Summary
Background
Fragmentation of collagen fibrils, the major structure protein in skin, is a hallmark of dermal aging. Matrix metalloproteinases (MMPs) are largely responsible for fragmentation of collagen fibrils. However, the alteration of all known mammalian MMPs and the mechanism underlying altered expression of MMPs in chronologically aged human skin are less understood.
Objectives
To quantify gene expression of all 23 known mammalian MMPs in sun-protected young and aged human skin in vivo, and investigate the potential mechanism underlying age-related alteration of multiple MMPs.
Methods
MMPs mRNA expression levels and MMPs activity in sun-protected young and aged human skin in vivo were determined by real-time RT-PCR and in situ zymography, respectively. The relative contributions to elevated MMPs in epidermis and dermis were quantified by laser capture microdissection (LCM) coupled real-time RT-PCR. Dermal fibroblast morphology and collagen fibrils fragmentation in human skin in vivo were assessed by second harmonic generation microscopy and atomic force microscopy, respectively. In vitro cell morphology was assessed by CellTracker® fluorescent dye and Phalloidin staining. Protein levels were determined by ProteinSimple capillary electrophoresis immunoassay.
Results
Among all 23 known mammalian MMPs, multiple MMPs are elevated in aged human skin dermis. Consistent with this finding, increased MMPs activity and collagen fibrils fragmentation were observed in aged skin dermis. As dermal fibroblasts are the major MMPs producing cells in the dermis, reduction of dermal fibroblast size, which is observed in aged human skin, contributes to elevation of age-related multiple MMPs. Reduction of fibroblast size up-regulates c-Jun/c-Fos and activates AP-1, the major regulator of multiple MMPs.
Conclusions
Combined actions of the wide variety of MMPs that are constitutively elevated in aged dermis may be involved in progressive degradation of dermal collagen fibrils. Age-related elevations of multiple MMPs are likely resulted from the reduction of fibroblast size via activation of AP-1.
Keywords: matrix metalloproteinases, skin aging, fibroblast, cell size, AP-1
Introduction
Collagen is the most abundant protein in mammals, making up about one-third of the whole-body protein content 1. In human skin, collagen is the main component of the dermis that provides structural and functional support of the skin 2. During aging, dermal collagen fibrils undergo progressive alterations due to age-related fragmentation and disorganization of collagen fibrils 3,4. Fragmentation of collagen fibrils is essentially responsible for aged-appearing skin, the most visible signs of aging such as wrinkles and fragile atrophic skin. Alterations of dermal collagen impair skin structural integrity and are associated with age-related disorders, such as increased fragility 5, impaired vasculature support 6,7, poor wound healing 8,9, and promotion of skin cancer 10,11.
In human skin, dermal fibroblasts are responsible for collagen homeostasis 12. Dermal fibroblasts are embedded in a collagen-rich microenvironment and physically interact with collagen fibrils to maintain normal cell shape and size. Accumulating evidence demonstrates that cell shape and size regulate essential cell functions 13,14. Cell shape and size are largely regulated by interactions of cells with surrounding extracellular matrix (ECM). The ECM provides both binding sites for cells and mechanical resistance to cellular traction forces 14,15. In young healthy skin, dermal fibroblasts interact with intact collagen fibrils; and exert traction forces to achieve normal cell shape and size. However, in aged dermis collagen fibrils are fragmented, which impairs fibroblast-collagen interaction and results in reduced fibroblast spreading, shape and size 3,16,17. While cell shape and size are known to regulate many cellular functions, the molecular basis of their impact on dermal fibroblast function and dermal aging are not well understood.
Matrix metalloproteinases (MMPs) are a large family of proteinases that are essentially responsible for fragmentation of collagen fibrils 18,19. We previously reported that dermal fibroblasts in aged human skin expresses elevated MMP-1, which leads to initiation of collagen fibril fragmentation 3,20. However, the complete profile of all 23 known mammalian MMPs in aged human skin is unknown. Here we investigated the expression of all 23 known mammalian MMPs in sun-protected young and aged human skin in vivo. We found that multiple MMPs are constitutively elevated in aged human skin dermis. As reduction of fibroblast size is a prominent feature of aged human skin 3,16,17, we found that reduction of fibroblast size activates transcription factor AP-1, the major regulator of multiple MMPs 21-23, leading to up-regulation of multiple age-related MMPs observed in aged human skin. Our data suggest insights into mechanisms of collagen fibrils fragmentation, which is a defining feature of the pathophysiology of human dermal aging.
Methods
Procurement of human skin samples and laser capture microdissection (LCM)
All procedures involving human subjects were approved by the University of Michigan Institutional Review Board, and all subjects provided written informed consent. Sun-protected buttock human skin punch biopsies were obtained from clinically normal adult volunteers; 25-30 years for young group (N=12, mean age 26±3 years) males and 80+ years for aged group (N=12, mean age 83±4 years). Skin samples were 4mm in diameter, full thickness skin. To quantify the relative levels of epidermal and dermal MMPs, epidermis and dermis were captured by laser capture microdissection (LCM)on (15μm), and were collected in lysis buffer (RNeasy Micro kit, Qiagen), followed by RNA extraction and RT-PCR, as described below.
Cell culture and three-dimensional collagen lattice cell cultures
Young (21-30 years) and aged (>80 years) human skin primary dermal fibroblasts were isolated from punch biopsies of sun-protected buttock skin by digestion skin with bacterial collagenase (Worthington Biochemical Corporation, Lakewood, NJ). Cells were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum (Invitrogen, Carlsbad, CA), penicillin (100 U/ml) and streptomycin (100 μg/ml) in a humidified incubator with 5% CO2 at 37°C. Cells were utilized between passages 4 and 9, and replicate experiments used fibroblasts from different donors. In some cases, dermal fibroblasts were cultured in three-dimensional collagen lattices, based on previous publication with minor modification17. Briefly, neutralized collagen lattices were prepared by mixing appropriate volume of rat tail type I collagen (BD Biosciences) with medium cocktail [DMEM, NaHCO3 (44mmol/L), l-glutamine (4 mmol/L), folic acid (9 mmol/L), and neutralized with 1 N NaOH to pH 7.2] to yield a final concentration of 2mg/ml. 0.5×106 cells were suspended in 2 ml collagen solution in 35mm culture dish, and placed in an incubator at 37°C for 30 minutes to allow polymerization of the collagen. For the constrained culture system, cells were cultured in collagen gel in which a nylon mesh (0.5 mm pore size) was placed on the bottom of culture dish to provide cytoskeletal stability and tension. For the unconstrained culture system, cells were cultured in collagen lattices without nylon mesh. The collagen gels were then incubated with 2 ml media (DMEM, 10% FBS) at 37°C, 5% CO2. For analyses, cells were harvested by digesting collagen gel with bacterial collagenase (1 mg/ml, Sigma, St. Louis, MO, USA) for 30 minutes at 37°C. Fibroblasts were collected by centrifugation and recovery of viable fibroblasts (>80%) was confirmed by trypan blue staining. For latrunculin-A (Lat-A) treatment, cells were treated with Lat-A at a concentration of 30 nM for 24 hours or the indicated times.
