Abstract
Aim
Studies have linked the use of inhaled corticosteroids (ICSs) to excess pneumonia risk in chronic obstructive pulmonary disease patients. The risk in asthma patients remains unclear. The objective of the present study was to examine the risk of pneumonia with ICSs in asthma patients aged 12–35 years.
Methods
We formed a cohort of asthma patients treated from 1990 to 2007 using Quebec health insurance databases. Subjects were considered currently exposed if they had had an ICS dispensed within the 60 days prior to their pneumonia index event or matched person‐moment. Secondary analyses investigated the risk of pneumonia according to ICS dose and type. Rate ratios (RRs) and rate differences (RDs) were both estimated through a quasi‐cohort approach.
Results
The cohort included 152 412 subjects, of whom 1928 had a pneumonia event during follow‐up. There was an increased risk of pneumonia associated with current use of ICSs [RR 1.83; 95% confidence interval (CI) 1.57, 2.14] or an excess risk of 1.44 cases per 1000 person‐years (RD 1.44; 95% CI 1.03, 1.85). There was an excess pneumonia risk with low doses (RR 1.60; 95% CI 1.06, 2.45), moderate doses (RR 1.53; 95% CI 1.12, 2.08) and high doses (RR 1.96; 95% CI 1.64, 2.34) of ICSs, and with budesonide (RR 2.67; 95% CI 2.05, 3.49) and fluticasone (RR 1.93; 95% CI 1.58, 2.36), specifically relative to no use. When accounting for potential protopathic bias, the risk with current use of ICSs was attenuated (RR 1.48; 95% CI 1.22, 1.78).
Conclusion
ICS use in asthma patients appears to be associated with an increased risk of pneumonia and is present for both budesonide and fluticasone.
Keywords: adverse events, asthma, cohort study, inhaled corticosteroids, pneumonia, quasi‐cohort
What is Already Known about this Subject
Inhaled corticosteroid (ICS) use increases risk of pneumonia in chronic obstructive pulmonary disease patients.
One type of ICS was found not to increase the risk of pneumonia in asthma patients.
What this Study Adds
ICS use appears to be associated with an increased risk of pneumonia in asthma patients.
Higher doses of ICSs are associated with a higher risk of pneumonia.
Both fluticasone and budesonide appear to be associated with a risk of pneumonia.
Tables of Links
| TARGETS |
|---|
| Nuclear hormone receptors 2 |
| Glucocorticoid receptor http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2534 |
These Tables list key protein targets and ligands in this article that are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY 1, and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 2.
Introduction
Inhaled corticosteroids (ICSs) are the first‐line treatment for patients with persistent asthma. ICSs have been shown to have a good efficacy and safety profile 3. However, complications have been found to be linked to ICS use, including a reduction in bone density, ocular hypertension, skin bruising, diabetes onset and progression, suppression of growth in children and decreased immunity 4, 5, 6, 7. Furthermore, the Towards a Revolution in COPD Health (TORCH) trial 8, as well as several large observational studies, found an excess pneumonia risk in chronic obstructive pulmonary disease (COPD) patients treated with ICSs 9, 10.
While the increase in the risk of pneumonia in association with ICS use in COPD patients seems clear, the risk in asthma patients remains contentious. In a meta‐analysis of randomized controlled trials involving asthma patients using budesonide or budesonide/formoterol, O′Byrne et al. 11 found a significant decrease in the hazard ratio (HR) of pneumonia adverse events, and an inconclusive effect on pneumonia severe adverse events. However, Zhang et al. 12 found an almost four‐fold increase in Streptococcus pneumoniae upper respiratory tract colonization in children using ICSs. In a case–control study with 6857 cases of pneumonia or lower respiratory tract infection (LRTI) among subjects with asthma aged 18–80 years, McKeever et al. 13 found an increased risk with ICS use, especially at higher doses. As a result of these contradictory findings, we conducted a population‐based cohort study of treated patients with asthma to assess whether ICS use is associated with an increased risk of pneumonia, and to evaluate whether the risk varied according to the dose and type of ICS.
Methods
Source of data
The source population was identified from the administrative health databases of the Régie de l'assurance maladie du Québec (RAMQ). This database captures the physician visits, procedures, hospitalizations and demographics for the entire population of the province, consisting of more than 7 million people. The provincial pharmacy claims databases are also included in the RAMQ database, consisting of approximately 42% of the entire Quebec population. This is because the public insurance is available only to people over the age of 65, people without access to a private plan, welfare recipients and children of persons covered by the public plan 14, 15. As a result, the people of highest income may be underrepresented in this database, as they tend to have private plans; nevertheless, the RAMQ still includes a large number of people of average socioeconomic status 16. Furthermore, the RAMQ databases have previously been used extensively to conduct epidemiological studies of the risk and benefits of ICSs 7, 9, 10, 17, 18, 19, 20, 21, and have been proven valid and comprehensive 22, 23, 24.
Study design
The source population for the present study consisted of all subjects with at least one prescription for a respiratory medication between 1 January 1990 and 31 December 2007. These included: any form of β‐agonist, theophylline, ipratropium bromide, tiotropium, sodium cromoglycate, nedocromil, ketotifen, leukotriene antagonists or ICSs. The study population of patients with asthma was formed by restricting the cohort to subjects aged 12–35 years at cohort entry, to stand the best chance of eliminating COPD patients, as there is often overlap in diagnoses between asthma and COPD among older subjects 25. Subjects with any mention of COPD prior to cohort entry were also excluded. To ensure a new‐user cohort, cohort entry was defined as the date of dispensing of the first prescription, with at least 12 months of no prior respiratory medication use. All subjects in the cohort were followed up from cohort entry to first hospitalization for pneumonia, death, end of RAMQ drug coverage or 31 December 2007, whichever came first.
