Abstract
Purpose
Physical activity (PA) has been consistently associated with improved self-esteem in breast cancer survivors. However, this relationship is poorly understood. The purpose of this study was to examine whether changes in PA and self-efficacy influenced changes in self-esteem in breast cancer survivors across six-months. Increases in PA were hypothesized to result in increases in self-efficacy which were hypothesized to influence increases in physical self-worth and global self-esteem.
Methods
Breast cancer survivors (n=370; Mage = 56.04) wore accelerometers to measure PA and completed measures of self-efficacy (e.g., exercise and barriers self-efficacy), physical self-worth, and global self-esteem at baseline and 6 months.
Results
The hypothesized model provided a good fit to the data (χ2 =67.56, df = 26, p <.001; CFI = .98; SRMR = .05). Women with higher activity at baseline reported significantly higher levels of barrier (β = .29) and exercise (β = .23) self-efficacy. In turn, more efficacious women reported significantly higher physical self-worth (β = .26, .16). Finally, higher physical self-worth was significantly associated with greater global self-esteem (β = .47). Relationships were similar among changes in model constructs over 6 months. After controlling for covariates, the hypothesized model provided an excellent fit to the data (χ2 =59.93, df = 33, p =.003; CFI = .99; SRMR = .03).
Conclusion
Our findings provide support for the role played by PA and self-efficacy in positive self-esteem, a key component of well-being. Highlighting successful PA mastery experiences is likely to enhance self-efficacy and improve self-esteem in this population.
Keywords: cancer, oncology, physical activity, self-esteem, self-efficacy
Introduction
Increases in early detection combined with advances in medical care have led to a dramatic increase in the 5-year survival rate of breast cancer, which research suggests now ranges from 85–98% [1]. Consequently, there are currently over 2.8 million women living with a history of breast cancer in the U.S. alone [2], a figure that is expected to rise to 4 million by the year 2020 [3]. Breast cancer and its treatment are associated with a host of negative consequences ranging from increased risk of developing comorbid conditions to cancer recurrence [4, 5], making it imperative to maintain adequate health status in survivors. Self-esteem, defined as the overall affective evaluation of one’s worth or value [6, 7], is a primary health indicator in breast cancer survivors [8]. Following a breast cancer diagnosis, self-esteem often declines [9], which may be due in part to physical changes from surgery and chemotherapy including scarring, hair loss, and weight gain. However, this construct has been identified as an important factor in influencing health-related quality of life and well-being, allowing survivors to continue to thrive after diagnosis and treatment [8]. Thus, it is critical to maintain, if not improve, self-esteem in breast cancer survivors.
Self-esteem is a hierarchical, multidimensional construct with global self-esteem at its apex, undergirded by physical self-worth at the domain level of esteem [10–12]. The effects of physical activity on self-esteem are more likely to be stronger at the proximal domain level, making it important to examine self-esteem from this hierarchical perspective when examining changes in self-esteem in the context of physical activity research. Thus, any physical activity influence on global self-esteem is likely to be mediated by physical self-worth.
Physical activity is a lifestyle behavior that has consistently and significantly been associated with improvements in self-esteem in breast cancer survivors [13]. However, the potential mechanisms underlying this relationship are poorly understood. A number of studies in older adults and community dwelling adults have demonstrated that self-efficacy mediates the effects of physical activity on the components of self-esteem [14–16]. Using this perspective to further examine underlying factors in the physical activity-self-esteem relationship may be useful and relevant given that approximately 60% of breast cancer survivors are 65 years of age or older [17]. In the context of breast cancer survivors, Phillips and colleagues [18] found physical activity indirectly influenced quality of life through self-efficacy and other cancer-specific health status measures. The authors called for further investigation into other psychosocial constructs associated with health status, such as self-esteem. However, there has been limited examination of the role of self-efficacy in the association between physical activity and self-esteem in this cancer cohort.