RNA isolation, laser capture microdisection, quantitative real-time RT-PCR
Total RNA was extracted, using a commercial kit (RNeasy midikit, Qiagen, Chatsworth, CA), and reverse transcription was performed using Taqman Reverse Transcription kit (Applied Biosystems, Foster City, CA). Real-time RT-PCR was performed using a Taqman Universal PCR Master Mix kit (Applied Biosystems, Foster City, CA) and 7300 Real-Time PCR System (Applied Biosystems, Foster City, CA). PCR procedures were performed using a robotic workstation (Biomek 2000; Beckman Coulter, Inc., Hialeah, FL) to ensure accuracy and reproducibility. All primers and probes were purchased from Applied Biosystems (Assays-on-Demand™ Gene Expression Products). Multiplex PCR reactions contained primers and probes for the target gene and 36B4, a ribosomal protein used as an internal normalization control for quantitation.
ProteinSimple capillary electrophoresis immunoassay
Dermal fibroblasts were lysed in whole cell extraction buffer (25mM HEPES [pH 7.7]; 0.3 M NaCl; 1.5 mM MgCl2; 0.2 mM EDTA; 0.1% Triton X-100; 0.5 mM DTT' 20 mM β-glycerolphosphate; 0.1 mM Na3VO4; 2 μg/ml leupeptin; and 100 μg/ml PMSF). Whole cells extract was prepared by centrifugation and protein concentration was determined by the Bio-Rad protein assay (Bio-Rad laboratories, Hercules, CA, USA). ProteinSimple capillary electrophoresis immunoassay was performed according to the ProteinSimple user manual. In brief, whole cell extract samples (800 ng/lane) were mixed with a master mix (ProteinSimple) to a final concentration of 1× sample buffer, 1× fluorescent molecular weight markers, and 40 mM dithiothreitol (DTT) and then heated at 95 °C for 5 min. The samples, blocking reagent, primary antibodies, HRP-conjugated secondary antibodies, chemiluminescent substrate, and separation and stacking matrices were also dispensed to designated wells plate. The electrophoresis and immunodetection steps took place in the capillary system (ProteinSimple Wes, ProteinSimple, Santa Clare, CA, USA) and were fully automated with instrument default settings. The digital image was analyzed and quantified with Compass software (ProteinSimple, Santa Clare, CA, USA) after normalization by and β-actin (loading control).
CellTracker, Phalloidin staining, Immunohistology, and second harmonic generation microscopy
Cell morphology in 3D collagen lattices was assessed by incubation of cultures with CellTracker® fluorescent dye (Molecular Probes, Eugene, OR, USA) for one hour. The cells were washed with PBS and were fixed in 2% paraformaldehyde for 30 minutes. Cell morphology in monolayer culture was assessed by Phalloidin staining. Cells were washed with PBS and were fixed in 2% paraformaldehyde for 30 minutes followed by Phalloidin stain (Sigma, St. Louis, MO, USA) for one hour. Images were obtained using Zeiss fluorescence microscopy. Second harmonic generation microscopy was performed using a Leica SP8 Confocal Microscope with 2-Photon, at University of Michigan Microscopy and Image Analysis Laboratory.
Transfection AP-1 reporter activity assay
Primary adult human dermal fibroblasts were transiently transfected with AP-1 reporter construct (pAP1-TA-Luc) purchased from BD Biosciences Clontech (Palo Alto, CA). Cells were transfected by electroporation using human dermal fibroblasts nucleofector kit (Amaxa Biosystems, Gaithersburg, MD) according to the manufacturer's protocol. After 48 hours of transfection, AP-1 luciferase activity was measured by luciferase assay using an enhanced luciferase assay kit (PharMingen International, San Diego, CA) according to the manufacturer's protocol. β-galactosidase expression vector was used as an internal control for transfection efficiency and aliquots containing identical β-galactosidase activity were used for each luciferase assay.
Zymography
In situ zymography was performed as described previously 24. Briefly, frozen skin sections were placed on glass slides coated with fluorescently-labeled collagen (Elastin products, Owensville, MO) as substrate. Skin sections were incubated for 24 hours to allow MMPs in the tissue to degrade the fluorescein-labeled collagen in the slide.
Atomic force microscopy (AFM) imaging
OCT embedded human skin samples were sectioned (50 μm) and mounted on glass coverslips (1.2 mm diameter, Fisher Scientific Co., Pittsburgh, PA). These AFM samples were allowed to air dry for at least 24 hours before AFM analysis25. The scan positions of the collagen lattices were determined by light optical image. Images were obtained by AFM with ScanAsyst mode (Dimension Icon, Bruker-AXS, Santa Barbara, CA) in air using a silicon etched cantilever (NSC15/AIBS, MikroMasch, San Jose, CA) with a full tip cone angle ∼40° and the tip radius of curvature ∼10nm. AFM images were acquired at a scan rate of 0.977 Hz, 512×512 pixel resolutions. AFM imaging was conducted at the Electron Microbeam Analysis Laboratory (EMAL), University of Michigan College of Engineering, and analyzed using Nanoscope Analysis software (Nanoscope Analysis v120R1sr3, Bruker-AXS, Santa Barbara, CA).
Statistical analysis
Statistical significance between groups was determined with the Student's t-test. All p-values are two-tailed and considered significant when p < 0.05.
Results
Elevation of multiple MMPs in aged human skin dermis in vivo
Measurement of all 23 known mammalian MMPs indicated that eight MMPs were significantly elevated in sun-protected (buttock) aged (83±4 years) human skin, compared to sun-protected young (26±3 years) skin (relative fold-increase from high to low: MMP-10, MMP-1, MMP-9, MMP-27, MMP-24, MMP-11, MMP-3, MMP-23) (Fig 1A). MMPs and tissue inhibitors of metalloproteinases (TIMPs) are often coordinately regulated as a means to control excess MMP activity, we also investigated whether TIMPs are elevated in aged skin. However, no differences in mRNA levels of all four known tissue inhibitors of metalloproteinases (TIMPs) were found between young and aged human skin (Fig 1B). To quantify the relative contributions to elevated MMPs, epidermis and dermis were separated by laser capture microdissection (LCM) (Fig 1C). Figure 1D shows that all MMPs that are elevated in aged skin were primarily expressed in the dermis (MMP-10, 88%; MMP-1, 78%; MMP-9, 70%; MMP-27, 91%; MMP-24, 91%; MMP-11, 92%; MMP-3, 84%; MMP-23, 81%). To access MMPs activity, we performed in situ zymography, in which unfixed skin sections are placed over a layer of fluorescently-labeled collagen. As shown in Fig 1E, elevated MMPs activity in aged skin resulted in breakdown of the collagen, resulting in loss of fluorescence. These data demonstrate that multiple MMPs are elevated in sun-protected aged human skin dermis, suggesting that the combined actions of the wide variety of MMPs may contribute to degradation of dermal collagen fibrils.
Figure 1. Elevated expression of multiple MMPs in aged human dermis.