A population‐based quasi‐cohort approach was used to allow better representation of the underlying cohort used for the nested case–control analysis 26. All outcome events were selected, along with their exposure classification at the time of the event. The outcome events were defined as a first hospitalization with a diagnosis of pneumonia of any cause identified in the RAMQ hospitalization database [International Classification of Diseases, Ninth Revision (ICD‐9) codes 480–487.0 inclusive; ICD 10 codes J10.0, J11.0, J12‐J18] during the cohort follow‐up. These hospitalizations include any admissions or deaths due to pneumonia, as well as those with primary or secondary diagnoses of pneumonia. The dates of these hospital admissions were defined as the index pneumonia person‐moment (all events and exposures are examined for each person on each day). These outcome events were matched to 10 event‐free person‐days based on age (±1 year), cohort entry (±1 month) and follow‐up (±1 month). When fewer than 10 event‐free person‐days were available, all available index person‐moments were used to match to the outcome index person‐moment.
All prescriptions for ICSs, alone or in a combination inhaler, dispensed between the cohort entry date and the index person‐moment, were identified. Formulations of ICSs were noted to allow classification into subgroups of budesonide, fluticasone and others, consisting of beclomethasone dipropionate, ciclesonide and mometasone. The estimation of equivalent amount of doses was based on the Canadian asthma consensus report, as well as the National Asthma Education Expert Panel report 2 27, 28. Accordingly, the equivalent doses for ICSs are beclomethasone 100 μg, budesonide 80 μg, triamcinolone 200 μg, fluticasone 50 μg and flunisolide 200 μg. The converted doses for each subject are classified into categories of high (fluticasone ≥500 μg day–1), moderate (fluticasone 250–499 μg day–1) and low (fluticasone <250 μg day–1) 27.
Data analysis
Both crude quasi‐rate ratios (RRs) as well as crude and adjusted quasi‐rate differences (RDs) of pneumonia associated with ICS use were estimated using methods proposed by Suissa 26. Adjusted quasi‐RRs with 95% confidence interval (CIs) were estimated by conditional logistic regression to account for matching. Covariates adjusted for included age (matched by design), gender, severity of disease and other comorbidity associated with a risk of pneumonia. Severity of disease was assessed independently of ICS use, via the number of other respiratory medications prescribed and the number of hospitalizations related to asthma in the prior year. For oral corticosteroids, only use within the last 3 months was considered as this would be an indication of severe asthma or recent asthma exacerbation, and would thus be critical to the risk of pneumonia. We also excluded antibiotic prescriptions in the 30 days prior to the index pneumonia event or matched person‐moment as it could represent initial therapy for pneumonia. Other conditions correlated to the risk of pneumonia were measured based on prescriptions for conditions of interest which may cause immune system depression or loss of mobility. These included medications prescribed for diabetes (insulin or oral hypoglycaemic agents), cardiovascular diseases (cardiotropic drugs, antihypertensive agents, diuretics or vasodilators), central nervous system conditions (benzodiazepines, major tranquillizers, anticonvulsants or drugs for parkinsonism) and rheumatic diseases (gold salts, methotrexate, azathioprine, hydroxychloroquine or chloroquine). Use of non‐steroidal anti‐inflammatory drugs, antidepressants and narcotics were also adjusted for.
Subjects were considered current users of ICSs if they had a prescription dispensed within the 60 days prior to the pneumonia index event or matched person‐moment, and non‐users otherwise. The primary analysis assessed the risk of pneumonia with all ICS use. Secondary analyses assessed differences in risk by formulation and dose of ICS. Exposed users were further classified into users of fluticasone, budesonide or others, and each ICS dose was further classified into high, moderate and low using their fluticasone‐equivalent dosage. All analyses were carried out with non‐exposed subjects as the reference group.
Sensitivity analyses were undertaken first to explore possible misclassification of the outcome due to misdiagnosis of pneumonia, by including only hospitalizations with a primary diagnosis of pneumonia, or death from pneumonia while in hospital. Secondly, we included an analysis exploring possible protopathic bias 29, a scenario where it may have been possible that a patient was only prescribed a respiratory medication upon early signs or symptoms of pneumonia which may have been misinterpreted as asthma. In the present analysis, we excluded the last 15 days immediately prior to the pneumonia index event or matched person‐moment. In other words, a subject was only considered to be exposed to ICSs if their prescription had taken place between 60 and 16 days, inclusive, prior to the pneumonia or matching person‐moment. Thirdly, we separated the non‐users of ICSs into past‐ and never users, defined as having a prescription in the 61–365 days prior to the pneumonia index event or matched person‐moment and not having a prescription within the year prior to the event or matching person‐moment, respectively. To ensure that we were comparing current users of ICSs to non‐ICS users who were being actively treated for asthma, we created a comparison group consisting of subjects currently using other respiratory medication in the 60 days prior to the event or matching person‐moment. Respiratory medications other than ICSs included any form of β‐agonist, theophylline, ipratropium bromide, tiotropium, sodium cromoglycate, nedocromil, ketotifen or leukotriene antagonists. This analysis was also repeated accounting for protopathic bias by not considering the last 15 days of therapy prior to the pneumonia index event or matched person‐moment. In a similar vein, in order to ensure that all subjects had actively treated asthma at cohort entry, we included a sensitivity analysis where cohort members were more strictly defined as having had three respiratory medication prescriptions including ICSs, on at least two occasions, over the span of 1 year. All analyses were conducted using SAS V.9.4 (Cary, NC). We obtained ethics approval for the study from the Research Ethics Committee of the Jewish General Hospital, Montreal, Quebec, Canada.
