are found to be associated with p53 activity, including haloethylamines (e.g., chlorambucil), urethane derivatives (e.g., mitomycin C), and 1,4-dihydroxybenzene (e.g. daunomycin). Two of the thirteen have over 50% of chemicals active in the p53RE assay (i.e., aziridine and mustards). These two alerts were also identified when using all of the Leadscope fingerprints. For the complete set of chemicals in fingerprints with enriched p53 activity, refer to Supporting Information File (“FP_SAR” and “SA_SAR”).
p53 activity in relation to cell viability
To parse out the relationship between p53RE response and cytotoxicity, we looked at the dose-response curves for each event to determine if the p53 response was independent of cytotoxicity. Specifically, if cytotoxicity was observed, we assessed whether it occurred at concentrations above those that induced an increase in p53 signaling. Only about 33% of actives (120/365) showed some degree of decrease in cell viability over the concentration range tested (typically 1.2 nM to 92 μM); the distribution of the ratios of the EC50 values for the cell viability data and the p53RE ratio data is shown in Figure 4a. For comparison, the EC50 ratio distribution of the 339 inconclusive chemicals (i.e., non-increasing signals in the reporter gene readout for p53 activity despite a significant increase in the ratio data after normalizing the reporter gene readout by the background) that showed a decrease in cell viability was also plotted. The EC50 ratio distribution of the active compounds tended to be right-shifted (i.e., cytotoxicity is less potent than the p53RE ratio data) and occurred over a wider dose range compared to the EC50 ratio distribution for the inconclusive chemicals, where the cytotoxicity response tended to be more potent than the p53RE ratio data. Focusing on the compounds that showed the greatest separation of EC50 values for cytotoxicity and p53RE activation, there are 29 actives with EC50 values for cytotoxicity at least 3.2-fold (0.5 log10 unit or ~2.5 standard deviations from the mean) less potent than the p53RE activity (i.e., cytotoxicity occurred at doses well above those that induced p53 signaling), including, e.g., mitomycin C (positive control), 5-HPP-33 (a thalidomide derivative that stabilizes microtubules), and nitrogen mustard (potent alkylating agent). Only seven chemicals had EC50 values for cytotoxicity that were ≥ 3.2-fold more potent than the p53RE activity (i.e., the p53 response may have been induced by cytotoxicity); included in this group are compounds such as 6-mercaptopurine (antimetabolite) and fluorosalan (disinfectant). For the complete list of all 36 of these chemicals, refer to Supporting Information, Table 3.
For the 242 actives (67%, 242/365) with no decrease in cell viability over the concentration range tested, we identified a group of chemicals that showed a distinct pattern of non-monotonic response, i.e., significantly increasing signal at low concentrations followed by a return to baseline at higher concentrations (“humped” response curve) (Figure 4b). In total, 29 actives with at least four data points showing a non-monotonic response were identified. Twenty-six compounds showed a “single-humped” pattern of concentration response curve; these included, for example, vincristine, plicamycin, and nocodazole. Three of the 29 compounds showed a “two-humped” pattern of concentration response curve; these included the structurally-related compounds paclitaxel, docetaxel, and epothilone B. Most of these 29 compounds are known tubulin inhibitors. Chromomycin A3 is the only chemical in this group that hasn’t been reported previously to be a tubulin inhibitor but it was highly potent in the p53RE assay. For the complete list of these 29 chemicals, see Supporting Information, Table 4.
Discussion
In this study, we used a qHTS platform to profile the Tox21 10K compounds for their potential to activate the p53 signaling pathway. The high level of data reproducibility observed for the 88 duplicate internal plate replicates as well as for all compounds among the three independent runs indicates that the screening was robust. The use of both nutlin-3 (a p53-MDM2 complex binder and a non-genotoxicant) and MMC (an alkylating and cross-linking genotoxicant) as reference compounds in this assay provides evidence that activity can be measured both for genotoxic and non-genotoxic compounds that induce activation of the p53RE through markedly different mechanisms.