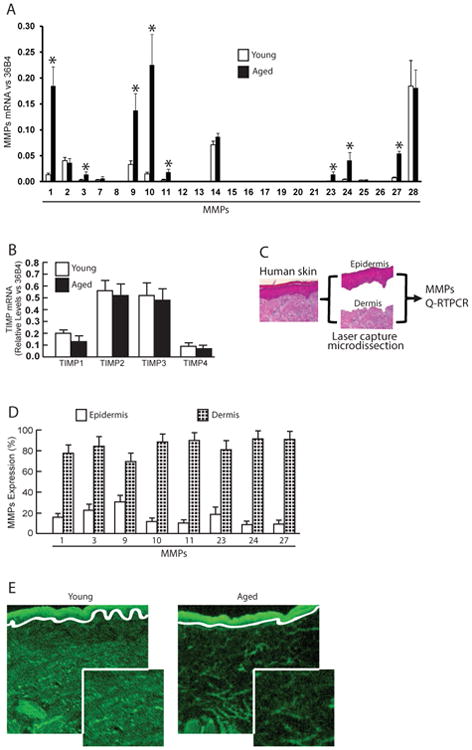
Adult human skin punch biopsies were obtained from sun-protected buttock skin. (A) Elevated multiple MMPs in aged (83±4 years) compared to young (26±3 years) human skin. N=12 each group. Mean ± SEM. *p<0.05. (B) No change in TIMP mRNA expression in young and aged human skin. N=12 each group. Mean ± SEM. (C) Schematic representation of the dissection of human skin epidermis and dermis by Laser Capture Microdissection (LCM, see Methods for details). (D) Elevated MMPs in the dermis of aged human skin. Epidermis and dermis were captured by LCM and total RNA was prepared form epidermis and dermis. N=8, Mean ± SEM. All MMPs mRNA levels were quantified by real-time RT-PCR and were normalized by the housekeeping gene (36B4, internal control). (E) Elevated collagenase activity in the dermis of aged human skin determined by in situ zymography (see Methods for details). Loss of green fluorescence in aged dermis indicates degradation of fluorescein-collagen substrate. White lines indicate boundary between the epidermis (top) and dermis (bottom). N=6.
Age-related reduction of dermal fibroblast size up-regulates multiple MMPs observed in aged human skin
Next, we investigated the potential mechanisms of age-related elevation of multiple MMPs using primary dermal fibroblasts, since dermal fibroblasts are major MMPs producing cells in the dermis and primarily responsible for collagen turnover. To this end, we first isolated the dermal fibroblasts from sun-protected young (mean age 26±3 years) and aged (mean age 83±4 years) human skin and examined the mRNA expression of all known mammalian MMPs in standard monolayer culture. Interestingly, we found that all 23 MMPs are similarly expressed in the fibroblasts isolated from young and aged skin (Fig 2A). These data suggest that age-related elevation of multiple MMPs might be induced by epigenetics rather than intrinsic genetic alteration.
Figure 2.
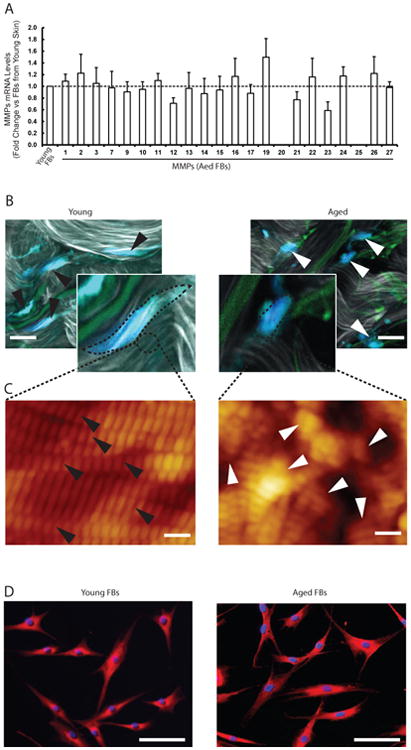
(A) MMPs are similarly expressed in the fibroblasts isolated from aged (83±4 years) and young (26±3 years) skin. Dermal fibroblasts were isolated from sun-protected young and aged buttock skin. MMPs mRNA expression levels were quantified by real-time RT-PCR and were normalized by the housekeeping gene (36B4, internal control). N=6. Mean ± SEM. (B) Reduced dermal fibroblast size is a prominent feature of aged dermal fibroblasts in human skin in vivo. Representative images of dermal fibroblasts in aged (78 years, right panel) and young (26 years, left panel) human skin. Skin was sectioned, and dermal fibroblasts were identified by immunostaining with collagen chaperone heat shock protein 47 (blue). Gray/white color is collagen fibrils and green color is elastin. Note spread fibroblasts in young skin versus contracted fibroblasts in aged human skin. Images were obtained by multiphoton laser scanning fluorescence microscopy. N=6 for each group. The black and white arrow heads indicate dermal fibroblasts in young and aged human skin, respectively Bars=25μm. (C) Collagen fibrils in aged skin dermis were fragmented and disorganized. Nanoscale collagen fibrils were imaged by atomic force microscopy. The black and white arrow heads indicate intact and fragmented/disorganized collagen fibrils, respectively. Images are representative of six independent experiments. Bars=100 nm (D) The morphology of the dermal fibroblasts from young (left panel) and aged (right panel) were similar in standard monolayer culture in vitro. Cells were stained with CellTracker® fluorescent dye and were imaged by fluorescence microscopy. Red fluorescence delineates cell cytoplasm; blue fluorescence delineates nuclei. Bars=100 μm. Images are representative of the dermal fibroblasts from five young and aged individuals.
We previously reported that a prominent characteristic of aged dermal fibroblasts in human skin in vivo is reduced size; with decreased elongation and a more rounded, collapsed morphology due to collagen fragmentation 3,16,17. These data prompted us to explore the connection between reduced fibroblast size and age-related elevation of multiple MMPs. Using second harmonic generation microscopy, we confirmed that the fibroblasts in aged dermis revealed reduced size and collapsed morphology (Fig 2B, upper right panel), compared to elongated and stretched fibroblasts from young skin dermis (Fig 2B, upper left panel). Atomic force microscopy indicated that collagen fibrils in young skin dermis were intact, tightly packed and well organized (Fig 2C, lower left panel). In contrast, collagen fibrils in aged skin dermis were fragmented, sparse and disorganized (Fig 2C, lower right panel), suggesting that fragmented and disorganized collagen fibrils appear to be unable to support normal fibroblasts morphology. Consequently, we confirmed that the morphology of the isolated dermal fibroblasts from young (Fig 2D, left panel) and aged (Fig 2D, right panel) were similar in standard monolayer culture in vitro, suggesting that dermal ECM microenvironment is largely responsible for fibroblasts shape and size.
