Abstract
Genetic factors significantly influence susceptibility for substance abuse and mood disorders. Rodent studies have begun to elucidate a role of Cav1.3 L-type Ca2+ channels in neuropsychiatric-related behaviors, such as addictive and depressive-like behaviors. Human studies have also linked the CACNA1D gene, which codes for the Cav1.3 protein, with bipolar disorder (BD). However, the neurocircuitry and the molecular mechanisms underlying the role of Cav1.3 in neuropsychiatric phenotypes are not well established. In the present study, we directly manipulated Cav1.3 channels in Cav1.2 dihydropyridine (DHP) insensitive mutant mice and found that VTA Cav1.3 channels mediate cocaine-related and depressive-like behavior through a common nucleus accumbens (NAc) shell calcium permeable AMPA receptor (CP-AMPAR) mechanism that requires GluA1 phosphorylation at S831. Selective activation of VTA Cav1.3 with (±)-BayK-8644 (BayK) enhanced cocaine conditioned place preference (CPP) and cocaine psychomotor activity while inducing depressive-like behavior, an effect not observed in S831A phospho-mutant mice. Infusion of the CP-AMPAR-specific blocker Naspm into the NAc shell reversed the cocaine and depressive-like phenotypes. In addition, activation of VTA Cav1.3 channels resulted in social behavioral deficits. In contrast to the cocaine- and depression-related phenotypes, GluA1/A2 AMPARs in the NAc core mediated social deficits, independent of S831-GluA1 phosphorylation. Using a candidate gene analysis approach, we also identified single nucleotide polymorphisms in the CACNA1D gene associated with cocaine dependence (CD) in human subjects. Together, our findings reveal novel, overlapping mechanisms through which VTA Cav1.3 mediates cocaine-related, depressive-like and social phenotypes suggesting that Cav1.3 may serve as a target for the treatment of neuropsychiatric symptoms.
INTRODUCTION
Altered reward brain circuitry and reward processing is associated with multiple psychiatric disorders1. In particular, substance abuse disorders are often co-morbid with mood disorders particularly bipolar disorder (BD)2 and major depressive disorder3. Common genetic risk factors4–5, overlapping neurocircuitry6, 7 and convergence of cellular and molecular mechanisms7, 8 have been suggested to underlie this co-morbidity. In particular, recent findings provide evidence that human variants in the CACNA1D gene, which codes for the Cav1.3 subunit of L-type Ca2+ channels (LTCCs), can confer risk for the development of neuropsychiatric disorders9, 10 including BD11, 12. Emerging data on impact of human mutations on Cav1.3 physiology13 and from animal studies14–16 suggests that enhanced Cav1.3 activity may contribute to neuropsychiatric disorders. This is in agreement with a crucial role of this channel in neuronal signaling10, 17, 18 underlying drug taking and emotional behaviors. However, as for other risk genes, the precise Cav1.3–dependent mechanisms and neurocircuits that contribute to the neurobiology of neuropsychiatric-related behavioral phenotypes remain unknown.
The VTA-NAc pathway plays a central role in mediating the effects of cocaine19, depressive-like20, 21 and social behavior22. LTCCs in the VTA regulate DA burst firing activity23, 24, a property known to mediate reward behaviors25, depressive-like behaviors26, social interaction22 and responses to stress20, 21. We and others have shown that Cav1.3 that is expressed in VTA dopamine neurons27, 28 is required for the short- and the long-term effects of cocaine14–16. Acute activation of Cav1.3 by systemic injection of BayK, has also been shown to induce depressive-like behavior28. Collectively, these findings suggest a role of Cav1.3 channels in addictive behavior and mood disorders.
Emerging evidence is establishing neuropsychiatric disorders as synaptic diseases. Several studies have reported enhanced glutamatergic AMPAR transmission, in particular increase in synaptic GluA2-lacking Ca2+ permeable AMPARs (CP-AMPARs herein) in medium spiny neurons of the NAc, as a key mechanism underlying cocaine-induced long-lasting behavioral plasticity29–35. Enhanced AMPAR transmission in the NAc has also been reported to drive depressive-like and social behavior36, suggesting that modulation of AMPAR transmission could represent one common mechanism linking addiction and mood disorders.
Given the evidence of enhanced VTA-NAc activity in drug taking and mood-related behaviors19, 21, 22, Cav1.3 channel properties in VTA dopamine cells24 and Cav1.3 gain of function mutations in neurological disease10, we hypothesized that enhanced activity of VTA Cav1.3 channels would drive cocaine-related behaviors and mood-related phenotypes such as depressive-like, anxiety-like and social behavior. To this end, we utilized Cav1.2 DHP insensitive (Cav1.2 DHP−/−) mutant mice. A single point mutation in the Cav1.2 α1-subunit of these mice does not affect Cav1.2 function and expression, but renders them insensitive to (±)-BayK-8644 and strongly reduces their sensitivity to DHP Ca2+ channel blockers, such as nifedipine28, 37. As successfully demonstrated in previous studies15, 38 this allows us to specifically pharmacologically manipulate Cav1.3 channels in the VTA in these mice. Subsequently, we pharmacologically assessed AMPARs in the NAc and utilized AMPAR subunit GluA1 phosphorylation deficient mutant mice to assess the necessity of GluA1 phosphorylation in cocaine and mood-related behaviors. Finally, we tested genetic variants within and around CACNA1D for association with cocaine dependence (CD; as defined by DSM-IV criteria) using a genome-wide association dataset.
MATERIALS AND METHODS
Detailed methods are provided in Supplementary Information.
Animals
All experimental procedures were conducted in accordance with Weill Cornell Medicine IACUC guidelines. Male C57BL/6 (Jackson Laboratories, Bar Harbor, Maine), Cav1.3 knockout (KO)39, Cav1.2 DHP-insensitive28 and GluA1 S831A phospho-mutant mice40 all on C57BL/6J background, were 8–10 weeks old at the start of the experiments. Animals were maintained on a 12-hr light/dark cycle (from 7A.M. to 7P.M.).
Drugs and antibodies
Cocaine HCl, nifedipine (Nif), (±)-BayK-8644 (BayK), and Naspm were obtained from Sigma (St. Louis, MO). NBQX was obtained from Tocris Bioscience (Minneapolis, MN). Nif and BayK were dissolved in 0.9% saline containing 1.5% DMSO and 1.5% Tween80. Naspm and NBQX were dissolved in 0.9% saline. Antibodies listed in Supplementary Table 1.
Subcellular fractionation
Mice were euthanized by rapid decapitation and bilateral VTA (18-gauge stainless-steel stylet), NAc (18-gauge stainless-steel stylet) or PFC (17-gauge stainless-steel stylet) tissue was obtained. Total protein lysates were isolated as previously described41. For postsynaptic density (PSD) fractions, subcellular fractionation was performed as published before42.
Coimmunoprecipiation assay
Crude membrane fractions were generated from bilateral VTA tissue pooled from four mice. Co-imunoprecipitation was performed as published in Calin-Jageman et al. (2007)43.
Immunoblot Analysis
Immunoblotting and quantitation was performed as previously described in Schierberl et al. (2011)15. Tubulin was used as a loading control for all PSD immunoblots.
Guide cannula surgery
For delivery of pharmacological drugs into the VTA or the NAc, guide cannula were implanted bilaterally in adult male mice as previously published15. Coordinates and drug concentrations are included in Supplementary Table 2.
Delivery of viral vectors into the VTA or NAc
AAV stereotaxic surgery and delivery of the AAV (Supplementary Table 3) was performed as previously published15.
Cocaine conditioned place preference test
A three-chamber place preference protocol (Med Associates Inc., St. Albans, VT, USA) was used as previously described in Tropea et al. 200844. On Day1 mice were allowed to freely explore all three chambers (20 min). On Day 2–4 (conditioning sessions), a biased procedure was used wherein mice were paired with cocaine (10 mg/kg, i.p.) for 20 min in the morning on the less preferred side, and paired with saline for 20 min in the afternoon on the opposite side. On Day 5 (WD1) mice were allow to freely explore the CPP box for 20 min and time spent in cocaine-paired side (sec) was recorded. For some experiments, mice were re-tested on day 34 (WD30).
Sucrose Preference Test (SPT)
Individually housed mice were habituated to two 50 ml bottles, one containing water and the other 1% sucrose for one day. For two days, body weight and mass of water and sucrose consumed was monitored. Results are presented as sucrose preference calculated as (sucrose consumed (g)/(sucrose consumed (g) + water consumed (g)))*100.
Forced Swim Test (FST)
Mice were placed in a 2 liter beaker filled with 1600 ml of water between 25–26°C for 10 minutes. Immobility time was scored using the computer-assisted software ButtonBox v5.1 (Behavioral Research Solutions, Dallas Texas).
Three-Chambered Social Interaction test
Social approach was performed as published in Inan et al. 201645. Following a 10 min habituation, mice were allowed to freely explore the apparatus containing an inanimate object and a stranger mouse and time spent within a predefined contact zone was recorded.
Elevated Plus Maze (EPM) test
EPM test was performed as previously reported by Lee et al. (2012)46. Data is reported as the percent of time spent in the open arms which was calculated as: (time in open arms (s)/total time (s))*100.
Human Genetics
To examine whether genetic variants within and around CACNA1D contribute to CD susceptibility in humans, we conducted a candidate gene association analysis for CACNA1D with CD using a genome-wide association study (GWAS) data set from the Study of Addiction: Genetics and Environment (SAGE), previously described in detail47 and as detailed in the Supplementary Methods.
Statistical Analyses
All statistical analyses were conducted using Graphpad Prism 6 (GraphPad Software, La Jolla, CA). A two-tailed unpaired t-test was performed after confirmation of normal distribution of the data using the Shapiro-Wilk test. If data were not normally distributed, the Mann Whitney nonparametric test was performed as indicated in the text. For experiments with four groups, a two-way analyses of variance (ANOVA) for main effect of experimental variables was performed followed by the Bonferroni-Dunn post hoc test. Statistical tests are specified in the results or in the figure legends and data were considered to be statistically significant for values of P ≤ 0.05.