The 10,000+ compounds in the Tox21 collection have been undergoing detailed chemical analysis for purity and identity, a feature unique to the Tox21 program. These detailed analytical data, available for most of the compounds in the library, were applied to exclude results generated from compounds with suboptimal purity or inappropriate identity. In addition, signals that resulted from assay interference (e.g., compound auto-fluorescence) were flagged. In total, after eliminating the data from unreliable compounds, 4.7% (365/7,849) of the compounds tested were considered to be active in the p53RE assay. Within the group of active compounds, specific chemical classes showed characteristic patterns of responses. For example, the anthracycline anticancer drugs tended to be very efficacious (>150% of the maximal activity of the positive control, mitomycin C) and potent (POD < 0.1 μM). The vinca alkaloid anti-tubulin drugs tended also to be very potent (POD < 0.01 μM) but only moderately efficacious (~50% of the maximal activity of the positive control mitomycin C). From the chemical-gene interaction data obtained from the CTD database, we found that active compounds in the p53RE reporter gene assay are associated with chemical-induced increased expression of specific downstream genes in the p53 signaling pathway. These genes are involved in processes relating to cell cycle arrest, apoptosis, and the negative feedback loop for p53, indicating that the compounds associated with them are likely to be p53 activators. In contrast, we found that compound activity in the p53RE assay is not associated with the activities of downstream genes in the p53 signaling pathway relating to inhibition of the IGF-1 pathway or metastasis/angiogenesis. This observation of specific gene-chemical associations provides additional support for the association of p53 actives with genotoxicity since the IGF-1 pathway is associated with cell growth and anabolic effects, not genotoxicity (Girnita et al., 2014). In addition, actives in the p53RE assay are over-represented in chemical annotations related to DNA/tubulin interference and anticancer, antimetabolite, and antiparasitic drug classes, all of which contain a high proportion of known genotoxicants. The results of these chemical-gene analyses together provide added confidence that the p53 assay is identifying relevant chemicals.
Interestingly, the gonadal hormone chemical class was enriched with p53 actives (e.g., diethylstilbestrol, 17α-ethinylestradiol), and a search of the CTD showed these actives have interactions with genes in the p53 signaling pathway. For example, diethylstilbestrol (DES) has been shown to induce expression of CDKN1A (a.k.a., P21), which is a potent cyclin-dependent kinase inhibitor that has a role in DNA damage repair as well as G1 cell cycle arrest following any of a variety of cellular stresses. In addition, DES is associated in CTD with increased expression of BAX, GADD45A, and MDM2, among other p53 downstream genes. In traditional genotoxicity assays, DES has shown clear evidence of chromosomal damage (primarily aneuploidy) in several in vitro studies (Johnson et al., 2010; Fauth et al., 2000; de Stoppelaar et al., 2000; Migliori et al., 1996). Estradiol and ethinyl estradiol (synthetic derivative of estradiol) are associated in CTD with increased expression of BAX, CDKN1A, GADD45A, and MDM2, among numerous other p53 downstream genes. Results of traditional genotoxicity studies with 17α-ethinylestradiol using the micronucleus endpoint in vivo have yielded contradictory results (e.g., Shelby et al., 1997; Dhillon and Dhillon, 1997), while one study showed weak evidence of aneuploidy induction (centromere positive micronuclei) in human lymphocytes treated in vitro (Bukvic et al., 2000). Thus, the activity of these hormonal compounds in the p53RE assay may reflect a potential for multiple biological effects including genotoxicity which are dependent upon the context in which the exposures occur.