Based on above observations, we investigated the relationship between reduced fibroblast size and age-related elevation of multiple MMPs using young dermal fibroblasts (mean age 26±3 years). First, we modulated dermal fibroblast size by culturing young dermal fibroblasts in three dimensional (3D) collagen lattices, under constrained and unconstrained 3D collagen lattices to model young and aged dermal fibroblasts, respectively. A red fluorescent dye (Cell Tracker), which is taken up into the cell cytoplasm was used to assess the morphology of fibroblasts in 3D collagen lattices. Dermal fibroblasts cultured in constrained collagen lattices displayed spread flattened appearance (Fig. 3A, left). In contrast, fibroblasts in unconstrained collagen gels, had reduced cytoplasmic area, and a contracted appearance (Fig 3B, right), similar to fibroblasts in aged human skin in vivo (Fig 2B, upper right panel). Quantification indicated that fibroblast size was reduced by approximately 50% in unconstrained collagen gel (Fig 3A, right panel). Interestingly, measurement of all 23 mammalian MMPs indicated that the majority MMPs that were found to be elevated in aged dermis in vivo, except MMP-23 and MMP-24, were elevated by reduction of fibroblasts size (Fig 3B). To further confirm this fibroblast-size-dependent elevation of MMPs, we modulated young dermal fibroblasts size by disrupting the actin cytoskeleton with latrunculin-A (Lat-A), which rapidly blocks actin polymerization 26. As expected, disruption of the actin cytoskeleton resulted in loss of actin cytoskeletal fibers and reduced fibroblasts size approximately 70% (Fig 3C, right panel). Importantly, reduction of fibroblast size resulted in elevation of multiple MMPs that were found to be elevated in aged dermis in vivo (Fig. 2E). As shown in Table 1, reduction of fibroblasts size either in 3D collagen lattices or in monolayer culture resulted in elevation of the majority MMPs that were elevated in aged dermis in vivo, except MMP-23 and MMP-24.
Figure 3. Age-related reduction of dermal fibroblast size up-regulates multiple MMPs observed in aged human skin.
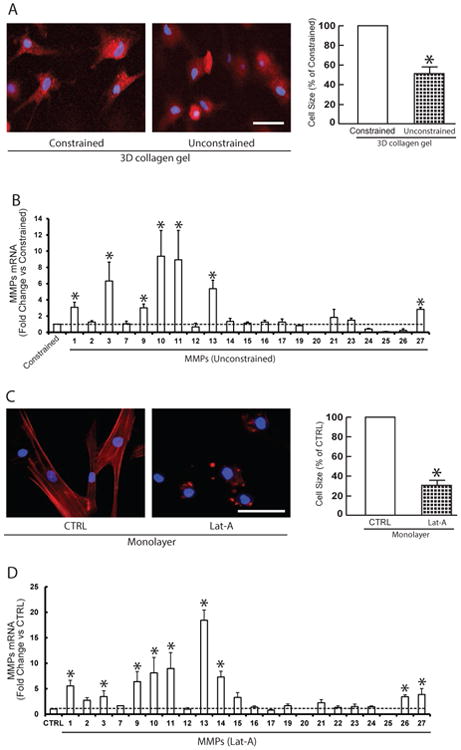
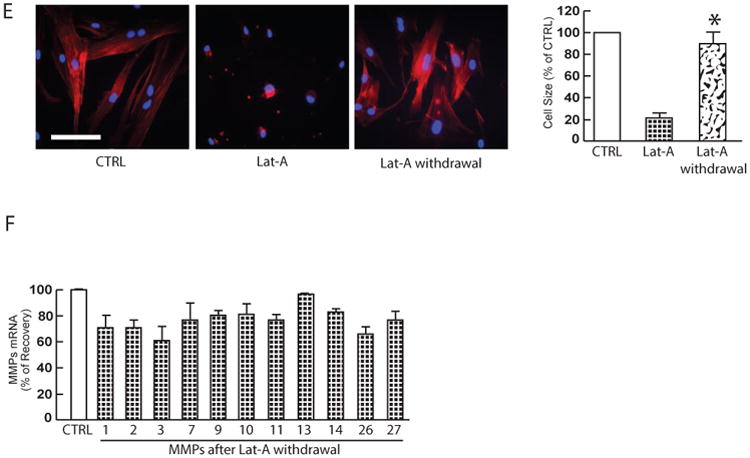
(A) To model young and aged dermal fibroblasts, dermal fibroblasts from young skin (26±3 years) were cultured under conditions of constrained (left panel) and unconstrained (right panel) 3D collagen lattices (see Methods for details). Cells were stained with CellTracker® fluorescent dye and were imaged by fluorescence microscopy. Red fluorescence delineates cell cytoplasm; blue fluorescence delineates nuclei. Relative cell surface areas were quantified using ImageJ software and data were expressed as % of control (non-contracted gel). Mean ± SEM, N =3, *p<0.05. Bars = 100 μm (B) The majority age-related MMPs were elevated by reduction of fibroblasts size. Total RNA was extracted from dermal fibroblasts cultured from 3D collagen lattices. MMPs mRNA expression levels were quantified by real-time RT-PCR and were normalized by the housekeeping gene (36B4, internal control). N=4. Mean ± SEM. *p<0.05. (C) Dermal fibroblasts from young skin (26±3 years) were treated with Lat-A (30 nM) for 24 hours. Dermal fibroblasts were stained with phalloidin and were imaged by fluorescence microscopy. Red fluorescence delineates cell cytoplasm; blue fluorescence delineates nuclei. Relative cell surface areas were quantified using ImageJ software and data were expressed as % of control (DMSO). Mean ± SEM, N=6, *p<0.05. Bars = 100 μm. (D) The majority age-related MMPs were elevated by reduction of fibroblast size via disruption of the actin cytoskeleton. Total RNA was extracted from dermal fibroblasts. MMPs mRNA expression levels were quantified by real-time RT-PCR and were normalized by the housekeeping gene (36B4, internal control). N=5. Mean ± SEM. *p<0.05. (E) Restoration of dermal fibroblast size after withdrawn Lat-A. Lat-A was withdrawn (right panel) 24 hours after Lat-A (30 nM) treatment (middle panel) by replacing with fresh culture medium, and the cells were further incubated for 48 hours. Dermal fibroblasts were stained with phalloidin and relative cell surface areas were quantified using ImageJ software. Data were expressed as % of control (DMSO, left panel). Mean ± SEM, N=6, *p<0.05. Bars = 100 μm. (F) Restoration of dermal fibroblast size reverses elevated MMPs. MMPs mRNA expression levels were quantified by real-time RT-PCR and were normalized by the housekeeping gene (36B4, internal control). MMPs mRNA expression levels after Lat-A withdrawn were expressed as % of control cells (DMSO). N=5. Mean ± SEM.
Table 1. Age-related reduction of dermal fibroblast size up-regulates multiple MMPs as observed in aged human skin Reduced Cell Size.
| Aged Skin | 3D Collagen Gel | Monolayer | |
|---|---|---|---|
| MMP-1 | + | + | + |
| MMP-2 | |||
| MMP-3 | + | + | + |
| MMP-7 | |||
| MMP-8 | |||
| MMP-9 | + | + | + |
| MMP-10 | + | + | + |
| MMP-11 | + | + | + |
| MMP-12 | |||
| MMP-13 | + | + | |
| MMP-14 | + | ||
| MMP-15 | |||
| MMP-16 | |||
| MMP-17 | |||
| MMP-18 | |||
| MMP-19 | |||
| MMP-20 | |||
| MMP-21 | |||
| MMP-22 | |||
| MMP-23 | + | ||
| MMP-24 | + | ||
| MMP-25 | |||
| MMP-26 | + | ||
| MMP-27 | + | + | + |
| MMP-28 |
Next, we assessed whether elevated MMPs are reversible by restoration of fibroblast size. For these studies, Lat-A-containing media was withdrawn 24 hours after its addition, and the cells were extensively washed, and fresh culture media was added. With removal of Lat-A, fibroblasts converted from a small, rounded morphology (Fig. 3E middle panel) to typical elongated morphology (Fig. 3E, right panel). Consistent with recovery of cellular size, elevated MMPs were normalized to basal level (Fig. 3F). These data suggest that reduced fibroblast size as observed in aged human skin likely contributes to age-related elevation of multiple MMPs.