RESULTS
Cav1.3 forms functional channels at the VTA postsynaptic density
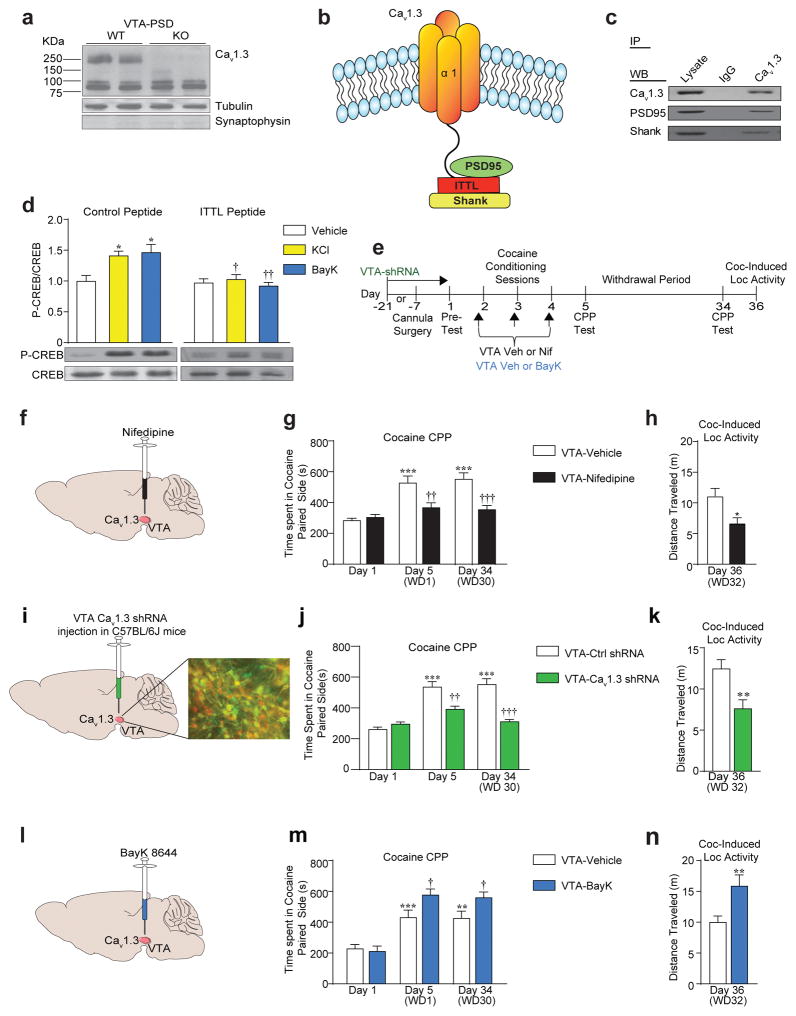
Cav1.3 mRNA is highly expressed in rat VTA dopamine neurons27. Cav1.3 protein is enriched in PSD fractions prepared from mouse VTA (Figure 1a) and associates with the PDZ scaffolding protein PSD-95 and Shank3 (Figure 1b, c). This interaction through its C-terminal class I PDZ binding domain (ITTL) is necessary for activity-dependent signaling in VTA slices, as revealed by lack of phosphorylation of the transcription factor CREB, a downstream target of Cav1.3 channels38, in the presence of a dominant-negative peptide to ITTL (Figure 1d). Thus, Cav1.3 protein is present in functional signaling complexes in the mouse VTA.
Figure 1.
Pharmacological inhibition and activation of VTA Cav1.3 channels oppositely regulates cocaine behaviors. (a) Western blot showing the presence of Cav1.3 protein in VTA PSD fractions from wildtype (WT) and Cav1.3 knockout (KO) mice. (b) Schematic of Cav1.3 showing its ITTL in the C-terminal cytoplasmic domain and its interaction with PSD-95 and Shank. (c) Western blots showing immunoprecipitated PSD95 and Shank with Cav1.3 antibody in VTA protein lysates. (d) Disruption of Cav1.3 PDZ domain ITTL with an inhibitory peptide blocks KCl- (Two-way ANOVA, [Peptide x KCl, F(1, 20) = 4.831, P=0.0399]; Bonferroni post hoc, Control Peptide: Vehicle vs KCl *P<0.05, KCl: Control Peptide vs ITTL Peptide †P<0.05) and BayK- (Two-way ANOVA, [Peptide x BayK, F(1, 20) = 7.593, P=0.0122]; Bonferroni post hoc test: Control Peptide: Vehicle vs BayK *P<0.05, BayK: Control Peptide vs ITTL Peptide ††P<0.01) induced Ser 133 P-CREB phosphorylation in VTA slices from Cav1.2 DHP−/− mice. For all groups, n = 6. (e, f, i, l) Schematic timeline of behavioral protocol and VTA infusion of either Veh or Nif, Veh or BayK in Cav1.2DHP−/− mice, or Cav1.3 shRNA in C57BL/6 mice. (g-h) Intra-VTA microinjection of Nif in Cav1.2 DHP−/− mice administered prior to each cocaine conditioning session attenuated (g) expression of CPP on Day 5 (WD1) and Day 34 (WD30) (Two-way ANOVA, [VTA infusion x day, F(2,60) = 7.595, P=0.0011]; Bonferroni post hoc test: Veh: Day 1 vs Day 5 ***P<0.001, Veh: Day 1 vs Day 34 ***P<0.001, Day 5: Veh vs Nif ††P<0.01, Day 34: Veh vs Nif †††P<0.001. Veh n = 12, Nif n = 10), and (h) the cocaine-induced locomotor activity measured on day 36 (t(17) = 2.86, *P=0.0108. Veh n = 10, Nif n = 9). (i) Inset, image shows green fluorescent protein (GFP-green), tyrosine hydroxylase (TH-red) and dual-labeled (yellow) cells. (j, k) Intra-VTA stereotaxic delivery of Cav1.3 shRNA (21 days before the start of CPP) attenuated (j) the expression of CPP tested on day 5 and 34 (Two-Way ANOVA, [VTA injection x day, F(2,51) = 14.09, P< 0.0011]; Bonferroni post hoc test: Ctrl shRNA: Day 1 vs Day 5 ***P<0.001, Ctrl shRNA Day 1 vs Day 34 ***P<0.001, Day 5: Ctrl shRNA vs Cav1.3 shRNA ††P<0.01, Day 34: Ctrl shRNA vs Cav1.3 shRNA †††P<0.001. Ctrl shRNA n= 10, Cav1.3 shRNA n = 9), and (k) cocaine-induced locomotor activity measured on day 36 (t-test, t(17) = 3.066, **P=0.0070. Ctrl shRNA n = 10, Cav1.3 shRNA n = 9). (m, n) Intra-VTA infusion of BayK prior to each cocaine conditioning session enhanced (m) expression of cocaine CPP on day 5 and 34 (Two-way ANOVA, [VTA infusion x day F(2,48) = 3.206, P=0.0493]; Bonferroni post hoc test: Veh: Day 1 vs Day 5 ***P<0.001, Veh: Day 1 vs Day 34 **P<0.01, Day 5: Veh vs BayK †P<0.05, Day 34: Veh vs BayK †P<0.05. Veh n = 9, BayK n = 9), and (n) enhanced cocaine-induced locomotor response on day 36 (t(16) = 3.955, **P=0.0011. Veh n = 9, BayK n = 9). Error bars represent ± s.e.m.
VTA Cav1.3 channel activation enhances cocaine-related behaviors
We have reported that blocking VTA Cav1.3 channels with nifedipine blocks cocaine psychomotor sensitization15. Similarly, VTA nifedipine infusions in Cav1.2 DHP−/− mice (Figure 1e–f, Supplementary Figure 1a) attenuated cocaine CPP, a measure of cocaine reward, twenty-four hours (WD1 herein) and 30 days (WD30 herein) after the last conditioning session (Figure 1g). These mice also exhibited a lower cocaine-induced locomotor response (Figure 1h), an effect replicated by VTA Cav1.3shRNA-mediated Cav1.3 knockdown (Figure 1e, i–k). Of note, blocking (Supplementary Figure 1b–d) or knocking down (Supplementary Figure 1e–f) Cav1.3 channels in the NAc had no effect on cocaine CPP.
To test if pharmacological activation of VTA Cav1.3 channels enhanced cocaine-induced behaviors, Cav1.2 DHP−/− mice received BayK infusions into the VTA (Figure 1e, 1l), which further increased cocaine CPP (Figure 1m) and cocaine-induced locomotor activity (Figure 1n). Thus, cocaine-induced behaviors require VTA Cav1.3 channels and VTA Cav1.3 activation is capable of enhancing cocaine-mediated behaviors.