Comparing chemical activity in the p53RE assay with the results from traditional genotoxicity assays (excluding genotoxicants requiring metabolic activation), we found that the p53RE assay has low sensitivity (< 20%) but moderate PPV (~65%). Similar results were found using the data from the QSAR model predictions, but with a slightly lower PPV, perhaps due in part to the inclusion of metabolism in the QSAR models for genotoxicity. The low sensitivity of the p53RE qHTS assay for known genotoxicants is not necessarily surprising, since the p53 signaling pathway is just one of several possible responses to genotoxicity. To further explore the value of qHTS assays in identifying DNA damaging agents among large compound collections, additional analyses are in progress. A companion paper (Hsieh J-H et al., in preparation) will extend these analyses from the single p53RE assay to all five of the Tox21 qHTS assays that measure some aspect of DNA damage/repair. The comprehensive analysis to be presented in the next paper will aim to address the question of how well the results of these high-throughput in vitro assays, in various combinations, compare to results from the traditional in vitro genotoxicity assays, and whether these qHTS DNA damage assay data can be used to predict genotoxicity, as defined by traditional assays for bacterial mutation and chromosomal damage, and serve as replacements for these traditional assays. Alternatively, the comprehensive five-assay data analysis may support the use of these high-throughput screens not for prediction but for prioritization of compounds for in-depth follow-up characterization of genotoxicity potential, based on their response patterns in the qHTS assays. This prioritization for genotoxicity potential would focus the detailed characterization efforts on those compounds most likely to exhibit biological liabilities, thereby promoting the most efficient use of resources.
Several factors might affect the comparison of results between the qHTS p53RE assay and traditional genotoxicity assays. The first factor to consider is the concentration used in the qHTS assay versus that used in the traditional genotoxicity assays. When in vitro bacterial mutagenicity or mammalian clastogenicity tests are used for hazard identification, compounds are typically tested at much higher concentrations (historically, up to 1–10 mM for in vitro mammalian genotoxicity assays and 5,000 μg/plate for bacterial mutagenicity assays, unless precluded by toxicity or solubility [e.g., OECD, 471; OECD, 473; ICH S2(R1), 2011]). To investigate the role of dose, data from a set of Tox21 chemicals that were positive in the BM test (-S9) and for which detailed concentration information existed, were collected (Supporting Information, Methods). We found a significant association between activity in the p53RE assay and the lowest effective dose (LED) that induced mutagenicity in bacteria (p-value < 0.003, Supporting Information, Table 5), suggesting that some compounds that were positive in the BM assay had LEDs that were higher than the highest concentration tested in the p53RE assay, thus possibly accounting for their inactivity in the p53RE assay. In addition, by incorporating information on the number of genotoxicity studies that had been conducted with a particular compound, we created a set of 25 unique chemicals (“common mutagens” or consensus mutagens) that were consistently positive in a number of genotoxicity assays in the absence of S9 and that had a lowest effective concentration in the BM test < 100 μM. Of these 25 consensus mutagens, 23 had a confirmed call in the p53RE assay (either active or inactive) and 2 were inconclusive. Of the 23 compounds with a confirmed call, 5 were active in the p53RE assay (Supporting Information, Table 6), including 3′-azido-3′-deoxythymidine, captan, 5-azacytidine, 2-aminoanthracene, and potassium dichromate. Thus, the adjusted sensitivity of the p53RE assay is 22% (5/23), higher than the sensitivity of the p53RE assay when dose levels in the bacterial mutagenicity assay are not taken into account (Table 3).
Other factors to consider in comparing the results of the p53RE assay with results from traditional genotoxicity assays are, differences in cell types (the p53RE assay uses transformed HCT-116 cells) and duration of chemical exposure (16 h in the p53RE assay, which is less than one cell cycle). Another consideration in the comparison of qHTS p53RE assay results with those from traditional assays is that bacterial mutation or mammalian cell chromosomal damage are the end results of unrepaired DNA damage while activation of the p53 response element is an early molecular event in response to DNA damage. While on the surface, the detection of initial damage might be expected to result in an increased number of compounds identified in the p53RE assay compared with traditional genotoxicity assays, the substantial differences in the assay protocols and endpoints they evaluate cannot be dismissed. The BM test measures induction of fixed mutations in bacteria, and chromosome damage assays (chromosomal aberrations and micronuclei) measure changes in chromosome structure or number in daughter cells following exposure of the parental cell. Therefore, a better correlation might be seen between the p53RE assay results and results from assays such as the comet assay, which measures early DNA damage shortly after chemical exposure. To investigate this hypothesis, selected sets of compounds from the Tox21 library are currently being tested in an in vitro comet assay to assess their potential for DNA damage in two different cell types, one that is p53 competent and the other one deficient in p53 activity, and to compare the results with those obtained with the qHTS p53RE assay.