Reduced fibroblast size up-regulates c-Jun/c-Fos and activates AP-1, the major regulator of multiple MMPs
We next investigated the mechanism by which reduced fibroblast size up-regulates multiple MMPs expression. We focused on transcription factors c-Jun and c-Fos, which typically comprise the AP-1 transcription factor complex. AP-1 transcription factor has been shown to principal regulator of multiple MMPs transcription, under a variety of conditions21,22,27. As shown in Figure 4, reduction of fibroblast size in either 3D collagen lattices (non-contraction vs contraction) or monolayer culture (DMSO vs latrunculin-A) resulted in significant increase of c-Jun mRNA (Fig 4A) and protein (Fig 4B), and c-Fos mRNA (Fig 4C) and protein (Fig 4D). Furthermore, reduction of fibroblast size markedly increased AP-1 reporter activities (Fig 4E). Finally, we asked that whether c-Jun and c-Fos expression are elevated in aged human skin dermis in vivo, the major source of elevated multiple MMPs. To address this question, the dermis was prepared by cutting off epidermis at a depth of 1 mm by cryostat. Indeed, both c-Jun and c-Fos mRNA (Fig 5A) and protein (Fig 5B) levels were elevated in aged dermis, compared to young dermis. These data suggest that reduction of fibroblast size may elevate multiple MMPs via up-regulation of c-Jun/c-Fos and activation of AP-1, the major regulator of multiple MMPs.
Figure 4. Reduced fibroblast size up-regulates c-Jun/c-Fos and activates AP-1, the major regulator of multiple MMPs.
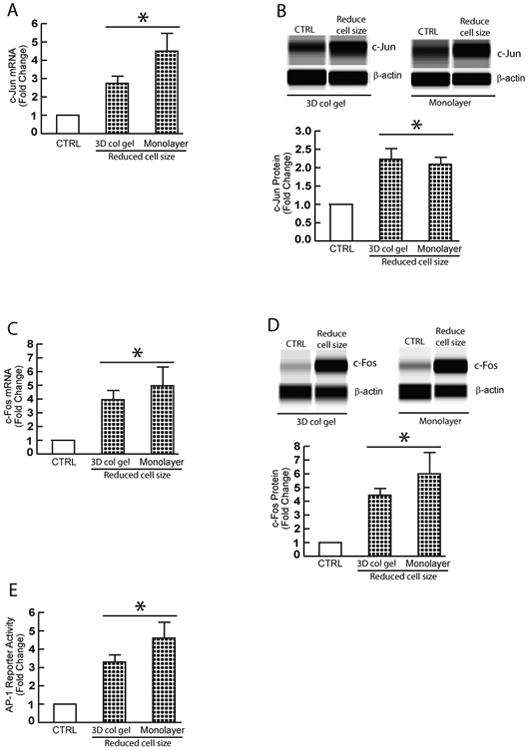
Dermal fibroblasts from young skin (26±3 years) were cultured in 3D collagen lattices or monolayer under conditions of reduced cell size, as described in Method. (A) Reduction of fibroblast size upregulates c-Jun mRNA expression. (B) Reduction of fibroblast size upregulates c-Jun protein expression. (C) Reduction of fibroblast size upregulates c-Fos mRNA expression. (D) Reduction of fibroblast size upregulates c-Fos protein expression. mRNA levels were quantified by real-time RT-PCR and were normalized by the housekeeping gene (36B4, internal control). Mean ± SEM, N=3, *p<0.05. Protein levels were determined by ProteinSimple capillary electrophoresis immunoassay (see Materials and methods for details) and normalized by β-actin (loading control). Insert shows representative digital images. Mean ± SEM, N=3, *p<0.05. (E) Dermal fibroblasts were transfected with AP-1 reporter construct (pAP-1-TA-Luc) or empty vector (CTRL). Cell lysates were prepared 48 hours after transfection. Reporter activity was determined by luciferase assay. Data are Mean ± SEM, N=3, *p<0.05.
Figure 5. Elevated expression of c-Jun and c-Fos expression in aged human skin dermis.
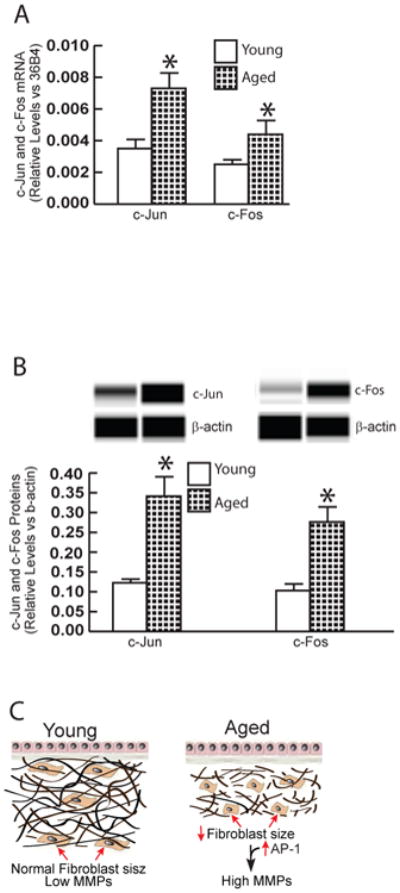
Adult human skin punch biopsies were obtained from young (26±3 years) and aged (83±4 years) sun-protected buttock skin. Dermis was prepared by cutting off epidermis at a depth of 1 mm by cryostat. (A) Total RNA was prepared from dermis and mRNA levels were quantified by real-time RT-PCR and were normalized by the housekeeping gene (36B4, internal control). Mean ± SEM, N=6, *p<0.05. (B) Dermal protein levels were determined by ProteinSimple capillary electrophoresis immunoassay (see Methods for details) and normalized by β-actin (loading control). Insert shows representative digital images. Mean ± SEM, N=6, *p<0.05. (C) Reduced fibroblast size elevates age-related MMPs (see details in Discussion).