Numerous studies have indicated that exercise training can increase self-esteem, although the measurement of this construct is typically conducted at the global level. For example, Courneya et al., [19] conducted a small randomized controlled exercise trial and reported that aerobic exercise training resulted in significant increases in global self-esteem, but these increases were unrelated to increases in cardiopulmonary function. Self-efficacy was not assessed. A cross-sectional study [20] found that physical self-efficacy mediated the physical activity-global self-esteem relationship. However, the measure used to assess self-efficacy has since been empirically demonstrated to be more representative of self-esteem than self-efficacy [21]. Musanti [22] compared the effects of several exercise modalities on multi-dimensional self-esteem in a small sample of breast cancer survivors and showed differential effects of resistance and aerobic training on components of self-esteem. However, no measures of self-efficacy were assessed in this study and the author stressed the need for subsequent examination of the physical activity-physical self-esteem association in breast cancer survivors to examine the potential mediating role of self-efficacy.
The purpose of the present study was to prospectively examine the relationship between physical activity and self-esteem in a large, geographically diverse sample of breast cancer survivors over a six-month period. Specifically, we hypothesized that changes in physical activity levels across time would be indirectly associated with changes in global self-esteem via changes in self-efficacy and physical self-worth. Changes in self-efficacy were hypothesized to have an indirect effect on changes in global self-esteem via changes in physical self-worth, and in turn, physical self-worth would have a direct effect on global self-esteem.
Materials and Methods
Study design details and participant information have been reported elsewhere [23]. Briefly, participants (n=1527) were breast cancer survivors recruited nationally through the Army of Women© who volunteered to participate in a 6-month on-line, prospective study of physical activity and well-being. Eligibility criteria included being at least 18 years of age, a past diagnosis of breast cancer, English-speaking, and access to a computer. For the present study, analyses included only those 486 women randomly selected to wear an accelerometer. Of this subsample, 370 (76%) provided full data at both time points. Sample characteristics are shown in Table 1. All procedures were approved by the University of Illinois Institutional Review Board, and informed consent was obtained from all individual participants included in the study.
Table 1.
Sample characteristics (n=486)
| Variable | Mean (SD) |
|---|---|
| Age (years) | 56.23 (9.35) |
| Race/Ethnicity % | |
| Nonwhite | 3.0% |
| Hispanic | 1.5% |
| ≥College Education % | 65.2% |
| Time since diagnosis % | |
| <5 years | 47.1% |
| 5 to <10 years | 28.9% |
| ≥10 years | 24.0% |
| Stage at diagnosis % | |
| 0 | 19.9% |
| I/II | 66.5% |
| III/IV | 13.6% |
| Total number of comorbidities % | |
| None | 28.9% |
| 1–2 | 43.9% |
| ≥ 3 | 27.2% |
| Menopausal Status at diagnosis | |
| No | 52.2% |
| Yes | 47.8% |
| Treatment Type | |
| Surgery + chemo + radiation | 38.3% |
| Surgery + chemo | 17.9% |
| Surgery + radiation | 26.7% |
| Surgery only | 15.4% |
| Other | 1.7% |
Measures
Demographics
Participants self-reported marital status, age, race, ethnicity, occupation, annual household income, and highest level of education.
Health & Cancer History
Participants indicated whether or not (yes or no) they had ever been diagnosed with 18 different comorbidities (i.e., diabetes, cardiovascular disease, obesity). Items with a positive response were summed to calculate total number of comorbidities. Information regarding breast cancer was also collected (i.e., time since diagnosis, stage of cancer, treatment type) and body mass index (BMI) was calculated using self-reported height and weight. Treatment type was categorized as follows: 1) surgery + chemotherapy + radiation; 2) surgery + chemotherapy; 3) surgery + radiation; 4) surgery only; 5) other.
Physical Activity
Physical activity was assessed using Actigraph accelerometers (Actigraph, Pensacola, FL: model GT1M or GT3X). Participants were instructed to wear the accelerometer on their non-dominant hip (i.e., if participants were right-handed, they would wear the accelerometer on their left hips) during waking hours and record the time worn on a log sheet. Data retained for analyses met a wear time validation criteria of ≥ 10 hours of wear time per day for at least 3 valid days when scored with an interruption period of 60 minutes [24]. These data were then downloaded as activity counts, which represent raw accelerations summed over a specific epoch length (e.g., 60 seconds) and subsequently processed into activity intensities in ActiLife software package (Version 6; Actigraph, Pensacola, FL) using adult-specific intensity (counts/min) cut-points as follows: sedentary (<100 counts/minute), light (100–759 counts/minute), moderate (1952–5724 counts/minute), vigorous (5725–9498 counts/minute), and very vigorous (≥9499 counts/minute) [25]. Moderate and vigorous intensity cut points were summed to create a measure of moderate to vigorous physical activity (MVPA). Each minute of wear time was classified according to these intensity cut-points. Estimated average daily minutes spent in each activity intensity category was calculated by dividing the number of minutes spent in each category by the total number of valid days worn per participant. For the analyses reported herein, variables for average MVPA per day at baseline and 6 months were used.