VTA Cav1.3 channels are necessary for a GluA1 S831 phosphorylation-dependent increase in CP-AMPARs in the NAc shell
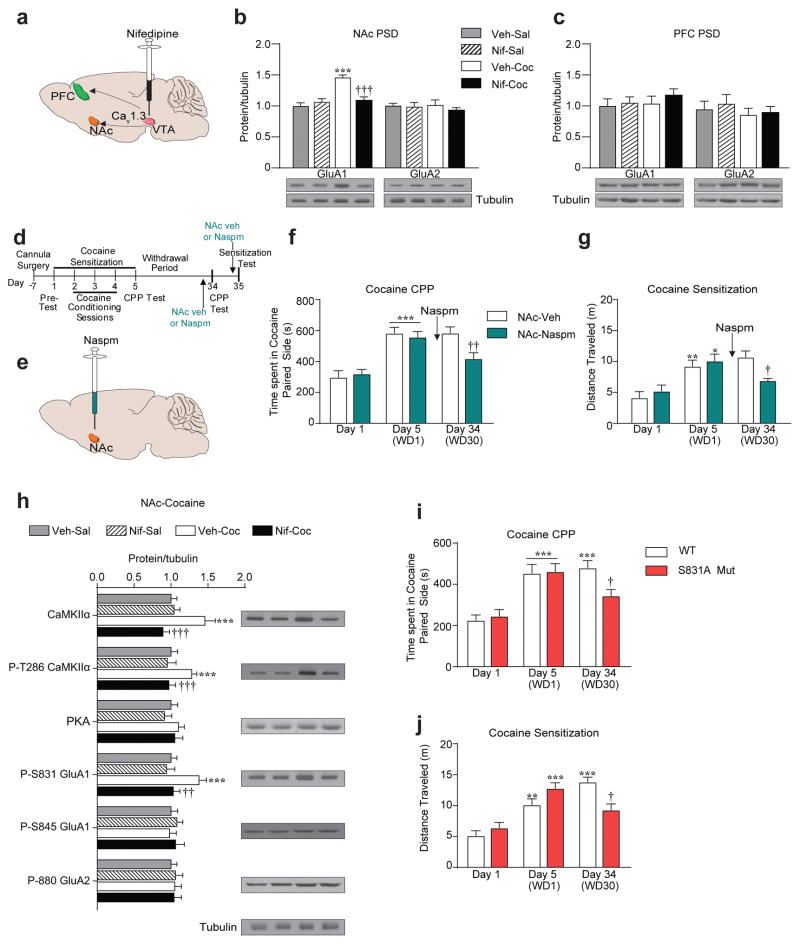
Increase in synaptic AMPAR transmission by redistribution of AMPAR subunits, GluA1 and GluA2 has been observed in the NAc following cocaine treatment and behaviors33. We measured levels of GluA1 and GluA2 in PSD fractions, indicative of synaptic levels, from NAc (shell and core combined) at WD1 and WD30 and role of VTA Cav1.3 channels, therein (Figure 2a). Cocaine CPP increased PSD GluA1 but not GluA2 levels in the NAc at WD30 (Figure 2b) but not WD1 (Supplementary Figure 2c–d). The GluA1 increase was blocked in VTA nifedipine (Figure 2b) and VTA Cav1.3shRNA (Supplementary Figure 2a) pretreated mice. Cocaine had no effect on PSD GluA1 levels in the PFC, a region that also receives VTA projections (Figure 2a, c and Supplementary Figure 2b).
Figure 2.
VTA Cav1.3 channels mediate long-term increase in CP-AMPARs at the NAc PSD of cocaine-exposed mice. (a) Schematic representation of VTA projection to the NAc and PFC. (b) Cocaine administration increased GluA1 but not GluA2 protein levels at the NAc PSD at WD30 that was blocked by Nif pretreatment (GluA1: Two-way ANOVA, [pre-treatment x post-treatment, F(1,22) = 18.82, P< 0.0003]. Bonferroni post hoc test: Veh-Sal vs Veh-coc ***P<0.001, Veh-coc vs Nif-coc †††P<0.001; Veh-Sal n = 7, Nif-Sal n = 5, Veh-Coc n = 7, Nif-Coc n = 8). (c) No difference in GluA1 or GluA2 levels was seen in the PFC PSD (Veh-Sal n = 7, Nif-Sal, n = 5, Veh-Coc, n = 7, Nif-Coc, n = 8). ***P <0.0001, †††P < 0.001. (d) Experimental timeline of Naspm microinjection. (e) Schematic of Naspm infusion in the NAc shell. (f-g) Naspm infusion in the NAc shell prior to (f) cocaine CPP test on day 34 attenuated expression of cocaine CPP (Day 1 and 5, Two-way ANOVA, [day, F(1,36) = 51.05, P<0.001]; Bonferroni post hoc test: Veh and Naspm groups: Day 5 vs Day 1 ***P<0.001; Day 34, t-test, t(18)=2.881, ††P=0.0099. Veh n = 8, Naspm n = 12) and (g) cocaine-induced locomotor activity test on day 34 attenuated expression of psychomotor sensitization (Day 1 and 5, Two-way ANOVA, [day, F(1,28) = 23.76, P<0.001]; Bonferroni post hoc test: Veh group: Day 5 vs Day 1 **P<0.01, Naspm group: Day 1 vs. Day 5 *P<0.05; Day 34, t-test, t(14)= 2.767, †P=0.0151. Veh n = 8, Naspm n = 12). (h) VTA Nif pretreatment in Cav1.2DHP−/− mice blocked cocaine-induced increase in CaMKIIα, P-T286 CaMKIIα and P-S831 GluA1 in the NAc of cocaine exposed mice examined 30 days later (Two-way ANOVA, [CaMKIIα: pretreatment x posttreatment, F(1,23) = 9.091, P=0.0062]; Bonferroni post hoc test: Veh-Sal vs Veh-Coc ***P<0.001, Veh-Coc vs Nif-Coc †††P<0.001; [P-T286 CaMKIIα: F(1,23) = 13.11, P=0.0014]; Veh-Sal vs Veh-Coc ***P<0.001, Veh-Coc vs Nif-Coc †††P<0.001; [P-S831 GluA1: F(1,23) = 4.247, P=0.05]; Veh-Sal vs Veh-Coc ***P<0.001, Veh-Coc vs Nif-Coc ††P<0.01. Veh-Sal n = 7, Nif-Sal n = 6, Veh-Coc n = 7, Nif-Coc n = 7). (i) S831A GluA1 phospho-mutant mice presented a blunted cocaine CPP response (Two-Way ANOVA, [day x genotype, F(2,50) = 3.211, P=0.0488]; Bonferroni post hoc test: WT and S831A: Day1 vs Day 5 ***P<0.001, WT: Day1 vs Day 34 ***P<0.001, Day 34: WT vs. S831A †P<0.05. WT n = 10, S831A n = 9), and (j) blunted cocaine-induced locomotor activity on Day 34 (Two-Way ANOVA, [day x genotype, F(2,48) = 8.863, P=0.0005]; Bonferroni post hoc test: WT: Day1 vs Day 5 **P<0.01, S831A: Day1 vs Day 5 ***P<0.001, WT: Day 1 vs Day 34 ***P<0.001, Day 34: WT vs S831A †P<0.05. WT n = 8, S831A n = 10). Error bars represent ± s.e.m.
The increase in GluA1 and not GluA2 at WD30 suggested the possibility of accumulation of GluA2-lacking CP-AMPARs. Thus, we infused the CP-AMPAR blocker, Naspm into the NAc shell 20 min before the WD30 CPP test (Figure 2d, e) and observed an attenuation of cocaine CPP (Figure 2f). Similarly, Naspm in the NAc shell blocked the expression of cocaine psychomotor sensitization on WD30 (Figure 2g). Thus, cocaine results in upregulation of CP-AMPARs within the NAc shell following extended withdrawal, an adaptation that requires activation of VTA Cav1.3 channels.
Since GluA1 synaptic function and trafficking is regulated by phosphorylation at Ser 831 (S831) via CaMKinase IIα (CaMKIIα), and Ser 845 (S845) via Protein Kinase A (PKA)48, we measured levels of these proteins in NAc PSD fractions at WD30. Cocaine treated mice had higher CaMKIIα, P-CaMKIIα and P-S831 GluA1, an effect that was absent in VTA nifedipine pretreated mice (Figure 2h). No differences in levels of S845 or PKA were seen (Figure 2h). To test the causal role of S831-GluA1 phosphorylation in inducing the cocaine behaviors, we utilized S831 phosphorylation deficient mutant mice (S831A)40. WT and S831A mice exhibited similar levels of cocaine preference at WD1 (Figure 2i) and similar psychomotor sensitization on cocaine Day 5 (Figure 2j). However, at WD30, S831A mice had a blunted CPP response (Figure 2i) and a blunted psychomotor response (Figure 2j). Collectively, the above experiments demonstrated that activation of VTA Cav1.3 channels are necessary for mediating the protracted accumulation of CP-AMPARs in the NAc shell via upregulation of CaMKIIα and S831-GluA1 phosphorylation.