Results
A total of 1 410 169 patients dispensed a respiratory medication were identified between 1 January 1990 and 31 December 2007. After excluding patients outside the age range of 12–35 years, prevalent users and those without enough information in the database, the study cohort consisted of 152 412 subjects (Figure 1). This study cohort was followed up for an average of 4.8 years; subjects were, on average, 24.2 years old at cohort entry and 34.9% were male.
Figure 1.
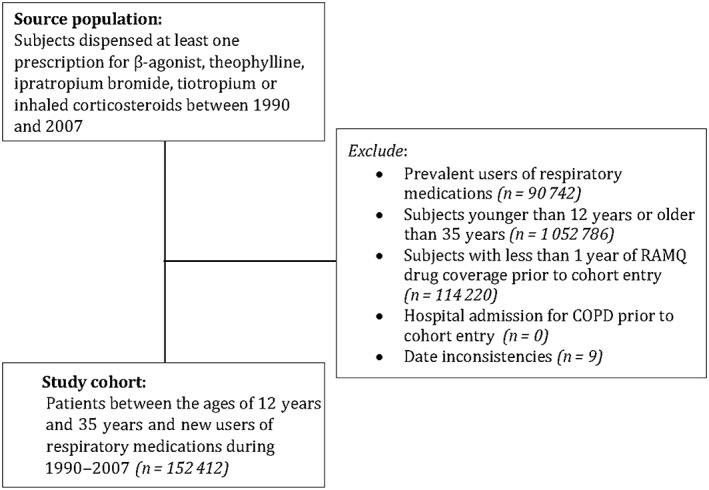
Flow diagram describing the assembly of the study cohort using the Régie de l'assurance maladie du Québec (RAMQ) healthcare insurance database. COPD, chronic obstructive pulmonary disease
A total of 1928 patients with the outcome of pneumonia hospitalization were then identified. These included all diagnosis entries for pneumonia, including primary, secondary, admission and death. The quasi‐cohort consisted of 19 275 person‐days (10‐to‐one matching with one patient having only five control person‐moments), selected by incidence density random sampling from a total of 267.3 million person‐days of follow‐up in the full study cohort. Table 1 shows the baseline characteristics of the quasi‐cohort by exposure. The non‐users of ICSs were slightly older and there were fewer males at cohort entry: 30.8 years old and 29.8% male, as opposed to 30.4 years of age and 31.0% male in users of ICSs. In addition, the ICS users had a higher prevalence of markers of asthma severity, and the non‐users had a higher prevalence of other comorbidity.
Table 1.
Baseline characteristics of the quasi‐cohort based on exposure
| Variables | Users | Non‐users |
|---|---|---|
| Number of subjects | 1769, (100.0) | 17506, (100.0) |
| Age (years), mean ± SD | 30.37 ± 8.34 | 30.82 ± 7.89 |
| Sex, n (%) men | 549, (31.03) | 5213, (29.78) |
| Hospitalizations in the year prior to index person‐moment | ||
| Hospitalization for asthma, n (%) | 9, (0.51) | 18, (0.10) |
| Number of hospitalizations for asthma (mean ± SD) | 0.01 ± 0.08 | 0.00 ± 0.03 |
| Hospitalization for pneumonia in the year prior to cohort entry, n (%) | 5, (0.28) | 30, (0.17) |
| Respiratory medication use in the year prior to index person‐moment | ||
| Respiratory drugs (no. of prescriptions), mean ± SD | 3.37 ± 5.89 | 0.85 ± 2.12 |
| Long‐acting muscarinic‐receptor antagonists, n (%) b | 2, (0.11) | 5, (0.03) |
| LAMA (no. of prescriptions), mean ± SD | 0.01 ± 0.26 | 0.00 ± 0.13 |
| LABAs, n (%) b | 141, (7.97) | 360, (2.06) |
| LABAs (no. of prescriptions), mean ± SD | 0.41 ± 1.88 | 0.06 ± 0.59 |
| SAMAs, n (%) b | 85, (4.80) | 209, (1.19) |
| SAMAs (no. of prescriptions), mean ± SD | 0.26 ± 1.70 | 0.03 ± 0.48 |
| SABAs, n (%) b | 1287, (72.75) | 6352, (36.28) |
| SABAs (no. of prescriptions), mean ± SD | 2.53 ± 3.93 | 0.68 ± 1.65 |
| Theophylline, n (%) b | 75, (4.24) | 916, (5.23) |
| Theophylline (no. of prescriptions), mean ± SD | 0.15 ± 1.31 | 0.07 ± 0.44 |
| Oral corticosteroids, a n (%) b | 173, (9.78) | 265, (1.51) |
| Oral corticosteroids (no. of prescriptions), a mean ± SD | 0.12 ± 0.48 | 0.02 ± 0.26 |
| Other medication use in the year prior to index person‐moment | ||
| Antibiotics (no. of prescriptions), c mean ± SD | 1.45 ± 2.08 | 1.16 ± 1.91 |
| Diabetes drugs, n (%) | 72, (4.07) | 393, (2.24) |
| Cardiac drugs, n (%) | 204, (11.53) | 1523, (8.70) |
| Central nervous system drugs, n (%) | 552, (31.20) | 4725, (26.99) |
| Osteoporosis drugs, n (%) | 19, (1.07) | 160, (0.91) |
| Rheumatic Disease drugs, n (%) | 4, (0.23) | 81, (0.46) |
| NSAIDs, n (%) | 542, (30.64) | 4756, (27.17) |
| Antidepressants, n (%) | 367, (20.75) | 2622, (14.98) |
| Narcotics, n (%) | 399, (22.56) | 3387, (19.35) |
LABA, long‐acting β‐agonists; LAMA, long‐acting muscarinic‐receptor antagonists; NSAID, nonsteroidal anti‐inflammatory drug; SABA, short‐acting β‐agonists; SAMA, short‐acting muscarinic‐receptor antagonists; SD, standard deviation
Use in 3 months prior to index person‐moment
Not included in analyses
30 days prior to index person‐moment is excluded