Self-Efficacy
Two measures of self-efficacy were used in this study. The Exercise Self-Efficacy Questionnaire (EXSE) [26] is designed to determine individuals’ beliefs in their capabilities to successfully complete 30+ minutes of exercise five times per week over the next 12 weeks. The second measure was the Barriers-Specific Self-Efficacy Questionnaire (BARSE) [27] which assessed individuals’ beliefs in their capabilities to successfully engage in exercise three times a week for 40+ minutes despite commonly reported barriers to participation. For each measure, participants were asked to indicate their confidence for engaging in the behavior on a 100-point percentage scale increasing in 10-point increments, ranging from 0% (not at all confident) to 100% (highly confident). Average confidence ratings were summed to yield a total efficacy score, with higher scores indicating greater self-efficacy. Internal consistencies were excellent (EXSE α = 0.99; BARSE α = 0.95).
Physical Self-Worth
Physical self-worth (PSW) was assessed using the Physical Self-Worth Subscale of the Physical Self-Perception Scale [28]. This instrument is designed to measure self-esteem relative to this specific domain in a hierarchical, multidimensional fashion. Participants were asked to indicate the degree to which each statement (e.g. “I feel confident in the physical side of myself”) is characteristic or true of them on a four-point scale, ranging from 1 (not at all true) to 4 (completely true). Negatively worded items are recoded, and then all items are summed resulting in a total esteem score. Higher scores are indicative of greater physical self-worth (range = 6–24). Internal consistency was excellent (α= 0.90).
Global Self-Esteem
Global self-esteem was measured using the Rosenberg Self-Esteem Scale [6]. Participants indicated their degree of agreement with each of the 10 statements (e.g. “On the whole I am satisfied with myself) on a five-point scale, ranging from 1 (strongly agree) to 5 (strongly disagree). Negatively worded items are recoded, and then all items are then summed to yield a total self-esteem score. Higher scores are indicative of greater overall self-esteem (range = 10–50). Once again, internal consistency was very good (α = 0.83).
Data Analysis
We conducted a panel analysis within a covariance modeling framework to test the hypothesized model. This is an appropriate approach for testing hypothesized, theoretically-based relationships among constructs across defined periods of time. The panel model has repeated observations of constructs and relationships among constructs across time periods, which allow the dynamics of relationship changes within a time series to be examined [29]. As an example, in our model in Figure 1, we can examine the relationship between physical activity and self-efficacy at time one (i.e., baseline) and then, with observations over time, we are able to determine the relationship between changes in physical activity and changes in self-efficacy that are independent of the baseline relationship and other variables in the model. Due to 24% missing data, we used the full-information maximum likelihood (FIML) estimator feature in the Mplus software program (Version 7.0) [30]. The FIML estimator provides both accurate parameter estimates and fit indices with simulated missing data [31, 32].
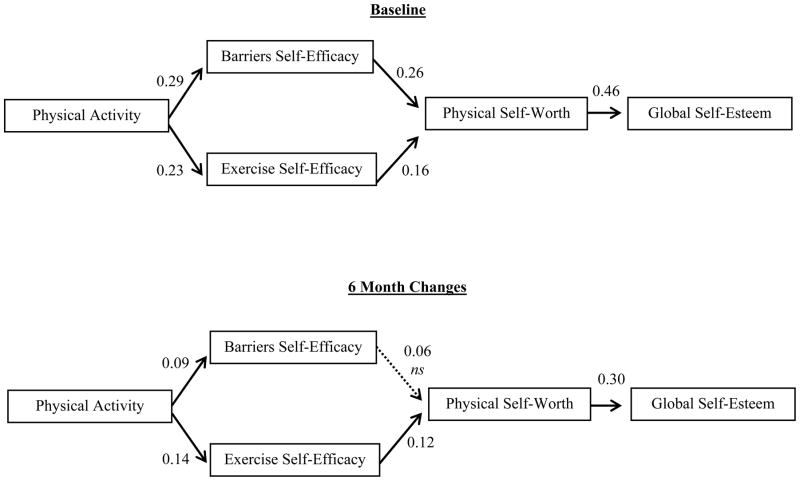
Figure 1.