Activation of Cav1.3 channels in the VTA promotes depressive-like behavior, cocaine-related behavior, and induces deficits in social behavior
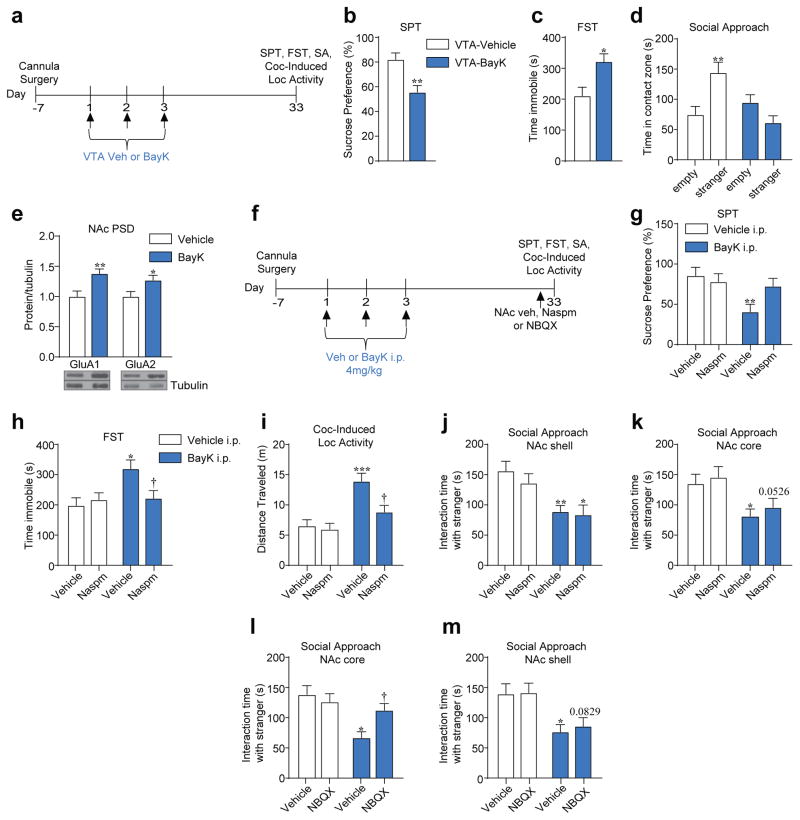
To determine whether VTA Cav1.3 channel manipulation is sufficient to alter mood-related and social behavior we used a repeated Cav1.3 channel activation protocol (BayK, once a day for 3 days, Figure 3a) in the VTA of Cav1.2DHP−/− mice, which has previously been shown to induce long-term behavioral changes in response to cocaine14. Repeated VTA-BayK infusion induced anhedonia in the sucrose preference test (SPT) and increased immobility in the forced swim test (FST), when tested 30 days after the last BayK infusion (Figure 3b, c). In a separate cohort, we also observed that BayK effects emerged as early as 24 hours after the last BayK infusion (Supplementary Figure 3a–b). VTA-BayK treatment also decreased social approach behavior in the three-chamber social interaction test (Figure 3d, Supplementary Figure 3c). As previously reported14, VTA-BayK also potentiated cocaine-induced locomotor activity when measured 30 days after the last BayK infusion (Supplementary Figure 3g) but not 24 hours later (Supplementary Figure 3d). VTA-BayK infusions had no effect on anxiety-like behavior or basal locomotor activity at either time point (Supplementary Figure 3e–f, h–i). Of note, repeated systemic BayK administration in Cav1.2DHP−/− mice had a similar effect on SPT, FST, social and cocaine-related behaviors, with no effect on EPM and basal locomotor activity (Supplementary Figure 3j–o). Thus, activation of Cav1.3 channels in the VTA is sufficient to induce mood-related and cocaine behaviors that are long lasting. In contrast to VTA Cav1.3 activation, inhibition of Cav1.3 with repeated nifedipine had no effect on depressive-like, social or cocaine induced-locomotion when measured 24 hours after the last nifedipine infusion (Supplementary Figure 4a–d) and had a milder effect 30 days later (Supplementary Figure 4g–j) with no effect on anxiety-like behavior or basal locomotion (Supplementary Figure 4e–f, k–l).
Figure 3.
Repeated VTA BayK 8644 treatment results in depressive-like behavior, social interaction deficits and enhanced cocaine psychomotor activity. (a) Experimental timeline of VTA-BayK infusion and behavioral testing. (b–d) Repeated administration of BayK in the VTA decreased sucrose preference (b, t(18) = 3.486, **P=0.0026. Veh n = 10, BayK n = 10), increased immobility time in FST (c, t(12) = 2.725, *P=0.0184. Veh n = 7, BayK n = 7), and impaired social approach behavior (d, Two-Way ANOVA, [genotype x contact zone, F(1,36) = 13.56, P=0.0008]; Veh: stranger vs empty cup **P<0.01. Veh n = 10, BayK n = 10). (e) BayK administration in the VTA increased GluA1 and GluA2 protein in the NAc PSD when tested 30 days later (GluA1: t(17) = 3.247, **P=0.0047. Veh n = 9, BayK: n = 10; GluA2: t(17) = 2.366, *P=0.0301. Veh n = 9, BayK n = 10). (f) Experimental timeline. (g–i) Naspm infusion in the NAc shell prior to (g) SPT (Two-Way ANOVA, [pre-treatment (Veh or BayK): F(1,34) = 6.351, P=0.016]; Bonferroni post hoc test: Veh-Veh vs. BayK-Veh **P=0.0079. Veh-Veh n = 8, Veh-Naspm, n = 9, BayK-Veh n = 10, BayK-Naspm n = 11) or (h) FST (Two-Way ANOVA, [pretreatment x posttreatment, F(1,34) = 4.903, P=0.0336]; Bonferroni post hoc test: Veh-Veh vs BayK-Veh *P<0.05, BayK-Veh vs BayK-Naspm †P<0.05. Veh-Veh n = 8, Veh-Naspm n = 9, BayK-Veh n = 10, BayK-Naspm n = 11), rescued depressive-like behavior and (i) decreased the enhanced cocaine-induced locomotor response (Two-Way ANOVA, [pre-treatment (Veh or BayK): F(1,34) = 19.96, P<0.0001]; post-treatment (Veh or Naspm): F(1,34) = 6.117, P=0.0185]; Bonferroni post hoc test: Veh-Veh vs BayK-Veh ***P<0.001, BayK-Veh vs BayK-Naspm †P<0.05. Veh-Veh n = 8, Veh-Naspm n = 9, BayK-Veh n = 10, BayK-Naspm n = 11), observed in BayK treated mice. (j–m) Naspm infusion in the (j) NAc shell (Two-way ANOVA, [pre-treatment (Veh or BayK): F(1,34) = 16.83, P< 0.0002]; Bonferroni post hoc test: Veh-Veh vs BayK-Veh **P<0.01, Veh-Naspm vs BayK-Naspm *P<0.05. Veh-Veh n = 9; Veh-Naspm n = 9, BayK-Veh n = 10, BayK-Naspm n = 10), or (k) in the NAc core (Two-way ANOVA, [pre-treatment (Veh or BayK): F(1,32) = 11.67, P< 0.0017]; Bonferroni post hoc test: Veh-Veh vs BayK-Veh *P<0.05, Veh-Naspm vs BayK-Naspm P=0.0526. Veh-Veh n = 8, Veh-Naspm n = 8, BayK-Veh n = 10, BayK-Naspm n = 10) had no effect on BayK-induced social approach deficit, whereas NBQX infusion in (l) the NAc core (Two-Way ANOVA, [pretreatment x posttreatment, F (1,35) = 5.403, P=0.0260]; Bonferroni post hoc test: Veh-Veh vs BayK-Veh *P<0.01, BayK-Veh vs BayK-NBQX †P<0.05. Veh-Veh n = 9, Veh-NBQX n = 9, BayK-Veh n = 10, BayK-NBQX n = 11), but not in (m) the NAc shell [pre-treatment (BayK or Veh), F(1,28) = 15.74, P=0.0005]; Post-treatment (NBQX or Veh), F(1,28) = 0.1436, P = 0.7076]; Bonferroni post hoc test: Veh-Veh vs BayK-Veh *P<0.05. Veh-NBQX vs BayK-NBQX P=0.0829. Veh-Veh n = 8, Veh-NBQX n = 8, BayK-Veh n = 8, BayK-NBQX n = 8), rescued the BayK- induced social approach deficit. Error bars represent ± s.e.m.
CP-AMPARs in the NAc shell mediate BayK-induced cocaine and depressive-like behavior
Formation of CP-AMPARs in the NAc has been reported in models of chronic stress36 and chronic neuropathic pain-induced depressive-like behaviors49. Thus, we measured levels of GluA1 and GluA2 in NAc (shell and core) PSD fractions 30 days following VTA-BayK infusions and both were higher in BayK infused mice (Figure 3e). Interestingly, even though BayK induced depressive-like and social behavioral deficits 24 hours after the last BayK administration, neither GluA1 nor GluA2 were significantly altered at 24 hours (Supplementary Figure 5a). To test the potential role of CP-AMPARs in VTA BayK-induced behaviors at the 30d time point, given overlapping mechanisms8, Naspm was administered into the NAc shell 30 mins prior to behavioral testing (Figure 3f), a manipulation that blocked VTA BayK-induced decrease in SPT (Figure 3g) and increase in immobility during FST (Figure 3h) (no effect on vehicle treated mice). Naspm additionally attenuated enhanced cocaine-induced locomotor activity in BayK-treated mice (Figure 3i). Interestingly, Naspm in the NAc shell had no effect on social approach behavior (Figure 3j) nor in the NAc core (Figure 3k), whereas blocking GluA1/A2 AMPARs with NBQX in the NAc core (Figure 3l, Supplementary Figure 5b) but not in the NAc shell (Figure 3m), reversed the deficit in social approach. Thus, long-term effect of repeated VTA-BayK on depressive behavior results from increase in CP-AMPARs in the NAc shell, whereas its effect on social behavior results from increase in GluA1/A2 AMPARs in the NAc core.
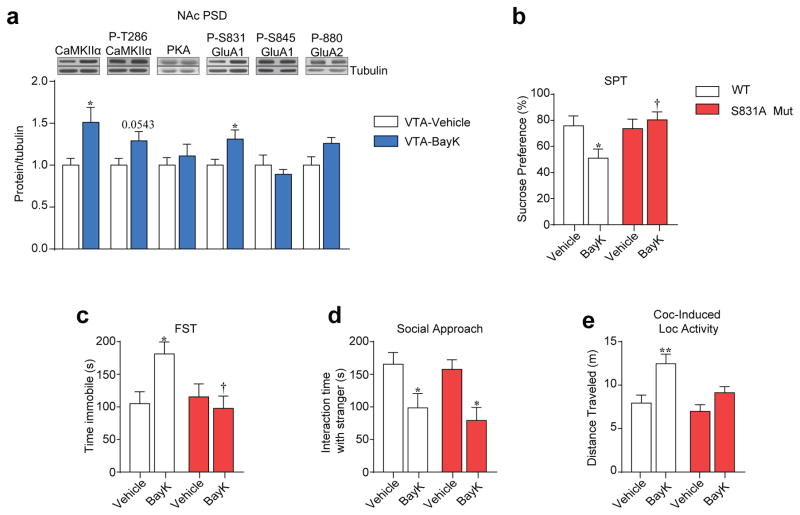
Next, we tested if repeated VTA-BayK alters protein kinase and GluA1 phosphorylation levels in the NAc. Similar to cocaine exposure (Figure 2h), we found that VTA-BayK infusion increased levels of NAc CaMKIIα, and S831-GluA1 30 days following BayK infusion (Figure 4a). To test the causal role of S831-GluA1 phosphorylation on behaviors, we utilized S831A-GluA1 phospho-mutant mice. At baseline, S831A did not differ from WT mice in the SPT (Supplementary Figure 6a), FST (Supplementary Figure 6b), social behavior (Supplementary Figure 6c), EPM (Supplementary Figure 6d), or basal locomotor activity (Supplementary Figure 6e). In contrast, VTA BayK-induced anhedonia (Figure 4b), increased immobility (Figure 4c), and enhanced cocaine-induced locomotor activity (Figure 4e) were not observed in S831A mice. Interestingly, WT and mutant mice exhibited similar social approach behavior (Figure 4d), demonstrating a causal role of S831-GluA1 phosphorylation in the NAc for VTA BayK-induced depressive-like and cocaine-related behaviors, but not social behavior. VTA-BayK infusions had no effect on anxiety-like behavior or basal locomotor activity in either genotype (Supplementary Figure 6f–g).