Table 2 shows that, after adjustment for differences in covariates, current ICS use was associated with an 83% increase in the rate of pneumonia hospitalization (RR 1.83; 95% CI 1.57, 2.14), or an excess risk of 1.44 cases per 1000 person‐years (RD 1.44; 95% CI 1.03, 1.85). Again, using non‐users as the reference group, low‐dose ICS was found to be associated with a 60% increase in risk (RR 1.60; 95% CI 1.06, 2.45); moderate dose ICS was associated with a 53% increase in risk (RR 1.53; 95% CI 1.12, 2.08); and high‐dose ICS was associated with a 96% increase in risk (RR 1.96; 95% CI 1.64, 2.34). The risk of pneumonia was also found to be increased with use of budesonide by 2.67 times (RR 2.67; 95% CI 2.05, 3.49), and fluticasone by 1.93 times (RR 1.93; 95% CI 1.58, 2.36), while the other ICS group yielded a nonstatistically significant increase (RR 1.23; 95% CI 0.92, 1.63).
Table 2.
Main analysis comparing the risk of pneumonia in ICS users with that in non‐users
| No. with pneumonia | No. quasi‐cohort (person‐days) | Quasi‐rates per 1000 person‐years | Rate ratio | ||
|---|---|---|---|---|---|
| Crude | Adjusteda (95% CI) | ||||
| Total number | 1928 | 19 275 | 2.63 | ||
| Non‐users b | 1515 | 17 442 | 2.29 | 1.00 | Ref |
| Current users | 413 | 1833 | 5.93 | 2.59 | 1.83 (1.57, 2.14) |
| Secondary analysis – dose c | |||||
| Low dose | 31 | 180 | 4.54 | 1.98 | 1.60 (1.06, 2.45) |
| Moderate dose | 72 | 342 | 5.54 | 2.42 | 1.53 (1.12, 2.08) |
| High dose | 310 | 1311 | 6.23 | 2.72 | 1.96 (1.64, 2.34) |
| Secondary analysis – type | |||||
| Budesonide | 96 | 362 | 6.98 | 3.05 | 2.67 (2.05, 3.49) |
| Fluticasone | 240 | 970 | 6.52 | 2.85 | 1.93 (1.58, 2.36) |
| Other ICSs | 77 | 501 | 4.05 | 1.77 | 1.23 (0.92, 1.63) |
CI, confidence interval; ICS, inhaled corticosteroid
Adjusted for all of the factors listed in Table 1 unless otherwise indicated.
Reference group (Ref) for primary and secondary analyses; refers to no use of ICS (fluticasone, budesonide, beclomethasone, flunisolide or triamcinolone) in the 60 days prior to the index person‐moment.
Daily dose in fluticasone equivalents, in μg day–1; low (fluticasone <250 μg day–1); moderate (fluticasone 250–499 μg day–1); high (fluticasone ≥500 μg day–1).
Sensitivity analyses
All results from sensitivity analyses included in the study are shown in Table 3. By excluding subjects with pneumonia as a secondary hospitalization diagnosis, current ICS exposure was found to be associated with a 91% increase in the rate of pneumonia (RR 1.91; 95% CI 1.56, 2.33). Moderate and high doses of ICS were also associated with an excess risk, as well as all types of ICS.
Table 3.
Sensitivity analyses
| No. with pneumonia | No. quasi‐cohort (person‐days) | Quasi‐rates per 1000 person‐years | Rate ratio | ||
|---|---|---|---|---|---|
| Crude | Adjusteda (95% CI) | ||||
| Sensitivity analysis 1: excluding outcomes of hospitalization with pneumonia as secondary diagnosis | |||||
| Non‐users b | 933 | 10 872 | 1.41 | 1.00 | Ref |
| Current users | 268 | 1133 | 3.88 | 2.76 | 1.91 (1.56, 2.33) |
| Sensitivity analysis 2: excluding last 15 days prior to index person‐moment | |||||
| Non‐users b | 1662 | 18 190 | 2.41 | 1.71 | Ref |
| Current users | 266 | 1085 | 6.46 | 2.68 | 1.48 (1.22, 2.78) |
| Sensitivity analysis 3: classifying non‐users into past users and never users | |||||
| Never users c | 1201 | 14 676 | 2.15 | 1.00 | Ref |
| Past users | 314 | 2766 | 2.99 | 1.39 | 1.12 (0.96, 1.30) |
| Current users | 413 | 1833 | 5.93 | 2.75 | 1.88 (1.60, 2.20) |
| Sensitivity analysis 4: stratifying non‐ICS users into current and non‐current users of other respiratory medications | |||||
| Non‐ICS users currently using other respiratory medications | |||||
| Current users | 413 | 1833 | 3.69 | 1.82 | 1.61 (1.38, 1.89) |
| Non‐users b | 1257 | 10 157 | 2.03 | Ref | Ref |
| Non‐ICS users not currently using other respiratory medications | |||||
| Current users | 413 | 1833 | 2.81 | 6.36 | 4.81 (3.56, 6.51) |
| Non‐users b | 258 | 7285 | 0.44 | Ref | Ref |
| Sensitivity analysis 5: stratifying non‐ICS users and excluding last 15 days prior to index‐person moment | |||||
| Non‐ICS users currently using other respiratory medications | |||||
| Current users | 214 | 969 | 3.62 | 1.67 | 1.32 (1.09, 1.60) |
| Non‐users b | 1456 | 11 021 | 2.16 | Ref | Ref |
| Non‐ICS users not currently using other respiratory medications | |||||
| Current users | 214 | 969 | 2.49 | 6.24 | 3.07 (2.08, 4.51) |
| Non‐users b | 258 | 7285 | 0.40 | Ref | Ref |
| Sensitivity analysis 6: restricting asthma patients to those with three prescriptions on at least two occasions over the span of 1 year | |||||
| Current users | 189 | 946 | 4.58 | 2.47 | 1.74 (1.37, 2.21) |
| Non‐users b | 389 | 4801 | 1.86 | Ref | Ref |
CI, confidence interval
Adjusted for all of the factors listed in Table 1 unless otherwise indicated.