Panel model testing hypothesized paths for baseline and 6 month follow-up. Coefficients reported herein are standardized estimates all are significant at p < .05 except where shown.
Model specification
Figure 1 shows the hypothesized panel model tested and included (1) paths from physical activity to exercise and barriers self-efficacy at both baseline and 6 months; (2) paths from self-efficacy to physical self-worth at both baseline and 6 months; and (3) paths from physical self-worth to global self-esteem at both baseline and 6 months. Models were initially tested without covariates and then reran controlling for age, income, education level, BMI, stage at diagnosis, number of comorbidities, time since diagnosis, treatment received, and menopausal status. As is common in panel analysis, stability coefficients [33] were also calculated. These coefficients represent correlations between the same variables (e.g., physical activity at baseline and 6 months) measured across time while controlling for the influence of other variables in the model. For the sake of clarity, we do not show these paths in the figures. Model fit was assessed using the chi-square statistic (χ2), standardized root mean residual (SRMR), and comparative fit index (CFI). We determined good model-data fit with SRMR values ≤ 0.08 and CFI ≥ 0.95, simultaneously [34, 35]. Additionally, modification indices were examined for potential reciprocal relationships as well as other relationships among model constructs.
Results
The hypothesized model provided a good fit to the data (χ2 =67.56, df = 26, p < .001; CFI = 0.98; SRMR = 0.05). Included in the model was a bidirectional correlation between barrier self-efficacy and exercise self-efficacy, as past work examining changes in self-efficacy over time suggests the need for this addition [36]. This model is shown in Figure 1. The top panel depicts the relationships between variables at baseline, while the bottom panel represents the relationships between changes in these variables over the 6 month time period controlling for baseline associations and stability among constructs across time. Overall, the stability coefficients were acceptable for physical activity (β=0.63), exercise and barriers self-efficacy (β=0.53; β=0.63), physical self-worth (β=0.66), and overall self-esteem (β=0.64).
Women with higher levels of physical activity at baseline reported significantly higher levels of self-efficacy (BARSE, β = 0.29, p<0.001; EXSE, β = 0.23, p<0.001). In turn, more efficacious women reported significantly greater levels of physical self-worth (BARSE, β = 0.26, p<0.001; EXSE, β = 0.16, p=0.004). Finally, women with greater reported levels of physical self-worth also reported significantly higher levels of global self-esteem (β = 0.46, p<0.001). Across time, increases in physical activity were associated with significant increases in barriers (β = 0.09, p=0.037) and exercise self-efficacy (β = 0.14, p=0.002) but only increases in exercise self-efficacy were associated with increases in physical self-worth (β = 0.12, p=0.018). Finally, increases in physical self-worth were associated with improvements in global self-esteem (β = 0.30, p<0.001).
The model was re-run to include the aforementioned covariates and continued to provide an excellent fit to the data (χ2 =63.00, df = 35, p =0.003; CFI = 0.99; SRMR = 0.03). Correlations between model constructs and covariates are depicted in Table 2. Relationships between model constructs at baseline and six months were unchanged with the addition of the covariates. Regarding the relationships between covariates and the model constructs, several interesting relationships emerged. At both time points, older women had significantly higher levels of global self-esteem (baseline, β = 0.14, p=0.007; 6 months, β = 0.12, p=0.023) and lower levels of physical activity (baseline, β = −0.23, p<0.001; 6 months, β = −0.19, p=0.001) compared to their younger counterparts. Additionally, women with greater BMI and more comorbidities had significantly lower levels of physical activity (ps<0.001for both), self-efficacy (ps<0.001 for both), physical self-worth (ps<0.001 for both) and global self-esteem (p=0.003 and 0.032 for BMI at baseline and follow-up, respectively and ps<0.001 for comorbidities) at both time points.
Table 2.