Discussion
MMPs are primarily responsible for fragmentation of dermal collagen fibrils, the hallmark of dermal aging 3,4,12. MMPs are a large family of proteinases that are capable of degrading every type of ECM protein 18. We demonstrate here that multiple MMPs become elevated during the aging process in human skin dermis. Elevated MMPs in aged dermis can be divided into following groups: collagenase (MMP-1), gelatinase-B (MMP-9), stromelysins (MMP-3), MMP-10, MMP-11, membrane-associated: MMP-23, MMP-24, and recently identified MMP-27. It is well-known that ultraviolet (UV) from the sun causes premature skin aging (photoaging) by transiently inducing only three MMPs in human skin in vivo (MMP-1, MMP-3, and MMP-9) 28-30. We demonstrate here that compared to acute UV irradiation, a larger variety of MMPs, including UV-inducible MMPs, are constitutively elevated in aged skin. Interestingly, compared to acute UV irradiation, in which the epidermis is the primary source of transiently induced MMPs 30, the dermis is the major source of elevated MMPs in chronologically aged sun-protected skin. These observations suggest that although chronologically aged and photoaged skin share many common molecular features, such as MMPs-mediated collagen fragmentation, the primary sources (epidermis vs dermis) and cell types (keratinocytes vs fibroblasts) of elevated MMPs are different. Also, our investigations appear to reveal that collagen fragmentation in aged skin dermis results from constitutive elevation of a wide variety of dermal MMPs, rather than a transient elevation of limited epidermal MMPs as observed in acute UV irradiation in human skin.
The production of these MMPs is the responsibility of dermal fibroblasts, which are the major MMP-producing cells and control collagen homeostasis in the dermis. We demonstrate here that reduced fibroblast size up-regulates multiple MMPs as observed in aged human skin dermis in vivo. Reduced fibroblast size is a prominent feature of dermal fibroblasts in aged human skin 3,16,17. We found that isolated primary dermal fibroblasts from aged (>80 years) or young (25-30 years) individuals are indistinguishable from each other with respect to morphology (Fig 2D) and expression of all known mammalian MMPs (Fig 2A). In contrast, human dermal fibroblasts, obtained from individuals of any age (21-86 years of age), cultured in the conditions of reduced cell size, result in elevation of multiple MMPs (Table 1). These data suggest that the elevation of multiple MMPs in aged human skin arises, at least in part, from reduced size of dermal fibroblasts. We are unable to confirm fibroblast-size-dependent increase of MMP-23 and MMP-24, which are elevated in aged human skin dermis. As both MMP-23 and MMP-24 are cell membrane-associated MMPs (type II transmembrane MMPs), we found that the basal mRNA expression levels of these two MMPs are extremely low in dermal fibroblasts, suggesting that dermal fibroblasts are not major source of elevated MMP-23 and MMP-24 in aged human skin dermis.
Further investigation indicates that reduced fibroblast size is closely associated with elevated c-Jun/c-fos and increased transcription factor AP-1 activity, the major driving for multiple MMPs 21,22,27. Transcription factor AP-1, typically composed of c-Jun and c-Fos is one of the first mammalian transcription factors to be identified 31,32. We and others, previously reported that stress-activated MAP Kinase pathways and c-Jun mRNA and protein are increased in aged, compared to young human skin in vivo 17,33,34. In this study, we confirmed that c-Jun and c-Fos expression are significantly elevated in aged human skin dermis in vivo, the major source of elevated multiple MMPs. These data suggest that reduced fibroblast size induces c-Jun and c-Fos, which in turn elevates multiple MMPs in aged human skin dermis.
In skin dermis, fibroblast spreading and size, which are mediated by cytoskeletal and intracellular structural machinery, largely depends on cellular interactions with surrounding ECM microenvironment. In young human skin dermis, binding of fibroblasts to intact collagen fibrils allows generation of traction forces that are necessary for spreading, mechanical stability, normal function. However, in aged human skin dermis, collagen fibril binding sites are lost and mechanical resistance to traction forces is reduced due to fragmentation. In this state, the ECM microenvironment is unable to provide sufficient mechanical stability to maintain normal cell spreading/mechanical force 3,16,17. Therefore, age-related fragmentation of the collagen fibril microenvironment deleteriously alters fibroblast size and function.
Also, cell shape and size impacts multiple cellular processes including signal transduction, gene expression, and metabolism 14,35-37. Currently, mechanisms by which reduced cells size induces c-Jun/AP-1 are not well-understood. AP-1 activity is regulated by a wide range of stimuli including reactive oxygen species (ROS) 32. We previously reported that fibroblasts that have reduced spreading/size due to fragmentation of surrounding collagen fibrils display increase levels of ROS 17. ROS is considered to be a major driving force for the aging process 38,39. Indeed, we observed that protein oxidation, a marker of oxidative stress, is increased in aged human dermis in vivo 17. These data suggest that elevated ROS may be mediate induction of c-Jun/AP-1 activity in response to reduced fibroblast spreading and size. Clearly, further studies are needed to understand the connections among fibroblast size, oxidative stress, AP-1 activity, and MMPs.
We have previously reported that reduction of fibroblast size by disruption of cytoskeleton reduced cellular mechanical force, which substantially induced MMP-1 expression40 and consequent collagen fibril fragmentation17. Reduced mechanical force induced transcription factor c-Jun to bind to a canonical AP-1 binding site in the MMP-1 proximal promoter. Blocking c-Jun function with dominant negative mutant c-Jun significantly reduced induction of MMP-1 expression in response to reduced cell size and mechanical force. Current study added new information that reduced fibroblast size not only induces MMP-1, but also induces multiple MMPs (Fig.1A). Furthermore, we demonstrated that restoration of fibroblast size led to reverse elevated multiple MMPs to a basal level (Fig. 3F). We also previously reported that reduced fibroblast size inhibits type I procollagen synthesis, the major structural protein in skin, via impairment of TGF-β/Smad signaling41. Further investigation reveals that reduced fibroblast size specifically down-regulates TGF-β type II receptor, and this down-regulation largely mediates the reduction of type I procollagen and other ECM production. Taken together, these data suggest that reduced fibroblast size could be a marker of cellular aging and of aging human skin dermis.
Impaired wound healing is common in the elderly and presents a significant clinical and economic problem. It has been reported that fibroblast dysfunction is a key factor in the non-healing of chronic wounds in the elderly42,43. Dermal fibroblasts are largely responsible for collagen turnover and remodeling in healing of wounds. Although MMPs play essential and beneficial roles in normal wound healing, MMPs must be present at the right amount and in the right time frame (duration) during the wound healing process. Substantial evidence has amassed that MMPs in general are highly elevated in the delayed wound healing44-47, and that treatments that lower MMP activities accelerate healing of wounds48-50. It is conceivable that constitutive elevations of multiple MMPs, as identified in current paper, result in a hostile tissue microenvironment that promotes age-related skin diseases, such as delayed wound healing in elderly. Clearly, additional studies are warranted to uncover the precise molecular mechanism(s) by which constitutive elevations of multiple MMPs may contribute to impaired wound healing in elderly.
In summary, aged dermis constitutively expresses elevated levels of several MMPs, which likely lead to chronic, progressive degradation of the dermal collagen in aged human skin. We propose a novel mechanism by which age-related reduction of fibroblast size activates AP-1, which in turn elevates multiple MMPs as observed in aged human skin (Fig 5C). This mechanism provides a foundation for understanding the cellular and molecular basis of age-related collagen fragmentation, the characteristic feature of aged human skin.
What is already known about this topic?
Collagen is the major structure protein in skin, and its fragmentation is a hallmark of dermal aging.
Matrix metalloproteinases (MMPs) are largely responsible for fragmentation of collagen fibrils.