Through SAR analysis, we have identified several structural fingerprints that are enriched with and specific to activity in the qHTS p53 assay. For the p53 actives in these fingerprints with sufficient genotoxicity test data in the Leadscope database to allow conclusions to be drawn regarding their activity, the majority are either known genotoxicants or probable genotoxicants based on the chemical class to which they belong (e.g., anticancer drugs). Thus, those fingerprints tend to have high PPVs for genotoxicity. The fingerprints related to the antitubulins (vinca alkaloids and 2-amino-benzimidazoles) do not have high PPVs because the p53RE actives in these fingerprints are too data poor to allow definitive conclusions be drawn on their activity, but the chemicals in this class are known to be aneugens that induce numerical changes in karyotype complement via spindle fiber disruption, and aneugens are considered to be genotoxic compounds. We conducted a separate association analysis using 106 mutagenic structural alerts derived through curation of the literature and statistical analysis from Leadscope. In this analysis, consistent with the low sensitivity of the p53RE assay for bacterial mutagens, we found only 13 of the 106 mutagenic structural alerts to be associated with p53 activity (again, dose factors in because these alerts were generated from BM test data that may have used much higher concentrations). In two of the 13 mutagenic structural alerts (the aziridines and mustards), more than 50% of the chemicals were active in the p53RE assay.
Since p53 activation is linked to DNA damage and repair, as well as other cellular stresses, all of which may lead to cell death, we investigated the relationship between potency of the p53 activation response and potency of the cytotoxicity response (Figure 4a; Supporting Information, Table 2). Analysis revealed that for compounds active in the p53RE assay, when cytotoxicity occurs concomitantly within the tested concentration range, cytotoxicity generally tends to be less potent than the p53RE response (i.e., cytotoxicity tends to occur at higher concentrations than the p53RE response). Representative chemicals demonstrating this activity pattern include mitomycin C (potent DNA cross-linking agent, positive control), 5HPP-33 (thalidomide derivative, microtubule depolymerizing agent), topotecan (a topoisomerase inhibitor), and nitrogen mustard (potent alkylating agent), all of which elicited cytotoxicity at higher concentrations than the concentrations that induced p53 activity. For compounds with this pattern of response, we suspect that cytotoxicity results from induction of apoptosis following DNA damage. For p53RE actives with more potent cytotoxicity responses compared with p53RE activity (notably fewer chemicals in this category), representative chemicals include 6-mercaptopurine (an antimetabolite with demonstrated genotoxic activity in traditional assays), bromodiolone (a rodenticide anticoagulant), Closantel (antihelmintic), Romidepsin (HDAC inhibitor that induces apoptosis), bisphenol fluorenone (heat resistant polymer), and fluorosalan (an antibacterial compound that inhibits NF-κB signaling).
For p53RE actives with no observable cytotoxicity within the concentration range tested, we identified a group of chemicals with unusual dose-response patterns (i.e., the “humped” structure, see Figure 4b). Initially, the “one-humped” response pattern was taken to indicate activation of the p53RE at lower concentrations followed by sharp onset of cytotoxicity with increasing concentrations. However, no evidence of cytotoxicity was observed, and for the “two-humped” response pattern, cytotoxicity as a cause for the rapid downturn in the p53 response obviously could not be invoked. Upon further investigation, almost all of the compounds producing these unusual dose-response patterns were found to be antitubulins. It is known that in responding to certain types of cellular insult, p53 translocates from the cytoplasm to the nucleus via the cytoplasmic microtubule apparatus (Giannakakou et al. 2000; Li and Martinez 2011). We suspect that the chemicals displaying the one-humped dose response pattern initially activated the p53RE at concentrations that did not appreciably disrupt microtubule infrastructure but then, with increasing concentration, they caused disruption of the cytoplasmic microtubule structure, preventing p53 translocation and thus blocking activation of the response elements in the nucleus. The two-humped pattern is more difficult to explain. The three compounds that induced a double-humped p53 dose response curve all stabilize microtubules rather than disrupt their aggregation. Thus, there may be a characteristic dynamic involved in the p53 response with these kinds of compounds that differs from the response seen with compounds that disrupt tubulin polymerization. Among the chemicals demonstrating these unusual humped response patterns are several vinca alkaloids, taxols, and benzimidazoles, along with a novel compound, chromomycin A3, which has not previously been reported to possess antitubulin activity.