Correlations between constructs and covariates.
| Age | Income | Education | BMI | Stage At Diagnosis | Time Since Diagnosis | Total Comorbidities | Menopausal Status | Treatment Type | |
|---|---|---|---|---|---|---|---|---|---|
| Baseline | |||||||||
| AvgMVPA | −0.23** | 0.15** | 0.21** | −0.31** | −0.11* | 0.09 | −0.30** | −0.18** | −0.00 |
| EXSE | −0.04 | 0.16** | 0.10 | −0.34** | −0.14** | 0.03 | −0.33** | −0.03 | 0.06 |
| BARSE | 0.03 | −0.11* | 0.05 | −0.26** | −0.04 | 0.01 | −0.24** | 0.01 | 0.07 |
| PSW | 0.07 | 0.14** | 0.16** | −0.42** | −0.04 | 0.03 | −0.35** | 0.00 | −0.03 |
| GSE | 0.14** | 0.14** | 0.04 | −0.16** | −0.12* | 0.07 | −0.20** | −0.20** | 0.02 |
| 6 months | |||||||||
| AvgMVPA | −0.19** | 0.11* | 0.19** | −0.22** | −0.12* | 0.07 | −0.27** | −0.08 | 0.03 |
| EXSE | 0.03 | 0.07 | 0.12* | −0.29** | −0.13* | 0.09 | −0.32** | 0.03 | 0.09 |
| BARSE | 0.03 | 0.11 | 0.09 | −0.28** | −0.10 | 0.08 | −0.31** | 0.05 | 0.09 |
| PSW | 0.08 | 0.11 | 0.07 | −0.36** | −0.12* | 0.07 | −0.34** | 0.00 | 0.09 |
| GSE | 0.12* | 0.11* | 0.07 | −0.12* | −0.09 | 0.05 | −0.18** | 0.01 | 0.11* |
indicates significance at p<0.05;
indicates significance at p<0.01;
AvgMVPA, average daily minutes of moderate to vigorous physical activity; EXSE, exercise self-efficacy; BARSE, barrier self-efficacy; PSW, physical self-worth; GSE, global self-esteem; treatment type, 1=surgery + chemo + radiation, 2=surgery + chemo, 3=surgery + radiation, 4=surgery only, 5=other
Discussion
The purpose of this study was to examine how physical activity and self-efficacy influence changes in domain specific and global self-esteem across a six-month period in breast cancer survivors. In the present study, women with higher levels of physical activity engagement exhibited greater self-efficacy. In turn, more efficacious women reported higher levels of physical self-worth, and greater physical self-worth was associated with greater global self-esteem. These relationships held constant at the 6 month follow up period as well. However, changes in barrier self-efficacy were no longer significantly associated with changes in physical self-worth. For both time points, the hypothesized model provided an excellent fit to the data when controlling for age, income, education level, BMI, stage at diagnosis, number of comorbidities, and time since diagnosis.
Although numerous reports exist in the breast cancer literature documenting the physical activity and self-esteem relationship, this is the first study, to our knowledge, that has examined the role played by self-efficacy in mediating this relationship. Our findings suggest that this relationship might be better understood by examining more proximal and modifiable psychosocial factors such as self-efficacy. Greater levels of exercise self-efficacy and barrier self-efficacy were significantly associated with higher levels of physical self-worth, a finding consistent with past research within an exercise framework [16]. Additionally, the measures of self-efficacy used in this study have been validated and shown to be reliable for assessing self-efficacy in a multitude of populations, unlike past research examining the relationship between physical activity and self-efficacy for improving esteem in breast cancer survivors.
In the present study, we followed the recommendation of McAuley et al. [37] in that we included more than one measure of self-efficacy in an effort to determine the veracity of the proposed mediational role of this construct. Clearly, the exercise specific measure, rather than the barriers efficacy measure, does play an important mediational role both in a cross-sectional and longitudinal sense. Such findings suggest that physical activity programs and exercise training regimens designed for breast cancer survivors would do well to provide participants with as many successful physical activity mastery experiences as possible in an effort to enhance and maintain self-efficacy and thereby improve self-esteem. Although there is evidence to suggest that increases in self-efficacy brought about by exercise training influence multidimensional and hierarchical self-esteem in middle-aged and older adults [14–16], no such evidence exists in exercise trials of breast cancer survivors. Rather than considering esteem variables as simple indicators of quality of life improved by exercise participation in breast cancer survivors, it will be important to examine underlying psychosocial, biological, and physiological parameters that are changed by exercise training and may underlie the physical activity-self-esteem relationship. Future researchers would do well to examine such factors that may work independently or in concert with self-efficacy to better understand mechanisms for improving self-esteem, and ultimately quality of life, in breast cancer survivors. Additionally, further investigation into the mediating and moderating roles of such constructs is warranted.