However, the expression of all known mammalian MMPs and the mechanism underlying altered expression of MMPs in chronologically aged human skin are less understood.
What does this study add?
Among all 23 known mammalian MMPs, multiple MMPs are elevated in aged human skin dermis.
As dermal fibroblasts are the major MMPs producing cells in the dermis, reduction of dermal fibroblast size, which is observed in aged human skin, contributes to elevation of age-related multiple MMPs.
Reduction of fibroblast size up-regulates c-Jun/c-Fos and activates AP-1, the major regulator of multiple MMPs.
These data provide a foundation for understanding the cellular and molecular basis of age-related collagen fragmentation, the characteristic feature of aged human skin.
Acknowledgments
We thank Ken Calderone, Yuan Shao, and Patrick Robichaud for technical assistance and Diane Fiolek for graphics and administrative assistance. This work was supported by the National Institute of Health (AG19364 to G Fisher and T Quan).
Footnotes
Author contribution: ZQ and RMB performed the experiments. TQ designed, analyzed the data, and wrote the manuscript.
References
- 1.Di Lullo GA, Sweeney SM, Korkko J, et al. Mapping the ligand-binding sites and disease-associated mutations on the most abundant protein in the human, type I collagen. J Biol Chem. 2002;277:4223–31. doi: 10.1074/jbc.M110709200. [DOI] [PubMed] [Google Scholar]
- 2.Uitto J. Connective tissue biochemistry of the aging dermis. Age-related alterations in collagen and elastin. Dermatol Clin. 1986;4:433–46. [PubMed] [Google Scholar]
- 3.Fisher GJ, Varani J, Voorhees JJ. Looking older: fibroblast collapse and therapeutic implications. Arch Dermatol. 2008;144:666–72. doi: 10.1001/archderm.144.5.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quan T, Fisher GJ. Role of Age-Associated Alterations of the Dermal Extracellular Matrix Microenvironment in Human Skin Aging: A Mini-Review. Gerontology. 2015;61:427–34. doi: 10.1159/000371708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ma W, Wlaschek M, Tantcheva-Poor I, et al. Chronological ageing and photoageing of the fibroblasts and the dermal connective tissue. Clin Exp Dermatol. 2001;26:592–9. doi: 10.1046/j.1365-2230.2001.00905.x. [DOI] [PubMed] [Google Scholar]
- 6.Ashcroft GS, Horan MA, Ferguson MW. The effects of ageing on cutaneous wound healing in mammals. J Anat. 1995;187(Pt 1):1–26. [PMC free article] [PubMed] [Google Scholar]
- 7.Gilchrest BA, Stoff JS, Soter NA. Chronologic aging alters the response to ultraviolet-induced inflammation in human skin. The Journal of investigative dermatology. 1982;79:11–5. doi: 10.1111/1523-1747.ep12510417. [DOI] [PubMed] [Google Scholar]
- 8.Eaglstein WH. Wound healing and aging. Clin Geriatr Med. 1989;5:183–8. [PubMed] [Google Scholar]
- 9.Ashcroft GS, Mills SJ, Ashworth JJ. Ageing and wound healing. Biogerontology. 2002;3:337–45. doi: 10.1023/a:1021399228395. [DOI] [PubMed] [Google Scholar]
- 10.Rogers HW, Weinstock MA, Feldman SR, et al. Incidence Estimate of Nonmelanoma Skin Cancer (Keratinocyte Carcinomas) in the US Population, 2012. JAMA Dermatol. 2015;151:1081–6. doi: 10.1001/jamadermatol.2015.1187. [DOI] [PubMed] [Google Scholar]
- 11.Pickup MW, Mouw JK, Weaver VM. The extracellular matrix modulates the hallmarks of cancer. EMBO Rep. 2014;15:1243–53. doi: 10.15252/embr.201439246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quan T. Skin connective tissue aging and dermal fibroblasts. Dermal Fibroblasts: Histological Perspectives, Characterization and Role in Disease. 2013:31–55. [Google Scholar]
- 13.Hynes RO. The extracellular matrix: not just pretty fibrils. Science. 2009;326:1216–9. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ingber DE. Cellular mechanotransduction: putting all the pieces together again. FASEB J. 2006;20:811–27. doi: 10.1096/fj.05-5424rev. [DOI] [PubMed] [Google Scholar]
- 15.Geiger B, Spatz JP, Bershadsky AD. Environmental sensing through focal adhesions. Nat Rev Mol Cell Biol. 2009;10:21–33. doi: 10.1038/nrm2593. [DOI] [PubMed] [Google Scholar]
- 16.Varani J, Schuger L, Dame MK, et al. Reduced fibroblast interaction with intact collagen as a mechanism for depressed collagen synthesis in photodamaged skin. J Invest Dermatol. 2004;122:1471–9. doi: 10.1111/j.0022-202X.2004.22614.x. [DOI] [PubMed] [Google Scholar]
- 17.Fisher GJ, Quan T, Purohit T, et al. Collagen fragmentation promotes oxidative stress and elevates matrix metalloproteinase-1 in fibroblasts in aged human skin. Am J Pathol. 2009;174:101–14. doi: 10.2353/ajpath.2009.080599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bonnans C, Chou J, Werb Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol. 2014;15:786–801. doi: 10.1038/nrm3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hegedus L, Cho H, Xie X, et al. Additional MDA-MB-231 breast cancer cell matrix metalloproteinases promote invasiveness. Journal of cellular physiology. 2008;216:480–5. doi: 10.1002/jcp.21417. [DOI] [PubMed] [Google Scholar]
- 20.Qin Z, Fisher GJ, Quan T. Cysteine-rich protein 61 (CCN1) domain-specific stimulation of matrix metalloproteinase-1 expression through αVβ3 integrin in human skin fibroblasts. Journal of Biological Chemistry. 2013;288:12386–94. doi: 10.1074/jbc.M112.424358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chakraborti S, Mandal M, Das S, et al. Regulation of matrix metalloproteinases: an overview. Mol Cell Biochem. 2003;253:269–85. doi: 10.1023/a:1026028303196. [DOI] [PubMed] [Google Scholar]
- 22.Gutman A, Wasylyk B. The collagenase gene promoter contains a TPA and oncogene-responsive unit encompassing the PEA3 and AP-1 binding sites. EMBO J. 1990;9:2241–6. doi: 10.1002/j.1460-2075.1990.tb07394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Birkedal-Hansen H, Moore WG, Bodden MK, et al. Matrix metalloproteinases: a review. Crit Rev Oral Biol Med. 1993;4:197–250. doi: 10.1177/10454411930040020401. [DOI] [PubMed] [Google Scholar]
- 24.Fisher GJ, Wang ZQ, Datta SC, et al. Pathophysiology of premature skin aging induced by ultraviolet light. N Engl J Med. 1997;337:1419–28. doi: 10.1056/NEJM199711133372003. [DOI] [PubMed] [Google Scholar]