Figure 4.
Repeated BayK-induced depressive-like and cocaine behavior is dependent on phosphorylation of GluA1 at S831. (a) Repeated BayK infusion in the VTA increased CaMKIIα and P-S831 GluA1 phosphorylation in the NAc examined 30 days later (CaMKIIα: t(12) = 2.589, *P=0.0237; P-T286 CaMKIIα: t(12) = 2.132, P=0.0543; P-831 GluA1: t(12) = 2.378, *P=0.0349. VTA-Veh n = 7, VTA-BayK n = 7). (b, c) Repeated BayK infusion in the VTA of WT mice but not S831A mice resulted in depressive-like behavior as revealed in (b) the SPT (Two-Way ANOVA, [genotype x pretreatment, F(1,32) = 4.983, P=0.0327]; Bonferroni post hoc test: WT: Veh vs BayK *P<0.05, BayK: WT vs S831A †P<0.05. WT-Veh n = 9, WT-BayK n = 10, S831A-Veh n = 8, S831A-BayK n = 9) and (c) FST (Two-Way ANOVA, [genotype x pretreatment, F(1,32) = 6.145, P=0.0186]; Bonferroni post hoc test: WT:Veh vs BayK *P<0.05, BayK:WT vs S831A Mut †P<0.05. WT-Veh n = 9, WT-BayK n = 10, S831A-Veh n = 8, S831A-BayK n = 9). (d) Repeated BayK infusion in the VTA of WT and S831A mutant mice induced social approach deficits in both genotypes (Two way ANOVA, [pretreatment, F(1,32) = 14.10, P=0.0007]; Bonferroni post hoc test: WT: Veh vs BayK *P<0.05, S831A: Veh vs BayK *P<0.05. WT-Veh n = 9, WT-BayK n = 10, S831A-Veh: n = 8, S831A-BayK n = 9). (e) Repeated BayK treatment in the VTA resulted in higher cocaine-induced locomotor activity in WT but not S831A mutant mice (Two-Way ANOVA, [genotype F(1,32) = 13.58, P=0.0008], Bonferroni post hoc test: WT: Veh vs BayK **P<0.01. WT-Veh n = 9, WT-BayK n = 10, S831A-Veh n = 8, S831A-BayK n = 9). Error bars represent ± s.e.m.
Significant association of CACNA1D SNPs with cocaine dependence
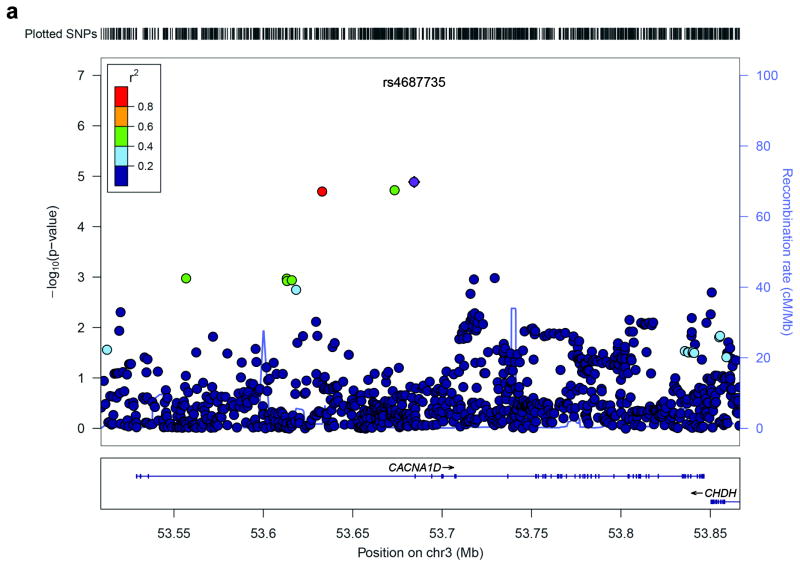
Based on our findings of a role for Cav1.3 channels in rodent cocaine behaviors, we tested whether variants within and around CACNA1D were associated with CD using the genomewide association study (GWAS data set from the Study of Addiction: Genetics and Environment (SAGE)). To examine as many variants as possible, we imputed the 1000 Genomes Project variants into CACNA1D. Specifically, we tested 947 single nucleotide polymorphism (SNPs) within and around CACNA1D in European-Americans (EA; 493 cases and 1,058 controls) and followed analyses of any significant findings in African-Americans (AA; 521 cases and 369 controls). We performed an association test in a logistic regression framework including sex, age and top three principle components as covariates. We found the strongest association with CD for intronic SNP rs4687735 in EAs (p = 1.3 × 10−5, OR = 2.46, 95% Confidence Interval (CI) = 1.65–3.67), an imputed intronic SNP (imputation quality score = 0.82) with a risk allele ‘T’ frequency of 6.5% in cases and 3.5% in controls, which remains significant after Bonferroni correction (p = 0.012). In addition, another two imputed SNPs, rs2926559 and rs898407, which are in high linkage disequilibrium (LD) with rs4687735, also showed strong association with CD (rs2926559, p=0.018 and rs898407, p=0.019 after Bonferroni correction). Figure 5 illustrates the association test results for 947 SNPs within and around CACNA1D in EAs.
Figure 5.
Regional association plot of SNPs in and around CACNA1D. (a) SNPs are plotted with their −log10 (p-value) on the y-axis along with their physical position (NCBI build 36) on the x-axis. The SNPs are color coded according to their correlations (r2) with the most significant SNP rs4687735 shown in purple. The light blue line and right y-axis indicates the observed recombination rates in the HapMap CEU samples.
We observed a trend association in the same direction as in EAs for rs4687735 in AAs (p = 0.14, OR = 2.62, 95% CI = 0.68–10.15), with risk allele ‘T’ frequency of 1.1% in cases and 0.45% in controls (imputation quality score = 0.88). Meta-analysis of the EA and AA samples showed stronger association between rs4687735 and CD (p=4.0×10−6, OR = 2.47, 95% CI = 1.68–3.63). We also observed trend associations for the rs2926559 (p = 0.13, OR = 2.42, 95% CI = 0.72–8.07) and rs898407 (p = 0.14, OR = 2.51, 95% CI = 0.68–9.23) in AAs. Supplementary Table 5 shows association results for EAs, AAs and meta-analysis for 778 SNPs that exist in both EAs and AAs.
To evaluate the association evidence at the gene level, we further computed a gene-based p-value for the EA and AA samples using GATES, a gene-based association test using extended Simes procedure50. We found significant association evidence in both EA (p=0.003) and AA (p=0.008).
DISCUSSION
Here, we demonstrate that VTA Cav1.3 channel activation robustly mediates cocaine-related, depression-related and social behavior via distinct NAc AMPAR mechanisms with no effect on anxiety-like behavior. These findings provide novel insight into VTA Cav1.3 to NAc mechanisms that likely underlie behavioral phenotypes in multiple neuropsychiatric illnesses. We propose that Cav1.3 activation may mediate co-morbid phenotypes in addiction and mood disorders. Our identification of CACNA1D SNPs associated with cocaine dependence, in combination with previous human genetic data linking CACNA1D with BD11, 12, suggests a potential role for VTA CACNA1D in mediating co-morbid behavioral endophenotypes in addiction and BD. The data also support further investigation of Cav1.3 as a potential therapeutic target for neuropsychiatric disorders with substance abuse, depression or social deficit-related phenotypes.
The current findings add to the emerging evidence for convergent mesolimbic dopamine pathway mechanisms mediating behavioral deficits in reward-related and mood disorders2, 7, 51. Our study is the first to examine Cav1.3 mechanisms across drug-related, depression-related and social-related behaviors and the first to link Cav1.3 animal studies to human GWAS data. The ability of VTA Cav1.3 activation to enhance cocaine behaviors after extended withdrawal is consistent with previous demonstration of VTA LTCC activation potentiating cocaine-induced psychomotor response14 and with a critical role of VTA Cav1.3 LTCCs in cocaine behaviors15, 16. The observed depressive-like effects of VTA Cav1.3 activation are consistent with our previous demonstration where global Cav1.3 activation resulted in depressive-like behavior28, while Cav1.3−/− mice showed an antidepressant-like response52. We additionally show for the first time that VTA Cav1.3 channel activation results in social behavioral deficits. The novelty of the present finding is our identification of the VTA as a critical brain locus mediating these Cav1.3-dependent depressive-like and social behavioral phenotypes in parallel with cocaine-induced phenotypes. Thus, we demonstrate for the first time that enhancing VTA Cav1.3 activation is a common mechanism that can induce behavioral alterations associated with substance abuse and mood-related psychiatric illnesses. Indeed, multiple investigations over the last several years have begun to elucidate specific VTA mechanisms that are activated by stress and by drugs of abuse6, 8, 51.
Importantly, our findings provide novel pathway-specific molecular insights regarding the mechanisms underlying these reward, mood, and social interaction-related behaviors. Our data show that activation of VTA Cav1.3 channels is sufficient to increase CP-AMPARs in the NAc shell; their activation, in turn, is necessary for cocaine CPP and enhanced locomotor response measured after greater than 1 month, but not 1 day, of withdrawal. These findings are generally consistent with a role for cocaine-evoked CP-AMPARs in the NAc shell in certain models of addictive behavior, although most such evidence has been obtained after cocaine self-administration31–35, 53–55. Cocaine-evoked CP-AMPARs in NAc core also mediate cocaine seeking after cocaine self-administration and prolonged withdrawal30, 54–56. It will be interesting in future studies to further examine NAc core versus shell AMPAR mechanisms in the model used in this study.