Reference group (Ref); refers to no use of ICS (fluticasone, budesonide, beclomethasone, flunisolide or triamcinolone) in the 60 days prior to the index person‐moment.
Reference group; refers to no use of ICS (fluticasone, budesonide, beclomethasone, flunisolide or triamcinolone) in the 365 days prior to the index person‐moment.
When accounting for potential protopathic bias by excluding the last 15 days of medication use, exposure to ICSs was found to be still associated with a 48% increased risk of pneumonia (RR 1.48; 95% CI 1.22, 1.78), and high doses, budesonide and fluticasone remained associated with an increased, but attenuated risk of pneumonia.
By further classifying the non‐users of ICSs into past and never users, current use was found to be associated with a similar higher risk (RR 1.88; 95% CI 1.60, 2.20), and past use with a nonsignificant risk (RR 1.12; 95% CI 0.96, 1.30).
When only including as non‐users of ICSs subjects currently using other respiratory medications, current use of ICSs was found to be associated with a smaller but nevertheless significant increase in risk (RR 1.61; 95% CI 1.38, 1.89). By further excluding the last 15 days prior to the index person‐moment, with non‐users currently using other respiratory medications as the reference group, the risk of pneumonia remained with current use of ICSs, although the effect was more modest (RR 1.32; 95% CI 1.09, 1.60).
Finally, by limiting the study to subjects with three or more prescriptions for respiratory medications at cohort entry, the results were similar, showing an increase in risk with current use of ICSs, albeit with wider confidence intervals due to the smaller cohort size (578 subjects with a total of 5747 person‐days); RR 1.74; 95% CI 1.37, 2.21. Figure 2 summarizes the RR estimates of all sensitivity analyses as compared with the primary analysis using a forest plot.
Figure 2.
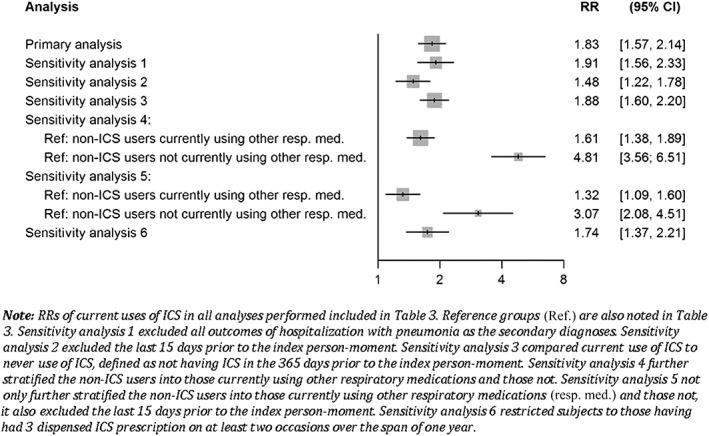
Forest plot of rate ratios (RRs) of hospitalization for pneumonia. CI, confidence interval; ICS, inhaled corticosteroid
Discussion
In a large cohort of over 150 000 patients treated with asthma medications, we found that current ICS use is associated with a significant 83% increase in the risk of being hospitalized for pneumonia. This risk was greatest with higher doses, with the dispensing of 500 μg or more of fluticasone‐equivalent per day being associated with a 96% increase. This risk remained significant with lower doses of ICSs. This risk was also found to be significant in relation to the use both of budesonide (167% increase in risk) and fluticasone (93% increase in risk). While the risk appears to be greater with budesonide, the confidence intervals overlap, such that we could not conclude that there is a difference in risk between these two agents. With the quasi‐cohort approach, we were also able to estimate rate differences, allowing conclusions to be made on the absolute scale. Per 1000 person‐years, the overall use of ICSs was found to be associated with 1.44 extra cases of hospitalized pneumonia. When considering the possibility that prescriptions for an ICS near a pneumonia event might actually be due to early signs of pneumonia (protopathic bias), and when restricting the comparison group to subjects with more active treatment for respiratory disease, the risk of pneumonia with current use of ICSs remained but was attenuated at 32% (95% CI of 9%, 60%). The results of these sensitivity analyses addressing confounding by indication as well as protopathic bias are likely to be more representative of the true magnitude of the effect of ICSs on pneumonia risk in asthma patients with similar levels of severity.