- 25.Quan T, Qin Z, Voorhees JJ, et al. Cysteine-rich protein 61 (CCN1) mediates replicative senescence-associated aberrant collagen homeostasis in human skin fibroblasts. J Cell Biochem. 2012 doi: 10.1002/jcb.24179. [DOI] [PubMed] [Google Scholar]
- 26.Gieni RS, Hendzel MJ. Mechanotransduction from the ECM to the genome: are the pieces now in place? J Cell Biochem. 2008;104:1964–87. doi: 10.1002/jcb.21364. [DOI] [PubMed] [Google Scholar]
- 27.Benbow U, Brinckerhoff CE. The AP-1 site and MMP gene regulation: what is all the fuss about? Matrix biology: journal of the International Society for Matrix Biology. 1997;15:519–26. doi: 10.1016/s0945-053x(97)90026-3. [DOI] [PubMed] [Google Scholar]
- 28.Brenneisen P, Sies H, Scharffetter-Kochanek K. Ultraviolet-B irradiation and matrix metalloproteinases: from induction via signaling to initial events. Annals of the New York Academy of Sciences. 2002;973:31–43. doi: 10.1111/j.1749-6632.2002.tb04602.x. [DOI] [PubMed] [Google Scholar]
- 29.Fisher GJ, Datta SC, Talwar HS, et al. Molecular basis of sun-induced premature skin ageing and retinoid antagonism. Nature. 1996;379:335–9. doi: 10.1038/379335a0. [DOI] [PubMed] [Google Scholar]
- 30.Quan T, Qin Z, Xia W, et al. Matrix-degrading metalloproteinases in photoaging. J Investig Dermatol Symp Proc. 2009;14:20–4. doi: 10.1038/jidsymp.2009.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Angel P, Karin M. The role of Jun, Fos and the AP-1 complex in cell-proliferation and transformation. Biochim Biophys Acta. 1991;1072:129–57. doi: 10.1016/0304-419x(91)90011-9. [DOI] [PubMed] [Google Scholar]
- 32.Shaulian E, Karin M. AP-1 as a regulator of cell life and death. Nat Cell Biol. 2002;4:E131–6. doi: 10.1038/ncb0502-e131. [DOI] [PubMed] [Google Scholar]
- 33.Chung JH, Kang S, Varani J, et al. Decreased extracellular-signal-regulated kinase and increased stress-activated MAP kinase activities in aged human skin in vivo. J Invest Dermatol. 2000;115:177–82. doi: 10.1046/j.1523-1747.2000.00009.x. [DOI] [PubMed] [Google Scholar]
- 34.Shin MH, Rhie GE, Kim YK, et al. H2O2 accumulation by catalase reduction changes MAP kinase signaling in aged human skin in vivo. J Invest Dermatol. 2005;125:221–9. doi: 10.1111/j.0022-202X.2005.23823.x. [DOI] [PubMed] [Google Scholar]
- 35.Alenghat FJ, Nauli SM, Kolb R, et al. Global cytoskeletal control of mechanotransduction in kidney epithelial cells. Exp Cell Res. 2004;301:23–30. doi: 10.1016/j.yexcr.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 36.Silver FH, Siperko LM, Seehra GP. Mechanobiology of force transduction in dermal tissue. Skin Res Technol. 2003;9:3–23. doi: 10.1034/j.1600-0846.2003.00358.x. [DOI] [PubMed] [Google Scholar]
- 37.Wang N, Butler JP, Ingber DE. Mechanotransduction across the cell surface and through the cytoskeleton. Science. 1993;260:1124–7. doi: 10.1126/science.7684161. [DOI] [PubMed] [Google Scholar]
- 38.Stadtman ER. Protein oxidation and aging. Science. 1992;257:1220–4. doi: 10.1126/science.1355616. [DOI] [PubMed] [Google Scholar]
- 39.Golden TR, Hinerfeld DA, Melov S. Oxidative stress and aging: beyond correlation. Aging Cell. 2002;1:117–23. doi: 10.1046/j.1474-9728.2002.00015.x. [DOI] [PubMed] [Google Scholar]
- 40.Qin Z, Voorhees JJ, Fisher GJ, et al. Age-associated reduction of cellular spreading/mechanical force up-regulates matrix metalloproteinase-1 expression and collagen fibril fragmentation via c-Jun/AP-1 in human dermal fibroblasts. Aging Cell. 2014;13:1028–37. doi: 10.1111/acel.12265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fisher GJ, Shao Y, He T, et al. Reduction of fibroblast size/mechanical force down-regulates TGF-beta type II receptor: implications for human skin aging. Aging Cell. 2016;15:67–76. doi: 10.1111/acel.12410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mendez MV, Stanley A, Park HY, et al. Fibroblasts cultured from venous ulcers display cellular characteristics of senescence. J Vasc Surg. 1998;28:876–83. doi: 10.1016/s0741-5214(98)70064-3. [DOI] [PubMed] [Google Scholar]
- 43.Wall IB, Moseley R, Baird DM, et al. Fibroblast dysfunction is a key factor in the non-healing of chronic venous leg ulcers. J Invest Dermatol. 2008;128:2526–40. doi: 10.1038/jid.2008.114. [DOI] [PubMed] [Google Scholar]
- 44.Wysocki AB, Staiano-Coico L, Grinnell F. Wound fluid from chronic leg ulcers contains elevated levels of metalloproteinases MMP-2 and MMP-9. J Invest Dermatol. 1993;101:64–8. doi: 10.1111/1523-1747.ep12359590. [DOI] [PubMed] [Google Scholar]
- 45.Weckroth M, Vaheri A, Lauharanta J, et al. Matrix metalloproteinases, gelatinase and collagenase, in chronic leg ulcers. J Invest Dermatol. 1996;106:1119–24. doi: 10.1111/1523-1747.ep12340167. [DOI] [PubMed] [Google Scholar]
- 46.Lobmann R, Ambrosch A, Schultz G, et al. Expression of matrix-metalloproteinases and their inhibitors in the wounds of diabetic and non-diabetic patients. Diabetologia. 2002;45:1011–6. doi: 10.1007/s00125-002-0868-8. [DOI] [PubMed] [Google Scholar]
- 47.Yager DR, Zhang LY, Liang HX, et al. Wound fluids from human pressure ulcers contain elevated matrix metalloproteinase levels and activity compared to surgical wound fluids. J Invest Dermatol. 1996;107:743–8. doi: 10.1111/1523-1747.ep12365637. [DOI] [PubMed] [Google Scholar]
- 48.Veves A, Sheehan P, Pham HT. A randomized, controlled trial of Promogran (a collagen/oxidized regenerated cellulose dressing) vs standard treatment in the management of diabetic foot ulcers. Arch Surg. 2002;137:822–7. doi: 10.1001/archsurg.137.7.822. [DOI] [PubMed] [Google Scholar]
- 49.Cullen B. The role of oxidized regenerated cellulose/collagen in chronic wound repair. Part 2. Ostomy Wound Manage. 2002;48:8–13. [PubMed] [Google Scholar]
- 50.Lobmann R, Zemlin C, Motzkau M, et al. Expression of matrix metalloproteinases and growth factors in diabetic foot wounds treated with a protease absorbent dressing. J Diabetes Complications. 2006;20:329–35. doi: 10.1016/j.jdiacomp.2005.08.007. [DOI] [PubMed] [Google Scholar]