While several exercise studies in breast cancer survivors have examined effects on self-esteem [19, 20], many have failed to measure self-esteem at the domain level (i.e. physical self-worth). Marsh and Sonstroem [38] have previously discussed the importance of focusing on physical self-esteem when interested in the potential effects of physical activity. Our findings support this perspective such that women with higher levels of physical activity displayed, indirectly through self-efficacy, higher levels of physical self-worth. Women with higher levels of physical self-worth then, in turn, exhibited higher levels of global self-esteem, further supporting the hierarchical nature of self-esteem where the domain level influences the global level. Future studies might include measures of subdomain levels on the esteem hierarchy (i.e. perceptions of physical strength, body attractiveness, physical condition) to extend the results found herein and further delineate how physical activity and self-efficacy further influence self-esteem.
This study has several strengths. First, a relatively large and geographically diverse subset of breast cancer survivors was sampled to further explore the poorly understood relationship between self-esteem and physical activity. Second, this study highlights the important role that self-efficacy plays in improving self-esteem in breast cancer survivors, and provides insight into this relationship that might drive future intervention designs. Third, we used a reliable, objective measure of physical activity, accelerometry. Fourth, we were able to study how changes in free-living physical activity influence changes in self-esteem across time in a longitudinal model. Finally, we believe this to be the first study, to our knowledge, that explored underlying psychosocial mechanisms to further understand the association between physical activity and self-esteem in breast cancer survivors.
There are a number of limitations that should be considered when interpreting the data reported herein. While this sample was geographically diverse and large in size, it was rather homogeneous and comprised mostly of Caucasian and healthy participants. Future studies might target more racially diverse survivors as well as those with compromised health to determine if the results found in this study can be replicated and/or magnified. Furthermore, this study was observational in nature, therefore future interventional trials are needed to determine if the same effects can be produced and maintained after a randomized, controlled exercise program.
In conclusion, these findings provide support for the role of self-efficacy in understanding the relationship between physical activity and self-esteem in breast cancer survivors. As medical advancements and screening methods progress and the population continues to age, the number of women diagnosed and surviving breast cancer will continue to increase making it imperative to maintain important health indicators in this cancer cohort. Self-esteem plays an important role in well-being and quality of life [8], yet is often negatively impacted after cancer diagnosis and treatment. Results from this study highlight the importance of physical activity, a low-cost behavior, for enhancing self-esteem in cancer survivors and lead us to be optimistic that self-esteem can be successfully targeted through self-efficacy for ultimately improving cancer survivorship.
Table 3.
Mean values (SD) for all model constructs at baseline and 6 months.
| Variable | Baseline | 6 months | Significance |
|---|---|---|---|
|
| |||
| M (SD) | M (SD) | p value | |
| Avg MVPA | 21.56 (18.59) | 19.12 (20.51) | <0.001** |
| EXSE | 74.94 (31.87) | 70.73 (33.26) | 0.008** |
| BARSE | 49.02 (23.53) | 48.67 (24.49) | 0.737 |
| PSW | 17.45 (4.52) | 17.47 (4.62) | 0.903 |
| GSE | 40.49 (5.97) | 40.54 (5.58) | 0.893 |
AvgMVPA, average daily minutes of moderate to vigorous physical activity; EXSE, exercise self-efficacy; BARSE, barrier self-efficacy; PSW, physical self-worth; GSE, global self-esteem
Acknowledgments
Funding: This work is supported by award #F31AG034025 from the National Institute on Aging awarded to Siobhan M. (White) Phillips as well as Ann Carlson Khan endowed professorship awarded to Edward McAuley who was also supported by grant #AG020118 from the National Institute on Aging.
This work is supported by award #F31AG034025 from the National Institute on Aging awarded to Siobhan M. (White) Phillips as well as Shahid and Ann Carlson Khan endowed professorship awarded to Edward McAuley who was also supported by grant #AG020118 from the National Institute on Aging.