Overall, our findings disagreed with the conclusions of the only other study published that directly assessed this relationship; O'Byrne et al. 11 found an HR of 0.52 (95% CI 0.36, 0.76) for pneumonia as an adverse event, and 1.29 (95% CI 0.53, 3.12) for pneumonia as a serious adverse event. This discrepancy may be due to the fact that the study by O'Byrne et al. obtained outcome data from clinical trials, where pneumonia was recorded as a secondary outcome. This means that these cases are likely to have been milder than those in our study, where our outcome definition was hospitalization for pneumonia. In addition, because patients in clinical trials are more closely monitored, they may be healthier and have better‐controlled asthma, putting them at lower risk of being hospitalized for pneumonia. A recent meta‐analysis of clinical trials found a lower risk of pneumonia with ICSs, although withdrawals were highly imbalanced between groups 30. Furthermore, the benefit was no longer significant when excluding the largest trial which included as pneumonia events outcomes such as mucus plugging and atelectasis that may be benefitted by the use of ICSs 30.
However, our findings were comparable with those of other observational studies that have alluded to this relationship 30. For example, Zhang et al.'s 12 cross‐sectional study in children found a large and significant increase in the risk of oropharyngeal colonization with S. pneumoniae in asthmatic children associated with ICS use, and, while colonization does not necessarily lead to pneumonia, it is likely to increase the risk, especially as S. pneumoniae remains a common cause of bacterial pneumonia in children and adults 31. The case–control study by McKeever et al. 13 also found that ICS use is associated with an increase in the risk of pneumonia as well as LRTIs. Furthermore, the latter study found a dose–response relationship in the 18–40‐year‐old population, where the results suggested that the risks are higher with higher doses. Similarly, an increased risk in pneumonia associated with ICS use was also found in COPD studies using RAMQ databases 9, 10. While ICSs are not expected to have the same efficacy and safety profile in COPD patients as in asthma patients, the association between ICSs and pneumonia in COPD patients may be instructive. In these studies, the overall increase in the risk of pneumonia hospitalization was found to be approximately 70%, which is comparable with the risk we found in the present analysis. While COPD patients are more likely to acquire pneumonia as the result of a higher likelihood of lower‐airway bacterial colonization 32, 33, the relative risk between ICS users and non‐users in COPD and in asthma patients appears to be comparable.
However, the findings of an association between the risk of pneumonia and the different ICSs do not agree with those of previous studies. While previous studies generally found fluticasone to be associated with a higher risk 13, 34, our results showed that budesonide and fluticasone are both similarly associated with a significant increase in the risk of pneumonia. However, in the McKeever et al. study 13, only fluticasone was found to be associated with a significant increase in the risk of pneumonia and LRTI in the 18–40‐year‐old population 13. In addition, in the COPD case–control study by Suissa et al. 10, budesonide was found to be associated with a lower, but still significant 17% increase in risk (95% CI 1.09, 1.26), while fluticasone had a much higher increase in risk (RR 2.01; 95% CI 1.93, 2.10). Other COPD studies included in the Kew et al. 34 meta‐analysis also showed fluticasone to be associated with an overall higher risk of pneumonia. While the pathophysiological differences between asthma and COPD may have contributed to this discrepancy, it is likely that our different outcomes of interest also led to different results. While we included all hospitalizations with pneumonia diagnoses as the outcome of interest, the McKeever et al. study [13] also included LRTI as the outcome, and the COPD studies included in the Kew et al. meta‐analyses 34 were all randomized controlled trials where the cases of pneumonia were principally milder.
The present study had both strengths and limitations. Using the number of prescriptions for all classes of asthma medications, and asthma hospitalizations present in the year prior to index person‐moment, as well as pneumonia hospitalizations and oral corticosteroids use prior to cohort entry, we were able to adjust for both the severity of asthma and other comorbidities, both of which can bias the relationship between ICS use and pneumonia as an outcome. The possibility of residual confounding arising from unmeasured factors remains a concern. For example, in the present study, had the data been available, smoking and other environmental exposures would have been important factors to consider.
Similarly, due to the nature of the study, pneumonia outcomes were identified based on diagnosis codes and not confirmed through further clinical testing. It is therefore possible that these cases of pneumonia were in fact episodes of misdiagnosed asthma exacerbation. However, this is unlikely to have explained our results, as previous studies have shown that regular ICS use decreases the risk of asthma exacerbations 35, 36, 37. Furthermore, previous studies have generally shown that the use of database hospital codes in identifying pneumonia outcomes is valid and accurate 38, 39, 40. Patients with asthma in the present study were identified based on only one prescription of respiratory medication. It is likely that some included subjects may not have had asthma, and were only medicated for a temporary condition, would be less likely to receive ICSs and would inherently be at lower risk of pneumonia than patients with true asthma 41. Such a bias should have been minimized by adjusting for the number of prescriptions for respiratory medications and asthma hospitalizations in the past year. Furthermore, the association persisted after restricting the analysis to patients more likely to have active asthma. Restricting our study to subjects under the age of 35 years will have minimized the inclusion of patients with COPD.
Other strengths of the study stem from our consideration of potential protopathic bias. By excluding the 15 days prior to the index person‐moment, we were able to provide results that may better represent the magnitude of the relationship between ICSs and pneumonia in the asthmatic population. In addition, by further classifying the non‐users into past and never users, we were able further to understand the duration of the effect that ICSs would have had on the risk of pneumonia. As was shown in the sensitivity analysis, only current users of ICSs show a significant increase in the risk of pneumonia. Lastly, by using the quasi‐cohort approach, we were able not only to represent better the relationship between users and non‐users, but also to estimate the risk difference, thus allowing a better representation of the risk on the absolute scale. This can facilitate physicians and decision makers better to understand the magnitude of the impact of the association.