In conclusion, the p53RE qHTS assay can not only identify activators of the p53RE, many of which are genotoxic, but the assay can also, in some instances, provide information on mechanism of action for the compound based on the response patterns observed across a broad range of doses accompanied by measurements of cytotoxicity. The assay is admittedly insensitive, based on the number of known direct-acting mutagens that are detected, but compounds that do show strong activity in the assay are enriched with genotoxicants. Using this assay alone or as a component of a test battery allows for prioritizing compounds for in-depth follow-up testing to more effectively focus the limited resources that are available for toxicological characterization of the many compounds still lacking such data.
Supplementary Material
Acknowledgments
The authors are grateful to Drs. Maria Shatz, Daniel Shaughnessy, and John Bucher for providing a critical review of this manuscript and helpful comments. This project was supported by Interagency Agreement NTR12003-001-0100 from the National Institute of Environmental Health Sciences/Division of the National Toxicology Program to the NIH Center for Advancing Translational Sciences, National Institutes of Health.
Footnotes
Conflict of interest statement: The authors declare they have no conflicts of interest.
Statement of Author Contributions
All authors made substantial contributions to the conception and design of this study, or acquisition, analysis, and interpretation of the resulting data. Similarly, all authors were involved in either drafting this manuscript or revising it critically for important intellectual content. All authors have read and provided final approval of the submitted version of the manuscript.
References
- Behl M, Hsieh JH, Shafer TJ, Mundy WR, Rice JR, Boyd WA, Freedman JH, Hunter ES, 3rd, Jarema KA, Padilla S, Tice RR. Use of alternative assays to identify and prioritize organophosphorus flame retardants for potential developmental and neurotoxicity. Neurotoxicol Teratol. 2015;52(Pt B):181–93. doi: 10.1016/j.ntt.2015.09.003. [DOI] [PubMed] [Google Scholar]
- Bukvic N, Susca F, Bukvic D, Fanelli M, Guanti G. 17-alpha-ethinylestradiol and norgestrel in combination induce micronucleus increases and aneuploidy in human lymphocyte and fibroblast cultures. Teratog Carcinog Mutagen. 2000;20(3):147–59. doi: 10.1002/(sici)1520-6866(2000)20:3<147::aid-tcm6>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Clewell RA, Sun B, Adeleye Y, Carmichael P, Efremenko A, McMullen PD, Pendse S, Trask OJ, White A, Andersen ME. Profiling Dose-Dependent Activation of p53-Mediated signaling Pathways by Chemicals with Distinct Mechanisms of DNA Damage. Toxicol Sci. 2014;142:56–73. doi: 10.1093/toxsci/kfu153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis AP, Grondin CJ, Lennon-Hopkins K, Saraceni-Richards C, Sciaky D, King BL, Wiegers TC, Mattingly CJ. The Comparative Toxicogenomics Database’s 10th year anniversary: update 2015. Nucleic Acids Res. 2015;43(Database issue):D914–20. doi: 10.1093/nar/gku935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Stoppelaar JM, Faessen P, Zwart E, Hozeman L, Hodemaekers H, Mohn GR, Hoebee B. Isolation of DNA probes specific for rat chromosomal regions 19p, 19q and 4q and their application for the analysis of diethylstilbestrol-induced aneuploidy in binucleated rat fibroblasts. Mutagenesis. 2000;15(2):165–75. doi: 10.1093/mutage/15.2.165. [DOI] [PubMed] [Google Scholar]