Footnotes
Conflict of Interest: None of the authors have any potential conflicts of interest.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
References
- 1.Bray F, McCarron P, Parkin DM. The changing global patterns of female breast cancer incidence and mortality. Breast Cancer Res. 2004;6(6):229–39. doi: 10.1186/bcr932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch. Surveillance, Epidemiology, and End Results (SEER) Program Research Data (2004–2010) 2014. [Google Scholar]
- 3.DeSantis CE, Lin CC, Mariotto AB, et al. Cancer treatment and survivorship statistics. CA Cancer J Clin. 2014;64:252–271. doi: 10.3322/caac.21235. [DOI] [PubMed] [Google Scholar]
- 4.Hewitt M, Rowland JH, Yancik R. Cancer survivors in the United States: age, health, and disability. J Gerontol A Biol Sci Med Sci. 2003;58:82–91. doi: 10.1093/gerona/58.1.m82. [DOI] [PubMed] [Google Scholar]
- 5.Meadows AT, Friedman DL, Neglia JP, et al. Second neoplasms in survivors of childhood cancer: findings from the Childhood Cancer Survivor Study cohort. J Clin Oncol. 2009;27:2356–2362. doi: 10.1200/JCO.2008.21.1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosenberg M. Society and the adolescent self-image. Princeton, NJ: Princeton University Press; 1965. [Google Scholar]
- 7.Blascovich J, Tomaka J. Measures of self-esteem. Measures of personality and social psychological attitudes. 1991;1:115–160. [Google Scholar]
- 8.Mustian KM, Katula JA, Gill DL, Roscoe JA, Land D, Murphy K. Tai Chi Chuan, health-related quality of life and self-esteem: A randomized trial with breast cancer survivors. Supp Care Cancer. 2004;12:871–876. doi: 10.1007/s00520-004-0682-6. [DOI] [PubMed] [Google Scholar]
- 9.Courneya KS, Friedenreich CM. Physical exercise and quality of life following cancer diagnosis: a literature review. Ann Behav Med. 1999;21(2):171–179. doi: 10.1007/BF02908298. [DOI] [PubMed] [Google Scholar]
- 10.Shavelson RJ, Hubner JJ, Stanton GC. Self-concept: Validation of construct interpretations. Rev Edu Res. 1976:407–441. [Google Scholar]
- 11.Marsh HW, Shavelson R. Self-concept: Its multifaceted, hierarchical structure. Edu Psychol. 1985;20(3):107–123. [Google Scholar]
- 12.Fox KR. Self-esteem, self-perceptions and exercise. International Journal of Sport Psychology. 2000 [Google Scholar]
- 13.Speck RM, Courneya KS, Mâsse LC, Duval S, Schmitz KH. An update of controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. J Cancer Surviv. 2010;4(2):87–100. doi: 10.1007/s11764-009-0110-5. [DOI] [PubMed] [Google Scholar]
- 14.McAuley E, Blissmer B, Katula J, Duncan TE, Mihalko SL. Physical activity, self-esteem, and self-efficacy relationships in older adults: a randomized controlled trial. Ann Behav Med. 2000;22(2):131–139. doi: 10.1007/BF02895777. [DOI] [PubMed] [Google Scholar]
- 15.McAuley E, Mihalko SL, Bane SM. Exercise and self-esteem in middle-aged adults: multidimensional relationships and physical fitness and self-efficacy influences. J Behav Med. 1997;20(1):67–83. doi: 10.1023/a:1025591214100. [DOI] [PubMed] [Google Scholar]
- 16.Opdenacker J, Delecluse C, Boen F. The longitudinal effects of a lifestyle physical activity intervention and a structured exercise intervention on physical self-perceptions and self-esteem in older adults. J Sport Exerc Psychol. 2009;31(6):743. doi: 10.1123/jsep.31.6.743. [DOI] [PubMed] [Google Scholar]
- 17.Parry C, Kent EE, Mariotto AB, Alfano CM, Rowland JH. Cancer survivors: a booming population. Cancer Epidemiol Biomarkers Prev. 2011;20(10):1996–2005. doi: 10.1158/1055-9965.EPI-11-0729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Phillips SM, McAuley E. Physical activity and quality of life in breast cancer survivors: the role of self-efficacy and health status. Psychooncology. 2014;23(1):27–34. doi: 10.1002/pon.3366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Courneya KS, Mackey JR, Bell GJ, Jones LW, Field CJ, Fairey AS. Randomized controlled trial of exercise training in postmenopausal breast cancer survivors: cardiopulmonary and quality of life outcomes. J Clin Oncol. 2003;21(9):1660–1668. doi: 10.1200/JCO.2003.04.093. [DOI] [PubMed] [Google Scholar]