In conclusion, the present study showed that the risk of pneumonia – in particular, serious pneumonia leading to hospitalization – in asthma patients is indeed elevated in relation to ICS use. Both budesonide and fluticasone are associated with a statistically significant increase in the risk of pneumonia. Interestingly, while the conclusions of our study are generally consistent with those of the previous literature, our study further suggests that the risk profile of budesonide is in fact similar to that of fluticasone. However, provided that it is ensured that only patients actively treated for asthma are included, and that the use of ICSs is not in fact a marker for early signs of pneumonia, the increase in risk is relatively small. In future studies, it would be helpful further to validate the diagnosis of pneumonia, identify subjects with a confirmed diagnosis of asthma and adjust for smoking.
Competing Interests
Dr Samy Suissa has received research grants and participated in advisory board meetings and/or as a speaker at conferences for Boehringer Ingelheim, AstraZeneca, Merck & Co, Novartis and Pfizer.
Qian, C. J. , Coulombe, J. , Suissa, S. , and Ernst, P. (2017) Pneumonia risk in asthma patients using inhaled corticosteroids: a quasi‐cohort study. Br J Clin Pharmacol, 83: 2077–2086. doi: 10.1111/bcp.13295.
References
- 1. Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SP, et al. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucl Acids Res 2016; 44 (Database Issue): D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alexander SPH, Cidlowski JA, Kelly E, Marrion N, Peters JA, Benson HE, et al. The concise Guide to PHARMACOLOGY 2015/16: nuclear hormone receptors. Br J Pharmacol 2015; 172: 5956–5978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chapman KR. The impact of budesonide and other inhaled corticosteroid therapies in the management of asthma in children and adults. Clin Ther 2003; 25 (Suppl. C): C2–14. [DOI] [PubMed] [Google Scholar]
- 4. Lipworth BJ. Systemic adverse effects of inhaled corticosteroid therapy: a systematic review and meta‐analysis. Arch Intern Med 1999; 159: 941–955. [DOI] [PubMed] [Google Scholar]
- 5. Ernst P, Suissa S. Systemic effects of inhaled corticosteroids. Curr Opin Pulm Med 2012; 18: 85–89. [DOI] [PubMed] [Google Scholar]
- 6. Pandya D, Puttanna A, Balagopal V. Systemic effects of inhaled corticosteroids: an overview. Open Respir Med J 2014; 8: 59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Suissa S, Kezouh A, Ernst P. Inhaled corticosteroids and the risks of diabetes onset and progression. Am J Med 2010; 123: 1001–1006. [DOI] [PubMed] [Google Scholar]
- 8. Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, et al., TORCH investigators . Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med 2007; 356: 775–789. [DOI] [PubMed] [Google Scholar]
- 9. Ernst P, Gonzalez AV, Brassard P, Suissa S. Inhaled corticosteroid use in chronic obstructive pulmonary disease and the risk of hospitalization for pneumonia. Am J Respir Crit Care Med 2007; 176: 162–166. [DOI] [PubMed] [Google Scholar]
- 10. Suissa S, Patenaude V, Lapi F, Ernst P. Inhaled corticosteroids in COPD and the risk of serious pneumonia. Thorax 2013; 68: 1029–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. O'Byrne PM, Pedersen S, Carlsson LG, Radner F, Thoren A, Peterson S, et al. Risks of pneumonia in patients with asthma taking inhaled corticosteroids. Am J Respir Crit Care Med 2011; 183: 589–595. [DOI] [PubMed] [Google Scholar]
- 12. Zhang L, Prietsch SO, Mendes AP, Von Groll A, Rocha GP, Carrion L, et al. Inhaled corticosteroids increase the risk of oropharyngeal colonization by Streptococcus pneumoniae in children with asthma. Respirology 2013; 18: 272–277. [DOI] [PubMed] [Google Scholar]
- 13. McKeever T, Harrison TW, Hubbard R, Shaw D. Inhaled corticosteroids and the risk of pneumonia in people with asthma: a case‐control study. Chest 2013; 144: 1788–1794. [DOI] [PubMed] [Google Scholar]
- 14. Pomey MP, Forest PG, Palley HA, Martin E. Public/private partnerships for prescription drug coverage: policy formulation and outcomes in Quebec's universal drug insurance program, with comparisons to the Medicare prescription drug program in the United States. Milbank Q 2007; 85: 469–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. RAMQ . In: Prescription drug insurance – eligibility, ed Maladie Rdla . Quebec, Canada: Gouvernement du Québec, 2014. [Google Scholar]
- 16. Blais L, Beauchesne MF, Levesque S. Socioeconomic status and medication prescription patterns in pediatric asthma in Canada. J Adolesc Health 2006; 38: 607 e9–607 16. [DOI] [PubMed] [Google Scholar]
- 17. Garbe E, Suissa S, LeLorier J. Association of inhaled corticosteroid use with cataract extraction in elderly patients. JAMA 1998; 280: 539–543. [DOI] [PubMed] [Google Scholar]
- 18. Garbe E, LeLorier J, Boivin JF, Suissa S. Inhaled and nasal glucocorticoids and the risks of ocular hypertension or open‐angle glaucoma. JAMA 1997; 277: 722–727. [PubMed] [Google Scholar]