We further demonstrate that similar to cocaine, VTA Cav1.3-induced depressive-like behavior results from increase in CP-AMPARs in the NAc shell. While the role of CP-AMPARs in depressive-like behavior has not been clearly delineated in the literature, recent studies have revealed changes in CP-AMPAR expression that are associated with exposure to a stress protocol that induces anhedonia36. Vialou V. et al. 201057 also showed that mice susceptible to social defeat stress show higher levels of GluA1 expression in the NAc, with no change in GluA2 suggestive of an increase in CP-AMPARs. Additionally, CP-AMPARs in the NAc have been found to emerge in a chronic neuropathic pain-induced depression model49. Collectively these studies support the conclusion that VTA Cav1.3 activation promotes cocaine and depressive-like behavior via enhancing synaptic CP-AMPARs. One mechanism suggested to contribute to increases in CP-AMPAR levels at the synapse is S831-GluA1 phosphorylation by CaMKIIα48. We demonstrate the necessity of S831-GluA1 phosphorylation for VTA Cav1.3-mediated enhancement of cocaine and depressive-like behaviors, highlighting VTA Cav1.3 mechanistic convergence. The ability of VTA Cav1.3 activation to increase NAc CaMKIIα activity also argues for a converging CaMKIIα mechanism. Previous studies have found an increase in CaMKIIα in the NAc of rats following 14 days of withdrawal from non-contingent cocaine administration58 and in the NAc of cocaine-dependent humans58, as well as following social defeat stress59. Additionally, a SNP in the CaMK2A gene has been linked to faster transition to severe cocaine use in humans60. A direct link between CaMKIIα and formation of CP-AMPARs remains to be established, however concurrent increase in phosphorylated CaMKII and CP-AMPARs at the NAc PSD following extended withdrawal from cocaine self-administration has been observed61.
We additionally demonstrate that activation of VTA Cav1.3 channels also promotes social deficits however via enhancing GluA1/A2 AMPARs in the NAc consistent with enhanced AMPAR transmission associated with social deficits62 including in the NAc core63. To the best of our knowledge, this is the first time that Cav1.3 channels have been associated with social behavior. The recruitment of AMPARs in the NAc core versus CP-AMPARs in the NAc shell in our models of cocaine and depressive-like phenotypes suggests the possibility of recruitment of different VTA-NAc neurons mediating diverse behavioral phenotypes. This is not surprising given the highly heterogeneous nature of the VTA afferents to the NAc and other regions of the brain26, 64, a question that will be addressed in future studies. Additionally, the higher but not significant levels of S880 P-GluA2 in the PSD of NAc (core+shell) is intriguing given that this phosphorylation event has been suggested to endocytose GluA2 subunits65, 66. However, States et al. (2008)67 have reported that S880 phosphorylation can stabilize GluA2 at the PSD, that could be the case in the NAc core where we find higher GluA1/GluA2 AMPARs. In future studies, it will be interesting to further explore the role of S880 GluA2 phosphorylation in GluA2 trafficking and social behavior.
The precise VTA Cav1.3 mechanisms that promote cocaine and depressive-like behaviors remain to be answered. Preliminary findings from our laboratory suggest that Cav1.3/CaMKII/ERK2 signaling within the VTA mediates long-term molecular changes in the NAc via a CREB-dependent mechanism. CREB in the VTA has been suggested to play a role in cocaine-induced plasticity68, an area of research that remains underexplored. One potential candidate downstream of the Cav1.3/CREB pathway in the VTA that may mediate the transition from the VTA to NAc is the neurotrophic factor BDNF, a downstream target of LTCCs69, 70 and of CREB71, 72 that regulates both cocaine73 and depressive-like behavior74. Phasic firing of VTA dopamine neurons promotes cocaine-25 and depressive behaviors26, a dopamine neuron property that also facilitates BDNF release in the NAc8. BDNF in the NAc via its postsynaptic TrkB receptor, regulates CaMKII-mediated S831 GluA1 phosphorylation75, AMPAR trafficking76, 77 and cocaine and depressive behaviors73, 74, 78. Thus, BDNF produced in the VTA and released in the NAc serves as a promising candidate in mediating the effect of VTA Cav1.3 channels. Our unpublished data supports this hypothesis as we find that VTA Cav1.3 channel activation increases Bdnf transcription in the VTA. Future studies will address VTA Cav1.3-mediated Bdnf release and postsynaptic effects in the NAc on AMPAR signaling and cocaine and mood-related behaviors.
Candidate gene association analysis in humans supports the role of genetic variations within CACNA1D in CD. To the best of knowledge, this is the first report describing a genetic association between CACNA1D and CD in two independent human samples, which corroborates the functional aspects of Cav1.3 in the mice models of cocaine behavior. Considering the fact that CACNA1D SNPs can confer risk for BD11, 12 as well as the association of human CACNA1D gain of function mutations associated with neurological disorders10, including autism13, characterized by significant mood dysregulation, an important follow-up investigation would be to examine via targeted sequencing whether rare variants within CACNA1D are also associated with CD. Further fine-mapping of this gene may contribute to the identification of functional causal variants underlying CD.
In summary, findings of this study support enhanced Cav1.3 channel activity within the VTA-NAc pathway as one potential mechanism underlying drug abuse and mood disorders, with implications for co-morbid neuropsychiatric-related behavioral phenotypes, in particular those resulting from disease-associated CACNA1D SNPs. The study provides a novel framework to further understand the pathophysiology of mental illness that can aid in designing therapeutic studies using available Cav1.3-selective LTCC blockers79.
Supplementary Material
Acknowledgments
This work was supported by the National Institute of Health Grants 5R01DA029122-04 (AMRajadhyaksha), R01AA022994 (SH), R21DA038048 (NAA and AMRajadhyaksha) and the NIDA Diversity Supplement 3R01DA029122-04S2 (AMartínez-Rivera).
The SAGE GWAS dataset used for the analyses described in this manuscript were obtained from http://www.ncbi.nlm.nih.gov/sites/entrez?Db=gap through dbGaP accession number phs000092.v1.p. Funding support for the Study of Addiction: Genetics and Environment (SAGE) was provided through the NIH Genes, Environment and Health Initiative [GEI] (U01 HG004422). SAGE is one of the genomewide association studies funded as part of the Gene Environment Association Studies (GENEVA) under GEI. Assistance with phenotype harmonization and genotype cleaning, as well as with general study coordination, was provided by the GENEVA Coordinating Center (U01 HG004446). Assistance with data cleaning was provided by the National Center for Biotechnology Information. Support for collection of datasets and samples was provided by the Collaborative Study on the Genetics of Alcoholism (COGA; U10 AA008401), the Collaborative Genetic Study of Nicotine Dependence (COGEND; P01 CA089392), and the Family Study of Cocaine Dependence (FSCD; R01 DA013423). Funding support for genotyping, which was performed at the Johns Hopkins University Center for Inherited Disease Research, was provided by the NIH GEI (U01HG004438), the National Institute on Alcohol Abuse and Alcoholism, the National Institute on Drug Abuse, and the NIH contract “High throughput genotyping for studying the genetic contributions to human disease” (HHSN268200782096C).
Footnotes
Conflict of Interest
None
Author Contributions
AM-R, JH, TFT, TPG, NAA and AMR contributed to the overall experimental design, and data interpretation. AM-R, NAA and AMR wrote the manuscript. AL, RLH, and JS provided reagents and edited the manuscript. MK and RCR performed research, analyzed data and edited the paper. SH contributed to the design and performed the GWAS and wrote the manuscript.