- Dhillon VS, Dhillon IK. Genotoxicity evaluation of estradiol. Mutat Res. 1995;345(1–2):87–95. doi: 10.1016/0165-1218(95)90073-x. [DOI] [PubMed] [Google Scholar]
- Fauth E, Scherthan H, Zankl H. Chromosome painting reveals specific patterns of chromosome occurrence in mitomycin C- and diethylstilboestrol-induced micronuclei. Mutagenesis. 2000;15(6):459–67. doi: 10.1093/mutage/15.6.459. [DOI] [PubMed] [Google Scholar]
- Fox JT, Sakamuru S, Huang R, Teneva N, Simmons SO, Xia M, Tice RR, Austin CP, Myung K. High-throughput genotoxicity assay identifies antioxidants as inducers of DNA damage response and cell death. Proc Natl Acad Sci U S A. 2012;109(14):5423–8. doi: 10.1073/pnas.1114278109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannakakou P, Sackett DL, Ward Y, Webster KR, Blagosklonny MV, Fojo T. p53 is associated with cellular microtubules and is transported to the nucleus by dynein. Nat Cell Biol. 2000;2(10):709–17. doi: 10.1038/35036335. [DOI] [PubMed] [Google Scholar]
- Girnita L, Worrall C, Takahashi S, Seregard S, Girnita A. Something old, something new and something borrowed: emerging paradigm of insulin-like growth factor type 1 receptor (IGF-1R) signaling regulation. Cell Mol Life Sci. 2014;71(13):2403–27. doi: 10.1007/s00018-013-1514-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladek A, Liehr JG. Mechanism of genotoxicity of diethylstilbestrol in vivo. J Biol Chem. 1989;264(28):16847–52. [PubMed] [Google Scholar]
- Hsieh JH, Sedykh A, Huang R, Xia M, Tice RR. A Data Analysis Pipeline Accounting for Artifacts in Tox21 Quantitative High-Throughput Screening Assays. J Biomol Screen. 2015;20(7):887–97. doi: 10.1177/1087057115581317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang R, Southall N, Wang Y, Yasgar A, Shinn P, Jadhav A, Nguyen DR, Austin CP. The NCGC pharmaceutical collection: a comprehensive resource of clinically approved drugs enabling repurposing and chemical genomics. Sci Transl Med. 3(80):80ps16. doi: 10.1126/scitranslmed.3001862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ICH. S2(R1). Guidance on Genotoxicity Testing and Data Interpretation for Pharmaceuticals Intended for Human Use. 2011 http://www.ich.org. [PubMed]
- Ji K, Kogame T, Choi K, Wang X, Lee J, Taniguchi Y, Takeda S. A novel approach using DNA-repair-deficient chicken DT40 cell lines for screening and characterizing the genotoxicity of environmental contaminants. Environ Health Perspect. 2009;117(11):1737–44. doi: 10.1289/ehp.0900842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson GE, Jenkins GJ, Thomas AD, Doak SH. Vinblastine and diethylstilboestrol tested in the in vitro mammalian cell micronucleus test (MNvit) at Swansea University UK in support of OECD draft Test Guideline 487. Mutat Res. 2010;702(2):189–92. doi: 10.1016/j.mrgentox.2009.07.014. [DOI] [PubMed] [Google Scholar]
- Levine AJ, Oren M. The first 30 years of p53: growing ever more complex. Nat Rev Cancer. 2009;9(10):749–58. doi: 10.1038/nrc2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Martinez JD. P53 is transported into the nucleus via an Hsf1-dependent nuclear localization mechanism. Mol Carcinog. 2011;50(2):143–52. doi: 10.1002/mc.20713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menendez D, Nguyen TA, Freudenberg JM, Mathew VJ, Anderson CW, Jothi R, Resnick MA. Diverse stresses dramatically alter genome-wide p53 binding and transactivation landscape in human cancer cells. Nucleic Acids Res. 2013;41(15):7286–301. doi: 10.1093/nar/gkt504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliore L, Cocchi L, Scarpato R. Detection of the centromere in micronuclei by fluorescence in situ hybridization: its application to the human lymphocyte micronucleus assay after treatment with four suspected aneugens. Mutagenesis. 1996;11(3):285–90. doi: 10.1093/mutage/11.3.285. [DOI] [PubMed] [Google Scholar]