- 20.Baldwin MK, Courneya KS. Exercise and self-esteem in breast cancer survivors: An application of the exercise and self-esteem model. J Sport Exerc Psychol. 1997;19:347–358. [Google Scholar]
- 21.Hu L, McAuley E, Elavsky S. Does the physical self-efficacy scale assess self-efficacy or self-esteem. J Sport Exerc Psychol. 2005;27(2):152–170. [Google Scholar]
- 22.Musanti R. A study of exercise modality and physical self-esteem in breast cancer survivors. Med Sci Sports Exerc. 2012;44(2):352–361. doi: 10.1249/MSS.0b013e31822cb5f2. [DOI] [PubMed] [Google Scholar]
- 23.Phillips SM, McAuley E. Social cognitive influences on physical activity participation in long-term breast cancer survivors. Psychooncology. 2013;22(4):783–791. doi: 10.1002/pon.3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40(1):181–188. doi: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- 25.Freedson PS, Melanson E, Sirard J. Calibration of the Computer Science and Applications, Inc. accelerometer. Med Sci Sports Exerc. 1998;30(5):777–781. doi: 10.1097/00005768-199805000-00021. [DOI] [PubMed] [Google Scholar]
- 26.McAuley E, Lox C, Duncan TE. Long-term maintenance of exercise, self-efficacy, and physiological change in older adults. Series B: J Gerontol Ser B-Psychol Sci. 1993;48(4):218–224. doi: 10.1093/geronj/48.4.p218. [DOI] [PubMed] [Google Scholar]
- 27.McAuley E. The role of efficacy cognitions in the prediction of exercise behavior in middle-aged adults. J Behav Med. 1992;15:65–88. doi: 10.1007/BF00848378. [DOI] [PubMed] [Google Scholar]
- 28.Fox KR, Corbin CB. The physical self-perception profile: Development and preliminary validation. J Sport Exerc Psychol. 1989;11:408–430. [Google Scholar]
- 29.Halaby CN. Panel models in sociological research: Theory into practice. Annu Rev Sociol. 2004:507–544. [Google Scholar]
- 30.Muthén LK, Muthén BO. Mplus. The comprehensive modelling program for applied researchers: User’s guide. 2012;5 [Google Scholar]
- 31.Arbuckle JL. Full information estimation in the presence of incomplete data. Advanced structural equation modeling: Issues and techniques. 1996;243:277. [Google Scholar]
- 32.Enders CK, Bandalos DL. The relative performance of full information maximum likelihood estimation for missing data in structural equation models. Struct Equ Modeling. 2001;8(3):430–457. [Google Scholar]
- 33.Kessler RC, Greenberg DF. Linear panel analysis: Models of quantitative change. Academic Press; Ann Arbor, MI: 1981. [Google Scholar]
- 34.Hu LT, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Struct Equ Modeling. 1999;6(1):1–55. [Google Scholar]
- 35.Browne MW, Cudeck R. Alternative ways of assessing model fit. Sage Focus Editions. 1993;154:136–136. [Google Scholar]
- 36.McAuley E, Mailey EL, Mullen SP, Szabo AN, Wójcicki TR, White SM, et al. Growth trajectories of exercise self-efficacy in older adults: influence of measures and initial status. Health Psychol. 2011;30(1):75. doi: 10.1037/a0021567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McAuley E, Elavsky S, Motl RW, Konopack JF, Hu L, Marquez DX. Physical activity, self-efficacy, and self-esteem: Longitudinal relationships in older adults. Series B: J Gerontol Ser B-Psychol Sci. 2005;60(5):268–275. doi: 10.1093/geronb/60.5.p268. [DOI] [PubMed] [Google Scholar]
- 38.Marsh HW, Sonstroem RJ. Importance ratings and specific components of physical self-concept: Relevance to predicting global components of self-concept and exercise. Journal of Sport & Exercise Psychology. 1995 [Google Scholar]