- 19. Ernst P, Baltzan M, Deschenes J, Suissa S. Low‐dose inhaled and nasal corticosteroid use and the risk of cataracts. Eur Respir J 2006; 27: 1168–1174. [DOI] [PubMed] [Google Scholar]
- 20. Suissa S, Baltzan M, Kremer R, Ernst P. Inhaled and nasal corticosteroid use and the risk of fracture. Am J Respir Crit Care Med 2004; 169: 83–88. [DOI] [PubMed] [Google Scholar]
- 21. Brassard P, Vutcovici M, Ernst P, Patenaude V, Sewitch M, Suissa S, et al. Increased incidence of inflammatory bowel disease in Quebec residents with airway diseases. Eur Respir J 2015; 45: 962–968. [DOI] [PubMed] [Google Scholar]
- 22. Tamblyn R, Lavoie G, Petrella L, Monette J. The use of prescription claims databases in pharmacoepidemiological research: the accuracy and comprehensiveness of the prescription claims database in Quebec. J Clin Epidemiol 1995; 48: 999–1009. [DOI] [PubMed] [Google Scholar]
- 23. Blais L, Vilain A, Kettani FZ, Forget A, Lalonde G, Beauchesne MF, et al. Accuracy of the days' supply and the number of refills allowed recorded in Quebec prescription claims databases for inhaled corticosteroids. BMJ Open 2014; 4: e005903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Blais L, Lemiere C, Menzies D, Berbiche D. Validity of asthma diagnoses recorded in the medical services database of Quebec. Pharmacoepidemiol Drug Saf 2006; 15: 245–252. [DOI] [PubMed] [Google Scholar]
- 25. Lacasse Y, Montori VM, Lanthier C, Maltis F. The validity of diagnosing chronic obstructive pulmonary disease from a large administrative database. Can Respir J 2005; 12: 251–256. [DOI] [PubMed] [Google Scholar]
- 26. Suissa S. The quasi‐cohort approach in pharmacoepidemiology: upgrading the nested case‐control. Epidemiology 2015; 26: 242–246. [DOI] [PubMed] [Google Scholar]
- 27. Boulet LP, Becker A, Berube D, Beveridge R, Ernst P. Canadian asthma Consensus report, 1999. Canadian Asthma Consensus Group CMAJ 1999; 161 (11 Suppl): S1–61. [PMC free article] [PubMed] [Google Scholar]
- 28. National Heart Lung and Blood Institute . Guidelines for the diagnosis and management of asthma : expert panel report 2. Bethesda, MD: US Dept. of Health and Human Services, Public Health Service, National Heart, Lung, and Blood Institute, 1999. [Google Scholar]
- 29. Horwitz RI, Feinstein AR. The problem of ‘protopathic bias’ in case‐control studies. Am J Med 1980; 68: 255–258. [DOI] [PubMed] [Google Scholar]
- 30. Bansal V, Mangi MA, Johnson MM, Festic E. Inhaled corticosteroids and incident pneumonia in patients with asthma: systematic review and meta‐analysis. Acta Med Acad 2015; 44: 135–158. [DOI] [PubMed] [Google Scholar]
- 31. CDC . FastStats – pneumonia [online]. Center for Disease Prevention. Available at http://www.cdc.gov/nchs/fastats/pneumonia.htm (last accessed 15 May 2015).
- 32. Monso E, Ruiz J, Rosell A, Manterola J, Fiz J, Morera J, et al. Bacterial infection in chronic obstructive pulmonary disease. A study of stable and exacerbated outpatients using the protected specimen brush. Am J Respir Crit Care Med 1995; 152 (4 Pt 1): 1316–1320. [DOI] [PubMed] [Google Scholar]
- 33. Marin A, Monso E, Garcia‐Nunez M, Sauleda J, Noguera A, Pons J, et al. Variability and effects of bronchial colonisation in patients with moderate COPD. Eur Respir J 2010; 35: 295–302. [DOI] [PubMed] [Google Scholar]
- 34. Kew KM, Seniukovich A. Inhaled steroids and risk of pneumonia for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2014; (3): CD010115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Suissa S, Ernst P. Inhaled corticosteroids: impact on asthma morbidity and mortality. J Allergy Clin Immunol 2001; 107: 937–944. [DOI] [PubMed] [Google Scholar]
- 36. Suissa S, Ernst P, Benayoun S, Baltzan M, Cai B. Low‐dose inhaled corticosteroids and the prevention of death from asthma. N Engl J Med 2000; 343: 332–336. [DOI] [PubMed] [Google Scholar]
- 37. Suissa S, Ernst P, Kezouh A. Regular use of inhaled corticosteroids and the long term prevention of hospitalisation for asthma. Thorax 2002; 57: 880–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Meropol SB, Metlay JP. Accuracy of pneumonia hospital admissions in a primary care electronic medical record database. Pharmacoepidemiol Drug Saf 2012; 21: 659–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kern DM, Davis J, Williams SA, Tunceli O, Wu B, Hollis S, et al. Validation of an administrative claims‐based diagnostic code for pneumonia in a US‐based commercially insured COPD population. Int J Chron Obstruct Pulmon Dis 2015; 10: 1417–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cadieux G, Tamblyn R. Accuracy of physician billing claims for identifying acute respiratory infections in primary care. Health Serv Res 2008; 43: 2223–2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Talbot TR, Hartert TV, Mitchel E, Halasa NB, Arbogast PG, Poehling KA, et al. Asthma as a risk factor for invasive pneumococcal disease. N Engl J Med 2005; 352: 2082–2090. [DOI] [PubMed] [Google Scholar]