References
- 1.Whitton AE, Treadway MT, Pizzagalli DA. Reward processing dysfunction in major depression, bipolar disorder and schizophrenia. Curr Opin Psychiatry. 2015;28(1):7–12. doi: 10.1097/YCO.0000000000000122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Post RM, Kalivas P. Bipolar disorder and substance misuse: pathological and therapeutic implications of their comorbidity and cross-sensitisation. Br J Psychiatry. 2013;202(3):172–176. doi: 10.1192/bjp.bp.112.116855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kendler KS, Prescott CA, Myers J, Neale MC. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Arch Gen Psychiatry. 2003;60(9):929–937. doi: 10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- 5.Goldman D, Oroszi G, Ducci F. The genetics of addictions: uncovering the genes. Nat Rev Genet. 2005;6(7):521–532. doi: 10.1038/nrg1635. [DOI] [PubMed] [Google Scholar]
- 6.Nestler EJ, Carlezon WA., Jr The mesolimbic dopamine reward circuit in depression. Biol Psychiatry. 2006;59(12):1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 7.Luthi A, Luscher C. Pathological circuit function underlying addiction and anxiety disorders. Nat Neurosci. 2014;17(12):1635–1643. doi: 10.1038/nn.3849. [DOI] [PubMed] [Google Scholar]
- 8.Russo SJ, Nestler EJ. The brain reward circuitry in mood disorders. Nat Rev Neurosci. 2013;14(9):609–625. doi: 10.1038/nrn3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kabir ZD, Lee AS, Rajadhyaksha AM. L-type Ca2+ channels in mood, cognition and addiction: Integrating human and rodent studies with a focus on behavioural endophenotypes. J Physiol. 2016 doi: 10.1113/JP270673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pinggera A, Striessnig J. Cav 1.3 (CACNA1D) L-type Ca2+ channel dysfunction in CNS disorders. J Physiol. 2016 doi: 10.1113/JP270672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ament SA, Szelinger S, Glusman G, Ashworth J, Hou L, Akula N, et al. Rare variants in neuronal excitability genes influence risk for bipolar disorder. Proc Natl Acad Sci U S A. 2015;112(11):3576–3581. doi: 10.1073/pnas.1424958112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ross J, Gedvilaite E, Badner JA, Erdman C, Baird L, Matsunami N, et al. A Rare Variant in CACNA1D Segregates with 7 Bipolar I Disorder Cases in a Large Pedigree. Mol Neuropsychiatry. 2016;2(3):145–150. doi: 10.1159/000448041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pinggera A, Lieb A, Benedetti B, Lampert M, Monteleone S, Liedl KR, et al. CACNA1D de novo mutations in autism spectrum disorders activate Cav1.3 L-type calcium channels. Biol Psychiatry. 2015;77(9):816–822. doi: 10.1016/j.biopsych.2014.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Licata SC, Freeman AY, Pierce-Bancroft AF, Pierce RC. Repeated stimulation of L-type calcium channels in the rat ventral tegmental area mimics the initiation of behavioral sensitization to cocaine. Psychopharmacology (Berl) 2000;152(1):110–118. doi: 10.1007/s002130000518. [DOI] [PubMed] [Google Scholar]
- 15.Schierberl K, Hao J, Tropea TF, Ra S, Giordano TP, Xu Q, et al. Cav1.2 L-type Ca(2)(+) channels mediate cocaine-induced GluA1 trafficking in the nucleus accumbens, a long-term adaptation dependent on ventral tegmental area Ca(v)1. 3 channels. J Neurosci. 2011;31(38):13562–13575. doi: 10.1523/JNEUROSCI.2315-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Degoulet M, Stelly CE, Ahn KC, Morikawa H. L-type Ca(2)(+) channel blockade with antihypertensive medication disrupts VTA synaptic plasticity and drug-associated contextual memory. Mol Psychiatry. 2016;21(3):394–402. doi: 10.1038/mp.2015.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stanika RI, Flucher BE, Obermair GJ. Regulation of Postsynaptic Stability by the L-type Calcium Channel CaV1. 3 and its Interaction with PDZ Proteins. Curr Mol Pharmacol. 2015;8(1):95–101. doi: 10.2174/1874467208666150507103716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rajadhyaksha AM, Kosofsky BE. Psychostimulants, L-type calcium channels, kinases, and phosphatases. Neuroscientist. 2005;11(5):494–502. doi: 10.1177/1073858405278236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pascoli V, Terrier J, Hiver A, Luscher C. Sufficiency of Mesolimbic Dopamine Neuron Stimulation for the Progression to Addiction. Neuron. 2015;88(5):1054–1066. doi: 10.1016/j.neuron.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 20.Tye KM, Mirzabekov JJ, Warden MR, Ferenczi EA, Tsai HC, Finkelstein J, et al. Dopamine neurons modulate neural encoding and expression of depression-related behaviour. Nature. 2013;493(7433):537–541. doi: 10.1038/nature11740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chaudhury D, Walsh JJ, Friedman AK, Juarez B, Ku SM, Koo JW, et al. Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature. 2013;493(7433):532–536. doi: 10.1038/nature11713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gunaydin LA, Grosenick L, Finkelstein JC, Kauvar IV, Fenno LE, Adhikari A, et al. Natural neural projection dynamics underlying social behavior. Cell. 2014;157(7):1535–1551. doi: 10.1016/j.cell.2014.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Y, Dore J, Chen X. Calcium influx through L-type channels generates protein kinase M to induce burst firing of dopamine cells in the rat ventral tegmental area. J Biol Chem. 2007;282(12):8594–8603. doi: 10.1074/jbc.M610230200. [DOI] [PubMed] [Google Scholar]
- 24.Liu Y, Harding M, Pittman A, Dore J, Striessnig J, Rajadhyaksha A, et al. Cav1.2 and Cav1. 3 L-type calcium channels regulate dopaminergic firing activity in the mouse ventral tegmental area. J Neurophysiol. 2014;112(5):1119–1130. doi: 10.1152/jn.00757.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Covey DP, Roitman MF, Garris PA. Illicit dopamine transients: reconciling actions of abused drugs. Trends Neurosci. 2014;37(4):200–210. doi: 10.1016/j.tins.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walsh JJ, Han MH. The heterogeneity of ventral tegmental area neurons: Projection functions in a mood-related context. Neuroscience. 2014;282:101–108. doi: 10.1016/j.neuroscience.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rajadhyaksha A, Husson I, Satpute SS, Kuppenbender KD, Ren JQ, Guerriero RM, et al. L-type Ca2+ channels mediate adaptation of extracellular signal-regulated kinase 1/2 phosphorylation in the ventral tegmental area after chronic amphetamine treatment. J Neurosci. 2004;24(34):7464–7476. doi: 10.1523/JNEUROSCI.0612-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sinnegger-Brauns MJ, Hetzenauer A, Huber IG, Renström E, Wietzorrek G, Berjukov S, et al. Isoform-specific regulation of mood behavior and pancreatic beta cell and cardiovascular function by L-type Ca 2+ channels. J Clin Invest. 2004;113(10):1430–1439. doi: 10.1172/JCI20208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kourrich S, Rothwell PE, Klug JR, Thomas MJ. Cocaine experience controls bidirectional synaptic plasticity in the nucleus accumbens. J Neurosci. 2007;27(30):7921–7928. doi: 10.1523/JNEUROSCI.1859-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y, et al. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454(7200):118–121. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mameli M, Halbout B, Creton C, Engblom D, Parkitna JR, Spanagel R, et al. Cocaine-evoked synaptic plasticity: persistence in the VTA triggers adaptations in the NAc. Nat Neurosci. 2009;12(8):1036–1041. doi: 10.1038/nn.2367. [DOI] [PubMed] [Google Scholar]
- 32.McCutcheon JE, Wang X, Tseng KY, Wolf ME, Marinelli M. Calcium-permeable AMPA receptors are present in nucleus accumbens synapses after prolonged withdrawal from cocaine self-administration but not experimenter-administered cocaine. J Neurosci. 2011;31(15):5737–5743. doi: 10.1523/JNEUROSCI.0350-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pierce RC, Wolf ME. Psychostimulant-induced neuroadaptations in nucleus accumbens AMPA receptor transmission. Cold Spring Harb Perspect Med. 2013;3(2):a012021. doi: 10.1101/cshperspect.a012021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pascoli V, Terrier J, Espallergues J, Valjent E, O’Connor EC, Luscher C. Contrasting forms of cocaine-evoked plasticity control components of relapse. Nature. 2014;509(7501):459–464. doi: 10.1038/nature13257. [DOI] [PubMed] [Google Scholar]
- 35.Terrier J, Luscher C, Pascoli V. Cell-Type Specific Insertion of GluA2-Lacking AMPARs with Cocaine Exposure Leading to Sensitization, Cue-Induced Seeking, and Incubation of Craving. Neuropsychopharmacology. 2016;41(7):1779–1789. doi: 10.1038/npp.2015.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lim BK, Huang KW, Grueter BA, Rothwell PE, Malenka RC. Anhedonia requires MC4R-mediated synaptic adaptations in nucleus accumbens. Nature. 2012;487(7406):183–189. doi: 10.1038/nature11160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hetzenauer A, Sinnegger-Brauns MJ, Striessnig J, Singewald N. Brain activation pattern induced by stimulation of L-type Ca2+-channels: contribution of Ca(V)1.3 and Ca(V)1.2 isoforms. Neuroscience. 2006;139(3):1005–1015. doi: 10.1016/j.neuroscience.2006.01.059. [DOI] [PubMed] [Google Scholar]
- 38.Giordano TP, Tropea TF, Satpute SS, Sinnegger-Brauns MJ, Striessnig J, Kosofsky BE, et al. Molecular switch from L-type Ca v 1.3 to Ca v 1.2 Ca2+ channel signaling underlies long-term psychostimulant-induced behavioral and molecular plasticity. J Neurosci. 2010;30(50):17051–17062. doi: 10.1523/JNEUROSCI.2255-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Platzer J, Engel J, Schrott-Fischer A, Stephan K, Bova S, Chen H, et al. Congenital deafness and sinoatrial node dysfunction in mice lacking class D L-type Ca2+ channels. Cell. 2000;102(1):89–97. doi: 10.1016/s0092-8674(00)00013-1. [DOI] [PubMed] [Google Scholar]
- 40.Lee HK, Takamiya K, Han JS, Man H, Kim CH, Rumbaugh G, et al. Phosphorylation of the AMPA receptor GluR1 subunit is required for synaptic plasticity and retention of spatial memory. Cell. 2003;112(5):631–643. doi: 10.1016/s0092-8674(03)00122-3. [DOI] [PubMed] [Google Scholar]