- Nikolova T, Dvorak M, Jung F, Adam I, Krämer E, Gerhold-Ay A, Kaina B. The γH2AX assay for genotoxic and nongenotoxic agents: comparison of H2AX phosphorylation with cell death response. Toxicol Sci. 2014;140(1):103–17. doi: 10.1093/toxsci/kfu066. [DOI] [PubMed] [Google Scholar]
- Nishihara K, Huang R, Zhao J, Shahane SA, Witt KL, Smith-Roe SL, Tice RR, Takeda S, Xia M. Identification of genotoxic compounds using isogenic DNA repair deficient DT40 cell lines on a quantitative high throughput screening platform. Mutagenesis. 2016;31(1):69–81. doi: 10.1093/mutage/gev055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NTP (National Toxicology Program) [last accessed on (2/21/2017)];NTP Vision and Roadmap. 2016 available at https://ntp.niehs.nih.gov/about/vision/index.html.
- OECD. Test No. 471: Bacterial Reverse Mutation Test. OECD Publishing; Paris: 1997. http://dx.doi.org/10.1787/9789264071247-en. [Google Scholar]
- OECD. Test No. 474: Mammalian Erythrocyte Micronucleus Test. OECD Publishing; Paris: 2014. http://dx.doi.org/10.1787/9789264224292-en. [Google Scholar]
- Riley T, Sontag E, Chen P, Levine A. Transcriptional control of human p53-regulated genes. Nat Rev Mol Cell Biol. 2008;9(5):402–12. doi: 10.1038/nrm2395. [DOI] [PubMed] [Google Scholar]
- Sedykh A, Zhu H, Tang H, Zhang L, Richard A, Rusyn I, Tropsha A. Use of in vitro HTS-derived concentration-response data as biological descriptors improves the accuracy of QSAR models of in vivo toxicity. Environ Health Perspect. 2011;119(3):364–70. doi: 10.1289/ehp.1002476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelby MD, Tice RR, Witt KL. 17-beta-estradiol fails to induce micronuclei in the bone marrow cells of rodents. Mutat Res. 1997;395(1):89–90. doi: 10.1016/s1383-5718(97)00148-4. [DOI] [PubMed] [Google Scholar]
- Sohn TA, Bansal R, Su GH, Murphy KM, Kern SE. High-throughput measurement of the Tp53 response to anticancer drugs and random compounds using a stably integrated Tp53-responsive luciferase reporter. Carcinogenesis. 2002;23(6):949–57. doi: 10.1093/carcin/23.6.949. [DOI] [PubMed] [Google Scholar]
- Tice RR, Austin CP, Kavlock RJ, Bucher JR. Improving the human hazard characterization of chemicals: a Tox21 update. Environ Health Perspect. 2013;121(7):756–65. doi: 10.1289/ehp.1205784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto KN, Hirota K, Kono K, Takeda S, Sakamuru S, Xia M, Huang R, Austin CP, Witt KL, Tice RR. Characterization of environmental chemicals with potential for DNA damage using isogenic DNA repair-deficient chicken DT40 cell lines. Environ Mol Mutagen. 2011;52(7):547–61. doi: 10.1002/em.20656. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The concentration-response data can be downloaded and visualized in https://tripod.nih.gov/tox21/assays/ and https://ntp.niehs.nih.gov/sandbox/tox21-curve-visualization/. The activity data used in this study is available in the Supporting Information File.