- 41.Tropea TF, Kabir ZD, Kaur G, Rajadhyaksha AM, Kosofsky BE. Enhanced dopamine D1 and BDNF signaling in the adult dorsal striatum but not nucleus accumbens of prenatal cocaine treated mice. Front Psychiatry. 2011;2:67. doi: 10.3389/fpsyt.2011.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Knackstedt LA, Moussawi K, Lalumiere R, Schwendt M, Klugmann M, Kalivas PW. Extinction training after cocaine self-administration induces glutamatergic plasticity to inhibit cocaine seeking. J Neurosci. 2010;30(23):7984–7992. doi: 10.1523/JNEUROSCI.1244-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Calin-Jageman I, Yu K, Hall RA, Mei L, Lee A. Erbin enhances voltage-dependent facilitation of Ca(v)1.3 Ca2+ channels through relief of an autoinhibitory domain in the Ca(v)1.3 alpha1 subunit. J Neurosci. 2007;27(6):1374–1385. doi: 10.1523/JNEUROSCI.5191-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tropea TF, Kosofsky BE, Rajadhyaksha AM. Enhanced CREB and DARPP-32 phosphorylation in the nucleus accumbens and CREB, ERK, and GluR1 phosphorylation in the dorsal hippocampus is associated with cocaine-conditioned place preference behavior. J Neurochem. 2008;106(4):1780–1790. doi: 10.1111/j.1471-4159.2008.05518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Inan M, Zhao M, Manuszak M, Karakaya C, Rajadhyaksha AM, Pickel VM, et al. Energy deficit in parvalbumin neurons leads to circuit dysfunction, impaired sensory gating and social disability. Neurobiol Dis. 2016;93:35–46. doi: 10.1016/j.nbd.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 46.Lee AS, Ra S, Rajadhyaksha AM, Britt JK, De Jesus-Cortes H, Gonzales KL, et al. Forebrain elimination of cacna1c mediates anxiety-like behavior in mice. Mol Psychiatry. 2012;17(11):1054–1055. doi: 10.1038/mp.2012.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bierut LJ, Agrawal A, Bucholz KK, Doheny KF, Laurie C, Pugh E, et al. A genome-wide association study of alcohol dependence. Proc Natl Acad Sci U S A. 2010;107(11):5082–5087. doi: 10.1073/pnas.0911109107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huganir RL, Nicoll RA. AMPARs and synaptic plasticity: the last 25 years. Neuron. 2013;80(3):704–717. doi: 10.1016/j.neuron.2013.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goffer Y, Xu D, Eberle SE, D’Amour J, Lee M, Tukey D, et al. Calcium-permeable AMPA receptors in the nucleus accumbens regulate depression-like behaviors in the chronic neuropathic pain state. J Neurosci. 2013;33(48):19034–19044. doi: 10.1523/JNEUROSCI.2454-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li MX, Gui HS, Kwan JS, Sham PC. GATES: a rapid and powerful gene-based association test using extended Simes procedure. Am J Hum Genet. 2011;88(3):283–293. doi: 10.1016/j.ajhg.2011.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Polter AM, Kauer JA. Stress and VTA synapses: implications for addiction and depression. Eur J Neurosci. 2014;39(7):1179–1188. doi: 10.1111/ejn.12490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Busquet P, Nguyen NK, Schmid E, Tanimoto N, Seeliger MW, Ben-Yosef T, et al. CaV1.3 L-type Ca2+ channels modulate depression-like behaviour in mice independent of deaf phenotype. Int J Neuropsychopharmacol. 2010;13(4):499–513. doi: 10.1017/S1461145709990368. [DOI] [PubMed] [Google Scholar]
- 53.Lee BR, Ma YY, Huang YH, Wang X, Otaka M, Ishikawa M, et al. Maturation of silent synapses in amygdala-accumbens projection contributes to incubation of cocaine craving. Nat Neurosci. 2013;16(11):1644–1651. doi: 10.1038/nn.3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ma YY, Lee BR, Wang X, Guo C, Liu L, Cui R, et al. Bidirectional modulation of incubation of cocaine craving by silent synapse-based remodeling of prefrontal cortex to accumbens projections. Neuron. 2014;83(6):1453–1467. doi: 10.1016/j.neuron.2014.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wolf ME. Synaptic mechanisms underlying persistent cocaine craving. Nat Rev Neurosci. 2016;17(6):351–365. doi: 10.1038/nrn.2016.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Loweth JA, Scheyer AF, Milovanovic M, LaCrosse AL, Flores-Barrera E, Werner CT, et al. Synaptic depression via mGluR1 positive allosteric modulation suppresses cue-induced cocaine craving. Nat Neurosci. 2014;17(1):73–80. doi: 10.1038/nn.3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vialou V, Robison AJ, Laplant QC, Covington HE, 3rd, Dietz DM, Ohnishi YN, et al. DeltaFosB in brain reward circuits mediates resilience to stress and antidepressant responses. Nat Neurosci. 2010;13(6):745–752. doi: 10.1038/nn.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Robison AJ, Vialou V, Mazei-Robison M, Feng J, Kourrich S, Collins M, et al. Behavioral and structural responses to chronic cocaine require a feedforward loop involving DeltaFosB and calcium/calmodulin-dependent protein kinase II in the nucleus accumbens shell. J Neurosci. 2013;33(10):4295–4307. doi: 10.1523/JNEUROSCI.5192-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Robison AJ, Vialou V, Sun HS, Labonte B, Golden SA, Dias C, et al. Fluoxetine epigenetically alters the CaMKIIalpha promoter in nucleus accumbens to regulate DeltaFosB binding and antidepressant effects. Neuropsychopharmacology. 2014;39(5):1178–1186. doi: 10.1038/npp.2013.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Easton AC, Lourdusamy A, Havranek M, Mizuno K, Solati J, Golub Y, et al. alphaCaMKII controls the establishment of cocaine’s reinforcing effects in mice and humans. Transl Psychiatry. 2014;4:e457. doi: 10.1038/tp.2014.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ferrario CR, Loweth JA, Milovanovic M, Ford KA, Galinanes GL, Heng LJ, et al. Alterations in AMPA receptor subunits and TARPs in the rat nucleus accumbens related to the formation of Ca(2)(+)-permeable AMPA receptors during the incubation of cocaine craving. Neuropharmacology. 2011;61(7):1141–1151. doi: 10.1016/j.neuropharm.2011.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Araki R, Ago Y, Hasebe S, Nishiyama S, Tanaka T, Oka S, et al. Involvement of prefrontal AMPA receptors in encounter stimulation-induced hyperactivity in isolation-reared mice. Int J Neuropsychopharmacol. 2014;17(6):883–893. doi: 10.1017/S1461145713001582. [DOI] [PubMed] [Google Scholar]
- 63.Neuhofer D, Henstridge CM, Dudok B, Sepers M, Lassalle O, Katona I, et al. Functional and structural deficits at accumbens synapses in a mouse model of Fragile X. Front Cell Neurosci. 2015;9:100. doi: 10.3389/fncel.2015.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Taylor SR, Badurek S, Dileone RJ, Nashmi R, Minichiello L, Picciotto MR. GABAergic and glutamatergic efferents of the mouse ventral tegmental area. J Comp Neurol. 2014;522(14):3308–3334. doi: 10.1002/cne.23603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim CH, Chung HJ, Lee HK, Huganir RL. Interaction of the AMPA receptor subunit GluR2/3 with PDZ domains regulates hippocampal long-term depression. Proc Natl Acad Sci U S A. 2001;98(20):11725–11730. doi: 10.1073/pnas.211132798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Seidenman KJ, Steinberg JP, Huganir R, Malinow R. Glutamate receptor subunit 2 Serine 880 phosphorylation modulates synaptic transmission and mediates plasticity in CA1 pyramidal cells. J Neurosci. 2003;23(27):9220–9228. doi: 10.1523/JNEUROSCI.23-27-09220.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.States BA, Khatri L, Ziff EB. Stable synaptic retention of serine-880-phosphorylated GluR2 in hippocampal neurons. Mol Cell Neurosci. 2008;38(2):189–202. doi: 10.1016/j.mcn.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Olson VG, Zabetian CP, Bolanos CA, Edwards S, Barrot M, Eisch AJ, et al. Regulation of drug reward by cAMP response element-binding protein: evidence for two functionally distinct subregions of the ventral tegmental area. J Neurosci. 2005;25(23):5553–5562. doi: 10.1523/JNEUROSCI.0345-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ghosh A, Carnahan J, Greenberg ME. Requirement for BDNF in activity-dependent survival of cortical neurons. Science. 1994;263(5153):1618–1623. doi: 10.1126/science.7907431. [DOI] [PubMed] [Google Scholar]
- 70.Tao X, Finkbeiner S, Arnold DB, Shaywitz AJ, Greenberg ME. Ca2+ influx regulates BDNF transcription by a CREB family transcription factor-dependent mechanism. Neuron. 1998;20(4):709–726. doi: 10.1016/s0896-6273(00)81010-7. [DOI] [PubMed] [Google Scholar]
- 71.Hong EJ, McCord AE, Greenberg ME. A biological function for the neuronal activity-dependent component of Bdnf transcription in the development of cortical inhibition. Neuron. 2008;60(4):610–624. doi: 10.1016/j.neuron.2008.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.West AE, Chen WG, Dalva MB, Dolmetsch RE, Kornhauser JM, Shaywitz AJ, et al. Calcium regulation of neuronal gene expression. Proc Natl Acad Sci U S A. 2001;98(20):11024–11031. doi: 10.1073/pnas.191352298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li X, Wolf ME. Multiple faces of BDNF in cocaine addiction. Behav Brain Res. 2015;279:240–254. doi: 10.1016/j.bbr.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wook Koo J, Labonte B, Engmann O, Calipari ES, Juarez B, Lorsch Z, et al. Essential Role of Mesolimbic Brain-Derived Neurotrophic Factor in Chronic Social Stress-Induced Depressive Behaviors. Biol Psychiatry. 2015 doi: 10.1016/j.biopsych.2015.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Caldeira MV, Melo CV, Pereira DB, Carvalho R, Correia SS, Backos DS, et al. Brain-derived neurotrophic factor regulates the expression and synaptic delivery of alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor subunits in hippocampal neurons. J Biol Chem. 2007;282(17):12619–12628. doi: 10.1074/jbc.M700607200. [DOI] [PubMed] [Google Scholar]
- 76.Reimers JM, Loweth JA, Wolf ME. BDNF contributes to both rapid and homeostatic alterations in AMPA receptor surface expression in nucleus accumbens medium spiny neurons. Eur J Neurosci. 2014;39(7):1159–1169. doi: 10.1111/ejn.12422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li X, Wolf ME. Brain-derived neurotrophic factor rapidly increases AMPA receptor surface expression in rat nucleus accumbens. Eur J Neurosci. 2011;34(2):190–198. doi: 10.1111/j.1460-9568.2011.07754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McGinty JF, Whitfield TW, Jr, Berglind WJ. Brain-derived neurotrophic factor and cocaine addiction. Brain Res. 2010;1314:183–193. doi: 10.1016/j.brainres.2009.08.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zamponi GW. Targeting voltage-gated calcium channels in neurological and psychiatric diseases. Nat Rev Drug Discov. 2016;15(1):19–34. doi: 10.1038/nrd.2015.5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.